Abstract
The popularity of fruits vinegar (FsV) has been increased recently as a healthy drink wealthy in bioactive compounds that provide several beneficial properties. This review was designed in the frame of valorization of fruits vinegar as a by-product with high value added by providing overall information on its biochemical constituents and beneficial potencies. It contains a cocktail of bioactive ingredients including polyphenolic acids, organic acids, tetramethylperazine, and melanoidins. Acetic acid is the most abundant organic acid and chlorogenic acid is the major phenol in apple vinegar. The administration of fruits vinegar could prevent diabetes, hypercholesterolemia, oxidative stress, cancer, and boost immunity as well as provide a remarkable antioxidant ability. The production techniques influence the quality of vinegar, and consequently, its health benefits.
Keywords: fruits vinegar, bioactive compounds, quality characteristics, biological properties, vinegar production
1. Introduction
Fruits vinegar (FsV) is a popular natural product with multiple use purposes. It is remarkably appreciated and included in many people’s daily diet [1,2,3]. Fruits fermentation produces a bio liquid that contains several functional molecules [4,5] such as organic acids [5,6], polyphenols [7,8], melanoidins [9,10], and tetramethylpyrazine [11,12]. The production technique used has an impact on the quality of vinegar, while the vinegar-making process plays an important role in the removal and/or formation of new components. The traditional technique promotes the development of aroma and flavor due to the slow process of production [4,13,14].
Polyphenols and organic acids, mainly acetic acid, plays an important role in the beneficial properties provided by fruits vinegar [15]. Previously, it is shown that the administration of apple vinegar curbs the installation of hyperglycemia and hyperlipidemia induced by hypercaloric fed enriched in d-glucose in male and female rats [16]. Clinical studies demonstrated that apple vinegar regulates gene expression via the Mitogrn-Activated Protein Kinase (MAPK) pathway [17,18,19], and control blood glucose and lipids levels [19]. Interestingly, it contains a wide range of functional substances that exert their effects in synergy [3,20,21]. This review aimed to itemize the chemical composition of FsV and its therapeutics application scientifically proved.
2. Methodology
All references used in the current review were collected via search engines including Google Scholar, Scopus, Science Direct, Web of Science, and Pub-Med using the following keywords: Fruits vinegar, Beneficial properties and vinegar, effects of fruits vinegar. The articles (203 articles) were evaluated for relevance and their scientific importance, and 119 articles were used to prepare the present review. Other articles were excluded for the purpose treated that they didn’t fall in the scope of the present review.
3. Quality Characteristics
FsV quality depends on the procedure conditions and raw matter. Vinegar-making process techniques influence the organoleptic properties of final vinegar [4]. The quality characteristics of local products determination are very important to promote vinegar marketing. It is named as natural means; it is completely safe and healthy [3,22]. The quality analysis provides the composition of each product in wanted and unwanted ingredients that can potentially be toxic. The quality evaluation requires a panel of analysis including determination of components content and sensory analysis. The acidity is considered a key factor to determine the vinegar quality and its content is at least 5 or 6 degrees [23,24,25]. The alcohol content is set to the maximum at 0.5 degrees for fruits vinegar and 1% for alcohol vinegar [23,25]. The same values are required by the Codex Alimentarius Norm 79/19 [26].
The physicochemical properties of FsV in terms of pH, acidity, electrical conductivity, and °Brix values are summarized in Table 1. The pH of fruits vinegar ranged from 2.40 to 3.90 [27,28,29,30]. Electrical conductivity (EC) is an important parameter to evaluate the ability of vinegar to allow the passage of electrical current and its relation with the mineral content of the sample. The values of EC depend on the raw matter used to produce vinegar [27,30]. The acidity is an important criterion to examine the quality of vinegar. High values of acidity observed in apple vinegar made in Turkey may be caused by the hyperoxygenation that occurs during the second step of the production of vinegar [29]. The second criterion used to determine vinegar quality is the ethanol content, the values reported for different vinegars range from 0.01 to 2.53 degrees. High values of ethanol content were observed in date vinegar from Iraq [31].
Table 1.
Range values of physicochemical characteristics of vinegar.
| Vinegar | Source | pH | Conductivity (mS/cm) | Acidity Titrable (%) |
Ethanol (°) |
Reference |
|---|---|---|---|---|---|---|
| Apple vinegar | Morocco | 3.18–3.83 | 2.11–2.90 | 0.24–5.6 | - | [28] |
| Apple vinegar | Algeria | - | - | 0.73 ± 0.06 | - | [32] |
| Pomegranate vinegar | - | - | 0.98 ± 0.01 | - | ||
| Prickly pear vinegar | - | - | 0.31 ± 0.02 | - | ||
| Date vinegar | Iraq | 2.85–3.07 | 1.81–7.48 | 2.85–7.24 | 0.01–1.44 | [31] |
| Date vinegar | Syria | 2.99–3.23 | 5.43–3.91 | 4.22–5.26 | 1.07–2.53 | |
| Ginger vinegar | 3.26 | 3.86 | 5.04 | 2.88 | ||
| Grape vinegar | 2.95 | 2.98 | 4.63 | 0.50 | ||
| Garlic vinegar | 3.12 | 4.26 | 4.98 | 0.01 | ||
| Grape vinegar | Lebanon | 2.49–2.86 | 1.31–2.84 | 5.34–6.18 | 0.01–0.18 | |
| Apple vinegar | Turkey | 2.71 | 1.72 | 5.17 | 0.09 | |
| Grape vinegar | 2.99 | 3.45 | 5.11 | 0.01 | ||
| Vegetable vinegar | USA | 2.40 | 1.57 | 6.12 | 0.16 | |
| Grape vinegar | 2.53 | 1.56 | 5.40 | 0.18 | ||
| Sugarcane vinegar | KSA | 2.43 | 1.57 | 6.36 | 0.01 | |
| Grape vinegar | Turkey | 2.70–3.90 | - | 0.32–5.72 | - | [29] |
| Apple vinegar | 2.71–3.56 | - | 0.66–7.20 | - | ||
| Artichoke vinegar | 3.79 ± 0.00 | - | 1.22 ± 0.03 | - | ||
| Pomegranate | 2.88–3.69 | - | 1.04–3.38 | - | ||
| Apple-lemon | 3.64 ± 0.01 | - | 1.36 ± 0.03 | - | ||
| Hawthorne vinegar | 3.76 ± 0.02 | - | 0.82 ± 0.03 | - | ||
| Lemon vinegar | 2.63 ± 0.02 | - | 4.34 ± 0.07 | - | ||
| Sour sherry vinegar | 3.05 ± 0.01 | - | 5.50 ± 0.07 | - | ||
|
Eucommia ulmoides leaves vinegar |
China | - | - | 1.6–4.7 | - | [33] |
| Wood vinegars | Thailand | 2.90–3.50 | - | 2.72–4.92 | - | [34] |
| Blueberry vinegar | Brazil | 2.94–2.98 | - | 4.2–4.8 | 0.1–0.2 | [35] |
| Apple vinegar | Romania | - | - | 3.9–9 | - | [27] |
| Dates vinegar | Malaysia | 2.70–2.77 | - | 1.19–5.86 | - | [36] |
| Citrus vinegar | Italy | 2.65–3.24 | - | 2.96–13.32 | 0 | [37] |
4. Chemical Characteristics of FsV
FsV has been the subject of numerous research studies during the last decades. Recently, studies are being carried out to determine and identify the phenolic composition of vinegar (Table 2). The nature and quantity of bioactive compounds present in vinegar are closely linked to the raw matter used to produce vinegar, the technique selected to produce vinegar, and the nature of microorganisms involved in the fermentation process [9,38,39]. The active molecules naturally present or new generated not only confer organoleptic properties such as astringency, taste, and color parameters [40,41]. Bioactive ingredients of vinegar play also an important role in the prevention and treatment of different human ailments [3,9,22,42].
Table 2.
Phytochemical profile of fruits vinegar.
| Variety | Country | Method of Vinegarmaking | Methods | Bioactive Compounds Identified | References |
|---|---|---|---|---|---|
| Grape vinegar | Turkey | Artisanal and industrial | HPLC-DAD | Gallic acid (16.36–18.23 mg/L), catechin (13.76–27.50 mg/L), epicatechin (4.96–8.20 mg/L), caffeic acid (6.30–10.30 mg/L), chlorogenic acid (0.16–3.73 mg/L), syringic acid (0.33–0.70 mg/L), p-coumaric acid (0.23–0.56 mg/L), and ferulic acid (0.06–0.35 mg/L) | [4] |
| Grape vinegar | Industrial | HPLC-PDA | Gallic acid (6 ± 2 mg/100 mL) and p-hydroxybenzoic acid (0.90 ± 0.05 mg/100 mL) | [7] | |
| Apple vinegar | Gallic acid (0.8 ± 0.04 mg/100 mL), p-hydroxybenzoic acid (0.2 ± 0.1 mg/100 mL), catechin (2.4 ± 0.1 mg/100 mL), syringic acid (0.12 ± 0.02 mg/100 mL), caffeic acid (0.40 ± 0.01 mg/100 mL), and p-coumaric acid (0.08 ± 0.01 mg/100 mL) | ||||
| Apple vinegar | Artisanal | HPLC-DAD | Gallic acid (61.24 ± 2.21 mg/L), chlorogenic acid (347.70 ± 31.94 mg/L), catechin (68.20 mg/L), and caffeic acid (17.21 ± 0.33 mg/L) | [45] | |
| Pomegranate vinegar | Gallic acid (67.80 ± 2.88 mg/L), catechin (47 ± 1.10 mg/L), and caffeic acid (13.41 ± 0.60 mg/L) | ||||
| Aromatic vinegar * | China | Artisanal | HPLC | Gallic acid, p-hydroxybenzoic acid, vanillic acid, catechin, caffeic acid, chlorogenic acid, syringic acid, ethyl gallate, p-coumaric acid, ferulic acid, sinapic acid, and rutin. | [46] |
| Grape vinegar | Turkey | Industrial | LC-DAD-ESI-MS/MS | Gallic acid (7.45–21.84 mg/L), tyrosol (11.54–17.68 mg/L), protocatechuic acid (7.21–11.05 mg/L), caftaric acid (1.76–15.83 mg/L), cholorogenic acid (0.09–1.77 mg/L), coutaric acid (0–1.95 mg/L) caffeic acid (0.11–2.58 mg/L), ferulic acid (0.01–0.21 mg/L), fertaric acid (0.03–0.83 mg/L), vanilic acid (0–2.58 mg/L), p-coumaric acid (0.02–0.45 mg/L), syringic acid (1.24–9.04 mg/L), procyanidin B2 (0.09–3.11 mg/L), catechin (3.73–27.11 mg/L), epicatechin (0.57–15.13 mg/L), quercetin-3-O-galactoside (0.04–0.39 mg/L), kaempferol-3-O-rutinoside (0–0.04 mg/L), rutin (0.02–0.20 mg/L), isorhamnetin-3-O-glucoside (0.05–0.09 mg/L), and quercetin (0.06–0.69 mg/L). |
[8] |
| Apple vinegar | Gallic acid (0.47–2.57 mg/L), protocatechuic acid (1.15–6.35 mg/L), cholorogenic acid (2.96–16.29 mg/L), caffeic acid (0.19–1.77 mg/L), vanilic acid (0.63–3.42 mg/L), p-coumaric acid (0.13–0.81 mg/L), procyanidin B2 (0.12–1.35 mg/L), catechin (0.14–0.95 mg/L), epicatechin (0.04–1.36 mg/L), luteolin-3-O-rutinoside (0.30–1.98 mg/L), isorhamnetin-3-O-rutinoside (0.10–0.63 mg/L), isorhamnetin-3-O-glucoside (0.08–0.48 mg/L), kaempferol-3-O-glucoside (0.03–0.20 mg/L), quercetin-3-O-rhamnoside (0.20–3.41 mg/L), quercetin (0.20–1.41 mg/L), rutin (0.04–0.29 mg/L), luteolin (0.27–1.63 mg/L), apigenin0.02–0.13 mg/L), phloretin (0.59–7.86 mg/L), and phloridzin (7.64–44.35 mg/L). | ||||
| Apple vinegar | Japan | Industrial | LC-MS | Chlorogenic acid (3.1–19.6 mg/100 mL), 4-p-coumaric acid (0–0.21 mg/100 mL), isomer of p-coumaroyquinic acid (0–1.3 mg/100 mL), 5-hydroxymethylfurfural (2.7–4.1 mg/100 mL), protocatechic acid (0–0.41 mg/100 mL), p-hydroxybenzoic acid (0–0.77 mg/100 mL), caffeic acid (0–0.76 mg/100 mL), isomer of chlorogenic acid (0–3.1 mg/100 mL), and p-coumaric acid (0–0.21 mg/100 mL) | [47] |
| Persimmon vinegar | China | Artisanal | HPLC | Gallic acid (22.91 ± 1.22 mg/L), (+/−)-catechin hydrate (0.16 ± 0.89 mg/L), chlorogenic acid (0.06 ± 0.12 mg/L), caffeic acid (0.04 ± 0.06 mg/L), p-coumaric acid (0.03 ± 0.21 mg/L), trans-ferulic acid (0.02 ± 0.11 mg/L), (-)-epicatechin gallate (0.13 ± 0.09 mg/L), and phloridzin (0.38 ± 0.12 mg/L) | [48] |
| Apple vinegar | Gallic acid (0.35 ± 0.02 mg/L), vanillic acid (0.06 ± 0.04 mg/L), chlorogenic acid (6.56 ± 0.43 mg/L), caffeic acid (3.03 ± 0.02 mg/L), p-coumaric acid (0.33 ± 0.28 mg/L), trans-ferulic acid (0.24 ± 0.07 mg/L), (-)-epicatechin gallate (0.77 ± 0.34), and phloridzin (1.76 ± 0.34 mg/L). | ||||
| Kiwifruit vinegar | Gallic acid (9.67 ± 0.59 mg/L), (+/−)-catechin hydrate (1.47 ± 0.34 mg/L), vanillic acid (1.77 ± 0.23 mg/L), chlorogenic acid (3.12 ± 0.21 mg/L), caffeic acid (0.04 ± 0.05 mg/L), p-coumaric acid (0.34 ± 0.01 mg/L), trans-ferulic acid (0.01 ± 0.03 mg/L), and phloridzin (0.49 ± 0.02 mg/L) | ||||
| Apple vinegar | Brazil | Industrial | HPLC-PDA | Phloretin-2′-β-d-glucoside (4.81–15.55 mg/L), 5-caffeoylquinic acid (20.62–26.85 mg/L), caffeic acid (0.51–3.87 mg/L), p-coumaric acid (1.16–2.03 mg/L), quercetin-3-rutinoside (2.69–4.65 mg/L), quercetin-3-d-galactoside (0.73–9.75 mg/L), quercetin-3-β-d-glucoside (1.58–3.45 mg/L), quercetin-3-d-xyloside (1.62–2.54 mg/L), quercetin-O-α-l-arabinofuranoside (0.85–1.34 mg/L), and quercetin-3-O-rhamnoside (1.13–3.37 mg/L). | [49] |
| Apple vinegar | China | Industrial | HPLC-PDA | Chlorogenic acid (0.11–10.91 µg/mL), protocatechuic acid (0.08–1.54 µg/mL), and p-coumaric acid (0.10–0.17 µg/mL | [5] |
| Red wine vinegar | Gallic acid (4.10–9.99 µg/mL), protocatechuic acid (0.47–1.38 µg/mL), p-coumaric acid (0.81–1.39 µg/mL), and caffeic acid (1.48–1.73 µg/mL) | ||||
| White wine vinegar | Protocatechuic acid (0.16–0.32 µg/mL), p-coumaric acid (0–0.18 µg/mL), caffeic acid (0–0.32 µg/mL), and ferulic acid (0–0.31 µg/mL) | ||||
| Balsamic vinegar | Gallic acid (7.50–12.56 µg/mL), protocatechuic acid (0–3.29 µg/mL), p-coumaric acid (1.17–1.97 µg/mL), and caffeic acid (0–3.58 µg/mL) | ||||
| Sour cherry vinegar | Turkey | Industrial | HPLC | Gallic acid (160–170 mg/mL), chlorogenic acid (45–55 mg/mL), p-coumaric acid (17–23 mg/mL), caffeic acid (3.5–4 mg/mL), ferulic acid (1.3–4.6 mg/mL), catechin (0.7–1 mg/mL), and epicatechin (1.7–3.5 mg/mL) | [50] |
| Palm vinegar | Thailand | Artisanal | LC-MS | Gallic acid (14.14 ± 0.07 µg/mL), catechin (8.61 ± 0.32 µg/mL), rutin (6.67 ± 0.03 µg/mL), isoquercetin (11.27 ± 0.12 µg/mL), and quercetin (10.33 ± 0.16 µg/mL) | [51] |
| Brow beer vinegar | Italy | Industrial | HPLC-DAD-ESI(+)-MS | Protocatechuic acid O-glucoside (7.42 ± 0.03 mg/L), 3-caffeoylquinic acid (40.01 ± 1.13 mg/L), (4-Hydroxyphenyl) acetic acid (11.84 ± 0.02 mg/L), 4-vinylguaiacol (10.22 ± 0.04 mg/L), Catechin 7 O-glucoside (8.84 ± 0.02 mg/L), 4-hydroxybenzoic acid (38.23 ± 0.05 mg/L), (3-hydroxyphenyl)acetic acid (18.95 ± 0.04 mg/L), catechin 5 O-glucoside (7.24 ± 0.06 mg/L), coumaric acid O-glucoside (4.90 ± 0.05 mg/L), cerulic acid O-glucoside (4.33 ± 0.02 mg/L), gallic acid (5.72 ± 0.04 mg/L), vanilic acid O-glucoside (10.25 ± 0.03 mg/L), gallocatechin (7.66 ± 0.10 mg/L), sinapic acid O-glucoside (14.03 ± 0.12 mg/L), catechin O-diglucoside (8.41 ± 0.04 mg/L), kaempferol O-glucoside (6.28 ± 0.04 mg/L), feruloylquinic acid (6.60 ± 0.15 mg/L), chlorogenic acid (18.30 ± 0.02 mg/L), (+)-catechin (7.89 ± 0.04 mg/L), (−)-epicatechin (7.78 ± 0.12 mg/L), caffeic acid (10.58 ± 0.08 mg/L), sinapic acid (15.5 ± 0.06 mg/L), apigenin O-glucoside (6.15 ± 0.02 mg/L), quercetin O-glucoside (7.05 ± 0.06 mg/L), cohumulone I (4.44 ± 0.02 mg/L), cohumulone II (6.58 ± 0.10 mg/L), 8-prenylnaringenin (2.33 ± 0.02 mg/L), 6-prenylnaringenin (1.86 ± 0.02 mg/L), humulone (5.62 ± 0.08 mg/L), and isohumulone (4.14 ± 0.03 mg/L) | [52] |
| Pineapple vinegar | Industrial | UHPLC-QTOF-MS | Catechol, peonidin, (+)-catechin 3-O-gallate, m-coumaric acid, 7,3’,4’-trihydroxyflavone, 4-vinylsyringol, ferulic acid, mullein, genistin, 3,4-dihydroxyphenylglycol, 4-ethylcatechol, 6-prenylnaringenin, gallic acid, kaempferol 3-O-xylosyl-glucoside, 6,8-Dihydroxykaempferol, spinacetin 3-O-glucosyl-(1-6)-[apiosyl(1-2)]-glucoside, and malvidin 3-O-arabinoside | [53] | |
| Cherry vinegar | Spain | Industrial | UPLC-DAD | Gallic acid (2.08–2.99 mg/L), HMF (6.96–9.48 mg/L), protocatechuic acid (2.12–2.43 mg/L), caftaric acid (2.05–2.81 mg/L), furoic acid (2.46–16.53 mg/L), protocatechualdehyde (0.046–0.263 mg/L), cis-p-Coutaric acid (1.83–2.25 mg/L), trans-p-Coutaric acid (1.15–1.55 mg/L), tyrosol (24.6–28.9 mg/L), catequin (0.165–0.334 mg/L), caffeic acid (0.184–0.308 mg/L), vanillic acid (2.66–3.44 mg/L), syringic acid (2.16–5.44 mg/L), vanillin (1.05–2.97 mg/L), cis-p-coumaric acid (0.174–0.481 mg/L), syringaldehyde (0.50–5.12 mg/L), coniferyl aldehyde (0.959–2.85 mg/L), and sinapaldehyde (16.1–19.1 mg/L) | [54] |
| Sugarcane vinegar | China | Industrial | UPLC-MS | Benzoic acid (1.027 ± 0.07 mg/L), ferulic acid (1.1240.063 mg/L), quinic acid (0.031 ± 0.002 mg/L), chlorogenic acid (1.217 ± 0.063 mg/L), apigenin (0.004 ± 0 mg/L), kaempferol (0.003 ± 0.0001 mg/L), caffeic acid (0.005 ± 0.0001 mg/L), luteolin (0.005 ± 0.0001 mg/L), and p-coumaric acid (0.027 ± 0.0001 mg/L) | [55] |
| Citrus vinegar | Italy | Industrial | UPLC-UV | Gallic acid (2.62–5.63 mg/L), neochlorogenic acid (2.69–5.83 mg/L), chlorogenic acid (2.95–58.51 mg/L), vanillic acid (0.47–3.64 mg/L), caffeic acid (1.39–3.64 mg/L), epicatechin (0–2.91 mg/L), procyanidin (0–9.43 mg/L), rutin (1.76–146.3 mg/L), quercetin (0.23–8.62 mg/L), eriocitrin (0.27–13.20 mg/L), neoeriocitrin (53.41–513.30 mg/L), narirutin (3.05–18.24 mg/L), naringin (61.19–700.56 mg/L), hesperidin (12.15–92.12 mg/L), neohesperidin (63.51–366.93 mg/L), didymin (1.73–9.82 mg/L), and hesperetin (0–15.54 mg/L) | [37] |
* Vinegar mixed with other aromatic plants.
FsV is considered a useful product in traditional medicine for the management of different illnesses such as diabetes [3,16,22]. As previously described, FsV is an excellent source of multiple bioactive ingredients such as phenolic acids, minerals, organic acids, and tetramethylpyrazine [9,11,29,43]. Phytochemicals present in FsV have numerous pharmacological properties including antidiabetic, antihyperlipidemic, antimicrobial, and anticancer [4,9,44].
4.1. Organic Acids
Multiple organic acids were found in FsV including volatile organic acid (acetic acid, formic acid, propionic acid, butyric acid, and quinic acid) and non-volatile organic acids (lactic acid, malic acid, pyroglutamic acid, citric acid, and succinic acid) (Table 3) [29]. As a food-grade product, vinegar’s quality depends on its various characteristics, including aroma, which is the most important quality criterion [55]. Aroma is related to the presence of organic acids in FsV that are present naturally in raw matter or newly generated during the fermentation process. As stated in Table 2, acetic acid is the most abundant acid in vinegar with a proportion of about 92.64–93.22% [29,55], and followed by succinic acid with a percentage of 3.92 to 6.43%, while, oxalic acid and malic acid were present at lowest quantities [56]. The content of organic acids changed during the production process of vinegar, and it showed the lowest content of organic acids during alcoholic fermentation with a gradual increase which was maintained during the second phase (acetic fermentation) [46]. It proved that these bioactive compounds exhibit several health benefits [24,57,58,59,60,61,62]. Acetic acid as the main organic acid in vinegars is the key factor of numerous beneficial properties through the activation of the MAPK pathway, which induces reduction of blood glucose, increases glycogen storage, reduces triglycerides levels, increases insulin sensitivity, and decreases insulin resistance [18]. The aforementioned features revealed that acetic acid is the main acid of vinegar that can ameliorate metabolic disorders and ameliorate disease markers [16,18,22,63,64,65,66,67,68,69,70,71].
Table 3.
Organic acids in FsV.
| Vinegars | Country | Organic Acids * | References |
|---|---|---|---|
| Apple vinegar | China | Tartaric acid, malic acid, lactic acid, citric acid, succinic acid | [5] |
| Red wine vinegar | Tartaric acid, succinic acid | ||
| White wine vinegar | Tartaric acid, succinic acid | ||
| Balsamic vinegar | Tartaric acid, malic acid | ||
| Apple vinegar | Spain | Propionic acid, isobutyric acid, butyric acid, isovaleric acid | [72] |
| baby corn silk vinegar | Thailand | Dimethyl ether, 2-Ethylthio-2-methoxy-3-oxo-n-phenylbutanamide, 1-Butanol, 3-methyl-, acetate, Cyclobutane,1,1,2,3,3-pentamethyl-Isoamylalcohol, pentanoic acid, Butanedioic acid, diethyl ester, 3-Cyclohexene-1-methanol, 4-rimethyl -ethyl 2-phenylacetate, 4-[1′-Phenylethenyloxymethyl] pyridine, hexanoic acid, 1H-Pyrrolizine-7-carboxylic acid, 2-(formyloxy), octaboic acid | [73] |
| Sour cherry vinegar | Turkey | Isobutyric acid, isovaleric acid, hexanoic acid, octanoic acid, nonanoic acid, decanoic acid, dodecanoic acid, tetradecanoic acid, methyl acetate, ethyl acetate, ethyl propanoate, isobutyl acetate, isoamyl acetate, ethyl caproate, ethyl caprylate, ethyl decanoate, benzyl acetate, phenethyl acetate, 2-ethyl acetate, 2-ethyl hexanoic acid, ethanol, isobutyl alcohol, hexanol, nonanol, benzyl alcohol, phenethyl alcohol, 1-dodecanol | [50] |
| Pineapple vinegar | Thailand | Methyl ester, ethyl acetate, isobutyl acetate, isobutanol, isopentyl alcohol (3-methyl butanol), acetoin, benzaldehyde, propanoic acid, butanoic acid, isobutyric acid, 4-methylbenzaldehyde, methylbutanoic acid, naphthalene, phenylethel acetate, phenylethyl alcohol | [74] |
* Acetic acid is present in all FsV.
4.2. Mineral Content
The mineral composition of FsV is related to the raw matter. It plays an important role of human nutrition. Minerals are involved in several physiological functions such as blood pressure regulation, muscles contraction, blood cells production, maintaining bones and teeth healthy, sleep modulator, and maintenance of nerve functioning [75,76,77]. Vinegars are the potential source of mineral elements including potassium, sodium, calcium, iron, zinc, magnesium, phosphorus, and copper [78,79]. Zhang et al. [80] reported concentrations of different minerals in wood vinegar as 7.66 ± 0.80 (K), 13 ± 0.78 (Ca), 1.98 ± 0.34 (Mg), 3751 ± 60 (Fe), 23.7 ± 0.43 (Mn), and 0.166 ± 0.16 (Zn) mg/kg. Another study also found 153.8 ± 5.22 and 131.2 ± 4.29 (K), 21.85 ± 1.502 and 15.37 ± 0.734 (Na), 30.04 ± 0.522 and 6.715 ± 0.2967 (Ca), and 10.90 ± 0.087 and 6.901 ± 0.1194 mg/g for grape vinegar and apple vinegar respectively [81]. Various reports evoked the concentration of minerals present in vinegars are presented in Table 4.
Table 4.
Mineral composition of FsV (mg/L).
| Vinegars | Country | K | Na | Ca | Zn | Mg | Fe | P | Ni | Cr | Co | Mn | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Banana vinegar | Romania | - | 14.69–186.06 | 11.89–148.94 | 0.569–3.61 | 7.12–113.31 | - | - | 0.07–0.15 | 0.04–3.63 | 0.01–0.02 | 0.09–4.01 | [82] |
| Apple vinegar | Turkey | 802.24 ± 114 | 360.21 ± 250.380 | 104.75 ± 28.695 | ND | 65.60 ± 7.565 | 1.31 ± 0.585 | 48.06 ± 17.044 | 0.01 ± 0.013 | - | - | 0.18 ± 0.130 | [79] |
| Rice vinegar | 0.43 ± 0.056 | ND | ND | ND | ND | 0.62 ± 0.103 | 0.22 ± 0.020 | 0.01 ± 0.003 | - | - | 0.22 ± 0.011 | ||
| Sour cherry vinegar | 1058.93 ± 103.502 | 303.20 ± 38.562 | 670.80 ± 30.811 | 0.32 ± 0.013 | 142.60 ± 46.11 | 10.68 ± 0.591 | 63.14 ± 11.078 | 0.12 ± 0.008 | - | - | 0.67 ± 0.009 | ||
| Date vinegar | 1384.93 ± 132.745 | 181.40 ± 25.787 | 1136 ± 105.112 | 0.02 ± 0.024 | 195.60 ± 22.235 | 10.44 ± 2.526 | 70.32 ± 36.123 | 0.16 ± 0.008 | - | - | 0.28 ± 0.018 | ||
| Balsamic vinegar | 1557.73 ± 416.841 | 264.96 ± 26.766 | 188.28 ± 46.997 | 0.36 ± 0.162 | 127.04 ± 18.470 | 6.94 ± 1.498 | 182.60 ± 50.577 | 0.03 ± 0.019 | - | - | 1.31 ± 0.180 | ||
| Apple vinegar | Morocco | 32.403–41.863 | 0.039–0.199 | 1.569–2.620 | 0.014–4.212 | 1.572–1.746 | 0.499–0.581 | - | - | - | - | 0.045–0.053 | [78] |
| Date vinegar | Algeria | 0.14–2.73 | 23.6–30.9 | 0.24–0.79 | - | 0.16–1.92 | 0.22–1.74 | - | - | - | - | - | [83] |
| Date vinegar | Iraq | 1958 | 148 | 293 | 1.29 | 50 | 1.15 | - | - | - | 0.069 | 0.49 | [31] |
| Wood vinegar | China | 7.66 ± 0.80 | - | 13 ± 0.78 | 0.166 ± 0.16 | 1.98 ± 0.34 | 3751 ± 60 | - | - | - | - | 23.7 ± 0.43 | [80] |
5. Beneficial Properties of FsV
The double fermentation of fruits furnishes a healthy product that contains an amount of biologically active components. Literature data reported that fruits vinegar consumption is positively counteracting against numerous diseases [3,32,39,42,62,67,84,85,86].
5.1. Antihyperglycemic Effect
The beneficial antihyperglycemic effects of FsV are partitioned as follows:
5.1.1. Animal Studies
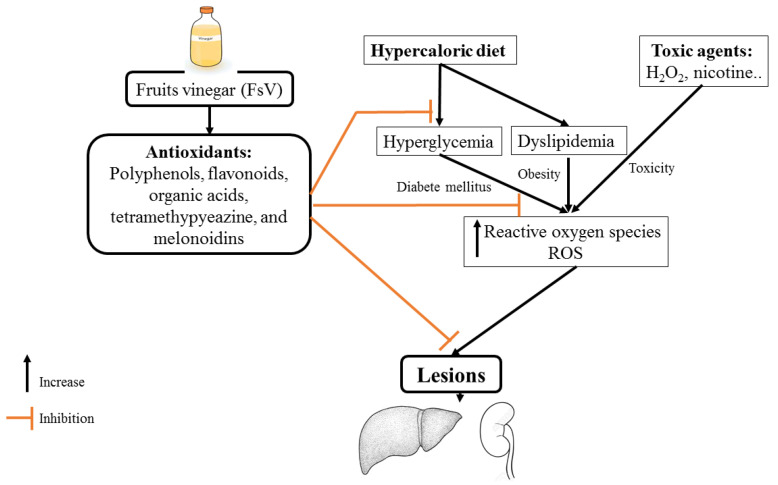
Investigations of the antihyperglycemic activity of FsV were started in the late 80s. In fact, the research conducted by [87] has demonstrated that the co-administration of 2% acetic acid with meals (starch intake) decreased significantly glycemia. Subsequently, multiple studies also found that the administration of apple vinegar reduced blood glucose levels. This effect may be due in part to the stimulation of glucose uptake and enhancement of the action of insulin in skeletal muscle [63]. These beneficial effects have been attributed to acetic acid which acts via MAPK pathway (Figure 1). It is activated through a cascade of phosphorylation/dephosphorylation of proteins which induces inhibition of gene expression [18]. Subsequently, reduction of glucose-6-phosphatase, reduction of Phosphoenolpyruvate carboxykinase (PEPCK), and reduction of Sterol Regulatory Element-Binding Protein-1 (SERBP-1) which decrease blood glucose levels, stimulation of glucose storage, amelioration of insulin sensitivity, and decreases insulinoresistance (Figure 1) [18]. Recently, the administration of apple vinegar at a dose of 2 mL daily by gavage for five weeks decreases the risk of developing persistent hyperglycemia induced by a hypercaloric diet [16]. It was previously reported that the organic acids counteract hydrolyzing enzymes activities such as sucrase, trehalase, maltase, and lactase [88,89].
Figure 1.
Mechanism of action possible of apple vinegar on nephro-hepatic functions against hydrogen peroxide and d-glucose toxicities.
5.1.2. Human Studies
Research conducted on humans was initiated early in ancient Greece medicine, vinegar is prescribed as a remedy for several ailments [30]. Healthy subjects who consumed vinegar, which contains 1 g of acetic acid, combined with meals enriched with carbohydrates limited glycemic response due to its acidity without affecting gastric emptying [90]. Furthermore, vinegar consumption reduced the area under the insulin response curve by 20% after ingestion of sucrose, and the glycemic response was reduced by 30% [89]. Additionally, the ability of vinegar to stimulate glucose uptake by the forearm muscles and blood flow rates were investigated using the arteriovenous difference technique. Authors reported that the administration of vinegar decreases postprandial glycemia and improves the insulin action on skeletal muscle, which enhances glucose disposal [63]. Studies focused on the safety and tolerance of vinegar intake reported that vinegar consumption could induce bowel movements and flatulence. On other hand, Johnston et al. reported the hypoglycemic incidence on one of the patients treated with vinegar [22,58].
5.2. Antihyperlipidemic Effect
Vinegar is used in traditional medicine to treat dyslipidemia, which promotes the development of cardiovascular diseases [91]. The administration of apple vinegar during 8 weeks ameliorates lipid profile (cholesterol, low-density lipoprotein (LDL), and triglycerides) [91]. Additionally, mice fed with a hyper-fat diet and treated with synthetic acetic acid vinegar or nipa vinegar reduced total cholesterol, triglycerides, LDL, and leptin levels [92]. In another study, the treatment of 3T3-L1 cells with tomato vinegar decreased the triglycerides content by 45.71% as compared to cells untreated with tomato vinegar. It suppressed lipid accumulation in 3T3-L1 cells and inhibited adipogenic differentiation, which involves reducing fat accumulation mass and hepatic steatosis [93]. The molecular mechanism involved in the control of body fat by pomegranate vinegar was evoked for the first time by [94] who proved that the pomegranate vinegar activated AMPK in adipose tissue. The activation of AMPK inhibits anabolic pathways through suppression of SERBP-1c (Figure 1) [18,19,94]. Acetic acid is the main component present in vinegar. It has been shown that the acetic acid downregulates the expression of lipogenic genes through the activation of AMPK, subsequently inducing diminution of fatty acid synthase and Acetyl-CoA carboxylase levels [95,96]. The interaction of the phytochemical content of vinegar could be an excellent AMPK activator that may control lipids metabolism (Figure 1).
5.3. Antimicrobial Effect
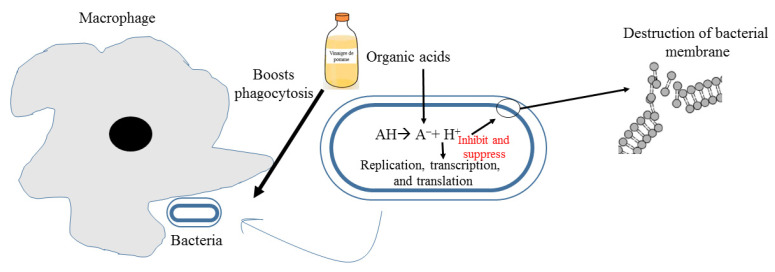
Bacterial resistance poses major health threats around the world on inconceivable scales. Vinegar has been shown to have potent antibacterial activity against resistant bacteria [28,85,97,98,99]. Recently, Yagnik et al. studied the effect of apple vinegar on the multiplication of two resistant bacterial strains (methicillin-resistant Staphylococcus aureus and Escherichia coli resistant to cefepime and cefepime-enmetazobactam combined). Proteomic analysis of both bacteria after treatment with apple vinegar shows the absence of key enzymes for DNA replication, glycolytic and respiratory proteins. Proteins absent after apple vinegar treatment for E. coli are 30 s ribosomal proteins, DNA-directed RNA polymerase alpha subunit, elongation factor TU and G OS-E. coli, formate C-acetyltransferase 1 OS E-coli, chaperone protein, 60 kDa chaperone OS E. coli. Concerning S. aureus, proteins not detected after treatment with apple vinegar are elongation factor TU and phosphoglycerate kinase [99]. Vinegar contains different organic acids that enter through bacterial membrane inciting internal pH decrease, protonation of macromolecules, and destabilization of the cell membrane by liberation of proton H+ (Figure 2) [100,101,102]. Other studies have shown a remarkable antibacterial potency of vinegar as compared with different detergents against several microorganisms [33,103]. On the other hand, antifungal activity of vinegar was established against Candida albicans spp. involved in dental stomatitis [104]. The combination of apple vinegar with the Endovac irrigation system shows promising results in irradiating Enterococcus faecalis (ATCC29212)[44].
Figure 2.
Mechanism of action possible of apple vinegar against pathogenic bacteria.
5.4. Antioxidant Effect
The ability to counteract the deleterious effects of free radicals was the main property searched in a natural product. This property gives vinegar an important preventive and therapeutic effects. Various compounds were found in the vinegar viz polyphenols that have an interesting antioxidant potential due to their power to scavenge free radicals, chelate transition metal ions, and reduce oxidants (Figure 1) [7,8]. Furthermore, melanoidins are among the bioactive compounds in vinegar. Due to their negative charge and macromolecular properties, they have a strong ability to chelate transition metal ions preventing metal-induced oxidation reactions [105]. In addition, it contains a large system of conjugated π bonds and abundant reducing ketone structures, which gives it good free radical scavenging capacity and high reducing power [106]. Likewise, tetramethylpurazine has an antioxidant effect by stabilizing the levels of reactive oxygen species and increasing the levels of antioxidant enzymes including superoxide dismutase and catalase [43]. The antioxidant properties of fruits vinegar were previously studied. A panel of tests was used to determine the antioxidant ability of fruits vinegar. The study conducted by [25] revealed that the pomegranate vinegar exhibited high antioxidant activity as compared to Rioja wine vinegar, Young Sherry vinegar, Reserva Sherry vinegar, and Gran Reserva sherry vinegar. Additionally, the authors concluded that the antioxidant activity of pomegranate vinegar decreased during alcoholic fermentation by 17.6% during acetic fermentation. In a study developed with persimmon vinegar, apple vinegar, polished rice vinegar, and unpolished rice vinegar, researchers compared the antioxidant ability of vinegars and found that persimmon vinegar exhibited high superoxide radical-scavenging activity, DPPH radical-scavenging activity, hydroxyl radical-scavenging activity, and antioxidant activity against lipid peroxidation in tuna homogenates [107]. In vivo studies have shown that fruits vinegar increased enzymatic antioxidants including superoxide dismutase (SOD) (7 fold), glutathione peroxidase (GPX) (4.81 fold), glutathione reductase (GRx) (1.66 fold), and total antioxidant status (TAS) (3.45 fold). Additionally, fruits vinegar proved its ability to decrease TBARS levels both in serum and liver by 61.20% and 43.83% against hyper fat diet [32]. In the same line, Halima et al. [88] evoked that oral administration of fruit vinegar reduced malondialdehyde levels and increased enzymatic antioxidants in hyper fat-diet rats. Fruits vinegars can be useful for the attenuation of oxidative stress through enhancing enzymatic and non-enzymatic antioxidants levels.
5.5. Anti-Inflammatory Effect
Fruit vinegar had multiple uses as a healthy product. It is used against inflammation thanks to its ability to reduce the levels of inflammatory cytokines [39,108]. Previously, it proved that the pear vinegar ameliorates histological disorganization induced by dextran sodium sulfate (DSS) in an experimental animal model. Research experiments have proven that pear vinegar has an interesting ability in reducing serum IL-6 and IL-1ß concentrations [109]. Studies have been suggested vinegar as a potential liquid to overcome inflammation. In opposite, Ross and Poluhowich proposed that the administration of apple cider vinegar was ineffective against inflammation induced by injection of Freund’s complete adjuvant, which induces rheumatoid arthritis [110]. Recently, Beh et al. (2017) reported that the administration of Nipa vinegar inhibited the genes expression of nuclear factor-kappa B (NF-Kb) and inducible nitric oxide synthase (iNOS) in the liver of mice fed high-fat-diet, subsequently decreasing nitric oxide (NO) synthesis in live [92]. The consumption of apple vinegar inhibits cyclooxygenase-2, which suppresses proinflammatory markers expression and leads to diminish cytokines levels [111]. An experiment in vitro revealed that the co-culture of 3T3-L1 and Raw264.7 cells using the contact method with Cudrania tricuspidata fruits vinegar suppressed inflammatory markers expression decreasing nitric oxide (NO), inducible nitric oxide synthase (iNOS), tumor necrosis alpha (TNF-α), interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1) [112].
5.6. Other Effects
Fruits vinegar contains diverse and different amounts of bioactive compounds which make it an active product against several diseases. Mounting evidence proved that fermented food counteracts several diseases including cancer [113]. Fruits vinegar contains also microbial components such as Lipopolysaccharides (LPS) that are generated during vinegar aging by the destruction of microorganisms by an elevation of acidity [114]. LPS modulates the host macrophage network to regulate numerous disorders such as diabetes, dyslipidemia, allergy, and cancer [115]. Additionally, it was proven that vinegar enhances phagocytic activity to ingest resistant microbes such as Staphylococcus aureus and Escherichia coli, which could reinforce the immune system to eradicate pathogen microbes and control the inflammation process [99]. Mimura et al. [116] investigated whether sugar can vinegar exerts an induction effect of apoptosis in human leukemia cells including HL-60, THP-1, Molt-4, U-937, Jurkat, Raji, and K-562. The finding led to the conclusion that sugar can vinegar contains lipophilic components with the ability to induce apoptosis in leukemia human cells. Study conducted by Seki et al. [117] revealed that the incorporation of vinegar in mice diet at a dose of 0.5% during 72 days decreased significantly tumor sizes and prolonged the life spans of mice implemented with sarcoma 180 and colon 38 tumor cells. Oxidative stress plays an important role in the installation of cancer through modifications of DNA. Furthermore, fruits vinegar exerts a good antioxidant ability as described above that could explain its antioxidative effect thereby protecting cells against reactive oxygen species damages.
Fruits vinegar consumption furnishes tremendous beneficial properties. It has been proven that vinegar prevents angiotensin-converting enzymes and reduced blood pressure in spontaneously hypertensive rats [95,118]. The mechanism of action was studied by Na et al. [119], who evoked that the vinegar decreased blood pressure through down-regulating AT1R expression through the AMPK/PGC-1/PPARγ pathway in spontaneously hypertensive rats at a dose of 7 mL/kg during 8 weeks of treatment. The experiment revealed that the treatment with vinegar inhibits the expression of angiotensin II receptor type 1 (AT1R) and increases the expression of PPARγ coactivator 1 alpha and PPARγ, which explains the antihypertensive effect of fruits vinegar.
6. Conclusions
Vinegar is a healthy and wealthy product with multiple functional properties including antidiabetic, antihyperlipidemic, antimicrobial, and anti-inflammatory effects. Different techniques have unraveled the presence of different bioactive compounds in vinegar which are related to the raw material used to produce vinegar. Thus, bioactive ingredients are present or newly generated during brewing process. The synergetic effect between different components of vinegar provides several properties as mentioned above.
Author Contributions
Conceptualization, D.O. and I.E.A.; methodology, D.O. and H.L.; validation, B.L.; writing—original draft preparation, D.O. and H.M.; writing—review and editing, M.B. and A.E.G.; Data curation C.H. and R.C.; supervision, B.L. and I.E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly supported by Cosmetosciences, a global training and research program dedicated to the cosmetic industry. Located in the heart of the Cosmetic Valley, this program led by University of Orléans is funded by the Région Centre-Val de Loire.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ramírez-Guzmán K.N., Torres-León C., Martinez-Medina G.A., de la Rosa O., Hernández-Almanza A., Alvarez-Perez O.B., Araujo R., González L.R., Londoño L., Ventura J. Fermented Beverages. Elsevier; Amsterdam, The Netherlands: 2019. Traditional Fermented Beverages in Mexico; pp. 605–635. [Google Scholar]
- 2.Robledo-Márquez K., Ramírez V., González-Córdova A.F., Ramírez-Rodríguez Y., García-Ortega L., Trujillo J. Research Opportunities: Traditional Fermented Beverages in Mexico. Cultural, Microbiological, Chemical, and Functional Aspects. Food Res. Int. 2021;147:110482. doi: 10.1016/j.foodres.2021.110482. [DOI] [PubMed] [Google Scholar]
- 3.Bounihi A., Bitam A., Bouazza A., Yargui L., Koceir E.A. Fruit Vinegars Attenuate Cardiac Injury via Anti-Inflammatory and Anti-Adiposity Actions in High-Fat Diet-Induced Obese Rats. Pharm. Biol. 2017;55:43–52. doi: 10.1080/13880209.2016.1226369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budak H.N., Guzel-Seydim Z.B. Antioxidant Activity and Phenolic Content of Wine Vinegars Produced by Two Different Techniques. J. Sci. Food Agric. 2010;90:2021–2026. doi: 10.1002/jsfa.4047. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q., Tang G.-Y., Zhao C.-N., Gan R.-Y., Li H.-B. Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants. 2019;8:78. doi: 10.3390/antiox8040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J., Li L., Yue Q., Zhang Q. Determination of Organic Acids in Apple Vinegar by Ion Exclusion Chromatography. [(accessed on 22 April 2021)];China Brew. 2005 12 Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZNGZ200512018.htm. [Google Scholar]
- 7.Bakir S., Toydemir G., Boyacioglu D., Beekwilder J., Capanoglu E. Fruit Antioxidants during Vinegar Processing: Changes in Content and In Vitro Bio-Accessibility. Int. J. Mol. Sci. 2016;17:1658. doi: 10.3390/ijms17101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelebek H., Kadiroğlu P., Demircan N.B., Selli S. Screening of Bioactive Components in Grape and Apple Vinegars: Antioxidant and Antimicrobial Potential. J. Inst. Brew. 2017;123:407–416. doi: 10.1002/jib.432. [DOI] [Google Scholar]
- 9.Chen H., Chen T., Giudici P., Chen F. Vinegar Functions on Health: Constituents, Sources, and Formation Mechanisms. Compr. Rev. Food Sci. Food Saf. 2016;15:1124–1138. doi: 10.1111/1541-4337.12228. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Z., Zhang Y., Wang W., Huang Z., Wang J., Li X., Sun S. Structural Characterisation and Antioxidant Activity of Melanoidins from High-Temperature Fermented Apple. Int. J. Food Sci. Technol. 2020;56:2471–2480. doi: 10.1111/ijfs.14881. [DOI] [Google Scholar]
- 11.Chen J.-C., Chen Q.-H., Guo Q., Ruan S., Ruan H., He G.-Q., Gu Q. Simultaneous Determination of Acetoin and Tetramethylpyrazine in Traditional Vinegars by HPLC Method. Food Chem. 2010;122:1247–1252. doi: 10.1016/j.foodchem.2010.03.072. [DOI] [Google Scholar]
- 12.Wu J., Zhao H., Du M., Song L., Xu X. Dispersive Liquid–Liquid Microextraction for Rapid and Inexpensive Determination of Tetramethylpyrazine in Vinegar. Food Chem. 2019;286:141–145. doi: 10.1016/j.foodchem.2019.01.159. [DOI] [PubMed] [Google Scholar]
- 13.Budak N.H., Kumbul Doguc D., Savas C.M., Seydim A.C., Kok Tas T., Ciris M.I., Guzel-Seydim Z.B. Effects of Apple Cider Vinegars Produced with Different Techniques on Blood Lipids in High-Cholesterol-Fed Rats. J. Agric. Food Chem. 2011;59:6638–6644. doi: 10.1021/jf104912h. [DOI] [PubMed] [Google Scholar]
- 14.Raspor P., Goranovič D. Biotechnological Applications of Acetic Acid Bacteria. Crit. Rev. Biotechnol. 2008;28:101–124. doi: 10.1080/07388550802046749. [DOI] [PubMed] [Google Scholar]
- 15.Budak N.H., Aykin E., Seydim A.C., Greene A.K., Guzel-Seydim Z.B. Functional Properties of Vinegar. J. Food Sci. 2014;79:R757–R764. doi: 10.1111/1750-3841.12434. [DOI] [PubMed] [Google Scholar]
- 16.Ousaaid D., Laaroussi H., Bakour M., ElGhouizi A., Aboulghazi A., Lyoussi B., ElArabi I. Beneficial Effects of Apple Vinegar on Hyperglycemia and Hyperlipidemia in Hypercaloric-Fed Rats. [(accessed on 4 November 2020)]. Available online: https://www.hindawi.com/journals/jdr/2020/9284987/ [DOI] [PMC free article] [PubMed]
- 17.Kondo T., Kishi M., Fushimi T., Kaga T. Acetic Acid Upregulates the Expression of Genes for Fatty Acid Oxidation Enzymes in Liver to Suppress Body Fat Accumulation. J. Agric. Food Chem. 2009;57:5982–5986. doi: 10.1021/jf900470c. [DOI] [PubMed] [Google Scholar]
- 18.Sakakibara S., Yamauchi T., Oshima Y., Tsukamoto Y., Kadowaki T. Acetic Acid Activates Hepatic AMPK and Reduces Hyperglycemia in Diabetic KK-A (y) Mice. Biochem. Biophys. Res. Commun. 2006;344:597–604. doi: 10.1016/j.bbrc.2006.03.176. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita H., Maruta H., Jozuka M., Kimura R., Iwabuchi H., Yamato M., Saito T., Fujisawa K., Takahashi Y., Kimoto M. Effects of Acetate on Lipid Metabolism in Muscles and Adipose Tissues of Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2009;73:570–576. doi: 10.1271/bbb.80634. [DOI] [PubMed] [Google Scholar]
- 20.Lafraxo H., Bakour M., Laaroussi H., El Ghouizi A., Ousaaid D., Aboulghazi A., Lyoussi B. The Synergistic Beneficial Effect of Thyme Honey and Olive Oil against Diabetes and Its Complications Induced by Alloxan in Wistar Rats. Evid.-Based Complement. Alternat. Med. 2021;2021:9949056. doi: 10.1155/2021/9949056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laaroussi H., Bakour M., Ousaaid D., Aboulghazi A., Ferreira-Santos P., Genisheva Z., Teixeira J.A., Lyoussi B. Effect of Antioxidant-Rich Propolis and Bee Pollen Extracts against d-Glucose Induced Type 2 Diabetes in Rats. Food Res. Int. 2020;138:109802. doi: 10.1016/j.foodres.2020.109802. [DOI] [PubMed] [Google Scholar]
- 22.Launholt T.L., Kristiansen C.B., Hjorth P. Safety and Side Effects of Apple Vinegar Intake and Its Effect on Metabolic Parameters and Body Weight: A Systematic Review. Eur. J. Nutr. 2020;59:2273–2289. doi: 10.1007/s00394-020-02214-3. [DOI] [PubMed] [Google Scholar]
- 23.Décret n°2-10-385 Réglementation de La Fabrication et Du Commerce Des Vinaigres. [(accessed on 22 April 2021)];2011 Available online: http://www.onssa.gov.ma/images/reglementation/reglementation-sectorielle/vegetaux-et-produits-dorigine-vegetaux/Produits-dorigine-vegetale/Produits_alimentaires/DEC.2-10-385.FR.pdf.
- 24.Tesfaye W., Morales M.L., García-Parrilla M.C., Troncoso A.M. Wine Vinegar: Technology, Authenticity and Quality Evaluation. Trends Food Sci. Technol. 2002;13:12–21. doi: 10.1016/S0924-2244(02)00023-7. [DOI] [Google Scholar]
- 25.Kharchoufi S., Gomez J., Lasanta C., Castro R., Sainz F., Hamdi M. Benchmarking Laboratory-Scale Pomegranate Vinegar against Commercial Wine Vinegars: Antioxidant Activity and Chemical Composition. J. Sci. Food Agric. 2018;98:4749–4758. doi: 10.1002/jsfa.9011. [DOI] [PubMed] [Google Scholar]
- 26.FAO-WHO Commission DU Codex Alimentarius Treizième Session Rome Décembre 1979 Rapport de la Onzieme Session du Comite de Coordination pour L’europe Innsbruck, 28 Mai–1er Juin 1979. 1979. [(accessed on 22 April 2021)]. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/de/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-701-13%252Fal79_38f.pdf.
- 27.Dabija A., Hatnean C.A. Study Concerning the Quality of Apple Vinegar Obtained through Classical Method. J. Agroaliment. Process. Technol. 2014;20:304–310. [Google Scholar]
- 28.Ousaaid D., Imtara H., Laaroussi H., Lyoussi B., Elarabi I. An Investigation of Moroccan Vinegars: Their Physicochemical Properties and Antioxidant and Antibacterial Activities. [(accessed on 10 February 2021)]. Available online: https://www.hindawi.com/journals/jfq/2021/6618444/
- 29.Ozturk I., Caliskan O., Tornuk F., Ozcan N., Yalcin H., Baslar M., Sagdic O. Antioxidant, Antimicrobial, Mineral, Volatile, Physicochemical and Microbiological Characteristics of Traditional Home-Made Turkish Vinegars. LWT Food Sci. Technol. 2015;63:144–151. doi: 10.1016/j.lwt.2015.03.003. [DOI] [Google Scholar]
- 30.Solieri L., Giudici P. Vinegars of the World. Springer; Berlin/Heidelberg, Germany: 2009. Vinegars of the World; pp. 1–16. [Google Scholar]
- 31.Matloob M.H. Zahdi Date Vinegar: Production and Characterization. Am. J. Food Technol. 2014;9:231–245. doi: 10.3923/ajft.2014.231.245. [DOI] [Google Scholar]
- 32.Bouazza A., Bitam A., Amiali M., Bounihi A., Yargui L., Koceir E.A. Effect of Fruit Vinegars on Liver Damage and Oxidative Stress in High-Fat-Fed Rats. Pharm. Biol. 2016;54:260–265. doi: 10.3109/13880209.2015.1031910. [DOI] [PubMed] [Google Scholar]
- 33.Jia C.-F., Yu W.-N., Zhang B.-L. Manufacture and Antibacterial Characteristics of Eucommia ulmoides Leaves Vinegar. Food Sci. Biotechnol. 2019;29:657–665. doi: 10.1007/s10068-019-00712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theapparat Y., Chandumpai A., Leelasuphakul W., Laemsak N., Ponglimanont C. Physicochemical Characteristics of Wood Vinegars from Carbonization of Leucaena leucocephala, Azadirachta Indica, Eucalyptus camaldulensis, Hevea brasiliensis and Dendrocalamus asper. Agric. Nat. Resour. 2014;48:916–928. [Google Scholar]
- 35.Da Silva Fonseca M., Santos V.A.Q., Calegari G.C., Dekker R.F.H., Barbosa-Dekker A.d.M., da Cunha M.A.A. Blueberry and Honey Vinegar: Successive Batch Production, Antioxidant Potential and Antimicrobial Ability. Braz. J. Food Technol. 2018;21:e2017101. doi: 10.1590/1981-6723.10117. [DOI] [Google Scholar]
- 36.Yusoff H., Saw J.W., Fadzilah I. Physicochemical Properties, Total Phenolic Content, and Antioxidant Capacity of Homemade and Commercial Date (Phoenix dactylifera L.) Vinegar. Int. Food Res. J. 2017;24:2557–2562. [Google Scholar]
- 37.Giuffrè A.M., Zappia C., Capocasale M., Poiana M., Sidari R., Di Donna L., Bartella L., Sindona G., Corradini G., Giudici P., et al. Vinegar Production to Valorise Citrus bergamia By-Products. Eur. Food Res. Technol. 2019;245:667–675. doi: 10.1007/s00217-018-3189-y. [DOI] [Google Scholar]
- 38.Patrignani F., D’Alessandro M., Vannini L., Lanciotti R. Sustainability of the Food System. Elsevier; Amsterdam, The Netherlands: 2020. Use of Functional Microbial Starters and Probiotics to Improve Functional Compound Availability in Fermented Dairy Products and Beverages; pp. 167–180. [Google Scholar]
- 39.Xia T., Zhang B., Duan W., Zhang J., Wang M. Nutrients and Bioactive Components from Vinegar: A Fermented and Functional Food. J. Funct. Foods. 2020;64:103681. doi: 10.1016/j.jff.2019.103681. [DOI] [Google Scholar]
- 40.De Jong C., Hazelwood L.A., Dijkstra A., Pepin L. Flavour Science. Elsevier; Amsterdam, The Netherlands: 2014. Use of the Micro-Scale Platform for High Throughput Screening of Flavor Characteristics in Strains (Yeast/LAB) for Alcoholic Beverages; pp. 355–359. [Google Scholar]
- 41.Lee J.H., Choi K.H., Kim S.H., Park K.S., Park S.H., Kim J.S., Kang S.A., Cheong C., Jang K.H. Physicochemical Characteristics and Electric Conductivity of Various Fruit Wines. Int. Food Res. J. 2013;20:2987–2993. [Google Scholar]
- 42.Xia T., Zhang J., Yao J., Zhang B., Duan W., Zhao C., Du P., Song J., Zheng Y., Wang M. Shanxi Aged Vinegar Protects against Alcohol-Induced Liver Injury via Activating Nrf2-Mediated Antioxidant and Inhibiting TLR4-Induced Inflammatory Response. Nutrients. 2018;10:805. doi: 10.3390/nu10070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J., Tian J., Ge H., Liu R., Xiao J. Effects of Tetramethylpyrazine from Chinese Black Vinegar on Antioxidant and Hypolipidemia Activities in HepG2 Cells. Food Chem. Toxicol. 2017;109:930–940. doi: 10.1016/j.fct.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 44.El-Sayed T.S., Nour El-Deen M.M., Rokaya M.E., Sherif M.M. Evaluation of the Antibacterial Effect of Apple Vinegar as a Root Canal Irrigant Using Endovac Irrigation System. Al-Azhar Dent. J. Girls. 2019;6:53–59. doi: 10.21608/adjg.2019.28400. [DOI] [Google Scholar]
- 45.Aykın E., Budak N.H., Güzel-Seydim Z.B. Bioactive Components of Mother Vinegar. J. Am. Coll. Nutr. 2015;34:80–89. doi: 10.1080/07315724.2014.896230. [DOI] [PubMed] [Google Scholar]
- 46.Duan W., Xia T., Zhang B., Li S., Zhang C., Zhao C., Song J., Wang M. Changes of Physicochemical, Bioactive Compounds and Antioxidant Capacity during the Brewing Process of Zhenjiang Aromatic Vinegar. Molecules. 2019;24:3935. doi: 10.3390/molecules24213935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura K., Ogasawara Y., Endou K., Fujimori S., Koyama M., Akano H. Phenolic Compounds Responsible for the Superoxide Dismutase-like Activity in High-Brix Apple Vinegar. J. Agric. Food Chem. 2010;58:10124–10132. doi: 10.1021/jf100054n. [DOI] [PubMed] [Google Scholar]
- 48.Ren M., Wang X., Tian C., Li X., Zhang B., Song X., Zhang J. Characterization of Organic Acids and Phenolic Compounds of Cereal Vinegars and Fruit Vinegars in China. J. Food Process. Preserv. 2017;41:e12937. doi: 10.1111/jfpp.12937. [DOI] [Google Scholar]
- 49.Bortolini D.G., Benvenutti L., Demiate I.M., Nogueira A., Alberti A., Zielinski A.A.F. A New Approach to the Use of Apple Pomace in Cider Making for the Recovery of Phenolic Compounds. LWT. 2020;126:109316. doi: 10.1016/j.lwt.2020.109316. [DOI] [Google Scholar]
- 50.Özen M., Özdemir N., Filiz B.E., Budak N.H., Kök-Taş T. Sour Cherry (Prunus cerasus L.) Vinegars Produced from Fresh Fruit or Juice Concentrate: Bioactive Compounds, Volatile Aroma Compounds and Antioxidant Capacities. Food Chem. 2020;309:125664. doi: 10.1016/j.foodchem.2019.125664. [DOI] [PubMed] [Google Scholar]
- 51.Chatatikun M., Kwanhian W. Phenolic Profile of Nipa Palm Vinegar and Evaluation of Its Antilipidemic Activities. Evid.-Based Complement. Alternat. Med. 2020;2020:6769726. doi: 10.1155/2020/6769726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mudura E., Coldea T.E., Socaciu C., Ranga F., Pop C.R., Rotar A.M., Pasqualone A. Brown Beer Vinegar: A Potentially Functional Product Based on Its Phenolic Profile and Antioxidant Activity. J. Serb. Chem. Soc. 2018;83:19–30. doi: 10.2298/JSC170803107M. [DOI] [Google Scholar]
- 53.Roda A., Lucini L., Torchio F., Dordoni R., De Faveri D.M., Lambri M. Metabolite Profiling and Volatiles of Pineapple Wine and Vinegar Obtained from Pineapple Waste. Food Chem. 2017;229:734–742. doi: 10.1016/j.foodchem.2017.02.111. [DOI] [PubMed] [Google Scholar]
- 54.Jiménez-Sánchez M., Durán-Guerrero E., Rodríguez-Dodero M.C., Barroso C.G., Castro R. Use of Ultrasound at a Pilot Scale to Accelerate the Ageing of Sherry Vinegar. Ultrason. Sonochem. 2020;69:105244. doi: 10.1016/j.ultsonch.2020.105244. [DOI] [PubMed] [Google Scholar]
- 55.Chen G.-L., Zheng F.-J., Lin B., Lao S.-B., He J., Huang Z., Zeng Y., Sun J., Verma K.K. Phenolic and Volatile Compounds in the Production of Sugarcane Vinegar. ACS Omega. 2020;5:30587–30595. doi: 10.1021/acsomega.0c04524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao Y., Jo Y., Chung N., Gu S.-Y., Jeong Y.-J., Kwon J.-H. Physicochemical Qualities and Flavor Patterns of Traditional Chinese Vinegars Manufactured by Different Fermentation Methods and Aging Periods. Prev. Nutr. Food Sci. 2017;22:30–36. doi: 10.3746/pnf.2017.22.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnston C.S., Quagliano S., White S. Vinegar Ingestion at Mealtime Reduced Fasting Blood Glucose Concentrations in Healthy Adults at Risk for Type 2 Diabetes. J. Funct. Foods. 2013;5:2007–2011. doi: 10.1016/j.jff.2013.08.003. [DOI] [Google Scholar]
- 58.Johnston K.L., Clifford M.N., Morgan L.M. Possible Role for Apple Juice Phenolic Compounds in the Acute Modification of Glucose Tolerance and Gastrointestinal Hormone Secretion in Humans. J. Sci. Food Agric. 2002;82:1800–1805. doi: 10.1002/jsfa.1264. [DOI] [Google Scholar]
- 59.Johnston C.S., Buller A.J. Vinegar and Peanut Products as Complementary Foods to Reduce Postprandial Glycemia. J. Am. Diet. Assoc. 2005;105:1939–1942. doi: 10.1016/j.jada.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Shah Q.A., Bibi F., Shah A.H. Anti-Microbial Effects of Olive Oil and Vinegar against Salmonella and Escherichia coli. Pac. J. Sci. Technol. 2013;14:479–486. [Google Scholar]
- 61.Shishehbor F., Mansoori A., Sarkaki A.R., Jalali M.T., Latifi S.M. Apple Cider Vinegar Attenuates Lipid Profile in Normal and Diabetic Rats. Pak. J. Biol. Sci. PJBS. 2008;11:2634–2638. doi: 10.3923/pjbs.2008.2634.2638. [DOI] [PubMed] [Google Scholar]
- 62.Tripathi S., Mazumder P.M. Apple Cider Vinegar (ACV) and Their Pharmacological Approach towards Alzheimer’s Disease (AD): A Review. Indian J. Pharm. Educ. Res. 2020;54:s67–s74. doi: 10.5530/ijper.54.2s.62. [DOI] [Google Scholar]
- 63.Mitrou P., Petsiou E., Papakonstantinou E., Maratou E., Lambadiari V., Dimitriadis P., Spanoudi F., Raptis S.A., Dimitriadis G. Vinegar Consumption Increases Insulin-Stimulated Glucose Uptake by the Forearm Muscle in Humans with Type 2 Diabetes. J. Diabetes Res. 2015;2015:175204. doi: 10.1155/2015/175204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naseem E., Shamim M., Khan N.I. Cardioprotective Effects of Herbal Mixture (Ginger, Garlic, Lemon, Apple Cider Vinegar & Honey) in Experimental Animal Models of Hyperlipidemia. Int. J. Biol. Res. 2016;4:28–33. [Google Scholar]
- 65.Nazıroğlu M., Güler M., Özgül C., Saydam G., Küçükayaz M., Sözbir E. Apple Cider Vinegar Modulates Serum Lipid Profile, Erythrocyte, Kidney, and Liver Membrane Oxidative Stress in Ovariectomized Mice Fed High Cholesterol. J. Membr. Biol. 2014;247:667–673. doi: 10.1007/s00232-014-9685-5. [DOI] [PubMed] [Google Scholar]
- 66.Omar N.A.A., Allithy A.N.E.A., Faleh F.M., Mariah R.A., Ayat M.M.A., Shafik S.R., Elshweikh S.A., Baghdadi H., Sayed S.M.E. Apple Cider Vinegar (A Prophetic Medicine Remedy) Protects against Nicotine Hepatotoxicity: A Histopathological and Biochemical Report. Am. J. Cancer Prev. 2015;3:122–127. [Google Scholar]
- 67.Öztürk A., Özdemir Y., Göksel Z. Apple Vinegar and Its Therapeutic Effects. TABAD Tarım Bilimleri Araştırma Dergisi. 2009;2:155–158. [Google Scholar]
- 68.Petsiou E.I., Mitrou P.I., Raptis S.A., Dimitriadis G.D. Effect and Mechanisms of Action of Vinegar on Glucose Metabolism, Lipid Profile, and Body Weight. Nutr. Rev. 2014;72:651–661. doi: 10.1111/nure.12125. [DOI] [PubMed] [Google Scholar]
- 69.Salbe A.D., Johnston C.S., Buyukbese M.A., Tsitouras P.D., Harman S.M. Vinegar Lacks Antiglycemic Action on Enteral Carbohydrate Absorption in Human Subjects. Nutr. Res. 2009;29:846–849. doi: 10.1016/j.nutres.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 70.Setorki M., Asgary S., Eidi A., KHazaei M. Acute Effects of Vinegar Intake on Some Biochemical Risk Factors of Atherosclerosis in Hypercholesterolemic Rabbits. Lipids Health Dis. 2010;9:10. doi: 10.1186/1476-511X-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thinathayalan D., Yuan B.T.K., Kaur J., Albert Y., Yan N.J. The Effects of Apple Cider Vinegar on Weight, Blood Pressure, Blood Glucose Level and Heart Rate of 60 MMMC Medical Students Randomized Controlled Trial. Med. J. 2019;6:88. [Google Scholar]
- 72.Mateos-Aparicio I., de la Peña R.J., Pérez-Cózar M.L., Rupérez P., Redondo-Cuenca A., Villanueva-Suárez M.J. Apple By-Product Dietary Fibre Exhibits Potential Prebiotic and Hypolipidemic Effectsin High-Fat Fed Wistar Rats. Bioact. Carbohydr. Diet. Fibre. 2020;23:100219. doi: 10.1016/j.bcdf.2020.100219. [DOI] [Google Scholar]
- 73.Krusong W., Sriphochanart W., Suwapanich R., Mekkerdchoo O., Sriprom P., Wipatanawin A., Massa S. Healthy Dried Baby Corn Silk Vinegar Production and Determination of Its Main Organic Volatiles Containing Antimicrobial Activity. LWT. 2020;117:108620. doi: 10.1016/j.lwt.2019.108620. [DOI] [Google Scholar]
- 74.Tanamool V., Chantarangsee M., Soemphol W. Simultaneous Vinegar Fermentation from a Pineapple By-Product Using the Co-Inoculation of Yeast and Thermotolerant Acetic Acid Bacteria and Their Physiochemical Properties. 3 Biotech. 2020;10:115. doi: 10.1007/s13205-020-2119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeda A., Tamano H. The Impact of Synaptic Zn2+ Dynamics on Cognition and Its Decline. Int. J. Mol. Sci. 2017;18:2411. doi: 10.3390/ijms18112411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cherasse Y., Urade Y. Dietary Zinc Acts as a Sleep Modulator. Int. J. Mol. Sci. 2017;18:2334. doi: 10.3390/ijms18112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akram M., Munir N., Daniyal M., Egbuna C., Găman M.-A., Onyekere P.F., Olatunde A. Functional Foods and Nutraceuticals. Springer; Berlin/Heidelberg, Germany: 2020. Vitamins and Minerals: Types, Sources and Their Functions; pp. 149–172. [Google Scholar]
- 78.Ousaaid D., Mansouri I., Rochdi M., Lyoussi B., El Arabi I. Etude Des Paramètres Physico-Chimiques et de L’Activité Antioxydante de Trois Vinaigres de Cidre Traditionnels Issus de Trois Variétés de Pomme de La Région de Midelt Au Maroc. Elwahat Recherches Etudes. 2017;10:37–50. doi: 10.54246/1548-010-001-011. [DOI] [Google Scholar]
- 79.Akpinar-Bayizit A., Turan M.A., Yilmaz-Ersan L., Taban N. Inductively Coupled Plasma Optical-Emission Spectroscopy Determination of Major and Minor Elements in Vinegar. Not. Bot. Horti Agrobot. Cluj-Napoca. 2010;38:64–68. [Google Scholar]
- 80.Zhang Y., Wang X., Liu B., Liu Q., Zheng H., You X., Sun K., Luo X., Li F. Comparative Study of Individual and Co-Application of Biochar and Wood Vinegar on Blueberry Fruit Yield and Nutritional Quality. Chemosphere. 2020;246:125699. doi: 10.1016/j.chemosphere.2019.125699. [DOI] [PubMed] [Google Scholar]
- 81.Kahraman H.A., Tutun H., Keyvan E., Balkan B.M. Investigation of Chemical, Antibacterial and Antiradical Properties of Home-Made Apple and Grape Vinegars. Ankara Üniversitesi Veteriner Fakültesi Dergisi. 2021 doi: 10.33988/auvfd.865309. [DOI] [Google Scholar]
- 82.Prisacaru A.E., Ghinea C., Apostol L.C., Ropciuc S., Ursachi V.F. Physicochemical Characteristics of Vinegar from Banana Peels and Commercial Vinegars before and after In Vitro Digestion. Processes. 2021;9:1193. doi: 10.3390/pr9071193. [DOI] [Google Scholar]
- 83.Sabrina B., Mohamed D.O.E.H. Contribution a L’Etude des Caracteristiques Physico-Chimiques et Biochimiques de Quelques Types de Vinaigres Traditionnels de Dattes Obtenues a Partir de Quelques Varietes de la Region de Ouargla. Ann. Sci. Technol. 2010;2:80–86. [Google Scholar]
- 84.Tripathi S., Kumari U., Mazumder P.M. Ameliorative Effects of Apple Cider Vinegar on Neurological Complications via Regulation of Oxidative Stress Markers. J. Food Biochem. 2020;44:e13504. doi: 10.1111/jfbc.13504. [DOI] [PubMed] [Google Scholar]
- 85.Yagnik D., Serafin V., Shah A.J. Antimicrobial Activity of Apple Cider Vinegar against Escherichia Coli, Staphylococcus Aureus and Candida Albicans; Downregulating Cytokine and Microbial Protein Expression. Sci. Rep. 2018;8:1732. doi: 10.1038/s41598-017-18618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J., Tian Z.G., Wang J.H., Wang A.R. Advances in Antimicrobial Molecular Mechanism of Organic Acids. Acta Vet. Zootech. Sin. Chin. J. Anim. Vet. Sci. 2011;42:323–328. [Google Scholar]
- 87.Ebihara K., Nakajima A. Effect of Acetic Acid and Vinegar on Blood Glucose and Insulin Responses to Orally Administered Sucrose and Starch. Agric. Biol. Chem. 1988;52:1311–1312. [Google Scholar]
- 88.Halima B.H., Sonia G., Sarra K., Houda B.J., Fethi B.S., Abdallah A. Apple Cider Vinegar Attenuates Oxidative Stress and Reduces the Risk of Obesity in High-Fat-Fed Male Wistar Rats. J. Med. Food. 2017;21:70–80. doi: 10.1089/jmf.2017.0039. [DOI] [PubMed] [Google Scholar]
- 89.Ogawa N., Satsu H., Watanabe H., Fukaya M., Tsukamoto Y., Miyamoto Y., Shimizu M. Acetic Acid Suppresses the Increase in Disaccharidase Activity That Occurs during Culture of Caco-2 Cells. J. Nutr. 2000;130:507–513. doi: 10.1093/jn/130.3.507. [DOI] [PubMed] [Google Scholar]
- 90.Brighenti F., Castellani G., Benini L., Casiraghi M.C., Leopardi E., Crovetti R., Testolin G. Effect of Neutralized and Native Vinegar on Blood Glucose and Acetate Responses to a Mixed Meal in Healthy Subjects. Eur. J. Clin. Nutr. 1995;49:242–247. [PubMed] [Google Scholar]
- 91.Bahesheti Z., Chan Y.H., Nia H.S., Hajihosseini F., Nazari R., Shaabani M. Influence of Apple Cider Vinegar on Blood Lipids. Life Sci. J. 2012;9:2431–2440. [Google Scholar]
- 92.Beh B.K., Mohamad N.E., Yeap S.K., Ky H., Boo S.Y., Chua J.Y.H., Tan S.W., Ho W.Y., Sharifuddin S.A., Long K. Anti-Obesity and Anti-Inflammatory Effects of Synthetic Acetic Acid Vinegar and Nipa Vinegar on High-Fat-Diet-Induced Obese Mice. Sci. Rep. 2017;7:6664. doi: 10.1038/s41598-017-06235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee S., Lee J.-A., Park G.-G., Jang J.-K., Park Y.-S. Semi-Continuous Fermentation of Onion Vinegar and Its Functional Properties. Molecules. 2017;22:1313. doi: 10.3390/molecules22081313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park J.E., Kim J.Y., Kim J., Kim Y.J., Kim M.J., Kwon S.W., Kwon O. Pomegranate Vinegar Beverage Reduces Visceral Fat Accumulation in Association with AMPK Activation in Overweight Women: A Double-Blind, Randomized, and Placebo-Controlled Trial. J. Funct. Foods. 2014;8:274–281. doi: 10.1016/j.jff.2014.03.028. [DOI] [Google Scholar]
- 95.Kondo S., Tayama K., Tsukamoto Y., Ikeda K., Yamori Y. Antihypertensive Effects of Acetic Acid and Vinegar on Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 2001;65:2690–2694. doi: 10.1271/bbb.65.2690. [DOI] [PubMed] [Google Scholar]
- 96.Yamashita H., Fujisawa K., Ito E., Idei S., Kawaguchi N., Kimoto M., Hiemori M., Tsuji H. Improvement of Obesity and Glucose Tolerance by Acetate in Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2007;71:1236–1243. doi: 10.1271/bbb.60668. [DOI] [PubMed] [Google Scholar]
- 97.Hindi N.K. In Vitro Antibacterial Activity of Aquatic Garlic Extract, Apple Vinegar and Apple Vinegar-Garlic Extract Combination. Am. J. Phytomed. Clin. Ther. 2013;1:42–51. [Google Scholar]
- 98.Hindi N.K.K., Al-Mahdi Z.K.A., Chabuck Z.A.G. Antibacterial Activity of the Aquatic Extractof Fresh, Dry Powder Ginger, Apple Vinegar Extract of Fresh Ginger and Crud Oil of Ginger (Zingiberofficinale) against Different Types of Bacteria in Hilla City, Iraq. Prostate. 2014;3:6. [Google Scholar]
- 99.Yagnik D., Ward M., Shah A.J. Antibacterial Apple Cider Vinegar Eradicates Methicillin Resistant Staphylococcus aureus and Resistant Escherichia coli. Sci. Rep. 2021;11:1854. doi: 10.1038/s41598-020-78407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alakomi H.-L., Skyttä E., Saarela M., Mattila-Sandholm T., Latva-Kala K., Helander I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000;66:2001–2005. doi: 10.1128/AEM.66.5.2001-2005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brul S., Coote P. Preservative Agents in Foods: Mode of Action and Microbial Resistance Mechanisms. Int. J. Food Microbiol. 1999;50:1–17. doi: 10.1016/S0168-1605(99)00072-0. [DOI] [PubMed] [Google Scholar]
- 102.Hirshfield I.N., Terzulli S., O’Byrne C. Weak Organic Acids: A Panoply of Effects on Bacteria. Sci. Prog. 2003;86:245–269. doi: 10.3184/003685003783238626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.AL-Salihi S.S. Antibacterial Activity of Some Disinfectants and Detergents on Some Pathogenic Bacteria. J. Pharm. Sci. Res. 2019;11:590–597. [Google Scholar]
- 104.Mota A.C.L.G., de Castro R.D., de Araújo Oliveira J., de Oliveira Lima E. Antifungal Activity of Apple Cider Vinegar on Candida Species Involved in Denture Stomatitis. J. Prosthodont. 2015;24:296–302. doi: 10.1111/jopr.12207. [DOI] [PubMed] [Google Scholar]
- 105.Wang H., Liu J., Liu Z. Effect of Enzymatic Digestion, Chemical and Boiled Water Extraction Techniques on Apparent Antioxidant Bioactivities of Apple Peel. J. Food Meas. Charact. 2019;13:959–966. doi: 10.1007/s11694-018-0010-3. [DOI] [Google Scholar]
- 106.Wang H.-Y., Qian H., Yao W.-R. Melanoidins Produced by the Maillard Reaction: Structure and Biological Activity. Food Chem. 2011;128:573–584. doi: 10.1016/j.foodchem.2011.03.075. [DOI] [Google Scholar]
- 107.Sakanaka S., Ishihara Y. Comparison of Antioxidant Properties of Persimmon Vinegar and Some Other Commercial Vinegars in Radical-Scavenging Assays and on Lipid Oxidation in Tuna Homogenates. Food Chem. 2008;107:739–744. doi: 10.1016/j.foodchem.2007.08.080. [DOI] [Google Scholar]
- 108.Ho C.W., Lazim A.M., Fazry S., Zaki U.K.H.H., Lim S.J. Varieties, Production, Composition and Health Benefits of Vinegars: A Review. Food Chem. 2017;221:1621–1630. doi: 10.1016/j.foodchem.2016.10.128. [DOI] [PubMed] [Google Scholar]
- 109.Wakuda T., Azuma K., Saimoto H., Ifuku S., Morimoto M., Arifuku I., Asaka M., Tsuka T., Imagawa T., Okamoto Y. Protective Effects of Galacturonic Acid-Rich Vinegar Brewed from Japanese Pear in a Dextran Sodium Sulfate-Induced Acute Colitis Model. J. Funct. Foods. 2013;5:516–523. doi: 10.1016/j.jff.2012.10.010. [DOI] [Google Scholar]
- 110.Ross C.M., Poluhowich J.J. The Effect of Apple Cider Vinegar on Adjuvant Arthritic Rats. Nutr. Res. 1984;4:737–741. doi: 10.1016/S0271-5317(84)80049-4. [DOI] [Google Scholar]
- 111.Golzarand M., Ebrahimi-Mamaghani M., Arefhosseini S.R., Asgarzadeh A.A. Effect of Processed Berberis Vulgaris in Apple Vinegar on Blood Pressure and Inflammatory Markers in Type 2 Diabetic Patients. Iran. J. Diabetes Metab. Disord. 2008;7:15–20. [Google Scholar]
- 112.Choi J.-H., Park S.-E., Yeo S.-H., Kim S. Anti-Inflammatory and Cytotoxicity Effects of Cudrania Tricuspidata Fruits Vinegar in a Co-Culture System with RAW264. 7 Macrophages and 3T3-L1 Adipocytes. Foods. 2020;9:1232. doi: 10.3390/foods9091232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tasdemir S.S., Sanlier N. An Insight into the Anticancer Effects of Fermented Foods: A Review. J. Funct. Foods. 2020;75:104281. doi: 10.1016/j.jff.2020.104281. [DOI] [Google Scholar]
- 114.Hashimoto M., Obara K., Ozono M., Furuyashiki M., Ikeda T., Suda Y., Fukase K., Fujimoto Y., Shigehisa H. Separation and Characterization of the Immunostimulatory Components in Unpolished Rice Black Vinegar (Kurozu) J. Biosci. Bioeng. 2013;116:688–696. doi: 10.1016/j.jbiosc.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 115.Kohchi C., Inagawa H., Nishizawa T., Yamaguchi T., Nagai S., Soma G.-I. Applications of Lipopolysaccharide Derived from Pantoea Agglomerans (IP-PA1) for Health Care Based on Macrophage Network Theory. J. Biosci. Bioeng. 2006;102:485–496. doi: 10.1263/jbb.102.485. [DOI] [PubMed] [Google Scholar]
- 116.Mimura A., Suzuki Y., Toshima Y., Yazaki S., Ohtsuki T., Ui S., Hyodoh F. Induction of Apoptosis in Human Leukemia Cells by Naturally Fermented Sugar Cane Vinegar (Kibizu) of Amami Ohshima Island. Biofactors. 2004;22:93–97. doi: 10.1002/biof.5520220118. [DOI] [PubMed] [Google Scholar]
- 117.Seki T., Morimura S., Shigematsu T., Maeda H., Kida K. Antitumor Activity of Rice-Shochu Post-Distillation Slurry and Vinegar Produced from the Post-Distillation Slurry via Oral Administration in a Mouse Model. Biofactors. 2004;22:103–105. doi: 10.1002/biof.5520220120. [DOI] [PubMed] [Google Scholar]
- 118.Ohnami K. Effects of Kurosu on the Blood Pressure of the Spontaneously Hypertension Rats. Kiso Rinsho. 1985;19:237–241. [Google Scholar]
- 119.Na L., Chu X., Jiang S., Li C., Li G., He Y., Liu Y., Li Y., Sun C. Vinegar Decreases Blood Pressure by Down-Regulating AT1R Expression via the AMPK/PGC-1α/PPARγ Pathway in Spontaneously Hypertensive Rats. Eur. J. Nutr. 2016;55:1245–1253. doi: 10.1007/s00394-015-0937-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.