Abstract
The regulation of RNA polymerase II (pol II) transcription requires a complex and context-specific array of proteins and protein complexes, as well as nucleic acids and metabolites. Every major physiological process requires coordinated transcription of specific sets of genes at the appropriate time, and a breakdown in this regulation is a hallmark of human disease. A proliferation of recent studies has revealed that many general transcription components, including sequence-specific, DNA-binding transcription factors, Mediator, and pol II itself, are capable of liquid-liquid phase separation, to form condensates that partition these factors away from the bulk aqueous phase. These findings hold great promise for next-level understanding of pol II transcription; however, many mechanistic aspects align with more conventional models, and whether phase separation per se regulates pol II activity in cells remains controversial. In this review, we describe the conventional and condensate-dependent models, and why their similarities and differences are important. We also compare and contrast these models in the context of genome organization and pol II transcription (initiation, elongation, and termination), and highlight the central role of RNA in these processes. Finally, we discuss mutations that disrupt normal partitioning of transcription factors, and how this may contribute to disease.
Graphical Abstract

Introduction
RNA polymerase II (pol II) transcription requires an array of proteins, metabolites, and nucleic acids to converge at a specific location in the nucleus. Given the sheer size of the human genome, multiple levels of nuclear organization help direct pol II to appropriate genomic locations at the correct time. One relatively new organizational mechanism involves formation of condensates (also called membraneless organelles) through a process called liquid-liquid phase separation (LLPS). LLPS is a spontaneous, thermodynamically driven process in which two co-existing liquid phases emerge from one homogenous mixture. One phase will have a higher concentration of select solutes (i.e. proteins, nucleic acids, and/or metabolites), surrounded by a less dense bulk aqueous phase [1]. Weak attractive interactions drive phase separation, which occurs when a given solute reaches a concentration above a critical point (called Csat: the saturating concentration of a solute that drives phase separation). Importantly, increasing the solute concentration beyond Csat doesn’t alter its concentration in the bulk aqueous phase; rather, it partitions to the condensate, which increases in size. Condensation or “demixing” of the solute(s) occurs because the combined attractive forces between solute molecules outweigh the entropic cost associated with demixing [2–4].
One common feature among factors that undergo LLPS is multivalency [5]. Polymers such as proteins or nucleic acids are good examples, because they can expose chemical groups (e.g. polyanionic phosphate backbone or repetitive sequences containing positively charged arginine residues) that can permit formation of low-affinity interaction networks, provided these chemical groups are accessible to surrounding molecules (see below). We emphasize that such mutivalent interactions are distinct from high-affinity protein-protein or protein-nucleic acid interactions, which typically involve structured domains and may contribute only peripherally to LLPS. Weak multivalent interactions are transient, and can occur through a variety of physical means, such as charge-charge, dipole-dipole, π-cation, and π-π stacking interactions (Figure 1). A second common feature among proteins that phase separate is the presence of one or more intrinsically disordered regions (IDRs) [6]. The sequence composition of an IDR is often repetitive and of low complexity: that is, consisting of only a subset of the 20 amino acids. Such low complexity IDRs can nevertheless be diverse in terms of their physical properties, showing enrichment in one or several combinations of polar, positively charged, negatively charged, or aromatic residues.
Figure 1: Molecular interactions that can drive phase separation.
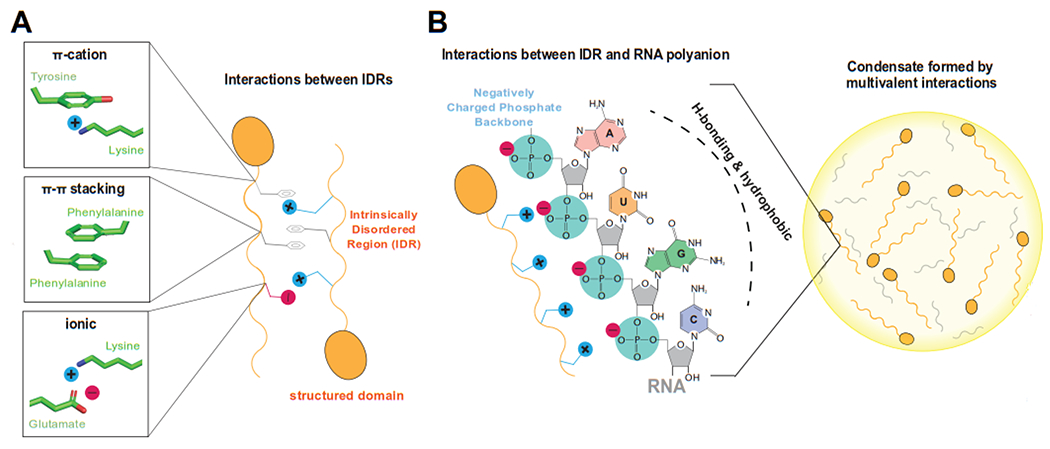
A) Two different proteins (orange) with structured and intrinsically disordered regions (IDR) are shown schematically, with different chemical properties due to their different amino acid sequences. Structural details of potential transient, low-affinity interactions between the IDRs are shown at left, representing simplified schematics for pi-cation, pi-pi, or ionic interactions. B) A simplified schematic of a phase separated condensate is shown at right, with disordered proteins and RNA partitioned within the condensate. At left are representative ionic interactions that could transiently occur between a protein IDR and the RNA phosphate backbone. Note that the RNA bases (A, U, G, C) are hydrophobic and can also participate in intermolecular interactions through hydrogen bonding (H-bonding).
The physical basis for LLPS is well understood, and we direct the reader to additional resources that describe the process in more detail [2, 4, 7–11]. That said, the complexity of biological systems, in which chemical environments can change and precise concentrations and composition often cannot be known, leaves many unanswered questions. Indeed, although condensate formation via LLPS is widely accepted as biologically relevant [1, 12], the magnitude and scope of its regulatory roles in pol II transcription remain controversial [13, 14]. Much of the controversy derives from the challenges associated with assessing condensate formation in cells, and the heterotypic nature of cellular condensates, which can change condensate properties compared with simplified model systems [15].
Condensates and hubs: How are they different and why does it matter?
The factors that regulate pol II transcription are diverse and include both proteins and nucleic acids. Pol II initiation is controlled by the pre-initiation complex (PIC), which is about 4.0 MDa in size and consists of TFIIA, TFIIB, TFIID, TFIIE, TFIIF, TFIIH, Mediator, and pol II [16]. The PIC assembles at specific sequences on genomic DNA (e.g. promoters), and DNA binding acts to cluster and concentrate protein complexes at transcription start sites (Figure 2A). Assembly and activation of the PIC is further controlled by sequence-specific, DNA-binding transcription factors (TFs). Two main models have emerged to describe how pol II activity is regulated in human cells, which we call the condensate and hub models. The condensate model invokes formation of phase separation, whereas the hub model does not. In this review we compare and contrast these models, noting that most aspects of each are not mutually exclusive.
Figure 2: Speculative models for condensate-dependent regulation of pol II transcription.
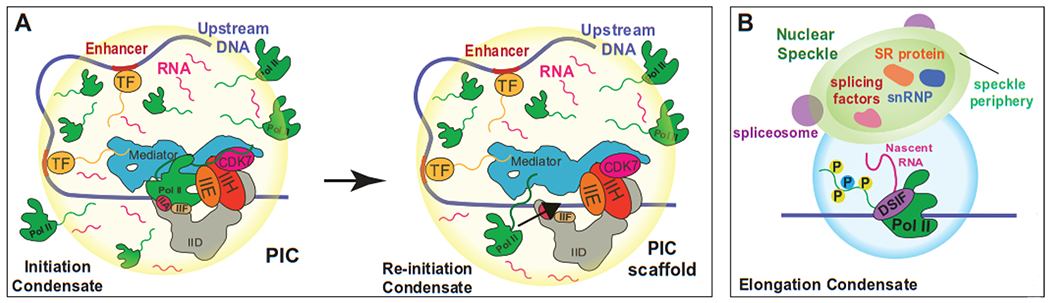
A) At some genes, low levels of eRNAs combined with high local concentration of TFs, Mediator, pol II, and other factors may cause condensate formation. The condensate may favor high-level activation (vs. bulk aqueous phase) that could be important during developmental or stress responses or for robust expression of lineage-specific genes. After pol II initiation and promoter escape (left), a second pol II could then engage the PIC scaffold to re-initiate transcription (right). Re-initiation would be enhanced by high local pol II concentration, driven by pol II CTD-dependent phase separation. B) After initiation, promoter escape, and promoter-proximal pause release, pol II separates from the PIC and enters an elongation state. During elongation, pol II associates with a different set of protein complexes (e.g. DSIF) and the pol II CTD is highly phosphorylated. This distinct environment may enable compartmentalization of elongation and initiation condensates. Such compartmentalization could promote more efficient elongation and RNA processing, perhaps through associated nuclear speckles.
Evidence for pol II transcriptional condensates or hubs was obtained in the 1990s, from labeling pol II with gold nanoparticles for EM visualization or labeling nascent RNA for visualization by fluorescence microscopy [17–19]. These experiments supported a “transcription factory” concept, in which a few hundred [18, 19] to a few thousand [17] sites of active transcription were observed in human cell nuclei. It was estimated that individual foci contained four to several dozen pol II complexes each; however, the transcriptional foci had variable intensities and it was therefore difficult to estimate the total number of pol II foci in the nucleus. The transcription factory model has been supported by later experiments using increasingly sophisticated and diverse experimental approaches [20–24].
Transcription factories are consistent with both the condensate and hub models for pol II transcription in human cells. Each of these models shares the following features: i) pol II clustering at a limited number of sites in the nucleus, presumably together with other transcription regulatory factors (e.g. Mediator, TFIID, P-TEFb, TFs, and so on); ii) pol II clusters (or foci) can contain many enhancers and promoters for multiple genes; iii) gene promoters or enhancers are recruited to pol II clusters upon activation, and iv) pol II clusters are adjacent to nuclear speckles, which represent sites for co-transcriptional splicing (Figure 2B). Finally, v) each model involves high local concentration of pol II and transcription factors (perhaps 1000-fold higher than elsewhere in the nucleoplasm) to promote transcription initiation and re-initiation (Figure 2A). High local concentration of pol II and transcription factors can be established through clustered binding sites for TFs and PIC factors and co-localization of these sites (e.g. through enhancer-promoter looping) in the nucleus. This clustering is enabled through a combination of high-affinity interactions (e.g. TF-Mediator or TF-DNA) and low-affinity interactions.
A key difference for the condensate model is that it requires establishment of a distinct liquid phase at sites of active transcription [25, 26]. Liquid-liquid phase separation (LLPS) is a well-established means to concentrate and compartmentalize proteins in the absence of a membrane, and major components of the pol II transcriptional apparatus have been shown to undergo LLPS through physiologically relevant in vitro experiments [27–32]. However, in vitro assays cannot match the complexity of the cellular environment. Whereas cell-based assays involving fluorescence microscopy, FRAP, and FISH have generally supported the in vitro results, it is difficult to prove condensate formation in cells. Existing microscopy methods generally lack the required resolution [33], especially given that many transcriptional condensates are likely small in size. This difficulty is compounded by the requirement to confirm that condensate formation i) overlaps in time and space with sites of active pol II transcription and ii) directly regulates pol II transcriptional output. By contrast, it is less experimentally demanding to claim clustering and higher local concentrations of factors in the absence of LLPS.
So why does it matter whether pol II transcription occurs within condensates or hubs? It matters because condensates represent a distinct liquid phase. The most consequential biological manifestation of this distinct phase is that the physical interactions between macromolecules (i.e. proteins or nucleic acids) and the solvent are different within condensates [2]. For example, the water content can be reduced in condensates [34], which would lower the dielectric constant in biological systems. A lower dielectric constant would increase the electrostatic forces between molecules (attractive or repulsive), according to Coulomb’s law, giving them outsized influence compared with a typical aqueous environment. Because electrostatic forces encompass ionic bonds, cation-pi interactions, hydrogen bonds, and dipole-dipole interactions, it is reasonable to assume that a lowered dielectric constant would alter the structure and function of proteins and nucleic acids within a condensate. Consistent with this concept, enzymes have shown enhanced activity within condensates [35], histone octamers were shown to partially unfold [36], and double-stranded DNA was shown to spontaneously unwind into single strands [37] within biologically relevant condensate environments.
Another motivation for understanding whether condensates contribute to regulation of pol II function (either through activation or repression) is the potential therapeutic implications [12, 38]. Whereas inhibition of specific protein-protein or protein-DNA interactions are promising and proven strategies to modulate aberrant gene expression patterns in vivo, selectively targeting such interactions is a daunting task. If specific transcriptional condensates can be selectively disrupted, it could provide an alternative or complementary means to manipulate pol II transcription for therapeutic purposes.
Condensates, hubs, and transcription regulation through genome organization
Some of the most compelling evidence for nuclear condensates derives from genome organization [39]. Transcription itself appears to be sufficient to form nuclear bodies at specific genomic locations [40] and accumulating RNA from transcriptionally active regions can act to physically separate the heterochromatin and euchromatin compartments [41–43]. Ribosomal RNA genes are sequestered in the nucleolus, which is a multi-layered condensate that is four-fold more viscous than water [44]. Other well-known examples of nuclear condensates are nuclear speckles, histone bodies, and Cajal bodies [45]. These represent sites of RNA splicing, histone gene transcription, and snRNP assembly, respectively. Also, the surface tension associated with a distinct liquid phase (i.e. condensate) can act to pull together separate regions of the genome [43], potentially to facilitate formation of enhancer-promoter loops.
The haploid human genome consists of 3 billion base pairs (bp) of DNA, and a longstanding question has been how TFs, pol II, PIC factors, and other regulatory proteins locate and coordinately assemble at the “correct” sites on genomic DNA. This is of course routinely accomplished, but it is a complex task, especially during cellular responses to developmental cues or stress, which can trigger a precise remodeling of gene expression programs within minutes. How is this accomplished? At a basic level, this task is achieved through genome organization. The nucleoplasm is organized into transcriptionally active or dormant regions, designated euchromatin or heterochromatin, respectively. These regions do not mix because chromatin adopts a gel-like state in the nucleus [46]. Heterochromatin regions are characterized by specific histone marks (e.g. H3K9me3, H3K27me3) and low transcriptional activity. Repressor proteins bind histone marks in these regions and reinforce transcriptional silencing. A number of these repressor proteins have shown evidence to form condensates in vitro and in cells, including HP1 [47–49] and the polycomb complex [50–52]. Each of these complexes serves important roles in genome organization [53, 54]. DNA methylation also acts to silence gene expression by antagonizing TF binding and by interacting with repressor proteins, such as MeCP2, which is also capable of phase separation [55]. Active chromatin regions (i.e. euchromatin) contain distinct histone marks (e.g. H3K4me3, H3K27Ac, H3K36me3) and these sites are bound by chromatin remodelers, co-activator complexes (e.g. CBP/p300, BRD4), and PIC factors (TFIID).
The separation of active and inactive genomic regions reduces the sequence space that TFs, pol II, and PIC factors must search to find their target sites on the human genome. Packaging of genomic DNA into nucleosomes also reduces the sites of open, accessible DNA available for TF binding. However, additional mechanisms are required. Whereas TFs scan the genome through non-specific DNA binding [56], a functional outcome (e.g. activation of transcription) is typically produced only when they bind at sites highly occupied by other TFs, such as enhancers and promoters. Binding can be cooperative [57, 58] and the high density of TFs, and their disordered activation domains, favors stable recruitment of chromatin remodelers, co-activators (e.g. CBP/p300), and PIC factors such as Mediator. Juxtaposition of enhancers and promoters in space, through formation of chromatin loops, further increases the local concentration of TFs and PIC factors such as pol II and Mediator.
TFs and PIC factors such as Mediator, pol II, and TFIID contain low-complexity sequences that are intrinsically disordered, which favors condensate formation (Table 1). In fact, Mediator, pol II, and many TFs have been shown to phase separate in vitro and this is also supported by data in cells [27–32, 59, 60]. At transcriptionally active genes (e.g. within transcription factories), the combination of clustered TF binding sites and the juxtaposition of enhancers and promoters creates a high density of disordered, low-complexity protein domains. These domains are anchored to the gel-like chromatin [46] through high-affinity DNA binding of TFs and the PIC factor TFIID. In the absence of condensate formation, the clustered sites and high density of disordered PIC domains (e.g. from pol II CTD, Mediator, and TFIID) and TF activation domains would promote transcription activation in part through longer residence times for TFs [56, 57, 61] and PIC factors such as Mediator. We speculate that a high density of enhancer-bound TFs would retain Mediator because multiple TFs may bind Mediator simultaneously. Longer residence times for PIC factors at gene promoters would increase the likelihood that all factors would correctly assemble to allow promoter opening and transcription initiation to occur.
Table 1: Pscore values for selected TFs (A) and PIC factors (B).
For Mediator, subunits for the kinase module are shown in orange. Pscore [11] values for pathogenic TBP polyQ repeat lengths are shown in red.
| A | |
|---|---|
| DNA-binding TF | PScore |
| HSF1 | 1.5 |
| p53 | 2.0 |
| MYC | 2.1 |
| HOXD13 | 2.5 |
| SOX2 | 3.3 |
| OCT4 | 4.4 |
| β-Catenin | 5.5 |
| EWSR1/FLI1 fusion | 6.0 |
| EWSR1 | 9.3 |
| B | |
| Pol II | PScore |
| RPB1 | 7.3 |
| Pol II CTD | 7.4 |
| Mediator Subunits | PScore |
| MED1 | 4.0 |
| MED4, MED6-11 | <3.0 |
| MED14 | 4.2 |
| MED15 | 5.4 |
| MED16-31 | <3.0 |
| MED12 | 3.9 |
| MED12L | 2.3 |
| MED13 | 3.7 |
| MED13L | 4.6 |
| CDK19 | 3.4 |
| CDK8 | 2.4 |
| CCNC | <0 |
| TFIID subunits | PScore |
| TBP (38Q) | 2.1 |
| TBP (47Q) | 4.9 |
| TBP (53Q) | 6.1 |
| TAF1-2 | <3.0 |
| TAF3 | 4.5 |
| TAF4 | 5.1 |
| TAF5 | 5.5 |
| TAF11 | 3.4 |
| TAF6-10, TAF12-13 | <3.0 |
The clustering of TFs and PIC factors could also trigger phase separation [25, 26], and emerging evidence suggests that condensate formation contributes to pol II transcriptional activation in cells [62–65]. One way that condensate formation could help regulate cellular transcription is to shorten the time that TFs and PIC factors must scan the genome before binding their “correct” target sites. This concept is supported by recent data from the Wu lab, in which PIC factor diffusion was measured in live yeast cells [66]; moreover, Barkai et al. showed that TF IDRs alone are sufficient to localize TFs near their target sites even in the absence of a DNA-binding domain [59]. Other studies have shown that the pol II CTD acts to promote clustering at enriched sites [27, 65, 67], with evidence that this is mediated at least in part through phase separation [68]. In this way, condensates could act as a “magnet” to allow cell type- and signal-specific factors to more rapidly locate target sites and therefore more rapidly respond to biological stimuli.
The formation liquid condensates at sites of active pol II transcription remains controversial [13, 69], and in some cases different teams of investigators have drawn different conclusions. For instance, Erdel et al. studied transcriptional repression and concluded that HP1α acted via chromatin compaction but lacked true hallmarks of phase separation [70], in contrast to earlier reports [47–49]. As another example, the Tjian and Darzacq labs analyzed TF-dependent pol II activation and concluded that a hub/transcription factory model was more plausible, although condensate formation was not excluded [71]. This model included high-density TF clustering and TF retention through dynamic, multivalent interactions. Although some hallmarks of phase separation were noted, other characteristics such as refractive index changes were evident only at super-physiological TF expression levels [71]. These same investigators noted that herpes virus replication compartments resembled phase separated condensates, but a series of super-resolution experiments revealed similar diffusion rates through the compartments. These data suggested that replication compartments acted in a more conventional way: high local concentration of factors, enabled by viral DNA that was not chromatinized and therefore more accessible. This, in turn, allowed the viral replication compartments to out-compete the host genome for DNA-binding factors [72].
The controversy over condensate-dependent regulation of transcription persists in part because it is extremely challenging to prove that liquid phase separated domains form at precise genomic locations, coincident with predicted changes in pol II activity. Likewise, it is difficult to prove that such phase separated domains are not forming and are not contributing to pol II function in cell nuclei. Models and diagnostic methods that infer cellular condensate formation will continue to be revised and improved. For example, the heterotypic nature of cellular condensates [15] precludes any straightforward comparison with simplified model systems, and computational simulations now suggest that molecular diffusion coefficients can in fact be similar across phase boundaries [73]. Also, it has been shown that RNA can greatly reduce the protein concentration (Csat) required for condensate formation [74], which is highly relevant for transcriptional condensates in cell nuclei.
Condensates, hubs, and RNA polymerase II transcription
The pre-initiation complex (PIC) is recruited to transcription start sites genome-wide, and regulates pol II initiation (Figure 2A). Recruitment of the PIC to the appropriate sites on the genome is mediated by sequence-specific, DNA-binding transcription factors (TFs). TFs, in turn, recruit Mediator (among other factors), which interacts directly with the pol II enzyme and helps control its function at gene promoters [75]. Among the eight PIC factors, Mediator, TFIID, and pol II contain subunits that have a high propensity to phase separate (Table 1B), based upon the Pscore metric [11]. Furthermore, Mediator and pol II can each form condensates in vitro at physiologically relevant concentrations [27, 28], and Mediator and pol II can coexist within the same condensate [30]. On its own, the pol II CTD can form condensates and this property appears to contribute to pol II clustering in cells [27, 65, 67, 68]. Additionally, the length of the pol II CTD modulates pol II clustering, with decreased Csat corresponding to increased CTD length [27]. The pol II CTD has increased in length across evolutionary time, with 52 heptad repeats of the general consensus sequence YSPTSPS in humans and 26 repeats in yeast (S. cerevisiae). The increased pol II CTD length likely evolved for several reasons, but one may be to enhance its condensate-forming properties.
Clustering of pol II enzymes appears to be common in human cells, and this is consistent with the “transcription factory” model in which genes migrate to pol II hubs upon activation [76]. Transcriptional bursting is also consistent with pol II clustering. It is now understood that cellular transcription occurs in bursts [77], in which multiple pol II enzymes initiate from the same promoter within a short time frame, followed by extended dormant periods [78]. Clustering of pol II enzymes within transcription hubs (presumably with hypo-phosphorylated CTD) would promote this behavior by maintaining a high local concentration of pol II at active gene promoters (Figure 2A).
Transcriptional bursting is consistent with a condensate model as well. Clusters of pol II and promoter-bound PICs could promote condensate formation, and bursting could be a consequence of a switch-like change in activity triggered by phase separation. Although speculative, accumulating evidence supports condensate-dependent acceleration enzymatic reactions [35, 79], including in the context of pol II transcription [62, 63, 65, 80]. Moreover, the structural states of proteins and nucleic acids can be altered within condensates, and this could contribute to bursting activity. For example, promoter DNA may be maintained in an “open” state to allow rapid re-initiation by additional pol II complexes. Pol II will initiate more rapidly from pre-melted DNA templates, and there is evidence that stable open complexes promote high-level expression of snRNA genes and heat shock genes [81, 82]. Other studies have shown that double-stranded DNA spontaneously unfolds to single strands in a condensate environment [37].
Condensate formation at genes within active pol II clusters would be promoted further by high local concentrations of TFs bound to enhancers and promoters. Whereas TF DNA-binding domains are structured and relatively rigid, TF activation domains are disordered and can phase separate [26]. TF activation domains interact with Mediator and can form mixed condensates in vitro and probably also in cells, at least at highly expressed genes [28, 83]. In addition, co-activators that augment signal-specific gene activation (e.g. β-catenin) were shown to preferentially incorporate into TF-Mediator condensates in vitro and in cells [32]. Although formation of bona-fide condensates is difficult to confirm in cells, Klein et al. [38] showed that increasing concentrations of the Mediator subunit MED1 increased its cellular condensate size, an expectation for liquid phase-separated condensates [14]. Work from the Young lab and others has highlighted the phase separation capacity of disordered TF activation domains; furthermore, mutations in TF activation domains that disrupt its phase separation properties led to diminished activity in cells [28, 32, 84]. An expectation based upon these results is that addition of an IDR would increase TF-dependent condensate formation and enhance TF-dependent transcription activation. This has been recently demonstrated in mammalian cells using rationally designed TFs [63, 65].
Mediator and pol II colocalize in stable clusters in live cells and have characteristics consistent with condensate formation via LLPS [85]. The size of some Mediator clusters in cells was estimated to be greater than 300 nm in diameter, large enough to encompass numerous PICs and co-localized promoter and enhancer regions [85]. Pol II clusters that co-localize with Mediator and TFs in cells likely regulate transcription initiation, whereas separate localized clusters of factors help control pol II elongation and co-transcriptional splicing. Nuclear speckles are enriched in splicing factors and display hallmarks of liquid phase separated condensates in cells [86, 87]. Upon the transition from initiation (at gene promoters) to elongation (in gene bodies), the pol II CTD becomes highly phosphorylated. Notably, this phosphorylation disrupts high-affinity pol II CTD-Mediator binding and work from Guo et al. showed that CTD phosphorylation promoted pol II exit from Mediator condensates; however, phosphorylated pol II could then preferentially associate with nuclear speckles [30]. In this way, the phosphorylation of the pol II CTD promotes a transition from promoter-associated initiation/PIC condensates to elongation/splicing condensates (Figure 2B). In agreement, data from multiple labs have shown that condensates containing unmodified pol II CTD dissolve upon CTD phosphorylation [27, 30, 67].
At gene promoters, phosphorylation of the pol II CTD is carried out by kinases such as the TFIIH-associated CDK7 and the P-TEFb kinase CDK9. Whereas TFIIH is a PIC factor, P-TEFb appears to associate with pol II immediately after initiation, at a promoter-proximal pause stage [88]. P-TEFb contains Cyclin T1 (CCNT1), which possesses an intrinsically disordered histidine-rich domain. Data from Lu et al. demonstrated that this domain could modulate condensate-forming properties of P-TEFb [31]. The CCNT1 histidine-rich domain was also shown to increase P-TEFb association with nuclear speckles. Collectively, the results from Boehning et al., Guo et al., and Lu et al. suggest that pol II initiation and elongation/splicing factors can be compartmentalized in cells [27, 30, 31], as a means to effectively regulate these distinct transcriptional stages [89].
We emphasize that the pol II CTD mediates direct, high-affinity protein-protein interactions that are distinct in the unphosphorylated vs. phosphorylated forms, and the CTD interactome is consistent with initiation vs. elongation and splicing factors, respectively [90]. Thus, differential roles for the pol II CTD during transcription initiation and elongation can be explained through conventional biochemical interactions involving proteins in their native folded states (i.e. not within condensates). Additional experiments are needed to determine the relative contribution of condensates in the regulation of pol II initiation, elongation, and splicing. Furthermore, it remains to be established how uniform condensate-dependent regulation may be at active genes. Potentially, condensates may contribute to pol II regulation only at a subset of genes in any given cell, such as those that are highly expressed and associated with super-enhancers [83].
RNA-dependent regulation of transcriptional hubs or condensates
RNA is a flexible, anionic polymer that can fold into an array of three-dimensional shapes through base pair hybridization. The ionic phosphate backbone of RNA is attached to polar sugar moieties that are linked to hydrophobic RNA bases (A, G, C, U; Figure 1B). The chemical diversity of RNA polymers is expanded by potential modifications (e.g. methylation or A-to-I editing) that alter its structural and chemical properties. These biochemical features enable RNA to form condensates on its own [91], as well as modulate condensate properties of complex mixtures [92]. For example, at low levels, RNA promotes condensate formation by increasing the number of low-affinity multivalent interfaces, whereas at high concentrations, RNA can dissolve condensates through charge-charge repulsion [93–95].
In cells, RNA is a key component of condensates such as stress granules, nucleoli, and nuclear speckles [45]. Structured RNAs tend to be excluded from condensates [96], whereas disordered RNAs are favored, presumably because the hydrophobic A, C, G, or U bases remain exposed for transient, low-affinity interactions with other proteins, metabolites, or nucleic acids. Notably, mRNAs tend to be less structured than other cellular RNAs such as tRNA or snRNA [97]. Although mRNA structural disorder likely evolved to allow splicing and splice site recognition [97], it could also be important for condensate formation. Several labs have shown that ATP-dependent RNA helicases, which unwind RNA secondary structures, can control RNA flux at condensate boundaries [98, 99]. Also, distinct pre-mRNA sequences (presumably adopting different structural states) can alter condensate properties to favor incorporation of certain types of proteins or nucleic acids while excluding others [100, 101].
About fifteen years ago it was discovered that pol II transcription was pervasive throughout the genome [102]. Whereas specific biological roles for all transcribed RNA species is unlikely, a general abundance of nuclear RNA probably helps keep RNA-bindng proteins (RBPs) soluble in the nucleus [94], and also appears to physically segregate transcriptionally active chromatin compartments [41, 42]. About ten years ago it was discovered that enhancer sequences are transcribed by pol II [103]. Such “eRNAs” (enhancer RNAs) are transcribed bidirectionally; that is, each DNA strand (sense and antisense) is transcribed. Bidirectional eRNA transcripts are typically short (less than 1kb), unstable, and commonly originate near TF binding sites in the enhancer [104, 105].
Biological roles for eRNAs have remained elusive, but several possible functions in pol II transcription have recently been identified. One function supports the condensate model and the other best supports the hub model. Several labs have shown that eRNAs can promote condensate formation in vitro and data support a similar role in cells [62, 106]. Inhibition of cellular transcription was also shown by Henninger et al. to increase the size of transcriptional condensates [62], an observation consistent with LLPS [14, 107] and congruent with condensate dissolution at high RNA concentrations [94]. Because too much RNA can dissolve condensates, the low-level expression of eRNAs, combined with their short half-lives, is consistent with a role in condensate formation. Alternatively, pol II-transcribed eRNAs have been shown to sequester enhancer-binding TFs near enhancer DNA, to effectively prolong TF occupancy by increasing local concentration [108, 109]. The potential for eRNA to “capture” dissociated TFs suggests a means to increase TF-enhancer residence time in cells, because the eRNA-bound TF would be retained near the enhancer and could re-bind enhancer DNA. This concept helps reconcile unexpected results obtained from live cell imaging experiments, which have shown that TF residence times at enhancers can range from about 15 seconds [57] to dozens of minutes [61]. Based upon in vitro binding assays with purified TFs and DNA, typical residence times were expected to be much longer.
The reduced dwell times for TFs bound to enhancer DNA in cells vs. in vitro suggests distinct dissociation mechanisms. One plausible explanation is that TF dissociation is enhanced in cells because of competition by other TFs. Competition would be facilitated by a high local concentration of TFs at the enhancer. Enhancer-bound TFs interact not only with DNA, but also with regulatory factors such as Mediator or chromatin modifying complexes. The Wright lab has shown that dissociation rates for TF activation domains can be increased due to competition by other TFs [110]. In particular, binding of a second TF activation domain to the CBP/p300 coactivator caused conformational changes that promoted more rapid dissociation of the original bound TF. Similar competitive mechanisms could favor rapid turnover of TF-DNA and/or TF-coactivator interactions in cells, which could be offset through TF sequestration by eRNAs [108, 109].
Finally, we note that pol II transcripts are poly-adenylated (polyA) at their 3’-ends, and the typical polyA tail length is 50-100 nucleotides in human cells [111]. Homopolymeric RNA sequences can form condensates on their own [91], and purine condensates (e.g. polyA) possess distinct biophysical properties compared with pyrimidine (e.g. polyU) condensates [112]. Potentially, the attachment of polyA tails onto pol II transcripts bestows phase separation properties for the RNA that are distinct from non-polyA transcripts; although speculative, such polyA-dependent properties may influence mRNA biogenesis, nuclear export, and/or translation.
Whether acting through a hub or a condensate, RNA has emerged as a general regulator of pol II transcription [41]. In this section we have highlighted how RNA indirectly impacts pol II activity, through nuclear organization, TF tethering, and/or modulation of condensate properties. RNA can play more direct roles in pol II transcription through formation of secondary structures [97, 113] or through binding and regulation of cofactor complexes (e.g. P-TEFb, steroid receptor coactivator), but these topics are beyond the scope of this review.
Transcriptional condensates and human disease
Emerging evidence suggests that mutations that disrupt the condensate-forming properties of proteins contribute to disease pathogenesis, and several excellent reviews have addressed this topic [12, 114]. In this section, we focus on a subset of examples that relate to TFs and pol II transcription.
TFs are the primary drivers of gene expression patterns in cells, and as a consequence their expression or activity is commonly disrupted in human diseases [115]. Repeat expansions in TFs can disrupt their normal capacity to form condensates and are linked to multiple human diseases and inherited disorders [116]. Two primary classes of disease-associated expansions are polyalanine and poly-glutamine repeats, which can occur in TF IDRs [117]. Poly-alanine repeats in the HOXD13 TF are linked to synpolydactyly syndrome [118]. The IDR of HOXD13 was shown to promote LLPS and colocalization with Mediator condensates [119]. HOXD13 mutants with polyalanine repeats had a lower Csat but were less efficiently incorporated within Mediator condensates [84]. This altered partitioning correlated with changes in gene expression patterns compared with wild type HOXD13. Additionally, repeat expansions in the TATA-box binding protein (TBP) may affect its phase separation properties. TBP is a subunit within the large TFIID complex, which deposits TBP about 30 bp upstream of transcription start sites, genome-wide. TBP possesses a poly-glutamine tract with varying lengths, ranging from 25-42 repeats in the general population. Greater than 42 repeats in TBP is associated with spinocerebellar ataxia type 17 [84], suggesting that altered phase separation may contribute to disease pathogenesis. In agreement, the Pscore algorithm [11] predicts a high probability for TBP to phase separate with a polyQ tract longer than 42 residues (Table 1B).
Aberrant tethering of IDRs to TF DNA-binding domains, through chromosomal translocations, can also disrupt normal TF function and may cause disease. Ewing sarcoma is a pediatric bone cancer that is caused by translocation of an RNA-binding protein EWSR1 with the DNA-binding domain of the FLI1 TF [120]. The Rivera lab showed that mutant EWS-FLI1 fusion proteins aberrantly recruited the BRG1/BRM-associated factor (BAF) chromatin remodeling complex to enhancers, which activated a set of genes to promote oncogenesis. Notably, this gain of function activity for EWS-FLI1 required residues important for phase separation [29]. These and other studies [28, 32, 59, 60, 83] suggest that phase separation is a basic aspect of TF function, and that impairment of TF condensate properties could be pathogenic. Recently, the Hnisz lab generated a catalog of IDRs for about 1500 human TFs [84], which serves as a valuable resource for further research.
Concluding remarks
In this review, we have summarized some of the mechanisms by which pol II transcription is regulated in human cells. The data support an underlying nuclear and chromatin architecture that favors activation at some genomic loci and repression at others. During transcription activation, juxtaposition of enhancer and promoter elements yields a high local concentration of proteins and RNA. This general feature is consistent with both the hub and condensate models.
Although the controversy surrounding pol II transcriptional hubs and condensates assumes that only one model is correct, we propose that both models are correct. We speculate that condensates act primarily at highly expressed genes, such as those controlled by super-enhancers. At other genes, more conventional mechanisms consistent with hubs and transcription factories may predominate. Similarly, transcriptional repression is likely to be variably dependent upon molecular condensates. Although the hub and condensate models have many shared features, condensates can better reconcile evidence of rapid, genome-wide changes in transcription during stress [121–123], and condensates appear to be more responsive to regulation by post-translational modifications [47, 123].
Many new and counter-intuitive discoveries will continue to advance our understanding of pol II transcription in the years ahead. We anticipate that technological advances will result in new and more rigorous ways to evaluate condensates in live cells, and will better enable spatial and temporal matching to sites of active transcription. We emphasize, however, that cellular systems are enormously complex, and the precise composition and concentration of factors within any given condensate cannot be accurately determined. By contrast, in vitro assays can reconstitute systems with defined components (e.g. proteins, nucleic acids, metabolites) at defined concentrations, pH, and temperature. Moreover, in vitro assays have yielded results consistent with cellular condensate behaviors [3, 8, 46, 124] and are therefore a good complement to cell-based assays. Because condensate formation appears to be a general property of proteins [107, 125, 126], it will be important to study native proteins and protein complexes at physiologically relevant concentrations and under physiologically relevant conditions (in vitro and in cells) whenever possible. Protein IDRs can act to drive phase separation but can also mediate stable, high-affinity protein-protein interactions; consequently, distinguishing whether IDR mutations cause changes in condensate behavior and/or altered protein-protein interactions will be an enduring challenge.
Highlights.
The precise role of condensates in regulation of human pol II transcription remains unclear
Conventional models share many features with condensate-dependent regulation
Condensates invoke new mechanisms for transcription regulation, and therapeutic strategies
Acknowledgments
Research in the Taatjes lab is supported in part by the NIH (R35 GM139550) and the NSF (MCB-1818147). M.P. was supported in part by the NIH (T32 GM065103).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CREDIT statement:
Megan Palacio: Visualization; Writing – Original draft, review & editing
Dylan J Taatjes: Writing – Original draft, review & editing
Declaration of interest:
Competing interest statement: D.J.T. is a member of the scientific advisory board at Dewpoint Therapeutics.
References
- [1].Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017;18:285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bergeron-Sandoval LP, Safaee N, Michnick SW. Mechanisms and Consequences of Macromolecular Phase Separation. Cell. 2016;165:1067–79. [DOI] [PubMed] [Google Scholar]
- [3].Wang J, Choi JM, Holehouse AS, Lee HO, Zhang X, Jahnel M, et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell. 2018;174:688–99 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu H, Fuxreiter M. The Structure and Dynamics of Higher-Order Assemblies: Amyloids, Signalosomes, and Granules. Cell. 2016;165:1055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, et al. Protein Phase Separation: A New Phase in Cell Biology. Trends in cell biology. 2018;28:420–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wright PE, Dyson HJ. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol. 2015;16:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brangwynne CP, Tompa P, Pappu RV. Polymer physics of intracellular phase transitions. Nat Phys. 2015;11:899–904. [Google Scholar]
- [8].Lin YH, Forman-Kay JD, Chan HS. Theories for Sequence-Dependent Phase Behaviors of Biomolecular Condensates. Biochemistry. 2018;57:2499–508. [DOI] [PubMed] [Google Scholar]
- [9].Martin EW, Mittag T. Relationship of Sequence and Phase Separation in Protein Low-Complexity Regions. Biochemistry. 2018;57:2478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ruff KM, Roberts S, Chilkoti A, Pappu RV. Advances in Understanding Stimulus-Responsive Phase Behavior of Intrinsically Disordered Protein Polymers. Journal of molecular biology. 2018;430:4619–35. [DOI] [PubMed] [Google Scholar]
- [11].Vernon RM, Chong PA, Tsang B, Kim TH, Bah A, Farber P, et al. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. eLife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tsang B, Pritisanac I, Scherer SW, Moses AM, Forman-Kay JD. Phase Separation as a Missing Mechanism for Interpretation of Disease Mutations. Cell. 2020;183:1742–56. [DOI] [PubMed] [Google Scholar]
- [13].McSwiggen DT, Mir M, Darzacq X, Tjian R. Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev. 2019;33:1619–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peng A, Weber SC. Evidence for and against Liquid-Liquid Phase Separation in the Nucleus. Noncoding RNA. 2019;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Riback JA, Zhu L, Ferrolino MC, Tolbert M, Mitrea DM, Sanders DW, et al. Composition-dependent thermodynamics of intracellular phase separation. Nature. 2020;581:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schier AC, Taatjes DJ. Structure and mechanism of the RNA polymerase II transcription machinery. Genes Dev. 2020;34:465–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Iborra FJ, Pombo A, Jackson DA, Cook PR. Active RNA polymerases are localized within discrete transcription “factories’ in human nuclei. Journal of cell science. 1996;109 (Pt 6):1427–36. [DOI] [PubMed] [Google Scholar]
- [18].Jackson DA, Hassan AB, Errington RJ, Cook PR. Visualization of focal sites of transcription within human nuclei. EMBO J. 1993;12:1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wansink DG, Schul W, van der Kraan I, van Steensel B, van Driel R, de Jong L. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J Cell Biol. 1993;122:283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, et al. In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol. 2007;14:796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ghamari A, van de Corput MP, Thongjuea S, van Cappellen WA, van Ijcken W, van Haren J, et al. In vivo live imaging of RNA polymerase II transcription factories in primary cells. Genes Dev. 2013;27:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mitchell JA, Fraser P. Transcription factories are nuclear subcompartments that remain in the absence of transcription. Genes Dev. 2008;22:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, et al. Active genes dynamically colocalize to shared sites of ongoing transcription. Nature genetics. 2004;36:1065–71. [DOI] [PubMed] [Google Scholar]
- [25].Hnisz D, Shrinivas K, Young RA, Chakraborty AK, Sharp PA. A Phase Separation Model for Transcriptional Control. Cell. 2017;169:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sabari BR, Dall’Agnese A, Young RA. Biomolecular Condensates in the Nucleus. Trends Biochem Sci. 2020;45:961–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boehning M, Dugast-Darzacq C, Rankovic M, Hansen AS, Yu T, Marie-Nelly H, et al. RNA polymerase II clustering through carboxy-terminal domain phase separation. Nat Struct Mol Biol. 2018;25:833–40. [DOI] [PubMed] [Google Scholar]
- [28].Boija A, Klein IA, Sabari BR, Dall’Agnese A, Coffey EL, Zamudio AV, et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell. 2018;175:1842–55 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Boulay G, Sandoval GJ, Riggi N, Iyer S, Buisson R, Naigles B, et al. Cancer-Specific Retargeting of BAF Complexes by a Prion-like Domain. Cell. 2017;171:163–78 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Guo YE, Manteiga JC, Henninger JE, Sabari BR, Dall’Agnese A, Hannett NM, et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature. 2019;572:543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lu H, Yu D, Hansen AS, Ganguly S, Liu R, Heckert A, et al. Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II. Nature. 2018;558:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zamudio AV, Dall’Agnese A, Henninger JE, Manteiga JC, Afeyan LK, Hannett NM, et al. Mediator Condensates Localize Signaling Factors to Key Cell Identity Genes. Mol Cell. 2019;76:753–66 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Patange S, Ball DA, Karpova T, Larson DR. Towards a ‘spot on’ understanding of transcription in the nucleus. Journal of molecular biology. 2021:167016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reichheld SE, Muiznieks LD, Keeley FW, Sharpe S. Direct observation of structure and dynamics during phase separation of an elastomeric protein. Proc Natl Acad Sci U S A. 2017;114:E4408–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].O’Flynn BG, Mittag T. The role of liquid-liquid phase separation in regulating enzyme activity. Curr Opin Cell Biol. 2021. ;69:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sanulli S, Trnka MJ, Dharmarajan V, Tibble RW, Pascal BD, Burlingame AL, et al. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature. 2019;575:390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nott TJ, Craggs TD, Baldwin AJ. Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. Nat Chem. 2016;8:569–75. [DOI] [PubMed] [Google Scholar]
- [38].Klein IA, Boija A, Afeyan LK, Hawken SW, Fan M, Dall’Agnese A, et al. Partitioning of cancer therapeutics in nuclear condensates. Science. 2020;368:1386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Feric M, Misteli T. Phase Separation in Genome Organization across Evolution. Trends in cell biology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shevtsov SP, Dundr M. Nucleation of nuclear bodies by RNA. Nat Cell Biol. 2011;13:167–73. [DOI] [PubMed] [Google Scholar]
- [41].Creamer KM, Kolpa HJ, Lawrence JB. Nascent RNA scaffolds contribute to chromosome territory architecture and counter chromatin compaction. Mol Cell. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hilbert L, Sato Y, Kuznetsova K, Bianucci T, Kimura H, Julicher F, et al. Transcription organizes euchromatin via microphase separation. Nature communications. 2021;12:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shin Y, Chang YC, Lee DSW, Berry J, Sanders DW, Ronceray P, et al. Liquid Nuclear Condensates Mechanically Sense and Restructure the Genome. Cell. 2018;175:1481–91 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, et al. Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell. 2016;165:1686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wiedner HJ, Giudice J. It’s not just a phase: function and characteristics of RNA-binding proteins in phase separation. Nat Struct Mol Biol. 2021;28:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Strickfaden H, Tolsma TO, Sharma A, Underhill DA, Hansen JC, Hendzel MJ. Condensed Chromatin Behaves like a Solid on the Mesoscale In Vitro and in Living Cells. Cell. 2020;183:1772–84 e13. [DOI] [PubMed] [Google Scholar]
- [47].Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, et al. Liquid droplet formation by HP1alpha suggests a role for phase separation in heterochromatin. Nature. 2017;547:236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Strom AR, Emelyanov AV, Mir M, Fyodorov DV, Darzacq X, Karpen GH. Phase separation drives heterochromatin domain formation. Nature. 2017;547:241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang L, Gao Y, Zheng X, Liu C, Dong S, Li R, et al. Histone Modifications Regulate Chromatin Compartmentalization by Contributing to a Phase Separation Mechanism. Mol Cell. 2019;76:646–59 e6. [DOI] [PubMed] [Google Scholar]
- [50].Fasciani A, D’Annunzio S, Poli V, Fagnocchi L, Beyes S, Michelatti D, et al. MLL4-associated condensates counterbalance Polycomb-mediated nuclear mechanical stress in Kabuki syndrome. Nature genetics. 2020;52:1397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kent S, Brown K, Yang CH, Alsaihati N, Tian C, Wang H, et al. Phase-Separated Transcriptional Condensates Accelerate Target-Search Process Revealed by Live-Cell Single-Molecule Imaging. Cell reports. 2020;33:108248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Plys AJ, Davis CP, Kim J, Rizki G, Keenen MM, Marr SK, et al. Phase separation of Polycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev. 2019;33:799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schwartz YB, Cavalli G. Three-Dimensional Genome Organization and Function in Drosophila. Genetics. 2017;205:5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zenk F, Zhan Y, Kos P, Loser E, Atinbayeva N, Schachtle M, et al. HP1 drives de novo 3D genome reorganization in early Drosophila embryos. Nature. 2021;593:289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li CH, Coffey EL, Dall’Agnese A, Hannett NM, Tang X, Henninger JE, et al. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature. 2020;586:440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liu Z, Legant WR, Chen BC, Li L, Grimm JB, Lavis LD, et al. 3D imaging of Sox2 enhancer clusters in embryonic stem cells. eLife. 2014;3:e04236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen J, Zhang Z, Li L, Chen BC, Revyakin A, Hajj B, et al. Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell. 2014;156:1274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Panne D, Maniatis T, Harrison SC. An atomic model of the interferon-beta enhanceosome. Cell. 2007;129:1111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Brodsky S, Jana T, Mittelman K, Chapal M, Kumar DK, Carmi M, et al. Intrinsically Disordered Regions Direct Transcription Factor In Vivo Binding Specificity. Mol Cell. 2020;79:459–71 e4. [DOI] [PubMed] [Google Scholar]
- [60].Gaglia G, Rashid R, Yapp C, Joshi GN, Li CG, Lindquist SL, et al. HSF1 phase transition mediates stress adaptation and cell fate decisions. Nat Cell Biol. 2020;22:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Stavreva DA, Garcia DA, Fettweis G, Gudla PR, Zaki GF, Soni V, et al. Transcriptional Bursting and Co-bursting Regulation by Steroid Hormone Release Pattern and Transcription Factor Mobility. Mol Cell. 2019;75:1161–77 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Henninger JE, Oksuz O, Shrinivas K, Sagi I, LeRoy G, Zheng MM, et al. RNA-Mediated Feedback Control of Transcriptional Condensates. Cell. 2021;184:207–25 e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schneider N, Wieland FG, Kong D, Fischer AAM, Horner M, Timmer J, et al. Liquid-liquid phase separation of light-inducible transcription factors increases transcription activation in mammalian cells and mice. Sci Adv. 2021;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Shrinivas K, Sabari BR, Coffey EL, Klein IA, Boija A, Zamudio AV, et al. Enhancer Features that Drive Formation of Transcriptional Condensates. Mol Cell. 2019;75:549–61 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wei MT, Chang YC, Shimobayashi SF, Shin Y, Strom AR, Brangwynne CP. Nucleated transcriptional condensates amplify gene expression. Nat Cell Biol. 2020;22:1187–96. [DOI] [PubMed] [Google Scholar]
- [66].Nguyen VQ, Ranjan A, Liu S, Tang X, Ling YH, Wisniewski J, et al. Spatiotemporal coordination of transcription preinitiation complex assembly in live cells. Mol Cell. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kwon I, Kato M, Xiang S, Wu L, Theodoropoulos P, Mirzaei H, et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell. 2013;155:1049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Quintero-Cadena P, Lenstra TL, Sternberg PW. RNA Pol II Length and Disorder Enable Cooperative Scaling of Transcriptional Bursting. Mol Cell. 2020;79:207–20 e8. [DOI] [PubMed] [Google Scholar]
- [69].Erdel F, Rippe K. Formation of Chromatin Subcompartments by Phase Separation. Biophys J. 2018;114:2262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Erdel F, Rademacher A, Vlijm R, Tunnermann J, Frank L, Weinmann R, et al. Mouse Heterochromatin Adopts Digital Compaction States without Showing Hallmarks of HP1-Driven Liquid-Liquid Phase Separation. Mol Cell. 2020;78:236–49 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chong S, Dugast-Darzacq C, Liu Z, Dong P, Dailey GM, Cattoglio C, et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science. 2018;361:eaar2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McSwiggen DT, Hansen AS, Teves SS, Marie-Nelly H, Hao Y, Heckert AB, et al. Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. eLife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bo S, Hubatsch L, Bauermann J, Weber CA, Julicher F. Stochastic dynamics of single molecules across phase boundaries. bioRXiv. 2021. [Google Scholar]
- [74].Guillen-Boixet J, Kopach A, Holehouse AS, Wittmann S, Jahnel M, Schlussler R, et al. RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell. 2020;181:346–61 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Allen BL, Taatjes DJ. The Mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol. 2015;16:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cook PR, Marenduzzo D. Transcription-driven genome organization: a model for chromosome structure and the regulation of gene expression tested through simulations. Nucleic Acids Res. 2018;46:9895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wan Y, Anastasakis DG, Rodriguez J, Palangat M, Gudla P, Zaki G, et al. Dynamic imaging of nascent RNA reveals general principles of transcription dynamics and stochastic splice site selection. Cell. 2021;184:2878–95 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rodriguez J, Larson DR. Transcription in Living Cells: Molecular Mechanisms of Bursting. Annu Rev Biochem. 2020;89:189–212. [DOI] [PubMed] [Google Scholar]
- [79].Peeples W, Rosen MK. Mechanistic dissection of increased enzymatic rate in a phase-separated compartment. Nature chemical biology. 2021;17:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zuo L, Zhang G, Massett M, Cheng J, Guo Z, Wang L, et al. Loci-specific phase separation of FET fusion oncoproteins promotes gene transcription. Nature communications. 2021;12:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Li J, Liu Y, Rhee HS, Ghosh SK, Bai L, Pugh BF, et al. Kinetic competition between elongation rate and binding of NELF controls promoter-proximal pausing. Mol Cell. 2013;50:711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pavelitz T, Bailey AD, Elco CP, Weiner AM. Human U2 snRNA genes exhibit a persistently open transcriptional state and promoter disassembly at metaphase. Mol Cell Biol. 2008;28:3573–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sabari BR, Dall’Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 2018;361:eear3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Basu S, Mackowiak SD, Niskanen H, Knezevic D, Asimi V, Grosswendt S, et al. Unblending of Transcriptional Condensates in Human Repeat Expansion Disease. Cell. 2020;181:1062–79 e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cho WK, Spille JH, Hecht M, Lee C, Li C, Grube V, et al. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science. 2018;361:412–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ilik IA, Malszycki M, Lubke AK, Schade C, Meierhofer D, Aktas T. SON and SRRM2 are essential for nuclear speckle formation. eLife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kim J, Han KY, Khanna N, Ha T, Belmont AS. Nuclear speckle fusion via long-range directional motion regulates speckle morphology after transcriptional inhibition. Journal of cell science. 2019;132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Core L, Adelman K. Promoter-proximal pausing of RNA polymerase II: a nexus of gene regulation. Genes Dev. 2019;33:960–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Cramer P. Organization and regulation of gene transcription. Nature. 2019;573:45–54. [DOI] [PubMed] [Google Scholar]
- [90].Ebmeier CC, Erickson B, Allen BL, Allen MA, Kim H, Fong N, et al. Human TFIIH Kinase CDK7 Regulates Transcription-Associated Chromatin Modifications. Cell reports. 2017;20:1173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Van Treeck B, Protter DSW, Matheny T, Khong A, Link CD, Parker R. RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc Natl Acad Sci U S A. 2018;115:2734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yamamoto T, Yamazaki T, Hirose T. Phase separation driven by production of architectural RNA transcripts. Soft Matter. 2020;16:4692–8. [DOI] [PubMed] [Google Scholar]
- [93].Banerjee PR, Milin AN, Moosa MM, Onuchic PL, Deniz AA. Reentrant Phase Transition Drives Dynamic Substructure Formation in Ribonucleoprotein Droplets. Angew Chem Int Ed Engl. 2017;56:11354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Maharana S, Wang J, Papadopoulos DK, Richter D, Pozniakovsky A, Poser I, et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science. 2018;360:918–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Portz B, Shorter J. Biochemical Timekeeping Via Reentrant Phase Transitions. Journal of molecular biology. 2021;433:166794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bracha D, Walls MT, Brangwynne CP. Probing and engineering liquid-phase organelles. Nat Biotechnol. 2019;37:1435–45. [DOI] [PubMed] [Google Scholar]
- [97].Scharfen L, Neugebauer KM. Transcription regulation through nascent RNA folding. Journal of molecular biology. 2021:166975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hondele M, Sachdev R, Heinrich S, Wang J, Vallotton P, Fontoura BMA, et al. DEAD-box ATPases are global regulators of phase-separated organelles. Nature. 2019;573:144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Tauber D, Tauber G, Khong A, Van Treeck B, Pelletier J, Parker R. Modulation of RNA Condensation by the DEAD-Box Protein eIF4A. Cell. 2020;180:411–26 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Langdon EM, Qiu Y, Ghanbari Niaki A, McLaughlin GA, Weidmann CA, Gerbich TM, et al. mRNA structure determines specificity of a polyQ-driven phase separation. Science. 2018;360:922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, et al. RNA Controls PolyQ Protein Phase Transitions. Mol Cell. 2015;60:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kapranov P, Willingham AT, Gingeras TR. Genome-wide transcription and the implications for genomic organization. Nature reviews Genetics. 2007;8:413–23. [DOI] [PubMed] [Google Scholar]
- [103].Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Azofeifa JG, Allen MA, Hendrix JR, Read T, Rubin JD, Dowell RD. Enhancer RNA profiling predicts transcription factor activity. Genome research. 2018;28:334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Franco HL, Nagari A, Malladi VS, Li W, Xi Y, Richardson D, et al. Enhancer transcription reveals subtype-specific gene expression programs controlling breast cancer pathogenesis. Genome research. 2018;28:159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nair SJ, Yang L, Meluzzi D, Oh S, Yang F, Friedman MJ, et al. Phase separation of ligand-activated enhancers licenses cooperative chromosomal enhancer assembly. Nat Struct Mol Biol. 2019;26:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Alberti S, Gladfelter A, Mittag T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell. 2019;176:419–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Holmes ZE, Hamilton DJ, Hwang T, Parsonnet NV, Rinn JL, Wuttke DS, et al. The Sox2 transcription factor binds RNA. Nature communications. 2020;11:1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sigova AA, Abraham BJ, Ji X, Molinie B, Hannett NM, Guo YE, et al. Transcription factor trapping by RNA in gene regulatory elements. Science. 2015;350:978–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Berlow RB, Dyson HJ, Wright PE. Hypersensitive termination of the hypoxic response by a disordered protein switch. Nature. 2017;543:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chang H, Lim J, Ha M, Kim VN. TAIL-seq: genome-wide determination of poly(A) tail length and 3′ end modifications. Mol Cell. 2014;53:1044–52. [DOI] [PubMed] [Google Scholar]
- [112].Alshareedah I, Kaur T, Ngo J, Seppala H, Kounatse LD, Wang W, et al. Interplay between Short-Range Attraction and Long-Range Repulsion Controls Reentrant Liquid Condensation of Ribonucleoprotein-RNA Complexes. J Am Chem Soc. 2019;141:14593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Saldi T, Riemondy K, Erickson B, Bentley DL. Alternative RNA structures formed during transcription depend on elongation rate and modify RNA processing. Mol Cell. 2021;81:1789–801 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Alberti S, Dormann D. Liquid-Liquid Phase Separation in Disease. Annual review of genetics. 2019;53:171–94. [DOI] [PubMed] [Google Scholar]
- [115].Bradner JE, Hnisz D, Young RA. Transcriptional Addiction in Cancer. Cell. 2017;168:629–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Goodman LD, Bonini NM. New Roles for Canonical Transcription Factors in Repeat Expansion Diseases. Trends in genetics : TIG. 2020;36:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Pelassa I, Cora D, Cesano F, Monje FJ, Montarolo PG, Fiumara F. Association of polyalanine and polyglutamine coiled coils mediates expansion disease-related protein aggregation and dysfunction. Hum Mol Genet. 2014;23:3402–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Muragaki Y, Mundlos S, Upton J, Olsen BR. Altered growth and branching patterns in synpolydactyly caused by mutations in HOXD13. Science. 1996;272:548–51. [DOI] [PubMed] [Google Scholar]
- [119].Nakamura K, Jeong SY, Uchihara T, Anno M, Nagashima K, Nagashima T, et al. SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein. Hum Mol Genet. 2001;10:1441–8. [DOI] [PubMed] [Google Scholar]
- [120].Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–5. [DOI] [PubMed] [Google Scholar]
- [121].Cai D, Feliciano D, Dong P, Flores E, Gruebele M, Porat-Shliom N, et al. Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat Cell Biol. 2019;21:1578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Jalihal AP, Pitchiaya S, Xiao L, Bawa P, Jiang X, Bedi K, et al. Multivalent Proteins Rapidly and Reversibly Phase-Separate upon Osmotic Cell Volume Change. Mol Cell. 2020;79:978–90 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Rawat P, Boehning M, Hummel B, Aprile-Garcia F, Pandit AS, Eisenhardt N, et al. Stress-induced nuclear condensation of NELF drives transcriptional downregulation. Mol Cell. 2021;81:1013–26 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hardenberg M, Horvath A, Ambrus V, Fuxreiter M, Vendruscolo M. Widespread occurrence of the droplet state of proteins in the human proteome. Proc Natl Acad Sci U S A. 2020;117:33254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Fuxreiter M, Vendruscolo M. Generic nature of the condensed states of proteins. Nat Cell Biol. 2021;23:587–94. [DOI] [PubMed] [Google Scholar]
- [126].Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 2014;15:384–96. [DOI] [PubMed] [Google Scholar]


