Abstract
The role of senescent cells has been implicated in various tissue dysfunction associated with aging, obesity, and other pathological conditions. Currently, most transgenic mouse models only target p16Ink4a-highly-expressing (p16high) cells. Here, we generated a p21-Cre mouse model, containing a p21 promoter driving inducible Cre, enabling us to examine p21Cip1-highly-expressing (p21high) cells, a previously unexplored cell population exhibiting several characteristics typical of senescent cells. By crossing p21-Cre mice with different floxed mice, we managed to monitor, sort, image, eliminate, or modulate p21high cells in vivo. We showed p21high cells can be induced by various conditions, and percentages of p21high cells varied from 1.5 to 10% across different tissues in 23-month-old mice. Intermittent clearance of p21high cells improved physical function in 23-month-old mice. Our study demonstrates that the p21-Cre mouse model is a valuable and powerful tool for studying p21high cells to further understand the biology of senescent cells.
Editor summary:
Wang et al, report a mouse model which can target p21-highly-expressing senescent cells. Using this model, they are able to monitor, sort, image, eliminate, or modulate these cells in vivo, which could be a valuable tool to study senescent cells.
Introduction
Cellular senescence is a cell fate characterized by essentially irreversible proliferative arrest1. A variety of stimuli, including DNA damage, dysfunctional telomeres, oncogenic proteins, fatty acids, reactive oxygen species (ROS), mitogens, and cytokines, can act alone or in combination to drive cells into the cellular senescence fate through pathways involving p16/Rb (retinoblastoma), p53/p21, and probably others2. These contribute to the widespread changes in gene expression that underlie senescence-associated growth arrest, the senescence-associated secretory phenotype (SASP), resistance to apoptosis, and changes in morphology2,3. In these respects, cellular senescence can be considered a state of major cellular programming in addition to differentiation, proliferation, or apoptosis. Intracellular autocrine loops reinforce progression to irreversible replicative arrest, heterochromatin formation, and initiation of the pro-inflammatory SASP over a span of days to weeks2,4.
Senescent cell burden increases in various tissues with aging5 and multiple chronic conditions6. Depending on specific tissues and varied pathological states, the percent of senescent cells can vary from 1-20%7–9. In spite of relatively small percentages, senescent cells cause substantial tissue dysfunction10. Senescent cells can elicit damage in both an autocrine and paracrine fashion. In addition to intracellular dysfunction induced by autocrine signaling, senescent cells can be “contagious” and induce cellular senescence and the SASP in nearby non-senescent cells9,11–14. Senescent cells can also directly impair function of healthy stem cells8. The paracrine signaling from senescent cells, possibly through the SASP, ROS, or other factors, amplifies damage within tissues, which might partially explain why small number of senescent cells can be so harmful.
Currently, the INK-ATTAC15 and p16-3MR16 mouse models are the two most commonly used transgenic models to investigate the role of senescent cells in vivo. Both models were designed using the p16 promoter to drive an inducible suicide gene, by which p16Ink4a-highly-expressing (p16high cells) can be eliminated in vivo. By leveraging these two models, the causal role of p16high cells has been suggested in a number of pathological conditions including osteoporosis17, metabolic dysfunction8,18, osteoarthritis19, neurodegenerative diseases20, cardiac dysfunction7, kidney dysfunction7, vasomotor dysfunction21, atherosclerosis22, liver steatosis23, pulmonary fibrosis24, stem cell dysfunction8,25, and lifespan reduction7. Recently, two more senescence-related transgenic mouse models were reported26,27, both of which had knock-in Cre inserted into the native p16 locus. Although valuable, all of these models only target p16high cells. Since not all p16high cells are senescent28,29, and not all senescent cells express high levels of p161, it has been increasingly perceived by the field that p16 might not be a fully sensitive or specific marker for senescent cells. Models that target other cellular senescence markers are needed to expand our understanding of the underlying complexity and heterogeneity of senescence biology especially when studied in vivo.
p16 and p21Cip1 (p21) are two major regulators, as well as being the two most recognized and used cellular markers for senescent cells30. However, unlike p16high cells, the role of p21-highly-expressing (p21high) cells in vivo remains largely unknown at this time. In this study, we generated a p21-Cre transgenic mouse model containing a p21 promoter driving an inducible Cre, allowing us to investigate p21high cells in vivo. By crossing this model with floxed mouse models, we were able to monitor, image, sort, eliminate, and modulate p21high cells in vivo. The p21-Cre mouse model holds great promise as a valuable and potentially powerful tool for examining the role and underlying mechanisms of p21high cells in aging and in various diseased conditions associated with cellular senescence.
Results
p16high and p21high cells are two distinct cell populations.
Senescent cells are highly heterogeneous in their biological properties, tissue distribution and responses to varied therapies31. Although p16 and p21 have been widely used as two major cellular markers for senescent cells, very little is known about possible overlap of p16high cells and p21high cells or their respective population diversity. To explore this, we leveraged a single cell transcriptomic (SCT) atlas database, Tabula Muris Senis32, which includes transcriptomic data from a range of tissues in 18-30 months old mice. We visualized the p21 and p16 expression levels using the browser based interactive platform (https://tabula-muris-senis.ds.czbiohub.org/). In aged visceral fat, p21high cells are mainly endothelial cells, mesenchymal stem cells, and myeloid cells, while p16high cells are scarce. In liver, p21high cells are mainly Kupffer cells, and myeloid cells, while p16high cells, are mainly Natural Killer (NK) cells and a different population of Kupffer cells. In heart, p21high cells are mainly endothelial cells, while p16high cells are mainly leukocytes (Extended Data Fig.1). Thus, p16high cells and p21high cells are indeed two distinct populations, at least in aged tissues. These findings emphasize the need for models targeting p21high cells.
Generation of p21-Cre mouse model.
To generate the p21-Cre mouse model, we synthesized a 7 kb DNA fragment (Fig.1a) containing a 3225 bp mouse p21 promoter fragment followed by a bicistronic message consisting of the coding sequence for Cre recombinase (Cre) fused to a tamoxifen-inducible estrogen receptor (ERT2) domain33. Cre-ERT2 is normally retained in the cytoplasm (inactive) in the absence of an inducer. Upon addition of tamoxifen or 4-hydroxytamoxifen (4-OHT), the Cre-ERT2 translocates to the nucleus where it acts preferentially on loxP sites. An internal ribosome entry site (IRES) followed by an open reading frame (ORF) coding for enhanced green fluorescent protein (GFP) was also added to facilitate detection of p21high cells. The 3225 bp mouse p21 promoter fragment is well conserved between human and mouse, and contains 3 p53-binding sites responsive to DNA damage34. Integrase-mediated transgenesis (IMT)35 was used to generate the p21-Cre transgenic mice. The synthesized fragment (p21-Cre) was first subcloned into the vector pBT378 containing 2 attB sites, then was microinjected into the pronucleus of recipient zygotes containing attP sites in the Hipp11 (H11, chromosome 11) locus, which has a high recombination rate and results in stable expression of a single copy of the transgene36. Site-specific recombination occurred between the attB sites in the construct and attP sites in the H11 genomic locus, leading to insertion of p21-Cre transgene into the H11 locus.
Figure 1. Generation of p21-Cre mouse model.
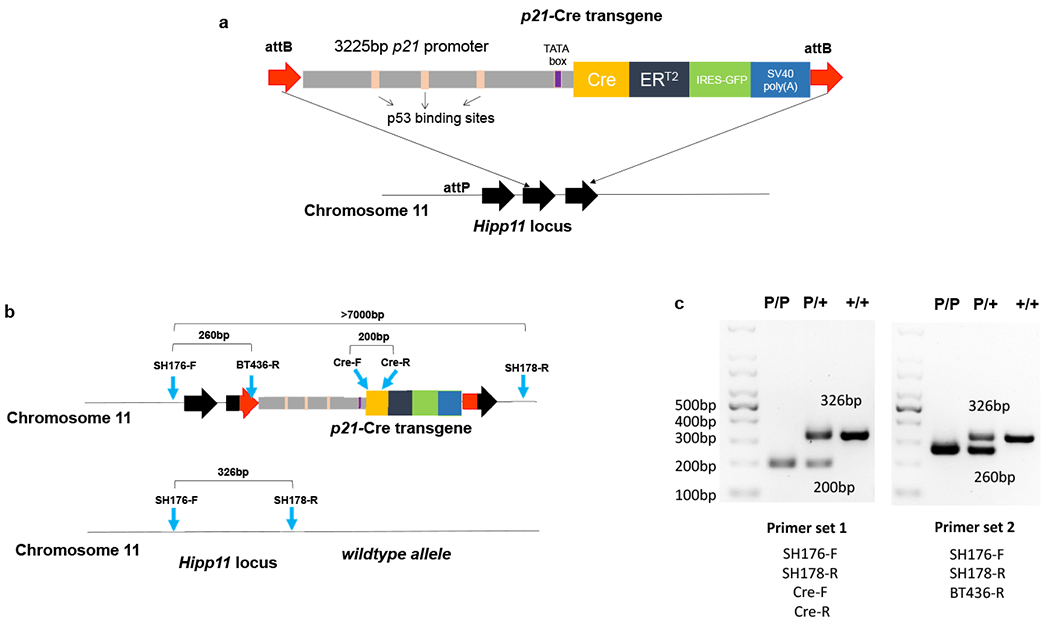
(a) Schematic of p21-Cre transgene. (b) Schematic of genotyping primers. (c) PCR results for two sets of genotyping primers. Set 1 was repeated in more than 2,000 mice. Set 2 was repeated in ~100 mice.
One advantage of this site-specific transgenic approach using the the H11 locus is that it is far less likely to interfere or disrupt any endogenous gene as opposed to the use of random insertion or insertion into targeting gene locus. In addition, this approach allows us to design genotyping primers to distinguish +/+, p21-Cre/+, and p21-Cre/p21-Cre genotypes (Fig.1b). As shown in Fig.1c, we validated two different sets of genotyping primers. For set 1, SH176-F and SH178-R generated a 326bp band for the wildtype (WT) allele, while Cre-F and Cre-R generated a 200bp band for the p21-Cre transgene allele. For set 2, SH176-F and SH178-R generated a 326bp band for the WT allele while SH176-F and BT436-R generated a 260bp band for the p21-Cre transgene allele.
Whole-body live imaging of p21high cells in vivo.
To validate function of the transgene, we first crossed the p21-Cre mouse with floxed knock-in firefly luciferase (LUC) mice37. Floxed knock-in LUC mice contain a loxP-flanked STOP fragment between the Gt(ROSA)26Sor (ROSA) promoter and LUC, which prevents LUC expression without presence of Cre (Fig.2a). We generated p21-Cre/+; LUC/+ (PL) mice (Fig.2a), which contain one copy of p21-Cre in chromosome 11 and one copy of LUC in chromosome 6. This PL mouse model allows us to detect p21high cells in live mice through bioluminescence imaging (BLI) in a temporal manner. Doxorubicin (DOXO) is a potent DNA damaging agent, which can induce p21 expression38 and accumulation of p21high cells. We treated PL mice with DOXO and tested whether we could detect p21high cells in vivo. We observed little signal in either the DOXO- or PBS-treated groups without tamoxifen treatment (Fig.2b), indicating that Cre or STOP fragment leakage is minimal. After 2 tamoxifen treatments to induce Cre and subsequent LUC activity in p21high cells, DOXO-treated PL mice had more BLI signal compared to PBS-treated ones (Fig.2b), suggesting the transgene works properly. Metabolic stress and obesity are also known to induce cellular senescence13 and p21 expression39. We next examined whether we can detect p21high cells in PL mice under metabolic stress. We fed PL mice with a high fat diet (HFD) for 4 months, and then treated these mice with 2 doses of tamoxifen. We found HFD significantly increased BLI signals in PL mice when compared to regular chow diet (RCD), indicating accumulation of p21high cells with obesity (Fig.2c). Moreover, we examined whether p21high cells are induced with aging. We treated 3-month-old (young) and 23-month-old (old) PL mice with 2 doses of tamoxifen, and found that BLI signals were significantly higher in old PL mice than young mice (Fig.2d). Thus, all 3 conditions (chemotherapy drugs, obesity, and aging) can induce p21high cells, which can be monitored by BLI in live mice.
Figure 2. Whole-body live imaging of p21high cells in vivo using bioluminescence imaging.
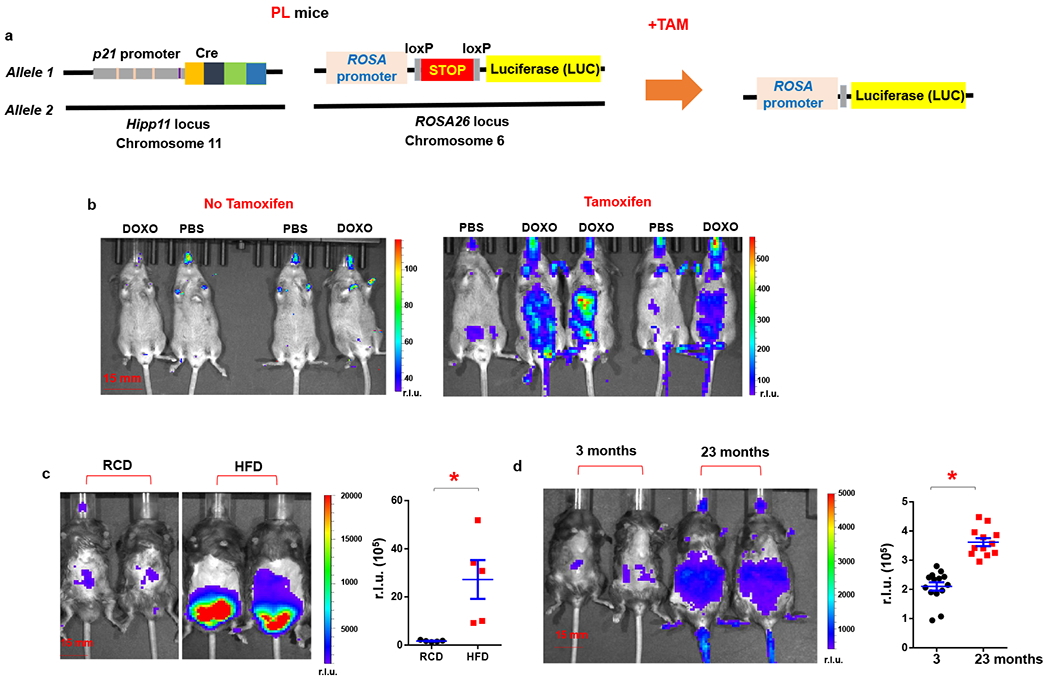
(a) Schematic of PL mice. (b) Representative images of LUC activity in DOXO or PBS treated PL mice (both male and female). Mice were given a single dose of DOXO (20 mg/kg) or PBS. Tamoxifen (2 mg/mouse) was given immediately after DOXO treatment and 16 h later, total 2 doses. BLI was performed 24 h after the DOXO administration. r.l.u., relative luciferase units. (c) Representative images of LUC activity in RCD or HFD-fed PL male mice, and quantification of LUC activity. n=5 for both groups. * p=0.013 (d) Representative images of LUC activity in young and old PL mice (both male and female), and quantification of LUC activity. n=14 for young and n=12 for old mice. Results are shown as means ± s.e.m. * p< 0.001; two-tailed, unpaired Student’s t-test.
p21high cells accumulate in various tissues with aging.
To image p21high cells using fluorescence in vivo at the tissue level, we crossed the p21-Cre mouse with floxed knock-in tdTomato mice40, which contain a loxP-flanked STOP fragment between the CMV early enhancer/chicken β actin (CAG) promoter and tdTomato (Fig.3a). We generated p21-Cre/+; tdTomato/+ (PT) mice (Fig.3a). Compared to the GFP included in the transgene (driven by p21 promoter), tdTomato (driven by the strong CAG promoter) generates a much brighter red fluorescent signal with less confounding by an autofluorescent background, which makes it highly suitable for in vivo fluorescence imaging. We first examined the p21 protein level in tdTomato+ cells and tdTomato− cells. We found tdTomato+ cells had more p21+ cells and higher p21 protein level than tdTomato− cells, indicating the enrichment of p21 expression in tdTomato+ cells (Extended Data Fig.2a–c). We then treated young (3-month-old) and old (23-month-old) PT mice with 2 doses of tamoxifen and collected a number of tissues for fluorescence imaging. Most of the tissues from old PT mice contained more tdTomato+ p21high cells, including visceral fat, brain, intestine, heart, liver, and skeletal muscle (Fig.3b). The percentages of p21high cells in these old tissues ranged from 1.5 to 10%, comparable to the percent of senescent cells in aged tissues. No reliably tdTomato+ cells were seen in aged kidneys due to high autofluorescence, and very few tdTomato+ cells were observed in the aged lung. Importantly, very few tdTomato+ cells were seen in all the tissues we collected from young and healthy PT mice, indicating that our p21-Cre mouse model might only target age- or disease-specific cells such as senescent cells without affecting most cells in young mice. p21high cells can also be detected by flow cytometry. Consistent with the fluorescence imaging, flow cytometry analysis revealed that the tdTomato+ p21high cells% in visceral fat and liver was higher in old mice than young PT mice (Fig.4a). We also carried out flow cytometry analysis using GFP and found similar results (Fig.4b), indicating that both tdTomato and GFP can be used to detect p21high cells using flow cytometry. While using BLI to detect p21high cells in dissected tissues from old mice, p21high cells were mainly found in visceral fat (Extended Data Fig.3), indicating the better sensitivity of fluorescence to detect p21high cells. In addition to aging, both DOXO treatment and HFD induced tdTomato+ p21high cells accumulation in visceral fat (Figs.4c and 4d) similarly to the findings using BLI (Figs.2b and 2c). Notably, p21-Cre is only activated (indicated by tdTomato) in a very small percentage (~0.1%) of DOXO-treated mouse embryonic fibroblasts (MEFs) with 2 copies of p21-Cre transgene, and the p21-Cre activity is higher in MEFs with 2 copies of transgene compared to 1 copy (Extended Data Fig.4). These findings indicate that the transgene is indeed working in vitro, and the low activity is likely due to low p21 promoter activity in cells in vitro. Altogether, these results suggest that p21high cells specifically accumulate in various tissues with aging or other conditions and can be precisely detected in our mouse models.
Figure 3. p21high cells accumulate in various tissues with aging.
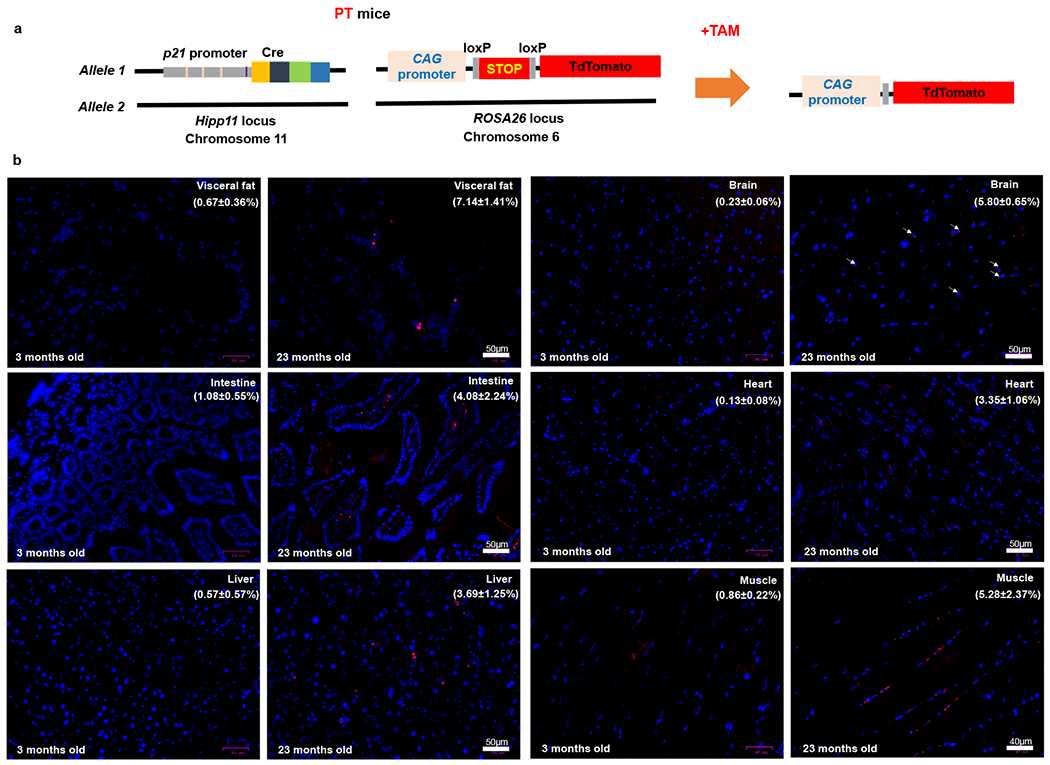
(a) Schematic of PT mice. (b) Representative micrographs of 6 tissues in young and old PT (male and female) mice. Red: tdTomato, Blue: DAPI. The percentages of tdTomato+ cells are shown as means ± s.e.m. Experiments were repeated in 3 mice.
Figure 4. p21high cells can be induced by aging, chemotherapy, and obesity.
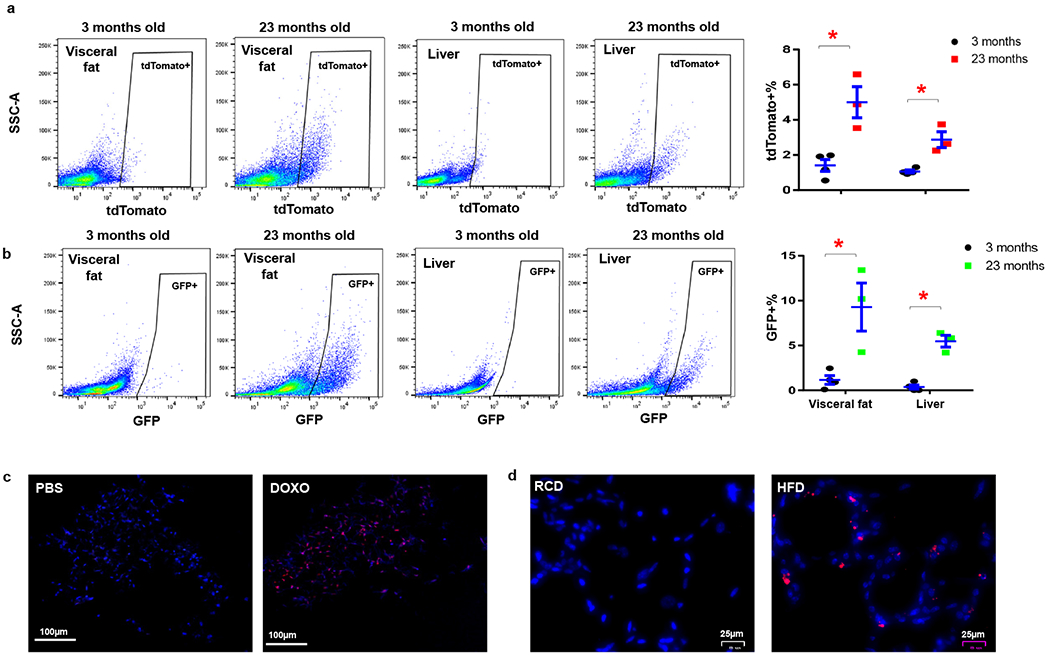
(a) Flow cytometry analysis and proportion of tdTomato+ p21high cells in 3-month-old (n=4) and 23-month-old (n=3) PT mice. * p=0.008 for fat, p=0.005 for liver; (b) Flow cytometry analysis and proportion of GFP+ p21high cells in 3-month-old (n=4) and 23-month-old (n=3) PT mice. Results are shown as means ± s.e.m. * p=0.017 for fat, p<0.001 for liver; two-tailed, unpaired Student’s t-test. (c) Representative micrographs of visceral fat in 3-month-old PT mice treated with PBS or DOXO. Red: tdTomato, Blue: DAPI. Experiments were repeated in 3 mice. (d) Representative micrographs of visceral fat in 5-month-old PT mice fed with RCD or HFD for 2 months. Red: tdTomato, Blue: DAPI. Experiments were repeated in 4 mice.
p21high cells exhibit features of cellular senescence.
In a previous study, we showed that p21high adipose-derived mesenchymal stem cells (ADSCs) accumulate in aged mice41. By single cell transcriptomics, these naturally occurring p21high cells appear to exhibit altered pathways commonly observed in senescent cells, including Senescent Cell Anti-Apoptotic Pathways (SCAPs; increased cell survival and decreased apoptosis) and NF-κB, IL6/JAK, mTOR, FOXO, and HMGB1 pathways41. Here, we further characterized p21high cells using our p21-Cre mouse model in vivo. We fed PL mice a HFD for 5 months to induce p21high cells in visceral fat (Fig.4d), isolated the stromal vascular fraction (SVF) from fat, and separated GFP+ p21high cells and GFP- non- p21high cells using fluorescence-activated cell sorting (FACS). Compared to GFP- cells, GFP+ p21high cells express 4-fold higher levels of p21 as well as 6-fold and 4-fold higher level of two major SASP components, Il6 and Cxcl1. Notably, p16 mRNA levels are not statistically different between these 2 cell populations (Fig.5a). We also leveraged imaging flow cytometry (ImageStreamX) technology, which combines flow cytometry with high content image analysis, to further characterize these GFP+ p21high cells at the single cell level. Enlarged size, senescence-associated-β-galactosidase (SA-β-gal), and proliferation arrest are 3 major features of senescent cells both in vitro and in vivo30. By analyzing the bright field (BF) images, GFP+ cells exhibit a larger cell size compared to GFP- cells (Fig.5b). We next stained SVF with SA-β-gal staining buffer and quantified SA-β-gal+ cells using a newly published method42. More than 60% of GFP+ cells were SA-β-gal+ compared to 40% in GFP- cells (Fig.5c). We also injected a thymidine analog, 5-ethyl-2′-deoxyuridine (EdU), into the mice, and assessed the incorporation of EdU using imaging flow cytometry 20 hours later. GFP- cells had more EdU+ cells (6%) than GFP+ cells (2%; Fig.5d), suggesting GFP+ cells had reduced proliferation rates. Loss of Lamin B1 is another commonly used marker for senescent cells43. By flow cytometry analysis, we found most GFP- cells (97%) expressed Lamin B1, while Lamin B1 was absent in 43% of GFP+ cells (Fig.5e). Altogether, p21high cells exhibited several characteristics typical of senescent cells, including higher expression of p21, the SASP, enlarged size, SA-β-gal-positivity, reduced proliferation, loss of Lamin B1, and a number of altered senescence-associated pathways.
Figure 5. p21high cells exhibit features of cellular senescence.
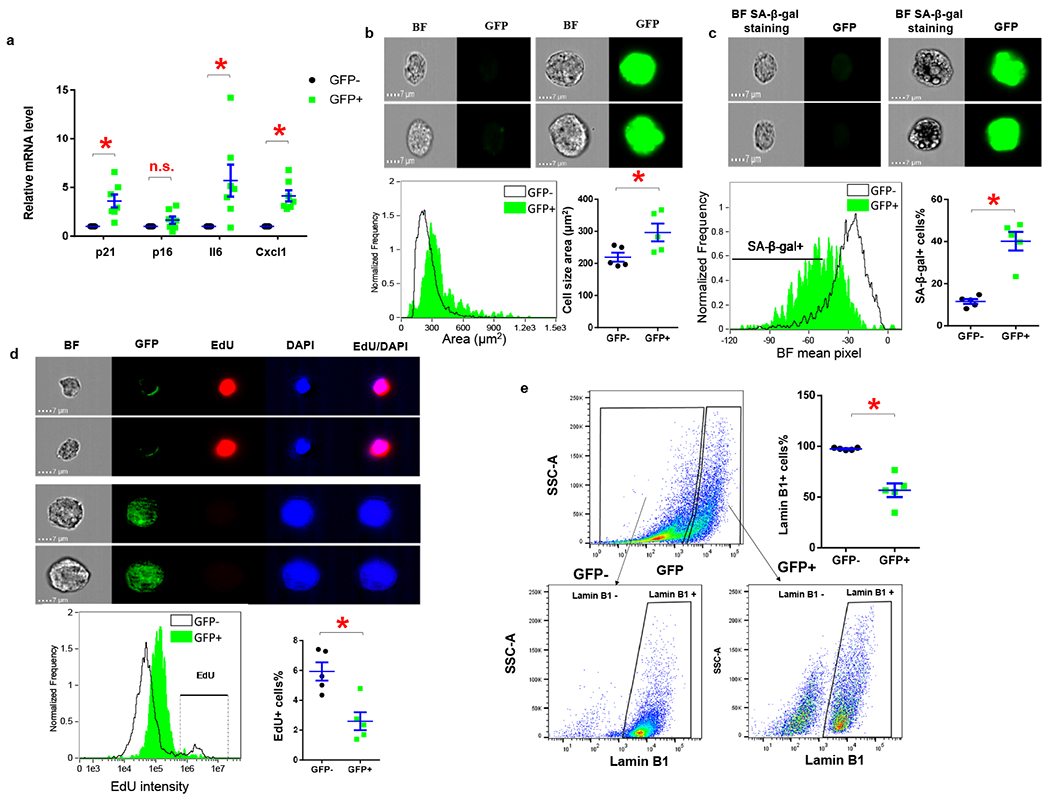
(a) Relative mRNA expression in GFP+ p21high cells and GFP- cells sorted from SVF from obese PL mice. * p< 0.05; two-tailed, paired Student’s t-test. p=0.008 for p21; p=0.1365 for p16; p=0.029 for Il6; p=0.002 for Cxcl1. (b) Representative images, cell size distribution and average cell size of GFP+ and GFP- cells. * p=0.006; two-tailed, paired Student’s t-test. (c) Representative images, mean SA-β-gal staining pixel distribution, and SA-β-gal+ cells as a percentage of GFP+ and GFP- cells. * p=0.003; two-tailed, paired Student’s t-test. (d) Representative images, mean EdU staining intensity distribution, and EdU+ cells percentage of GFP+ and GFP- cells. * p=0.019; two-tailed, paired Student’s t-test. (e) Flow cytometry analysis of Lamin B1 in GFP+ and GFP- cells. For A, n=7 for both groups. For b-e, n=5 for both groups. Results are shown as means ± s.e.m. * p=0.004; two-tailed, paired Student’s t-test.
Inducible elimination of p21high cells.
To enable elimination of p21high cells in a temporal manner in vivo, we crossed PL mice with floxed diphtheria toxin A (DTA) mice44, which contain a floxed-STOP cassette followed by DTA driven by the ROSA promoter (Fig.6a). We generated p21-Cre/+; LUC/DTA (PLD) mice (Fig.6a), which contain one copy of p21-Cre at the Hipp11 locus, one copy of LUC at the ROSA locus, and one copy of DTA at the ROSA locus. Diphtheria toxin has two subunits, A and B. Subunit B is responsible for binding the receptor and internalization of DTA. Once inside cells, DTA inhibits eukaryotic translation elongation factor 2 (eEF2), leading to protein translation block and ensuing apoptosis45. Since PLD mice have only DTA without subunit B, it is theoretically unlikely that DTA leakage from p21high cells (if any) could enter nearby non-p21high cells and kill them. Thus, the PLD mice allow us to specifically kill (by DTA) as well as monitor p21high cells (by LUC) in vivo. To validate clearance, we fed both PL and PLD mice a HFD for 4 months. After 2 doses of tamoxifen treatment, HFD-fed PL mice had high BLI signals, while the BLI signals were much lower in PLD mice (Fig.6b), demonstrating successful clearance of p21high cells from obese mice. Two doses of tamoxifen treatment also reduced BLI signal in 23-month-old PLD mice compared to PL mice (Fig.6c), suggesting that p21high cells can be eliminated using our model with aging as well.
Figure 6. Inducible elimination of p21high cells using diphtheria toxin A.
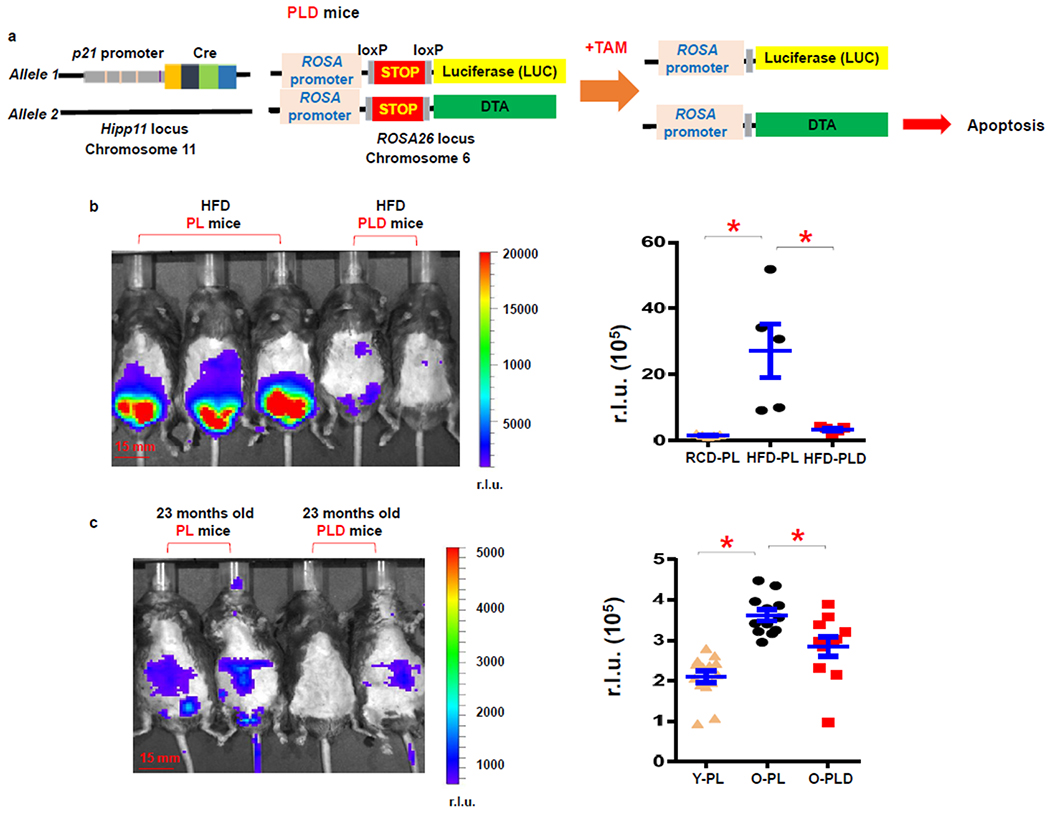
(a) Schematic of PLD mice. (b) Representative images and quantification of LUC activity in PL and PLD male mice fed with a HFD. n=5 for all groups. * p< 0.05; two-tailed, unpaired Student’s t-test. p=0.013 for RCD-PL vs. HFD-PL; p=0.018 for HFD-PL vs. HFD-PLD. (c) Representative images and quantification of LUC activity in 23-month-old PL and PLD mice (both male and female). n=14 for Y-PL, n= 12 for O-PL, and n=11 for O-PLD mice. Y, young; O, old. Results are shown as means ± s.e.m. * p< 0.05; two-tailed, unpaired Student’s t-test. p<0.001 for Y-PL vs. O-PL; p=0.009 for O-PL vs. O-PLD.
Genetic inhibition of the SASP in p21high cells in vivo.
The role of senescent cells has been extensively examined across a range of pathological conditions, while the underlying mechanisms have rarely been investigated in vivo. The SASP has long been speculated as one of the major mechanisms responsible for the harmful effects of senescent cells2. Here, we leveraged our p21-Cre mouse model to suppress the SASP exclusively in p21high cells. The NF-κB pathway serves as a master regulator of the SASP46, and Rela (v-rel reticuloendotheliosis viral oncogene homolog A, or p65) is a crucial subunit for NF-κB activation47. Both Rela and the NF-κB pathway are highly activated in p21high preadipocytes isolated from aged mice41. To inactivate the NF-κB pathway in p21high cells, we crossed p21-Cre mice with floxed Rela mice48, in which exon 1 of the Rela gene is flanked by loxP sites (Fig.7a). We generated p21Cre/+; Relafl/fl (P-Rela) mice, which contain one copy of p21-Cre and two copies of floxed Rela and +/+; Relafl/fl (Rela) mice, which only have two copies of floxed Rela (Fig.7b). We fed these mice a HFD for 3 months to induce p21high cells. SVF cells were collected from visceral fat after 2 doses of tamoxifen administration to allow Rela mutation detection. We designed primers that generated a 164bp Rela mutant band and a 160bp WT band. Rela mutant bands were detected only in P-Rela SVF cells, while WT bands were detected in both P-Rela and Rela SVF cells (Fig.7c). The Rela mutation was further confirmed by sequencing the Rela mutant bands. In addition, Rela mutant bands were much fainter than WT bands, and strong WT bands were also observed in P-Rela SVF cells, indicating that only a small percent of P-Rela SVF cells (p21high cells) had the Rela mutation. We next interrogated whether the Rela mutation lead to SASP inhibition. We crossed CAG-Cre mice49, which carry a constitutively active CAG promoter driving Cre, with floxed Rela mice to generate CAG-Cre/+; Relafl/fl (CAG-Rela) mice. We isolated ear fibroblasts from these mice and induced senescence using DOXO. After 4-OHT treatment to induce Cre, expression levels of Rela and several key SASP components were reduced by 40-70% in senescent CAG-Rela cells compared to senescent WT cells (Fig.7d). Thus, the SASP of senescent cells can be genetically suppressed in our models.
Figure 7. Genetic inhibition of SASP in p21high cells in vivo.
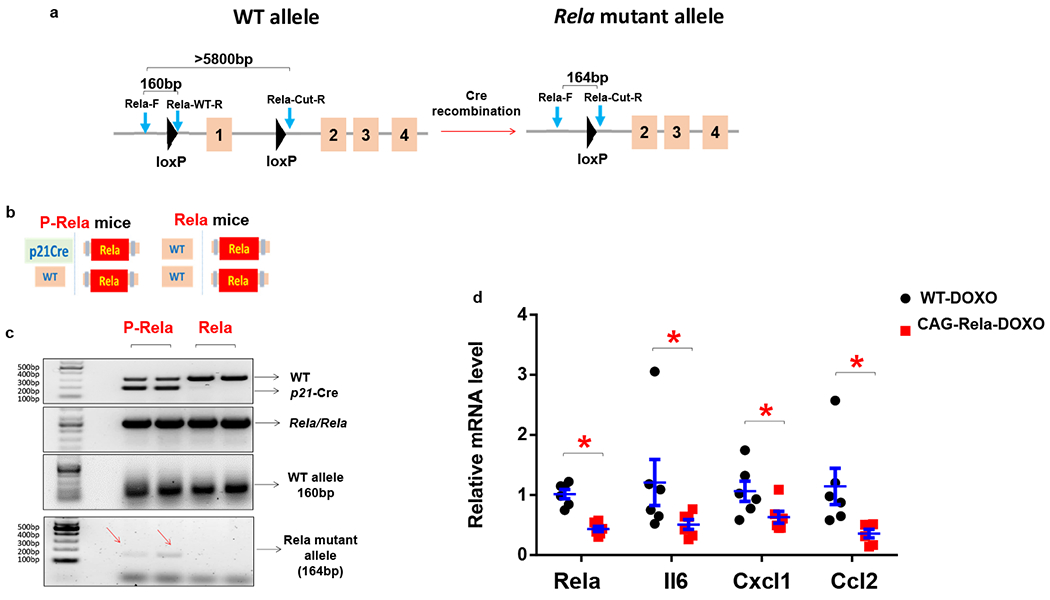
(a) Schematic of floxed Rela mice. (b) Schematic of P-Rela and Rela mice. (c) PCR results using Rela-F, Rela-WT-R, Rela-Cut-R primers for SVF from P-Rela and Rela mice. Experiments were repeated in 3 mice. (d) Relative mRNA expression in senescent WT and CAG-Rela ear fibroblasts. n=6 for both groups. Results are shown as means ± s.e.m. * p< 0.05; two-tailed, unpaired Student’s t-test. p<0.001 for Rela; p=0.037 for Il6; p=0.033 for Cxcl1; p=0.005 for Ccl2.
Clearance of p21high cells improves physical function.
Physical function declines with aging, leading to physical frailty, compromised systemic homeostasis, and increased vulnerability to stresses50. We next investigated whether p21high cells play a causal role in physical frailty with aging. We treated 20-month-old PL and PLD mice with 2 doses of tamoxifen per month for 3 months (Fig.8a) to eliminate p21high cells in PLD mice (Fig.6c). We assessed physical frailty following the frailty criteria widely used in clinic practice51,52, including weight loss, walking speed, grip strength, physical endurance, food intake, and daily activity. No difference was observed in body weight, maximal walking speed, or grip strength between 20-month-old PL and PLD mice before tamoxifen treatment. After 3 months of intermittent tamoxifen administration (total 6 doses), maximal walking speed, grip strength, hanging endurance, daily food intake, and daily activity were all significantly higher in PLD mice than PL mice, while body weight changes were not statistically different (Fig.8b–g). These results demonstrate that clearance of p21high cells alleviate physical frailty in old mice.
Figure 8. Clearance of p21high cells improves physical function in old mice.
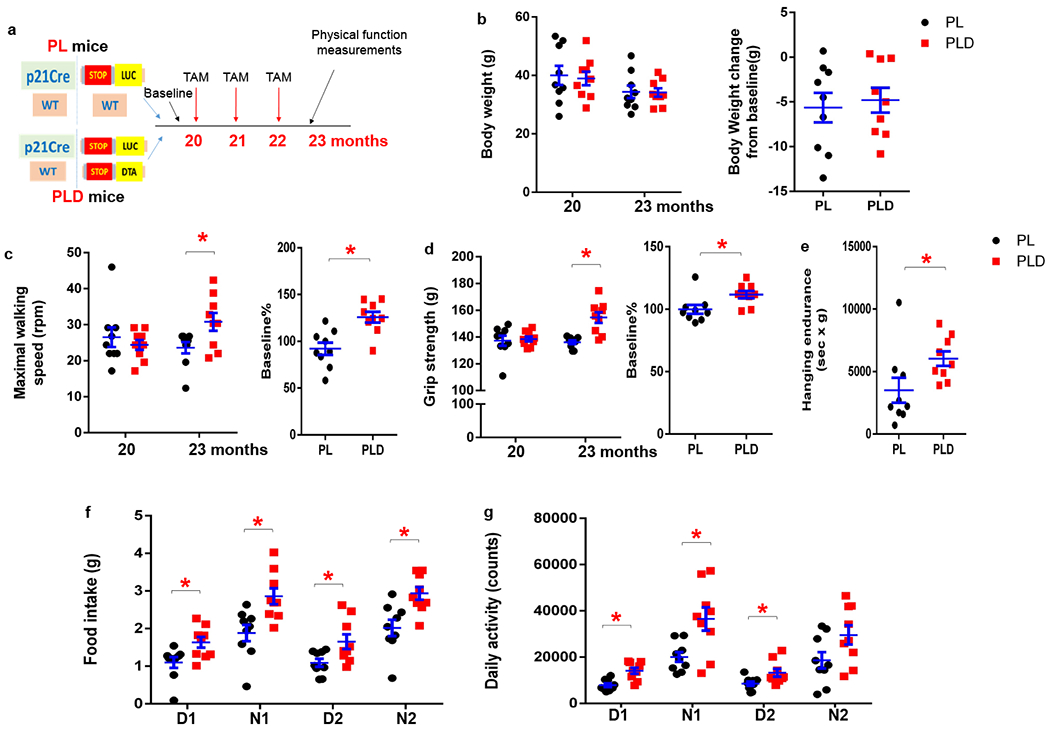
(a) Experimental design. (b) Bodyweight, (c) Maximal walking speed, and (d) Grip strength was measured in PL and PLD mice (both male and female) at age 20 months (before tamoxifen treatment) and 23 months (after tamoxifen treatment, p=). (e) Hanging endurance, (f) Food intake, and (g) Daily activity was measured at age 23 months. n=9 for both groups. Results are shown as means ± s.e.m. * p< 0.05; two-tailed, unpaired Student’s t-test. For c, p=0.026 for 23 months; p=0.001 for baseline%. For d, p<0.001 for 23 months; p=0.020 for baseline%. For e, p=0.044. For f, p=0.017, 0.006, 0.022, 0.004 for D1, N1, D2, N2 respectively. For g, p=0.001, 0.001, 0.026, 0.062 for D1, N1, D2, N2 respectively.
Discussion
In this technical report, we present a mouse model that enables us to investigate the role and underlying mechanisms of p21high cells in various conditions, including obesity and aging. Senescent cells are highly heterogeneous, and no single marker has been shown to be sufficient to define senescent cells31. Up to now, to our knowledge, most mouse models in the field15,16,26,27 were designed to exclusively target p16high cells, limiting our perspective on senescence biology. Here, we characterized a previously unexplored senescent cell population, p21high cells. We found that p21high cells accumulate in tissues with aging and obesity, and that these cells exhibit a number of typical features of senescent cells. Moreover, single cell transcriptomic analysis reveals that p21high cells and p16high cells are two distinct cell populations in a number of aged tissues, suggesting that the role of p21high cells in various age-related conditions could be different from p16high cells. Thus, future investigations using this model focusing on p21high cells could provide key novel insights into the biology of aging, pathogenesis of chronic diseases of aging and the discovery of novel geroscience-guided interventions.
Both p21 and p16 are cell cycle regulators and are widely expressed across a range of cells and tissues at relatively low levels. It is possible that models leveraging these two markers for senescent cells might target some non-senescent cells, especially in the models using a knock-in strategy. Since senescent cells accumulate exponentially in aged mice (older than 20-month-old)53–55, it is critically important to precisely identify the cells targeted by these models in aged tissues, not just in young to middle-aged tissues. The p21-Cre mouse model presented here was made by introducing a transgene containing the p21 promoter driving Cre, which is likely to have a high threshold for Cre activation. Using PT mice, we found that percentages of p21high cells varied from 1.5 to 10% across a number of tissues in 23-month-old mice, which is consistent with the percent of senescent cells in aged mice42, while few tdTomato+ p21high cells (<1%) were observed in young tissues. Moreover, p21high cells targeted by this model showed typical features of senescent cells (Fig.5). Therefore, our p21-Cre mouse model seems to only target a small number of cells, which only accumulate with aging or other pathological conditions. Importantly, intermittent clearance of these cells was sufficient to improve age-associated physical dysfunction (Fig.8), consistent with findings using senolytic drugs13.
The p21-Cre mouse model offers various opportunities to study p21high cells in vivo. As demonstrated in this report, we can monitor, sort, image, eliminate, or modulate p21high cells by crossing this model with different floxed mice. Inducible Cre allows us to intermittently clear p21high cells in later life, in contrast to continuous clearance, and is more clinically relevant. Notably, our strategy of eliminating p21high cells is distinct from interference with p21 gene function. As a cyclin-dependent kinase inhibitor, p21 plays an essential role in a wide range of cellular events. Inactivation or suppression of the p21 gene in vivo is likely to have severe side effects, such as tumorigenesis56. Our strategy here is to leverage p21 as a marker to eliminate p21high cells. In addition, the p21-Cre transgene was specifically inserted into H11 locus, rather than the native p21 locus. Theoretically, this is less likely to affect function and regulation of the p21 gene. PL mice also offer a unique opportunity for us to assess p21high senescent cell burden in live mice. PL mice are distinct from p2138,57 or p1616,54 reporter mice, which are currently used for monitoring the load of senescent cells in vivo19,25,58. The expression of LUC from all these reporter mice is driven by either the p16 or p21 promoter. Since p16 and p21 expression levels may fluctuate and are highly variable across different cell types, the BLI signals observed in these reporter mice likely indicate the p16 or p21 promoter activity in vivo, rather than the absolute numbers of p16high or p21high cells. Our PL mouse model is mechanistically distinct. After a STOP fragment is excised by Cre in p21high cells in PL mice, expression of LUC in these cells is driven by Gt(ROSA)26Sor promoter, which is ubiquitously and stably active in most prenatal and postnatal cells and tissues59. Therefore, compared to p21 or p16 reporter mice, the BLI signals in PL mice more likely represent the number of p21high cells in vivo. This makes PL mice more suited for screening drugs that can kill p21high cells, because a reduction in BLI signals in PL mice indicates clearance of p21high cells, rather than inhibition of p21 promoter activity. This is important since drugs that reduce p21 promoter activity (thus interfere p21 gene function as a tumor suppressor) will likely initiate tumorigenesis56. Thus, these mice can be indispensable models for screening novel senolytic drugs to kill p21high cells in vivo and greatly accelerate next-generation senolytic drug development and senescence biomarker discovery.
The underlying mechanisms of how senescent cells cause tissue dysfunction are largely unknown in vivo. The SASP has been considered to be one major mechanism and a number of SASP inhibitors can improve healthspan in aged mice, including JAK inhibitors8,9,60, metformin61,62, and rapamycin63–65. However, it is difficult to distinguish effects of these inhibitors on senescent cells from other cells. The p21-Cre mouse model allows us to suppress the SASP only in p21high cells, thus is a cleaner system for examining the contribution of the SASP to age-related disorders. Moreover, using this tool, we can modulate specific SASP components or multiple genes of interest (any gene located outside chromosome 11, since the p21-Cre is located on chromosome 11) specifically in p21high cells, which will be valuable for the senescence field.
In summary, our study demonstrates that the p21-Cre mouse model is a valuable and powerful model for studying p21high cells and could initiate a new avenue of research to further understand the biology of senescent cells.
Methods
p21-Cre mouse model generation.
A 7 kb DNA fragment was synthesized by GenScript (Piscataway, NJ), containing a 3225 bp mouse p21 promoter fragment followed by a bicistronic message consisting of the coding sequence for Cre fused to a tamoxifen-inducible estrogen receptor (ERT2) domain. An internal ribosome entry site (IRES) followed by an open reading frame (ORF) coding for enhanced green fluorescent protein (EGFP) was also added into the transgene. The synthesized fragment (p21-ER-Cre) was subcloned into vector pBT378 for generating p21-Cre mice through Integrase-mediated transgenesis (IMT). Briefly, the p21-ER-Cre-pBT378 construct was microinjected into the pronucleus of recipient zygotes containing attP sites in the Hipp11 locus, which has a high recombination rate and results in stable expression of a single copy of the transgene (done at the Transgenic Research Center in Stanford University). Site-specific recombination occurred between the attB sites in the plasmid and attP sites in the H11 genomic locus. Positive embryonic stem (ES) cell clones were then implanted into C57BL/6 females. A positive founder mouse was confirmed by PCR using a set of primers flanking the recombined 5’- and 3’- attP/attB sites in the H11 locus, as well as sets of specific primers that amplify unique sequences within the transgene. The investigators are happy to share the p21-Cre mouse model with the research community. Please contact Dr. Ming Xu at mixu@uchc.edu to request the mouse model. The mice will be shipped after a Material Transfer Agreement (MTA) is approved by Mayo Clinic. For non-profit research purpose, the requestor will be only responsible for the shipping cost.
Mouse models and drug treatments.
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at UConn Health. All mice were maintained in a pathogen-free facility at 23–24 °C with humidity of 30-60% under a 12-h light, 12-h dark regimen with free access to a RCD (Teklad global 18% protein, Envigo #2918, Indianapolis, IN), or a 60% (by calories) HFD (D12492, irradiated; Research Diets, New Brunswick, NJ) and water. For tamoxifen treatment, tamoxifen (Sigma-Aldrich, St Louis, MO) was dissolved in corn oil, and was administrated to mice by intraperitoneal (i.p.) injection one dose (2 mg per mouse) daily for two consecutive days. For DOXO treatment, DOXO (Sigma-Aldrich) was given to mice (20 mg/kg) once by i.p. injection. Tamoxifen was given one dose at the same time of DOXO administration, and one more dose was given 16 h after. Floxed tdTomato mice (#007914), floxed LUC mice (#005125), floxed DTA mice (#009669), CAG-Cre mice (#004682), and Relafl/fl mice (#024342) were all purchased from the Jackson Laboratory.
Single cell RNA-seq analysis.
Single cell RNA-seq data was collected from the Tabula Muris Senis single cell transcriptomic atlas for aged mice32. Data were visualized using the provided browser based platform available at https://tabula-muris-senis.ds.czbiohub.org/. In brief, single cell RNA seq data was examined from visceral adipose tissue (FACS method), liver (droplet method), and heart (droplet method). To account for differences in animal ages available for each tissue, all available data from animals 18 months or older was included in our analysis (Fat: 18 and 24 months; Liver: 18, 21, 24, 30 months; Heart: 18, 21, 24, 30 months). Expression profiles were then captured for p21 and p16 in each tissue.
Tissue dissociation.
Visceral fat and liver tissues were minced with scissors, and digested in PBS containing 1 mg/ml type II collagenase (Sigma-Aldrich) and 10 μg/ml DNase I (Sigma-Aldrich) at 37°C for 1 h. After digestion, stromal vascular fraction (SVF) cells were separated from visceral fat. SVF cells and liver cells were washed with PBS/2% FBS and filtered through 100 μm cell strainer. Cells were then incubated with ACK lysing buffer (Thermo Fisher Scientific, Waltham, MA) at room temperature for 10 min to remove red blood cells. Cells were washed with PBS/2% FBS for further experiments.
SA-β-gal staining.
SA-β-gal staining was assayed as previously described9,42. Briefly, purified SVF cells were fixed in 4% paraformaldehyde (PFA, Thermo Fisher Scientific) for 15 min at room temperature. Cells were washed with PBS, then incubated with SA-β-gal staining solution containing 1 mg/ml X-gal (Teknova, Hollister, CA), 40 mM citric acid/sodium phosphate at pH 6.0, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl2 at 37 °C in a humidified chamber, and protected from light. After 16 h incubation, cells were washed in ice-cold PBS to stop the enzymatic reaction and resuspended in PBS buffer for ImageStreamX analysis.
EdU staining.
Mice fed HFD for 5 months were treated with 1 mg/kg 5-ethynyl-2′-deoxyuridine (EdU, Cayman Chemical, Ann Arbor, MI) via intraperitoneal injection. After 20 h, SVF cells were isolated and fixed in 4% PFA for 15 min at room temperature. After being washed with PBS/1% BSA, cells were permeabilized by 0.3% Triton X-100, and stained with 100 mM Tris-HCl (pH 7.5), 2 mM CuSO4, 10 mM ascorbic acid, 2 μM Alexa Fluor 647 azide (Thermo Fisher Scientific) for 30 min at room temperature. Cells were washed with PBS/1% BSA twice, incubated with 0.1 μg/ml DAPI (Sigma-Aldrich) for 5 min, and analyzed by ImageStreamX.
Imaging flow cytometry analysis.
SVF cells stained with SA-β-gal, EdU, or p21 antibody were imaged by ImageStreamX Mark II (Amnis, Seattle, WA) and analyzed by IDEAS 6.2 software (Amnis). To focus cells, samples were gated using gradient RMS values of brightfield channel. Cells were further gated using brightfield area and aspect ratio to select single cells. GFP- and GFP+ SVF cells were measured for cell areas using brightfield area. Mean pixel of brightfield was used to calculate SA-β-gal intensity in GFP- and GFP+ SVF cells as previously described42. Low passage wildtype mouse ear fibroblasts stained with SA-β-gal were used as control. Notably, SA-β-gal staining did not significantly affect the GFP signal in SVF cells from obese PL mice. To measure EdU, the intensity of Alexa Fluor 647 channel was compared between GFP- and GFP+ SVF cells. Wildtype young mice SVF cells with staining buffer but without EdU injection were used as control. tdTomato− and tdTomato+ SVF cells were measured for p21 expression using the intensity of Alexa Fluor 647 channel. The SVF cells from same old PT mice but without anti-p21 primary antibody were used as control for p21 expression.
Cell culture.
Mouse embryonic fibroblasts (MEFs) were isolated from embryos between embryonic day 13.5-14.5 of gestation with their head, tail, limbs and visceral organs removed. The embryo carcass was washed with PBS, minced thoroughly with scissors before being dissociated in 0.05% trypsin-EDTA (Thermo Fisher Scientific) at 37°C for 15 min. Then embryo tissue was pipetted up and down, and filtered through cell strainer. Single cells were seeded into culture plates in DMEM high glucose (Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS, Corning, Corning, NY) and 1% penicillin-streptomycin (Thermo Fisher Scientific). Cells were cultured at 37°C in a humidified 5% CO2 and 3% O2 incubator. To test the p21-Cre activity in vitro, MEFs were treated with 0.5 μM DOXO for 24 h and 0.5 μM 4-OHT (Sigma-Aldrich) for 3 days. Ear fibroblasts were isolated as described previously66. To induce senescence, ear fibroblasts were treated with 0.5 μM DOXO for 24 h, and considered to be senescent 10 days after.
Immunofluorescence staining.
SVF cells were fixed in 4% PFA for 15 min at room temperature. Cells were then washed with PBS prior to a 10 min incubation in permeabilization buffer (0.2% Triton X-100, 1% BSA in PBS). Next, the cells were washed with PBS and blocked for 1 h in PBS/1% BSA solution. After blocking, cells were incubated with anti-Lamin B1 primary antibody (1:100, 12987-1-AP, Proteintech, Rosemont, IL) or anti-p21 primary antibody (1:40, 14-6715-81, Thermo Fisher Scientific) in PBS/1% BSA overnight at 4 °C. The next day, cells were washed with PBS and incubated for 1 h at room temperature with Alexa Fluor 647-conjugated anti-rabbit secondary antibody (A-21244, Thermo Fisher Scientific) diluted 1:200 in PBS/1% BSA. Stained cells were then washed with PBS and analyzed by flow cytometry or ImageStreamX.
Flow cytometry analysis.
SVF cells and liver cells were isolated from visceral fat and liver tissues, and washed with PBS/2% FBS. Cells were stained with 0.1 μg/ml of DAPI for 5 min and detected by BD LSR II flow cytometer (BD Biosciences, San Jose, CA). Data analysis was performed on the FlowJo V10.7 software. SVF cells and liver cells from wildtype young mice were used as control for tdTomato and GFP expression. For Lamin B1 expression, the SVF cells from same obese PL mice but without anti-Lamin B1 primary antibody were used as control.
Fluorescence-activated cell sorting (FACS).
SVF cells were isolated from mice fed HFD for 5 months, cells were sorted into GFP- and GFP+ populations through BD FACSAria II flow cytometer (BD Biosciences). Sorted cells were used to detect p21, p16 and SASP expression.
Bioluminescence imaging.
Mice were anesthetized with isoflurane (Piramal Critical Care, Bethlehem, PA) gas and injected intraperitoneally with 3 mg D-luciferin (Gold Biotechnology, St. Louis, MO) in 200 μl PBS. 5 min after injection, mice were placed in IVIS spectrum in vivo imaging system (PerkinElmer, Waltham, MA) and bioluminescence images were subsequently captured with 3 min exposure time. Region of interest (ROI) was manually selected in a consistent way for individual mouse in same cohort. Bioluminescent signal of ROI was calculated using the Living Image 4.5.5 software (PerkinElmer). Bioluminescence images of brain, lung, heart, pancreas, spleen, kidney, liver, intestine, inguinal fat, visceral fat, and muscle tissues were also captured from young and old PL mice.
Histological analysis.
Visceral fat, liver, intestine, brain, heart, muscle tissues were fixed with 4% PFA overnight at 4°C. After being washed with PBS, tissues were then transferred to 30% sucrose (Sigma-Aldrich) overnight at 4°C, and embedded in OCT compound (Thermo Fisher Scientific). Liver, intestine, brain, heart, muscle tissues in OCT blocks were cut into 6 μm thickness sections on Leica CM3050 S cryostat, and visceral fat tissues were cut into 10 μm thickness sections. Nuclei were counterstained with Hoechst 33342 (Thermo Fisher Scientific) and imaged on a fluorescence microscope (Zeiss, Jena, Germany). To quantify tdTomato+ cells, three sections from each mouse were scanned and then counted using ImageJ (v1.51g) software.
Physical function measurements.
Physical function measurements were performed as previously described13. To test muscle strength and neuromuscular function, maximal walking speed, grip strength and grid hanging test were performed in aged mice. Maximal walking speed was tested by accelerating 4 lane RotaRod system (Columbus Instruments, Columbus, OH). Mice were trained on the RotaRod for consecutive 2 d at a constant speed of 4 r.p.m. for 300 s. The day after the last day of training, mice were acclimatized to the testing room for 30 min. RotaRod test was started at 4 r.p.m. and accelerated from 4 to 47.2 r.p.m. in 6 min. The speed was recorded when the mouse fell off, the average of 4 trials were calculated and normalized to the baseline speed. Forelimb grip strength (g) was assessed by a grip strength test meter (Bioseb, France). Results were averaged over 10 trials. Grid hanging test was performed on the grid which was placed onto a holding apparatus at a 35 cm distance from the floor, a soft pad was used to avoid injuries. Hanging time was recorded when the mouse fell off, the average of 3 trials were calculated and normalized to body weight as hanging duration (sec) × body weight (g). A threshold at 7 min was set to consider the maximum hanging time.
Comprehensive laboratory animal monitoring system (CLAMS).
Daily activity, food and water intake, metabolic performance, temperature were measured for the 23 months old PL and PLD mice on a CLAMS system equipped with Oxymax open-circuit calorimeter (Columbus Instruments, Columbus, OH). Physiological and behavioral parameters for individual mouse were monitored over a 24 h period (12 h light/dark cycle), and results were analyzed by CLAX software (Columbus Instruments).
DNA preparation and PCR.
Rela and P-Rela mice were sacrificed 24 h after tamoxifen injection for 3 doses. Genomic DNA was extracted by lysing the SVF cells in an alkaline reagent containing 25 mM NaOH and 0.2 mM EDTA at 94°C for 2 h and neutralizing with equal volume of 40 mM Tris-HCl (pH 7.4), followed by purifying with isopropanol and 75% ice-cold ethanol (Sigma-Aldrich). Equal amount of DNA for each sample was adopted to react with GoTaq Green Master Mix (Promega, Madison, WI) and indicated genotyping primers, respectively. PCR products were separated on a 2% agarose gel with SYBR safe DNA gel stain (Thermo Fisher Scientific) running in TAE buffer (Bio-Rad, Hercules, CA) and visualized by ChemiDoc MP imaging system (Bio-Rad). All primers were purchased from Integrated DNA Technologies (IDT, Coralville, IA). Primer sequences (5′ to 3′) are listed below.
Cre-F: ACCAGCCAGCTATCAACTCG;
Cre-R: TACATTGGTCCAGCCACC;
SH176-F: TGGAGGAGGACAAACTGGTCAC;
SH178-R: TTGTTCCCTTTCTGCTTCATCTTGC;
BT436-R: ATCAACTACCGCCACCTCGAC;
Rela-F: GACACGCTGAACTTGTGGCCGTTTA;
Rela-WT-R: TATCATGTCTGGATCAATTCATAAC;
Rela-Cut-R: TTTCGACCTGCAGCCAATAAGCT
RNA extraction and real-time PCR.
Cells were collected, lysed in TRIzol reagent (Thermo Fisher Scientific) and then extracted with chloroform, isopropanol and 75% ice cold ethanol (Sigma-Aldrich). RNA was dissolved in RNase free water, and reverse transcribed to cDNA with M-MLV reverse transcriptase kit (Thermo Fisher Scientific). Quantitative real-time PCR was conducted in four or five duplicates using PerfeCTa FastMix II (Quantabio, Beverly, MA) on CFX96 Real-Time PCR detection system (Bio-Rad). The relative mRNA level of target genes was normalized to TATA-binding protein (Tbp) and calculated via the 2−ΔΔCT method. Probes and primers for Tbp, p21, p16, Il6, Cxcl1, Ccl2 and Rela were purchased from Integrated DNA Technologies (IDT, Coralville, IA).
Statistics and Reproducibility
Sample sizes were determined using power analysis based on the means and variation of pilot experiments and previous publications9,13. No data was excluded. Mice were assigned to experimental groups based on their genotypes. Investigators were blinded to allocation during experiments and outcome assessments. Data was analyzed using Prism 7 (Graphpad Inc.).
Data Availability Statement
The source data are published with the manuscript and are available from the corresponding author upon reasonable request. The link for Tabula Muris Senis database is https://tabula-muris-senis.ds.czbiohub.org/.
Extended Data
Extended Data Fig. 1. p16high and p21high cells are two distinct cell populations in aged tissues.
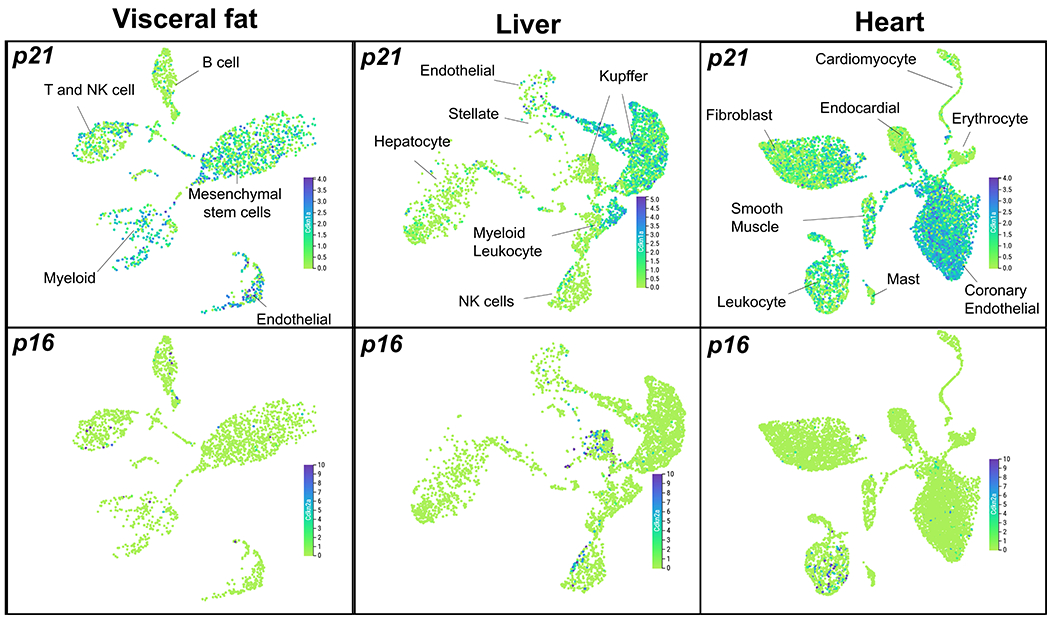
Uniform manifold approximation and projection (UMAP) plots showing expression levels of p21 (cdkn1a) and p16 (cdkn2a) in visceral fat, liver and heart in 18-30 months old mice. The figures were generated using the Tabula Muris Senis interactive platform (https://tabula-muris-senis.ds.czbiohub.org/).
Extended Data Fig. 2. p21 expression in tdTomato+ and tdTomato− cells in old mice.
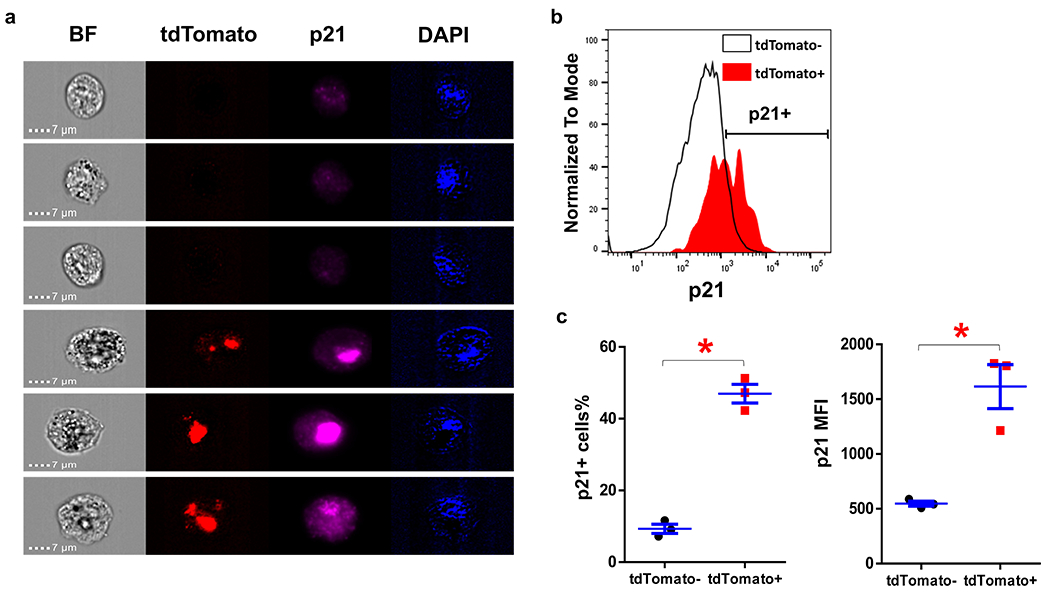
(a) Representative images of tdTomato+ and tdTomato− SVF cells in old PT mice. (b) p21 staining intensity of tdTomato+ and tdTomato− SVF cells detected by flow cytometry. (c) p21+ cells percentage and p21 mean fluorescence intensity (MFI) of tdTomato+ and tdTomato− SVF cells. For c, n=3 for both groups. Results were shown as mean ± s.e.m. * p< 0.05; two-tailed, paired Student’s t-test. p=0.009 for p21+ cells%; p=0.028 for p21 MFI.
Extended Data Fig. 3. Tissue distribution of p21high cells.
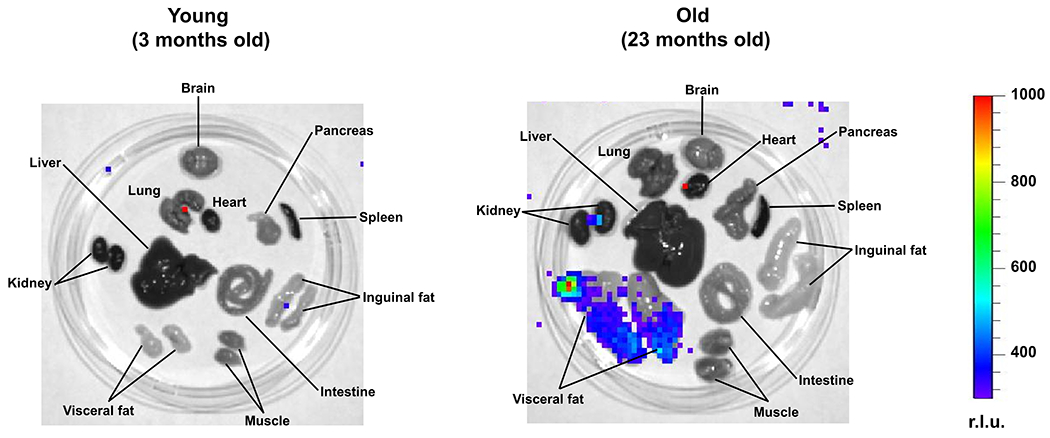
Representative images of LUC activity in various tissues from young and old mice. r.l.u., relative luciferase units.
Extended Data Fig. 4. The activity of p21-Cre is low in cells in vitro.
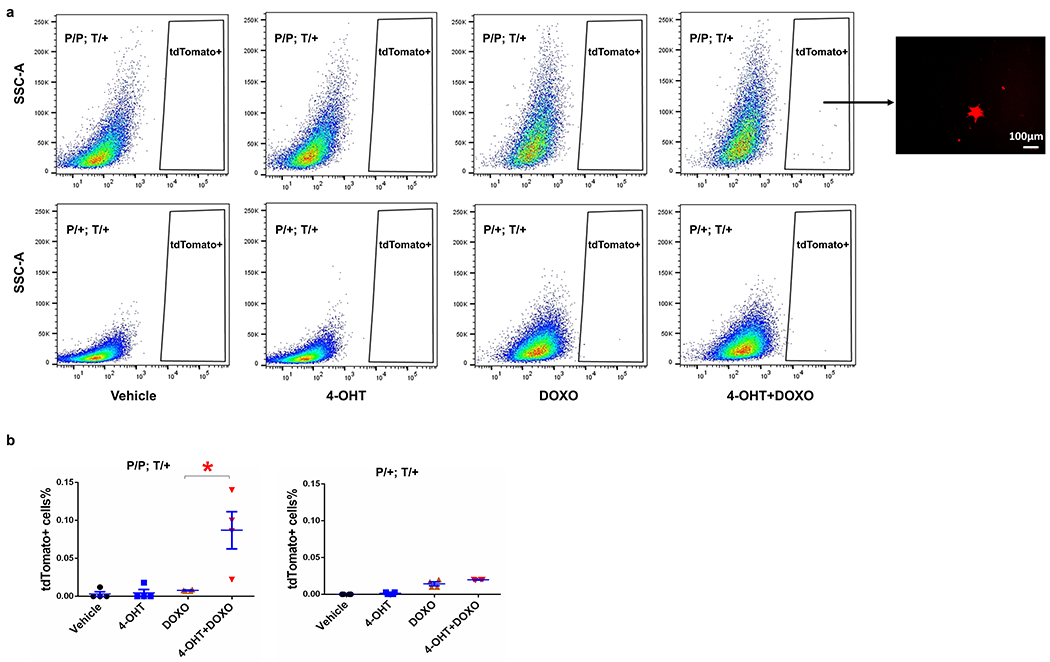
(a) Flow cytometry analysis of tdTomato (T) in MEFs (P/P; T/+ and P/+; T/+). Representative image of tdTomato+ cells can be only seen in P/P; T/+ MEFs treated with both 4-OHT and DOXO. (b) tdTomato+ cells percentage in MEFs. For b, n=4 for all groups. Results were shown as mean ± s.e.m. * p= 0.018; two-tailed, unpaired Student’s t-test.
Supplementary Material
Acknowledgements
The authors are grateful to colleagues in the UConn Center on Aging for helpful and constructive discussion, Zhifang Hao for histology service, and Stefanie Farkas for administrative assistance. This work was supported in part by the Regenerative Medicine Initiative for Diabetes-Career Development Award from Mayo Clinic (M.X.), Glenn Foundation for Medical Research and AFAR Grant for Junior Faculty (M.X.), Robert and Arlene Kogod (J.L.K.), the Connor Group (J.L.K.), Robert J. and Theresa W. Ryan (J.L.K.), the Noaber Foundation (J.L.K.), Travelers Chair in Geriatrics and Gerontology (G.A.K.), and NIH grants R37AG013925 (J.L.K.), P01AG062413 (J.L.K.), R33AG061456 (J.L.K., T.T., G.A.K.), AG063528 (M.X.), AG066679 (M.X.), and AG068860 (M.X.).
Footnotes
Competing Interests
None of the authors have competing financial or non-financial interests.
Peer review Information:
Nature Aging thanks Valery Krizhanovsky, and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Reference
- 1.Gorgoulis V et al. Cellular Senescence: Defining a Path Forward. Cell 179, 813–827, doi: 10.1016/j.cell.2019.10.005 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Tchkonia T, Zhu Y, van Deursen J, Campisi J & Kirkland JL Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. The Journal of clinical investigation 123, 966–972, doi: 10.1172/JCI64098 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkland JL & Tchkonia T Cellular Senescence: A Translational Perspective. EBioMedicine 21, 21–28, doi: 10.1016/j.ebiom.2017.04.013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuilman T et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031, doi: 10.1016/j.cell.2008.03.039 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Wang C et al. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8, 311–323, doi: 10.1111/j.1474-9726.2009.00481.x (2009). [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Armstrong JL, Tchkonia T & Kirkland JL Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Current opinion in clinical nutrition and metabolic care 17, 324–328, doi: 10.1097/MCO.0000000000000065 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Baker DJ et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189, doi: 10.1038/nature16932 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu M et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 4, e12997, doi: 10.7554/eLife.12997 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu M et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proceedings of the National Academy of Sciences of the United States of America 112, E6301–6310, doi: 10.1073/pnas.1515386112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ & Robbins PD The Clinical Potential of Senolytic Drugs. Journal of the American Geriatrics Society 65, 2297–2301, doi: 10.1111/jgs.14969 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acosta JC et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature cell biology 15, 978–990, doi: 10.1038/ncb2784 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson G et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11, 345–349, doi: 10.1111/j.1474-9726.2012.00795.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M et al. Senolytics improve physical function and increase lifespan in old age. Nature medicine 24, 1246–1256, doi: 10.1038/s41591-018-0092-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Silva PFL et al. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 18, e12848, doi: 10.1111/acel.12848 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker DJ et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236, doi: 10.1038/nature10600 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demaria M et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental cell 31, 722–733, doi: 10.1016/j.devcel.2014.11.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farr JN et al. Targeting cellular senescence prevents age-related bone loss in mice. Nature medicine 23, 1072–1079, doi: 10.1038/nm.4385 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer AK et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell, e12950, doi: 10.1111/acel.12950 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon OH et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nature medicine 23, 775–781, doi: 10.1038/nm.4324 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bussian TJ et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582, doi: 10.1038/s41586-018-0543-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roos CM et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977, doi: 10.1111/acel.12458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Childs BG et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477, doi: 10.1126/science.aaf6659 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogrodnik M et al. Cellular senescence drives age-dependent hepatic steatosis. Nature communications 8, 15691, doi: 10.1038/ncomms15691 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer MJ et al. Cellular senescence mediates fibrotic pulmonary disease. Nature communications 8, 14532, doi: 10.1038/ncomms14532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang J et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature medicine 22, 78–83, doi: 10.1038/nm.4010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosse L et al. Defined p16(High) Senescent Cell Types Are Indispensable for Mouse Healthspan. Cell Metab 32, 87–99 e86, doi: 10.1016/j.cmet.2020.05.002 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Omori S et al. Generation of a p16 Reporter Mouse and Its Use to Characterize and Target p16(high) Cells In Vivo. Cell Metab 32, 814–828 e816, doi: 10.1016/j.cmet.2020.09.006 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Hall BM et al. p16(Ink4a) and senescence-associated beta-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging (Albany NY) 9, 1867–1884, doi: 10.18632/aging.101268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frescas D et al. Murine mesenchymal cells that express elevated levels of the CDK inhibitor p16(Ink4a) in vivo are not necessarily senescent. Cell Cycle 16, 1526–1533, doi: 10.1080/15384101.2017.1339850 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Micco R, Krizhanovsky V, Baker D & d’Adda di Fagagna F Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol, doi: 10.1038/s41580-020-00314-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy AL et al. A Blueprint for Characterizing Senescence. Cell 183, 1143–1146, doi: 10.1016/j.cell.2020.10.032 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabula Muris C A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595, doi: 10.1038/s41586-020-2496-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feil R, Wagner J, Metzger D & Chambon P Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 237, 752–757 (1997). [DOI] [PubMed] [Google Scholar]
- 34.el-Deiry WS et al. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res 55, 2910–2919 (1995). [PubMed] [Google Scholar]
- 35.Tasic B et al. Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proceedings of the National Academy of Sciences of the United States of America 108, 7902–7907, doi: 10.1073/pnas.1019507108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hippenmeyer S et al. Genetic mosaic dissection of Lis1 and Ndel1 in neuronal migration. Neuron 68, 695–709, doi: 10.1016/j.neuron.2010.09.027 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safran M et al. Mouse reporter strain for noninvasive bioluminescent imaging of cells that have undergone Cre-mediated recombination. Mol Imaging 2, 297–302 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Tinkum KL et al. Bioluminescence imaging captures the expression and dynamics of endogenous p21 promoter activity in living mice and intact cells. Molecular and cellular biology 31, 3759–3772, doi: 10.1128/MCB.05243-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schafer MJ et al. Exercise Prevents Diet-Induced Cellular Senescence in Adipose Tissue. Diabetes 65, 1606–1615, doi: 10.2337/db15-0291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madisen L et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140, doi: 10.1038/nn.2467 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang B et al. Transplanting cells from old but not young donors causes physical dysfunction in older recipients. Aging Cell, e13106, doi: 10.1111/acel.13106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biran A et al. Quantitative identification of senescent cells in aging and disease. Aging Cell 16, 661–671, doi: 10.1111/acel.12592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freund A, Laberge RM, Demaria M & Campisi J Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23, 2066–2075, doi: 10.1091/mbc.E11-10-0884 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voehringer D, Liang HE & Locksley RM Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J Immunol 180, 4742–4753 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oppenheimer NJ & Bodley JW Diphtheria toxin. Site and configuration of ADP-ribosylation of diphthamide in elongation factor 2. The Journal of biological chemistry 256, 8579–8581 (1981). [PubMed] [Google Scholar]
- 46.Chien Y et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev 25, 2125–2136, doi: 10.1101/gad.17276711 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen LF & Greene WC Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5, 392–401, doi: 10.1038/nrm1368 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Heise N et al. Germinal center B cell maintenance and differentiation are controlled by distinct NF-kappaB transcription factor subunits. J Exp Med 211, 2103–2118, doi: 10.1084/jem.20132613 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi S & McMahon AP Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Developmental biology 244, 305–318, doi: 10.1006/dbio.2002.0597 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Fried LP et al. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nature Aging 1, 36–46, doi: 10.1038/s43587-020-00017-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fried LP et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56, M146–156 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Justice JN et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563, doi: 10.1016/j.ebiom.2018.12.052 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ovadya Y et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nature communications 9, 5435, doi: 10.1038/s41467-018-07825-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burd CE et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 152, 340–351, doi: 10.1016/j.cell.2012.12.010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karin O, Agrawal A, Porat Z, Krizhanovsky V & Alon U Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nature communications 10, 5495, doi: 10.1038/s41467-019-13192-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin-Caballero J, Flores JM, Garcia-Palencia P & Serrano M Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res 61, 6234–6238 (2001). [PubMed] [Google Scholar]
- 57.Ohtani N et al. Visualizing the dynamics of p21(Waf1/Cip1) cyclin-dependent kinase inhibitor expression in living animals. Proceedings of the National Academy of Sciences of the United States of America 104, 15034–15039, doi: 10.1073/pnas.0706949104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yousefzadeh MJ et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28, doi: 10.1016/j.ebiom.2018.09.015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao X, Fujiwara Y, Chapdelaine A, Yang H & Orkin SH Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood 97, 324–326 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Xu M, Tchkonia T & Kirkland JL Perspective: Targeting the JAK/STAT pathway to fight age-related dysfunction. Pharmacol Res 111, 152–154, doi: 10.1016/j.phrs.2016.05.015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moiseeva O et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell 12, 489–498, doi: 10.1111/acel.12075 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Martin-Montalvo A et al. Metformin improves healthspan and lifespan in mice. Nature communications 4, 2192, doi: 10.1038/ncomms3192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison DE et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395, doi: 10.1038/nature08221 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herranz N et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nature cell biology 17, 1205–1217, doi: 10.1038/ncb3225 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laberge RM et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nature cell biology 17, 1049–1061, doi: 10.1038/ncb3195 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu M et al. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J Gerontol A Biol Sci Med Sci 72, 780–785, doi: 10.1093/gerona/glw154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source data are published with the manuscript and are available from the corresponding author upon reasonable request. The link for Tabula Muris Senis database is https://tabula-muris-senis.ds.czbiohub.org/.


