Abstract
Aflatoxin B1 (AFB1) is one of the most toxic mycotoxins. It has been reported that dietary exposure to AFB1 is related to the low growth performance, immunosuppression, and high susceptibility to infectious diseases of chickens. The aim of the present study was to evaluate the protective effects of Lactobacillus salivarius on broiler chickens challenged with AFB1. First, AFB1 degradation ability of Lactobacillus salivarius was measured by a high-performance liquid chromatography (HPLC) method. Then, the Arbor Acres broiler chickens were randomly assigned to experimental groups. The effects of Lactobacillus salivarius supplementation on the growth performance, liver function, and meat quality were measured, and immune response was also determined after vaccination with attenuated infectious bursal disease virus (IBDV) vaccine of broilers challenged with AFB1. Besides, resistance to Salmonella Pullorum infection along with AFB1 exposure was determined in broilers. The results showed that Lactobacillus salivarius could effectively degrade AFB1. Lactobacillus salivarius supplementation improved growth performance, liver function, and meat quality of broilers challenged with AFB1. In addition, Lactobacillus salivarius supplementation resulted in enhanced specific antibody and IFN-γ production, and lymphocyte proliferation in broilers challenged with AFB1 after IBDV vaccine immunization. Furthermore, Lactobacillus salivarius supplementation enhanced Salmonella Pullorum infection resistance in broilers challenged with AFB1. Our results revealed a tremendous potential of Lactobacillus salivarius as feed additive to degrading AFB1 and increasing broilers production performance in poultry production.
Key words: AFB1, Lactobacillus salivarius, feed additive, chickens
INTRODUCTION
Aflatoxins, primarily produced by fungi Aspergillus flavus and Aspergillus parasiticus, are one of the most widespread pollutants in poultry feed in both developing and developed countries (Rushing and Selim, 2019; Hernández-Ramírez et al., 2021). At present, many aflatoxins have been identified, among which Aflatoxin B1 (AFB1) is the most toxic for chicken and has been classified as a Class I carcinogen by the International Agency for Research on Cancer (Elwan et al., 2021; Li et al., 2021). AFB1 is often found in corn and peanut, which is the major energy sources for the poultry feed, and the permitted level of AFB1 is very low in poultry feed, and thus poultry feed is at a high risk of contamination with AFB1 (Fouad et al., 2019). It has been reported that the most vulnerable body organ of chicken to AFB1 is the liver (Li et al., 2021). AFB1 can induce unbalanced lipid metabolism, inhibit the activity of antioxidant enzymes, and increase pro-inflammatory cytokines and hepatocyte apoptosis levels (Rosa et al., 2001; Fouad et al., 2019; Li et al., 2021). In addition, dietary exposure to AFB1 is associated with immune dysfunction of chickens, making the broilers more susceptible to infectious diseases (Qureshi et al., 1998; Shivachandra et al., 2003). It has been reported that AFB1 could induce apoptosis in immune organs, and cause significant decrease in the production of immunoglobulin (Ig), including IgA, IgG, and IgM, as well as the production of T and B lymphocytes (Fouad et al., 2019). Therefore, it is necessary to explore preventive approaches to reduce the negative effects caused by AFB1 in poultry.
Until now, there are mainly 3 methods to reduce harmful effects of AFB1 including physical (absorption, heating, and irradiation), chemical (ozone, ammonia fumigation, and solvent extraction) and biological (probiotics and enzymes) detoxification methods (Guo et al., 2021). In the process of poultry production, physical and chemical detoxification methods are complicated, costly, time-consuming, and easy to pollute the environment. In addition, high temperature and other physical and chemical detoxification methods can also easily lead to the loss of nutritional value of feed (Zhu et al., 2016; Vila-Donat et al., 2018; Guo et al., 2021). Compared to physical and chemical detoxification methods, biological method has little impact on the nutritional losses and expensive equipment independent, which is convenient for large-scale use in poultry production. Researcher demonstrated several detoxification probiotics have been reported, including Saccharomyces, Bacillus, and Lactobacillus for biological detoxification of AFB1 (Farzaneh et al., 2016; Chlebicz and Śliżewska, 2020; Ragoubi et al., 2021; Zhu et al., 2021). It was reported that Saccharomyces cerevisiae RC016 and Bacillus subtilis ANSB060 as feed additives reduced liver injury and residual AFB1 levels of chicken's exposure to AFB1-contaminated diets (Ma et al., 2012; Poloni et al, 2020). A Lactobacillus plantarum FJS003 also showed excellent antifungal properties and AFB1 detoxification activity, which is characterized by the removal ratio of AFB1 nearly 90% and inhibitory ratio to Aspergillus flavus spores’ growth is nearly 100% (Zhu et al., 2021). Due to the beneficial effects on growth performance and disease resistance, probiotics have been used in poultry feed industry for decades (Wang et al., 2020). Probiotics also have incomparable advantages due to their low production cost and high availability (Gao et al., 2017). Therefore, it is a reliable direction for poultry production to screen out probiotics which can not only degrade AFB1 but also promote growth and disease resistance as feed additives.
It has been reported that the antibody levels against Newcastle disease virus and avian influenza (H5N1) were markedly reduced by poultry diets contaminated with AFB1 (Fouad et al., 2019). Infectious bursal disease virus (IBDV) and Salmonella Pullorum are both common and important pathogens in chicken farms. Little information is available on whether AFB1 exposure can reduce antibody titer of IBDV after vaccination and increase susceptibility to Salmonella Pullorum. In recent study, we isolated a strain of Lactobacillus salivarius Erya and confirmed that the Lactobacillus salivarius Erya exerted multiple beneficial effects in laying hens including growth promotion, stress resistance and immunity improvement (Wang et al., 2020). Therefore, the present study further explored whether Lactobacillus salivarius Erya as feed additive could alleviate the harmful effects including poor performance, liver injury, immunosuppression, and Salmonella Pullorum susceptibility caused by AFB1 in broilers.
MATERIALS AND METHODS
Effects of Incubation Time on AFB1 Degradation Rate by Lactobacillus salivarius
Lactobacillus salivarius Erya was grown in MRS liquid medium for 24 h at 37°C as described previously (Wang et al., 2020). The Lactobacillus was adjusted at a concentration of 107 CFU/mL and incubated with 2.5 µg/mL AFB1 aerobically cultured at 37°C for 0, 12, 24, 36, 48 and 72 h, respectively. The concentration of AFB1 was measured using a previously published method with some modifications (Wang et al., 2019). Briefly, chloroform was used to extract AFB1 at least 3 times, the extracts were condensed in methanol, then filtered by 0.22 µm organic system membrane and detected by high-performance-liquid chromatography (HPLC, Waters 2695-2489) equipped with a C18 column (5 µm, 4.6 × 150 mm, Waters). The mobile phase was composed of a mixture of solvent (water/acetonitrile, 40/60, v/v), and separation was performed at a flow rate of 1.0 mL/min. The time of analysis was 45 min. The volume of injection was 10 µL.
Chickens
All animal trials were conducted under the approval of Laboratory Animal Ethics Committee of Shanxi Agricultural University (Shanxi, China) in accordance with Laboratory Animal-Guideline for ethical review of animal welfare (GB/T35892-2018, National Standards of the People's Republic of China). Healthy 1-day-old commercial Arbor Acres (AA) broilers were obtained from a commercial hatchery (RenNong, Taigu, Shanxi, China). Room temperature was set to 34 ± 2˚C for the first week and gradually reduced to 23 ± 2˚C. Water was provided ad libitum and fed basal diet (Table 1). All broilers were checked against Salmonella Pullorum and other major pathogens before starting the experiments.
Table 1.
List of ingredients in the basal diet.
| Ingredients | 1–21d | 21–42 d | Calculated nutrient levels | 1–21 d | 21–42 d |
|---|---|---|---|---|---|
| Corn | 55.200 | 59.188 | Metabolizable energy (Mcal/kg) | 3.00 | 3.15 |
| Soybean meal | 36.992 | 31.900 | Crude protein (g/kg) | 220.00 | 200.00 |
| Soybean oil | 3.705 | 5.150 | Total lysine (g/kg) | 11.50 | 10.50 |
| CaHPO4 | 1.955 | 1.698 | Total methionine (g/kg) | 6.20 | 5.00 |
| Limestone flour | 1.190 | 1.095 | Total methionine + cysteine (g/kg) | 9.10 | 7.60 |
| NaCl | 0.350 | 0.350 | Available phosphorus (g/kg) | 4.50 | 4.00 |
| DL-Methionine | 0.268 | 0.248 | Calcium (g/kg) | 10.00 | 9.00 |
| L-Lysine HCl | 0.020 | 0.034 | |||
| Vitamin premixa | 0.020 | 0.020 | |||
| Mineral premixb | 0.200 | 0.200 | |||
| Choline chloride | 0.100 | 0.100 |
Provided the following (per kg of complete diet): vitamin A, 12,500 IU; vitamin D3, 2,500 IU; vitamin E, 15 IU; vitamin K, 2.65 mg; vitamin B1, 2 mg; vitamin B12, 0.02 mg; biotin, 0.35 mg; folic acid, 1.25 mg; pantothenic acid, 12 mg; nicotinic acid, 50 mg.
Provided the following (per kg of complete diet): Cu (as copper sulfate), 8 mg; Zn (as zinc sulfate), 75 mg; Fe (as ferrous sulfate), 80 mg; Mn (as manganese sulfate), 80 mg; I (as potassium iodide), 0.35 mg; Se, (as sodium selenite) 0.15 mg.
Lactobacillus salivarius and Culture Condition
The Lactobacillus salivarius Erya (Genbank no. MT378407) was used in the present study, isolated from the feces of healthy chickens, which showed multiple beneficial effects in chickens such as improved growth promotion and heat stress tolerance, and enhanced immunity according to a previous study (Wang et al., 2020). A single colony of the Lactobacillus salivarius was cultured in MRS broth (Solarbio, Beijing, China) for 18 h under aerobic conditions at 37°C, then the Lactobacillus salivarius was added in the basal diet to a final concentration of 107, 108, and, 109 CFU/kg of feed.
Trial 1-Effects of Lactobacillus salivarius Supplementation on the Growth Performance, Liver Function and Meat Quality of Broilers Challenged with Aflatoxin B1
Animal Experiments Design Four hundred 1-day-old broilers were randomly divided into 8 experimental groups in 5 replicates (10 chickens/replicate). Broilers in control group were fed basal diet (Table 1); broilers in Lactobacillus salivarius group were fed basal diet supplemented with Lactobacillus salivarius of 107, 108 and 109 CFU/kg of feed, respectively. Indicated group were fed 1 mg/kg AFB1-contaminated basal diet. Samples including serum, liver tissues, breast muscle tissues, and thigh muscle tissues were collected at 6 wk of age for further analysis.
Growth Performance At d 42, all broilers were euthanized by cervical dislocation. Initial body weight, final body weight and feed consumption were recorded, then the feed conversion ratio (FCR) was calculated with the following formula: FCR = total feed consumption (g)/total gain weight (g). Heads, feet, and organs of chickens were removed manually and weighed to calculate the eviscerated yield percentage (EYP). Breast and thigh muscles of chickens were removed manually and weighed to calculate the breast muscle percentage (BMP) and thigh muscle percentage (TMP). A total of 400 chickens, including 8 experimental groups (50 chickens per group) were selected for growth performance evaluation.
Liver Function Assay Whole blood of each bird was collected at room temperature at an oblique position, then centrifuged at 1,200 rpm for 10 min, the supernatant was used to detect alanine aminotransferase (ALT, C009-3-1), aspartate aminotransferase (AST, C010-2-1) activities (Jiancheng Institute of Bioengineering, Nanjing, China) by using commercial kits. Liver tissues were collected and homogenized with 4 volumes of PBS, and centrifuged at 1000 rpm for 5 min. The supernatant used for malondialdehyde (MDA, A003-1-2) content, catalase (CAT, A007-1-1) activity, superoxide dismutase (SOD, A001-3-2) activity evaluation (Jiancheng Institute of Bioengineering, Nanjing, China) by using commercial kits. A total of 400 chickens, including 8 experimental groups (50 chickens per group) were selected for liver function assay.
Meat Quality Analysis Drip loss was measured as previously described (Zhang et al., 2020), breast muscle tissues, thigh muscle tissues were collected and weighed (W1), and then suspended in sealed plastic bags and stored at 4°C. After 24 h, all muscle tissues were reweighed (W2). Drip loss was calculated as (W1-W2)/W1 × 100%. Shear force was measured by using a digital muscle tenderness meter (C-LM3, Tenovo International, Beijing, China). A total of 400 chickens, including 8 experimental groups (50 chickens/group) were selected for meat quality analysis.
Trial 2-Effects of Lactobacillus salivarius Supplementation on the IBDV Immune Responses of Broilers Challenged With Aflatoxin B1
Animal Experiments Design Two hundred 1-day-old broilers were randomly divided into 4 experimental groups in 5 replicates (10 chickens/replicate). Broilers in control group were fed basal diet (Table 1); broilers in Lactobacillus salivarius group were fed basal diet supplemented with Lactobacillus salivarius of 108 CFU/kg of feed. Indicated groups were fed 1 mg/kg AFB1-contaminated basal diet. All broilers were vaccinated with an attenuated IBDV vaccine (Strain B87, Zhejiang EBVAC Bioengineering, Hangzhou, China) at d 14. Samples including serum and spleen tissues were collected 14-d postimmunization.
Serum Specific Antibody Detection Serum-specific IBDV antibody of chickens were detected by a commercial detection kit (IDEXX R Laboratory, Inc., Westbrook, ME) according to the manufacturer's recommended procedure. The relative level of IBDV antibody titer was measured by detecting the S/P value with the following formula: [(average value of sample)-(average value of negative control)]/[(average value of positive control)-(average value of negative control). Endpoint titer was calculated with the following formula: Log 10 titer = 1.09 (Log 10 S/P) + 3.36. The value was represented as positive when S/P ratio is > 0.2 and negative when S/P ratio is ≤0.2. A total of 200 chickens, including 4 experimental groups 50 chickens/group were selected for IBDY special antibody detection.
Serum IFN-γ Levels Detection Whole blood was collected at room temperature at an oblique position, then centrifuged at 1200 rpm for 10 min, the supernatant was used to detect IFN-γ levels by using commercial kits (LS-F4229-1, LifeSpan BioSciences, Seattle, WA). A total of 200 chickens, including 4 experimental groups (50 chickens/group) were selected for serum IFN-γ levels detection.
Lymphocyte Proliferation Index Measurement Lymphocyte proliferation experiment was carried out as previously described with some modifications (Wang et al., 2020). Briefly, the chicken spleen samples were collected and minced with Hank's solution, then cell suspension were obtained by mesh screen. The cells were washed with the full Hank's solution and cultured in RPMI 1640 medium. Cells were inoculated on 96-well plates at the same concentration in each well, and then the cells were incubated with 5 μg/mL Concanavalin (ConA, Solarbio, Beijing, China). The cells were cultured at 37°C and 5% CO2 for 24 h. Cell proliferation was detected by MTT method. Stimulation index (SI) was calculated with the following formula: SI = (stimulation hole value - blank hole value)/(unstimulated hole value-blank hole value). A total of 40 chickens, including 4 experimental groups (10 chickens/group) were selected for serum IFN-γ levels detection.
Trial 3-Effects of Lactobacillus salivarius Supplementation on the Salmonella Pullorum Infection Resistance of Broilers Challenged With Aflatoxin B1
Animal Experiments Design Four hundred 1-day-old broiler chickens were randomly divided into 8 experimental groups in 5 replicates (10 chickens/replicate). Broilers in the control group were fed basal diet (Table 1); broilers in Lactobacillus salivarius group were fed basal diet supplemented with Lactobacillus salivarius of 108 CFU/kg of feed. Indicated group were fed 1 mg/kg AFB1-contaminated basal diet on d 14, indicated group were orally administered 107 CFU Salmonella Pullorum (CVCC1789, China veterinary culture collection center). The broilers that survived 14-d post-Salmonella Pullorum challenge were counted (the moribund chickens euthanized by cervical dislocation and recorded as mortality). Samples including ileum tissues, ileal contents and feces were collected 14-d post-Salmonella Pullorum challenge.
Salmonella Pullorum Detection in Ileal Contents The quantification of Salmonella Pullorum abundance was performed as previously described with some modifications (Rubio et al., 2017; Deng et al., 2021). Briefly, DNA was extracted from the feces using TIANamp Stool DNA Kit (Tiangen, Beijing, China) according to the manufacturer's recommended procedure. The ratA gene copy of Salmonella Pullorum was detected by quantitative PCR (qPCR) using a standardized PCR amplicon to establish a standard curve as previously described (Rubio et al., 2017), qPCR was performed on LightCycler 480 instrument (Roche, Switzerland), and the reaction mixture were 12.5 μL TB Green Fast qPCR Mix (2 ×) (Takara, Beijing, China), 10 μM of each forward and reverse primer (Table 2), 2 μL template DNA and 8.5 μL ddH2O. The amplification conditions were 1 cycle of 94°C for 15 s, 40 cycles of 94°C for 10 s, 50°C for 10 s, 74°C for 35 s, and finally 1 cycle of 74°C for 2 min. A total of 120 chickens, including 8 experimental groups (15 chickens/group) were selected for Salmonella Pullorum detection in ileal contents.
Table 2.
Primers used in qRT-PCR.
| Gene | Primer sequence (5’-3’) | References |
|---|---|---|
| β-actin | F: GAGAAATTGTGCGTGACATCA R: CCTGAACCTCTCATTGCCA |
(Wang et al., 2020) |
| Occludin | F: TCGTGCTGTGCATCGCCATC R: CGCTGGTTCACCCCTCCGTA |
(Wang et al., 2020) |
| ZO-1 | F: GCGCCTCCCTATGAGGAGCA R: CAAATCGGGGTTGTGCCGGA |
(Wang et al., 2020) |
| Claudin-1 | F: TGGAGGATGACCAGGTGAAGA R: CGAGCCACTCTGTTGCCATA |
(Wang et al., 2020) |
| Salmonella Pullorum | F: CCGCCTGCGCGATGGCTTTA R: TCTGGTTGACGGCGTGGGGA |
(Rubio et al., 2017) |
Secretory IgA, TNF-α and IL-1β Detection Ileal content was collected and homogenized with 9 volumes of PBS, and then centrifuged at 3,000 rpm for 15 min. The supernatant used to secretory IgA (SIgA) detection by a commercial kit (ANG-E32006C-1) according to the manufacturer's instructions (Angle Gene, Nanjing, China). Ileum tissues were collected and homogenized with 4 volumes of PBS, and centrifuged at 1000 rpm for 5 min. The supernatant used for TNF-α (H052-1) and IL-1β (H002) detection (Jiancheng Institute of Bioengineering, Nanjing, China) by using commercial kits. A total of 120 chickens, including 8 experimental groups (15 chickens/group) were selected for SIgA, TNF-α, and IL-1β detection.
Quantitative Real-time PCR Analysis The quantitative real-time PCR (qRT-PCR) was performed as described previously (Wang et al., 2020). Briefly, total RNA was extracted using Triquick Reagent (Trizol Substitute) (Solarbio, Beijing, China) according to the manufacturer's instructions. 500 ng total RNA were reverse transcribed using the PrimeScript RT Master Mix (Takara, Beijing, China). The mRNA expression levels were measured by TB Green Fast qPCR Mix (2 ×) (Takara, Beijing, China) on a Roche 480 real-time PCR system thermocycler. Each sample was analyzed in triplicates and the mRNA expression of the target genes was analyzed by 2−△△Ct method (Bustin et al., 2009), following normalization with β-actin gene. The primers are shown in Table 2. A total of 48 chickens, including 8 experimental groups (6 chickens/group) were selected for Claudin-1, Occludin and ZO-1 mRNA expression levels detection.
Fecal Shorter-chain Fatty Acids Detection The fecal shorter-chain fatty acids (SCFAs, mainly acetate, propionate and butyrate) concentrations were detected as previously described (Wang et al., 2020). The standard substance of acetate, propionate and butyrate (Sigma-Aldrich, Shanghai, China) were used to construct the standard curve by diluted into a series of concentrations of the working solution using ultrapure water. Fecal samples were incubated with 800 μL sodium chloride solution and 100 μL of 3 mM hydrochloric acid sodium chloride solution, and centrifuged at 13,000 rpm for 15 min. The content of SCFAs in the fecal samples was detected using a gas chromatography (GC) system (7890B, Agilent, Beijing, China). The initial oven temperature was set at 80˚C for 0.5 min, then increased 5˚C per min for 10 min and held for 2 min, then adjusts the temperature by rising 20˚C per min for 5 min and held for 1 min. The flow rates of hydrogen and air were 40 mL and 450 mL per min, respectively. A total of 120 chickens, including 8 experimental groups (15 chickens/group) were selected for SCFAs detection.
Statistical Analysis
The data are expressed as mean ± SD. Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software). Statistical significance was calculated by 2-way ANOVA with Tukey tests for multiple-group comparisons. The level of significance was set at P < 0.05.
RESULTS
AFB1 Degradation Activity of Lactobacillus salivarius
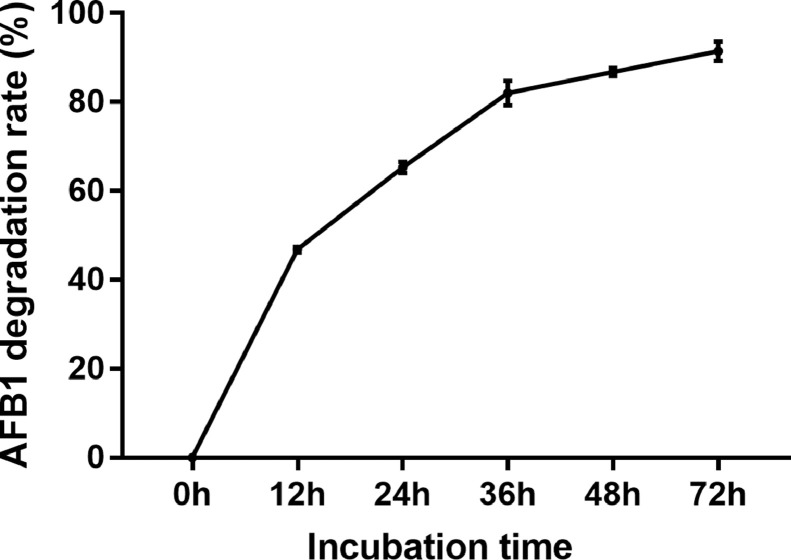
Lactobacillus salivarius was incubated with AFB1 and AFB1 degradation rate was determined. In the first 12 h, AFB1 degradation rates increased rapidly from 0 to 46.9%, then reached to 65.3% at 24 h, 86.7% at 48 h, and 91.5% at 72 h (Figure 1). The results indicated that Lactobacillus salivarius Erya could significantly degrade AFB1.
Figure 1.
Effect of Lactobacillus salivarius incubation time on AFB1 degradation rate. The experiments were performed in the presence of 2.5 µg/mL AFB1 at 37°C, pH = 7.0. Bars represent means ± SD of 3 independent experiments.
Effects of Lactobacillus salivarius Supplementation on Growth Performance
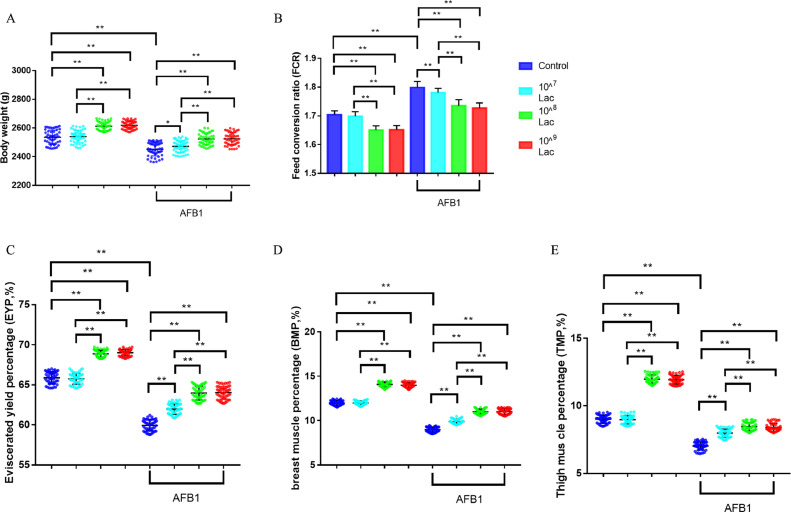
At 6 wk of age, the body weight was respectively 3.0% and 3.2% greater in 108 and 109 CFU/kg Lactobacillus salivarius group compared to the control group, the FCR respectively was 3.1% and 3.1% lower (Figures 2A, B, all P < 0.01). Compared to the control group, broilers fed with AFB1-contaminated diet significantly reduced body weight by 3.3% and increased FCR by 5.5% at 6 wk of age (Figures 2A, B, all P < 0.01). Compared to the AFB1-contaminated diet group, treatment of broilers with 107, 108 and 109 CFU/kg Lactobacillus salivarius appeared to respectively increase the body weight by 1.2%, 2.9%, 2.9% (Figure 2A, all P < 0.05), and respectively reduce the FCR by 1.3%, 3.5%, 3.7% (Figure 2B, all P < 0.01). In addition, the Lactobacillus salivarius diet improved dressing percentage of broilers.
Figure 2.
Effects of dietary Lactobacillus salivarius supplementation on growth performance of broilers challenged with AFB1. (A) Effects of dietary supplementation with Lactobacillus salivarius on body weight at 6 wk of age (n = 50). (B) Effects of dietary supplementation with Lactobacillus salivarius on feed conversion ratio (FCR) at 6 wk of age (n = 50). (C) Effects of dietary supplementation with Lactobacillus salivarius on eviscerated yield percentage (EYP) at 6 wk of age (n = 50). (D) Effects of dietary supplementation with Lactobacillus salivarius on breast muscle percentage (BMP) at 6 wk of age (n = 50). (E) Effects of dietary supplementation with Lactobacillus salivarius on thigh muscle percentage (TMP) at 6 wk of age (n = 50). Two-way ANOVA for repeated measurements, followed by Tukey tests. *Indicates P < 0.05; ⁎⁎ Indicates P < 0.01. Lac, Lactobacillus salivarius.
The EYP, BMP, TMP were significantly increased in the 108 CFU/kg and 109 CFU/kg Lactobacillus salivarius group compared to control group (Figures 2C–E, all P < 0.01). Compared to the control group, broilers fed with AFB1-contaminated diet significantly reduced EYP, BMP, TMP by 9.0%, 25.1%, 21.9% (Figures 2C–E, all P < 0.01). Compared to the AFB1-contaminated diet group, treatment of broilers with 107, 108 and 109 CFU/kg Lactobacillus salivarius significantly improved dressing percentage of broilers challenged with AFB1, which was characterized by elevated EYP, BMP, TMP of broilers (Figures 2C–E, all P < 0.01).
Effects of Lactobacillus salivarius Supplementation on Liver Function of Broilers
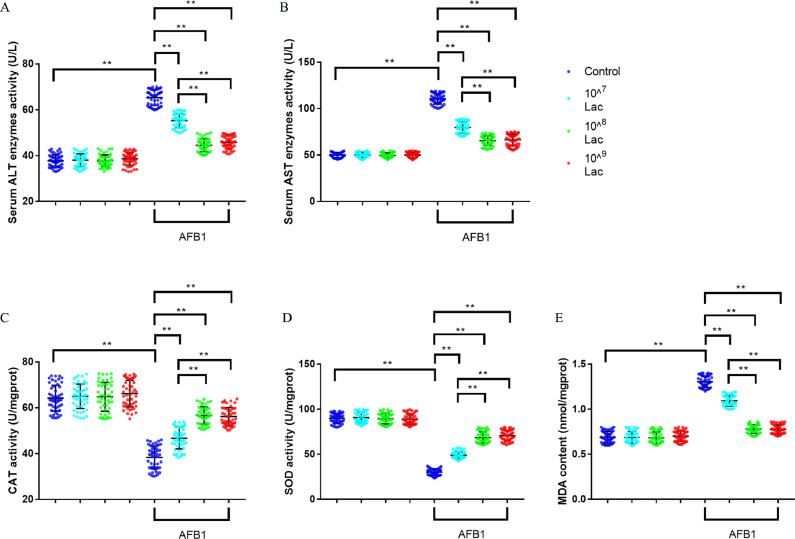
Compared to the control group, broilers fed with AFB1-contaminated diet brought a 73.1% increase in ALT level and a 120.4% increase in AST level (Figures 3A, B, all P < 0.01), which indicated that AFB1 caused severe liver damage in broilers. While, 107, 108, and 109 CFU/kg Lactobacillus salivarius supplementation markedly decreased serum ALT and AST levels in AFB1-treated broilers (Figures 3A, B, all P < 0.01), which indicated that Lactobacillus salivarius supplementation alleviated AFB1-induced severe liver damage in broilers. In addition, broilers fed with AFB1-contaminated diet showed significantly increased liver MDA content by 89.5%, reduced CAT and SOD activity by 40.3% and 66.5%, respectively (Figures 3C–E, all P < 0.01), while broilers fed with 107, 108 and 109 CFU/kg Lactobacillus salivarius showed significantly higher antioxidant enzyme activities and lower MDA content (Figures 3C–E, all P < 0.01), which indicated that Lactobacillus salivarius alleviated AFB1-induced oxidative stress in liver of broilers.
Figure 3.
Effects of dietary Lactobacillus salivarius supplementation on liver function of broilers challenged with AFB1. (A) Serum ALT activity (n = 50). (B) Serum AST activity (n = 50). (C) Liver CAT activity (n = 50). (D) Liver SOD activity (n = 50). (E) Liver MDA concentration (n = 50). Two-way ANOVA for repeated measurements, followed by Tukey tests. ⁎⁎Indicates P < 0.01. Lac, Lactobacillus salivarius.
Effects of Lactobacillus salivarius Supplementation on Meat Quality
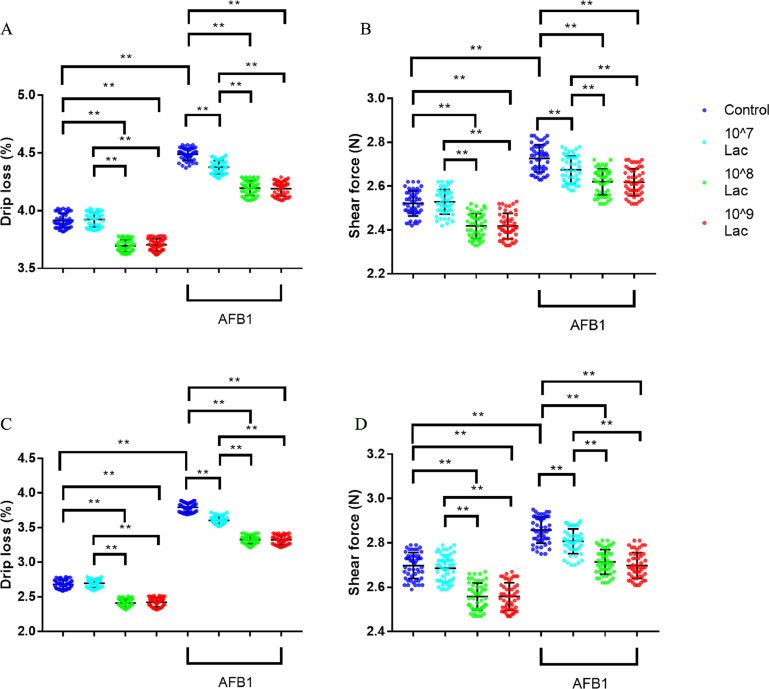
In general, the lower the drip loss and shear force, the better the meat quality. At 6 wk of age, the drip loss of breast muscle was respectively 5.6% and 5.4% lower in 108 and 109 CFU/kg Lactobacillus salivarius group compared to the control group, the shear force of breast muscle respectively was 4.0% and 4.1% lower (Figures 4A, B, all P < 0.01). The drip loss of thigh muscle was respectively 10.1% and 9.7% lower in 108 and 109 CFU/kg Lactobacillus salivarius group compared to the control group, the shear force of thigh muscle respectively was 5.1% and 5.0% lower (Figures 4C, D, all P < 0.01). Compared to the control group, broilers fed with AFB1-contaminated diet significantly increased drip loss of breast and thigh muscles by 16.6% and 6.1% respectively, and significantly increased shear force of breast and thigh muscles by 41.1% and 6.2% respectively (Figures 4A–D, all P < 0.01). Treatment of broilers with 107, 108 and 109 CFU/kg Lactobacillus salivarius alleviated the adverse effects of AFB1 on drip loss and shear force of breast and thigh muscles (Figures 4A–D, all P < 0.01), and thus improved the meat quality of broilers challenged with AFB1.
Figure 4.
Effects of dietary Lactobacillus salivarius supplementation on meat quality of broilers challenged with AFB1. (A, B) Drip loss and shear force of breast muscle (n = 50), respectively. (C, D) Drip loss and shear force of thigh muscle (n = 50), respectively. Two-way ANOVA for repeated measurements, followed by Tukey tests. ⁎⁎Indicates P < 0.01. Lac, Lactobacillus salivarius.
Effects of Lactobacillus salivarius Supplementation on Immune Responses
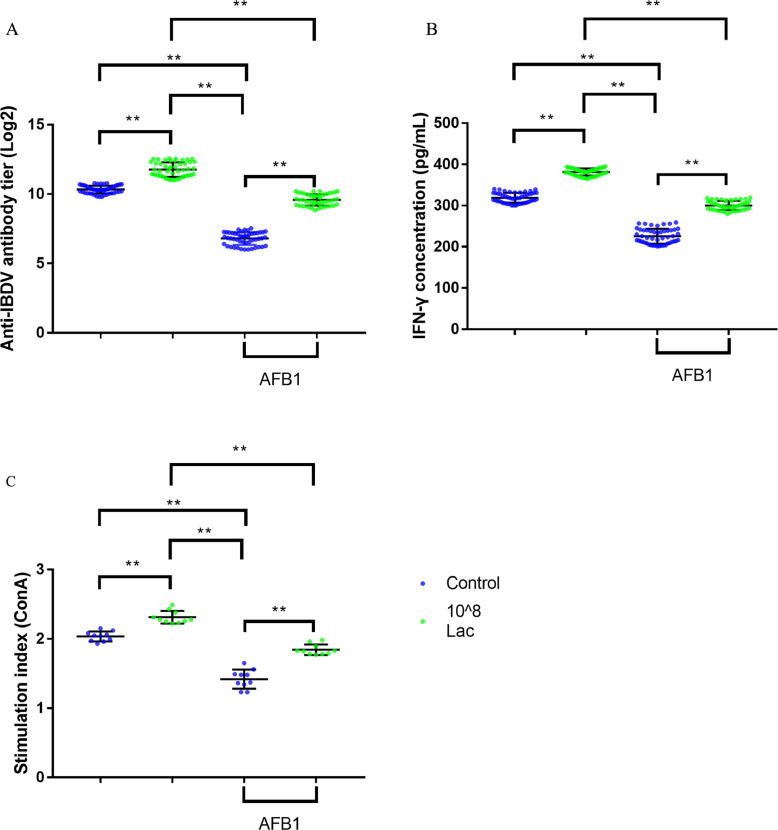
Compared to the control group, there were significantly reduced levels of serum specific antibody levels of IBDV and serum IFN-γ in AFB1-treated group post 14 d vaccine immunization (Figures 5A, B, all P < 0.01). While, markedly increased levels of serum specific antibody levels of IBDV and serum IFN-γ were noted in Lactobacillus salivarius supplementation group post 14 d immunization compared to the control and AFB1-treated group (Figures 5A, B, all P < 0.01). Compared to the control group, broilers fed with AFB1-contaminated diet significantly reduced lymphocyte proliferation induced by ConA 14-d immunization (Figure 5C, P < 0.01). Compared to the control and AFB1-treated group, Lactobacillus salivarius supplementation significantly enhanced lymphocyte proliferation induced by ConA (Figure 5C, P < 0.01). These results indicated that Lactobacillus salivarius can not only alleviated the immune suppression caused by AFB1, but also enhanced the immune response as an immune adjuvant.
Figure 5.
Effects of dietary Lactobacillus salivarius supplementation on immune response of broilers challenged with AFB1. (A) Serum anti-IBDV specific antibody titers, which were measured 14-d postimmunization (n = 50). Log 2 titers below 8.63 (which corresponds to S/P ratio < 0.2) are considered negative and above 8.63 (S/P ratio > 0.2) are considered positive. (B) Serum IFN-γ levels, which were measured 14-d postimmunization (n = 50). (C) Lymphocyte proliferation response in chickens, which were measured 14-d postimmunization (n = 10). Two-way ANOVA for repeated measurements, followed by Tukey tests. ⁎⁎Indicated P < 0.01. Lac, Lactobacillus salivarius.
Effects of Lactobacillus salivarius Supplementation on Salmonella Pullorum Infection
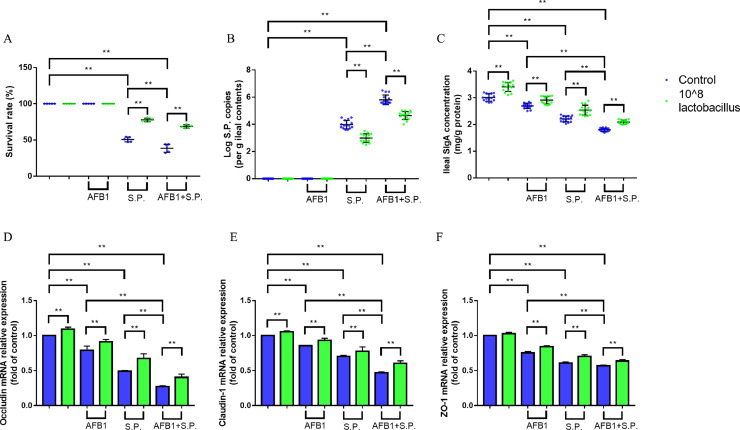
Compared to the control group, broilers fed with AFB1-contaminated diet did not affect survive rate (Figure 6A). However, Salmonella Pullorum challenge significantly reduced survive rate of broilers and increased Salmonella Pullorum colonization in digestive tract (Figures 6A, B, all P < 0.01). The mRNA expression levels of Occludin, Claudin-1, and ZO-1, and intestinal SIgA content reflect the intestinal barrier function state, AFB1 or Salmonella Pullorum challenge significantly reduced the mRNA expression levels of Occludin, Claudin-1, and ZO-1, and intestinal SIgA content (Figures 6C–F, all P < 0.01). AFB1 or Salmonella Pullorum challenge also significantly increased pro-inflammatory cytokines TNF-α and IL-1β levels, and decreased intestinal beneficial metabolites acetic acid, propionic acid, and butyric acid (Table 3, all P < 0.01). Moreover, AFB1 aggravated Salmonella Pullorum infection, which is characterized by further decreased survive rate and the beneficial metabolites, worse barrier function, increased Salmonella Pullorum colonization in digestive tract and pro-inflammatory cytokines (Figure 6 and Table 3, all P < 0.01). Treatment of broilers with 108 CFU/kg Lactobacillus salivarius could alleviate the adverse effect of AFB1 or/and Salmonella Pullorum challenge (Figure 6 and Table 3, all P < 0.01).
Figure 6.
Effects of dietary Lactobacillus salivarius supplementation on Salmonella Pullorum infection resistance of broilers challenged with AFB1. (A) Survival rate of broilers following Salmonella Pullorum challenge (n = 5). Each point represents the result from an independent experiment and bars represent mean ± SD. (B) Salmonella Pullorum colonization in ileal contents at 14 d post-Salmonella Pullorum infection (n = 15). (C) Secretory IgA (sIgA) concentration of ileum mucosa, which was measured 14 d post-Salmonella Pullorum challenge (n = 15). (D–F) Tight junction related gene mRNA expression of ileum, which were measured 14 d post-Salmonella Pullorum challenge (n = 6). Two-way ANOVA for repeated measurements, followed by Tukey tests. ⁎⁎ indicates P < 0.01. Lac, Lactobacillus salivarius. S.P., Salmonella Pullorum.
Table 3.
Effects of Lactobacillus salivarius on shorter-chain fatty acids (SCFAs) levels of feces and proinflammatory cytokines of ileal tissues.
| Con | 108 Lac | Con + AFB1 | 108 Lac + AFB1 | Con+ S.P. | 108 Lac + S.P. | Con + AFB1+ S.P. | 108 Lac + AFB1 +S.P. | |
|---|---|---|---|---|---|---|---|---|
| Acetic acid (mmol/g feces) | 69.56 ± 3.45 | 80.20 ± 2.92⁎⁎ | 60.90 ± 2.84⁎⁎ | 75.77 ± 3.32## | 53.49 ± 1.79⁎⁎ | 66.49 ± 3.03&& | 42.63 ± 1.55⁎⁎ | 58.89 ± 6.87^^ |
| Propionic acid (mmol/g feces) | 21.66 ± 1.37 | 26.93 ± 3.23⁎⁎ | 19.06 ± 1.76 ⁎⁎ | 26.72 ± 1.60## | 15.79 ± 1.41⁎⁎ | 22.63 ± 1.76 && | 13.85 ± 1.14 ⁎⁎ | 19.03 ± 1.15^^ |
| Butyric acid (mmol/g feces) | 44.33 ± 2.81 | 51.79 ± 1.05⁎⁎ | 40.68 ± 2.06⁎⁎ | 51.09 ± 1.59 ## | 34.47 ± 1.76⁎⁎ | 48.77 ± 1.01&& | 30.09 ± 1.65⁎⁎ | 40.82 ± 2.02^^ |
| IL-1β (pg/mL) | 2.01 ± 0.12 | 1.99 ± 0.92 | 3.06 ± 0.25⁎⁎ | 2.53 ± 0.21## | 3.89 ± 0.17⁎⁎ | 3.22 ± 0.16&& | 7.10 ± 0.32⁎⁎ | 4.83 ± 0.26^^ |
| TNF-α (pg/mL) | 7.99 ± 0.10 | 8.03 ± 0.32 | 9.89 ± 0.29⁎⁎ | 9.04 ± 0.17## | 11.85 ± 0.42⁎⁎ | 10.93 ± 0.62&& | 13.99 ± 0.34⁎⁎ | 12.72 ± 0.52^^ |
The data are expressed as mean ± SD of n = 15 chickens per group.
P < 0.01 for control group versus other groups.
P < 0.01 for Con + AFB1 group versus 108 Lac + AFB1 group.
P < 0.01 for Con+ S.P. group versus 108 Lac + S.P. group.
P < 0.01 for Con + AFB1+ S.P. group versus 108 Lac + AFB1 +S.P. group.
Abbreviations: Con, Control group; Lac, Lactobacillus salivarius; S.P., Salmonella Pullorum.
DISCUSSION
Broilers are extremely vulnerable to the toxic and carcinogenic action of AFB1, resulting in large economic losses to farmers due to decreased growth performance, increased susceptibility to disease, and other negative effects (Micco et al., 1988; Peng et al., 2014). Undoubtedly, preventing AFB1 contamination of feed is the top choice; however, since prevention is not always possible (Śliżewska et al., 2019), thus, it is necessary to develop a cost-effective way to detoxify AFB1 contaminated feed. The use of probiotics as feed additives in poultry industry has increased considerably for decades (Wang et al., 2020). In addition to promoting growth, probiotics have been found to degrade AFB1. For example, Lactobacillus plantarum FJS003 exhibited great potential and immense value in detoxifying AFB1 because of Lactobacillus plantarum FJS003 could inhibit Aspergillus flavus growth (inhibitory rate is 42.8%) and degrade AFB1 (degradation rate is 89.5%) (Zhu et al., 2021). In the present study, we evaluated the ability of Lactobacillus salivarius Erya to degrade AFB1. Lactobacillus salivarius Erya could alleviate the harmful effects of AFB1 effectively, and decreased AFB1 content by 65.3% and 91.5% after aerobically cultured for 24 h and 72 h, respectively. On the one hand, Lactobacillus could secrete heat-resistant enzyme to degrade AFB1. On the other hand, Lactobacillus has ability to bind AFB1 (Zhu et al., 2021). However, the exact mechanisms of AFB1 degradation involved the Lactobacillus salivarius Erya used in the present study are still unclear, which need further study.
In recent study, multiple beneficial effects, such as growth promotion and stress resistance, of Lactobacillus salivarius Erya were confirmed in laying hens (Wang et al., 2020). In the present study, dietary supplementation of the 108 and 109 CFU/kg Lactobacillus salivarius Erya improved body weight, feed conversion ratio, and improved dressing percentage of broilers, which indicated that this Lactobacillus salivarius Erya has great potential as a feed additive. In addition, 107, 108 and 109, CFU/kg Lactobacillus salivarius Erya alleviated the adverse effects of AFB1 on growth performance and dressing percentage, which is perhaps via degrading AFB1 to reduced AFB1 exposure to broilers, and inhibiting gut pathogens, thus decreasing the nutrient consumption required for maintaining immunological function (Gao et al., 2017). Compared to 108 and 109 CFU/kg Lactobacillus salivarius group, there were significant differences in growth performance of chickens fed with the diet of 107 CFU/kg Lactobacillus salivarius, which was similar with previous studies and indicated that Lactobacillus salivarius Erya need to reach a certain amount before they can be beneficial to the host (Lee et al., 2020; Wang et al., 2020).
Liver is the major accumulation organ of AFB1, oxidative stress has been stated to have a vital role in AFB1-induced hepatotoxicity (Singh et al., 2015). AFB1 can promote the accumulation of reactive oxygen species (ROS) and free radicals that exceed antioxidant activity of chickens, thereby increasing lipid peroxidation and apoptosis in liver (Fouad et al., 2019). Previous studies have confirmed the antioxidant activity of various Lactobacillus salivarius (Peran et al., 2005; Lee et al., 2020). For instance, administration of the Lactobacillus salivarius CECT5713, isolated from human milk, alleviated oxidative stress, and thus facilitated the recovery of the inflamed tissue in a rat colitis model (Peran et al., 2005). In addition, Lactobacillus salivarius BP121, isolated from isolated from infant feces could protect against cisplatin-induced acute kidney injury (AKI) by decreasing inflammation and oxidative stress in an AKI rat model (Lee et al., 2020). In our recent study, the Lactobacillus salivarius Erya, isolated from the feces of healthy chickens, exhibited good performance to reduce oxidative stress in both acute heat stress and circular heat stress condition in laying hens (Wang et al., 2020). Similarly, Lactobacillus salivarius Erya also enhanced antioxidative capacity of liver in the present study to reduce AFB1-induced liver injury in broilers in a dose-dependent manner. In the present study, AFB1 caused meat quality reduction which was characterized by increased drip loss and shear force. Previous studies showed that both low and high dose AFB1 in feeds could increase MDA content in in poultry meat (Fouad et al., 2019), and this could be the reason of AFB1-induced meat quality reduction.
AFB1 can induce oxidative stress and apoptosis in immune organs, which were characterized by increased MDA content and suppressed antioxidant enzymes activities (Fouad et al., 2019; Peng et al., 2017). This could be responsible for immune organs malfunction by AFB1, thus leading to significant reduction of antibodies and the numbers of lymphocytes (Fouad et al., 2019; Peng et al., 2017). In the present study, we also evaluated 108 CFU/kg Lactobacillus salivarius Erya as feed additive that effect immune responses in chickens exposed to AFB1. The results confirmed that 108 CFU/kg Lactobacillus salivarius Erya supplementation alleviated the immunosuppression caused by AFB1 to a certain extent and promoted the production of specific IBDV antibodies. This may be because Lactobacillus salivarius not only can elevate antioxidative ability to resist oxidative stress, but also promote the development of immune organs (Peran et al., 2005; Lee et al., 2020; Wang et al., 2020). Moreover, the results also indicated that 108 CFU/kg Lactobacillus salivarius group showed an enhanced immune response after IBDV vaccine immunization compared to the control group in chickens without AFB1 treatment. This is consistent with previous findings that Lactobacillus can be used as immune adjuvant to promote antigen-specific antibody production (Aleksandra et al., 2016; Choi et al., 2018; Fouad et al., 2019).
In addition to immunosuppression, AFB1 can also induce intestinal dysfunction including disruption of intestinal barrier and gut microbiota disorder (Chen et al., 2016; Chang et al., 2020), which provide opportunities for Salmonella infection. In the present study, AFB1 aggravated Salmonella infection, while 108 CFU/kg Lactobacillus salivarius Erya supplement alleviated AFB1 or/and Salmonella caused intestinal injury. This may be due to AFB1 that could decrease the expression levels of tight junction protein related genes Occludin, Claudin-1 and ZO-1, which indicated that the physical barriers of intestinal mucosa were destroyed and the ability to resist intestinal pathogen infection was weakened (Wang et al., 2020). While, 108 CFU/kg Lactobacillus salivarius Erya supplementation not only can degrade AFB1, but also improve gut barrier function (Wang et al., 2020). Besides regulating gut barrier, Lactobacillus can not only directly inhibit the growth of Salmonella through its own metabolism (Kowalska et al., 2020), but also play an important role against Salmonella infection by regulating intestinal microbiota (Deng et al., 2021). In the present study, 108 CFU/kg Lactobacillus salivarius Erya supplementation significantly increased the beneficial metabolites SCFAs levels. SCFAs, mainly acetic acid, propionic acid and butyric acid, are the metabolic products of gut microbiota. High levels of SCFAs inhibit the growth of gut pathogenic bacteria, especially, gut commensal-derived propionic acid enhances colonization resistance to Salmonella infection (Jacobson et al., 2018).
In conclusion, the present study confirmed that the Lactobacillus salivarius Erya supplementation improved growth performance and liver function, enhanced immune response and ameliorated the negative effects of AFB1 and Salmonella infection. These findings support Lactobacillus salivarius Erya as an effective feed additive in the poultry industry.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (STIP, 2021L161) and the project of Science and Technology Innovation Fund of Shanxi Agricultural University (2021BQ75).
DISCLOSURES
All authors declared that there are no potential conflicts of interests.
REFERENCES
- Aleksandra I., Marijana S., Emilija M., Elisabeth B., Elisabeth S., Ivana L., Radmila D., Nadine S., Johannes H., Talin A. A probiotic adjuvant Lactobacillus rhamnosus enhances specific immune responses after ocular mucosal immunization with chlamydial polymorphic membrane protein C. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S., Benes V., Garson J., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M., Shipley G., Vandesompele J., Wittwer C. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Chang J., Wang T., Wang P., Yin Q., Liu C., Zhu Q., Lu F., Gao T. Compound probiotics alleviating aflatoxin B 1 and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotoxicol. Environ. Saf. 2020;194 doi: 10.1016/j.ecoenv.2020.110420. [DOI] [PubMed] [Google Scholar]
- Chen X., Naehrer K., Applegate T. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016;95:1312–1325. doi: 10.3382/ps/pew022. [DOI] [PubMed] [Google Scholar]
- Chlebicz A., Śliżewska K. In vitro detoxification of Aflatoxin B1, deoxynivalenol, fumonisins, T-2 toxin and zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast. Probiotics. Antimicrob. Proteins. 2020;12:289–301. doi: 10.1007/s12602-018-9512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D., Jung S., Kang J., Nam Y., Lim S., Kim K., Shin H. Immune-enhancing effect of nanometric Lactobacillus plantarum nF1 (nLp-nF1) in a mouse model of cyclophosphamide-induced immunosuppression. J. Microbiol. Biotechnol. 2018;28:218–226. doi: 10.4014/jmb.1709.09024. [DOI] [PubMed] [Google Scholar]
- Deng Z., Han D., Wang Y., Wang Q., Yan X., Wang S., Liu X., Song W., Ma Y. Lactobacillus casei protects intestinal mucosa from damage in chicks caused by Salmonella Pullorum via regulating immunity and the Wnt signaling pathway and maintaining the abundance of gut microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwan H., Xie C., Miao L., Dong X., Zou X., Mohany M., Mohany M., Al-Rejaie S., Al-Rejaie S. Methionine alleviates aflatoxinb1-induced broiler chicks embryotoxicity through inhibition of caspase-dependent apoptosis and enhancement of cellular antioxidant status. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh M., Shi Z., Ahmadzadeh M., Hu L., Ghassempour A. Inhibition of the Aspergillus flavus growth and Aflatoxin B1 contamination on pistachio nut by fengycin and surfactin-producing Bacillus subtilis UTBSP1. Plant. Pathol. J. 2016;32:209–215. doi: 10.5423/PPJ.OA.11.2015.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad A., Ruan D., El-Senousey H., Chen W., Jiang S., Zheng C. Harmful effects and control strategies of Aflflatoxin B1 produced by Aspergillus flflavus and Aspergillus parasiticus strains on poultry: review. Toxins (Basel) 2019;11:176. doi: 10.3390/toxins11030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P., Ma C., Sun Z., Wang L., Huang S., Su X., Xu J., Zhang H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome. 2017;5:91. doi: 10.1186/s40168-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Chang J., Wang P., Yin Q., Liu Chao., Xu X., Dang X., Hu X., Hu X., Wang Q. Effects of compound probiotics and aflatoxin-degradation enzyme on alleviating aflatoxin-induced cytotoxicity in chicken embryo primary intestinal epithelium, liver and kidney cells. AMB Express. 2021;11:35. doi: 10.1186/s13568-021-01196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Ramírez J., Merino-Guzmán R., Téllez-Isaías G., Vázquez-Durán A., Méndez-Albores A. Mitigation of AFB1-related toxic damage to the intestinal epithelium in broiler chickens consumed a yeast cell wall fraction. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.677965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A., Lam L., Rajendram M., Tamburini F., Honeycutt J., Pham T., Treuren W., Pruss K., Stabler S., Lugo K., Bouley D., Vilches-Moure J., Smith M., Sonnenburg J., Bhatt A., Huang K., Monack D. A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell. Host. Microbe. 2018;24 doi: 10.1016/j.chom.2018.07.002. 296-307.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska J., Nowak A., Śliżewska K., Stańczyk M., Łukasiak M., Dastych J. Anti-Salmonella potential of new Lactobacillus strains with the application in the poultry industry. Pol. J. Microbiol. 2020;69:5–18. doi: 10.33073/pjm-2020-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Liu R., Wei G., Guo G., Yu H., Zhang Y., Ishfaq M., Fazilani S., Zhang X. Curcumin protects against Aflatoxin B1-induced liver injury in broilers via the modulation of long non-coding RNA expression. Ecotoxicol. Environ. Saf. 2021;208 doi: 10.1016/j.ecoenv.2020.111725. [DOI] [PubMed] [Google Scholar]
- Lee T., Park D., Kim Y., Lee I., Kim S., Oh C., Kim J., Yang J., Jo S. Lactobacillus salivarius BP121 prevents cisplatin-induced acute kidney injury by inhibition of uremic toxins such as lndoxyl sulfate and p-cresol sulfate via alleviating dysbiosis. Int. J. Mol. Med. 2020;45:1130–1140. doi: 10.3892/ijmm.2020.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Gao X., Zhou T., Zhao L., Fan Y., Li X., Lei Y., Ji C., Zhang J. Protective effect of Bacillus subtilis ANSB060 on egg quality, biochemical and histopathological changes in layers exposed to aflatoxin B1. Poult. Sci. 2012;91:2852–2857. doi: 10.3382/ps.2012-02474. [DOI] [PubMed] [Google Scholar]
- Micco C., Miraglia M., Onori R., Brera C., Mantovani A., Ioppolo A., Stasolla D. Long-term administration of low doses of mycotoxins to poultry. 1. Residues of aflatoxin B1 and its metabolites in broilers and laying hens. Food Addit. Contam. 1988;5:303–308. doi: 10.1080/02652038809373708. [DOI] [PubMed] [Google Scholar]
- Peng X., Zhang S., Fang J., Cui H., Zuo Z., Deng J. Protective roles of sodium selenite against aflatoxin B1-induced apoptosis of jejunum in broilers. Int. J. Environ. Res. Public. Health. 2014;11:13130–13143. doi: 10.3390/ijerph111213130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Bai S., Ding X., Zhang K. Pathological impairment, cell cycle arrest and apoptosis of thymus and bursa of fabricius induced by aflatoxin-contaminated corn in broilers. Int. J. Environ. Res. Publ. Health. 2017;14:77. doi: 10.3390/ijerph14010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peran L., Camuesco D., Comalada M., Nieto A., Concha A., Diaz-Ropero M., Olivares M., Xaus J., Zarzuelo A., Galvez J. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rat colitis. World J. Gastroenterol. 2005;11:5185–5192. doi: 10.3748/wjg.v11.i33.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloni V., Magnoli A., Fochesato A., Cristofolini A., Caverzan M., Merkis C., Montenegro M., Cavaglieri L. A Saccharomyces cerevisiae RC016-based feed additive reduces liver toxicity, residual aflatoxin B1 levels and positively influences intestinal morphology in broiler chickens fed chronic aflatoxin B1-contaminated diets. Anim. Nutr. 2020;6:31–38. doi: 10.1016/j.aninu.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi M., Brake J., Hamilton P., Hagler Jr W., Nesheim S. Dietary exposure of broiler breeders to aflatoxin results in immune dysfunction in progeny chicks. Poult. Sci. 1998;77:812–819. doi: 10.1093/ps/77.6.812. [DOI] [PubMed] [Google Scholar]
- Ragoubi C., Quintieri L., Greco D., Mehrez A., Maatouk I., D'Ascanio V., Landoulsi A., Avantaggiato G. Mycotoxin removal by Lactobacillus spp. and their application in animal liquid feed. Toxins (Basel) 2021;13:185. doi: 10.3390/toxins13030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa C., Miazzo R., Magnoli C., Salvano M., Chiacchiera S., Ferrero S., Saenz M., Carvalho E., Dalcero A. Evaluation of the efficacy of bentonite from the south of Argentina to ameliorate the toxic effects of aflatoxin in broilers. Poult. Sci. 2001;80:139–144. doi: 10.1093/ps/80.2.139. [DOI] [PubMed] [Google Scholar]
- Rubio M., Filho R., Almeida A., Junior A. Development of a multiplex qPCR in real time for quantification and differential diagnosis of Salmonella gallinarum and Salmonella Pullorum. Avian. Pathol. 2017;46:644–651. doi: 10.1080/03079457.2017.1339866. [DOI] [PubMed] [Google Scholar]
- Rushing B., Selim M. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food. Chem. Toxicol. 2019;124:81–100. doi: 10.1016/j.fct.2018.11.047. [DOI] [PubMed] [Google Scholar]
- Shivachandra S., Sah R., Singh S., Kataria J., Manimaran K. Immunosuppression in broiler chicks fed aflatoxin and inoculated with fowl adenovirus serotype-4 (FAV-4) associated with hydropericardium syndrome. Vet. Res. Commun. 2003;27:39–51. doi: 10.1023/a:1022058623634. [DOI] [PubMed] [Google Scholar]
- Singh K., Maurya B., Trigun S. Activation of oxidative stress and inflammatory factors could account for histopathological progression of aflatoxin-B1 induced hepatocarcinogenesis in rat. Mol. Cell. Biochem. 2015;401:185–196. doi: 10.1007/s11010-014-2306-x. [DOI] [PubMed] [Google Scholar]
- Śliżewska K., Cukrowska B., Smulikowska S., Cielecka-Kuszyk J. The effect of probiotic supplementation on performance and the histopathological changes in liver and kidneys in broiler chickens fed diets with Aflatoxin B1. Toxins (Basel) 2019;11:112. doi: 10.3390/toxins11020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila-Donat P., Marín S., Sanchis V., Ramos A. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food. Chem. Toxicol. 2018;114:246–259. doi: 10.1016/j.fct.2018.02.044. [DOI] [PubMed] [Google Scholar]
- Wang J., Ishfaq M., Guo Y., Chen C., Li J. Assessment of probiotic properties of Lactobacillus salivarius isolated from chickens as feed additives. Front. Vet. Sci. 2020;7:415. doi: 10.3389/fvets.2020.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wu J., Liu Z., Shi Y., Liu J., Xu X., Hao S., Mu P., Deng F., Deng Y. Aflatoxin B 1 degradation and detoxification by Escherichia coli CG1061 isolated from chicken cecum. Front. Pharmacol. 2019;9:1548. doi: 10.3389/fphar.2018.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wang C., Zhao X., Chen K., Geng Z. Effect of L-theanine on meat quality, muscle amino acid profiles, and antioxidant status of broilers. Anim. Sci. J. 2020;91:e13351. doi: 10.1111/asj.13351. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Hassan Y., Watts C., Zhou T. Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients-a review of recent patents. Anim. Feed. Sci. Technol. 2016;216:19–29. [Google Scholar]
- Zhu Y., Xu Y., Yang Q. Antifungal properties and AFB1 detoxification activity of a new strain of Lactobacillus plantarum. J. Hazard. Mater. 2021;414 doi: 10.1016/j.jhazmat.2021.125569. [DOI] [PubMed] [Google Scholar]