Abstract
Background
hospital level healthcare in the home guided by comprehensive geriatric assessment (CGA) might provide a less costly alternative to hospitalisation for older people.
Objective
to determine the cost-effectiveness of CGA admission avoidance hospital at home (HAH) compared with hospital admission.
Design/intervention
a cost-effectiveness study alongside a randomised trial of CGA in an admission avoidance HAH setting, compared with admission to hospital.
Participants/setting
older people considered for a hospital admission in nine locations across the UK were randomised using a 2:1 randomisation schedule to admission avoidance HAH with CGA (N = 700), or admission to hospital with CGA when available (N = 355).
Measurements
quality adjusted life years, resource use and costs at baseline and 6 months; incremental cost-effectiveness ratios were calculated. The main analysis used complete cases.
Results
adjusting for baseline covariates, HAH was less costly than admission to hospital from a health and social care perspective (mean −£2,265, 95% CI: −4,279 to −252), and remained less costly with the addition of informal care costs (mean difference −£2,840, 95% CI: −5,495 to −185). There was no difference in quality adjusted survival. Using multiple imputation for missing data, the mean difference in health and social care costs widened to −£2,458 (95% CI: −4,977 to 61) and societal costs remained significantly lower (−£3,083, 95% CI: −5,880 to −287). There was little change to quality adjusted survival.
Conclusions
CGA HAH is a cost-effective alternative to admission to hospital for selected older people.
Keywords: cost-effectiveness, comprehensive geriatric assessment, admission avoidance hospital at home, older people
Key Points
Comprehensive geriatric assessment in an admission avoidance hospital at home can be a cost-effective alternative to hospital.
We found benefits in terms of fewer days in hospital and lower residential care costs.
Evidence of cost-effectiveness from a randomised trial that recruited over 1,000 older people.
Background
Combining comprehensive geriatric assessment (CGA) with admission avoidance hospital at home (HAH) could be a cost-effective solution to concerns about the suitability of a hospital environment for an older population, and relieve pressure on bed based hospital care [1–4]. Although the benefits of CGA guided hospital care are well established [5], evidence of the cost-effectiveness of implementing CGA in other healthcare settings and at different levels of intensity is mixed [6, 7]. Delivering healthcare to older people in the right place to optimise living at home has had variable success [4, 8, 9]. Multi-component community based interventions with an element of assessment can improve patient outcomes, depending on the healthcare services usually available [10].
Despite continued interest in the provision of urgent healthcare in the home as an alternative to hospitalisation, and a long standing expectation that this will improve patient health outcomes and reduce health service cost [11–13], there is limited evidence to support the cost-effectiveness of this approach [14, 15]. Wide scale implementation of such services has also been constrained by the practical difficulties of designing and delivering services that cut across primary and secondary care, might involve social care and require different workforce and funding arrangements [14, 16–20]. We aimed to strengthen the evidence base by conducting a cost-effectiveness analysis alongside a multi-site randomised trial of a CGA admission avoidance HAH service as an alternative to admission to hospital, to aid decision-making about investing in health services for older people.
Methods
Design1
This cost-effectiveness analysis was conducted within a multi-site open parallel participant randomised trial that used a 2:1 ratio (2 CGAHAH: 1 acute inpatient hospital care). The trial protocol [21] was approved by the England and Wales Research Ethics Committee [14/WA/1081] and Scotland REC [14/SS/1046], the information sheets and consent forms were approved by the Northern Ireland sub-committee of the Health and Social Care Board. The trial is registered with ISRCTN, number 60477865.
The University of Oxford was the Sponsor. We recruited participants between 14th March 2015 and 18th June 2018. We collected data on participant characteristics, health service use over the previous 6 months to adjust for differences in previous utilisation of health services, measures of outcome at baseline, quality of life measured by the EuroQol (EQ-5D-5L) at 6 months and clinical outcome data at one and 6 months (reported elsewhere) [22]. We included data on the resources used by those who died up to the follow-up time that preceded their death.
Setting and participants1
Trained research nurses, working with a consultant geriatrician at nine sites across the UK (see Appendix Table 1,Supplementary data are available in Age and Ageing online), identified potentially eligible participants who had been considered for an unplanned hospital admission and were referred to an admission avoidance CGAHAH service. Eligibility criteria were (i) aged 65 years and older; (ii) willing and able to give informed consent, or, if lacking capacity to consent, having a relative or friend who was the ‘personal consultee’ or an Independent Mental Capacity Advocate to advise on whether they believed participation would be in accordance with the values and interests of the individual and (iii) able and willing in the local principal investigator’s opinion to comply with the requirements of the research. The presence of a caregiver was not a requirement for enrolment. Participants were excluded if they had an acute coronary syndrome, suspected stroke, required acute surgical assessment, were receiving end of life care, refused admission to CGAHAH, were considered by the clinical staff to be too high risk for home-based care or were living in a residential care setting. The consent process took into account the Mental Capacity Act (2005) in England and Wales, the Mental Capacity Act (2016) in Northern Ireland and the Adults with Incapacity Act (2000) in Scotland.
Randomisation and intervention1
Eligible participants who provided informed consent were randomly allocated using a validated secure online system. Randomisation was stratified by site, gender and score (<3.5, ≥3.5) on the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) [23]. Patients were assessed in the community, using an acute frailty admission model, or in an acute admissions unit and transferred to the CGAHAH service (Appendix Figure A1, Supplementary data are available in Age and Ageing online). The CGAHAH services had access to social workers, homecare, district nursing, community rehabilitation, community mental health services and acute hospital services such as diagnostic tests and transfer to hospital. The core workforce usually included consultant geriatricians, junior doctors, nurse practitioners, health care assistants or support workers, physiotherapists, occupational therapists and community pharmacists. There were at least daily virtual ward rounds. We anticipated that ~80% of participants randomised to the control hospital group (i.e. admitted to hospital) would receive geriatrician-led care.
Costs and health outcomes
We estimated costs per participant from healthcare, social care and societal perspectives including the productivity loss of informal carers costed by the hour using the National Living Wage [24]. The costs of the intervention included length of stay in an acute assessment unit at the time of recruitment, and initial CGAHAH or hospital length of stay (duration of the intervention) immediately after randomisation. The cost per bed day of admission to CGAHAH was calculated by dividing each site’s annual total spent budget in 2017/18 for CGAHAH by the total number of bed days (i.e. number of patients multiplied by the average length of stay per patient) in the same year. CGAHAH budgets included staff costs, medicines, equipment, transport and overheads. The unit cost of hospital care was set at the weighted national average of non-elective short stay, non-elective long stay and elective admissions that were relevant to the trial population (e.g. excluding admissions to neonatal units) and was applied to all admissions over the 6 months follow-up (Appendix Table 2, Supplementary data are available in Age and Ageing online) [25]. We did not discount costs as the time horizon was from baseline to 6 months. Site research nurses completed a Health Resource Use questionnaire (HRU) and Case Report Form (CRF) with details of admissions to hospital, residential care and CGAHAH obtained from participants’ medical records. Participants and their caregivers provided an estimate of the number of consultations with primary care physicians, the amount of informal care, travel and loss of earnings. We checked adverse event data for hospital and residential care admissions, and extreme values against data sources (Appendix Table 3, Supplementary data are available in Age and Ageing online). Costs are reported in pounds sterling, in 2017/18 prices, inflated when necessary to 2017/2018 prices using a standard health care inflation index. The main health outcome was quality adjusted life years (QALYs) measured by the EQ-5D-5L at baseline and six months. We converted all EQ-5D-5L responses to utility values using an approved crosswalk algorithm [24] and combined these with survival data to calculate QALYs over the 6 months, estimated as the area under the curve per patient.
Cost-effectiveness analysis
We followed the National Institute for Health and Social Care Excellence (NICE) guidance for conducting a cost-effectiveness analysis alongside a randomised trial [24], and report the results according to the consolidated health economic evaluation reporting standards (CHEERS) statement [26]. The time-horizon of the analysis was 6 months. The main economic analysis was on complete cases, with participants analysed on an intention to treat basis. We used multilevel mixed-effects linear regression models to estimate differences in mean costs and QALYs (i.e. incremental costs and QALYs) between the two groups, after adjusting for baseline gender, known cognitive decline, baseline utilities and pre-randomisation costs and site as a random effect. We estimated the incremental cost-effectiveness ratio (ICER) as the difference in cost per QALY gained. We used non-parametric bootstrapping with replacement to assess uncertainty in the ICER, by estimating the mean costs and QALYs per group and their mean between-group differences in each of 5,000 bootstrapped samples, reporting 95% confidence intervals for costs and QALYs using the percentile method and plotting ICERs on cost-effectiveness planes. We generated cost-effectiveness acceptability curves (CEACs) to display the probability of CGAHAH being cost-effective at different levels of willingness-to-pay for an additional QALY.
Sensitivity analyses
We assessed the impact of missing cost and EQ-5D-5L utility data on the estimated ICERs, by rerunning the main analysis using mean imputation to impute missing baseline costs and utilities and multiple imputation with chained equations for missing costs and utilities at 6 months using baseline costs, utilities, gender and age [27, 28]. The multiple imputation process was partitioned by treatment group and 20 imputed datasets were generated, following standard practice that suggests generating a number of imputed datasets equal to the percentage of missingness [29]. The imputed datasets were used in the bootstrapping process, similar to the main analysis. All analyses were conducted using Stata, version 15. [30]
Role of the funding source
This research was supported by the National Institute of Health Research (grant number 12/209/66).
Results
Between 9th February 2015 and 18th June 2018, 4,805 potential participants were screened for eligibility, 2,169 (45%) were not eligible, 1581 (33%) were potentially eligible and did not participate in the study, and 700 were randomised to CGAHAH and 355 to hospital care using a 2:1 allocation ratio. The first participant was recruited on 14th March 2015. Twenty three participants were not included in the analysis due to withdrawing consent to use their data (N = 10), a deterioration in health that prevented data collection (N = 4), previously recruited (N = 4), lived outside the CGAHAH area (N = 1), <65 years (N = 1) or withdrew after randomisation with incomplete data (N = 3; see Figure 1) [22]. Thirty-seven participants allocated to CGAHAH were immediately admitted to hospital due to a further decline in health, and of those randomised to hospital 76/345 (22.0%) were instead admitted to CGAHAH due to participant preference for CGAHAH or a high rate of hospital bed occupancy diverted participants to CGAHAH1. For the main economic analysis, based on complete cases, we excluded 124 patients in the CGAHAH group and 71 patients in the control group with incomplete information on costs and/or EQ-5D-5L utilities.
Figure 1.
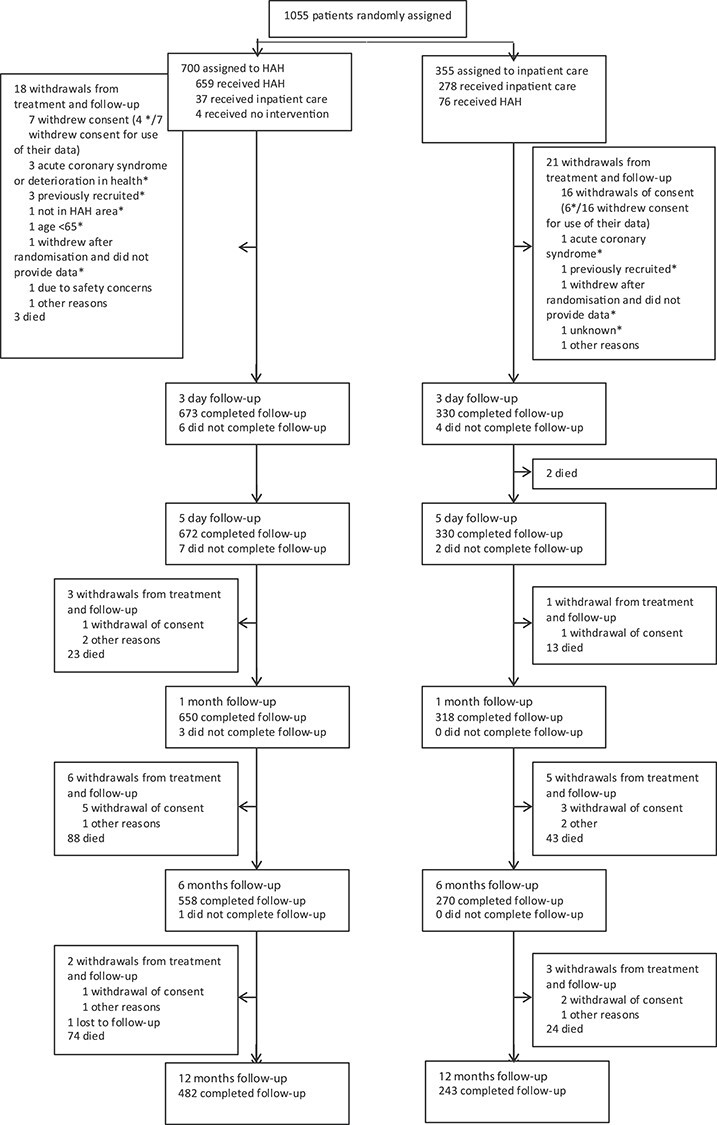
CONSORT flow diagram of trial participants.1
A higher proportion of complete cases compared with non-complete cases in the hospital group were female (Table 1). Most participants had seen their primary care physician in the 6 months prior to recruitment, and 41% (233/563) of the CGAHAH group and 49% (134/274) of the hospital group had at least one prior admission to hospital. Detailed numbers of patients with missing data are given in Appendix Table 4, Supplementary data are available in Age and Ageing online.
Table 1.
Baseline characteristics of complete and incomplete cases by treatment group
| CGAHAH | Hospital | |||
|---|---|---|---|---|
| Complete cases (n = 563) | Non-complete cases (n = 124) | Complete cases (n = 274) | Non-complete cases (n = 71) | |
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Age (years) | 83 (7) | 85 (6) | 83 (7) | 85 (7) |
| Female (%) | 60.04% | 63.71% | 62.41% | 52.11% |
| EQ-5D-5L utilities* | 0.531 (0.270) | 0.553 (0.304) | 0.528 (0.302) | 0.552 (0.294) |
| EQ-5D-5L visual analogue scale score** | 56.431 (21.270) | 57.026 (21.841) | 56.018 (22.158) | 53.831 (26.394) |
| Number of health problems recorded at baseline (derived from the Charlson co-morbidity score) | 1.721 (1.203) | 1.653 (1.275) | 1.588 (1.205) | 1.155 (0.905) |
| Prior health service use | ||||
| Number (%) who had attended A&E in the 6 months prior to recruitment | 91 (16%) | 7 (6%) | 43 (16%) | 9 (13%) |
| Number (%) who had an admission to hospital in the 6 months prior to recruitment | 233 (41%) | 60 (48%) | 134 (49%) | 29 (41%) |
| Number (%) who had an admission to short-term residential care in the 6 months prior to recruitment | 10 (2%) | 5 (4%) | 7 (3%) | 2 (3%) |
| Number (%) who had seen their primary care physician in the 6 months prior to recruitment*** | 499 (89%) | 100 (81%) | 247 (90%) | 52 (73%) |
*Out of the non-complete cases, 100 out of 124 patients had complete information for calculation of EQ-5D-5L utilities in the CGAHAH group and 56 out of 71 patients in the Hospital group.
**As the main economic evaluation only estimated complete cases, the number of patients for complete cases was 559 out of 563 patients in the CGAHAH group and 272 out of 274 patients in the Hospital group who had complete information for the calculation of EQ-5D-5L utility. Out of the incomplete cases, 115 out of 124 had complete information in the CGAHAH group and 59 out of 71 patients in the Hospital group for calculation of EQ-5D-5L utilities.
***This is the number for patients who had seen their primary care physician at the surgery, at home or by telephone.
Initial post-randomisation mean length of stay in the CGAHAH group was 7.17 (SD 5.62) days of CGAHAH care, and 1.43 (SD 4.84) days in hospital due to 29 participants allocated to CGAHAH crossing over to hospital treatment immediately after randomisation (Table 2). Patients randomised to hospital had a mean of 4.92 (SD 7.64) days in hospital, significantly less than the CGAHAH group (mean difference −3.49 days, 95% CI −4.35 to −2.64) and incurred an average of 3.84 (SD 7.12) days of CGAHAH due to 74 participants crossing over to CGAHAH immediately after randomisation. During the first month each group had additional days in CGAHAH (CGAHAH 0.17 [SD 1.64] versus hospital 0.28 [SD 1.39], mean difference −0.11, 95% CI −0.33 to 0.12) and in hospital (CGAHAH 2.20 [SD 5.62] versus hospital 1.66 [SD 5.41], mean difference 0.54, 95% CI −0.27 to 1.34). At 6 months follow-up, the mean total days in hospital had increased to 9.47 (SD 18.41) in the CGAHAH group and 10.58 (SD 19.49) in the hospital group, a non-significant mean difference of −1.12 days (95% CI: −3.83 to 1.59). There was no evidence of a difference at 6 months in subsequent CGAHAH length of stay (mean difference −0.12, 95% CI −0.61 to 0.37). Mean days in residential care at 6 months were 3.43 (SD 16.85) in the CGAHAH group and 6.14 (SD 25.59) in the hospital group (mean difference −2.71 days, 95% CI −5.6 to 0.21). Total hours of unpaid help at 6 months were 594.89 (SD 1093.63) hours in the CGAHAH group and 657.64 (SD 1170.87) hours in the hospital group (mean difference of −62.76 hours, 95% CI −224.61 to 99.09; Table 2).
Table 2.
Resource use by treatment group from baseline to 1 month follow-up and baseline to 6 month follow-up, complete cases
| Baseline to 1 month follow-up | Baseline to 6 month follow-up | |||||
|---|---|---|---|---|---|---|
| CGAHAH (N = 563) | Hospital (N = 274) | Difference in means | CGAHAH (N = 563) | Hospital (N = 274) | Difference in means | |
| Mean (SD) | Mean (SD) | Mean (SE) [95% CI] | Mean (SD) | Mean (SD) | Mean (SE) [95% CI] | |
| Health and social care | ||||||
| Intervention | ||||||
| Initial admissions (number of days)† | ||||||
| Hospital-at-home | 7.17 (5.62) | 3.84 (7.12) | 3.33 (0.45) [2.44, 4.22] | |||
| Hospital‡ | 1.43 (4.84) | 4.92 (7.64) | −3.49 (0.44) [−4.35, −2.64] | |||
| Subsequent admissions (number of days) | ||||||
| Hospital-at-home‡ | 0.17 (1.64) | 0.28 (1.39) | −0.11 (0.12) [−0.33, 0.12] | 0.69 (3.14) | 0.81 (3.90) | −0.12 (0.25) [−0.61, 0.37] |
| Hospital | 2.20 (5.62) | 1.66 (5.41) | 0.54 (0.41) [−0.27, 1.34] | |||
| Hospital admissions (number of days)* | 9.47 (18.41) | 10.58 (19.49) | −1.12 (1.38) [−3.83, 1.59] | |||
| Primary Care | 7.33 (13.53) | 6.05 (9.06) | 1.28 (0.90) [−0.49, 3.05] | |||
| Outpatient | 2.63 (4.01) | 2.97 (4.57) | −0.34 (0.31) [−0.95, 0.26] | |||
| Home care (number of times)** | 135.91 (306.75) | 117.29 (234.18) | 18.63 (20.99) [110.48, 149.15] | |||
| Residential Care (number of days)*** | 3.43 (16.85) | 6.14 (25.59) | −2.71 (1.48) [−5.62, 0.21] | |||
| Informal care | ||||||
| Total hours of unpaid help over last 6 months | 594.89 (1093.63) | 657.64 (1170.87) | −62.76 (82.46) [−224.61, 99.09] | |||
†Initial admissions include 74 patients allocated to the hospital group that switched group to CGAHAH and 29 patients allocated to CGAHAH that switched to hospital; the average CGAHAH initial length of stay in the hospital group of 3.8 days is allocated to the 74 patients but averaged over 274 patients in the hospital group.
‡The average hospital length of stay in the CGAHAH group of 1.4 days is allocated to the 29 patients who switched to hospital and average over 563 patients who were allocated to CGAHAH.
‡This estimate includes participants who received CGAHAH following discharge from hospital.
*In the analysis, we use hospital admissions at 6 months as this measure includes the initial and subsequent hospital admissions at 1 month and 6 months.
**This resource use is part of personal social services from the HRU questionnaire.
***Although the number of residential care days is averaged over the whole sample of patients in the CGAHAH and hospital group, this value is allocated to 37 patients in the CGAHAH group and 27 patients in the hospital group.
After adjusting for baseline covariates, the difference between the groups in health and social care costs at six months was −£2,265 (95% CI: −4,279 to −252), widening from the societal perspective to −£2,840 (95% CI: −5,495 to −185; Table 3). There were small non-significant differences in utility values and QALYs between the two groups (Table 4). Quality of life declined in both groups from baseline to 6 months, with equal proportions (15%) dying by 6 months follow-up. Combining these health outcomes to calculate quality adjusted survival produced no evidence of any difference in QALYs over the 6 months following randomisation.
Table 3.
Costs (in UK £s, 2017–18 prices) and health outcomes by group from baseline to 1 month follow-up and baseline to 6 month follow-up, complete cases
| Baseline to 1 month follow-up | Bassline to 6 month follow-up | |||||
|---|---|---|---|---|---|---|
| CGAHAH (N = 563) |
Hospital (N = 274) |
Difference in means | CGAHAH (N = 563) |
Hospital (N = 274) |
Difference in means | |
| Mean (SD) | Mean (SD) | Mean (SE) [95% CI] | Mean (SD) | Mean (SD) | Mean (SE) [95% CI] | |
| Health and social care | ||||||
| Intervention (initial admissions)† | ||||||
| Hospital-at-home | 764 (683) | 346 (644) | 418 (49) [ 321, 515] | |||
| Hospital | 978 (3,317) | 3,377 (317) | −2,399 (298) [−2,984, −1,814] | |||
| Total intervention cost | 1,742 (3,234) | 3,723 (5,095) | −1,981 (290) [−2,551, −1,411] | |||
| Subsequent admissions | ||||||
| Hospital-at-home | 27 (265) | 37 (224) | −10 (19) [−46, 27] | 99 (521) | 110 (498) | −11 (38) [−85, 63] |
| Hospital | 1,509 (3,854) | 1,141 (3,707) | 368 (280) [−182, 918] | |||
| Hospital admissions* | 6,492 (12,627) | 7,259 (13,370) | −767 (948) [−2,628, 1,094] | |||
| Primary care | 178 (255) | 168 (225) | 10 (18) [−25, 46] | |||
| Outpatient | 389 (586) | 438 (658) | −48 (45) [−137, 40] | |||
| Other community services | 838 (2,502) | 647 (1,409) | 191 (162) [−128, 509] | |||
| Home care** | 3,670 (8,282) | 3,167 (382) | 503 (567) [−610, 1,616] | |||
| Residential care | 567 (2,780) | 1,013 (4,223) | −446 (245) [−927, 34] | |||
| Informal care | ||||||
| Total unpaid help | 4,462 (8,202) | 4,932 (8,781) | −471 (618) [−1,685, 743] | |||
| Unadjusted costs | ||||||
| Total health care costs†,*** | 7,060 (12,789) | 7,864 (13,486) | −805 (959) [−2,687, 1,078] | |||
| Total health and social care costs† | 13,975 (17,248) | 16,521 (17,639) | −2,547 (1,280) [−5,059, −34] | |||
| Total societal costs† | 18,437 (19, 057) | 21, 453 (18,902) | −3,017 (1,400) [−5,765, −269] | |||
| Adjusted costs | ||||||
| Total health and social care costs+ | 15,124 | 17,390 | −2,265 (1,027) [−4,279, −252] | |||
| Total societal costs+ | 19,067 | 21,907 | −2,840 (1,354) [−5,495, −185] | |||
*In the analysis, we use hospital admissions at 6 months as this measure includes subsequent hospital admissions at 1 and 6 months.
†Total unadjusted health and social care and societal costs were also significantly different using non-parametric testing such as the Wilcoxon (Mann–Whitney) test.
†The total intervention costs include 74 patients allocated to the hospital group that crossed over to CGAHAH and 29 patients allocated to CGAHAH crossed over to hospital, the cost incurred for initial hospital-at-home admission by the hospital arm is averaged over 274 patients, although the costs arise from the 74 patients who crossed over from the hospital group to CGAHAH. Similarly, the costs incurred for initial hospital admissions from the CGAHAH are averaged over 563 patients, although arise from the 29 patients who crossed over.
**Home care services are part of personal social services, data collected from the HRU questionnaire.
Total adjusted costs were adjusted for baseline gender known cognitive decline, baseline utilities and pre-randomisation costs and site as a random effect.
***Health care costs include primary care, outpatient attendance and hospital admissions.
Table 4.
Quality of life at baseline and 6 months, mortality and quality adjusted life years to 6 month follow-up, by treatment group, complete cases
| CGAHAH (N = 563) |
Hospital (N = 274) |
Difference in means | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SE) [95% CI] | |
| EQ-5D-5L utility | |||
| At baseline | 0.531 (0.270) | 0.528 (0.302) | 0.003 (0.021) [−0.037, 0.044] |
| At 6 months | 0.451 (0.324) | 0.457 (0.340) | −0.006 (0.024) [−0.053, 0.041] |
| Number of health problems recorded on the Charlson Index at 6 months | 1.561 (1.350) | 1.507 (1.296) | 0.054 (0.098) [−0.139, 0.245] |
| Mortality at 6 months | |||
| Alive (%) | 85% | 85% | |
| Dead (%)* | 15% | 15% | |
| Quality Adjusted Life Years from baseline to 6 months (unadjusted): | 0.246 (0.123) | 0.246 (0.132) | −0.001 (0.009) [−0.019, 0.017] |
| Quality Adjusted Life Years from baseline to 6 months (adjusted)+: | 0.245 | 0.247 | −0.002 (0.006) [−0.013, 0.010] |
*The difference in mortality at 6 months was not statistically significant (Pearson χ2 test P-value = 0.854).
QALYs were adjusted for baseline gender, known cognitive decline, baseline utilities and site as a random effect.
Uncertainty around the observed differences in costs and QALYs is illustrated on the cost-effectiveness plane in Figure 2, with the point estimate for differences in costs and QALYs shown as a grey dot, and black dots representing 5,000 pairs of incremental costs and QALYs. The difference in costs largely falls below the X-axis, indicating CGAHAH is very likely to be cost-saving, while the difference in QALYs is more evenly distributed around the Y-axis indicating large uncertainty about the effect of CGAHAH on QALYs. The joint distribution of differences in costs and QALYs falls mainly below a willingness to pay threshold of £20,000 per QALY (represented by the dashed line) adopted by NICE, indicating that the probability of the CGAHAH intervention being cost-effective at that threshold is 97%.
Figure 2.
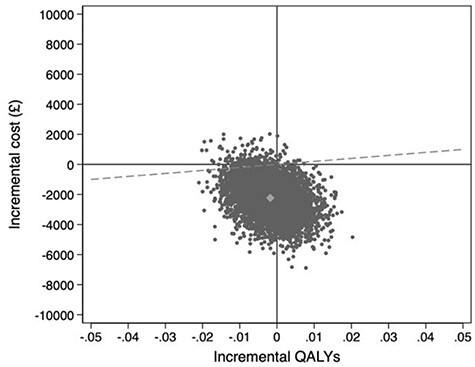
Cost-effectiveness plane showing incremental costs (health and social care perspective) and QALYs, complete cases, using baseline covariate adjustment.
Appendix Figure A2, Supplementary data are available in Age and Ageing online presents a cost-effectiveness plane from a societal perspective, in which the probability of CGAHAH being cost-effective at the £20,000 threshold was 98%. Appendix Figures A3 and A4, Supplementary data are available in Age and Ageing online report CEACs of the probability of cost-effectiveness when the willingness to pay threshold is altered. Appendix Tables 5 and 6, Supplementary data are available in Age and Ageing online provide details of resource use and costs in the 6 months prior to recruitment and resource use and costs for the complete cases; Appendix Tables 7 and 8, Supplementary data are available in Age and Ageing online details for the available cases; Appendix Table 9,Supplementary data are available in Age and Ageing online descriptive statistics for resource use for available cases; Appendix Table 10, Supplementary data are available in Age and Ageing online costs for available cases and Appendix Table 11, Supplementary data are available in Age and Ageing online data on quality of life, mortality and QALYs for available cases.
Results of sensitivity analyses
Using multiple imputation for all missing data, there was a non-significant increase in the mean difference in health and social care costs from £2,265 to £2,458 (95% CI: −4,977 to 61), the reduction in societal costs remained significant (−£3,083, 95% CI: −5,880 to −287). Differences in QALYs remained small and non-significant (Appendix Table 12, Supplementary data are available in Age and Ageing online; Appendix Figures A5–A8, Supplementary data are available in Age and Ageing online).
Discussion
Our results indicate that admission avoidance CGAHAH as an alternative to hospitalisation for an older population considered eligible for urgent healthcare in their home is likely to be cost-effective. For complete cases, we found that allocation to CGAHAH resulted in three fewer days in hospital, a difference that was reduced to one day at 6 months follow-up. We also found some evidence that the group allocated to CGAHAH were less likely to have been admitted to long-term residential care at six months follow-up, a difference that could by definition have longer-term cost implications.
When combined with lower residential care costs in the CGAHAH group and with no apparent adverse effects on quality of life or informal care requirements, CGAHAH was a cost-effective alternative to hospitalisation.
Strength of our study is that we recruited and randomised over 1,000 older people to CGAHAH or hospital, the largest randomised trial in this area that we are aware of, and tested the effect of delivering CGA guided healthcare in an admission avoidance HAH setting. We prospectively collected data on the use of health and social care and hours of informal care. These resources contributed to our results, illustrating the benefits of adopting a broader perspective when assessing the cost-effectiveness of health system interventions for an older population. As this was a pragmatic effectiveness trial, we anticipated that as in actual clinical practice participants might not adhere to the allocated intervention and that the primary intention to treat analysis would yield a more conservative treatment effect than an analysis based on adherence. Participants allocated to CGA HAH who were immediately admitted to the hospital experienced a further decline in health, and those assigned to the hospital but were instead admitted to CGA HAH expressed a strong preference for receiving care at home or a high rate of hospital bed occupancy prevented admission to hospital.
Sensitivity analyses did not qualitatively alter our results, indicating that they are robust to any impact of missing data. A limitation of our analysis may be that we calculated the cost of CGAHAH from the service budget at each site, and did not conduct a ‘bottom-up’ costing exercise entailing collection of detailed information from each site on actual resources involved, such as hours of time required from different staff categories to deliver the CGAHAH service. However, any differences between the allocated budget and actual delivery costs would have to be improbably large to alter the overall results. Participants’ estimates of the amount of informal care they received each week may not have been accurate, but given the population’s age and on-going care needs they probably reasonably approximated the actual hours of care they received. We were unable to investigate whether CGAHAH has a differential impact on the health outcomes and cost to disadvantaged communities, and future studies could usefully investigate this.
Analyses of service delivery interventions can be hard to generalise across health systems due to differences in healthcare staff and personal care arrangements. However, the CGAHAH interventions in our study were broadly similar across the different sites for the main drivers of cost (staff costs, medical supplies and overheads) and similar to HAH resources reported by others [14, 15]. Participants meeting the study entry criteria were an older population in frequent contact with healthcare services. Other factors that might limit generalizability include recruiting the majority of participants from an acute assessment unit that participants could continue to access their usual primary care services during their CGAHAH episode of care, and the availability of specialist geriatrician services.
Despite a rising number of older people living with multiple health conditions, many HAH services have focused on single procedures or conditions, for example the delivery of intravenous antibiotics or chronic obstructive pulmonary disease [14, 31]. This might reflect the challenge in realigning healthcare for people with two or more long-term health conditions who experience a decline in functional and cognitive capacity, and require integrated care across health and social care sectors [32–34]. Providing adequate support to informal carers, who inevitably become involved in the delivery of healthcare in the home, is crucial to prevent burdening older people and their support networks [35, 36].
Providing CGAHAH as an alternative to admission to hospital for older people, with a focus on multi-dimensional assessment, is one option that might reduce reliance on hospitalisation and residential care and at a lower cost. In this study, we investigated two different locations (hospital and home) in which CGA was delivered to older people presenting with an acute deterioration in their health. We conclude that CGAHAH is a reasonable and cost-effective alternative to admission to hospital for selected older people who present with an acute deterioration in their health.
Data sharing
The data set that generated the results reported in this manuscript will be made available to external researchers subject to the constraints of the consent under which data were collected https://www.ndph.ox.ac.uk/files/about/ndph-data-access-policy-1.pdf. Research data requests should be submitted to the corresponding author for consideration by the research team.
Supplementary Material
Acknowledgements
The authors acknowledge the funding received by the National Institute for Health Research that supported this research, the contribution made by those who consented to participate in the study, and the support of the Nuffield Department of Population at the University of Oxford. In addition, we acknowledge the contributions from Joyce Epstein as a patient representative, other patient representatives and members of the Trial Steering Committee: Professor Rowan Harwood (Chair), Professor Mark Mullee, Dr Sarah Pendlebury, Dr Mikey Dunn, Dr Niro Siriwardena, Natasha Curry; and Professor Mike Clarke (Chair), Professor Graeme MacLennan and Dr Isobel Reading as members of the Data Monitoring Committee; research nurses Angie Bowring, Audrey McAlpine, Lubena Mirza, Bernie Welsh and the contribution of other research nurses who supported the recruitment of participants and collection of data; the following who supported the study at Guy’s and St Thomas’s NHS Foundation Trust: Dr Darmiga Thayabaran, Dr Esther Hindley, Dr Natasha Thorley, Dr Mollika Chakravorty; Dr Jan Ritchie, site PI at Belfast; and Dr Pat McCaffrey, site PI Soutern Health and Social Care Trust.
The views expressed are those of the authors and not necessarily those of the National Institute of Health Research or the Department of Health and Social Care.
Footnotes
From Annals of Internal Medicine, Shepperd S, Butler C, Cradduck-Bamford A, Ellis G, Gray A, Hemsley A, Khanna P, Langhorne P, Mort S, Ramsay S, Schiff R, Stott DJ, Wilkinson A, Yu LM, Young J. Is Comprehensive Geriatric Assessment Admission Avoidance Hospital at Home an Alternative to Hospital Admission for Older Persons? : A Randomized Trial. Ann Intern Med. 2021 Jul;174(7):889–898. doi: 10.7326/M20-5688. Epub 2021 Apr 20. PMID: 33872045. Copyright © 2021 American College of Physicians. All rights reserved. Reprinted with the permission of the American College of Physicians, Inc.
Contributor Information
Surya Singh, Health Economics Research Centre, Nuffield Department of Population Health Sciences, Richard Doll Building, Old Road Campus, University of Oxford, Oxford OX3 7LF, UK.
Alastair Gray, Health Economics Research Centre, Nuffield Department of Population Health Sciences, Richard Doll Building, Old Road Campus, University of Oxford, Oxford OX3 7LF, UK.
Sasha Shepperd, Nuffield Department of Population Health Sciences, Richard Doll Building, Old Road Campus, University of Oxford, Oxford OX3 7LF, UK.
David J Stott, Institute of Cardiovascular and Medical Sciences, University of Glasgow, New Lister Building Glasgow Royal Infirmary G31 2ER, Glasgow, UK.
Graham Ellis, University Hospital Monklands, NHS Lanarkshire, Monkscourt Avenue, Airdrie, ML6 0JS, UK.
Anthony Hemsley, Royal Devon and Exeter NHS Foundation Trust, Exeter, Devon EX2 5DW, UK.
Pradeep Khanna, Aneurin Bevan University Health Board, Newport, South Wales, NP20 4SZ, UK.
Scott Ramsay, St John’s Hospital, NHS Lothian, Howden W Rd, Howden, Livingston EH54 6PP, UK.
Rebekah Schiff, Guy’s and St Thomas’ NHS Foundation Trust, Westminster Bridge Rd, Bishop's, London SE1 7EH, UK.
Apostolos Tsiachristas, Health Economics Research Centre, Nuffield Department of Population Health Sciences, Richard Doll Building, Old Road Campus, University of Oxford, Oxford OX3 7LF, UK.
Angela Wilkinson, Victoria Hospital, NHS Fife, Hayfield Rd, Kirkcaldy, KY2 5AH, UK.
John Young, Academic Unit of Elderly Care and Rehabilitation, University of Leeds, Bradford Royal Infirmary, Duckworth Lane, Bradford, BD9 6RJ, UK.
Declaration of Sources of Funding
The National Institute for Health Research Health Service and Delivery Research programme (12/209/66)
Declaration of Conflicts of Interest
Alastair Gray reports grants from National Institute of Health Research during the conduct of the study. Sasha Shepperd reports membership of the NIHR Health Services Research and Delivery Research programme Commissioned Prioritisation Committee during the conduct of this randomised trial, grants from the National Institute of Health Research (NIHR) and the MRC. Graham Ellis is employed part-time as a clinical advisor on ageing and health to NHS Scotland's Chief Medical Officer and has advised the Scottish Cabinet Secretary for Health and Sport regarding implementation of Hospital at Home in Scotland.
References
- 1. Prince MJ, Wu F, Guo Y et al. The burden of disease in older people and implications for health policy and practice. Lancet 2015; 385: 549–62. [DOI] [PubMed] [Google Scholar]
- 2. Department of Health & Social Care NHS England . Reducing Emergency Admissions. London: National Audit Office. 2018. https://www.nao.org.uk/wp-content/uploads/2018/02/Reducing-emergency-admissions.pdf (6 April 2021, date last accessed. [Google Scholar]
- 3. OECD . Fiscal Sustainability of Health Systems: Bridging Health and Finance Perspectives. OECD Publising; Paris; 2015. 10.1787/9789264233386-en (6 April 2021, date last accessed). [DOI] [Google Scholar]
- 4. Finklestein A, Zhou A, Taubman S, J D. Health care hotspotting — a randomized, controlled trial. N Engl J Med 2020; 382: 152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ellis G, Gardner M, Tsiachristas A et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017: CD006211. 10.1002/14651858.CD006211.pub3 (12 November 2021, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kocman D, Regen E, Phelps K et al. Can comprehensive geriatric assessment be delivered without the need for geriatricians? A formative evaluation in two perioperative surgical settings. Age Ageing 2019; 48: 644–9. [DOI] [PubMed] [Google Scholar]
- 7. Conroy SP, Stevens T, Parker SG, Gladman JR. A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: 'interface geriatrics'. Age Ageing 2011; 40: 436–43. [DOI] [PubMed] [Google Scholar]
- 8. Howard R, Gathercole R, Bradley R et al. The effectiveness and cost-effectiveness of assistive technology and telecare for independent living in dementia: a randomised controlled trial. Age Ageing 2021; 50: 882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong AKC, Wong FKY, So C et al. Cost-effectiveness of a preventive self-care health management program for community-dwelling older adults: a randomised controlled trial. Age Ageing 2020; 50: 440–6. [DOI] [PubMed] [Google Scholar]
- 10. Beswick AD, Rees K, Dieppe P et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet 2008; 371: 725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thiyagarajan JA, Araujo de Carvalho I, Pena-Rosas JP et al. Redesigning care for older people to preserve physical and mental capacity: WHO guidelines on community-level interventions in integrated care. PLoS Med 2019; 16: e1002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imison C, Curry N, Holder H, et al. Shifting the Balance of Care. London: The Nuffield Trust; 2017. https://www.nuffieldtrust.org.uk/files/2017-02/shifting-the-balance-of-care-report-web-final.pdf (6 April 2021, date last accessed). [Google Scholar]
- 13. Al-Janabi A, Al-Wahdani B, Ammar W et al. Bellagio declaration on high-quality health systems: from a quality moment to a quality movement. Lancet Glob Health 2018; 6: e1144–e5. [DOI] [PubMed] [Google Scholar]
- 14. Shepperd S, Iliffe S, Doll HA et al. Admission avoidance hospital at home. Cochrane Database Syst Rev 2016: CD007491. 10.1002/14651858.CD007491.pub2 (12 November 2021, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levine DM, Ouchi K, Blanchfield B et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med 2019; 172: 77–85. [DOI] [PubMed] [Google Scholar]
- 16. Brody AA, Arbaje AI, DeCherrie LV, Federman AD, Leff B, Siu AL. Starting up a hospital at home program: facilitators and barriers to implementation. J Am Geriatr Soc 2019; 67: 588–95. [DOI] [PubMed] [Google Scholar]
- 17. Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med 2016; 176: 1693–702. [DOI] [PubMed] [Google Scholar]
- 18. Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med 2018; 178: 1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jessup R, Putrik P, Buchbinder R et al. Identifying alternative models of healthcare service delivery to inform health system improvement: scoping review of systematic reviews. BMJ Open 2020; 10: e036112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nundy SPK. Hospital at home to support COVID-19 surge - time to bring down the walls? JAMA 2020; 1: e200504. https://jamanetwork.com/channels/health-forum/fullarticle/2765661. [DOI] [PubMed] [Google Scholar]
- 21. Shepperd S, Cradduck-Bamford A, Butler C et al. A multi-Centre randomised trial to compare the effectiveness of geriatrician-led admission avoidance hospital at home versus inpatient admission. Trials 2017; 18: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shepperd S, Butler C, Cradduck-Bamford A et al. Is comprehensive geriatric assessment admission avoidance hospital at home an alternative to hospital admission for older persons? : A randomised trial. Ann Intern Med 2021; 174: 889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jorm AF. The informant questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr 2004; 16: 275–93. [DOI] [PubMed] [Google Scholar]
- 24. National Institute for Health and Care Excellence . Guide to Methods of Technology Appraisal 2013. https://wwwniceorguk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (6 April 2021, date last accessed). [PubMed]
- 25. Department of Health and Social Care (DHSC) . National Schedule of Reference Costs 2017/2018: Highlights, Analysis, and Introduction to the Data. London: DHSC; (accessed 6 April 2021). [Google Scholar]
- 26. Husereau D, Drummond M, Petrou S et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMC Med 2013; 11: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmaco Econ 2014; 32: 1157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005; 24: 993–1007. [DOI] [PubMed] [Google Scholar]
- 29. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011; 30: 377–99. [DOI] [PubMed] [Google Scholar]
- 30. Stata Statistical Software : https://www.stata.com/. 2017.
- 31. Wedzicha JAEC-C, Miravitlles M, Hurst JR et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2017; 49: 1600791. [DOI] [PubMed] [Google Scholar]
- 32. Sezgin D, O'Caoimh R, Liew A. The effectiveness of intermediate care including transitional care interventions on function, healthcare utilisation and costs: a scoping review. Eur Geriatr Med 2020; 11: 961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sezgin D, O'Caoimh R, O'Donovan MR et al. Defining the characteristics of intermediate care models including transitional care: an international Delphi study. Aging Clin Exp Res 2020; 32: 2399–410. 10.1007/s40520-020-01579-z. [DOI] [PubMed] [Google Scholar]
- 34. Liao JM, Navathe A, Press MJ. Hospital-at-home care programs-is the hospital of the future at home? JAMA Intern Med 2018; 178: 1040–1. [DOI] [PubMed] [Google Scholar]
- 35. Mäkelä P, Stott D, Godfrey M, Ellis G, Schiff R, Shepperd S. The work of older people and their informal caregivers in managing an acute health event in a hospital at home or hospital inpatient setting. Age Ageing 2020; 19: 569. 10.1186/s13063-018-2929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murphy C, De Laine C, Macaulay M, Hislop Lennie K, Fader M. Problems faced by people living at home with dementia and incontinence: causes, consequences and potential solutions. Age Ageing 2021; 50: 944–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


