Abstract
Coronavirus disease 2019 (COVID-19) is the third deadly coronavirus infection of the 21st century that has proven to be significantly more lethal than its predecessors, with the number of infected patients and deaths still increasing daily. From December 2019 to July 2021, this virus has infected nearly 200 million people and led to more than 4 million deaths. Our understanding of COVID-19 is constantly progressing, giving better insight into the heterogeneous nature of its acute and long-term effects. Recent literature on the long-term health consequences of COVID-19 discusses the need for a comprehensive understanding of the multisystemic pathophysiology, clinical predictors, and epidemiology to develop and inform an evidence-based, multidisciplinary management approach. A PubMed search was completed using variations on the term post-acute COVID-19. Only peer-reviewed studies in English published by July 17, 2021 were considered for inclusion. All studies discussed in this text are from adult populations unless specified (as with multisystem inflammatory syndrome in children). The preliminary evidence on the pulmonary, cardiovascular, neurological, hematological, multisystem inflammatory, renal, endocrine, gastrointestinal, and integumentary sequelae show that COVID-19 continues after acute infection. Interdisciplinary monitoring with holistic management that considers nutrition, physical therapy, psychological management, meditation, and mindfulness in addition to medication will allow for the early detection of post-acute COVID-19 sequelae symptoms and prevent long-term systemic damage. This review serves as a guideline for effective management based on current evidence, but clinicians should modify recommendations to reflect each patient's unique needs and the most up-to-date evidence. The presence of long-term effects presents another reason for vaccination against COVID-19.
Abbreviations and Acronyms: 6MWT, 6-minute walk test; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; IL, interleukin; KD, Kawasaki disease; MIS-A, multisystem inflammatory syndrome in adults; MIS-C, multisystem inflammatory syndrome in children; VTE, venous thromboembolism events
Coronavirus disease 2019 (COVID-19) is the third deadly coronavirus infection of the 21st century and has proven to be significantly more deadly than its predecessors, with the number of infections and deaths still increasing daily.1 Since its emergence in December 2019, this virus has spread across the globe infecting nearly 200 million people and leading to more than 4 million deaths as of July 17, 2021.2
Knowledge of COVID-19, which is caused by severe acute respiratory coronavirus 2 (SARS-CoV-2), is constantly progressing with the discovery of new scientific and clinical evidence. Comparison between the timeline, common symptoms, systematic effects, and risk factors for adverse events of acute versus chronic COVID-19 symptoms can be found in Table 1 .
Table 1.
Comparison Between the Timeline, Common Symptoms, Systematic Effects, and Risk Factors for Adverse Events of Acute Versus Chronic COVID-19 Symptomsa
| Features | Acute COVID-19 | Post-acute COVID-19 |
|---|---|---|
| Initiation of symptoms 3, 4, 5, 6, 7, 8 | 2 to 14 days after exposure | 4 weeks after initial response |
| Pathophysiology9, 10, 11, 12, 13, 14, 15 | Direct viral toxicity via ACE2/TMPRSS2 expressing cells (alveolar epithelial type II cells, heart, kidneys, gastrointestinal tract, lung tissue, and nasal olfactory cells) Immune-mediated dysregulation of RAAS pathway Microvascular dysfunction via endothelial damage Dysregulation of the innate immune system with lymphopenia Prolonged activation of type 1 interferons and endothelial cell damage lead to thromboembolic events Decrease in lymphocytes may allow for viral particles to persist, resulting in excessive inflammation that can increase symptom severity and need for ICU admission |
Presence of autoreactive antibodies Inflammatory and metabolic changes to parenchyma, and supporting structures during initial infection Sequelae mediated by hospitalization interventions |
| Common symptoms3, 4, 5, 6, 7,16, 17, 18, 19, 20 | Fever, dry cough, and shortness of breath >50% of patients | Fatigue, pneumonia, myalgias, headache, thromboembolic conditions, and MIS |
| Systems commonly involved16,17,19,21 | Respiratory, renal (AKI), hematological (thromboembolism) | Respiratory, cardiovascular, neurologic, MIS, hematological Less frequently involved: renal, endocrine, gastrointestinal, or integumentary |
| Laboratory markers and clinical indications of severe disease16,20,22,23 | Progressive elevation of cardiac troponins, hepatic enzymes, and serum creatinine, along with advanced lymphocytopenia and increased neutrophils ARDS development for severe cases |
See Table 2 for systematic breakdown of lab markers and risk factors |
| Risk factors for adverse consequences4,16,23, 24, 25 | Hypertension, diabetes, mechanical ventilation, ICU admission, advanced age, frailty, immune response | Ages 40-49 (OR, 15.3) years Hospitalization during acute infection (OR, 2.9) Clinical findings from the initial infection (variable) |
| Risk factors for death26 | Hospitalization, 85+ years old (OR, 11.36 years), pneumonia, diabetes, heart failure, CKD | Not yet determined |
ACE2, angiotensin-converting enzyme 2; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; ICU, intensive care unit; MIS, multisystem inflammatory syndrome; OR, odds ratio; RAAS, renin-angiotensin aldosterone system; TMPRSS2, transmembrane serine protease 2.
The long-term sequelae of COVID-19 (referred to as long-COVID, post-acute COVID-19, post-COVID condition, and post-COVID sequelae interchangeably) are defined as an inflammatory or host response towards virus that occurs approximately 4 weeks after initial infection and continues for a yet uncharacterized duration (Supplemental Table, available online at http://www.mayoclinicproceedings.org).8 This review aims to report the up-to-date epidemiology, pathophysiology, clinical predictors, management recommendations, and the unanswered questions and future directions for the systematic effects of long-term COVID-19 infections that clinicians can refer to when managing COVID-19 long-haulers (Table 2 ).
Table 2.
| System | Clinical findings or diagnostic criteria 17,19 | Risk factors 27, 28, 29 | Management recommendations 30, 31, 32, 33, 34, 35, 36, 37 | Clinical trial based pharmacology 30,38, 39, 40, 41, 42, 43, 44 |
|---|---|---|---|---|
| Pulmonary | Fatigue, dyspnea CT: ground-glass opacities and fibrotic changes |
Severe disease, ICU admission, or severe hospital stay Lower limit 6MWT, higher CT severity score, increased D-dimer or urea nitrogen Advanced age, male, history of cigarette smoking |
Initial evaluation 4-6 weeks with pulmonary function testing and 6MWT Follow-up imaging at 12 wks Dyspnea: pulmonary and cardiac workup |
Maximum initial dose 0.5 mg/kg oral prednisolone: FVC, radiological and symptomatic improvement 2403 mg daily pirfenidone: FVC, 6WMT, and disease progression improvement 150 mg of twice daily nintedanib: FVC improvement Pulmonary rehabilitation: pulmonary function improvements Ongoing clinical trials to determine potential pharmacological candidates, use current recommended guidelines for most likely differential diagnosis |
| Cardiovascular | Chest pain, heart palpitations CMR: myocardial inflammation, myocarditis, pericarditis, cardiac fibrosis Echocardiogram: impaired LVEF, pericarditis, myocarditis, other cardiac anomalies Increased troponin level |
Severe disease, high viral load, pneumonia during hospitalization Increased troponin levels, decrease in LVEF Hydroxychloroquine with or without ritonavir/lopinavir and azithromycin use in acute COVID-19 |
Initial evaluation with noninvasive technology (point-of-care ECG, transthoracic echocardiogram, laboratory tests for CRP, and troponin-T) Escalate to invasive testing and referral if abnormalities are detected in the initial evaluation |
Ongoing clinical trials to determine potential pharmacological candidates, use current recommended guidelines for most likely differential diagnosis Continue RAAS modifying drug use (ACE-inhibitors, ARBs, etc) Avoid amiodarone as it may exacerbate pulmonary fibrosis |
| Neurological | Neuropsychiatric: anxiety, depression, PTSD, OCD, insomnia Cognitive: headaches, brain fog, memory loss, nonrestorative sleep Peripheral: anosmia, ageusia, fatigue, malaise, POTS |
Severity of illness, ICU admission, MIS-C Medications such as lopinavir-ritonavir and corticosteroid use (rare) |
Standard screening tools be used for neuropsychiatric conditions by primary care providers to evaluate for disorders such as anxiety, depression, PTSD, and OCD No specific published guidelines on screening protocols for neurological disorders |
Standard therapies with referral to neurological specialists for refractory conditions or imaging abnormalities Beta-blockers, diet, and exercise: beneficial in reducing POTS symptoms |
| Hematological | Pulmonary embolism, VTE, ATE | Disease severity, length of acute infection, and ICU admission Low fibrinogen and higher D-dimer on admission, and DI ≥1.5-fold Increased age, cancer, and corticosteroid use (critically ill) |
In-patient (all): CBC, coagulation studies PT and aPTT, fibrinogen and D-Dimer Outpatient (symptomatic or at-risk): same as in-patient Proceed to further invasive testing and imaging studies under standard protocols recommended for the suspected differential diagnosis if abnormalities are present |
Thromboprophylaxis in those with high clot burden or at risk of thromboembolic events if there is no bleeding risk Ongoing clinical trials to determine potential pharmacological candidates, use current recommended guidelines for most likely differential diagnosis |
| Renal | AKI, wide spectrum of glomerular and tubular diseases | Same as hematological system High-risk APOL1 variant (collapsed glomerulopathy) |
Refer to a nephrologist if AKI is persistent or there is severe dysfunction | Standard therapies with referral to specialists as needed |
| Endocrine | New-onset diabetes, worsening pre-existing diabetes, DKA, subacute thyroiditis, Graves thyrotoxicosis | High viral loads, severe disease | Initial evaluation for new-onset diabetes should include testing for antibodies to beta-islet cells and CRP and rule out risk factors for type 2 diabetes Monitor labs to rule out new-onset thyroid autoimmune diseases (Graves and Hashimoto thyroiditis) vs COVID-19 thyroiditis Refer to an endocrine specialist as necessary |
Standard therapies with referral to specialists as needed |
| Gastrointestinal | Loss of appetite, nausea, acid reflux, diarrhea, abdominal distension, belching, vomiting, and bloody stools | High viral loads, severe disease | Fecal cultures to check for gut dysbiosis, refer to a gastrointestinal specialist as necessary | Dietary changes or fecal transplantation Standard therapies with referral to specialists as needed |
| Integumentary | Hair loss, skin rash, urticarial lesions, angioedema | Unknown | Standard protocols with referral to specialists as needed | Standard therapies with referral to specialists as needed |
| MIS-C | (Age ≤ 21 y): fever ≥ 38.0°C or subjective fever for ≥24 h, laboratory inflammation evidence, severe illness requiring hospitalization with ≥ 2 organ involvement | Presenting to the emergency department or admitted to the general ward Increased D-dimer, troponin, BNP, pro-BNP,CRP, and ferritin In patients ≥5 y old, identify as Black |
Tier 1: CBC, CMP, ESR, CRP and SARS-CoV-2 PCR/serology If tier 1 results reach the diagnostic threshold then tier 2: cardiac enzymes (BNP, troponin T, etc), hematological/coagulation factors (D-dimer, fibrinogen, PTT, blood smear, etc), cardiac studies (ECG and echocardiogram), LDH, triglycerides, urinalysis, and cytokine panel In shock without clear etiology: tiers 1 and 2 |
1st line in shock: IVIG 2 gm/kg and MPIV at 1-2 mg/kg per day Refractory disease with shock: MPIV 10-30 mg/kg per day or high dose anakinra No shock: IVIG 2 gm/kg per day Refractory Disease, No shock: MPIV 1-2 mg/kg per day or high-dose anakinra |
6MWT, 6-minute walk test; ACE, angiotensin-converting enzyme; AKI, acute kidney injury; APOL1, apolipoprotein L1; aPTT, activated partial prothrombin time; ARB, angiotensin receptor blocker; ATE, arterial thromboembolism; BNP, brain natriuretic peptide; CBC, complete blood count; CMP, complete metabolic panel; CMR, cardiac magnetic resonance; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; DI, D-dimer increment; DKA, diabetic ketoacidosis; ECG, electrocardiogram; ESR, erythrocyte sedimentation rate; FVC, forced vital capacity; ICU, intensive care unit; IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; LVEF, left ventricular ejection fraction; MIS-C, multisystem inflammatory syndrome in children; MPIV, methylprednisone intravenous; OCD, obsessive compulsive disorder; PCR, polymerase chain reaction; POTS, postural orthostatic tachycardia syndrome; PT, prothrombin time; PTSD, post-traumatic stress disorder; RAAS, renin angiotensin-aldosterone system; SARS-CoV-2, severe acute respiratory syndrome; VTE, venous thromboembolism.
High-risk or symptomatic individuals are the priority for screening.
A PubMed search was completed using the terms “long-COVID,” “post-acute COVID-19,” “post-COVID condition,” “post-COVID sequelae,” or “long-term sequelae of COVID-19.” Only peer-reviewed studies in English published by July 17, 2021, were considered for inclusion. All studies discussed in this text are from adult populations unless specified (as with multisystemic inflammatory syndrome in children [MIS-C]).
Pulmonary System
Epidemiology
The evidence of the epidemiology of post–COVID-19 pulmonary sequelae comes from primary studies that look at persistent symptomology, acute manifestations of COVID-19, as well as extrapolations from follow-up from previous coronaviruses. Overall, the most common symptoms of long-term pulmonary sequelae experienced by those with COVID-19 include fatigue, dyspnea, and/or cough. The prevalence of these symptoms and the severity of abnormalities on imaging depend on the severity of acute illness and intensive care unit (ICU) admission status. A breakdown of the major studies looking at post-acute COVID-19 pulmonary effects can be found in Table 3 .
Table 3.
Epidemiological Studies of Post-Acute COVID-19 Broken Down by System
| Author | Study type | Key findings | Key takeaways |
|---|---|---|---|
| Pulmonary | |||
| Ahmed et al45 | Meta-analysis on SARS/MERS 6 months post-discharge | DLCO, FVC, TLC abnormalities more prevalent than abnormalities in FEV1 TLC and FVC abnormalities resolve with time, but DLCO impairment remained in 24.35% for >6 months |
|
| Carvalho-Schneider et al46 | Prospective follow-up study of 150 patients after 60 days | Instances of grade 2-4 dyspnea decrease with time (at the onset-42.2%, Day 30-10.7% vs. Day 60- 7.7% post-infection) Increased odds of persistent symptoms in those between ages 40 and 60 y, admitted to hospitals, and abnormal auscultation |
|
| Halpin et al47 | Prospective study of 100 subjects in the United Kingdom after 4 weeks | Fatigue (72% ICU vs 60.3% non-ICU) and dyspnea (65.6% ICU vs 42.6% non-ICU) | |
| Huang et al17 | Ambidirectional cohort study of 1733 patients after 6 months | Severe disease: 4.6 times more likely to have lung diffusion impairments and 2.69 times more likely to report fatigue 1+ major lung abnormality in 50% of patients after 6 months; most common is ground-glass opacities |
|
| Zhao et al48 | 39 patients after 3 months | Persistent fibrotic changes on CT in 25% of mild to moderate and 65% of severe COVID-19 patients Increases in urea nitrogen and D-dimer levels at admission were prognostic indicators of impaired lung diffusion |
|
| Cardiovascular | |||
| Carfi et al19 | Italian study with 143 patients after 2 months | 22% experience chest pain |
|
| Huang et al17 | Ambidirectional cohort study of 1733 patients after 6 months | Chest pain and palpitations to be <5% | |
| Sultanian et al49 | Observational study of 3026 patients in Sweden | COVID-19 was also involved in at least 16% of IHCAs and 10% of all OHCAs, 2.3-fold increase in 30-day mortality due to IHCA and 3.4-fold in OHCA |
|
| Wu et al50 | 12-year follow-up study of SARS patients | 40% continued to have cardiovascular complications | |
| Puntmann et al51 | Cohort study of 100 patients 60 days post COVID-19 infection | 60% of participants had ongoing myocardial inflammation on CMR and 78% had some cardiac involvement independent of pre-existing conditions or illness severity Endomyocardial biopsy in severe patients revealed active lymphocytic infiltration without viral genome presence |
|
| Rajpal et al52 | 26 college athletes after 11-53 days | Myocarditis and pericarditis in 4 of 26 and 2 of 26 participants, respectively | |
| Neurological | |||
| Taquet et al53 | Retrospective cohort study with 236,371 patients after 6 months | 23% to 37% had 1+ neuropsychiatric disorders Rates of neuropsychiatric disorder varied dependent on disease severity: Encephalopathy > ICU > hospitalized/nonhospitalized Patients with COVID-19 are 5.28-4.52x more likely to develop myoneural junction diseases than other respiratory infections |
|
| Mazza et al54 | Self-rating among patients at a 1-mo follow-up | Anxiety (42%), insomnia (40%), depression (31%), PTSD (28%), and obsessive compulsive disorders (20%) | |
| Garrigues et al55 | 120 patients, 100 d after an initial infection | Memory loss was the third most common persistent symptom at 34% Cognitive symptom manifestation did not differ between ICU and hospitalized patients |
|
| National Institute of Neurological Disorders and Stroke56 | Retrospective study, 20 patients, with persistent neurological and cardiovascular complaints | 15 had POTS, 3 neurocardiogenic syncope, and 2 orthostatic hypotension 85% had persistent residual autonomic symptoms, and 12 could not return to work after 6-8 mo
|
|
| Lechien et al57 | Multicenter European study of 1363 subjects after 6 months | 5% of patients had prolonged anosmia with the highest prevalence in those with mild COVID 19 | |
| Hematological | |||
| Guan et al3 | Retrospective study of 1099 people in China | Lymphocytopenia 83.2%, thrombocytopenia in 36.2%, and leukopenia in 33.7% on admission Elevated C-reactive protein (60.7%) and D-dimer (46.7%) Severe disease had more pronounced laboratory anomalies |
|
| Roncon et al58 | Systematic review | 23.4% incidence of pulmonary embolism in ICU vs 14.7% in general ward Segmental/subsegmental arteries more frequently involved than main/lobar arteries |
|
| Rashidi et al59 | Multicenter prospective study of 1529 participants | Venous thromboembolism is at 0.2% | |
| Fan et al60 | Case reports 40-90 d after positive serology | Arterial thromboembolic events have been seen in young, asymptomatic COVID-19 subjects | |
| Townsend et al61 | Prospective study of 150 participants in Ireland | Increased D-Dimer levels in 25.3% of patients up to 4 months More common in hospitalized patients and 50+ y old; also seen in 29% of patients exclusively managed in outpatient settings |
|
| Renal | |||
| Nugent et al62 | Cohort study of 1612 patients in the United States | eGFR declined faster with COVID-19–associated AKI vs other causes (adjusted hazard ratio, 0.57; 95% CI, 0.35-0.92) |
|
| Gupta et al63 | Multicenter cohort study with 2215 adults, 60 days post-discharge | 56% of patients discharged RRT (35% with COVID-19 AKI) unable to get off of it | |
| Stevens et al64 | Cohort study of 115 patients | 84% with RRT during acute infection recovered kidney function | |
| Kudose et al65 | Kidney biopsies done at Columbia University | 15 (88%) AKI, 9 (53%) nephrotic-range proteinuria, 5 (29%) collapsing glomerulopathy, 4 (24%) isolated acute tubular injury, 2 (12%) membranous glomerulopathy, 1 (6%) anti-GBM nephritis, 1 crescentic transformation of lupus nephritis, and 1 minimal change disease Collapsing glomerulopathy and minimal change disease showed the presence of the APOL1 gene variant |
|
| Endocrine | |||
| Zhou et al66 | Retrospective study from China | 6.4% of COVID-19 patients with DKA, but only 35.7% had diabetes |
|
| Multiple studies67, 68, 69, 70, 71 | Various case reports | Subacute thyroiditis and Graves thyrotoxicosis, and new diagnoses of DM diagnosed in COVID-19 patients | |
| Gastrointestinal | |||
| Weng et al72 | Follow-up study of 117 patients after 90 days | Loss of appetite (24%), nausea (18%), acid reflux (18%), diarrhea (15%), and abdominal distension (14%) with <10% of patients reporting belching, vomiting, and bloody stools |
|
| Integumentary | |||
| Huang et al17 | Ambidirectional cohort study of 1733 patients after 6 months | Hair loss (20% of patients) and skin rash which resolved in 97% of patients |
|
| Galván Casas et al73 | Prospective study of 375 cases in Spain | Urticarial lesions made up 19% of COVID-19 cutaneous manifestations | |
| Argolo et al74 | Retrospective studies in Argentina | 30% of patients reported worsening chronic urticaria during the pandemic | |
| Multiple studies75, 76, 77 | Case reports | Oral angioedema with or without urticaria on the tongue and/or lips 1-2 weeks into the acute disease course | |
AKI, acute kidney injury; APOL1, apolipoprotein L1; CMR, cardiac magnetic resonance; COVID-19, coronavirus disease 2019; CT, computed tomography; DKA, diabetic ketoacidosis; DLCO, diffusing capacity of the lung for carbon monoxide; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GBM, glomerular basement membrane ; ICU, intensive care unit; IHCA, in-hospital cardiac arrest; MERS, Middle East respiratory syndrome; OHCA, out-of-hospital cardiac arrest; POTS, postural orthostatic tachycardia syndrome; PTSD, post-traumatic stress syndrome; RRT, renal replacement therapy; SARS, severe acute respiratory syndrome; TLC, total lung capacity.
Pathophysiology
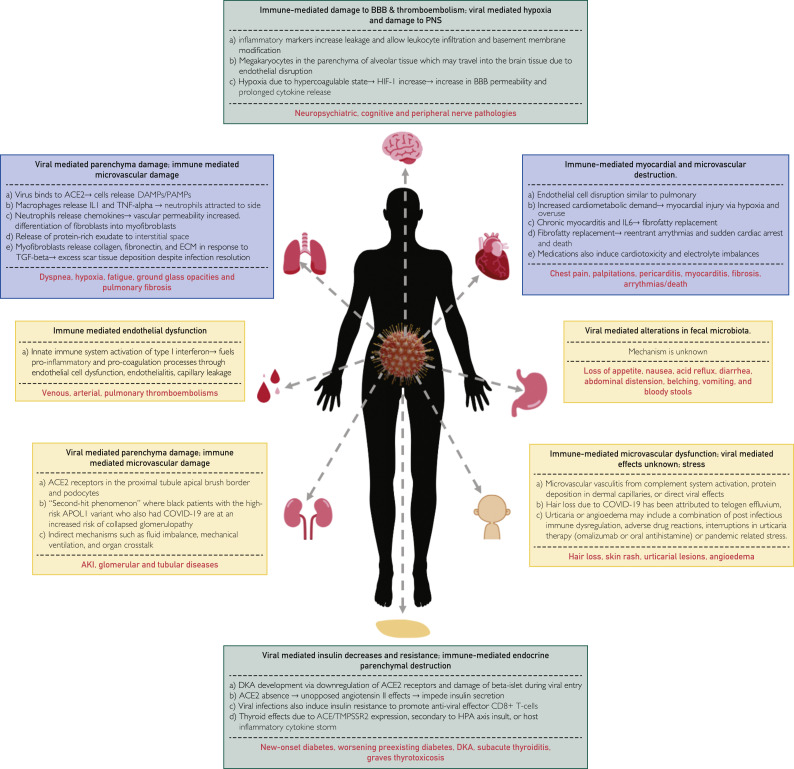
A summary of the pathophysiology of pulmonary sequelae due to post-acute COVID-19 can be found in Figure 1 . An analysis of 41 post-mortem samples in Italy found extensive alveolar damage in 100% of subjects, lung macro- and microvasculature thrombosis in 71% of individuals, and thrombi stage of the organization consistent with local origin. Pneumocytes and endothelial cells contained viral ribonucleic acid even in the later stages of the disease, but viral infection was not detected in other organs.95 The mechanism for thrombotic changes in this disease can also be found in Figure 1.
Figure 1.
Pathophysiology of pulmonary, cardiac, neurological, hematological, renal, gastrointestinal, integumentary, and endocrine effects of post-acute coronavirus disease 2019 and its subsequent clinical manifestations.15,17,28,29,48,78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94 BBB = blood brain barrier; ACE = angiotensin-converting enzyme; ACE2 = angiotensin-converting enzyme 2; AKI = acute kidney injury; APOL1 = apolipoprotein L1; COVID-19 = coronavirus disease 2019; DAMP = damage-associated molecular patterns; DKA = diabetic ketoacidosis; ECM = extracellular matrix; HIF-1 = hypoxia-inducible factor 1; HPA = hypothalamic-pituitary-adrenal; IL = interleukin; PAMP = pathogen-associated molecular pattern; PNS = peripheral nervous system; TGF = tumor growth factor; TMPSSR2 = type 2 transmembrane protein; TNF = tumor necrosis factor.
Clinical Predictors
A comprehensive list of risk factors for the development of pulmonary manifestations for post-acute COVID-19 can be found in Table 2. In a study of 1733 patients from China, patients categorized as having a more severe hospital stay were more likely to have diffusion impairments (4.6 times) and report fatigue (2.69 times) than less severe counterparts (Supplemental Figure 1, available online at http://www.mayoclinicproceedings.org).17 There is also an increased prevalence of fatigue and dyspnea in ICU patients than non-ICU groups 4 weeks after acute infection resolution.47 Six-minute walk testing (6MWT) results can provide prognostic factors for illness severity as those with more severe disease had a higher proportion of subjects below the lower limit of the normal range (Supplemental Figure 1).17 The odds for developing pulmonary fibrosis were significantly increased in subjects with increased urea nitrogen (odds ratio [OR], 7.149) and D-dimer levels (OR, 1.066) at admission.48
Management Recommendations
Priority for prognostic screening for pulmonary sequelae of post-acute COVID-19 should be given to those who are symptomatic or show appropriate clinical predictors. Management recommendations for those that meet these criteria can be found in Table 2.
For high-risk patients (see Table 2), the British Thoracic Society recommends an initial evaluation at 4 to 6 weeks post-discharge and in-person visit/follow-up imaging within 12 weeks.31 Radiological imaging is recommended for all COVID-19 patients 12 weeks after discharge regardless of symptom status. However, the study from Liu et al.96 of 150 patients from China shows that radiological resolution of pulmonary abnormalities occurs in greater than or equal to 50% of patients within 3 weeks of discharge; therefore, imaging of nonsymptomatic patients may be unnecessary.
Steroid use in the setting of post-acute COVID-19 has mixed results; therefore, steroid-sparing therapies should be considered when possible.17 An observational study in the United Kingdom has shown some rapid functional improvements with corticosteroid therapy.38 Of the 837 patients assessed by telephone at 4 weeks after infection resolution, 35 (4.8%) were initially diagnosed with interstitial lung disease and significant functional deficits. These 35 patients were brought in for outpatient evaluation where 30 were diagnosed with persistent interstitial lung changes and were offered steroid treatment with a maximum dose of 0.5 mg/kg oral prednisolone for 3 weeks. Improvements were noted in transfer factor (31.6%±27.6%, P<0.001) and forced vital capacity (9.6%±13.0%, P=0.014) with treatment, and significant symptomatic and radiological improvement.
Unanswered Questions and Future Directions
Questions that remain include whether patients with significant lung involvement ever go back to pre–COVID-19 baseline, both symptomatically and by objective measures of lung function. In addition, we also do not know how long it takes for symptoms to resolve completely or for lung function to fully recover. Future studies involving medications that can help decrease permanent lung damage such as pulmonary fibrosis would be beneficial.
Cardiovascular System
Epidemiology
A breakdown of the major studies looking at post-acute COVID-19 cardiac effects can be found in Table 3. Increases in cardiac troponin levels may indicate underlying cardiovascular pathology and is correlated with increased severity of acute illness and use of mechanical ventilation during hospitalization, cardiac dysfunction, arrhythmias, and death.97
Pathophysiology
The pathophysiology behind the cardiovascular sequelae of post-acute COVID-19 can be found in Figure 1. These findings indicate immune-mediated pathogenesis in the cardiovascular sequelae of post-acute COVID-19 instead of viral pathogenesis that leads to cardiac fibrosis. Imbalances in electrolytes can also interfere with electrical conduction in myocytes resulting in fatal rhythmic abnormalities. Hydroxychloroquine and azithromycin may cause QT prolongation which can lead to torsade de pointes and fatal arrhythmias.98 In patients at risk of cardiovascular symptoms of COVID-19, medication lists and electrolyte levels should be closely monitored to reduce instances of arrhythmias.
Clinical Predictors
History of increased troponin, disease severity, higher viral load, and pneumonia increase the risk of cardiovascular sequelae in patients who have had COVID-19. Patients with elevated troponin levels are also at an increased risk due to higher white blood cell counts and longer prothrombin times.97 In a meta-analysis of 591 patients with severe acute respiratory syndrome (SARS), Corrales-Medina et al99 found that hospitalization with pneumonia increases the risk of cardiovascular disease 2.1 times within the first year and 1.86 times in 10 years. Hospitalization records in COVID-19 patients should be evaluated for pneumonia during active infection and flagged for cardiac follow-up. A cytokine response panel consisting of 6 pro-inflammatory cytokines (tumor necrosis factor [TNF]-alpha, interferon-gamma, C-C motif chemokine ligand 5, interleukin 6 [IL-6], IL-8, and IL-18) were elevated in 16 patients with viral loads greater than 1000 copies per real-time polymerase chain reaction (rt-PCR) and cardiac fibrosis.100 These lab markers may serve as prognostic indicators of post–COVID-19 patients that may have cardiac sequelae due to fibrosis. Cardiac fibrosis is central to the pathophysiology of heart failure (HF), which explains the increased incidence of acute HF in COVID-19 and acute decompensation in patients with chronic-HF in this study of 3080 patients in Spain.43
Prolonged corticosteroid use may increase viral shedding, thus increasing the odds of developing cardiovascular and other systemic sequelae. A multicenter randomized control trial of 86 patients with COVID-19 found that early use of corticosteroids (methylprednisolone) prolongs viral shedding in patients with COVID-19 pneumonia by suppressing immune cells.101 In this single-blind prospective trial, patients were either given 1 mg/kg per day of methylprednisolone in 100 mL 0.9% saline or 100 mL 0.9% normal saline for 7 days within 72 hours of being admitted to the hospital. Viral RNA was detectable in the methylprednisolone group (11 days; interquartile range, 6 to 16 days), which was significantly longer than that in the control group (8 days; interquartile range, 2 to 12 days; P=0.030) with throat cultures. CD3+ T cells and CD8+ T cells in the methylprednisolone group were also lower than the control group (P<0.05). However, this study also found no significant differences in other secondary outcomes or incidence of clinical deterioration.
Corticosteroid use was beneficial in reducing patient mortality in patients with acute respiratory distress syndrome (ARDS) (HR, 0.38).102 A landmark Randomised Evaluation of COVID-19 Therapy (RECOVERY) open-label trial with 6425 hospitalized COVID-19 patients who were assigned to either take dexamethasone 6 mg once a day for 10 days or have “usual” care. Reduction in mortality with glucocorticoid therapy varied greatly depending on the level of respiratory support at the time of randomization. Mortality was lower in treatment groups in patients receiving invasive mechanical ventilation (29.3% vs 41.4%; rate ratio [RR], 0.64) or oxygen without invasive mechanical ventilation (23.3% vs 26.2%; RR, 0.82) but not in subjects receiving no respiratory support (17.8% vs 14.0%; RR, 1.19).103 Although corticosteroids may cause systemic sequelae issues in the long term, it would not be reasonable to delay treatment given the mortality benefits in patients who need respiratory support or who have ARDS.
Management Recommendations
Major concerns in the cardiovascular sequelae of COVID-19 are acute coronary syndrome, myocarditis, arrhythmias, cardiogenic shock, and medication-induced heart conditions. Full cardiac workup should be limited to patients with active symptoms or at increased risk. Point-of-care electrocardiogram (ECG), transthoracic echocardiogram, laboratory tests for C-reactive protein (CRP) and troponin-T, and pro-inflammatory markers (TNF-alpha, C-C motif chemokine ligand 5, IL-6, IL-8, IL-18, and interferon-gamma) should be measured in symptomatic patients. Decreases in left ventricular ejection fraction, abnormal increases in the aforementioned laboratory tests, and any electrical abnormalities warrant invasive measures such as cardiac catheterization, transesophageal echocardiography, and cardiac magnetic resonance if they are of clinical benefit.104 Athletes presenting with clinical findings of myocarditis at the onset of illness (disproportionate dyspnea on exertion, chest pain, unexpected elevations in serum troponins, arrhythmias/atrioventricular block, etc) should stop aerobic activity and athletic participation and undergo an echocardiogram, 24-hour Holter monitoring, and exercise ECG within 3 to 6 months after initial illness. They can resume training if cardiac and inflammatory biomarkers, electrical activity, and ventricular systolic function have returned to normal.105
The current clinical trial–based pharmacology for management of cardiac sequelae in post-acute COVID-19 can be found in Table 2. Corticosteroids may improve cardiac function in viral-induced myocarditis if viral RNA is undetectable due to concerns of prolonged viral shedding.106
Unanswered Questions and Future Directions
There are many unanswered questions on the long-term cardiovascular effects of COVID-19. This is an evolving question due to the novelty of COVID-19. We do not know if post–COVID-19 arrhythmias are permanent or temporary. We do not know how long patients will need to avoid proarrhythmic drugs. We also do not know if post–COVID-19–associated myocarditis or cardiomyopathy and related congestive HF will fully recover. The duration of treatment with angiotensin-converting enzymes or beta blockers in these patients is also unknown. Another unknown is how long should limitations on strenuous activity remain. Future studies should focus on determining risk factors for long-term cardiovascular side effects and the development of mitigation measures to prevent these sequelae.
Neurological System
Epidemiology
Survivors of COVID-19 report prolonged anosmia/ageusia, headaches, “brain fog”/memory loss, fatigue, malaise, and nonrestorative sleep.16 , 17 , 19 , 47 A breakdown of the major studies of post-acute COVID-19 neurological effects can be found in Table 3. The prevalence of brain fog lacks significant scientific validity due to the lack of objective evidence.
Pathophysiology
A summary of the pathophysiology of neurological effects of post-acute COVID-19 can be found in Figure 1.
Clinical Predictors
The severity of illness, ICU admission, MIS-C, and certain medication during acute infection all increase risk of neurological sequelae in the post-acute COVID-19 period. A retrospective cohort study with 236,371 patients found substantial neurological and psychiatric morbidity among patients with severe illness.53 Intensive care unit admissions are also associated with increased neuropsychiatric symptom manifestation (depression, anxiety, etc) but not cognitive symptoms (memory loss and headache). Instances of neuropsychiatric diseases increase with encephalopathy during the acute phase of illness (Supplemental Figure 1).53 Case reports of children with MIS-C show unexpectedly high instances of neurological involvement (40%) as well.107 Persistent anosmia after infection resolution may be a prognostic sign of a milder disease because it is prevalent in people with less severe COVID-19. Increased production of immunoglobulin A limits viral spread in those with mild COVID-19, but it escalates local inflammatory reactions in the olfactory system.57
Medications used in acute COVID-19 such as lopinavir-ritonavir and corticosteroids have neurological side effects that can manifest and persist during the acute or post-acute phase of illness. Ototoxicity-mediated sensorineural hearing loss is associated with lopinavir-ritonavir treatment, although this effect is rare and resolves approximately 20 weeks after discontinuation.108 A multicenter cohort study in France unrelated to COVID-19 with 88 patients showed prednisone use greater than 20 mg/day caused delirium and manic mood changes in 52% of patients.109 A controlled, open-label trial of 6425 patients that looked at dexamethasone (6 mg once a day for 10 days) as a potential treatment for patients hospitalized with COVID-19 had four adverse reactions, one of which included psychosis.103 The neurological side effects of corticosteroids are infrequent but can still cause significant neuropsychological and peripheral symptoms. Corticosteroids are frequently used in hospital and outpatient settings during acute COVID-19 infection, so clinicians must consider the neurological effects of these medications when monitoring patients in the outpatient setting.
Management Recommendations
There are no specific published guidelines on screening protocols for neurological disorders caused by COVID-19 other than for postural orthostatic tachycardia syndrome (POTS). Primary care providers should use standard screening tools to evaluate for common disorders such as anxiety, depression, post-traumatic stress disorder (PTSD), and obsessive compulsive disorder (OCD).54 Standard therapies should be implemented for all conditions with referral to neurological specialists for refractory conditions or imaging abnormalities. Diagnostic criteria for POTS are light-headedness or fainting accompanied by an increase in heart rate greater than 30 beats/min or heart rate greater than or equal to 120 beats/min after 10 minutes of rising.56 Beta blockers are effective in managing this condition in combination with diet and exercise.41
Unanswered Questions and Future Directions
As in the case of pulmonary and cardiovascular manifestations, the duration of symptoms is still unknown. The effectiveness of rehabilitation exercises in recovering function is not clear. Depression post–COVID-19 must be distinguished from depression due to pandemic-related secondary issues, including work, school, and economic factors. Olfactory rehabilitation exercises have been shown to improve identification, discrimination, and threshold for odor detection.110 However, it is unclear how effective olfactory rehabilitation exercises are for people who have not spontaneously regained sense of smell after COVID-19 infection.
Hematological System
Epidemiology
A breakdown of the major studies looking at post-acute COVID-19 hematological effects can be found in Table 3. Common hematological findings of an acute COVID-19 infection are lymphocytopenia, neutrophilia, eosinopenia, and thrombocytopenia.111 , 112 Lymphocytopenia, thrombocytopenia, elevated CRP, and elevated procalcitonin have also been identified as markers of severe disease in other studies, which increases the risk of thromboembolic events.113 , 114 However, the analysis from Zuin et al113 of post-acute thromboembolic events shows that the rate of thrombotic events is lower post-discharge than during the acute infection; therefore, it is a rarer complication of COVID-19.
Pathophysiology
A summary of the pathophysiology of hematological effects of post-acute COVID-19 can be found in Figure 1.
Thromboembolic events observed after infection resolution are immune-mediated rather than true thromboembolic events.113 The increase in thrombotic risk due to SARS-CoV-2 is limited to acute illness even in those with severe disease that require admission.59 The multicenter prospective study from Rashidi et al59 of 1529 patients did not find a high rate of symptomatic venous thromboembolism (VTE) after hospital discharge. Of the 3.3% of patients who died after discharge, only 0.2% deaths could be attributed to VTE. Severely ill patients may have an increased risk for bleeding due to thrombocytopenia and depletion of coagulation factors, but it has been shown that thromboembolic events occur at higher rates than bleeding complications in acute COVID-19.88 Consequently, extended thromboprophylaxis after discharge may not provide any net benefit clinically, but randomized clinical trials are needed for additional verification.
Clinical Predictors
Acute illness severity and ICU admissions are risk factors for coagulation abnormality–induced thromboembolisms. Severe disease is associated with an increased risk of VTE and arterial thromboembolic events, which increases short-term morbidity and mortality.113 , 114 Intensive care unit admission associated with increased pulmonary embolism as compared with non-ICU patients and independent factors include age, cancer, length of acute infection phase, low fibrinogen and higher D-dimer on admission, and D-dimer increment (DI) greater than or equal to 1.5-fold. Fibrinogen, D-dimer, and DI predicted instances of VTE in the acute phase with a 93% sensitivity and 0.71% specificity.58 , 115 Patients with any of the mentioned clinical factors should be monitored for coagulation abnormalities that can present in any system and prioritized for post-illness follow-up when clinical indications are present.
Management Recommendations
Guidelines for management of thromboembolic conditions due to COVID-19 can be found in Table 2. These are based on recommendations from the American College of Cardiology and American Society of Hematology on care for patients in the hospital; in out-patient cases, post-hospital thromboprophylaxis should also be given to at-risk patients and those that have undergone prolonged corticosteroid therapy who do not have an active bleeding risk to reduce risk of VTE. Extended use of thromboprophylaxis or use in all patients is not recommended at this time.59
Unanswered Questions and Future Directions
Unanswered questions include the mechanisms by which COVID-19 leads to long-term lipid abnormalities which can lead to atherogenesis. The role of statins in the maintenance of vascular health and integrity is unknown with regard to COVID-19.
Multisystem Inflammatory Syndromes
Epidemiology
Multi-inflammatory syndrome (MIS) sequelae are rare but severe complications following acute COVID-19 infection in previously symptomatic and asymptomatic individuals that present a few weeks after initial infection.116 Although this syndrome was first documented in children younger than 21 years old (MIS-C), there is an increasing number of case reports that show adults (21 to 50 years old) may also develop these sequelae (MIS-A).117
The diagnostic criteria for MIS-C can be found in Table 2. Similarities have been observed between MIS-C, Kawasaki disease (KD), and toxic shock syndrome with common clinical features such as persistent fever, systemic hyperinflammation, multiorgan involvement with prominent and severe gastrointestinal (GI) symptoms, erythematous rashes, conjunctivitis, and inflammatory changes in the oral mucosa.118 As a result, the pathophysiology of COVID-19 MIS-C may be revealed from studying these diseases. Critical differences between KD and MIS-C include ethnicities of those predominantly affected, organ systems attacked, and laboratory findings.118, 119, 120 Sixty-two percent of MIS-C patients are Hispanic, Latino, or Black as of June 2021, whereas most KD patients are of Asian heritage.116 , 118 Patients with MIS-C are also characterized by myocardial dysfunction, neuropsychological/central nervous system findings, and severe GI symptoms compared with the mild GI symptoms of KD and lack of neurological and cardiovascular shock clinical characteristics.118 Patients with MIS-C also show increased D-dimer and troponin levels, lymphocytopenia, and thrombocytopenia.119 , 120 In contrast, toxic shock syndrome has nearly identical symptom presentation, laboratory findings, and drug response and only differs in ethnicity, affecting those of White descent more, and does not show increased troponin levels.119 , 120
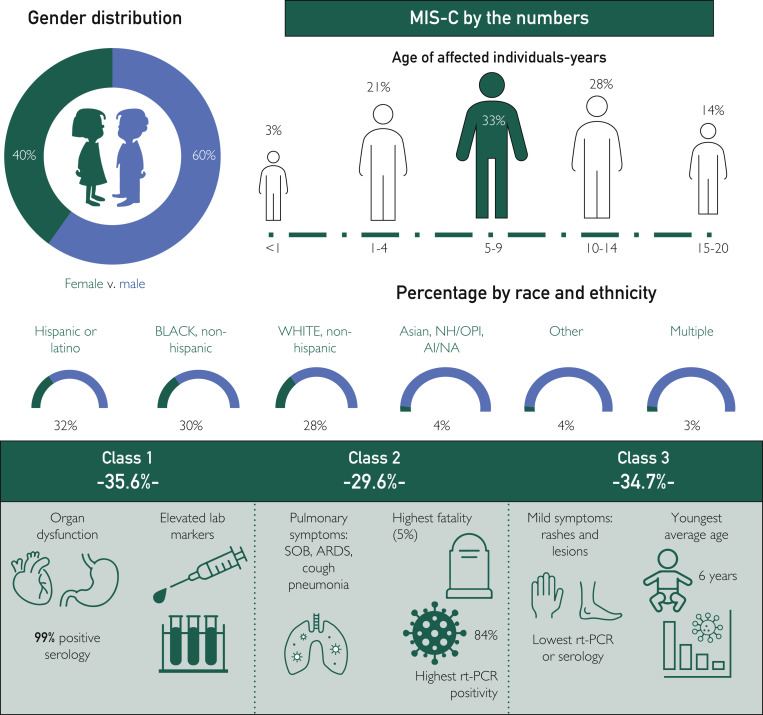
Epidemiological information and subdivisions of the three distinct classes of MIS-C compiled from the Centers for Disease Control and Prevention (CDC) database for 4018 reported cases of MIS-C as of June 2, 2021, can be found in Figure 2 .121 , 122 Diagnosis of MIS-A still lacks clear criteria, but has been described by the CDC using case reports from 16 patients between the ages of 21 and 50 years.123 This criteria includes age 21 years and older, being hospitalized for 24 hours or longer, fever greater than or equal to 38.0°C or a subjective fever for 24 hours or longer or within the first 3 days of hospitalization, meeting at least one primary clinical criteria (severe cardiac illness, rash, and nonpurulent conjunctivitis), and may have secondary criteria (new-onset neurological symptoms, shock/hypotension that is not from medical therapy such as medications or dialysis, abdominal symptoms, and thrombocytopenia).124 They must also have laboratory evidence of SARS-CoV-2 infection either by rt-PCR or serology and evidence of inflammation.124 Little epidemiological data is currently available about MIS-A, and there are no registered Cochrane trials ongoing to study MIS-A specifically.
Figure 2.
Multisystem inflammatory syndrome in children (MIS-C) by the numbers. Epidemiological images derived from the Centers for Disease Control and Prevention data on 4018 reported cases of MIS-C as of June 2, 2021.121 Disease classifications from 570 MIS-C case reports from March 2 to July 18, 2020.122 Percentages by race and ethnicity may exceed 100%. Multiple race identities could be selected. Race/ethnicity data were not reported for 276 of 4018 cases. Percentages of Asian, NH/OPI, and AI/NA were rounded up to 1%. AI = American Indian; ARDS = acute respiratory distress syndrome; NA = Native Alaskan; NH = Native Hawaiian; OPI = Other Pacific Islander; rt-PCR = real-time polymerase chain reaction; SOB = shortness of breath.
Pathophysiology
The pathophysiology behind the sequelae remains relatively unknown due to the small sample sizes of MIS-C and MIS-A. Reports show abnormalities in the coagulation cascade similar to adults at increased thrombosis risk after COVID-19.30 In addition, most patients with MIS symptoms are antibody-positive but do not have an active viral infection.30 Pediatric hospitalizations due to COVID-19 are on the rise again in unvaccinated children due to the increased transmission rates of the delta variant.117 More data should be available soon and the mechanism of MIS can be further explored.
Clinical Predictors
Symptoms of MIS have been reported in both the pediatric (MIS-C) and adult (MIS-A) populations, but clinical predictors for MIS-C are currently more reliable due to their earlier characterization. Clinical predictors for MIS-C can be found in Table 2.
Management Recommendations
The American College of Rheumatology updated information regarding MIS-C diagnosis, and treatment guidelines were released in December 2020 and are continually being revised as more information becomes available.30 Treatment guidelines for MIS-C can be found in Table 2.
Unanswered Questions and Future Directions
Little epidemiological data are currently available for MIS-A, and there are no registered Cochrane trials ongoing to study MIS-A specifically. Therefore, more case studies must be identified to develop an accurate epidemiological picture before analyzing the pathophysiology and management recommendations. For MIS-C, there is a greater database of cases, but this too must be expanded to further characterize pathophysiology and management recommendations.
Other Long-Term Systematic Effects
Other rare long-term systemic effects of COVID-19 involve the renal, endocrine, integumentary, and GI systems. Pertinent information about the pathophysiology, clinical predictors, and management recommendations can be found in Figure 1 and Table 2, respectively. A breakdown of the major studies looking at post-acute COVID-19 effects of these systems can be found in Table 3. As of June 2021, 683 trials are registered with the Cochrane COVID-19 study registrar which should further clarify the prevalence of these infrequent systemic complications (available at https://covid-19.cochrane.org).
Unanswered Questions and Future Directions
Endocrine
Elevated blood sugar levels have been observed in many patients during acute COVID-19 infection. How long does this hyperglycemic effect last? Should all at-risk patients be placed on low-sugar diets? Other questions include the duration of thyroid function monitoring and whether or not fertility is affected post–COVID-19 infection.
Gastrointestinal
Fecal microbiota alterations have been associated with prolonged persistent fecal levels of SARS-CoV-2 and increased COVID-19 severity through modulation of host immune responses.125 , 126 How aggressive we should be in restoring gut microbiome post–COVID-19 is unclear and is a candidate for future studies.
Skin
Increased cases of urticaria have been reported after COVID-19. Urticaria is observed after many other viral infections. Is COVID-19 different in this regard, or does it lead to longer duration of chronic urticaria, such as that seen in autoimmune or chronic idiopathic urticaria?
Effective Multidisciplinary Management of Long-Term Effects of Covid-19
Patients with severe disease, increased risk factors, as well as symptomatic and/or hospitalized individuals should be prioritized for in-person or telemedicine visits (Supplemental Figure 2, available online at http://www.mayoclinicproceedings.org). Long-term management should focus on a holistic approach that involves physical therapy, nutrition, and psychological interventions in addition to medication-based methods.
Rehabilitation in post-acute COVID-19 involves appropriate management of the consequences of ventilatory support, prolonged immobilization, and bed rest. These include impaired lung function, physical deconditioning that leads to impaired muscle function, and cognitive impairments.127 Patients should resume regular activities 6 to 8 weeks post-discharge, but this may be more challenging in patients who have severe disease, extended hospital stays, or prolonged symptoms.19 , 128 Even if a formal exercise and exertional desaturation assessment cannot be conducted in patients with COVID-19, they should perform low- or moderate-intensity exercise to reduce muscle deterioration and improve pulmonary function.128 A randomized control trial of 150 participants showed an increased rate of 6MWT results in participants with critical illness who participated in outpatient physiotherapy after ICU discharge.129 A 6-week respiratory rehabilitation program in elderly COVID-19 patients shows significant improvements in forced expiratory volume-1 (FEV1), forced vital capacity (FVC), FEV1/FVC percentage, diffusing capacity for carbon monoxide percentage, and 6MWT.130 Neurological rehabilitation may also be beneficial in patients with residual COVID-19 neurological dysfunction, but research on this is minimal. Clinicians should schedule a 6- to 8-week follow-up appointment for hospitalized patients to measure lung function, exercise capacity, and cognitive function.128 For nonsevere patients, a comprehensive physical should be performed if symptomatic, but a mental-health assessment should be conducted for all.
Nutrition after COVID-19 infections plays a vital role in aiding recovery. Studies show that 50% or more of elderly in-patient participants with COVID-19 are malnourished with an additional 25.7% at risk of malnourishment.131 Inflammation, ageusia, decreased appetite, prolonged bed rest, and gut-microbiome disturbances during acute and post-acute COVID-19 leave patients deficient in key micro- and macronutrients necessary for physical reconditioning and recovery. Nutrition evaluations and consultations with registered dieticians should be considered in muscle loss, GI abnormalities, and/or vitamin deficiencies.
With all the challenges of the pandemic that patients may face, mental health becomes a significant concern. The physical effects of long-term COVID-19 sequelae can often be intertwined with the psychological outlook of patients and efforts should be made to ensure that mental health is preserved. To this end, complementary therapies such as acupuncture, massage, low-impact yoga, and Tai Chi may be of benefit.
Conclusion
The findings in this review show that the effects of COVID-19 do not end with acute infection resolution. In all patients, interdisciplinary monitoring is required to detect post-acute COVID-19 symptoms before long-term systemic damage occurs. Patients post–COVID-19 may visit many different providers because of multisystem involvement and multiple symptoms. Integrated health care settings are crucial to facilitate communication between providers about health care plans and progress and avoid unnecessary duplicate tests, thus saving patients time and health care costs. Health care systems have already begun establishing dedicated COVID-19 care clinics that facilitate multidisciplinary care. Policy and practices also need to be established to effectively harness and optimize collaboration between medical specialties, multiple health care professionals, and patients.
This review discusses the most up-to-date evidence-based knowledge on management of common systemic sequelae of post-acute COVID-19. More information about post-acute COVID-19 continues to be discovered as the pandemic continues. This will help develop a detailed explanation of the mechanisms behind symptoms and classify the risk factors that predispose patients to adverse outcomes. Guidelines will need to be continuously updated to reflect the most up-to-date body of knowledge. Every patient, symptom, and circumstance is unique; thus, the guidelines recommended in this article should be modified according to each patient’s needs and the clinicians’ discretion.
The clear association between COVID-19 and long lasting sequelae that has the potential to affect multiple organ systems is another reason why everyone should be vaccinated. No longer can any demographic be confident that their illness may be mild and that they will completely recover from it. The best way to prevent the acute illness and long-term effects of COVID-19 is not to get the infection in the first place. Social distancing measures, masking, and vaccines are effective ways of lowering the risk of infection.
Acknowledgment
The authors thank Rani Vatti, MD, for her valuable suggestions and review of this paper.
Footnotes
Potential Competing Interests: The authors report no potential competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Čivljak R., Markotić A., Kuzman I. The third coronavirus epidemic in the third millennium: what’s next? Croat Med J. 2020;61(1):1–4. doi: 10.3325/cmj.2020.61.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int Accessed July 17, 2021.
- 3.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin J., You C., Lin Q., Hu T., Yu S., Zhou X.-H. Estimation of incubation period distribution of COVID-19 using disease onset forward time: a novel cross-sectional and forward follow-up study. Sci Adv. 2020;6(33) doi: 10.1126/sciadv.abc1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta S.D., Talwar A., Lee J.T. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. JAMA. 2020;324(22):2251–2252. doi: 10.1001/jama.2020.22717. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cañas C.A. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med Hypotheses. 2020;145:110345. doi: 10.1016/j.mehy.2020.110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong A.K.H., Woodhouse I., Schneider F., Kulpa D., Silvestri G., Maier C. Broad Auto-Reactive IgM Responses Are Common In Critically Ill COVID-19 Patients. Cell Rep Med. 2021;2(6):100321. doi: 10.1016/j.xcrm.2021.100321. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8160082/. Accessed July 25, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carsana L., Sonzogni A., Nasr A., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fodoulian L., Tuberosa J., Rossier D., et al. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. iScience. 2020;23(12):101839. doi: 10.1016/j.isci.2020.101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gkogkou E., Barnasas G., Vougas K., Trougakos I.P. Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol. 2020;36:101615. doi: 10.1016/j.redox.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrill J.T., Erkan D., Winakur J., James J.A. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol. 2020;16(10):581–589. doi: 10.1038/s41584-020-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno-Pérez O., Merino E., Leon-Ramirez J.M., et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Office for National Statistics Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK - Office for National Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/4june2021 Accessed June 29, 2021.
- 19.Carfì A., Bernabei R., Landi F., et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X., Xue S., Xu J., et al. Clinical characteristics and related risk factors of disease severity in 101 COVID-19 patients hospitalized in Wuhan, China. Acta Pharmacol Sin. 2021 doi: 10.1038/s41401-021-00627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goërtz Y.M.J., Van Herck M., Delbressine J.M., et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):00542–2020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abers M.S., Delmonte O.M., Ricotta E.E., et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021;6(1) doi: 10.1172/jci.insight.144455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geriatric Medicine Research Collaborative Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing. 2021;50(3):617–630. doi: 10.1093/ageing/afab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estiri H., Strasser Z.H., Klann J.G., Naseri P., Wagholikar K.B., Murphy S.N. Predicting COVID-19 mortality with electronic medical records. NPJ Digit Med. 2021;4(1):15. doi: 10.1038/s41746-021-00383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scappaticcio L., Pitoia F., Esposito K., Piccardo A., Trimboli P. Impact of COVID-19 on the thyroid gland: an update. Rev Endocr Metab Disord. 2020:1–13. doi: 10.1007/s11154-020-09615-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7688298/ Accessed July 17, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlieb M., Long B. Dermatologic manifestations and complications of COVID-19. Am J Emerg Med. 2020;38(9):1715–1721. doi: 10.1016/j.ajem.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H., Larsen C.P., Hernandez-Arroyo C.F., et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol. 2020;31(8):1688–1695. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson L.A., Canna S.W., Friedman K.G., et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021;73(4):e13–e29. doi: 10.1002/art.41616. https://pubmed.ncbi.nlm.nih.gov/33277976/ Accessed July 19, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George P.M., Barratt S.L., Condliffe R., et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75(11):1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 32.Geoffrey D. Barnes Feature | Thrombosis and COVID-19: FAQs For Current Practice. Am Coll Cardiol. 2020:1–5. https://www.acc.org/latest-in-cardiology/articles/2020/04/17/14/42/thrombosis-and-coronavirus-disease-2019-covid-19-faqs-for-current-practice Accessed June 25, 2021. [Google Scholar]
- 33.American Society of Hematology COVID-19 and Coagulopathy. https://www.hematology.org/covid-19/covid-19-and-coagulopathy Accessed June 25, 2021.
- 34.Ruggeri R.M., Campennì A., Siracusa M., Frazzetto G., Gullo D. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones (Athens) 2021;20(1):219–221. doi: 10.1007/s42000-020-00230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiMeglio L.A., Evans-Molina C., Oram R.A. Type 1 diabetes. Lancet. 2018;391(10138):2449–2462. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh T.S., Rampelli S., Jeffery I.B., et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69(7):1218. doi: 10.1136/gutjnl-2019-319654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Salhy M., Hatlebakk J.G., Gilja O.H., Bråthen Kristoffersen A., Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69(5):859–867. doi: 10.1136/gutjnl-2019-319630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myall K.J., Mukherjee B., Castanheira A.M., et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18(5):799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King T.E., Bradford W.Z., Castro-Bernardini S., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 40.Richeldi L., du Bois R.M., Raghu G., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 41.Moon J., Kim D.-Y., Lee W.-J., et al. Efficacy of propranolol, bisoprolol, and pyridostigmine for postural tachycardia syndrome: a randomized clinical trial. Neurotherapeutics. 2018;15(3):785–795. doi: 10.1007/s13311-018-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puchner B., Sahanic S., Kirchmair R., et al. Beneficial effects of multi-disciplinary rehabilitation in postacute COVID-19: an observational cohort study. Eur J Phys Rehabil Med. 2021;57(2):189–198. doi: 10.23736/S1973-9087.21.06549-7. [DOI] [PubMed] [Google Scholar]
- 43.Rey J.R., Caro-Codón J., Rosillo S.O., et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail. 2020;22(12):2205–2215. doi: 10.1002/ejhf.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sobol S.M., Rakita L. Pneumonitis and pulmonary fibrosis associated with amiodarone treatment: a possible complication of a new antiarrhythmic drug. Circulation. 1982;65(4):819–824. doi: 10.1161/01.cir.65.4.819. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed H., Patel K., Greenwood D.C., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5) doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho-Schneider C., Laurent E., Lemaignen A., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halpin S.J., McIvor C., Whyatt G., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y.-M., Shang Y.-M., Song W.-B., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sultanian P., Lundgren P., Strömsöe A., et al. Cardiac arrest in COVID-19: characteristics and outcomes of in- and out-of-hospital cardiac arrest. A report from the Swedish Registry for Cardiopulmonary Resuscitation. Eur Heart J. 2021;42(11):1094–1106. doi: 10.1093/eurheartj/ehaa1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Q., Zhou L., Sun X., et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017;7(1):9110. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazza M.G., De Lorenzo R., Conte C., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garrigues E., Janvier P., Kherabi Y., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Institute of Neurological Disorders and Stroke Postural Tachycardia Syndrome Information Page. https://www.ninds.nih.gov/Disorders/All-Disorders/Postural-Tachycardia-Syndrome-Information-Page Accessed July 2, 2021.
- 57.Lechien J.R., Chiesa-Estomba C.M., Beckers E., et al. Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. J Intern Med. 2021;290(2):451–461. doi: 10.1111/joim.13209. [DOI] [PubMed] [Google Scholar]
- 58.Roncon L., Zuin M., Barco S., et al. Incidence of acute pulmonary embolism in COVID-19 patients: systematic review and meta-analysis. Eur J Intern Med. 2020;82:29–37. doi: 10.1016/j.ejim.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rashidi F., Barco S., Kamangar F., et al. Incidence of symptomatic venous thromboembolism following hospitalization for coronavirus disease 2019: Prospective results from a multi-center study. Thromb Res. 2021;198:135–138. doi: 10.1016/j.thromres.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan B.E., Umapathi T., Chua K., et al. Delayed catastrophic thrombotic events in young and asymptomatic post COVID-19 patients. J Thromb Thrombolysis. 2021;51(4):971–977. doi: 10.1007/s11239-020-02332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Townsend L., Fogarty H., Dyer A., et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost. 2021;19(4):1064–1070. doi: 10.1111/jth.15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nugent J., Aklilu A., Yamamoto Y., et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta S., Hayek S.S., Wang W., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevens J.S., King K.L., Robbins-Juarez S.Y., et al. High rate of renal recovery in survivors of COVID-19 associated acute renal failure requiring renal replacement therapy. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0244131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kudose S., Batal I., Santoriello D., et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suwanwongse K., Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: causality or coincidence? A report of three cases. J Med Virol. 2021;93(2):1150–1153. doi: 10.1002/jmv.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khatri A., Charlap E., Kim A. Subacute thyroiditis from COVID-19 infection: a case report and review of literature. Eur Thyroid J. 2021;9(6):324–328. doi: 10.1159/000511872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattar S.A.M., Koh S.J.Q., Rama Chandran S., Cherng B.P.Z. Subacute thyroiditis associated with COVID-19. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-237336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harris A., Al Mushref M. Graves’ thyrotoxicosis following SARS-CoV-2 infection. AACE Clin Case Reports. 2021;7(1):14–16. doi: 10.1016/j.aace.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weng J., Li Y., Li J., et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol. 2021;6(5):344–346. doi: 10.1016/S2468-1253(21)00076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galván Casas C., Català A., Carretero Hernández G., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Argolo P., Pereira G., Pereira G.F., Kalil J., Motta A., Agondi R. Clinical conditions of patients with chronic urticaria during the pandemic caused by the 2019 novel coronavirus disease (COVID-19) J Allergy Clin Immunol. 2021;147(suppl 2) AB26-AB26. [Google Scholar]
- 75.Royer P.-Y., Zayet S., Jacquin-Porretaz C., et al. Angioedema and COVID-19: a new dermatological manifestation? Infect Dis Reports. 2021;13(1):23–25. doi: 10.3390/idr13010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abasaeed Elhag S.A., Ibrahim H., Abdelhadi S. Angioedema and urticaria in a COVID-19 patient: a case report and review of the literature. JAAD Case Rep. 2020;6(10):1091–1094. doi: 10.1016/j.jdcr.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Azmy V., Benson J., Love K., Steele R. Idiopathic nonhistaminergic acquired angioedema in a patient with coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125(5):600–602. doi: 10.1016/j.anai.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bharat A., Querrey M., Markov N.S., et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020;12(574) doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ojo A.S., Balogun S.A., Williams O.T., Ojo O.S. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies. Pulm Med. 2020;2020:6175964. doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janeway C.A.J. Pillars article: approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peretto G., Sala S., Rizzo S., et al. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75(9):1046–1057. doi: 10.1016/j.jacc.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 84.Xiong T.-Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41(19):1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erickson M.A., Rhea E.M., Knopp R.C., Banks W.A. Interactions of SARS-CoV-2 with the blood–brain barrier. Int J Mol Sci. 2021;22(5):2681. doi: 10.3390/ijms22052681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nauen D.W., Hooper J.E., Stewart C.M., Solomon I.H. Assessing brain capillaries in coronavirus disease 2019. JAMA Neurol. 2021;78(6):760–762. doi: 10.1001/jamaneurol.2021.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duarte-Neto A.N., Monteiro R.A.A., da Silva L.F.F., et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77(2):186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leentjens J., van Haaps T.F., Wessels P.F., Schutgens R.E.G., Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents—lessons after 1 year. Lancet Haematol. 2021;8(7):e524–e533. doi: 10.1016/S2352-3026(21)00105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nadim M.K., Forni L.G., Mehta R.L., et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16(12):747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X., Zheng J., Guo L., et al. Fecal viral shedding in COVID-19 patients: clinical significance, viral load dynamics and survival analysis. Virus Res. 2020;289:198147. doi: 10.1016/j.virusres.2020.198147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mieczkowska K., Deutsch A., Borok J., et al. Telogen effluvium: a sequela of COVID-19. Int J Dermatol. 2021;60(1):122–124. doi: 10.1111/ijd.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reddy P.K., Kuchay M.S., Mehta Y., Mishra S.K. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes Metab Syndr. 2020;14(5):1459–1462. doi: 10.1016/j.dsx.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Šestan M., Marinović S., Kavazović I., et al. Virus-induced interferon-γ causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity. 2018;49(1):164–177.e6. doi: 10.1016/j.immuni.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Bussani R., Schneider E., Zentilin L., et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020;61:103104. doi: 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X., Zhou H., Zhou Y., et al. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J Infect. 2020;81(1):e95–e97. doi: 10.1016/j.jinf.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mercuro N.J., Yen C.F., Shim D.J., et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corrales-Medina V.F., Alvarez K.N., Weissfeld L.A., et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313(3):264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lindner D., Fitzek A., Bräuninger H., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang X., Feng Y.-M., Ni J.-X., et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration. 2021;100(2):116–126. doi: 10.1159/000512063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kociol R.D., Cooper L.T., Fang J.C., et al. Recognition and initial management of fulminant myocarditis. Circulation. 2020;141(6):e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 105.Maron B.J., Udelson J.E., Bonow R.O., et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis. Circulation. 2015;132(22):e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 106.Chen H.S., Wang W., Wu S.N., Liu J.P. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev. 2013;2013(10):CD004471. doi: 10.1002/14651858.CD004471.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dufort E.M., Koumans E.H., Chow E.J., et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williams B. Ototoxicity may be associated with protease inhibitor therapy. Clin Infect Dis. 2001;33(12):2100–2102. doi: 10.1086/324361. [DOI] [PubMed] [Google Scholar]
- 109.Fardet L., Flahault A., Kettaneh A., et al. Corticosteroid-induced clinical adverse events: frequency, risk factors and patient’s opinion. Br J Dermatol. 2007;157(1):142–148. doi: 10.1111/j.1365-2133.2007.07950.x. [DOI] [PubMed] [Google Scholar]
- 110.Sorokowska A., Drechsler E., Karwowski M., Hummel T. Effects of olfactory training: a meta-analysis. Rhinology. 2017;55(1):17–26. doi: 10.4193/Rhino16.195. [DOI] [PubMed] [Google Scholar]
- 111.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun S., Cai X., Wang H., et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. 2020;507:174–180. doi: 10.1016/j.cca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zuin M., Rigatelli G., Zuliani G., Roncon L. The risk of thrombosis after acute-COVID-19 infection. QJM. 2021;114(9):619–620. doi: 10.1093/qjmed/hcab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li J.-Y., Wang H.-F., Yin P., et al. Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID-19 patients: a multicenter retrospective study. J Thromb Haemost. 2021;19(4):1038–1048. doi: 10.1111/jth.15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Center for Disease Control and Prevention (CDC) HAN Archive — 00442 | Health Alert Network (HAN) https://emergency.cdc.gov/han/2020/han00432.asp Accessed June 21, 2021.
- 117.COVID-NET Laboratory Confirmed COVID-19 Associated Hospitalizations. https://gis.cdc.gov/grasp/covidnet/COVID19_5.html Accessed August 9, 2021.
- 118.Noval Rivas M., Porritt R.A., Cheng M.H., Bahar I., Arditi M. COVID-19-associated multisystem inflammatory syndrome in children (MIS-C): a novel disease that mimics toxic shock syndrome-the superantigen hypothesis. J Allergy Clin Immunol. 2021;147(1):57–59. doi: 10.1016/j.jaci.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Whittaker E., Bamford A., Kenny J., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheung E.W., Zachariah P., Gorelik M., et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324(3):294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Centers for Disease Control and Prevention (CDC) Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. https://www.cdc.gov/mis-c/cases/index.html Accessed June 21, 2021.
- 122.Godfred-Cato S., Bryant B., Leung J., et al. COVID-19–associated multisystem inflammatory syndrome in children — United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morris S.B., Schwartz N.G., Patel P., et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Center for Disease Control and Prevention (CDC) Multisystem Inflammatory Syndrome in Adults (MIS-A) https://www.cdc.gov/mis-c/mis-a/hcp.html . Accessed June 21, 2021.
- 125.Zuo T., Zhang F., Lui G.C.Y., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yeoh Y.K., Zuo T., Lui G.C.-Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pan American Health Organization (PAHO). W Rehabilitation Considerations during the COVID-19 Outbreak. www.paho.org/coronavirus Accessed June 28, 2021.
- 128.Spruit M.A., Holland A.E., Singh S.J., Tonia T., Wilson K.C., Troosters T. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society and American Thoracic Society–coordinated international task force. Eur Respir J. 2020;56(6):2002197. doi: 10.1183/13993003.02197-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Denehy L., Skinner E.H., Edbrooke L., et al. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care. 2013;17(4):R156. doi: 10.1186/cc12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu K., Zhang W., Yang Y., Zhang J., Li Y., Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;39:101166. doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li T., Zhang Y., Gong C., et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74(6):871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.