Abstract
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor involved in the regulation of biological responses to more planar aromatic hydrocarbons, like TCDD. We previously described the sequence of events following exposure of male rats to a dioxin-like polychlorinated biphenyl (PCB) congener, 3,3’,4,4’,5-pentachlorobiphenyl (PCB126), that binds avidly to the AhR and causes various types of toxicity including metabolic syndrome, fatty liver, and disruption of energy homeostasis. The purpose of this study was, to investigate the role of AhR to mediate those toxic manifestations following sub-acute exposure to PCB126 and to examine possible sex differences in effects. For this goal, we created an AhR knockout (AhR-KO) model using CRISPR/Cas9. Comparison was made to the wild type (WT) male and female Holtzman Sprague Dawley rats. Rats were injected with a single IP dose of corn oil vehicle or 5 µmol/kg PCB126 in corn oil and necropsied after 28 days. PCB126 caused significant weight loss, reduced relative thymus weights, and increased relative liver weights in WT male and female rats, but not in AhR-KO rats. Similarly, significant pathologic changes were visible which included necrosis and regeneration in female rats, micro- and macro-vesicular hepatocellular vacuolation in males, and a paucity of glycogen in livers of both sexes in WT rats only. Hypoglycemia and lower IGF1, and reduced serum non-esterified fatty acids (NEFAs) was found in serum of both sexes of WT rats, low serum cholesterol levels only in the females, and no changes in AhR-KO rats. The expression of genes encoding enzymes related to xenobiotic metabolism (e.g. CYP1A1), gluconeogenesis, glycogenolysis, and fatty acid oxidation were unaffected in the AhR-KO rats following PCB126 exposure as opposed to WT rats where expression was significantly upregulated (PPARα, females only) or downregulated suggesting a disrupted energy homeostasis. Interestingly, Acox2, Hmgcs, G6Pase and Pc were affected in both sexes, the gluconeogenesis and glucose transporter genes Pck1, Glut2, Sds, and Crem only in male WT-PCB rats. These results show the essential role of the AhR in glycogenolysis, gluconeogenesis, and fatty acid oxidation, i.e. in the regulation of energy production and homeostasis, but also demonstrate a significant difference in the effects of PCB126 in males verses females, suggesting higher vulnerability of glucose homeostasis in males and more changes in fatty acid/lipid homeostasis in females. These differences in effects, which may apply to more/all AhR agonists, should be further analyzed to identify health risks to specific groups of highly exposed human populations.
Keywords: PCB126, liver toxicity, gene expression, glucose homeostasis, fatty acid oxidation, energy homeostasis, sex differences, AhR knockout rats
1. Introduction
Polychlorinated biphenyls (PCBs) are toxic industrial chemicals of global environmental health concern due to their detrimental effects on human health, including liver diseases, obesity, diabetes, cardiovascular diseases, hypertension, and other metabolic disorders (Cave et al. 2010; Crinnion 2011; Donat-Vargas et al. 2014; Park et al. 2016; Silverstone et al. 2012; Taylor et al. 2013). PCBs also affect the female reproductive system to cause reproductive and developmental toxicity in humans and animals (Bae et al. 1999; Fein et al. 1984; Loch-Caruso 2002). The production and sale of these Group 1 carcinogens was banned in the late 1970’s, but PCBs persist in some closed systems uses, and in the environment due to their high chemical and thermodynamic stability, and they bioaccumulate in fatty tissues due to their lipophilicity and resistance to biodegradation (Cancer 2014; Grimm et al. 2015; Lauby-Secretan et al. 2013; Matthews and Dedrick 1984). Ongoing routes of exposure to PCBs are through ingestion, inhalation, and dermal/occupational exposure (Ampleman et al. 2015; Winneke et al. 1998).
Among the 209 different PCB congeners, 3, 3´, 4, 4´, 5-pentachlorobiphenyl (PCB126) is the most potent dioxin-like toxicant. PCB126 was only a minor component of commercial PCB mixtures (Frame et al. 1996), but much of the toxicity of PCB exposure is due to this congener (Hansen 1999; Iwata et al. 2004). Human exposure to PCB126 mainly comes from food, specifically fish, but also from game, meats, and plants (Chambers et al. 2012; EFSA Panel on Contaminants in the Food Chain et al. 2018; Hong and Bush 1990; Kumar et al. 2016; Mendola et al. 1997; Nakatani and Yamano 2017; San Martin et al. 2016; Wang et al. 2018; Warenik-Bany et al. 2016; Weber et al. 2018). PCB126 avidly binds to and activates the aryl hydrocarbon receptor (AhR). AhR is a transcription factor which is expressed in thymus, spleen, liver, heart, kidney, ovary and some cells in the immune system (Harrill et al. 2013; Sorg 2014). AhR is in an inactive state outside of the nucleus where it is bound to a multiprotein complex of binding partners consisting of HSP90, AIP, and p23 (Beischlag et al. 2008; Hankinson 1995; Sorg 2014). Binding of ligands like 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) or dioxin-like compounds like PCB126, causes the complex to transfer to the nucleus where it dimerizes with AhR nuclear translocator (ARNT) and connects to dioxin/xenobiotic response elements (DREs/XREs) in the upstream promoters of AhR-regulated genes (Baba et al. 2001; Denison et al. 2011) leading to the expression of various genes, like aryl hydrocarbon receptor repressor (Ahrr) and xenobiotic metabolizing enzymes (CYP1A1, CYP1A2, CYP1B1) (DeVito et al. 1994; Korkalainen et al. 2004). After dissociating from the DREs, AhR is transported to the cytoplasm and degraded via the ubiquitin-proteasome pathway (Ma and Baldwin 2002; Pollenz 2002; Roberts and Whitelaw 1999). There are a wide variety of man-made and naturally occurring ligands which can activate AhR signaling (Denison et al. 2011; Nguyen and Bradfield 2008). The toxicity of TCDD is mediated through the AhR (Fernandez-Salguero et al. 1996; Gonzalez and Fernandez-Salguero 1998; Harrill et al. 2013) and similarly the toxicity of PCB126 may mostly or exclusively be mediated through the AhR by this canonical pathway (Sorg 2014).
The liver is not only a major site for detoxification of xenobiotics but also for glucose and lipid metabolism to maintain energy homeostasis during feeding/fasting conditions and in production and storage of glucose in different physiological states (Sherwin 1980). During excess glucose levels in the body, the liver synthesizes glycogen (glycogenesis) as a storage form of glucose and increases lipogenesis as response to insulin. When the body needs glucose during fasting or starvation, glycogen is broken down to produce glucose (glycogenolysis) in early fasting. The liver can also produce glucose from lactate, pyruvate, and some amino acids (gluconeogenesis) during prolonged starvation. To maintain energy homeostasis, the body also meets energy requirements through fatty acid oxidation in the liver (Lippi et al. 1994; Pilkis and Granner 1992; Zhang et al. 2014). Activation of multiple transcription factors and their coactivators within the liver maintain the transcription of enzymes critical to balance energy homeostasis during feeding and fasting conditions (Rui 2014). Downregulation of genes regulating glucose homeostasis and fatty acid oxidation by toxicants like PCB126 can lead to impairments in hepatic energy metabolism resulting in hypoglycemia (low serum glucose levels). In addition, PCB126 causes fatty liver and various types of liver diseases (Gadupudi et al. 2018; Gadupudi et al. 2016b; Gadupudi et al. 2016c; Shen et al. 2019; Wahlang et al. 2017), which may further endanger energy homeostasis. Although effects of PCB126 on liver toxicity and impairment of energy metabolism are known, there is a significant knowledge gap in our understanding of the mechanism of action of PCB126 induced liver toxicity.
As PCB126 is a dioxin-like PCB, we hypothesized that the liver toxicity and disturbance of energy homeostasis by PCB126 is mostly but possibly not completely mediated through the AhR. We also hypothesized that sex differences exist. To test our hypothesis, we used an AhR knock out (AhR-KO) rat model and compared wild type (WT) and AhR-KO and male and female rats with and without exposure for 28 days after a single ip dose of PCB126 (5 µmol/kg body weight) (Gadupudi et al. 2018). We report here that all measured changes in gene expression involved in energy metabolism/homeostasis were dependent on a functioning AhR, but that several differences between effects in male and female exist.
2. Materials and Methods
2.1. Chemicals
3, 3´, 4, 4´, 5-pentachlorobiphenyl (PCB126) was produced by the Suzuki coupling method. The purity of PCB126 was >99% as determined by gas chromatography-mass spectrometry (Joshi et al. 2011). Authentication of PCB126 was achieved through stringent guidelines, which were established by the Iowa Superfund Research Program (Li et al. 2018). The synthesis, characterization, and purity of PCB126 were previously described (Gadupudi et al. 2018). To prepare the dose of PCB126 and vehicle injections, stripped corn oil (Acros, New Jersey, USA; CAS: 8001–30-7 lot#: A0234007) was used.
2.2. Animal studies
All protocols and procedures were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center. The AhR knock out (AhR-KO) rat model was generated using CRISPR/Cas9-mediated disruption of the AhR basic helix loop helix DNA binding domain. These AhR mutant rats are available at the Rat Resource & Research Center (RRRC# 831; strain name SD-Ahrem1Soar; University of Missouri, Columbia, MO; www.rrrc.us). AhR-KO and wild type (WT) male and female Holtzman Sprague Dawley rats used in this study were ~ 4-weeks of age and had body weights ranging from 70–130 g at the start of the experiment. All rats were fed a standard rodent diet (Teklad No. 8604, Envigo, Indianapolis, IN) and had access to feed and water at libitum. Each rat was singly housed in a controlled environment (14:10 h light-dark cycle). For this experiment litters were produced by breeding an AhR heterozygote male x AhR heterozygous female. Littermates were then distributed to different groups and assigned to control or treatment groups at random.
Both wild type (WT, n=28) and AhR-KO (n=25) male and female rats were injected with a single IP dose of corn oil/vehicle (5 ml/kg body weight) or PCB126 in corn oil (5 µmol/kg body weight; 1.63 mg/kg). A single IP administration at this dosage was used previously in several studies (Gadupudi et al. 2018; Gadupudi et al. 2016a; Gadupudi et al. 2016c) showing increase in liver triglycerides and induction of CYP enzymes in the liver of Sprague-Dawley rats. The dose was sufficient to produce an effect, but minimal enough to avoid a high mortality rate. Considering human exposure, it is known that PCB126 contributes the most toxicity equivalence (TEQ) among the dioxin-like PCBs (DL-PCBs), PCDDs and PCDFs (PCDD/DFs), accounting for over 50% of the TEQ from human food intake (EFSA Panel on Contaminants in the Food Chain et al. 2018). Therefore, although PCB126 is a minor component of PCBs measured in environmental matrices, it is a major contributor to PCB toxicity. Although most of the human exposure to PCB126 happens through the diet, IP injection was chosen for this study. PCB126 is a toxic compound. Handling it for mixing with diet increases the risk of exposure for researcher/personnel, animal cages and environment. It is also more problematic to achieve the exact dose of PCB126 for each animal through feed or by gavage. IP injection is a very safe and exact exposure method that also allowed us to compare results with previous experiments and was therefore chosen for this study. Overall, this study had a total of 8 groups, 4 groups for each sex, one each for male and female rats for WT-vehicle (Male, n=7; Female, n=7), WT-PCB126 (Male, n=3; Female, n=5), AhR-KO-vehicle (Male, n=7; Female, n=5), and AhR-KO-PCB126 (Male, n=7; Female, n=6) (Supplementary Fig-1). At the beginning of the study, the total number of rats was 53, but 6 rats died (male, n=4; female, n=2) in the WT-PCB126 groups during the study. The body weights of all rats were monitored regularly. After the 28-day PCB126 exposure, all rats were sacrificed using carbon-dioxide asphyxiation followed by thoracotomy. Liver and other organs were collected, weighed, and processed for further analysis as described below. Additional details of the experimental design may be found in our previous paper (Klenov et al. 2021; Williams et al. 2020).
2.3. Blood Collection and Analysis
After euthanasia, whole blood was collected through cardiac puncture into non-anticoagulant coated tubes. Blood was allowed to clot at room temperature. The clot was removed by centrifugation at 1500 x g for 10 min. Serum was aliquoted and frozen at −80°C for further analysis. Serum glucose levels were measured by Comparative Clinical Pathology Services LLC (CPath, Columbia, MO). Serum non-esterified fatty acids (NEFA) and cholesterol were measured by IDEXX BioAnalytics, Westbrook, Maine. All animals were sampled for outcome assessment.
2.4. Histological examination of liver
Male and female rat livers were collected postmortem and either fixed in 10% neutral buffered formalin for approximately 3 days or frozen in OCT. Fixed tissues were routinely processed, embedded and sectioned at 4 µm thickness followed by hematoxylin and eosin (H&E) staining, and periodic acid-Schiff (PAS) staining to identify glycogen. Frozen sections were cut at 5 µm thickness and stained with Oil-Red-O to identify lipid in liver sections. All animals were sampled for outcome assessment. Slides were examined by a board-certified veterinary pathologist.
2.5. Analysis of gene expression
Total RNAs were isolated from rat hepatic tissue (10–20mg) using the RNeasy Plus Mini kit from Qiagen Inc. Absorbance of isolated RNA was determined at 260 and 280 nm. Purity ratios (A260/A280 and A260/230) of RNA samples were between 1.8 and 2.0. Complementary DNAs (cDNAs) were synthesized from total RNAs with a high-capacity cDNA reverse transcription kit from Applied Biosystems Inc. Real Time quantitative PCR measurements were performed using a SYBR Green Master Mix kit supplied by Applied Biosystems Inc. Primers sequences are provided in Supplementary Table-1 and were synthesized by Integrated DNA Technologies Inc. (Coralville, Iowa). The program for the amplification reaction started at 95°C for 10 min followed by 40 cycles of 2 step PCR cycle at 95°C for 15 s and 60°C for 1 min using an Eppendorf RealPlex2 Mastercycler (Hamburg, Germany). Each sample was run in duplicate. The reference gene hypoxanthine-guanine phosphoribosyl transferase 1 (Hprt1) was used to normalize transcript levels. For measuring expression of different genes, 18 samples for males (n=5 for AhR-Vehicle, n=5 for AhR-PCB126, n=5 for WT-Vehicle, and n=3 for WT=PCB) and 19 samples for females (n=4 for AhR-Vehicle, n=5 for AhR-PCB126, n=5 for WT-Vehicle, and n=5 for WT=PCB) were used. These animals were chosen randomly for each group. There was no bias in sample selection. The mean of the WT-vehicle group was used to normalize transcript expression in both male and female samples. The standard curve based method was used to quantify the relative transcript levels (Larionov et al. 2005).
2.6. Statistical analysis
Values were reported as mean ± SE. Statistical differences between WT and AhR-KO male and female rats with various treatment groups were determined using two-way ANOVA followed by Tukey-Kramer post hoc test. There was no eliminated outlier in sample distribution. P values less than 0.05 were reported as statistically significant. GraphPad Prism software was used for the preparation of all graphs and statistical analyses.
3. Results and Discussion
The goal of this study was to examine the effects of PCB126 on energy homeostasis, and to elucidate the role of the AhR with special emphasis on potential sex differences.
3.1. PCB126 affects rat’s survival, growth, and organ weights 28 days after exposure
Previous studies have shown that in male SD rats PCB126 significantly reduced body weights compared to controls (Gadupudi et al. 2018; Gadupudi et al. 2016b; Gadupudi et al. 2016c; Lai et al. 2010). In this study with Holzman-SD rats, average body weights were significantly reduced by 33% and 27%, respectively, in WT male and female rats exposed to PCB126. In contrast, there were no significant differences in body weights between the AhR-KO control and PCB126-treated groups in either sex (Supplementary Fig-2A, B). WT-PCB126 rats showed a reduction in weight gain, resulting in a significant growth retardation over time (Supplementary Fig-2C, D). On the18th day following PCB126 injection, the first death of a WT rat was observed and a total of 6 WT rats (4 male rats and 2 female rats) exposed to PCB126 died before the end of study. However, no death or growth retardation was observed in AhR-KO rats. This indicates that the AhR was essential for this toxicity and that both sexes were affected similarly.
Dioxin and dioxin-like compounds like PCB126 cause thymic atrophy (decreased thymus weights) by negatively impacting the proliferation of T-lymphocyte progenitor cells in the thymus of rats and mice (Gadupudi et al. 2018; Gadupudi et al. 2016b; Lai et al. 2010; Laiosa et al. 2003; Shen et al. 2019). Our experiment demonstrates similar effects of PCB126 on relative thymus weights of both male and female WT rats, which were 6-fold and almost 12-fold reduced, respectively, while AhR-KO rats showed no change in thymus weights (Supplementary Fig-3A, B), indicating that the effects of PCB126 on thymic atrophy is mediated by AhR and sex independent.
The liver is a major target organ of dioxin-like compounds. PCB126 caused hypertrophy and enlarged hepatocytes in male SD rats after only 3 days of exposure (Wang et al. 2011) and following 6 days of exposure in a high fat diet experiment (Gadupudi et al. 2016b). In this study, relative liver weights were significantly higher in WT-PCB126 rats for both sexes, showing an increase of 15% and 23%, respectively (Supplementary Fig-3C, D). However, PCB126 exposure did not affect relative liver weights in AhR-KO rats. In addition, PCB126 exposure was also a potent inducer of hepatic CYP1A1 expression. Relative transcript levels of CYP1A1 in liver were almost 1000-fold increased in male and female WT-PCB126 rats. These results are consistent with previous reports in male rats (Gadupudi et al. 2018; Gadupudi et al. 2016b; Lai et al. 2011; Sorg 2014; Zhang et al. 2012). CYP1A1 in AhR-KO rats was very low and not induced by PCB126 (Supplementary Fig-4A, B). No sex difference was visible for these effects.
Zn deficiency can cause growth retardation which is accompanied by an increase in spleen weight, and we had previously observed a reduction of Zn levels in the livers of PCB126 exposed rats (Klaren et al. 2016; Kumari et al. 2019). However, there were no significant changes in spleen weight for all groups of male and female rats exposed to vehicle or PCB126 (data not shown), suggesting that Zn deficiency was not the cause of growth retardation.
3.2. PCB126 induces liver vacuolation, necrosis, and regeneration
PCB126 not only increased liver weights, but also caused histopathologic changes in livers of all 8 WT rats after 28 days exposure. Previous studies showed that PCB126 caused necrosis, regeneration, micro and macro vesicular hepatocellular vacuolation, each indicative of liver injury in male rats and mice (Gadupudi et al. 2016b; Gadupudi et al. 2016c; Nayak et al. 1996; Wahlang et al. 2017). We observed multifocal and coalescing micro- and macro-vesicular hepatocellular cytoplasmic vacuolation in all 3 surviving WT PCB126 exposed male rats. In WT female rats, PCB126 caused multifocal and coalescing micro- and macro-vesicular hepatocellular cytoplasmic vacuolation in 5 of 5 rats. Also, in WT female rats PCB126 affected the portal zones, including increased distribution of neutrophils and hepatocellular mitotic figures (regeneration) and small to sometimes large zones of hepatocellular necrosis in 2 of 5 rats. PCB126 exposed AhR-KO rats showed no significant change in liver histology/pathology (Fig-1). These results indicate that PCB126 induced liver toxicity is mediated by AhR activation and that sex-specific differences in pathology exist.
Fig-1. PCB126 caused AhR– and sex-dependent pathologies in livers of WT rats (H&E Staining).
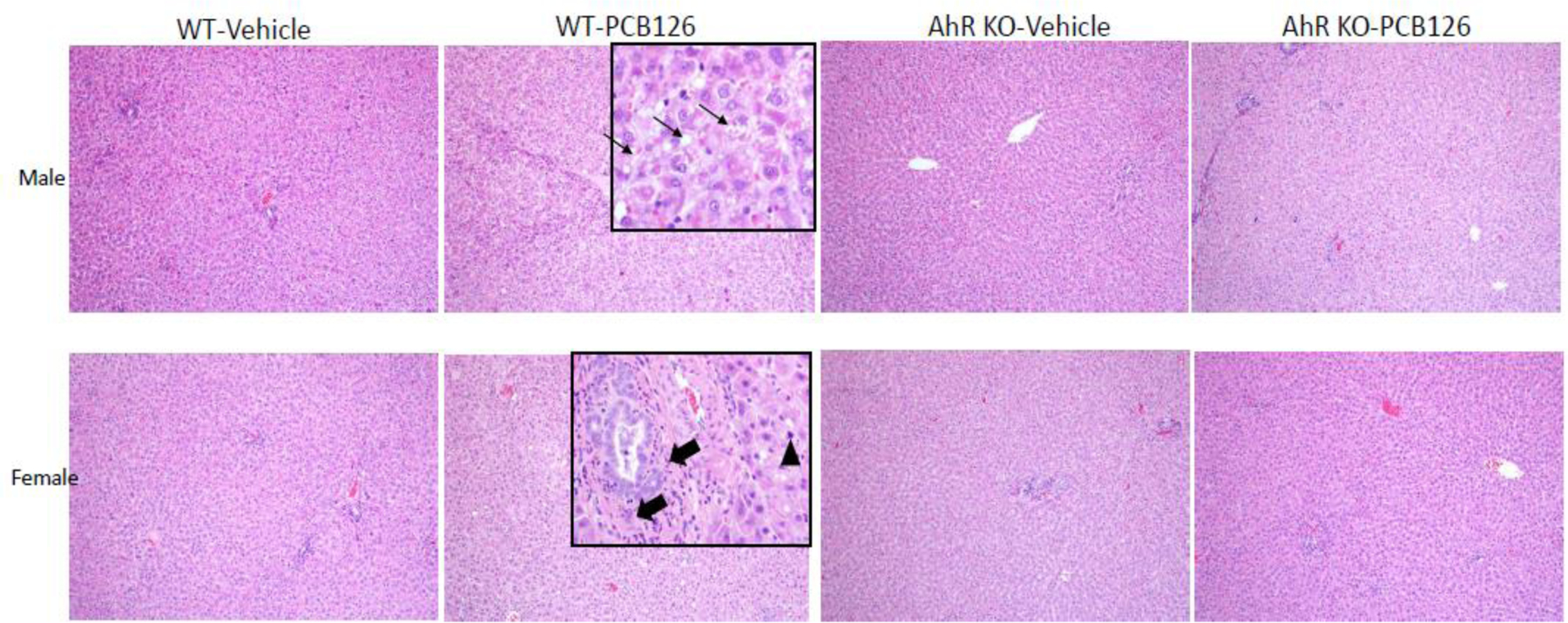
Microvesicular and macrovesicular hepatocellular cytoplasmic vacuolation in male WT rats and hepatocellular necrosis and mitotic figures (regeneration) in female WT rats were observed after PCB126 exposure. No significant histologic changes were identified in AhR KO rats or WT rats treated with vehicle alone (n=5 to 7 per group; n=3 for male WT-PCB126). (Thin arrows: microvesicular hepatocellular vacuolation; Thick arrows: periportal inflammation; Arrowhead: mitotic figure)
3.3. PCB126 affects glucose and energy homeostasis
The blood glucose level is an important indicator of glucose homeostasis. Previous studies showed that PCB126 causes hypoglycemia in rats and mice (Gadupudi et al. 2018; Gadupudi et al. 2016b; Shen et al. 2019). Similarly, we found significantly decreased serum glucose levels in WT-PCB126 exposed male and female rats, but PCB126 did not affect blood glucose levels in AhR-KO rats (Fig-2A, B).
Fig-2. PCB126 significantly reduced serum glucose and the liver transcript level of Insulin-like growth factor 1 (IGF-1) of WT male and female rats, not in AhR-KO rats.
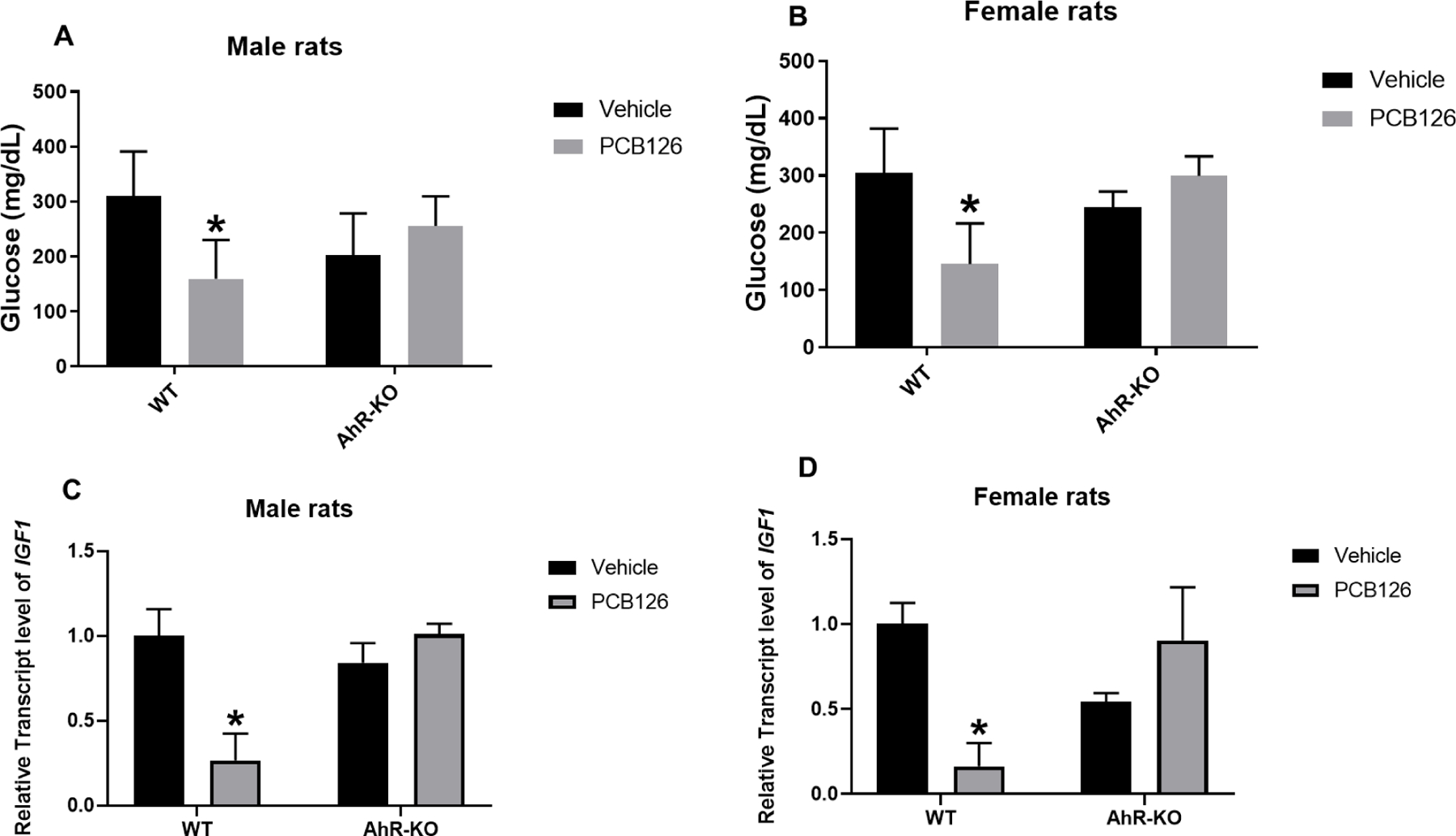
Serum glucose level for male (A) and female rats (B), relative liver transcript level of IGF-1 in male (C) and female rats (D) reported as mean ± SE. Statistical differences between WT control and treatment groups, and AhR-KO control and treatment groups were analyzed by Two-way ANOVA (*P <0.05; n=5 to 7 per group; n=3 for male WT-PCB126 for serum glucose level; and n= 3 to 5 for gene expression).
Hypoglycemia can occur in normal subjects with prolonged fasting, in diabetic patients with insulin therapy or any hypoglycemic drugs, or during hepatic failure or in patients with type 1 glycogen storage disease (De Herder 2004; Shehadeh et al. 1998; Suh et al. 2007). Severe and prolonged hypoglycemia can cause neuronal death and brain damage (Auer 2004). To maintain fine control of blood glucose, the liver produces and stores glucose, and also synthesizes endocrine factors like insulin-like growth factor 1 and 2 (IGF1 and IGF2), and IGF binding proteins (IGFBPs) (Postic et al. 2004). IGF1 is a key mediator of growth hormone actions for post-natal growth in animals and humans (Laron 2001). IGF1 controls intermediary metabolism, glucose uptake, glycogen storage and lipogenesis (Clemmons 2004b; Rotwein and Chia 2010) and regulates insulin sensitivity (Clemmons 2004a). Considering the critical roles of IGF1 in regulating energy metabolism, the expression of IGF1 gene was measured in this study. Hepatic IGF1 was significantly downregulated in WT male and female rats treated with PCB126, but no significant changes were seen in AhR-KO rat groups (Fig-2C, D). Therefore, our study shows that AhR-KO rats are protected from the PCB126 induced hypoglycemia and insulin resistance, and that IGF1 may be the mechanism for these toxic effects in rats.
There are three main sources of blood glucose: i) diet, ii) glycogenolysis (breakdown of liver glycogen during early fasting), and iii) gluconeogenesis (derivation from amino acids during prolonged starvation). To maintain energy homeostasis, the body also meets energy requirements through fatty acid oxidation in the liver (Lippi et al. 1994; Pilkis and Granner 1992; Zhang et al. 2014). The rats used in this study were not food restricted or fasted. To understand why PCB126 exposed rats were hypoglycemic, we measured the expression of genes encoding proteins critical to the regulation of gluconeogenesis, glycogenolysis, and fatty acid oxidation.
3.3.1. PCB126 reduces liver glycogen and disrupts glycogenolysis
Glycogen is a primary storage form of glucose in the liver. Glycogen is broken down during fasting to liberate glucose in a process called glycogenolysis (Radziuk and Pye 2001). Low serum glucose levels in PCB126 exposed rats may reflect reduced liver glycogen levels or disruption in the glycogenolysis pathway. Pygl encodes glycogen phosphorylase, the rate limiting enzyme for glycogenolysis. The transcript levels of Pygl were significantly downregulated in PCB126-exposed WT male and female rats but unchanged in AhR-KO groups (Fig-3B, C). Thus, the effect of PCB126 on Pygl required the presence of a functional AhR. The presence of glycogen was measured in liver by PAS staining. A relative paucity of PAS staining (low glycogen) was observed in centrilobular hepatocytes in WT-PCB126 groups compared to controls. However, PCB126-exposed and control AhR-KO rats did not show any significant differences in liver PAS staining (Fig-3A). Diminished Pygl expression and depleted glycogen following PCB126 exposure of WT male rats were also observed in our previous study (Gadupudi et al. 2018). These results indicate that PCB126 induced a reduction of hepatic glycogen storage and glycogenolysis which is mediated by AhR and sex independent. This suggests that WT rats were either unable to store glycogen and/or were forced to deplete this energy source during the 28 days of the exposure, either way depriving the rats of this important source of glucose.
Fig-3: Low liver glycogen levels in WT-PCB126 rats and reduced glycogenolysis pathway gene.
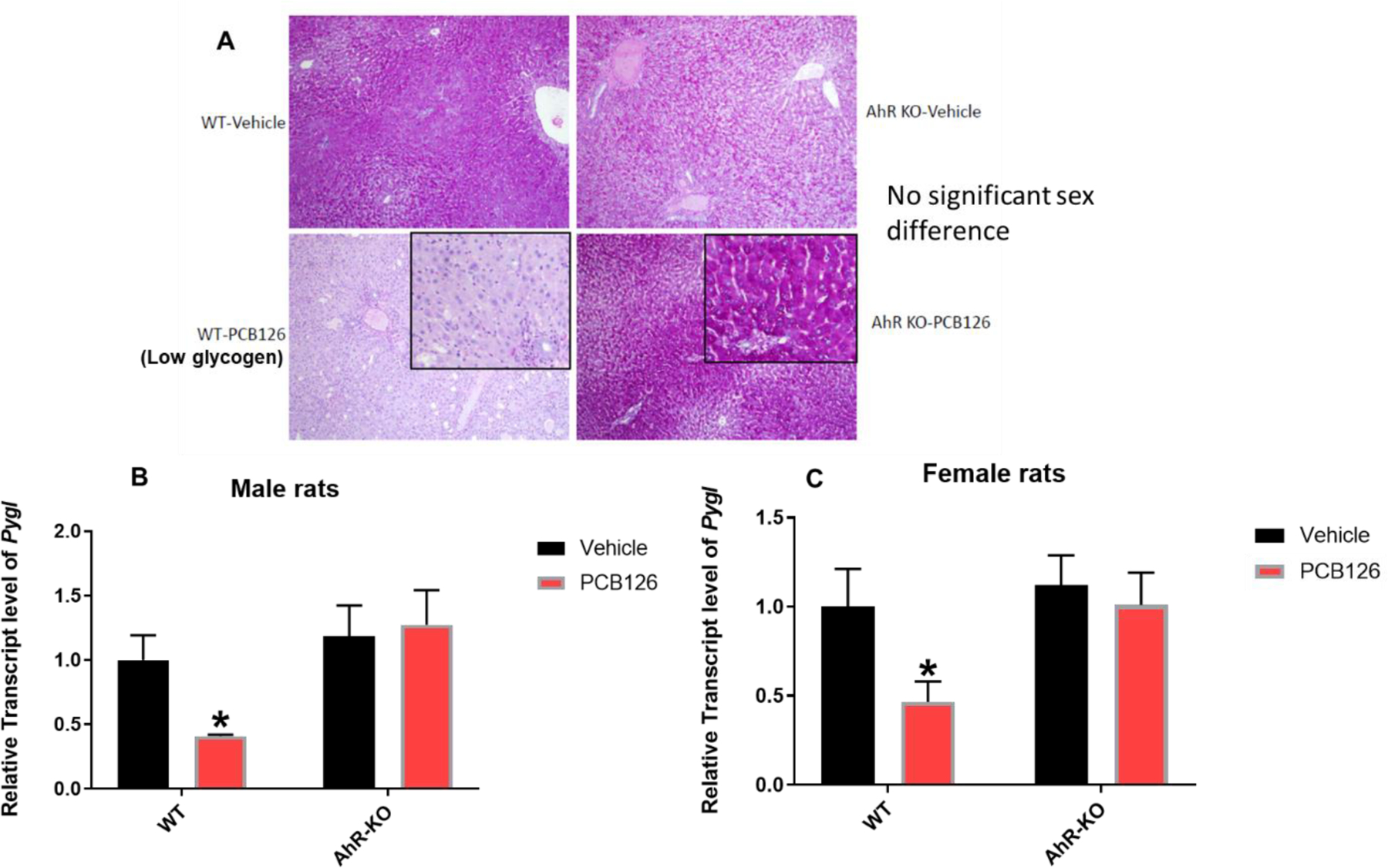
PAS staining showed low glycogen in the liver of WT rats after PCB126 exposure (A). Liver transcript level of Glycogen Phosphorylase (Pygl) in male (B) and female rats (C) reported as mean ± SE. Statistical differences between WT control and treatment groups, and AhR-KO control and treatment groups were analyzed by Two-way ANOVA (*P <0.05; n=5 to 7 per group; n=3 for male WT-PCB126 for histology; and n= 3 to 5 for gene expression).
3.3.2. PCB126 diminishes the expression of genes related to gluconeogenesis
During starvation and/or after a meal with high protein, high fat, and low carbohydrate content, gluconeogenesis, the synthesis of glucose from gluconeogenic amino acids, pyruvate, propionate, or glycerol, is the major source of glucose (Exton 1972; Hanson and Owen 2013). TCDD produced a fasting or starvation-like state in which food intake and gluconeogenesis were both decreased (Stahl et al. 1993; Yoon et al. 2001) and in a previous study we observed changes in expression of genes related to gluconeogenesis in both fed and fasted animals (Gadupudi et al. 2018). Thus, impaired gluconeogenesis could represent another target for PCB126 impact on energy homeostasis. The rate limiting enzyme of gluconeogenesis is phosphoenol pyruvate carboxykinase (PEPCK-C). Relative transcript levels of hepatic Pepck-c/Pck1 were significantly reduced in male WT-PCB126 exposed rats but not in male AhR-KO rats. Surprisingly, PCB126 exposure did not affect hepatic Pepck-c/Pck1 expression in female WT or AhR-KO rats (Fig-4A, B). Another important enzyme for gluconeogenesis is glucose-6-phosphatase (G6Pase). Relative transcript levels of G6Pase were significantly downregulated in male and female WT-PCB126 exposed rats but not in PCB126 exposed AhR-KO rats (Fig-4C, D). Inhibition of gluconeogenesis, specifically in male rats, and the lack of generated glucose from glycogen stores in the liver can be expected to reduce the glucose level in liver cells. In addition, or as a consequence, relative transcript levels of glucose transporter 2 (GLUT2) which helps to transport glucose from liver to blood was significantly downregulated in male WT PCB126-exposed rats, but not in female WT PCB126-exposed rats or in PCB126 exposed AhR-KO rats (Fig-4E, F).
Fig-4. PCB126 downregulated the transcript level of genes related to gluconeogenesis and glucose transporter in male liver, less so in female liver.
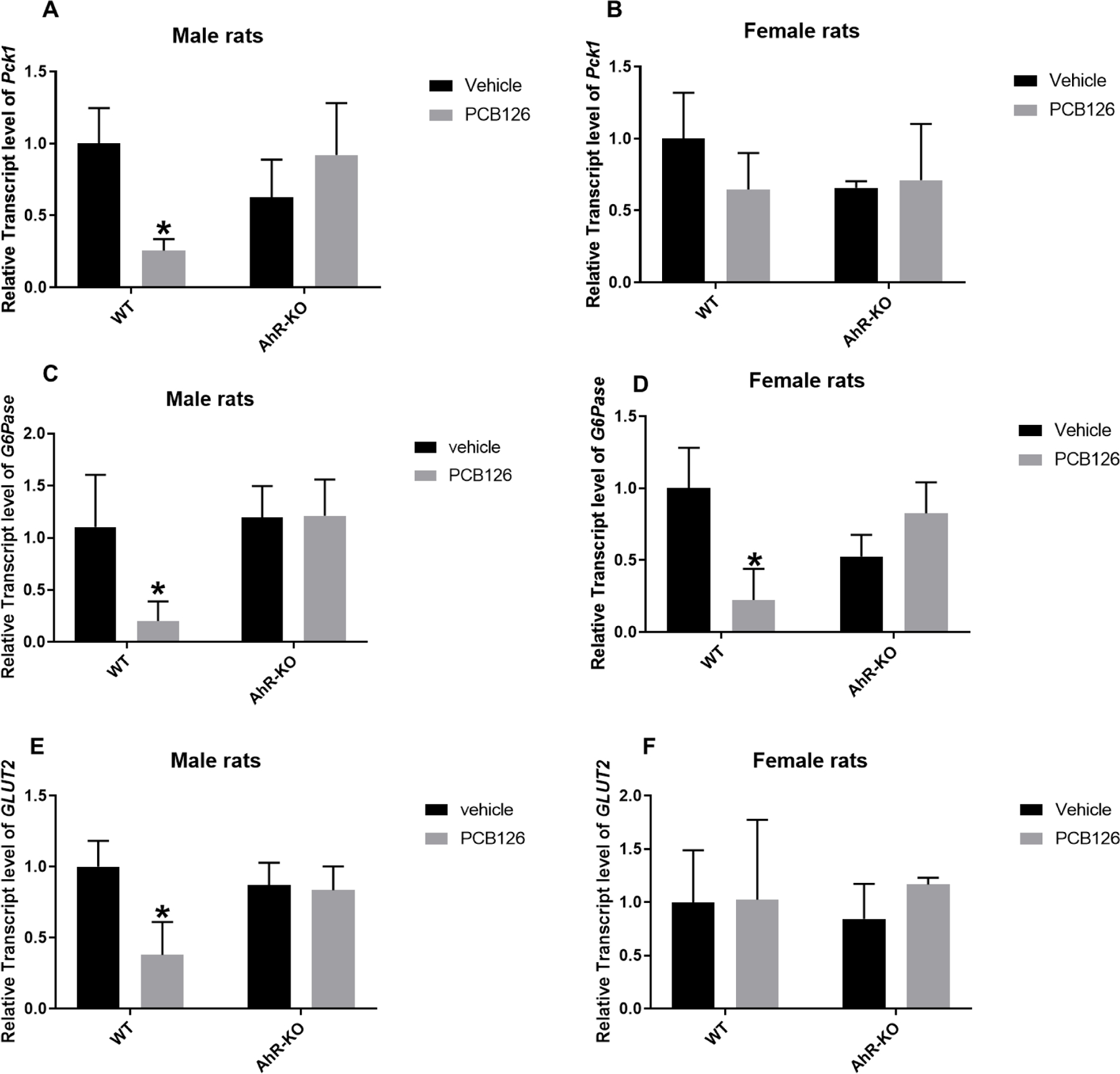
Relative transcript level of Pck1 for male (A) and female rats (B), of G6Pase for male (C) and female rats (D), of GLUT2 for male (E) and female rats (F) reported as mean ± SE. Statistical differences between WT control and treatment groups, and AhR-KO control and treatment groups were analyzed by Two-way ANOVA (*P <0.05; n= 3 to 5 for each group).
Pyruvate carboxylase (Pc), serine dehydratase (Sds), and lactate dehydratase A (LdhA) are anaplerotic enzymes that replenish TCA cycle intermediates for gluconeogenesis in the liver. Sds and LdhA use serine and lactate, respectively, as metabolic precursors for the TCA cycle, while Pc uses pyruvate (gluconeogenic amino acids) (Gadupudi et al. 2018; Kornberg 1966; Owen et al. 2002). Relative transcript level of Pc was significantly reduced in WT-PCB126 exposed male and female rats, but not in AhR-KO rats after PCB126 exposure. In WT male rats, PCB126 significantly downregulated the expression of Sds, while no significant changes were observed in WT female rats and AhR-KO rats. Ldh-A transcript levels did not differ significantly in any group (Fig-5A-F).
Fig-5: Some liver gluconeogenesis gene transcript levels were reduced by PCB126 in WT rats.
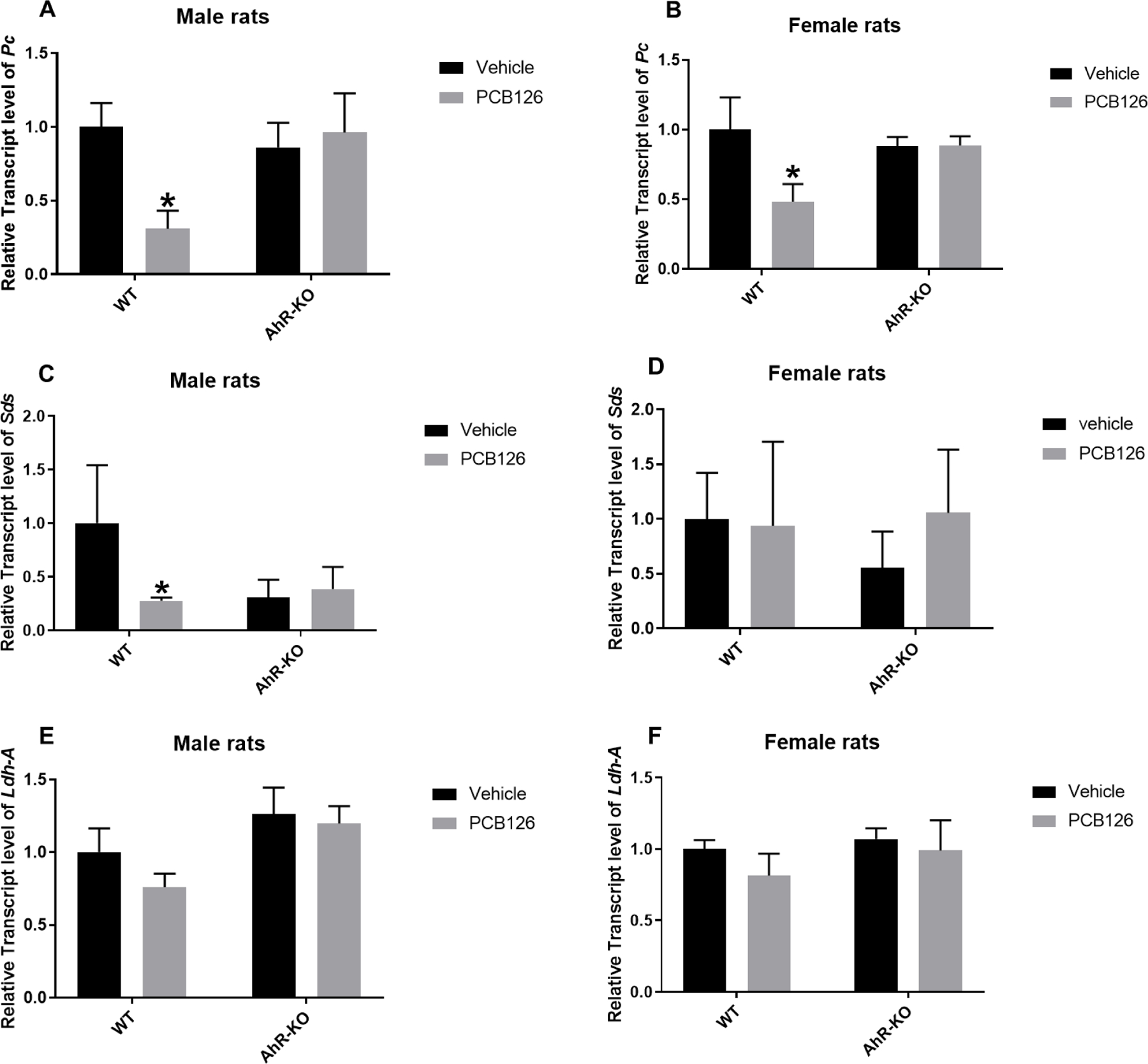
Relative transcript level of Pc in male (A) and female (B), of Sds in male (C) and female (D), and of Ldh-A in male (E) and female (F) rat livers reported as mean ± SE. Statistical differences between WT control and treatment groups, and AhR-KO control and treatment groups were analyzed by Two-way ANOVA (*P <0.05; n= 3 to 5 for each group).
The expression of gluconeogenic genes is triggered by glucagon and glucocorticoids during starvation. Cyclic AMP (cAMP) response element binding (CREB) protein stimulates gluconeogenic PEPCK gene transcription and also plays an important role in activating gluconeogenesis in liver (Liu et al. 1991). If the CREB gene is disrupted or a dominant-negative CREB inhibitor is overexpressed, the expression of gluconeogenic enzymes is diminished resulting in hypoglycemia (Herzig et al. 2001). CREB activity is regulated by the nuclear receptor coactivators PGC-1, CREM, and ICER which interact with CREB to produce stable transcription complexes which activate or repress the transcription of genes related to gluconeogenesis (Herzig et al. 2001). In our experiment, relative transcript levels for Creb1 (Fig-6A, B) and of Icer (Fig-6C, D), which is a corepressor, were not significantly affected by PCB126 exposure in WT and AhR-KO rats. However, relative transcript levels of coactivators, Pgc1α and Crem were decreased in WT male rats after PCB126 exposure, but not in female rats or AhR-KO rats exposed to PCB126 (Fig-6E-H). These results are consistent with PCB126 exposure negatively affecting hepatic gluconeogenesis through modulation of CREB1 transcriptional coactivators in male rats to reduce gluconeogenesis in an AhR-dependent way while again females seemed less affected. Thus, PCB126 significantly decreased transcript levels for some genes critical to gluconeogenesis in WT rats, but not in AhR-KO rats (Fig-7). Several sex differences were observed. Although, the precise mechanisms responsible for the sex differences are not known, elevated circulating estrogen levels in females and/or interactions between AhR and estrogen receptor (ER) (Ahmed et al. 2009; Klinge et al. 2000; Pighon et al. 2011) may be contributing factors. The other effects of PCB126 exposure on gene expression are consistent with our previous studies, which used only male WT rats (Gadupudi et al. 2018; Gadupudi et al. 2016a).
Fig-6: Among tested coactivators of gluconeogenesis only Crem was downregulated by PCB126 and only in male rat livers.
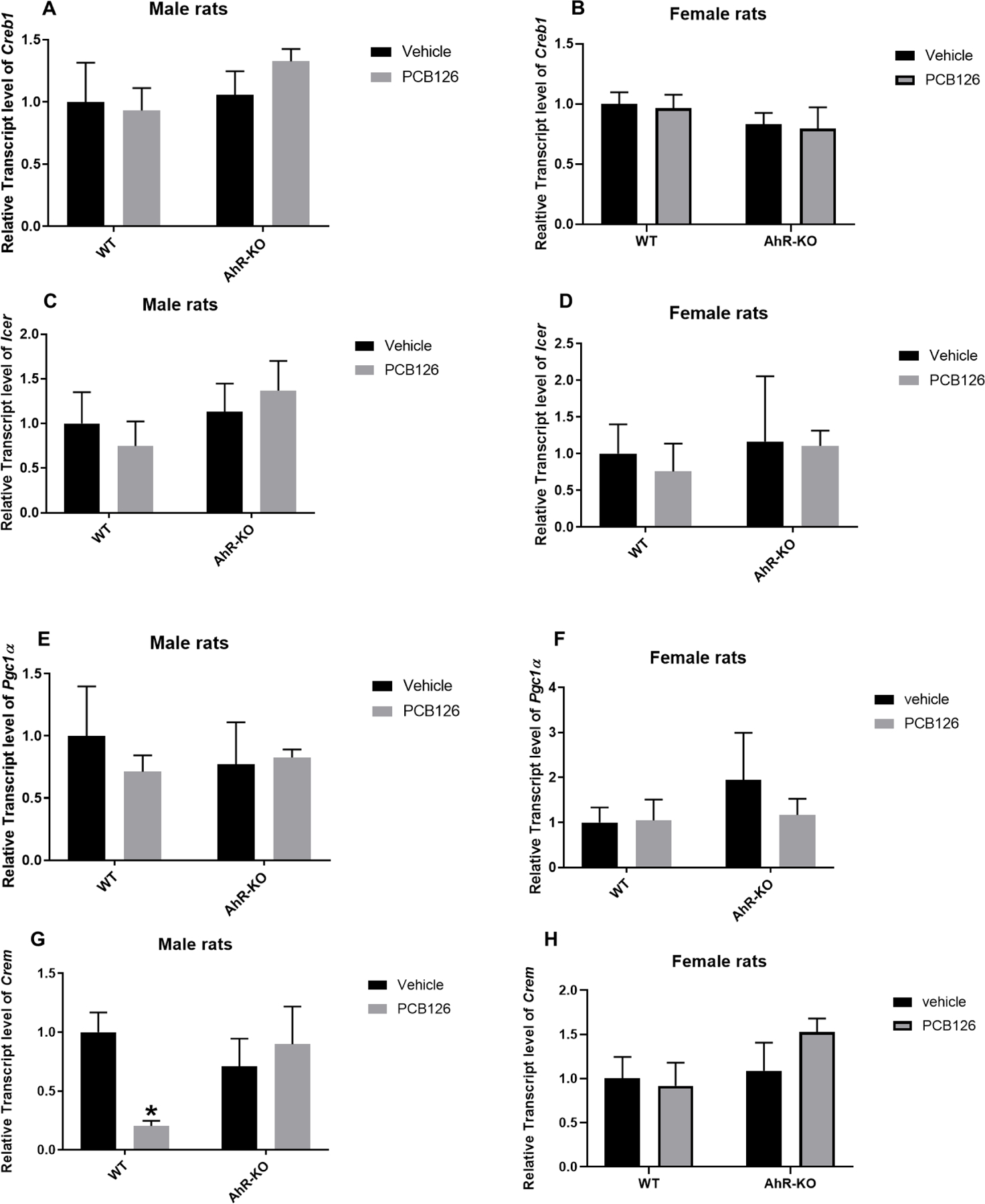
Relative liver transcript level of Creb1 in male (A) and female rats (B), of Icer in male (C) and female rats (D), of Pgc1-α in male (E) and female rats (F), and of Crem in male (G) and female rat (H) reported as mean ± SE. Statistical differences between WT control and treatment groups, and AhR-KO control and treatment groups were analyzed by Two-way ANOVA (*P <0.05; n= 3 to 5 for each group).
Fig-7. PCB126 mediated AhR activation caused decreased serum glucose levels and lower transcript levels of genes involved in liver gluconeogenesis and glucose transport, particularly in male rats.
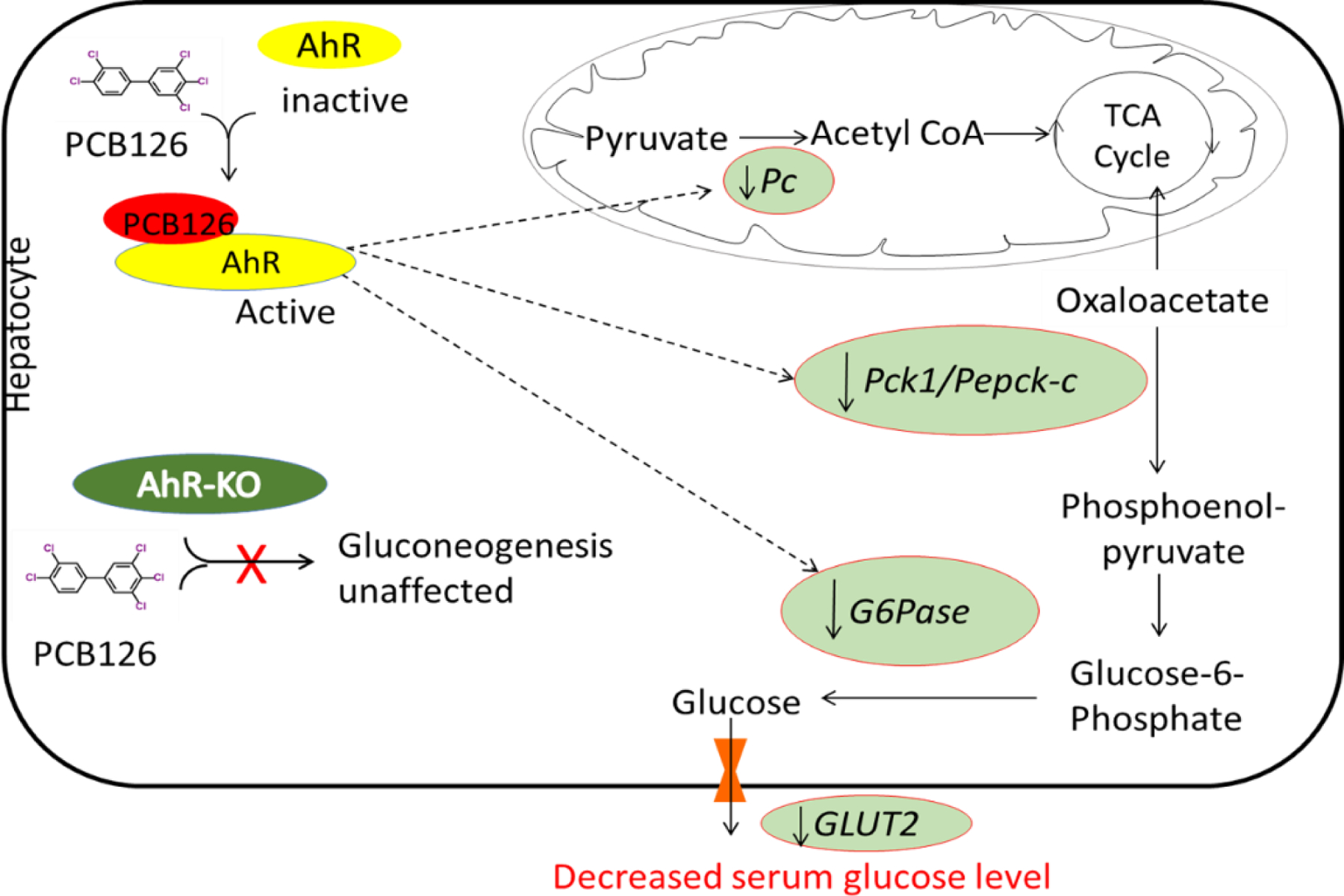
The specific mechanism of how AhR mediates the suppression of genes related to gluconeogenesis after PCB126 exposure is still not clear. However, Diani-Moore et al explained that TiPARP (TCDD-inducible poly (ADP-ribose) polymerase, PARP7), an AhR target gene, can mediate the toxicity of TCDD by suppressing hepatic gluconeogenesis and expression of gluconeogenic genes like PEPCK and G6Pase. TiPARP also affects PGC1α acetylation, decreasing the levels of Pgc1α which is a coactivator of PEPCK and G6Pase (Diani-Moore et al. 2010; Diani-Moore et al. 2013). In this study, the role of TiPARP was not measured. However, as PCB126 is a DL-PCB, TiPARP may have a central role in AhR mediated impaired liver gluconeogenesis by PCB126. To understand the mechanisms related to TiPARP and AhR for PCB126-induced impaired hepatic gluconeogenesis, further analysis is needed.
3.3.3. PCB126 exposure reduces fatty acid oxidation, including low serum non-esterified fatty acids (NEFAs) and cholesterol
A dose of 5 µmol/kg PCB126 reduced weight after 7 days exposure with a normal diet (Wang et al. 2011) or within 28 days on defined AIN-93G diet (Gadupudi et al. 2018). However, exposure to a similar PCB126 dose showed no significant effects on body weights when the animals received a modified AIN-93G diet containing 10% total fat content (Gadupudi et al. 2016a; Gadupudi et al. 2016c). These studies suggest that PCB126 adverse effect on weight may be ameliorated with dietary fats. Fatty acid oxidation affects energy metabolism during fasting or excessive exercise. The oxidation of fatty acids occurs in various cell compartments, in mitochondria, peroxisomes and endoplasmic reticulum. Peroxisomal β-oxidation is a significant source of metabolic energy (Moreno-Fernandez et al. 2018; Talley and Mohiuddin 2020). Peroxisome-proliferator activated receptor-α (Pparα) is a ligand-activated transcription factor that regulates genes controlling β-oxidation in peroxisomes and mitochondria (Pawlak et al. 2015). Transcript levels of Pparα were significantly upregulated in WT-PCB126-exposed female rats, and not affected in males and AhR-KO rats (Fig-8A, B). Pparα has an androgen response element in the promoter region which enhances gene expression in males that may play a role in this sex dimorphic effect (Park and Choi 2017). Alternatively, the upregulation of Pparα may be a compensatory effect. Our finding is in contrast to studies with mice, where Pparα was reported to be downregulated in females but not males (Wahlang et al. 2019), or upregulated in males (females were not examined) at the end of the circadian dark phase (Shen et al. 2019). Thus species, circadian time, dose, and sex may all be involved in Pparα regulation and need further examination. The rate limiting enzyme of peroxisomal β-oxidation is acyl CoA-oxidase 1 (Acox1). Acox1 and Hmgcs2 are transcriptional targets of Pparα. The transcript level of Acox1 and Hmgcs2 were significantly reduced in WT male and female rats exposed to PCB126, but not in AhR-KO rats (Fig-8C, D, E, F). In our earlier work we investigated several PCBs and PBBs, including PCB126, in male rats. This coplanar PCB, more than the other PCBs and PBBs, lowered the activities of catalase, FAO (Acox1) and peroxisomal β-oxidation, while also lowering the total CYP4A isozyme expression (Robertson et al. 2007). While the Acox1 is known to be regulated by PPARα, the expression of Hmgcs2 can be regulated by other nuclear receptors, LXRα, CAR and PXR, in order to maintain the homeostasis in liver metabolism (López-Velázquez et al. 2012). In addition, Hmgcs2 is known to have regulatory roles in ketogenesis (Wang et al. 2019).
Fig-8. PCB126 exposed WT rats had lower transcript levels of genes involved in β-oxidation but female rats had increased PPARα levels.
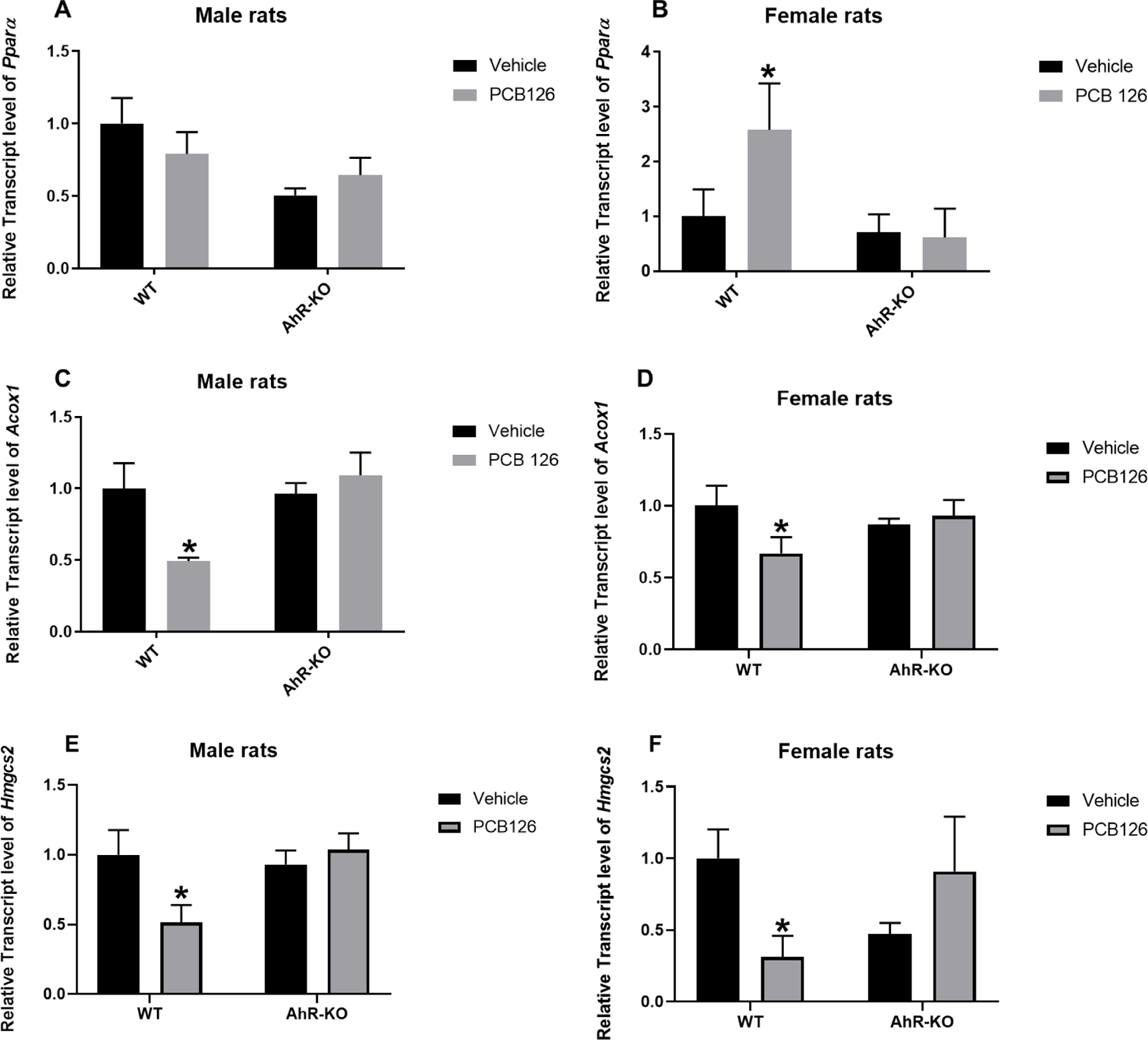
Relative transcript level of Pparα in male (A) and female rats (B), of Acox1 in male (C) and female rats (D), and of Hmgcs2 in male (E) and female rats (F) reported as mean ± SE. Statistical differences between WT control and treatment groups, and AhR-KO control and treatment groups were analyzed by Two-way ANOVA (*P <0.05; n= 3 to 5 for each group).
In this study, PCB126 exposure resulted in significantly reduced serum NEFAs in WT male and female rats but not in AhR-KO rats (Fig-9A, B) and also reduced serum cholesterol in female WT rats (Fig-9C, D), but not in male or AhR-KO rats. Low serum NEFAs and cholesterol are linked to reduced fatty acid oxidation and altered lipid metabolism in the liver (Groop et al. 1991; Hexeberg et al. 1994) . Based on the results in this study, it is evident that the AhR plays a clear role in PCB126 mediated decreased levels of NEFAs and cholesterol, however, it is unclear if these are direct effects of AhR on the regulation of genes involved in lipid/cholesterol metabolism or indirect effects that result from its interaction with other nuclear receptors in the liver. The rate-limiting step in cholesterol biosynthesis is the enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) which reduces HMG-CA to mevalonate. Whether reduced serum cholesterol is a result of lowered cholesterol synthesis, lowered transport, or greater cholesterol utilization is still unknown and needs further analysis. Cholesterol is the starting point of steroid synthesis in the adrenal glands and PCB126 was shown to significantly change adrenal steroidogenesis (Li and Wang 2005). Adrenal steroids have a strong influence on lipid metabolism, glucose tolerance, and obesity and it can be hypothesized that this mechanism may have an indirect effect on energy homeostasis in the liver. Considering the acute effects of PCB126 on glycogenolysis and gluconeogenesis that resulted in reduced glucose levels and survival, the changes in lipid and cholesterol metabolism may also be compensatory mechanisms to overcome the energy deprived states. This would also explain the lack of significantly altered hepatic lipid accumulation in this study (data not shown).
Fig-9. PCB126 reduced serum non-esterified fatty acids (NEFAs) and cholesterol in WT rats, not in AhR-KO rats.
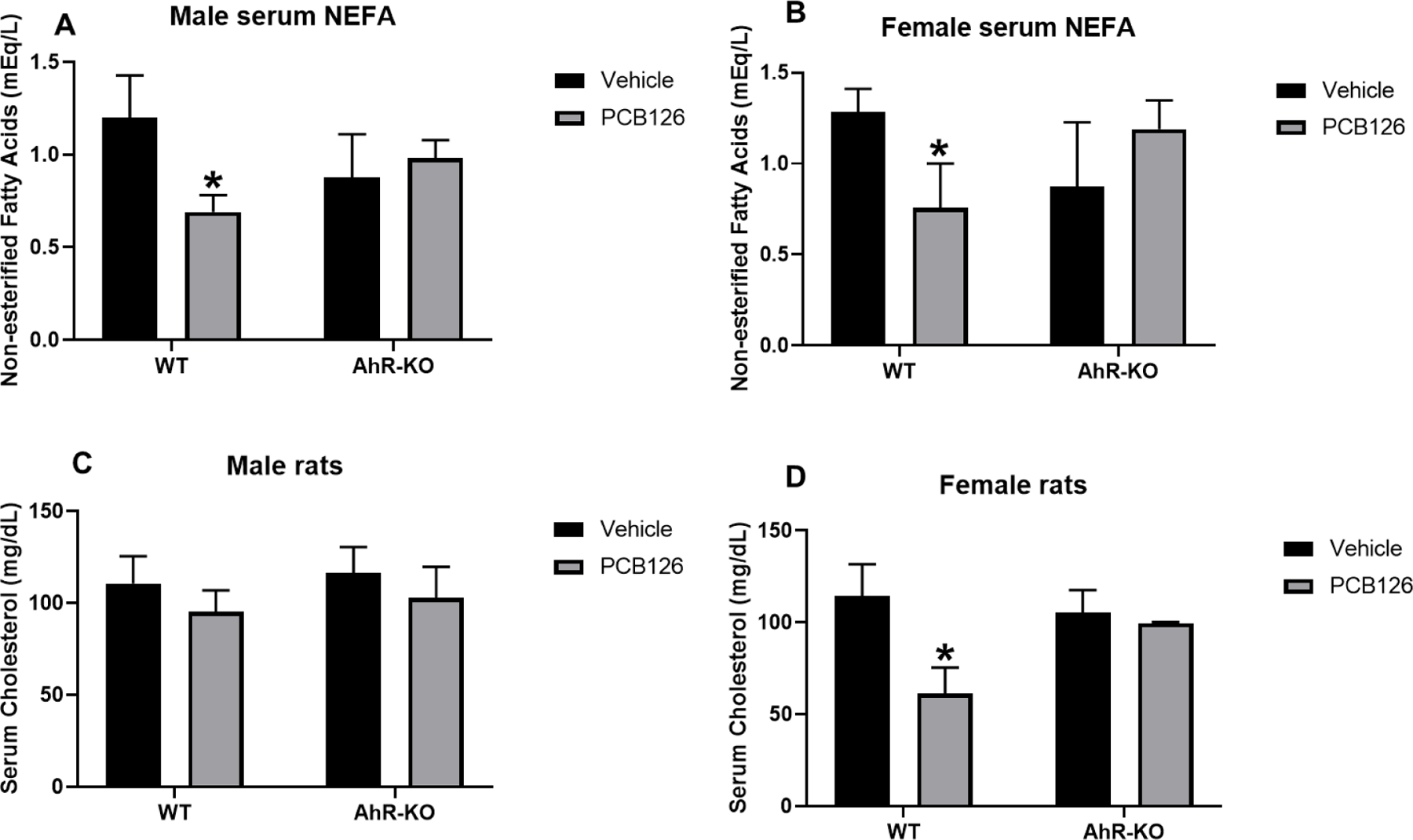
Serum NEFAs level of male (A) and female rats (B), serum cholesterol level of male (C) and female rats (D), reported as mean ± SE. Statistical differences between WT control and treatment groups, and AhR-KO control and treatment groups were analyzed by Two-way ANOVA (*P <0.05; n= 3 to 7 for each group).
This study and a recently published report with high dose TCDD (Hoyeck et al. 2020) suggest a significant sex difference in susceptibility to disturbed glucose homeostasis caused by AhR agonists, affecting predominantly males while females in our study exhibited reduced serum cholesterol levels and increased PPARalpha transcription. The probable cause for these sex differences is the estrogen receptor (ER) and AhR-ER crosstalk. Activation of AhR results in interferences of ER and AhR on binding at the promoter and/or enhancer regions and in addition to the reduction in estradiol due to induced phase I and II metabolizing enzymes (reviewed in (Matthews and Gustafsson 2006; Tarnow et al. 2019). Persistent AHR agonists like TCDD and PCB126 have therefore an anti-estrogenic effect. ER knock-out studies have shown this complex interaction between ER and AhR (see reviews above). Our studies indicate that this interaction is even more complicated during pregnancy (unpublished results). Overall, PCB126 and TCDD have significant toxic effects in general and on the female reproductive system (Iqbal et al. 2021; Klenov et al. 2021), but male rats were more at risk to die of wasting. We hypothesize that the stronger dysregulation of glucose homeostasis in males and possibly lower fat depots as temporary energy source may be causative factors. This needs further experimental analysis.
Since disturbed energy homeostasis and wasting were major effects of PCB126, diet and/or nutritional status may influence this toxicity of AhR ligands. Our previous studies indicate that a rodent diet with higher fat content may ameliorate somewhat the toxicity of PCB126 in rats (Gadupudi et al. 2018; Gadupudi et al. 2016a; Gadupudi et al. 2016c) and a similar effect was reported for TCDD in mice (Matteo et al. 2021). The blood half-life of TCDD was increased by a high-fat diet but only in obese mice, not in lean mice (Emond et al. 2018), and high doses of TCDD acted like an obesogen in mice on a high-fat diet but not on a normal diet, with adverse consequences on the liver (Brulport et al. 2017; Duval et al. 2017; Zhu et al. 2008). The dose of the AhR agonist and the toxic effect, wasting or only disturbance of homeostasis, may also play a role. Even more confusing, a study with PCB77 found disturbed glucose homeostasis in obese male but not female WT mice during weight loss while in AhR deficient mice the females and not the males showed impaired glucose tolerance during weight loss (Jackson et al. 2019). This interaction between dose, AhR agonist, body weight, diet and nutritional status clearly need more research to identify vulnerable populations.
4. Conclusions
Considering the ongoing exposure to PCB126 and other dioxin-like pollutants through food consumption and occupational exposure, understanding the targets and mechanisms of toxicity of PCB126 in humans and animals is paramount. Our study found that 28 days exposure to PCB126 caused weight loss, affected organs, such as liver and thymus, reduced serum glucose levels and lipids, altered expression of genes related to gluconeogenesis, glycogenolysis and fatty acid oxidation to disrupt energy homeostasis (Fig-10), and finally some rats died likely due to lack of energy sources. AhR activation was essential for these toxicities. However, there are also clear sex differences, males showing more disturbances in glucose/glycogen homeostasis while females had more changes in lipid/fatty acid homeostasis, a probable cause for the different pathological changes in liver and the higher toxicity in males. Considering the multitude of compounds that act as AhR ligands, including man-made compounds like pesticides and polycyclic aromatic hydrocarbons, an additive effect that might reach harmful levels should be considered. The findings of this study may help to understand causes and mechanisms of disturbed energy homeostasis and metabolic disorders and help to develop biomarkers of risk.
Fig-10. PCB126 significantly imbalanced the energy homeostasis in rats, which depended on AhR activation.
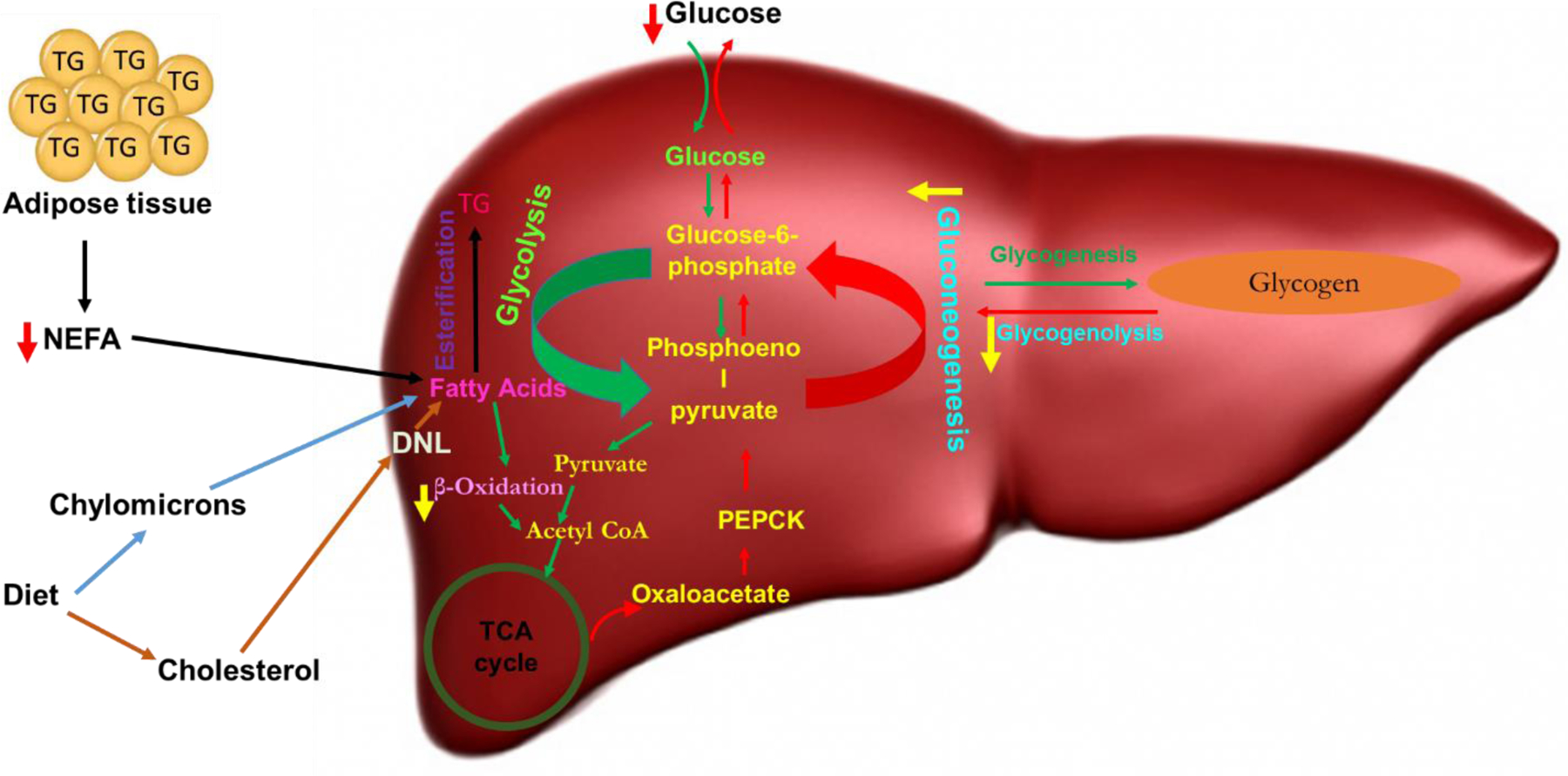
The scheme depicts the effects of PCB126 on gluconeogenesis, glycogenolysis, and fatty acid oxidation in the liver resulting in an imbalance in energy homeostasis after 28 days exposure that is mediated by AhR activation. Sex differences in gene regulation suggest a larger role of ß-oxidation in female and gluconeogenesis in male rats (DNL-De Novo Lipogenesis; TG- Triglyceride; NEFA- non-esterified fatty acid).
Supplementary Material
Highlights:
PCB126 caused weight loss, hypoglycemia, lower IGF1, reduced NEFA levels in rats
Sex-specific liver pathology and changes in gene expression were observed
PCB downregulated genes in glucose production pathways, particularly in male rats
Genes of fatty acid oxidation and serum cholesterol were more affected in females
Knocking out AhR completely prevented PCB126 alterations
Acknowledgements
We are thankful to Drs. Xueshu Li and Hans-Joachim Lehmler of the Iowa Superfund Research Program Synthesis Core for supplying PCB126, and the University of Iowa Comparative Pathology Laboratory for histology services. We would also like to thank Dr. Gopi Gadupudi and Ms. Laura Gosse-Dean for helpful comments.
Funding
This study was supported by the Iowa Superfund Research Program with funding from the National Institute of Environmental Health Sciences (NIEHS: P42 ES 013661 and ES029280), the Iowa Superfund Research Program Training Core, and the Center for Health Effects of Environmental Contamination (CHEEC). The opinions expressed are solely those of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest
There is no conflict of interest to disclose.
Supplementary Data
The schematic depicts the experimental design. The table provides information about the primers used for measuring gene expression.
References:
- Ahmed S, Valen E, Sandelin A and Matthews J 2009. Dioxin increases the interaction between aryl hydrocarbon receptor and estrogen receptor alpha at human promoters. Toxicological Sciences 111, 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampleman MD, Martinez A.s., DeWall J, Rawn DF, Hornbuckle KC and Thorne PS 2015. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environmental science & technology 49, 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer RN 2004. Hypoglycemic brain damage. Forensic science international 146, 105–110. [DOI] [PubMed] [Google Scholar]
- Baba T, Mimura J, Gradin K, Kuroiwa A, Watanabe T, Matsuda Y, Inazawa J, Sogawa K and Fujii-Kuriyama Y 2001. Structure and expression of the Ah receptor repressor gene. Journal of Biological Chemistry 276, 33101–33110. [DOI] [PubMed] [Google Scholar]
- Bae J, Peters-Golden M and Loch-Caruso R 1999. Stimulation of pregnant rat uterine contraction by the polychlorinated biphenyl (PCB) mixture Aroclor 1242 may be mediated by arachidonic acid release through activation of phospholipase A2 enzymes. Journal of Pharmacology and Experimental Therapeutics 289, 1112–1120. [PubMed] [Google Scholar]
- Beischlag TV, Morales JL, Hollingshead BD and Perdew GH 2008. The aryl hydrocarbon receptor complex and the control of gene expression. Critical Reviews™ in Eukaryotic Gene Expression 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brulport A, Le Corre L and Chagnon MC 2017. Chronic exposure of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces an obesogenic effect in C57BL/6J mice fed a high fat diet. Toxicology 390, 43–52. [DOI] [PubMed] [Google Scholar]
- Cancer, I.A.f.R.o. 2014. Polychlorinated biphenyls and polybrominated biphenyls. IARC Monogr 107. [PMC free article] [PubMed] [Google Scholar]
- Cave M, Appana S, Patel M, Falkner KC, McClain CJ and Brock G 2010. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environmental health perspectives 118, 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RC, Davis DD, Habeck EA, Roy NK and Wirgin I 2012. Toxic effects of PCB126 and TCDD on shortnose sturgeon and Atlantic sturgeon. Environmental toxicology and chemistry 31, 2324–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR 2004a. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. The Journal of clinical investigation 113, 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR 2004b. Role of insulin-like growth factor iin maintaining normal glucose homeostasis. Hormone research in paediatrics 62, 77–82. [DOI] [PubMed] [Google Scholar]
- Crinnion WJ 2011. Polychlorinated biphenyls: persistent pollutants with immunological, neurological, and endocrinological consequences. Alternative Medicine Review 16. [PubMed] [Google Scholar]
- De Herder W 2004. Insulinoma. Neuroendocrinology 80, 20–22. [DOI] [PubMed] [Google Scholar]
- Denison MS, Soshilov AA, He G, DeGroot DE and Zhao B 2011. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicological sciences 124, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito MJ, Ma X, Babish JG, Menache M and Birnbaum LS 1994. Dose-response relationships in mice following subchronic exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin: CYP1A1, CYP1A2, estrogen receptor, and protein tyrosine phosphorylation. Toxicology and applied pharmacology 124, 82–90. [DOI] [PubMed] [Google Scholar]
- Diani-Moore S, Ram P, Li X, Mondal P, Youn DY, Sauve AA and Rifkind AB 2010. Identification of the aryl hydrocarbon receptor target gene TiPARP as a mediator of suppression of hepatic gluconeogenesis by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin and of nicotinamide as a corrective agent for this effect. Journal of Biological Chemistry 285, 38801–38810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diani-Moore S, Zhang S, Ram P and Rifkind AB 2013. Aryl hydrocarbon receptor activation by dioxin targets phosphoenolpyruvate carboxykinase (PEPCK) for ADP-ribosylation via 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-inducible poly(ADP-ribose) polymerase (TiPARP). J Biol Chem 288, 21514–21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donat-Vargas C, Gea A, Sayon-Orea C, Carlos S, Martinez-Gonzalez M and Bes-Rastrollo M 2014. Association between dietary intakes of PCBs and the risk of obesity: the SUN project. J Epidemiol Community Health 68, 834–841. [DOI] [PubMed] [Google Scholar]
- Duval C, Teixeira-Clerc F, Leblanc AF, Touch S, Emond C, Guerre-Millo M, Lotersztajn S, Barouki R, Aggerbeck M and Coumoul X 2017. Chronic Exposure to Low Doses of Dioxin Promotes Liver Fibrosis Development in the C57BL/6J Diet-Induced Obesity Mouse Model. Environ Health Perspect 125, 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain, Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M and Edler L 2018. Risk for animal and human health related to the presence of dioxins and dioxin‐like PCBs in feed and food. EFSA Journal 16, e05333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond C, DeVito MJ, Diliberto JJ and Birnbaum LS 2018. The Influence of Obesity on the Pharmacokinetics of Dioxin in Mice: An Assessment Using Classical and PBPK Modeling. Toxicological Sciences 164, 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J 1972. Gluconeogenesis. Metabolism 21, 945–990. [DOI] [PubMed] [Google Scholar]
- Fein GG, Jacobson JL, Jacobson SW, Schwartz PM and Dowler JK 1984. Prenatal exposure to polychlorinated biphenyls: effects on birth size and gestational age. The Journal of pediatrics 105, 315–320. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM and Gonzalez FJ 1996. Aryl-hydrocarbon receptor-deficient mice are resistant to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicology and applied pharmacology 140, 173–179. [DOI] [PubMed] [Google Scholar]
- Frame GM, Cochran JW and Bøwadt SS 1996. Complete PCB congener distributions for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener‐specific analysis. Journal of High Resolution Chromatography 19, 657–668. [Google Scholar]
- Gadupudi GS, Elser BA, Sandgruber FA, Li X, Gibson-Corley KN and Robertson LW 2018. PCB126 Inhibits the Activation of AMPK-CREB Signal Transduction Required for Energy Sensing in Liver. Toxicol Sci 163, 440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi GS, Klaren WD, Olivier AK, Klingelhutz AJ and Robertson LW 2016a. PCB126-induced disruption in gluconeogenesis and fatty acid oxidation precedes fatty liver in male rats. Toxicological Sciences 149, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi GS, Klaren WD, Olivier AK, Klingelhutz AJ and Robertson LW 2016b. PCB126-Induced Disruption in Gluconeogenesis and Fatty Acid Oxidation Precedes Fatty Liver in Male Rats. Toxicol Sci 149, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi GS, Klingelhutz AJ and Robertson LW 2016c. Diminished phosphorylation of CREB is a key event in the dysregulation of gluconeogenesis and glycogenolysis in PCB126 hepatotoxicity. Chemical research in toxicology 29, 1504–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FJ and Fernandez-Salguero P 1998. The aryl hydrocarbon receptor: studies using the AHR-null mice. Drug Metabolism and Disposition 26, 1194–1198. [PubMed] [Google Scholar]
- Grimm FA, Hu D, Kania-Korwel I, Lehmler H-J, Ludewig G, Hornbuckle KC, Duffel MW, Bergman Å and Robertson LW 2015. Metabolism and metabolites of polychlorinated biphenyls. Critical reviews in toxicology 45, 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop LC, Bonadonna RC, Shank M, Petrides AS and DeFronzo RA 1991. Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. The Journal of clinical investigation 87, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson O 1995. The aryl hydrocarbon receptor complex. Annual review of pharmacology and toxicology 35, 307–340. [DOI] [PubMed] [Google Scholar]
- Hansen L 1999. The ortho side of PCBs: Occurrence and disposition Kluwer Academic Publishers. Boston.[Google Scholar] [Google Scholar]
- Hanson RW and Owen OE 2013. Gluconeogenesis [Google Scholar]
- Harrill JA, Hukkanen RR, Lawson M, Martin G, Gilger B, Soldatow V, LeCluyse EL, Budinsky RA, Rowlands JC and Thomas RS 2013. Knockout of the aryl hydrocarbon receptor results in distinct hepatic and renal phenotypes in rats and mice. Toxicology and applied pharmacology 272, 503–518. [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C and Puigserver P 2001. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183. [DOI] [PubMed] [Google Scholar]
- Hexeberg S, Hexeberg E, Willumsen N and Berge RK 1994. A study on lipid metabolism in heart and liver of cholesterol- and pectin-fed rats. Br J Nutr 71, 181–192. [DOI] [PubMed] [Google Scholar]
- Hong C-S and Bush B 1990. Determination of mono-and non-ortho coplanar PCBs in fish. Chemosphere 21, 173–181. [Google Scholar]
- Hoyeck MP, Blair H, Ibrahim M, Solanki S, Elsawy M, Prakash A, Rick KRC, Matteo G, O’Dwyer S and Bruin JE 2020. Long-term metabolic consequences of acute dioxin exposure differ between male and female mice. Scientific Reports 10, 1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Pierce SH, Kozai K, Dhakal P, Scott RL, Roby KF, Vyhlidal CA and Soares MJ 2021. Evaluation of Placentation and the Role of the Aryl Hydrocarbon Receptor Pathway in a Rat Model of Dioxin Exposure. Environmental Health Perspectives 129, 117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H, Watanabe M, Okajima Y, Tanabe S, Amano M, Miyazaki N and Petrov EA 2004. Toxicokinetics of PCDD, PCDF, and coplanar PCB congeners in Baikal seals, Pusa sibirica: age-related accumulation, maternal transfer, and hepatic sequestration. Environmental science & technology 38, 3505–3513. [DOI] [PubMed] [Google Scholar]
- Jackson EN, Thatcher SE, Larian N, English V, Soman S, Morris AJ, Weng J, Stromberg A, Swanson HI, Pearson K and Cassis LA 2019. Effects of Aryl Hydrocarbon Receptor Deficiency on PCB-77-Induced Impairment of Glucose Homeostasis during Weight Loss in Male and Female Obese Mice. Environ Health Perspect 127, 77004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Vyas S, Duffel M, Parkin S and Lehmler H-J 2011. Synthesis of sterically hindered polychlorinated biphenyl derivatives. Synthesis 7, 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaren WD, Gibson-Corley KN, Wels B, Simmons DL, McCormick ML, Spitz DR and Robertson LW 2016. Assessment of the Mitigative Capacity of Dietary Zinc on PCB126 Hepatotoxicity and the Contribution of Zinc to Toxicity. Chem Res Toxicol 29, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenov V, Flor S, Ganesan S, Adur M, Eti N, Iqbal K, Soares MJ, Ludewig G, Ross JW, Robertson LW and Keating AF 2021. The Aryl hydrocarbon receptor mediates reproductive toxicity of polychlorinated biphenyl congener 126 in rats. Toxicol Appl Pharmacol 426, 115639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge CM, Kaur K and Swanson HI 2000. The aryl hydrocarbon receptor interacts with estrogen receptor alpha and orphan receptors COUP-TFI and ERRα1. Archives of biochemistry and biophysics 373, 163–174. [DOI] [PubMed] [Google Scholar]
- Korkalainen M, Tuomisto J and Pohjanvirta R 2004. Primary structure and inducibility by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) of aryl hydrocarbon receptor repressor in a TCDD-sensitive and a TCDD-resistant rat strain. Biochemical and biophysical research communications 315, 123–131. [DOI] [PubMed] [Google Scholar]
- Kornberg H 1966. Anaplerotic sequences and their role in metabolism. Essays Biochem 2, 1–31. [Google Scholar]
- Kumar N, Ramirez-Ortiz D, Solo-Gabriele HM, Treaster JB, Carrasquillo O, Toborek M, Deo S, Klaus J, Bachas LG and Whitall D 2016. Environmental PCBs in Guánica Bay, Puerto Rico: implications for community health. Environmental Science and Pollution Research 23, 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari D, Nair N and Bedwal RS 2019. Morphological changes in spleen after dietary zinc deficiency and supplementation in Wistar rats. Pharmacol Rep 71, 206–217. [DOI] [PubMed] [Google Scholar]
- Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, Haschek WM, Ludewig G and Robertson LW 2010. Acute toxicity of 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status. Environ Int 36, 918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai IK, Chai Y, Simmons D, Watson WH, Tan R, Haschek WM, Wang K, Wang B, Ludewig G and Robertson LW 2011. Dietary selenium as a modulator of PCB 126–induced hepatotoxicity in male Sprague-Dawley rats. Toxicological Sciences 124, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiosa MD, Wyman A, Murante FG, Fiore NC, Staples JE, Gasiewicz TA and Silverstone AE 2003. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. The Journal of Immunology 171, 4582–4591. [DOI] [PubMed] [Google Scholar]
- Larionov A, Krause A and Miller W 2005. A standard curve based method for relative real time PCR data processing. BMC bioinformatics 6, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laron Z 2001. Insulin-like growth factor 1 (IGF-1): a growth hormone. Molecular Pathology 54, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock R and Straif K 2013. International Agency for Research on Cancer Monograph Working Group IARC, Lyon, France. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol 14, 287–288. [DOI] [PubMed] [Google Scholar]
- Li LA and Wang PW 2005. PCB126 induces differential changes in androgen, cortisol, and aldosterone biosynthesis in human adrenocortical H295R cells. Toxicol Sci 85, 530–540. [DOI] [PubMed] [Google Scholar]
- Li X, Holland EB, Feng W, Zheng J, Dong Y, Pessah IN, Duffel MW, Robertson LW and Lehmler H-J 2018. Authentication of synthetic environmental contaminants and their (bio) transformation products in toxicology: polychlorinated biphenyls as an example. Environmental Science and Pollution Research, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi D, Caleri D, Bucalossi A and Rotella CM 1994. The role of liver in glucose homeostasis in type 2 diabetes: the modernity of Claude Bernard’s studies. Diabetes/metabolism reviews 10, 63–74. [DOI] [PubMed] [Google Scholar]
- Liu J, Park EA, Gurney AL, Roesler WJ and Hanson RW 1991. Cyclic AMP induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription is mediated by multiple promoter elements. Journal of Biological Chemistry 266, 19095–19102. [PubMed] [Google Scholar]
- Loch-Caruso R 2002. Uterine muscle as a potential target of polychlorinated biphenyls during pregnancy. International journal of hygiene and environmental health 205, 121–130. [DOI] [PubMed] [Google Scholar]
- López-Velázquez JA, Carrillo-Córdova LD, Chávez-Tapia NC, Uribe M and Méndez-Sánchez N 2012. Nuclear Receptors in Nonalcoholic Fatty Liver Disease. Journal of Lipids 2012, 139875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q and Baldwin KT 2002. A cycloheximide-sensitive factor regulates TCDD-induced degradation of the aryl hydrocarbon receptor. Chemosphere 46, 1491–1500. [DOI] [PubMed] [Google Scholar]
- Matteo G, Hoyeck MP, Blair HL, Zebarth J, Rick KRC, Williams A, Gagné R, Buick JK, Yauk CL and Bruin JE 2021. Prolonged Low-Dose Dioxin Exposure Impairs Metabolic Adaptability to High-Fat Diet Feeding in Female but Not Male Mice. Endocrinology 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H and Dedrick R 1984. Pharmacokinetics of PCBs. Annual Review of Pharmacology and Toxicology 24, 85–103. [DOI] [PubMed] [Google Scholar]
- Matthews J and Gustafsson J-Å 2006. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nuclear Receptor Signaling 4, nrs.04016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendola P, Buck GM, Sever LE, Zielezny M and Vena JE 1997. Consumption of PCB-contaminated freshwater fish and shortened menstrual cycle length. American Journal of Epidemiology 146, 955–960. [DOI] [PubMed] [Google Scholar]
- Moreno-Fernandez ME, Giles DA, Stankiewicz TE, Sheridan R, Karns R, Cappelletti M, Lampe K, Mukherjee R, Sina C and Sallese A 2018. Peroxisomal β-oxidation regulates whole body metabolism, inflammatory vigor, and pathogenesis of nonalcoholic fatty liver disease. JCI insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T and Yamano T 2017. Polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans and dioxin-like coplanar polychlorinated biphenyls in mackerel obtained from the Japanese market, 1999–2003. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 34, 1562–1572. [DOI] [PubMed] [Google Scholar]
- Nayak N, Sathar SA, Mughal S, Duttagupta S, Mathur M and Chopra P 1996. The nature and significance of liver cell vacuolation following hepatocellular injury—an analysis based on observations on rats rendered tolerant to hepatotoxic damage. Virchows Archiv 428, 353–365. [DOI] [PubMed] [Google Scholar]
- Nguyen LP and Bradfield CA 2008. The search for endogenous activators of the aryl hydrocarbon receptor. Chemical research in toxicology 21, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Kalhan SC and Hanson RW 2002. The key role of anaplerosis and cataplerosis for citric acid cycle function. Journal of Biological Chemistry 277, 30409–30412. [DOI] [PubMed] [Google Scholar]
- Park HJ and Choi JM 2017. Sex-specific regulation of immune responses by PPARs. Exp Mol Med 49, e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Lim J. e., Park H and Jee SH 2016. Body burden of persistent organic pollutants on hypertension: a meta-analysis. Environmental Science and Pollution Research 23, 14284–14293. [DOI] [PubMed] [Google Scholar]
- Pawlak M, Lefebvre P and Staels B 2015. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. Journal of hepatology 62, 720–733. [DOI] [PubMed] [Google Scholar]
- Pighon A, Gutkowska J, Jankowski M, Rabasa-Lhoret R and Lavoie J-M 2011. Exercise training in ovariectomized rats stimulates estrogenic-like effects on expression of genes involved in lipid accumulation and subclinical inflammation in liver. Metabolism 60, 629–639. [DOI] [PubMed] [Google Scholar]
- Pilkis SJ and Granner D 1992. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annual review of physiology 54, 885–909. [DOI] [PubMed] [Google Scholar]
- Pollenz RS 2002. The mechanism of AH receptor protein down-regulation (degradation) and its impact on AH receptor-mediated gene regulation. Chemico-biological interactions 141, 41–61. [DOI] [PubMed] [Google Scholar]
- Postic C, Dentin R and Girard J 2004. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes & metabolism 30, 398–408. [DOI] [PubMed] [Google Scholar]
- Radziuk J and Pye S 2001. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes/metabolism research and reviews 17, 250–272. [DOI] [PubMed] [Google Scholar]
- Roberts BJ and Whitelaw ML 1999. Degradation of the basic helix-loop-helix/Per-ARNT-Sim homology domain dioxin receptor via the ubiquitin/proteasome pathway. Journal of Biological Chemistry 274, 36351–36356. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Berberian I, Borges T, Chen L-C, Chow CK, Glauert HP, Filser JG and Thomas H 2007. Suppression of Peroxisomal Enzyme Activities and Cytochrome P450 4A Isozyme Expression by Congeneric Polybrominated and Polychlorinated Biphenyls. PPAR Research 2007, 015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotwein P and Chia DJ 2010. Gene regulation by growth hormone. Pediatric Nephrology 25, 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L 2014. Energy metabolism in the liver. Compr Physiol 4: 177–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martin BV, Pizarro-Aranguiz N, Garcia-Mendoza D, Araya-Jordan C, Maddaleno A, Abad E and Galban-Malagon CJ 2016. A four-year survey in the farming region of Chile, occurrence and human exposure to polychlorinated dibenzo-p-dioxins and dibenzofurans, and dioxin -like polychlorinated biphenyls in different raw meats. Sci Total Environ 573, 1278–1286. [DOI] [PubMed] [Google Scholar]
- Shehadeh Ν, Davis E and Jones T 1998. Hypoglycemia in children with diabetes: incidence, counterregulation and cognitive dysfunction. Journal of Pediatric Endocrinology and Metabolism 11, 177–182. [DOI] [PubMed] [Google Scholar]
- Shen X, Chen Y, Zhang J, Yan X, Liu W, Guo Y, Shan Q and Liu S 2019. Low-dose PCB126 compromises circadian rhythms associated with disordered glucose and lipid metabolism in mice. Environment international 128, 146–157. [DOI] [PubMed] [Google Scholar]
- Sherwin RS 1980. Role of liver in glucose homeostasis. Diabetes Care 3, 261–265. [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Foushee HR, Shelton C, Pavuk M and Consortium AEHR 2012. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environmental health perspectives 120, 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg O 2014. AhR signalling and dioxin toxicity. Toxicology letters 230, 225–233. [DOI] [PubMed] [Google Scholar]
- Stahl BU, Beer DG, Weber LW and Rozman K 1993. Reduction of hepatic phosphoenolpyruvate carboxykinase (PEPCK) activity by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) is due to decreased mRNA levels. Toxicology 79, 81–95. [DOI] [PubMed] [Google Scholar]
- Suh SW, Hamby AM and Swanson RA 2007. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia 55, 1280–1286. [DOI] [PubMed] [Google Scholar]
- Talley JT and Mohiuddin SS 2020. Biochemstry, Fatty Acid Oxidation. StatPearls [Internet], StatPearls Publishing. [PubMed] [Google Scholar]
- Tarnow P, Tralau T and Luch A 2019. Chemical activation of estrogen and aryl hydrocarbon receptor signaling pathways and their interaction in toxicology and metabolism. Expert Opinion on Drug Metabolism & Toxicology 15, 219–229. [DOI] [PubMed] [Google Scholar]
- Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, DeVito M, Jacobs D, Köhrle J, Lee D-H and Rylander L 2013. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environmental health perspectives 121, 774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Barney J, Thompson B, Wang C, Hamad OM, Hoffman JB, Petriello MC, Morris AJ and Hennig B 2017. Editor’s highlight: PCB126 exposure increases risk for peripheral vascular diseases in a liver injury mouse model. Toxicological Sciences 160, 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Jin J, Hardesty JE, Head KZ, Shi H, Falkner KC, Prough RA, Klinge CM and Cave MC 2019. Identifying sex differences arising from polychlorinated biphenyl exposures in toxicant-associated liver disease. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 129, 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Robertson LW, Wang K and Ludewig G 2011. Species difference in the regulation of cytochrome P450 2S1: lack of induction in rats by the aryl hydrocarbon receptor agonist PCB126. Xenobiotica; the fate of foreign compounds in biological systems 41, 1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yuan H, Jin J, Li P, Ma Y and Wang Y 2018. Polychlorinated biphenyl concentrations in pooled serum from people in different age groups from five Chinese cities. Chemosphere 198, 320–326. [DOI] [PubMed] [Google Scholar]
- Wang Y-H, Liu C-L, Chiu W-C, Twu Y-C and Liao Y-J 2019. HMGCS2 Mediates Ketone Production and Regulates the Proliferation and Metastasis of Hepatocellular Carcinoma. Cancers 11, 1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenik-Bany M, Strucinski P and Piskorska-Pliszczynska J 2016. Dioxins and PCBs in game animals: Interspecies comparison and related consumer exposure. Environ Int 89–90, 21–29. [DOI] [PubMed] [Google Scholar]
- Weber R, Gonser S, Kohler J, Korner W, Herold C, Haag R, Krapp M and Peichl L 2018. Biomonitoring of polychlorinated biphenyls in Bavaria/Germany-long-term observations and standardization. Environ Sci Pollut Res Int 25, 16344–16354. [DOI] [PubMed] [Google Scholar]
- Williams AE, Watt J, Robertson LW, Gadupudi G, Osborn ML, Soares MJ, Iqbal K, Pedersen KB, Shankar K, Littleton S, Maimone C, Eti NA, Suva LJ and Ronis MJJ 2020. Skeletal Toxicity of Coplanar Polychlorinated Biphenyl Congener 126 in the Rat Is Aryl Hydrocarbon Receptor Dependent. Toxicol Sci 175, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winneke G, Bucholski A, Heinzow B, Kramer U, Schmidt E, Walkowiak J, Wiener JA and Steingruber HJ 1998. Developmental neurotoxicity of polychlorinated biphenyls (PCBS): cognitive and psychomotor functions in 7-month old children. Toxicol Lett 102–103, 423–428. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR and Granner DK 2001. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sargis RM, Volden PA, Carmean CM, Sun XJ and Brady MJ 2012. PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. PloS one 7, e37103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu D, Huang H, Chen S, Wang L, Zhu L, Jiang X, Ruan X, Luo X and Cao P 2014. Regulation of glucose homeostasis and lipid metabolism by PPP1R3G-mediated hepatic glycogenesis. Molecular endocrinology 28, 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu BT, Gallo MA, Burger CW Jr., Meeker RJ, Cai MX, Xu S and Conney AH 2008. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin administration and high-fat diet on the body weight and hepatic estrogen metabolism in female C3H/HeN mice. Toxicol Appl Pharmacol 226, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


