Abstract
The ability to visualize cell and tissue morphology at a high magnification using scanning electron microscopy (SEM) has revolutionized plant sciences research. In plant–insect interactions studies, SEM-based imaging has been of immense assistance to understand plant surface morphology including trichomes [plant hairs; physical defense structures against herbivores], spines, waxes, and insect morphological characteristics such as mouth parts, antennae, and legs, that they interact with. While SEM provides finer details of samples, and the imaging process is simpler now with advanced image acquisition and processing, sample preparation methodology has lagged. The need to undergo elaborate sample preparation with cryogenic freezing, multiple alcohol washes, and sputter coating makes SEM imaging expensive, time consuming, and warrants skilled professionals, making it inaccessible to majority of scientists. Here, using a desktop version of SEM (SNE- 4500 Plus Tabletop), we show that the “plug and play” method can efficiently produce SEM images with sufficient details for most morphological studies in plant–insect interactions. We used leaf trichomes of Solanum genus as our primary model, and oviposition by tobacco hornworm (Manduca sexta; Lepidoptera: Sphingidae) and fall armyworm (Spodoptera frugiperda; Lepidoptera: Noctuidae), and leaf surface wax imaging as additional examples to show the effectiveness of this instrument and present a detailed methodology to produce the best results with this instrument. While traditional sample preparation can still produce better resolved images with less distortion, we show that even at a higher magnification, the desktop SEM can deliver quality images. Overall, this study provides detailed methodology with a simpler “no sample preparation” technique for scanning fresh biological samples without the use of any additional chemicals and machinery.
Keywords: ecology, insect-plant interactions, microscopy
Introduction
While we can clearly see leaves, roots, and flowers with our naked eye, plants and animals have functionally complex and morphologically diverse structures which necessitates the need of advanced microscopy techniques to comprehend microscopic structures such as tissues and cells [5]. These structures tend to have complex morphological variations, which can only be visualized by advanced and powerful microscopy [6]. For example, while pollen is visible, individual pollen grains typically range in size between 25 and 50 μm [7] and can be studied more effectively with microscopy. Similarly, insect antennae and trichomes also range in micrometers and are difficult to observe in detail with the naked eye. Therefore, to understand the ultra-structure and to penetrate beyond the visible surface morphology, various microscopy tools are routinely employed [8]. Light microscope is popular and commonly used for this purpose but is limited by extremely low resolution and limited specimen compatibility [9].
Alternatively, a scanning electron microscope (SEM) can acquire images ranging from magnifications of ×10 to ×500 000, using secondary electrons [10], X-rays [11], and electron beams [12] that interact with the specimen while collecting the scattered radiation to produce an image [13]. For the past few decades, SEM has revolutionized imaging by helping to understand the microstructure of biological samples, cell lines, superconductors, micro-crystallization of nano particles, and many more [14]. Plant–insect interactions studies utilize SEM quite often because the field routinely examines morphological traits of insects and plants and their interactions between them, and among other components of the environment [1–3, 15, 16]. For example, plants morphological traits including trichomes, stomata, waxes, and pollens show tremendous variation like their insect counterparts including their antennae, eggs, mouth parts, legs, and wings vary immensely among them [17, 18], commonly imaged using SEM.
As the use of SEM has exponentially increased in last few decades, the technological advances in microscopy have also improved [19–22]. However, due to the lack of innovation in sample preparation methodology, SEM imaging remains a time-consuming and expensive process [13, 23]. As a result of the time-consuming and expensive sample preparation associated with SEM prior to the actual scanning of sample, SEM is still limited to core facilities across various research and educational institutions. Due to the lack of a simpler protocol, microscopists follow lengthy and complex procedures of sample preparation and image processing involving fixation of samples using critical buffers (e.g. glutaraldehyde) overnight, tissue drying through sequential alcohol washes, critical point drying, and sputter coating of samples, which not only adds up cost and efforts, but can also take minimum of 6–10 h to get samples ready to be processed for imaging ([23]; Table 1). Moreover, SEM demands skilled personnel and technical expertise to acquire quality images, thus limiting its use to core facilities or big-budget laboratories with the technicians available. It is quite evident that SEM imaging warrants methodology that can retain its quality in imaging, but it can also shorten the timeline.
Table 1:
A flowchart representing the basic steps involved in image acquisition on traditional SEM

|
To overcome intensive and expensive methodology associated with traditional SEM, we have been using the SNE-4500 Plus, a tabletop SEM (Nanoimages LLC, Pleasanton, CA, USA) that requires almost no sample preparation before imaging. The Desktop SEM (DSEM) can capture images up to ×200 000 magnification, while providing precise 3-D imaging of morphology and dimensions of structures seen in the samples. The DSEM is equally capable of capturing miniature plant features and ultra-structures of objects, thus providing detailed morphological characterization. For instance, one of the biggest advantages of DSEM is its ability to use fresh specimens (having high water content such as leaves, an almost impossible task with a traditional SEM), cutting back on elaborative, and tedious sample preparation time. Since DSEM requires no sample preparation before imaging and only takes 15 min for image acquisition (Table 2), it allows for more throughput processing unlike traditional SEM (Table 1). Moreover, it is user-friendly as it requires little or no technical expertise, and its cost effectiveness makes it possible for individual laboratories to possibly acquire and can also serve as a learning opportunity for students who can use it on a routine basis by themselves without supervision.
Table 2:
A flowchart representing the basic steps involved in image acquisition on SNE-4500 plus tabletop SEM with representative images of SNE-4500 parts

|
DSEM is even more beneficial in plant–insect interactions studies, where plant and insect images are routinely studied at microscopic levels [24, 25]. Previously, we have documented that trichomes not only act as a mechanical barrier to the movement and feeding of caterpillars but can also damage the peritrophic membrane of caterpillars and are even present in the frass pellets of these caterpillars [23, 26]. Furthermore, to test for subtle differences in treatments (e.g. damaged versus undamaged; inbred versus outbred plants; [23]), we require fast and throughput imaging, an almost impossible feat with traditional high-performance SEM. Resorting to simple light microscopy on the other hand can lead to missing key details. For example, trichomes can be classified into glandular and non-glandular types, based on the presence or absence of glandular top, and silverleaf nightshade (Solanum elaeagnifolium Cav.; Solanaceae), a worldwide invasive weed, has been found to have a dense mat of non-glandular stellate trichomes [27, 28]. However, using the DSEM, we recently found that in addition to non-glandular stellate trichomes, also possess a low density of glandular trichomes, almost impossible to detect through light microscopy. And, in a recent study [4], we imaged 11 Solanaceae species, and leaves for each leaf surface (adaxial and abaxial) to determine the statistical significance between treatment groups (species; leaf surface)—clearly showing the need for extensive and thorough imaging which could have only been possible with SEM needing no prior sample preparation. DSEM can also distinguish the sub-parts of these microscopic hairs. In addition, DSEM also aided us to observe epicuticular wax along with trichomes present on the leaf surface in species like Solanum glaucescens Zucc. We show how DSEM can acquire images of various biological samples and their morphological features with precision, without the costs, or time associated with traditional SEM.
Materials and methods
Instrument-specific methodology for image acquisition
There are four major steps in imaging using DSEM. The first step “Pre-sample preparation DSEM operation” involves preparing the DSEM before placing the sample in the vacuum chamber. The second step “Sample preparation” includes setting up the sample stage without the use of chemicals and other machinery. The third step “Post-sample preparation DSEM operation” involves the steps to be followed post sample set up in the machine. The final step “Image capturing and processing using ‘Nanoeye’” involves steps to be followed to acquire SEM image post sample insertion and vacuum build up in machine.
Below is the description of these major steps.
Pre-sample preparation DSEM operation
Before turning on the DSEM and computer, the vacuum pressure should range from 90 to 120 psi on the air compressor (rotary vane pump). Confirm that the vacuum pumps are functional for the entire duration of imaging and that the fastened stage with/without sample is under vacuum despite the fact it is turned off to avoid any debris entering the machine. Allow the vacuum to build in DSEM by pressing the “Exchange” button. A stable green light indicates that full vacuum has been established into the column containing stage inside the DSEM. After the establishment of full vacuum, press the “Exchange” switch again to initiate release of vacuum from DSEM. Then, launch “Nanoeye” software associated with DSEM on the computer linked to DSEM. Then, pull out the motorized stage smoothly, fully open the door, loosen the stage using hex key (Fig. 1A-1), and take out sample along with aluminum stub using the SEM mount forceps (Fig. 1A-2). Select the “Calibration” icon on the Motor control panel of “Nanoeye” software and allow the stage to come to its originally assigned 3-D position.
Figure 1:
Frequently used tools for sample preparation and handling while operating DSEM: (A) 1—Hex key to loosen or tighten the screws of stage while taking out or putting the sample stage (aluminum stub with sample mounted on it) in vacuum chamber. 2—SEM mount forceps to handle the aluminum stubs without damaging the sample. (B) Aluminum stubs of diameters 1, 15 mm; 2, 25 mm; 3, 40 mm; and 4, 40 mm stub to handle multiple (1-4) 15-mm stubs at one time. (C) Double-sided carbon tape used to fix sample on the aluminum stubs, and (D) Machine jig to measure the diameter and height of sample stage with sample mounted on it.
Sample preparation
Any fresh biological/non-biological sample can be used for image acquisition in SNE-4500 Plus. A general principle for sample preparation is to get an excised sample of diameter up to 80 mm and thickness up to 50 mm (based on size of the stage). Then, fix the sample onto a double-sided conductive carbon tape (Fig. 1C) glued to an aluminum stub of suitable diameter (15 mm, 25 mm, and 40 mm) depending on the size of the sample (Fig. 1B).
Post-sample preparation in DSEM operation
The diameter and height of stub along with sample should be recorded using the machine jig (Fig. 1D) by aligning the specimen stage with the groove in the middle. The recorded parameters should be entered into the “Nanoeye” software. It is imperative that the height entered is 2–3 mm more than the recorded height to avoid contact of sample to electron gun. In the case of single aluminum stub (15 mm, 25 mm, or 40 mm in diameter), it can be directly placed on the motorized stage and stage should be fastened using a hex key. Use the “Camera” button on the motor control panel of software screen to capture the image of the stage as it will later act as a map to capture magnified images of different regions of the specimen. It is important to note that the center of x–y coordinates on the camera navigation should align with the center of the fastened stage. Press “Exchange” switch while keeping the door slightly pushed for few seconds using one hand. After loading a fresh unprepared sample, the user has approximately 5 min to acquire images.
Image capturing and processing using “nanoeye”
On the Start Page of Nanoeye software screen, select 5KV voltage, SE detector, and high/low vacuum (we used high vacuum for most samples). Then, press “START” to start scanning of specimen inside vacuum chamber. After pressing start button, check to see that the emission current rises to 110 µA. The initial page of Image Window shows the fast-scanning mode with minimum resolution. Use X, Y, Z, R, and T on Motor control panel to select the region/coordinates of sample to be scanned at certain rotation and angle. By default, the Z is an equal height of fastened stage, and X, Y, T, and R is 0. When selecting x–y coordinates, double click on camera navigation and later minor changes in the coordinates can be made using X–Y motor operation. Initially, increase the magnification up to ×500–1000 to focus rather than focusing at a magnification lower than ×500. Then, decrease the magnification as per requirement and the image to be captured. Select 10–30% spot size on Image Control as per requirement before changing the scan mode. Select the slow photo 2 scan mode (on the extreme bottom right; Image Control) for highest resolution publication quality image. Then select “Auto” on the brightness/contrast focus area on Image Control. On the bottom bar of Image Window, company label, researcher’s name and specimen label can be modified. Monitor the complete scanning on the screen and just before the scanning is completed, click the “Camera” icon on bottom right corner of Image Control, and save the image at a designated storage location. To measure the dimensions of the various components in the image, pause the scanning by clicking on pause icon on bottom bar and click M. Tools. Select length to measure two-point/multi-point length and markings (arrow, square, and rectangle) to mark the components of image. Click on the operation button on top right bar of image control and a dropdown menu will appear. Select “Stop” to stop scanning. Once “Stop” is selected on the Nanoeye page, the power switch can be pressed to stop operation of DSEM and Nanoeye window will be closed. A detailed flowchart comparing traditional SEM and DSEM is presented, respectively, in Tables 1 and 2.
Results and discussion
Here, we document a detailed procedure of a possible alternative to traditional electron microscopy that removes the major bottlenecks while sustaining image quality. Using SNE-4500 Plus Tabletop Desktop SEM, we captured images of fresh leaf samples of different species of Solanaceae (Figs 2B–D, 3A, and 4), Cucurbitaceae (Figs 2A and 3B), Asteraceae (Fig. 3C), and Poaceae (Fig. 3D) plant families to study their surface features (e.g. trichomes, stomata, and waxes) (Fig. 3B). We varied magnifications to estimate the density of trichomes depending on the characteristics of plant families (Fig. 3). Additionally, we captured images of insect eggs (Figs 2E and 4A), pollen grains (Fig. 2D), and caterpillars using DSEM. Images of fall armyworm (Spodoptera frugiperda; Lepidoptera; Noctuidae) and tobacco hornworm (Manduca sexta; Lepidoptera; Sphingidae) (Fig. 4A) eggs laid by adult moths on tomato (Solanum lycopersicum: Solanaceae) leaf surface were collected to assess any damage caused by trichomes present on the leaf surface to eggs.
Figure 2:
Schematic representation of the wide diversity of functions performed by high-tech, no sample prep DSEM in plant–insect interactions studies including (A) density of trichomes present on cucumber (Cucumis sativa: Cucurbitaceae) leaf at estimated at ×180 magnification, (B) waxes and glandular trichomes (gland on the top of hair) of Solanum glaucescens (Solanaceae) magnified at ×150, (C) potato tree (Solanum grandiflorum; Solanaceae) magnified at ×60 for measurement of dimensions of different trichome types, (D) pollen grains of silverleaf nightshade (Solanum elaeagnifolium: Solanaceae) flowers magnified at ×250, (E) surface interphase of squash bugs (Anasa tristis; Hemiptera: Coreidae) eggs and plant surface of cucumber magnified at ×90, and (F) microstructural details of presence of undigested trichomes of bottle gourd (Lagenaria siceraria: Cucurbitaceae) embedded in frass pellets of cabbage loopers Trichoplusia ni (Lepidoptera: Noctuidae) magnified at ×700.
Figure 3:
Desktop SEM images of various kinds of trichomes present in different plant families including (A) Solanaceae (tomato; Solanum lycopersicum) at ×140, (B) Cucurbitaceae (bottle gourd; L. siceraria) at ×200, (C) Asteraceae (sunflower; Helianthus annuus), and (D) Poaceae (sorghum; Sorghum bicolor).
Figure 4:
Desktop SEM showing shrunken (A) egg of fall armyworm (Spodoptera frugiperda) on tomato (S. lycopersicum; Solanaceae) leaf surface at ×75 and (B) glandular trichome of African eggplant (Solanum macrocarpon; Solanaceae) at ×1000, indicating that although the surface features of a biological sample are visible, but not fixing sample and leaving out sputter coating can lead to deviation of image from its original structure.
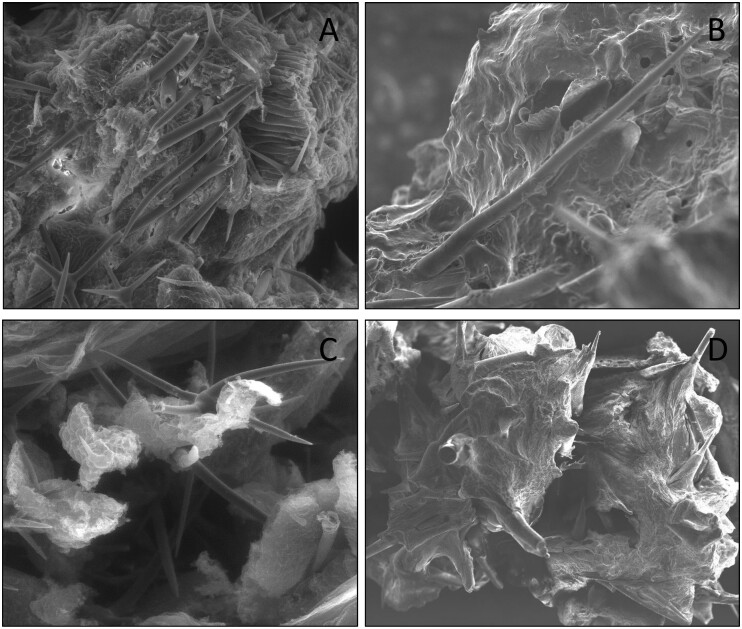
In DSEM imaging of insect eggs, we did observe some structural distortion (shrinkage) after placing them under vacuum in DSEM (Fig. 4A). However, aldehyde fixing and sputter coating would resolve this issue. Previously, Kariyat et al. [23] had captured images of M. sexta caterpillars’ peritrophic matrix and frass pellets to study the effects of trichomes of horsenettle (Solanum carolinense: Solanaceae) post-feeding (Fig. 5A and C). This was done with a traditional Cambridge S360 SEM (Huck Institutes of Life Sciences, Microscopy Core Facility, Pennsylvania State University, USA) and used typical SEM preparation protocol which includes overnight fixation of the sample at 4°C in 25% glutaraldehyde solution, dehydration rinses through series of ethanol solutions, critical point drying, and sputter coating of critically dried sample (Fig. 5A and C and Table 1). However, without using the abovementioned method, we captured images of frass pellets of cabbage loopers (Trichoplusia ni; Lepidoptera: Noctuidae) to detect the presence of undigested trichomes when the caterpillars were fed on cucumber (Cucumissativus; Cucurbitaceae) and bottle gourd (Lagenaria siceraria; Cucurbitaceae) leaves. Interestingly, we obtained similar results of the trichomes being embedded in these frass pellets (Figs 2F and 5B and D) to the images earlier captured by Kariyat et al. [23]. Additionally, plant waxes, one of first line of defenses encountered by herbivores, were also observed very clearly in our SEM images from S.glaucescens (Fig. 2B). Additionally, the resolution of DSEM is 5 nm which is comparable to traditional SEMs with nanoscopic resolution. This strengthens our point that DSEM is equally capable of capturing minute details from the specimens with no sample prep which was earlier thought to be only captured by expensive high-tech SEMs which use extensive sample preparation methodology.
Figure 5:
SEM of undigested trichomes embedded in frass pellets of (A and C) tobacco hornworm (Manduca sexta: Sphingidae) caterpillar fed on trichome-rich plant material of horsenettle (Solanum carolinense; Solanaceae). Scanning of sample was carried out at ×300 and ×400 using traditional Cambridge S360 SEM following conventional sample fixation with glutaraldehyde, tissue dehydration with ethanol washes, critical point drying of sample and sputter coating, and (B and D) cabbage looper (Trichoplusia ni: Noctuidae) after caterpillar fed on cucumber (Cucumis sativa; Cucurbitaceae) and bottle gourd (Lagenaria siceraria; Cucurbitaceae) plant material and scanning of sample done at ×450 and ×150 using no sample preparation desktop SEM.
While sample preparation and image acquisition are much easier and can be accomplished quickly, there are some concerns. We found that it is difficult to estimate the gland cell number and the exact shape of the gland of glandular trichomes, and eggs of insects due to the damage sustained after placing the sample under vacuum in DSEM (Fig. 4). Alternatively, we found that shape of non-glandular trichomes of tomato was not distorted under vacuum. Sputter coating and would have possibly produced better images of glandular trichomes. Using a more efficient method for capturing SEM images by placing the sample on the stage can produce similar results as produced by using more complicated and traditional SEM. This is especially true for plant–insect interactions studies where routine imaging of plant and insect parts could be fast tracked using DSEM. Overall, we demonstrate that using DSEM, which is fast, easy to operate, and inexpensive, can produce quality images similar to other SEM, with significantly lower costs of purchase, maintenance, and methodology.
Acknowledgments
The authors thank Tom Eubanks with SEM preparation.
Conflict of interest
The authors declare that there are no personal, professional, or financial conflicts among them that have or can affect the work of described in the manuscript.
Funding
The study was funded by ‘Rising Star Award' to Rupesh Kariyat and ‘Presidential Graduate Research Assistanship' to Sakshi Watts.
Data availability statement
The data underlying this article are available in the article.
References
- 1. Kaur I, Kariyat RR.. Eating barbed w ire: direct and indirect defensive roles of non‐glandular trichomes. Plant Cell Environ 2020;43:2015–8. doi: 10.1111/pce.13828. [DOI] [PubMed] [Google Scholar]
- 2. Kaur J, Chavana J, Soti P. et al. Arbuscular mycorrhizal fungi (AMF) influences growth and insect community dynamics in Sorghum–Sudangrass (Sorghum × drummondii). Arthropod Plant Interact 2020;14:301–15. doi: 10.1007/s11829-020-09747-8. [DOI] [Google Scholar]
- 3. Kaur J, Kariyat R.. Role of trichomes in plant stress biology. In: Núñez-Farfán J, Valverde P (eds) Evolutionary Ecology of Plant–Herbivore Interaction, Springer, Cham. 2020, 15–35. doi: 10.1007/978-3-030-46012-9_2. [DOI] [Google Scholar]
- 4. Watts S, Kariyat R.. Picking sides: feeding on the abaxial leaf surface is costly for caterpillars. Planta 2021;253:77. doi: 10.1007/s00425-021-03592-6. [DOI] [PubMed] [Google Scholar]
- 5. Caldwell AS, Iyer-Pascuzzi A.. Scanning electron microscopy technique for viewing plant–microbe interactions at tissue and cell-type resolution. Phytopathology 2019;109:1302–11. doi: 10.1094/phyto-07-18-0216-r. [DOI] [PubMed] [Google Scholar]
- 6. Palaiologou P, Goggin DS, Chatelet R. et al. Serial block‐face scanning electron microscopy reveals novel intercellular connections in human term placental microvasculature. J Anat 2020;237:241–9. doi: 10.1111/joa.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelly JK, Rasch A, Kalisz S.. A method to estimate pollen viability from pollen size variation. Am J Bot 2002;89:1021–3. doi: 10.3732/ajb.89.6.1021. [DOI] [PubMed] [Google Scholar]
- 8. Mustafa A, Ensikat H-J, Weigend M.. Stinging hair morphology and wall biomineralization across five plant families: conserved morphology versus divergent cell wall composition. Am J Bot 2018;105:1109–22. doi: 10.1002/ajb2.1136. [DOI] [PubMed] [Google Scholar]
- 9. Wollman AJ, Nudd R, Hedlund EG. et al. From Animaculum to single molecules: 300 years of the light microscope. Open Biol 2015;5:150019. doi: 10.1098/rsob.150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu Y, Inada H, Nakamura K. et al. Imaging single atoms using secondary electrons with an aberration-corrected electron microscope. Nat Mater 2009;8:808–12. [DOI] [PubMed] [Google Scholar]
- 11. Kotula PG, Keenan MR, Michael JR.. Automated analysis of SEM X-ray spectral images: a powerful new microanalysis tool. Microsc Microanal 2003;9:1–17. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura R, Matsumoto A, Ishimaru M.. Explosive crystallization of sputter-deposited amorphous germanium films by irradiation with an electron beam of SEM-level energies. J Appl Phys 2021;129:215301. [Google Scholar]
- 13. Inkson BJ. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization. In: Gerhard Hübschen, Iris Altpeter, Ralf Tschuncky, Hans-Georg Herrmann (eds.), Materials Characterization using Nondestructive Evaluation (NDE) Methods, Woodhead Publishing, 2016, 17–43. doi: 10.1016/b978-0-08-100040-3.00002-x. [DOI] [Google Scholar]
- 14. Zhang W, Dong Z, Zhu L. et al. Direct observation of the release of nanoplastics from commercially recycled plastics with correlative raman imaging and scanning electron microscopy. ACS Nano 2020;14:7920–6. doi: 10.1021/acsnano.0c02878. [DOI] [PubMed] [Google Scholar]
- 15. Pathan AK, Bond J, Gaskin RE.. Sample preparation for SEM of plant surfaces. Mater Today 2010;12:32–43. doi: 10.1016/s1369-7021(10)70143-7. [DOI] [Google Scholar]
- 16. McCully ME, Canny MJ, Huang CX.. Invited review: cryo-scanning electron microscopy (CSEM) in the advancement of functional plant biology. Morphological and anatomical applications. Funct Plant Biol 2009;36:97–124. doi: 10.1071/fp08304. [DOI] [PubMed] [Google Scholar]
- 17. Krenn HW. Form and function of insect mouthparts. In: Krenn HW (ed.), Insect Mouthparts, Spring International Publishing Cham, 2019, 9–46. doi: 10.1007/978-3-030-29654-4_2. [DOI] [Google Scholar]
- 18. Singh S, Kumari B.. Roving survey of major insect pests of rice crop (Oryza sativa L.) in different blocks of Patna district of Bihar state, India. J Entomol Zool Stud 2020;8:1115–20. [Google Scholar]
- 19. Sujata K, Jennings HM.. Advances in scanning electron microscopy. MRS Bull 1991;16:41–5. doi: 10.1557/s0883769400057390. [DOI] [Google Scholar]
- 20. Stokes DJ. Recent advances in electron imaging, image interpretation and applications: environmental scanning electron microscopy. Philos Trans A Math Phys Eng Sci 2003;361:2771–87. doi: 10.1098/rsta.2003.1279. [DOI] [PubMed] [Google Scholar]
- 21. Newbury DE, Ritchie NW.. Performing elemental microanalysis with high accuracy and high precision by scanning electron microscopy/silicon drift detector energy-dispersive X-ray spectrometry (SEM/SDD-EDS). J Mater Sci 2015;50:493–518. doi: 10.1007/s10853-014-8685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weigend M, Mustafa A, Ensikat H-J.. Calcium phosphate in plant trichomes: the overlooked biomineral. Planta 2018;247:277–85. doi: 10.1007/s00425-017-2826-1. [DOI] [PubMed] [Google Scholar]
- 23. Kariyat RR, Smith JD, Stephenson AG. et al. Non-glandular trichomes of Solanum carolinense deter feeding by Manduca sexta caterpillars and cause damage to the gut peritrophic matrix. Proc R Soc B 2017;284:20162323. doi: 10.1098/rspb.2016.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silva EO, Feio AC, Cardoso-Gustavson P. et al. Extrafloral nectaries and plant–insect interactions in Passiflora L. Brazil J Bot volume 2017;40:331–40. [Google Scholar]
- 25. Watts S, Kariyat RR. Morphological characterization of trichomes show enormous variation in shape, density, and dimensions across the leaves of 14 Solanum species. AoB Plants2021. [DOI] [PMC free article] [PubMed]
- 26. Kariyat RR, Raya CE, Chavana J. et al. Feeding on glandular and non-glandular leaf trichomes negatively affect growth and development in tobacco hornworm (Manduca sexta) caterpillars. Arthropod Plant Interact 2019;13:321–33. doi: 10.1007/s11829-019-09678-z. [DOI] [Google Scholar]
- 27. Kariyat RR, Chavana J.. Field data on plant growth and insect damage on the noxious weed Solanum elaeagnifolium in an unexplored native range. Data Brief 2018;19:2348–51. doi: 10.1016/j.dib.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chavana J, Singh S, Vazquez A. et al. Local adaptation to continuous mowing makes the noxious weed Solanum elaeagnifolium a superweed candidate by improving fitness and defense traits. Sci Rep 2021;11. doi: 10.1038/s41598-021-85789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.