ABSTRACT
Background
High meat consumption might play a role in promoting low-grade systemic inflammation, but evidence is limited.
Objectives
We examined cross-sectional associations of habitual meat consumption with serum C-reactive protein (CRP) and total white blood cell count (WBCC) in British adults.
Methods
We included 403,886 men and women (aged 38–73 y) participating in the UK Biobank who provided information on meat intake (via touchscreen questionnaire) and a nonfasting blood sample at recruitment (2006–2010). For a subset of participants (∼5%), an additional blood sample was collected (median 4.4 y later). We used multivariable linear regression models to estimate associations of meat intake (total meat, unprocessed red meat, processed meat, and poultry) with logCRP and logWBCC.
Results
The difference in the serum CRP (mg/L) for each 50-g/d higher intake for total meat was 11.6% (95% CI: 11.1, 12.0%), for processed meat was 38.3% (95% CI: 36.0, 40.7%), for unprocessed red meat was 14.4% (95% CI: 13.6, 15.1%), and for poultry was 12.8% (95% CI: 12.0, 13.5%). The difference in the WBCC (×10–9L) for each 50 g/d higher intake of total meat was 1.5% (95% CI: 1.4, 1.6%), for processed meat was 6.5% (95% CI: 6.1, 6.9%), for unprocessed red meat was 1.6% (95% CI: 1.4, 1.7%), and for poultry was 1.6% (95% CI: 1.4, 1.7%). All associations were attenuated after adjustment for adiposity; by 67% with BMI (in kg/m2) and by 58% with waist circumference for total meat and CRP, and by 53% and 47%, respectively, for WBCC, although associations remained statistically significant. Findings of sensitivity analyses in 15,420 participants were similar prospectively, except there were no associations between unprocessed red meat and WBCC.
Conclusions
Higher meat consumption, particularly of processed meat, was positively associated with inflammatory markers in these British adults; however, the magnitudes of associations are small and predominantly due to higher adiposity.
Keywords: inflammation, meat intake, cohort study, UK Biobank, C-reactive protein, white blood cell count
Introduction
Systemic low-grade inflammation, characterized by increases in inflammatory biomarkers such as C-reactive protein (CRP), white blood cell count (WBCC), interleukin 6 (IL-6), and Tumour Necrosis Factor alpha (TNF-α) (1), has been associated with a higher risk of some chronic diseases such as type 2 diabetes (2) and all-cause mortality (3). It has been suggested that high meat consumption might play a role in inflammatory processes, possibly through its high amounts of heme iron (4), saturated fat content (5), and advanced glycation end products (AGEs) (6). Another possibility is that an association of meat and inflammation is confounded or mediated by increased adiposity (central or general), which has been found to be related to meat intake (7) and inflammation, with genetic evidence suggesting that the relation of adiposity and inflammation is causal (8).
The available evidence for associations of meat intake with markers of systemic inflammation is inconsistent, based on small studies (<17 k), and mostly focused on red meat. Most (9–13) but not all previous studies (14, 15) have found a positive association between red meat (9–13), processed meat (11, 12, 15), and CRP before adjustment for adiposity, and no association with poultry (15). Of the studies that adjusted for adiposity, most (10–13) but not all (9) reported that the association of meat with CRP was no longer significant, suggesting that the association may be due to higher adiposity.
The aim of the current study was to assess the associations of habitual consumption of different types of meat (including total meat, unprocessed red meat, processed meat, and poultry) with CRP and WBCC in a large cohort of British adults, and to clarify the role of adiposity.
Methods
Study population
This cross-sectional study was based on 403,886 men and women aged between 38 and 73 y, registered with the National Health Service in England, Wales, and Scotland, and enrolled in the UK Biobank cohort study between 2006 and 2010 (16). The study was conducted in accordance with the Helsinki Declaration of 1975 as revised in 1983, and approved by the National Information Governance Board for Health and Social Care and the National Health Service North West Multicentre Research Ethics Committee (16/NW/0274), and participants provided informed consent.
Exposure, outcome, and covariate collection
Usual dietary intake was collected at recruitment using a touchscreen questionnaire that included 29 questions on diet, assessing the consumption frequency of each listed food (as of 22 July 2021; https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/TouchscreenQuestionsMainFinal.pdf). Unprocessed red meat was defined as the sum of the responses to 3 questions on red meat, which included beef, lamb/mutton, and pork, while processed meat (e.g. bacon, ham, sausages, meat pies, kebabs, burgers, chicken nuggets), and poultry intake (including chicken, turkey, or other poultry) were based on 1 question each. To investigate the combined effects, all meat types were summed as total meat intake. Meat intakes were categorized into groups based on weekly intake frequency depending on data distribution, as reported previously (17). We calculated meat intake in grams by assigning a portion size of 120 g for unprocessed red meat, 50 g for processed meat, and 130 g for poultry (18).
All participants provided a nonfasting blood sample at recruitment and a subsample of participants (n = 20,345; 21% of those invited) who lived within a 35-km radius of the UK Biobank Co-ordinating Centre in Stockport, England, provided an additional nonfasting blood sample a median of 4.4 y later (min 2.1 y, max 7.0 y) between 2012 and 2013 (as of 25 May, 2021; https://biobank.ctsu.ox.ac.uk/∼bbdatan/Repeat_assessment_doc_v1.0.pdf). Samples were subsequently kept at 4°C during shipping to the purpose-built laboratory for UK Biobank in Stockport, England (as of 25 May 2021; https://biobank.ndph.ox.ac.uk/∼bbdatan/biomarkers.pdf); complete blood cell counts (including WBCC in ×10–9 cells/L) were conducted within 24 h of venipuncture using a Coulter Counter (Beckman Coulter), and serum CRP concentrations (mg/L) were measured later in stored samples using high-sensitivity immunoturbidimetry. The average within-laboratory coefficients of variation (ratio of the SD to the mean) for CRP were 2.31% for low concentrations, 1.70% for medium concentrations, and 1.69% for high concentrations (as of 25 May 2021; https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf).
All covariates except for waist circumference, weight, and height were ascertained via the touchscreen questionnaire. Waist circumference was measured using a Wessex nonstretchable sprung tape (passed around the smallest part of the trunk (i.e., the natural indent) or the umbilicus if the natural indent was not found), height using a stadiometer, and weight using a Tanita BC418MA body composition analyzer to perform part of a Bioimpedance Analysis (BIA) or a standard scale in participants that did not participate in BIA. All measurements were conducted by trained staff according to standard procedures. (as of 15 September 2021; https://biobank.ndph.ox.ac.uk/ukb/ukb/docs/Anthropometry.pdf).
For all analyses, study participants were excluded if they had withdrawn from the study (n = 829), had missing data on CRP and WBCC at baseline (n = 45,965), or missing data on meat intake (n = 6806) or any covariates (n = 45,831) resulting in an analytical sample of 403,886 participants (Supplemental Figure 1).
Statistical analysis
We used multivariable linear regression models to investigate the associations of habitual meat intake with log(CRP) and log(WBCC); CRP and WBCC were logarithmically transformed to satisfy model assumptions and normalize distributions. For trend analyses per 50 g/d higher intake, β coefficients were exponentiated to yield percentage differences and corresponding 95% CIs. For categorical meat intakes, associations were expressed as geometric means with 95% CIs using the margins postestimation command in Stata. We used 4 models to assess the effects of potential confounders (models 1 and 2) and adiposity (models 3 and 4) on observed associations. In model 1, adjustments were made for age and sex, and model 2 was additionally adjusted for baseline smoking status, ethnicity, Townsend deprivation index, employment, qualification level, total fruit and vegetable intake, fiber intake from bread and breakfast cereals, total fish consumption, total physical activity, alcohol intake, and menopausal status in women. In models 3 and 4, we additionally adjusted the full model (model 2) for BMI (in kg/m2) and waist circumference (in cm), respectively.
The relation between meat intake and inflammation may vary by sex (12). Therefore, we assessed heterogeneity by sex in the associations of meat intake (per 50 g/d higher intake) with logCRP and logWBCC by adding an interaction term to test for statistical significance using likelihood ratio tests and by stratifying results. We conducted sensitivity analyses in a subsample of 15,420 adults with serum biomarker measures at follow-up, 4.4 (median) y after baseline. All analyses were performed using Stata Release 16.1, StataCorp LLC.
Results
Table 1 shows characteristics of the analytical sample and participants by categories of total meat intake. With higher meat intake the proportions of participants who were men, former or current smokers, less physically active, or consumed more alcohol were higher, and intakes of fruit and vegetables and cereal fiber were lower. Furthermore the proportions of participants of white European ethnicity, who were affluent, had a lower level of education, and who were retired were higher in the highest (≥7 times/wk) meat intake category than in the lowest (<3 times/wk) meat intake category but there was no clear trend across categories. Participants in the highest (≥7 times/wk) meat intake category had a 2.1 kg/m2 higher mean BMI and 5.2 cm higher waist circumference compared with participants who reported the lowest meat intakes (<3 times/wk), with a trend across meat intake categories.
TABLE 1.
Characteristics of the analytical sample by total meat intake frequency1
| Total meat intake frequency | |||||
|---|---|---|---|---|---|
| Characteristics | Analytical sample (n = 403,886) | <3 times/wk (n = 49,330) | 3 to <5 times/wk (n = 103,599) | 5 to <7 times/wk (n = 133,549) | ≥7 times/wk (n = 117,408) |
| Sex | |||||
| Women | 213,511 (52.9) | 33,362 (67.6) | 61,751 (59.6) | 71,196 (53.3) | 47,202 (40.2) |
| Men | 190,375 (47.1) | 15,968 (32.4) | 41,848 (40.4) | 62,353 (46.7) | 70,206 (59.8) |
| Age, y | 56.7 ± 8.1 | 55.9 ± 8.1 | 57.6 ± 7.9 | 56.9 ± 8.0 | 56.1 ± 8.2 |
| Ethnicity | |||||
| White | 385,694 (95.5) | 44,986 (91.2) | 99,617 (96.2) | 129,136 (96.7) | 111,955 (95.4) |
| Nonwhite | 18,192 (4.5) | 4344 (8.8) | 3982 (3.8) | 4413 (3.3) | 5453 (4.6) |
| Townsend deprivation | |||||
| Most affluent | 84,218 (20.9) | 8291 (16.8) | 22,101 (21.3) | 29,413 (22.0) | 24,413 (20.8) |
| Most deprived | 74,431 (18.4) | 11,445 (23.2) | 18,123 (17.5) | 22,390 (16.8) | 22,473 (19.1) |
| Qualification | |||||
| College or university degree/vocational qualification | 247,738 (61.3) | 32,989 (66.9) | 62,517 (60.3) | 80,382 (60.2) | 71,850 (61.2) |
| National examination at ages 17–18 y | 22,617 (5.6) | 2727 (5.5) | 5519 (5.3) | 7540 (5.6) | 6831 (5.8) |
| National examination at age 16 y | 67,283 (16.7) | 6,895 (14.0) | 17,266 (16.7) | 23,433 (17.5) | 19,689 (16.8) |
| Other/unknown | 66,248 (16.4) | 6719 (13.6) | 18,297 (17.7) | 22,194 (16.6) | 19,038 (16.2) |
| Employment | |||||
| In paid employment | 233,897 (57.9) | 30,486 (61.8) | 56,895 (54.9) | 76,288 (57.1) | 70,228 (59.8) |
| Retired | 127,058 (31.5) | 13,116 (26.6) | 36,183 (34.9) | 43,632 (32.7) | 34,127 (29.1) |
| Not in paid employment | 42,931 (10.6) | 5728 (11.6) | 10,521 (10.2) | 13,629 (10.2) | 13,053 (11.1) |
| Smoking | |||||
| None | 221,188 (54.8) | 28,493 (57.8) | 57,738 (55.7) | 73,557 (55.1) | 61,400 (52.3) |
| Former | 142,320 (35.2) | 16,556 (33.6) | 36,386 (35.1) | 47,479 (35.6) | 41,899 (35.7) |
| Current <15 cigarettes/d | 11,593 (2.9) | 1462 (3.0) | 2887 (2.8) | 3640 (2.7) | 3604 (3.1) |
| Current ≥15 cigarettes/d | 15,532 (3.8) | 1306 (2.6) | 3344 (3.2) | 4760 (3.6) | 6122 (5.2) |
| Current, unknown amount | 13,253 (3.3) | 1513 (3.1) | 3244 (3.1) | 4113 (3.1) | 4383 (3.7) |
| Physical activity level, MET h/wk | |||||
| <5 | 50,223 (12.4) | 5485 (11.1) | 12,829 (12.4) | 16,706 (12.5) | 15,203 (12.9) |
| ≥100 | 40,603 (10.1) | 4904 (9.9) | 9825 (9.5) | 12,859 (9.6) | 13,015 (11.1) |
| Alcohol intake | |||||
| <1 g/d | 43,135 (10.7) | 7166 (14.5) | 11,788 (11.4) | 13,745 (10.3) | 10,436 (8.9) |
| ≥25 g/d | 81,306 (20.1) | 5471 (11.1) | 16,450 (15.9) | 26,532 (19.9) | 32,853 (28.0) |
| Non-drinkers | 29,758 (7.4) | 6524 (13.2) | 7842 (7.6) | 8122 (6.1) | 7270 (6.2) |
| Fruit and vegetable intake, servings2/d | 4.7 ± 2.6 | 5.4 ± 3.0 | 4.7 ± 2.5 | 4.6 ± 2.4 | 4.5 ± 2.5 |
| Estimated cereal fiber intake, g/d | 4.6 ± 2.9 | 4.7 ± 3.1 | 4.6 ± 2.9 | 4.6 ± 2.9 | 4.5 ± 3.0 |
| Fish intake, times/wk | |||||
| 0–1 | 101,960 (25.2) | 18,485 (37.5) | 23,676 (22.9) | 30,026 (22.5) | 29,773 (25.4) |
| <2 | 88,992 (22.0) | 7115 (14.4) | 23,952 (23.1) | 30,890 (23.1) | 27,035 (23.0) |
| <3 | 96,942 (24.0) | 7950 (16.1) | 27,020 (26.1) | 34,284 (25.7) | 27,688 (23.6) |
| ≥3 | 115,992 (28.7) | 15,780 (32.0) | 28,951 (27.9) | 38,349 (28.7) | 32,912 (28.0) |
| Menopausal status | |||||
| Premenopausal | 52,284 (24.5) | 9041 (27.1) | 12,968 (21.0) | 17,215 (24.2) | 13,060 (27.7) |
| Postmenopausal | 161,227 (75.5) | 24,321 (72.9) | 48,783 (79.0) | 53,981 (75.8) | 34,142 (72.3) |
| BMI3, kg/m2 | 27.3 (27.3, 27.3) | 26.0 (26.0, 26.1) | 26.9 (26.9, 27.0) | 27.5 (27.5, 27.5) | 28.1 (28.0, 28.1) |
| Waist circumference3, cm | 90.2 (90.2, 90.3) | 87.2 (87.1, 87.4) | 89.2 (89.2, 89.3) | 90.0 (90.5, 90.6) | 92.0 (92.0, 92.1) |
Values are presented as n (%) of participants, means (95% CIs), or means ± SDs. All associations P < 0.001 based on ANOVA for characteristics presented as means ± SDs and Pearson's chi for those presented as n (%).
Each serving of fruit and vegetable is equivalent to 1 piece of fresh fruit (approximately 80 g), 2 pieces of dried fruit (approximately 15 g) or 2 heaped tablespoons of vegetables (approximately 50 g) (28).
Arithmatic means adjusted for sex and age.
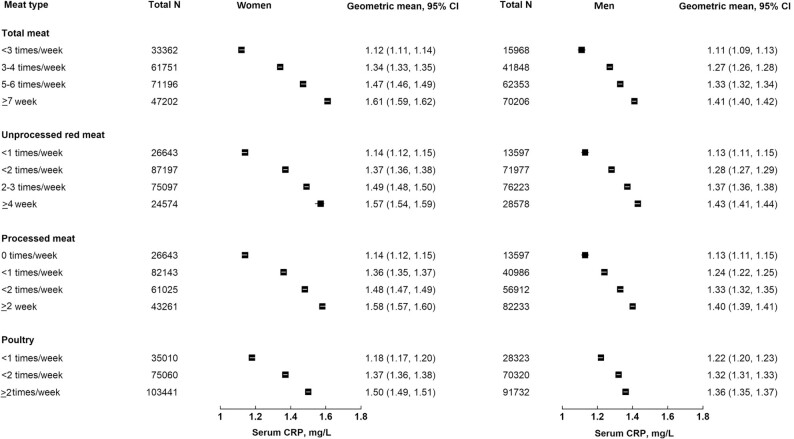
In multivariable adjusted models (model 2), each additional 50 g/d intake of meat was associated with higher CRP. The difference in the serum CRP (mg/L) for each 50 g/d higher intake of total meat was 11.6% (95% CI: 11.1, 12.0%), of processed meat was 38.3% (95% CI: 36.0, 40.7%), of unprocessed red meat was 14.4% (95% CI: 13.6, 15.1%), and of poultry was 12.8% (95% CI: 12.0, 13.5%). (Table 2). There were significant interactions by sex for all associations with CRP (P < 0.001). In stratified results both women and men showed positive associations, with larger associations observed in women [the difference in the serum CRP (mg/L) for each 50 g/d higher intake of total meat in women: 15.2% (95% CI: 14.5,15.9%), in men: 7.9% (95% CI:7.4,8.5%); see Table 2 for meat subtypes). Figure 1 shows geometric means for CRP in women and men by categories of meat intakes (based on model 2).
TABLE 2.
Mean percentage difference (95% CI) in serum CRP and WBCC per 50 g/d higher meat intake by level of adjustment (women, n = 213,511; men, n = 190,375)1
| Model 12 | Model 23 | Model 2 + BMI4 | Model 2 + WC5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Meat type per 50 g/d | Difference, % (95% CI) | P-heterogeneity6 | Difference, % (95% CI) | P-heterogeneity6 | Difference, % (95% CI) | P-heterogeneity6 | Change due to BMI, %7 | Mean change, % (95% CI) | P-heterogeneity6 | Change due to WC, %7 |
| Serum CRP, mg/L | ||||||||||
| Total meat8 | 12.6 (12.1, 13.0) | <0.001 | 11.6 (11.1, 12.0) | <0.001 | 3.8 (3.5, 4.2) | <0.001 | 67.2 | 4.9 (4.5, 5.2) | <0.001 | 57.8 |
| Women | 15.4 (14.7, 16.0) | 15.2 (14.5, 15.9) | 4.7 (4.2, 5.2) | 69.1 | 6.1 (5.5, 6.6) | 59.9 | ||||
| Men | 9.7 (9.1, 10.3) | 7.9 (7.4, 8.5) | 2.9 (2.4, 3.5) | 63.3 | 3.4 (2.9, 3.9) | 57.0 | ||||
| Unprocessed red meat8 | 16.9 (16.1, 17.6) | <0.001 | 14.4 (13.6, 15.1) | <0.001 | 5.7 (5.1, 6.4) | <0.001 | 60.4 | 6.6 (5.9, 7.2) | <0.001 | 54.2 |
| Women | 18.9 (17.7, 20.1) | 18.4 (17.3, 19.5) | 6.8 (5.9, 7.7) | 63.0 | 8.1 (7.2, 9.1) | 56.0 | ||||
| Men | 14.9 (13.9, 15.9) | 10.6 (9.6, 11.5) | 4.7 (3.9, 5.6) | 55.7 | 5.0 (4.1, 5.8) | 52.8 | ||||
| Processed meat8 | 53.2 (50.7, 55.8) | <0.001 | 38.3 (36.0, 40.7) | <0.001 | 14.6 (12.8, 16.4) | <0.001 | 61.9 | 13.1 (11.3, 14.8) | <0.001 | 65.8 |
| Women | 69.5 (65.0, 74.3) | 56.7 (52.5, 61.0) | 15.9 (13.1, 18.7) | 72.0 | 15.9 (13.1, 18.7) | 72.0 | ||||
| Men | 43.0 (40.0, 46.0) | 25.9 (23.3, 28.5) | 13.2 (11.0, 15.5) | 49.0 | 10.7 (8.5, 12.9) | 58.7 | ||||
| Poultry8 | 11.1 (10.3, 11.8) | <0.001 | 12.8 (12.0, 13.5) | <0.001 | 2.5 (1.9, 3.2) | <0.001 | 80.5 | 4.9 (4.2, 5.5) | <0.001 | 61.7 |
| Women | 17.2 (16.0, 18.3) | 18.2 (17.1, 19.4) | 4.3 (3.4, 5.2) | 76.4 | 6.8 (5.9, 7.7) | 62.6 | ||||
| Men | 4.1 (3.0, 5.1) | 6.9 (5.8, 7.9) | 0.6 (−0.3, 1.6) | 91.3 | 2.3 (1.3, 3.2) | 66.7 | ||||
| WBCC, ×109 cells/L | ||||||||||
| Total meat8 | 1.6 (1.5, 1.7) | <0.001 | 1.5 (1.4, 1.6) | <0.001 | 0.7 (0.6, 0.8) | 0.168 | 53.3 | 0.8 (0.7, 0.9) | 0.037 | 46.7 |
| Women | 1.8 (1.7, 2.0) | 1.7 (1.6, 1.9) | 0.8 (0.7, 0.9) | 52.9 | 0.9 (0.7, 1.0) | 47.1 | ||||
| Men | 1.5 (1.4, 1.7) | 1.2 (1.1, 1.4) | 0.6 (0.5, 0.7) | 50.0 | 0.6 (0.5, 0.8) | 50.0 | ||||
| Unprocessed red meat8 | 2.1 (1.9, 2.3) | 0.015 | 1.6 (1.4, 1.7) | 0.041 | 0.7 (0.6, 0.9) | 0.707 | 56.3 | 0.7 (0.6, 0.9) | 0.333 | 56.3 |
| Women | 2.1 (1.8, 2.3) | 1.8 (1.6, 2.1) | 0.8 (0.6, 1.0) | 55.6 | 0.9 (0.6, 1.1) | 50.0 | ||||
| Men | 2.2 (2.0, 2.4) | 1.3 (1.1, 1.5) | 0.6 (0.4, 0.8) | 53.8 | 0.6 (0.4, 0.8) | 53.8 | ||||
| Processed meat8 | 9.5 (9.0, 9.9) | <0.001 | 6.5 (6.1, 6.9) | <0.001 | 4.4 (4.0, 4.8) | 0.039 | 32.3 | 4.1 (3.7, 4.5) | 0.005 | 36.9 |
| Women | 10.6 (9.9, 11.3) | 8.0 (7.3, 8.7) | 4.9 (4.3, 5.6) | 38.8 | 4.7 (4.1, 5.4) | 41.3 | ||||
| Men | 8.9 (8.3, 9.5) | 5.4 (4.9, 6.0) | 4.0 (3.5, 4.5) | 25.9 | 3.6 (3.1, 4.2) | 33.3 | ||||
| Poultry8 | 1.0 (0.8, 1.1) | <0.001 | 1.6 (1.4, 1.7) | <0.001 | 0.5 (0.4, 0.7) | 0.003 | 68.8 | 0.7 (0.6, 0.9) | 0.003 | 56.3 |
| Women | 1.7 (1.5, 2.0) | 2.0 (1.8, 2.2) | 0.8 (0.6, 1.0) | 60.0 | 0.9 (0.7, 1.2) | 55.0 | ||||
| Men | 0.2 (0.0, 0.5) | 1.1 (0.9, 1.3) | 0.3 (0.1, 0.6) | 72.7 | 0.5 (0.3, 0.8) | 54.5 | ||||
BMI, Body mass index; CRP, C-reactive protein; MET, metabolic equivalent; WBCC, white blood cell count; WC, waist circumference.
Model 1 adjusted for age. The percentage difference refers to an increase of in CRP/WBCC for every 50 g/d higher meat intake.
Model 2: model 1 + baseline smoking status (never, former, current smoker < 15 cigarettes/d, ≥15 cigarettes/d, unknown amount), ethnicity (nonwhite), Townsend deprivation index (quintiles from least to most deprived), employment (employed or self-employed, retired, unemployed) and qualification level (college or university degree or vocational qualification, national examination at ages 17–18, national examination at age 16, other or unknown), total fruit and vegetable intake (<3, 3–3.99, 4–5.99, ≥6 servings/d), bread and cereal fiber intake (sex-specific quintiles), total fish consumption (0–1, >1 to <2, 2 to <3, ≥3 times/wk), total physical activity (<5, 5–9.9, 10–14.9, 15–24.9, 25–34.9, 35–49.9, 50–74.9, 75–99.9, ≥100 metabolic equivalent h/wk), alcohol intake (<1, 1 < 5, 5 < 10, 10 < 15, 15 < 20, 20 < 25, ≥25, nondrinkers) and menopausal status (premenopausal/postmenopausal) in women.
Model 2 + baseline BMI (continuous).
Model 2 + baseline WC (continuous).
P-heterogeneity based on a likelihood-ratio test comparing the model with and without an interaction for sex.
BMI/WC percentage change is the proportion of the main association (model 2) attenuated after adjustment for adiposity.
Association for women and men combined, all models additionally adjusted for sex.
FIGURE 1.
Adjusted geometric means of serum CRP (mg/L) and 95% CI by meat types and sex. Adjusted for age, baseline smoking status (never, former, current smoker <15 cigarettes/d, ≥15 cigarettes/d, unknown amount), ethnicity (white, nonwhite), Townsend deprivation index (quintiles from least to most deprived), employment (employed or self-employed, retired, unemployed), and qualification level (college or university degree or vocational qualification, national examination at ages 17–18 y, national examination at age 16 y, other or unknown), total fruit and vegetable intake (<3, 3–3.99, 4–5.99, ≥6 servings/d), bread and cereal fiber intake (sex-specific quintiles), total fish consumption (0–1, >1 to <2, 2 to <3, ≥3 times/wk), total physical activity (<5, 5–9.9, 10–14.9, 15–24.9, 25–34.9, 35–49.9, 50–74.9, 75–99.9, ≥100 MET h/wk), alcohol intake (<1, 1 to <5, 5 to <10, 10 to <15, 15 to <20, 20 to <25, ≥ 25, nondrinkers) and menopausal status (premenopausal, postmenopausal) in women. CRP, C-reactive protein; MET, metabolic equivalent.
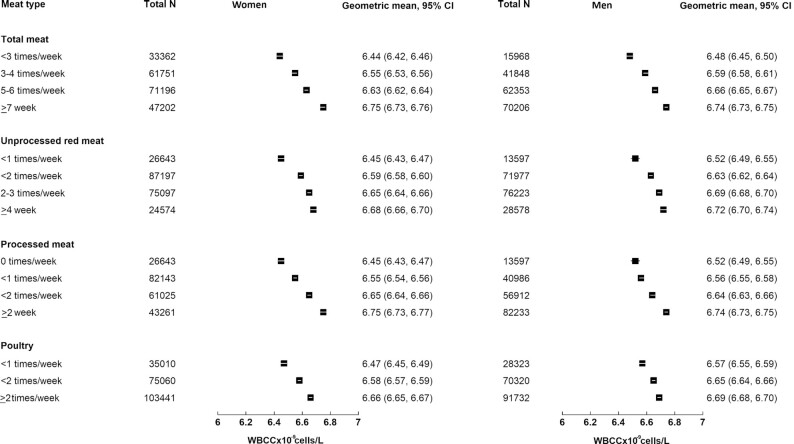
Each additional 50 g/d intake of meat was associated with higher WBCC. The difference in the WBCC (×10–9L) for each 50 g/d higher intake of total meat was 1.5% (95% CI: 1.4, 1.6%), of processed meat was 6.5% (95% CI: 6.1, 6.9%), of unprocessed red meat was 1.6% (95% CI: 1.4, 1.7%), and of poultry was 1.6% (95% CI: 1.4, 1.7%) (Table 2). There were significant interactions by sex for all associations with WBCC (P < 0.001 for total meat, processed meat, and poultry and 0.041 for unprocessed red meat). In stratified results both women and men showed a positive association, with larger associations observed in women [difference in the WBCC (×10–9L) for each 50 g/d higher intake of total meat in women: 1.7% (95% CI: 1.6,1.9%), in men: 1.2% (95% CI:1.1,1.4%); see Table 2 for meat subtypes]. Figure 2 shows geometric means for WBCC in women and men by categories of meat intakes (based on model 2).
FIGURE 2.
Adjusted geometric means of WBBC (×109 cells/L) and 95% CI by meat types and sex. Adjusted for age, baseline smoking status (never, former, current smoker <15 cigarettes/d, ≥15 cigarettes/d, unknown amount), ethnicity (white, nonwhite), Townsend deprivation index (quintiles from least to most deprived), employment (employed or self-employed, retired, unemployed) and qualification level (college or university degree or vocational qualification, national examination at age 17–18 y, national examination at age 16 y, other or unknown), total fruit and vegetable intake (<3, 3–3.99, 4–5.99, 6+ servings/d), bread and cereal fiber intake (sex-specific quintiles), total fish consumption (0–1, >1 to <2, 2 to <3, ≥3 times/wk), total physical activity (<5, 5–9.9, 10–14.9, 15–24.9, 25–34.9, 35–49.9, 50–74.9, 75–99.9, ≥100 MET h/wk), alcohol intake (<1, 1 to <5, 5 to <10, 10 to <15, 15 to <20, 20 to <25, ≥25, nondrinkers) and menopausal status (premenopausal, postmenopausal) in women. MET, metabolic equivalent; WBCC, white blood cell count.
When additionally adjusting for BMI or waist circumference, we observed similar magnitudes of attenuation for the 2 measures of adiposity. For total meat, the associations were attenuated by 67% for BMI and 58% for waist circumference for CRP, and by 53% and 47% for WBCC based on the estimates (see Table 2 for estimates by subtypes).
In sensitivity analyses using biomarkers at follow-up in a subsample with follow-up biomarker data, baseline intakes of total, unprocessed red, processed meat, and poultry were all positively associated with CRP at follow-up. Similarly, baseline intakes of total, processed meat, and poultry were also positively associated with WBCC at follow-up. However, there was a difference in effect estimates and attenuation with smaller % differences in CRP per 50 g/d intake of processed meat and larger effect estimates for total and unprocessed red meat and poultry intake before and after adjustment for adiposity, and smaller percentage differences in WBCC per 50 g/d intake for unprocessed red meat but little differences for other meat types (Supplemental Table 1).
Discussion
Overall, we found positive associations between any meat intake and 2 inflammatory markers, with larger magnitudes of associations for processed meat, and in women, in this large study of British adults.
Our findings are in line with several previous studies that found small positive associations between red meat (9–13), processed meat (11, 12, 15), and CRP. Previous studies have not found associations between poultry intake and inflammatory markers (15), and to our knowledge no previous studies have investigated associations between meat intake and WBCC.
In most previous studies, adjustment for adiposity attenuated the associations to null (10–12). This was not the case in the present study where associations attenuated substantially (>50%) but remained statistically significant; this might be related to the large size and therefore high power of our study. The remaining associations between meat and inflammatory markers were relatively small (ranging from 0.6 to 15.9% for CRP, mg/L and from 0.3–9% to 4.9% for WBCC, × 10 cells/L) and could have been due to residual confounding by other aspects of adiposity such as time exposed to excess weight. In comparison, associations for other lifestyle factors (such as smoking) have been estimated to be around twice as large as what we observed for meat after adjustment for adiposity (19, 20).
Our study findings support the hypothesis that increased adiposity might play a principal role in the association between meat intake, CRP, and WBCC. However, there might also be some independent effects, due for example to meat's heme iron content (4, 21), high saturated fat content (5, 22), and/or AGEs (23), which have each been suggested to be associated with inflammation (mostly assessed by measuring CRP), but none of these putative mediating effects are established.
To our knowledge, this is the largest investigation of habitual meat intake and markers of inflammation to date, but this study has some limitations. UK Biobank study participants are not representative of the UK general population, with UK Biobank participants showing more favorable health behaviors (24). This selection bias could have led to reduced variation in meat intake and inflammatory markers, with those with the least favorable conditions such as very high meat intake and BMI potentially missing from the sample and the results described potentially underestimating a real association. Additionally, UK Biobank did not measure other inflammatory markers (e.g., IL-6 and TNF-α), so these could not be considered in the present study. Moreover, information on some potential confounders was not available, for instance presence of acute infection or details on fasting or nonfasting status. Therefore, there may be residual confounding by these factors (25, 26). Another limitation was the method of dietary assessment; the touchscreen questionnaire did not allow the calculation of total dietary intake to control for potential over- or underreporting. We attempted to account for other dietary factors by adjusting for intakes of total fruit, vegetable, and cereal fiber and of fish, but residual confounding by other aspects of the diet could still operate (27). Moreover, information on diet and adiposity was collected at the same time point. As a result we could not conduct a formal mediation analysis. Future work in this area could assess if the proportion of the association that is attributed to adiposity differs when conducting a mediation analysis (27). The main analysis was cross-sectional, and therefore we cannot assess temporality in all participants; however, we found that most of the associations were similar in a prospective sensitivity analysis in a subsample with follow-up biomarker data.
In this study of British adults, higher meat consumption, particularly of processed meat, was positively associated with inflammatory markers. However, the magnitudes of the associations are small and predominantly due to higher adiposity, and the modest associations remaining after adjustment may be due to residual confounding by other aspects of adiposity.
Supplementary Material
Acknowledgments
We thank Aurora Perez-Cornago (Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford), the application holder.
The authors’ responsibilities were as follows—AK, KP: designed the research; AK, KP, LH: conducted research and analyzed the data; AK, KP: wrote the first manuscript; AK, KP, LH, TYNT, TK: interpreted the findings; and all authors: read and approved the final manuscript.
Notes
This work was supported by the Wellcome Trust; Our Planet Our Health; Livestock, Environment and People - LEAP (grant number 205212/Z/16/Z); and the UK Medical Research Council (grant number MR/M012190/1). TYNT was supported by a Nuffield Department of Population Health Intermediate Fellowship; LH was supported by a Lord Crewe fellowship of Lincoln College Oxford. The funders had no role in the design, analysis, or writing of this article.
KP and LH are joint first authors.
Author disclosures: The authors report no conflicts of interest.
This research was conducted using the UK Biobank (UKB) resource under application number 24494.
Supplemental Figure 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AGE, advanced glycation end product; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, Tumour Necrosis Factor alpha; WBCC, white blood cell count
Contributor Information
Keren Papier, Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom.
Lilian Hartman, John Radcliffe Hospital, Medical Sciences Division, University of Oxford, Oxford, United Kingdom; Lincoln College, University of Oxford, Oxford, United Kingdom.
Tammy Y N Tong, Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom.
Timothy J Key, Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom.
Anika Knuppel, Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom.
Data Availability
The data, codebook and analytic code described in the manuscript will be made available for bona fide researchers who apply to use the UK Biobank data set by registering and applying at http://www.ukbiobank.ac.uk/register-apply.
References
- 1. Calder PC, Ahluwalia N, Albers R, Bosco N, Bourdet-Sicard R, Haller D, Holgate ST, Jönsson LS, Latulippe ME, Marcos A. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr. 2013;109(S1):S1–S34. [DOI] [PubMed] [Google Scholar]
- 2. Cheng L, Zhuang H, Yang S, Jiang H, Wang S, Zhang J. Exposing the causal effect of c-reactive protein on the risk of type 2 diabetes mellitus: a mendelian randomization study. Frontiers in Genetics. 2018;9(657):e00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Welsh C, Welsh P, Mark PB, Celis-Morales CA, Lewsey J, Gray SR, Lyall DM, Iliodromiti S, Gill JMR, Pell J et al. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK Biobank. Arterioscler Thromb Vasc Biol. 2018;38(6):1415–23. [DOI] [PubMed] [Google Scholar]
- 4. de Oliveira Otto MCC, Alonso A, Lee D-H, Delclos GL, Jenny NS, Jiang R, Lima JA, Symanski E, Jacobs DR Jr., Nettleton JA. Dietary micronutrient intakes are associated with markers of inflammation but not with markers of subclinical atherosclerosis. J Nutr. 2011;141(8):1508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clarke R, Shipley M, Armitage J, Collins R, Harris W. Plasma phospholipid fatty acids and CHD in older men: Whitehall Study of London Civil Servants. Br J Nutr. 2008;102(2):279–84. [DOI] [PubMed] [Google Scholar]
- 6. Chen G, Scott Smith J. Determination of advanced glycation end products in cooked meat products. Food Chem. 2015;168:190–5. [DOI] [PubMed] [Google Scholar]
- 7. Schlesinger S, Neuenschwander M, Schwedhelm C, Hoffmann G, Bechthold A, Boeing H, Schwingshackl L. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2019;10(2):205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Timpson NJ, Nordestgaard BG, Harbord RM, Zacho J, Frayling TM, Tybjærg-Hansen A, Davey Smith G. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal mendelian randomization. Int J Obes. 2011;35(2):300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr. 2009;139(2):335–9. [DOI] [PubMed] [Google Scholar]
- 10. Montonen J, Boeing H, Fritsche A, Schleicher E, Joost H-G, Schulze MB, Steffen A, Pischon T. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr. 2013;52(1):337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ley SH, Sun Q, Willett WC, Eliassen AH, Wu K, Pan A, Grodstein F, Hu FB. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr. 2014;99(2):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chai W, Morimoto Y, Cooney RV, Franke AA, Shvetsov YB, Le Marchand L, Haiman CA, Kolonel LN, Goodman MT, Maskarinec G. Dietary red and processed meat intake and markers of adiposity and inflammation: the multiethnic cohort study. J Am Coll Nutr. 2017;36(5):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mazidi M, Kengne AP, George ES, Siervo M. The association of red meat intake with inflammation and circulating intermediate biomarkers of type 2 diabetes is mediated by central adiposity. Br J Nutr. 2021;125(9):1043–50. [DOI] [PubMed] [Google Scholar]
- 14. Hobbs-Grimmer DA, Givens DI, Lovegrove JA. Associations between red meat, processed red meat and total red and processed red meat consumption, nutritional adequacy and markers of health and cardio-metabolic diseases in British adults: a cross-sectional analysis using data from UK National Diet and Nutrition Survey. Eur J Nutr. 2021;60(6):2979–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Woudenbergh GJ, Kuijsten A, Tigcheler B, Sijbrands EJ, Van Rooij FJ, Hofman A, Witteman JC, Feskens EJ. Meat consumption and its association with C-reactive protein and incident type 2 diabetes: the Rotterdam study. Diabetes Care. 2012;35(7):1499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collins R. What makes UK Biobank special?. Lancet North Am Ed. 2012;379(9822):1173–4. [DOI] [PubMed] [Google Scholar]
- 17. Knuppel A, Papier K, Fensom GK, Appleby PN, Schmidt JA, Tong TYN, Travis RC, Key TJ, Perez-Cornago A. Meat intake and cancer risk: prospective analyses in UK biobank. Int J Epidemiol. 2020;49(5):1540–52. [DOI] [PubMed] [Google Scholar]
- 18. Perez-Cornago A, Pollard Z, Young H, van Uden M, Andrews C, Piernas C, Key TJ, Mulligan A, Lentjes M. Description of the updated nutrition calculation of the Oxford WebQ questionnaire and comparison with the previous version among 207,144 participants in UK Biobank. medRxiv. 2020:2020.11.30.20240713. doi: 10.1101/2020.11.30.20240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kianoush S, Yakoob MY, Al-Rifai M, DeFilippis AP, Bittencourt MS, Duncan BB, Bensenor IM, Bhatnagar A, Lotufo PA, Blaha MJ. Associations of cigarette smoking with subclinical inflammation and atherosclerosis: eLSA-Brasil (the Brazilian Longitudinal Study of Adult Health). J Am Heart Assoc. 2017;6(6):e005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tani S, Kawauchi K, Atsumi W, Matsuo R, Ashida T, Imatake K, Suzuki Y, Yagi T, Takahashi A, Matsumoto N et al. Association among daily fish intake, white blood cell count, and healthy lifestyle behaviors in an apparently healthy Japanese population: implication for the anti-atherosclerotic effect of fish consumption. Heart Vessels. 2021;36(7):924–33. [DOI] [PubMed] [Google Scholar]
- 21. Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K, Corpet D. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–600. [DOI] [PubMed] [Google Scholar]
- 22. Public Health England. National Diet and Nutrition Survey Results from Years 7 and 8 (combined) of the Rolling Programme (2014/2015 to 2015/2016). London: 2018. https://www.gov.uk/government/statistics/ndns-results-from-years-7-and-8-combined. [Google Scholar]
- 23. Zhang Q, Wang Y, Fu L. Dietary advanced glycation end-products: perspectives linking food processing with health implications. Compr Rev Food Sci Food Saf. 2020;19(5):2559–87. [DOI] [PubMed] [Google Scholar]
- 24. Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Yang Q, Liao Q, Li M, Zhang P, Santos HO, Kord-Varkaneh H, Abshirini M. Effects of intermittent fasting diets on plasma concentrations of inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Nutrition. 2020;79-80:110974. [DOI] [PubMed] [Google Scholar]
- 26. Kościelniak BK, Charchut A, Wójcik M, Sztefko K, Tomasik PJ. Impact of fasting on complete blood count assayed in capillary blood samples. Lab Med. 2017;48(4):357–61. [DOI] [PubMed] [Google Scholar]
- 27. Fewell Z, Davey Smith G, Sterne JAC. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166(6):646–55. [DOI] [PubMed] [Google Scholar]
- 28. Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in UK Biobank: a prospective study. Int J Epidemiol. 2020;49(1):246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, codebook and analytic code described in the manuscript will be made available for bona fide researchers who apply to use the UK Biobank data set by registering and applying at http://www.ukbiobank.ac.uk/register-apply.