Dear Editor,
Although the main repercussions of SARS-CoV-2 infection are described in the lung parenchyma, preliminary studies highlight the liver as a particularly affected organ, with increased laboratory markers of liver injury and function, as well as histopathological evaluation [1]. Besides that, despite the global collective effort, the increasing number of macroscopic and microscopic liver findings in autopsies have not yet been fully mapped in COVID-19 infection [2], [3], [4]. Thus, a question arises: what are the most common histopathological changes caused by COVID-19?
According to the literature, the most common histopathological changes associated with SARS-CoV-2 were hepatic steatosis (HS), thrombosis or/and portal and periportal inflammatory infiltrate, Kupffer cell hyperstimulation and cholestasis [2], [3], [4], [5], [6], [7], [8], [9], [10]. We summarize the main histopathological findings of COVID-19 in the liver in Figs. 1 and 2 .
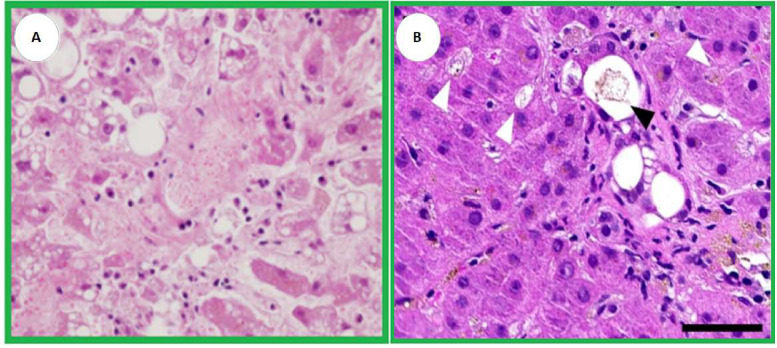
Fig. 1.
[Adapted] A. Macrovesicular and microvesicular steatoses are demonstrated with platelet-fibrin thrombi are present in the sinusoidal spaces and central vein (H&E, ×400) [5]. B. Ductular (black arrowhead) and canalicular (white arrowheads) cholestasis (H&E; scale bar 50µm) [10].
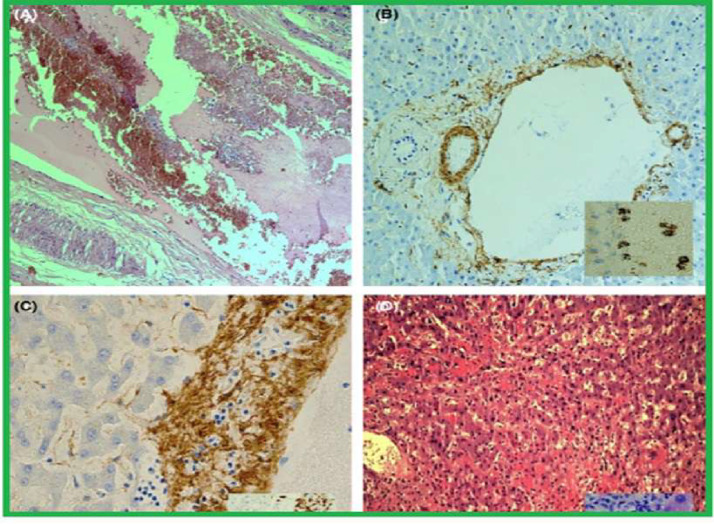
Fig. 2.
A: Example of genuine thrombosis: portal with clearly enlarged lumen obliterated by red cell mixed andstratified with lymphocytes and granulocytes (H&E,100 ×); B: smooth musde layer of portal vein lamina media extremely irregular fragmented (SMA, 100 ×); SAR-CoV-2 virions are demonstrated within vessel lumen and onendothelial cells (ISH) (inset); C: medium layer of a portal vein, partially lost and infiltrated by inflammatory cellslymphocytes, also attaching endothelial layer (AMA, 400×); CD3-positive lymphocytes attack endotelium andmedium vessel layer (inset); D: severe confluent haemorrhagic necrosis in a patient with elevation of ALT> 10 N(H&E, 100×); inset showing liver necrosis by apoptosis (H&E, 400×) [Adapted] [3].
About hepatic steatosis (HS) and COVID-19, a systematic review including research studies from seven countries suggested an estimated prevalence of HS of 51% in histopathological analyses [2,5] (Fig. 1). Sonzogni et al. demonstrated a similar incidence. According to the authors, in 48 liver autopsy samples from patients with SARS-CoV-2, 54% had HS [3]. The high prevalence may be associated with some factors, according to the literature: (I) the aggression/direct effect of the virus on liver cells [4]; (II) the effect of the virus as a co-factor of existing comorbidities (e.g. diabetes mellitus, obesity, and cardiovascular diseases); (III) the effect of the virus as a catalyst for reduced hepatic flow by septic shock in the “Cytokine storm” caused by it; and (IV) thrombosis caused by the hypercoagulable status of SARS-CoV-2 [1].
When we analyze the vascular processes and local inflammatory reaction in the liver by SARS-CoV-2, the incidence of thrombosis of the portal system in patients with COVID-19 varies according to the literature from 45.3% [1] to 70.7% [4] (Fig. 2). There is evidence that SARS-CoV-2 stimulates a prothrombotic state due to increased clotting, decreased fibrinolysis, and immunological effects. The endothelial damage and disruption of intercellular junctions caused by COVID-19 expose the subendothelial matrix containing tissue factor (TF) and collagen. This activates the coagulation cascade and results in the generation of thrombin and the conversion of fibrinogen into fibrin which, together with platelet aggregates, form blood clots [1], [2], [3]. In addition to numerous microthrombi located in hepatic sinusoids, centrilobular vein, and portal vein being observed, as shown in Fig. 2, other vascular changes were also observed during autopsies of the organ of patients affected by COVID-19, such as obstruction of the venous outflow and portal vasculits [4].
In fact, during the SARS-CoV-2 infection process, studies show an intense inflammatory process in the portal and lobular regions [5], [6], [7], [8] (Fig. 2). It is important to point out that, in an acute injury, aggregates of macrophages and lymphocytes are observed in the hepatic lobe, as well as a chronic infiltrate of inflammatory cells close to the portal regions. A study carried out with 48 patients showed that 100% of the sample had signs of an inflammatory process, with 67% [5] having portal inflammation and 50% lobular inflammation [3].
Regarding cell proliferation, two findings were relevant in the literature. The first one concerns the proliferation of Kupffer cells, whose function is to resolve the inflammatory process and wound healing [1,6]. Such cells act in the mononuclear phagocytic system and are central to the hepatic and systemic response to pathogens [6]. In this context, regarding the changes triggered by the new coronavirus (SARS-CoV-2) in the liver, the result of a study with 11 autopsies showed that Kupffer cells were activated in all patients, having a nodular proliferation of 70% [7]. Furthermore, data from a systematic review and meta-analysis found that Kupffer cell hyperplasia was present in 13.5% of the reports, being the 5th most relevant histological finding [2]
Regarding the ability of SARS-CoV-2 to cause cholestasis the literature points out that in patients with COVID-19, histopathological signs that suggest cholestasis are frequently described, such as the proliferation of the bile ducts and intra-canalicular bile plugs [9,10] (Fig. 1). From the pathophysiological perspective of the infection, a biphasic pattern develops with an initial increase in transaminases, followed by an increase in hepatic cholestatic enzymes that can aggravate ductal injury and boost pro-inflammatory cholangiocellular growth factors. Histopathological findings of mild and focal intralobular cholestasis correspond to 38% of the cases (n=15/40), of which 10% had the canalicular phenomenon in Lagana et al research [9]. On the other hand, in a parallel study, 32% and 27% of the individuals developed medium and severe cholestasis, respectively, during hospitalization in the intensive care unit (n=9/40) [10].
Finally, it is important to emphasize that the quality of published studies still lacks greater and better theoretical support. Thus, there is weakness in the data presented. Therefore, the objective of this publication is to stimulate the discussion and carry out more robust research aiming to map the most relevant liver histopathological alterations caused by COVID-19.
Declaration of Competing Interest
The authors declare that they have no competing interests in paper “LIVER HISTOPATHOLOGICAL CHANGES AND COVID-19: WHAT DOES LITERATURE HAVE TO TELL US?” by Jorge Lucas de Sousa Moreira, Sarah Maria Bacurau Barbosa, Jacyanne Gino Vieira, Nicolly Castelo Branco Chaves and Jucier Gonçalves Júnior.
References
- 1.Moreira J., Barbosa S., Gonçalves Júnior J. Pathophysiology and molecular mechanisms of liver injury in severe forms of COVID-19: an integrative review. Clin Res Hepatol Gastroenterol. 2021;45 doi: 10.1016/j.clinre.2021.101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Díaz L., Idalsoaga F., Cannistra M., Candia R., Cabrera D., Barrera F., et al. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19: A systematic review and meta-analysis of autopsy data. World J Gastroenterol. 2020;26:7693–7706. doi: 10.3748/wjg.v26.i48.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonzogni A., Previtali G., Seghezzi M., Grazia Alessio M., Gianatti A., Licini L., et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lax S., Skok K., Zechner P., Kessler H., Kaufmann N., Koelblinger C., et al. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao C., Rapkiewicz A., Maghsoodi-Deerwester M., Gupta M., Cao W., Palaia T., et al. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID-19) Hum Pathol. 2021;109:59–68. doi: 10.1016/j.humpath.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirayama Y., Daniels N., Evans S., Clarke D., Purvis S., Oliver C., et al. High Prevalence of Pre-Existing Liver Abnormalities Identified Via Autopsies in COVID-19: identification of a New Silent Risk Factor? Diagnostics. 2021;11:1703. doi: 10.3390/diagnostics11091703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Xiao S. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92:1491–1494. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- 8.Lax S., Skok K., Zechner P., Kessler H., Kaufmann N., Koelblinger C., et al. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagana S., Kudose S., Iuga A., Lee M., Fazlollahi L., Remotti H., et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bütikofer S., Lenggenhager D., Wendel Garcia P., Maggio E., Haberecker M., Reiner C., et al. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41:2404–2417. doi: 10.1111/liv.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]