Abstract
BACKGROUND:
Use of pyrethroid insecticides is a pivotal strategy for mosquito control globally. Commonly known for its insecticidal activity by acting on voltage-gated sodium channels, volatile pyrethroids, such as bioallethrin and transfluthrin, are used in mosquito coils, emanators, and other vaporizers to repel mosquitoes and other biting arthropods. However, whether specific olfactory receptor neurons are activated by pyrethroids to trigger spatial repellency remains unknown.
RESULTS:
We took behavioral and electrophysiological approaches to elucidate the mechanism of bioallethrin repellency in Aedes aegypti, a major vector of dengue, yellow fever, Zika and chikungunya viruses. We found that bioallethrin elicits spatial (i.e., non-contact) repellency and activates a specific type of olfactory receptor neurons in mosquito antennae. Furthermore, bioallethrin repellency is significantly reduced in a mosquito mutant of Orco, an obligate olfactory co-receptor that is essential for the function of odorant receptors (Ors). These results indicate that activation of specific Or(s) by bioallethrin contributes to bioallethrin repellency. In addition, bioallethrin repellency was reduced in a pyrethroid-resistant strain which carries two mutations in the sodium channel gene that are responsible for knockdown resistance (kdr) to pyrethroids, indicating a role of activation of sodium channels in bioallethrin repellency.
CONCLUSION:
Results from this study show that bioallethrin repellency is likely the result of co-activation of Or(s) and sodium channels. These findings not only contribute to the understanding of the modes of action of volatile pyrethroids in spatial repellency, but also provide a framework for developing new repellents based on the dual-target mechanism revealed.
Keywords: Pyrethroids, spatial repellency, olfaction, sodium channels
1. INTRODUCTION
Many vector-borne human diseases, including malaria, dengue, Zika and West Nile encephalitis, are transmitted by mosquitoes. Current mosquito control strategies rely heavily on insecticide interventions due to the absence of vaccines or therapy for the majority of these vector-borne diseases. Many vector control products are pyrethroid-based, due to their high efficacy against mosquitoes and relatively low mammalian toxicity.1–3 It has been well established that pyrethroids target voltage-gated sodium channels to exert their insect-killing action. Sodium channels are large transmembrane proteins that selectively conduct sodium ions through cell membrane, which is critical for initiation and propagation of action potentials in excitable cells.4 Pyrethroids alters sodium channel gating by prolonging the opening of sodium channels, resulting in repetitive firing and/or membrane depolarization in the nervous system, leading to rapid insect “knockdown”.1,5,6 Large-scale and repeated use of pyrethroids has led to the development of resistance to pyrethroids in mosquito populations around the world.3,7–11 One major type of pyrethroid resistance is known as knockdown resistance (kdr) which is caused by mutations in the sodium channels.1,5,6,12–14 Besides their insect-killing activity, pyrethroids also induce avoidance behavioral responses in mosquitoes. Both excito-repellency (also called contact repellency or irritancy) and contact-independent spatial repellency in mosquitoes have been reported (e.g., Bibbs and Kaufman15, Chareonviriyaphap et al.16, Kongmee et al.17, Sukkanon et al.18, Wagman et al.19, Yang et al.20). However, our understanding of the underlying mechanisms of pyrethroid repellency remains limited.
Allethrin, the first commercial pyrethroid, is structurally similar to the natural insecticides pyrethrins I and II from pyrethrum extract from dried flowers of certain Chrysanthemum species. It was first introduced in 1952 as a mixture of eight stereoisomers. It is more effective than pyrethrins in mosquito coils and thermal fumigation due to its increased volatility and thermostability.21,22 Subsequently, several partially resolved mixtures of isomers of allethrin became commercially available, including bioallethrin (also known as d-trans allethrin) which contains two of the eight stereoisomers of allethrin (i.e., the esters from 1R-trans-chrysanthemic acid and the racemic alcohol) (Fig. 1(A)). Allethrin, particularly, bioallethrin, remains a common active ingredient in mosquito coils, emanators, vaporizer mats and other repellent devices. However, in the past decades, almost all of the studies on the effect on mosquitoes caused by allethrin or allethrin-containing mosquito coils focused on their activities on knockdown and/or lethality.23–28 Whether allethrin and/or bioallethrin evokes spatial repellency in mosquitoes and the mechanism underlying spatial repellency remains largely unknown.
Figure 1. Bioallethrin elicits spatial repellency in Ae. aegypti mosquitoes.
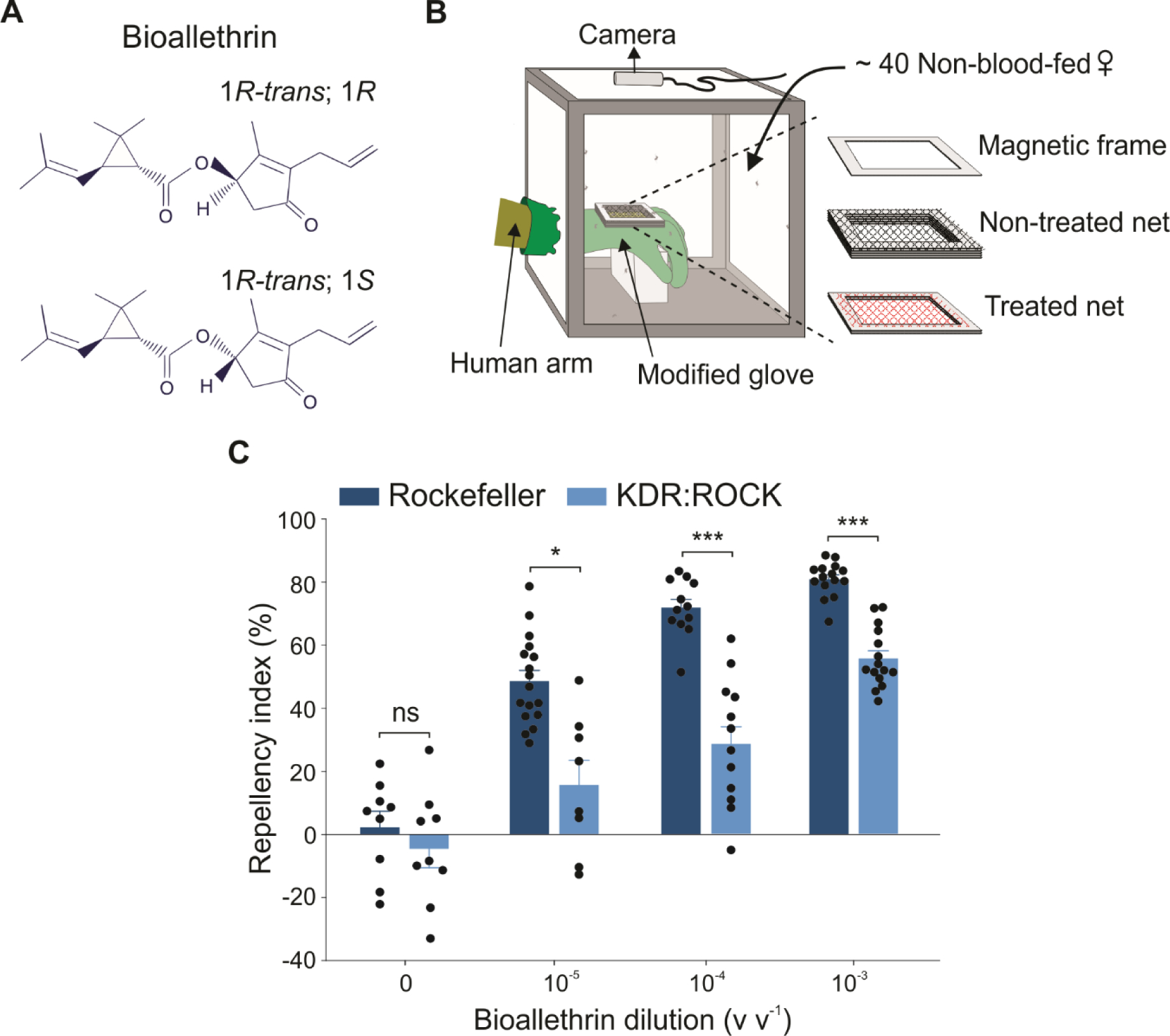
(A) Chemical structure of bioallethrin. Bioallethrin (or d-trans allethrin) is a mixture of two allethrin isomers (i.e., 1R-trans; 1R and 1R-trans; 1S). (B) A schematic drawing illustrating the hand-in-cage setup used to evaluate spatial repellency. (C) Concentration-dependent bioallethrin repellency in Rockefeller (wild-type) and KDR:ROCK (pyrethroid-resistant) mosquitoes. Two-tailed Student’s t-test, control (0): t = 0.212, df = 8, P = 0.837; 10−5 dilution: t = 3.024, df = 8, P = 0.016, 10−4 dilution: t = 6.171, df = 11, P < 0.0001; 10−3 dilution: t = 8.552, df = 14, P < 0.0001; ns = not significant; *P < 0.05, ***P < 0.001; n = 10 cages for Rockefeller and 9 for KDR:ROCK in control (0), n = 17 cages for Rockefeller and 9 cages for KDR:ROCK in 10−5 dilution, n = 12 cages for Rockefeller and 13 cages for KDR:ROCK in 10−4 dilution, and n = 15 cages for both Rockefeller and KDR:ROCK in 10−3 dilution. The 10−5, 10−4, and 10−3 dilutions correspond to 0.114, 1.14, and 11.4 μg cm−2, respectively. The control represents the baseline activity in response to the solvent. Data are presented as mean ± SEM. The dots over bars represent individual replicate values. Experiments were conducted independently by two additional researchers.
Perception of many volatiles in insects is achieved by activation of odorant receptors (Ors), a major group of chemoreceptor proteins expressed in olfactory receptor neurons (ORNs) which are housed in olfactory sensilla in the major olfactory appendages, such as antennae and maxillary palps.29 An earlier study Bohbot et al.30 reported that an experimental pyrethroid (TL-I-73) inhibited the response of specific Ors activated by volatiles indole and R-(−)-1-octen-3-ol in Xenopus oocytes. More recent studies show that pyrethrum and/or pyrethrins elicit olfactory responses in Anopheles gambiae, Ae. aegypti and Ae. albopictus.20,31,32 Further analysis with single sensillum recording (SSR) revealed that pyrethrins activate a specific type of ORNs in Ae. aegypti antennae.31 In addition, D-allethrin, containing four of the eight stereoisomers of allethrin, and vapothrin, another volatile pyrethroid (also known as empenthrin), have been reported to elicit electroantennogram (EAG) responses from the German cockroach (Blattella germanica).33
These recent findings prompted us to address the following questions in this study (i) Can bioallethrin elicit non-contact repellency (i.e., spatial repellency) in Ae. aegypti mosquitoes? (ii) Does bioallethrin activate antennal olfactory receptor neurons? (iii) Is bioallethrin repellency Or-mediated; and (iv) Is activation of sodium channels involved in bioallethrin repellency? For that, we carried out electrophysiological and behavioral analyses in Ae. aegypti mosquitoes. Our results indicate that bioallethrin repellency is the result of a unique dual action of bioallethrin on both Or(s) and sodium channels. Our study established a new framework for understanding the modes of action of volatile pyrethroids in spatial repellency against mosquitoes.
2. MATERIALS AND METHODS
2.1. Mosquitoes and chemicals
Four Ae. aegypti mosquito strains were used in our study: Rockefeller, KDR:ROCK, Orlando, and orco−/−. Rockefeller is an insecticide-susceptible wild-type strain; and KDR:ROCK is a pyrethroid-resistant strain carrying two kdr mutations (S996P and V1016G) and is near-isogenic to Rockefeller.34 Both Rockefeller and KDR:ROCK were kindly provided by Jeffrey G. Scott (Cornel University). Orlando (kindly provided by Leslie Vosshall, Rockefeller University) is another insecticide-susceptible wild-type strain from which an Orco mutant, orco−/− (orco16 from BEI Resources, NIAID, NIH), was generated. In orco−/−, the orco gene was mutated resulting in impaired Or-mediated olfactory responses.35
Mosquitoes were maintained in the Insect Toxicology and Neurobiology Laboratory in the Department of Entomology, Michigan State University (East Lansing, MI, USA) at 27 °C, with 70 – 80% humidity and 12 hours of photoperiod. Larvae were fed with beef liver powder (NOW Foods®, Bloomingdale, Illinois), and adults were fed with 10% aqueous sucrose. Adult females were blood-fed five days after emergence using defibrinated sheep blood (Colorado Serum Company). All the odorants used in this study and their sources are provided in Table S1.
2.2. Purification of bioallethrin from Sigm-Aldrich
Thin-layer chromatography (TLC), developed with 1:9 ethyl acetate in hexane and visualized with anisaldehyde stain was used to determine the presence of trace impurtities in the commercial samples of bioallethrin. Impurities were identified using gas chromatography-mass spectrometry (GC-MS). Bioallethrin commercial samples were anlyzed by an Aglient Intuvo 9000 GC equiped with an 5977B MSD, an Aglient 7693A series Autoinjector, a split/splitlles capillary injector port and an J&W DB-5 Intuvo (30 m × 0.25 mm i.d × 0.25 μm) column. The oven temperature program was: 70°C (2 min) and 10°C min−1 to 250°C (10 min). Helium was used as the carrier gas at a flow rate of 1.2 mL min−1. Injection volume was 1 μL and sample injection was carried out splitless at 250°C. The mass spectrometer was used in electron ionization mode (EI) and in scan mode with ionization energy of 70 eV. The transfer line temperature was kept at 200°C. The primary impurity in the commercial bioallethrin samples were determined to be d-trans-chrysanthemic acid, an organic compound commonly used for synthesizing bioallethrin.
Bioallethrin from Sigma-Aldrich was purified using column chromatography on a Buchi C-810 flash chromatography system. A 25-gram silica FlashPure column (Buchi cat. #11067705) was used to purify 750 mg bioallethrin using a gradient of 1:99 to 10:90 hexane:methyl tert-butyl ether as the eluent. Elution was detected by monitoring absorption in the 230–254 nm range. TLC was used to determine which fractions contained bioallethrin; these fractions were then concentrated under vacuum. Residual solvent from the concentrated bioallethrin was removed under high vacuum (100 mTorr) for 6 hours to yield purified bioallethrin as a colorless oil (635 mg, 85% recovery, > 99.9% purity).
2.3. Behavioral (hand-in-cage) assay
To assess spatial (i.e., non-contact) repellency by bioallethrin, we used a hand-in-cage assay similar to that described in Liu et al.31. The setup includes a 30 cm × 30 cm × 30 cm mosquito cage (BioQuip, Rancho Dominguez, CA), a digital camera (e-con Systems Inc, San Jose, CA, model: e-CAM51A) mounted on the top of the cage and connected to a laptop computer; and a human hand inside a modified nitrile glove (Ansell Protective Products, Coshoton, OH, part number: 37–155), as illustrated in Fig. 1(B). The nitrile rubber glove was cut on its backside to create a window (6 cm × 5 cm). A piece of magnetic frame (slightly larger than the dimension of the window) was glued onto the cut window, serving as a base for stacking more magnetic frames (Fig. 1(B)). One piece of test compound-treated polyester netting (Shason Textile Inc., part number: WS-B532–111, white; 6.5 cm × 5.5 cm) was placed on this fixed magnetic frame, which was ~3.0 mm above the glove. The second piece of the netting was untreated and placed ~8.0 mm above the treated net using a stack of four magnetic frames. The stacked magnetic frames were further secured with a binder clip. The stacking creates sufficient space between the treated net and the untreated net so that mosquitoes that land on the open window were not able to contact the treated net or contact and pierce the skin of the hand in the glove. The hand makes no contact with the treated net (Fig. 1(B)).
The assay was conducted under controlled condition of humidity (30 to 50%) and temperature (27 – 30°C). Twenty-four hours before an assay, four to nine days-old females (about 30–50, mated, non-blood fed) were transferred into cages. The cages were kept in an incubator where mosquitoes were provided only with water in a cotton ball. Immediately before the assay, the bottom net was treated with 500 μl of of either solvent (acetone) or test compound (bioallethrin) in a glass Petri dish, in an adjacent room. After acetone was fully evaporated (approximately 7 min), the net was assembled into a modified glove (Fig. 1(B)). A researcher (i.e., tester) put on the modified glove and introduced the hand in the modified glove into the mosquito cage to initiate the assay. The number of mosquito landings on the test window (top net) was recorded by the digital camera for five minutes. The number of mosquito landings during the second to fifth min was counted. For each cage, solvent control was tested first, and then followed with a treatment. Cages that gave a low landing number (i.e., < 50% of usual landing from other cages) in a solvent trial were not used for further testing. The treatment was bioallethrin diluted at 10−5, 10−4, and 10−3 (i.e., 0.114, 1.14, and 11.4 μg cm−2). The time interval of assays between control and treatment was at least 1.5 hours, allowing the mosquitoes to fully recover from the first trial. Controls were also done from two trials of solvent 1.5 hours apart to make sure that mosquitoes continue landing in the second trial at the same rate. Percentage repellency was determined for each cage using the following equation: Percentage repellency = [1− (cumulative number of landings on the window of treatment/cumulative number of landings on the window of solvent treatment)] × 100). When testing two mosquito strains for direct comparison, cages from both mosquito lines were randomly tested. Each experiment was repeated at least by two different testers (i.e., hands) and performed between 12:00 pm and 4:00 pm.
2.4. Electrophysiology recordings
2.4.1. Electroantennogram (EAG)
The methodology for EAG is similar to those previously described in Liu et al.31 with minor modifications. For that, the head of non-blood-fed female mosquito (Rockefeller, KDR:ROCK, Orlando and orco−/− strains) with four to nine days-old was excised and the tip of its right antennae was removed before the head be connected to a reference electrode. The reference electrode an its handle, with the head connected on its tip, was placed under a microscope (Nikon Eclipse FN1, Japan, 100×). Under the microscope view, and with the aid of two micromanipulators, the cut tip of the antenna was connected to a second (recording) electrode. The recording electrode was connected to a high-impedance AC/DC 10× preamplifier (Syntech, Germany) (Fig. 2(A)). Chloridized 0.25 mm silver wires in glass electrodes filled half way with 0.1% KCl (Sigma-Aldrich®, Inc., USA, CAS #7447-40-7, molecular weight 75.55 g/mol, lot #5LCC9757,) and 0.5% polyvinylpyrrolidone (PVP) (Sigma-Aldrich®, Inc., USA, CAS #9003-39-8, molecular weight 40,000 g/mol, lot #WXBD0775V) were used for both reference and recording electrodes. Preparation was bathed in a high humidity air stream flowing at 1.2 l min−1 to which a stimulus pulse of 0.5 l min−1 was delivered for 0.5 s. Any change in antennal deflection induced by the stimuli or control puffs was recorded for 10 s starting 2 s prior the stimulation. For delivery, 1 μl of undiluted purified bioallethrin or other odorants were applied in the inner walls of a glass Pasteur pipette, as stimulus cartridge. Purified and humidified air was delivered to the antennae through a glass tube (10-mm inner diameter) perforated by a small hole 10 cm away from the end of the tube, into which the tip of the Pasteur pipette could be inserted. An empty pipette cartridge (i.e., blank) served as control. The distance between the end of the glass tube and the antennae was about 1 cm. The maximum amplitude response (−mV) was obtained by averaging the results for each antenna/compound combination from EAGPro (Syntech, Germany).
Figure 2. Bioallethrin induces antennal response in Ae. aegypti mosquitoes.
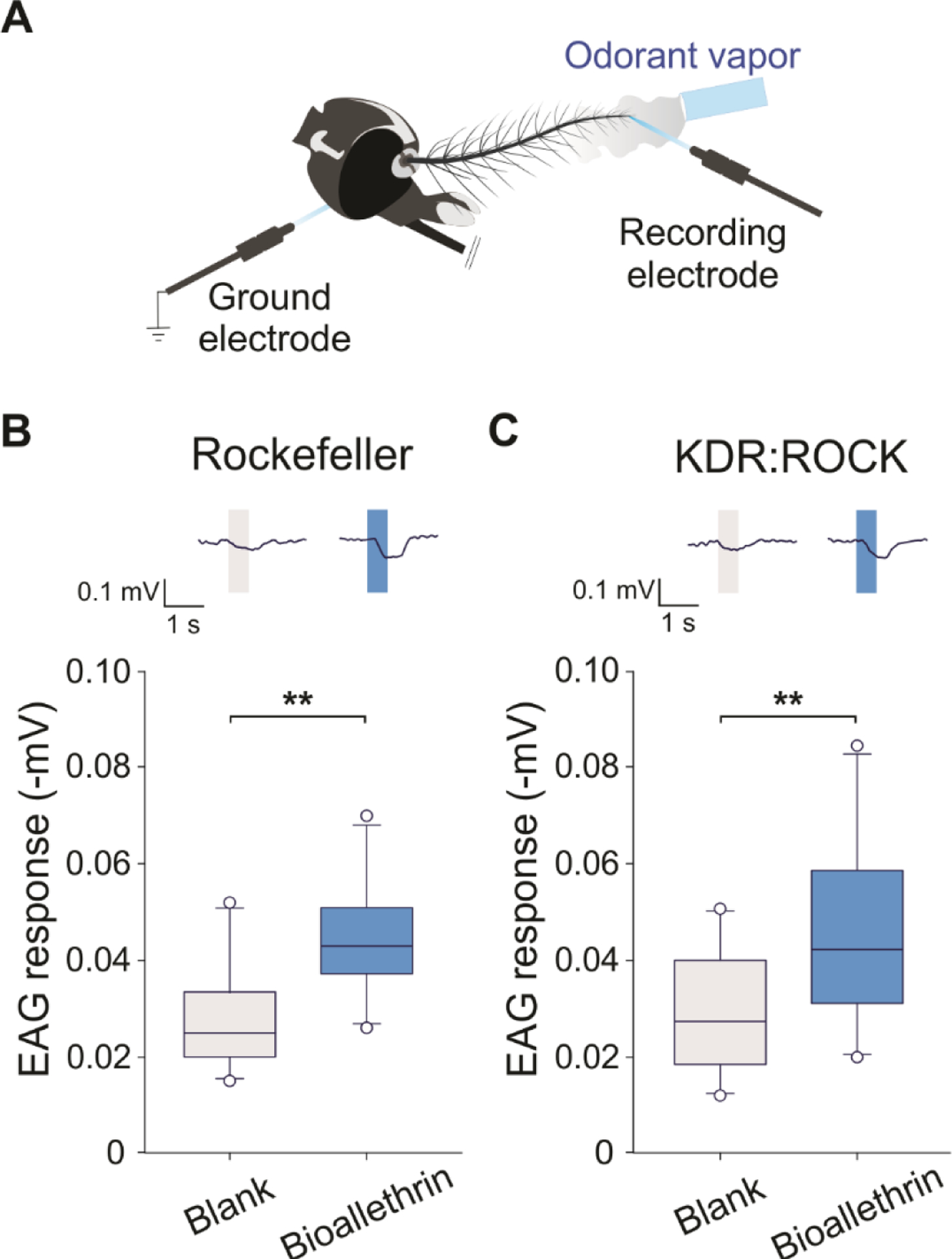
(A) A schematic drawing illustrating electroantennogram (EAG) recordings. (B) and (C) EAG responses of Rockefeller (wild-type) and KDR:ROCK (pyrethroid resistant), respectively, to purified bioallethrin (volume of 1.0 μL of undiluted compound into the odorant cartridge). The blank represents the control (0 μL). Representative traces are shown above the plot. Paired t-test on Wilcoxon Signed Rank Test (Z = 2.803, P = 0.002) for Rockefeller and KDR:ROCK strains. **P < 0.01. n = 10 insect antennae for each strain. Boxes represent the 25th, 50th and 75th, whiskers the 10th and 90th, and open circles the 5th and 95th percentiles of the data.
2.4.2. Single sensillum recording (SSR)
SSR was performed using the methodology described in Liu et al.31 with slight modifications. A four- to nine-day old non-blood-fed female mosquito was trapped with its head protruding from the cut end of a 200 μl disposable pipette tip. This preparation was mounted on a microscope slide (76 × 26 mm) with aid of deltal wax. One mosquito antenna was fixed to a coverslip using a double-sided tape. After mounted, the slide was placed under a microscope (Nikon Eclipse FN1, Japan), and the antenna was viewed at high magnification (1000×). Two tungsten microelectrodes were sharpened in 20% KNO2 (Fisher Scientific®, Inc., USA, CAS #7758-09-0, molecular weight 85.1 g/mol, lot #192084) at 2–10 V. The reference electrode (ground electrode) was inserted into the compound eye and the other was connected to a preamplifier (10×, Syntech, Kirchzarten, Germany) and inserted into the shaft of an olfactory sensillum (mainly in 8th to 13th flagellomeres). Controlled manipulation of the recording electrode was performed using a micromanipulator (Burleigh PCS-6000, CA). The preamplifier was connected to an analog-to-digital signal converter (IDAC-4, Syntech, Germany), which in turn was connected to a computer for signal recording and visualization. The activity of co-located ORNs in each sensillum was assessed based on the differences in spike amplitude. The ORN with the larger spike amplitude was designated as neuron A and the one with the smaller spike amplitude as neuron B.36 Signals were recorded for 10 s starting 3 s before stimulation, and the action potentials were counted off-line over a 500-ms period before and after stimulation. The spontaneous firing rates observed in the preceding 500 ms were subtracted from the total spike rates observed during the 500-ms stimulation, and counts were recorded in units of spikes s−1.
The sensilla were functionally classified based on response to DEET and/or other 14 volatiles (Table S1) as detailed in Liu et al.31 with slight modifications. Briefly, 0.1 μl (which is equavelent to 10 μl of 10−2 solution in Liu et al.31) of each undiluted volatile was applied in the inner walls of a glass Pasteur pipette, as stimulus cartridge. Once the sst-1 sensilla were identified, their responses to 0.1 and 1.0 μL of purified bioallethrin were also examined. An empty pipette cartridge (i.e., blank) served as control. The airflow across the antennae was maintained constant at 20 ml s−1 throughout the experiment. Purified and humidified air was delivered to the preparation through a glass tube (10-mm inner diameter) perforated by a small hole 10 cm away from the end of the tube, into which the tip of the Pasteur pipette could be inserted. The stimulus was delivered to sensilla by inserting the tip of the stimulus cartridge into this hole and diverting a portion of the air stream (0.5 l min−1) to flow through the stimulus cartridge for 500 ms using a stimulus controller (Syntech, Germany). The distance between the end of the glass tube and the antennae was ≤ 1 cm.
2.5. Statistical analysis
All statistical analysis and figure plotting was done using SigmaPlot 12.5 (Systat Software). Unpaired Student’s t-tests were used to compare two sets of data. Paired t-test with Wilcoxon Signed Rank Test was used for paired comparison of a treatment against a control on a same individual. For paired comparison of multiple treatments on a same individual against control, a Friedman RM ANOVA on Ranks or Two Way RM ANOVA was used with Dunnett’s multiple comparison against a control treatment.
3. RESULTS
3.1. Bioallethrin elicits spatial (i.e., non-contact) repellency in a hand-in-cage assay
Here we used a hand-in-cage assay (Liu et al.31, as illustrated in Fig 1(B)) which was designed to evaluate spatial repellency. In this assay, the mosquitoes released into the cage are attracted to the human hand (Fig. 1(B)). Mosquitoes from a wild-type strain, Rockefeller, frequently landed on the untreated net (i.e., top net) when the bottom net close to the hand was not treated with any compounds. However, when the bottom net was treated with bioallethrin from Sigma Aldrich, the number of Rockefeller mosquitoes that landed on the untreated net was significantly reduced in a concentration-dependent manner (Fig. 1(C)). We found about 81% of repellency at the highest bioallethrin dilution (10−3 v v−1 which corresponds to 11.4 μg of bioallethrin cm−2) we used and did not observe any abnormal flight and locomotive activities of mosquitoes during the assay.
We also evaluated bioallethrin repellency in a pyrethroid-resistant mosquito strain, KDR:ROCK.34 This resistant strain was derived from a field collected pyrethroid resistant strain (Singapore37) carrying two kdr mutations (S996P and V1016G) in the sodium channel. The Singapore strain was crossed to the Rockefeller strain for four generations to generate the KDR:ROCK strain.34 The double mutation of S996P and V1016G conferred mosquitoes various levels of resistance to 17 tested pyrethroids including bioallethrin.34 It has also been confirmed in the Xenopus oocyte expression assay that the double mutation reduced the sensitivity of Ae. aegypti mosquito sodium channels to pyrethroids by 100-fold.38 Here we showed that bioallethrin repellency was reduced in KDR:ROCK mosquitoes compared to that in Rockefeller mosquitoes (Fig. 1(C)).
3.2. Bioallethrin activates specific olfactory receptor neurons (ORNs) in mosquito antenna
Detection of spatial repellency by bioallethrin prompted us to conduct further electrophysiological analysis to determine whether bioallethrin evokes any olfactory responses. We first conducted electroantennogram (EAG) recordings from mosquito antennae (Fig. 2(A)), which measures compounded olfactory responses from antennal ORNs. Given that the purity of bioallethrin from Sigma-Aldrich was low, 95.4%, impurities in this commercial sample could potentially elicit EAG responses. To avoid such a problem, the bioallethrin from Sigma-Aldrich was analyzed by gas chromatography–mass spectrometry and thin-layer chromatography, and subsequently purified. Indeed, we found that besides bioallethrin (95.4%), d-trans-chrysanthemic acid (4.6%), an organic compound used as a starting material for synthesizing bioallethrin, was detected in this commercial product. We removed the impurity and used purified bioallethrin for subsequent experiments. As shown in Fig. 2(B–C), bioallethrin elicited EAG signals in both Rockefeller and KDR:ROCK mosquitoes. Furthermore, the amplitude of the EAG was not significantly different between Rockefeller and KDR:ROCK (t = −0.19, df = 18, P = 0.85), indicating that the two kdr mutations do not impair the olfactory sensory response to bioallethrin.
We then conducted single sensillum recording (SSR) (Fig. 3(A)) to determine which type of ORNs is activated by bioallethrin in Rockefeller and KDR:ROCK mosquitoes. Trichoid (olfactory) sensilla on the antennae of Ae. aegypti mosquitoes were classified initially based on the morphological characteristics: short sharp-tipped (sst), long sharp-tipped (lst) and short blunt-tipped (sbt).36 Each sensillum generally houses two neurons, neuron A which generates larger spikes and neuron B which generates smaller spikes. Functional characterization of these olfactory sensilla in response to a panel of volatiles (most of them are repellents) in our recent study31 identified three types of sst and six types of sbt sensilla based on their odorant response profiles.31 In that same study, we found that sst-1 is the only type of sst sensilla that is activated by pyrethrum (pyrethrin I and II)36 and also N,N-diethyl-meta-toluamide (DEET) (Liu et al., published data). Here we confirmed that pyrethrum and DEETactivate sst-1 sensilla (Fig. S2). Furthermore, sst-1 sensilla were also activated by bioallethrin in both Rockefeller and KDR:ROCK mosquitoes (Fig. 3). More specifically, neuron A in sst-1 sensilla was activated by bioallethrin, whereas neuron B in sst-1 sensilla was not activated (Fig. 3(B)). Furthermore, the activation by bioallethrin was concentration-dependent and not significantly different between Rockefeller and KDR:ROCK mosquitoes (Two-way RM ANOVA: treatment F(2,22) = 124.14, P < 0.001; mosquito strain F(1,22) = 0.352, P = 0.565; treatment vs mosquito F(2,22) = 0.30, P = 0.744; Fig. 3(C)).
Figure 3. Neuron A in sst-1 sensilla is activated by bioallethrin.
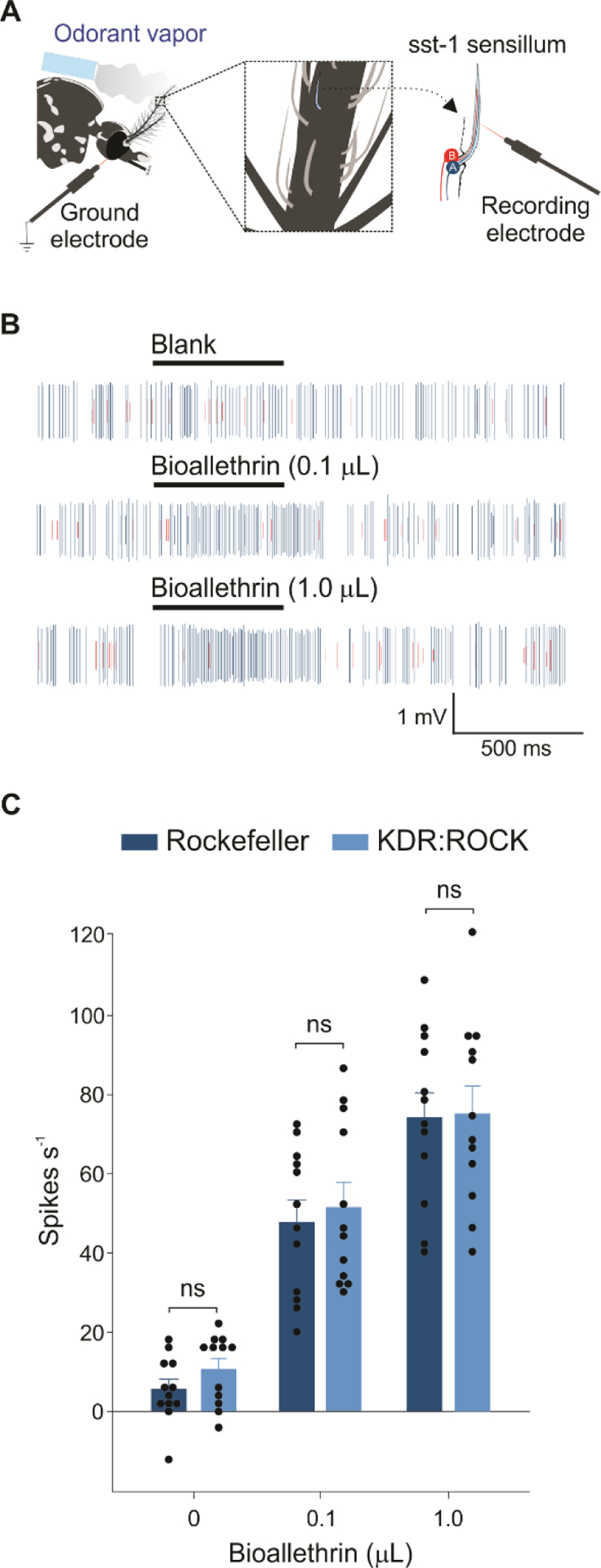
(A) A schematic drawing illustrating single sensillum recordings (SSR) from Ae. aegypti mosquito antennae. (B) Representative SSR traces indicating increased firing of neuron A of sst-1 sensilla in response to control (blank) and purified bioallethrin (0.1 and 1.0 μL) in Rockefeller mosquitoes. (C) SSR responses of sst-1A neurons to blank control (0 μL) and purified bioallethrin (0.1 and 1.0 μL) from Rockefeller and KDR:ROCK strains. A Two Way RM ANOVA with Dunnett’s multiple comparison was performed to compare the effect of treatment, mosquito strains, and their interactions (Two-way RM ANOVA: treatment F(2,22) = 124.14, P < 0.001; mosquito strain F(1,22) = 0.352, P = 0.565; treatment vs mosquito F(2,22) = 0.30, P = 0.744). n = 12 sensilla for each strain. Data are presented as mean ± SEM. The dots over bars represent individual replicate values.
3.3. Bioallethrin-induced EAG and repellency are impaired in orco−/− mosquitoes
An obligate olfactory receptor coreceptor (Orco) is essential for Or-mediated odorant detection by forming odor-gated cation channels with ligand-selective Ors.39,40 Or-mediated odorant responses are impaired in Orco mutant (orco−/−) mosquitoes.34 The orco−/− mosquitoes cannot detect human odors that activate Ors, but can still find live hosts possibly by detecting CO2 via gustatory receptor (Gr)-mediated olfactory pathways and other odor cues, such as lactic acid, via ionotropic receptor (Ir)-mediated olfactory pathways35,41,42 and heat.43 To confirm that repellency by bioallethrin is Or-mediated, we examined the response of orco−/− mosquitoes to bioallethrin in both EAG and hand-in-cage experiments. The EAG response to bioallethrin was not detected in orco−/− mutant mosquitoes but detected from the wild-type Orlando mosquitoes from which the orco−/− mutant was generated35 (Fig. 4(A–B)). We also examined the EAG response to L-(+)-lactic acid which activates Irs, not Ors, on mosquito antennae,42 in orco−/− and Orlando mosquitoes. Unlike bioallethrin, L-(+)-lactic acid elicited EAG responses from both orco−/− and Orlando mosquitoes and the amplitude of the EAG signals was not significantly different between the two strains (t = 1.39, df = 12, P = 0.19) (Fig. S1(C–D)). Furthermore, we found that bioallethrin repellency was significantly reduced in orco−/− mosquitoes compared to Orlando mosquitoes (Fig. 4(C)). Similarly, repellency by geranyl acetate, an Or-mediated repellent,31 was also reduced (Fig. S3).
Figure 4. Bioallethrin-induced EAG and repellency are impaired in orco−/− mosquitoes.
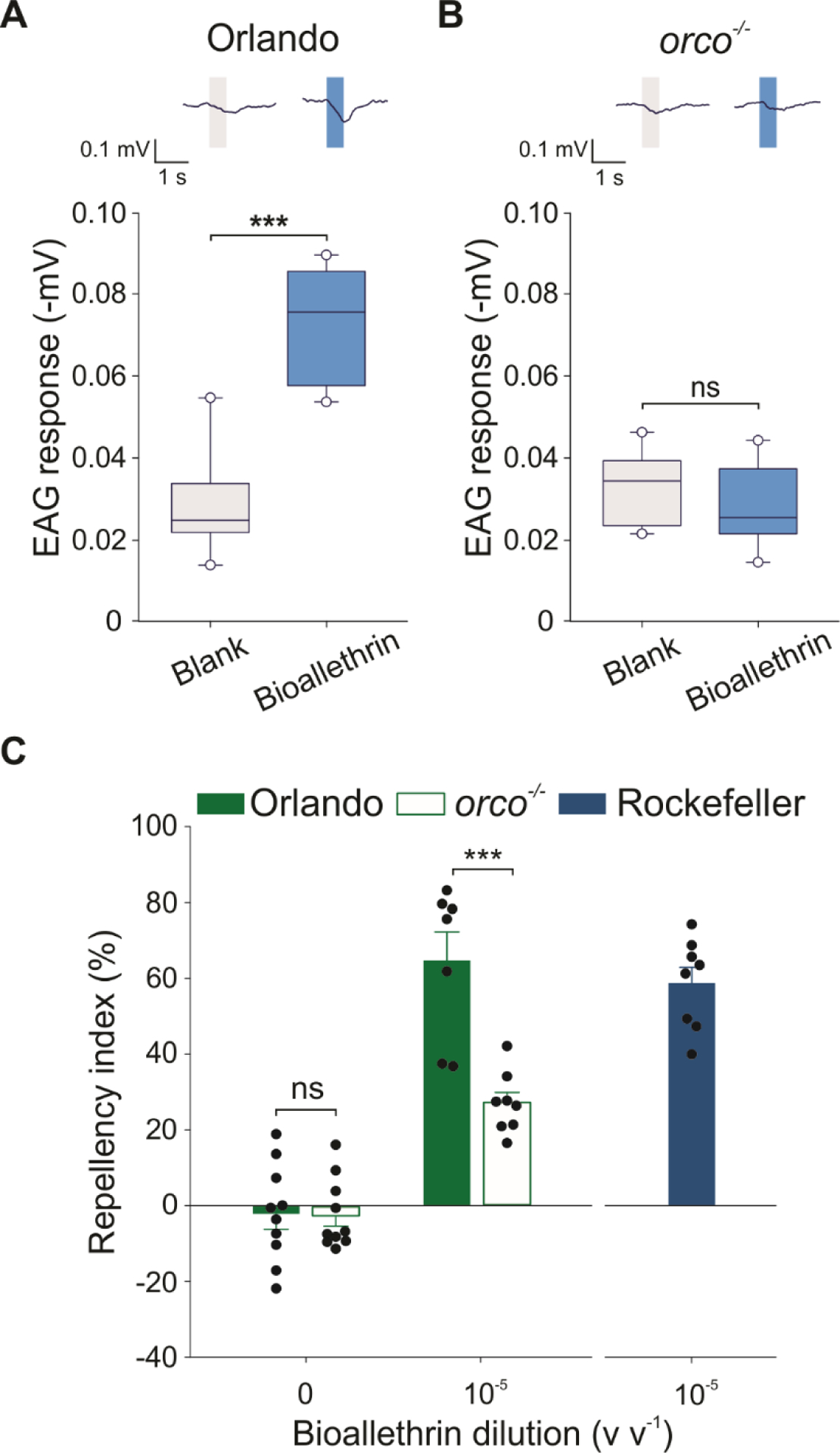
(A) and (B) EAG responses of Orlando (wild-type) and orco−/− mutant strains, respectively, to purified bioallethrin (volume of 1.0 μL of undiluted compound into the odorant cartridge). The blank represents the control (0 μL). Representative traces are shown above the plot. Two-tailed Paired Student’s t-test, Orlando: t = −6.837, df = 6, P < 0.001, orco−/−: t = 1.233, df = 6, P = 0.264; ns = not significant, **P < 0.01. n = 7 insect antennae for each strain. Boxes represent the 25th, 50th and 75th, whiskers the 10th and 90th, and open circles the 5th and 95th percentiles of the data (C) Repellency by purified bioallethrin in Orlando (wild-type), orco−/− mutant, and Rockefeller (wild-type) mosquitoes. Two-tailed Student’s t-test, control (0): t = 0.046, df = 18, P = 0.963, 10−5 dilution: t = 4.92, df = 13, P = 0.0002; ns = not significant, ***P < 0.001; n = 10 cages for both mosquito strains in control (0), n = 7 cages for Orlando, 8 cages for orco−/−, and 8 cages for Rockefeller in 10−5 dilution. The 10−5 dilution corresponds to 114 ng cm−2. The control represents the baseline activity in response to the solvent. Data are presented as mean ± SEM. The dots over bars represent individual replicate values.
4. DISCUSSION
Our behavioral and electrophysiological analyses in Ae. aegypti mosquitoes show that bioallethrin elicits spatial repellency and activates specific antennal ORNs. Furthermore, bioallethrin repellency is reduced in both orco−/− mutants and pyrethroid-resistant mosquitoes. Together these findings provide experimental evidence for the involvement of both Or(s) and sodium channels in the repellency mediated by bioallethrin, an active ingredient in repellent products used globally against Ae. aegypti.
Detection of EAG by bioallethrin is reminiscent of EAG elicited by D-allethrin and vapothrin (empenthrin) in the German cockroach.33 While it is not yet known if D-allethrin or vapothrin-mediated repellency is dependent on activation of Or(s), we found in this study that bioallethrin repellency is reduced in orco−/− mutant mosquitoes. Orco/Or complexes function as ligand-gated cation channels, activation of which by specific odorants drives odor perception in insects.40 Activation of specific ORNs by bioallethrin and reduced bioallethrin repellency in the orco−/− mutant indicate bioallethrin activate specific Or(s), not Orco; and bioallethrin repellency in Ae. aegypti is Or-mediated. An important next step is to identify the specific Or(s) that are activated by bioallethrin. Our discovery that sst-1A neuronsare responsive to bioallethrin suggests that the bioallethrin-responsive Or(s) is localized in sst-1A neurons. Interestingly, sst-1A neurons are also responsive to pyrethrins, which are components of the natural repellent pyrethrum.31 Although it remains to be determined if the ability of the mosquito olfactory system to sense natural pyrethrins is a result of ecological interactions, we speculate that mosquito repellency exhibited by some synthetic pyrethroids, such as bioallethrin in this study, likely reflects their structural mimicry of natural pyrethrins.
Our previous studies showed that repellency by pyrethrum and transfluthrin was reduced in pyrethroid-resistant KDR:ROCK mosquitoes, which is near isogenic to the parental pyrethroid-susceptible strain ROCK.31,44 Here we found that bioallethrin repellency is also reduced in KDR:ROCK mosquitoes. More generally, reduced repellency for volatile pyrethroids have been reported in other pyrethroid-resistant strains carrying different kdr mutations.19,20,31,44 For example, Wagman et al.19 showed that reduced transfluthrin spatial repellency was associated with increased frequency of the V106G kdr mutation. Yang et al.20 reported that repellency of transfluthrin and metofluthrin was reduced in another pyrethroid-resistant strain, Puerto Rico, which carries three kdr mutations, V410L, V1016G and F1534C45, as well as has enhanced P450-mediated pyrethroid detoxification mechanism of resistance46. Taken together, these results suggest that activation of sodium channels is involved in pyrethrum and pyrethroid repellency and the kdr mutations likely reduced the activation of sodium channels that is required for eliciting repellency.
We found that repellency of neither DEET nor geranyl acetate (a plant-derived mosquito repellent) that did not act on sodium channels was not affected in KDR:ROCK mosquitoes,31,44 indicating that the S996P and V1016G kdr mutations may specifically affect repellency by pyrethrum, transfluthrin and bioallethrin. It remains to be examined if all kdr mutations have such specificity. Yang et al.20 found that repellency of not only pyrethoids, but also DEET, 2-undecanone and IR3535, was reduced in the Puerto Rico strain, and suggested that the reduced repellency to pyrethroids and non-pyrethroid repellents in Puerto Rico mosquitoes was the result of a general fitness cost associated with the kdr mutations. In contrast, another recent study47 reported that a pyrethroid-resistant An. gambiae strain carrying the L1014F kdr mutation exhibited enhanced repellency by DEET, geraniol, carvacrol, culminaldehyde and cinnamaldehyde, none of which activates sodium channels, when compared to a pyrethroid-susceptible strain. Future research shall determine whether these different results are caused by the use of different pyrethroid-resistant Ae. aegypti strains with different genetic backgrounds, different mosquito species and/or different behavioral assays. More importantly, it will be exciting to elucidate how activation of sodium channels by pyrethrin/pyrethroids is integrated with the Or-mediated repellency at the molecular and mechanistic levels.
5. CONCLUSION
Behaviroal and electrophysiological analyses in this study show the involvement of both Or(s) and sodium channels in bioallethrin spatial repellency. Our study laid a foundation for further elucidation into the mode of action of volatile pyrethroids that exhibit spatial repellency, and also generated an exciting new paradigm for the development of new mosquito repellents that are based on co-activation of Or-mediated repellent pathways and voltage-gated sodium channels.
Supplementary Material
ACKNOWLEDGMENTS
The study was funded by a grant from the National Institutes of Health (GM115475) to K.D. Support is also acknowledged from Iowa Agricultural Experiment Station, Ames, Iowa; We thank Jeffery G. Scott for providing the KDR:ROCK strain for this study. We thank Tays Paiva da Rosa, Amanda Carlos Túler, and Hannah Green for assistance with behavioral experiments. W.V. was supported by the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) Foundation (Financial code 001); E.E.O. was supported by CAPES-PrInt (8887.311952/2018-00).
Footnotes
CONFLICT OF INTEREST DECLARATION
The autors declare no conflict of interest.
REFERENCES
- 1.Narahashi T, Neuroreceptors and ion channels as the basis for drug action: past, present, and future, J Pharmacol Exp Ther 294:1–26 (2000). [PubMed] [Google Scholar]
- 2.WHO, Vector control operations framework for Zika virus, World Health Organization; (2016). [Google Scholar]
- 3.WHO, Test procedures for insecticide resistance monitoring in malaria vector mosquitoes, World Health Organization; (2016). [Google Scholar]
- 4.Catterall WA, Voltage-gated sodium channels at 60: structure, function and pathophysiology, J Physiol 590:2577–2589 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, et al. , Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment, Toxicology 171:3–59 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, et al. , Molecular biology of insect sodium channels and pyrethroid resistance, Insect Biochem Mol Biol 50:1–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enayati A and Hemingway J, Malaria management: past, present, and future, Annu Rev Entomol 55:569–591 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Du Y, Wu S, Nomura Y, Zhu G, Zhorov BS, et al. , Molecular evidence of sequential evolution of DDT-and pyrethroid-resistant sodium channel in Aedes aegypti, PLoS Negl Trop Dis 13:e0007432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yunta C, Grisales N, Nász S, Hemmings K, Pignatelli P, Voice M, et al. , Pyriproxyfen is metabolized by P450s associated with pyrethroid resistance in An. gambiae, Insect Biochem Mol Biol 78:50–57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddi K, Tomé HV V, Du Y, Valbon WR, Nomura Y, Martins GF, et al. , Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: a potential challenge for mosquito control, Sci Rep 7:46549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu N, Insecticide resistance in mosquitoes: impact, mechanisms, and research directions, Annu Rev Entomol 60:537–559 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Enayati AA, Vatandoost H, Ladonni H, Townson H, and Hemingway J, Molecular evidence for a kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi, Med Vet Entomol 17:138–144 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Rinkevich FD, Du Y, and Dong K, Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids, Pestic Biochem Physiol 106:93–100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, Du Y, Nomura Y, Zhorov BS, and Dong K, Chronology of sodium channel mutations associated with pyrethroid resistance in Aedes aegypti, Arch Insect Biochem Physiol 104:e21686 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bibbs CS and Kaufman PE, Volatile pyrethroids as a potential mosquito abatement tool: a review of pyrethroid-containing spatial repellents, J Integr Pest Manag 8 (2017). [Google Scholar]
- 16.Chareonviriyaphap T, Bangs MJ, Suwonkerd W, Kongmee M, Corbel V, and Ngoen-Klan R, Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand, Parasit Vectors 6:280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kongmee M, Boonyuan W, Achee NL, Prabaripai A, Lerdthusnee K, and Chareonviriyaphap T, Irritant and repellent responses of Anopheles harrisoni and Anopheles minimus upon exposure to bifenthrin or deltamethrin using an excito-repellency system and a live host, J Am Mosq Control Assoc 28:20–29 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Sukkanon C, Nararak J, Bangs MJ, Hii J, and Chareonviriyaphap T, Behavioral responses to transfluthrin by Aedes aegypti, Anopheles minimus, Anopheles harrisoni, and Anopheles dirus (Diptera: Culicidae), PLoS One 15:e0237353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagman JM, Achee NL, and Grieco JP, Insensitivity to the spatial repellent action of transfluthrin in Aedes aegypti: a heritable trait associated with decreased insecticide susceptibility, PLoS Negl Trop Dis 9:e0003726 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Norris EJ, Jiang S, Bernier UR, Linthicum KJ, and Bloomquist JR, Reduced effectiveness of repellents in a pyrethroid-resistant strain of Aedes aegypti (Diptera: culicidae) and its correlation with olfactory sensitivity, Pest Manag Sci 76:118–124 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Katsuda Y, Pyrethroids research and development centennial in Japan, J Pestic Sci 7:317–327 (1982). [Google Scholar]
- 22.Yamamoto I and Casida JE, Syntheses of 14C-labeled pyrethrin I, allethrin, phthalthrin, and dimethrin on a submillimole scale, Agric Biol Chem 32:1382–1391 (1968). [Google Scholar]
- 23.Bibbs CS, Fulcher A, and Xue R-D, Allethrin-based mosquito control device causing knockdown, morbidity, and mortality in four species of field-caught mosquitoes (Diptera: Culicidae), J Med Entomol 52:739–742 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Chen CD, Chin AC, Lau KW, Low VL, Lee HL, Lee PKY, et al. , Bioefficacy Evaluation of Commercial Mosquito Coils Containing Metofluthrin, d-Allethrin, d-Trans Allethrin, and Prallethrin Against Aedes albopictus (Diptera: Culicidae) in Malaysia, J Med Entomol 55:1651–1655 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Chin AC, Chen CD, Low VL, Lee HL, Azidah AA, Lau KW, et al. , Comparative efficacy of commercial mosquito coils against Aedes aegypti (Diptera: Culicidae) in Malaysia: a nationwide report, J Econ Entomol 110:2247–2251 (2017). [DOI] [PubMed] [Google Scholar]
- 26.El-garj FMA, Avicor SW, Wajidi MFF, and Jaal Z, Comparative efficacy of spatial repellents containing d-allethrin and d-trans allethrin against the major dengue vector Aedes aegypti (Linnaeus), Asian Biomed 9:313–320 (2017). [Google Scholar]
- 27.Katsuda Y, Leemingsawat S, Thongrungkiat S, Prummonkol S, Samung Y, Kanzaki T, et al. , Control of mosquito vectors of tropical infectious diseases:(3) susceptibility of Aedes aegypti to pyrethroid and mosquito coils, Southeast Asian J Trop Med Public Health 40:929 (2009). [PubMed] [Google Scholar]
- 28.Msangi S, Mwang’onde BJ, Mahande AM, and Kweka EJ, Field evaluation of the bio-efficacy of three pyrethroid based coils against wild populations of anthropophilic mosquitoes in northern Tanzania, J Glob Infect Dis 2:116, s (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson BS and Stensmyr MC, Evolution of insect olfaction, Neuron 72:698–711 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Bohbot JD, Fu L, Le TC, Chauhan KR, Cantrell CL, and Dickens JC, Multiple activities of insect repellents on odorant receptors in mosquitoes, Med Vet Entomol 25:436–444 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Liu F, Wang Q, Xu P, Andreazza F, Valbon WR, Bandason E, et al. , A dual-target molecular mechanism of pyrethrum repellency against mosquitoes, Nat Commun 12:2553 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan R, Zhou Q, Xu Z, Wu Y, Zhu G, Wang M, et al. , Pyrethrins elicit olfactory response and spatial repellency in Aedes albopictus, Pest Manag Sci 77:3706–3712 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Boné E, González-Audino PA, and Sfara V, Spatial Repellency Caused by Volatile Pyrethroids is Olfactory-Mediated in the German Cockroach Blattella germanica (Dictyoptera: Blattellidae), Neotrop Entomol 49:275–283 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Smith LB, Kasai S, and Scott JG, Voltage-sensitive sodium channel mutations S989P+ V1016G in Aedes aegypti confer variable resistance to pyrethroids, DDT and oxadiazines, Pest Manag Sci 74:737–745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, et al. , orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET, Nature 498:487–491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghaninia M, Ignell R, and Hansson BS, Functional classification and central nervous projections of olfactory receptor neurons housed in antennal trichoid sensilla of female yellow fever mosquitoes, Aedes aegypti, Eur J Neurosci 26:1611–1623 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasai S, Komagata O, Itokawa K, Shono T, Ng LC, Kobayashi M, et al. , Mechanisms of pyrethroid resistance in the dengue mosquito vector, Aedes aegypti: target site insensitivity, penetration, and metabolism, PLoS Negl Trop Dis 8:e2948 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirata K, Komagata O, Itokawa K, Yamamoto A, Tomita T, and Kasai S, A single crossing-over event in voltage-sensitive Na+ channel genes may cause critical failure of dengue mosquito control by insecticides, PLoS Negl Trop Dis 8:e3085 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, and Vosshall LB, Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction, Neuron 43:703–714 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, and Touhara K, Insect olfactory receptors are heteromeric ligand-gated ion channels, Nature 452:1002–1006 (2008). [DOI] [PubMed] [Google Scholar]
- 41.McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, and Vosshall LB, Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans, Cell 156:1060–1071 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raji JI, Melo N, Castillo JS, Gonzalez S, Saldana V, Stensmyr MC, et al. , Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway, Curr Biol 29:1253–1262 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greppi C, Laursen WJ, Budelli G, Chang EC, Daniels AM, Van Giesen L, et al. , Mosquito heat seeking is driven by an ancestral cooling receptor, Science (80-) 367:681–684 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreazza F, Valbon WR, Wang Q, Liu F, Xu P, Bandason E, et al. , Sodium channel activation underlies transfluthrin repellency in Aedes aegypti, PLoS Negl Trop Dis 15:e0009546 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinch M, Rodriguez SD, Mitra S, Kandel Y, Moore E, and Hansen IA, Low Levels of Pyrethroid Resistance in Hybrid Offspring of a Highly Resistant and a More Susceptible Mosquito Strain, J Insect Sci 20:1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reid WR, Thornton A, Pridgeon JW, Becnel JJ, Tang F, Estep A, et al. , Transcriptional analysis of four family 4 P450s in a Puerto Rico strain of Aedes aegypti (Diptera: Culicidae) compared with an Orlando strain and their possible functional roles in permethrin resistance, J Med Entomol 51:605–615 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Deletre E, Martin T, Duménil C, and Chandre F, Insecticide resistance modifies mosquito response to DEET and natural repellents, Parasit Vectors 12:1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


