Abstract
Background
It remains unclear whether the novel biomarker cysteine‐rich angiogenic inducer 61 (CCN1) adds incremental prognostic value to the GRACE 2.0 (Global Registry of Acute Coronary Events) risk score and biomarkers high‐sensitivity Troponin T, hsCRP (high‐sensitivity C‐reactive protein), and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) in patients with acute coronary syndromes.
Methods and Results
Patients referred for coronary angiography with a primary diagnosis of acute coronary syndromes were enrolled in the Special Program University Medicine – Acute Coronary Syndromes and Inflammation cohort. The primary/secondary end points were 30‐day/1‐year all‐cause mortality and the composite of all‐cause mortality or myocardial infarction as used in the GRACE risk score. Associations between biomarkers and outcome were assessed using log‐transformed biomarker values and the GRACE risk score (versions 1.0 and 2.0). The incremental value of CCN1 beyond a reference model was assessed using Harrell’s C‐statistics calculated from a Cox proportional‐hazard model. The P value of the C‐statistics was derived from a likelihood ratio test. Among 2168 patients recruited, 1732 could be analyzed. CCN1 was the strongest single predictor of all‐cause mortality at 30 days (hazard ratio [HR], 1.77 [1.31, 2.40]) and 1 year (HR, 1.81 [1.47, 2.22]). Adding CCN1 alone to the GRACE 2.0 risk score improved C‐statistics for prognostic accuracy of all‐cause mortality at 30 days (0.87–0.88) and 1 year (0.81–0.82) and when combined with high‐sensitivity Troponin T, hsCRP, NT‐proBNP for 30 days (0.87–0.91), and for 1‐year follow‐up (0.81–0.84). CCN1 also increased the prognostic value for the composite of all‐cause mortality or myocardial infarction.
Conclusions
CCN1 predicts adverse outcomes in patients with acute coronary syndromes adding incremental information to the GRACE risk score, suggesting distinct underlying molecular mechanisms.
Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01000701.
Keywords: acute coronary syndrome, biomarkers, inflammation, risk
Subject Categories: Biomarkers, Inflammation
Nonstandard Abbreviations and Acronyms
- CCN1
CCN family member 1, syn. Cyr61, cysteine‐rich angiogenic inducer 61
- GRACE
Global Registry of Acute Coronary Events
- hsTnT
high‐sensitivity Troponin T
- SPUM‐ACS
Special Program University Medicine – Acute Coronary Syndromes and Inflammation
Clinical Perspective
What Is New?
Circulating biomarker cysteine‐rich angiogenic inducer 61 adds independent information to risk stratification in patients with acute coronary syndrome beyond Global Registry of Acute Coronary Events risk score, high‐sensitivity Troponin T, high‐sensitivity C‐reactive protein, and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide).
What Are the Clinical Implications?
Present data support further exploration of matricellular protein cysteine‐rich angiogenic inducer 61 as a novel biomarker reflecting a distinct pathobiology in acute coronary syndromes.
Cardiovascular disease is the major cause of death, with one fifth of all cases attributable to coronary artery disease. 1 Acute coronary syndromes (ACS) constitute the acute manifestation of coronary artery disease caused by plaque rupture or erosion. 2 Risk stratification remains of great importance because 1 of 6 patients experiences an adverse cardiovascular event within 1 year after an ACS despite percutaneous coronary intervention and optimal medical treatment. 3
Current guidelines recommend biomarkers comprising Troponin T (TnT) measured by high sensitive assays and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) along with clinical scores (ie, GRACE [Global Registry of Acute Coronary Events] risk score) for assessment of risk for adverse cardiovascular events in patients with ACS. 4 , 5 , 6 , 7 CRP (C‐reactive protein) may further improve risk stratification; however, to a lesser extent. 5 We recently demonstrated that the prognostic accuracy of the GRACE score (version 1.0) for major adverse outcomes is improved when combining it with the biomarkers high‐sensitivity Troponin T (hsTnT), hsCRP (high‐sensitivity C‐reactive protein), and NT‐proBNP. 8 The updated version 2.0 of the GRACE risk score uses nonlinear functions enabling better short‐ and long‐term risk discrimination with results expressed as percentage risks, thus facilitating clinical use. 9
Cysteine‐rich angiogenic inducer 61 (CCN1 by official unified nomenclature 10 ) is a secreted matricellular protein with a variety of functions in angiogenesis, inflammation, and wound healing. 11 , 12 , 13 , 14 , 15 We have recently identified CCN1 as a novel biomarker of myocardial injury with increased levels measured in patients with ST‐ segment–elevation myocardial infarction (STEMI) and non–ST‐segment–elevation myocardial infarction (NSTEMI) compared with patients with stable coronary artery disease. 3 We considered the 95th percentile (589.01 ng/L) in patients with stable coronary artery disease as a clinically relevant cut‐off. 3 However, it remained unclear whether CCN1 adds further information to risk stratification of patients with ACS when combining conventional cardiovascular biomarkers hsTnT, hsCRP, and NT‐proBNP with the GRACE 2.0 risk score.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patients
Patients were enrolled in the Special Program University Medicine – Acute Coronary Syndromes and Inflammation (SPUM‐ACS, ClinicalTrials.gov Identifier: NCT01000701) biomarker cohort 1 between December 2009 and October 2012 if they had a primary diagnosis of an ACS and were referred for coronary angiography to 1 of 4 university hospitals in Switzerland. 8 All patients had signed a written consent form, and the study was conducted with permission from the local ethical committees in accordance with the Declaration of Helsinki. Blood was drawn from the inguinal arterial sheath at coronary angiography before percutaneous coronary intervention. Clinical and demographic parameters along with data on coronary anatomy and procedural aspects were ascertained and entered into an electronic database.
Biomarkers
Concentrations of CCN1 in serum were measured in stored duplicates of single serum aliquots using a semi‐automated solid phase enzyme‐linked immunosorbent assay (EIA‐5108, DRG Instruments GmbH, Marburg, Germany). The inter‐ and intra‐assay coefficients of variation of CCN1 in SPUM‐ACS patients were 3.21% and 3.46%, respectively. hsTnT and NT‐proBNP were measured in serum aliquots on a Cobas e 602 reader, hsCRP on a Cobas c 501 autoanalyzer (all Roche Diagnostics, Mannheim, Germany) with assay characteristics as reported by the manufacturer.
Clinical Risk Score Calculation
GRACE 1.0
The GRACE 1.0 score was used to calculate both in‐hospital and long‐term predictions of mortality and to assess the degree of disease severity in patients included in the current study. The GRACE 1.0 parameters used to assess the score for in‐hospital mortality comprised age, initial heart rate, systolic blood pressure, serum creatinine, Killip class, cardiac arrest on admission, elevated cardiac markers (conventional troponins as per local laboratories), and ST‐segment deviation. 16 The GRACE 1.0 parameters used to calculate the score for long‐term mortality comprised age, heart rate, systolic blood pressure, initial serum creatinine, history of congestive heart failure, history of myocardial infarction (MI), elevated cardiac markers (conventional troponins as per local laboratories), ST‐segment depression, and no in‐hospital percutaneous coronary intervention. 17 The GRACE 1.0 scores were calculated using a program written in Stata statistical software (Version 13, Stata Corp, and College Station, TX), and we used the standard scoring of GRACE 1.0, as mentioned in the reference publications. 16 , 17
GRACE 2.0
The original GRACE risk score (GRACE 1.0) used linear scores in combination with a nomogram to estimate the overall score. To improve prognostic accuracy, especially for 1‐year risk periods, the updated GRACE 2.0 score uses nonlinear functions for age, systolic blood pressure, serum creatinine, and congestive heart failure. 9 The GRACE 2.0 scores were calculated using a program implemented in R Core Team (2019) (A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R‐project.org/). The code is implemented based on GRACE ACS risk score 2.0 calculator documentation.
Outcomes
The primary end point was all‐cause mortality within 30‐day and 1‐year follow‐up. The secondary end point was the composite of all‐cause mortality or recurrent MI, both end points as defined in the GRACE risk score. Additional outcomes comprising repeat revascularization, cerebrovascular events (transient ischemic attack or stroke), and stent thrombosis were also assessed. 8 All events were adjudicated by 3 independent senior cardiologists using prespecified forms.
Statistical Analysis
This study aimed to assess the performance of CCN1 in combination with GRACE risk scores (GRACE 1.0 and GRACE 2.0), hsTnT, NT‐proBNP, and hsCRP in predicting clinical outcomes. Clinical characteristics were presented as means with SDs and P values from t tests for continuous variables. Categorical variables were shown as counts with percentages and P values from or Fisher exact tests. Time‐to‐first event or composite events were analyzed throughout, censoring patients at 30‐day or 1‐year, or last valid contact date. For the development of the prediction model(s) we followed 7 steps proposed by Steyerberg. 18 These steps comprise (1) consideration of the research question and initial data inspection, (2) coding of the predictors, (3) model specification, (4) model estimation, (5) evaluation of model performance, (6) internal validation, and (7) model presentation.
We used Cox proportional‐hazards models to evaluate possible associations between the outcomes all‐cause mortality and all‐cause mortality or recurrent MI (at 30‐day and 1‐year follow‐up) and log‐transformed GRACE 2.0 risk scores, biomarkers (CCN1, hsTnT, NT‐proBNP, and hsCRP), and continuous GRACE 1.0 risk scores. We report unadjusted hazard ratios (HRs) because potential confounders are part of the GRACE scores. The added predictive ability of a new predictor combined with an existing model was assessed by Harrell’s C‐statistics calculated from a Cox regression model and Integrated Discrimination Improvement index, which is based on a logistic model. 19 The P value of the C‐statistics derived from a likelihood ratio test comparing the Cox proportional‐hazards model(s) with and without the new marker(s). The internal validation of the models is done using bootstrapping methods with 100 replications with samples of 1732 patients.
Two‐sided P values were reported throughout, and P values <0.05 were considered statistically significant. Statistical analyses were performed using Stata statistical software (Version 16.1, Stata Corp, and College Station, TX). The forest plots are produced using the “forestplot” function from the “rmeta” package of the R 3.6.1 R (Foundation for Statistical Computing, Vienna, Austria; https://www.R‐project.org/.)
Results
Among 2168 patients with ACS enrolled, 1732 (79.9%) had available biomarker measurements. Complete follow‐up data were available in 99.1% of the analyzed patients at 30‐day, and 95.7% of patients at 1‐year follow‐up (Figure 1). Baseline characteristics showed a predominance of NSTEMI (43.1%) and STEMI (52.9%) entities, respectively. GRACE 1.0 risk scores for all‐cause mortality were 144.17±32.97 (in‐hospital) and 123.20±26.18 (long‐term), and GRACE 2.0 risk scores were 6.39±8.6% (in‐hospital) and 13.01±13.42% (Table 1). A comparison between patients with and without biomarker analyses revealed no differences in baseline characteristics except for periprocedural medications (Table S1). Adherence to discharge medications was good (96.5% at 1 year from 99.2% at discharge for aspirin and 93.5% at 1 year from 98.1% at discharge for statins; Table S2). The primary end point all‐cause death occurred in 32 cases (1.8%), and the secondary end point (composite of all‐cause death or MI) occurred in 58 cases (3.3%) (Table S3).
Figure 1. Flow diagram.
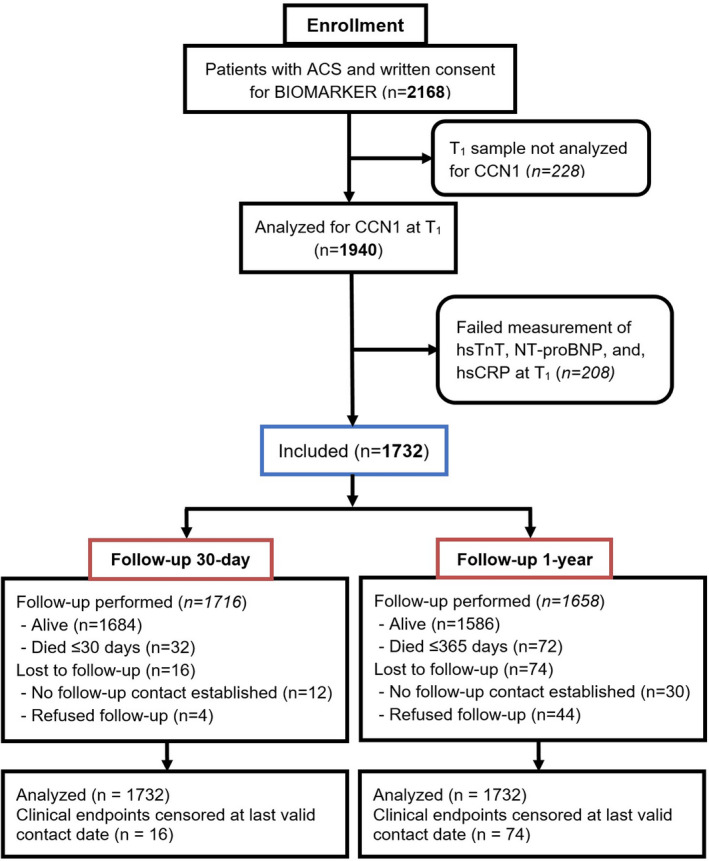
The flow diagram shows patient enrollment and follow‐up throughout the study. T1 signifies blood drawn performed at coronary angiography. ACS indicates acute coronary syndromes; CCN1, cysteine‐rich angiogenic inducer 61; hsCRP, high‐sensitivity C‐reactive protein; hsTnT, high‐sensitivity Troponin T; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Table 1.
Baseline Characteristics of Patients With ACS (n=1732)
| Parameters | n=1732 Analyzed |
|---|---|
| Age, y | n=1732, 63.82±12.27 |
| Sex, female | n=1732, 367 (21.2%) |
| Body weight, kg | n=1713, 80.33±15.20 |
| Body mass index, kg/m² | n=1711, 27.16±4.35 |
| Medical history | |
| Diabetes mellitus | n=1732, 313 (18.1%) |
| Hypertension | n=1732, 1018 (58.8%) |
| Hypercholesterolemia | n=1732, 1072 (61.9%) |
| Current smoker | n=1701, 674 (39.6%) |
| Family history of CAD | n=1711, 432 (25.2%) |
| Chronic kidney disease* | n=1728, 220 (12.7%) |
| History of stroke or TIA | n=1732, 64 (3.7%) |
| Previous myocardial infarction | n=1730, 266 (15.4%) |
| Previous PCls | n=1731, 307 (17.7%) |
| Previous CABG | n=1732, 101 (5.8%) |
| Clinical presentation | |
| Unstable angina | n=1732, 69 (4.0%) |
| NSTEMI | n=1732, 747 (43.1%) |
| STEMI | n=1732, 916 (52.9%) |
| Index procedure | |
| PCI | n=1732, 1564 (90.3%) |
| Any drug‐eluting stent | n=1633, 1229 (75.3%) |
| Any bare‐metal stent | n=1633, 292 (17.9%) |
| PTCA alone | n=1633, 186 (11.4%) |
| CABG | n=1633, 65 (4.0%) |
| Periprocedural medications | |
| Unfractionated heparin | n=1729, 1656 (95.8%) |
| LMWH | n=1732, 82 (4.7%) |
| Bivalirudin | n=1732, 78 (4.5%) |
| Glycoprotein IIb/IIIa antagonists | n=1732, 445 (25.7%) |
| GRACE risk score | |
| GRACE 1.0 | |
| In‐hospital | n=1732, 144.17±32.97 |
| Long‐term | n=1732, 123.20±26.18 |
| GRACE 2.0 † | |
| In‐hospital death (%) | n=1732, 6.39±8.60 |
| In‐hospital death/MI (%) | n=1732, 18.26±8.70 |
| 1‐y death (%) | n=1732, 13.01±13.42 |
| 1‐y death/MI (%) | n=1732, 18.29±13.03 |
Depicted are counts (%) or means±SDs. ACS indicates acute coronary syndromes; CABG, coronary artery bypass graft; CAD, coronary artery disease; GRACE, Global Registry of Acute Coronary Events; LMWH, low‐molecular weight heparin; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCIs, percutaneous coronary interventions; PTCA, percutaneous transluminal coronary angioplasty; STEMI, ST‐segment–elevation myocardial infarction; and TIA, transient ischemic attack.
Based on creatinine‐estimated glomerular filtration rate clearance of <60 mL/min per 1.73 m², using the Modification of Diet in Renal Disease (MDRD) formula.
GRACE 2.0 returns percentage probability of observing a respective event for each patient.
CCN1 Provides Independent Information to Predict Adverse Outcomes
Following univariable Cox models showing significant associations for the GRACE risk score (version 1.0 and 2.0) and individual biomarkers (CCN1, hsTnT, NT‐proBNP, and hsCRP) (Table S4), multivariable Cox models for GRACE risk score, CCN1, hsTnT, NT‐proBNP, and hsCRP for 30‐day and 1‐year follow‐up were performed in 1732 patients. Unlike any of the other biomarkers, CCN1 was independently associated with all‐cause mortality and the composite of all‐cause mortality or MI both short‐ and long‐term, respectively (Figure 2). The result of bootstrapping for the multivariable Cox model was consistent with the actual model (Table S5).
Figure 2. Forrest plot illustration of the relative prognostic accuracy of GRACE risk scores (version 1.0 in blue color and 2.0 in red color) and biomarkers for short‐term (A and B) and long‐term (C and D) adverse outcomes (n=1732).
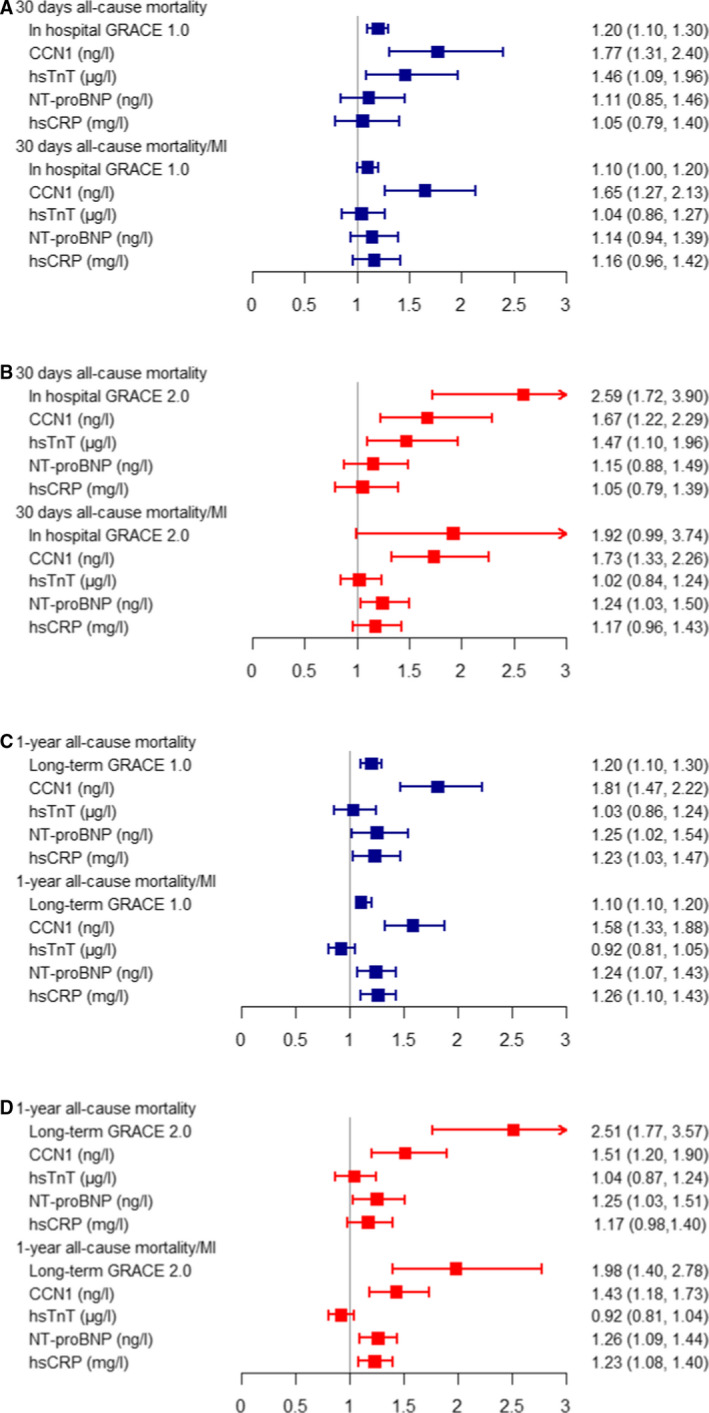
For GRACE 2.0 scores, and the biomarkers, natural logarithm was used, and hazard ratios were reported per 1 log‐unit increase. CCN1 indicates cysteine‐rich angiogenic inducer 61; GRACE, Global Registry of Acute Coronary Events; hsCRP, high‐sensitivity C‐reactive protein; hsTnT, high‐sensitivity Troponin T; MI, myocardial infarction; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
CCN1 Provides Incremental Prognostic Accuracy Beyond Current Risk Stratification
In the comparative analysis (c‐statistic) of individual biomarkers (CCN1, hsTnT, NT‐proBNP, and hsCRP) and combinations thereof against the GRACE risk score (version 1.0 and 2.0) as the reference model, CCN1 consistently improved prognostic accuracy beyond the GRACE risk score to predict all‐cause mortality and the composite of all‐cause mortality or MI both short‐ and long‐term, respectively (Table 2). These data for CCN1 were corroborated when integrated discrimination improvement index analysis was used (Table 3). Albeit statistically significant, the improvement of model performance in GRACE 1.0 risk score based on the Integrated Discrimination Improvement analysis was small for CCN1, especially with respect to predictions of 30‐day all cause‐mortality and slightly better for 1‐year all‐cause mortality. A similar increment in prognostic accuracy for both end points and time intervals using both methods (c‐statistics and Integrated Discrimination Improvement) was found for hsTnT, NT‐proBNP, and hsCRP. The best prognostic accuracy (up to c‐statistic 0.91) was obtained when combining all 3 biomarkers with the GRACE risk score with the highest increment observed to predict all‐cause mortality both short‐ and long‐term, respectively (Table 2). In a subgroup analysis, unlike in patients with NSTEMI, CCN1 did not add information to improve predictions of all‐cause mortality in patients with STEMI (Table S6 through S9).
Table 2.
Accuracy of Risk Prediction for All‐Cause Mortality and All‐Cause Mortality/MI (n=1732)
| 30‐D All‐Cause Mortality | GRACE 1.0* | GRACE 2.0 † | ||
|---|---|---|---|---|
| C‐Statistic | P Value | C‐Statistic | P Value | |
| In‐hospital GRACE | 0.867 | … | 0.868 | … |
| GRACE+CCN1 | 0.876 | 0.001 | 0.876 | 0.002 |
| GRACE+hsTnT | 0.877 | <0.001 | 0.886 | <0.001 |
| GRACE+NT‐proBNP | 0.875 | 0.007 | 0.876 | 0.002 |
| GRACE+hsCRP | 0.875 | 0.092 | 0.866 | 0.036 |
| GRACE+CCN1+hsTnT | 0.886 | <0.001 | 0.895 | <0.001 |
| GRACE+CCN1+hsTnT+NT‐proBNP | 0.897 | <0.001 | 0.905 | <0.001 |
| GRACE+CCN1+hsTnT+NT‐proBNP+hsCRP | 0.898 | <0.001 | 0.906 | <0.001 |
| 30‐d all‐cause mortality/MI | ||||
| In‐hospital GRACE | 0.715 | … | 0.666 | … |
| GRACE+CCN1 | 0.716 | 0.003 | 0.667 | 0.001 |
| GRACE+hsTnT | 0.717 | 0.046 | 0.667 | 0.011 |
| GRACE+NT‐proBNP | 0.725 | 0.017 | 0.695 | <0.001 |
| GRACE+hsCRP | 0.726 | 0.030 | 0.676 | 0.002 |
| GRACE+CCN1+hsTnT | 0.718 | 0.001 | 0.676 | <0.001 |
| GRACE+CCN1+hsTnT+NT‐proBNP | 0.727 | <0.001 | 0.716 | <0.001 |
| GRACE+CCN1+hsTnT+NT‐proBNP+hsCRP | 0.738 | <0.001 | 0.726 | <0.001 |
| 1‐y all‐cause mortality | ||||
| Long‐term GRACE | 0.765 | … | 0.805 | … |
| GRACE+CCN1 | 0.795 | <0.001 | 0.816 | 0.012 |
| GRACE+hsTnT | 0.786 | 0.001 | 0.806 | 0.004 |
| GRACE+NT‐proBNP | 0.797 | <0.001 | 0.827 | <0.001 |
| GRACE+hsCRP | 0.787 | <0.001 | 0.816 | <0.001 |
| GRACE+CCN1+hsTnT | 0.806 | <0.001 | 0.807 | <0.001 |
| GRACE+CCN1+hsTnT+NT‐proBNP | 0.827 | <0.001 | 0.836 | <0.001 |
| GRACE+CCN1+hsTnT+NT‐proBNP+hsCRP | 0.838 | <0.001 | 0.838 | <0.001 |
| 1‐y all‐cause mortality/MI | ||||
| Long‐term GRACE | 0.676 | … | 0.686 | … |
| GRACE+CCN1 | 0.696 | <0.001 | 0.685 | 0.045 |
| GRACE+hsTnT | 0.675 | 0.051 | 0.676 | 0.093 |
| GRACE+NT‐proBNP | 0.697 | <0.001 | 0.706 | <0.001 |
| GRACE+hsCRP | 0.695 | <0.001 | 0.707 | <0.001 |
| GRACE+CCN1+hsTnT | 0.699 | <0.001 | 0.676 | 0.027 |
| GRACE+CCN1+hsTnT+NT‐proBNP | 0.726 | <0.001 | 0.716 | <0.001 |
| GRACE+CCN1+hsTnT+NT‐proBNP+hsCRP | 0.736 | <0.001 | 0.728 | <0.001 |
CCN1 indicates cysteine‐rich angiogenic inducer 61; GRACE, Global Registry of Acute Coronary Events; hsCRP, high‐sensitivity C‐reactive protein; hsTnT, high‐sensitivity Troponin T; MI, myocardial infarction; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
We used continuous GRACE 1.0 risk scores. P values were based on likelihood ratio tests.
For GRACE 2.0 and the biomarkers, a natural logarithm was used.
Table 3.
IDI for All‐Cause Mortality and All‐Cause Mortality/MI (n=1732)
| 30‐D All‐Cause Mortality | GRACE 1.0* | GRACE2.0 † | ||
|---|---|---|---|---|
| IDI Value | P Value | IDI Value | P Value | |
| In‐hospital GRACE risk score | Reference | … | Reference | … |
| GRACE+CCN1 | 0.030 | 0.049 | 0.021 | 0.055 |
| GRACE+hsTnT | 0.036 | <0.001 | 0.039 | 0.004 |
| GRACE+NT‐proBNP | 0.017 | 0.062 | 0.024 | 0.044 |
| GRACE+hsCRP | 0.006 | 0.259 | 0.011 | 0.184 |
| GRACE+CCN1+hsTnT | 0.035 | 0.089 | 0.028 | 0.086 |
| GRACE+CCN1+hsTnT+NT‐proBNP | 0.037 | 0.062 | 0.029 | 0.073 |
| GRACE+CCN1+hsTnT+NT‐proBNP+hsCRP | 0.036 | 0.070 | 0.030 | 0.082 |
| 30‐d all‐cause mortality/MI | ||||
| In‐hospital GRACE risk score | Reference | … | Reference | … |
| GRACE+CCN1 | 0.015 | 0.024 | 0.015 | 0.026 |
| GRACE+hsTnT | 0.009 | <0.001 | 0.010 | <0.001 |
| GRACE+NT‐proBNP | 0.008 | 0.027 | 0.019 | 0.001 |
| GRACE+hsCRP | 0.006 | 0.102 | 0.011 | 0.010 |
| GRACE+CCN1+hsTnT | 0.017 | 0.025 | 0.018 | 0.032 |
| GRACE+CCN1+hsTnT+NT‐proBNP | 0.019 | 0.012 | 0.018 | 0.018 |
| GRACE+CCN1+hsTnT+NT‐proBNP+hsCRP | 0.022 | 0.009 | 0.021 | 0.012 |
| 1‐y all‐cause mortality | ||||
| Long‐term GRACE risk score | Reference | … | Reference | … |
| GRACE+CCN1 | 0.034 | 0.002 | 0.011 | 0.024 |
| GRACE+hsTnT | 0.014 | 0.004 | 0.015 | <0.001 |
| GRACE+NT‐proBNP | 0.028 | <0.001 | 0.022 | 0.008 |
| GRACE+hsCRP | 0.017 | 0.006 | 0.015 | 0.024 |
| GRACE+CCN1+hsTnT | 0.036 | 0.001 | 0.013 | 0.019 |
| GRACE+CCN1+hsTnT+NT‐proBNP | 0.038 | <0.001 | 0.019 | 0.010 |
| GRACE+CCN1+hsTnT+NT‐proBNP+hsCRP | 0.043 | <0.001 | 0.022 | 0.007 |
| 1‐y all‐cause mortality/MI | ||||
| Long‐term GRACE risk score | Reference | … | Reference | … |
| GRACE+CCN1 | 0.017 | 0.001 | 0.005 | 0.025 |
| GRACE+hsTnT | 0.005 | 0.004 | 0.005 | <0.001 |
| GRACE+NT‐proBNP | 0.020 | <0.001 | 0.019 | <0.001 |
| GRACE+hsCRP | 0.018 | <0.001 | 0.017 | <0.001 |
| GRACE+CCN1+hsTnT | 0.018 | <0.001 | 0.006 | 0.017 |
| GRACE+CCN1+hsTnT+NT‐proBNP | 0.020 | <0.001 | 0.011 | 0.006 |
| GRACE+CCN1+hsTnT+NT‐proBNP+hsCRP | 0.025 | <0.001 | 0.014 | 0.002 |
CCN1 indicates cysteine‐rich angiogenic inducer 61; GRACE, Global Registry of Acute Coronary Events; hsCRP, high‐sensitivity C‐reactive protein; hsTnT, high‐sensitivity Troponin T; IDI, Integrated Discrimination Improvement; MI, myocardial infarction; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
We used continuous GRACE 1.0 risk scores. P values were based on likelihood ratio tests.
For GRACE 2.0 and the biomarkers, a natural logarithm was used.
Dichotomized Analysis Based on Cut‐off Demonstrates Worse Survival for Patients With Elevated CCN1 Levels
Using the previously identified cut‐off from patients with stable coronary artery disease, we describe the baseline characteristics including biomarker levels and outcomes for patients with ACS with low versus high CCN1 levels (Table S10 and Figure S1).
Discussion
We here report for the first time that the novel biomarker CCN1 provides independent incremental prognostic information in patients with ACS for all‐cause mortality beyond the clinical GRACE 2.0 risk score.
Specifically, serum levels of CCN1 were independently associated with all‐cause mortality occurring during 30‐day and 1‐year follow‐up, respectively. Furthermore, CCN1 had independent prognostic accuracy in predicting the composite of all‐cause mortality or MI, both during short‐ and long‐term follow‐up. Indeed, the novel serum biomarker CCN1 turned out to be a reliable biomarker to predict all‐cause mortality and the composite of all‐cause mortality or MI during the first year after an ACS when compared with hs‐TnT, NT‐proBNP, and hs‐CRP. CCN1 retains its high prognostic accuracy irrespective of which version of the GRACE risk score is used (GRACE 1.0 or GRACE 2.0), adding independent information.
In a post hoc subgroup analysis, CCN1 retained its incremental value in predicting adverse outcomes in patients with NSTEMI, unlike in patients with STEMI. In light of the rather low event rate in the entire cohort, a subgroup analysis is prone to a type I error. Thus, the observed failure of CCN1 to predict all‐cause mortality (n=39) and the composite of all‐cause mortality and/or MI (n=62) in the first 12 months after STEMI (n=916 patients) is likely attributable to the low event rate rather than reflecting a true biological difference. The lack of a high event rate is a limitation that calls for a larger validation study to provide a clear answer to whether there is a difference between the NSTEMI and STEMI subgroups. From a clinical point of view, patients with NSTEMI constitute the entity among patients with ACS with the greatest heterogeneity, and thus, a new biomarker reflecting specific pathophysiology with prognostic impact appears highly desirable. Two recent studies in patients with NSTEMI managed with early revascularization showed that NT‐proBNP was better in predicting adverse long‐term outcomes than Troponin I and T measured with high‐sensitive assays, 20 , 21 corroborating our data obtained in the SPUM‐ACS cohort with the vast majority of patients receiving percutaneous coronary intervention (90.3%). Prior studies also reported improved risk stratification in patients with NSTEMI when adding NT‐proBNP to clinical scores (Thrombolysis in Myocardial Infarction or GRACE, respectively) in a multimarker approach. 22 , 23 Our current data show that combining CCN1 with the GRACE risk score and cardiovascular biomarkers (hsTnT, hsCRP, and NT‐proBNP) further improved prognostic accuracy, albeit improvements were small in magnitude. Of note, when analyzed against the updated version 2.0 of the GRACE risk score as a reference, CCN1 remained a good predictor of risk; however, it was smaller in magnitude.
Our data show that CCN1 unmasks a distinct pathophysiology translating into adverse outcome, which is currently not reflected by the established biomarkers hsTnT, hsCRP, and NT‐proBNP. In our SPUM‐ACS cohort, the incremental prognostic accuracy of CCN1 when added to the GRACE risk score is moderate from a clinical standpoint. This may be attributable to the high rate of patients who were planned for an early invasive strategy (rate of percutaneous coronary intervention: 90.3%). It would be interesting to learn whether CCN1 may inform the clinician on optimal patient management before treatment decisions are made using a separate validation cohort. Indeed, the role of the GRACE risk score in risk stratification of patients with ACS has recently been challenged by a neutral study outcome in the AGRIS (Australian GRACE Risk Score Intervention Study) in the design of a cluster randomized trial. 24 In this trial, receipt of early invasive treatment was higher in the randomized arm where the GRACE risk score was calculated compared with the standard care arm, unlike the combination of 3 individual outcomes (early invasive treatment, discharge prescription of 4 of 5 guideline‐recommended pharmacotherapies, and cardiac rehabilitation referral) defined as the primary end point, which was not different. Of note, death or MI were not different at 12 months between the 2 arms. However, interpretation of this trial is hampered by the fact that it was stopped prematurely before reaching the planned sample size because of slow recruitment with futility to detect a difference between groups based on an interim analysis. 25
Our data warrant external validation in separate cohorts to better assess the potential value of CCN1 in a clinical routine setting. In our study, most deaths for 30‐day mortality were cardiac in origin (29/32); however, for 1‐year mortality, a different pattern emerges (57/72). Future work needs to clarify the role of CCN1 as a potential marker for the prediction of recurrent cardiovascular events as opposed to it being rather an unspecific marker of increased risk of death. We demonstrate that CCN1 is a novel independent prognostic factor: in other words a marker of risk. However, at this stage we do not aim to postulate that CCN1 is a risk factor where an incremental stepwise increase in concentration translates into a change in outcome as has been demonstrated (ie, for hsTnT). CCN1 is a matricellular protein expressed in response to ischemic injury 3 and is involved in wound healing and myocardial fibrosis. 11 , 12 , 13 , 14 , 15 Future research directed at the role of CCN1 as a potential causal event in the sequelae of MI appears highly promising in order to define its value as a novel therapeutic target.
Limitations
The SPUM‐ACS cohort comprises well‐characterized patients with ACS with independently adjudicated follow‐up throughout 1‐year follow‐up. The current analysis provides biomarker measurements at the time of coronary angiography. Temporal increases of NT‐proBNP and hsCRP were found to be associated with adverse outcomes. 26 We cannot provide biomarker kinetics of CCN1 as part of the current SPUM‐ACS study. However, in light of the acute release kinetics of CCN1 in patients with ACS with peak levels obtained in the first 6 hours, 3 it appears unlikely that analyses at additional time points would affect the prognostic impact of CCN1 taken just before percutaneous coronary intervention. Because these data are derived from 1 study, cross‐validation in other cohorts is needed to assess the added value of CCN1 in risk prediction of patients with NSTEMI and STEMI, respectively.
Conclusions
Serum levels of CCN1 predict adverse outcomes in patients with ACS and provide independent added value to current risk stratification tools.
Sources of Funding
Funding was obtained from the Swiss National Science Foundation (SPUM 33CM30‐124112 and 32473B_163271); the Swiss Heart Foundation; the Foundation Leducq and the Foundation for Cardiovascular Research – Zurich Heart House, Zurich. The SPUM consortium (TFL as PI) received support from Roche Diagnostics, Rotkreuz, Switzerland (providing the kits for high‐sensitivity troponin T, CRP, and NT‐proBNP), Eli Lilly, Indianapolis (USA), AstraZeneca, Zug; Medtronic, Münchenbuchsee; Merck Sharpe and Dome (MSD), Lucerne; Sanofi‐Aventis, Vernier; and St. Jude Medical, Zurich (all Switzerland).
Disclosures
The authors have no conflicts of interest to report beyond the stated funding institutions and companies.
Supporting information
Table S1–S10
Figure S1
Acknowledgments
We express our gratitude to the independent clinical event committee for SPUM‐ACS. Furthermore, we would like to thank the local study nurses, the core lab technicians, the central data monitors, the electronic data capturing system (2mt GmbH Ulm, Germany), and the members of the local catheter teams for their valuable work.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37:3232–3245. DOI: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 2. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. DOI: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 3. Klingenberg R, Aghlmandi S, Liebetrau C, Räber L, Gencer B, Nanchen D, Carballo D, Akhmedov A, Montecucco F, Zoller S, et al. Cysteine‐rich angiogenic inducer 61 (cyr61): a novel soluble biomarker of acute myocardial injury improves risk stratification after acute coronary syndromes. Eur Heart J. 2017;38:3493–3502. DOI: 10.1093/eurheartj/ehx640. [DOI] [PubMed] [Google Scholar]
- 4. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. Management of Acute Coronary Syndromes in Patients Presenting without Persistent STSEotESoC . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the european society of cardiology (ESC). Eur Heart J. 2015;2016(37):267–315. DOI: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 5. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. DOI: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 6. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA , Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;61:485–510. DOI: 10.1016/j.jacc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 7. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130:2354–2394. DOI: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 8. Klingenberg R, Aghlmandi S, Räber L, Gencer B, Nanchen D, Heg D, Carballo S, Rodondi N, Mach F, Windecker S, et al. Improved risk stratification of patients with acute coronary syndromes using a combination of hsTnT, NT‐proBNP and hsCRP with the GRACE score. Eur Heart J Acute Cardiovasc Care. 2018;7:129–138. DOI: 10.1177/2048872616684678. [DOI] [PubMed] [Google Scholar]
- 9. Fox KA, FitzGerald G, Puymirat E, Huang W, Carruthers K, Simon T, Coste P, Monsegu J, Gabriel Steg P, Danchin N, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated grace risk score. BMJ Open. 2014;4:e004425. DOI: 10.1136/bmjopen-2013-004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perbal B, Tweedie S, Bruford E. The official unified nomenclature adopted by the hgnc calls for the use of the acronyms, CCN1–6, and discontinuation in the use of CYR61, CTGF, NOV and WISP 1–3 respectively. J Cell Commun Signal. 2018;12:625–629. DOI: 10.1007/s12079-018-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. DOI: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hinkel R, Trenkwalder T, Petersen B, Husada W, Gesenhues F, Lee S, Hannappel E, Bock‐Marquette I, Theisen D, Leitner L, et al. MRTF‐A controls vessel growth and maturation by increasing the expression of CCN1 and CCN2. Nat Commun. 2014;5:3970. DOI: 10.1038/ncomms4970. [DOI] [PubMed] [Google Scholar]
- 13. Jun JI, Kim KH, Lau LF. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat Commun. 2015;6:7386. DOI: 10.1038/ncomms8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. DOI: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J Am Coll Cardiol. 2016;67:2018–2028. DOI: 10.1016/j.jacc.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 16. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van de Werf F, Avezum A, Goodman SG, Flather MD, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. DOI: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 17. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6‐month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. DOI: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 18. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925–1931. DOI: 10.1093/eurheartj/ehu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. discussion 207–112. DOI: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 20. Kvisvik B, Morkrid L, Rosjo H, Cvancarova M, Rowe AD, Eek C, Bendz B, Edvardsen T, Gravning J. High‐sensitivity troponin T vs I in acute coronary syndrome: prediction of significant coronary lesions and long‐term prognosis. Clin Chem. 2017;63:552–562. DOI: 10.1373/clinchem.2016.261107. [DOI] [PubMed] [Google Scholar]
- 21. Lindholm D, James SK, Bertilsson M, Becker RC, Cannon CP, Giannitsis E, Harrington RA, Himmelmann A, Kontny F, Siegbahn A, et al. Biomarkers and coronary lesions predict outcomes after revascularization in non‐ST‐elevation acute coronary syndrome. Clin Chem. 2017;63:573–584. DOI: 10.1373/clinchem.2016.261271. [DOI] [PubMed] [Google Scholar]
- 22. Widera C, Pencina MJ, Bobadilla M, Reimann I, Guba‐Quint A, Marquardt I, Bethmann K, Korf‐Klingebiel M, Kempf T, Lichtinghagen R, et al. Incremental prognostic value of biomarkers beyond the GRACE (Global Registry of Acute Coronary Events) score and high‐sensitivity cardiac troponin T in non‐ST‐elevation acute coronary syndrome. Clin Chem. 2013;59:1497–1505. DOI: 10.1373/clinchem.2013.206185. [DOI] [PubMed] [Google Scholar]
- 23. Scirica BM, Sabatine MS, Jarolim P, Murphy SA, de Lemos JL , Braunwald E, Morrow DA. Assessment of multiple cardiac biomarkers in non‐ST‐segment elevation acute coronary syndromes: observations from the MERLIN‐TIMI 36 Trial. Eur Heart J. 2011;32:697–705. DOI: 10.1093/eurheartj/ehq468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chew DP, Hyun K, Morton E, Horsfall M, Hillis GS, Chow CK, Quinn S, D’Souza M, Yan AT, Gale CP, et al. Objective risk assessment vs standard care for acute coronary syndromes: a randomized clinical trial. JAMA Cardiol. 2021;6:304–313. DOI: 10.1001/jamacardio.2020.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harrington RA, Ohman EM. Risk stratification science goes to a new level. JAMA Cardiol. 2021;6:314–315. DOI: 10.1001/jamacardio.2020.6325. [DOI] [PubMed] [Google Scholar]
- 26. Chan MY, Neely ML, Roe MT, Goodman SG, Erlinge D, Cornel JH, Winters KJ, Jakubowski JA, Zhou C, Fox KAA, et al. Temporal biomarker profiling reveals longitudinal changes in risk of death or myocardial infarction in non‐ST‐segment elevation acute coronary syndrome. Clin Chem. 2017;63:1214–1226. DOI: 10.1373/clinchem.2016.265272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S10
Figure S1


