Abstract
Frailty is a state of heightened vulnerability and susceptibility to physiologic stressors that increases with age. It has shown increasing utility in predicting a range of adverse health outcomes. Here, we characterize a 67-item deficit-accumulation frailty index (FI) in 19 110 community-dwelling individuals in the ASPirin in Reducing Events in the Elderly clinical trial. Participants aged 65–98 years were recruited from the United States and Australia and were without diagnosed dementia and cardiovascular disease, and major physical disability. The median FI score was .10 (interquartile range: .07–.14) at baseline, and the prevalence of frailty (FI > .21) increased from 8.1% to 17.4% after 6 years. FI was positively associated with age, and women had significantly higher scores than men at all ages. The FI was negatively correlated with gait speed (r = −.31) and grip strength (r = −.46), and strongly associated with a modified Fried’s frailty phenotype (p < .0001, for all comparisons). Frailty was associated with the primary composite outcome capturing independent life lived free of major disability and dementia, and increased the rate of persistent physical disability (hazard ratio: 21.3, 95% confidence interval: 15.6–28.9). It added significantly to the predictive capacity of these outcomes above age, sex, and ethnicity alone. The FI is thus a useful biomarker of aging even among relatively healthy older individuals and provides important information about an individual’s vulnerability to and risk of disease.
Keywords: Biomarker, Physical function, Preventive health care
Frailty is a cumulative decline across multiple physiological systems which results in reduced functional reserve and increased vulnerability to stressors, illness, and injury (1,2). In both clinical and community-based populations, frailty is also associated with a heightened risk of hospitalizations, physical dependency, and disease (3–5). Frailty is the most common underlying syndrome observed preceding death in older adults (6).
With the aging population, the prevalence of frailty is increasing. Indeed, some estimates have suggested that around a quarter of individuals older than 85 years are frail (7), and this number is expected to grow. Frailty is now recognized as an important public health concern (8); however, it is still not routinely assessed in clinical practice. Identifying patients with frailty is essential to help inform clinical decisions and treatment options (9) and in primary care to identify individuals where timely interventions could help reduce the consequences of frailty on quality of life, health, and the costs of care (10).
There are many operational definitions of frailty (5,11), and one of the most common is the deficit-accumulation frailty index (FI) (2). The FI is a multidimensional measure which assesses the accumulation of health deficits with aging across a range of domains of functioning (physical, psychological, and social) (12). The exact deficits that are included in the FI are not fixed and can, thus, be adapted to information which is available. The FI focuses primarily on the number of deficits, with less emphasis on the severity of an individual deficit. The FI has been shown to predict adverse health outcomes more accurately than chronological age (13) and to characterize age-related decline in health better than other biological measures of aging (14).
ASPirin in Reducing Events in the Elderly (ASPREE) was a randomized, placebo-controlled trial to determine the effect of low-dose aspirin on disability-free survival (DFS) in older community-dwelling adults. Individuals were predominantly aged 70 years and older, without established cardiovascular disease, major cognitive impairments, or functional limitations at recruitment. The ASPREE population thus represents a unique cohort in which to examine the FI and how it changes over time.
The aims of this study were to adapt the deficit-accumulation FI approach to determine frailty status in ASPREE participants, to characterize the FI in terms of its association with age and physical health, and to compare it to the modified Fried’s frailty phenotype. This study also aimed to validate the ASPREE FI by determining its predictive capacity for dementia-free survival and DFS and persistent physical disability over 5 years.
Method
Study Population
Full details regarding the ASPREE study design and findings from the main trial have been published previously (15,16). In brief, between 2010 and 2014, the study recruited 19 114 older community-dwelling individuals from general practitioners in Australia (87% of participants) and from clinical-based mailing lists, electronic medical screening in clinics, and media advertisements in the United States (13% of participants). Eligible individuals were aged 70 years and older (or 65+ years for U.S. African Americans and Hispanic/Latinos) and without a self-report or physician diagnosis of dementia, a Modified Mini-Mental State Examination score less than 78 (to exclude potentially undiagnosed dementia) (17), established cardiovascular or cerebrovascular disease or a previous event, an inability or a lot of difficulty to perform independently any of 6 basic activities of daily living (ADLs), and serious illness that was likely to cause death within the next 5 years.
At baseline, in-person interviews collected information on detailed health, medical history, and lifestyle factors. Clinical assessments of physical and cognitive function were performed, and anthropometric and biological measures were taken. Over follow-up, regular 6-monthly phone contact was maintained with participants to track the occurrence of study endpoints and other clinical events, and participants were seen in person at annual visits for clinical assessments and examinations. The current analysis uses data gathered during the randomized trial phase of ASPREE, which ended in June 2017 (18). ASPREE currently remains an ongoing (ASPREE-eXTension) observational cohort.
Ethics approval for ASPREE was granted by multiple Institutional Review Boards in the United States and Australia, and the trial was registered (NCT01038583 at clinicaltrials.gov). The study was undertaken in accordance with the Declaration of Helsinki and all participants provided written informed consent.
Grip Strength, Gait Speed, and Fried’s Frailty Phenotype
During in-person physical assessments, grip strength was measured and gait speed was assessed. Grip strength (kg force [kgf]) was measured in the seated position using handheld dynamometers. Participants completed a maximum of 3 measures on each hand with a 15- to 20-second rest in between. The average grip strength on the dominant hand was used in this analysis.
Gait speed (m/s) was calculated from the average of 2 timed walks over a 3-m distance. It was performed inside, on a flat surface, with at least 1 m spare at the end, and participants were instructed to walk at their usual pace.
A modified Fried’s frailty phenotype was defined at baseline based on the presence of 3 or more of the following 5 criteria (1): slow gait speed based on walking 15-feet (lowest quintile according to sex and height); weak grip strength assessed by handheld dynamometer (lowest quintile according to sex and weight); low body mass index (<20 kg/m2); self-reported exhaustion based on a question from the Center for Epidemiologic Studies Depression scale; and low physical activity based on self-report of no walking outside the home or walked for less than 10 minutes without sitting down to rest in the past 2 weeks (19). The total frailty score ranged from 0 (none of the criteria) to 5 (all). To facilitate comparison with the FI, the total frailty score was rescaled to the unit level (ie, 0→0, 1→.2, 2→.4, 3→.6, 4→.8, 5→1, as described previously (20)). Fried’s frailty was defined as having 3 or more of these conditions, and Fried’s prefrailty as the presence of only 1 or 2, according to the standard definition (1).
Frailty Index
The FI provides a measure of frailty based on the accumulation of health deficits across multiple systems. There is no fixed criteria regarding the exact number or type of deficits to include in the FI. It has been suggested, however, that a minimum of 30 deficits should be included across several different systems and health indicators, including chronic conditions, physical limitations, cognitive deficits, and general health (21). Furthermore, in order for a deficit to be included in the FI, it should fulfill a number of criteria: is acquired and generally at older ages or accumulates with aging, is biologically and/or clinically meaningful and is associated with adverse health outcomes and is not prevalent across all older individuals. Furthermore, the value of one deficit is not considered when assessing another, and the deficits should occur across a variety of organ systems and physiological functions.
In ASPREE, we used a standard procedure to construct the FI (22). This involved selecting health variables (diseases, symptoms, signs, disabilities) that were ascertained, measured, or reported at baseline and assessed at most annual follow-up visits (or reported through more regular phone contact with participants). Specifically, the ASPREE FI included 67 deficits: 11 health conditions that were ascertained through self-report, detailed medical history, or by the use of prescription medications, of which 6 were adjudicated endpoints of the ASPREE trial; 13 disease indicators which were obtained from blood measures, clinical assessments, and self-report; 26 deficits reflecting difficulty in completing the ADLs (23), physical-related items from the 12-item Short-Form questionnaire (SF-12), or other physical activity limitations from the Fitness and Arthritis in Seniors Trial (FAST) Functional Performance Measure (24) and the Lifestyle Interventions and Independence for Elders study (25); 11 mental and psychosocial deficits (from the SF-12 and FAST); and 6 measures of cognitive function and physical performance (grip strength and gait speed). The full list of deficits is provided in Supplementary Table 1.
For each individual item, the presence of a deficit was coded as 1, and its absence as 0. For a number of items for which there was a degree of deficit, item scores intermediate between 0 and 1 were used. The deficit-accumulation approach assigns equal weights to all 67 included items, and the FI is calculated as the sum of all deficits, divided by the number of items where data were available (eg, 67 items in the case of no missing data). An overall score thus ranges from 0 to 1, with a higher score indicating a greater number of deficits. The FI was calculated for all participants with data for at least 50 items (eg, no more than 25% missing items), to avoid excessive reliance on imputation, or bias in the calculation of the FI between participants. The same procedure was used to create the FI at baseline and at each annual visit.
Various cutpoints for the FI have been used in the literature to define groups that can be classified as frail, prefrail, and not frail. There is currently no consensus on the optimal cutpoints to use, and the choice may depend on both the deficits included and the population being considered. To simplify the interpretation of the results, we used cutpoints to define frail as a FI > .21 and prefrail as > .10 and ≤ .21, as used in other studies (26,27). We also considered cutpoints based on a .05 increase in FI scores (FI = 0; 0 < FI ≤ .05; .05 < FI ≤ .10; .10 < FI ≤ .15; .15< FI ≤ .25; .25 < FI ≤ .30; FI > .30), as well as distributional cutpoints.
DFS and Persistent Physical Disability
The primary endpoint in ASPREE was a composite measure to capture life span free from dementia or disability, and termed “disability-free survival” (18). It was defined as the time to the first of any of the following 3 events: death, dementia (Diagnostic and Statistical Manual of Mental Disorders IV criteria) (28), or persistent physical disability (29). These 3 events were adjudicated by an international panel of clinical experts who reached a consensus on whether the event had occurred or not using comprehensive clinical source documentation.
Given the strong established link between frailty and disability (30), we also examined more specifically physical disability to validate our FI. When a participant first reported having an inability to perform or severe difficulty in performing at least one of the 6 basic ADLs (walking across a room, bathing, dressing, transferring from chair or bed, eating, toileting) (23), or if they required assistance to perform the ADL, this was defined as incident physical disability. Confirmation after 6 months of the same ADL disability, which was thus more likely to reflect permanent rather than transient disability, was defined as persistent physical disability (29). Persistent physical disability was also recorded if the Katz ADL questions could not be administered but the participant was eligible for admission to a nursing care facility for a physical disability (in Australia).
Statistical Analysis
Standard descriptive statistics were generated for the FI at each year, including the 99% submaximal score.
Sex-stratified graphs of the mean FI for each year of age (eg, 65 years included all participants aged from 65.0 to 65.9 years) were generated. Potential ethno-racial differences were also examined. Sex-specific linear regression analyses were used to estimate the increase of the FI with age. Several mathematical functions (linear, quadratic, and log linear) were evaluated to find the best-fitting function according to R-squared.
The correlation between FI score at baseline with grip strength and gait speed was determined using Pearson’s correlation coefficient. Analysis of variance was used to determine the association between the FI and Fried’s frailty phenotype. A composite measure was also constructed to categorize participants based on their frailty status according to both the FI and Fried’s frailty phenotype.
To assess the predictive validity of the FI (22), we used longitudinal data for the primary composite endpoint and persistent disability over follow-up. Univariate and multivariate (age, sex, and ethnic/racial group) adjusted Cox proportional hazard models were fitted to assess the association between FI-defined not-frail, prefrail, and frail groups and DFS, as well as using cutpoints based on a .05 increase in FI scores. The predictive capacity of the FI and Fried’s frailty phenotype was compared, using continuous measures (eg, with rescaling of Fried to the unit level) and categorical groupings; and the FI-Fried’s composite measure was also examined. The analysis was repeated for persistent disability. Hazard ratios (HRs) with 95% confidence intervals (CIs) are reported. Proportional hazards assumptions were checked using Schoenfeld residuals. Sex- and ethno-racial group–stratified analyses were also performed to ensure associations were consistent across these subgroups.
Receiver operating characteristic curves for DFS and disability were generated, and the discriminative performance of FI scores over a basic model including only age, sex, and ethnicity was estimated with the C statistic (31). Stata version 16.1 was used for all analyses (StataCorp LP, College Station, TX).
Results
A total of 19 114 participants were enrolled into the ASPREE study and 56.4% were women. Across the country and ethno-racial groups, there were 16 362 White Australians, 1 088 White U.S. adults, 901 African American, 488 Hispanic/Latino, and 275 self-reporting as another ethno-racial groups or with mixed race. The median age of participants was 74 years (interquartile range [IQR]: 71.6–77.7).
At baseline, 96.2% of the participants were missing data for fewer than 4 of the deficits included in the FI (Supplementary Table 1), and 4 participants were excluded as they did not have data for at least 50 deficit items. Participants were followed annually, with the FI score calculated each year over a median of 4.7 years (IQR: 3.6–5.7 years).
The distribution of FI scores at baseline was positively skewed (Supplementary Figure 1A), with a similar pattern over follow-up, but with a progressive shift towards higher FI scores with each year. Supplementary Figure 1B shows an example, comparing the distribution of FI scores at baseline and at year 4.
The mean FI score at baseline was .11 (SD .6), which increased gradually over the follow-up period (Table 1 and Supplementary Table 2). The 99th percentile limit to deficit accumulation, considered the submaximal score, was .32 at baseline and was .47 in year 6. Using the prespecified cutoffs, at baseline, 40.6% of participants were classified as prefrail (.1 > FI score < .21), and 8.1% as frail (FI score ≥ .21). After 6 years, the percentage of prefrail participants was similar (41.1%), and the proportion who were frail had more than doubled (17.4%).
Table 1.
Summary Statistics of the ASPREE FI Score Across Follow-up
| Baseline | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | |
|---|---|---|---|---|---|---|---|
| N | 19 110 | 18 090 | 17 301 | 15 099 | 11 361 | 7 182 | 2 683 |
| Mean (SD) | .11 (0.06) | .11 (.07) | .12 (.08) | .12 (.08) | .13 (.09) | .14 (.09) | .14 (.09) |
| Median (25%–75%) | .10 (.07–.14) | .10 (.06–.15) | .10 (.07–.16) | .10 (0.07–.16) | .11 (.07–.18) | .11 (.07–.18) | .12 (.07–.18) |
| 99% submaximal | .32 | .37 | .41 | .40 | .44 | .44 | .47 |
| FI prefraila (%) | 40.6% | 36.7% | 38.6% | 38.4% | 38.7% | 39.0% | 41.1% |
| FI frailb (%) | 8.1% | 10.4% | 13.2% | 13.7% | 16.9% | 17.5% | 17.4% |
Notes: ASPREE = ASPirin in Reducing Events in the Elderly; FI = frailty index.
aFI score > .1 and ≤.21.
bFI score > .21.
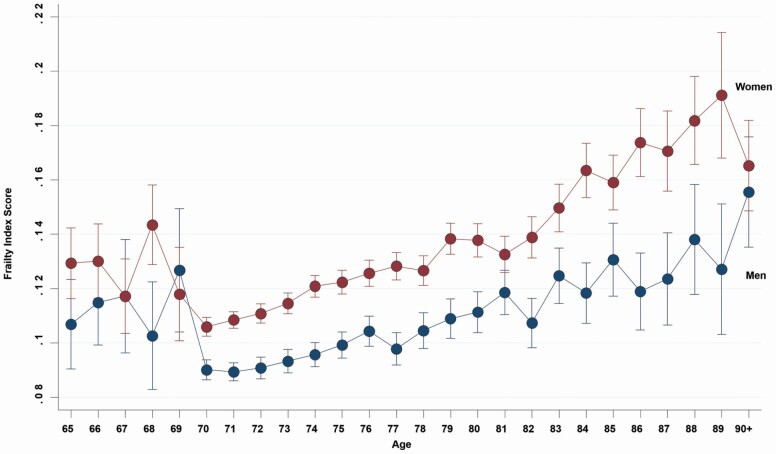
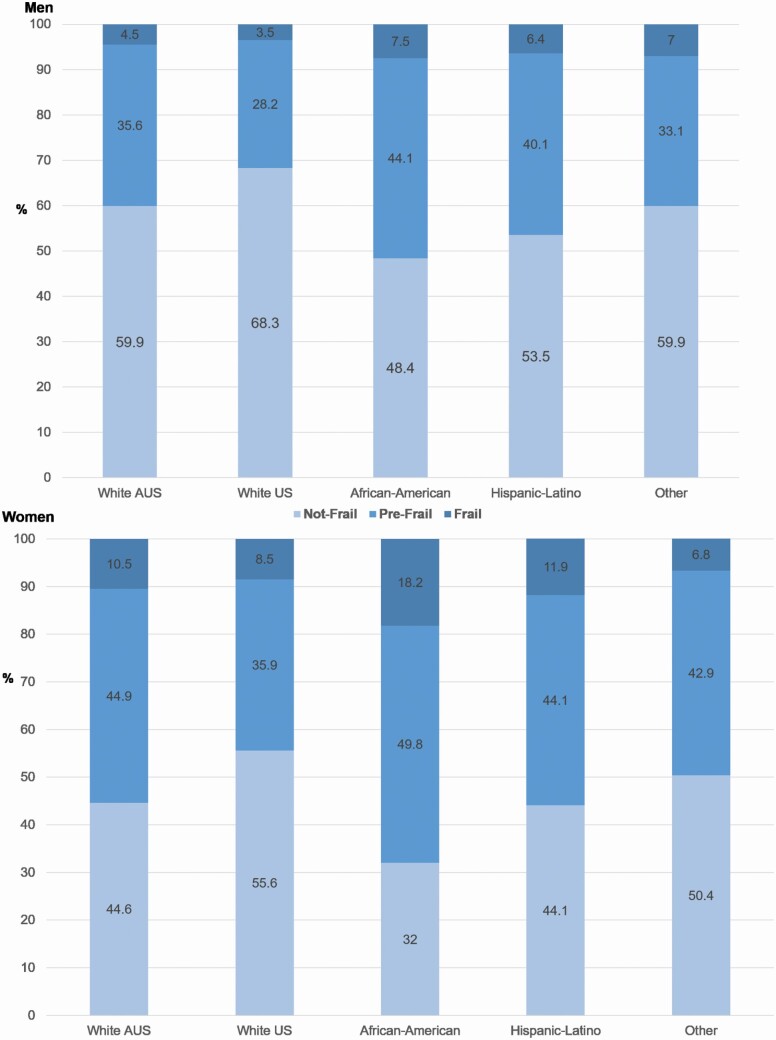
Overall, there was a positive relationship between increasing age at baseline and FI score, which was best fit by a quadratic line in the overall population (R2 = .046), although there was no increase in fit over a linear relationship when minorities aged 65–70 years were excluded (Supplementary Figure 2). Women had consistently higher scores than men (Figure 1), and older women had higher scores by on average .0030 (95% CI: .0028–.0033) for each year of age, with a similar relationship in men (.0020; 95% CI: .0018–.0023 per year of age). The eligibility criteria of ASPREE were such that participants aged 65–69 years were only from U.S. minority groups, which likely explains higher FI scores at these ages in Figure 1. Indeed, African American U.S. men and, in particular, women had a higher proportion of individuals classified as frail at baseline (Figure 2).
Figure 1.
Age- (collapsed to a single year) and gender-specific mean frailty index scores at baseline. Note the only individuals aged 65–69 years who were eligible for inclusion in ASPirin in Reducing Events in the Elderly were from African American and Hispanic/Latino populations in the United States.
Figure 2.
The proportion (%) of participants who are defined as not frail, prefrail (FI > .10 and ≤ .21), and frail (FI > .21) using the frailty index (FI), and according to sex and ethno-racial group. The numbers on the bars indicate the percentage in each group.
At baseline, significant correlations indicated a tendency for lower FI scores to be observed with greater grip strength (r = −.31, p < .0001) and gait speed (r = −.46, p < .0001). Likewise, there was a strong association between frailty status defined using the FI score and the Fried’s frailty phenotype categories (p < .0001), with 61% participants having the same classification for both frailty measures, and less than 1% was classified as frail by one measure and not frail by the other (Supplementary Table 3). Individuals classified as not frail according to the Fried’s phenotype had a mean FI score of .09 (SD .05), those individuals prefrail a mean score of .14 (SD .07), and frail a mean FI score of .23 (SD .08) (Supplementary Figure 3).
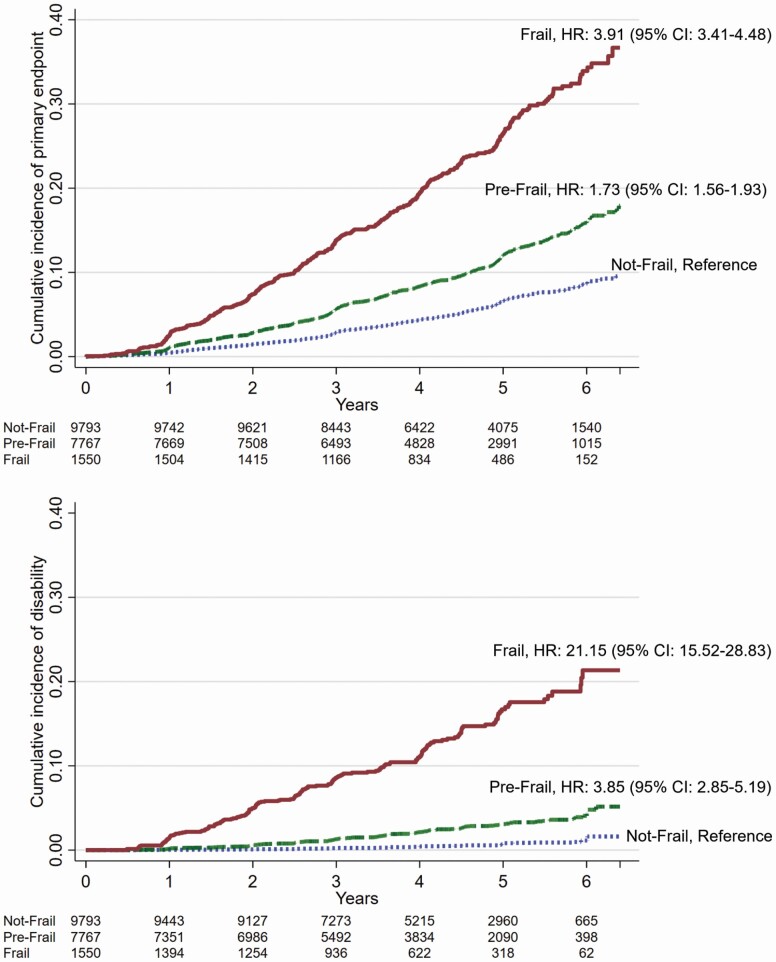
During follow-up, 1 835 participants reached the primary composite endpoint, and 412 participants reached persistent physical disability. A cumulative incidence function was used to display the risk of DFS or persistent physical disability stratified by frailty group. The incidence of DFS increased with increasing FI frailty group (Figure 3). Further investigation using 8 groups defined by a .05 increase in FI score showed a clear dose–response relationship between being in a higher-frailty group and an increased risk of reaching the DFS endpoint (Supplementary Figure 4). Similar findings were observed for persistent physical disability (Supplementary Table 4) and when distributional cutoffs were used (data not shown). There was no evidence of sex interactions in any of the analyses (all p > .2).
Figure 3.
Cumulative incidence of primary composite outcome (death, dementia, or persistent physical disability, upper graph) and persistent disability (lower graph) according to frailty status at baseline based on frailty index score (prefrail > .10 and ≤ .21; frail > .21). The hazard ratio (HR) and 95% confidence interval (CI) are adjusted for age, gender, and ethno-racial group, and in all cases, p < .001.
In Cox proportional hazards regression analyses using continuous FI scores, the FI was positively associated with the risk of DFS and persistent physical disability, even after adjustment for age, sex, and ethno-racial group (Supplementary Table 5). To enable interpretation of the effect size in relation to the number of deficits, we reparametrized the HRs, hence for each additional deficit from the list of 67 items, the corresponding increase in risk would be given by HR: 1.11 (95% CI: 1.09–1.12), p < .0001 for the primary composite endpoint, and HR: 1.23 (95% CI: 1.20–1.25), p < .0001 for persistent physical disability. There was no evidence that these associations differed between men and women or across ethno-racial groups (Supplementary Table 6).
Compared to a Cox regression model including age, sex, and ethnicity, the discriminative ability to predict DFS was almost equivalent with FI alone (Harrell’s C statistic .66 vs .65), and the addition of FI score to a model with age, sex, and ethnicity increased the C statistic to .71. Likewise, the discriminative ability for persistent physical disability with the FI was .82, compared to .65 for a model with age, sex, and ethnicity; and the addition to FI to this model increased the C statistic to .84. Area under the curve demonstrated a significant increase in discriminative ability for the primary composite endpoint and disability (both p < .0001) with the addition of FI to a model with age, sex, and ethno-racial group alone.
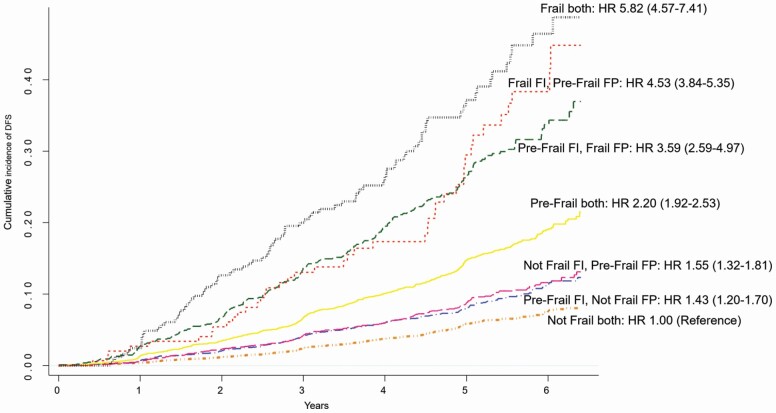
When the FI and Fried’s phenotype frailty measures were considered together in adjusted models, they both remained significantly associated with the risk of DFS and disability, indicating they contribute independently to risk (Supplementary Table 7). In comparative analyses, FI and the Fried’s phenotype similarly predicted DFS, when using either a continuous or categorical measure (Supplementary Table 8). However, the FI, particularly for frail individuals, was a stronger predictor of DFS, and especially disability, than the Fried’s phenotype. Finally, a cumulative incidence function was used to display the risk of DFS stratified by the 9 group composite frailty measures, classifying participants based on frailty status according to the FI and Fried’s phenotype (Figure 4). There was strong agreement between the 2 measures in terms of DFS risk, with individuals who were defined as frail by both measures having the highest mortality risk than the other subgroups (87% of participants reached the DFS endpoint), prefrail individuals according to both criteria had an intermediate risk (31% reaching the DFS endpoint), and nonfrail individuals had the lowest risk (11% reaching the DFS endpoint). Participants with discordant classification between the 2 measures had a risk of mortality that was in between the concordant categories; however, there were very few participants classified as nonfrail by one measure and frail by another (Supplementary Table 9).
Figure 4.
Cumulative incidence of primary composite outcome (death, dementia, or persistent physical disability) according to frailty status at baseline based on both the frailty index (FI) score (prefrail > .10 and ≤ .21; frail > .21) and the Fried’s phenotype (FP) score (prefrail = 1 or 2; frail ≥ 3 of the 5 components). The hazard ratio (HR) and 95% confidence interval (CI) are adjusted for age, gender, and ethno-racial group, and in all cases, p < .001. The most discordant groups between the 2 measures, “Not Frail FI, Frail FP” and “Frail FI, Not Frail FP” are not shown due to very low numbers (2 and 16 events, respectively; see Supplementary Table 9 for full details).
The longitudinal trajectory of FI according to the primary composite endpoint is shown in Supplementary Figure 5. A sharp mean increase in the FI over time for participants reaching the endpoint was observed, while the remaining participants have a comparatively very small mean increase in FI from baseline to year 6.
Discussion
Here, we described the adaptation of a FI in the ASPREE trial using a deficit-accumulation model which comprised 67 items and included a range of different diseases and symptoms, physiological markers, and clinical assessments. ASPREE participants were community-dwelling older adults free of major physical disability, major cognitive impairments, or known cardiovascular disease at study enrolment and were followed for up to 6 years. While it is well established that FI predicts mortality risk across diverse populations and settings (7,13,32–35), we have demonstrated for the first time that the FI is also a strong predictor of DFS, a composite endpoint that attempts to capture independent life lived free of major disability and dementia. The FI also predicted persistent physical disability and, alone, was comparable in predictive capacity to a model containing age, sex, and ethnicity, indicating the FI is a good aging index.
We assessed the construct validity of our FI index through its positive relationship with chronological age at baseline. Women had higher frailty scores than men at all ages, and African American participants also had higher frailty scores than White, Hispanic-Latino, and other ethno-racial groups. The concurrent validity of our FI was supported by an inverse correlation between the FI and both grip strength and gait speed. Furthermore, the FI was strongly associated with an alternative measure of frailty, the Fried’s frailty phenotype (1), and had slightly higher predictive capacity for DFS and disability than the Fried’s frailty phenotype. However, when considered together, individuals with frailty defined by both measures were at the highest risk of reaching the endpoint.
This is one of the first studies with longitudinal FI scores on such a large group of individuals, and thus provides valuable insights into how frailty operates in individuals who have reached older age in relatively good health. As anticipated, individuals who reached the DFS endpoint had a step increase in FI over the follow-up period, while for the remaining participants, there was only a small increase in FI over time.
The characteristics of the ASPREE FI largely align with reports of other FIs that have been developed. However, the FI scores of participants (mean FI .11, median .10) were slightly lower than those reported in other studies, which have ranged from around .14 (32) to .19 (36). This is likely driven by the inclusion criteria for ASPREE. In the Systolic Blood Pressure Intervention Trial (SPRINT) for example, participants had a mean age of 68 years and were at an increased cardiovascular disease risk, and the median FI at baseline was .16 (27). Likewise, we found that only 8% of individuals were frail at baseline (mean age 74 years) which is at the lower end of existing prevalence estimates (65–70 years: 5%–15%, 70–80 years: 8%–17%, and 80+ years: >16%) (37).
The FI has consistently been shown to have a fixed submaximal limit of around 0.70 (38), or roughly two-thirds of the total deficits (22), such that an individual can only “support” a certain proportion of deficits (39). This has been demonstrated in both community and institutionalized samples (39), and in studies where the majority of individuals have been followed until death (32). In ASPREE, the 99th percentile was .32 at baseline, and rose to .47 in year 6 when the mean age of participants was approximately 80 years. Only 2 participants were observed to exceed this limit, with an FI of .73 and .78, and both reached the DFS endpoint.
Another consistent finding in the literature which was confirmed in our study is that women on average have higher frailty scores than men (26,36,37,40,41). Whether this is uniform across all ages is less clear. A couple of studies have found that older women have higher scores than men but the curves start to come together at about age 80 years (33,42). In contrast, the ESTHER-study found the reverse pattern, with no differences at younger ages, but older women (>72 years) having higher FI scores than men (34). All 3 studies, however, lacked data on potential sex differences for individuals older than 80 years. Our study demonstrated that at least up to 90 years, women still have on average higher frailty scores than men. These sex differences are supported by research demonstrating that women experience physiological dysregulation with age more quickly than men (43), and also have more disability for given health conditions than men (44).
The FI is a clinically relevant tool as it enables more tailored treatment for individuals and person-centered care (45). Individuals who are frail have a greater risk of adverse health outcomes (9), are more likely to be hospitalized and have complications following surgery, and are more susceptible to medication side-effects (46). Knowing the frailty status of an individual can thus permit appropriate stratification of the population based on risk, thus informing decision making around treatment and care (47).
Screening for frailty may be particularly useful in older adults (48), especially given that frailty is considered potentially preventable. Early identification is a key to enable effective strategies targeted to at-risk individuals, to prevent and slow the progression of frailty. Interventions shown to reduce frailty have included lifestyle changes focused on exercise and nutrition and reducing polypharmacy (45). A benefit of the FI is that it can be derived from existing clinical data available in medical records and does not require additional measures that are not routinely performed in clinical practice, such as gait speed and strength testing. Frailty status can directly inform the best preventive or therapeutic interventions to reduce frailty for a given individual (49). Further, the FI approach of quantifying frailty provides important information about the underlying deficits which are contributing to high frailty scores.
Strengths of our study are the use of a large number of items across a broad range of deficits in the development of the FI, which included information that was directly measured (physical and biological measures), as well as detailed information from medical records which complemented that obtained from face-to-face assessments and self-report questionnaires. We measured the FI at baseline and over 6 subsequent waves, and the large sample enabled us to examine not only sex differences but also the potential differences across ethnic/racial groups. Finally, we were able to demonstrate the construct and concurrent validity of our FI and its predictive validity for DFS.
The ASPREE study used community-based recruitment and individuals with known cardiovascular disease, major physical disability, dementia, or major life-limiting illness were not eligible to participate. Despite this, we have shown that more than 8% of participants would be classified as frail using standard FI cutoffs, and 41% as prefrail. This indicates that despite the inclusion criteria of ASPREE, the proportion of frail individuals is not markedly dissimilar from that reported in other clinical trials and cohorts, and that the sample is heterogenous with a range of underlying conditions and health status.
Further research is needed to determine predictors of the interindividual change in FI trajectories over time. While at the population level, there is a clear increase in frailty with age, frailty is also a dynamic state (50) and some individuals may have improvements in FI scores over time. Longitudinal research into frailty is considered a high priority (8) and the depth and breadth of information available in ASPREE for such a large multiethnic sample, across multiple timepoints, provides this unique opportunity.
Aging is an inevitable process that occurs with the accumulation of cellular damage and a reduced capacity to repair this damage. Beyond the physical changes that are observed with aging, progressive decline in functioning of many physiological systems (51) is accompanied by a heightened susceptibility, reduction in physical function, and risk of disease. Our study provides further support for frailty as a useful biomarker of aging, which contributes important information about an individual’s susceptibility and risk above that contributed by age alone. Clinically, the FI is useful to inform decision making around treatment and care. The FI can also provide new insights into the aging process, which may lead to opportunities for interventions to slow the decline of health and function with aging. This is becoming increasingly pertinent as the world’s population ages.
Supplementary Material
ASPREE Investigator Group listed on www.aspree.org.
Funding
The work was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (U01AG029824 and U19AG062682); the National Health and Medical Research Council (NHMRC) of Australia (334047 and 1127060); Monash University and the Victorian Cancer Agency; and an NHMRC Dementia Research Leader Fellowship to J.R. (1135727).
Conflict of Interest
None declared.
Author Contributions
J.R., S.E., M.E.E., A.R.M.S.E., and R.L.W. designed the study. J.R. analyzed and interpreted the data and drafted the manuscript. R.W. helped interpret the data and provided critical comments, together with A.B.N. A.M.M., R.C.S., S.G.O., S.F., L.J.B., S.A.W., J.D.W., A.B.N., J.J.M., and R.L.W. obtained the data. All authors approved the final submitted version.
References
- 1. Fried LP, Tangen CM, Walston J, et al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 2. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanlon P, Fauré I, Corcoran N, et al. . Frailty measurement, prevalence, incidence, and clinical implications in people with diabetes: a systematic review and study-level meta-analysis. Lancet Healthy Longev. 2020;1:e106–e116. doi: 10.1016/s2666-7568(20)30014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saifuddin E, Woods R, Britt C, Espinoza SE, Ernst ME, Ryan J. The association between frailty and all-cause mortality in community-dwelling older individuals: an umbrella review. J Frail Aging. 2021. doi: 10.14283/jfa.2021.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. . Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17(12):1163.e1–1163.e17. doi: 10.1016/j.jamda.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 6. Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362(13):1173–1180. doi: 10.1056/NEJMoa0909087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–687. doi: 10.1111/j.1532-5415.2010.02764.x [DOI] [PubMed] [Google Scholar]
- 8. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi: 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 9. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fairhall N, Sherrington C, Kurrle SE, et al. . Economic evaluation of a multifactorial, interdisciplinary intervention versus usual care to reduce frailty in frail older people. J Am Med Dir Assoc. 2015;16(1):41–48. doi: 10.1016/j.jamda.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 11. Buta BJ, Walston JD, Godino JG, et al. . Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. doi: 10.1016/j.arr.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. In search of an integral conceptual definition of frailty: opinions of experts. J Am Med Dir Assoc. 2010;11:338–343. doi: 10.1016/j.jamda.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 13. Romero-Ortuno R, Kenny RA. The frailty index in Europeans: association with age and mortality. Age Ageing. 2012;41(5):684–689. doi: 10.1093/ageing/afs051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitnitski A, Howlett SE, Rockwood K. Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci. 2017;72(7):877–884. doi: 10.1093/gerona/glw089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ASPREE IG. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36:555–564. doi: 10.1016/j.cct.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McNeil JJ, Woods RL, Nelson MR, et al. . Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) Study. J Gerontol A Biol Sci Med Sci. 2017;72(11):1586–1593. doi: 10.1093/gerona/glw342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan J, Woods RL, Britt C, et al. . Normative performance of healthy older individuals on the Modified Mini-Mental State (3MS) examination according to ethno-racial group, gender, age, and education level. Clin Neuropsychol. 2019;33(4):779–797. doi: 10.1080/13854046.2018.1488996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McNeil JJ, Woods RL, Nelson MR, et al. . Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379:1499–1508. doi: 10.1056/NEJMoa1800722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolfe R, Murray AM, Woods RL, et al. . The ASPirin in Reducing Events in the Elderly trial: statistical analysis plan. Int J Stroke. 2018;13(3):335–338. doi: 10.1177/1747493017741383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56(5):898–903. doi: 10.1111/j.1532-5415.2008.01656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 22. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x [DOI] [PubMed] [Google Scholar]
- 24. Rejeski WJ, Ettinger WH Jr., Schumaker S, James P, Burns R, Elam JT. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthr Cartil. 1995;3(3):157–167. doi: 10.1016/s1063-4584(05)80050-0 [DOI] [PubMed] [Google Scholar]
- 25. Rejeski WJ, Fielding RA, Blair SN, et al. . The Lifestyle Interventions and Independence for Elders (LIFE) pilot study: design and methods. Contemp Clin Trials. 2005;26(2):141–154. doi: 10.1016/j.cct.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 26. Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep. 2013;24(9):10–17. [PubMed] [Google Scholar]
- 27. Pajewski NM, Williamson JD, Applegate WB, et al. . Characterizing frailty status in the systolic blood pressure intervention trial. J Gerontol A Biol Sci Med Sci. 2016;71(5):649–655. doi: 10.1093/gerona/glv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ryan J, Storey E, Murray AM, et al. . Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology. 2020;95(3):e320–e331. doi: 10.1212/WNL.0000000000009277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woods RL, Espinoza S, Thao LTP, et al. . Effect of aspirin on activities of daily living disability in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. Published online December 26, 2020. doi: 10.1093/gerona/glaa316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Espinoza SE, Quiben M, Hazuda HP. Distinguishing comorbidity, disability, and frailty. Curr Geriatr Rep. 2018;7(4):201–209. doi: 10.1007/s13670-018-0254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845.doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 32. Armstrong JJ, Mitnitski A, Launer LJ, White LR, Rockwood K. Frailty in the Honolulu-Asia Aging Study: deficit accumulation in a male cohort followed to 90% mortality. J Gerontol A Biol Sci Med Sci. 2015;70(1):125–131. doi: 10.1093/gerona/glu089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan J, Yu C, Guo Y, et al. . Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health. 2020;5(12):e650–e660. doi: 10.1016/S2468-2667(20)30113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saum KU, Dieffenbach AK, Müller H, Holleczek B, Hauer K, Brenner H. Frailty prevalence and 10-year survival in community-dwelling older adults: results from the ESTHER cohort study. Eur J Epidemiol. 2014;29(3):171–179. doi: 10.1007/s10654-014-9891-6 [DOI] [PubMed] [Google Scholar]
- 35. Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61(9):1537–1551. doi: 10.1111/jgs.12420 [DOI] [PubMed] [Google Scholar]
- 36. Hoogendijk EO, Theou O, Rockwood K, Onwuteaka-Philipsen BD, Deeg DJH, Huisman M. Development and validation of a frailty index in the Longitudinal Aging Study Amsterdam. Aging Clin Exp Res. 2017;29(5):927–933. doi: 10.1007/s40520-016-0689-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12(2):719–736. doi: 10.1016/j.arr.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 38. Bennett S, Song X, Mitnitski A, Rockwood K. A limit to frailty in very old, community-dwelling people: a secondary analysis of the Chinese Longitudinal Health and Longevity Study. Age Ageing. 2013;42(3):372–377. doi: 10.1093/ageing/afs180 [DOI] [PubMed] [Google Scholar]
- 39. Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127(5):494–496. doi: 10.1016/j.mad.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 40. Mitnitski A, Song X, Skoog I, et al. . Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53(12):2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x [DOI] [PubMed] [Google Scholar]
- 41. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26. doi: 10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 42. Lachmann R, Stelmach-Mardas M, Bergmann MM, et al. . The accumulation of deficits approach to describe frailty. PLoS One. 2019;14(10):e0223449. doi: 10.1371/journal.pone.0223449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arbeev KG, Cohen AA, Arbeeva LS, et al. . Optimal versus realized trajectories of physiological dysregulation in aging and their relation to sex-specific mortality risk. Front Public Health. 2016;4:3. doi: 10.3389/fpubh.2016.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whitson HE, Landerman LR, Newman AB, Fried LP, Pieper CF, Cohen HJ. Chronic medical conditions and the sex-based disparity in disability: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65(12):1325–1331. doi: 10.1093/gerona/glq139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi: 10.1016/S0140-6736(19)31785-4 [DOI] [PubMed] [Google Scholar]
- 46. Afilalo J, Alexander KP, Mack MJ, et al. . Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63(8):747–762. doi: 10.1016/j.jacc.2013.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hao Q, Zhou L, Dong B, Yang M, Dong B, Weil Y. The role of frailty in predicting mortality and readmission in older adults in acute care wards: a prospective study. Sci Rep. 2019;9(1):1207. doi: 10.1038/s41598-018-38072-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morley JE, Vellas B, van Kan GA, et al. . Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10–12. doi: 10.1093/ageing/aft160 [DOI] [PubMed] [Google Scholar]
- 50. Espinoza SE, Jung I, Hazuda H. Frailty transitions in the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc. 2012;60(4):652–660. doi: 10.1111/j.1532-5415.2011.03882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Q, Wang S, Milot E, et al. . Homeostatic dysregulation proceeds in parallel in multiple physiological systems. Aging Cell. 2015;14:1103–1112. doi: 10.1111/acel.12402 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.