Abstract
Background:
The prevalence of childhood asthma, rhinoconjunctivitis, and eczema in the city of Patras, Greece, has been followed in four consecutive surveys since 1991. After a continuous rise in the prevalence of all three of these disorders, a plateau was reached for asthma between 2003 and 2008, whereas the prevalence of rhinoconjunctivitis and eczema continued to increase.
Objective:
To investigate these trends in the same population into the following decade.
Methods:
We repeated two methodologically identical cross-sectional parental questionnaire surveys in 2013 and 2018 among 8–9-year-old schoolchildren (N = 2554 and N = 2648, respectively). In 2018, spirometry and fractional exhaled nitric oxide (FeNO) measurements were also performed.
Results:
Current asthma (i.e., wheeze/asthma in the past 2 years) decreased from 6.9% in 2008 to 5.2% in 2013 and 4.3% in 2018 (p for trend < 0.001). The prevalence of lifetime (“ever had”) rhinoconjunctivitis also declined (5.1% in 2008, 4.4% in 2013, 3.0% in 2018; p for trend < 0.001), whereas that of lifetime eczema increased (10.8%, 13.6%, and 16.1%, respectively; p for trend < 0.001). The relative risk of current asthma in children with ever-had rhinoconjundtivitis was 7.73 in 2008, 6.00 in 2013, and 6.69 in 2018, whereas the relative risk in those with ever-had eczema was 5.15, 2.80, and 2.22, respectively. Among children with asthma, those with rhinoconjunctivitis had lower forced expiratory volume in the first second of expiration and higher FeNO values than those with eczema.
Conclusion:
The prevalence of asthma and rhinoconjunctivitis declined during the past decade in Greek schoolchildren, whereas the prevalence of eczema continued to rise. Nevertheless, the relationship between rhinoconjunctivitis and asthma remained strong, whereas the association between eczema and asthma appears to have weakened.
Keywords: asthma, atopic dermatitis, children, eczema, epidemiology, hay fever, prevalence, questionnaire survey, respiratory allergy, rhinoconjunctivitis
A dramatic increase in the prevalence of childhood asthma, rhinoconjunctivitis (“hay fever”), and eczema was noted throughout the 1980s and 1990s in Westernized societies.1 This “asthma and allergy epidemic,” however, seems to have decelerated into the new millennium.2,3 In the International Study of Asthma and Allergies in Childhood,4 a plateau or even a decline in the rates of asthma and atopic disorders was noted in the early 2000s in high prevalence countries.5–7 Similar trends were reported in two independent U.S. studies8,9 and in the Aberdeen, Scotland surveys the longest cross-sectional study of asthma, hay fever, and eczema in children.10,11 By using identical parental questionnaires in four repeated cross-sectional surveys conducted in 1991, 1998, 2003, and 2008, among 8–10-year-old schoolchildren in Patras, Greece, we reported a continuous increase in the prevalence of rhinoconjunctivitis and eczema12,13; the prevalence of asthma also increased from 1991 to 2003, followed by a plateau between 2003 and 200814,15 and a decline in 2013 and 2018.16
In the present study, when using identical methods, we further examined the prevalence of allergic asthma, rhinoconjunctivitis, and eczema in the two most recent surveys in 2013 and 2018. We hypothesized that, similar to the international trends, the prevalence of allergic respiratory disorders and eczema would eventually stabilize or, perhaps, decline in the past decade in Greek schoolchildren. Furthermore, in the 2018 survey, we performed fractional exhaled nitric oxide (FeNO) measurements to assess atopic inflammation in relation to reported symptoms and spirometric evidence of airway obstruction.
METHODS
Standard Questionnaire and Study Design
A standardized parental questionnaire, identical to that of previous surveys,12–15 was distributed to third and fourth grade schoolchildren (8 and 9 years old) in the city of Patras, in 2013 and 2018. It included simple questions on current (i.e., in the past 2 years) and lifetime (i.e., “ever had”) physician-diagnosed wheeze/asthma, rhinoconjunctivitis, and eczema. The exact wording of the questionnaire remained unchanged in the six cross-sectional surveys (1991, 1998, 2003, 2008, 2013, 2018) and is presented in Appendix 1. The method of distribution and collection of the questionnaires has been described elsewhere; all six surveys were conducted in the months of January and February.12–16 Similar to previous surveys, the presence of a history of (“ever had”) rhinoconjunctivitis and/or eczema in schoolchildren with current wheeze/asthma was termed “allergic asthma”; children with no such history were termed “non-allergic”.10,12,13,16 Telephone confirmation was sought for all questionnaires with at least one positive answer and in an equal number of randomly selected negative questionnaires.16
Spirometry and FeNO Measurement
In the 2018 survey, all the participants who responded to telephone confirmation were invited for spirometry and FeNO measurement between June 18 and September 7, 2018, at the pediatric respiratory unit of the University Hospital of Patras. They were required to have interrupted any asthma and/or systemic antihistamine medication and to be free of respiratory symptoms for at least 2 weeks. Topical treatment interruption for allergic rhinitis and/or rhinosinusitis and/or eczema was not a prerequisite. Spirometry was performed with a Micro 5000 spirometer (Medisoft, Sorinnes, Belgium) according to standard guidelines.17 Forced expiratory volume at 1 second (FEV1) values were assessed according to Global Lung Initiative normative data.18 The FeNO measurement preceded spirometry; it was conducted at a flow of 50 mL/s with a FeNO+ device (Medisoft), according to the established guidelines.19
Ethics
Formal approval was obtained for both surveys from the ethics committee of the University Hospital of Patras, the Regional Directorate of Primary Education, and the Greek Ministry of Education. Parental written consent forms were distributed and collected with the questionnaires. Spirometry and FeNO measurement were performed after additional written parental consent and child's verbal assent. Appropriate management was delivered, and treatment was prescribed as needed for the occasional child who, although asymptomatic, was unexpectedly found to have worrisome lung function and/or an increased FeNO value. Such children were referred to their physician who was also notified by the member of our team who conducted the measurements.
Statistics
The prevalence of wheeze/asthma, rhinoconjunctivitis, and eczema were calculated as the ratio of cases to the total number of responders. Logistic regression analysis with adjustment for sex was used to calculate the prevalence change ratio (the relative prevalence change) between consecutive surveys. One-way analysis of variance with the Tukey post hoc test for multiple comparisons was used to compare FEV1 and FeNO values among the different groups. Statistical analyses were performed by using the IBM SPSS version 27 (IBM Corp., Armonk, NY). A p value of <0.05 was considered as statistically significant.
RESULTS
Overall, 3086 questionnaires in 2013 and 3135 in 2018 were distributed to 44 public primary schools of the metropolitan area of Patras; the same 44 schools were surveyed in 1998, 2003, and 2008. The target sample represented 89.8% of the local population within the age range of the study in 2013 and 91.2% in 2018. The collection rate was 85.6% in 2013 and 86.7%, in 2018. After excluding those with missing or inconsistent responses, 2554 questionnaires were analyzed in 2013 and 2648 were analyzed in 2018. A detailed study chart is shown in Appendix 2.
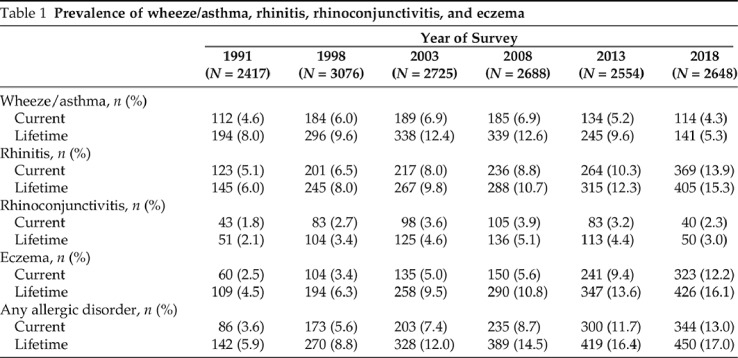
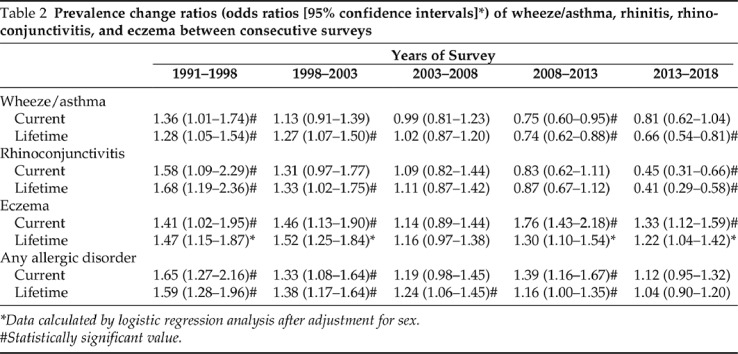
The prevalence of current and lifetime wheeze/asthma, rhinoconjunctivitis, and eczema over the 27-year follow-up period is shown in Table 1 and Appendix 3. After its initial rise from 4.6% (95% confidence interval [CI], 3.8 – 5.4%) in 1991 to 6.9% (95% CI, 5.9 – 7.9%) in 2003, current wheeze/asthma reached a plateau between 2003 and 2008, and declined to 4.3% (95% CI, 3.5 – 5.1%) in 2018 (2008–2018 p for trend < 0.001). Similarly, the prevalence of lifetime rhinoconjunctivitis increased from 2.1% (95% CI, 1.5 – 2.7%) in 1991 to 5.1% (95% CI, 4.3 – 5.9%) in 2008 and decreased to 3% (95% CI, 2.4 – 3.6%) in 2018 (2008–2018 p for trend < 0.001). Conversely, the prevalence of lifetime eczema increased continuously throughout the surveillance period, from 4.5% (95% CI, 3.7 – 5.3%) in 1991 to 16.1% (95% CI, 14.7 – 17.5%) in 2018 (Table 1). The prevalence change ratios between consecutive surveys are shown in Table 2.
Table 1.
Prevalence of wheeze/asthma, rhinitis, rhinoconjunctivitis, and eczema
Table 2.
Prevalence change ratios (odds ratios [95% confidence intervals]*) of wheeze/asthma, rhinitis, rhinoconjunctivitis, and eczema between consecutive surveys
Data calculated by logistic regression analysis after adjustment for sex.
#Statistically significant value.
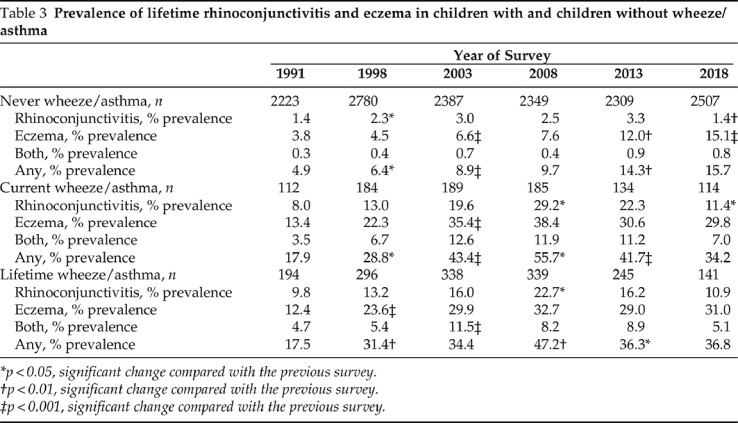
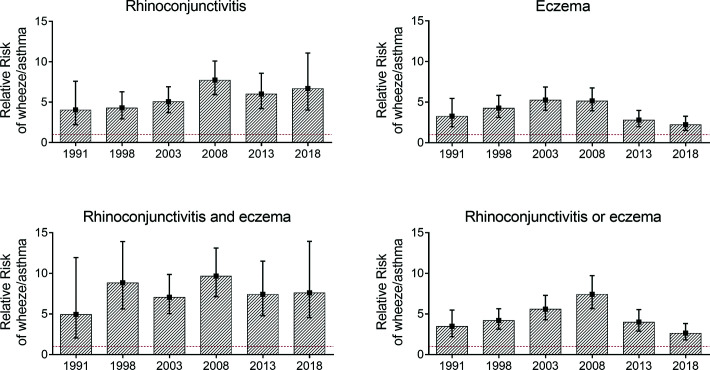
Changes in the prevalence of rhinoconjunctivitis and eczema in children with or without wheeze/asthma are shown in Table 3. After a continuous increase from 1991 to 2008, the prevalence of rhinoconjunctivitis decreased in both children with asthma and children without asthma from 2008 to 2018, whereas that of eczema continued to rise in those without asthma and stabilized among those with asthma. The relative risk of wheeze/asthma among children with rhinoconjunctivitis increased, from 4.05 (95% CI, 2.20–7.60) in 1991 to 7.73 (95% CI, 5.93–10.1) in 2008 and remained high in both 2013 (6.00 [95% CI, 4.20–8.58]) and 2018 (6.69 [95% CI, 4.03–11.1]) surveys, despite the decrease in the prevalence of rhinoconjunctivitis during that period (Fig. 1, Appendix 4). The relative risk of wheeze/asthma among children with eczema also increased, from 3.27 (95% CI, 1.97–5.45) in 1991 to 5.25 (95% CI, 4.01–6.87) in 2003 but then progressively declined, to 2.22 (95% CI, 1.50–3.27) in 2018. The combination of rhinoconjunctivitis and eczema was associated with the highest risk of wheeze/asthma in all six surveys (Fig. 1, Appendix 4).
Table 3.
Prevalence of lifetime rhinoconjunctivitis and eczema in children with and children without wheeze/asthma
*p < 0.05, significant change compared with the previous survey.
†p < 0.01, significant change compared with the previous survey.
‡p < 0.001, significant change compared with the previous survey.
Figure 1.
Risk of current wheeze with or without asthma in children with rhinoconjunctivitis and/or eczema.
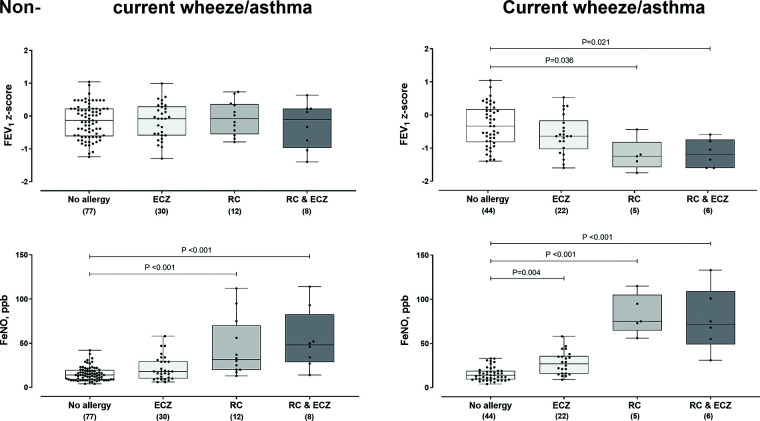
Overall, 127 with a positive response to a question regarding current or non-current wheeze with or without diagnosed asthma and/or rhinoconjunctivitis and/or eczema and 77 children with negative response to all questions of the questionnaire underwent spirometry and FeNO measurements in 2018. Of the positive responders, 77 were classified with current wheeze/asthma, 66 with eczema, 31 with rhinoconjunctivitis (14 with both allergic disorders) (Fig. 2). There were no differences in the median FEV1 z-scores among those with non-current wheeze/asthma with a history (“ever had”) rhinoconjunctivitis, eczema, or both, compared with those without a history of these two allergic diseases (no allergy); however, children with a history of rhinoconjunctivitis or rhinoconjunctivitis and eczema had higher median FeNO values among those with non-current wheeze/asthma. Among children with current wheeze/asthma, those with a history of rhinoconjunctivitis or rhinoconjunctivitis and eczema had lower FEV1 and higher FeNO median values than those without such a history (“no allergy”). In those with a history of eczema alone, the values of neither FEV1 nor FeNO differed from those of children with “no allergy” (Fig. 2), although there was a trend toward higher FeNO levels (post hoc p = 0.161).
Figure 2.
Forced expiratory volume at 1 second (FEV1) and fractional exhaled nitric oxide (FeNO) in children with non-current wheeze/asthma and in those with current wheeze/asthma in relation to the presence of eczema (ECZ) and/or rhinoconjunctivitis (RC). Between-groups comparisons were performed with Kruskal-Wallis test and the Dunn post hoc test.
DISCUSSION
After a continuous rise since 1991 and a phase of plateau between 2003 and 2008 (four surveys), the prevalence of current wheeze/asthma has significantly declined among Greek schoolchildren as shown in two surveys during the past decade.16 Concomitant measurements of rhinoconjunctivitis and eczema revealed an increasing prevalence for both disorders between 1991 and 2008, with a subsequent diverging trend that is presented in this study; the prevalence of rhinoconjunctivitis decreased between 2008 and 2018, in parallel with the prevalence of wheeze/asthma, whereas that of eczema continued to rise. Nevertheless, ever-had rhinoconjunctivitis remained a more important risk factor for current wheeze/asthma than ever-had eczema, whereas their coexistence rendered the highest risk. Moreover, rhinoconjunctivitis, but not eczema alone, was associated with lower FEV1 and higher FeNO values among schoolchildren with asthma. During the 27 years of surveillance, persistent or recurrent rhinitis apart from colds has also continued to increase.
An increasing prevalence of childhood asthma, rhinoconjunctivitis, and eczema throughout the1990s,1–3,10–14 with a subsequent stabilization or even decrease among high-prevalence countries, has repeatedly been reported in the literature.5–11 However, this “general” trend has significant geographical and temporal variation, especially when it comes to allergic disorders. In three cross-sectional surveys over a period of 20 years in Australia, a stabilization of wheeze, current asthma, airway hyperresponsiveness, hay fever, and eczema was noted in the early 2000s.20 In the Aberdeen studies (seven surveys between 1964 and 2014 in the city of Aberdeen, Scotland), after a rise until the end of the previous century, there was a deceleration and a subsequent decrease in the prevalence of wheeze/asthma and eczema but a continuous increase of rhinoconjunctivitis.11,21
In Sweden, three surveys in 1979, 1991, and 2007 showed a progressive increase in the prevalence of rhinoconjunctivitis but a leveling off of asthma and eczema between 1991 and 2007.22 However, a study that used Swedish and Danish national registries found an increase in the incidence of asthma, a decrease in rhinoconjunctivitis, and no change in eczema during the first decade of the new millennium.23 Striking geographic and temporal inconsistencies were also observed in the International Study of Asthma and Allergies in Childhood phase I and phase III studies.5–7 The divergence in trends of asthma and allergies into the first decade of the new millennium has been previously discussed in more detail.12,13,16
Despite the significant decrease in the prevalence of rhinoconjunctivitis during the past decade, this disorder continued to be associated with a high risk of wheeze/asthma in our population; the risk was even higher in children who reported both rhinoconjunctivitis and eczema. However, the risk of wheeze/asthma in those who reported eczema alone declined, despite the continuing increase in the prevalence of eczema in the past decade; in 2018, the risk of wheeze/asthma was almost threefold lower in children with eczema than in those with rhinoconjunctivitis. Importantly, in the 2018 survey, FEV1 and FeNO values of those with asthma were adversely affected, particularly in children with a history of rhinoconjunctivitis but equivocally so in those with eczema alone. The results of studies with regard to the FeNO increase in children with eczema in the absence of respiratory allergy are controversial.24,25
In our study, there was a trend toward higher FeNO values in those with noncurrent wheeze and with eczema alone compared with those with noncurrent wheeze without any allergic disorder (Fig. 2), although the statistical analysis did not reveal a significant post hoc difference between these two groups. A larger, more focused study is required to resolve this issue. To our knowledge, this was the first time that FEV1 and FeNO measurements were included in such an epidemiologic study in children. Taken together, the above findings are in accord with the close relationship between allergic rhinitis and asthma in childhood26–28; they also confirm the concordance of reported symptoms with lung function abnormalities and airway inflammation, which thus indirectly validated our questionnaire.
Similar to other reports that document diverging trends between asthma and allergies,21–23 the decrease of asthma and rhinoconjunctivitis in association with the continuing increase in eczema cannot be considered consistent with the “hygiene hypothesis,” even in its most current versions.29 However, the strong influence of rhinoconjunctivitis on lung function and on the level of bronchial inflammation in individuals with asthma offers further epidemiologic support to the hypothesis of “nose-lung united airway disease.”26–28
Our study had some limitations. First, its cross-sectional design could not address the causality of associations. Second, although more elegant questionnaires30,31 could have been used for rhinoconjunctivitis and eczema, we opted to adhere to the exact phrasing of our previous questionnaires for reasons of comparability. Third, all individuals with current wheeze, whether diagnosed with asthma, are considered as having “asthma” in the risk-stratification analysis; however, it should be remembered that these are children with physician-diagnosed recurrent wheeze at the age of 6–7 years, i.e., most likely with asthma. Fourth, as already mentioned in the case of eczema, the small number of children in the “allergy” subgroups of children with current and non-current wheeze/asthma (Fig. 2) may have prevented statistical significance of the differences in spirometric values and/or FeNO measurements between groups to emerge. Fifth, the finding that chronic or recurrent rhinitis has continued to rise in the most recent decade may raise concerns of underdiagnosis of allergic rhinitis. The term “rhinitis,” even when specified as chronic or recurrent, which occurs “apart from colds,” refers to a heterogeneous group of diseases, such as overlapping upper airway tract infections identified as “a constantly runny nose,” vasomotor rhinitis, and anatomic causes, which may present with troublesome nasal symptoms and, thus, may exaggerate the prevalence of upper airway allergic disease.31 We elected to use the more restrictive term of rhinoconjunctivitis in all our surveys in accordance with other epidemiologic literature,10 and, in the present study, we continue to do so, also for comparability purposes.
The question on what drives the changing prevalence of asthma and allergies, despite well-characterized risk factors and the divergence of the epidemiologic expressions of the “atopic march,” remains unanswered.16 Atmospheric pollution is an unequivocally established risk factor of asthma,32 which drastically declined in the large cities of the United Kingdom in the 1960s through 1980s; however, it was during this period that asthma increased most steeply.33 In Greece, for the most part of the second decade of the new century (years of financial crisis and austerity), a rapid decay of air quality was noted due to the use of low-cost wood and biomass for the heating of homes in urban environments34; however, it was during this time that the drop of wheeze/asthma prevalence among schoolchildren was noted in the city of Patras. With regard to indoor cigarette smoking, we found (unpublished data) that the number of packs smoked yearly in the homes of schoolchildren in Patras had not changed during 1998–2018, a time during which wheeze/asthma prevalence rose, stabilized, and then declined. In the absence of an overall biologic hypothesis that can explain these trends, we propose that many well-established risk factors, which operate solely or in various combinations in space and time, may be responsible for the diversity of available epidemiologic observations; the possibility of yet unknown factors cannot be excluded.
CONCLUSION
Our findings in six repeated cross-sectional surveys showed that, after a marked increase since 1991, the prevalence of asthma and rhinoconjunctivitis declined during the past decade in schoolchildren in Patras, Greece, whereas the prevalence of eczema continued to increase. Nevertheless, the relationship between rhinoconjunctivitis and asthma remained strong throughout the 27-year surveillance period, whereas the association between eczema and asthma seemed to have weakened. Our spirometric and FeNO measurements in the 2018 survey, despite the small numbers in various subgroups, corroborated these findings and validated our parental questionnaire as an epidemiologic tool in the assessment of respiratory allergies and eczema in Greek schoolchildren.
ACKNOWLEDGMENTS
We thank the children, their parents, and the schoolteachers for their enthusiasm in participating in our surveys, and we are thankful for all our colleagues who have been involved in conducting them in these three decades. We devote this work to the memory of George Russel, Pediatrician, Ph.D., founding member of the Aberdeen asthma and allergies epidemiologic studies, whose generosity and wisdom have been an inspiration to many of us throughout this endeavor.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
No external funding sources reported
Supplemental data available at www.IngentaConnect.com
REFERENCES
- 1. Galassi C, De Sario M, Biggeri A, et al. Changes in the prevalence of asthma and allergies among children and adolescents in Italy: 1994–2002. Pediatrics. 2006; 117:34–42. [DOI] [PubMed] [Google Scholar]
- 2. Devenny A, Wassall H, Ninan T, et al. Respiratory symptoms and atopy in children in Aberdeen: questionnaire studies of a defined school population repeated over 35 years. BMJ. 2004; 329:489–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burr ML, Wat D, Evans C, et al. Asthma prevalence in 1973, 1988 and 2003. Thorax. 2006; 61:296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). Eur Respir J. 1998; 12:315–335. [DOI] [PubMed] [Google Scholar]
- 5. Pearce N, Aït-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007; 62:758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Björkstén B, Clayton T, Ellwood P, et al. Worldwide time trends for symptoms of rhinitis and conjunctivitis: phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008; 19:110–124. [DOI] [PubMed] [Google Scholar]
- 7. Odhiambo JA, Williams H, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC phase three. J Allergy Clin Immunol. 2009; 124:1251–1258.e23. [DOI] [PubMed] [Google Scholar]
- 8. Jackson KD, Howie LJD, Akinbami L. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. 2013; 121:1–8. [PubMed] [Google Scholar]
- 9. Akinbami L, Simon AE, Rossen LM. Changing trends in asthma prevalence. Pediatrics. 2016; 137:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Osman M, Tagiyeva N, Wassall HJ, et al. Changing trends in sex specific prevalence rates for childhood asthma, eczema, and hay fever. Pediatr Pulmonol. 2007; 42:60–65. [DOI] [PubMed] [Google Scholar]
- 11. Barnish MS, Tagiyeva N, Devereux G, et al. Diverging prevalence and different risk factors for childhood asthma and eczema: a cross-sectional study. BMJ Open. 2015; 5:e008446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anthracopoulos MB, Antonogeorgos G, Liolios E, et al. Increase in chronic or recurrent rhinitis, rhinoconjunctivitis and eczema among schoolchildren in Greece: three surveys during 1991–2003. Paediatr Allergy Immunol. 2009; 20:180–6. [DOI] [PubMed] [Google Scholar]
- 13. Anthracopoulos MB, Fouzas S, Pandiora A, et al. Prevalence trends of rhinoconjunctivitis, eczema, and atopic asthma in in Greek schoolchildren: four surveys during 1991–2008. Allergy Asthma Proc. 2011; 32:56–62. [DOI] [PubMed] [Google Scholar]
- 14. Anthracopoulos M, Karatza A, Liolios E, et al. Prevalence of asthma among schoolchildren in Patras, Greece: three surveys over 20 years. Thorax. 2001; 56:569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anthracopoulos MB, Pandiora A, Fouzas S, et al. Sex-specific trends in prevalence of childhood asthma over 30 years in Patras, Greece. Acta Paediatr. 2011; 100:1000–1005. [DOI] [PubMed] [Google Scholar]
- 16. Ntzounas A, Giannakopoulos I, Lambropoulos P, et al. Changing trends in the prevalence of childhood asthma over 40 years in Greece. Pediatr Pulmonol. 2021; 56:3242–3249. [DOI] [PubMed] [Google Scholar]
- 17. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338. [DOI] [PubMed] [Google Scholar]
- 18. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values of spirometry for the 3-95-yr age range: the Global Lung Function 2012 equations. Eur Respir J. 2012; 40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toelle BG, Ng K, Belousova E, et al. Prevalence of asthma and allergy in schoolchildren in Belmont, Australia: three cross sectional surveys over 20 years. BMJ. 2004; 328:386–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McNeill G, Tagiyeva N, Aucott L, et al. Changes in the prevalence of asthma, eczema, and hay fever in pre-pubertal children: a 40-year perspective. Paediatr Perinat Epidemiol. 2009; 23:506–512. [DOI] [PubMed] [Google Scholar]
- 22. Hicke-Roberts A, Åberg N, Wennegren G, et al. Allergic rhinoconjunctivitis continued to increase in Swedish children up to 2007, but asthma and eczema levelled off from 1991. Acta Paediatr. 2017; 106:75–80. [DOI] [PubMed] [Google Scholar]
- 23. Henriksen L, Simonsen J, Haerskjold A, et al. Incidence rates of atopic dermatitis, asthma, and allergic rhinoconjunctivitis in Danish and Swedish children. J Allergy Clin Immunol. 2015; 136:360–366.e2. [DOI] [PubMed] [Google Scholar]
- 24. Profita M, La Grutta S, Carpagnano E, et al. Noninvasive methods for the detection of upper and lower airway inflammation in atopic children. J Allergy Clin Immunol. 2006; 118:1068–1074. [DOI] [PubMed] [Google Scholar]
- 25. Zinelli C, Caffarelli C, Strid J, et al. Measurement of nitric oxide and 8-isoprostane in exhaled breath of children with atopic eczema. Clin Exp Dermatol. 2009; 34:607–612. [DOI] [PubMed] [Google Scholar]
- 26. Licari A, Castagnoli R, Denicolò CF, et al. The nose and the lung: united airway disease. Front Pediatr. 2017; 5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Skylogianni E, Triga M, Douros K, et al. Small-airway dysfunction precedes the development of asthma in children with allergic rhinitis. Allergol Immunopathol (Madr). 2018; 46:313–321. [DOI] [PubMed] [Google Scholar]
- 28. Paller AS, Spergel JM, Mina-Osorio P, et al. The atopic march and atopic multimorbidity: many trajectories, many pathways. J Allergy Clin Immunol. 2019; 143:46–55. [DOI] [PubMed] [Google Scholar]
- 29. Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017; 10:1076–1083. [DOI] [PubMed] [Google Scholar]
- 30. Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiological study. Ann Allergy Asthma Immunol. 2021; 126:417–428.e2. [DOI] [PubMed] [Google Scholar]
- 31. Yum HY, Ha EK, Shin YH, et al. Prevalence, comorbidities, diagnosis, and treatment of nonallergic rhinitis: real-world comparison with allergic rhinitis. Clin Exp Pediatr. 2021; 64:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gauderman WJ, Urman R, Avol E, et al. Association of improved air quality with lung development in children. N Engl J Med 2015; 372:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fowler D, Brimblecombe P, Burrows J, et al. A chronology of global air quality. Philos Trans A Math Phys Eng Sci. 2020; 378:20190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valavanidis A, Vlachogianni T, Loridas S, et al. Atmospheric pollution in urban areas in Greece and economic crisis. Trends in air quality and atmospheric pollution data, research and adverse health effects. Available online at https://www.researchgate.net/publication/284415702_Atmospheric_Pollution_in_Urban_Areas_of_Greece_and_Economic_Crisis_Trends_in_Air_Quality_and_Atmospheric_Pollution_Data_Research_and_Adverse_Health_Effects/link/5652e16c08aeafc2aabac950/download; accessed September 29, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.