Abstract
Cannabis use is highly prevalent in patients with schizophrenia and worsens the course of the disorder. To understand how exposure to cannabis changes schizophrenia-related oscillatory disruptions, we investigated the impact of administering cannabis vapor containing either Δ9-tetrahydrocannabinol (THC) or balanced THC/cannabidiol (CBD) on oscillatory activity in the neonatal ventral hippocampal lesion (NVHL) rat model of schizophrenia. Male Sprague Dawley rats underwent lesion or sham surgeries on postnatal day 7. In adulthood, electrodes were implanted targeting the cingulate cortex (Cg), the prelimbic cortex (PrLC), the hippocampus (HIP), and the nucleus accumbens (NAc). Local field potential recordings were obtained after rats were administered either the “THC-only” cannabis vapor (8–18% THC/0% CBD) or the “Balanced THC:CBD” cannabis vapor (4–11% THC/8.5–15.5% CBD) in a cross-over design with a 2-week wash-out period between exposures. Compared to controls, NVHL rats had reduced baseline gamma power in the Cg, HIP, and NAc, and reduced HIP-Cg high-gamma coherence. THC-only vapor exposure broadly suppressed oscillatory power and coherence, even beyond the baseline reductions observed in NHVL rats. Balanced THC:CBD vapor, however, did not suppress oscillatory power and coherence, and in some instances enhanced power. For NVHL rats, THC-only vapor normalized the baseline HIP-Cg high-gamma coherence deficits. NHVL rats demonstrated a 20 ms delay in HIP theta to high-gamma phase coupling, which was not apparent in the PrLC and NAc after both exposures. In conclusion, cannabis vapor exposure has varying impacts on oscillatory activity in NVHL rats, and the relative composition of naturally occurring cannabinoids may contribute to this variability.
Keywords: cannabinoid, oscillations, preclinical, psychosis, electomes, oscillopathies
Introduction
Lifetime rates of cannabis use and cannabis use disorder in patients with schizophrenia are reported to be as high as 80% and 50%, respectively.1 Concerningly, this use is associated with worsened clinical outcomes for patients through symptom exacerbation, treatment noncompliance, as well as increased rates of hospitalization and relapse1–3; however, patients also report positive outcomes including enhanced socializing and reduced anxiety.4–6 The varying outcomes of cannabis use for patients with schizophrenia may reflect different methods of use, or the phytochemical diversity of the cannabis plant.7–9 With over 113 constituents, or “cannabinoids”, in the cannabis plant, their relative composition dictates the effects produced.10 Scientific investigation has mainly focused on two cannabinoids due to their abundance in the plant and relevance for biomedical research: Δ9-tetrahydrocannabinol (THC), the main psychoactive constituent that binds with endogenous cannabinoid type 1 receptors (CB1Rs) to produce the rewarding and psychotomimetic effects.10–13 Cannabidiol (CBD), which has some psychoactive effects, does not produce the psychotomimetic effects seen with THC.14,15 CBD also acts as a negative allosteric modulator of CB1Rs and may also act as a partial agonist of both the cannabinoid type 2 and dopamine type 2 receptors, with growing evidence that it may, at least partially, interact agonistically with serotonin 1A receptors.16–21 The independent effects of these cannabinoids differ from the combined effects, and these differential effects further vary by administration route.22–24 Interestingly, CBD often mitigates the psychoactive effects of THC and may have antipsychotic properties.25–28
Consistent with this, Wall et al.7 recently used magnetic resonance imaging (MRI) to measure functional connectivity changes in the default mode network (DMN, measured as increased functional connectivity of the posterior cingulate cortex), executive control network (ECN, negatively correlated with DMN activity and measured as reduced connectivity in the posterior cingulate cortex), and salience network (SAL, measured as increased connectivity within the anterior insula), after healthy subjects were administered either vaporized placebo cannabis, cannabis with THC (8 mg) and CBD (10 mg), or cannabis without CBD (THC: 8 mg). Cannabis vapor reduced DMN connectivity relative to placebo, while not significantly affecting ECN connectivity. Only vapor without CBD impacted SAL connectivity, reducing it relative to vapor with CBD. Reduced DMN activity was correlated with the self-reported psychoactive effects of THC whereas exposure to vapor with CBD had a restorative effect.7
Altered neural circuit activity is also apparent in mesocorticolimbic brain regions of rodents used to model schizophrenia and in experimentally naïve rats after cannabinoid exposure.29 Local field potential (LFP) recordings from Wistar rats selectively bred as a model of schizophrenia to exhibit maximal disruptions after post-weaning social isolation and subchronic ketamine administration (20 mg/kg, intraperitoneal [IP]), demonstrate enhanced upper theta (4–8 Hz) and beta (13–30 Hz) spectral power in the parietal cortex, as well as reduced delta (0.6–4 Hz) and high-gamma (71–100 Hz) power.30 In contrast, LFP recordings from rats used to model schizophrenia that underwent neonatal ventral hippocampal lesions (NVHL rats) demonstrate reduced evoked delta, theta, and beta power31 compared to controls, as well as reduced theta (5–15 Hz) and beta (20–30 Hz) coherence in the hippocampus (HIP), with intact coherence in the medial prefrontal cortex (PFC).32 Additionally, NVHL rats do not exhibit stereotypical increases in phase-locking of auditory evoked potentials to increasing stimulus frequencies, measured in the temporal cortex.33 Importantly, the dysfunctions modeled in NVHL rats are not singularly due to the lesion but are instead the result of the neurodevelopmental insult produced by the lesion. Compared to controls, NVHL rats demonstrate impaired working memory measured using a T-maze decision making task, whereas adult rats that underwent ventral hippocampal lesions demonstrate intact working memory.34 NVHL rats also demonstrate enhanced neuronal activity in striatal and cortical regions, measured as enhanced ΔFosB expression compared to controls, while this difference was absent in rats lesioned in early adulthood.35 NVHL rats also demonstrate enhanced firing of medial PFC pyramidal neurons in response to electrical stimulation of the ventral tegmental area (VTA), an effect that is absent in rats lesioned in adulthood.36
Similar to the observations from rodents used to model schizophrenia, we previously published data demonstrating acute THC (10 mg/kg) vapor exposure in experimentally naïve rats suppresses LFP gamma (30–100 Hz) power and coherence in the dorsal striatum, the PFC, and the orbitofrontal cortex.37 LFP recordings from the HIP and entorhinal cortex of experimentally naïve rats administered CB1R agonist CP-55,940 (0.3 mg/kg, IP) also demonstrate reduced evoked theta (4–7 Hz) and gamma (30–80 Hz) power, which was corroborated in healthy humans administered THC (0.035 mg/kg, IV) using whole-brain electroencephalography (EEG); human subjects were also administered CBD (5 mg, IV), either alone or combined with THC, and while the combined treatment produced similar effects to THC alone, CBD alone had no effects on brain activity.38
Considering the clinical and preclinical evidence of altered neural circuit activity associated with cannabinoid exposure and the symptoms of schizophrenia, as well as reports of the oppositional effects between THC and CBD on neural circuit activity, we herein aimed to (1) assess baseline differences in corticolimbic LFPs between NVHL rats and sham-surgery (sham) controls, and (2) assess LFP changes in NVHL and sham rats resulting from cannabis vapor exposure, with and without CBD. We hypothesized that NVHL rats demonstrate reduced corticolimbic oscillatory activity, reflecting observations from preclinical and clinical studies of schizophrenia. We further hypothesized that exposure to cannabis vapor without CBD will worsen oscillatory dysfunctions in NVHL rats compared to sham rats, while exposure to cannabis vapor with CBD may ameliorate some of the lesion-induced deficits. We targeted the prelimbic cortex (PrLC), the cingulate cortex (Cg), the HIP, and the nucleus accumbens (NAc) due to their involvement in mesocorticolimbic signaling pathways, drug-seeking behavior, and the pathophysiology of schizophrenia. To maintain translational relevance, we administered cannabinoids as they naturally exist in the cannabis plant, by exposing NVHL and sham rats to vaporized cannabis flower (i.e., dried plant matter) using a consumer-grade cannabis vaporizer.
Methods
Animals
Ten male Sprague Dawley rat pups with lactating dam were purchased from Charles River (Senneville, Quebec, Canada). Rats were housed in a colony room maintained on a 12-hour light:dark cycle with ad libitum access to food and water. Prior to the start of experiments, rats were habituated to the experimental environment for 2 min daily and for a total of 5 days. All treatments were performed during the light phase of the 12-hour reverse light:dark cycle. All procedures complied with the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care, 1993) and set by the Animal Care Committee at the University of Guelph.
The Neonatal Ventral Hippocampal Lesion Rat Model of Schizophrenia
The NVHL rat was produced as previously described.39,40 Considered a valuable heuristic model of schizophrenia and co-occurring substance use,41 including in the context of cannabis use and schizophrenia,42,43 this neurodevelopmental model involves bilaterally lesioning the ventral hippocampi of rat pups using ibotenic acid. This model has (a) face validity, in that it produces behavioral and neural circuit changes reflecting those observed in schizophrenia, on a similar neurodevelopmental timeline; (b) construct validity, as it is based on the neurodevelopmental hypothesis of schizophrenia and involves an early neurodevelopmental insult to the ventral hippocampus, consistent with anterior hippocampal dysfunctions in patients; and (c) predictive validity, in that NVHL rats respond to antipsychotics in a manner reflective of outcomes observed in humans with schizophrenia.39,44 On postnatal day 7 (PND7), rat pups were anesthetized via hypothermia, injected with either 0.3 μL of ibotenic acid (Sigma–Aldrich, Oakville, Ontario, Canada; for NVHL rats) or artificial cerebrospinal fluid (aCSF, for sham rats) into the ventral hippocampus (anterior–posterior [AP]: −3 mm; medial-lateral [ML]: ± 3.5 mm; dorsal-ventral [DV]: −5 mm) at a rate of 0.1 μL/min over 3 min, followed by a two-minute wait period. Animals were housed with dam in polyethylene cages (Allentown, Allentown, NJ, USA) until weaning, and pair-housed until the electrode implantation surgeries.
Electrode Implantation Surgeries
Electrode implantation was performed as previously described.45 Custom-built, 10-channel electrode microarrays were constructed using Delrin templates46,47 and polyimide-insulated stainless-steel wires (791600, 0.008”, A-M Systems, Carlsborg, Washington, USA) attached to a single-row connector. All arrays had an electrode impedance of less than 2 MΩ. Rats were anesthetized with isoflurane (5%), affixed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA), and electrode arrays were implanted bilaterally into the prelimbic cortex (PrLC; AP: +3.24 mm, ML: ±0.6 mm, DV: −3.8 mm), the cingulate cortex (Cg; AP: +1.9 mm, ML: ±0.5 mm, DV: −2.8 mm), the CA1 region of the hippocampus (HIP; AP: −3.5 mm, ML: ±2.5 mm, DV: −2.6 mm), and the nucleus accumbens (NAc; AP: +1.9 mm, ML: ±1.2 mm, DV: −6.6 mm). Animals recovered in their home cage for a minimum of 7 days prior to experimentation.
Vaporized THC/CBD Administration
Cannabis flower vapor administration was performed using our customized OpenVape apparatus with a Utillian 420 (Utillian, Toronto, Ontario, Canada) dried herb vaporizer.48–50 A randomized, within-study crossover design was used as performed in human subjects and in our previous study with experimentally naïve rats22,37 (figure 1A). Rats were randomly assigned to two groups (N = 5/group) for exposure to either cannabis vapor with 8%–18% THC and 0% CBD (Summer Fling, Aurora, Edmonton, Alberta, Canada; “THC-only” exposure group) or 4%–11% THC and 8.5%–15.5% CBD (Balance, Solei, Leamington, Ontario, Canada; “Balanced THC:CBD” exposure group). Ground cannabis flower was vaporized at 200°C and channeled into a mouse cage (40 × 20 × 20 cm) holding two rats at a time. For each minute of the first 5 min the rats were in the chamber, 10 puffs were delivered for 2 s total with a four-second interpuff interval. Rats remained in the chamber for another 5 min such that the duration of exposure was 10 min total. The chamber was vented with clean air by removal of the cage lid. All vapor administration procedures occurred under a fume hood to remove any side-stream vapor.
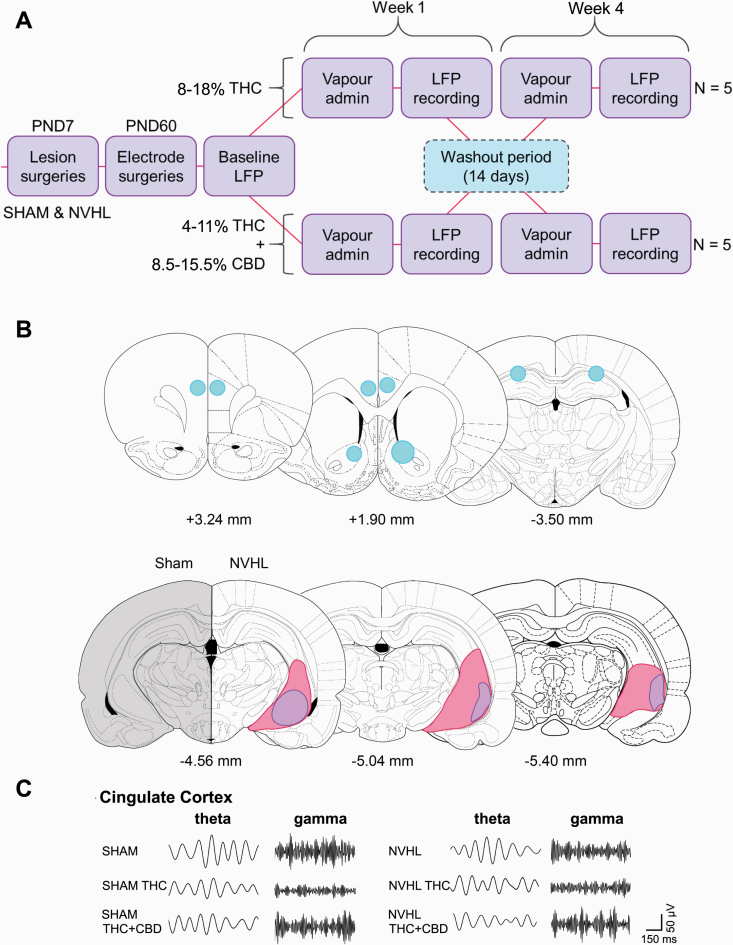
Fig. 1.
Experimental design, lesion and electrode verification, and representative local field potential tracing. A) Cross-over design with a two-week wash-out period between exposures B) Electrode placements (top) and lesion (bottom) showing electrode termini (blue) in the PrLC (left), Cg + NAc (middle), and HIP (right), as well as lesion extent in all NVHL rats (purple represents size of smallest and pink represents size of largest lesions), compared to sham controls. C) Representative tracings from the Cg showing changes in theta and gamma frequencies at baseline and after cannabis vapor exposure for both sham and NVHL rats. Figures were made using the brain schematics available from Paxinos and Watson (2007).51
Electrophysiology
LFP recordings (W2100-system, Multichannel Systems, Kusterdingen, Germany) were performed in awake, freely moving rats exploring clear plexiglass boxes (45 cm × 45 cm × 45 cm). Recordings were taken from five animals with two electrodes/animal/region resulted in a final sample size of 10. Baseline recordings were collected 24 h before the first vapor exposure, and immediately before each cannabis vapor exposure, for 30 min at a rate of 1000 samples/s. MATLAB (MathWorks, Natick, MA, USA) routines using the Chronux software package were used to analyze the spectral power of each brain region, as well as coherence and theta–gamma cross-correlation between brain regions. Five-minute epochs were used, with epochs segmented, detrended, denoised, and low-pass filtered to remove frequencies greater than 100 Hz. Continuous multitaper spectral power for data normalized to total spectral power and coherence (tapers = [5 9]) was calculated for delta (1–4 Hz), theta (>4–12 Hz), beta (>12–30 Hz), low gamma (>30–60 Hz), and high gamma (>60–100 Hz).
Histology
After experimentation, rats were euthanized by carbon dioxide + isoflurane before their brains were extracted and flash frozen. Brains were subsequently sectioned (at 40 μm) using a cryostat, mounted on slides, and stained with thionin. The Cg, PrLC, HIP, and NAc were examined microscopically to confirm electrode placement and bilateral lesioning. Rats with unilateral or extrahippocampal lesions or misplace electrodes would be removed from the analyses.
Data Analysis
Analysis was performed on one-minute time bins with data curves presented as normalized data with jack-knife estimates of standard error of the mean (SEM). Data were log-transformed to better exhibit group differences. Prior to all analyses, normality was assessed using the Shapiro–Wilk test. Sham or NVHL group comparisons were performed using a repeated measures ANOVA with treatment as the within-subjects factor, followed by paired t-tests. A Student’s t-test was used to compare NVHL exposure groups to baseline sham data. Computations were performed using IBM SPSS 24 software and are expressed as means ± SEM or percent change from sham baseline ± SEM.
Results
Lesions and electrode placements were verified microscopically in NVHL and sham rats. All NVHL rats showed bilateral lesions of the ventral hippocampus and the electrodes were verified to be in the correct locations (figure 1B). No animals were removed from the study nor were the electrodes ever inactive. Representative traces of theta and gamma oscillations obtained from the Cg are shown in figure 1C.
To confirm that our study was appropriately powered, we evaluated effect sizes for high gamma power and coherence in each brain region, where we saw the largest variations in the data. We had large effect sizes (>1.1, N = 8–10, power = 0.95) that were associated with a minimum 25% change in power/coherence.
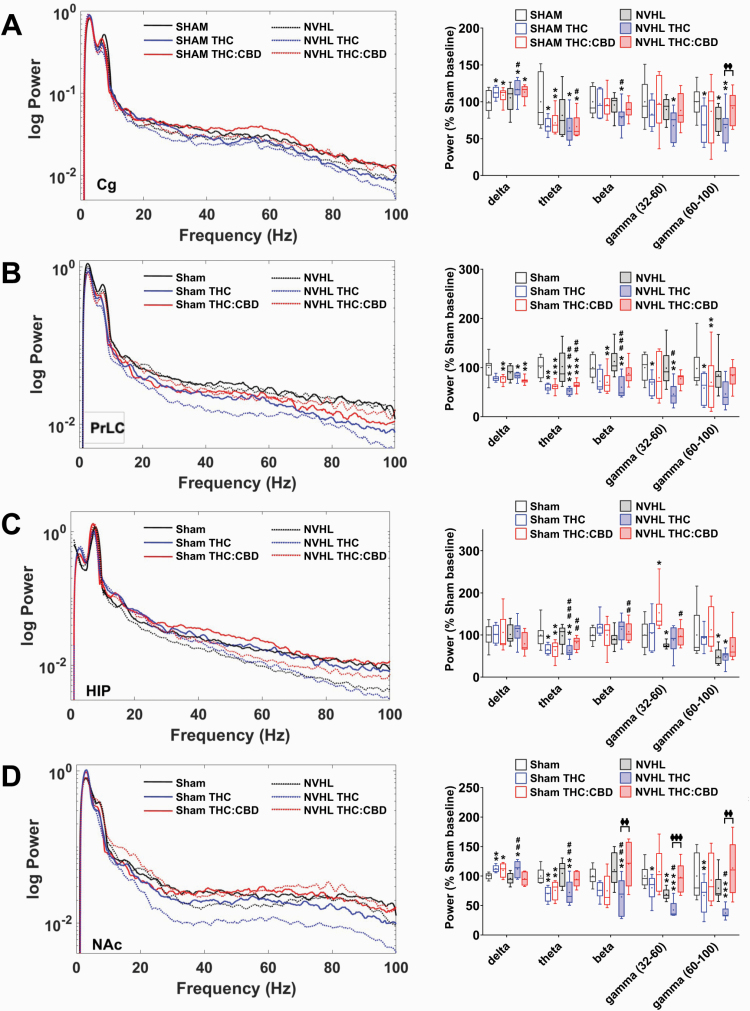
Baseline gamma deficits in spectral power exist in the Cg, HIP, and NAc of NVHL rats, when compared to sham rats, and these deficits are selectively modified by cannabis vapor exposure (figure 2). In the Cg, the only baseline deficit apparent between NVHL and sham rats was with high-gamma power (P = .021, figure 2A). Balanced THC:CBD vapor exposure attenuated this high-gamma baseline deficit in NVHL rats to levels above baseline (figure 2A). THC-only vapor exposure reduced Cg beta (P = .001) and low-gamma power (P = .019) only in NVHL rats, as well as high-gamma power in the sham rats (P = .016), effects that were no longer apparent after Balanced THC:CBD vapor exposure. Both exposures enhanced delta power and reduced theta power in the Cg of all rats (sham delta: F[2,14] = 6.7, P = .009, theta: F[2,14] = 9.2, P = .003; NVHL delta: F[2,14] = 3.5, P = 0.059, theta: F[2,14] = 7.5, P = .008; figure 2A).
Fig. 2.
NVHL rats exhibit reduced baseline spectral power compared to sham rats, and cannabis vapor differentially modulates this dysfunction. A) Spectral power tracing (left) and quantification (right) in the Cg showing baseline deficits in high-gamma power for NVHL rats (N = 5 rats, 10 recordings per region) compared to sham rats (N = 5 rats, 10 recordings per region); THC-only vapor reduced Cg beta and low-gamma power only in NVHL rats, an effect that was no longer apparent after Balanced THC:CBD vapor exposure; Balanced THC:CBD vapor exposure also attenuated the high-gamma baseline deficit in NVHL rats; both exposures enhanced delta, and reduced theta, power in the Cg of all rats; B) In NVHL rats, THC-only vapor exposure suppressed PrLC beta power while the THC:CBD-vapor exposure did not; for all rats, both exposures reduced theta power, and delta power to a lesser degree, while THC-only vapor exposure reduced both low- and high-gamma power; Balanced THC:CBD vapor exposure did not have any effect; C) In the HIP, NVHL rats showed baseline deficits in low- and high-gamma power, compared to sham rats whereas Balanced THC:CBD vapor exposure appeared to marginally improve baseline deficits; both exposures did not affect delta power and robustly suppressed theta power in NHVL and sham rats. D) In the NAc, NVHL rats showed baseline deficits in low-gamma power, compared to sham controls; THC-only vapor exposure reduced spectral power across all other frequencies in both NVHL and sham rats; THC:CBD exposure did not change from baseline measures any frequencies for NVHL rats, and selectively changed the gamma frequency band for sham rats. *P < .05; **P < .01 compared to sham control baseline; ##P < .05 compared to NVHL rat baseline; φP < .05 compared THC-only treated rats to THC:CBD treated rats.
In the PrLC, baseline power was similar between NVHL and sham rats across all frequency bands (figure 2B). For NVHL rats, THC-only vapor exposure suppressed PrLC beta power while the Balanced THC:CBD vapor exposure did not have this effect (NVHL beta: F[2,14] = 8.9, P = .003). For all rats, both exposures reduced theta power, and delta power to a lesser degree (sham delta: F[2,14] = 5.6, P = .016, theta: F[2,14 = 32.3, P < .001; NVHL delta: F[2,14] = 3.3, P = .067, theta: F[2,14] = 20.6, P < .001), while THC-only vapor exposure reduced low-gamma power (sham low gamma: F[2,14] = 6.7, P = .009, high gamma: F[2,14] = 4.4, P = .032; NVHL low gamma: F[2,14] = 4.0, P = .042). Balanced THC:CBD vapor exposure did not have any effect.
In the HIP of NVHL rats, baseline deficits in low-gamma (P = .044) and high-gamma (P = .047) power were observed, compared to sham controls (figure 2C). These significant differences between sham and NVHL rats were no longer present after the Balanced THC:CBD exposure and the THC-only vapor exposure did not produce any differences from baseline for either NVHL or sham rats. Similarly, both exposures did not affect delta power in NHVL or sham rats. Both exposures, however, robustly suppressed theta power in all rats (sham theta: F[2,14] = 32.3, P < .001; NVHL theta: F[2,14] = 20.6, P < .001).
In the NAc, NVHL rats had baseline deficits in low-gamma power, compared to sham controls (P = .001, figure 2D). THC-only vapor exposure increased NAc delta power for all rats while the Balanced THC:CBD exposure enhanced delta power for sham rats only (sham delta: F[2,14] = 4.7, P = .027; NVHL delta: F[2,14] = 6.6, P = .010). Notably, THC-only vapor exposure reduced spectral power across all other frequencies in both NVHL and sham rats, while the Balanced THC:CBD exposure did not produce any significant change from baseline for NVHL rats across all frequencies, or for sham rats in the gamma frequency band; this exposure; however, reduced theta power in sham rats (sham theta: F[2,14] = 13.2, P = .001; low gamma: F[2,14] = 4.8, P = .026; high gamma: F[2,14] = 9.9, P = .002; NVHL theta: F[2,14] = 7.8, P = 0.005; beta: F[2,14] = 10.3, P = .002; low gamma: F[2,14] = 8.0, P = .005; high gamma: F[2,14] = 15.6, P = .001).
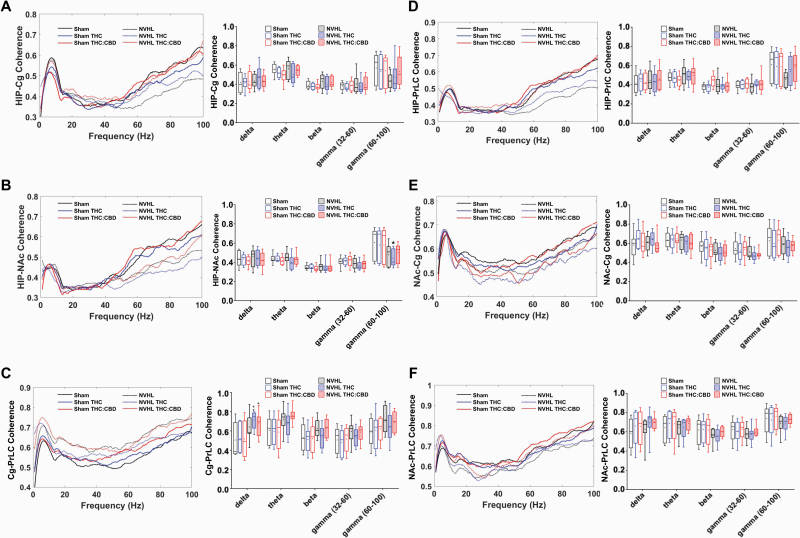
Assessing oscillatory coherence between target brain regions revealed that baseline differences exist between NVHL and sham rats and are modifiable by cannabinoid exposure (figure 3). Compared to sham rats, NVHL rats exhibited reduced high-gamma power between the HIP-Cg (P = .041, figure 3A). THC-only exposure reduced HIP-NAc high-gamma coherence in NVHL rats (P = .018, figure 3B). Both cannabis vapor exposures enhanced Cg-PrLC delta coherence in NVHL rats to levels above sham baseline coherence (THC: P = .005; THC:CBD: P = 0.039; figure 3C). Cannabis vapor exposure did not alter HIP-PrLC (figure 3D), NAc-Cg (figure 3E), or NAc-PrLC (figure 3F) coherence.
Fig. 3.
NVHL rats exhibit reduced baseline coherence within and between cortical and limbic regions, with variable impacts of cannabis vapor. A) Both vapor exposures enhanced HIP-Cg high-gamma coherence in the NVHL rats (N = 5 rats, 10 recordings per region) to levels that were no longer different from sham (N = 5 rats, 10 recordings per region) baseline coherence; B) THC-only vapor exposure reduced HIP-NAc high-gamma coherence in NVHL rats; C) Both types of cannabis-vapor exposures enhanced Cg-PrLC delta coherence in NVHL rats to levels above baseline coherence in sham rats; Cannabis vapor exposure did not alter D) HIP-PrLC, E) NAc-Cg, or F) NAc-PrLC coherence. *P < .05; **P < .01 compared to sham control baseline.
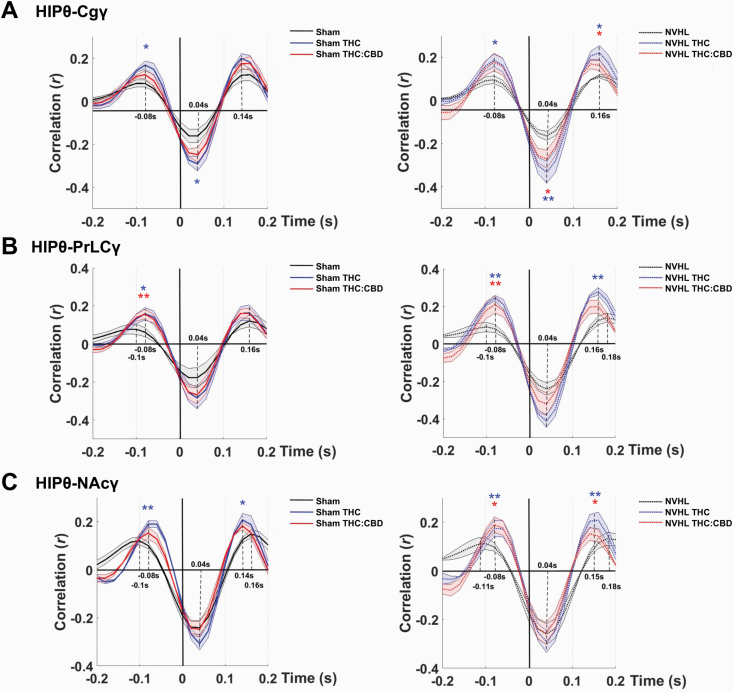
Cross-correlation analysis revealed baseline deficits in NVHL rats of phase coupling between HIP theta and gamma in the Cg, PrLC, or NAc that are sensitive to the effects of cannabis vapor exposure (figure 4). Comparing theta-gamma coupling strength between NVHL and sham rats revealed similar baseline values for all region-pair comparisons; however, compared to sham rats, NVHL rats exhibited a 20 ms delay in coupling of HIP theta to Cg, PrLC, and NAc high-gamma. This delay was not apparent in the PrLC and NAc region-pairs after both vapor exposures (figure 4B,C) but was observed in the Cg (figure 4A). For all rats, the THC-only vapor exposure also enhanced the HIP theta-gamma coupling strength between all regions. This effect was also observed to a lesser extent after the Balanced THC:CBD vapor exposure.
Fig. 4.
NVHL rats exhibit baseline deficits in phase coupling between HIP theta and Cg, PrLC, or NAc gamma, and cannabis vapor exposure selectively alters this delay. A–C) NVHL rats (N = 5 rats, 10 recordings per region) exhibited a 20 ms delay in coupling of HIP theta to high-gamma in the Cg, PrLC, and NAc; both vapor exposures reversed this delay in the B) PrLC and C) NAc, but not in the A) Cg; For all rats, the THC-only vapor exposure also enhanced the HIP theta–gamma coupling strength in all regions, an effect that was also observed to a lesser extent after Balanced THC:CBD vapor exposure; NVHL rats exhibited baseline theta–gamma coupling strength similar to sham rats (N = 5 rats, 10 recordings per region), for all pairwise comparisons. *P < .05; **P < .01 compared to sham control baseline. θ = theta frequency band; γ = gamma frequency band.
Discussion
This study assessed differences in baseline oscillatory activity between the NVHL rat model of schizophrenia and sham animals, as well as changes in oscillatory activity after exposure to THC-containing cannabis vapor with or without CBD. Our results (summarized in figure 5) demonstrate that NVHL rats have reduced baseline gamma power and coherence compared to sham-surgery controls, mirroring observations from clinical studies in patients with schizophrenia. Interestingly, baseline measures of delta, theta, and beta power from NVHL rats were not different from sham rats. This contradicts existing preclinical evidence of reduced theta (5–15 Hz) and beta (20–30 Hz) coherence in the HIP of NVHL rats, along with intact PFC coherence (which was corroborated in this study), as well as reduced theta (4–12 Hz) power in the PrLC and HIP of DISC1 knock-out mice or the enhanced theta and beta power in the parietal cortex of selectively bred Wistar rats that was reported above.30,32,52 Moreover, whole-brain EEG recordings of cortical oscillatory activity demonstrate that delta, theta, alpha, and beta power is enhanced in patients with schizophrenia compared to healthy controls, along with enhanced delta and alpha coherence between various brain regions.53–55 Given that alterations observed in NVHL rats are not due directly to the lesion but are instead a result of the neurodevelopmental insult during the neonatal period,34–36 it could be that the contradictory results are caused by different neurodevelopmental trajectories influenced by varying genetic and environmental factors. The discordance between the present study and existing preclinical studies may also be due to the use of different rodent strains to produce these models (Long Evans rats, C57BL/6J mice, and Wistar rats, respectively, in the papers cited above), while the variance between clinical and preclinical studies may be explained by the inherent differences in oscillatory activity that exist between rodents and humans.56 Head-to-head studies are required to completely characterize the strain- and species-related differences.
Fig. 5.
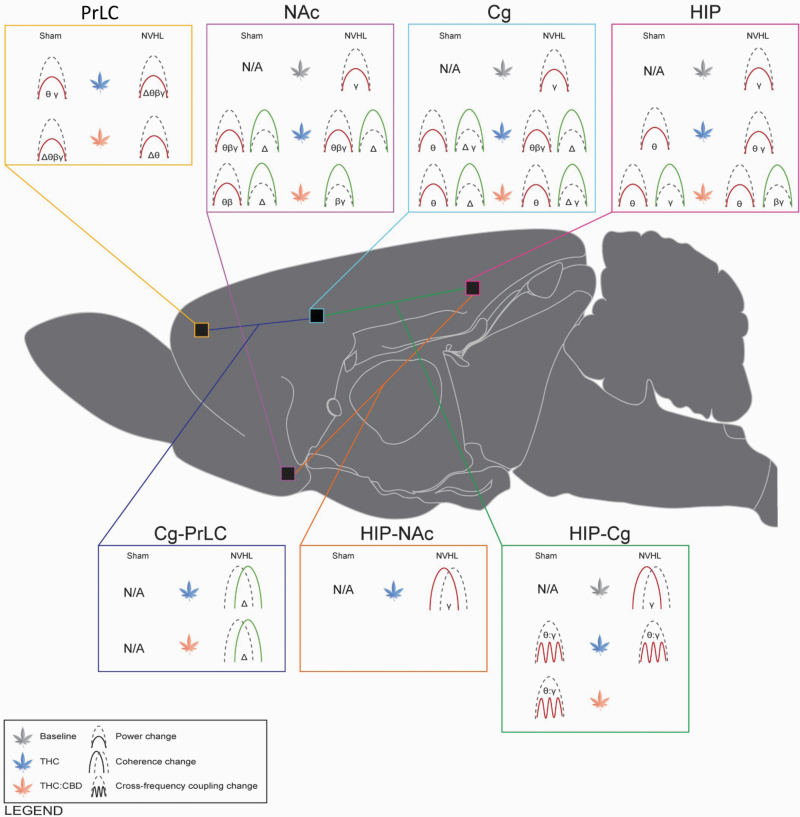
Graphical summary of power, coherence and phase coupling changes associated with NVHL lesions and cannabis vapor exposure. Cannabis vapor exposure produced constituent- and region-dependent disruptions in neural circuit oscillatory activity, often reducing theta, beta, and gamma power while enhancing delta power. Cannabis vapor exposure also enhanced coherence in cortical regions while reducing coherence and phase coupling between limbic regions. Cannabis vapor exposure further exacerbated baseline NAc, Cg, HIP gamma power deficits apparent in NVHL rats (N = 5 rats, 10 recordings per region), compared to sham controls (N = 5 rats, 10 recordings per region). PrLC = prelimbic cortex; NAc = nucleus accumbens; Cg = cingulate cortex; HIP = hippocampus. Δ = delta frequency band; θ = theta frequency band; β = beta frequency band; γ = gamma frequency band. Green = increase; red = decrease. Icons represent comparisons to sham baseline values and thus “N/A” is used for baseline sham findings. Figure was made using the brain schematics available from Swanson (2018).67
For all rats in this study, the THC-only vapor exposure suppressed spectral power in all brain regions and across all frequencies except delta, while the Balanced THC:CBD vapor exposure reduced theta power in all brain regions (except the NAc for NVHL rats), delta power in the PrLC, and beta power in the NAc (selectively in sham rats). THC modulates brain signals and behavior in otherwise healthy subjects, and in patients with schizophrenia. When administered intravenously (IV) to healthy subjects, THC (2.5 mg, 5 mg) produces transient states of psychosis57–59 that dose-dependently (at 0.03 mg/kg, but not 0.015 mg/kg, IV) correlate with reduced coherence of gamma frequency oscillations (30–100 Hz).60 When THC (1.25 mg; IV) is administered to patients with schizophrenia, it differentially alters cortical oscillations, reducing slow-wave spectral power (below 27 Hz) and enhancing gamma frequency power (above 27 Hz),61 while also transiently exacerbating the positive, negative, and cognitive symptoms of schizophrenia including learning, memory, and perceptual deficits (2.5 mg, 5 mg; IV THC).9,62 This is contrasted by evidence that synthetic THC (dronabinol; 5 mg to 20 mg/day) reduces psychotic symptoms in patients.63–65 To further characterize the changes in neural circuit activity correlated with cannabis use and the symptoms of schizophrenia, we previously assessed DMN functional connectivity in patients with co-occurring schizophrenia and cannabis use disorder, compared to healthy controls, using MRI scans taken before and after subjects smoked cannabis (3.6% THC) or ingested THC (15 mg).66 Before cannabis exposure, patients exhibited DMN hyperconnectivity compared to controls, and this hyperconnectivity was correlated with positive symptom severity; patients also exhibited reduced anticorrelation between the DMN and ECN. Cannabis exposure reduced hyperconnectivity and increased anticorrelation. Working memory performance was also assessed and found to be positively correlated with the magnitude of anticorrelation in controls and patients that were administered cannabis.68
In NVHL rats, gamma power remained largely unchanged by the Balanced THC:CBD vapor exposure, although this exposure enhanced Cg and NAc gamma power in NVHL rats toward sham baseline levels, and enhanced Cg delta power for all rats. Interestingly, the enhancing or neutral effects of the Balanced THC:CBD vapor exposure was most pronounced in NVHL rats, compared to sham rats, which did not display the baseline deficits observed in the NVHL rats. After both exposures, Cg-PrLC delta coherence was enhanced for NVHL rats, whereas the THC-only exposure exacerbated HIP-NAc coherence. The baseline 20 ms-delay deficit in phase coupling observed in NVHL rats was also no longer apparent after both exposures, for all regions but the Cg. Taken together, while the exposure to THC-containing cannabis vapor without CBD broadly suppressed oscillatory power similarly for all rats, NVHL rats may be differentially susceptible to shifts in oscillatory power and coherence induced by THC-containing cannabis vapor exposure when CBD is present in the plant matter.
These data support past observations that CBD opposes some of the effects of THC. Intracranial infusion of THC (100 ng) directly into the ventral HIP (vHIP) of anesthetized male Sprague Dawley rats enhances downstream VTA neuronal firing and bursting rates, as well as beta and gamma power. Rats that were infused with CBD (100 ng) or co-infused with THC + CBD (100 ng each) demonstrated no difference in oscillatory power when compared to vehicle-treated controls.24 Phosphorylated ERK was up-regulated after THC administration and down-regulated after THC + CBD administration, whereas CBD alone did not change phosphorylated ERK expression. That said, more research is needed to fully characterize the causal mechanisms underlying this oppositional effect and the relative contribution of each cannabinoid (as well as other potential components (e.g., terpenes) of the cannabis plant that could have differed between our two cannabis types). Some variance may also arise from the use of different administration routes,69 as we chose a protocol with greater translational relevance (i.e., vapor inhalation rather than IV injection),69 or the doses administered, as previous research using the PCP rat model of schizophrenia demonstrates through single-unit recordings in the VTA, before and after two doses of THC (0.1 mg/kg and 1 mg/kg, IP), that baseline deficits in the number of active VTA neurons in PCP rats are reversed only by exposure to the low dose. Both the low and high dose decreased the number of active neurons in control rats.70 PCP rats administered THC (1 mg/kg, IP) also demonstrate dysfunctional PrLC activity, evidenced by an absence of the THC-induced disinhibition of neuronal firing observed in controls rats.71
Our results corroborate existing clinical observations of oral CBD (600 mg) normalizing mediotemporal, mediotemporal-striatal, and prefrontal cortical activity, measured using functional MRI in patients with schizophrenia; importantly, this normalization of brain activity corresponded with improvements in self-reported symptom severity,72 extending the growing body of literature on the antipsychotic potential of CBD.73 Preclinical evidence also demonstrates that antipsychotic administration normalizes aberrant PFC delta (1–4 Hz) and theta (4–12 Hz) power and coherence of the methylazoxymethanol rat model of schizophrenia.74 Considering this, we plan to investigate the electrophysiological changes produced after exposure to a “CBD-only” cannabis flower vapor (i.e., exposure to cannabis flower vapor containing a moderate to high amount of CBD and near-zero amounts of THC) in future studies. We expect that the CBD-only exposure will not reduce oscillatory power and coherence in NVHL and sham rats, while it may in some instances enhance activity; particularly for NVHL rats.
Relevant to this study, the oppositional effects of CBD on THC-induced changes are evident in our target brain regions (recently reviewed by Gunasekera et al.75). CB1R activation in the PrLC of rats via administration of CP-55,940 (0.7 mg/kg, IP) and agonist WIN55,212-2 (1.2 mg/kg, IP) alters local excitatory and inhibitory signaling76 while administration of THC (0.3–10 mg/kg, IP) and CBD (15, 30, or 60 nmol, intracranial infusion; 1 and 3 mg/kg, IP) attenuates schizophrenia-like behaviors, including fear memory and anxiety-related behaviors.77,78 Although preclinical studies of cannabinoid activity in the Cg remains focused on analgesia and pain mechanisms, evidence exists showing CB1R activation in the Cg via local injection of agonist arachidonyl-2-chloroethylamide (ACEA) impairs decision-making in rats, reducing motivation for seeking large rewards, measured using a T-maze decision-making task.79 In clinical studies, MRI assessments of Cg volume in patients with schizophrenia using or abstaining from cannabis reveals patients using cannabis have reduced grey-matter volume, compared to patients not using cannabis and to healthy controls.80 Furthermore, postmortem analysis of brain samples from patients with schizophrenia reveal increased CB1R binding in the anterior Cg,81 although this is contested.82 Numerous preclinical and clinical investigations implicate the HIP in the effects of cannabis and of schizophrenia, with reproduced evidence that CP-55,940 administration (0.3 mg/kg, IP; 0.1 mg/kg, IV) in rats reduces HIP theta (4–10 Hz) and gamma (30–90 Hz) oscillatory power.38,83 Furthermore, NVHL rats demonstrate shifts in baseline oscillatory coherence in favor of slow-wave frequencies, with reports of enhanced HIP theta (10 Hz) and reduced gamma (50 Hz) phase-locking in the HIP,33 mirroring clinical observations cited above61; however, reduced HIP theta (5–15 Hz) power in NVHL rats is also reported.32 Finally, preclinical evidence exists demonstrating rats used to model schizophrenia have aberrant, high frequency (>100 Hz) oscillations in this region, and this is mediated by the endocannabinoid system (ECS).84,85 Additionally, ECS modulation via CB1R antagonism with rimonabant (0.3 mg/kg, IV) reduces stereotypical enhancements of NAc gamma power (30–70 Hz) in a rat model of amphetamine-induced psychosis.86
Additionally, the differential sensitivity of patients with schizophrenia to cannabinoid exposure may be due in part to the involvement of the endocannabinoid system (ECS) in the etiology of the disorder. Patients with schizophrenia have altered ECS functioning, evidenced by elevated levels of the endocannabinoid anandamide and postmortem CB1R expression in patients.87,88 Exposure to exogenous cannabinoids may shift the homeostatic norm in patients such that it acutely restores system function in some regions to levels associated with typical functioning, while concurrently exacerbating dysfunctions in other regions. Importantly, our results posit a question of whether advising patients to consume cannabis with balanced THC:CBD levels could be a viable harm reduction strategy.
This study is limited in that the slightly lower (yet overlapping) THC concentration in the Balanced THC:CBD strain, as compared to THC-only strain, may have contributed to some of the differences between the two strains. Since the cannabis products on the market only provide an approximate range for the concentrations of THC and CBD, matching THC concentrations between strains proved difficult; thus, the exposures were matched by the total amount of cannabis flower that was vaporized. Similarly, the other constituents in the selected cannabis strains were not matched and could have contributed to the differential effects of the strains,89 where the differences between the THC-only and Balanced THC:CBD exposures may be related to the presence of another phytocannabinoid or terpene, and not purely due to the higher CBD concentration in the Balanced flower. Additionally, our selection of a within-subject design means that rats were exposed to cannabis vapor twice and there could be residual effects of prior exposure.37 However, for this study we selected a two-week washout period, and we did not observe any significant differences between the baseline recordings and recordings captured before the vapor exposure two weeks later, thus confirming that the washout period duration was sufficient. Lastly, only male rats were used in this study. Although cannabis use in schizophrenia is primarily observed in male patients,90 previous studies have suggested that considerable sex differences exist in the oscillatory biomarkers associated with psychopathology,45 warranting the assessment of sex as a biological variable in the measures assessed here in future studies.
Another limitation of this present study is the lack of behavioral investigations to associate with our measures of altered corticolimbic oscillations. Given the crossover design in the present study, we wanted to limit the impact of potential behavioral testing on subsequent circuit function assessment, and therefore we decided to focus our attention on characterizing the oscillatory changes induced in NVHL rats by cannabis exposure, prior to assessing behavioral changes. Future investigations will be performed using the same protocol to detect any changes in schizophrenia-like behaviors after acute and chronic cannabis vapor exposure, as past investigations indicate that THC-induced reductions in oscillatory activity relate to deficits in sensorimotor gating and working memory.38,91 We will also aim to identify which electrophysiological features are related to behavioral deficits.46 Patients with schizophrenia exhibit dysfunctional PFC gamma band activity during high and low cognitive demands that correlate with psychotic symptoms.92 Similarly, reduced evoked theta (3–7 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–100 Hz) power is associated with deficits in working memory.93 Reductions in evoked beta (20–30 Hz) and gamma (30–50 Hz) power are also associated with P50 gating deficits, a hallmark feature in EEG traces from patients with schizophrenia that relates to symptomatic sensory processing deficits.94 Finally, impaired theta–gamma phase coupling is apparent in patients with schizophrenia, compared to healthy controls, and is associated with working memory impairments.95 Interestingly, theta–gamma phase coupling was improved in patients with schizophrenia that were using cannabis in addition to nicotine, compared to patients only using nicotine.96 To conclude, the results of this study reveal that cannabis has a varying impact on oscillatory activity and the administration route, dose, and relative composition of natural or synthetic cannabinoids may be partly responsible for the varying cannabis-related outcomes reported in patients with schizophrenia.
Acknowledgments
The authors graciously acknowledge the contributions of M. Asfandyaar Talhat, Bryana Hallam, and Chuyun (Judy) Chen for their assistance with the execution of experiments. This work was supported by Canadian Institutes of Health Research (CIHR) Project Grant award to J.Y.K. (PJT-436591) and a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to M.L.P. (401359).
References
- 1.Volkow ND. Substance use disorders in schizophrenia–clinical implications of comorbidity. Schizophr Bull. 2009;35(3):469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.oti DJ, Kotov R, Guey LT, Bromet EJ. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drake RE, Brunette MF. Complications of severe mental illness related to alcohol and drug use disorders. Recent Developments in Alcoholism: The Consequences of Alcoholism Medical Neuropsychiatric Economic Cross-Cultural. Boston, MA: Springer US; 1998:285–299. [DOI] [PubMed] [Google Scholar]
- 4.Buadze A, Stohler R, Schulze B, Schaub M, Liebrenz M. Do patients think cannabis causes schizophrenia? — A qualitative study on the causal beliefs of cannabis using patients with schizophrenia. Harm Reduct J. 2010;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekker N, Linszen DH, De Haan L. Reasons for cannabis use and effects of cannabis use as reported by patients with psychotic disorders. Psychopathology. 2009;42(6):350–360. [DOI] [PubMed] [Google Scholar]
- 6.Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4(5):231–244. [DOI] [PubMed] [Google Scholar]
- 7.Wall MB, Pope R, Freeman TP, et al. . Dissociable effects of cannabis with and without cannabidiol on the human brain’s resting-state functional connectivity. J Psychopharmacol. 2019;33(7):822–830. [DOI] [PubMed] [Google Scholar]
- 8.Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, Vandrey R. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: a crossover trial. JAMA Netw Open 2018;1(7):e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza DC, Sewell RA, Ranganathan M. Cannabis and psychosis/schizophrenia: human studies. Eur Arch Psychiatry Clin Neurosci. 2009;259(7):413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin MR, Ali DW. Pharmacology of medical cannabis. Adv Exp Med Biol. 2019;1162:151–165. [DOI] [PubMed] [Google Scholar]
- 11.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86(8):1646– 1647. [Google Scholar]
- 12.Howlett AC, Barth F, Bonner TI, et al. . International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. [DOI] [PubMed] [Google Scholar]
- 13.Pertwee RG. The central neuropharmacology of psychotropic cannabinoids. Pharmacol Ther. 1988;36(2-3):189–261. [DOI] [PubMed] [Google Scholar]
- 14.Schier AR, Ribeiro NP, Silva AC, Hallak JE, Crippa JA, Nardi AE, Zuardi AW. Cannabidiol, a Cannabis sativa constituent, as an anxiolytic drug. Braz J Psychiatry 2012;34 Suppl 1:S104–110. [DOI] [PubMed] [Google Scholar]
- 15.De Gregorio D, McLaughlin RJ, Posa L, et al. . Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160(1):136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037–1043. [DOI] [PubMed] [Google Scholar]
- 20.Galaj E, Xi ZX. Possible receptor mechanisms underlying cannabidiol effects on addictive-like behaviors in experimental animals. Int J Mol Sci 2020;22(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rock EM, Bolognini D, Limebeer CL, et al. . Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 2012;165(8):2620–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan CJA, Freeman TP, Hindocha C, Schafer G, Gardner C, Curran HV. Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Transl. Psychiatry 2018;8(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohleder C, Pahlisch F, Graf R, Endepols H, Leweke FM. Different pharmaceutical preparations of Δ9-tetrahydrocannabinol differentially affect its behavioral effects in rats. Addict Biol. 2020;25(3):e12745. [DOI] [PubMed] [Google Scholar]
- 24.Hudson R, Renard J, Norris C, Rushlow WJ, Laviolette SR. Cannabidiol counteracts the psychotropic side-effects of δ-9-tetrahydrocannabinol in the ventral hippocampus through bidirectional control of ERK1-2 phosphorylation. J Neurosci. 2019;39(44):8762–8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solowij N, Broyd SJ, Beale C, et al. . Therapeutic effects of prolonged cannabidiol treatment on psychological symptoms and cognitive function in regular cannabis users: a pragmatic open-label clinical trial. Cannabis Cannabinoid Res. 2018;3(1):21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Taylor A, Wright S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry 2018;175(3):225–231. [DOI] [PubMed] [Google Scholar]
- 27.Osborne AL, Solowij N, Babic I, Lum JS, Huang X-F, Newell KA, Weston-Green K. Cannabidiol improves behavioural and neurochemical deficits in adult female offspring of the maternal immune activation (poly I:C) model of neurodevelopmental disorders. Brain Behav Immun. 2019;81:574–587. [DOI] [PubMed] [Google Scholar]
- 28.Kozela E, Krawczyk M, Kos T, Juknat A, Vogel Z, Popik P. Cannabidiol improves cognitive impairment and reverses cortical transcriptional changes induced by ketamine, in schizophrenia-like model in rats. Mol Neurobiol. 2019;57(3):1733–1747. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins BW, Khokhar JY. Cannabis use and mental illness: understanding circuit dysfunction through preclinical models. Front Psychiatry. 2021;12:597725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horvath G, Petrovszki Z, Kekesi G, et al. . Electrophysiological alterations in a complex rat model of schizophrenia. Behav Brain Res. 2016;307:65–72. [DOI] [PubMed] [Google Scholar]
- 31.Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30(50):17102–17110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H, Dvorak D, Fenton AA. Targeting neural synchrony deficits is sufficient to improve cognition in a schizophrenia-related neurodevelopmental model. Front Psychiatry. 2014;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vohs JL, Chambers RA, O’Donnell BF, Krishnan GP, Morzorati SL. Auditory steady state responses in a schizophrenia rat model probed by excitatory/inhibitory receptor manipulation. Int J Psychophysiol. 2012;86(2):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipska B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology 2002;27(1): 47–54. [DOI] [PubMed] [Google Scholar]
- 35.Powell KJ, Binder TL, Hori S, Nakabeppu Y, Weinberger DR, Lipska BK, Robertson GS. Neonatal ventral hippocampal lesions produce an elevation of ΔFosB-like protein(s) in the rodent neocortex. Neuropsychopharmacology 2006;31(4): 700–711. [DOI] [PubMed] [Google Scholar]
- 36.O’Donnell P. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cerebral Cortex 2002;12(9): 975–982. [DOI] [PubMed] [Google Scholar]
- 37.Nelong TF, Jenkins BW, Perreault ML, Khokhar JY. Extended attenuation of corticostriatal power and coherence after acute exposure to vapourized Δ9-tetrahydrocannabinol in rats. Can J Addic 2019;10(3):60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skosnik PD, Hajós M, Cortes-Briones JA, et al. . Cannabinoid receptor-mediated disruption of sensory gating and neural oscillations: a translational study in rats and humans. Neuropharmacology. 2018;135:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brady AM. The Neonatal Ventral Hippocampal Lesion (NVHL) Rodent Model of Schizophrenia. Curr Protoc Neurosci 2016;77(1):9 55 51–59 55 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khokhar JY, Todd TP. Behavioral predictors of alcohol drinking in a neurodevelopmental rat model of schizophrenia and co-occurring alcohol use disorder. Schizophr Res. 2018;194:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng E, McGirr A, Wong AH, Roder JC. Using rodents to model schizophrenia and substance use comorbidity. Neurosci Biobehav Rev. 2013;37(5):896–910. [DOI] [PubMed] [Google Scholar]
- 42.Gallo A, Bouchard C, Rompré PP. Animals with a schizophrenia-like phenotype are differentially sensitive to the motivational effects of cannabinoid agonists in conditioned place preference. Behav Brain Res. 2014;268:202–212. [DOI] [PubMed] [Google Scholar]
- 43.Gallo A, Bouchard C, Fortier E, Ducrot C, Rompré PP. Cannabinoids reward sensitivity in a neurodevelopmental animal model of schizophrenia: a brain stimulation reward study. Eur Neuropsychopharmacol. 2014;24(9):1534–1545. [DOI] [PubMed] [Google Scholar]
- 44.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204(2):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thériault RK, Manduca JD, Perreault ML. Sex differences in innate and adaptive neural oscillatory patterns link resilience and susceptibility to chronic stress in rats. J Psychiatry Neurosci. 2021;46(2):E258–E270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dwiel LL, Khokhar JY, Connerney MA, Green AI, Doucette WT. Finding the balance between model complexity and performance: Using ventral striatal oscillations to classify feeding behavior in rats. PLoS Comput Biol. 2019;15(4):e1006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doucette WT, Dwiel L, Boyce JE, Simon AA, Khokhar JY, Green AI. Machine learning based classification of deep brain stimulation outcomes in a rat model of binge eating using ventral striatal oscillations. Front Psychiatry. 2018;9:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE. A vapourized Delta(9)-tetrahydrocannabinol (Delta(9)-THC) delivery system part I: development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. J Pharmacol Toxicol Methods 2014;70(1):120–127. [DOI] [PubMed] [Google Scholar]
- 49.Manwell LA, Ford B, Matthews BA, Heipel H, Mallet PE. A vapourized Delta(9)-tetrahydrocannabinol (Delta(9)-THC) delivery system part II: comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J Pharmacol Toxicol Methods 2014;70(1):112–119. [DOI] [PubMed] [Google Scholar]
- 50.Frie JA, Underhill J, Zhao B, de Guglielmo G, Tyndale RF, Khokhar JY. OpenVape: an Open-Source E-Cigarette Vapour Exposure Device for Rodents. eNeuro 2020;7(5):ENEURO.0279-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. San Diego, CA: Academic Press; 2007. [Google Scholar]
- 52.Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464(7289):763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong LE, Summerfelt A, Mitchell BD, O’Donnell P, Thaker GK. A shared low-frequency oscillatory rhythm abnormality in resting and sensory gating in schizophrenia. Clin Neurophysiol. 2012;123(2):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kam JW, Bolbecker AR, O’Donnell BF, Hetrick WP, Brenner CA. Resting state EEG power and coherence abnormalities in bipolar disorder and schizophrenia. J Psychiatr Res. 2013;47(12):1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narayanan B, O’Neil K, Berwise C, et al. . Resting state electroencephalogram oscillatory abnormalities in schizophrenia and psychotic bipolar patients and their relatives from the bipolar and schizophrenia network on intermediate phenotypes study. Biol Psychiatry. 2014;76(6):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobs J. Hippocampal theta oscillations are slower in humans than in rodents: implications for models of spatial navigation and memory. Philos Trans R Soc Lond B Biol Sci. 2014;369(1635):20130304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Souza DC, Perry E, MacDougall L, et al. . The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558–1572. [DOI] [PubMed] [Google Scholar]
- 58.Morrison PD, Zois V, McKeown DA, Lee TD, Holt DW, Powell JF, Kapur S, Murray RM. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med 2009;39(10):1607–1616. [DOI] [PubMed] [Google Scholar]
- 59.Murray RM, Englund A, Abi-Dargham A, et al. . Cannabis-associated psychosis: Neural substrate and clinical impact. Neuropharmacology. 2017;124:89–104. [DOI] [PubMed] [Google Scholar]
- 60.Cortes-Briones J, Skosnik PD, Mathalon D, et al. . Delta9-THC disrupts gamma (gamma)-band neural oscillations in humans. Neuropsychopharmacology 2015;40(9): 2124–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nottage JF, Stone J, Murray RM, Sumich A, Bramon-Bosch E, Ffytche D, Morrison PD. Delta-9-tetrahydrocannabinol, neural oscillations above 20 Hz and induced acute psychosis. Psychopharmacology (Berl) 2015;232(3): 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D’Souza DC, Abi-Saab WM, Madonick S, et al. . Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57(6):594–608. [DOI] [PubMed] [Google Scholar]
- 63.Potvin S, Joyal CC, Pelletier J, Stip E. Contradictory cognitive capacities among substance-abusing patients with schizophrenia: a meta-analysis. Schizophr Res. 2008;100(1–3): 242–251. [DOI] [PubMed] [Google Scholar]
- 64.Schwarcz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255–258. [DOI] [PubMed] [Google Scholar]
- 65.Crippa JAS, Zuardi AW, Hallak JEC. Uso terapêutico dos canabinoides em psiquiatria. Revista Brasileira de Psiquiatria 2010;32(suppl 1):556–566. [PubMed] [Google Scholar]
- 66.Whitfield-Gabrieli S, Fischer AS, Henricks AM, Khokhar JY, Roth RM, Brunette MF, Green AI. Understanding marijuana’s effects on functional connectivity of the default mode network in patients with schizophrenia and co-occurring cannabis use disorder: A pilot investigation. Schizophr Res. 2018;194: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swanson LW. Brain maps 4.0-Structure of the rat brain: an open access atlas with global nervous system nomenclature ontology and flatmaps. J Comp Neurol 2018;526(6), 935–943. doi: 10.1002/cne.24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu C, Chang T, Du Y, Yu C, Tan X, Li X. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ Toxicol Pharmacol. 2019;70:103202. [DOI] [PubMed] [Google Scholar]
- 69.Moore CF, Stiltner JW, Davis CM, Weerts EM. Translational models of cannabinoid vapor exposure in laboratory animals. Behav Pharmacol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seillier A, Martinez AA, Giuffrida A. Differential effects of Δ9-tetrahydrocannabinol dosing on correlates of schizophrenia in the sub-chronic PCP rat model. PLOS One. 2020;15(3):e0230238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aguilar DD, Giuffrida A, Lodge DJ. THC and endocannabinoids differentially regulate neuronal activity in the prefrontal cortex and hippocampus in the subchronic PCP model of schizophrenia. J Psychopharmacol. 2016;30(2):169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Neill A, Wilson R, Blest-Hopley G, Annibale L, Colizzi M, Brammer M, Giampietro V, Bhattacharyya S. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020:1–11. [DOI] [PubMed] [Google Scholar]
- 73.Seillier A. The endocannabinoid system as a therapeutic target for schizophrenia: Failures and potentials. Neurosci Lett. 2021;759:136064. [DOI] [PubMed] [Google Scholar]
- 74.Foute Nelong T, Manduca JD, Zonneveld PM, Perreault ML. Asenapine maleate normalizes low frequency oscillatory deficits in a neurodevelopmental model of schizophrenia. Neurosci Lett. 2019;711:134404. [DOI] [PubMed] [Google Scholar]
- 75.Gunasekera B, Davies C, Martin-Santos R, Bhattacharyya S. The Yin and Yang of Cannabis: a systematic review of human neuroimaging evidence of the differential effects of Δ9-tetrahydrocannabinol and cannabidiol. Biol. Psychiatry: Cognit. Neurosci. Neuroimaging 2020;6(6):636–645. [DOI] [PubMed] [Google Scholar]
- 76.den Boon FS, Werkman TR, Schaafsma-Zhao Q, Houthuijs K, Vitalis T, Kruse CG, Wadman WJ, Chameau P. Activation of type-1 cannabinoid receptor shifts the balance between excitation and inhibition towards excitation in layer II/III pyramidal neurons of the rat prelimbic cortex. Pflugers Arch 2015;467(7):1551–1564. [DOI] [PubMed] [Google Scholar]
- 77.Fogaça MV, Reis FM, Campos AC, Guimarães FS. Effects of intra-prelimbic prefrontal cortex injection of cannabidiol on anxiety-like behavior: involvement of 5HT1A receptors and previous stressful experience. Eur Neuropsychopharmacol. 2014;24(3):410–419. [DOI] [PubMed] [Google Scholar]
- 78.Stern CAJ, Gazarini L, Vanvossen AC, Zuardi AW, Galve-Roperh I, Guimaraes FS, Takahashi RN, Bertoglio LJ. Δ9-Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol. 2015;25(6):958–965. [DOI] [PubMed] [Google Scholar]
- 79.Khani A, Kermani M, Hesam S, Haghparast A, Argandoña EG, Rainer G. Activation of cannabinoid system in anterior cingulate cortex and orbitofrontal cortex modulates cost-benefit decision making. Psychopharmacology 2015;232(12):2097–2112. [DOI] [PubMed] [Google Scholar]
- 80.Szeszko PR, Robinson DG, Sevy S, et al. . Anterior cingulate grey-matter deficits and cannabis use in first-episode schizophrenia. Br J Psychiatry. 2007;190:230–236. [DOI] [PubMed] [Google Scholar]
- 81.Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(2):355–360. [DOI] [PubMed] [Google Scholar]
- 82.Koethe D, Llenos IC, Dulay JR, Hoyer C, Torrey EF, Leweke FM, Weis S. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm (Vienna) 2007;114(8):1055–1063. [DOI] [PubMed] [Google Scholar]
- 83.Hajós M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biol Psychiatry. 2008;63(11):1075–1083. [DOI] [PubMed] [Google Scholar]
- 84.Hunt MJ, Falinska M, Kasicki S. Local injection of MK801 modifies oscillatory activity in the nucleus accumbens in awake rats. J Psychopharmacol. 2010;24(6):931–941. [DOI] [PubMed] [Google Scholar]
- 85.Goda SA, Olszewski M, Piasecka J, Rejniak K, Whittington MA, Kasicki S, Hunt MJ. Aberrant high frequency oscillations recorded in the rat nucleus accumbens in the methylazoxymethanol acetate neurodevelopmental model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2015;61:44–51. [DOI] [PubMed] [Google Scholar]
- 86.Morra JT, Glick SD, Cheer JF. Cannabinoid receptors mediate methamphetamine induction of high frequency gamma oscillations in the nucleus accumbens. Neuropharmacology. 2012;63(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leweke FM, Mueller JK, Lange B, Fritze S, Topor CE, Koethe D, Rohleder C. Role of the endocannabinoid system in the pathophysiology of schizophrenia: implications for pharmacological intervention. CNS Drugs 2018;32(7):605–619. [DOI] [PubMed] [Google Scholar]
- 88.Navarrete F, Garcia-Gutierrez MS, Jurado-Barba R, Rubio G, Gasparyan A, Austrich-Olivares A, Manzanares J. Endocannabinoid system components as potential biomarkers in psychiatry. Front Psychiatry 2020;11:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cabral GA, Rogers TJ, Lichtman AH. Turning over a new leaf: cannabinoid and endocannabinoid modulation of immune function. J Neuroimmune Pharmacol. 2015;10(2):193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lindamer LA, Bailey A, Hawthorne W, Folsom DP, Gilmer TP, Garcia P, Hough RL, Jeste DV. Gender differences in characteristics and service use of public mental health patients with schizophrenia. Psychiatr Serv 2003;54(10):1407–1409. [DOI] [PubMed] [Google Scholar]
- 91.O’Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol. 2006;20(5):611–621. [DOI] [PubMed] [Google Scholar]
- 92.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103(52):19878–19883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, Linden DE, Rodriguez E. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci 2009;29(30): 9481–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nguyen AT, Hetrick WP, O’Donnell BF, Brenner CA. Abnormal beta and gamma frequency neural oscillations mediate auditory sensory gating deficit in schizophrenia. J Psychiatr Res. 2020;124:13–21. [DOI] [PubMed] [Google Scholar]
- 95.Barr MS, Rajji TK, Zomorrodi R, Radhu N, George TP, Blumberger DM, Daskalakis ZJ. Impaired theta-gamma coupling during working memory performance in schizophrenia. Schizophr Res. 2017;189:104–110. [DOI] [PubMed] [Google Scholar]
- 96.Barr M, Zomorrodi R, Goodman M, et al. . Differential effects of cannabis versus tobacco on theta-gamma coupling in schizophrenia. Biol Psychiatry 2017;81(10):S274–S275. [Google Scholar]