Abstract
Objectives
We investigate whether obesity accounts for widening socioeconomic disparities in pain.
Methods
Based on nationally-representative samples of Americans aged 25–74 in 1995–96 and 2011–14, we use logistic regression to model period change in headaches, backaches, and joint aches as well as physical limitations and to determine whether those changes vary by a multidimensional measure of socioeconomic status.
Results
Prevalence of backaches, joint aches, physical limitations, and obesity increased between the mid-1990s and the early 2010s, particularly among more disadvantaged Americans. Socioeconomic disparities in frequent backaches, frequent joint pain, and physical limitations more than doubled over this period. We estimate that obesity and health conditions may account for nearly a quarter of the widening disparity in frequent backaches and about half of the widening disparity in frequent joint pain and physical limitations.
Discussion
Widening disparities in backaches, joint pain, and physical limitations have coincided with growing obesity.
Keywords: Socioeconomic disparities, Pain, Physical Function, Obesity, United States
INTRODUCTION
Reported pain in the US has increased since the early 1990s (Nahin et al., 2019; Zimmer & Zajacova, 2020). Pain has a detrimental effect on physical function and quality of life (Duenas et al., 2016) and imposes enormous health care and productivity costs (Gaskin & Richard, 2012). Prior research has highlighted growing socioeconomic disparities in mental health (Goldman et al., 2018), drug misuse (Glei et al., 2020), physical function (Zajacova & Montez, 2017), and mortality (Chetty et al., 2016). A similar pattern for pain would provide further evidence of widening inequality in health and wellbeing. A recent study suggested that socioeconomic status (SES) is inversely associated with the increase in pain (Glei et al., 2020); three other studies noted growing educational or income disparities in pain (Case et al., 2020; Cutler et al., 2020; Zajacova et al., Forthcoming).
Rising pain does not appear to be merely a result of population aging: increases have been observed at all adult ages and in both sexes (Institute of Medicine, 2011). While it is possible that the increase reflects changes over time in reporting or perception, prior studies have reported a recent increase in physical/functional limitations in the US (Iezzoni et al., 2014)—particularly at working ages (Freedman et al., 2013; Martin & Schoeni, 2014) and among those with less than a high school degree (Zajacova & Montez, 2017). Those findings suggest that rising pain may represent more than a statistical artifact.
Another potential contributor to rising pain is obesity (Institute of Medicine, 2011; Stokes et al., 2020; Zimmer & Zajacova, 2020). The age-adjusted prevalence of obesity (i.e., body mass index greater than 30) among US adults grew from 15% in 1976–80 to 42% in 2017–18 (Fryar et al., 2018; Hales, 2020). Evidence regarding recent trends in the socioeconomic disparity in obesity among US adults is mixed (Ljungvall & Zimmerman, 2012; Ogden et al., 2010; Yu, 2016), although Frederick et al. (2014) reports that obesity has risen faster among adolescents with less educated parents. Obesity could increase pain through two primary mechanisms (Okifuji & Hare, 2015): 1) excess weight—particularly overloading the lower back, hip, and knee joints—amplifies the mechanical stresses on the body, which may cause structural damage and wear-and-tear on the joints leading to osteoarthritis and pain; and 2) obesity is associated with chronic low-grade inflammation, which may also induce pain. Prior studies have demonstrated that obesity is associated with various types of pain including lower back pain (Shiri et al., 2010; Walsh et al., 2018), joint pain (Walsh et al., 2018), and headaches (Chai et al., 2014), but the relationship may be stronger for lower back pain than headaches (Wright et al., 2010). Previous research suggest that obesity accounts for 19–32% of the recent increase in pain (Case et al., 2020; Stokes et al., 2020; Zajacova et al., Forthcoming), but none of those studies quantified the extent to which obesity may account for widening SES disparities in pain.
Here we address the overarching question: does obesity account for widening socioeconomic disparities in pain among Americans? We evaluate period differences in the frequency of headaches as well as backaches and joint aches because, if rising pain is related to obesity, then we would expect to see a larger increase over time in musculoskeletal pain (e.g., back, joints) than in headaches. Unlike prior studies that used a proxy measure of SES such as education or income (Case et al., 2020; Zajacova et al., Forthcoming), we use a multidimensional measure of SES that enables us to evaluate changes1 over time in pain for fixed quantiles (i.e., percentiles) of the population, thereby avoiding the problem of lagged selection bias (Dowd & Hamoudi, 2014) that biases analyses of period trends by education. To measure obesity, we include a measure of abdominal obesity (i.e., waist circumference) as well as body mass index (BMI).
METHODS
Data
The data came from two cross-sectional waves of Midlife in the United States (MIDUS), each of which targeted a national probability sample of non-institutionalized, English-speaking adults aged 25–74 in the contiguous United States. In 1995–96, respondents were selected by random digit dialing with oversampling of older people and men (Brim et al., 2016); 3487 respondents completed the phone interview (70% response rate) and 3034 also completed mail-in self-administered questionnaires (SAQ). In 2011–14, a new refresher cohort was drawn from the national population using a sampling frame that included both landlines and cell phones (Palit et al., 2016a); 3577 individuals participated in the phone interview (59% response rate) and 2598 also completed the SAQ. We restricted our analyses to respondents who completed the SAQ (pooled analysis sample: N=5632). The MIDUS survey protocols were reviewed and approved by the Education and Social/Behavioral Science Institutional Review Board at University of Wisconsin-Madison. The data used in this analysis are publicly available from the Inter-university Consortium for Political and Social Research (https://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/2760; https://www.icpsr.umich.edu/web/ICPSR/studies/36532).
Measures
Pain
Respondents were asked how often, during the past 30 days, they experienced: 1) headaches; 2) lower backaches; and 3) aches/stiffness in joints. The six response categories ranged from “not at all” to “almost every day.” For each of the three outcomes, we created an additional dichotomous measure indicating frequent pain, defined as pain “almost every day.” Although MIDUS did not distinguish between chronic and acute pain, frequent pain is likely to be more debilitating than occasional pain and may be closer to the construct of “chronic pain.” MIDUS did not capture all types of pain, but prior literature has suggested that lower back and joint pain are likely to be the most prevalent types of pain at the population level (Johannes et al., 2010).
Physical function
Respondents were asked, “How much does your health limit you in doing each of the following? Lifting or carrying groceries; climbing several flights of stairs; bending, kneeling, or stooping; walking more than a mile; walking several blocks; walking one block; vigorous activity (e.g., running, lifting heavy objects); moderate activity (e.g., bowling, vacuuming).” Each question had four response categories (i.e., not at all, a little, some, a lot). Like prior studies (e.g., Iezzoni et al., 2014; Martin & Schoeni, 2014), we created a dichotomous variable indicating whether the respondent reported any limitation on at least one of the eight physical tasks. To better capture impairment that may be more strongly correlated with frequent pain, we coded a second binary measure to indicate whether the respondent reported “a lot” of limitation on any of those same tasks (hereafter referred to as “major limitation”).
Obesity
The respondent was asked to report current height, weight, and waist circumference as well as weight one year prior to the survey. We computed BMI (kg/m2) based on current height and the higher of the two weight measures to help avoid the problem that someone may have lost weight owing to a recent illness. Then, we categorized BMI using the World Health Organization cutoffs for underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), class I obesity (30–34.9), class II obesity (35–39.9), and class III obesity (40+).
Health conditions
We included 11 health conditions that were likely to be associated with obesity and potentially cause pain. Dichotomous variables indicated whether the respondent ever had heart trouble suspected or confirmed by a doctor and whether s/he ever had cancer. The respondent was also asked: “In the past 12 months, have you experienced or been treated for any of the following?” (as worded in the questionnaire): 1) Asthma, bronchitis, or emphysema; Tuberculosis; Other lung problems (we combined these 3 sets of conditions); 2) Arthritis, rheumatism, or other bone or joint disease; 3) Recurring stomach trouble, indigestion, or diarrhea; 4) Gallbladder trouble; 5) Persistent foot trouble (e.g., bunions, ingrown toenails); 6) Lupus or other autoimmune disorders; 7) Diabetes or high blood sugar; 8) Multiple sclerosis, epilepsy, or other neurological disorders; and 9) Stroke. The relationship between obesity and these health conditions was likely to be bidirectional: obesity may have precipitated illness, which in turn could have caused weight loss (e.g., obesity contributed to the development of type II diabetes, which caused sudden weight loss). Thus, failure to control for diabetes could underestimate the association between obesity and pain. Of course, these health conditions also represent key pathways through which obesity may increase pain. By including both obesity and health conditions, we were able to more fully capture the potential effects of obesity on pain.
Control variables
We controlled for age, sex, race,2 smoking history, and a composite measure of relative socioeconomic status (SES), all of which may confound the relationship between obesity and perceived pain. For example, the inverse association between smoking and obesity is well-established, and several studies describe mechanisms by which smoking can influence pain sensitivity (Aamodt et al., 2006; Ditre et al., 2011; Waldie et al., 2008). Other reviews (e.g., Campbell & Edwards, 2012; Mossey, 2011) suggest there may be racial differences in pain sensitivity and/or under-reporting of pain. To test for a period effect, we included a dichotomous variable for survey wave. As described in detail in Supplementary Material, we created the SES index based on education, occupation, income, and assets, which we then converted to a percentile rank representing the individual’s position within the distribution at that wave. For ease of interpretation, we reverse-coded and rescaled the SES variable to range from 0 (top percentile) to 1 (bottom percentile), such that a one-unit change denotes the difference between the bottom and top percentile of SES.
Analytic Strategy
We used standard practices of multiple imputation to handle missing data (see Supplementary Material for details). All analyses used post-stratification weights (Brim et al., 2019; Palit et al., 2016b) to ensure that the weighted samples show very similar distributions (in terms of age, sex, race, education and marital status) as the corresponding Current Population Survey.
To assess the change in pain levels between the mid-1990s and early-2010s and determine whether those changes vary by SES, we began by examining the frequency distribution for each type of pain by wave and SES quintile. We performed similar analyses for physical function to determine whether the pattern mirrored the changes in pain.
Next, we used a logit model to regress each of the dichotomous measures for frequent pain of each type and physical limitations on sociodemographic characteristics, smoking history, survey wave, and an interaction between period and SES. We compared linear, quadratic, and quintile specifications for SES, but the linear specification produced the best model fit (according to the Bayesian Information Criterion); there was no evidence of non-linearity. Thus, Model 1 evaluated the magnitude of the SES disparity in 1995–96 (i.e., the main effect of SES), the difference between 1995–96 and 2011–14 among those in the top percentile of SES (i.e., main effect of period), and the change in the SES disparity over time (i.e., interaction between period and SES).
To explore whether obesity and health conditions might account for widening disparities in pain and physical function, we first examined the period change by low versus high SES (median split). We expected to find greater increases in obesity and adverse health conditions among those with low SES than those with high SES. Next, we graphed BMI (distribution across categories) and waist circumference (box plot) by period and SES quintile. Finally, we added the measures of obesity (Model 2) and health conditions (Model 3) to the logit models to determine the extent to which they explained the widening SES disparity in pain.
Unlike linear models, we could not simply compare the coefficients across models to quantify the extent to which selected variables account for the period increase in pain/physical limitations or widening of the SES disparity. The coefficients from nested nonlinear models are not comparable because of rescaling; therefore, we used the Karlson-Holm-Breen method to obtain those estimates (Karlson et al., 2012).
We performed four sensitivity analyses. First, because smoking was likely to confound the relationship between obesity and health, we re-estimated the models, restricting the sample to never smokers. Second, in order to exclude people who may recently have lost weight because of serious illness, we refitted the models after excluding respondents who reported a history of cancer, heart trouble, stroke, diabetes, lung problems, autoimmune, or neurological disorders. Third, we explored robustness to the exclusion of outliers on BMI and waist circumference. Finally, we fitted an alternative version of Model 3 that included only the health conditions expected to be strongly associated with obesity: diabetes, heart disease, stroke, and arthritis.
RESULTS
As shown in Figure 1, there were substantial increases between 1995–96 and 2011–14 in reported frequency of lower backaches and joint aches, but little change in headaches. Change in backaches and joint pain were much greater at lower levels of SES, while there was little SES variation in headaches. Among those with low SES, the percentage reporting frequent lower backaches doubled (11% in 1995–96 versus 23% in 2011–14) and frequent joint aches nearly doubled (15% versus 29%, respectively; eTable 1). For those with high SES, the period differences were much smaller: 7% in 1995–96 vs. 9% in 2011–14 for back pain; 11% vs. 17%, respectively, for joint pain.
Figure 1.
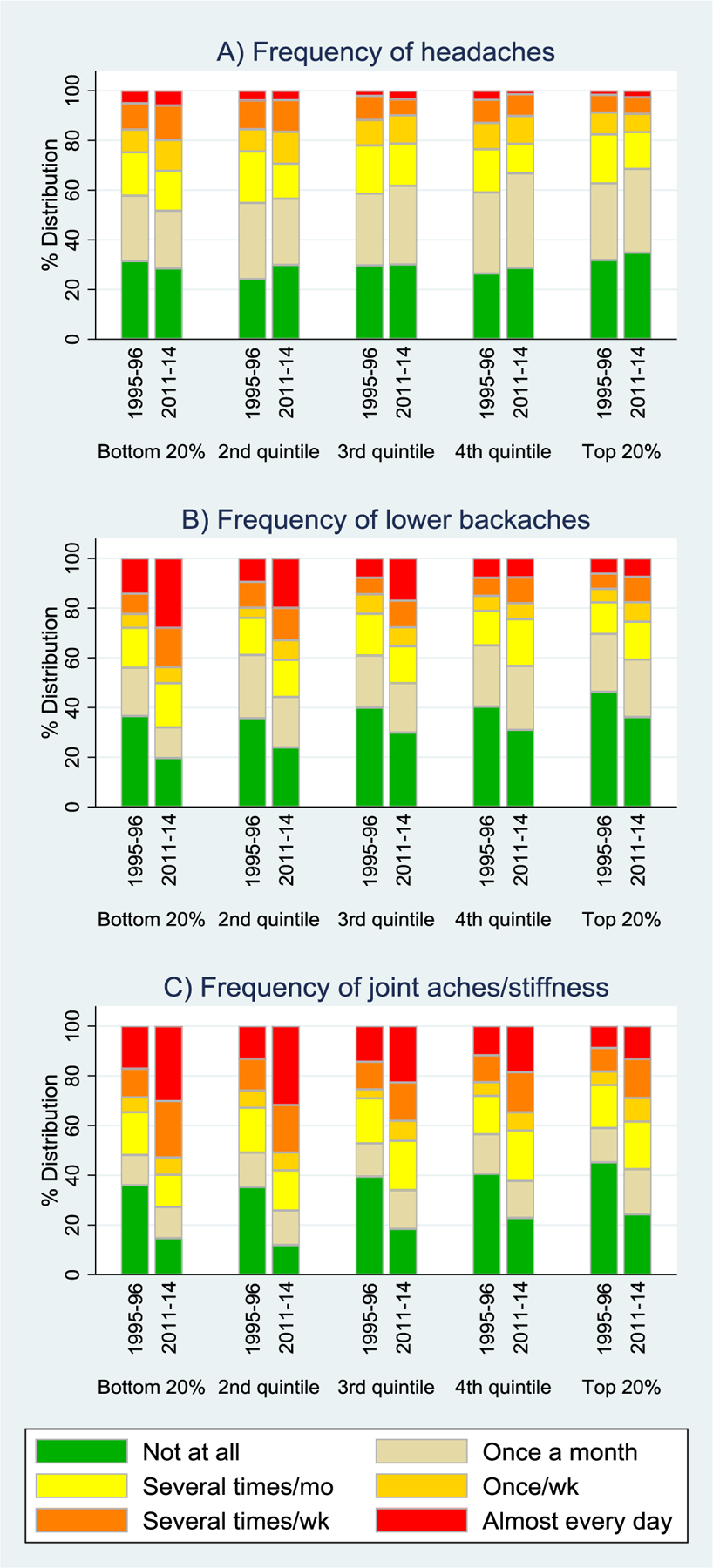
Pain by Period and SES quintile: A) Headaches; B) Lower Backaches; C) Joint aches/stiffness
As shown in Figure 2, physical limitations increased, particularly at lower levels of SES, in a manner similar to the patterns observed for back and joint pain. The percentage who reported any physical limitation increased from 71% in the mid-1990s to 79% in the early-2010s among those with low SES, whereas there was a much smaller change among their more advantaged counterparts (60% vs. 63%, respectively; eTable 1). The pattern was similar for prevalence of a major limitation.
Figure 2.
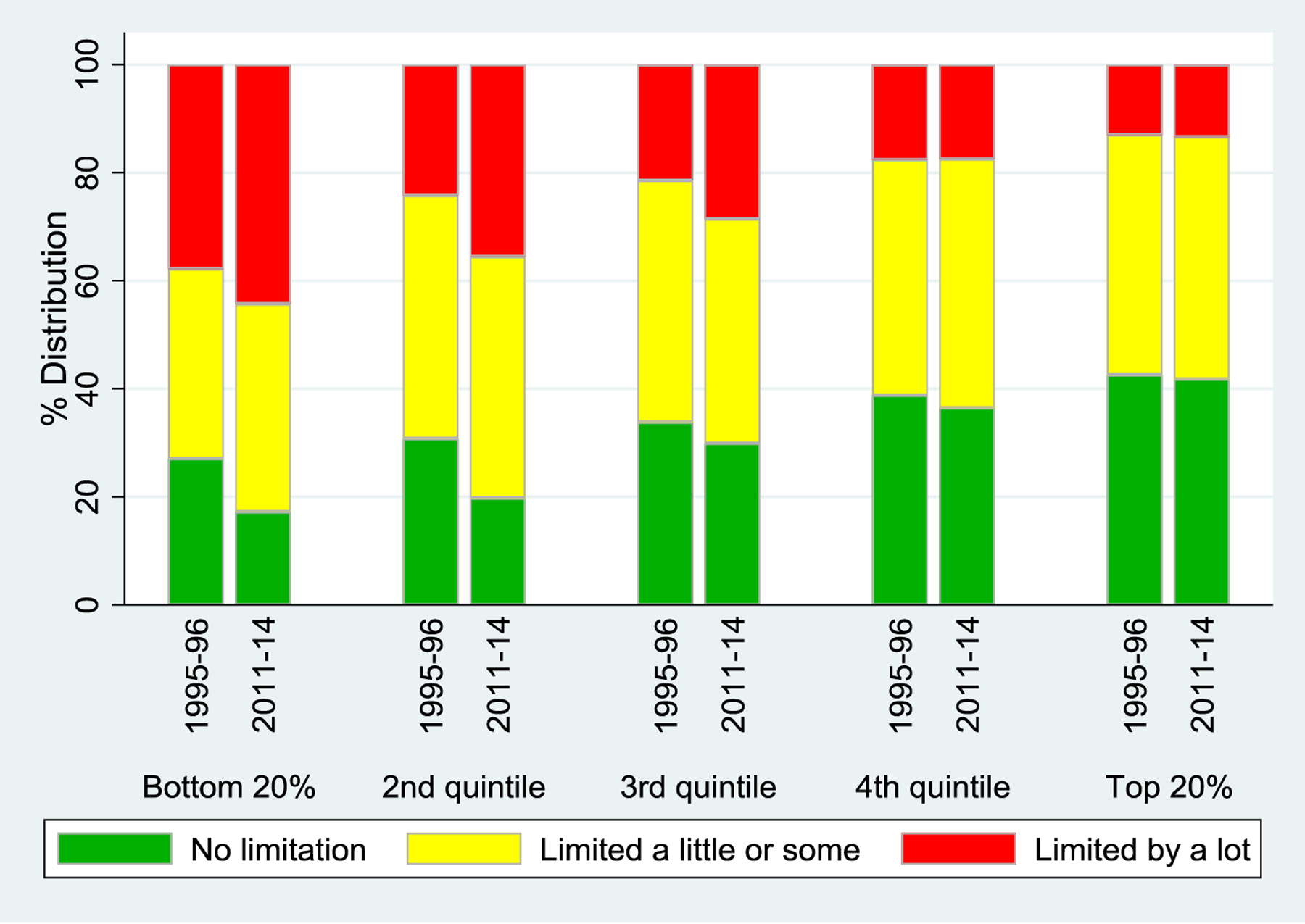
Limitations on Any of 8 Physical Tasks by Period and SES quintile
After controlling for sociodemographic characteristics and smoking history, we found no significant period effect for headaches at any level of SES (eTable 2, Model 1), but the period effect for the other outcomes was much larger at lower levels of SES and thus, SES disparities widened (eTables 3–6). For example, the interaction between period and SES in eTable 3 (OR=2.69, 95% CI 1.33–5.46) indicated that the period increase in the odds of frequent backache was nearly 2.7 times as high for someone in the bottom percentile of SES than it was for someone in the top percentile of SES (represented by the main effect of period). The main effect of SES implied that the odds of frequent backache in 1995–96 were 3.2 times as high for someone in the bottom percentile of SES relative to someone in the top percentile (OR=3.17, 95% CI 1.90–5.28). To simplify interpretation, Table 1 shows the odds ratios for: the period effects at the bottom (A) and top (B) of the SES continuum, the SES disparity in 1995–96 (C) and 2011–14 (D), and the degree to which the SES disparity widened over that period (E).
Table 1.
Odds ratios for selected effects and the percent of those effects explaineda by obesity and health conditions: Period effects for those in the bottom (A) and top (B) top percentile of SES, SES disparity in 1995–96 (C) and 2011–14 (D), and widening of the disparity over that period (E)
| Frequent Pain | Physical Limitations | |||||
|---|---|---|---|---|---|---|
| Headaches | Backaches | Joints | Any | Major | ||
| A) Period effect at bottom percentile of SES | ||||||
| Odds ratio (Model 1)b | 1.28 | 2.84*** | 2.65*** | 1.98*** | 1.70** | |
| % explained by obesity (Model 2) | c | 13.8 | 30.4 | 73.5 | 81.3 | |
| % explained by obesity & health (Model 3) | c | 10.3 | 21.4 | 71.3 | 88.3 | |
| B) Period effect at top percentile of SES | ||||||
| Odds ratio (Model 1)d | 0.92 | 1.06 | 1.28 | 0.71** | 0.76 | |
| % explained by obesity (Model 2) | c | c | c | −19.2 | c | |
| % explained by obesity & health (Model 3) | c | c | c | 30.3 | c | |
| C) SES disparity in 1995–96 | ||||||
| Odds ratio (Model 1)e | 3.26** | 3.17*** | 2.24*** | 2.23*** | 4.16*** | |
| % explained by obesity (Model 2) | 8.3 | 5.4 | 18.9 | 27.4 | 12.7 | |
| % explained by obesity & health (Model 3) | 19.3 | 21.7 | 51.2 | 52.1 | 26.1 | |
| D) SES disparity in 2011–14 | ||||||
| Odds ratio (Model 1)f | 4.52** | 8.52*** | 4.62*** | 6.23*** | 9.27*** | |
| % explained by obesity (Model 2) | 23.3 | 8.7 | 27.1 | 37.7 | 24.8 | |
| % explained by obesity & health (Model 3) | 43.6 | 22.7 | 49.2 | 55.6 | 39.9 | |
| E) Widening of the SES disparity | ||||||
| Odds ratio (Model 1)g | 1.39 | 2.69** | 2.06* | 2.79*** | 2.23** | |
| % explained by obesity (Model 2) | c | 12.5 | 35.9 | 45.2 | 44.9 | |
| % explained by obesity & health (Model 3) | c | 23.5 | 47.4 | 58.3 | 63.4 | |
Note. SES, socioeconomic status.
The percent explained is calculated using the Karlson-Holm-Breen (KHB) method (Karlson et al., 2012) with the Stata user-written program “khb” (Kohler et al., 2011). KHB estimates are computed for each multiple imputation dataset and then we use the Stata program “micombine” written by Gérman Rodríguez to combine the estimates. The contribution of individual variables is computed as the mean of the estimates across the 5 multiple imputation datasets.
Represents the odds ratio (OR) of the main effect for period (ORPeriod) multiplied by the OR of the interaction between period and SES (ORPeriod×SES) from Model 1 (eTables 2–6). We present the OR for the bottom (A) and top (B) percentiles of SES to demonstrate the full range, but the OR for any quantile of the distribution can be computed as: , where X represents the quantile of interest. For example, the odds of frequent joint pain for someone at the 50th percentile would be: exp[ln(1.28) + 0.5 * ln(2.06)] = 1.83.
We do not show the percent explained in cases where the odds ratio is not significant. In such cases, the results can be erratic because the denominator is small.
Represents the OR of the main effect for period.
The OR of the main effect for SES, which represents the odds of the outcome for someone in the bottom relative to the top percentile of SES in 1995–96. The corresponding odds ratio for any two quantiles (X and Y) of SES can be computed as: (ORSES)((Y−X)/100). For example, the odds of frequent joint pain for someone in the 25th percentile of SES relative to the 75th percentile of SES in 1995–96 would be (2.24)0.5 = 1.50.
Represents the OR of the main effect for SES (ORSES) multiplied by the OR of the interaction between period and SES (ORPeriod×SES). The corresponding odds ratio for any two quantiles (X and Y) of SES in 2011–14 can be computed as: (ORSES * ORPeriod×SES)((Y−X)/100). For example, the odds of frequent joint pain for someone in the 25th relative to the 75th percentile of SES in 2011–14 would be (4.61)0.5 = 2.15.
Represents the OR of the interaction between period and SES, which can be interpreted as the change in the SES disparity between 1995–96 and 2011–14, or alternatively, as the relative difference between the period effect for the bottom versus the top percentile of SES. This OR is equivalent to the ratio of the ORs of the period effects for the bottom (A) relative to the top percentile of SES (B). It is also equivalent to the ratio of the ORs for the effect of SES at 2011–14 (D) relative to 1995–96 (C).
p<0.001,
p <0.01,
p<0.05
The odds of frequent backaches and frequent joint aches more than doubled between 1995–96 and 2011–14 among those in the bottom percentile of SES (Table 1, Panel A). The odds of any physical limitation nearly doubled, while the odds of a major limitation were 70% higher in 2011–14 than in 1995–96 among those with the lowest SES. In contrast, the corresponding period effects for someone in the top percentile of SES were much smaller (Panel B). Consequently, the SES disparity widened considerably. For example, the odd ratios for the SES disparity in frequent backaches was 3.2 in 1995–96 (Panel C), but rose to 8.5 by 2011–14 (Panel D). The disparity in the odds of frequent backaches, frequent joint aches, and physical limitations were more than twice as a high in 2011–14 than in 1995–96 (Panel E). The widening of disparity for frequent headaches was smaller and not significant.
Mean levels of both BMI and waist circumference were higher in 2011–14 than in 1995–96 (eTable 1), but the aggregate change was much greater for those with low SES than for those with high SES. Graphs by SES quintile further demonstrated that BMI (Figure 3) and waist circumference (eFigure 1) increased over time, especially at lower levels of SES.
Figure 3.
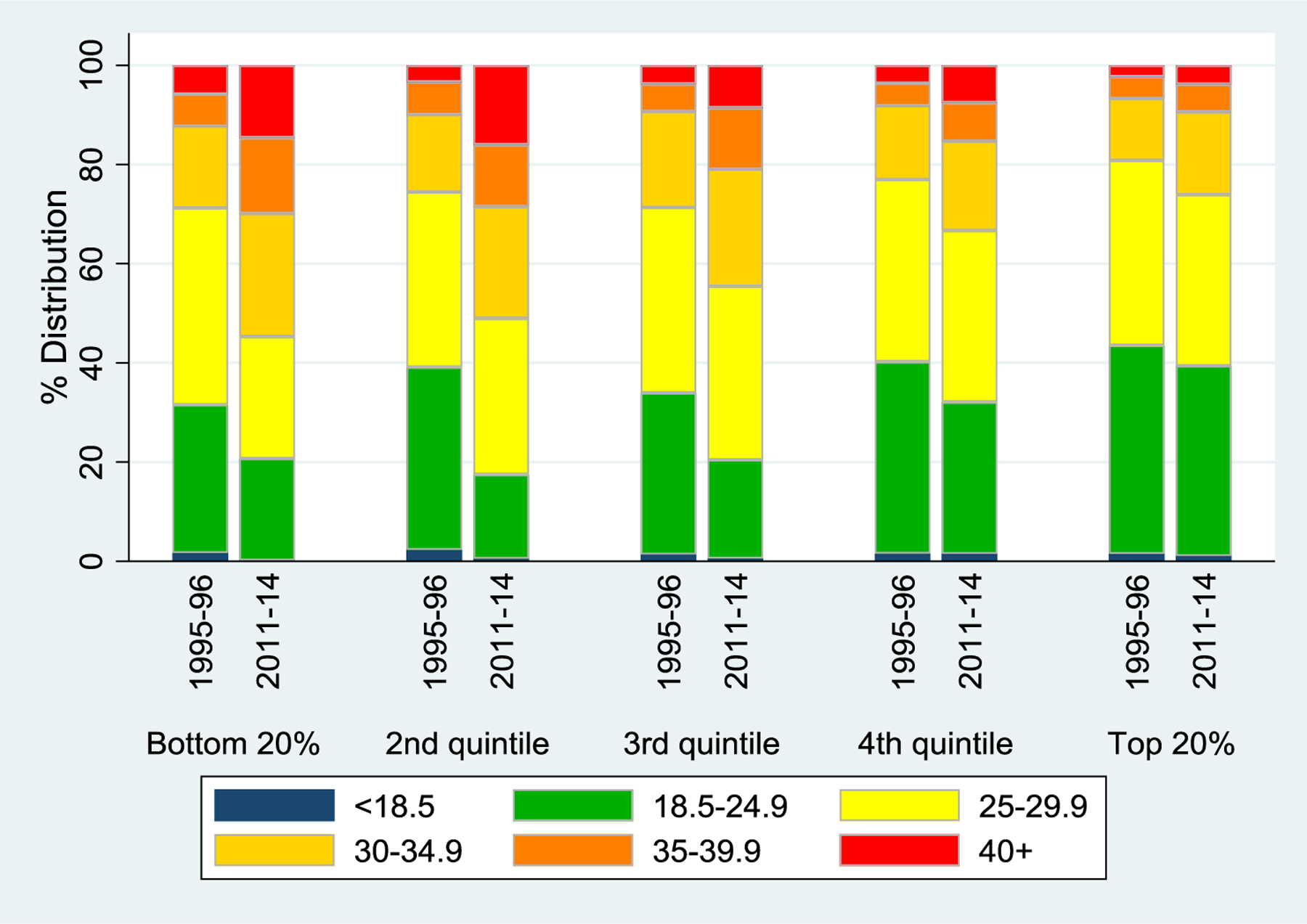
BMI by Period and SES Quintile
When the obesity measures were added to Model 2, neither BMI nor waist circumference was significantly associated with headaches (eTable 2) or backaches (eTable 3), but BMI was positively associated with joint pain (eTable 4). Both BMI and waist circumference were related to the two measures of physical limitations (eTables 5 and 6). For those in the bottom percentile of SES, obesity accounted for 14% of the period increase in backaches and 30% of the increase in joint aches, but an even greater share of the increase in any physical limitation (74%) and a major limitation (81%; Table 1A). Obesity explained a much larger share of the SES disparity in 2011–14 (Table 1D) than in 1995–96 (Table 1C). Finally, we estimated the degree to which obesity accounted for widening of the SES disparity over that same period (13% for backaches, 36% for joint pain, and 45% for physical limitations; Table 1E).
Among the selected health conditions (eTable 1), only diabetes exhibited a pattern that was highly consistent with that of pain and physical limitations: prevalence increased over time, especially among those with low SES (+10 percentage points vs. +3 percentage points for those with high SES). The prevalence of other conditions increased over time for those with low SES, but was virtually unchanged (i.e., neurological disorder, autoimmune disorder) or declined (i.e., lung problems, arthritis) among those with high SES. Thus, the SES differentials in the trends in prevalence of these health conditions were consistent with the larger increase in pain and physical limitations among those with low SES.]
The remaining conditions were unlikely to explain why pain and physical limitations increased over time at lower levels of SES. There was little SES differential in increased prevalence of heart trouble. Cancer increased over time, but more so at high SES, perhaps because of SES differences in diagnosis. Prevalence declined over time for the other conditions (i.e., stroke, recurring stomach problems; gallbladder trouble; persistent foot trouble).
When health conditions were added to Model 3, we found that arthritis was strongly associated with frequent backaches (eTable 3) and even more so for frequent joint aches (eTable 4). Arthritis was also associated with physical limitations, but the effect size was weaker (eTables 5 and 6). Neurological disorders had the strongest effect on physical limitations, but they were not significantly associated with frequent pain of any type. Health conditions did not yield added value for explaining the period increase in backaches or joint aches: the combined contribution of obesity and health conditions was actually less than that of obesity alone (Table 1A). In contrast, these conditions made an incremental contribution to the increase in a major physical limitation, largely because of the increased prevalence of diabetes, lung problems, and neurological disorders among those with low SES.
Health conditions also helped account for the widening SES disparity (Table 1E). Together, obesity and the health conditions accounted for 24% of the widening disparity in frequent backaches and an even larger share for joint aches (47%) and physical limitations (58–63%). Among the health conditions, the biggest contributor to the widening SES disparity was arthritis (results not shown), for which prevalence increased at low SES while declining at high SES (eTable 1). Lung disorders also made a notable contribution to the widening disparity in physical function because lung disorders increased at low SES while decreasing at high SES.
Additional sensitivity analyses demonstrate that restricting the analysis to never smokers exacerbated the widening SES disparities in pain and physical function, but obesity generally accounted for a smaller share of the widening SES disparity. After excluding respondents who reported a history of selected serious illnesses, the share of SES widening that could be accounted for by obesity was generally smaller. Exclusion of outliers on BMI and waist circumference had no substantive effect on the results. Finally, when controls for health conditions were limited to diabetes, heart trouble, stroke, and arthritis, they accounted for a smaller percentage of SES widening. (See Supplementary Material for more details regarding the sensitivity analyses.) In sum, these tests of robustness suggested that declines in smoking may have tempered SES widening, and some of the explanatory power of health conditions may not necessarily be a result of obesity.
DISCUSSION
Our results suggest that widening SES disparities in back pain, joint aches, major physical limitations, and disability are linked with the obesity epidemic. In contrast, there was little change in reported frequency of headaches, which is also consistent with the idea that rising pain is driven by obesity. We expected obesity to have a bigger effect on musculoskeletal pain than on headaches because of the additional mechanical stresses that excess weight imposes on muscles, bones, and connective tissue, and because obesity is associated with up-regulation of pro-inflammatory cytokines secreted by adipose tissue, such as leptin, which has been linked with osteoarthritis (Walsh et al., 2018; Yan et al., 2018).
Some researchers have suggested that rising levels of reported pain may reflect changes in reporting (Institute of Medicine, 2011; Zimmer & Zajacova, 2020). If so, why would it have affected those with low SES much more than those with high SES, and why don’t we see the same pattern for headaches? The fact that the patterns of physical function were similar to patterns for back and joint pain also counters the notion that it reflects increased pain sensitivity or greater expectation for pain relief.
Deteriorating mental health has also been cited as a potential explanation for rising pain, but it is difficult to determine the direction of causation because the relationship between emotional distress and pain is bi-directional. Mental health may also have a bi-directional relationship with obesity (Luppino et al., 2010). Thus, it is not clear whether we should treat mental health as an obesity-pain confounder or as an intermediate mediator. It would be pointless to add mental health to the model because even if it attenuated the period effect, we would not be able to say whether that was because mental distress exacerbated pain or because pain caused mental distress (i.e., reverse-causality).
Case et al. (2020) argue that rising pain may be a consequence of deteriorating social and economic conditions faced by less-educated Americans, but that hypothesis does not preclude the possibility that obesity acts as a mediator. Indeed, Deaton (2017) suggested that “if obesity is the cause [of an increase in heart disease mortality], as many argue, some of these deaths might also be classed as deaths of despair (p. 2)…heavy drinking, obesity, increasing social isolation, drugs, and suicide are plausible outcomes of these cumulative processes that deprive white working class lives of their meaning (p. 4).”
Even if the obesity epidemic is a proximate determinant of the rise in pain, we need a better understanding of its underlying causes if we want to combat the problem effectively. Obesity is the result of an imbalance between energy intake (i.e., diet) and expenditure (i.e., physical activity). Most research on the obesity epidemic has focused on these “Big Two” (Cardel et al., 2011), but other factors such as diet composition (Cordain et al., 2005) and exposure to endocrine-disrupting compounds and other environmental obesogens (Grün & Blumberg, 2006) may play a role. Obesogens may affect lipid metabolism by interfering with the endocrine system, hypothalamic-pituitary-adrenal axis, or adipose tissue biology (Grün & Blumberg, 2009). Food, food packaging, and the water supply expose us to thousands of compounds; food preparation can result in further exposure to potential obesogens such as perfluorochemicals (PFCs) from non-stick cookware and bisphenol A (BPA) from microwaving plastic containers (Simmons et al., 2014). Although there are plausible biological mechanisms as well as animal studies that support the obesogen hypothesis, there is not yet strong evidence that any environmental contaminant causes obesity (Simmons et al., 2014).
Factors that may have contributed to a change in (the amount and/or composition of) energy intake include the marketing, affordability, and availability of cheap, convenient high-calorie processed food versus healthy, whole foods. The US subsidizes corn, soybeans, wheat, rice, sorghum, dairy, and livestock, much of which is converted into high-fat meat and dairy products, refined grains, sugar-sweetened beverages, and processed foods (Siegel et al., 2016). One study found an inverse relationship between indicators of SES (i.e., education and income) and the proportion of calories derived from major subsidized food commodities; furthermore, higher subsidy scores were associated with increased risk of obesity and abdominal adiposity (Siegel et al., 2016). Cutler et al. (2003) argue that the increase in calorie consumption since 1980 resulted from the shift to mass-produced, pre-prepared food, which lowered the time costs of food consumption (e.g., between 1965 and 1995, the amount of time devoted to preparing and cleaning up after meals fell by up to 50% among women). Given resource limitations and increased likelihood of living in a “food desert” (Dutko et al., 2012), disadvantaged Americans may be more likely to consume cheap, processed food, which affects nutritional content and may result in greater exposure to obesogens.
Lavizzo-Mourey & McGinnis (2003, p. 1386) argue that transformations of the built environment—which influence how we work, where we live, and how we get around—have effectively “engineered physical activity out of Americans’ lives.” We have become increasing dependent on cars, and communities are often designed to optimize vehicle flow rather than to allow safe pedestrian and bike routes (Lavizzo-Mourey & McGinnis, 2003). A study of US children showed that parental education was positively associated with neighborhood environmental conditions (e.g., access to sidewalks, parks, and recreation centers), which in turn had a negative effect on the risk of childhood obesity (Singh et al., 2010). Studies focused on adults have also documented a link between SES, the availability of places to exercise, and obesity (Lovasi et al., 2009). Thus, Americans with low SES status may be particularly vulnerable to obesity in part because their neighborhood environment is less conducive to physical activity.
Limitations
We cannot determine the direction of causation from the cross-sectional data in this study. It is possible that pain causes or exacerbates obesity, in which case our analyses may over-estimate the proportion of the rise in pain explained by obesity. Nonetheless, we can say that obesity, back/joint pain, and physical limitations appeared to follow similar changes over time. Regardless of causal direction, the close relationships among obesity, pain, and physical function suggest the need to look deeper for underlying causes that may affect all these outcomes.
We must also acknowledge the limitations of our measures. The questions in the initial wave of MIDUS do not allow us to distinguish between acute and chronic pain. Our obesity measures are based on self-reports. Based on comparisons of self-reported values versus anthropometric measurements among the subset of MIDUS refresher who participated in the examination component, we know that respondents tend to under-report weight and over-report height, and thus BMI tends to be under-estimated. However, we find no evidence that reporting of BMI or waist circumference varies significantly by SES or prevalence of frequent pain. Thus, measurement error is unlikely to bias our estimates regarding the associations between SES, obesity, and pain. Furthermore, we do not have full information regarding the development of obesity over the respondent’s lifetime. Unmeasured illness that causes both weight loss and pain could downwardly bias estimates of the relationship between obesity and pain. Although we included many health conditions likely to be associated with obesity, their inclusion may overestimate the contribution of obesity. If some of those health conditions cause pain but were not a result of obesity, then their inclusion would inflate our estimates. For example, arthritis is a major contributor to the widening SES disparity. Although arthritis increased over time among those with low SES, it declined among those with high SES even as obesity increased. Thus, obesity cannot explain the decline in arthritis at high levels of SES.
Conclusions
Prevalence of backaches, joint aches, physical limitations, and obesity were higher in the early 2010s than in the mid-1990s, particularly among more disadvantaged Americans. Overall, the socioeconomic disparity in frequent backaches, frequent joint aches, and physical limitations more than doubled between 1995–96 and 2011–14. Our estimates suggest that obesity and health conditions may account for nearly a quarter of the widening SES disparity in frequent backaches and about half of the widening disparity in frequent joint pain and physical limitations. If widening SES disparities in backaches, joint pain, and physical limitations are linked with growing obesity, then we need to better understand the underlying factors that caused the rapid increase in obesity over recent decades in order to develop effective interventions to combat obesity. The past year has demonstrated the power of vaccines, but there is no vaccine for obesity on the horizon.
Supplementary Material
FUNDING
This work was supported by the National Institute on Aging [grant numbers P01 AG020166, U19 AG051426] and the Graduate School of Arts and Sciences, Georgetown University.
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
In this paper, we use the term “change” to refer to a period difference in aggregate-level values rather than within-individual changes.
We did not include ethnicity because the 1995–96 wave of MIDUS did not ask respondents to report their ethnicity.
Data Availability:
The MIDUS data used in this analysis are publicly available from the Inter-university Consortium for Political and Social Research (ICPSR, https://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/2760; https://www.icpsr.umich.edu/web/ICPSR/studies/36532).
REFERENCES
- Aamodt AH, Stovner LJ, Hagen K, Bråthen G, & Zwart J (2006). Headache prevalence related to smoking and alcohol use. The Head-HUNT Study. European Journal of Neurology, 13(11), 1233–1238. 10.1111/j.1468-1331.2006.01492.x [DOI] [PubMed] [Google Scholar]
- Brim OG, Baltes PB, Bumpass LL, Cleary PD, Featherman DL, Hazzard WR, Kessler RC, Lachman ME, Markus HR, Marmot MG, Rossi AS, Ryff CD, & Shweder RA (2016). National Survey of Midlife Development in the United States (MIDUS), 1995–1996. Technical Report on Methodology. Inter-university Consortium for Political and Social Research. 10.3886/ICPSR02760.v11 [DOI] [Google Scholar]
- Brim OG, Baltes PB, Bumpass LL, Cleary PD, Featherman DL, Hazzard WR, Kessler RC, Lachman ME, Markus HR, Marmot MG, Rossi AS, Ryff CD, & Shweder RA (2019). National Survey of Midlife Development in the United States (MIDUS 1), 1995–1996: Documentation of Post-Stratification Weights Created at MIDUS 1. Inter-university Consortium for Political and Social Research [distributor], Version 16. 10.3886/ICPSR02760.v16 [DOI] [Google Scholar]
- Campbell CM, & Edwards RR (2012). Ethnic differences in pain and pain management. Pain Management, 2(3), 219–230. 10.2217/pmt.12.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardel M, Dulin-Keita A, & Casazza K (2011). Contributors to Pediatric Obesity in Adolescence: More than just Energy Imbalance. The Open Obesity Journal, 3(2), 17–26. 10.2174/1876823701103010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, Deaton A, & Stone AA (2020). Decoding the mystery of American pain reveals a warning for the future. Proceedings of the National Academy of Sciences. 10.1073/pnas.2012350117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai NC, Scher AI, Moghekar A, Bond DS, & Peterlin BL (2014). Obesity and Headache: Part I – A Systematic Review of the Epidemiology of Obesity and Headache. Headache, 54(2), 219–234. 10.1111/head.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, Bergeron A, & Cutler D (2016). The association between income and life expectancy in the United States, 2001–2014. JAMA, 315(16), 1750–1766. 10.1001/jama.2016.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, & Brand-Miller J (2005). Origins and evolution of the Western diet: Health implications for the 21st century. The American Journal of Clinical Nutrition, 81(2), 341–354. 10.1093/ajcn.81.2.341 [DOI] [PubMed] [Google Scholar]
- Cutler DM, Glaeser EL, & Shapiro JM (2003). Why Have Americans Become More Obese? Journal of Economic Perspectives, 17(3), 93–118. [Google Scholar]
- Cutler DM, Meara E, & Stewart S (2020). Socioeconomic Status and the Experience of Pain: An Example from Knees (No. w27974). National Bureau of Economic Research. 10.3386/w27974 [DOI] [Google Scholar]
- Deaton A (2017). Economic Aspects of the Opioid Crisis: Testimony before the Joint Economic Committee of the United States Congress. Hearing. [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, & Meagher MM (2011). Pain, Nicotine, and Smoking: Research Findings and Mechanistic Considerations. Psychological Bulletin, 137(6), 1065–1093. 10.1037/a0025544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd J, & Hamoudi A (2014). Is life expectancy really falling for groups of low socio-economic status? Lagged selection bias and artefactual trends in mortality. International Journal of Epidemiology, 43(4), 983–988. 10.1093/ije/dyu120 [DOI] [PubMed] [Google Scholar]
- Duenas M, Ojeda B, Salazar A, Mico JA, & Failde I (2016). A review of chronic pain impact on patients, their social environment and the health care system. Journal of Pain Research, 9(Journal Article), 457–467. 10.2147/JPR.S105892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutko P, Ploeg MV, & Farrigan T (2012). Characteristics and Influential Factors of Food Deserts (ERR-140; p. 36). Department of Agriculture, Economic Research Service. [Google Scholar]
- Frederick CB, Snellman K, & Putnam RD (2014). Increasing socioeconomic disparities in adolescent obesity. Proceedings of the National Academy of Sciences, 111(4), 1338–1342. 10.1073/pnas.1321355110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman VA, Spillman BC, Andreski PM, Cornman JC, Crimmins EM, Kramarow E, Lubitz J, Martin LG, Merkin SS, Schoeni RF, Seeman TE, & Waidmann TA (2013). Trends in late-life activity limitations in the United States: An update from five national surveys. Demography, 50(2), 661–671. 10.1007/s13524-012-0167-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryar CD, Carroll MD, & Ogden CL (2018). Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults Aged 20 and Over: United States, 1960–1962 Through 2015–2016 (Health E-Stat). National Center for Health Statistics. https://www.cdc.gov/nchs/data/hestat/obesity_adult_15_16/obesity_adult_15_16.pdf [Google Scholar]
- Gaskin DJ, & Richard P (2012). The Economic Costs of Pain in the United States. The Journal of Pain, 13(8), 715–724. 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Glei DA, Stokes A, & Weinstein M (2020). Changes in mental health, pain, and drug misuse since the mid-1990s: Is there a link? Soc Sci Med, 246(Journal Article), 112890. 10.1016/j.socscimed.2020.112789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N, Glei DA, & Weinstein M (2018). Declining mental health among disadvantaged Americans. Proceedings of the National Academy of Sciences of the United States of America, 115(28), 7290–7295. 10.1073/pnas.1722023115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grün F, & Blumberg B (2006). Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology, 147(6 Suppl), S50–55. 10.1210/en.2005-1129 [DOI] [PubMed] [Google Scholar]
- Grün F, & Blumberg B (2009). Endocrine disrupters as obesogens. Molecular and Cellular Endocrinology, 304(1–2), 19–29. 10.1016/j.mce.2009.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM (2020). Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. 360, 8. [PubMed] [Google Scholar]
- Iezzoni LI, Kurtz SG, & Rao SR (2014). Trends in U.S. adult chronic disability rates over time. Disability and Health Journal, 7(4), 402–412. 10.1016/j.dhjo.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. (2011). Relieving pain in America: A blueprint for transforming prevention, care, education, and research. National Academies Press. 10.17226/13172 [DOI] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, & Dworkin RH (2010). The prevalence of chronic pain in United States adults: Results of an internet-based survey. The Journal of Pain, 11(11), 1230–1239. [DOI] [PubMed] [Google Scholar]
- Karlson KB, Holm A, & Breen R (2012). Comparing regression coefficients between same-sample nested models using logit and probit: A new method. Sociological Methodology, 42(1), 286–313. [Google Scholar]
- Kohler U, Karlson KB, & Holm A (2011). Comparing coefficients of nested nonlinear probability models. The Stata Journal, 11(3), 420–438. [Google Scholar]
- Lavizzo-Mourey R, & McGinnis JM (2003). Making the case for active living communities. American Journal of Public Health, 93(9), 1386–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungvall Å, & Zimmerman FJ (2012). Bigger bodies: Long-term trends and disparities in obesity and body-mass index among U.S. adults, 1960–2008. Social Science & Medicine, 75(1), 109–119. 10.1016/j.socscimed.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Lovasi GS, Hutson MA, Guerra M, & Neckerman KM (2009). Built Environments and Obesity in Disadvantaged Populations. Epidemiologic Reviews, 31(1), 7–20. 10.1093/epirev/mxp005 [DOI] [PubMed] [Google Scholar]
- Luppino FS, Wit L. M. de, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, & Zitman FG (2010). Overweight, Obesity, and Depression: A Systematic Review and Meta-analysis of Longitudinal Studies. Archives of General Psychiatry, 67(3), 220–229. 10.1001/archgenpsychiatry.2010.2 [DOI] [PubMed] [Google Scholar]
- Martin LG, & Schoeni RF (2014). Trends in disability and related chronic conditions among the forty-and-over population: 1997–2010. Disability and Health Journal, 7(1, Supplement), S4–S14. 10.1016/j.dhjo.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossey JM (2011). Defining Racial and Ethnic Disparities in Pain Management. Clinical Orthopaedics and Related Research, 469(7), 1859–1870. 10.1007/s11999-011-1770-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahin RL, Sayer B, Stussman BJ, & Feinberg TM (2019). Eighteen-Year Trends in the Prevalence of, and Health Care Use for, Noncancer Pain in the United States: Data from the Medical Expenditure Panel Survey. The Journal of Pain, 20(7), 796–809. 10.1016/j.jpain.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Lamb MM, Carroll MD, & Flegal KM (2010). Obesity and Socioeconomic Status in Adults: United States, 2005–2008. 50, 8. [PubMed] [Google Scholar]
- Okifuji A, & Hare BD (2015). The association between chronic pain and obesity. Journal of Pain Research, 8(Journal Article), 399–408. 10.2147/JPR.S55598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palit CD, Radler B, & Lein V (2016a). Midlife in the United States (MIDUS Refresher), 2011–2014: MIDUS refresher sampling and weighting. Inter-university Consortium for Political and Social Research [distributor]. [Google Scholar]
- Palit CD, Radler B, & Lein V (2016b). Midlife in the United States (MIDUS Refresher), 2011–2014: MIDUS Refresher Sampling and Weighting. ICPSR36532–v2 (Issue September 12). 10.3886/ICPSR36532.v2 [DOI] [Google Scholar]
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, & Viikari-Juntura E (2010). The association between obesity and low back pain: A meta-analysis. American Journal of Epidemiology, 171(2), 135–154. 10.1093/aje/kwp356 [DOI] [PubMed] [Google Scholar]
- Siegel KR, Bullard KM, Imperatore G, Kahn HS, Stein AD, Ali MK, & Narayan KM (2016). Association of Higher Consumption of Foods Derived From Subsidized Commodities With Adverse Cardiometabolic Risk Among US Adults. JAMA Internal Medicine, 176(8), 1124–1132. 10.1001/jamainternmed.2016.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AL, Schlezinger JJ, & Corkey BE (2014). What Are We Putting in Our Food That Is Making Us Fat? Food Additives, Contaminants, and Other Putative Contributors to Obesity. Current Obesity Reports, 3(2), 273–285. 10.1007/s13679-014-0094-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Siahpush M, & Kogan MD (2010). Neighborhood Socioeconomic Conditions, Built Environments, And Childhood Obesity. Health Affairs, 29(3), 503–512. 10.1377/hlthaff.2009.0730 [DOI] [PubMed] [Google Scholar]
- Stokes AC, Xie W, Lundberg DJ, Hempstead K, Zajacova A, Zimmer Z, Glei DA, Meara E, & Preston SH (2020). Increases in BMI and Chronic Pain for US Adults in Midlife, 1992 to 2016. SSM - Population Health, 100644. 10.1016/j.ssmph.2020.100644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldie KE, McGee R, Reeder AI, & Poulton R (2008). Associations between frequent headaches, persistent smoking, and attempts to quit. Headache, 48(4), 545–552. 10.1111/j.1526-4610.2007.01037.x [DOI] [PubMed] [Google Scholar]
- Walsh TP, Arnold JB, Evans AM, Yaxley A, Damarell RA, & Shanahan EM (2018). The association between body fat and musculoskeletal pain: A systematic review and meta-analysis. BMC Musculoskeletal Disorders, 19. 10.1186/s12891-018-2137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LJ, Schur E, Noonan C, Ahumada S, Buchwald D, & Afari N (2010). Chronic Pain, Overweight, and Obesity: Findings from a Community-Based Twin Registry. The Journal of Pain : Official Journal of the American Pain Society, 11(7), 628–635. 10.1016/j.jpain.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Zhang J, Yang H, & Sun Y (2018). The role of leptin in osteoarthritis. Medicine, 97(14). 10.1097/MD.0000000000010257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y (2016). Four Decades of Obesity Trends among Non-Hispanic Whites and Blacks in the United States: Analyzing the Influences of Educational Inequalities in Obesity and Population Improvements in Education. PLOS ONE, 11(11), e0167193. 10.1371/journal.pone.0167193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajacova A, Grol-Prokopczyk H, & Zimmer Z (Forthcoming). Pain trends among American adults 2002–2018: Patterns, disparities, and correlates. Demography. 10.31235/osf.io/vgp95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajacova A, & Montez JK (2017). Physical Functioning Trends among US Women and Men Age 45–64 by Education Level. Biodemography and Social Biology, 63(1), 21–30. 10.1080/19485565.2016.1263150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer Z, & Zajacova A (2020). Persistent, Consistent, and Extensive: The Trend of Increasing Pain Prevalence in Older Americans. The Journals of Gerontology.Series B, Psychological Sciences and Social Sciences, 75(2), 436–447. 10.1093/geronb/gbx162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MIDUS data used in this analysis are publicly available from the Inter-university Consortium for Political and Social Research (ICPSR, https://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/2760; https://www.icpsr.umich.edu/web/ICPSR/studies/36532).


