Key Points
Question
What is the discriminative accuracy of COPD screening instruments in 3 low- and middle-income country settings?
Findings
In this cross-sectional study that included 10 709 adults in Nepal, Peru, and Uganda, 3 screening instruments for COPD were feasible to administer and, using postbronchodilator spirometry as the reference standard, yielded area under receiver operating characteristic curves ranging from 0.72 to 0.79.
Meaning
This study demonstrated that 3 screening instruments for COPD were feasible to administer in low- and middle-income settings, although further research is needed to assess their performance in other settings and determine whether implementation is associated with improved clinical outcomes.
Abstract
Importance
Most of the global morbidity and mortality in chronic obstructive pulmonary disease (COPD) occurs in low- and middle-income countries (LMICs), with significant economic effects.
Objective
To assess the discriminative accuracy of 3 instruments using questionnaires and peak expiratory flow (PEF) to screen for COPD in 3 LMIC settings.
Design, Setting, and Participants
A cross-sectional analysis of discriminative accuracy, conducted between January 2018 and March 2020 in semiurban Bhaktapur, Nepal; urban Lima, Peru; and rural Nakaseke, Uganda, using a random age- and sex-stratified sample of the population 40 years or older.
Exposures
Three screening tools, the COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE; range, 0-6; high risk indicated by a score of 5 or more or score 2-5 with low PEF [<250 L/min for females and <350 L/min for males]), the COPD in LMICs Assessment questionnaire (COLA-6; range, 0-5; high risk indicated by a score of 4 or more), and the Lung Function Questionnaire (LFQ; range, 0-25; high risk indicated by a score of 18 or less) were assessed against a reference standard diagnosis of COPD using quality-assured postbronchodilator spirometry. CAPTURE and COLA-6 include a measure of PEF.
Main Outcomes and Measures
The primary outcome was discriminative accuracy of the tools in identifying COPD as measured by area under receiver operating characteristic curves (AUCs) with 95% CIs. Secondary outcomes included sensitivity, specificity, positive predictive value, and negative predictive value.
Results
Among 10 709 adults who consented to participate in the study (mean age, 56.3 years (SD, 11.7); 50% female), 35% had ever smoked, and 30% were currently exposed to biomass smoke. The unweighted prevalence of COPD at the 3 sites was 18.2% (642/3534 participants) in Nepal, 2.7% (97/3550) in Peru, and 7.4% (264/3580) in Uganda. Among 1000 COPD cases, 49.3% had clinically important disease (Global Initiative for Chronic Obstructive Lung Disease classification B-D), 16.4% had severe or very severe airflow obstruction (forced expiratory volume in 1 second <50% predicted), and 95.3% of cases were previously undiagnosed. The AUC for the screening instruments ranged from 0.717 (95% CI, 0.677-0.774) for LFQ in Peru to 0.791 (95% CI, 0.770-0.809) for COLA-6 in Nepal. The sensitivity ranged from 34.8% (95% CI, 25.3%-45.2%) for COLA-6 in Nepal to 64.2% (95% CI, 60.3%-67.9%) for CAPTURE in Nepal. The mean time to administer the instruments was 7.6 minutes (SD 1.11), and data completeness was 99.5%.
Conclusions and Relevance
This study demonstrated that screening instruments for COPD were feasible to administer in 3 low- and middle-income settings. Further research is needed to assess instrument performance in other low- and middle-income settings and to determine whether implementation is associated with improved clinical outcomes.
This cross-sectional study assesses the discriminative accuracy of 3 screening instruments in identifying chronic obstructive pulmonary disease in Nepal, Peru, and Uganda.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is the third leading cause of mortality globally, causing an estimated 3.23 million deaths in 2019.1,2 The majority of the morbidity and mortality from COPD occurs in low- and middle-income countries (LMICs), with significant economic effects.2
The primary risk factor for COPD in high-income countries is cigarette smoke exposure. COPD can be readily diagnosed using spirometry in adults with chronic respiratory symptoms in the context of an appropriate exposure history.3 LMICs, in contrast, have well-documented challenges in the diagnosis and treatment of COPD, including limited availability of quality-assured spirometry, respiratory specialists, and access to affordable medications, as well as a lack of guidelines tailored to local context.4,5,6,7 Complicating COPD diagnosis in LMICs, COPD can arise from or alongside additional risk factors including indoor and outdoor air pollution, impaired lung growth and development, chronic asthma, and posttuberculosis lung damage.8,9,10 There is a need to better identify and treat persons with COPD in LMIC settings.
Case-finding instruments for COPD comprising questionnaires, some of which also include peak expiratory flow (PEF), have reasonable precision in identifying symptomatic COPD in high-income settings. These instruments can facilitate timely diagnosis and treatment outside specialty settings.11,12 Case-finding has received a conditional recommendation in the international Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy.3 However, the value of such approaches in LMICs where the prevalence, etiology, natural history, and availability of treatment for COPD are different has not been tested.
The objective of this multicountry, population-based study was to assess the discriminative accuracy of 3 simple COPD screening tools in 3 diverse LMIC settings.
Methods
Study Setting
The protocol for the Global Excellence in COPD Outcomes 1 (GECo1) study has been published (protocol and statistical analysis plan available in Supplement 1 and Supplement 2, respectively). A random, age-and sex-stratified sample, based on previous census data, was recruited in 3 diverse LMIC settings: semiurban Bhaktapur, Nepal; urban Lima, Peru; and rural Nakaseke, Uganda. Sites were selected to test the performance of screening tools on different continents and at sites with different degrees of urbanization, COPD risk, and economic development.
The study was conducted between January 2018 and March 2020. All participants provided written informed consent. Ethics permissions were obtained from the University College London Research Ethics Committee, Johns Hopkins School of Medicine, Nepal Health Research Council, A.B. PRISMA in Peru, and the Uganda National Council for Science and Technology, Makerere School of Medicine.
Participants
Individuals were eligible if they were 40 years or older, able to perform spirometry, and full-time residents in the prespecified catchment areas (defined as having lived in the area for more than 6 months). Exclusion criteria were self-reported pregnancy, self-reported active pulmonary tuberculosis or receiving medications for pulmonary tuberculosis, or contraindications to spirometry (eye surgery, thoracic surgery, abdominal surgery, or myocardial infarction in the 3 months prior to study visit or measured blood pressure >180/100 mm Hg at the research assessment). Participants were recruited irrespective of symptoms, and a prior diagnosis of COPD did not exclude participants from the study.
Procedures
Field workers collected socioeconomic information, medical history, and data on exposure history to cigarettes and household air pollution. Current exposure to household air pollution was defined as when the primary source of fuel in the home was biomass (wood, dung, coal, or agricultural crop waste). Current smoking was defined as currently smoking tobacco products. Height and weight were recorded in triplicate and the median measurement was used. Field-workers also conducted prebronchodilator and postbronchodilator spirometry in accordance with American Thoracic Society/European Respiratory Society guidelines.13 Data were entered into REDCap using tablets (Asus Z380M ZenPad). Local and central quality assurance of spirometry was performed as described in the study protocol (Supplement 1). Participants with abnormal results were referred to local health centers and provided with basic information related to smoking and air pollution.
Screening Instruments
The performance of 3 existing screening instruments, 2 of which included PEF, was assessed against a reference-standard diagnosis of COPD using spirometry. The instruments were the COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE),11 the Lung Function Questionnaire (LFQ),12 and the COPD in LMICs Assessment (COLA-6) tool.14 The scores in each tool can be dichotomised to identify participants at risk of having COPD. CAPTURE has an overall range of 0 to 6 and defines high risk as a score of 5 or more or an intermediate score (2-5) with low PEF (<250 L/min for females or <350 L/min for males). It was developed to identify moderate to severe COPD and people at high risk for COPD exacerbation.11 The LFQ considers people with a score of 18 or less to be at risk for airflow obstruction (overall range, 0-25).12 A COLA-6 score of 4 or more is considered to indicate higher risk for airflow obstruction (overall range, 0-5); COLA-6 incorporates a measurement of PEF.14
Definition of COPD
COPD was defined as a ratio of postbronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) less than the fifth percentile of the Global Lung Function Initiative mixed ethnic population reference lower limit of normal (LLN), ie, a z score less than –1.645 using age as an integer.15 Reversibility was defined as a difference between prebronchodilator and postbronchodilator FEV1 of more than 200 mL, a percent increase greater than 12%, or both.15
Outcomes
The primary outcome was the discriminative accuracy of the 3 screening instruments for identifying COPD as measured by area under receiver operating characteristic (ROC) curves (AUCs) using the previously published thresholds. Secondary outcomes included the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Prevalence estimates were calculated as percentages and were weighted by population. COPD severity was assessed using the COPD Assessment Test (range, 0–40; minimum clinically important difference [MCID], 2; lower score represents lower severity), modified MRC Questionnaire (range, 0–4; MCID, 1; lower score represents less severe breathlessness) and questionnaires assessing the frequency of exacerbations over the preceding 12 months as per GOLD criteria.16,17,18,19 This enables classification of people with COPD into GOLD groups A through D.4 Clinically important disease was defined as GOLD group B, C, or D (indicating significant symptoms [B], significant exacerbation risk [C], or both significant symptoms and significant exacerbation risk [D]). Health-related quality of life was measured using EuroQol-5D instruments (EQ-5D-3L and EQ-5D-5L; range, 0–100; MCID, 0.028; higher scores indicate better quality of life).20
Time taken to deliver CAPTURE, COLA-6, and LFQ was measured across settings to assess feasibility, and data completeness was considered to inform fidelity across sites.
Statistical Methods
The sample size required to estimate the AUC within 1.5% (based on a 95% CI) was 9669 participants, assuming 90% sensitivity and 60% specificity and assuming 11% COPD prevalence from previous studies conducted in LMIC settings.10 To ensure an adequate sample size to support recruitment to a separate randomized clinical trial of supported self-management (GECo2), the sample size was increased to 10 500 participants.21
Using ROC analysis, the discriminative accuracy of CAPTURE, COLA-6, and LFQ in identifying cases of COPD was assessed using spirometry as the reference standard, reporting AUC with 95% CI for each study site. Comparisons of AUCs between the 3 possible pairs of diagnostic tools were performed using an algorithm suggested by DeLong et al.22 Using prepublished threshold values to identify participants at high risk of COPD, sensitivity, specificity, PPV, NPV, and accuracy were calculated with 95% CIs. Thresholds were also identified at each site for COLA-6 and the LFQ to maximize sensitivity to at least 90%. To allow for uniform sampling to population size, analyses were weighted based on census information. A preplanned secondary analysis was conducted examining discriminative accuracy of the instruments when identifying clinically important (GOLD group B-D) COPD and using a fixed FEV1:FVC ratio less than 0.7 (rather than LLN) as advocated by GOLD.3
Characteristics of individuals with and without COPD were compared using standard 2-sample tests (t tests and χ2 test), with linear regression used for a comparison of quality of life scores (adjusted for age, sex, current smoker [yes/no], current biomass exposure [yes/no], education level [primary incomplete/primary/secondary/higher education], and comorbidities [diagnosis of heart disease, yes/no; tuberculosis, yes/no; diabetes, yes/no]). Participant characteristics were compared between false-positive and true-negative results and between false-negative and true-positive results, as classified by each questionnaire, using t tests for continuous variables and χ2 tests for categorical variables. This analysis considered all 3 tools at all 3 sites, and results are reported when there was a statistically significant difference in the majority of scenarios (≥5/9 instances) and all statistically significant differences were in the same direction.
All P values were 2-sided, and P < .05 was taken as statistically significant. Because of the potential for type I error due to multiple comparisons, findings for secondary analyses and end points should be interpreted as exploratory. Each analysis used all available data, those with missing values were excluded. Analyses were conducted following a predefined analysis plan using STATA version 15 or above (StataCorp LLC) and R version 4.0.2 or above (R Foundation for Statistical Computing).
Results
Participant Characteristics
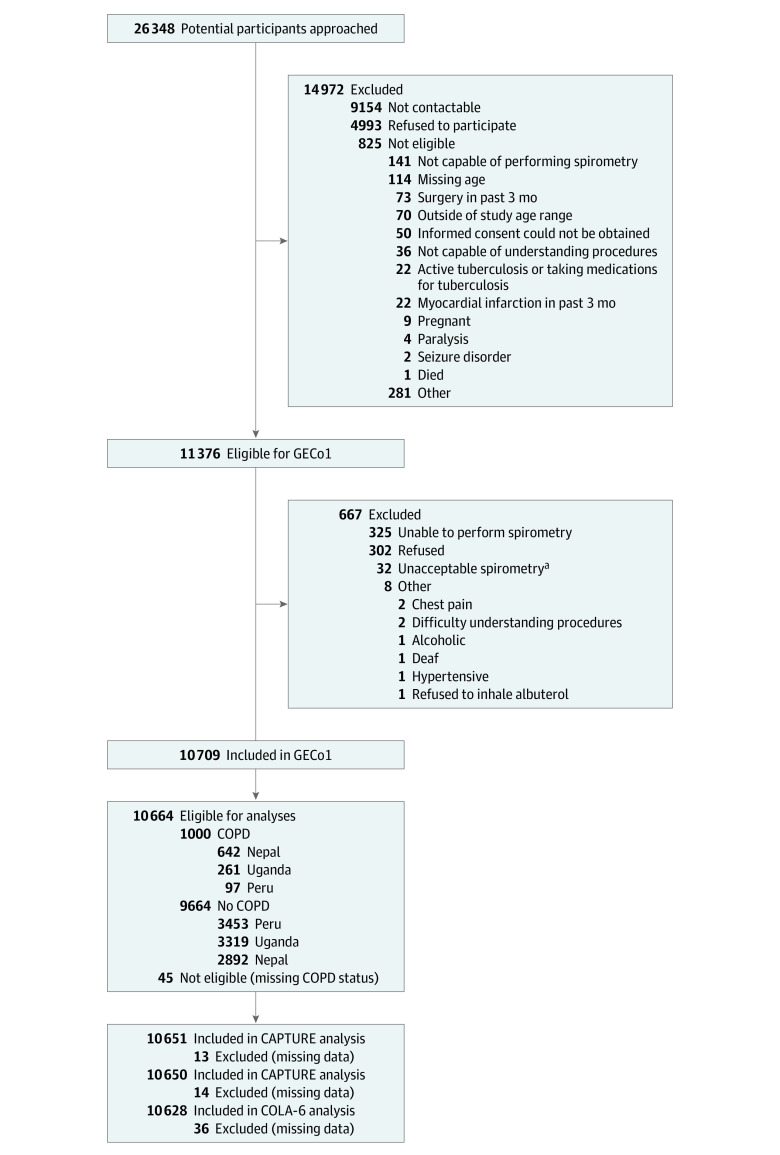
A total of 26 348 people were approached, of whom 11 376 were contactable and eligible (Figure 1) and 10 709 consented to participate. The characteristics of those consented are reported in Table 1. Overall, the mean age was 56.3 (SD, 11.7) years, and 50% of participants were women; 35% had ever smoked, and 30% were currently exposed to biomass smoke (but exposures varied across sites). Nearly all participants (99.2%) had data available for the primary analysis, and 96.1% of spirometry met Grade A through B American Thoracic Society/European Respiratory Society criteria (Supplement 1).
Figure 1. STARD Diagram of Participant Flow Through the GECo1 Study.
CAPTURE indicates COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk; COPD, chronic obstructive pulmonary disease; COLA-6, COPD in Low- and Middle-Income Countries Assessment; GECo1, Global Excellence in COPD Outcomes 1; LFQ, Lung Function Questionnaire; STARD, Standards for Reporting Diagnostic Accuracy Studies.
aGrade F according to American Thoracic Society/European Respiratory Society guidelines.13
Table 1. Characteristics of Study Participants in Bhaktapur, Nepal; Lima, Peru; and Nakaseke, Uganda.
| No. (%) | |||
|---|---|---|---|
| Nepal (n = 3534) | Peru (n = 3551) | Uganda (n = 3624) | |
| Age, mean (SD), ya | 56.2 (11.7) | 56.6 (11.3) | 56.1 (12.1) |
| Women | 1769 (50.1) | 1768 (49.8) | 1847 (51.0) |
| Men | 1765 (49.9) | 1783 (50.2) | 1777 (49.0) |
| Ever smoked | 1148 (32.5) | 1975 (55.6) | 598/3621 (16.5) |
| Current smoker | 355 (9.8) | 727/3550 (20.6) | 559/3621 (15.8) |
| Current biomass exposurea | 187 (5.3) | 16/3549 (0.5) | 2966/3608 (82.2) |
| BMI, mean (SD)b | 25.98 (4.1) | 29.67 (4.7) | 23.11 (4.7) [n = 3611] |
| Education | |||
| Never attended | 1686 (47.7) | 133/3545 (3.8) | 744/3605 (20.6) |
| Primary school completed | 464 (13.1) | 720/3545 (20.3) | 625/3605 (17.3) |
| Secondary/high school completed | 566 (16.0) | 1599/3545 (45.1) | 186/3605 (5.2) |
| Employment | |||
| Employed | 2522/2925 (86.2) | 1964/2793 (70.3) | 3136/3254 (96.4) |
| Unemployed | 68/2925 (2.3) | 137/2793 (4.9) | 49/3254 (1.5) |
| Other | 335/2925 (11.5) | 692/2793 (24.8) | 42/3254 (1.3) |
| Household size, mean (SD) | 5.13 (2.5) [n = 3553] | 4.93 (2.3) [n = 3550] | 4.85 (2.8) [n = 3621] |
| EQ-5Dc | 0.89 (0.2) | 0.78 (0.2) [n = 3549] | 0.92 (0.1) n = 3603] |
| Questionnaires, mean (SD)d | |||
| CAPTURE | 2.21 (1.4) | 1.43 (1.2) [n = 3549] | 1.28 (1.3) [n = 3604] |
| COLA-6 | 2.01 (1.3) | 1.82 (1.2) [n = 3545] | 2.89 (1.2) [n = 3578] |
| LFQ | 20.14 (2.9) | 19.73 (2.7) [n = 3548] | 20.40 (2.7) [n = 3604] |
| Medical history | |||
| Self-report asthma | 131 (3.7) | 255/3550 (7.2) | 27/3622 (0.8) |
| Self-report COPD | 55 (1.6) | 5/3549 (0.1) | 3/3622 (0.1) |
| Confirmed COPDe,f | 642 (18.2) | 97/3550 (2.7) | 261/3580 (7.3) |
| COPD severity | |||
| 1 (mild) | 218 (33.9) | 50 (51.5) | 77 (29.5) |
| 2 (moderate) | 310 (48.3) | 33 (34.0) | 149 (57.1) |
| 3 (severe) | 97 (15.1) | 12 (12.4) | 29 (11.1) |
| 4 (very severe) | 17 (2.7) | 2 (2.1) | 6 (2.3) |
| GOLD stagingg | |||
| A (low risk, fewer symptoms) | 453 (70.6) | 23 (23.7) | 30/260 (11.5) |
| B (low risk, more symptoms) | 175 (27.3) | 62 (63.9) | 140/260 (53.2) |
| C (high risk, fewer symptoms) | 2 (0.3) | 0 | 14/260 (5.3) |
| D (high risk, more symptoms) | 12 (1.87) | 12 (12.37) | 76/260 (29.3) |
| COPD Assessment Test, mean (SD)h | 7.32 (5.7) [n = 632] | 14.31 (7.0) [n = 91] | 20.89 (6.69) [n = 219] |
| mMRCi | |||
| 0 (better) | 249 (38.8) | 47 (48.5) | 121 (46.4) |
| 1 | 324 (50.5) | 36 (37.1) | 91 (34.9) |
| 2 | 56 (8.7) | 12 (12.4) | 34 (13.0) |
| 3 | 10 (1.6) | 2 (2.1) | 13 (5.0) |
| 4 (worse) | 3 (0.5) | 0 | 2 (0.8) |
| Exacerbations, median (IQR), yj | 0 (0-0) | 0 (0-1) | 0 (0-3) [n = 260] |
Abbreviations: BMI, body mass index; CAPTURE, COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk; COLA-6, COPD in Low- and Middle-Income Countries Assessment; COPD, chronic obstructive pulmonary disease; EQ-5D, EuroQol 5D; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LFQ, Lung Function Questionnaire; mMRC, Modified Medical Research Council Dyspnea Scale.
Individual use of biomass fuel daily for cooking, heating, or both, indoors or outdoors.
Calculated as weight in kilograms divided by height in meters squared.
EuroQol (EQ-5D-3L and EQ-5D-5L) has a range from 0 to 100, with higher scores conferring higher health-related quality of life.
CAPTURE uses a score 5 or more as high risk, or an intermediate score (2-5) with low peak expiratory flow (<250 L/min for women or <350 L/min for men) as high risk (range, 0-6); COLA-6 uses a score of 4 or more to be at risk for airflow obstruction (range, 0-5); LFQ uses a score of 18 or less to be at risk for airflow obstruction (range, 0-25).
Unweighted results.
COPD defined by airflow obstruction on postbronchodilator spirometry.
GOLD stage only reported for participants with confirmed COPD determined by highest CAT or mMRC score and exacerbations.4
Range, 0-40, with higher scores representing higher disease severity.
Range, 0-4, with higher scores representing more severe breathlessness.
Respiratory infections per year.
Prevalence, Severity, and Clinical Importance of COPD
The crude overall prevalence of COPD defined by spirometry was 9.4%, and this varied by site (Table 1). The weighted prevalence of COPD at the 3 sites was 17.7% in Bhaktapur, Nepal; 2.6% in Lima, Peru; and 6.9% in Nakaseke, Uganda. The overall self-reported prevalence of COPD was 0.6%, and 95.3% of participants with COPD were previously unaware of the diagnosis.
Overall, among 1000 participants with COPD, 494 (49.4%) had clinically important (GOLD groups B-D) disease but this varied by site, from 29.4% in Nepal to 76.3% in Peru; 16.4% had severe or very severe airflow obstruction (FEV1<50% predicted, GOLD grade 3 or 4),3 and disease severity also varied by setting, with Peru having a higher proportion of milder (GOLD grade 1) cases (51.5%) compared with Nepal (33.9%) and Uganda (29.5%) (P < .001 by χ2 test). The proportion of COPD cases that were very severe (GOLD grade 4) was 2.7% in Nepal, 2.1% in Peru, and 2.3% in Uganda. When COPD was defined using a fixed ratio (FEV1:FVC <0.70) rather than LLN, the unweighted prevalence of COPD at the 3 sites was 21.5% in Nepal, 4.1% in Peru, and 9.8% in Uganda.
Compared with persons without COPD, those with COPD were older (62.5 vs 55.6 years, P < .001), more likely to be men (59.2% vs 48.8%, P < .001), and more frequently current or past tobacco smokers (50.5% vs 33.2%, P < .001).
Individuals with COPD had lower quality of life (EQ-5D) scores compared with those who did not (0.78 [95% CI, 0.77-0.79] vs 0.89 [95% CI, 0.88-0.90]; P < .001), even after adjusting for age, sex, current smoking status, biomass exposure, education level, and comorbidities (adjusted mean difference, −0.07 [95% CI, −0.08 to −0.06]).23
Performance of COPD Screening Tools
Results from the ROC analyses for CAPTURE, COLA-6, and LFQ are summarized in Figure 2. The weighted AUCs for each questionnaire ranged from 0.717 (95% CI, 0.677-0.774) for LFQ in Peru to 0.791 (95% CI, 0.770-0.809) for COLA-6 in Nepal. Table 2 reports the sensitivity, specificity, PPV, NPV, and accuracy of the 3 screening approaches at each site, based on previously published thresholds to identify people at high risk for COPD. The performance based on thresholds for LFQ and COLA-6 to provide at least 90% sensitivity are also reported in Table 2.
Figure 2. Receiver Operating Characteristic Curves for CAPTURE, COLA-6, and LFQ to Identify Individuals at High Risk for Chronic Obstructive Pulmonary Disease in 3 Low- and Middle-Income Countries.
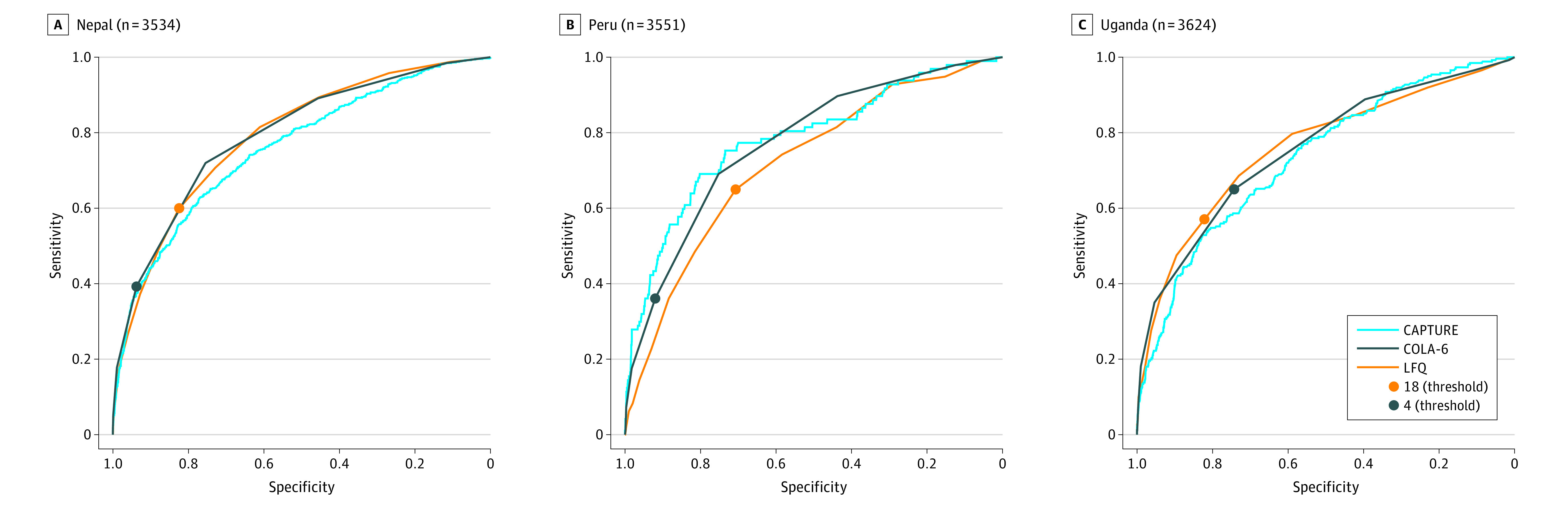
LFQ scores 18 or less and COLA-6 scores 4 or greater signify high risk. See Table 2 for thresholds optimized to 90% sensitivity. Optimization is not possible for CAPTURE, as the instrument does not have a single threshold and is scored in 2 stages. CAPTURE indicates COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk; COLA-6, COPD in Low- and Middle-Income Countries Assessment; LFQ, Lung Function Questionnaire.
Table 2. Weighted Discriminative Performance of CAPTURE, COLA-6, and LFQ to Identify COPD, by Threshold.
| CAPTURE, % (95% CI)a | COLA-6, % (95% CI)a | LFQ, % (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nepal | Peru | Uganda | Nepal | Peru | Uganda | Nepal | Peru | Uganda | |
| By prepublished threshold | |||||||||
| Threshold | ≥4 | ≥4 | ≥4 | ≤18 | ≤18 | ≤18 | |||
| Sensitivity | 64.2 (60.3-67.9) | 53.0 (42.4-63.4) | 50.2 (43.9-56.6) | 38.6 (34.9-42.4) | 34.8 (25.3-45.2) | 61.1 (54.7-67.3) | 59.1 (55.2-62.8) | 62.7 (51.9-72.6) | 54.08 (47.7-60.4) |
| Specificity | 74.9 (73.3-76.5) | 89.1 (88.0-90.1) | 79.9 (78.5-81.2) | 94.0 (93.1-94.8) | 92.1 (91.2-92.9) | 76.9 (75.8-78.0) | 83.1 (81.9-84.4) | 71.8 (70.3-73.2) | 83.9 (82.7-85.0) |
| PPV | 35.4 (32.7-38.2) | 11.4 (8.6-14.8) | 15.6 (13.2-18.2) | 58.0 (53.3-62.7) | 10.5 (7.4-14.3) | 16.4 (14.1-18.8) | 42.9 (39.6-46.2) | 5.58 (4.3-7.1) | 19.8 (17.0-22.9) |
| NPV | 90.7 (89.5-91.8) | 98.6 (98.2-99.0) | 95.6 (94.8-96.3) | 87.7 (86.5-88.8) | 98.2 (97.6-98.6) | 96.4 (95.6-97.1) | 90.4 (89.3-91.5) | 98.6 (98.1-99.1) | 96.1 (95.3-96.8) |
| Accuracy | 73.0 (71.5-74.5) | 88.2 (87.1-89.2) | 77.9 (74.5-79.2) | 84.2 (83.0-85.4) | 90.6 (89.6-91.5) | 75.8 (74.6-77.0) | 78.9 (77.6-80.2) | 71.5 (70.1-73.0) | 81.8 (80.6-83.0) |
| By threshold to achieve ≥90% sensitivityb | |||||||||
| Threshold | ≥1 | ≥1 | ≥2 | ≤22 | ≤21 | ≤22 | |||
| Sensitivity | 98.4 (97.2-99.3) | 97.9 (92.8-99.8) | 96.5 (93.5-98.4) | 95.7 (93.8-97.2) | 92.6 (85.1-97.0) | 90.4 (85.6-94.0) | |||
| Specificity | 11.7 (10.5-12.9) | 12.4 (11.3-13.5) | 10.7 (9.7-11.8) | 27.5 (26.0-29.1) | 30.4 (28.9-31.9) | 25.2 (23.7-26.7) | |||
Abbreviations: CAPTURE, COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk; COLA-6, COPD in Low- and Middle-Income Countries Assessment; COPD, chronic obstructive pulmonary disease; LFQ, Lung Function Questionnaire; NPV, negative predictive value; PPV, positive predictive value.
CAPTURE and COLA-6 include a measure of peak expiratory flow.
Optimized thresholds were identified at each site for COLA-6 and the LFQ to maximize sensitivity to 90% or greater. Optimization from receiver operating characteristic curve analysis is not possible for CAPTURE, because the instrument does not have a single threshold and is scored in 2 stages.
eFigure 2 in Supplement 3 provides a visual summary of the performance of the 3 approaches to screening at the 3 sites.
In a prespecified subanalysis, discriminative accuracy of the instruments improved when identifying GOLD group B through D COPD. For group B through D COPD, the weighted AUC ranged from 0.742 (95% CI, 0.707-0.777) to 0.895 (95% CI, 0.871-0.915). The analysis examining performance against a fixed FEV1:FVC ratio is provided in Supplement 3.
Characteristics Associated With Discriminative Accuracy
An analysis comparing false-positive with true-negative results, and false-negative with true-positive results, for CAPTURE, COLA-6, and LFQ is reported in Supplement 3. Considering the 3 tools at all 3 sites, participants with false-positive results were significantly older than those with true-negative results. In 6 of 9 instances, participants with false-positive results were significantly more likely than those with true-negative results to report a history of asthma, and in 5 of 9 instances they were significantly more likely to report a history of chronic bronchitis and have current exposure to biomass smoke. Conversely, participants with false-negative results were significantly more likely to have milder disease than those with true-positive results (as assessed by the proportion in GOLD group A and indices of FEV1 impairment). In 8 of 9 instances, participants with false-negative results were significantly more likely to be younger and in 5 of 9 instances were significantly less likely to have ever smoked and report a history of asthma.
Feasibility and Fidelity
Administration of the screening instruments in participants’ homes took a mean of 7.6 minutes (SD, 1.11): 7.8 minutes (SD, 0.42) for CAPTURE, 8.7 minutes (SD, 0.61) for COLA-6, and 6.5 minutes (SD, 0.28) for the LFQ, all inclusive of the introductory conversation. Questionnaire data were 99.5% complete (Table 1), demonstrating fidelity of use by field workers.
Discussion
In this multicountry, population-based study of COPD screening among 3 communities in Nepal, Peru, and Uganda, the screening instruments were feasible to administer, and the prevalence of COPD and instrument performance varied by location.
In this study, a high percentage of screen-identified COPD cases were clinically important, had severe or very severe airflow obstruction, or both. A minority of people had a history of smoking. Most participants were previously unaware of the diagnosis despite the high prevalence of clinically important disease and lower quality of life. In contrast, in high-income settings the prevalence of COPD ranges from 10% to 28% in ever smokers, and among undiagnosed populations newly identified cases are predominantly mild and moderate (84%-95%).24 Effective interventions to improve respiratory outcomes in COPD are increasingly available in many LMIC settings where smoking cessation, clean cook-stove interventions, influenza vaccination, and pulmonary rehabilitation have all been successfully implemented.25,26,27 Additionally, recent changes to the World Health Organization essential medicines list, including the addition of a long-acting antimuscarinic bronchodilator, make effective COPD diagnosis strategies in LMICs increasingly necessary.28
The 3 screening instruments evaluated in this study were feasible to deliver based on time-to-use by field researchers and completeness of data. A good screening test should be safe, affordable, easy to administer, reliable, and widely available.29,30 The BOLD investigators, in secondary analysis, modeled data from 9390 participants in 14 countries and suggested that COPD screening instruments were likely be cost-effective.31 The results presented here demonstrate that these instruments are able to detect clinically important disease in community settings. Quality-assured postbronchodilator spirometry is the reference standard diagnostic test, yet spirometry remains impractical as a first-line test in LMICs because of the need for equipment, training, and interpretation from skilled personnel. Thus, simple screening tools may be cost-effective to identify individuals who require further confirmatory testing, particularly where resources are scarce.
To justify widespread screening in a population, the disease should be prevalent, associated with significant morbidity and mortality, and have readily available and effective treatments. In many contexts, including many LMICs, COPD meets these criteria. For example, in the National Health and Wellness Survey in China, a high proportion of the population tested positive on the LFQ (16.6%), and this screen-positive group had impaired quality of life, lower productivity, and greater health care resource use.32 That study did not, however, perform confirmatory spirometry. There remains an evidence gap to understand if treatment for screen-detected COPD in LMICs is beneficial to individuals and society, although given the natural history of COPD, earlier detection to prevent progression is likely to be beneficial.33
Using simple tools to screen for COPD, or to identify people who need to go on to have confirmatory spirometry, would be a significant step to reducing the underdiagnosis of COPD in LMICs while providing more effective use of available resources. The consequence of testing false positive is the need for spirometry. However, the higher respiratory exposures and past history of respiratory disease reported in persons with false-positive results suggest that another condition may be present, with potential benefits from additional assessment. Persons with false-negative results had less severe COPD, and those who progress would likely be identified at subsequent screening rounds if such a model were adopted.
In choosing among screening instruments, a policy maker needs to consider the trade-offs between specificity (reducing health system costs from false-positive results) and sensitivity (accurately identifying more COPD cases). Two of these instruments require PEF, which may not be readily available and requires additional training. Similarly, while the established thresholds were not associated with high sensitivity, increasing sensitivity would create additional requirement for confirmatory spirometry and consequent cost. Programs designed to better target and reach individuals at risk of COPD could improve the cost-accuracy of these tools.
COPD morbidity and screening test performance varied by site, emphasizing that the utility of these tools is context dependent. Understanding local COPD prevalence and severity is crucial in determining whether the use of any of the screening strategies can be justified. For example, the Pulmonary Risk in South America study from 4 cities in Latin America reported a COPD prevalence of 9.3%; of these cases, 40% were GOLD grade 134 Among the 12 countries surveyed in the Burden of Lung Disease (BOLD) study, the prevalence of GOLD grade 2 or higher was 10.1%.35 Even within countries there will be significant differences, including urban-rural disparities in COPD prevalence and access to diagnostics and treatment.5
The strengths of this analysis include the large sample size, random population sample, quality assurance of postbronchodilator spirometry, and delivery in 3 diverse LMIC locations within a single study and at which there was a variable prevalence of COPD and factors predisposing to COPD. This allowed assessment of screening tool performance in diverse contexts.
Limitations
This study has several limitations. First, this study did not follow up individuals to determine any subsequent changes in respiratory health and health care access for identified cases, and long-term outcomes were not quantified to justify health system investments in screening. Second, evaluation of implementation in existing primary care systems will be necessary to assess acceptability, sustainability, and scalability, which were not evaluated in this study. Third, as demonstrated in this study and previous investigations, the prevalence of COPD is heterogeneous, and screening for COPD may not be appropriate in all LMIC settings.31 Fourth, the screening tools used in this study identify individuals at high risk for airflow obstruction. The presentation of COPD in LMICs is highly heterogeneous and can frequently overlap with other undiagnosed and untreated respiratory conditions.36
Conclusions
This study demonstrated that screening instruments for COPD were feasible to administer in 3 low- and middle-income settings. Further research is needed to assess instrument performance in other low- and middle-income settings and to determine whether implementation is associated with improved clinical outcomes.
GECo1 Protocols and Standard Operating Procedures
GECo1 Statistical Analysis Plan
eFigure 1. STARD Flow Diagram, Overall and for Nepal, Peru, and Uganda
eTables 1-12
eFigure 2. Performance of the 3 COPD Screening Tools at the 3 Sites, Weighted to the Population Data
eTables 13-25
Nonauthor Collaborators. GECo Study Investigators
References
- 1.Roth GA, Abate D, Abate KH, et al. ; GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736-1788. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Chronic obstructive pulmonary disease (COPD). Published 2020. Accessed September 2, 2021. https://www.who.int/news-room/fact-sheets
- 3.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2022 report. Published 2021. Accessed December 16, 2021. https://goldcopd.org/2022-gold-reports-2/
- 4.Beran D, Zar HJ, Perrin C, Menezes AM, Burney P; Forum of International Respiratory Societies Working Group Collaboration . Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countries. Lancet Respir Med. 2015;3(2):159-170. doi: 10.1016/S2213-2600(15)00004-1 [DOI] [PubMed] [Google Scholar]
- 5.Robertson NM, Nagourney EM, Pollard SL, et al. Urban-rural disparities in chronic obstructive pulmonary disease management and access in Uganda. Chronic Obstr Pulm Dis. 2019;6(1):17-28. doi: 10.15326/jcopdf.6.1.2018.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabyshova A, Hurst JR, Soriano JB, et al. Gaps in COPD guidelines of low- and middle-income countries: a systematic scoping review. Chest. 2021;159(2):575-584. doi: 10.1016/j.chest.2020.09.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurst JR, Buist AS, Gaga M, et al. Challenges in the implementation of chronic obstructive pulmonary disease guidelines in low- and middle-income countries: an official American Thoracic Society Workshop report. Ann Am Thorac Soc. 2021;18(8):1269-1277. doi: 10.1513/AnnalsATS.202103-284ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Checkley W, West KP Jr, Wise RA, et al. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med. 2010;362(19):1784-1794. doi: 10.1056/NEJMoa0907441 [DOI] [PubMed] [Google Scholar]
- 9.Checkley W, Pollard SL, Siddharthan T, et al. Managing threats to respiratory health in urban slums. Lancet Respir Med. 2016;4(11):852-854. doi: 10.1016/S2213-2600(16)30245-4 [DOI] [PubMed] [Google Scholar]
- 10.Siddharthan T, Grigsby MR, Goodman D, et al. Association between household air pollution exposure and chronic obstructive pulmonary disease outcomes in 13 low- and middle-income country settings. Am J Respir Crit Care Med. 2018;197(5):611-620. doi: 10.1164/rccm.201709-1861OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez FJ, Mannino D, Leidy NK, et al. ; High-Risk-COPD Screening Study Group . A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(6):748-756. doi: 10.1164/rccm.201603-0622OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yawn BP, Mapel DW, Mannino DM, et al. ; Lung Function Questionnaire Working Group . Development of the Lung Function Questionnaire (LFQ) to identify airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2010;5:1-10. [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force . Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 14.Siddharthan T, Wosu AC, Pollard SL, et al. ; LiNK Cohort Study Investigators . A novel case-finding instrument for chronic obstructive pulmonary disease in low- and middle-income country settings. Int J Chron Obstruct Pulmon Dis. 2020;15:2769-2777. doi: 10.2147/COPD.S268076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celli BR, MacNee W; ATS/ERS Task Force . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946. doi: 10.1183/09031936.04.00014304 [DOI] [PubMed] [Google Scholar]
- 16.Williams N. The MRC breathlessness scale. Occup Med (Lond). 2017;67(6):496-497. doi: 10.1093/occmed/kqx086 [DOI] [PubMed] [Google Scholar]
- 17.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 18.Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2(3):195-203. doi: 10.1016/S2213-2600(14)70001-3 [DOI] [PubMed] [Google Scholar]
- 19.Mahler DA, Witek TJ Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99-103. doi: 10.1081/COPD-200050666 [DOI] [PubMed] [Google Scholar]
- 20.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddharthan T, Pollard SL, Quaderi SA, et al. ; GECo Study Investigators . Effectiveness-implementation of COPD case finding and self-management action plans in low- and middle-income countries: Global Excellence in COPD Outcomes (GECo) study protocol. Trials. 2018;19(1):571. doi: 10.1186/s13063-018-2909-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 23.Bae E, Choi S-E, Lee H, Shin G, Kang D. Validity of EQ-5D utility index and minimal clinically important difference estimation among patients with chronic obstructive pulmonary disease. BMC Pulm Med. 2020;20(1):73. doi: 10.1186/s12890-020-1116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi: 10.1001/jama.2016.2654 [DOI] [PubMed] [Google Scholar]
- 25.Jones R, Kirenga BJ, Katagira W, et al. A pre-post intervention study of pulmonary rehabilitation for adults with post-tuberculosis lung disease in Uganda. Int J Chron Obstruct Pulmon Dis. 2017;12:3533-3539. doi: 10.2147/COPD.S146659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar BK, West R, Arora M, Ahluwalia JS, Reddy KS, Shahab L. Effectiveness of a brief community outreach tobacco cessation intervention in India: a cluster-randomised controlled trial (the BABEX Trial). Thorax. 2017;72(2):167-173. doi: 10.1136/thoraxjnl-2016-208732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortimer K, Balmes JR. Cookstove trials and tribulations: what is needed to decrease the burden of household air pollution? Ann Am Thorac Soc. 2018;15(5):539-541. doi: 10.1513/AnnalsATS.201710-831GH [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization . WHO model list of essential medicines—22nd list, 2021. Published September 30, 2021. Accessed December 14, 2021. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
- 29.Herman C. What makes a screening exam “good”? Virtual Mentor. 2006;8(1):34-37. [DOI] [PubMed] [Google Scholar]
- 30.Maxim LD, Niebo R, Utell MJ. Screening tests: a review with examples. Inhal Toxicol. 2014;26(13):811-828. doi: 10.3109/08958378.2014.955932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jithoo A, Enright PL, Burney P, et al. ; BOLD Collaborative Research Group . Case-finding options for COPD: results from the Burden of Obstructive Lung Disease study. Eur Respir J. 2013;41(3):548-555. doi: 10.1183/09031936.00132011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch M, Butt T, Guo W, et al. Characteristics and health burden of the undiagnosed population at risk of chronic obstructive pulmonary disease in China. BMC Public Health. 2019;19(1):1727. doi: 10.1186/s12889-019-8071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111-122. doi: 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 34.Sobrino E, Irazola VE, Gutierrez L, et al. Estimating prevalence of chronic obstructive pulmonary disease in the Southern Cone of Latin America: how different spirometric criteria may affect disease burden and health policies. BMC Pulm Med. 2017;17(1):187. doi: 10.1186/s12890-017-0537-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buist AS, McBurnie MA, Vollmer WM, et al. ; BOLD Collaborative Research Group . International variation in the prevalence of COPD (the BOLD study): a population-based prevalence study. Lancet. 2007;370(9589):741-750. doi: 10.1016/S0140-6736(07)61377-4 [DOI] [PubMed] [Google Scholar]
- 36.Siddharthan T, Gupte A, Barnes PJ. Chronic obstructive pulmonary disease endotypes in low- and middle-income country settings: precision medicine for all. Am J Respir Crit Care Med. 2020;202(2):171-172. doi: 10.1164/rccm.202001-0165ED [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GECo1 Protocols and Standard Operating Procedures
GECo1 Statistical Analysis Plan
eFigure 1. STARD Flow Diagram, Overall and for Nepal, Peru, and Uganda
eTables 1-12
eFigure 2. Performance of the 3 COPD Screening Tools at the 3 Sites, Weighted to the Population Data
eTables 13-25
Nonauthor Collaborators. GECo Study Investigators