Abstract
Mental disorders represent a worldwide public health concern. Psychotherapies and pharmacotherapies are recommended as first line treatments. However, evidence has emerged that their efficacy may be overestimated, due to a variety of shortcomings in clinical trials (e.g., publication bias, weak control conditions such as waiting list). We performed an umbrella review of recent meta‐analyses of randomized controlled trials (RCTs) of psychotherapies and pharmacotherapies for the main mental disorders in adults. We selected meta‐analyses that formally assessed risk of bias or quality of studies, excluded weak comparators, and used effect sizes for target symptoms as primary outcome. We searched PubMed and PsycINFO and individual records of the Cochrane Library for meta‐analyses published between January 2014 and March 2021 comparing psychotherapies or pharmacotherapies with placebo or treatment‐as‐usual (TAU), or psychotherapies vs. pharmacotherapies head‐to‐head, or the combination of psychotherapy with pharmacotherapy to either monotherapy. One hundred and two meta‐analyses, encompassing 3,782 RCTs and 650,514 patients, were included, covering depressive disorders, anxiety disorders, post‐traumatic stress disorder, obsessive‐compulsive disorder, somatoform disorders, eating disorders, attention‐deficit/hyperactivity disorder, substance use disorders, insomnia, schizophrenia spectrum disorders, and bipolar disorder. Across disorders and treatments, the majority of effect sizes for target symptoms were small. A random effect meta‐analytic evaluation of the effect sizes reported by the largest meta‐analyses per disorder yielded a standardized mean difference (SMD) of 0.34 (95% CI: 0.26‐0.42) for psychotherapies and 0.36 (95% CI: 0.32‐0.41) for pharmacotherapies compared with placebo or TAU. The SMD for head‐to‐head comparisons of psychotherapies vs. pharmacotherapies was 0.11 (95% CI: –0.05 to 0.26). The SMD for the combined treatment compared with either monotherapy was 0.31 (95% CI: 0.19‐0.44). Risk of bias was often high. After more than half a century of research, thousands of RCTs and millions of invested funds, the effect sizes of psychotherapies and pharmacotherapies for mental disorders are limited, suggesting a ceiling effect for treatment research as presently conducted. A paradigm shift in research seems to be required to achieve further progress.
Keywords: Psychotherapies, pharmacotherapies, mental disorders, randomized controlled trials, meta‐analyses, effect sizes, meta‐analytic evaluation
Mental disorders represent a worldwide public health concern1, 2. Psychotherapies and pharmacotherapies are recommended as first line treatments3, 4. However, evidence has recently emerged suggesting that the efficacy of both types of treatment may have been overestimated, due to several shortcomings of clinical trials, such as publication bias, researcher allegiance, or use of weak comparison groups (in particular, waiting list)5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16. A realistic estimate of the efficacy of psychotherapies and pharmacotherapies is important to obtain.
Meta‐analyses and systematic reviews of randomized controlled trials (RCTs) are thought to provide the highest level of evidence 17 . However, not only individual RCTs but also meta‐analyses may be affected by the above‐mentioned biases6, 18, 19. To avoid overestimating treatment efficacy, meta‐analyses need to take risk of bias systematically into account6, 18, 19, 20. Furthermore, estimates of efficacy depend upon the comparator against which treatments are tested. Waiting list conditions, for example, represent a relatively weak comparator, leading to larger effect sizes6, 8, 21, 22. Comparisons with placebo or treatment‐as‐usual (TAU) provide better estimates of the true efficacy of a treatment6, 22.
The most recent comprehensive review of meta‐analyses of both psychotherapies and pharmacotherapies in mental disorders, including 61 meta‐analyses, was published in 2014, reporting a medium effect size (standardized mean difference, SMD = 0.50) 8 . Some of the included meta‐analyses, however, used waiting list comparisons in the assessment of overall efficacy. In addition, the authors seem to have just averaged the extracted effect sizes, without performing a meta‐analytic evaluation including weighting effect sizes. Furthermore, a large number of studies and meta‐analyses have been published since 2014.
For all these reasons, we carried out an up‐to‐date umbrella review of recent meta‐analyses of psychotherapies and pharmacotherapies for the main mental disorders in adults which used placebo or TAU as comparison groups and formally assessed risk of bias or quality of studies. As the primary outcome, we used the effect size for target symptoms of the relevant disorder.
METHODS
The study protocol of this umbrella review was registered in advance at PROSPERO (International Prospective Register of Systematic Reviews), registration number: CRD42020155452.
Inclusion criteria
Meta‐analyses of RCTs comparing psychotherapies or pharmacotherapies to placebo or TAU in adults with mental disorders published since 2014 were eligible. We also considered meta‐analyses comparing psychotherapies vs. pharmacotherapies head‐to‐head, or their combination to either monotherapy. Only meta‐analyses which formally assessed risk of bias or quality of studies were included. If multiple meta‐analyses fulfilling the inclusion criteria were available for one condition, all of them were included. Reporting of SMD or other measures of between‐group effect size was required.
All types of pharmacotherapy or psychotherapy were eligible for inclusion. Meta‐analyses examining specific subgroups (e.g., treatment resistant depression, primary care patients, the elderly), psychiatric or somatic comorbidities (e.g., depression in cancer patients), specific settings (e.g., group therapy only, or inpatient therapy) or augmentation strategies (e.g., psychostimulants added to antipsychotic drugs in schizophrenia), or focusing on secondary outcomes (e.g., quality of life in depression) were not included. These inclusion criteria are consistent with the above‐mentioned 2014 review 8 , except for excluding waiting list comparisons and requiring meta‐analyses to have assessed risk of bias or quality of studies. Both standard and network meta‐analyses were eligible.
Combining data of patients receiving TAU or placebo with those of patients on waiting list has been shown to inflate effect sizes8, 22, 23. On the other hand, mixing data of patients on TAU with those receiving specific therapies (e.g., cognitive‐behaviour therapy, CBT) can be expected to underestimate the effect size of the treatment in question. Therefore, meta‐analyses mixing data of TAU or placebo with waiting list or active treatments were excluded.
Search for studies
We searched PubMed and PsycINFO and individual records of the Cochrane Library for meta‐analyses of RCTs of psychotherapies and/or pharmacotherapies for mental disorders in adults published between January 2014 and March 2021.
Four reviewers independently searched for studies. Decision on inclusion was made by consensus including another rater. Search terms were meta‐analy* or metaanaly* combined with the thesaurus of the individual databases concerning each disorder. To provide comparable results, we used the syntax applied in the previous most comprehensive review 8 .
Data extraction
We focused on effect sizes and 95% confidence intervals (CIs) for the target symptoms of the relevant disorder (primary outcome). We extracted between‐group SMDs and related measures (Cohen's d, Hedges' g) as reported in the meta‐analyses. Odds ratios (ORs) and hazard ratios (HRs) were converted to SMDs24, 25. Data on relative risk (RR) were extracted as reported. We used Cohen's convention of d=0.2, d=0.5 and d=0.8 as indicating small, medium and large effect sizes 26 , corresponding to success rate differences of 11%, 28% and 43%; numbers needed to treat of 9, 4 and 2; ORs of 1.43, 2.48 and 4.25; RRs of 1.22, 1.86 and 3.00; and HRs of 1.3, 1.9 and 2.824, 25, 27, 28, 29. Intention‐to‐treat data were preferred whenever available.
If meta‐analyses took risk of bias into account by, for example, additionally reporting data separately for low risk of bias studies or correcting for publication bias, we listed all reported effect sizes but preferably focused on the corrected or high quality data for interpreting results.
Rates of remission and response were included as secondary outcomes when available. Dichotomous variables have some limitations 30 , but complementarily to SMDs they can provide useful information about efficacy.
One author extracted data (type of treatment and disorder, number of studies, number of participants, type of comparator, risk of bias, adverse events/side effects, and effect sizes). Data were cross‐checked independently by two raters each.
Quality assessment
The quality of the included meta‐analyses was independently assessed by two raters. For the purpose of this review, we used the items 1 to 9 of the Checklist for Systematic Reviews and Research Syntheses31, 32, complemented by item 12 of AMSTAR 220 (“Was the impact of risk of bias in individual studies on results of the meta‐analysis taken into account?”) and an additional item addressing whether the meta‐analysis was registered. In case of disagreement between raters, consensus ratings were used.
Data synthesis
The results of the largest meta‐analyses for each condition, i.e. those including most RCTs, are presented and evaluated separately. Additionally, these independent meta‐analyses were included in second‐order meta‐analyses combining their summary effect sizes across all the different mental disorders 33 . This allowed to obtain a weighted effect of psychotherapy or pharmacotherapy across all mental disorders, and weighted effects for the benefits of combined therapy, and for the comparative efficacy of psychotherapy vs. pharmacotherapy. The analysis was performed by Comprehensive Meta‐Analysis (CMA, Version 3) using a random effects model based on SMDs and their CIs via the CMA analysis option “generic estimates”.
Heterogeneity was assessed using the I2 statistic. If meta‐analyses did not report an overall effect size, but effect sizes for specific treatments and comparisons, the effect sizes of the relevant comparisons were aggregated by CMA and the resulting overall SMDs were entered into the second‐order meta‐analyses across disorders. Only effect estimates based on at least two primary RCTs were used.
RESULTS
Included meta‐analyses
The search retrieved 23,601 items, reduced to 19,500 after removing duplicates, which were screened by titles and abstracts. Full‐text evaluation was carried out for 319 papers. One hundred and two meta‐analyses fulfilled the inclusion criteria (see Figure 1 and supplementary information). These encompassed 69 meta‐analytic comparisons of pharmacotherapies with placebo or TAU, 26 comparisons of psychotherapies with placebo or TAU, 11 comparisons of psychotherapies vs. pharmacotherapies head‐to‐head, and 13 comparisons of combined psychotherapy and pharmacotherapy to either monotherapy6, 12, 13, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134. The 102 meta‐analyses encompassed 3,782 RCTs (range: 2 to 522) and 650,514 patients (range: 65 to 116,477) (see supplementary information).
Figure 1.
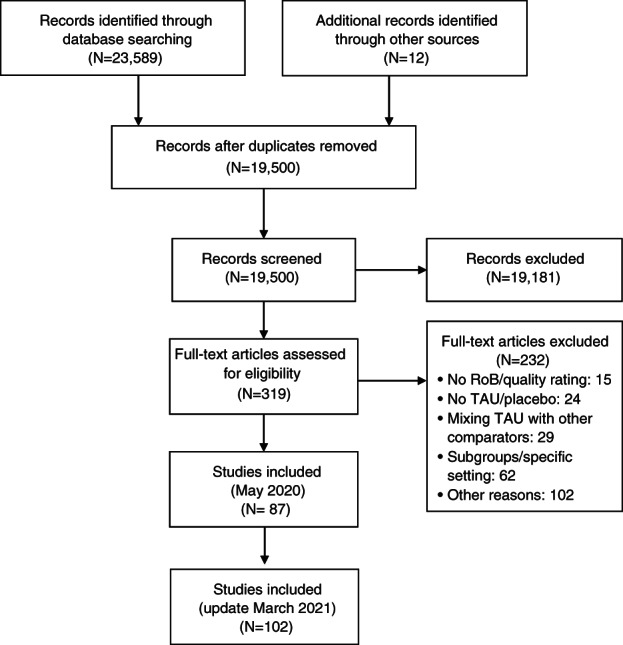
PRISMA flow chart. RoB – risk of bias, TAU – treatment as usual
Across all meta‐analyses, the mean number of positively rated items in the quality assessment was 8.71±1.43 (range: 4 to 11). The items 10 (item 12 of AMSTAR 2, addressing whether the meta‐analyses took the impact of bias on results into account) and 11 (study registration) were the least frequently fulfilled (48% and 47%, respectively). The quality of meta‐analyses was not significantly different between psychotherapies and pharmacotherapies (mean of positively rated items: 8.95±1.12 for psychotherapies, 8.68±1.54 for pharmacotherapies, t=0.74, p=0.46).
Psychotherapies and pharmacotherapies vs. TAU or placebo
In the largest meta‐analyses, the effect sizes of both psychotherapies and pharmacotherapies in comparison to TAU or placebo were small (SMD<0.50) for most disorders and treatments (see Figure 2 and supplementary information). Medium effect sizes were found only for pharmacotherapies of obsessive‐compulsive disorder (OCD) (SMD=0.56) 72 , bulimia nervosa (SMD=0.61) 80 , and somatoform disorders (SMD=0.50) 91 , and for psychotherapies of post‐traumatic stress disorder (PTSD) (SMD=0.54) 54 and borderline personality disorder (SMD=0.57) 93 . Large effect sizes were only reported for psychotherapy of OCD (SMD=1.03) 74 , with, however, a substantial proportion of patients taking concomitant pharmacotherapy72, 74. Overall, risk of bias was often high (see Figure 2 and supplementary information).
Figure 2.
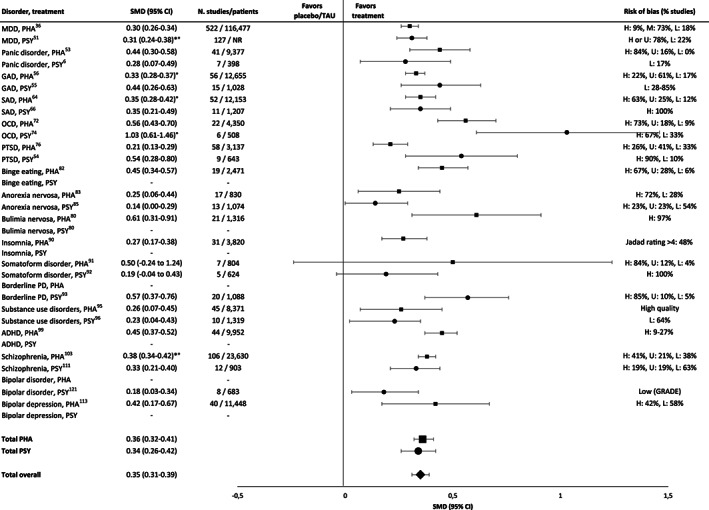
Effect sizes in the largest meta‐analyses of pharmacotherapies (squares) and psychotherapies (circles) in comparison to placebo or treatment‐as‐usual (TAU). PHA – pharmacotherapy; PSY – psychotherapy, SMD – standardized mean difference, * – adjusted for risk of bias, ° – adjusted for small‐study effects, MDD – major depressive disorder, GAD – generalized anxiety disorder, SAD – social anxiety disorder, OCD – obsessive‐compulsive disorder, PTSD – post‐traumatic stress disorder, PD – personality disorder, ADHD – attention‐deficit/hyperactivity disorder, H – high, M – medium, L – low, U – uncertain, NR – not reported. Where SMD is not provided, this means that no valid meta‐analysis reporting this value was available.
For psychotherapies and pharmacotherapies, second‐order random effects meta‐analyses in comparison to placebo or TAU yielded statistically significant but small SMDs of 0.34 (95% CI: 0.26‐0.42, I2=66.33%) and 0.36 (95% CI: 0.32‐0.41, I2=70.61%), respectively, across disorders (see Figure 2). For the aggregated data of psychotherapies and pharmacotherapies, the SMD was 0.35 (95 CI: 0.31‐0.39, I2=68.23%).
Depressive disorders
For psychotherapies of depressive disorders, the largest meta‐analysis reported a small SMD of 0.31, adjusted for biases, in comparison to TAU51 (see Figure 2). Taking all included meta‐analyses into account, psychotherapy achieved effect sizes (SMDs) between 0.11 and 0.61 in comparison to placebo or TAU6, 12, 37, 50, 51, 52, except for one outlying meta‐analysis reporting a large SMD post‐therapy (1.11), reduced to 0.27 at 3 to 12 month follow‐up and associated with a high risk of bias 52 . The majority of effect sizes were small (<0.50).
Only between 1% and 17% of studies of psychotherapy for depression were found to show a low risk of bias. When meta‐analyses took risk of bias into account, they consistently found a decrease in effect sizes (see supplementary information).
Across all forms of psychotherapy, remission from major depressive disorder (Hamilton Depression Rating Scale, HAM‐D <7) was achieved in 43% of patients, with no significant differences between the various psychotherapies 5 . Response (50% reduction of HAM‐D score) was achieved in 54% of patients 5 . TAU was superior to no treatment with regard to remission (33% vs. 23%), but inferior to psychotherapy (33% vs. 43%) 135 .
The largest meta‐analysis of pharmacotherapy for depressive disorders reported a SMD of 0.3036 (see Figure 2). All effect sizes (SMD) achieved by pharmacotherapy in comparison to placebo were below 0.50, ranging from 0.19 to 0.41. The exception was ketamine, which achieved large short‐term effects (0.83, 0.88) 24 hours and 3‐4 days after treatment, dropping to 0.31 after 7 days13, 34, 35, 36, 37, 38, 39, 41, 42, 43, 44, 45, 46, 47, 48, 49. Most effect sizes in terms of RRs were small as well (≤1.22).
The mean response rate for selective serotonin reuptake inhibitors (SSRIs) was 51% vs. 39% for placebo 35 , corresponding to a small effect size 27 .
Many trials of pharmacotherapy in depression showed a high risk of bias13, 35, 36, 42 (see supplementary information).
Anxiety disorders
In the largest meta‐analyses of anxiety disorders, psychotherapies achieved SMDs between 0.28 and 0.446,55,66 (see Figure 2). Overall, psychotherapies of anxiety disorders achieved SMDs compared to TAU or placebo between 0.01 and 0.726, 54, 55, 59, 65, 66, 71, except for two outlying effect sizes in generalized anxiety disorder (1.44, 1.32), each based on three studies only6, 55. Two effect sizes of psychotherapy (CBT) in social anxiety disorder were medium (0.72, 0.56) 66 , but most effect sizes were small (see supplementary information).
Overall, only 17% of psychotherapy studies in anxiety disorders were found to show a low risk of bias 6 .
In the largest meta‐analyses for anxiety disorders, pharmacotherapies achieved SMDs in comparison to placebo between 0.33 and 0.4553,56,64 (see Figure 2). Overall, effect sizes for pharmacotherapy were between 0.01 and 0.56, with the majority of effect sizes being small53, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 66, 67, 69, 70 (see supplementary information). RR ranged between 1.20 and 4.03, with most values being small, one medium (monoamine oxidase inhibitors), and one large (benzodiazepines, RR=4.03) 69 .
For social anxiety disorder and generalized anxiety disorder, pharmacotherapy yielded response rates of 52% and 56%, respectively, versus 32% and 41% with placebo59, 69.
Obsessive‐compulsive disorder
For psychotherapy (CBT) of OCD, the largest meta‐analysis reported a large SMD (1.03) 74 (see Figure 2). Considering all meta‐analyses, large SMDs in comparison to placebo were reported (0.91‐1.46)72, 74. At follow‐up of on average of 15.1 months after the end of treatment, SMDs decreased from 0.57 to 0.06 for all comparators 74 . Follow‐up results were not available for a comparison against placebo. Most psychotherapy trials included patients taking stable doses of antidepressants72, 74, possibly overestimating effect sizes in favour of psychotherapy 72 .
For pharmacotherapy of OCD, the largest meta‐analysis reported a medium effect size (SMD=0.56) 72 (see Figure 2). Considering all meta‐analyses, small to medium SMDs were reported (0.45‐0.66).
For most studies of psychotherapy and pharmacotherapy, the risk of bias was high (see Figure 2 and supplementary information).
Post‐traumatic stress disorder
For psychotherapy (CBT) of PTSD, the largest meta‐analysis reported a medium effect size compared to TAU (SMD=0.54) 54 (see Figure 2), which was stable at follow‐ups of up to 12 months after end of therapy 54 . For PTSD related to childhood maltreatment, a SMD of 0.50 in comparison to TAU/placebo was found, which was reduced to 0.21 after adjusting for small sample size 79 .
For pharmacotherapy of PTSD, the largest meta‐analysis reported a small SMD in comparison to placebo (0.21) 76 (see Figure 2). Considering all meta‐analyses, effect sizes achieved by pharmacotherapy in comparison to placebo were heterogeneous (SMDs: –0.10 to 0.97)75, 76, 77, 78. Risk of bias was often high77, 78. A large SMD was obtained with phenelzine (0.97), a medium one with mirtazapine (0.79), desipramine (0.52) and olanzapine (0.51), all based on only one RCT except for olanzapine 75 . For SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs), a medium SMD was reported (0.50) 77 . For all other drugs, SMDs were <0.50 (from –0.10 to 0.47).
Personality disorders
For psychotherapy of personality disorders, only a meta‐analysis of borderline personality disorder was available, which reported a medium SMD in comparison to TAU (0.57), with a high risk of bias in most studies (see Figure 2) 93 .
An update for developing a Cochrane report of pharmacotherapy in borderline personality disorder did not provide meta‐analytic results since the authors did not find robust evidence 136 .
Somatoform disorders
For psychotherapy of somatoform disorders, the largest meta‐analysis reported a small SMD (0.19, see Figure 2) in comparison to enhanced care, with high risk of bias due to lack of blinding 92 . For pharmacotherapy of somatoform disorders, the largest meta‐analysis reported a medium SMD (0.50, see Figure 2) in comparison with placebo 91 .
Considering all meta‐analyses, heterogeneous SMDs (from 0.13 to 0.91) were reported for pharmacotherapy, based on two or three RCTs, with a high risk of bias for most RCTs 91 .
Eating disorders
For psychotherapy of bulimia nervosa, no recent meta‐analysis fulfilled the inclusion criteria. For pharmacotherapy, the largest meta‐analysis reported a medium SMD in comparison with placebo (0.61, see Figure 2) 80 . Considering all meta‐analyses, considerable heterogeneity among classes of drugs were found (SMDs: 0.10‐1.00) 80 .
For psychotherapy of binge eating disorder, no meta‐analysis fulfilled the inclusion criteria. For pharmacotherapy, the largest meta‐analysis reported a small to medium SMD in comparison with placebo (0.45, see Figure 2) 82 . Considering all meta‐analyses, a small to medium effect size compared to placebo was found for pharmacotherapy (SMD=0.45, RR: 1.67, 2.61)81, 82. One of these meta‐analyses reported a high 82 , the other a medium to low risk of bias 81 .
For psychotherapy of anorexia nervosa, the largest meta‐analysis reported a small SMD in comparison with TAU (0.14, see Figure 2) 85 . Overall, the effect sizes in comparison to TAU or placebo were small (SMD=0.10‐0.31, RR: 0.97, 1.28)84, 85, 86. For pharmacotherapy, the largest meta‐analysis reported a small effect size (SMD=0.25) 83 .
Substance use disorders
For both psychotherapy and pharmacotherapy of substance use disorders, the largest meta‐analysis reported small SMDs in comparison with TAU or placebo (0.23 and 0.26, respectively, see Figure 2)95, 96. For psychotherapy, the effect size decreased at follow‐ups ≥8 months after end of treatment (SMD=0.05) 96 . Considering all meta‐analyses, small effect sizes were found for pharmacotherapy (SMDs: 0.07 to 0.35, RR: 0.32‐1.39)94, 95.
Insomnia
For psychotherapy, no recent meta‐analysis fulfilled the inclusion criteria. The quality of studies was found to be low 137 . For pharmacotherapy, the largest meta‐analysis reported a small SMD in comparison with placebo (0.27, see Figure 2) 90 . Overall, for pharmacotherapy of insomnia, small to medium SMDs were reported (0.07 to 0.58)88, 89, 90. One meta‐analysis provided effect sizes only for one of eight outcome measures, with large SMDs (0.88‐1.38), suggesting selective reporting 87 .
Attention‐deficit/hyperactivity disorder (ADHD)
For psychotherapy of ADHD in adults, no meta‐analysis could be included 134 . For pharmacotherapy, the largest meta‐analysis reported a small to medium SMD in comparison with placebo (0.45, see Figure 2) 99 . Considering all meta‐analyses, the effects of pharmacotherapy were heterogeneous (from 0.16 to 0.97)98, 99, 100, 101, 102. Large SMDs were found for amphetamines98, 100, 102, small to medium SMDs for methylphenidate100, 101. Risk of bias was often high or unclear, and level of evidence was low to very low98, 100.
Schizophrenia spectrum disorders
Results of psychotherapy in schizophrenia spectrum disorders were evaluated in the context of pharmacotherapy (i.e., patients usually received concomitant medication). The largest meta‐analysis reported a small SMD in comparison with TAU (0.33, see Figure 2) 111 . Considering all meta‐analyses, small effect sizes compared to nonspecific controls were found for overall symptoms, positive and negative symptoms (SMDs: 0.32, 0.24 and 0.08, respectively) 138 . In comparison to TAU, psychotherapy achieved small to medium SMDs for negative symptoms (0.15‐0.58)110, 112.
For psychotherapy, a response rate of 13% for overall symptoms and 25% for positive symptoms was found139 (a reduction of symptoms of at least 50% was required). The response rate decreased considerably if researcher allegiance (authors evaluated the therapy that they developed) was taken into account (from 13% to 4.9%) 139 .
For acute pharmacological treatment of schizophrenia, the largest meta‐analysis reported an overall SMD of 0.45 for target symptoms, reduced to 0.38 after adjusting for publication bias (Figure 2) 103 . These results are consistent with meta‐analyses on specific drugs, such as quetiapine (SMD=0.33), cariprazine (SMD: 0.32‐0.37), lurasidone (SMD: 0.34‐0.47), and aripiprazole and brexpiprazole (RR=1.1)104, 107, 108, 109. A large effect size was reported for clozapine (SMD=0.89) 103 . Large and medium SMDs were achieved by long‐acting injectable antipsychotics in the maintenance treatment of non‐affective psychoses (RR: 1.75‐3.70) 107 .
For the acute treatment of schizophrenia with pharmacotherapy, differences in response rates in comparison with placebo were small (23% vs. 14%) 14 .
Bipolar disorder
For psychotherapy of bipolar disorder, the largest meta‐analysis reported a small effect size in comparison to TAU (SMD=0.18, see Figure 2) 121 , with small effect sizes for both depression and mania symptoms (SMDs: 0.23 and 0.05, respectively) and for relapse prevention post‐therapy (RR: 1.52) 121 . At follow‐up 26 to 78 weeks post‐therapy, SMDs were 0.21 and 0.38; RR for relapse was 1.35 121 . Psychotherapy was given in the context of concomitant pharmacotherapy.
For the acute treatment of mania, the results for pharmacotherapy are heterogeneous. One meta‐analysis reported medium SMDs for cariprazine (0.51‐0.52) 119 , and another reported a small effect size for aripiprazole (SMD=0.16) 115 . These two meta‐analyses included only three RCTs. A third meta‐analysis reported a very large SMD (1.51) for tamoxifen 120 , based on two RCTs with small samples (16 and 66 cases, respectively), making the results questionable. This study represents a clear outlier.
For the acute treatment of bipolar depression, the largest meta‐analysis of pharmacotherapy reported heterogeneous results, with effect sizes (SMDs) between 1.41 and –1.84 113 . Large effect sizes were achieved by fluoxetine (1.41), divalproex (1.25), lurasidone (1.15), moclobemide (1.09), cariprazine (0.85) and imipramine (0.86) 113 , all of them based, however, on only 0‐3 direct comparisons. Some drugs achieved medium effect sizes (olanzapine, phenelzine, tranylcypromine). The effect sizes of all other drugs were small 113 . Quetiapine achieved an almost medium effect size (SMD=0.48) based on 11 direct comparisons 113 .
For the prevention of manic/hypomanic/mixed episodes, effect sizes of lithium were almost medium (RR=1.85) 114 . Medium effect sizes were reported for olanzapine (RR=2.88) and risperidone (RR=2.88); large effect sizes for aripiprazole (once monthly) and asenapine (RR: 3.31, 4.81) 114 . The results for asenapine are based on only one RCT 116 . For all other drugs, effect sizes were small 114 .
For the prevention of any mood episode relapse, a large effect size was found for asenapine (RR=3.82), with the caveat mentioned above 114 . Medium effect sizes were reported for quetiapine and olanzapine114, 116, 118. Small effect sizes for the prevention of any mood episode were achieved by lithium (RR: 1.60, 1.61) and several other drugs114, 118. Earlier meta‐analyses reported heterogeneous results for the prevention of any relapse by lithium (SMD: 1.12, 0.47) 140 .
For the prevention of depressive episodes, quetiapine and asenapine achieved medium effect sizes (RR: 2.08, 2.60). For all other drugs, including lithium (RR=1.26), effect sizes were small 114 . Small effect sizes were found for antidepressants (RR=1.56) 117 .
Psychotherapies vs. pharmacotherapies
Head‐to‐head comparisons of psychotherapies vs. pharmacotherapies yielded small effect sizes for all disorders examined, i.e., depressive disorders, anxiety disorders, PTSD and OCD (SMDs: 0.00‐0.24, see Figure 3)37, 66, 74, 126. A second‐order random effects meta‐analysis across the effect sizes of the largest meta‐analyses (Figure 3) yielded a non‐significant SMD of 0.11 (95% CI: –0.05 to 0.26, I2=61.99).
Figure 3.
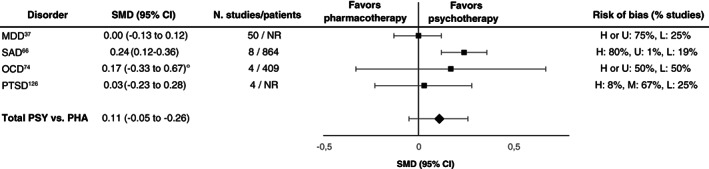
Effect sizes in the largest meta‐analyses for head‐to‐head comparisons of psychotherapies (PSY) vs. pharmacotherapies (PHA). SMD – standardized mean difference, ° – adjusted for small‐study effects, MDD – major depressive disorder, SAD – social anxiety disorder, OCD – obsessive‐compulsive disorder, PTSD – post‐traumatic stress disorder, H – high, M – medium, L – low, U – uncertain, NR – not reported
Considering all included meta‐analyses, no substantial differences in short‐term efficacy between psychotherapies and pharmacotherapies in depressive disorders, anxiety disorders and PTSD were found12, 37, 66, 122, 123, 124, 125, 126, with only a few exceptions. In OCD, psychotherapy achieved medium to large SMDs (0.61‐0.95) in comparison to SSRIs 72 , but most psychotherapy trials included patients taking stable doses of antidepressants, affecting results in favour of psychotherapy. Most studies of psychotherapy and pharmacotherapy in OCD had a high risk of bias 72 . With regard to long‐term efficacy, psychotherapy achieved a large SMD compared to pharmacotherapy in PTSD (0.83) 126 . For other disorders, no head‐to‐head comparisons fulfilled the inclusion criteria.
Combining psychotherapy and pharmacotherapy
In the largest meta‐analyses (Figure 4), effect sizes (SMDs) in favour of the combined treatment were small for most disorders, that is depressive disorders (0.37, 0.15) 37 , social anxiety disorder (combined vs. pharmacotherapy: 0.40) 66 , OCD (combined vs. psychotherapy: 0.25) and PTSD (0.09, 0.12) 126 . Effect sizes (SMDs) were medium in favour of the combined treatment vs. psychotherapy in social anxiety disorder (0.52) 66 and for the combined treatment vs. pharmacotherapy (SSRIs) in OCD (0.73), based on a network meta‐analysis including only one direct comparison with very small samples 72 . A large effect size was found only for the combined treatment vs. pharmacotherapy in ADHD (0.80) 134 , based on only two RCTs showing a high risk of bias in at least one domain.
Figure 4.
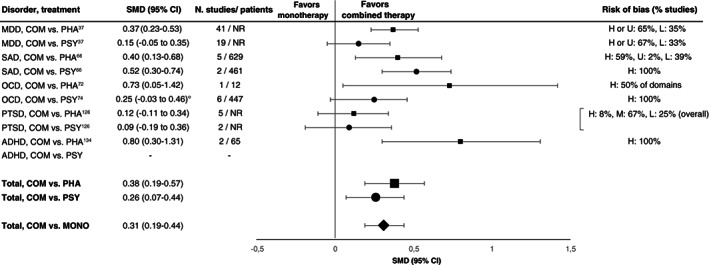
Effect sizes in the largest meta‐analyses for combined therapy vs. pharmacological (squares) or psychological (circles) monotherapy. SMD – standardized mean difference, ° – adjusted for small‐study effects, COM – combined therapy, PHA – pharmacotherapy, PSY – psychotherapy, MONO – monotherapy, MDD – major depressive disorder, SAD – social anxiety disorder, OCD – obsessive‐compulsive disorder, PTSD – post‐traumatic stress disorder, ADHD – attention‐deficit/hyperactivity disorder, H – high, M – medium, L – low, U – uncertain, NR – not reported
A second‐order random effects meta‐analysis across the effect sizes of the largest meta‐analyses yielded a statistically significant but small SMD of 0.31 (95% CI: 0.19‐0.44, I2=53.02) in favour of the combined treatment (Figure 4).
Considering all included meta‐analyses, most effect sizes (SMDs) achieved by the combined treatment compared to either monotherapy in depressive disorders, anxiety disorders, PTSD, OCD and ADHD were small (0.09‐0.48) when risk of bias was taken into account12, 37, 66, 72, 74, 126, 128, 129, 131, 132, 134. Exceptions were the superiority of the combined treatment in long‐term outcome of PTSD over pharmacotherapy (SMD=0.96, based on only two direct comparisons) 126 , and the superiority of the combined treatment over psychodynamic therapy in social anxiety disorder (SMD=0.68), based on a network meta‐analysis including zero direct comparisons for the condition 66 , making a study of inconsistencies impossible 141 .
In several of these meta‐analyses, risk of bias was high in several domains, or results were based on only a few or small studies66, 72, 126, 134.
DISCUSSION
In this field‐wide assessment of psychotherapies and pharmacotherapies for mental disorders in adults, we included evidence from 102 meta‐analyses with 3,782 RCTs and 650,514 patients. We found small benefits overall for both types of interventions, with an average SMD of 0.35 and moderate heterogeneity across conditions 142 . This finding challenges the result of the previous most comprehensive review, which reported an overall medium effect size (SMD=0.50) across psychotherapies and pharmacotherapies, based on 61 meta‐analyses with 852 RCTs and 137,126 patients 8 . This latter estimate seems to be due to including waiting list comparators and averaging effect sizes without performing a random effects meta‐analytic evaluation.
According to the results of this umbrella review and second‐order meta‐analyses, there is an additional gain of psychotherapy and pharmacotherapy in the treatment of mental disorders in adults, but this is small in terms of effect sizes 26 . Conditions for which very extensive evidence was available (e.g., depression) almost always had such modest effect sizes when only studies with low risk of bias were considered, or efforts were made to correct for bias. Medium or large effect sizes were found only for few conditions, and most of the effects sizes ≥0.50 were associated with a high risk of bias and/or limited evidence. Nevertheless, the argument still holds that, although there are some medications for general medical conditions with clearly higher effect sizes, psychotropic agents or psychotherapies are not generally less efficacious than those medications 140 .
Some limitations and features of this umbrella review should be discussed as they affect the interpretation of overall evidence. First, several meta‐analytic comparisons included only a few studies, affecting statistical power and external validity of results.
Second, the results of network meta‐analyses need some extra caution141, 143. It has been argued that these meta‐analyses can only provide observational evidence, since the comparisons between treatments are both direct and indirect, and the latter are not randomized 144 . As a related issue, transitivity (similar distribution of effect modifiers) can be controlled statistically only for known modifiers, in contrast to controlling all modifiers by randomization. Some of the network meta‐analyses included in this review encompassed only a few or even no direct comparisons of specific treatments66, 72, 75. Statistical power may be low if only a few studies with small samples and large heterogeneity are included141, 145. Thus, some inconsistencies between direct and indirect comparisons may not have been detected 145 , possibly affecting effect size estimates.
Third, we followed Cohen's convention of small, medium and large effect sizes 26 . However, the clinical relevance of these estimates is not clear. The clinical benefit of an intervention needs to be determined by comparison with a benchmark such as the minimal clinically important difference (MCID) 146 . For the HAM‐D, for example, a minimal clinically relevant improvement has been claimed by some authors to correspond to a 7‐point difference 147 or to an SMD of 0.88 148 . If this is correct, in psychotherapies or pharmacotherapies for depression, effect sizes of 0.30, 0.40 or even 0.50 correspond to a difference on the HAM‐D of 2 or 4 points (i.e. <7) which cannot be detected by clinicians and can therefore hardly be regarded as clinically significant. In schizophrenia, a SMD of 0.73 is required for a minimal clinical improvement of 15 points on the Positive and Negative Syndrome Scale (PANSS) 149 , implying that SMDs below 0.73 are not detectable by clinicians and may not be clinically significant.
For a better judgment, the CIs of the effect size estimates may be compared with the proposed MCID values 150 . It has been argued that, if the upper limit of the 95% CIs is smaller than the MCID, effect sizes can be regarded as “definitely clinically not important” 150 . For the vast majority of meta‐analyses on depression and schizophrenia, this would be the case if SMDs of 0.88 and 0.73 are used as MCID. However, even if the summary effect sizes are substantially smaller than the MCID, there is heterogeneity in treatment responses across patients. Therefore, a minority of patients may still achieve large benefits from treatment.
Fourth, identical effect sizes may have different clinical importance in different patient populations (e.g., according to disorders, gender or age) and for different outcomes (e.g., mortality) 151 . For outcomes including vital events (e.g., rates of suicide) small differences in success rates may be clinically important, whereas for continuous measures of (often transient) depression, anxiety or other symptoms, small differences of a few scale points may not 152 . Of the meta‐analyses on the treatment of depression, for example, only a few examined hard outcomes such as suicidal behaviour41, 42, 44. In the meta‐analyses on schizophrenia and bipolar disorder, data on suicidal behaviour were not reported, except for one meta‐analysis 118 . Future studies and meta‐analyses should include such important hard outcomes.
Fifth, TAU as a comparator was found to be superior to no treatment in depression (with regard to remission, 33% vs. 23%) 135 . However, TAU is a heterogeneous condition, and effect sizes achieved depend on the type of treatment actually delivered. Larger effect sizes may be achieved in comparison to weaker forms of TAU23, 153. This applies to psychological placebo as well 154 .
Sixth, the results of RCTs may not necessarily represent real‐world effectiveness 155 . In clinical practice, patients often suffer from multiple mental disorders, and treatments are usually tailored to the individual patients' needs. This applies, for example, to treatment duration. Most of the treatments included in this review were short‐term 6 . Data on longer‐term treatments are widely missing from RCT research.
In summary, a systematic re‐assessment of recent evidence across multiple meta‐analyses on key mental disorders provided an overarching picture of limited additional gain for both psychotherapies and pharmacotherapies over placebo or TAU. A ceiling seems to have been reached with response rates ≤50% and most SMDs not exceeding 0.30‐0.40. Thus, after more than half a century of research, thousands of RCTs and millions of invested funds, the “trillion‐dollar brain drain” 2 associated with mental disorders is presently not sufficiently addressed by the available treatments. This should not be seen as a nihilistic or dismissive conclusion, since undoubtedly some patients do benefit from the available treatments. However, realistically facing the situation is a prerequisite for improvement. Pretending that everything is fine will not move the field forward 156 , nor will conforming and producing more similar findings 157 .
A paradigm shift in research seems to be required to achieve further progress. Suggestions for such a shift have recently been made 11 , for example, for improving methodological quality and replicability (e.g., open science158, 159), improving available treatments – e.g., by personalized management160, 161, 162, defining specific targets and outcomes 163 , considering response to previous treatments (staging)164, 165, switching or augmentation strategies 166 – or developing new treatments (e.g., exploration of out‐of‐the box ideas and accidental discoveries 167 ). A focus on prevention (e.g., in educational or occupational settings)168, 169 may improve the situation as well.
Improving treatment strategies for mental disorders can be regarded as a central health challenge of the 21st century.
ACKNOWLEDGEMENTS
The authors are grateful to M. Huhn for providing effect sizes for the pharmacological treatment of schizophrenia. They thank J. Matzat, an official patient representative of the Federal Joint Committee in Germany, for approving the paper. They are also indebted to M. Wolf for his support in producing the figures, to S. Uysal for his help in the evaluation of the quality of the included meta‐analyses and the review of the extracted data, and to B. Leowald and L. Feix for support in searching for meta‐analyses. The paper is dedicated to the late H. Kächele, a pioneer of psychotherapy research. Supplementary information on the study is available at https://osf.io/yvg2c/.
REFERENCES
- 1. Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry 2016;3:171‐8. [DOI] [PubMed] [Google Scholar]
- 2. Smith K. Trillion‐dollar brain drain – enormous costs of mental health problems in Europe not matched by research investment. Nature 2011;478:15. [DOI] [PubMed] [Google Scholar]
- 3. Nathan PE, Gorman JM. A guide to treatments that work, 4th ed. New York: Oxford University Press, 2015. [Google Scholar]
- 4. National Institute for Health and Care Excellence . Mental health and behavioural conditions. https://www.nice.org.uk.
- 5. Cuijpers P, Karyotaki E, Weitz E et al. The effects of psychotherapies for major depression in adults on remission, recovery and improvement: a meta‐analysis. J Affect Disord 2014;159:118‐26. [DOI] [PubMed] [Google Scholar]
- 6. Cuijpers P, Cristea IA, Karyotaki E et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry 2016;15:245‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ioannidis JP. Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials? Philos Ethics Humanit Med 2008;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huhn M, Tardy M, Spineli LM et al. Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta‐analyses. JAMA Psychiatry 2014;71:706‐15. [DOI] [PubMed] [Google Scholar]
- 9. Leichsenring F, Abbass A, Hilsenroth MJ et al. Biases in research: risk factors for non‐replicability in psychotherapy and pharmacotherapy research. Psychol Med 2017;47:1000‐11. [DOI] [PubMed] [Google Scholar]
- 10. Ioannidis JP. Why most published research findings are false. PLoS Med 2005;2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leichsenring F, Steinert C, Ioannidis JPA. Toward a paradigm shift in treatment and research of mental disorders. Psychol Med 2019;49:2111‐7. [DOI] [PubMed] [Google Scholar]
- 12. Driessen E, Hollon SD, Bockting CL et al. Does publication bias inflate the apparent efficacy of psychological treatment for major depressive disorder? A systematic review and meta‐analysis of US National Institutes of Health‐funded trials. PLoS One 2015;10:e0137864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munkholm K, Paludan‐Muller AS, Boesen K. Considering the methodological limitations in the evidence base of antidepressants for depression: a reanalysis of a network meta‐analysis. BMJ Open 2019;9:e024886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leucht S, Leucht C, Huhn M et al. Sixty years of placebo‐controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta‐analysis, and meta‐regression of efficacy predictors. Am J Psychiatry 2017;174:927‐42. [DOI] [PubMed] [Google Scholar]
- 15. Turner EH, Matthews AM, Linardatos E et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 2008;358:252‐60. [DOI] [PubMed] [Google Scholar]
- 16. Dragioti E, Karathanos V, Gerdle B et al. Does psychotherapy work? An umbrella review of meta‐analyses of randomized controlled trials. Acta Psychiatr Scand 2017;136:236‐46. [DOI] [PubMed] [Google Scholar]
- 17. Oxford Centre for Evidence‐Based Medicine . Levels of evidence. https://www.cebm.ox.ac.uk.
- 18. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. New York: Wiley, 2009. [Google Scholar]
- 19. Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shea BJ, Reeves BC, Wells G et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furukawa TA, Noma H, Caldwell DM et al. Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta‐analysis. Acta Psychiatr Scand 2014;130:181‐92. [DOI] [PubMed] [Google Scholar]
- 22. Michopoulos I, Furukawa TA, Noma H et al. Different control conditions can produce different effect estimates in psychotherapy trials for depression. J Clin Epidemiol 2020;132:59‐70. [DOI] [PubMed] [Google Scholar]
- 23. Watts SE, Turnell A, Kladnitski N et al. Treatment‐as‐usual (TAU) is anything but usual: a meta‐analysis of CBT versus TAU for anxiety and depression. J Affect Disord 2015;175:152‐67. [DOI] [PubMed] [Google Scholar]
- 24. Azuero A. A note on the magnitude of hazard ratios. Cancer 2016;122:1298‐9. [DOI] [PubMed] [Google Scholar]
- 25. Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Stat Med 2000;19:3127‐31. [DOI] [PubMed] [Google Scholar]
- 26. Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum, 1988. [Google Scholar]
- 27. Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry 2006;59:990‐6. [DOI] [PubMed] [Google Scholar]
- 28. Olivier J, May WL, Bell ML. Relative effect sizes for measures of risk. Commun Stat Theory Methods 2017;46:6774‐81. [Google Scholar]
- 29. Kraemer HC, Neri E, Spiegel D. Wrangling with p‐values versus effect sizes to improve medical decision‐making: a tutorial. Int J Eat Disord 2020;53:302‐8. [DOI] [PubMed] [Google Scholar]
- 30. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aromataris E, Fernandez R, Godfrey CM et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015;13:132‐40. [DOI] [PubMed] [Google Scholar]
- 32. Joanna Briggs Institute . Checklist for Systematic Reviews and Research Syntheses. Adelaide: Joanna Briggs Institute, 2017. [Google Scholar]
- 33. Borenstein M, Hedges LV, Higgins JPT et al. Introduction to meta‐analysis. Chichester: Wiley, 2011. [Google Scholar]
- 34. Bai S, Guo W, Feng Y et al. Efficacy and safety of anti‐inflammatory agents for the treatment of major depressive disorder: a systematic review and meta‐analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2020;91:21‐32. [DOI] [PubMed] [Google Scholar]
- 35. Barth M, Kriston L, Klostermann S et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta‐regression and mediation analysis of placebo‐controlled trials. Br J Psychiatry 2016;208:114‐9. [DOI] [PubMed] [Google Scholar]
- 36. Cipriani A, Furukawa TA, Salanti G et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta‐analysis. Lancet 2018;391:1357‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cuijpers P, Noma H, Karyotaki E et al. A network meta‐analysis of the effects of psychotherapies, pharmacotherapies and their combination in the treatment of adult depression. World Psychiatry 2020;19:92‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo X, McCutcheon RA, Pillinger T et al. The magnitude and heterogeneity of antidepressant response in depression: a meta‐analysis of over 45,000 patients. J Affect Disord 2020;276:991‐1000. [DOI] [PubMed] [Google Scholar]
- 39. Humes EC, Brunoni AR. Comment on Fu, J . and Chen, Y. : The efficacy and safety of 5 mg/d vortioxetine compared to placebo for major depressive disorder: a meta‐analysis. Psychopharmacology 2017;234:903‐4. [DOI] [PubMed] [Google Scholar]
- 40. Fu J, Chen Y. The efficacy and safety of 5 mg/d vortioxetine compared to placebo for major depressive disorder: a meta‐analysis. Psychopharmacology 2015;232:7‐16. [DOI] [PubMed] [Google Scholar]
- 41. Henssler J, Kurschus M, Franklin J et al. Long‐term acute‐phase treatment with antidepressants, 8 weeks and beyond: a systematic review and meta‐analysis of randomized, placebo‐controlled trials. J Clin Psychiatry 2018;79:15r10545. [DOI] [PubMed] [Google Scholar]
- 42. Jakobsen JC, Katakam KK, Schou A et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta‐analysis and trial sequential analysis. BMC Psychiatry 2017;17:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kishi T, Meltzer HY, Matsuda Y et al. Azapirone 5‐HT1A receptor partial agonist treatment for major depressive disorder: systematic review and meta‐analysis. Psychol Med 2014;44:2255‐69. [DOI] [PubMed] [Google Scholar]
- 44. Koesters M, Ostuzzi G, Guaiana G et al. Vortioxetine for depression in adults. Cochrane Database Syst Rev 2017;7:CD011520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kryst J, Kawalec P, Mitoraj AM et al. Efficacy of single and repeated administration of ketamine in unipolar and bipolar depression: a meta‐analysis of randomized clinical trials. Pharmacol Rep 2020;72:543‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laoutidis ZG, Kioulos KT. Desvenlafaxine for the acute treatment of depression: a systematic review and meta‐analysis. Pharmacopsychiatry 2015;48:187‐99. [DOI] [PubMed] [Google Scholar]
- 47. Meeker AS, Herink MC, Haxby DG et al. The safety and efficacy of vortioxetine for acute treatment of major depressive disorder: a systematic review and meta‐analysis. Syst Rev 2015;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pae CU, Wang SM, Han C et al. Vortioxetine: a meta‐analysis of 12 short‐term, randomized, placebo‐controlled clinical trials for the treatment of major depressive disorder. J Psychiatry Neurosci 2015;40:174‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taylor D, Sparshatt A, Varma S et al. Antidepressant efficacy of agomelatine: meta‐analysis of published and unpublished studies. BMJ 2014;348:g1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cuijpers P, Turner EH, Mohr DC et al. Comparison of psychotherapies for adult depression to pill placebo control groups: a meta‐analysis. Psychol Med 2014;44:685‐95. [DOI] [PubMed] [Google Scholar]
- 51. Cuijpers P, Karyotaki E, Reijnders M et al. Was Eysenck right after all? A reassessment of the effects of psychotherapy for adult depression. Epidemiol Psychiatr Sci 2019;28:21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lopez‐Lopez JA, Davies SR, Caldwell DM et al. The process and delivery of CBT for depression in adults: a systematic review and network meta‐analysis. Psychol Med 2019;49:1937‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bighelli I, Castellazzi M, Cipriani A et al. Antidepressants versus placebo for panic disorder in adults. Cochrane Database Syst Rev 2018;4:CD010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Dis EAM, van Veen SC, Hagenaars MA et al. Long‐term outcomes of cognitive behavioral therapy for anxiety‐related disorders: a systematic review and meta‐analysis. JAMA Psychiatry 2020;77:265‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carl E, Witcraft SM, Kauffman BY et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta‐analysis of randomized controlled trials. Cogn Behav Ther 2020;49:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gomez AF, Barthel AL, Hofmann SG. Comparing the efficacy of benzodiazepines and serotonergic anti‐depressants for adults with generalized anxiety disorder: a meta‐analytic review. Expert Opin Pharmacother 2018;19:883‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. He H, Xiang Y, Gao F et al. Comparative efficacy and acceptability of first‐line drugs for the acute treatment of generalized anxiety disorder in adults: a network meta‐analysis. J Psychiatr Res 2019;118:21‐30. [DOI] [PubMed] [Google Scholar]
- 58. Kong W, Deng H, Wan J et al. Comparative remission rates and tolerability of drugs for generalised anxiety disorder: a systematic review and network meta‐analysis of double‐blind randomized controlled trials. Front Pharmacol 2020;11:580858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li X, Zhu L, Su Y et al. Short‐term efficacy and tolerability of venlafaxine extended release in adults with generalized anxiety disorder without depression: a meta‐analysis. PLoS One 2017;12:e0185865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maneeton N, Maneeton B, Woottiluk P et al. Quetiapine monotherapy in acute treatment of generalized anxiety disorder: a systematic review and meta‐analysis of randomized controlled trials. Drug Des Devel Ther 2016;10:259‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qin B, Huang G, Yang Q et al. Vortioxetine treatment for generalised anxiety disorder: a meta‐analysis of anxiety, quality of life and safety outcomes. BMJ Open 2019;9:e033161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang SM, Woo YS, Kim NY et al. Agomelatine for the treatment of generalized anxiety disorder: a meta‐analysis. Clin Psychopharmacol Neurosci 2020;18:423‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Y, Huang G, Yang S et al. Duloxetine in treating generalized anxiety disorder in adults: a meta‐analysis of published randomized, double‐blind, placebo‐controlled trials. Asia Pac Psychiatry 2016;8:215‐25. [DOI] [PubMed] [Google Scholar]
- 64. Curtiss J, Andrews L, Davis M et al. A meta‐analysis of pharmacotherapy for social anxiety disorder: an examination of efficacy, moderators, and mediators. Expert Opin Pharmacother 2017;18:243‐51. [DOI] [PubMed] [Google Scholar]
- 65. Heeren A, Mogoase C, Philippot P et al. Attention bias modification for social anxiety: a systematic review and meta‐analysis. Clin Psychol Rev 2015;40:76‐90. [DOI] [PubMed] [Google Scholar]
- 66. Mayo‐Wilson E, Dias S, Mavranezouli I et al. Psychological and pharmacological interventions for social anxiety disorder in adults: a systematic review and network meta‐analysis. Lancet Psychiatry 2014;1:368‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu X, Li X, Zhang C et al. Efficacy and tolerability of fluvoxamine in adults with social anxiety disorder: a meta‐analysis. Medicine 2018;97:e11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu H, Li X, Han B et al. Effects of cognitive bias modification on social anxiety: a meta‐analysis. PLoS One 2017;12:e0175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Williams T, Hattingh CJ, Kariuki CM et al. Pharmacotherapy for social anxiety disorder (SAnD). Cochrane Database Syst Rev 2017;10:CD001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sugarman MA, Loree AM, Baltes BB et al. The efficacy of paroxetine and placebo in treating anxiety and depression: a meta‐analysis of change on the Hamilton Rating Scales. PLoS One 2014;9:e106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Carpenter JK, Andrews LA, Witcraft SM et al. Cognitive behavioral therapy for anxiety and related disorders: a meta‐analysis of randomized placebo‐controlled trials. Depress Anxiety 2018;35:502‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Skapinakis P, Caldwell D, Hollingworth W et al. A systematic review of the clinical effectiveness and cost‐effectiveness of pharmacological and psychological interventions for the management of obsessive‐compulsive disorder in children/adolescents and adults. Health Technol Assess 2016;20:1‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Skapinakis P, Caldwell DM, Hollingworth W et al. Pharmacological and psychotherapeutic interventions for management of obsessive‐compulsive disorder in adults: a systematic review and network meta‐analysis. Lancet Psychiatry 2016;3:730‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Öst LG, Havnen A, Hansen B et al. Cognitive behavioral treatments of obsessive‐compulsive disorder. A systematic review and meta‐analysis of studies published 1993‐2014. Clin Psychol Rev 2015;40:156‐69. [DOI] [PubMed] [Google Scholar]
- 75. Cipriani A, Williams T, Nikolakopoulou A et al. Comparative efficacy and acceptability of pharmacological treatments for post‐traumatic stress disorder in adults: a network meta‐analysis. Psychol Med 2018;48:1975‐84. [DOI] [PubMed] [Google Scholar]
- 76. de Moraes Costa G, Zanatta FB, Ziegelmann PK et al. Pharmacological treatments for adults with post‐traumatic stress disorder: a network meta‐analysis of comparative efficacy and acceptability. J Psychiatr Res 2020;130:412‐20. [DOI] [PubMed] [Google Scholar]
- 77. Lee DJ, Schnitzlein CW, Wolf JP et al. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta‐analyses to determine first‐line treatments. Depress Anxiety 2016;33:792‐806. [DOI] [PubMed] [Google Scholar]
- 78. Hoskins M, Pearce J, Bethell A et al. Pharmacotherapy for post‐traumatic stress disorder: systematic review and meta‐analysis. Br J Psychiatry 2015;206:93‐100. [DOI] [PubMed] [Google Scholar]
- 79. Ehring T, Welboren R, Morina N et al. Meta‐analysis of psychological treatments for posttraumatic stress disorder in adult survivors of childhood abuse. Clin Psychol Rev 2014;34:645‐57. [DOI] [PubMed] [Google Scholar]
- 80. Svaldi J, Schmitz F, Baur J et al. Efficacy of psychotherapies and pharmacotherapies for bulimia nervosa. Psychol Med 2019;49:898‐910. [DOI] [PubMed] [Google Scholar]
- 81. Brownley KA, Berkman ND, Peat CM et al. Binge‐eating disorder in adults: a systematic review and meta‐analysis. Ann Intern Med 2016;165:409‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hilbert A, Petroff D, Herpertz S et al. Meta‐analysis of the efficacy of psychological and medical treatments for binge‐eating disorder. J Consult Clin Psychol 2019;87:91‐105. [DOI] [PubMed] [Google Scholar]
- 83. de Vos J, Houtzager L, Katsaragaki G et al. Meta analysis on the efficacy of pharmacotherapy versus placebo on anorexia nervosa. J Eat Disord 2014;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hay PJ, Claudino AM, Touyz S et al. Individual psychological therapy in the outpatient treatment of adults with anorexia nervosa. Cochrane Database Syst Rev 2015;7:CD003909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Solmi M, Wade TD, Byrne S et al. Comparative efficacy and acceptability of psychological interventions for the treatment of adult outpatients with anorexia nervosa: a systematic review and network meta‐analysis. Lancet Psychiatry 2021;8:215‐24. [DOI] [PubMed] [Google Scholar]
- 86. van den Berg E, Houtzager L, de Vos J et al. Meta‐analysis on the efficacy of psychological treatments for anorexia nervosa. Eur Eat Disord Rev 2019;27:331‐51. [DOI] [PubMed] [Google Scholar]
- 87. Liang L, Huang Y, Xu R et al. Eszopiclone for the treatment of primary insomnia: a systematic review and meta‐analysis of double‐blind, randomized, placebo‐controlled trials. Sleep Med 2019;62:6‐13. [DOI] [PubMed] [Google Scholar]
- 88. Kishi T, Nomura I, Matsuda Y et al. Lemborexant vs suvorexant for insomnia: a systematic review and network meta‐analysis. J Psychiatr Res 2020;128:68‐74. [DOI] [PubMed] [Google Scholar]
- 89. Kuriyama A, Honda M, Hayashino Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta‐analysis. Sleep Med 2014;15:385‐92. [DOI] [PubMed] [Google Scholar]
- 90. Winkler A, Auer C, Doering BK et al. Drug treatment of primary insomnia: a meta‐analysis of polysomnographic randomized controlled trials. CNS Drugs 2014;28:799‐816. [DOI] [PubMed] [Google Scholar]
- 91. Kleinstauber M, Witthoft M, Steffanowski A et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev 2014;11:CD010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. van Dessel N, den Boeft M, van der Wouden JC et al. Non‐pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev 2014;11:CD011142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Storebo OJ, Stoffers‐Winterling JM, Vollm BA et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev 2020;5:CD012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cheng YC, Huang YC, Huang WL. Gabapentinoids for treatment of alcohol use disorder: a systematic review and meta‐analysis. Hum Psychopharmacol 2020;35:1‐11. [DOI] [PubMed] [Google Scholar]
- 95. Donoghue K, Elzerbi C, Saunders R et al. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta‐analysis. Addiction 2015;110:920‐30. [DOI] [PubMed] [Google Scholar]
- 96. Magill M, Ray L, Kiluk B et al. A meta‐analysis of cognitive‐behavioral therapy for alcohol or other drug use disorders: treatment efficacy by contrast condition. J Consult Clin Psychol 2019;87:1093‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vanderkam P, Solinas M, Ingrand I et al. Effectiveness of drugs acting on adrenergic receptors in the treatment for tobacco or alcohol use disorders: systematic review and meta‐analysis. Addiction 2021;116:1011‐20. [DOI] [PubMed] [Google Scholar]
- 98. Castells X, Blanco‐Silvente L, Cunill R. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev 2018;8:CD007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cunill R, Castells X, Tobias A et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta‐analysis and meta‐regression in over 9000 patients. Psychopharmacology 2016;233:187‐97. [DOI] [PubMed] [Google Scholar]
- 100. Cortese S, Adamo N, Del Giovane C et al. Comparative efficacy and tolerability of medications for attention‐deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta‐analysis. Lancet Psychiatry 2018;5:727‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lenzi F, Cortese S, Harris J et al. Pharmacotherapy of emotional dysregulation in adults with ADHD: a systematic review and meta‐analysis. Neurosci Biobehav Rev 2018;84:359‐67. [DOI] [PubMed] [Google Scholar]
- 102. Maneeton N, Maneeton B, Suttajit S et al. Exploratory meta‐analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther 2014;8:1685‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huhn M, Nikolakopoulou A, Schneider‐Thoma J et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi‐episode schizophrenia: a systematic review and network meta‐analysis. Lancet 2019;394:939‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hutton P, Taylor PJ, Mulligan L et al. Quetiapine immediate release v. placebo for schizophrenia: systematic review, meta‐analysis and reappraisal. Br J Psychiatry 2015;206:360‐70. [DOI] [PubMed] [Google Scholar]
- 105. Kishi T, Ikuta T, Matsuda Y et al. Aripiprazole vs. brexpiprazole for acute schizophrenia: a systematic review and network meta‐analysis. Psychopharmacology 2020;237:1459‐70. [DOI] [PubMed] [Google Scholar]
- 106. McCutcheon RA, Pillinger T, Mizuno Y et al. The efficacy and heterogeneity of antipsychotic response in schizophrenia: a meta‐analysis. Mol Psychiatry 2021;26:1310‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ostuzzi G, Bertolini F, Del Giovane C et al. Maintenance treatment with long‐acting injectable antipsychotics for people with nonaffective psychoses: a network meta‐analysis. Am J Psychiatry 2021;178:424‐36. [DOI] [PubMed] [Google Scholar]
- 108. Zhao MJ, Qin B, Wang JB et al. Efficacy and acceptability of cariprazine in acute exacerbation of schizophrenia: meta‐analysis of randomized placebo‐controlled trials. J Clin Psychopharmacol 2018;38:55‐9. [DOI] [PubMed] [Google Scholar]
- 109. Zheng W, Cai DB, Yang XH et al. Short‐term efficacy and tolerability of lurasidone in the treatment of acute schizophrenia: a meta‐analysis of randomized controlled trials. J Psychiatr Res 2018;103:244‐51. [DOI] [PubMed] [Google Scholar]
- 110. Cella M, Preti A, Edwards C et al. Cognitive remediation for negative symptoms of schizophrenia: a network meta‐analysis. Clin Psychol Rev 2017;52:43‐51. [DOI] [PubMed] [Google Scholar]
- 111. Jauhar S, McKenna PJ, Radua J et al. Cognitive‐behavioural therapy for the symptoms of schizophrenia: systematic review and meta‐analysis with examination of potential bias. Br J Psychiatry 2014;204:20‐9. [DOI] [PubMed] [Google Scholar]
- 112. Lutgens D, Gariepy G, Malla A. Psychological and psychosocial interventions for negative symptoms in psychosis: systematic review and meta‐analysis. Br J Psychiatry 2017;210:324‐32. [DOI] [PubMed] [Google Scholar]
- 113. Bahji A, Ermacora D, Stephenson C et al. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: a systematic review and network meta‐analysis. J Affect Disord 2020;269:154‐84. [DOI] [PubMed] [Google Scholar]
- 114. Kishi T, Ikuta T, Matsuda Y et al. Mood stabilizers and/or antipsychotics for bipolar disorder in the maintenance phase: a systematic review and network meta‐analysis of randomized controlled trials Mol Psychiatry (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Li DJ, Tseng PT, Stubbs B et al. Efficacy, safety and tolerability of aripiprazole in bipolar disorder: an updated systematic review and meta‐analysis of randomized controlled trials. Prog Neuropsychopharmacol Biol Psychiatry 2017;79:289‐301. [DOI] [PubMed] [Google Scholar]
- 116. Lindstrom L, Lindstrom E, Nilsson M et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – A systematic review and meta‐analysis. J Affect Disord 2017;213:138‐50. [DOI] [PubMed] [Google Scholar]
- 117. Liu B, Zhang Y, Fang H et al. Efficacy and safety of long‐term antidepressant treatment for bipolar disorders – A meta‐analysis of randomized controlled trials. J Affect Disord 2017;223:41‐8. [DOI] [PubMed] [Google Scholar]
- 118. Miura T, Noma H, Furukawa TA et al. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta‐analysis. Lancet Psychiatry 2014;1:351‐9. [DOI] [PubMed] [Google Scholar]
- 119. Pinto JV, Saraf G, Vigo D et al. Cariprazine in the treatment of bipolar disorder: a systematic review and meta‐analysis. Bipolar Disord 2020;22:360‐71. [DOI] [PubMed] [Google Scholar]
- 120. Talaei A, Pourgholami M, Khatibi‐Moghadam H et al. Tamoxifen: a protein kinase C inhibitor to treat mania: a systematic review and meta‐analysis of randomized, placebo‐controlled trials. J Clin Psychopharmacol 2016;36:272‐5. [DOI] [PubMed] [Google Scholar]
- 121. Oud M, Mayo‐Wilson E, Braidwood R et al. Psychological interventions for adults with bipolar disorder: systematic review and meta‐analysis. Br J Psychiatry 2016;208:213‐22. [DOI] [PubMed] [Google Scholar]
- 122. Amick HR, Gartlehner G, Gaynes BN et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta‐analysis. BMJ 2015;351:h6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cuijpers P, Karyotaki E, Andersson G et al. The effects of blinding on the outcomes of psychotherapy and pharmacotherapy for adult depression: a meta‐analysis. Eur Psychiatry 2015;30:685‐93. [DOI] [PubMed] [Google Scholar]
- 124. Cuijpers P, Donker T, Weissman MM et al. Interpersonal psychotherapy for mental health problems: a comprehensive meta‐analysis. Am J Psychiatry 2016;173:680‐7. [DOI] [PubMed] [Google Scholar]
- 125. Imai H, Tajika A, Chen P et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev 2016;10: CD011170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Merz J, Schwarzer G, Gerger H. Comparative efficacy and acceptability of pharmacological, psychotherapeutic, and combination treatments in adults with posttraumatic stress disorder: a network meta‐analysis. JAMA Psychiatry 2019;76:904‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sonis J, Cook JM. Medication versus trauma‐focused psychotherapy for adults with posttraumatic stress disorder: a systematic review and meta‐analysis. Psychiatry Res 2019;282:112637. [DOI] [PubMed] [Google Scholar]
- 128. Burkner PC, Bittner N, Holling H et al. D‐cycloserine augmentation of behavior therapy for anxiety and obsessive‐compulsive disorders: a meta‐analysis. PLoS One 2017;12:e0173660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cuijpers P, Sijbrandij M, Koole SL et al. Adding psychotherapy to antidepressant medication in depression and anxiety disorders: a meta‐analysis. World Psychiatry 2014;13:56‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Driessen E, Dekker JJM, Peen J et al. The efficacy of adding short‐term psychodynamic psychotherapy to antidepressants in the treatment of depression: a systematic review and meta‐analysis of individual participant data. Clin Psychol Rev 2020;80:101886. [DOI] [PubMed] [Google Scholar]
- 131. Karyotaki E, Smit Y, Holdt Henningsen K et al. Combining pharmacotherapy and psychotherapy or monotherapy for major depression? A meta‐analysis on the long‐term effects. J Affect Disord 2016;194:144‐52. [DOI] [PubMed] [Google Scholar]
- 132. Ori R, Amos T, Bergman H et al. Augmentation of cognitive and behavioural therapies (CBT) with d‐cycloserine for anxiety and related disorders. Cochrane Database Syst Rev 2015;5:CD007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hoskins MD, Sinnerton R, Nakamura A et al. Pharmacological‐assisted psychotherapy for post‐traumatic stress disorder: a systematic review and meta‐analysis. Eur J Psychotraumatol 2021;12:1853379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lopez PL, Torrente FM, Ciapponi A et al. Cognitive‐behavioural interventions for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev 2018;3:CD010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kolovos S, van Tulder MW, Cuijpers P et al. The effect of treatment as usual on major depressive disorder: a meta‐analysis. J Affect Disord 2017;210:72‐81. [DOI] [PubMed] [Google Scholar]
- 136. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep 2015;17:534. [DOI] [PubMed] [Google Scholar]
- 137. Sakaluk JK, Kilshaw RE, Williams AJ et al. Evaluating the evidential value of empirically supported psychological treatments (ESTs): a meta‐scientific review. J Abnorm Psychol 2019;128:500‐9. [DOI] [PubMed] [Google Scholar]
- 138. Jauhar S, Laws KR, McKenna PJ. CBT for schizophrenia: a critical viewpoint. Psychol Med 2019; 49:1233‐6. [DOI] [PubMed] [Google Scholar]
- 139. Bighelli I, Huhn M, Schneider‐Thoma J et al. Response rates in patients with schizophrenia and positive symptoms receiving cognitive behavioural therapy: a systematic review and single‐group meta‐analysis. BMC Psychiatry 2018;18:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Leucht S, Hierl S, Kissling W et al. Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta‐analyses. Br J Psychiatry 2012;200:97‐106. [DOI] [PubMed] [Google Scholar]
- 141. Faltinsen EG, Storebo OJ, Jakobsen JC et al. Network meta‐analysis: the highest level of medical evidence? BMJ Evid Based Med 2018;23:56‐9. [DOI] [PubMed] [Google Scholar]
- 142. Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Leucht S, Chaimani A, Cipriani AS et al. Network meta‐analyses should be the highest level of evidence in treatment guidelines. Eur Arch Psychiatry Clin Neurosci 2016;266:477‐80. [DOI] [PubMed] [Google Scholar]
- 144. Cipriani A, Higgins JP, Geddes JR et al. Conceptual and technical challenges in network meta‐analysis. Ann Intern Med 2013;159:130‐7. [DOI] [PubMed] [Google Scholar]
- 145. Veroniki AA, Mavridis D, Higgins JP et al. Characteristics of a loop of evidence that affect detection and estimation of inconsistency: a simulation study. BMC Med Res Methodol 2014;14:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407‐15. [DOI] [PubMed] [Google Scholar]
- 147. Leucht S, Fennema H, Engel R et al. What does the HAMD mean? J Affect Disord 2013;148:243‐8. [DOI] [PubMed] [Google Scholar]
- 148. Moncrieff J, Kirsch I. Empirically derived criteria cast doubt on the clinical significance of antidepressant‐placebo differences. Contemp Clin Trials 2015;43:60‐2. [DOI] [PubMed] [Google Scholar]
- 149. Leucht S, Kane JM, Etschel E et al. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology 2006;31:2318‐25. [DOI] [PubMed] [Google Scholar]
- 150. Man‐Son‐Hing M, Laupacis A, O'Rourke K et al. Determination of the clinical importance of study results. J Gen Intern Med 2002;17:469‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA 2014;312:1342‐3. [DOI] [PubMed] [Google Scholar]
- 152. Hengartner MP, Ploderl M. Statistically significant antidepressant‐placebo differences on subjective symptom‐rating scales do not prove that the drugs work: effect size and method bias matter! Front Psychiatry 2018;9:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cuijpers P, Quero S, Papola D et al. Care‐as‐usual control groups across different settings in randomized trials on psychotherapy for adult depression: a meta‐analysis. Psychol Med 2021;51:634‐44. [DOI] [PubMed] [Google Scholar]
- 154. Baskin TW, Tierney SC, Minami T et al. Establishing specificity in psychotherapy: a meta‐analysis of structural equivalence of placebo controls. J Consult Clin Psychol 2003;71:973‐9. [DOI] [PubMed] [Google Scholar]
- 155. Sherman RE, Anderson SA, Dal Pan GJ et al. Real‐world evidence – What is it and what can it tell us? N Engl J Med 2016;375:2293‐7. [DOI] [PubMed] [Google Scholar]
- 156. Cuijpers P, Karyotaki E, Reijnders M et al. Is psychotherapy effective? Pretending everything is fine will not help the field forward. Epidemiol Psychiatr Sci 2019;28:356‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nicholson JM, Ioannidis JP. Research grants: conform and be funded. Nature 2012;492:34‐6. [DOI] [PubMed] [Google Scholar]
- 158. Open Science Collaboration. Estimating the reproducibility of psychological science. Science 2015;349:aac4716. [DOI] [PubMed] [Google Scholar]
- 159. Munafò MR, Nosek BA, Bishop DV et al. A manifesto for reproducible science. Nat Hum Behav 2017;1:0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Maj M, Stein DJ, Parker G et al. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry 2020;19:269‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Maj M, van Os J, De Hert M et al. the clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry 2021;20:4‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Stein DJ, Craske MG, Rothbaum BO et al. The clinical characterization of the adult patient with an anxiety or related disorder aimed at personalization of management. World Psychiatry 2021;20:336‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cuijpers P. Targets and outcomes of psychotherapies for mental disorders: an overview. World Psychiatry 2019;18:276‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Fava G, Tomba E, Sonino N. Clinimetrics: the science of clinical measurements. Int J Clin Pract 2012;66:11‐5. [DOI] [PubMed] [Google Scholar]
- 165. Steinert C, Kruse J, Leichsenring F. Long‐term outcome and non‐response in psychotherapy: are we short‐sighted. Psychother Psychosom 2016;85:235‐7. [DOI] [PubMed] [Google Scholar]
- 166. Markowitz JC, Milrod BL. What to do when a psychotherapy fails. Lancet Psychiatry 2015;2:186‐90. [DOI] [PubMed] [Google Scholar]
- 167. Nutt D. Help luck along to find psychiatric medicines. Nature 2014;515:165. [DOI] [PubMed] [Google Scholar]
- 168. Barlow J, Schrader McMillan A, Kirkpatrick S et al. Health‐led interventions in the early years to enhance infant and maternal mental health: a review of reviews. Child Adolesc Ment Health 2010;15:178‐85. [DOI] [PubMed] [Google Scholar]
- 169. Fusar‐Poli P, Correll CU, Arango C et al. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry 2021:20:200‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]


