Abstract
Lymphangiogenesis is a key process in cancer development and metastasis. Lymphatic vessel endothelial hyaluronan receptor 1 (LYVE‐1) is a widely used marker for lymphatic endothelial cells (LEC), which also mediates immune and cancer cell migration. Recently, LYVE‐1–positive tumor cells were shown to acquire LEC‐like phenotype and exploit this receptor for lymphatic dissemination. Furthermore, selective targeting of LYVE‐1 impaired the growth of cancer‐related vasculature and reduced metastasis in vivo, signifying its role in therapeutic and prognostic applications. Although numerous studies have investigated the role of LYVE‐1 in cancer, a unifying detailed review of its prognostic utility is lacking to date. Thus, we compiled and critically appraised evidence from clinical studies comprising a total of 2352 patients diagnosed with different types of cancer and using a variety of experimental approaches. Collectively, most studies revealed a significant association between LYVE‐1 overexpression and dismal outcome of at least one survival estimate. Furthermore, the importance of vasculature location, intra‐ or peritumoral, and the influence of various lymphangiogenesis‐related parameters, such as lymphatic vessel density and invasion, were discussed. However, the specificity of LYVE‐1 staining is challenged by its expression in non‐LEC cells, implying the need for double labelling to better estimate its prognostic significance. In conclusion, this is to our knowledge the first comprehensive systematic review on the prognostic value of LYVE‐1 in cancer. More well‐designed studies across different populations and the development of standardized protocols would be paramount for the consistency of LYVE‐1 findings and for its potential transferability to clinical practice in future.
Keywords: biomarker, cancer, lymphatic vessel endothelial hyaluronan receptor 1, metastasis, prognosis
In spite of the fast‐growing pipeline of novel biomarkers, there is unmet need for accurate and reliable molecules that can facilitate risk stratification and individualized treatment decisions of cancer patients. The present comprehensive review is the first study to compile evidence and analyse the utility of LYVE‐1 for the mapping of the lymphatic vasculature, which also provided useful information, including current limitations, regarding the potential transferability of this intriguing marker to clinical practice in the future.
Abbreviations
- CBM
ciliary body melanomas
- CI
confidence interval
- CM
conjunctival melanoma
- DDFS
distant disease–free survival
- DFS
disease‐free survival
- DMFS
distant metastasis–free survival
- ELISA
enzyme‐linked immunosorbent assay
- FFPE
formalin‐fixed paraffin‐embedded
- HCC
hepatocellular carcinoma
- HNSCC
head and neck squamous cell carcinoma
- HR
hazard ratio
- IHC
immunohistochemistry
- LEC
lymphatic endothelial cells
- LNM
lymph node metastasis
- LSFS
lymphatic spread–free survival
- LV
lymphatic vessels
- LVD
lymphatic vessel density
- LVI
lymphatic vessel invasion
- LYVE‐1
lymphatic vessel endothelial hyaluronan receptor 1
- LYVE‐1+ve
LYVE‐1–positive
- MAStARI
meta‐analysis of statistics assessment and review instrument tool
- MFS
metastasis‐free survival
- MSS
melanoma‐specific survival
- NSCLC
non–small cell lung cancer
- OS
overall survival
- OSCC
oral squamous cell carcinoma
- PCR
polymerase chain reaction
- PRISMA
preferred reporting items for systematic reviews and meta‐analyses
- PROSPERO
prospective register of systematic reviews
- REMARK
reporting recommendations for tumor marker prognostic studies
- RFS
relapse‐free survival
- RSSM
risk score staging model
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
1. INTRODUCTION
The lymphatic system comprises an intricate network of vessels and nodes that play key roles in immunity and homeostasis. Additionally, lymphatics were shown to influence tumor development and mediate the majority of tumor cell metastasis in carcinomas. 1 , 2 Metastasis is responsible for approximately 90% of cancer‐related fatalities. 3 In this regard, both intra‐ and peritumoral lymphatic vessels (LV) serve as low shear stress conduits for tumor cells to traverse to locoregional and distant lymph nodes. 1 Owing to the limited effectiveness of the available angiogenic inhibitors, the lymphatic pathway has emerged as a promising targeting alternative in cancer not only for therapeutic purposes but also for risk stratification and prognostication designs. 4 , 5
Since its discovery in 1999, lymphatic vessel endothelial hyaluronic acid receptor 1 (LYVE‐1) has been extensively used to distinguish lymphatic endothelial cells (LEC) from blood vessels. 6 LYVE‐1—a receptor for hyaluronic acid—was also shown to mediate vital processes ranging from the lymphatic trafficking of immune and cancer cells to the development of lymphatic vasculature. 7 , 8 , 9 Recently, we introduced a potential novel mechanism termed “lymphatic mimicry,” whereby LYVE‐1+ve tumor cells acquire LEC‐like phenotype and exploit this receptor for lymphatic dissemination. More importantly, LYVE‐1 knockdown in tumor cells not only inhibited their lymphogenic activity but also impaired tumor cell metastasis in vivo. 10 A recent preprint study reported that selective targeting of LYVE‐1+ve tumor‐associated macrophages significantly impairs the growth of cancer‐related vasculature and hence slows tumor development. 11 Furthermore, targeting LYVE‐1 revealed therapeutic and prognostic potential in common aggressive neoplasms including breast cancer, wherein lymph node metastasis (LNM) can influence the treatment modality and survival expectancy. 12 , 13
Numerous studies have investigated the association between LYVE‐1 status and the clinical outcome of cancer; however, results are variable. To date, a unifying detailed review is lacking with regard to the utility of LYVE‐1 for prognostication, which may guide the future implementation of this intriguing molecule in the clinical practice. Thus, this study systemically reviews the literature to consolidate, and critically appraise, the available evidence regarding the prognostic value of LYVE‐1 in patients diagnosed with different types of cancer.
2. METHODS
2.1. Protocol and registration
This systematic review was designed based on the “Preferred Reporting Items for Systematic reviews and Meta‐Analyses” (PRISMA) guidelines. The protocol was registered ahead of starting in the international prospective register of systematic reviews (PROSPERO).
2.2. Eligibility criteria
Included studies were original research articles that investigated the relationship between LYVE‐1 and the survival outcomes of patients diagnosed with cancer. We excluded case reports, letters, and review articles, as well as studies based on animal models, studies that were not in English, and studies lacking data regarding the patient's survival outcome.
2.3. Search strategy
A comprehensive search of Ovid Medline, PubMed, Scopus, Cochrane Library, and Web of Science databases was undertaken on the 19th of January 2021 to include all hits up to that date. The following search terms were used: (“hyaluronic acid” OR hyaluronan OR hyaluronate) AND (LYVE1 OR LYVE‐1 OR “Lymphatic vessel endothelial hyaluronan receptor 1”) AND (cancer OR neoplasm* OR carcinoma* OR malignan* OR tumor* OR sarcoma* OR leukemi* OR lymphoma* OR adenocarcinoma*) AND (prognos* OR predict* OR surviv* OR recur* OR mortal* OR metasta*). The search results were exported from each database into ProQuest RefWorks for deduplication and further screening. Literature search and the following review steps were all conducted by two reviewers (SK and RH). Differences, if any, were rechecked and resolved through discussion with a third reviewer (AS) until consensus was reached.
2.4. Data extraction and study items
Data extraction was performed using a previously designed retrieval form. The extracted data comprised the following elements: the first author's name, publication year, country of origin, method and sample used, cancer type and site, study period, follow‐up duration, patient information (location, number of patients, mean age, gender), number of LYVE‐1–positive (LYVE‐1+ve) samples, LYVE‐1 scoring method, antibody information, cutoff value, grade of diagnosis, survival analysis, the studied endpoint, main findings, and statistical information on prognosis including hazard ratios (HRs) with 95% confidence intervals (CI) and P‐values.
2.5. Reporting quality and bias assessment
A modified checklist from the “Reporting Recommendations for Tumor Marker Prognostic Studies” (REMARK) guidelines was utilized to assess the reporting quality whenever applicable. The adapted REMARK checklist included the following six items: (a) patient samples, (b) clinical data of the cohort, (c) immunohistochemistry (IHC), (d) prognostics, and (e) statistics, (f) classic prognostic factors. To assess the risk of bias in the included studies, we used the Meta‐analysis of Statistics assessment and Review Instrument tool (MAStARI). The options to answer each question were defined as: yes, no, unclear, or not applicable.
3. RESULTS
3.1. Search results and study selection
A search of the literature in the four databases resulted in 571 records. After deduplication, a total of 233 articles were screened for eligibility based on title and abstract, ultimately revealing 36 studies for further full‐text screening. Of these, 18 studies were deemed eligible to be included in this systematic review. The flowchart of the search procedure and results is shown in Figure 1.
FIGURE 1.
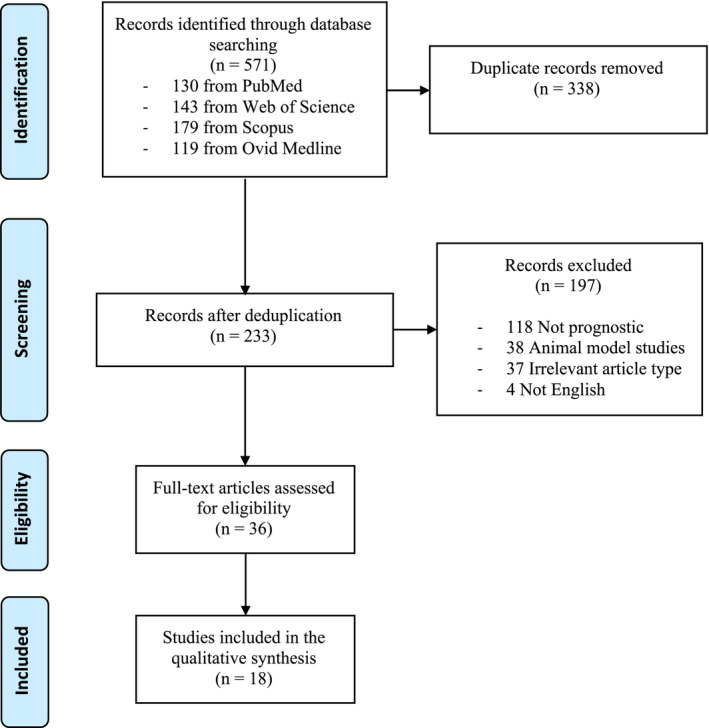
Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) flowchart of the study selection process
3.2. General study characteristics
The included studies (n = 18) were published between 2003 and 2021, and they were based in China (n = 5), Japan (n = 3), Germany (n = 4), Finland (n = 2), Poland (n = 1), Korea (n = 1), UK (n = 1), and jointly UK/Japan (n = 1). The selected studies comprised 2352 patients diagnosed with a range of different cancers, including breast cancer, 12 , 14 , 15 , 16 lung cancer, 17 , 18 , 19 , 20 melanomas, 21 , 22 , 23 , 24 head and neck squamous cell carcinoma (HNSCC), 25 , 26 , 27 human hepatocellular carcinoma (HCC), 28 ovarian carcinoma, 29 and endometrial carcinoma. 30 LYVE‐1 expression was evaluated using IHC on formalin‐fixed paraffin‐embedded (FFPE) samples (n = 14), enzyme‐linked immunosorbent assay (ELISA; n = 1), polymerase chain reaction (PCR; n = 4), and gene expression data downloaded from The Cancer Genome Atlas (n = 1). The baseline characteristics of the included studies are detailed in Table 1.
TABLE 1.
The baseline characteristics of the included studies
| Study | Country | Cancer type | Tumor stage/size (cm) | Cases | Age | Study period | Sample type | Compliance to remark |
|---|---|---|---|---|---|---|---|---|
| (12) | Finland | BC | I‐III | 180 | Median 57 | January 1987 ‐ December 1990 | FFPE | Fulfilled all items |
| (14) | Japan | BC | T1‐3 | 67 | Median 49 | January 1991 ‐ December 1991 | FFPE | Lacked item no. 5 |
| (16) | Japan/UK | BC | I‐III | 173/184 | Median 51/56 | 1991 ‐ 1993 | FFPE | Fulfilled all items |
| (15) | China | BC | I‐IV | 544 | 65 | ‐ | RSSM | Lacked items no. 1& 3 |
| (20) | China | NSCLC | N1‐2, T1‐3 | 82 | 55 | January 1995 ‐ November 2004 | FFPE | Fulfilled all items |
| (17) | Korea | NSCLC | I‐II | 40 | 62.8 | 2007 ‐ 2009 | FFPE | Lacked item no. 5 |
| (19) | Japan | LC | I‐IV | 58 | 71.3 | October 2008 ‐ March 2011 | Serum | Lacked item no. 3 |
| (18) | Poland | NSCLC | I‐IIIA | 140 | 62 | 2000 ‐ 2010 | FFPE | Lacked item no. 3 |
| (21) | Germany | Melanoma | II‐V a | 37 | 53.8, 54.9 | ‐ | FFPE | Lacked item no. 1 |
| (22) | Germany | CBM | T1d‐T3d | 20 | 69 | January 1995 ‐ May 2007 | FFPE | Fulfilled all items |
| (23) | Germany | CM | pT1a‐pT2b | 109 | 65 | 1986 ‐ 2007 | FFPE | Fulfilled all items |
| (24) | Germany | CM | pT1a‐pT2c | 60 | 65, 64, 66 | 1986 ‐ 2005 | FFPE | Lacked item no. 5 |
| (27) | Finland | HNSCC | I‐III | 97 | Median 66 | March 1989 ‐ March 1995 | FFPE | Fulfilled all items |
| (26) | China | OTSCC | I‐IV | 50 | 53.5 | 2000 ‐ 2007 | FFPE | Fulfilled all items |
| (25) | China | OSCC | I‐IV | 128 | Median 60 | Jan 2004 ‐ Oct 2008 | FFPE | Fulfilled all items |
| (28) | Japan | HCC | 4.2 (0.8‐17) | 173 | 63 | December 1993 ‐ May 2007 | FFPE | Lacked item no. 3 |
| (29) | UK /India | OC | I‐IV | 108 | Median 63 | 1990 ‐ 1998 | FFPE | Lacked item no. 3 |
| (30) | China | EC | I‐III | 102 | Median 52.9 | January 1997 ‐ July 2002 | FFPE | Lacked item no. 3 |
Abbreviations: BC, breast cancer; CBM, ciliary body melanomas; CM, conjunctival melanoma; EC, endometrial carcinoma; FFPE, formalin‐fixed paraffin‐embedded; HCC, human hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; LC, lung cancer; NSCLC, non–small cell lung cancer; OC, ovarian carcinoma; OSCC, oral squamous cell carcinoma; OTSCC, oral tongue squamous cell carcinoma; RSSM, risk score staging model.
Clark level of invasion.
3.3. Quality and risk of bias analysis
Quality assessment using the adapted REMARK checklist showed that eight studies were fully compliant with the selected items. 12 , 16 , 20 , 22 , 23 , 25 , 26 , 27 Three additional studies were fully compliant with the checklist excluding the IHC‐related item, which was not employed by these studies. 18 , 19 , 28 However, two studies lacked compliance with the first item, revealing insufficient information regarding patient samples. 15 , 21 Additionally, two studies did not disclose detailed information about the IHC (the third item) methodology, 29 , 30 whereas two other studies lacked sufficient reporting on statistics (the fifth item). 14 , 24 The compliance of each study with the adapted REMARK checklist is specified in Table 1.
The risk of bias was assessed by the MAStARI tool, revealing an excellent quality in the 18 included studies. The percentage of “yes” scores was as follows: 87.5% (n = 3), 22 , 23 , 29 75% (n = 12), 12 , 14 , 16 , 18 , 19 , 20 , 21 , 25 , 26 , 27 , 28 , 30 and 62.5% (n = 3). 15 , 17 , 24 The results of the MAStARI assessment tool are listed in Table S1.
3.4. Assessment of LVs in cancer patients
Characterizing LVs and assessing their importance have long been hampered by the lack of specific markers distinguishing them from blood vessels. Recently, several markers were identified for LVs, making it feasible to investigate this route in cancer patients. Herein, the lymphatic vessel density (LVD) or count (LVC) was identified as vascular lumen expressing LYVE‐1 (alone or together with other LV markers) within and around the tumor area. 12 , 14 , 16 , 17 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 29 , 30 In these studies, LVD was determined either by direct counting of the LVs, or indirectly by first determining the “hotspot” areas, and thence LVD was calculated. Alternatively, the lymphatic vessel invasion (LVI) was assessed in two studies. 16 , 20 In one study, the authors defined LVI as the presence of tumor cell emboli within LV spaces, which were identified by associated fibrin clot and/or LYVE‐1+ve staining. 16 In the other study, LVI was scored positive if at least one tumor cell cluster was visible inside the podoplanin+ve lumen. 20
Tumor‐associated lymphangiogenesis was investigated in two studies on non–small cell lung cancer (NSCLC) using different LV markers. Sun et al 20 employed podoplanin, LYVE‐1, or vascular endothelial growth factor receptor‐3 (VEGFR)‐3 to identify LVD. In this study, none of the podoplanin+ve spots were blood vessels as observed in VEGFR‐3+ve and LYVE‐1+ve vessels, revealing more specificity for LVs. However, only LYVE‐1 or VEGF‐D were used in a more recent study on NSCLC. 17 In ciliary body melanoma (CBM), Heindl et al 22 utilized two LV markers, LYVE‐1 and podoplanin, for evidence of red blood cells (RBC) lacking intraocular lymphatic vasculature. In two more studies by the same group, proliferating peritumoral (ie, at ≤500 µm from tumor border) and intratumoral LVD were identified as the number of LYVE‐1+ve/Ki‐67+ve and podoplanin+ve/Ki‐67+ve vessels per a square millimeter in conjunctival melanoma (CM). 23 , 24 The authors also considered invasion present if LYVE‐1+ve/Ki‐67+ve LV contained at least one cluster of tumor cells. 23
Of note, LYVE‐1 was the sole LVD marker in studies investigating HNSCC. Maula et al 27 defined intratumoral LVD as LYVE‐1+ve vessels within the tumor cell islets, while peritumoral LVD as those strictly located at the tumor margin, outside the carcinoma tissue. However, a precise description of intra‐ vs. peritumoral LYVE‐1+ve LVD was not disclosed in the other two studies. 25 , 26 Likewise, only LYVE‐1+ve vessels were considered as LVD in the ovarian and endometrial cancer studies. 29 , 30
3.5. LYVE‐1 immunoexpression in cancer patients
Of interest, LYVE‐1+ve vessels in breast cancer tissues were mainly identified in the peritumoral areas including the extralobular stroma and dermis, but less commonly within the tumor itself. 12 , 14 In contrast, small blood vessels were found in both intra‐ and extralobular stroma. 14 However, LYVE‐1+ve microvessels were found in intratumoral and peritumoral areas of lung cancer tissues and showed a strong correlation between LYVE‐1+ve and podoplanin+ve vessels. 20 In NSCLC tumors, half of the patient samples were deemed LYVE‐1+ve, which was significantly overexpressed in cases of lymphatic thromboembolisms. 17
Using double‐label immunofluorescence on melanoma samples, CD31+ve/LYVE‐1+ve LVs were observed in prominent hotspots throughout the samples, but intratumoral vessels were more common in metastatic than nonmetastatic melanomas. 21 Intraocular podoplanin+ve/LYVE‐1+ve vessels were found at the tumor borders, but not within the tumor, in more than half of CBM sections. 22 The same group showed that proliferating Ki‐67+ve/LYVE‐1+ve LVs were nonetheless seen in the intra‐ and peritumoral areas of melanoma samples. However, the intratumoral vessels were smaller, more branched, and significantly more proliferative than the peritumoral ones. 23 , 24
Noteworthy, LYVE‐1 expression was seen occasionally in macrophages and in the underlying connective tissue in samples obtained from patients with oral squamous cell carcinoma (OSCC). While most LVs were located at the interface between tumor and connective tissue (invasive front), intratumoral vessels were present in merely 13% of the samples. 27 Likewise, Ding et al 26 found that LYVE‐1+ve LVs in oral tongue SCC were more frequent at the peritumoral regions than within the tumor itself. In a recent study on HNSCC, intratumoral LYVE‐1+ve LVs were generally compressed with inconspicuous lumens compared with the more frequent, well‐delimited, and dilated peritumoral vessels. 25
Irregular and thin‐walled LYVE‐1+ve lymphatics were identified in ovarian carcinoma samples with more capsular than intratumoral vessels. 29 Similarly, LYVE‐1+ve cells were predominantly found in the peripheral regions of endometrial tumor nests compared with weak intratumoral staining. Furthermore, intratumoral LVs were considerably smaller and of irregular shape without a thick‐walled structure. 30
3.6. Cutoff values of LYVE‐1+ve vessels in cancer patients
The cutoff value for determining high versus low LVD was established as the scoring median value of LYVE‐1+ve LVs, 12 , 14 , 20 , 21 , 23 , 24 , 25 , 26 the scoring mean value, 29 , 30 or simply as the presence/absence of LYVE‐1+ve LVs. 22 , 27
3.7. LYVE‐1 transcriptional activity and serum level in cancer patients
Out of the 18 studies, three evaluated the transcriptional activity 15 , 18 , 28 and one evaluated the serum level of LYVE‐1. 19 A risk score staging model (RSMM) was employed by Liu et al 15 to identify certain prognostic genes in breast cancer patients. Of these, LYVE‐1 level was significantly higher in the tumor tissues. Aiming to identify angiogenic genes in HCC, Kitagawa et al 28 analyzed the expression profiles of 13 genes in the tumor nodules, which revealed a downregulated expression of LYVE‐1. However, the analysis results showed a substantial expression variability among patients. Consistent with these data, Kowalczuk et al 18 examined the transcriptional activity of lymphangiogenesis‐associated genes in NSCLC, where tumoral areas exhibited a significantly lower LYVE‐1 compared with tumor‐free tissues. In a different experimental approach, ELISA was used to determine the serum levels of LYVE‐1 in lung cancer patients, using a cutoff value of 1.553 pg/mL. 19 The methods used to measure LYVE‐1 in cancer patients are summarized in Table 2.
TABLE 2.
Methods used to detect LYVE‐1 in the included studies
| Study | Cancer type | Method | LYVE‐1 reagent information | LYVE‐1+ve target localization | Screening criteria | Cutoff |
|---|---|---|---|---|---|---|
| (12) | BC | PE, IHC | PC, rabbit | Mostly peritumoral | Counting LYVE‐1+ve LVD from tissue hotspots | Median value |
| (14) | BC | PE, IHC | PC, rabbit; MC, mouse | Mostly extralobular | Counting LYVE‐1/PCAB+ve LVs from tissue hotspots | Median value |
| (16) | BC | PE, IHC | PC, rabbit; MC, mouse | Mostly extralobular | Presence of tumor cells within fibrin‐ and/or LYVE‐1+ve LVs | ‐ |
| (15) | BC | RSSM | ‐ | ‐ | Screening dataset for seven prognostic genes including LYVE‐1 | Median risk score |
| (20) | NSCLC | PE, IHC | PC, rabbit; Angiobio | Intratumoral and peritumoral | Presence of LYVE‐1+ve/podoplanin+ve/VEGFR‐3+ve hotspots | Median value |
| (17) | NSCLC | IHC, PCR | Neo‐Markers | ‐ | The percentage score is multiplied by the intensity score | IHC score >6 |
| (19) | LC | ELISA | Duoset kit, R&D systems | ‐ | LYVE‐1 serum level was measured by commercial ELISA | 1.553 pg/mL |
| (18) | NSCLC | PE, mRNA | Micro fluid cards | ‐ | Analysis of 15 lymphangiogenic genes including LYVE‐1 | Median value |
| (21) | Melanoma | PE, IHC | PC, rabbit | Intratumoral and peritumoral | LYVE1+ve hotspots evaluated at high magnifications | ≤1.0 to >1.5% |
| (22) | CBM | PE, IHC | PC, rabbit; Acris Antibodies | Only peritumoral | LYVE1+ve and podoplanin+ve erythrocyte‐free LVs | Five positive LVs |
| (23) | CM | PE, IHC | PC, rabbit; Acris Antibodies | Intratumoral and peritumoral | LYVE‐1+ve and podoplanin+ve erythrocyte‐free LVs | Median value |
| (24) | CM | PE, IHC | PC, rabbit; Acris Antibodies | Intratumoral and peritumoral | LYVE‐1+ve/Ki‐67+ve and podoplanin+ve/Ki‐67+ve LVs | Median value |
| (27) | HNSCC | PE, IHC | PC, rabbit | Peritumoral; macrophages | LYVE‐1+ve LVs analyzed with microscopic ocular grid | Presence of LVs |
| (26) | OTSCC | PE, IHC | PC; Abcam | Intratumoral and peritumoral | LYVE1+ve hotspots evaluated at high magnifications | Median value |
| (25) | OSCC | PE, IHC | PC, goat; R&D systems | Intratumoral and peritumoral | LYVE1+ve hotspots evaluated at high magnifications | Median value |
| (28) | HCC | PE, PCR | Hs00272659; TaqMan | ‐ | Analysis of 13 lymphangiogenic genes including LYVE‐1 | P < .01 |
| (29) | OC | PE, IHC | PC, rabbit | Capsular and intratumoral | Counting LYVE‐1+ve LVD from tissue hotspots | Mean value |
| (30) | EC | PE, IHC | MC, mouse; R&D systems | Mostly peritumoral | Counting LYVE‐1+ve LVD from tissue hotspots | Mean value |
Abbreviations: BC, breast cancer; CBM, ciliary body melanomas; CM, conjunctival melanoma; EC, endometrial carcinoma; ELISA, enzyme‐linked immunosorbent assay; HCC, human hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; IHC, immunohistochemistry; LC, lung cancer; LV, lymphatic vessel; LVD, lymphatic vessel density; LYVE‐1, lymphatic vessel endothelial hyaluronan receptor 1; MC, monoclonal; NSCLC, non–small cell lung cancer; OC, ovarian carcinoma; OSCC, oral squamous cell carcinoma; OTSCC, oral tongue squamous cell carcinoma; PC, polyclonal; PCAB, polyclonal antibody; PCR, reverse‐transcription polymerase chain reaction; PE, paraffin‐embedded tissue samples; RSSM, risk score staging model; VEGFR‐3, vascular endothelial growth factor receptor‐3.
3.8. Survival endpoints
Multiple survival endpoints were measured including overall survival (OS), 12 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 26 , 28 , 29 , 30 progression‐free survival, 29 , 30 disease‐free survival (DFS), 21 , 28 relapse‐free survival (RFS) 14 , 16 , 23 , 24 ; disease‐specific survival, 25 , 27 lymphatic spread–free survival (LSFS), distant metastasis–free survival (DMFS), 23 , 24 and metastasis‐free survival (MFS). 22 Additionally, melanoma‐specific survival (MSS) was examined in studies by Heindl et al. 22 , 23 , 24 Also, distant disease–free survival (DDFS) was calculated as the time from the diagnosis date to the first occurrence of distant metastases. 12 The endpoints measured in each study are specified in Table 3.
TABLE 3.
The prognostic data of LYVE‐1 in the included studies
| Study | Cancer type | N | OS | Other survival endpoints | Prognostic effect | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||||
| (12) | BC | 180 | ‐ | 0.013 | ‐ | .0088 (DDFS) | Unfavorable |
| (14) | BC | 67 | ‐ | 0.045 | ‐ | .29 (RFS) | Unfavorable |
| (16) | BC | 173/184 | 0.8 (0.5‐1.3) | 0.51 | 1.1 (0.8‐1.4) | .41 (RFS) | No effect |
| (15) | BC | 544 | 1.22 (1‐1.5) | 0.04 | ‐ | ‐ | Unfavorable |
| (20) | NSCLC | 82 | RR = 2.04 (0.993‐4.19) | 0.00 | ‐ | ‐ | Unfavorable |
| (17) | NSCLC | 40 | ‐ | 0.96 | ‐ | ‐ | No effect |
| (19) | LC | 58 | 0.80 (0.68‐0.94) | 0.006 | ‐ | ‐ | Favorable |
| (18) | NSCLC | 140 | 1.27 (0.42‐3.83) | 0.66 | ‐ | ‐ | No effect |
| (21) | Melanoma | 37 | 1.55 | 0.0028 | ‐ | <.0001 (DFS) | Unfavorable |
| (22) | CBM | 20 | ‐ | ‐ | 8.91 | .008 (MSS) | Unfavorable |
| (23) | CM | 109 | ‐ | ‐ | ‐ | <.001 (RFS; LSFS; DMFS; MSS) | Unfavorable |
| (24) | CM | 60 | ‐ | ‐ | ‐ | <.05 (RFS; LSFS; DMFS; MSS) | Unfavorable |
| (27) | HNSCC | 97 | ‐ | ‐ | ‐ | .0009 (DSS) | Unfavorable |
| (26) | OTSCC | 50 | 1.52 (0.55‐4.21) | 0.41 | ‐ | ‐ | No effect |
| (25) | OSCC | 128 | ‐ | ‐ | OR = 1.29 (1.19‐1.40) | <.001 (DSS) | Unfavorable |
| (28) | HCC | 173 | 3.067 (1.507‐6.273); | 0.002 | 1.394 (0.864‐2.203) | .16 (DFS) | Favorable |
| (29) | OC | 108 | 1.02 (1.00‐1.04) | 0.41 | 1.02 (1.00‐1.05) | .5 (PFS) | No effect |
| (30) | EC | 102 | 0.3 (0.1‐0.8) | 0.019 | 0.2 (0.1‐0.6) | .003 (PFS) | Unfavorable |
Abbreviations: BC, breast cancer; CBM, ciliary body melanomas; CI, confidence interval; CM, conjunctival melanoma; DDFS, distant disease–free survival; DFS, disease‐free survival; DMFS, distant metastasis–free survival; DSS, disease‐specific survival; EC, endometrial carcinoma; HCC, human hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; HR, hazard ratio; LC, lung cancer; LSFS, lymphatic spread free survival; LYVE‐1, lymphatic vessel endothelial hyaluronan receptor 1; MSS, melanoma‐specific survival; NSCLC, non–small cell lung cancer; OC, ovarian carcinoma; OR, odds ratio; OS, overall survival; OSCC, oral squamous cell carcinoma; OTSCC, oral tongue squamous cell carcinoma; PFS, progression free survival; RFS, relapse free survival.
3.9. The prognostic value of LYVE‐1 in cancer patients
3.9.1. Breast cancer
A higher‐than‐median peritumoral LVD was associated with unfavorable OS in a cohort of 180 unilateral, invasive ductal breast carcinomas. 12 Furthermore, women with high peritumoral LVD had less rates of DDFS compared with those with a low peritumoral LVD only. In contrast, the intra‐tumoral LVD did not seem to affect the survival. However, LVD was not an independent prognostic factor in a multivariate survival analysis. 12 In another smaller study (n = 67), the authors found that LVI, but not LVD, was significantly associated with both LNM and unfavorable OS of breast cancer patients. 14 However, Kato et al 16 found that LVI was not significantly associated with survival in samples from Japanese/British patients (n = 173/184) diagnosed with primary invasive breast cancer. In another approach, Liu et al 15 analyzed gene expression data from 544 patients with breast cancer, whereby risk score staging classification was created to predict the clinical outcome. Among the seven identified prognostic genes, LYVE‐1 was a risk factor that predicted a shorter OS.
3.9.2. Lung cancer
Sun et al 20 showed that NSCLC patients (n = 82) with an overexpressed peritumoral, but not intratumoral, LVD had shorter OS. Additionally, the 5‐year survival rate was significantly reduced in LVI+ve patients compared with the LVI‐ve group. On the contrary, a smaller NSCLC study (n = 40) failed to reveal a significant association between LYVE‐1 and OS; however, LYVE‐1 overexpression was associated with lymphatic thromboembolism. 17 Although no correlation was found between the mRNA level of LYVE‐1 and OS, Kowalczuk et al 18 showed that LYVE‐1 was strongly downregulated in tumor tissues from 140 NSCLC patients. In an ELISA‐based study, Nunomiya et al 19 showed that low serum LYVE‐1 was associated with shorter OS in 58 patients with lung cancer.
3.9.3. Melanoma
The presence of intratumoral LYVE‐1+ve LVs in cutaneous melanoma (n = 37) was associated with poorer DFS when compared with intratumoral lymphatic‐free melanomas. Additionally, higher tumor lymphangiogenesis was a significant prognostic factor for reduced OS. 21 In the included studies by Heindl et al, 22 , 23 , 24 MSS rates increased significantly with the presence of intraocular LYVE‐1+ve/podoplanin+ve LVs. By multivariate Cox regression, presence of intraocular LVs was a strong prognostic predictor of mortality among CBM patients. 22 In patients with conjunctival malignant melanoma, high intratumoral LVD revealed shorter RFS, LSFS, DMFS, and MSS. 23 In agreement with this, patients with high intratumoral LVD revealed significantly lower RFS rates in conjunctival melanocytic intraepithelial neoplasia with atypia. Furthermore, CM patients with higher LVD had significantly lower RFS, LSFS, DMFS, and MSS. 24
3.9.4. Head and neck squamous cell carcinoma
Maula et al 27 showed that intratumoral LVD was significantly associated with poor disease‐specific survival in a cohort of 97 patients with HNSCC. Likewise, Chen et al 25 recently found that intratumoral, but not peritumoral, LVD was associated with poor disease‐specific survival of 97 OSCC patients. In contrast, Ding et al 26 reported no correlation between the expression of intratumoral LYVE‐1 and OS in 50 patients with oral tongue SCC. They did, however, find a significant association between higher intratumoral LYVE‐1+ve LVC and worse TNM status. 26
3.9.5. Hepatocellular carcinoma
One study evaluated the LYVE‐1 expression level in Japanese patients with HCC (n = 173). The authors found that LYVE‐1 overexpression was associated with a longer OS. Using multivariate Cox regression analysis, LYVE‐1 expression was concluded as an independent prognostic indicator of OS. However, interpatient variability has been observed in LYVE‐1 expression between HCC and noncancer samples. 28
3.9.6. Ovarian and endometrial cancers
Examining a cohort of 108 ovarian cancer patients with multivariate analysis, Sundar et al 29 found an association between LVD and OS. Similarly, a higher‐than‐mean peritumoral LVD was associated with worse progression‐free survival and OS of 102 patients with endometrial cancer. However, the intratumoral LVD did not seem to influence the survival in this cohort. 30
4. DISCUSSION
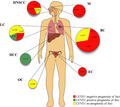
Lymphatic metastasis represents a key factor for risk stratification and decision‐making of stage‐based cancer therapeutic strategies. The recent identification of specific lymphatic markers—including LYVE‐1—has facilitated the exploitation of this route in clinical research. To our knowledge, this is the first systematic review to identify, critically appraise, and summarize clinical evidence from the literature assessing the prognostic influence of LYVE‐1 in cancer. Collectively, the 18 included studies comprised 2352 patients with seven different neoplasms and a variety of experimental approaches (Figure 2). In the majority of these reports (n = 11; 61.11%), higher expression of LYVE‐1 predicted dismal outcome of at least one survival estimate. However, some studies either found an opposite association (n = 2) or failed to observe any prognostic effect of LYVE‐1 in cancer (n = 5).
FIGURE 2.
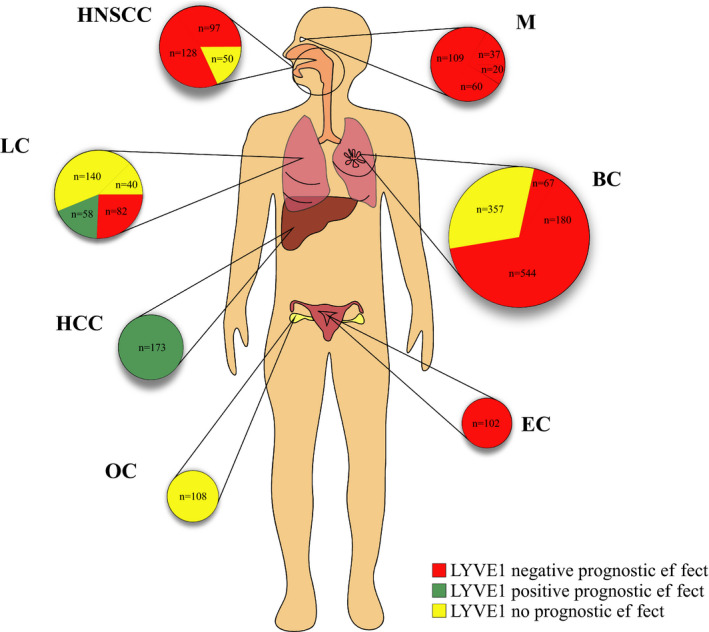
The included studies grouped by cancer type (circles) and studies (sectors). The size of the circle/sector is proportional to the number of patients (n) in each study. The color codes indicate the prognostic influence of LYVE‐1 per study as follows: red, indicates a negative or unfavorable prognostic effect; green, indicates a positive or favorable prognostic effect; yellow, indicates no prognostic effect. BC, breast cancer; EC, endometrial carcinoma; HCC, human hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; LC, lung cancer; M, melanomas; OC, ovarian cancer
A comprehensive PRISMA‐guided screening of diverse databases, PROSPERO‐registered protocol, and tailored quality‐assessment tools on studies across different populations are among the strengths of this review, making it an important contribution to the field. There are, however, a number of limitations that need to be considered. First, the number of included studies is relatively small with variable designs, scoring protocols, and outcome measures; thus, it was not possible to perform a meta‐analysis. Further, as the criteria used in each study to define LYVE‐1 expression were variable and the threshold was not standardized, we rather focused on comparing the findings qualitatively. Second, this systematic review is restricted to publications in English language only and, hence, may not represent all the available evidence. Third, the findings are derived from certain tumor types, and thus more studies are necessary before they can be generalized to other cancers.
In 2020, female breast cancer was the most frequently diagnosed tumor worldwide, surpassing lung cancer, with more than 2 million new cases. 31 Although Bono et al 12 concluded that LYVE‐1 overexpression is associated with poor outcomes in patients with ductal breast cancer, there were conflicting findings regarding the significance of LVI. 14 , 16 While LYVE‐1+ve LVI predicted LNM and OS outcomes in one study, such association was not observed in a larger cohort of Japanese and British patients, although the methods and detection rates were comparable in the two studies. 14 , 16 Nonetheless, a gene analysis study revealed LYVE‐1 as a risk‐associated factor, demonstrating a negative correlation with breast cancer survival time. 15
Reports from lung cancer, the second most prevalent malignancy, showed some prognostic utility of LYVE‐1, akin to breast cancer studies. LYVE‐1 expression in NSCLC tissues was associated with poor OS. 20 The sonic hedgehog signaling pathway plays a crucial role in tumor‐related vasculogenesis. In this regard, Hwang et al 17 investigated the clinical and pathological significances of this pathway with several LEC markers including LYVE‐1. While LYVE‐1 was not associated with distant metastasis, it was correlated with increased lymphatic thromboembolism, which is considered the first stage of LNM. 17 Notably, Nunomiya et al 19 found that serum LYVE‐1 was consistently reduced in lung cancer patients. However, it is worth noting that the control group, which had higher serum LYVE‐1, comprised patients with other inflammatory conditions including inflammatory nodules, pericardial cyst, pulmonary hamartoma, and foreign‐body granuloma. Although these data are interesting, it would be likewise important to assess the baseline level of LYVE‐1 in healthy individuals compared with cancer patients.
The importance of LV location was highlighted in the melanoma studies, where intratumoral lymphatics predicted poor survival. 21 , 23 , 24 Intraocular LVs were also detrimental to survival outcomes in CBM. 22 Such negative prognostic effect of intratumoral LVD was also revealed in two studies on HNSCC. 25 , 27 However, Ding et al 26 did not conclude a similar effect; instead, they found that high intratumoral LVD predicted worse TNM status. These findings seem in line with the importance of LNM in HNSCC, which represents a key factor for determination of appropriate management. 32 In ovarian cancer, LVD was associated with poor OS, while in endometrial carcinoma peritumoral LVD was an indicator of poor progression‐free survival and OS. 29 , 30 In contrast, LYVE‐1 overexpression predicted better OS in patients with HCC. 28 However, LYVE‐1 has also been detected in the normal liver sinusoids, posing a specificity challenge when identifying LV in the hepatic tissues. 33 Taken altogether, LYVE‐1+ve LVD and LVI were often associated with poor outcomes in the IHC‐based studies, signifying the prognostic value of this marker. Of particular importance, higher intratumoral LVD revealed prognostic utility in HNSCC studies. In OSCC, for instance, lymphatics are the primary route of metastasis and LNM is commonly evident even at the time of diagnosis. 34 Compared with blood vasculature, the leaky nature of LVs pose easier entry and hospitable environment for the traversing tumor cells. 35
In addition to LYVE‐1, a few other lymphatic‐specific markers were utilized in the included studies. Podoplanin, VEGFR‐3 and its two cognate ligands (VEGF‐C/‐D) were used in lung cancer and melanoma studies. 17 , 20 , 22 , 23 , 24 Besides their role in lymphangiogenesis, these molecules were shown to promote cancer‐related events such as endothelial‐mesenchymal transition, tumor cell invasion, and metastasis. 36 , 37 Overall, the use of such markers, including LYVE‐1, has proven to be indispensable for understanding the role of the lymphatic vascular system in promoting carcinogenesis, which ignited substantial research efforts in recent decades. 1 There are, however, challenges regarding the specificity of LEC markers that need to be overcome. One issue is that although LYVE‐1 is a widely used lymphatic marker, it bears a risk of detecting other irrelevant cell types including macrophages and cancer cells. 10 , 27 Likewise, podoplanin has been shown to stain alveolar type I cells and breast myoepithelium, which may be misinterpreted as LECs. 38 , 39 Therefore, it has been recommended to utilize a combination of at least two lymphatic endothelium markers to enhance the sensitivity and veracity of the detection. 33 , 40 , 41 In this review, five studies have supplemented LYVE‐1 with at least one additional lymphatic marker. 17 , 20 , 22 , 23 , 24
In conclusion, utilizing LYVE‐1 for the mapping of lymphatic vasculature, within and around tumor tissues, provided useful information regarding patient survival in most studies included in this review. However, more well‐designed studies examining the predictive value of LYVE‐1 in other cancer types are still needed to solidify its role as a reliable prognostic indicator. Furthermore, the development of a standardized detection methodology and definition of LV would be paramount for greater consistency of study results and for the potential transferability to clinical practice in the future.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this article.
Supporting information
Table S1
ACKNOWLEDGEMENTS
The authors would like to acknowledge the funders of this study: MD‐PhD Program, Faculty of Medicine, University of Helsinki; Paulo Foundation; Finnish Medical Foundation; Jane and Aatos Erkko Foundation; Minerva Foundation Institute for Medical Research; Cancer Society of Finland; Sigrid Jusélius Foundation; Helsinki University Central Hospital research funds; and Oulu University Hospital MRC grant.
Karinen S, Hujanen R, Salo T, Salem A. The prognostic influence of lymphatic endothelium–specific hyaluronan receptor 1 in cancer: A systematic review. Cancer Sci.2022;113:17–27. doi: 10.1111/cas.15199
Tuula Salo and Abdelhakim Salem jointly supervised this work.
REFERENCES
- 1. Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31(42):4499‐4508. [DOI] [PubMed] [Google Scholar]
- 2. Wong SY, Hynes RO. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle. 2006;5(8):812‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamakawa M, Doh SJ, Santosa SM, et al. Potential lymphangiogenesis therapies: learning from current antiangiogenesis therapies‐A review. Med Res Rev. 2018;38(6):1769‐1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou H, Lei PJ, Padera TP. Progression of metastasis through lymphatic system. Cells. 2021;10(3):627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banerji S, Ni J, Wang SX, et al. LYVE‐1, a new homologue of the CD44 glycoprotein, is a lymph‐specific receptor for hyaluronan. J Cell Biol. 1999;144(4):789‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev. 2009;230(1):216‐231. [DOI] [PubMed] [Google Scholar]
- 8. Johnson LA, Banerji S, Lawrance W, et al. Dendritic cells enter lymph vessels by hyaluronan‐mediated docking to the endothelial receptor LYVE‐1. Nat Immunol. 2017;18(7):762‐770. [DOI] [PubMed] [Google Scholar]
- 9. Makinen T, Norrmen C, Petrova TV. Molecular mechanisms of lymphatic vascular development. Cell Mol Life Sci. 2007;64(15):1915‐1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karinen S, Juurikka K, Hujanen R, et al. Tumour cells express functional lymphatic endothelium‐specific hyaluronan receptor in vitro and in vivo: Lymphatic mimicry promotes oral oncogenesis? Oncogenesis. 2021;10(3):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Opzoomer JW, Anstee JE, Dean I, et al. Macrophages orchestrate the expansion of a pro‐angiogenic perivascular niche during cancer progression. bioRxiv 2020.10.30.361907. [DOI] [PMC free article] [PubMed]
- 12. Bono P, Wasenius VM, Heikkilä P, Lundin J, Jackson DG, Joensuu H. High LYVE‐1‐positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin Cancer Res. 2004;10(21):7144‐7149. [DOI] [PubMed] [Google Scholar]
- 13. Hara Y, Torii R, Ueda S, et al. Inhibition of tumor formation and metastasis by a monoclonal antibody against lymphatic vessel endothelial hyaluronan receptor 1. Cancer Sci. 2018;109(10):3171‐3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kato T, Prevo R, Steers G, et al. A quantitative analysis of lymphatic vessels in human breast cancer, based on LYVE‐1 immunoreactivity. Br J Cancer. 2005;93(10):1168‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu L, Chen Z, Shi W, Liu H, Pang W. Breast cancer survival prediction using seven prognostic biomarker genes. Oncol Lett. 2019;18(3):2907‐2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kato T, Pezzella F, Steers G, et al. Blood vessel invasion and other variables as predictors of long‐term survival in Japanese and British patients with primary invasive breast cancer. Int J Clin Exp Pathol. 2014;7(11):7967‐7978. [PMC free article] [PubMed] [Google Scholar]
- 17. Hwang J, Kang MH, Yoo YA, et al. The effects of sonic hedgehog signaling pathway components on non‐small‐cell lung cancer progression and clinical outcome. World J Surg Oncol. 2014;12:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kowalczuk O, Laudanski J, Laudanski W, Niklinska WE, Kozlowski M, Niklinski J. Lymphatics‐associated genes are downregulated at transcription level in non‐small cell lung cancer. Oncol Lett. 2018;15(5):6752‐6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nunomiya K, Shibata Y, Abe S, et al. Relationship between serum level of lymphatic vessel endothelial hyaluronan receptor‐1 and prognosis in patients with lung cancer. J Cancer. 2014;5(3):242‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun JG, Wang Y, Chen ZT, et al. Detection of lymphangiogenesis in non‐small cell lung cancer and its prognostic value. J Exp Clin Cancer Res. 2009;28:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dadras SS, Paul T, Bertoncini J, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162(6):1951‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heindl LM, Hofmann TN, Adler W, et al. Intraocular tumor‐associated lymphangiogenesis a novel prognostic factor for ciliary body melanomas with extraocular extension? Ophthalmology. 2010;117(2):334‐342. [DOI] [PubMed] [Google Scholar]
- 23. Heindl LM, Hofmann‐Rummelt C, Adler W, et al. Prognostic significance of tumor‐associated lymphangiogenesis in malignant melanomas of the conjunctiva. Ophthalmology. 2011;118(12):2351‐2360. [DOI] [PubMed] [Google Scholar]
- 24. Heindl LM, Hofmann‐Rummelt C, Adler W, et al. Tumor‐associated lymphangiogenesis in the development of conjunctival melanoma. Invest Ophthalmol vis Sci. 2011;52(10):7074‐7083. [DOI] [PubMed] [Google Scholar]
- 25. Chen J, Zhang F, Hua M, Song X, Liu S, Dong Z. Prognostic value of lymphatic vessel density in oral squamous cell carcinoma. Life Sci. 2021;265: 118746. [DOI] [PubMed] [Google Scholar]
- 26. Ding L, Zhang Z, Shang D, et al. alpha‐Smooth muscle actin‐positive myofibroblasts, in association with epithelial‐mesenchymal transition and lymphogenesis, is a critical prognostic parameter in patients with oral tongue squamous cell carcinoma. J Oral Pathol Med. 2014;43(5):335‐343. [DOI] [PubMed] [Google Scholar]
- 27. Maula SM, Luukkaa M, Grénman R, Jackson D, Jalkanen S, Ristamäki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63(8):1920‐1926. [PubMed] [Google Scholar]
- 28. Kitagawa K, Nakajima G, Kuramochi H, Ariizumi SI, Yamamoto M. Lymphatic vessel endothelial hyaluronan receptor‐1 is a novel prognostic indicator for human hepatocellular carcinoma. Mol Clin Oncol. 2013;1(6):1039‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sundar SS, Zhang H, Brown P, et al. Role of lymphangiogenesis in epithelial ovarian cancer. Br J Cancer. 2006;94(11):1650‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao Y, Liu Z, Gao F, Meng X. High density of peritumoral lymphatic vessels is a potential prognostic marker of endometrial carcinoma: a clinical immunohistochemical method study. BMC Cancer. 2010;10:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 32. Xing Y, Zhang J, Lin H, et al. Relation between the level of lymph node metastasis and survival in locally advanced head and neck squamous cell carcinoma. Cancer. 2016;122(4):534‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mouta Carreira C, Nasser SM, di Tomaso E, et al. LYVE‐1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down‐regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61(22):8079‐8084. [PubMed] [Google Scholar]
- 34. Massano J, Regateiro FS, Januario G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(1):67‐76. [DOI] [PubMed] [Google Scholar]
- 35. Paduch R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol (Dordr). 2016;39(5):397‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quintanilla M, Montero‐Montero L, Renart J, Martin‐Villar E. Podoplanin in inflammation and cancer. Int J Mol Sci. 2019;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25(7):387‐395. [DOI] [PubMed] [Google Scholar]
- 38. Rabban JT, Chen YY. D2–40 expression by breast myoepithelium: potential pitfalls in distinguishing intralymphatic carcinoma from in situ carcinoma. Hum Pathol. 2008;39(2):175‐183. [DOI] [PubMed] [Google Scholar]
- 39. Ugorski M, Dziegiel P, Suchanski J. Podoplanin ‐ a small glycoprotein with many faces. Am J Cancer Res. 2016;6(2):370‐386. [PMC free article] [PubMed] [Google Scholar]
- 40. Kong LL, Yang NZ, Shi LH, et al. The optimum marker for the detection of lymphatic vessels. Mol Clin Oncol. 2017;7(4):515‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schledzewski K, Falkowski M, Moldenhauer G, et al. Lymphatic endothelium‐specific hyaluronan receptor LYVE‐1 is expressed by stabilin‐1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209(1):67‐77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


