Abstract
Background
Cocaine dependence is a public health problem characterised by recidivism and a host of medical and psychosocial complications. Cocaine dependence remains a disorder for which no pharmacological treatment of proven efficacy exists.
Objectives
To evaluate the efficacy and the acceptability of antipsychotic medications for cocaine dependence.
Search methods
This review is an update of a previous Cochrane review published in 2007. We searched up to 15 July 2015 in Cochrane Drugs and Alcohol Group Specialised Register (searched in CRSLive); the Cochrane Library (including the Cochrane Central Register of Controlled Trials (CENTRAL); the Database of Abstracts of Reviews of Effects (DARE)); PubMed; EMBASE; CINAHL and Web of Science. All searches included non‐English language literature.
Selection criteria
All randomised controlled trials and controlled clinical trials with focus on the use of any antipsychotic medication for the treatment of cocaine dependence.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
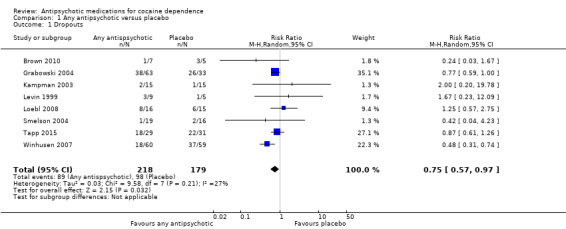
We included 14 studies (719 participants). The antipsychotic drugs studied were risperidone, olanzapine, quetiapine, lamotrigine, aripiprazol, haloperidol and reserpine. Comparing any antipsychotic drugs versus placebo, we found that antipsychotics reduced dropout: eight studies, 397 participants, risk ratio (RR) 0.75 (95% confidence interval (CI) 0.57 to 0.97), moderate quality of evidence. We found no significant differences for any of the other primary outcomes considered: number of participants using cocaine during the treatment, two studies, 91 participants: RR 1.02 (95% CI 0.65 to 1.62); continuous abstinence, three studies, 139 participants: RR 1.30 (95% CI 0.73 to 2.32); side effects, six studies, 291 participants: RR 1.01 (95% CI 0.93 to 1.10); and craving, four studies, 240 participants: RR 0.13 (‐1.08 to 1.35). For all of these comparisons we rated the quality of evidence as low.
Comparisons of single drug versus placebo or versus another drug are conducted in few trials with small sample sizes, limiting the reliability of the results. Among these comparisons, only quetiapine seemed to outperform placebo in reducing cocaine use, measured by grams per week: mean difference (MD) ‐0.54 (95% CI ‐0.92 to ‐0.16), by US dollars spent per week: MD ‐53.80 (95% CI ‐97.85 to ‐9.75), and by craving: MD ‐1.23 (95% CI ‐2.19 to ‐0.27), but results came from one study with 60 participants.
The major limitations of the studies were the high risk of attrition bias (40% of the included studies) and low quality of reporting, mainly for the risk of selection bias, performance and detection bias, that we rated as being at unclear risk for 75% to 80% of the studies. Furthermore, most of the included studies did not report results on important outcomes such as side effects, or use of cocaine during treatment and craving, which prevented the possibility of including them in statistical synthesis.
Authors' conclusions
At present, there is no evidence supporting the clinical use of antipsychotic medications in the treatment of cocaine dependence, although results come from only 14 trials, with small sample sizes and moderate to low quality of evidence.
Keywords: Humans, Antipsychotic Agents, Antipsychotic Agents/therapeutic use, Aripiprazole, Aripiprazole/therapeutic use, Benzodiazepines, Benzodiazepines/therapeutic use, Cocaine‐Related Disorders, Cocaine‐Related Disorders/drug therapy, Haloperidol, Haloperidol/therapeutic use, Lamotrigine, Olanzapine, Patient Dropouts, Patient Dropouts/statistics & numerical data, Quetiapine Fumarate, Quetiapine Fumarate/therapeutic use, Randomized Controlled Trials as Topic, Reserpine, Reserpine/therapeutic use, Risperidone, Risperidone/therapeutic use, Triazines, Triazines/therapeutic use
Plain language summary
Antipsychotic medications for cocaine dependence
Background
Cocaine dependence is often associated with medical, psychological and social problems for individual and public health, generating problems for the community. Users play a role in the spread of infectious diseases such as AIDS, hepatitis and tuberculosis, as well as in crime, violence and neonatal drug exposure. Use of drugs such as antidepressants, anticonvulsants and dopamine agonists to treat cocaine abuse or dependence is not supported by evidence from Cochrane reviews. The use of antipsychotic agents has also been considered, particularly because cocaine can induce hallucinations and paranoia that mimic psychosis.
Study characteristics
The review authors identified 14 randomised controlled trials involving 719 adults. One study was conducted in Italy, and the rest in the USA. They involve both inpatient and outpatient settings and had a duration of 14 to 168 days (mean 80 days). Eleven trials randomised participants to receive an antipsychotic drug or placebo using the following antipsychotic medications: risperidone (three studies, 1 to 4 mg/day and one study with injections of long‐acting risperidone at a dose of 25 mg/14 days); olanzapine (three studies, 2.5 to 20 mg/day); quetiapine (two studies, 400 and 800 mg/day); lamotrigine (one study, 400 mg/day); reserpine (one study, 50 mg/day). Three trials compared two drugs; olanzapine (10 mg/day) versus haloperidol (10 mg/day), olanzapine (20 mg/day) versus risperidone (9 mg/day) and aripiprazol (10 mg/day) versus ropirinol (4.5 mg/day).
Key results
The studies used different instruments or ways to assess the outcomes of interest, limiting the possibility for us to combine the data. When we grouped together all trial results comparing any antipsychotic drug to placebo, we found that antipsychotics slightly increase those who stayed in treatment but they were not effective in reducing cocaine use during treatment (two studies), in sustained abstinence (three studies), or in reducing the urge to consume cocaine (four studies). The single comparisons of each drug versus placebo or versus another drug were made in few trials with small sample sizes, limiting the reliability of the results. However, among these comparisons, only quetiapine seemed to perform better than placebo in reducing cocaine use and craving, but results came only from one study with 60 participants. Information was limited on the acceptability of treatment in terms of side effects, abstinence from cocaine use and withdrawal symptoms. Overall we found no evidence supporting the clinical use of antipsychotic medications in the treatment of cocaine dependence.
Quality of the evidence
The major limitations of the studies were the high number of people who withdrew from them and the lack of clear reporting of the methods used to conduct the studies. Moreover, the number of participants was small, and different ways of measuring and reporting results were used, limiting the possibility for us to combine the data. Overall we judged the quality of the evidence to be moderate for dropouts and low for all the other outcomes considered. The evidence is current up to 15 of July 2015.
Funding and conflict of interest reported by the studies
The majority of trials included in this review had funding from industrial sources or declared conflict of interests for some of the researchers due to different contractual collaborations with the pharmaceutical industry. Only five of the 14 included trials reported being funded exclusively by non‐industry sources, and of these just one (Grabowski 2004) disclosed no conflict of interest for the authors. Another study (Brown 2012) reported conflicts of interest for several authors and three studies (Levin 1999, Reid 2005 and Winhusen 2007) did not disclose conflict of interest of the authors. One included trial (Meini 2010) did not report information about funding sources, but disclosed no conflict of interest for the authors. The other eight studies included in this review were either funded by industry (Brown 2010; Hamilton 2009; Kampman 2003), or by a combination of industry and non‐industry grants (Akerele 2007; Loebl 2008; Smelson 2004; Smelson 2006; Tapp 2015), with three (Brown 2010; Hamilton 2009; Kampman 2003) disclosing conflicts of interests for the authors, and the rest without declaration on this issue.
Summary of findings
Summary of findings for the main comparison. Any antipsychotic versus placebo for cocaine dependence (Update).
| Any antipsychotic versus placebo for cocaine dependence | ||||||
| Patient or population: people with cocaine dependence Settings: outpatients or inpatients Intervention: Any antipsychotic versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any antipsychotic versus placebo | |||||
| Dropouts Number of participants who dropped out from the study Follow‐up: mean 12 weeks | Study population | RR 0.75 (0.57 to 0.97) | 397 (8 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 547 per 1000 | 411 per 1000 (312 to 531) | |||||
| Moderate | ||||||
| 500 per 1000 | 375 per 1000 (285 to 485) | |||||
| Side effects Number of participants with at least i side effect Follow‐up: mean 12 weeks | Study population | RR 1.01 (0.93 to 1.10) | 291 (6 studies) | ⊕⊕⊝⊝ low2 | ||
| 497 per 1000 | 502 per 1000 (462 to 546) | |||||
| Moderate | ||||||
| 465 per 1000 | 470 per 1000 (432 to 512) | |||||
| Number of participants using cocaine during the treatment (as days/week by urine tests or self report) Number of participants that reported the use of cocaine during the treatment Follow‐up: mean 10 weeks | Study population | RR 1.02 (0.65 to 1.62) | 91 (2 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 478 per 1000 | 488 per 1000 (311 to 775) | |||||
| Moderate | ||||||
| 596 per 1000 | 608 per 1000 (387 to 966) | |||||
| Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks) Number of participants that maintained negative cocaine screens for at least 2 ‐ 3 weeks Follow‐up: mean 12 weeks | Study population | RR 1.30 (0.73 to 2.32) | 139 (3 studies) | ⊕⊕⊝⊝ low1,5 | ||
| 197 per 1000 | 256 per 1000 (144 to 457) | |||||
| Moderate | ||||||
| 129 per 1000 | 168 per 1000 (94 to 299) | |||||
| Craving (Brief Substance Craving Scale) Brief Substance Craving Scale. Scale from: 0 to 4. Follow‐up: mean 11 weeks | The mean craving (brief substance craving scale) in the control groups was 2.39 score | The mean craving (Brief Substance Craving Scale) in the intervention groups was 0.13 higher (1.08 lower to 1.35 higher) | 240 (4 studies) | ⊕⊕⊝⊝ low6,7 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1All the studies were at unclear risk of selection bias. 2One study was at high risk of selection bias, and the others at unclear risk. One study was at high risk of performance, detection bias and attrition bias, three at unclear risk. 3All the studies were at unclear risk of selection bias; one study was at unclear risk of performance and detection bias. 4Only two studies with 91 participants. 5Only three studies with 139 participants. 6All the studies were at unclear risk of selection, performance and attrition bias. One study was at high risk of attrition bias. 7High heterogeneity (I²: 85%).
Background
Description of the condition
Cocaine is an alkaloid derived from the coca leaf, being commonly used as powder, for intranasal or intravenous use, or as crack, a free‐base form which is normally smoked. Cocaine dependence is a major public health problem, characterised by recidivism and a host of medical and psychosocial complications (EMCDDA 2014a).
There is a wide and well‐documented range of consequences associated with acute and chronic use of this drug, such as medical, psychological and social problems, including the spread of infectious diseases (e.g. AIDS, hepatitis and tuberculosis), criminal behaviour, violence and neonatal drug exposure (Higgins 1994). Both injection and non‐injection cocaine use are thought to increase the risk of HIV infection through high‐risk injecting and sexual behaviours (Sorensen 1991).
The illicit use of cocaine has become a persistent health problem worldwide. According to the estimates of the World Drug Report 2015 (UNDOC 2015) about 0.4 % of the global population uses cocaine, and in recent European national population surveys between 0.3% and 9% of the adult population report having tried cocaine at least once (i.e. lifetime prevalence), with Ireland (6.8%), Spain (8.8%) and the United Kingdom (9%) being at the upper end of this range. Recent cocaine use (last 12 months) is, in general, reported by less than 1% of adults; in most countries, the range is between 0.1% and 1%. After a peak in 2008, a decline in cocaine use has been observed in almost all countries, including those reporting high prevalence rates. In Spain and the United Kingdom recent prevalence rates are still around 2%. Although cocaine prevalence figures are much lower than comparable figures for cannabis, levels of use among younger adults can be higher than the population average. Lifetime experience among 15‐ to 34‐year‐olds ranges from 0.7% to 11.9%, with the highest levels again being found in Spain (11.1%) and the United Kingdom (11.9%). Recent use ranges from 0.2% to 3.6%, with Denmark, Italy and the Netherlands all having rates of about 2%; Spain and the United Kingdom over 3% and 3.6% respectively (EMCDDA 2014b). In the USA in 2013, an estimated 1.5 million people (0.6%) were current cocaine users (NSDUH 2014).The number of people entering treatment for the first time in their life for primary cocaine use has been decreasing in recent years in countries with traditionally high prevalence, from a peak of 38,000 in 2008 to 24,000 in 2013 (EMCDDA 2015). Differences exist between countries, with more than 70% of all cocaine users being reported by only three European countries: Spain, Italy, and the United Kingdom (EMCDDA 2015).
Description of the intervention
Although effective pharmacotherapy is available for alcohol and heroin dependence (Amato 2010; Faggiano 2003; Mattick 2014; Minozzi 2010; O'Brian 2001), none exists currently for cocaine dependence despite more than two decades of clinical trials primarily involving antidepressant, anticonvulsant and dopaminergic medications.
Cocaine effect seems to rely on its ability to increase the availability of monoamines (dopamine, serotonin and noradrenaline) in the brain. The dopamine increase in specific areas of the mesolimbic system, which is shared by cocaine with other drugs like heroin, alcohol, cannabis and nicotine, has been involved in rewarding effect of drugs and self‐administration behaviour in animals and humans (Di Chiara 1988; Drevets 1999; Drevets 2001; Volkow 2003).
There has been extensive research of optimal pharmacological approaches to the treatment of cocaine dependence, with consideration of both dopamine antagonists and agonists (Grabowski 1997; Kosten 1996).
Four Cochrane reviews have been published on the efficacy of antidepressants (Pani 2011), carbamazepine (Lima Reisser 2009), dopamine agonists (Minozzi 2015) and psychostimulants (Castells 2010) for the treatment of cocaine dependence, but none of them found clear support for the efficacy of these treatments.
Cocaine dependence remains a disorder for which no pharmacological treatment of proven efficacy exists, although considerable advances in the neurobiology of this addiction could guide future medication development.
How the intervention might work
Antipsychotics have been candidates for the treatment of addiction for their ability to block dopamine receptors and counterbalance the increase in dopaminergic activity related to drugs' effects. However, while the short‐term effect of cocaine is associated with dopamine increase in definite brain areas, acute and protracted withdrawal from this drug is associated with diminished dopaminergic neurotransmission. This reduced dopaminergic tone may underlie impaired hedonic function and increased craving, so maintaining the addictive behaviour (Dackis 2002; Kuhar 1996). On this basis, the supposed efficacy of dopamine antagonists in cocaine addiction could be questionable, since their use could even further reduce dopamine tone. While these observations apply well to classical neuroleptics, which exert their action essentially through the dopaminergic system, the so‐called 'atypical' ones, like risperidone and olanzapine, extend their action to other brain systems which have been involved in drug addiction. Particularly, their action on the serotoninergic system has been regarded with interest, given the involvement of serotonin neurotransmission in addictive behaviour (Filip 2005). The use of atypical neuroleptics in cocaine addiction has also been criticised due to their antagonist effect on dopamine receptors. However, their interference with the serotoninergic system and a less severe side‐effect profile (Berk 1999; Leucht 1999) could improve the compliance of patients and promote their retention in treatment. These assumptions would support the use of atypical neuroleptics such as olanzapine and aripripazol in the treatment of cocaine addiction.
Furthermore, cocaine use can lead to symptoms mimicking psychosis, such as hallucinations and paranoia, and the use of antipsychotics may relieve these symptoms. Some of the antipsychotics more commonly studied for this purpose are, for example, haloperidol, olanzapine, quetiapine, clozapine, risperidone and lamotrigine.
Why it is important to do this review
This review is an update of the original Cochrane review (Amato 2007). The original version on the efficacy of antipsychotic agents for the treatment of cocaine dependence did not find evidence supporting the clinical use of antipsychotic medications in the treatment of cocaine dependence.
This update reviews the current state of scientific evidence for the efficacy of antipsychotic pharmacotherapy for the treatment of cocaine dependence.
Objectives
To evaluate the efficacy and the acceptability of antipsychotic medications for cocaine dependence.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials and controlled clinical trials which focus on the use of any antipsychotic medication for cocaine dependence.
Types of participants
Cocaine‐dependent people as diagnosed by the Diagnostic and Statistical Manual of Mental Disorder (DSM‐IV‐R) or by specialists, since all trials have been performed prior to the publication of the DSM‐V Manual. Trials including participants with additional diagnoses of substance dependence were also eligible. We exclude trials in people under 18 years of age and in pregnant women, because of the substantially different approach to the clinical management of these people. People with comorbid mental health conditions were included and considered in a subgroup analysis.
Types of interventions
Experimental intervention:
Any antipsychotic medication, alone or in combination with any psychosocial intervention.
Control Intervention
Placebo
No intervention
Other pharmacological interventions
Any psychosocial intervention
Types of outcome measures
Primary outcomes
Dropouts from treatment, defined as the number of participants who did not complete the treatment
Acceptability of the treatment, defined as the number and type of side effects experienced during the treatment
Use of primary substance of abuse, defined as the number of participants that reported the use of cocaine during the treatment, and/or the number of participants with urine samples positive or negative for cocaine
Results at follow‐up, defined as the number of participants using cocaine at follow‐up
Secondary outcomes
Compliance
Craving as measured by validated scales, e.g. Brief Substance Craving Scale (BSCS), Visual Analogue Scale (VAS)
Severity of dependence as measured by validated scales, e.g. Addiction Severity Index (ASI), Clinical Global Impression scale (CGI‐S), Clinical Global Impression ‐ Observer Scale (CGI‐O)
Amount of cocaine use, as measured by grams used or money spent
Psychiatric symptoms/psychological distress, diagnosed using standard criteria, e.g. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) criteria, or measured by validated scales, e.g. Hamilton Depression Scale (HAM‐D), Profile of Mood States Scale (POMSS), Positive and Negative Syndrome Scale (PANSS)
Withdrawal symptoms, measured using validated scales such as the Cocaine Selective Severity Assessment (CSSA)
Search methods for identification of studies
Electronic searches
We have listed the search methods we used in the original review (Amato 2007) in Appendix 1
For the update performed up to 15 July 2015, we searched the following databases:
Cochrane Drugs and Alcohol Group (CDAG) Specialised Register (searched July 2015) using the search strategy outlined in Appendix 2;
Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 7) using the search strategy outlined in Appendix 3;
MEDLINE (PubMed) (October 2006 to July 2015) using the search strategy outlined in Appendix 4;
EMBASE (Elsevier, EMBASE.com) (October 2006 to July 2015) using the search strategy outlined in Appendix 5;
CINAHL (EBSCO HOST) (October 2006 to July 2015)) using the search strategy outlined in Appendix 6;
Web of Science (Thomson Reuters) (January 2006 to January 2015) using the search strategy outlined in Appendix 7.
Searching other resources
We also searched:
The reference lists of all relevant papers to identify further studies
Some of the main electronic sources of ongoing trials (National Research Register, meta‐Register of Controlled Trials, ClinicalTrials.gov)
We contacted investigators to request information about unpublished or incomplete trials.
All searches included non‐English language literature, and we assessed studies with English abstracts for inclusion. We had studies translated where we considered that they were likely to meet the inclusion criteria.
Data collection and analysis
Selection of studies
For the first review, one review author (LA) inspected the search hits by reading titles and abstracts. We obtained each potentially relevant study located in the search in full text, and two review authors (SM, LA) independently assessed them for inclusion. We resolved disagreements by discussion between all the review authors.
For the present update, two review authors (BII, SM) inspected the search hits by reading titles and abstracts. We obtained each potentially relevant study located in the search in full text, and two review authors (BII, SM) independently assessed them for inclusion. We resolved disagreements by discussion between all the review authors.
Data extraction and management
Two review authors (SM, BII) independently extracted data . In case of missing data about declared outcomes in the full article, we also consulted the information reported in the Clinical Trials Registry of the US National Institutes of Health (ClinicalTrials.gov). We resolved any disagreement by discussion. We summarised key findings narratively in the first instance, and assessed them for meta‐analysis where possible.
We extracted the following data from the identified publications:
Year of publication
Country
Inclusion and exclusion criteria
Mean characteristics of participants (age, sex, other substances of abuse, comorbidity)
Experimental and control treatment
Outcomes assessed
Duration of the study
Assessment of risk of bias in included studies
Two review authors (BII, SM) independently assessed risk of bias of the included studies.
We conducted the 'Risk of bias' assessment using the criteria recommended by the Cochrane Handbook (Higgins 2011). The recommended approach for assessing risk of bias in studies included in Cochrane reviews is a two‐part tool, addressing specific domains, namely sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias) and selective outcome reporting (reporting bias). The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement related to the risk of bias for that entry, in terms of low, high or unclear risk. To make these judgements we used the criteria indicated by the Handbook, adapted to the addiction field. See Appendix 8 for details.
We addressed the domains of sequence generation and allocation concealment (avoidance of selection bias) in the tool by a single entry for each study.
We considered blinding of participants and of outcome assessors (avoidance of detection bias) separately for objective outcomes (e.g. dropouts, abstinence measured by urinanalysis, participants relapsed at the end of follow‐up) and for subjective outcomes (e.g. duration and severity of signs and symptoms of withdrawal, craving, participant self‐reported use of substances, side effects, psychiatric symptoms, clinical global evaluation).
We assessed incomplete outcome data (avoidance of attrition bias) for all outcomes except for the dropout rates from the treatment, which is very often the primary outcome measure in trials on addiction; see Characteristics of included studies for a detailed description of how we assessed the risks of bias in this review.
Grading of evidence
We assessed the overall quality of the evidence for the primary outcome using the GRADE system. The GRADE Working Group developed a system for grading the quality of evidence (GRADE 2004; Guyatt 2008; Guyatt 2011; Schünemann 2006) which takes into account issues not only related to internal validity but also to external validity, such as directness of results. The 'Summary of findings' tables present the main findings of a review in a transparent and simple tabular format. In particular, they provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grades of evidence:
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
Grading is decreased for the following reasons:
Serious (‐1) or very serious (‐2) limitation to study quality.
Important inconsistency (‐1).
Some (‐1) or major (‐2) uncertainty about directness.
Imprecise or sparse data (‐1).
High probability of reporting bias (‐1).
Grading is increased for the following reasons:
Strong evidence of association ‐ significant relative risk of > 2 (< 0.5) based on consistent evidence from two or more observational studies, with no plausible confounders (+1).
Very strong evidence of association ‐ significant relative risk of > 5 (< 0.2) based on direct evidence with no major threats to validity (+2).
Evidence of a dose‐response gradient (+1).
All plausible confounders would have reduced the effect (+1).
Measures of treatment effect
We analysed dichotomous outcomes by calculating the risk ratio (RR) for each trial with uncertainty in each result expressed by 95% confidence intervals (CIs). We analysed continuous outcomes by calculating the mean difference (MD) with a 95% CI when studies used the same instrument to assess the outcome. We used the standardised mean difference (SMD) when studies used different instruments. For craving score, severity of dependence (Drug ASI, CGI‐O, depression (HAM‐D) and anxiety (Hamilton Anxiety Scale (HAM‐A)), we compared the postintervention mean scores of the experimental and control groups. Meta‐analysis of continuous outcomes of the old studies of ASI, CGI‐O, HAM‐D and HAM‐A had to be redone for postintervention outcomes, because the previous type of analysis comparing before‐and‐after changes was incorrect.
Unit of analysis issues
We have not used data presented as the number of positive urine tests over the total number of tests in the experimental and control group as a measure of substance abuse. This is because using tests instead of the participants as the unit of analysis violates the hypothesis of independence among observations. In fact, the results of tests done in each participant are not independent.
If there had been cross‐over trials to include, we would have considered only the results of the first phase of the study.
Assessment of heterogeneity
We analysed heterogeneity by using the I² statistic and the Chi² test. Cut‐off points included an I² value greater than 50% and a P value for the Chi² test less than 0.1.
Assessment of reporting biases
We planned to use a funnel plot (plotting the effect from each study against the sample size or effect standard error) to assess the potential for bias related to the size of the trials, which could indicate possible publication bias. However this was not possible because less than ten trials were included in the analyses
Data synthesis
We combined outcomes from the individual trials through meta‐analysis when possible (comparability of interventions and outcomes between trials), using a random‐effects model, because we expected some degree of heterogeneity among trials.
Subgroup analysis and investigation of heterogeneity
We first compared any antipsychotic versus placebo. We then performed subgroup analyses for single types of antipsychotics.
Sensitivity analysis
To incorporate our assessment of risk of bias into the review process, we first plotted the intervention effect estimates stratified for risk of selection bias. If we had found differences in results among studies at different risks of bias, we planned to perform sensitivity analysis by excluding from the analysis those studies at high risk of bias. We did not conduct these analyses, because we found no studies at high risk of selection bias. Neither did we conduct sensitivity analysis excluding studies with inadequate allocation concealment, because only one of the included studies had inadequate allocation concealment, and it was not included in meta‐analysis
Results
Description of studies
Results of the search
In the original review (Amato 2007), the bibliographic searches identified 97 reports; we excluded 80 studies on the basis of title and abstract, and retrieved 17 articles in full text, 8 of which we excluded, two were awaiting assessment and seven satisfied all the criteria to be included in the review.
For the present update, we identified 246 reports after removing duplicates, of which we excluded 221 on the basis of title and abstract; we retrieved 25 articles in full text for more detailed evaluation, 14 of which we excluded, and 11 articles (eight studies) satisfied all the criteria to be included in the review. SeeFigure 1.
1.
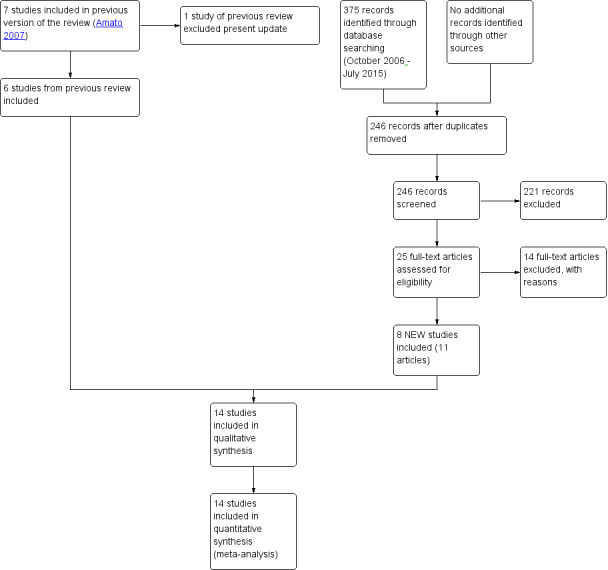
Study flow diagram. Review update 2015.
We excluded from the review one study which was included in the first version (Berger 1996). For substantive descriptions of studies see 'Characteristics of included studies' and 'Characteristics of excluded studies' tables.
Included studies
We include 14 studies (719 participants); six from the original review and eight studies from the update.
Duration of trials: The mean duration of the trials was 80 days (range 14 to 168 days).
Treatment regimens and setting: Thirteen studies were conducted in the USA and one in Italy. The antipsychotic medication used in the included studies were:
Risperidone: five studies (Akerele 2007; Grabowski 2004; Levin 1999; Loebl 2008; Smelson 2004), mean dose for four of the studies 2.27 mg/day (range 1 to 4 mg) and one study with injections of long‐acting risperidone at a dose of 25 mg/14 days;
Olanzapine: five studies (Akerele 2007; Hamilton 2009; Kampman 2003; Reid 2005; Smelson 2006), mean dose 14 mg/day (range 2.5 to 20 mg/day);
Haloperidol: one study (Smelson 2006), using a target dose of 10 mg/day;
Quetiapine: two studies (Brown 2010; Tapp 2015), using doses of 400 ‐ 800 and 400 mg/day respectively;
Lamotrigine: one study (Brown 2012), using a dose of 400 mg/day;
Reserpine: one study (Winhusen 2007), using a dose of 50 mg/day;
Aripiprazol: one study (Meini 2010), using a dose of 10 mg/day.
Twelve studies were conducted in the outpatient setting, two studies in an inpatient .
Participants: The studies covered 719 cocaine‐dependent participants; 469 from nine studies according to DSM‐IV criteria (DSM‐IV‐R) and 250 from five studies with different diagnostic criteria: 142 participants from two studies based on a reference to a previous diagnosis of dependence and recent use of cocaine; 28 participants from a study selected according to ICD‐9‐CM criteria; 20 diagnosed for another study using the Mini‐International Neuropsychiatric Interview (MINI); and one study with 30 participants requiring certification by a psychiatrist); 477/719 (66.3%) were men, but two studies (Smelson 2004; Smelson 2006) did not report data on gender; the mean age was 41.5 years.
Rating instruments used in the studies:
Acceptability of the treatment:
Side effects:
Abnormal Involuntary Movements Scale (NIMH 1988) used by Akerele 2007
Simpson‐Angus Scale (Simpson 1970) used by Akerele 2007
Craving:
Brief Substance Craving Scale (Somoza 1995) used by Kampman 2003, Reid 2005, Tapp 2015 and Winhusen 2007
Cocaine Craving Questionnaire (Tiffany 1993) used by Reid 2005, Brown 2010,Brown 2012 and Hamilton 2009
Visual Analogue Scale (McCormack 1988) used by Levin 1999 and Meini 2010
Voris Cocaine Craving Questionnaire (Smelson 1999) used by Smelson 2004 and Smelson 2006
Cocaine Craving Report (Weddington 1990) used by Akerele 2007
Cocaine Craving Scale (Halikas 1991) used by Loebl 2008
Use of cocaine:
Timeline Followback Interview (Sobell 1992) used by Brown 2012, Reid 2005, Tapp 2015 and Winhusen 2007
Severity of dependence:
Addiction Severity Index (McLellan 1992) used by Akerele 2007, Brown 2012, Grabowski 2004, Hamilton 2009, Kampman 2003, Loebl 2008, Reid 2005, and Winhusen 2007
Clinical Global Impression Scale (Guy 1976) used by Akerele 2007, Meini 2010 and Reid 2005
Psychiatric symptoms/psychological distress:
Anxiety
Hamilton Anxiety Rating Scale (Hamilton 1959) used by Kampman 2003 and Reid 2005
Depression
Hamilton Depression Rating Scale (Hamilton 1967) used by Akerele 2007, Brown 2012, Kampman 2003, Loebl 2008, Reid 2005, and Winhusen 2007
Quick Inventory of Depressive Symptomatology‐SR (Rush 2003) used by Brown 2010 and Brown 2012
Beck Depression Inventory (Beck 1996) used by Grabowski 2004
Psychopathology
Positive and Negative Syndrome Scale (Kay 1992) used by Akerele 2007 and Smelson 2006
Snaith‐Hamilton Pleasure Scale (Snaith 1995) used by Loebl 2008
Young Mania Rating Scale (Young 1978) used by Brown 2010 and Brown 2012
Withdrawal symptoms:
Cocaine Selective Severity Assessment (Kampman 1998) used by Kampman 2003 and Loebl 2008
Comparisons:
Any antipsychotic versus placebo: eight studies, 430 participants (Brown 2010; Grabowski 2004; Kampman 2003; Levin 1999; Loebl 2008; Smelson 2004; Tapp 2015; Winhusen 2007)
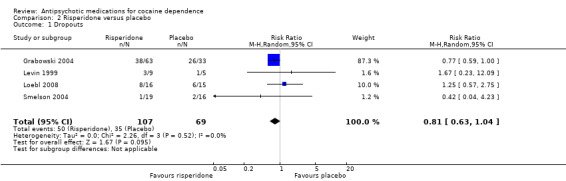
Risperidone versus placebo: four studies, 176 participants (Grabowski 2004; Levin 1999; Loebl 2008; Smelson 2004)
Olanzapine versus placebo: three studies, 146 participants (Hamilton 2009; Kampman 2003; Reid 2005)
Quetiapine versus placebo: two studies, 72 participants (Brown 2010; Tapp 2015)
Lamotrigine versus placebo: one study, 112 participants (Brown 2012)
Reserpine versus placebo: one study, 119 participants (Winhusen 2007)
Olanzapine versus haloperidol: one study, 31 participants (Smelson 2006)
Olanzapine versus risperidone: one study, 28 participants (Akerele 2007)
Aripiprazol versus ropirinole: one study, 28 participants (Meini 2010)
Grabowski 2004 has three arms, comparing risperidone 2 mg and 4 mg versus placebo; we have used the 33 participants in the placebo arm in both Analysis 1.1 and Analysis 2.1.
1.1. Analysis.
Comparison 1 Any antipsychotic versus placebo, Outcome 1 Dropouts.
2.1. Analysis.
Comparison 2 Risperidone versus placebo, Outcome 1 Dropouts.
Excluded studies
Twenty‐four studies did not meet the criteria for inclusion. The grounds for exclusion were the following: study design: ; objective of the studies and outcomes measures: 16 studies (Evans 2001; Farren 2000; Haney 2011; Price 1997; Sherer 1988; Ersche 2010; Lile 2008; Lile 2011; Lofwall 2014; Máñez 2010; Netjek 2008; Middleton 2009; Nuzzo 2012; Rush 2009; Stoops 2007; Landabaso 2009); impossible to extract usable data: six studies (Grabowski 2000; Rubio 2006a; Rubio 2006b; Landabaso 2003; Sayers 2005; Tsuang 2002). We now excluded one study (Grabowski 2006), included in the previous version as an ongoing trial which met the inclusion criteria, due to a modification in the study protocol in 2007. The pharmacological intervention had been modified, substituting an antipsychotic drug (aripiprazol) with an antidepressant (citalopram), thus rendering it ineligible for this update. Another study included in the previous version (Berger 1996) was excluded from the update because the outcome did not comply with our inclusion criteria.
Risk of bias in included studies
All the studies were randomised controlled trials.
Allocation
Random sequence generation: we judged five studies to be at low risk of bias (Brown 2012; Kampman 2003; Meini 2010; Tapp 2015; Winhusen 2007). All the other were at unclear risk of bias because no information was provided about the methods followed.
Allocation concealment: Only two studies (Hamilton 2009; Reid 2005) had an adequate allocation concealment. One study (Brown 2012) presented a high risk of bias due to an inadequate concealment of allocation. In all the other studies the concealment of allocation was unclear.
Blinding
Performance bias
Objective outcomes: we judged all the studies to be at low risk of bias, because we considered that lack of blinding was unlikely to bias the outcomes.
Subjective outcomes: we judged only three studies (Brown 2012; Hamilton 2009; Reid 2005) to be at low risk of performance bias. One study (Meini 2010) was open‐label and judged to be at high risk of bias. All the other studies simply stated that they were double‐blind without further description, so we judged them to be at unclear risk.
Detection bias
Objective outcome: we judged all the studies to be at low risk of bias, because we considered that lack of blinding was unlikely to bias the outcomes.
Subjective outcomes: we rated only two studies (Brown 2012; Reid 2005) at low risk of performance bias. One study (Meini 2010) was open‐label and judged to be at high risk of bias . None of the other studies reported any information on this domain, so we judged them to be at unclear risk.
Incomplete outcome data
We judged five studies (Akerele 2007; Meini 2010; Smelson 2006; Tapp 2015; Winhusen 2007) to be at high risk of attrition bias because more than 30% of participants dropped out and there was no imputing of missing data or intention‐to‐treat (ITT) analysis.
One study (Brown 2012) did not report sufficient information for us to evaluate the risk of attrition bias. We rated all the other studies at low risk of attrition bias.
Selective reporting
All but one study (Akerele 2007) reported on the primary outcomes prespecified in the Methods section.
2.
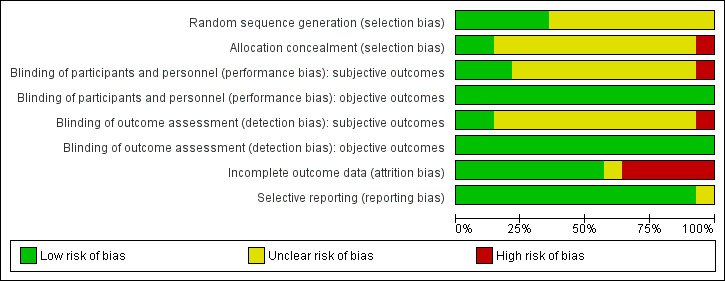
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
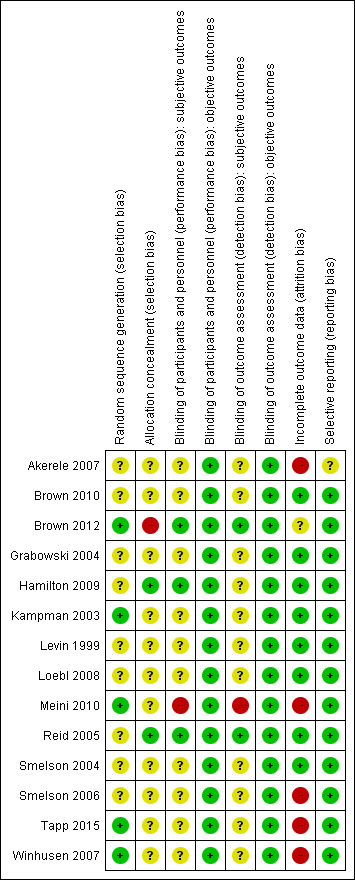
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
We summarised the results, with comparisons of quantitative data where possible, first for any antipsychotic drug versus placebo (see Table 1) and then comparing separately the different types of antipsychotic medications versus placebo, as well as separate analyses for olanzapine versus haloperidol, olanzapine versus risperidone, and aripiprazol versus ropinirol.
For some outcomes reported in the included studies, it was impossible to pool the data due to the different ways of reporting the results. Different rating instruments were used and for many of them the authors did not indicate the scores considered to represent boundaries of mild, moderate and severe, to allow comparison of results between studies.
Primary outcomes
Dropouts from the treatment
Measured as number of participants who did not complete the treatment
(01) Any antipsychotic versus placebo
Eight studies (Brown 2010; Grabowski 2004; Kampman 2003; Levin 1999; Loebl 2008; Smelson 2004; Tapp 2015; Winhusen 2007), 430 participants, see Analysis 1.1 and Table 1: Risk ratio (RR) 0.75 (95% confidence interval (CI) 0.57 to 0.97; I² = 27%); the results favour antipsychotic treatment.
(02) Risperidone versus placebo
Four studies (Grabowski 2004; Levin 1999; Loebl 2008; Smelson 2004), 176 participants, see Analysis 2.1, RR 0.81 (95% CI 0.63 to 1.04). The result shows a small but statistically non‐significant trend in favour of risperidone.
(03) Olanzapine versus placebo
One study (Kampman 2003), 30 participants, RR 2.00 (95% CI 0.20 to 19.78), see Analysis 3.1. No significant difference.
3.1. Analysis.
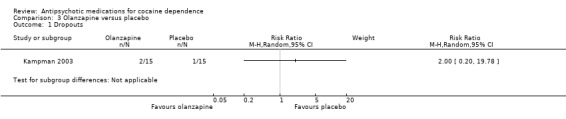
Comparison 3 Olanzapine versus placebo, Outcome 1 Dropouts.
(04) Quetiapine versus placebo
Two studies (Brown 2010; Tapp 2015), 72 participants, RR 0.64 (95% CI 0.20 to 2.03), see Analysis 4.1. No significant difference.
4.1. Analysis.
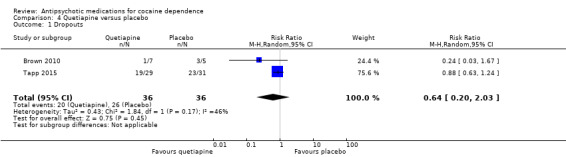
Comparison 4 Quetiapine versus placebo, Outcome 1 Dropouts.
(06) Reserpine versus placebo
One study (Winhusen 2007), 119 participants, RR 0.80 (95% CI 0.48 to 1.34), see Analysis 6.1. No significant difference.
6.1. Analysis.
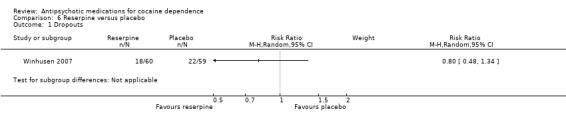
Comparison 6 Reserpine versus placebo, Outcome 1 Dropouts.
(07) Olanzapine versus haloperidol
One study (Smelson 2006), 31 participants, RR 1.50 (95% CI 0.63 to 3.57), see Analysis 7.1. No significant difference.
7.1. Analysis.
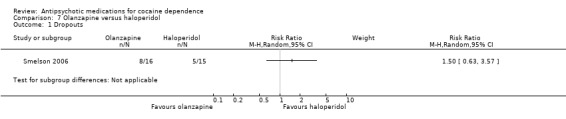
Comparison 7 Olanzapine versus haloperidol, Outcome 1 Dropouts.
(08) Olanzapine versus risperidone
One study (Akerele 2007), 28 participants, RR 2.00 (95% CI 0.78 to 5.14), see Analysis 8.1. No significant difference.
8.1. Analysis.
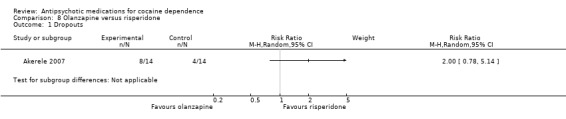
Comparison 8 Olanzapine versus risperidone, Outcome 1 Dropouts.
(09) Aripiprazol versus ropinirol
One study (Meini 2010), 28 participants, RR 1.35 (95% CI 0.61 to 2.99), see Analysis 9.1. No significant difference.
9.1. Analysis.
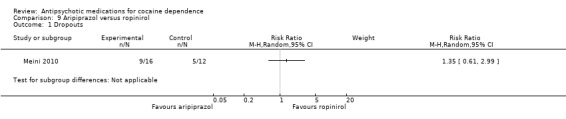
Comparison 9 Aripiprazol versus ropinirol, Outcome 1 Dropouts.
Acceptability of the treatment
Measured as number of participants presenting at least one side effect
(01) Any antipsychotic versus placebo
Six studies (Brown 2010; Brown 2012; Hamilton 2009;Meini 2010; Reid 2005; Tapp 2015), 291 participants, RR 1.01 (95% CI 0.93 to 1.10), see Analysis 1.2 and Table 1; no statistically significant difference.
1.2. Analysis.
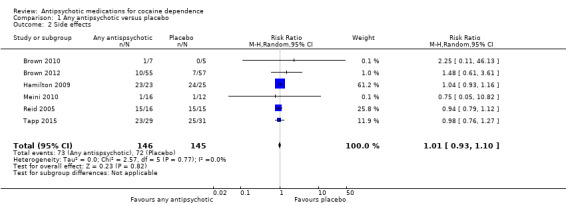
Comparison 1 Any antipsychotic versus placebo, Outcome 2 Side effects.
(03) Olanzapine versus placebo
Two studies (Hamilton 2009; Reid 2005), 79 participants, RR 1.01 (95% CI 0.92 to 1.11), see Analysis 3.2, no significant difference.
3.2. Analysis.
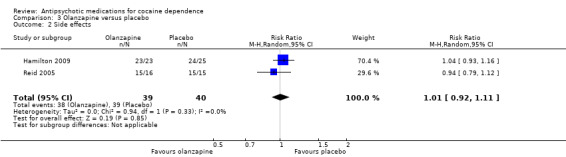
Comparison 3 Olanzapine versus placebo, Outcome 2 Side effects.
One study (Kampman 2003) reported that adverse events were evenly distributed between the olanzapine and placebo groups, without significant differences in the occurrence of any adverse event between the two groups.
(04) Quetiapine versus placebo
Two studies (Brown 2010; Tapp 2015), 72 participants, RR 0.99 (95% CI 0.77 to 1.27), see Analysis 4.2; no significant difference.
4.2. Analysis.
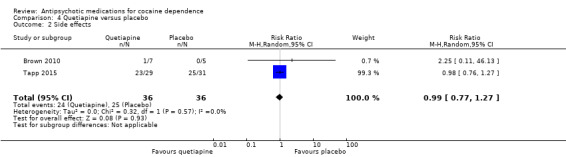
Comparison 4 Quetiapine versus placebo, Outcome 2 Side effects.
(05) Lamotrigine versus placebo
One study (Brown 2012), 112 participants, RR 1.48 (95% CI 0.61 to 3.61), see Analysis 5.1, no significant difference.
5.1. Analysis.
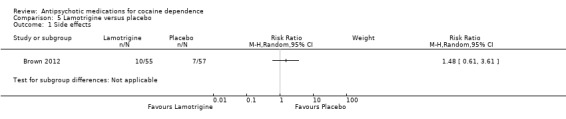
Comparison 5 Lamotrigine versus placebo, Outcome 1 Side effects.
(09) Aripiprazol versus ropinirol One study (Meini 2010), 28 participants, RR 0.75 (95% CI 0.05 to 10.82), see Analysis 9.2, no significant difference.
9.2. Analysis.
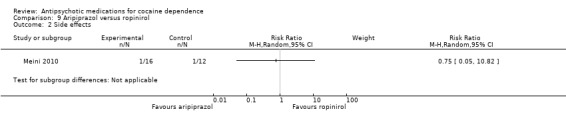
Comparison 9 Aripiprazol versus ropinirol, Outcome 2 Side effects.
Use of primary substance of abuse
(01) Any antipsychotic versus placebo
Measured as the number of participants that reported the use of cocaine during the treatment
Two studies (Reid 2005; Tapp 2015), 91 participants, RR 1.02 (95% CI 0.65 to 1.62), see Analysis 1.3 and Table 1; no significant difference.
1.3. Analysis.
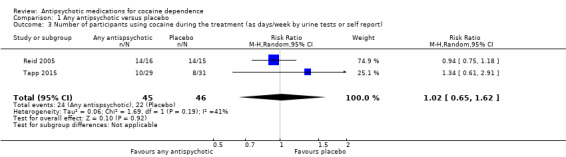
Comparison 1 Any antipsychotic versus placebo, Outcome 3 Number of participants using cocaine during the treatment (as days/week by urine tests or self report).
Measured as continuous abstinence (number of participants that maintained negative cocaine screens for at least 2 ‐ 3 weeks)
Three studies (Hamilton 2009; Reid 2005; Tapp 2015), 139 participants, RR 1.30 (95% CI 0.73 to 2.32), see Analysis 1.4 and Table 1; no significant difference.
1.4. Analysis.
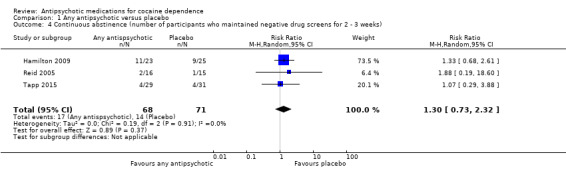
Comparison 1 Any antipsychotic versus placebo, Outcome 4 Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks).
(02) Risperidone versus placebo
Measured as the number of participants that maintained negative cocaine screens throughout the treatment period
One study (Loebl 2008) of 31 participants, reported that five participants in the risperidone group had negative cocaine screens at 50% or more of their visits throughout the treatment period, and that the 11 other participants were abstinent at 28% or fewer of their visits. Only one participant in the placebo group had negative cocaine screens at 50% or more of their visits. The difference was not statistically significant (Fisher exact test = 0.17). Participants in the risperidone group had a mean reduction in days of cocaine use in the preceding 30 days of 51% (standard deviation (SD) 45%), and in the placebo group of 9% (SD 117%). This difference was also not statistically significant.
(03) Olanzapine versus placebo
Measured as the number of participants that reported the use of cocaine during the treatment
One study (Reid 2005) of 31 participants measured self‐reported cocaine use in days/week, and did not find a statistically significant treatment effect, MD 0.80 (95% CI ‐0.61 to 2.21), seeAnalysis 3.3.
3.3. Analysis.
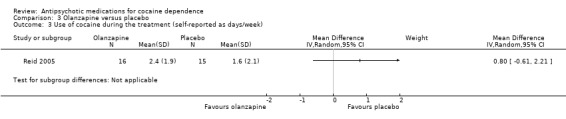
Comparison 3 Olanzapine versus placebo, Outcome 3 Use of cocaine during the treatment (self‐reported as days/week).
A second study (Kampman 2003) measured self‐reported cocaine use in days during the past 30 days in 30 participants, and reported similar results: MD 2.19 (95% CI ‐3.15 to 7.53), seeAnalysis 3.4.
3.4. Analysis.
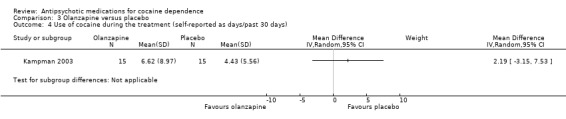
Comparison 3 Olanzapine versus placebo, Outcome 4 Use of cocaine during the treatment (self‐reported as days/past 30 days).
Measured as the number of participants that maintained negative cocaine screens throughout the treatment period
Two studies (Hamilton 2009; Reid 2005), 79 participants, RR 1.37 (95% CI 0.71 to 2.61; I² = 0%) see Analysis 3.5, did not find a statistically significant treatment effect on continuous abstinence.
3.5. Analysis.
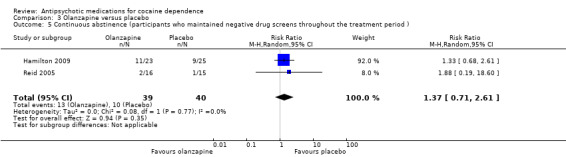
Comparison 3 Olanzapine versus placebo, Outcome 5 Continuous abstinence (participants who maintained negative drug screens throughout the treatment period ).
(04) Quetiapine versus placebo
Measured as the number of participants that reported the use of cocaine during the treatment
One study (Tapp 2015), 60 participants, measured self‐reported cocaine use in days/week. No statistically significant results were reported, MD ‐0.89 (95% CI ‐1.81 to 0.03), see Analysis 4.3.
4.3. Analysis.
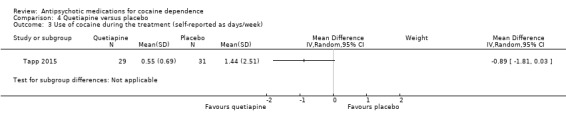
Comparison 4 Quetiapine versus placebo, Outcome 3 Use of cocaine during the treatment (self‐reported as days/week).
Measured as the number of participants that had negative cocaine screens for three consecutive weeks
One study (Tapp 2015), 60 participants, showed no differences in terms of end‐of‐trial abstinence (13.7% in the quetiapine group versus 12.9% in the placebo group (Wald statistic = 0.01, df = 1, P = 0.92)). By the end of the study, 30% of participants who completed the study had achieved this remission, but there was no statistically significant difference in the percentage of change by group.
(05) Lamotrigine versus placebo
One study (Brown 2012) with 112 participants reported, using a declining‐effects random regression model, no differences in treatment effect between groups (F = 1.1, P = 0.31) in the percentage of days of cocaine use as measured by the change from week 1 to week 12.
(06) Reserpine versus placebo
Measured by weekly proportion of self‐reported cocaine non‐use days, confirmed by negative cocaine screens
One study(Winhusen 2007) of 119 participants showed a higher average of non‐use days (7%) than the reserpine group across the treatment period, and found no difference between groups over the treatment period (GEE, P = 0.45).
Results at follow‐up
None of the included studies presented results on follow‐up in a way suitable for use in a meta‐analysis or narrative description.
Secondary outcomes
Compliance
(03) Olanzapine versus placebo
Measured by pill count
One study (Hamilton 2009), 48 participants, reported no significant differences in mean percentage of adherence to prescribed olanzapine (93.4%, SD 9.9%) and prescribed placebo (90.1%, SD 13.1%); these did not significantly differ by one‐way ANOVA (F(1,43) = 0.896, P = 0.349).
Another study (Kampman 2003), 30 participants, described similar results with an average percentage of prescribed pills taken by the olanzapine‐treated participants of 84.5% compared to 89.3% in the placebo‐treated group (t = 0.629, df = 28, not statistically significant).
A third study (Reid 2005) of 31 participants reported that compliance with medication treatment was not significantly different between any treatment groups (P = 0.832).
(06) Reserpine versus placebo
Measured by pill count
One study (Winhusen 2007) of 119 participants assessed this outcome, calculating a compliance score from the number of tablets dispensed minus the number returned or reported lost divided by the number of tablets prescribed. The mean of this compliance score for the reserpine group, 0.79 (SD = 0.23), was not significantly different from that of the placebo group at 0.74 (SD = 0.34), (t = 1.13, P = 0.26).
(08) Olanzapine versus risperidone
Measured by self‐reported ingestion of medication
One study (Akerele 2007), 28 participants, showed no difference in the percentage reduction of self‐reported missed dosage over all administered doses: Olanzapine group 7% and risperidone group 8% (t = 0.31, df = 20, P = 0.76).
Craving
The included studies used different scales to rate this outcome, limiting the possibility to pool data.
(01) Any antipsychotic versus placebo
Measured by Brief Substance Craving Scale
Four studies (Kampman 2003; Reid 2005; Tapp 2015; Winhusen 2007), 240 participants, MD 0.13 (95% CI ‐1.08 to 1.35), see Analysis 1.5 and Table 1, showed no significant difference, but heterogeneity was extremely high ( I2:85%) .
1.5. Analysis.
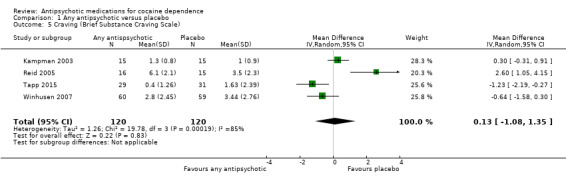
Comparison 1 Any antipsychotic versus placebo, Outcome 5 Craving (Brief Substance Craving Scale).
(02) Risperidone versus placebo
Measured by mean decrease of Visual Analogue Scale
In one study (Levin 1999),14 participants, using a scale from 0 to 100, the percentage reduction of the score after treatment was ‐31% in the risperidone group compared with ‐49% in the placebo group. The result was not y significant.
Measured by mean decrease in Minnesota Cocaine Craving Scale
One study (Loebl 2008) of 31 participants showed no treatment effect on intensity (F = 0.03, P = 0.86) or frequency (F = 1.69, P = 0.11) of craving, but described a trend for main effect in duration of craving episodes, with a smaller decrease in the risperidone group (56.6%) than in the placebo group (65.7%, F = 2.71, P = 0.11).
Measured by improvement on the Voris Cocaine Craving Questionnaire (VCCQ) subscale scores
One study (Smelson 2004) of 35 participants showed a significant main effect of time for the craving (F = 33.62, P = 0.01), mood (F = 5.78, P = 0.023), and sick (F = 4.264, P = 0.040) subscales of the VCCQ ( no further description is provided about which symptoms the term "sick" refers to), suggesting that participants in both groups improved over the course of the study.
(03) Olanzapine versus placebo
Measured by Brief Substance Craving Scale
Two studies (Kampman 2003; Reid 2005), 61 participants, see Analysis 3.6. MD 1.33 (95% CI ‐0.91 to 3.58; I² = 86%). There is no significant difference that could indicate a treatment effect, but heterogeneity was extremely high .
3.6. Analysis.
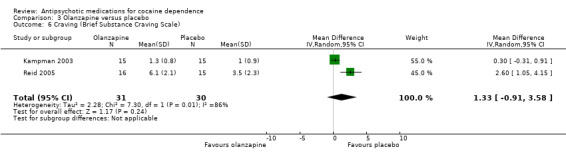
Comparison 3 Olanzapine versus placebo, Outcome 6 Craving (Brief Substance Craving Scale).
Another study (Hamilton 2009), 48 participants, did not find statistically significant differences between the olanzapine and placebo groups for the Craving Questionnaire.
(04) Quetiapine versus placebo
Measured by Brief Substance Craving Scale
One study (Tapp 2015), 60 participants, reported no differences between groups in terms of absence of cravings (34.5% in quetiapine group versus 29.0% in placebo group; Wald statistic = 0.21,df = 1, P = 0.65). See also Analysis 4.4. MD ‐1.23 (95% CI ‐2.19 to ‐0.27), which also shows no statistically significant difference.
4.4. Analysis.
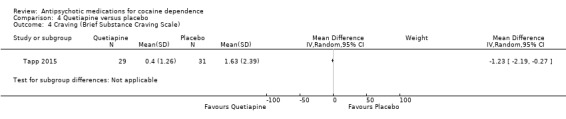
Comparison 4 Quetiapine versus placebo, Outcome 4 Craving (Brief Substance Craving Scale).
(05) Lamotrigine versus placebo
Measured by Cocaine Craving Questionnaire
One study (Brown 2012), 112 participants, reported using a declining‐effects random regression model, and found no differences in treatment effect between groups (F = 0.4, P = 0.53) as measured by the change from week 1 to week 12.
(06) Reserpine versus placebo
Measured by Brief Substance Craving Scale
One study (Winhusen 2007), 119 participants, see Analysis 6.2. MD ‐0.64 (95% CI ‐1.58 to 0.30), no statistically significant difference.
6.2. Analysis.
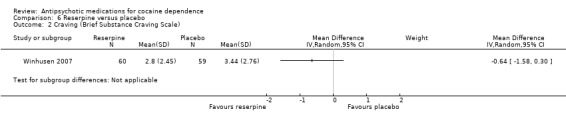
Comparison 6 Reserpine versus placebo, Outcome 2 Craving (Brief Substance Craving Scale).
(07) Olanzapine versus haloperidol
Measured by Voris Cocaine Craving Questionnaire
One study (Smelson 2006), 31 participants, see Analysis 7.3. MD ‐5.90 (95% CI ‐12.49 to 0.69), no statistically significant difference.
7.3. Analysis.
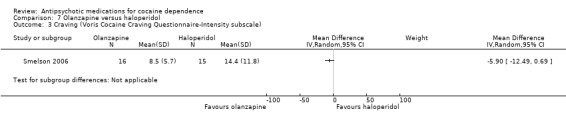
Comparison 7 Olanzapine versus haloperidol, Outcome 3 Craving (Voris Cocaine Craving Questionnaire‐Intensity subscale).
(08) Olanzapine versus risperidone
Measured by Cocaine Craving Report
One study (Akerele 2007), in an analysis of the cocaine‐dependent subgroup with 19 participants, reported no statistically significant differences between groups in terms of cocaine craving over time.
(09) Aripiprazol versus ropinirol
Measured by Voris Cocaine Craving Questionnaire
This small trial (Meini 2010) of 28 participants, found no statistically significant differences: MD ‐14.90 (95% CI ‐38.25 to 8.45), see Analysis 9.3.
9.3. Analysis.
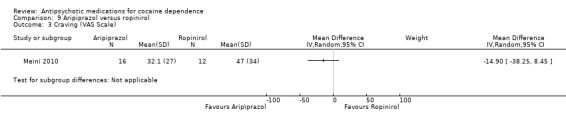
Comparison 9 Aripiprazol versus ropinirol, Outcome 3 Craving (VAS Scale).
Severity of dependence
(01) Any antipsychotic versus placebo
Measured by Addiction Severity Index (ASI)
Four studies (Kampman 2003; Loebl 2008; Reid 2005; Winhusen 2007), 211 participants, MD 0.01 (95% CI ‐0.01 to 0.04), see Analysis 1.6, showed no statistically significant difference in ASI global scores.
1.6. Analysis.
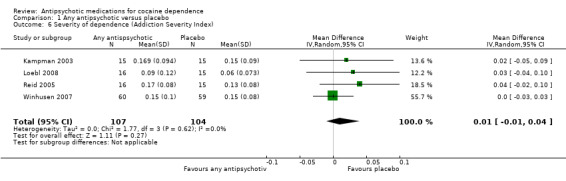
Comparison 1 Any antipsychotic versus placebo, Outcome 6 Severity of dependence (Addiction Severity Index).
Measured by Clinical Global Impression Scale (CGIS)
Three studies (Kampman 2003; Reid 2005; Winhusen 2007), 180 participants, MD 0.01 (95% CI ‐0.38 to 0.39), see Analysis 1.7, showed a similar result on CGIS scores.
1.7. Analysis.
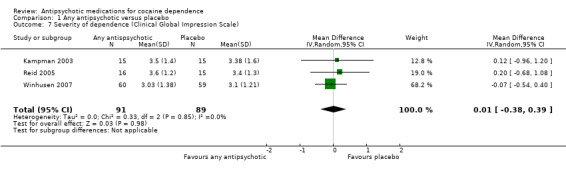
Comparison 1 Any antipsychotic versus placebo, Outcome 7 Severity of dependence (Clinical Global Impression Scale).
(02) Risperidone versus placebo
Measured by Addiction Severity Index (ASI)
One study (Loebl 2008) of 31 participants reported no main effect of treatment for cocaine selective ASI scores, and showed no statistically significant differences in drug composite scores: MD 0.03 (95% CI ‐0.04 to 0.10), see Analysis 2.2.
2.2. Analysis.
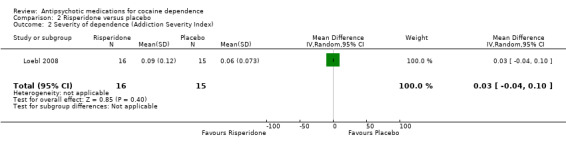
Comparison 2 Risperidone versus placebo, Outcome 2 Severity of dependence (Addiction Severity Index).
(03) Olanzapine versus placebo
Measured by Addiction Severity Index (ASI) Two studies (Kampman 2003; Reid 2005), 61 participants, see Analysis 3.7. MD 0.03 (95% CI ‐0.01 to 0.07) showed no statistically significant differences.
3.7. Analysis.
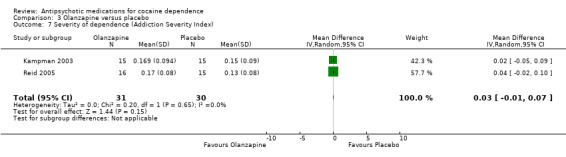
Comparison 3 Olanzapine versus placebo, Outcome 7 Severity of dependence (Addiction Severity Index).
One study (Hamilton 2009), 48 participants, identified no statistically significant differences between the olanzapine and placebo groups on any of the ASI subscale measures.
Measured by Clinical Global Impression Scale Using another measuring instrument, the above‐mentioned two studies (Kampman 2003; Reid 2005), 61 participants, see Analysis 3.8, MD 0.17 (95% CI ‐0.51 to 0.85) presented similar non‐significant results.
3.8. Analysis.
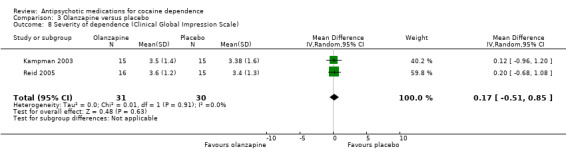
Comparison 3 Olanzapine versus placebo, Outcome 8 Severity of dependence (Clinical Global Impression Scale).
(06) Reserpine versus placebo
Measured by Addiction Severity Index One study (Winhusen 2007), 119 participants, see Analysis 6.3, MD 0.00 (95% CI ‐0.03 to 0.03) found no statistically significant difference.
6.3. Analysis.
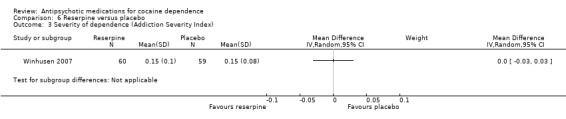
Comparison 6 Reserpine versus placebo, Outcome 3 Severity of dependence (Addiction Severity Index).
Measured by Clinical Global Impression Scale The same study (Winhusen 2007), 119 participants, see Analysis 6.4, presented similar results using another scale: MD ‐0.07 (95% CI ‐0.54 to 0.40).
6.4. Analysis.
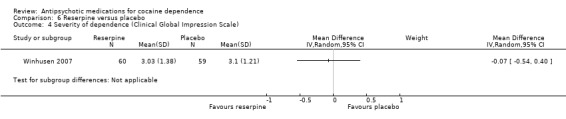
Comparison 6 Reserpine versus placebo, Outcome 4 Severity of dependence (Clinical Global Impression Scale).
(09) Aripiprazol versus ropinirol
Measured by Clinical Global Impression Scale
One study (Meini 2010), 28 participants, also reported statistically non‐significant results for addiction severity: MD ‐0.40 (95% CI ‐1.66 to 0.86). See Analysis 9.4.
9.4. Analysis.
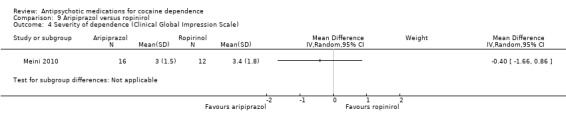
Comparison 9 Aripiprazol versus ropinirol, Outcome 4 Severity of dependence (Clinical Global Impression Scale).
Amount of cocaine use
(01) Any antipsychotic versus placebo
Measured by grams used during the last week
Two studies (Brown 2010; Tapp 2015), 72 participants, MD ‐0.54 (95% CI ‐0.92 to ‐0.16), see Analysis 1.8, showed a small statistically significant reduction in self‐reported grams of cocaine used during the past week in the antipsychotic treatment group. But again, the result is limited by the small sample size of the included studies.
1.8. Analysis.
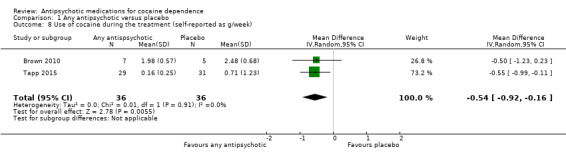
Comparison 1 Any antipsychotic versus placebo, Outcome 8 Use of cocaine during the treatment (self‐reported as g/week).
(03) Olanzapine versus placebo
Measured by money (US dollars) spent during past 30 days
One study(Kampman 2003), 30 participants, MD 218 (95% CI ‐61.96 to 497.96), see Analysis 3.9, showed no significant difference.
3.9. Analysis.
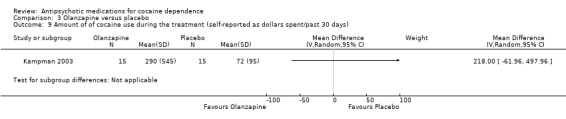
Comparison 3 Olanzapine versus placebo, Outcome 9 Amount of of cocaine use during the treatment (self‐reported as dollars spent/past 30 days).
(04) Quetiapine versus placebo
Measured by grams used during the last week
Two studies (Brown 2010; Tapp 2015), 72 participants, showed a very small statistically significant difference in favour of quetiapine when comparing the amount of cocaine use by grams per week: MD ‐0.54 (95% CI ‐0.92 to ‐0.16), see Analysis 4.5.
4.5. Analysis.
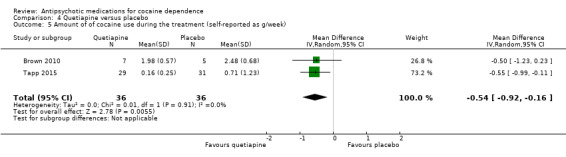
Comparison 4 Quetiapine versus placebo, Outcome 5 Amount of of cocaine use during the treatment (self‐reported as g/week).
Measured by money (US dollars) spent during the last week
One of these studies (Tapp 2015), 60 participants, also measured the amount of cocaine use by US dollars spent per week and found results in favour of quetiapine: MD ‐53.80 (95% CI ‐97.85 to ‐9.75), see Analysis 4.6. But the generalisability of this finding is low, due to the small sample size .
4.6. Analysis.
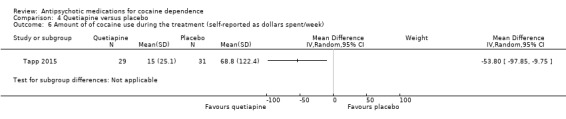
Comparison 4 Quetiapine versus placebo, Outcome 6 Amount of of cocaine use during the treatment (self‐reported as dollars spent/week).
(05) Lamotrigine versus placebo
One study (Brown 2012), 112 participants, using a declining‐effects random regression model, reported a statistically significant decrease in US dollars spent on cocaine in the lamotrigine group compared to the placebo group (F = 3.9, P = 0.05), as measured by the change from week 1 to week 12.
(09) Aripiprazol versus ropinirol
Measured by grams of self‐reported cocaine consume per week One study (Meini 2010), 28 participants, see Analysis 9.5, shows a small statistically significant effect in favour of aripiprazol: MD: ‐1.20 (95% CI ‐1.86 to ‐0.54), but the generalisability is low due the very small sample size.
9.5. Analysis.
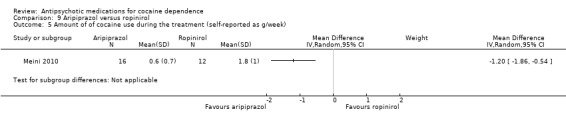
Comparison 9 Aripiprazol versus ropinirol, Outcome 5 Amount of of cocaine use during the treatment (self‐reported as g/week).
Psychiatric symptoms/psychological distress
Depression
(01) Any antipsychotic versus placebo
Measured by Hamilton Depression Rating Scale
Four studies (Brown 2010; Kampman 2003; Reid 2005; Winhusen 2007), 192 participants, MD ‐0.82 (95% CI ‐3.19 to 1.55), see Analysis 1.9, showed a statistically non‐significant reduction in Hamilton Depression Rating Scale scores over the treatment periods in favour of antipsychotic treatment.
1.9. Analysis.
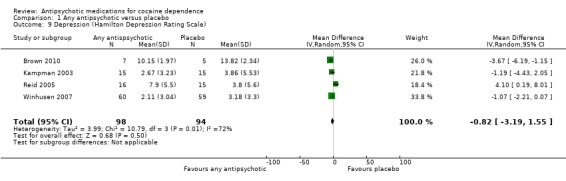
Comparison 1 Any antipsychotic versus placebo, Outcome 9 Depression (Hamilton Depression Rating Scale).
(02) Risperidone versus placebo
Measured by Hamilton Depression Rating Scale
One study (Loebl 2008) of 31 participants reported that no participants assigned to risperidone who completed the trial had an improvement in depressive symptoms. There was a mean increase in HAM‐D scores in the risperidone group of 7.4 (SD 8.8) and in the placebo group a decrease of ‐2.3 (SD 5.8,P = 0.018).
(03) Olanzapine versus placebo
Measured by Hamilton Depression Rating Scale Two studies (Kampman 2003; Reid 2005), 61 participants, see Analysis 3.10, MD 1.34 (95% CI ‐3.84 to 6.52; I² = 76%). No significant difference found, but heterogeneity was extremely high.
3.10. Analysis.
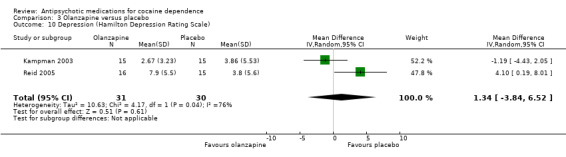
Comparison 3 Olanzapine versus placebo, Outcome 10 Depression (Hamilton Depression Rating Scale).
(04) Quetiapine versus placebo
Measured by Hamilton Depression Rating Scale
One small trial (Brown 2010) of 12 participants, see Analysis 4.7, MD ‐3.67 (95% CI ‐6.19 to ‐1.15) obtained results favouring quetiapine in reducing HAM‐D mean scores, but the very small sample size limits generalisability.
4.7. Analysis.
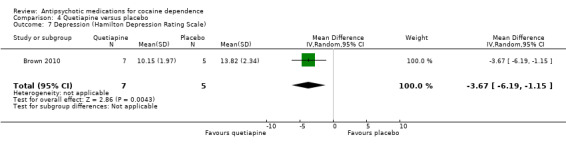
Comparison 4 Quetiapine versus placebo, Outcome 7 Depression (Hamilton Depression Rating Scale).
Measured by Quick Inventory of Depressive Symptomatology‐SR
One small trial (Brown 2010) of 12 participants, see Analysis 4.8, MD ‐1.27 (95% CI ‐9.61 to 7.07) showed no statistically significant difference.
4.8. Analysis.
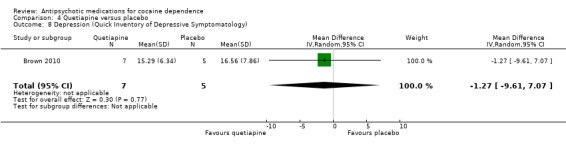
Comparison 4 Quetiapine versus placebo, Outcome 8 Depression (Quick Inventory of Depressive Symptomatology).
(05) Lamotrigine versus placebo
Measured by Hamilton Depression Rating Scale and Quick Inventory of Depressive Symptomatology‐SR
One study (Brown 2012), 112 participants,using a declining‐effects random regression model, reported no differences in score changes between groups in depressive symptoms measured by HAM‐D (F = 0.3, P = 0.57) and QIDS‐SR (F = 0.1, P = 0.89) from week 1 to week 12.
(06) Reserpine versus placebo
Measured by Hamilton Depression Rating Scale One study (Winhusen 2007), 119 participants, see Analysis 6.5, MD ‐1.07 (95% CI ‐2.21 to 0.07) No significant difference found,
6.5. Analysis.
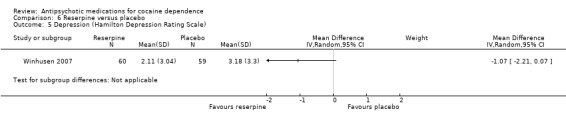
Comparison 6 Reserpine versus placebo, Outcome 5 Depression (Hamilton Depression Rating Scale).
(08) Olanzapine versus risperidone
Measured by Hamilton Depression Rating Scale
One study (Akerele 2007), 28 participants, see Analysis 8.2, showed no significant differences: MD 0.11 (95% CI ‐0.49 to 0.71).
8.2. Analysis.
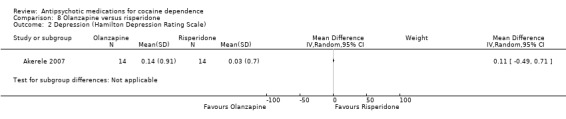
Comparison 8 Olanzapine versus risperidone, Outcome 2 Depression (Hamilton Depression Rating Scale).
Anxiety
(03) Olanzapine versus placebo
Measured by Hamilton Anxiety Rating Scale Two studies (Kampman 2003; Reid 2005), 61 participants, see Analysis 3.11, MD 1.37 (95% CI ‐3.02 to 5.75; I² = 86%) showed no significant difference, but heterogeneity was extremely high.
3.11. Analysis.
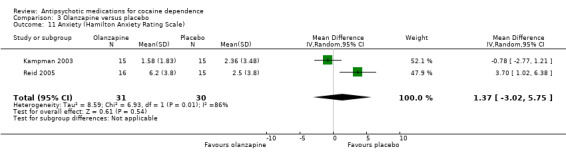
Comparison 3 Olanzapine versus placebo, Outcome 11 Anxiety (Hamilton Anxiety Rating Scale).
Psychopathology
(02) Risperidone versus placebo
Measured by Snaith‐Hamilton Pleasure Scale
One study (Loebl 2008), 31 participants, reported no effect of treatment.
(04) Quetiapine versus placebo
Measured by Young Mania Rating Scale (YMRS)
One small trial (Brown 2010) of 12 participants, see Analysis 4.9, MD ‐4.20 (95% CI ‐7.65 to ‐0.75) obtained results favouring quetiapine in reducing YMRS mean scores, but the small sample size limits generalisability.
4.9. Analysis.
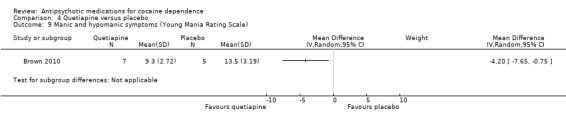
Comparison 4 Quetiapine versus placebo, Outcome 9 Manic and hypomanic symptoms (Young Mania Rating Scale).
(05) Lamotrigine versus placebo
Measured by Young Mania Rating Scale (YMRS)
One study (Brown 2012), 112 participants, using a declining‐effects random regression model, reported no differences in treatment effect between groups in manic and hypomanic symptoms (F = 0.5, P = 0.47) as measured by the change from week 1 to week 12.
(07) Olanzapine versus haloperidol
Measured by Positive and Negative Syndrome Scale (PANSS) One study (Smelson 2006), 31 participants, see Analysis 7.2, MD ‐6.10 (95% CI ‐10.93 to ‐1.27) showed results favouring haloperidol in reducing scores, but the small sample size limits generalisability.
7.2. Analysis.
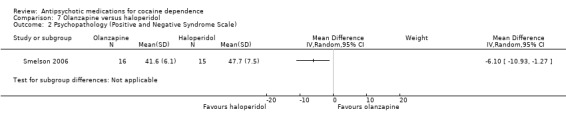
Comparison 7 Olanzapine versus haloperidol, Outcome 2 Psychopathology (Positive and Negative Syndrome Scale).
(08) Olanzapine versus risperidone
Measured by Positive and Negative Syndrome Scale
The Akerele 2007 study found a decrease in severity on the positive subscale over time for both groups (Z = 2.53, P = 0.01), but found no statistically significant differences between groups (Z = 0.49, P = 0.62) and no decrease in severity for the negative subscale over time (Z = 0.34, P = 0.73).
Withdrawal symptoms
(03) Olanzapine versus placebo
Measured by Cocaine Selective Severity Assessment
One study (Kampman 2003), 30 participants see Analysis 3.12, MD 5.60 (95% CI ‐0.31 to 11.51) found no statistically significant difference in withdrawal symptoms between groups.
3.12. Analysis.
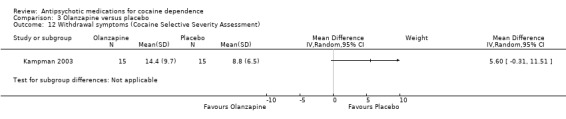
Comparison 3 Olanzapine versus placebo, Outcome 12 Withdrawal symptoms (Cocaine Selective Severity Assessment).
Discussion
Summary of main results
We include 14 trials. They tested risperidone, olanzapine, quetiapine, lamotrigine, reserpine, haloperidol and aripiprazol as antipsychotic agents for the treatment of cocaine dependence, comparing them with placebo and in three studies (Akerele 2007; Meini 2010; Smelson 2006) comparing two drugs.
Comparing any antipsychotic drugs versus placebo, we found moderate‐quality evidence that antipsychotics reduced dropouts; these findings came from eight studies with 397 participants (Brown 2010; Grabowski 2004; Kampman 2003; Levin 1999; Loebl 2008; Smelson 2004; Tapp 2015; Winhusen 2007). There were no significant differences for any of the other primary outcomes: number of participants using cocaine during the treatment (as days/week by urine tests or self report): continuous abstinence (number of participants who maintained negative drug screens for two to three weeks), and side effects (number of participants with at least one side effect), but the evidence was low and came from only two, three and four studies respectively. We also found low‐quality evidence showing no difference in craving as assessed by the BSCS, but these results are based on only four studies with 240 participants (Kampman 2003; Reid 2005; Tapp 2015; Winhusen 2007). The single comparisons of each drug versus placebo or versus another drug included few trials with small sample sizes, so limiting the reliability of the results. Among these comparisons, only quetiapine seemed to perform better than placebo in reducing cocaine use as measured by grams per week or US dollars spent per week, and in levels of craving assessed by the BSCS. These results came only from two studies, one with 60 participants (Tapp 2015) and another with 20 participants (Brown 2010).
Overall completeness and applicability of evidence
Thirteen of the 14 included studies were conducted in the USA; this limits the generalisability of the results, because health effects of various substances of abuse seem to be strongly dependent on social context, and the location of the conduct of the studies could act as an effect modifier in the estimation of the efficacy of treatment.
Most of the included studies did not report useful results on important outcomes such as side effects, use of cocaine during the treatment and craving. In those studies which did report them, it was not possible to undertake a cumulative analysis because of the great heterogeneity of the scales used in the primary studies and in the way in which results are reported.
Quality of the evidence
The major limitations of the studies were the high risk of attrition bias (40% of the included studies) and the low quality of reporting, mainly affecting the risk of selection bias, and performance and detection bias, which we rated as being at unclear risk for 75% to 80% of the studies. Overall we rated study quality as moderate for dropouts, and low for the other outcomes
Potential biases in the review process
Risk of publication bias by Funnel plot could not be assessed because less than ten trials were included in the analysis. We performed a very comprehensive search and contacted investigators to request information about unpublished or incomplete trials. However, publication bias could not be excluded because only small studies have been included in the analyses.
Authors' conclusions
Implications for practice.
The argument in favour of antipsychotics for treating cocaine use disorders is based largely on their use in treating psychiatric complications, which is not the same as treating the use disorder itself. Antipsychotics are not risk‐free medications; their side effects are not trivial and may be long‐lasting. We have not found positive effects on reduction in cocaine use. At present, there is no evidence supporting the clinical use of antipsychotic medications in the treatment of cocaine dependence, although results come from only 14 trials, with small sample sizes and moderate‐to‐low quality of evidence.
Implications for research.
Most of the included studies did not report useful results on important outcomes such as side effects, use of cocaine during the treatment, and craving. When studies did report them, no cumulative analysis was possible due to great heterogeneity among the scales used by the primary studies and in the way results were reported. This major problem needs to be addressed in future research through the use of instruments that allow for improved comparability of results and by following best‐practice recommendations for the reporting of results (i.e. GRADE).
Given the absence of promising results for reduction of drug use and the relevance of side effects, no further research seems to be justified to explore the effectiveness of this class of drug in people without psychiatric comorbidities or complications.
What's new
| Date | Event | Description |
|---|---|---|
| 9 June 2016 | Amended | added information about source of support |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 7 February 2016 | New citation required but conclusions have not changed | Conclusions not changed. |
| 7 February 2016 | New search has been performed | Searches updated. |
| 20 October 2008 | Amended | Contact details updated |
| 21 March 2008 | Amended | Converted to new review format. |
| 10 May 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Blanca I Indave undertook this update as her trainee's project at the EMCDDA under supervision of Marica Ferri. We would like to thank Marica Ferri for her support in particular on the assessment of the quality of the studies.We would like to acknowledge the previous contributions of Marina Davoli who was a co‐author on previous versions and helped with liaison on discussion and results writing. We thank Zuzana Mitrova, the trial search co‐ordinator, for her help in performing the bibliographic searches and retrieving the articles for the review.
Appendices
Appendix 1. Search strategies October 2006
In the first version of the review we identified relevant studies by searching the following sources from the earliest available date to 2006: MEDLINE (1966 toOctober 2006), EMBASE (1980 to October 2006), CINAHL (1982 to October 2006), Cochrane Drug and Alcohol Group Specialised Register (October 2006)
MEDLINE search strategy
1.exp cocaine‐related disorders/ 2.((cocaine$) adj2 (abuse$ or addict$ or dependen$)).ti,ab 3.exp cocaine/ or exp crack cocaine/ 4.cocaine.ti,ab 5.1 or 2 or 3 or 4 6.exp antipsychotic/ 7.antipsychotic$.ti,ab 8.exp serotonin antagonists/ 9.5‐HT2$.ti,ab 10.chlorpromazine.mp. or exp Chlorpromazine/ 11.fluphenazine.mp. or exp Fluphenazine/ 12.perphenazine.mp. or exp Perphenazine/ 13.prochlorperazine.mp. or exp Prochlorperazine/ 14.thioridazine.mp. or exp Thioridazine/ 15.trifluoperazine.mp. or exp Trifluoperazine/ 16.haloperidol.mp. or exp Haloperidol/ 17.droperidol.mp. or exp Droperidol/ 18.pimozide.mp. or exp Pimozide/ 19.clozapine.mp. or exp Clozapine/ 20.olanzapine.mp. 21.risperidone.mp. or exp Risperidone/ 22.quetiapine.mp. 23.ziprasidone.mp. 24.aripiprazole.mp. 25.symbax.ti,ab. 26.tetrabenazine.mp. or exp Tetrabenazine/ 27. OR 6/26 28. 5 and 27 combined with the phases 1 & 2 of the Cochrane Sensitive Search Strategy for the identification of RCTs as published in Appendix 5b2, Cochrane Handbook for Systematic Reviews of Interventions: 29.randomized controlled trial.pt. 30.randomized controlled trials/ 31.controlled clinical trial.pt. 32.random allocation/ 33.double blind method/ 34.single blind method/ 35.29/34 36.clinical trial.pt. 37.exp clinical trials/ 38.(clin$ adj trial$).ab,ti. 39.((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ab,ti 40.exp PLACEBOS/ 41.placebo$.ab,ti 42.random$.ab,ti 43.exp Research Design/ 44.36/43 45.35 or 44 46.4 and 7 and 12 47.30 and 29 48.limit 31 to human
EMBASE search strategy
exp drug abuse/
exp Cocaine Dependence/
((cocaine) adj2 (abuse$ or addict$ or dependen$)).ti,ab.
((drug or substance) adj2 (abuse$ or addict$ or dependen$)).ti,ab.
1 or 2 or 3 or 4
exp COCAINE DERIVATIVE/ or exp COCAINE/
cocaine.ti,ab.
6 or 7
antipsychotic.mp.
serotonin agents.mp. or exp Serotonin Receptor Affecting Agent/
exp CHLORPROMAZINE/ or chlorpromazine.mp.
fluphenazine.mp. or exp FLUPHENAZINE/
perphenazine.mp. or exp PERPHENAZINE/
exp PROCHLORPERAZINE/ or prochlorperazine.mp.
thioridazine.mp. or exp THIORIDAZINE/
exp TRIFLUOPERAZINE/ or trifluoperazine.mp.
haloperidol.mp. or exp HALOPERIDOL/
exp DROPERIDOL/ or droperidol.mp.
pimozide.mp. or exp PIMOZIDE/
clozapine.mp. or exp CLOZAPINE/
exp OLANZAPINE/ or olanzapine.mp.
risperidone.mp. or exp RISPERIDONE/
quetiapine.mp. or exp QUETIAPINE/
ziprasidone.mp. or exp ZIPRASIDONE/
aripiprazole.mp. or exp ARIPIPRAZOLE/
symbax.ti,ab.
tetrabenazine.mp. or exp TETRABENAZINE/
OR 9/27
5 and 8 and 28
random$.ti,ab.
placebo$.ti,ab.
((singl$ or doubl$ or trebl$ or tripl$) adj2 (blind$ or mask$)).mp.
(cross‐over$ or crossover$).tw.
randomized controlled trial/
phase‐2‐clinical‐trial/
phase‐3‐clinical‐trial/
double blind procedure/
single blind procedure/
crossover procedure/
Latin square design/
exp PLACEBOS/
multicenter study/
OR 30/42
29 and 43
limit 44 to human
CINAHL search strategy
exp "Substance Use Disorders"/
(cocaine adj2 (abuse$ or dependen$))
TX cocaine or MH cocaine
TX chlorpromazine or MH chlorpromazine
TX fluphenazine or MH fluphenazine
TX perphenazine
MH PROCHLORPERAZINE or TX prochlorperazine
TX thioridazine or MH thioridazine
TX trifluoperazine
TX haloperidol or MH HALOPERIDOL
MH DROPERIDOL or TX droperidol
TX pimozide
TX clozapine or MH CLOZAPINE
TX OLANZAPINE or MH olanzapine
TX risperidone. or MH RISPERIDONE
TX quetiapine or MH QUETIAPINE
TX ziprasidone
TX aripiprazole
TX symbax
TX tetrabenazine
random$.tw.
clini$.tw.
trial$.tw.
(clin$ adj2 trial$).tw.
(singl$ or doubl$ or tripl$ or trebl$).mp. and (mask$ or blind$).tw.
crossover.tw.
allocate$.tw.
assign$.tw.
(random$ adj2 (allocate$ or assign$)).tw.
exp Random Assignment/
exp Clinical Trials/
1 or 2 or 3
OR 4/20
OR 21/31
32 and 33 and 34
Cochrane Drug and Alcohol Group Specialised Register search strategy
We searched the free text "cocaine" in the field "Diagnosis" and "antipsychotic" in the field "Intervention" of the Register
Appendix 2. Cochrane Drug and Alcohol Group Specialised Register search strategy (2015)
July 15, 2015 (15 hits)
Publication Year from 2006
(cocaine*:XDI,TI AND (Antipsychotic* OR aripiprazole OR clozapine OR chlorpromazine OR droperidol OR olanzapine OR prochlorperazine OR trifluoperazine OR fluphenazine OR haloperidol OR perphenazine OR pimozide OR quetiapine OR risperidone OR tetrabenazine OR thioridazine OR ziprasidone))
Appendix 3. CENTRAL search strategy (2015)
The Cochrane Library
Issue 7, July 2015 ( 26 hits in CENTRAL; 3 hits in DARE)
MeSH descriptor: [Cocaine‐Related Disorders] explode all trees
cocaine* or crack:ti,ab,kw (Word variations have been searched)
#1 or #2
"antipsychotic":ti,ab,kw (Word variations have been searched)
"neuroleptic":ti,ab,kw (Word variations have been searched)
chlorpromazine:ti,ab,kw (Word variations have been searched)
"fluphenazine":ti,ab,kw (Word variations have been searched)
"perphenazine":ti,ab,kw (Word variations have been searched)
"prochlorperazine":ti,ab,kw (Word variations have been searched)
"thioridazine":ti,ab,kw (Word variations have been searched)
"trifluoperazine":ti,ab,kw (Word variations have been searched)
"haloperidol":ti,ab,kw (Word variations have been searched)
"droperidol":ti,ab,kw (Word variations have been searched)
"pimozide":ti,ab,kw (Word variations have been searched)
"clozapine":ti,ab,kw (Word variations have been searched)
"olanzapine":ti,ab,kw (Word variations have been searched)
"risperidone":ti,ab,kw (Word variations have been searched)
"quetiapine":ti,ab,kw (Word variations have been searched)
"ziprasidone":ti,ab,kw (Word variations have been searched)
"aripiprazole":ti,ab,kw (Word variations have been searched)
"tetrabenazine":ti,ab,kw (Word variations have been searched)
#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
#3 and #22 Publication Year from 2006
Appendix 4. MEDLINE search strategy (2015)
MEDLINE (via PubMed)
July 15, 2015 (71 hits)
Cocaine‐Related Disorders[Mesh]
((cocaine[tiab] OR crack[tiab]) AND (abuse*[tiab] OR dependen*[tiab] OR misuse[tiab] OR addict*[tiab]))
#1 or #2
"Antipsychotic Agents"[Mesh] OR "Antipsychotic Agents" [Pharmacological Action]
antipsychot*[tiab] OR anti‐psychot*[tiab] OR neuroleptic*[tiab]
aripiprazole
chlorpromazine[tiab] OR chlorpromazine[MeSH]
clozapine[tiab] OR clozapine[MeSH]
droperidol[tiab] OR droperidol[MeSH]
fluphenazine[tiab] OR fluphenazine[MeSH]
Haloperidol[tiab] OR Haloperidol[MeSH]
olanzapine
perphenazine[tiab] OR perphenazine[MeSH]
pimozide[tiab] OR pimozide[MeSH]
prochlorperazine[tiab] OR prochlorperazine[MeSH]
quetiapine
Risperidone[tiab] OR Risperidone[MeSH]
tetrabenazine[tiab]OR tetrabenazine[MeSH]
thioridazine[tiab] OR thioridazine[MeSH]
trifluoperazine[tiab] OR trifluoperazine[MeSH]
ziprasidone
#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
randomized controlled trial [pt]
controlled clinical trial [pt]
randomized [tiab]
placebo [tiab]
drug therapy [sh]
randomly [tiab]
trial [tiab]
groups [tiab]
#23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30
animals [mh] NOT humans [mh]
#31 NOT #32
#3 AND #22 AND #33 Filters: Publication date from 2006/10/01
Appendix 5. EMBASE search strategy (2015)
EMBASE (via embase.com)
July 15, 2015 (149 hits)
'cocaine dependence'/exp OR ((cocaine OR crack) NEAR/3 (abuse* OR dependen* OR addict*)):ab,ti AND ('neuroleptic agent'/exp OR antipsychotic*:ab,ti OR 'chlorpromazine':ab,ti OR 'chlorpromazine'/exp OR 'fluphenazine'/exp OR 'fluphenazine':ab,ti OR 'perphenazine'/exp OR 'perphenazine':ab,ti OR 'prochlorperazine'/exp OR 'prochlorperazine':ab,ti OR 'thioridazine'/exp OR 'thioridazine':ab,ti OR 'trifluoperazine'/exp OR 'trifluoperazine':ab,ti OR 'haloperidol'/exp OR 'haloperidol':ab,ti OR 'droperidol'/exp OR 'droperidol':ab,ti OR 'pimozide'/exp OR 'pimozide':ab,ti OR 'clozapine'/exp OR 'clozapine':ab,ti OR 'olanzapine'/exp OR 'olanzapine':ab,ti OR 'risperidone'/exp OR 'risperidone':ab,ti OR 'quetiapine'/exp OR 'quetiapine':ab,ti OR 'ziprasidone'/exp OR 'ziprasidone':ab,ti OR 'aripiprazole'/exp OR 'aripiprazole':ab,ti) AND ('crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR 'controlled clinical trial'/exp OR 'clinical trial'/exp OR placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti) OR 'randomized controlled trial'/exp) AND [1‐10‐2006]/sd
Appendix 6. CINAHL search strategy (2015)
CINAHL (via EBSCO HOST)
July 15, 2015 (17 hits)
(MH "Substance Use Disorders+")
TX((cocaine OR crack) N3 (abuse* OR dependen* OR addict*))
TI cocaine OR AB cocaine OR MH cocaine
S1 OR S2 OR S3
TX (antipsychotic* OR neuroleptic* OR aripiprazole OR clozapine OR chlorpromazine OR droperidol OR olanzapine OR prochlorperazine OR trifluoperazine OR fluphenazine OR haloperidol OR perphenazine OR pimozide OR quetiapine OR risperidone OR tetrabenazine OR thioridazine OR ziprasidone)
S4 AND S5
MH "Clinical Trials+"
PT Clinical trial
TI clinic* N1 trial* or AB clinic* N1 trial*
TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
TI randomi?ed control* trial* or AB randomi?ed control* trial*
MH "Random Assignment"
TI random* allocat* or AB random* allocat*
MH "Placebos"
TI placebo* or AB placebo*
MH "Quantitative Studies"
S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17
S6 AND S18 Exclude MEDLINE records
S6 AND S18 Publication Year from 2006
Appendix 7. Web of Science search strategy (2015)
WOS (via THOMSON REUTERS)
July 15, 2015 (94 hits)
TS=((cocaine OR crack) NEAR/6 (abuse* OR dependen* OR addict* OR disorder*))
TS=(antipsychotic* OR neuroleptic* OR aripiprazole OR clozapine OR chlorpromazine OR droperidol OR olanzapine OR prochlorperazine OR trifluoperazine OR fluphenazine OR haloperidol OR perphenazine OR pimozide OR quetiapine OR risperidone OR tetrabenazine OR thioridazine OR ziprasidone)
TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
#3 AND #2 AND #1Indexes=SCI‐EXPANDED, SSCI, A&HCI Timespan=2006‐2015
Appendix 8. criteria for risk of bias assessment
| Item | Judgment | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement | |
| 3. Blinding of participants and providers (performance bias) Objective outcomes |
Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 4. Blinding of participants and providers (performance bias) Subjective outcomes |
Low risk | Blinding of participants and providers ensured and unlikely that the blinding could have been broken; |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 5. Blinding of outcome assessor (detection bias) Objective outcomes |
Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 6.Blinding of outcome assessor (detection bias) Subjective outcomes |
Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 7. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop out |
Low risk | No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; Missing data have been imputed using appropriate methods All randomised patients are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of drop out not reported for each group); | |
| 8 Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| High risk | Not all of the study’s pre‐specified primary outcomes have been reported; One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk |
Data and analyses
Comparison 1. Any antipsychotic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 8 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.97] |
| 2 Side effects | 6 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.93, 1.10] |
| 3 Number of participants using cocaine during the treatment (as days/week by urine tests or self report) | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.62] |
| 4 Continuous abstinence (number of participants who maintained negative drug screens for 2 ‐ 3 weeks) | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.73, 2.32] |
| 5 Craving (Brief Substance Craving Scale) | 4 | 240 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐1.08, 1.35] |
| 6 Severity of dependence (Addiction Severity Index) | 4 | 211 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 7 Severity of dependence (Clinical Global Impression Scale) | 3 | 180 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.38, 0.39] |
| 8 Use of cocaine during the treatment (self‐reported as g/week) | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.92, ‐0.16] |
| 9 Depression (Hamilton Depression Rating Scale) | 4 | 192 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐3.19, 1.55] |
Comparison 2. Risperidone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 4 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.04] |
| 2 Severity of dependence (Addiction Severity Index) | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
Comparison 3. Olanzapine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Side effects | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 3 Use of cocaine during the treatment (self‐reported as days/week) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Use of cocaine during the treatment (self‐reported as days/past 30 days) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Continuous abstinence (participants who maintained negative drug screens throughout the treatment period ) | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.71, 2.61] |
| 6 Craving (Brief Substance Craving Scale) | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐0.91, 3.58] |
| 7 Severity of dependence (Addiction Severity Index) | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 8 Severity of dependence (Clinical Global Impression Scale) | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.51, 0.85] |
| 9 Amount of of cocaine use during the treatment (self‐reported as dollars spent/past 30 days) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10 Depression (Hamilton Depression Rating Scale) | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐3.84, 6.52] |
| 11 Anxiety (Hamilton Anxiety Rating Scale) | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐3.02, 5.75] |
| 12 Withdrawal symptoms (Cocaine Selective Severity Assessment) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 4. Quetiapine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.20, 2.03] |
| 2 Side effects | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.77, 1.27] |
| 3 Use of cocaine during the treatment (self‐reported as days/week) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Craving (Brief Substance Craving Scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Amount of of cocaine use during the treatment (self‐reported as g/week) | 2 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.92, ‐0.16] |
| 6 Amount of of cocaine use during the treatment (self‐reported as dollars spent/week) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Depression (Hamilton Depression Rating Scale) | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐3.67 [‐6.19, ‐1.15] |
| 8 Depression (Quick Inventory of Depressive Symptomatology) | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐9.61, 7.07] |
| 9 Manic and hypomanic symptoms (Young Mania Rating Scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 5. Lamotrigine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Side effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 6. Reserpine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Craving (Brief Substance Craving Scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Severity of dependence (Addiction Severity Index) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Severity of dependence (Clinical Global Impression Scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Depression (Hamilton Depression Rating Scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 7. Olanzapine versus haloperidol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Psychopathology (Positive and Negative Syndrome Scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Craving (Voris Cocaine Craving Questionnaire‐Intensity subscale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 8. Olanzapine versus risperidone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Depression (Hamilton Depression Rating Scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 9. Aripiprazol versus ropinirol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Side effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Craving (VAS Scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Severity of dependence (Clinical Global Impression Scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Amount of of cocaine use during the treatment (self‐reported as g/week) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akerele 2007.
| Methods | Randomised double‐blind parallel trial | |
| Participants | Participants: 28, mean age 36 years, predominantly men (89%), 54% African‐American, 32% Hispanic,14% white, 26 participants current cannabis abuse/dependence and 20 cocaine dependence. Inclusion criteria: DSM‐IV criteria for cocaine dependence and schizophrenia or schizoaffective disorder. |
|
| Interventions | (1) Risperidone 9 mg/day (14 participants) versus (2) olanzapine 20 mg/day (14 participants). Previously, in a 2‐week cross‐taper phase, participants were tapered off their previously prescribed medication onto the study medication with gradual increases in doses of risperidone (3 mg/day for days 1 – 3, 6 mg/day for days 4 – 7, 9 mg/day from day 8 until end of the study) and olanzapine (5 mg/day for days 1 – 3, 10 mg/day for days 4 – 7, 15 mg/day for days 8 – 12, 20 mg/day from day 13 until end of the study). Setting: Outpatient Duration: 10 weeks Country of origin: USA |
|
| Outcomes | Substance use (Quantitative Substance Use Inventory, urinalysis and self report), craving (Cocaine Craving Report), psychiatric decompensation (PANSS, HAM‐D, CGI), side effects (AIMS, Simpson, psychiatrist assessments), compliance, retention, percentage of study completers | |
| Notes | Dates when the study was conducted: not reported Funding from the National Institute of Drug Abuse and from MARSAD and Ely Lilly and Co, Indianapolis Confict of interest: Levin received support from Ortho. Mc Neil Pharmaceuticals, Ely Lilly & Company, UCB Pharma; he served as a consultant to Shire Phamaceuticals Inc, Astra Zeneca Pharmaceuticals and Ely Lilly & Company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information reported to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement: method of concealment is not described |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 43% of participants dropped out, with imbalance between groups; reasons for missing data provided separately for each group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; it seems that the published reports include all expected outcomes, but the study protocol is not available and we therefore do not know the prespecified outcomes. |
Brown 2010.
| Methods | Randomised double‐blind placebo‐controlled trial. | |
| Participants | Participants: 12, mean age 47.4 years, 50% men Inclusion criteria: current diagnosis of cocaine dependence and reported cocaine use within 1 week prior to baseline assessment and/or a cocaine‐positive urine drug screen (UDS) result at baseline. Bipolar I or II disorder Receiving mood stabiliser prior to initiation | |
| Interventions | (1) Quetiapine 400 mg to 800 mg/day (mean exit doses 428.57 mg/day) (7 participants) versus (2) placebo (5 participants) setting: Outpatient Duration: 12 weeks Country of origin: USA |
|
| Outcomes | Attrition, side effects, compliance, cocaine use , craving (CCQ), amount of cocaine used (self‐reported g/week), psychiatric decompensation (YMRS, HAM‐D, CGI, PRD‐III) | |
| Notes | Dates when the study was conducted: not reported Funding from an investigator‐initiated grant from Astra Zeneca No conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement; randomisation process is not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of concealment is not described |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information , but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Brown 2012.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants: 112, mean age 45.1 years in the lamotrigine group and 43.5 in the placebo group, 59.8% men Inclusion criteria: current diagnosis of cocaine dependence and reported cocaine use within 14 days before randomisation. Bipolar I, II or NOS disorder as determined by SCID‐CV. Baseline Hamilton rating scale for depression (HRSD17) score ≥ 10 |
|
| Interventions | (1) Lamotrigine initiated at 25 mg/day and increased to 200 mg/day over 5 weeks. After that increased to a maximum of 400 mg/day (55 participants) versus (2) placebo (57 participants)
Setting: Outpatient
Duration: 10 weeks Country of origin: USA |
|
| Outcomes | Attrition, side effects, compliance, craving (CCQ), amount of cocaine used (self‐reported % days of use and money spent on cocaine) | |
| Notes | Dates when the study was conducted: not reported Funding from the Stanley Medical Research Institute, grant number 05T‐704 Confict of interest: Dr Brown received support from Stanley Medical Research Institute, Sunovion Pharmaceuticals, Forest Research Institute, GlaxoSmithKline and Astra Zeneca; Dr Sunderajan from Bristol‐MyersSquibb, Lilly USA, LLC, and Takeda Pharmaceuticals North America; and Dr Carmody from Cyberonics and the Institute for Chronic Illness |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted by the study statistician through a computerised randomisation process |
| Allocation concealment (selection bias) | High risk | Randomisation was downloaded to a spreadsheet used by unblinded clinic staff to allocate medication |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | All direct care staff (i.e. study physicians and raters) were blinded |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | All direct care staff (i.e. study physicians and raters) were blinded |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | All direct care staff (i.e. study physicians and raters) were blinded |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | All direct care staff (i.e. study physicians and raters) were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 120 participants randomised but "The number of subjects available for analysis was 112 (those with at least one post baseline assessment)". Not specified from which groups the 8 participants (6.6%) dropped out |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Grabowski 2004.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 96, mean age 36.9 years, 59.4% men, 79.2% white, 10.4% Hispanic, 10.4% black; mean educational level 12.3 years; 68% unemployed, 25% employed, 7% student or retired; use of cocaine: 20.9% less than once/week, 6.3% once a week, 38.5% several times/week, 28.1% once a day, 6.3% greater than 3 times/day Inclusion criteria: meet DSM criteria for cocaine dependence, good medical health and no other psychiatric diagnoses | |
| Interventions | (1) Risperidone 2 mg/day (32 participants) (2) risperidone 4 mg/day (31 participants) versus (3) placebo (33 participants) Setting: Outpatient Duration: 24 weeks Country of origin: USA | |
| Outcomes | Retention, side effects, cocaine use, changes in blood pressure, psychiatric decompensation (BDI) | |
| Notes | Dates when the study was conducted: not reported Funding from NIDA Grants DA P50‐9262, DA RO1‐6143 AND DA RO1 16302 No conflicts of interest existed for any author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement; randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of allocation is not described |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Hamilton 2009.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 48, mean age 45.8 years, all male veterans, with 41 (85.4%) Africa‐American and 7 (14.6%) white. Means of cocaine use and money spent on drugs 30 days before study entry: 11.28 days and USD 357; reported routes of cocaine administration: 73.9% smoking, 10.9% nasal, 6.5% IV, 6.5% non‐IV injection, and 2.2% oral; other substances used 30 days before entry: alcohol (85.1%), heroin (6.4%), methadone (10.6%), other opioids (8.5%), sedative‐hypnotics (14.9%), and cannabis (25.5%), 87.2% reported using more than 1 substance per day;
Comorbid diagnoses: depression not otherwise specified (2.1%), dysthymic disorder (2.1%), post‐traumatic stress disorder (2.1%), obsessive‐compulsive disorder (4.2%), and bereavement (2.1%) Inclusion criteria: ≥ 18 years, diagnosis of cocaine dependence as determined by a clinician interview using a checklist of DSM‐IV criteria and active use of cocaine within the past 30 days, determined by urine testing or self report |
|
| Interventions | (1) starting 2.5 mg olanzapine per day, could be titrated up to a maximum daily dose of 20 mg (23 participants), versus (2) placebo (25 participants) Setting: Outpatient Duration: 16 weeks (20 weeks with follow‐up visit) Country of origin: USA |
|
| Outcomes | Cocaine use (urinalysis), craving (Craving Questionnaire), side effects (Barnes Akathisia Scale, Simpson‐Angus Scale, the AIMS, 7 ASI subscales), compliance (pill count), severity of dependence (ASI), attrition (number of sessions attended) | |
| Notes | Dates when the study was conducted: not reported Funding from an investigator‐initiated grant from Eli Lilly & Company, Indianapolis, Indiana No conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement; only described that participants were randomised to receive in double‐blind fashion either olanzapine or placebo (1:1 ratio) |
| Allocation concealment (selection bias) | Low risk | A support staff member not involved with the treatment of participants obtained the randomisation assignment by opening a sealed opaque envelope and conveyed the assignment to a research pharmacist. |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | A support staff member not involved with the treatment of participants obtained the randomisation assignment by opening a sealed opaque envelope and conveyed the assignment to a research pharmacist, who dispensed the study medication in coded containers and identical‐appearing placebo. |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | A support staff member not involved with the treatment of participants obtained the randomisation assignment by opening a sealed opaque envelope and conveyed the assignment to a research pharmacist, who dispensed the study medication in coded containers and identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants ( 6%) not included in the analysis for side effects |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Kampman 2003.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 30, mean age 41 years, 73.3% men, 93.3% African‐American, 3.3% white, 3.3% Native American; mean educational level 12.33 years; cocaine use: past 30 days mean 12.5 days, use lifetime mean 12 years, numbers of prior treatments mean 2.5; route of administration: 10% intranasal, 86.6% smoked, 10% intravenous Inclusion criteria: age 18 ‐ 60, cocaine addiction (certified by a psychiatrist), self‐reported at least USD 100 worth of cocaine use in the month prior to entry Exclusion criteria: dependence on any additional drug (except nicotine and alcohol), psychosis, dementia, use of psychotropic medications, unstable medical illness, pregnancy, hypersensitivity to olanzapine | |
| Interventions | (1) Olanzapine 10 mg/day (15 participants) versus (2) placebo (15 participants) Setting: Outpatient Duration: 12 weeks Country of origin: USA | |
| Outcomes | Retention, craving (BSCS), use of cocaine (self‐reported as days used in past 30 days ), amount of cocaine used (money spent in past 30 days), side effects, severity of dependence (ASI, CGI), anxiety (HAM‐A), depression (HAM‐D), withdrawal symptom (CSSA) | |
| Notes | Dates when the study was conducted: not reported Funding by a grant from the Eli Lilly Company No conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation method has been used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of allocation is not described |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/30 participants dropped out, balanced between groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Levin 1999.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 14, mean age 39.9 years, 71% men, 43% African‐American, 43% Hispanic, 14% white; mean educational level 13.6 years; cocaine use: past 30 days mean 16.1 days, mean amount spent last 30 days USD 70.3; route of administration: 50% intranasal, 50% intravenous/freebase Inclusion criteria: DSM‐IV criteria for cocaine dependence Exclusion criteria: physiologic dependence on alcohol, opiates or sedatives; current major depression or dysthymia or any other Axis I disorder requiring psychiatric treatment; pregnancy | |
| Interventions | (1) Risperidone mean 2.1 mg/day (9 participants) versus (2) placebo (5 participants) Setting: Outpatient Duration 12 weeks Country of origin: USA | |
| Outcomes | Dropouts, cocaine use (urinalysis and self report), craving (VAS), side effects | |
| Notes | Dates when the study was conducted: not reported Funding by grants K20 DA‐00214 (Dr. Levin), K02 DA‐00288 (Dr. Nunes), and P50 DA‐09236 from the National Institute on Drug Abuse No conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement; randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of allocation is not described |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; only reported that it is double‐blind |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data imputed in analysis and reasons balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported. |
Loebl 2008.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 31, mean age 44.1 in the risperidone group and 42.4 in the placebo group, all men. Mean baseline frequency of cocaine use 12.6 days in the past 30 days Inclusion criteria: men, age 18 to 60 years, with DSM‐IV criteria for cocaine dependence, use of cocaine by self report of at least once every week and 2 urine samples positive for cocaine Exclusion criteria: diagnostic criteria of schizophrenia, bipolar disorder, current severe major depressive disorder or HIV infection, head trauma with loss of consciousness, corrected QT interval > 450 msec or another unstable medical condition |
|
| Interventions | (1) Risperidone (long‐acting) 25 mg IM every other week (16 participants) and behavioural intervention, versus (2) placebo and behavioural intervention (15 participants) Setting: Outpatient Duration 12 weeks Country of origin: USA | |
| Outcomes | Dropouts, side effects (SATEEGI), cocaine use (urinary concentration of cocaine metabolites and self report), compliance, craving (University of Minnesota Cocaine Craving Scale), withdrawal symptoms (CSSA), severity of dependence (ASI), psychiatric symptoms (SHPS, HAM‐D) | |
| Notes | Dates when the study was conducted: October 2005‐ September 2006 Funding by a grant from Janssen Pharmaceutica to Dr. Evins and by an invest fellowship from the NIDA International programme to Dr. Loebl No conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement; only described that participants were randomised |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of concealment is not described |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Meini 2010.
| Methods | Randomised clinical trial (open label) | |
| Participants | Participants 28, 22 men, mean age 33.4 years. 75% were employed and 93% were living with their family or with their partner, 43% had more than 8 years of education Inclusion criteria: Out‐patients, age 18 to 65 years, diagnosis of cocaine dependence ICD‐9‐CM criteria (304.20 code), at least 3 preadmission urine samples positive for cocaine metabolites throughout the latest month preceding enrolment, carried out every 72 ‐ 96 hours. Exclusion criteria: diagnosis of cocaine abuse (305.60 ICD‐9‐CM code), or remission of cocaine dependence (304.23 ICD‐9‐CM code), or any other current Axis I Disorder (DSM‐IV‐TR), organic mental disorders or patients at serious suicidal risk or with significant auto‐aggressive behaviour. Also chronic medical illnesses, pregnancy or nursing, hyperglycaemia, lactose intolerance or malabsorption, malignant neuroleptic syndrome, epileptic seizures, any kind of pharmacological treatment, psychotherapy treatment, therapeutic communities treatment or detention. But not opioid dependence comorbidity if treated with a constant dosage of methadone, or remission of life‐time psychiatric disorders. |
|
| Interventions | (1) Aripriprazol 10 mg/day (16 participants) versus (2) ropinirole 1.5 mg x 3/day (12 participants) Setting: Outpatient Duration: 12 weeks Country of origin: Italy |
|
| Outcomes | Dropouts, side effects, craving (VAS), cocaine use (urinalysis), amount of cocaine used (self‐reported g/week), severity of dependence (CGI severity score) | |
| Notes | Dates when the study was conducted: between May 2008 and June 2009 Funding not reported The authors report no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by a statistician using an ad hoc procedure in SPSS and kept by an administrative staffer of the co‐ordinating centre not involved in participant recruitment. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; only specified that randomisation was concealed until inclusion criteria were determined and then communicated to the participating centres |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Open‐label design |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | High risk | Open‐label design and not otherwise specified |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46.6% dropout. ITT analysis performed only for percentage of urine positive |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Reid 2005.
| Methods | Randomised double‐blind placebo‐controlled trial. | |
| Participants | Participants 63, mean age 38.7 years, 50 men, 11 white, 51 black, 1 other; mean educational level 13 years, use of cocaine, lifetime men 14 years, last 30 days mean 16.8 days; route of administration: 20.8% intranasal, 76% smoked, 3% injected Inclusion and exclusion criteria: CREST criteria | |
| Interventions | Olanzapine 10 mg/day (16 participants), versus placebo (15 participants) Setting: Outpatient Duration: 8 weeks Country of origin: USA | |
| Outcomes | Dropouts, side effects, use of cocaine self‐reported (TLFB), craving (BSCS, CCQ), severity of dependence (ASI, CGIS), anxiety (HAM‐A), depression (HAM‐D) | |
| Notes | Dates when the study was conducted: not reported Funding by a contract from the National Institute on Drug Abuse: YO1 DA 50038 No conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement; randomisation process not described |
| Allocation concealment (selection bias) | Low risk | Study medications were dispensed by, and returned to, a non‐blinded pharmacist. All other study staff and investigators were blinded to treatment assignment |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | Study medications were dispensed by, and returned to, a non‐blinded pharmacist. All other study staff and investigators were blinded to treatment assignment |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Study medications were dispensed by, and returned to, a non‐blinded pharmacist. All other study staff and investigators were blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Smelson 2004.
| Methods | Randomised double blind controlled trial | |
| Participants | Participants 35, mean age 41.2, cocaine use in the last 30 days: mean 5.4; years of cocaine use: 6.3 Inclusion criteria: DSM‐IV criteria for cocaine dependence, use of at least 6 g of cocaine in the past month, responded to cue exposure increased craving. Exclusion criteria: DSM‐IV criteria for any additional AXIS I disorder and dependence (excluded nicotine), taking prescribed medication that could affect the central nervous system, history of seizures, pregnancy | |
| Interventions | Risperidone 1 mg/day (19 participants), placebo (16 participants). Cue exposure: videotape. Setting: Inpatient Duration: 2 weeks Country of origin: USA | |
| Outcomes | Dropouts, craving (VCCQ) | |
| Notes | Dates when the study was conducted: not reported The project was supported by the VISN 3 Mental Illness, Research and Clinical Center, the VA New Jersey Health Care System and Janssen Research Foundation No conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement; randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; only specified that randomisation was concealed until inclusion criteria were determined and then communicated to the participating centres |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.5% dropout, balanced between groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Smelson 2006.
| Methods | Randomised controlled trial | |
| Participants | Participants 31, mean age 42.9, cocaine use in the last 30 days: mean 8.4; age of first cocaine use mean 29.3
Inclusion criteria: DSM‐IV criteria for cocaine dependence and schizophrenia, showed any positive change in baseline craving following cocaine cue exposure Exclusion criteria: DSM‐IV criteria for any additional AXIS I disorder and dependence (excluded nicotine), taking prescribed medication that could affect the central nervous system, history of seizures, pregnancy, chronic disease of the central nervous system other than schizophrenia |
|
| Interventions | Olanzapine 10 mg/day (16 participants), haloperidol 10 mg/day (15 participants) Cue exposure videotape. Setting: Inpatient Duration: 6 weeks Country of origin: USA | |
| Outcomes | Dropouts, craving (VCCQ), psychopathology (PANSS), withdrawal symptoms (PANSS) | |
| Notes | Dates when the study was conducted: not reported Funding by an investigator‐initiated grant from Eli Lilly and grants from Substance Abuse and Mental Health Service Administration (H79 TI16576), National Institute of Complimentary and Alternative Medicine (R21 AT001350), and a VA HSR&D Merit Review (MR‐2‐09499) to DS, and National Institute of Drug Abuse (R01 DA15978 and RO1 DA 15537) to DZ. Conflict of interest; the authors received support from Eli Lilly (David Smelson), Astra Zeneca (David Smelson), Bristol Meyers Squib (David Smelson, Douglas Ziedonis), and Janssen (David Smelson, Douglas Ziedonis, Maureen Kaune), Forest (Douglas Ziedonis), and New Jersey Comprehensive Tobacco Control Program (Jill Williams) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement; only described that participants were randomised |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement; method of concealment is not described |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 42% dropout, balanced between groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Tapp 2015.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 60, mean age 47.9, 86.6% men Inclusion criteria: Has used cocaine within the 30 days prior to screening Exclusion criteria: DSM‐IV criteria for any psychotic disorder (bipolar, schizophrenia, etc.) or who were psychiatrically or medically unstable, pregnant or nursing |
|
| Interventions | (1) Quetiapine 400 mg/day (29 participants) versus placebo (31 participants) Setting:outpatient Duration: 12 weeks Country of origin: USA |
|
| Outcomes | Dropouts, side effects, compliance, craving (BSCS), cocaine use: end‐of‐trial abstinence measured by urinalysis and self‐reported use (TLFB), amount of cocaine used (self‐reported in dollars spent/week, days of cocaine use/week and g/week) | |
| Notes | Dates when the study was conducted: not reported Funded by the Seattle Institute for Biomedical and Clinical Research and in collaboration with the VA Puget Sound Health Care System and Astra Zeneca Confict of interest: Dr. Tapp received support from Astra Zeneca |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation computer software was used for a randomised block design with randomly assigned block sizes of 2, 4, and 6 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 68% dropped out, unbalanced in numbers or reasons reported between groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
Winhusen 2007.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants 119, mean age 41.2 in the reserpine group and 40.7 in the placebo group, 70% men Inclusion criteria: DSM‐IV criteria for cocaine dependence and at least 1 positive urine toxicology screen for cocaine during the 2‐week screening period, and to be seeking treatment for cocaine dependence | |
| Interventions | (1) Reserpine 0.50 mg (60 participants) versus placebo (59 participants) Setting:outpatient Duration: 12 weeks Country of origin: USA |
|
| Outcomes | Retention, side effects, cocaine non‐use days (self‐reported and by urine drug test), compliance, craving (BSCS), severity of addiction (ASI), depression (HAM‐D) | |
| Notes | Dates when the study was conducted: not reported Funded by the National Institutes of Health, National Institute on Drug Abuse through contract N01‐DA‐9‐8095 (E. Somoza) No conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation, balancing for gender and self report of cocaine use was used to assign eligible participants to reserpine or placebo within each study site |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | Insufficient information to permit judgement; it only reports that the study was double‐blind |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Insufficient information, but objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 34% dropout, balanced between groups |
| Selective reporting (reporting bias) | Low risk | All the study’s prespecified primary outcomes have been reported |
ASI: Addiction Severity Index BDI: Beck Depression Inventory BSCS: Brief Substance Craving Scale CCQ: Cocaine Craving Questionnaire CGIS: Clinical Global Impression Scale CREST:Cocaine Rapid Efficacy Screening Trial CSSA: Cocaine Selective Severity Assessment DSM: Diagnostic and Statistical Manual of Mental Disorders IDS‐C‐30: Clinical‐Rated Inventory of Depressive Symptomatology HAM‐A: Hamilton Anxiety Rating Scale HAM‐D: Hamilton Depression Rating Scale ITT: intention‐to‐treat PANSS: Positive and Negative Syndrome Scale SATEEGI: Systematic Assessment for Treatment Emergent Events General Inquiry SHPS: Snaith Hamilton Pleasure Scale SCQ‐10: Stimulant Craving Questionaire TLFB: Timeline Followback Interview VAS Scale: Visual Analogue Scale VCCQ: Voris Cocaine Craving Questionnaire WSRS: Within Session Rating Scale YMRS: Young Mania Rating Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berger 1996 | Excluded, as the outcome was not in the inclusion criteria: cross‐over study with 12 participants assessing craving up to 1 hour after the administration of a cue exposure. Previously an included study in Amato 2007 |
| Ersche 2010 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: attentional bias for stimulant‐related words was measured |
| Evans 2001 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular system and subjective response to cocaine |
| Farren 2000 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine |
| Grabowski 2000 | Excluded as it was impossible to extract useful data from the study: number of participants allocated to each group not stated |
| Grabowski 2006 | Excluded as the objective of the study was not in the inclusion criteria.This trial was included and classified as ongoing in the original review and has been excluded from this update, because of a modification of the study protocol before initiating the original trial in 2007, substituting the pharmacological intervention with an antipsychotic drug (aripiprazol) with one of an antidepressant (citalopram). |
| Haney 2011 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular system and subjective response to cocaine. This study was identified as ongoing in the original review. |
| Landabaso 2009 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: cocaine consumption assessed in opiate‐dependent patients in Methadone Maintenance Therapy (MMT) who use cocaine (not cocaine dependence) |
| Landabaso 2003 | Excluded as it was impossible to extract useful data from the study: no raw data for outcome measures, only P‐values |
| Lile 2008 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess the safety and tolerability of intranasal cocaine during maintenance with the drug. |
| Lile 2011 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular system and subjective response to cocaine. |
| Lofwall 2014 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the reinforcing efficacy of cocaine. |
| Middleton 2009 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug given by the researcher to assess their effects on the reinforcing efficacy of cocaine. |
| Máñez 2010 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: looking to assess the value of amisulpride as antipsychotic treatment. |
| Netjek 2008 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: results provided for drug use defined as cocaine or metamphetamine use, without reporting separately results for cocaine use. This study was identified as ongoing in the original review. |
| Nuzzo 2012 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: aripiprazole effects on cigarette smoking among cocaine users were measured. |
| Price 1997 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine. |
| Rubio 2006a | Excluded as it was impossible to extract useful data from the study: data not separately reported for cocaine‐dependent participants. |
| Rubio 2006b | Excluded as it was impossible to extract useful data from the study: data not separately reported for cocaine‐dependent participants. |
| Rush 2009 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular, nervous system and subjective response to cocaine. |
| Sayers 2005 | Excluded as it was impossible to extract useful data from the study: number of participants allocated to each group not stated and no raw data for outcome measures reported, only P‐values. |
| Sherer 1988 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their effects on the cardiovascular system and subjective response to cocaine. |
| Stoops 2007 | Excluded as the objective of the study and the outcomes were not in the inclusion criteria: drug and cocaine given simultaneously by the researcher to assess their safety, tolerability, and subject‐rated effects. |
| Tsuang 2002 | Excluded as only 4 participants have been included in the study and the results have been presented only for 3. |
Differences between protocol and review
We now excluded one study (Grabowski 2006), included in the previous version as an ongoing trial which met the inclusion criteria, due to a modification in the study protocol in 2007. The pharmacological intervention had been modified, substituting an antipsychotic drug (aripiprazol) with an antidepressant (citalopram), thus rendering it ineligible for this update. Another study included in the previous version (Berger 1996) was excluded from the update because the outcome did not comply with our inclusion criteria.
Meta‐analysis of continuous outcomes of the old studies of ASI, CGI‐O, HAM‐D and HAM‐A had to be redone for postintervention outcomes, because the previous type of analysis comparing before‐and‐after changes was incorrect.
Contributions of authors
In the first version LA wrote the background, inspected search hits by reading titles and abstracts, assessed full texts for inclusion, extracted data, made the analysis, wrote the results section, drafted conclusions. SM assessed full texts for inclusion, extracted data, assessed risk of bias, made the analysis, wrote the results section, PP helped with suggestion in writing the background and wrote the discussion.
In the present update BII, SM extracted data, assessed risk of bias, performed the analysis and wrote the main text of the review. LA and PP supervised and wrote the Discussion and the conclusions.
Sources of support
Internal sources
Department of Epidemiology, ASL RM E, Italy.
European Monitoring Centre for Drugs and Drug Addictionof support, Portugal.
External sources
National Institute of Health, Italy.
Declarations of interest
Blanca I Indave: None known.
Silvia Minozzi: None known.
Pier Paolo Pani: None known.
Laura Amato: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Akerele 2007 {published data only}
- Akerele E, Levin FR. Comparison of olanzapine to risperidone in substance‐abusing individuals with schizophrenia. The American Journal on Addictions 2007;16(4):260‐8. [DOI] [PubMed] [Google Scholar]
Brown 2010 {published data only}
- Brown ES, Gabrielson P, Gu P. A randomized, double‐blind, placebo‐controlled pilot study of quetiapine in outpatients with bipolar disorder and cocaine dependence. Bipolar Disorders 2009;11(S1):25. [Google Scholar]
Brown 2012 {published data only}
- Brown ES, Sunderajan P, Hu LT, Sowell SM, Carmody TJ. A randomized, double‐blind, placebo‐controlled trial of lamotrigine therapy in bipolar disorder, depressed or mixed phase and cocaine dependence. Neuropsychopharmacology 2012;37(11):2347‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grabowski 2004 {published data only}
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, et al. Agonist‐like or antagonist‐like treatment for cocaine dependence with methadone for heroin dependence: two double‐blind randomized clinical trials. Neuropsychopharmacology 2004;29(7):969‐81. [DOI] [PubMed] [Google Scholar]
Hamilton 2009 {published data only}
- Hamilton JD, Nguyen QX, Gerber RM, Rubio NB. Olanzapine in cocaine dependence: a double‐blind, placebo‐controlled trial. The American Journal on Addictions 2009;18(1):48‐52. [DOI] [PubMed] [Google Scholar]
Kampman 2003 {published data only}
- Kampman KM, Pettinati H, Lynch KG, Sparkman T, O'Brien CP. A pilot trial of olanzapine for the treatment of cocaine dependence. Drug and Alcohol Dependence 2003;70(3):265‐73. [DOI] [PubMed] [Google Scholar]
Levin 1999 {published data only}
- Levin FR, McDowell D, Evans SM, Brooks D, Spano C, Nunes EV. Pergolide mesylate for cocaine abuse: a controlled preliminary trial. The American Journal on Addiction 1999;8(2):120‐7. [DOI] [PubMed] [Google Scholar]
Loebl 2008 {published data only}
- Loebl T, Angarita GA, Pachas GN, Huang KL, Lee SH, Nino J, et al. A randomized, double‐blind, placebo‐controlled trial of long‐acting risperidone in cocaine‐dependent men. Journal of Clinical Psychiatry 2008;69(3):480‐6. [DOI] [PubMed] [Google Scholar]
Meini 2010 {published data only}
- Meini M, Moncini M, Cecconi D, Cellesi V, Biasci L, Simoni G, et al. Aripiprazole and ropinirole treatment for cocaine dependence: evidence from a pilot study. Current Pharmaceutical Design 2011;17(14):1‐8. [DOI] [PubMed] [Google Scholar]
- Meini M, Moncini M, Cecconi D, Cellesi V, Biasci L, Simoni G, et al. Safety, tolerability, and self‐rated effects of aripiprazole and ropinirole treatment for cocaine dependence: a pilot study. The American Journal on Addictions 2010;20(2):179‐80. [DOI] [PubMed] [Google Scholar]
Reid 2005 {published data only}
- Reid MS, Casadonte P, Baker S, Sanfilippo M, Braunstein D, Hitzemann R, et al. A placebo‐controlled screening trial of olanzapine, valproate and coenzyme Q10/L‐carnitine for the treatment of cocaine dependence. Addiction 2005;100(Suppl 1):43‐57. [DOI] [PubMed] [Google Scholar]
Smelson 2004 {published data only}
- Smelson DA, Williams J, Ziedonis D, Sussner BD, Losonczy MF, Engelhart C, et al. A double‐blind placebo controlled pilot study of risperidone for decreasing cue‐elicited craving in recently withdrawn cocaine dependent patients. Journal of Substance Abuse Treatment 2004;27(1):45‐9. [DOI] [PubMed] [Google Scholar]
Smelson 2006 {published data only}
- Smelson DA, Ziedonis D, Williams J, Losonczy MF, Williams J, Steinberg ML, et al. The efficacy of olanzapine for decreasing cue‐elicited craving in individuals with schizophrenia and cocaine dependence. Journal of Clinical Psychopharmacology 2006;26(1):9‐12. [DOI] [PubMed] [Google Scholar]
Tapp 2015 {published data only}
- NCT00631748. Quetiapine for the reduction of cocaine use. clinicaltrials.gov/ct2/show/results/NCT00631748 2014.
- Tapp A, Wood A, Sylvers P, Kilzieh N, Saxon A. Quetiapine for the treatment of cocaine dependence. Addictive Behaviors 2013;38:2733. [Google Scholar]
- Tapp A, Wood A, Sylvers P, Kilzieh N, Saxon A. Quetiapine for the treatment of cocaine dependence. European Psychiatry 2013;28(S1):273. [Google Scholar]
- Tapp A, Wood AE, Kennedy A, Sylvers P, Kilziehb N, Saxon AJ. Quetiapine for the treatment of cocaine use disorder. Drug and Alcohol Dependence 2015;149:18–24. [DOI] [PubMed] [Google Scholar]
Winhusen 2007 {published data only}
- Winhusen T, Somoza E, Sarid‐Segal O, Goldsmith RJ, Harrer JM, Coleman FS, et al. A double‐blind, placebo‐controlled trial of reserpine for the treatment of cocaine dependence. Drug and Alcohol Dependence 2007;91(2‐3):205‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Berger 1996 {published data only}
- Berger SP, Hall S, Mickallan JD, Reid MS, Crawford CA, Delucchi K, et al. Haloperidol antagonism of cue‐elicited cocaine craving. Lancet 1996;347(9000):504‐8. [DOI] [PubMed] [Google Scholar]
Ersche 2010 {published data only}
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Muller U, et al. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Archives of General Psychiatry 2010;67(6):632‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2001 {published data only}
- Evans SM, Walsh SL, Levin FR, Foltin RW, Fischman MW, Bigelow GE. Effect of flupenthixol on subjective and cardiovascular responses to intravenous cocaine in humans. Drug and Alcohol Dependence 2001;64(3):271‐83. [DOI] [PubMed] [Google Scholar]
Farren 2000 {published data only}
- Farren CK, Hameedi FA, Rosen MA, Woods S, Jatlow P, Kosten TR. Significant interaction between clozapine and cocaine in cocaine addicts. Drug and Alcohol Dependence 2000;59(2):153‐63. [DOI] [PubMed] [Google Scholar]
Grabowski 2000 {published data only}
- Grabowski J, Rhoades H, Silverman P, Schmitz JM, Stotts A, Creson D, et al. Risperidone for the treatment of cocaine dependence: randomized, double trial. Journal of Clinical Psychopharmacology 2000;20(3):305‐10. [DOI] [PubMed] [Google Scholar]
Grabowski 2006 {unpublished data only}
- NCT00218036. Pharmacotherapy dosing regimen in cocaine and opiate dependent individuals. clinicaltrials.gov/ct2/show/record/NCT00218036.
Haney 2011 {published data only}
- Haney M, Rubin E, Foltin RW. Aripiprazole maintenance increases smoked cocaine self‐administration in humans. Psychopharmacology 2011;216(3):379‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Landabaso 2003 {published data only}
- Landabaso M, Iraurgi I, Jimenez JM, Hormaechea JA, Sanz J, Larrazabal A, et al. Olanzapina and cocaine consumption in methadone maintenance programs: new results [Olanzapina y consumo de cocaina en programas de mantenimiento con metadona: nuevos resultados]. Psiquiatría Biológica 2003;10(5):160‐4. [Google Scholar]
Landabaso 2009 {published data only}
- Vazquez ML, Castillo II, Jimenez‐Lerma JM, Beldarrain JAH, Gutierrez‐Fraile M. Clinical trial on the use of olanzapine in reducing the consumption of cocaine in methadone maintenance programmes. Heroin Addiction and Related Clinical Problems 2009;11(2):21‐9. [Google Scholar]
Lile 2008 {published data only}
- Lile JA, Stoops W, Hays LR, Rush CR. The safety, tolerability and subject‐rated effects of acute intranasal cocaine administration during aripiprazole maintenance II: Increased aripipirazole dose and maintenance period. American Journal of Drug and Alcohol Abuse 2008;34(6):721‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lile 2011 {published data only}
- Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR. Discriminative stimulus, subject‐rated and cardiovascular effects of cocaine alone and in combination with aripiprazole in humans. Journal of Psychopharmacology (Oxford, England) 2011;25(11):1469‐79. [DOI] [PubMed] [Google Scholar]
Lofwall 2014 {published data only}
- Lofwall MR, Nuzzo PA, Campbell C, Walsh SL. Aripiprazole effects on self‐administration and pharmacodynamics of intravenous cocaine and cigarette smoking in humans. Experimental and Clinical Psychopharmacology 2014;22(3):238‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Máñez 2010 {published data only}
- Máñez AS, Muñoz CP, Aleixandre NL, Garcia ML, Tudela LL, Gómez FJ. Treatment with amisulpride of addicted patients to psychoactive substances and psychotic symptoms. Actas Españolas de Psiquiatría 2010;38(3):138‐48. [PubMed] [Google Scholar]
Middleton 2009 {published data only}
- Middleton L, Lofwall M, Nuzzo PA, Walsh SL. Safety of oral aripiprazole alone and in combination with intravenous cocaine in humans. Proceedings of the 71st Annual Scientific Meeting of the College on Problems of Drug Dependence; Reno/Sparks, Nevada, USA. 2009; Vol. 20‐5:102.
Netjek 2008 {published data only}
- Nejtek VA, Avila M, Chen LA, Zielinski T, Djokovic M, Podawiltz A, et al. Do atypical antipsychotics effectively treat co‐occurring bipolar disorder and stimulant dependence? A randomized, double‐blind trial. Journal of Clinical Psychiatry 2008;69(8):1257‐66. [DOI] [PubMed] [Google Scholar]
Nuzzo 2012 {published data only}
- Nuzzo PA, Lofwall MR, Walsh SL. Aripiprazole effects on cigarette smoking among cocaine users. Proceedings of the 74th Annual Scientific Meeting of the College on Problems of Drug Dependence, Palm Springs, CA , Abstract. 2012.
Price 1997 {published data only}
- Price LH, Pelton GH, McDougle CJ, Malison RT, Jatlow P, Carpenter L, et al. Effects of acute pretreatment with risperidone on responses to cocaine in cocaine addicts. Poster presented at the 36th Annual Meeting of the American College of Neuropsychopharmacology, Waikoloa, Hawai, USA. 1997.
Rubio 2006a {published data only}
- Rubio G, Martínez I, Recio A, Ponce G, López‐Muñoz F, Alamo C, et al. Risperidone versus zuclopenthixol in the treatment of schizophrenia with substance abuse comorbidity: a long‐term randomized, controlled, crossover study. The European Journal of Psychiatry 2006;20(3):133‐46. [Google Scholar]
Rubio 2006b {published data only}
- Rubio G, Martínez I, Ponce G, Jiménez‐Arriero MA, López‐Muñoz F, Alamo C. Long‐acting injectable risperidone compared with zuclopenthixol in the treatment of schizophrenia with substance abuse comorbidity. Journal of Clinical Psychiatry 2006;51(8):531‐9. [DOI] [PubMed] [Google Scholar]
Rush 2009 {published data only}
- Rush CR, Stoops WW, Lile JA, Lofwall MR, Hays LR. Aripiprazole as a pharmacotherapy for cocaine dependence. The American Journal on Addiction 2009;18(4):326. [Google Scholar]
Sayers 2005 {published data only}
- Sayers SL, Campbell EC, Kondrich J, Mann SC, Cornish J, O'Brien C, et al. Cocaine abuse in schizophrenic patients with olanzapine versus haloperidol. The Journal of Nervous and Mental Diseases 2005;139(6):379‐86. [DOI] [PubMed] [Google Scholar]
Sherer 1988 {published data only}
- Sherer MA, Kumor KM, Jaffe JH. Effects of intravenous cocaine are partially attenuated by haloperidol. Psychiatry Research 1988;27(2):117‐25. [DOI] [PubMed] [Google Scholar]
Stoops 2007 {published data only}
- Stoops W, Lile JA, Lofwall M, Rush CR. The safety, tolerability, and subject‐rated effects of acute intranasal cocaine administration during aripiprazole maintenance. The American Journal of Drug and Alcohol Abuse 2007;33(6):769‐76. [DOI] [PubMed] [Google Scholar]
Tsuang 2002 {published data only}
- Tsuang J, Marder SR, Han A, Hsieh W. Olanzapine treatment for patients with schizophrenia and cocaine abuse. Journal of Clinical Psychiatry 2002;63(12):1180‐1. [DOI] [PubMed] [Google Scholar]
Additional references
Amato 2010
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005063.pub3] [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory, 2nd edn. The Psychological Corporation: San Antonio, TX, 1996. [Google Scholar]
Berk 1999
- Berk M, Brooks S, Trandafir A. A comparison of olanzapine with haloperidol in cannabis‐induced psychotic disorder: a double blind, randomized controlled trial. International Clinical Psychopharmacology 1999;14(3):177‐80. [PubMed] [Google Scholar]
Castells 2010
- Castells X, Casas M, Pérez‐Mañá C, Roncero C, Vidal X, Capellà D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD007380.pub3] [DOI] [PubMed] [Google Scholar]
Dackis 2002
- Dackis CA, O'Brien CP. Cocaine dependence: the challenge for pharmacotherapy. Current Opinion in Psychiatry 2002;15:261‐8. [Google Scholar]
Di Chiara 1988
- Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America 1988;85(14):5274‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drevets 1999
- Drevets WC, Price JC, Kupfer DJ, Kinahan PE, Lopresti B, Holt D, et al. PET measures of amphetamine‐induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology 1999;21(6):694‐709. [DOI] [PubMed] [Google Scholar]
Drevets 2001
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine‐induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry 2001;49(2):81‐96. [DOI] [PubMed] [Google Scholar]
DSM‐IV‐R
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
EMCDDA 2014a
- European Monitoring Centre for Drug and Drug Addiction. Emergency health consequences of cocaine use in Europe. A review of the monitoring of drug‐related acute emergencies in 30 European countries.. www.emcdda.europa.eu/attachements.cfm/att_226037_EN_Cocaine_emergencies_report_final.pdf Accessed 24th February 2016.
EMCDDA 2014b
- European Monitoring Centre for Drugs and Drug Addiction. Data and statistics by topic. www.emcdda.europa.eu/data/2014 Accessed 24th February 2016.
EMCDDA 2015
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2015: Trends and developments. www.emcdda.europa.eu/publications/edr/trends‐developments/2015 2015.
Faggiano 2003
- Faggiano F, Vigna‐Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD002208] [DOI] [PubMed] [Google Scholar]
Filip 2005
- Filip M, Frankowska M, Zaniewska M, Golda A, Przegalinski E. The serotonergic system and its role in cocaine addiction. Pharmacological Reports 2005;57(6):685‐700. [PubMed] [Google Scholar]
Grabowski 1997
- Grabowski J, Roache JD, Scmitz JM, Rhoades H, Creson D, Korszun A. Replacement medication for cocaine dependence. Journal of Clinical Psychopharmacology 1997;17(6):485‐8. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. The GRADE working group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Assessment Manual for Psychopharmacology. Washington DC: US Department of Health Education and Welfare, 1976. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Halikas 1991
- Halikas JA, Kuhn KL, Crosby R, Carlson G, Crea F. The measurement of craving in cocaine patients using the Minnesota Cocaine Craving Scale. Comprehensive Psychiatry 1991;32(1):22‐7. [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. The British Journal Medical Psychology 1959;32(1):50‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1967
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal ofSocial and Clinical Psychology 1967;6(4):278‐96. [DOI] [PubMed] [Google Scholar]
Higgins 1994
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badjer GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry 1994;51(7):568‐76. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kampman 1998
- Kampman K, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D'Angelo L, et al. Reliability and validity of the cocaine selective severity assessment. Addictive Behavaviors 1998;23(4):449‐61. [DOI] [PubMed] [Google Scholar]
Kay 1992
- Karch SB. A Brief History of Cocaine. Vol. 2nd edition, New York NY: CRC Press, 1992. [Google Scholar]
Kosten 1996
- Kosten T, McCance E. A review of pharmacotherapies for substance abuse. American Journal of Addiction 1996;5(1):58‐65. [Google Scholar]
Kuhar 1996
- Kuhar MJ, Pilotte NS. Neurochemical changes in cocaine withdrawal. Trends in Pharmacological Sciences 1996;17(7):260‐4. [DOI] [PubMed] [Google Scholar]
Leucht 1999
- Leucht S, Pitschel‐Waltz G, Abraham D, Kissling W. Efficacy and extrapyramidal side effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta‐analysis of randomised controlled trials. Schizophrenia Research 1999;35(1):51‐68. [DOI] [PubMed] [Google Scholar]
Lima Reisser 2009
- Lima Reisser A, Lima MS, Soares BGO, Farrell M. Carbamazepine for cocaine dependence. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD002023.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattick 2014
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD002207.pub4] [DOI] [Google Scholar]
McCormack 1988
- McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales. A critical review. Psychological Medicine 1988;18(4):1007‐19. [DOI] [PubMed] [Google Scholar]
McLellan 1992
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse and Treatment 1992;9(3):199‐213. [DOI] [PubMed] [Google Scholar]
Minozzi 2010
- Minozzi S, Amato L, Vecchi S, Davoli M. Anticonvulsants for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005064.pub3] [DOI] [PubMed] [Google Scholar]
Minozzi 2015
- Minozzi S, Amato L, Pani PP, Solimini R, Vecchi S, Crescenzo F, et al. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD003352.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
NIMH 1988
- National Institute of Mental Health (NIMH), Psychopharmacology Research Branch. Abnormal Involuntary Movement Scale (AIMS). Psychopharmacology Bulletin 1988;24:781‐3. [PubMed] [Google Scholar]
NSDUH 2014
- Substance Abuse and Mental Health Services Administration. The NSDUH Report: substance use and mental health estimates from the 2013 National Survey on drug use and health: overview of findings. Summary of national findings (HHS Publication No. 14–4863, NSDUH Series H–48). Rockville MD. [PubMed]
O'Brian 2001
- O'Brian C. Drug addiction and drug abuse. In: Hardman JG, Lee CL editor(s). The Pharmacological Basis of Therapeutics. New York: MCGraw‐Hill, 2001:621‐42. [Google Scholar]
Pani 2011
- Pani PP, Trogu E, Vecchi S, Amato L. Antidepressants for cocaine dependence and problematic cocaine use. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD002950.pub3] [DOI] [PubMed] [Google Scholar]
Rush 2003
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN. The 16‐item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS‐C), and self‐report (QIDS‐SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry 2003;54(5):573–83. [DOI] [PubMed] [Google Scholar]
Schünemann 2006
- Schünemann HJ, Jaeschke R, Cook D, Bria W, El‐Solh A, Ernst A, et al. ATS Documents Development and Implementation Committee. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006;174(5):605‐14. [DOI] [PubMed] [Google Scholar]
Simpson 1970
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica. Supplementum 1970;212:11‐9. [DOI] [PubMed] [Google Scholar]
Smelson 1999
- Smelson DA, McGee‐Caulfield E, Bergstein P, Engelhart C. Initial validation of the Voris cocaine craving scale: a preliminary report. Journal of Clinical Psychology 1999;55(1):135‐9. [DOI] [PubMed] [Google Scholar]
Snaith 1995
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith‐Hamilton Pleasure Scale. British Journal of Psychiatry 1995;167(1):99‐103. [DOI] [PubMed] [Google Scholar]
Sobell 1992
- Sobell LCS, Sobell MB. Timeline follow‐back: a technique for assessing self‐reported alcohol consumption. In: Litten RA, Allen J editor(s). Measuring Alcohol Consumption. Totowa, NJ: The Humana Press, Inc, 1992:41–72. [Google Scholar]
Somoza 1995
- Somoza E, Dyrenforth S, Goldsmith J, Mezinskis J, Cohen M. In search of a universal drug craving scale. Annual Meeting of the American Psychiatric Association. 1995.
Sorensen 1991
- Sorensen JL, Wermuth LA, Gibson DR, Choi K, Guydish JR. Preventing AIDS in Drug Abusers and their Sexual Partners. New York: Guilford, 1991. [Google Scholar]
Tiffany 1993
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug and Alcohol Dependence 1993;34(1):19‐28. [DOI] [PubMed] [Google Scholar]
UNDOC 2015
- United Nations Office on Drugs and Crime. World Drug Report 2015. www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf (accessed 30 July 2015).
Volkow 2003
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights for imaging studies. Journal of Clinical Investigation 2003;111(10):1444‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weddington 1990
- Weddington WW, Brown BS, Haertzen CA, Cone EJ, Dax EM, Herning RI, et al. Changes in mood, craving, and sleep during short‐term abstinence reported by male cocaine addicts. A controlled, residential study. Archives of General Psychiatry 1990;47(9):861‐8. [DOI] [PubMed] [Google Scholar]
Young 1978
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry 1978;133:429–35. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Amato 2007
- Amato L, Minozzi S, Pani PP, Davoli M. Antipsychotic medications for cocaine dependence. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006306.pub2] [DOI] [PubMed] [Google Scholar]


