Abstract
Background
Coronavirus disease (COVID–19) has resulted in the death of more than 3.5 million people worldwide. While COVID–19 mostly affects the lungs, different comorbidities can have an impact on its outcomes. We performed an overview of reviews to assess the effect of Chronic Kidney Disease (CKD) on contracting COVID–19, hospitalization, mortality, and disease severity.
Methods
We searched published and preprint databases. We updated the reviews by searching for primary studies published after August 2020, and prioritized reviews that are most updated and of higher quality using the AMSTAR tool.
Results
We included 69 systematic reviews and 66 primary studies. Twenty-eight reviews reported on the prevalence of CKD among patients with COVID–19, which ranged from 0.4 to 49.0%. One systematic review showed an increased risk of hospitalization in patients with CKD and COVID–19 (RR = 1.63, 95% CI 1.03–2.58) (Moderate certainty). Primary studies also showed a statistically significant increase of hospitalization in such patients. Thirty-seven systematic reviews assessed mortality risk in patients with CKD and COVID–19. The pooled estimates from primary studies for mortality in patients with CKD and COVID–19 showed a HR of 1.48 (95% CI 1.33–1.65) (Moderate certainty), an OR of 1.77 (95% CI 1.54–2.02) (Moderate certainty) and a RR of 1.6 (95% CI 0.88–2.92) (Low certainty).
Conclusions
Our review highlights the impact of CKD on the poor outcomes of COVID–19, underscoring the importance of identifying strategies to prevent COVID–19 infection among patients with CKD.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40620-021-01206-8.
Keywords: COVID–19, SARS–CoV–2, Chronic kidney disease (CKD), Mortality, Hospitalization
Introduction
Since its emergence in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS–CoV–2) has caused over 240 million confirmed cases and more than 5 million deaths worldwide at the time of writing [1]. The World Health organization (WHO) declared it to be a global pandemic in March 2020. Multiple studies have assessed the association between different comorbidities and coronavirus disease 2019 (COVID–19) outcomes [2, 3]. COVID–19 preferentially affects the lungs with a potential to involve multiple organ systems, including the kidneys.
The global prevalence of chronic kidney disease (CKD) is estimated to be between 9 and 12% [4]. The incidence of CKD increases with age, and about 38% of the estimated CKD population is > 65 years of age [5]. The definition and classification of CKD was established and endorsed by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative and the international Kidney Disease Improving Global Outcomes (KDIGO) guideline group which categorizes CKD into five stages based on the estimated glomerular filtration rate (eGFR) (level) with further sub–classification of stage 5 into dialysis-dependent and dialysis-independent [6]. Advanced CKD is associated with a marked increase in the risk of all–cause mortality and morbidity [7]. Cardiovascular causes are estimated to account for 50% of the mortality in patients with CKD, while infections are recognized as a leading cause of non–cardiovascular morbidity and mortality in patients with advanced CKD [8–11].
In this updated overview of reviews, we aim to summarize the effect of CKD on different outcomes among patients with COVID–19. We reviewed available systematic reviews and large primary studies to assess COVID–19 incidence, severity, risk of hospitalization, and mortality among patients with CKD.
Materials and methods
The protocol for this overview was published online and is available on PROSPERO (International Prospective Register of Systematic Reviews). The registration number is CRD42021227974. There were no amendments from the pre-specified criteria reported in the protocol throughout the review process. The results are reported according to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [12].
Inclusion and exclusion criteria
We started by conducting an overview of systematic reviews that reported COVID–19 outcomes in patients with CKD from January 1, 2020 to January 5, 2021. After searching systematic reviews, we updated the search by identifying primary studies published after August 2020, which was the date of last search in the reviews. We included all published and unpublished studies of any design including retrospective, prospective, and cross-sectional observational studies. The review included studies of adult patients with suspected or confirmed COVID–19 who had CKD. The update focused on primary studies with more than 1000 patients with COVID–19. We excluded systematic reviews and primary studies focusing on children, pregnant women, kidney transplant recipients, and those with acute kidney injury. We prioritized the following PICO questions, which we addressed in the review:
PICO 1: What is the risk of patients with chronic kidney disease being infected by SARS–CoV2?
PICO 2: What is the risk of patients with chronic kidney disease being hospitalized for COVID–19?
PICO 3: What is the risk of severe COVID–19 disease and death amongst patients with chronic kidney disease?
Search strategy
The methods team searched the following electronic databases: Embase, PubMed, Epistemonikos, and Cochrane from January 1st, 2020 to January 5th, 2021. Additionally, the investigators searched MEDRXIV, SSRN, and LiTCOVID databases for preprints of unpublished reviews. The detailed search strategy is available in Appendix-1 (See Online Supplementary material). In addition, reviewers manually checked the reference lists of included studies to identify additional relevant publications. The investigators further extended the search to include primary studies that were not incorporated in the systematic reviews from September 1st, 2020 to January 10th, 2021. The investigators also included results from four registries in this review: Hilbrands 2020 [13], Holman 2020 [14], Jager 2020 [15], and Williamson 2020 [16]. Similarly, reviewers assessed the references of included primary studies to identify additional publications that were not captured in the original search.
Data collection
Four investigators (AA, RM, AG, SJ) independently performed title and abstract screening in pairs to identify eligible literature. When present, disagreements were resolved by a third investigator (RAM). After full-text screening, four reviewers extracted data from the included systematic reviews independently (AA, RM, AG, SJ).
We collected the following information from each review: study characteristics (author name, region/country, study design, inclusion and exclusion criteria), patient characteristics (number of patients with CKD, age, gender, comorbidities, and clinical setting), and CKD specifications (CKD stage and whether they included patients with end stage kidney disease (ESKD) or not). We extracted the adjusted effect estimates when available with 95% confidence interval (CI) including odds ratio (OR), relative risk (RR), and hazard ratio (HR) for the following outcomes: incidence of COVID–19 infection, hospitalization, severe illness, ICU admission, mechanical ventilation, mortality, and poor outcomes among patients with CKD and COVID–19 infection from both systematic reviews and primary studies.
Quality and risk of bias assessment
We evaluated the quality of included systematic reviews using the modified Assessment of the methodological quality of systematic reviews (AMSTAR) tool checklist [17], and applying the following criteria: availability of a study protocol, comprehensive search strategy, list of excluded studies and their reason for exclusion, risk of bias (RoB) assessment and evaluation of its impact, appropriate methods for statistical combination of results, and assessment of publication bias.
When more than one review addressed the same question, we prioritized reviews that fulfilled most of the following criteria: higher AMSTAR rating, peer reviewed, recent date of literature search, use of the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) assessment. We also prioritized reviews that addressed the outcomes of interest in the most direct way. Appendix 2A (See Online Supplementary material) provides details about the AMSTAR evaluation of all included systematic reviews.
We evaluated the risk of bias of the primary studies using the Quality in Prognostic Studies (QUIPS) tool [18]. The QUIPS tool covers six domains: selection bias, attrition bias, prognostic factor and outcome measurement, confounding, and bias related to statistical analysis or presentation of results. The quality of each study was categorized as low risk, moderate risk, and high risk for each of the six domains. Appendix 2B (See Online Supplementary material) provides details about the QUIPS RoB evaluation for the included primary studies.
Certainty of the evidence
We assessed the certainty in the evidence using the GRADE approach [19]. This approach has four levels of certainty; very low, low, moderate and high. Observational studies start at low certainty and can be downgraded for concerns of risk of bias, indirectness (applicability of the results to the question), inconsistency (heterogeneity between study results), imprecision, and publication bias, while it can be upgraded if there is a large effect, residual confounding effect, or dose-response gradient.
Statistical analysis
Quantitative and descriptive analyses were conducted. We summarized the characteristics of the included systematic reviews and primary studies. Moreover, overall ORs, HRs, and RRs, along with their respective 95% CIs of the mortality outcome in patients diagnosed with CKD versus without CKD diagnosis and COVID–19 were calculated from the additional primary studies and the studies included in the reviews using a random effect model when more than five studies were available. Study data was considered worthy of exploration of heterogeneity when the I2 statistic was more than 50%. Attempts were also made to explain heterogeneity based on the patients’ clinical characteristics. We explored potential publication bias for the mortality outcome in studies through funnel plots. Reviewers eyeballed the plots to assess their symmetry. Subgroup analyses were conducted according to CKD classification status (stage 3, 4, or 5). All analyses were performed using Review Manager (RevMan) software version 5.4.
Results
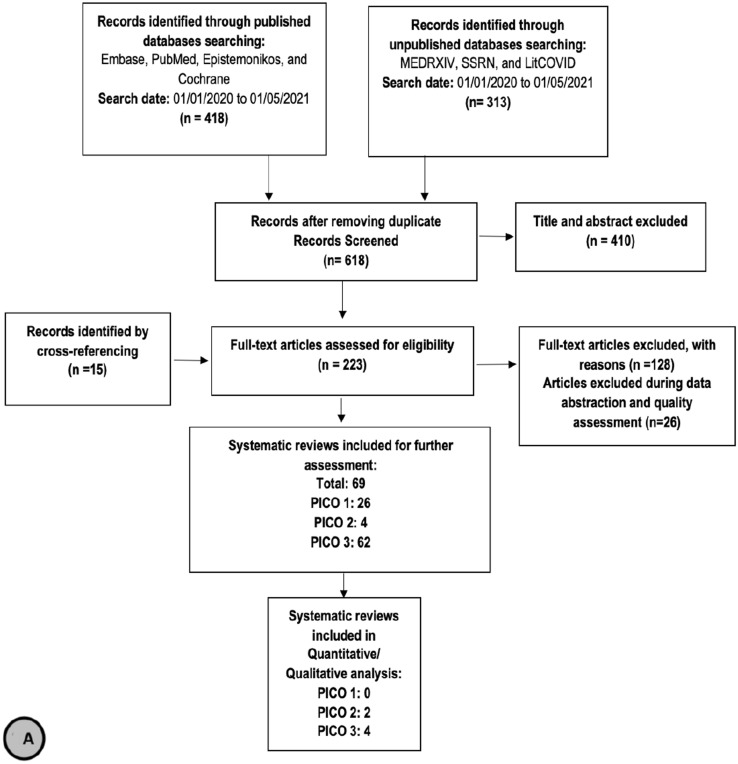
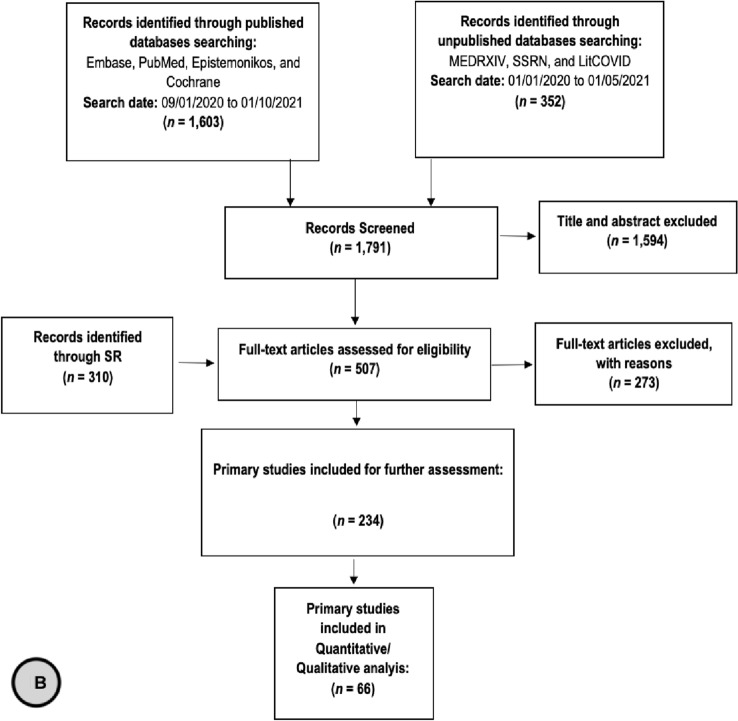
We Identified 731 records through published and unpublished databases. After removing duplicates, we screened a total of 618 reviews for title and abstract screening. Two hundred and twenty-three reviews were included for full text screening, during which a total of 69 systematic reviews were included for prioritization: twenty–six reviews reported indirect evidence that informed PICO–1, four reviews addressed the risk of hospitalization in patients with CKD and COVID–19 (PICO–2), and 62 reviews addressed the risk of mortality in patients with CKD and COVID–19 (PICO–3). After prioritization of the 69 reviews, we included six reviews in our final report (Figure 1A). We also identified and screened 1,791 primary studies for eligibility, of which 234 studies were used for further assessment and meta-analysis. We included 66 primary studies in our final report. Table 1 summarizes the effect estimates for the different outcomes in the included systematic reviews and primary studies. The characteristics as well as outcome measurements reported in the systematic reviews and primary studies are detailed in appendix 3 (Tables 3 A–D) (See Online Supplementary material).
Fig. 1.
Flow Chart of a systematic reviews and b primary studies included in the review
Table 1.
Summary of the effect estimates for the different outcomes in the included systematic reviews and primary studies
| Outcome | Systematic Reviews | Primary studies |
|---|---|---|
| Contracting COVID-19 | No direct evidence | No direct evidence |
| Hospitalization | RR 1.63 (95% CI 1.03–2.58) ⨁⨁⨁◯ |
RR 4.0 (95% CI 3.0–5.2) OR from 1.38 (95% CI 1.19–1.60) to 3.9 (95% CI 2.4– 6.3) HR 1.21 (95% CI 1.11–1.32), and 1.9 (95% CI 1.3– 2.9) |
| Mortality |
Pooled OR 1.77 (95% CI 1.54–2.02) ⨁⨁⨁◯ Pooled RR 1.6 (95% CI 0.88–2.92) ⨁⨁◯◯ Pooled HR 1.48 (95% CI 1.33–1.65) ⨁⨁⨁◯ RR 2.52 (95% CI 2.1–3.0) ⨁⨁⨁◯ |
Pooled OR 1.77 (95% CI 1.54–2.02) ⨁⨁⨁◯ Pooled RR 1.6 (95% CI 0.88–2.92) ⨁⨁◯◯ Pooled HR ESKD vs no ESKD 1.92 (95% CI 0.96–3.81) Pooled HR CKD III vs no CKD 1.46 (95% CI 1.41–1.51) |
| COVID-19 severity | RR 1.56 (95% CI 1.3–1.86) ⨁⨁⨁◯ | OR from 2.1 (95% CI 1.2–3.8) to 3.6 (95% CI 2.2–5.8) |
| ICU admission | OR 1.37 (95% CI 0.8–1.86) ⨁⨁◯◯ | Inconsistent evidence |
| Poor outcomes |
OR 5.32 (95% CI 1.86–15.19) RR 2.63 (95% CI 1.33–5.17) |
Pneumonia OR 1.66 (95% CI 1.38–2.00) Acute kidney injury OR 2.86 (95% CI 1.73–4.73) Longer hospital stay OR 1.62 (95% CI 1.27–2.06) |
CKD and contracting COVID–19
We did not identify any systematic reviews that directly inform on CKD and the risk of contracting Covid–19. We identified 28 systematic reviews [2, 20–46] that reported on the prevalence of CKD among patients with COVID–19, which ranged from 0.4 to 49.0% among different settings. Two [27, 32] reviews reported on the prevalence of ESKD in patients with COVID–19 infection which ranged from 2.3 to 30.9%. One review [33] reported an 8% incidence of COVID–19 infection in patients on chronic hemodialysis (95% CI 4.7–12.0%).
With regard to additional primary studies that were not included in the reviews, there was no convincing difference on the risk of acquiring COVID–19 infection in patients with and without CKD, with inconsistent results being present among different studies. While Rentsch et al. showed no difference in the OR for testing positive for COVID–19 infection in patients with and without CKD, OR 1.00 (95% CI 0.76–1.33) [47], Ji et al. reported an OR of 0.50 (95% CI 0.39–0.65) [48] when examining the relationship between CKD and the presence of COVID–19, and Corbett et al. [49] reported on the rate of COVID–19 infection over a six–week period in a large urban dialysis center in the United Kingdom with 1530 patients. During this period, 19.6% of the dialysis patients developed COVID–19 infection, with the majority of cases (96%) being in patients on in–center dialysis compared to home dialysis patients.
CKD and the risk of hospitalization among patients with COVID–19
We prioritized two of the four systematic reviews addressing this question [50, 51]. Fernandez Villalobos [50] provided the needed information to assess certainty in evidence. The risk of hospitalization appears to be increased in patients with COVID–19 infection and CKD compared to those without CKD, RR = 1.63 (95% CI 1.03–2.58) (Moderate certainty) [50] (Table 2).
Table 2.
Evidence profile for different outcomes
| No of studies | Certainty assessment | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | No of events | No of individuals | Relative (95% CI) | |||
| Hospitalization (Fernandez Villalobos 2020) [50] | |||||||||||
| 8 | Observational studies | Seriousa | Not seriousb | Not serious | Not serious | None | 176 | 362 | RR 1.63 (1.03–2.58) |
⨁⨁⨁◯ Moderate |
Important |
| Mortality in hospitalized patients (Dorjee 2020) [46] | |||||||||||
| 23 | Observational studies | Seriousc | Not seriousd | Not serious | Not serious | None |
RR 2.52 (2.1–3.0) |
⨁⨁⨁◯ Moderate |
Critical | ||
| Pooled Hazard ratio for mortality (Sept 2020 till Jan 2021) | |||||||||||
| 20 | Observational studies | Seriouse | Not seriousb | Not serious | Not serious | None | 17,163 | 1,718,678 |
HR 1.48 (1.33–1.65) |
⨁⨁⨁◯ Moderate |
Critical |
| Pooled Odds ratio for mortality (Sept 2020 till Jan 2021) | |||||||||||
| 24 | Observational studies | Seriousf | Not serious | Not serious | Not serious | None | 8929 | 26,267 | OR 1.77 (1.54–2.02) |
⨁⨁⨁◯ Moderate |
Critical |
| Pooled Risk ratio for mortality (Sept 2020 till Jan 2021) | |||||||||||
| 3 | Observational studies | Serious g | Not serious | Not serious | serioush | None | 9493 | 50,411 |
RR 1.6 (0.88–2.92) |
⨁⨁◯◯ Low |
Critical |
| Severe disease (Dorjee 2020) [46]i | |||||||||||
| 27 | Observational studies | Seriousj | Not seriousb | Not serious | Not serious | None |
RR 1.56 (1.3–1.86) |
⨁⨁⨁◯ Moderate |
Important | ||
| ICU admission (Degarege, 2020) [62] | |||||||||||
| 2 | Observational studies | Seriousk | Not serious | Not serious | Seriousl | None |
OR 1.37 (0.8–1.86) |
⨁⨁◯◯ Low |
Important | ||
aThe included studies were judged to be at high risk of bias in the domains of bias due to missing data and at moderate risk of bias in the domains of bias due to confounding and bias due to selection of participants and follow–up
bDespite the presence of high statistical heterogeneity as reflected by the I2 of > 80%, most of the effect estimates suggest the same direction of effect
cSome of the included studies were judged to be at high risk of bias in the domains of selection, comparability and outcome bias using Newcastle Ottawa tool
dEven though I2 is 72%, the effect estimates point toward increase mortality in patients with CKD
eDifferent included studies were judged to be at high risk of bias in the domains of study participation, prognostic factor measurement, outcome measurement and study confounding
fSome of the included studies were judged to be at high risk of bias in the domains of prognostic factor measurement and study confounding
gDominguez–Ramirez, which contributes to 33% of the weight, was judged to be at high risk of bias in the domain of prognostic factor measurement
hThe effect estimates cross the value of no effect suggesting both possible high and low risk
iSevere disease for any of 1) the study classified COVID–19 disease as severe or critical, 2) intensive care unit (ICU) admission, 3) acute respiratory distress syndrome, or 4) mechanical ventilation. Severe disease was defined by studies as respiratory rate > 30 per minute, oxygen saturation < 93%, and PaO2/FiO2 < 300 and/or lung infiltrates > 50% within 24–48 h
jSome of the included studies were judged to be at high risk of bias in the domains of selection, comparability and outcome bias using Newcastle Ottawa tool
kThe included studies with highest weight (98% weight) were judged to be at high risk of bias in the domains of selection bias and data collection
lThe effect estimates cross the value of no effect suggesting both possible high and low risk
Concerning additional primary studies that were not included in the reviews, most of the studies that reported on hospitalization in patients with COVID–19 infection and CKD showed a statistically significant increase in the risk of hospitalization. The majority of these studies calculated the OR for hospitalization, which ranged from 1.38 (95% CI 1.19–1.60) to 3.9 (95% CI 2.4–6.3) [47, 52–58]. One primary study reported a RR of 4.0 (95% CI 3.0–5.2) for hospitalization in patients with COVID–19 infection and CKD [59]. Two studies reported a HR of 1.21 (95% CI 1.11–1.32), and 1.9 (95% CI 1.3– 2.9) [60, 61].
Oetjens et al. analyzed the risk of hospitalization stratified by the advancement of CKD, showing an incremental increase in the odds of hospitalization in patients with COVID–19 infection with advancing CKD stage [54]. The OR for hospitalization in patients with CKD stage 5 and on dialysis was 11.07 (95% CI 4.54–26.97) in the Geisinger health care system, and 8.83 (95% CI 2.76–28.27) in United States Renal Data System (USRDS) data [54].
CKD and mortality among patients with COVID–19
We identified 62 systematic reviews assessing mortality risk in patients with CKD who contracted COVID–19 [2, 20, 24, 40, 43, 44, 46, 50, 51, 62–88]. Dorjee et al. [46] was the most updated systematic review with good AMSTAR quality which showed higher RR of mortality in patients with CKD and COVID–19 infection, RR = 2.52, (95% CI 2.11–3.00) (Moderate certainty) (Table 2).
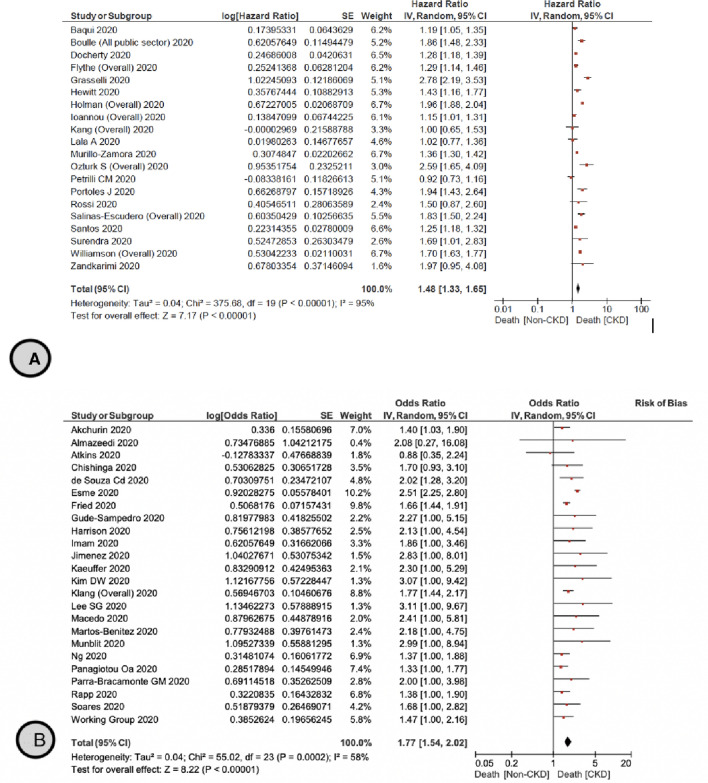
We identified 20 primary studies assessing the HR of mortality in patients with CKD and COVID–19 [14, 16, 57, 60, 90–104], with a pooled HR of 1.48 (95% CI 1.33–1.65) (Moderate certainty) compared to patients without CKD (Fig. 2A). Holman et al. showed that there is an increase in the HR of mortality with advanced CKD among patients with type 1 diabetes, with a HR of 2.07 (95% CI 1.48–2.89), 2.46 (95% CI 1.72–3.52), 3.71 (95% CI 2.47–5.58), and 8.35 (95% CI 5.50–12.70) in CKD stages 3A, 3B, 4, and 5, respectively [14]. Patients with type 2 diabetes had a similar increase in mortality risk with advanced CKD, with a HR of 1.39 (95% CI 1.30–1.49), 1.76 (95% CI 1.63–1.89), 2.31 (95% CI 2.10–2.54), and 4.91 (95% CI 4.34–5.56) in CKD stages 3A, 3B, 4, and 5, respectively [14]. This incremental increase in mortality was consistent with findings in Williamson et al. which showed higher HR 3.69 (95% CI 3.09–4.39) for mortality in patients with ESKD compared to those without ESKD [16].
Fig. 2.
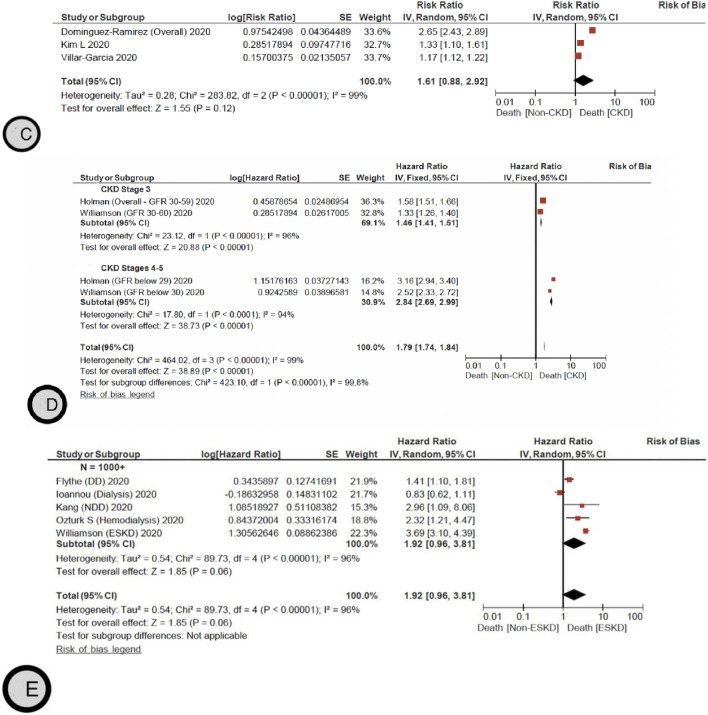
A The pooled hazard ratio for mortality for patients with and without CKD. B The pooled odds ratio for mortality for patients with and without CKD. C The pooled risk ratio for mortality for patients with and without CKD. D The pooled Hazard ratio for mortality for patients with CKD stage 3, 4–5 and without CKD. E The pooled Hazard ratio for mortality for patients with and without ESKD
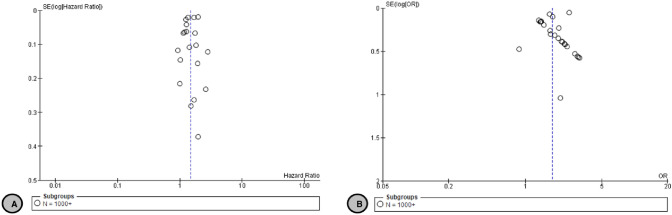
We also identified 24 additional primary studies that were not included in the reviews [52, 53, 55, 56, 58, 106–123] that examined the OR of mortality in patients with CKD and COVID–19 infection with a pooled OR of 1.77 (95% CI 1.54–2.02) (Moderate certainty) compared to patients without CKD (Fig. 2B). Ng et al. [123] reported specifically on patients with ESKD, showing a higher RR of mortality in this population, OR 1.37 (95% CI 1.09–1.73). Additionally, three primary studies [125–126] reported on the RR of mortality in patients with CKD and COVID–19 infection with a pooled RR of 1.6 (95% CI 0.88–2.92) (Low certainty) (Fig. 2C). Jager et al. [15] reported that the attributable mortality was 20% and the mortality risk was 21.1 (95% CI 18.6–23.9) times higher in dialysis patients diagnosed with COVID-19 compared with the 1.2% mortality in the matched control group of dialysis patients without COVID-19. There was no suspected publication bias in the studies reporting mortality (Fig. 3).
Fig. 3.
A Funnel plot of comparison: 1 Covid-19 and CKD Mortality Outcomes, outcome: 1.1 HR CKD vs Non-CKD Mortality. B Funnel plot of comparison: 1 Covid-19 and CKD Mortality Outcomes, outcome: 1.2 OR CKD vs Non-CKD Mortality
In a subgroup analysis, the pooled HR for mortality in patients with CKD stage 3 is 1.46 (95% CI 1.41–1.51) compared to patients without CKD [14, 16] (Fig. 2D). Interestingly, the pooled HR for mortality significantly increased in patients with CKD 4–5, HR 2.84 (95% CI 2.69–2.99) [14, 16] (Fig. 2D). This increased mortality risk was also persistent in patients with ESKD with a pooled HR of 1.92 (95% CI 0.96–3.81) compared to patients without ESKD [16, 60, 93, 101, 103] (Fig. 2E).
CKD and COVID–19 severity
We identified 32 systematic reviews [2, 20, 23, 24, 28, 30, 35, 36, 43, 46, 50, 51, 62, 82, 128–144] reporting on COVID–19 severity in patients with CKD. Dorjee et al. [46] was the most updated systematic review with good AMSTAR quality answering this question and it showed an increased risk of severe COVID–19 infection among patients with CKD with a RR of 1.6 (95% CI 1.3–1.9) (Moderate certainty) (Table 2).
Five additional primary studies that were not included in the reviews [48, 52, 107, 145, 146] examined the effect of CKD on the severity of COVID–19 infection, showing a higher OR for severe COVID–19 infection ranging from 2.1 (95% CI 1.2–3.8) to 3.6 (95% CI 2.2–5.8). However, the definition of severe COVID–19 infection was not consistent among different studies.
CKD and ICU admission among patients with COVID–19
We identified nine systematic reviews [2, 29, 40, 50, 62, 79, 137, 142, 143] reporting on ICU admission in patients with CKD and COVID–19 infection. Among them, Degarege A [62] was the most updated systematic review with the highest AMSTAR quality. Degarege reported a trend towards higher need for ICU admission in patients with CKD and COVID–19 infection with an OR of 1.37 (95% CI 0.88–1.86) (Low certainty) (Table 2).
The effect estimate for the need of ICU admission was inconsistent among additional primary studies that were not included in the reviews. While three studies [52, 108, 147] showed a statistically significant increase in the risk of ICU admission with an OR ranging from 1.7 to 3.6, the other five studies [47, 53, 55, 119, 126] did not provide convincing evidence for higher ICU admission need in patients with CKD and COVID–19 infection (Table 1).
CKD and mechanical ventilation among patients with COVID–19
We identified one relevant systematic review [79] that reported on the risk of mechanical ventilation need in patients with CKD and COVID–19 infection. The meta-analysis included one primary study which did not show convincing evidence that CKD increases the need for mechanical ventilation in this population based on 18 events only.
There was also no convincing evidence that CKD increases the risk of needing mechanical ventilation among patients with COVID–19 infection in five additional primary studies that were not included in the reviews [53, 60, 93, 123, 148]. The ORs for mechanical ventilation ranged from 0.97 to 1.32, and the HRs ranged from 0.87 to 1.16.
CKD and poor outcomes among patients with COVID–19
We identified four systematic reviews [22, 79, 83, 149] reporting on overall poor outcomes in patients with CKD and COVID–19 infection. Nandy, K. et al. [22] defined adverse events such as ICU admission, acute respiratory distress syndrome (ARDS), mechanical ventilation, pneumonia, and death. The OR for adverse events was higher in CKD patients compared to non-CKD OR 5.32 (95% CI 1.86–15.19). Pranata R [79] defined poor outcomes as mortality, severe COVID–19, ARDS, ICU care, and mechanical ventilation, and showed a RR of 2.63 (95% CI 1.33– 5.17) for the CKD population compared to non–CKD. Xiao, W et al. defined adverse outcomes as severe illness, critical illness or death and reported a pooled effect estimate of 1.64 (95% CI 1.28–2.09) [83]. Kunutsor et al. also reported a higher incidence of AKI in patients with baseline CKD [27].
Additional primary studies that were not included in the reviews showed that patients with CKD and COVID–19 infection are at higher risk of pneumonia, OR 1.66 (95% CI 1.38–2.00) [53] and acute kidney injury in the first 48 h, OR 2.86 (95% CI 1.73–4.73) [150]. One study reported longer hospital stay in patients with ESKD compared to non-dialysis CKD, OR 1.62 (95% CI 1.27–2.06) [123].
Discussion
COVID–19 has affected millions of people worldwide. Many chronic medical diseases were reported as risk factors for increased mortality and severity of COVID–19, such as diabetes [56], hypertension, chronic obstructive pulmonary disease, malignancies, and CKD [127]. In 2019, CKD affected approximately 15.0% of patients aged 65 years or older of the US Medicare population [151]. Some of the major causes of morbidity and mortality in patients with CKD are infections, sepsis, and bacteremia [152]. Infections in patients with CKD can cause longer duration of hospitalization, [153] and the mortality rate from pneumonia in patients with CKD is higher than that of patients without CKD [154]. In our review, we gathered evidence from all systematic reviews and primary studies to report the impact of CKD on COVID–19 mortality, hospitalization, incidence, ICU admission, disease severity, and adverse outcomes. We found that patients with CKD were more likely to have worse outcomes from COVID–19 compared to patients without CKD. This could be attributed to the attenuated immune system activation of both the innate and adaptive immunity systems which lead to an increased susceptibility to infections in patients with CKD [155].
We are reporting moderate certainty evidence that CKD increases the risk of COVID–19-related mortality and of disease severity. Importantly, this mortality risk is higher in patients with advanced CKD stage (Figs. 2 D, E).
In comparison with the impact of other comorbidities on COVID–19 mortality, a meta-analysis showed that cardiovascular disease, hypertension and diabetes were associated with increased mortality and severity of COVID–19: diabetes OR 2.50 (95% CI 1.74–3.59), and OR 2.35 (95% CI 1.80–3.06), hypertension OR 2.88 (95% CI 2.22–3.74), and OR 2.98 (95% CI 2.37–3.75), and cardiovascular disease OR 6.34 (95% CI 3.71–10.84), and OR 4.02 (95% CI 2.76–5.86), respectively for mortality and severity [156]. Another meta-analysis reported that patients with diabetes mellitus RR 1.48 (95% CI 1.02–2.15), cardiovascular diseases RR 2.25 (95% CI 1.60–3.17), malignancy RR 1.47 (95% CI 1.01–2.14), and hypertension RR 1.82 (95% CI 1.43–2.32) suffer a greater mortality risk compared to patients without these comorbidities [81].
Our findings show moderate certainty evidence that risk of hospitalization is increased in patients with COVID–19 infection and CKD compared to those without CKD. Similar to mortality, there is an incremental increase in the risk of hospitalization with advanced CKD stage [54]. In a case-control study that assessed risk factors associated with increased hospitalization in patients with CKD, the presence of comorbid ischemic heart disease was associated with a 3.5fold increase in admission rate (95% CI 2.14–5.9), while other factors included the presence of anemia, hypoalbuminemia, and late referral to a nephrologist [157].
The significance of CKD as an underlying condition for severe COVID–19 remains less well understood. In our review, we found one high quality systematic review [46] and five primary studies [48, 52, 107, 145, 146] that reported an increased risk of severe COVID–19 disease in patients with CKD, with an OR ranging from 2.1 (95% CI 1.2–3.8) to 3.6 (95% CI 2.2–5.8). However, the definition of severe COVID–19 infection was not clear and likely inconsistent in all studies. In one meta-analysis, no primary study reported CKD as a risk factor for COVID–19 severity, but a significant association was found, OR 3.03 (95% CI, 1.09–8.47) when pooling of data took place [128].
It is worthy to note that the results informing some of the outcomes were inconsistent among studies. For example, the effect estimates for the need of ICU admission and poor outcomes in patients with CKD and COVID–19 were inconsistent among the identified primary studies. Moreover, some inconsistencies in the inclusion of primary studies among the published reviews were noted. These discrepancies can be attributed to several possible factors, like the use of different definitions of CKD and disease severity, the use of different inclusion criteria in the systematic reviews, the difference in sample sizes and the timing of the studies, and different management and care that is provided to patients in each study. In addition, some studies did not adjust for all appropriate confounders, which may have played a role in the inconsistencies among results.
The findings in this review and in other studies have shed some light on the importance of implementing clear guidelines for the prevention and management of COVID–19 that are specific to patients with CKD. Because studies on these patients have shown an increased risk of mortality, hospitalization, and adverse outcomes of COVID–19, physicians should maintain a low threshold for hospital admission and close monitoring of patients with CKD who are not hospitalized, as well as early aggressive management to prevent complications. The findings should also guide us to prioritize patients with CKD during vaccine administration, regardless of their age and the advancement of their disease. Recent literature on vaccinated patients on maintenance hemodialysis showed that those patients have mounted an immune response to the vaccine, however, their antibody titers were lower than their controls [158, 159]. Studies examining the protective effect of the COVID–19 vaccine in CKD patients are underway.
Limitations
Some limitations to our overview of reviews could be noted. First, we relied on existing systematic reviews to identify studies published before September 2020. Given the inconsistency in studies included among the published reviews that is unexplained by the reviews’ inclusion and exclusion criteria, it is possible that some primary studies may have been missed. However, due to the extensive effort in identifying large and well–done studies, it is unlikely that any major study that would have a considerable impact on the conclusions has been missed. Some of the systematic reviews and primary studies were preprints, which lack the vigilant peer–review process. Another limitation is the high risk of bias in multiple domains in the included primary studies, and some primary studies did not consistently adjust for important confounders. In addition, the methods of diagnosing chronic kidney disease and measuring different confounders were not explicitly detailed in most of the included primary studies. In the mortality outcome, study data were considered worthy of exploration of heterogeneity when the I2 statistic was more than 50%. Attempts were also made to explain heterogeneity based on the patients’ clinical characteristics. However, due to lack of reporting of factors that may explain heterogeneity in the included studies, we were unable to explore them for all the outcomes in our analysis.
Conclusions
This overview of reviews addressed systematic reviews and primary studies that evaluated different outcomes in patients with CKD who contracted COVID–19. Our overview also evaluated the quality of both systematic reviews and individual studies. Evidence consistently demonstrated an increased risk of mortality and hospitalization in patients with CKD and COVID–19. The extent to which CKD increases the likelihood of the rate of infection, and other poor outcomes is not currently well understood, and the results are inconsistent among studies. The results shed some light on the significance of prioritizing patients with CKD for COVID–19 vaccination and critical care management. Further research studying the pathophysiology behind the effect of CKD on COVID–19 outcomes would provide deeper insight for the management of such patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Conception or design of the work: RM. Data collection and interpretation: All Authors, Data analysis: PT. Drafting the article: SJ, RM, AE, RM. Critical revision of the article: All Authors. Final approval of the version to be published: All authors.
Funding
This work informed a brief for the World Health organization and was funded through the Pan American Health Organization (PAHO).
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
Declarations
Conflict of interest
All the authors declared no conflict of interest/competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.https://covid19.who.int/. WHO website. In:2021.
- 2.Zhou Y, Yang Q, Chi J, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki N, Mohamed EA, Ibrahim S, Khan G. The influence of comorbidity on the severity of COVID-19 disease: systematic review and analysis. medRxiv. 2020;2020:20134478. [Google Scholar]
- 4.GBDCKD Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Chronic Kidney Disease in the United States. Atlanta GUDoHaHS, Centers for Disease Control and Prevention; 2021. Chronic Kidney Disease in the United States, 2021. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 6.https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf. KDIGO guidelines. 2021.
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 8.Dalrymple LS, Go AS. Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(5):1487–1493. doi: 10.2215/CJN.01290308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302(16):1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 10.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16(12):3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 11.Chang C-H, Fan P-C, Kuo G, et al. Infection in advanced chronic kidney disease and subsequent adverse outcomes after dialysis initiation: a nationwide cohort study. Sci Rep. 2020;10(1):2938. doi: 10.1038/s41598-020-59794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35(11):1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jager Kj KACNCCCS-AJEGLCFHMHAPKJL Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020 doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma C, Gu J, Hou P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. medRxiv. 2020;41:145. [Google Scholar]
- 21.Bennett S, Tafuro J, Mayer J, et al. Clinical features and outcomes of adults with coronavirus disease 2019: a systematic review and pooled analysis of the literature. Int J Clin Pract. 2021;2020:e13725. doi: 10.1111/ijcp.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nandy K, Salunke A, Pathak SK, et al. Coronavirus disease (COVID-19): A systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab Syndr. 2020;14(5):1017–1025. doi: 10.1016/j.dsx.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in patients with liver and kidney diseases: an early systematic review and meta-analysis. Trop Med Infect Dis. 2020 doi: 10.3390/tropicalmed5020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh AK, Gillies CL, Singh R, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1915–1924. doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan MMA, Khan MN, Mustagir MG, Rana J, Islam MS, Kabir MI. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: a systematic review and meta-analysis. J Glob Health. 2020;10(2):020503. doi: 10.7189/jogh.10.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang M, Wang D, Tang O, Selvin E. Prevalence of chronic disease in laboratory-confirmed covid-19 cases and US adults (2017–2018) Diabetes Care. 2020;43(10):e127. doi: 10.2337/dc20-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunutsor SK, Laukkanen JA. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. 2020;52(7):345–353. doi: 10.1080/07853890.2020.1790643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fathi M, Vakili K, Sayehmiri F, et al. Prognostic value of comormidity for severity of covid-19: a systematic review and meta-analysis study. medRxiv. 2020;12:6049. doi: 10.1371/journal.pone.0246190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang R, Elhusseiny KM, Yeh Y-C, Sun W-Z. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes—a systematic review and meta-analysis. medRxiv. 2020;16(2):e0246318. doi: 10.1371/journal.pone.0246318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng Y, Wang J, Wen K, Da W, Yang K, Zhou S, Tao Z, Liu H, Tao L. Clinical Features and Laboratory Examination to Identify Severe Patients with COVID-19: A Systematic Review and Meta-Analysis. BioMed Res Int. 2021;2021:6671291. doi: 10.1155/2021/6671291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajwa H, Riaz Y, Ammar M, Farooq S, Yousaf A. The dilemma of renal involvement in Covid-19: a systematic review. Cureus. 2020;12(6):e8632. doi: 10.7759/cureus.8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou S, Xu J, Xue C, Yang B, Mao Z, Ong ACM. Coronavirus-associated kidney outcomes in COVID-19, SARS, and MERS: a meta-analysis and systematic review. Ren Fail. 2020;43(1):1–15. doi: 10.1080/0886022X.2020.1847724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan E, Song J, Deane AM, Plummer MP. Global impact of coronavirus disease 2019 infection requiring admission to the icu: a systematic review and meta-analysis. Chest. 2021;159(2):524–536. doi: 10.1016/j.chest.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bajgain KT, Badal S, Bajgain BB, Santana MJ. Prevalence of comorbidities among individuals with COVID-19: a rapid review of current literature. Am J Infect Control. 2021;49(2):238–246. doi: 10.1016/j.ajic.2020.06.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Ren Q, Chen G, et al. Chronic kidney diseases and acute kidney injury in patients with COVID-19: evidence from a meta-analysis. Front Med (Lausanne) 2020;7:588301–588301. doi: 10.3389/fmed.2020.588301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu T, Zuo Z, Kang S, et al. Multi-organ dysfunction in patients with COVID-19: a systematic review and meta-analysis. Aging Dis. 2020;11(4):874–894. doi: 10.14336/AD.2020.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1):e35. [PMC free article] [PubMed] [Google Scholar]
- 38.Kaur N, Gupta I, Singh H, et al. Epidemiological and clinical characteristics of 6635 COVID-19 patients: a pooled analysis. SN Compr Clin Med. 2020 doi: 10.1007/s42399-020-00393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansrivijit P, Qian C, Boonpheng B, et al. Incidence of acute kidney injury and its association with mortality in patients with COVID-19: a meta-analysis. J Investig Med. 2020;68(7):1261–1270. doi: 10.1136/jim-2020-001407. [DOI] [PubMed] [Google Scholar]
- 40.Espinosa OA, Zanetti ADS, Antunes EF, Longhi FG, Matos TAd, Battaglini PF. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo. 2020;62:e43–e43. doi: 10.1590/S1678-9946202062043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baradaran A, Ebrahimzadeh MH, Baradaran A, Kachooei AR. Prevalence of comorbidities in COVID-19 patients: a systematic review and meta-analysis. Arch Bone Jt Surg. 2020;8(Suppl 1):247–255. doi: 10.22038/abjs.2020.47754.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goel S, Jain T, Hooda A, et al. Clinical characteristics and in-hospital mortality for COVID-19 across the globe. Cardiol Ther. 2020;9(2):553–559. doi: 10.1007/s40119-020-00189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang B, Luo Q, Zhang W, et al. The involvement of chronic kidney disease and acute kidney injury in disease severity and mortality in patients with COVID-19: a meta-analysis. Kidney Blood Press Res. 2021;46(1):17–30. doi: 10.1159/000512211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tisminetzky M, Delude C, Hebert T, Carr C, Goldberg RJ, Gurwitz JH (2020) Age, multiple chronic conditions, and COVID-19: a literature review. J Gerontol A Biol Sci Med Sci. Dec 24:glaa320. 10.1093/gerona/glaa320. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 45.Farha MN, Md Momin I. Prevalence of clinical manifestations and comorbidities of coronavirus (COVID-19) infection: a meta-analysis. Fortune J Health Sci. 2020 doi: 10.26502/fjhs009. [DOI] [Google Scholar]
- 46.Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS ONE. 2020 doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54–75 years. medRxiv. 2020;57:279. [Google Scholar]
- 48.Ji W, Huh K, Kang M, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020;35(25):e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corbett RW, Blakey S, Nitsch D, et al. Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol. 2020;31(8):1815–1823. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandez Villalobos NV, Ott JJ, Klett-Tammen CJ, et al. Quantification of the association between predisposing health conditions, demographic, and behavioural factors with hospitalisation, intensive care unit admission, and death from COVID-19: a systematic review and meta-analysis. medRxiv. 2020;8:e001343. [Google Scholar]
- 51.Wingert A, Pillay J, Gates M, et al. Risk factors for severe outcomes of COVID-19: a rapid review. medRxiv. 2020;38:5861. [Google Scholar]
- 52.Chishinga N, Gandhi NR, Onwubiko UN, et al (2020) Characteristics and Risk Factors for Hospitalization and Mortality among Persons with COVID-19 in Atlanta Metropolitan Area. medRxiv 2020.2012.2015.20248214
- 53.Martos-Benitez FD, Soler-Morejon CD, Garcia-Del BD. Chronic comorbidities and clinical outcomes in patients with and without COVID-19: a large population-based study using national administrative healthcare open data of Mexico. Intern Emerg Med. 2021 doi: 10.1007/s11739-020-02597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oetjens MT, Luo JZ, Chang A, et al. Electronic health record analysis identifies kidney disease as the leading risk factor for hospitalization in confirmed COVID-19 patients. PLoS ONE [Electronic Resource] 2020;15(11):e0242182. doi: 10.1371/journal.pone.0242182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Working group for the s, control of C-iS, Members of the Working group for the s, control of C-iS The first wave of the COVID-19 pandemic in Spain: characterisation of cases and risk factors for severe outcomes, as at 27 April 2020. Euro Surveill. 2020;25(50):12. doi: 10.2807/1560-7917.ES.2020.25.50.2001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atkins JL, Masoli JAH, Delgado J, et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J Gerontol Ser A. 2020;75(11):2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soares RdCM, Mattos LR, Raposo LM. Risk factors for hospitalization and mortality due to COVID-19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020;103(3):1184–1190. doi: 10.4269/ajtmh.20-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko JY, Danielson ML, Town M, et al. Risk factors for COVID-19-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2020;69:343. doi: 10.1093/cid/ciaa1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 Infection. JAMA Netw Open. 2020;3(9):e2022310–e2022310. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rossi PG, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R (2020) Characteristics and outcomes of a cohort of SARS-CoV-2 patients in the Province of Reggio Emilia, Italy. medRxiv. 2020:2020.2004.2013.20063545 [DOI] [PMC free article] [PubMed]
- 62.Degarege A, Naveed Z, Kabayundo J, Brett-Major D. Risk factors for severe illness and death in COVID-19: a systematic review and meta-analysis. MedRxiv. 2020;323:2052. doi: 10.3390/pathogens11050563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai R, Zhang J, Zhu Y, Liu L, Liu Y, He Q. Mortality in chronic kidney disease patients with COVID-19: a systematic review and meta-analysis. Int Urol Nephrol. 2021 doi: 10.1007/s11255-020-02740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, Wang Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Islam MS, Barek MA, Aziz MA, Aka TD, Jakaria M. Association of age, sex, comorbidities, and clinical symptoms with the severity and mortality of COVID-19 cases: a meta-analysis with 85 studies and 67299 cases. medRxiv. 2020;16:1678. doi: 10.1016/j.heliyon.2020.e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Izcovich A, Ragusa MA, Tortosa F, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE. 2020;121:62. doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan M, Khan MN, Mustagir MG, Rana J, Islam MS, Kabir MI. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: a systematic review and meta-analysis. medRxiv. 2020;26:26. doi: 10.7189/jogh.10.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo L, Fu M, Li Y, et al. The potential association between common comorbidities and severity and mortality of coronavirus disease 2019: a pooled analysis. Clin Cardiol. 2020 doi: 10.1002/clc.23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehraeen E, Karimi A, Barzegary A, et al. Predictors of mortality in patients with COVID-19-a systematic review. Eur J Integr Med. 2020;40:101226–101226. doi: 10.1016/j.eujim.2020.101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mesas AE, Cavero-Redondo I, Alvarez-Bueno C, et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS ONE. 2020 doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoo Jung Rhim, Jin Hyun Park, Yuna Lee et al (2020) Clinical, laboratory, and radiologic findings associated with mortality in COVID-19: a systematic review and meta-analysis. PREPRINT (Version 1) available at Research Square. 10.21203/rs.3.rs-39877/v1
- 72.Sepandi M, Taghdir M, Alimohamadi Y, Afrashteh S, Hosamirudsari H. Factors associated with mortality in Covid-19 patients a systematic review and meta-analysis. Iran J Public Health. 2020 doi: 10.18502/ijph.v49i7.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou S, Xu J, Xue C, Yang B, Mao Z, Ong ACM. Coronavirus-associated kidney outcomes in COVID-19, SARS, and MERS: a meta-analysis and systematic review. Ren Fail. 2020 doi: 10.1080/0886022X.2020.1847724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou X, Cheng Z, Shu D, et al. Characteristics of mortal COVID-19 cases compared to the survivors. Aging. 2020 doi: 10.18632/aging.202216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y, Ren Q, Chen G, et al. Chronic kidney diseases and acute kidney injury in patients with Covid-19: evidence from a meta-analysis. Front Med. 2020;7:588301. doi: 10.3389/fmed.2020.588301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B. association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2020 doi: 10.1159/000512592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12(13):12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noor FM, Islam MM. Prevalence and associated risk factors of mortality among COVID-19 patients: a meta-analysis. J Community Health. 2020;45(6):1270–1282. doi: 10.1007/s10900-020-00920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pranata R, Supriyadi R, Huang I, et al. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients a meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2020 doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi C, Wang L, Ye J, Gu Z, Wang S, Xia J, Xie Y, Li Q, Xu R, Lin N. Predictors of mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):663. doi: 10.1186/s12879-021-06369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS ONE [Electronic Resource]. 2020;15(8):e0238215. doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chidambaram V, Tun NL, Haque WZ, Majella MG, Sivakumar RK, Kumar A, Hsu AT, Ishak IA, Nur AA, Ayeh SK, Salia EL, Zil-E-Ali A, Saeed MA, Sarena APB, Seth B, Ahmadzada M, Haque EF, Neupane P, Wang KH, Pu TM, Ali SMH, Arshad MA, Wang L, Baksh S, Karakousis PC, Galiatsatos P. Factors associated with disease severity and mortality among patients with COVID-19: A systematic review and meta-analysis. PLoS One. 2020;15( 11):e0241541. doi: 10.1371/journal.pone.0241541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao W, Xu J, Liang X, Shi L, Zhang P, Wang Y, Yang H. Relationship between chronic kidney disease and adverse outcomes of coronavirus disease 2019: a meta-analysis based on adjusted risk estimates. Int Urol Nephrol. 2021;53(8):1723–1727. doi: 10.1007/s11255-020-02748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Awortwe C, Cascorbi I. Meta-analysis on outcome-worsening comorbidities of COVID-19 and related potential drug-drug interactions. Pharmacol Res. 2020 doi: 10.1016/j.phrs.2020.105250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bajgain KT, Badal S, Bajgain BB, Santana MJ. Prevalence of comorbidities among individuals with COVID-19: a rapid review of current literature. Am J Infect Control. 2020 doi: 10.1016/j.ajic.2020.06.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bajwa H, Riaz Y, Ammar M, Farooq S, Yousaf A. The dilemma of renal involvement in COVID-19: a systematic review. Cureus. 2020 doi: 10.7759/cureus.8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in patients with liver and kidney diseases: an early systematic review and meta-analysis. Trop Med Infect Dis. 2020 doi: 10.3390/tropicalmed5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiu P, Zhou Y, Wang F, et al. Clinical characteristics, laboratory outcome characteristics, comorbidities, and complications of related COVID-19 deceased: a systematic review and meta-analysis. Aging Clin Exp Res. 2020 doi: 10.1007/s40520-020-01664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murillo-Zamora E, Hernandez-Suarez CM. Survival in adult inpatients with COVID-19. Public Health. 2021;190:1–3. doi: 10.1016/j.puhe.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salinas-Escudero G, Carrillo-Vega MF, Granados-Garcia V, Martinez-Valverde S, Toledano-Toledano F, Garduno-Espinosa J. A survival analysis of COVID-19 in the Mexican population. BMC Public Health. 2020;20(1):1616. doi: 10.1186/s12889-020-09721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Flythe JE, Assimon MM, Tugman MJ, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2020;77(2):190–203. doi: 10.1053/j.ajkd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giorgi Rossi P, Marino M, Formisano D, et al. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia Italy. PLoS ONE. 2020;15(8):e0238281–e0238281. doi: 10.1371/journal.pone.0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Health. 2020;8(8):e1018–e1026. doi: 10.1016/S2214-109X(20)30285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boulle A, Davies MA, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province South Africa. Clin Infect Dis. 2020;7:e314. doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Santos MM, Lucena EES, Lima KC, Brito AAC, Bay MB, Bonfada D. Survival and predictors of deaths of patients hospitalized due to COVID-19 from a retrospective and multicenter cohort study in Brazil. Epidemiol Infect. 2019;194:119. doi: 10.1017/S0950268820002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Surendra H, Elyazar IR, Djaafara BA, et al. Clinical characteristics and mortality associated with COVID-19 in Jakarta, Indonesia: A hospital-based retrospective cohort study. Lancet Reg Health West Pac. 2021;9:100108. doi: 10.1016/j.lanwpc.2021.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Portolés J, Marques M, López-Sánchez P, et al. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant. 2020;35(8):1353–1361. doi: 10.1093/ndt/gfaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ozturk S, Turgutalp K, Arici M, et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35(12):2083–2095. doi: 10.1093/ndt/gfaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kang SH, Kim SW, Kim AY, Cho KH, Park JW, Do JY. Association between chronic kidney disease or acute kidney injury and clinical outcomes in COVID-19 patients. J Korean Med Sci. 2020;35(50):e434. doi: 10.3346/jkms.2020.35.e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zandkarimi E, Moradi G, Mohsenpour B. The prognostic factors affecting the survival of Kurdistan province COVID-19 patients: a cross-sectional study from February to May 2020. Int J Health Policy Manag. 2020 doi: 10.34172/ijhpm.2020.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020;28(9):1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Macedo MCF, Pinheiro IM, Carvalho CJL, et al. Correlation between hospitalized patients' demographics, symptoms, comorbidities, and COVID-19 pandemic in Bahia, Brazil. PLoS ONE. 2020;15(12):e0243966. doi: 10.1371/journal.pone.0243966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee SG, Park GU, Moon YR, Sung K. Clinical characteristics and risk factors for fatality and severity in patients with coronavirus disease in korea: a nationwide population-based retrospective study using the korean health insurance review and assessment service (HIRA) database. Int J Environ Res Public Health. 2020;17(22):18. doi: 10.3390/ijerph17228559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Munblit D, Nekliudov NA, Bugaeva P, et al. StopCOVID cohort: An observational study of 3480 patients admitted to the Sechenov University hospital network in Moscow city for suspected COVID-19 infection. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Esme M, Koca M, Dikmeer A, et al. Older adults with coronavirus disease 2019: a nationwide study in Turkey. J Gerontol A Biol Sci Med Sci. 2021;76(3):e68–e75. doi: 10.1093/gerona/glaa219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fried MW, Crawford JM, Mospan AR, et al. patient characteristics and outcomes of 11,721 patients with covid19 hospitalized across the United States. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gude-Sampedro F, Fernández-Merino C, Ferreiro L, et al. Development and validation of a prognostic model based on comorbidities to predict COVID-19 severity: a population-based study. Int J Epidemiol. 2021;50(1):64–74. doi: 10.1093/ije/dyaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17(9):e1003321. doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiménez E, Fontán-Vela M, Valencia J, et al. Characteristics, complications and outcomes among 1549 patients hospitalised with COVID-19 in a secondary hospital in Madrid, Spain: a retrospective case series study. BMJ Open. 2020;10(11):e042398. doi: 10.1136/bmjopen-2020-042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaeuffer C, Le Hyaric C, Fabacher T, et al. Clinical characteristics and risk factors associated with severe COVID-19: prospective analysis of 1045 hospitalised cases in North-Eastern France, March 2020. Euro Surveill. 2020 doi: 10.2807/1560-7917.ES.2020.25.48.2000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rapp JL, Lieberman-Cribbin W, Tuminello S, Taioli E. Male sex, severe obesity, older age, and chronic kidney disease are associated with COVID-19 severity and mortality in New York City. Chest. 2021;159(1):112–115. doi: 10.1016/j.chest.2020.08.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim DW, Byeon KH, Kim J, Cho KD, Lee N. The correlation of comorbidities on the mortality in patients with COVID-19: an observational study based on the Korean National Health insurance big data. J Korean Med Sci. 2020;35(26):e243. doi: 10.3346/jkms.2020.35.e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Panagiotou OA, Kosar CM, White EM, et al. risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2020.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Almazeedi S, Al-Youha S, Jamal MH, et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Souza CD, de Arruda Magalhães AJ, Lima AJ, et al. Clinical manifestations and factors associated with mortality from COVID-19 in older adults: Retrospective population-based study with 9807 older Brazilian COVID-19 patients. Geriatr Gerontol Int. 2020;20(12):1177–1181. doi: 10.1111/ggi.14061. [DOI] [PubMed] [Google Scholar]
- 121.Parra-Bracamonte GM, Parra-Bracamonte FE, Lopez-Villalobos N, Lara-Rivera AL. Chronic kidney disease is a very significant comorbidity for high risk of death in patients with COVID-19 in Mexico. Nephrology (Carlton) 2021;26(3):248–251. doi: 10.1111/nep.13827. [DOI] [PubMed] [Google Scholar]
- 122.Akchurin O, Meza K, Biswas S, et al. COVID-19 in Patients with CKD in New York City. Kidney360. 2021;2(1):63. doi: 10.34067/KID.0004142020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ng JH, Hirsch JS, Wanchoo R, et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98(6):1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Villar-García J, Vivanco-Hidalgo RM, Clèries M, et al. Risk factors for SARS-CoV-2 infection, hospitalisation, and death in Catalonia, Spain: a population-based cross-sectional study. medRxiv. 2020;9:1548. [Google Scholar]
- 125.Dominguez-Ramirez L, Rodriguez-Perez F, Sosa-Jurado F, Santos-Lopez G, Cortes-Hernandez P. The role of metabolic comorbidity in COVID-19 mortality of middle-aged adults. The case of Mexico. medRxiv. 2020;69:343. [Google Scholar]
- 126.Kim L, Garg S, O'Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barek MA, Aziz MA, Islam MS. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases. Heliyon. 2020;6(12):e05684. doi: 10.1016/j.heliyon.2020.e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52(6):1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Izcovich A, Ragusa MA, Tortosa F, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS ONE. 2020;15(11):e0241955. doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li J, He X, Yuan Y, et al. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. Am J Infect Control. 2021;49(1):82–89. doi: 10.1016/j.ajic.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luo L, Fu M, Li Y, et al. The potential association between common comorbidities and severity and mortality of coronavirus disease 2019: a pooled analysis. Clin Cardiol. 2020;43(12):1478–1493. doi: 10.1002/clc.23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mudatsir M, Fajar JK, Wulandari L, et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Res. 2020;9:1107. doi: 10.12688/f1000research.26186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Plasencia-Urizarri TM, Aguilera-Rodríguez R, Almaguer-Mederos LE. Comorbidities and clinical severity of COVID-19: systematic review and meta-analysis. Rev habanera cienc méd. 2020;19(supl.1):e3389–e3389. [Google Scholar]
- 134.Radwan NM, Mahmoud NE, Alfaifi AH, Alabdulkareem KI. Comorbidities and severity of coronavirus disease 2019 patients. Saudi Med J. 2020;41(11):1165–1174. doi: 10.15537/smj.2020.11.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tabrizi R, Lankarani KB, Nowrouzi-sohrabi P, et al. The role of comorbidities and clinical predictors of severe disease in COVID-19: a systematic review and meta-analysis. medRxiv. 2020;395:1225. [Google Scholar]
- 136.Toraih EA, Elshazli RM, Hussein MH, et al. Association of cardiac biomarkers and comorbidities with increased mortality, severity, and cardiac injury in COVID-19 patients: a meta-regression and decision tree analysis. J Med Virol. 2020;92(11):2473–2488. doi: 10.1002/jmv.26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12(7):6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang X, Fang X, Cai Z, et al. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash D C) 2020;2020:2402961. doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yanling Wu, Hu Li, Shengjin Li et al (2020) Clinical determinants of the severity of Coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PREPRINT (Version 1) available at Research Square [10.21203/rs.3.rs-56852/v1]
- 140.Liu YF, Zhang Z, Pan XL, et al. The chronic kidney disease and acute kidney injury involvement in COVID-19 pandemic: a systematic review and meta-analysis. PLoS ONE. 2021;16(1):e0244779. doi: 10.1371/journal.pone.0244779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zaki N, Mohamed EA, Ibrahim S, Khan G (2020) The influence of comorbidity on the severity of COVID-19 disease: a systematic review and analysis. medRxiv. 2020:2020.2006.2018.20134478
- 142.Awortwe C, Cascorbi I. Meta-analysis on outcome-worsening comorbidities of COVID-19 and related potential drug-drug interactions. Pharmacol Res. 2020;161:105250. doi: 10.1016/j.phrs.2020.105250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020;12(13):12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pranata R, Supriyadi R, Huang I, et al. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of covid-19 patients: a meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420959165. doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu D, Cui P, Zeng S, Wang S, Feng X, Xu S, Li R, Gao Y, Yu R, Wang Y, Yuan Y. Risk factors for developing into critical COVID-19 patients in Wuhan, China: a multicenter, retrospective, cohort study. Eclinicalmedicine. 2020;25:100471. doi: 10.1016/j.eclinm.2020.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim SR, Nam SH, Kim YR. Risk factors on the progression to clinical outcomes of Covid-19 patients in south korea: using national data. Int J Environ Res Public Health. 2020 doi: 10.3390/ijerph17238847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Omrani AS, Almaslamani MA, Daghfal J, Alattar RA, Elgara M, Shaar SH, Ibrahim TB, Zaqout A, Bakdach D, Akkari AM, Baiou A. The first consecutive 5000 patients with Coronavirus Disease 2019 from Qatar; a nation-wide cohort study. BMC Infect Dis. 2020;20(1):777. doi: 10.1186/s12879-020-05511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fried MW, Crawford JM, Mospan AR, Watkins SE, Munoz B, Zink RC, Elliott S, Burleson K, Landis C, Reddy KR, Brown RS., Jr Patient characteristics and outcomes of 11,721 patients with Covid19 hospitalized across the United States. Clin Infect Dis. 2020;2(10):e558–e565. doi: 10.1093/cid/ciaa1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bellou V, Tzoulaki I, Evangelou E, Belbasis L (2020) Risk factors for adverse clinical outcomes in patients with COVID-19: asystematic review and meta-analysis. medRxiv. 10.1101/2020.05.13.20100495
- 150.Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saran R, Robinson B, Abbott KC, et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 Suppl 1):A6–a7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 152.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58(4):1758–1764. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 153.Naqvi SB, Collins AJ. Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis. 2006;13(3):199–204. doi: 10.1053/j.ackd.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 154.Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120(6):1883–1887. doi: 10.1378/chest.120.6.1883. [DOI] [PubMed] [Google Scholar]
- 155.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.de Almeida-Pititto B, Dualib PM, Zajdenverg L, et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr. 2020;12:75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Salman B, Hussain M, Shafique K, Imtiaz S, Dhrolia MF. Risk factors of hospitalization among chronic kidney disease patients in tertiary care hospitals—a single-center experience. Saudi J Kidney Dis Transpl. 2018;29(5):1150–1158. doi: 10.4103/1319-2442.243973. [DOI] [PubMed] [Google Scholar]
- 158.Agur T, Ben-Dor N, Goldman S, et al. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients—a prospectivecohort study. Nephrol Dial Transplant. 2021 doi: 10.1093/ndt/gfab155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grupper A, Sharon N, Finn T, et al. Humoral RESPONSE TO THE Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021 doi: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Not applicable.