ABSTRACT
Background
Carbohydrate restriction shows promise for diabetes, but concerns regarding high saturated fat content of low-carbohydrate diets limit widespread adoption.
Objectives
This preplanned ancillary study aimed to determine how diets varying widely in carbohydrate and saturated fat affect cardiovascular disease (CVD) risk factors during weight-loss maintenance.
Methods
After 10–14% weight loss on a run-in diet, 164 participants (70% female; BMI = 32.4 ± 4.8 kg/m2) were randomly assigned to 3 weight-loss maintenance diets for 20 wk. The prepared diets contained 20% protein and differed 3-fold in carbohydrate (Carb) and saturated fat as a proportion of energy (Low-Carb: 20% carbohydrate, 21% saturated fat; Moderate-Carb: 40%, 14%; High-Carb: 60%, 7%). Fasting plasma samples were collected prerandomization and at 20 wk. Lipoprotein insulin resistance (LPIR) score was calculated from triglyceride-rich, high-density, and low-density lipoprotein particle (TRL-P, HDL-P, LDL-P) sizes and subfraction concentrations (large/very large TRL-P, large HDL-P, small LDL-P). Other outcomes included lipoprotein(a), triglycerides, HDL cholesterol, LDL cholesterol, adiponectin, and inflammatory markers. Repeated measures ANOVA was used for intention-to-treat analysis.
Results
Retention was 90%. Mean change in LPIR (scale 0–100) differed by diet in a dose-dependent fashion: Low-Carb (–5.3; 95% CI: –9.2, –1.5), Moderate-Carb (–0.02; 95% CI: –4.1, 4.1), High-Carb (3.6; 95% CI: –0.6, 7.7), P = 0.009. Low-Carb also favorably affected lipoprotein(a) [–14.7% (95% CI: –19.5, –9.5), –2.1 (95% CI: –8.2, 4.3), and 0.2 (95% CI: –6.0, 6.8), respectively; P = 0.0005], triglycerides, HDL cholesterol, large/very large TRL-P, large HDL-P, and adiponectin. LDL cholesterol, LDL-P, and inflammatory markers did not differ by diet.
Conclusions
A low-carbohydrate diet, high in saturated fat, improved insulin-resistant dyslipoproteinemia and lipoprotein(a), without adverse effect on LDL cholesterol. Carbohydrate restriction might lower CVD risk independently of body weight, a possibility that warrants study in major multicentered trials powered on hard outcomes. The registry is available through ClinicialTrials.gov: https://clinicaltrials.gov/ct2/show/NCT02068885.
Keywords: low-carbohydrate diet, saturated fat, cardiovascular disease risk factors, obesity, macronutrients, dietary trial
Introduction
For nearly a half century, advice to reduce saturated fat intake has been a major focus of dietary guidelines for public health and medical nutrition therapy (1). This advice is based in part on evidence from clinical trials showing that saturated fat increases plasma LDL cholesterol (2), a major risk factor for cardiovascular disease (CVD). Replacing saturated with unsaturated fat lowers LDL cholesterol in trials and reduces risk of cardiovascular and total mortality in cohort studies (3).
Conversely, when saturated fat is replaced by carbohydrate, particularly from processed sources (4), reducing intake does not decrease risk (3) and can have adverse effects on components of the metabolic syndrome, including high triglycerides, low HDL cholesterol, and other risk factors related to insulin resistance (5, 6). Low-carbohydrate diets, with saturated fat content far exceeding current guidelines, have become popular for diabetes management (7) based on preliminary evidence of efficacy (8, 9), albeit with concern for the potential of saturated fat to raise LDL cholesterol and consequently CVD risk. However, LDL cholesterol does not capture potentially important diet effects on CVD risk from insulin-resistant dyslipoproteinemia (10). Indeed, there is broad consensus regarding the need to assess multiple biomarkers beyond LDL cholesterol to clarify the relation between diet and CVD (11).
The aim of this study was to compare the effects on novel and conventional CVD risk factors of low-, moderate-, and high-carbohydrate diets varying in saturated fat content in a manner reflective of how these diets are typically consumed. We hypothesized that the low-carbohydrate diet would improve lipoprotein insulin resistance (LPIR) score, a metabolic marker that captures incipient effects of insulin resistance on lipoprotein metabolism and has been robustly associated with incident type 2 diabetes and premature coronary heart disease (10, 12–15). To enhance dietary adherence, we provided participants with fully prepared meals throughout the study.
Methods
Parent study
The Framingham State Food Study was a randomized controlled feeding trial conducted from August 2014 to May 2017, with the primary aim of examining macronutrient effects on energy metabolism (16, 17). Briefly, the study comprised Run-In and Test phases (Figure 1). During the Run-In phase, we restricted energy intake to promote 12 ± 2% weight loss over 9–10 wk and randomly assigned participants who achieved the target weight loss to low-, moderate-, and high-carbohydrate Test diets (Low-Carb, Moderate-Carb, High-Carb). The method for random assignment is presented in Supplemental Methods. During the 20-wk Test phase, we adjusted energy intake to maintain weight within ±2 kg of that achieved after weight loss and immediately prior to randomization. A partnership with Sodexo, the food service contractor at Framingham State University, was established for implementing the feeding protocol on campus (17). The institutional review board at Boston Children's Hospital approved the study protocol. Participants provided written informed consent. The protocol history is presented in the Supplemental Material. Results for the primary outcome were previously reported, that total energy expenditure was higher (∼200 kcal/d) on the low- compared with high-carbohydrate diet (18).
FIGURE 1.
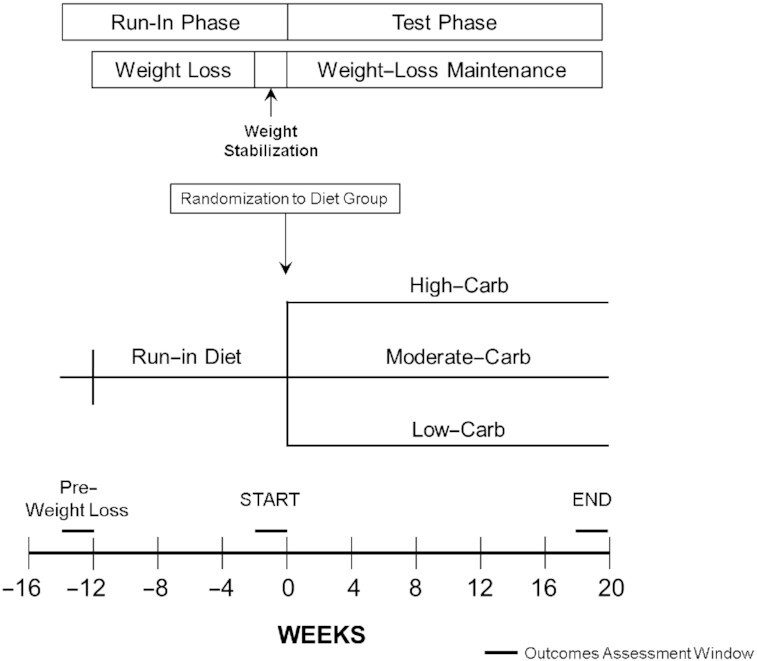
Study design.
We utilized the infrastructure of this trial to conduct a preplanned ancillary study focused on clinically relevant CVD risk factors. All individual outcomes were prespecified except lipoprotein(a) [Lp(a)], which was measured after review of initial data. The composite LPIR score was calculated from prespecified outcomes. We analyzed blood samples collected following an overnight fast at the following time points: pre-weight-loss (PRE), start of the trial (START, post-weight-loss, prerandomization), and end of the Test phase (END).
Participants
We enrolled adults aged 18 to 65 y with BMI ≥25 kg/m2, excluding those with known CVD or diabetes. Additional eligibility criteria are listed in Supplemental Table 1. At the time of enrollment, we collected demographic information including sex, date of birth, race (white, black, Asian, multiple, or other), and ethnic group (Hispanic or non-Hispanic).
Diets
The hypocaloric Run-In diet contained 45% of total energy from carbohydrate, 35% from fat, and 25% from protein. The Test diets, with protein controlled at 20% of total energy, were designed to vary in proportions of carbohydrate and fat by 3-fold (Low-Carb: 20%, 60%; Moderate-Carb: 40%, 40%; High-Carb: 60%, 20%). Saturated fat comprised 35% of total fat for each diet, also with a 3-fold difference across diets when expressed as a proportion of total energy (21%, 14%, 7%, respectively). Monounsaturated fat was 25%, 16%, and 8%, and polyunsaturated fat was 11%, 9%, and 5% as a proportion of total energy. Daily dietary fiber was 25, 30, and 35 g/2000 kcal, respectively, with added sugar relative to total carbohydrate controlled at 15% across diets. Glycemic load was 28, 80, and 135 g/2000 kcal, respectively. Additional details regarding dietary interventions are presented in the Supplemental Material, including nutrient profiles and sample menus for the Test diets in Supplemental Tables 2 and 3.
Outcomes
Research personnel assessing outcomes for this report were blinded to random group assignment. Lipoprotein particle subfractions were measured by proton NMR spectroscopy using the LipoProfile-4 algorithm (LabCorp, Inc.) (19). Outcomes included mean particle diameters [triglyceride-rich lipoprotein particles (TRL-P), high-density lipoprotein particles (HDL-P), low-density lipoprotein particles (LDL-P)] and subclass particle concentrations (sum of large and very large TRL-P, large HDL-P, small LDL-P, large LDL-P). The LIPR test is calculated from 6 component metabolic markers of insulin-resistant dyslipoproteinemia, including particle diameters (TRL-P, HDL-P, LDL-P) and concentrations (sum of large and very large TRL-P, large HDL-P, small LDL-P) (10, 15). This score is more strongly related to insulin resistance than each of the individual components (15, 20) and has been associated with incident type 2 diabetes mellitus (10, 12, 20) and coronary heart disease (13). We obtained NMR-derived plasma concentrations of triglycerides, HDL cholesterol, and LDL cholesterol, and also measured serum concentrations by direct enzymatic assays (Roche Diagnostics) using samples from the same blood draw. Correlations between the 2 methods exceeded r = 0.9 (P < 0.001) for all variables. Lp(a) was measured by turbidimetric assay insensitive to the number of kringle IV type-2 repeats (Roche Diagnostics) (21). We measured serum high-sensitivity C-reactive protein (hsCRP) using an immunoturbidimetric assay (Roche Diagnostics) and IL-6 using an ultrasensitive ELISA (R&D Systems). We measured total and high molecular weight (HMW) adiponectin using an ELISA (R&D Systems). We measured resting blood pressure 3 times by auscultation, averaging the second 2 measurements for analysis.
Statistical analyses
Patient characteristics at PRE and study outcomes at each time point were summarized with descriptive statistics (mean and SD for continuous variables, median and IQR for skewed continuous variables, count and percentage for categorical variables). An unadjusted repeated-measures ANOVA was used to evaluate change in LPIR from START to END in the intention-to-treat sample. Secondary outcomes were analyzed using the same model. A post hoc power analysis and rationale for fitting unadjusted models are presented in the Supplemental Methods. A partial F test was used to assess overall significance of diet in the model, and t tests were used to assess changes within each arm. Post hoc pairwise comparison between Low-Carb and High-Carb was equivalent to a test for linear trend across the 3 diets, recognizing equal increments in carbohydrate content across the 3 diets. Skewed variables were log- or log-plus-one–transformed for analysis. Least squares means and SEs were back-transformed to the original units for reporting. Studentized residual plots and Cook's distance were used to identify outliers and influential observations with potential to impact the validity of results. Pearson correlation coefficients (or Spearman coefficients for skewed variables) were calculated using PRE data to construct a correlation matrix of LPIR, components of LPIR, TRL triglycerides, total triglycerides, HDL cholesterol, and apoA-I (derived from NMR data). Two-sided P values ≤0.05 were considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc.).
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. Study participants received a written summary of their clinically relevant results.
Results
Participants
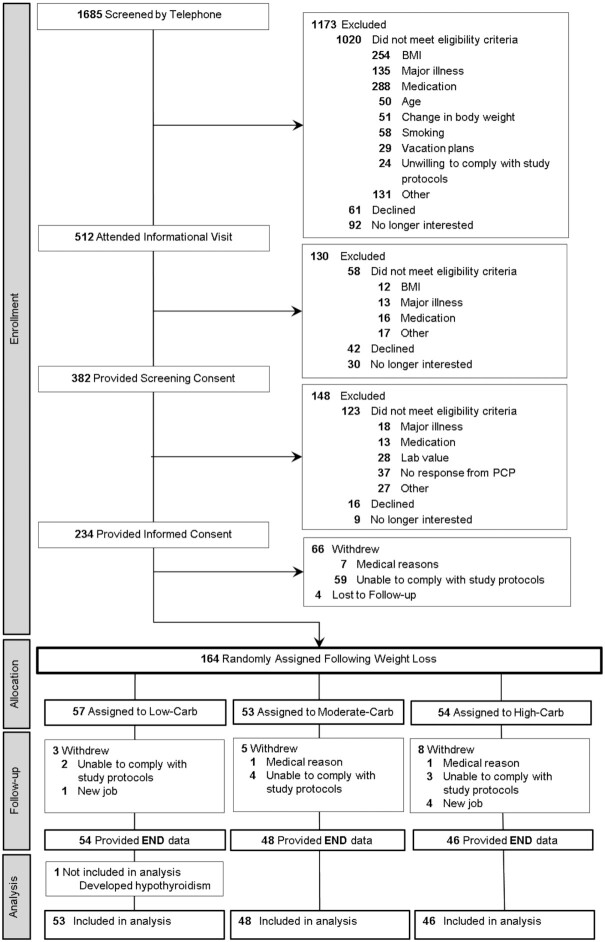
Figure 2 depicts the flow of participants through the trial, and Table 1 lists participant characteristics at PRE. Retention rate was 90%, with 148 participants completing the study and 147 included in the analysis [after a priori exclusion of 1 participant who developed hypothyroidism (16)]. Weight loss during the Run-In phase (mean ± SD) was 10.5% ± 1.7% for 164 participants randomly assigned to a diet group (and, similarly, 10.5% ± 1.6% for n = 147 completers). Mean change in body weight for the full cohort from START to END was –0.55 kg (–4 g/d), with no difference between diet groups (P = 0.79). Details of adverse events, which did not differ by diet group, are included in Supplemental Table 4.
FIGURE 2.
Participant flow. PCP, primary care practitioner.
TABLE 1.
Pre-weight-loss characteristics of study participants in the Framingham State Food Study1
| Characteristic | All randomized (n = 164) | Completers2 (n = 147) |
|---|---|---|
| Sex | ||
| Male | 49 (29.9) | 45 (30.6) |
| Female | 115 (70.1) | 102 (69.4) |
| Ethnic group | ||
| Hispanic | 25 (15.2) | 21 (14.3) |
| Non-Hispanic | 139 (84.8) | 126 (85.7) |
| Racial group | ||
| White | 128 (78.0) | 115 (78.2) |
| Black | 17 (10.4) | 16 (10.9) |
| Asian | 5 (3.0) | 5 (3.4) |
| Unknown/other | 14 (8.5) | 11 (7.5) |
| Age at first visit, y | 35.0 (23.6–50.1) | 35.7 (24.0–51.2) |
| Weight, kg | 91.5 ± 18.2 | 91.3 ± 18.3 |
| Height, cm | 167.7 ± 10.0 | 167.9 ± 10.1 |
| BMI, kg/m2 | 32.4 ± 4.8 | 32.2 ± 4.8 |
| BMI category | ||
| Overweight (≥25 to <30) | 65 (39.6) | 63 (42.9) |
Obesity (≥ ) ) |
99 (60.4) | 84 (57.1) |
| Body fat (% total mass) | 40.9 ± 6.2 | 40.7 ± 6.4 |
| Blood pressure, mmHg | ||
| Systolic | 123.3 ± 10.5 | 123.7 ± 10.7 |
| Diastolic | 76.5 ± 7.3 | 76.6 ± 7.4 |
| Blood lipids | ||
| Triglycerides, mg/dL | 112.0 (81.0–156.0) | |
| Total cholesterol, mg/dL | 166.4 ± 34.3 | |
| HDL-C, mg/dL | 51.1 ± 11.7 | |
| Non-HDL-C, mg/dL | 115.3 ± 32.6 | |
| LDL-C, mg/dL | 92.2 ± 25.9 | |
| Lipoprotein(a),3 mg/dL | 10.7 (5.9–34.0) |
For categorical variables, values are frequency (%). For continuous variables, values are mean ± SD if normally distributed and median (IQR) if skewed. HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
Among the completers (n = 148), 1 participant developed hypothyroidism and was an a priori exclusion from analyses of all outcome variables in this report.
Lipoprotein(a) was missing for 1 participant at pre-weight-loss.
Outcomes
Supplemental Table 5 shows descriptive data at PRE and START for study outcomes, and Table 2 presents changes from START to END. Figure 3 depicts percentage change from START to END for LPIR, Lp(a), and LDL cholesterol; and Supplemental Figure 1 depicts individual participant data for these variables.
TABLE 2.
Changes in outcome variables by diet group during the Test phase of the Framingham State Food Study1
| Change (END − START) by diet group | Linear trend across diet groups | |||||
|---|---|---|---|---|---|---|
| Variable | Low-Carb (n = 53) | Moderate-Carb (n = 48) | High-Carb (n = 46) | P value2 | Low-Carb − High-Carb | P value3 |
| Composite score | ||||||
| LPIR | –5.3 (–9.2, –1.5) | –0.02 (–4.1, 4.1) | 3.6 (–0.6, 7.7) | 0.009 | –8.9 (–14.6, –3.2) | 0.002 |
| Particle sizes | ||||||
| TRL-P, nm | 0.15 (–1.63, 1.92) | 1.52 (–0.35, 3.39) | 2.63 (0.72, 4.54) | 0.17 | –2.49 (–5.09, 0.12) | 0.06 |
| HDL-P, nm | 0.09 (0.03, 0.15) | 0.03 (–0.03, 0.10) | 0.05 (–0.02, 0.11) | 0.34 | 0.05 (–0.04, 0.13) | 0.29 |
| LDL-P, nm | 0.06 (–0.05, 0.18) | 0.16 (0.04, 0.28) | –0.01 (–0.14, 0.11) | 0.14 | 0.08 (–0.09, 0.25) | 0.38 |
| Particle concentrations | ||||||
| Large/very large TRL-P,4 % | –9.5 (–24.0, 7.7) | 10.9 (–7.6, 33.2) | 38.5 (14.9, 67.0) | 0.01 | –34.7 (–49.4, –15.6) | 0.001 |
| Large HDL-P, μmol/L | 0.71 (0.48, 0.95) | 0.36 (0.11, 0.60) | 0.32 (0.07, 0.58) | 0.045 | 0.39 (0.04, 0.73) | 0.03 |
| Small LDL-P, μmol/L | –193.9 (–289.2, –98.0) | –221.7 (–322.5, –120.9) | –107.5 (–210.4, –4.5) | 0.27 | –86.4 (–227.2, 54.3) | 0.23 |
| Large LDL-P, μmol/L | 55.3 (9.3, 101.3) | 54.6 (6.4, 102.9) | –1.1 (–50.4, 48.2) | 0.18 | 56.4 (–11.0, 123.8) | 0.10 |
| Lipoprotein(a),4,5 % | –14.7 (–19.6, –9.5) | –2.1 (–8.2, 4.3) | 0.2 (–6.0, 6.8) | 0.0005 | –14.9 (–22.0, –7.1) | 0.0004 |
| Blood lipids | ||||||
| Triglycerides,4 % | –9.2 (–15.6, –2.4) | 1.9 (–5.6, 10.0) | 7.6 (–0.5, 16.3) | 0.006 | –15.7 (–24.2, –6.2) | 0.002 |
| Total cholesterol, mg/dL | 16.1 (11.5, 20.7) | 18.5 (13.6, 23.3) | 16.2 (11.2, 21.1) | 0.73 | –0.1 (–6.8, 6.7) | 0.99 |
| HDL-C, mg/dL | 9.8 (8.0, 11.5) | 7.4 (5.6, 9.3) | 6.7 (4.8, 8.5) | 0.04 | 3.1 (0.5, 5.7) | 0.02 |
| Non-HDL-C, mg/dL | 6.3 (1.8, 10.9) | 11.0 (6.3, 15.8) | 9.5 (4.6, 14.4) | 0.35 | –3.2 (–9.8, 3.5) | 0.35 |
| LDL-C, mg/dL | 10.0 (6.3, 13.7) | 11.7 (7.8, 15.7) | 8.2 (4.2, 12.2) | 0.47 | 1.8 (–3.7, 7.3) | 0.52 |
| Adiponectin | ||||||
| Total,4 % | 33.6 (24.9, 42.9) | 17.4 (9.4, 26.0) | 23.0 (14.5, 32.3) | 0.03 | 8.6 (–1.6, 19.9) | 0.10 |
| High molecular weight,4 % | 42.9 (33.3, 53.1) | 27.6 (18.6, 37.2) | 27.8 (18.7, 37.7) | 0.04 | 11.8 (1.0, 23.7) | 0.03 |
| Inflammatory mediators | ||||||
| hsCRP,4,6 % | –9.9 (–24.2, 7.1) | –20.6 (–33.8, –4.8) | –1.7 (–18.6, 18.6) | 0.27 | –8.4 (–29.0, 18.3) | 0.50 |
| IL-6,4 % | –23.6 (–35.4, –9.6) | –20.1 (–33.0, –4.7) | –18.3 (–31.8, –2.2) | 0.86 | –6.5 (–26.9, 19.7) | 0.59 |
| Blood pressure, mmHg | ||||||
| Systolic7 | –1.5 (–4.4, 1.5) | 0.0 (–3.1, 3.1) | 1.8 (–1.3, 5.0) | 0.33 | –3.3 (–7.7, 1.0) | 0.13 |
| Diastolic7 | 2.2 (–0.4, 4.8) | 1.4 (–1.3, 4.1) | 2.2 (–0.6, 5.0) | 0.89 | 0.03 (–3.8, 3.8) | 0.99 |
Means (95% CI) were constructed and compared using unadjusted repeated measures ANOVA. HDL-C, HDL cholesterol; HDL-P, high-density lipoprotein particle; hsCRP, high-sensitivity C-reactive protein; LDL-C, LDL cholesterol; LPIR, lipoprotein insulin resistance; LDL-P, low-density lipoprotein particle; TRL-P, triglyceride-rich lipoprotein particle.
A partial F test was used to assess overall significance of diet in the repeated measures model.
Pairwise comparison between Low-Carb and High-Carb was equivalent to a test for linear trend across the 3 diets, recognizing equal increments in carbohydrate content across the 3 diets.
Skewed variables were log- or log-plus-one–transformed for analysis. For reporting, the least squares mean and 95% CIs from the model were retransformed to the original units as (exp(log estimate)) for log transformations or (exp(log estimate) − 1) for log-plus-one transformations. Changes are expressed in percentage units [100% × (exp(change in log) − 1)].
Because data were missing for 2 participants in Moderate-Carb, the lipoprotein(a) analyses included n = 46 for this group. Values were below the assay detection limit of 6.0 mg/dL for n = 38 at START (17 in Low-Carb, 7 in Moderate-Carb, 14 in High-Carb) and n = 45 at END (22 in Low-Carb, 10 in Moderate-Carb, 13 in High-Carb). We assigned values of 5.9 mg/dL. Consistent results were obtained when these data points were removed in a sensitivity analysis.
Four influential data points (1 in Low-Carb, 1 in Moderate-Carb, 2 in High-Carb) were not included in the analysis of hsCRP.
Blood pressure data were missing for 2 participants (1 in Low-Carb, 1 in High-Carb).
FIGURE 3.
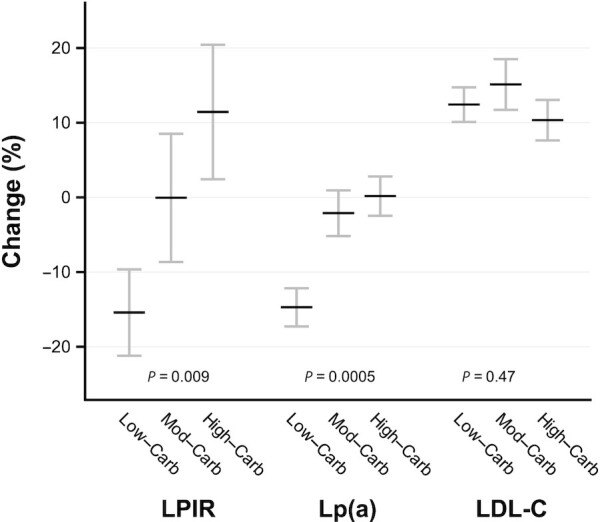
Change in LPIR, Lp(a), and LDL-C by diet group in the Framingham State Food Study. LDL-C increased in all groups, without difference by group, potentially reflecting adaptation to increased energy intake in the Test phase. The sample included n = 53 in Low-Carb, n = 48 in Moderate-Carb, and n = 46 in High-Carb. Because data were missing for 2 participants in Moderate-Carb, the Lp(a) analysis included n = 46 for this group. Means were constructed and compared using unadjusted repeated measures ANOVA. A partial F test was used to assess overall significance of diet in the repeated measures model. Lp(a) was log-transformed for analysis. For visualization, percentage change (mean with SE) was calculated from data in Table 2 and Supplemental Table 5. LDL-C, LDL cholesterol; Lp(a), lipoprotein(a); LPIR, lipoprotein insulin resistance.
At PRE, LPIR had strong correlations with its 6 constituent components, and strong to moderate correlations with other lipid variables related to insulin resistance (Supplemental Table 6). Change in LPIR differed by diet group (P = 0.009), with a decrease in Low-Carb (–5.3; 95% CI: –9.2, –1.5; P = 0.007), no change in Moderate-Carb (–0.02; 95% CI: –4.1, 4.1; P = 0.99), and a nonsignificant increase in High-Carb (3.6; 95% CI: –0.6, 7.7; P = 0.09). The components of the score that contributed most notably to the difference between Low-Carb and High-Carb were sum of large and very large TRL-P (lower for Low-Carb, P = 0.001) and large HDL-P (higher for Low-Carb, P = 0.03) concentrations. Other components of the score (TRL-P, HDL-P, and LDL-P sizes; small LDL-P concentration) and large LDL-P concentration did not differ individually by diet group.
Percentage change in Lp(a) differed by diet group (P = 0.0005), with a decrease in Low-Carb (–14.7; 95% CI: –19.6, –9.5; P < 0.0001) and no change in Moderate-Carb (–2.1; 95% CI: –8.2, 4.3; P = 0.51) and High-Carb (0.2; 95% CI: –6.0, 6.8; P = 0.96). Changes in triglycerides (P = 0.002) and HDL cholesterol (P = 0.02) favored Low-Carb compared with High-Carb. LDL cholesterol increased in all groups—potentially reflecting adaptation to increased energy intake in the Test phase—without difference by group. Changes in adiponectin (total, P = 0.03; HMW, P = 0.04) differed by diet group, and the pairwise comparison for HMW adiponectin favored Low-Carb (Table 2). Measures of chronic inflammation (hsCRP, IL-6) and blood pressure did not differ by group.
Discussion
Carbohydrate restriction shows promise for the treatment of diabetes and other prevalent chronic diet-related diseases (22). However, low-carbohydrate diets are characteristically high in saturated fat, raising concern for adverse effects. In the United States and Europe, saturated fat intake is strongly associated with LDL cholesterol and with cardiovascular morbidity and total mortality (3). Nevertheless, these observational findings derive from populations with relatively high intakes of carbohydrate. For example, in a 2-cohort study of mortality beginning in the 1980s, mean dietary carbohydrate as a proportion of energy intake ranged from 54% for women and 56% for men in the lowest quintile of saturated fat consumption, to 35% for women and 41% for men in the highest quintile (23). For this reason, experimental evidence is needed regarding how low-carbohydrate diets with high saturated fat content affect CVD risk factors.
In this feeding trial, we found that carbohydrate restriction had dose-dependent benefits for insulin-resistant dyslipoproteinemia, without adverse effects on total cholesterol, LDL cholesterol, LDL-P size, measures of chronic inflammation, or blood pressure. The low-carbohydrate diet also increased adiponectin, an adipocyte hormone that promotes insulin sensitivity and protects against atherogenesis (24). In addition, we found a potentially novel dietary effect on Lp(a), a major independent and causal risk factor for atherosclerosis (25). A recent review of trials ranging from 3 to 8 wk reported that “diet modestly affects Lp(a) and often in the opposing direction to LDL-C” (26), consistent with findings from the Delta Study in 1998 (27). Nevertheless, the prevailing view, as exemplified by a Scientific Statement from the National Lipid Association, holds that “Lifestyle therapy, including diet and physical exercise, has no significant effect on Lp(a) concentrations,” motivating the search for new pharmacological options (25). Lacking recognized treatment options, this important risk factor might not be consistently monitored in the clinical setting.
These findings suggests that a dietary strategy focused on carbohydrate restriction might not raise, and could potentially lower, CVD risk. To the extent that a low-carbohydrate diet results in greater weight loss, overall magnitude of CVD risk reduction could be greater than suggested here. These results are broadly consistent with small feeding trials and behavioral studies that report improvements in multiple cardiometabolic outcomes on low-carbohydrate diets, including triglycerides, HDL cholesterol, glycemia, blood pressure, liver fat, and body weight (28–31). Effects on these risk factors could mediate, to some degree, the associations between glycemic load and risk of CVD events and mortality observed in a recent 20-country study (32).
In contrast to our findings, some (33, 34) but not all (35) meta-analyses of clinical trials report higher LDL cholesterol on low-carbohydrate diets—heterogeneity that could relate to dietary composition, participant characteristics, study duration, or other design issues. In the DIETFITS trial (36), involving 609 participants assigned to a “healthy low-carbohydrate” or “healthy low-fat” diet (both with an emphasis on reducing intake of processed carbohydrates), LDL cholesterol increased by 5.7 mg/dL in the low-carbohydrate group over 12 mo. Interestingly, the association between change in saturated fat intake and LDL cholesterol was significant in the low-fat but not the low-carbohydrate group. Even so, cases of severe LDL cholesterol elevation have been reported on low-carbohydrate diets (37), characteristically involving individuals with genetic predisposition, those who consumed a more restrictive diet than was used in our study, or those who had recently experienced rapid weight loss (a cause of transient hypercholesterolemia (38).
Excluding these extreme examples, other effects of a low-carbohydrate diet might attenuate or counterbalance any risk associated with the moderate LDL cholesterol elevation that can occur in some individuals. Even at higher LDL cholesterol concentrations, lipid markers of insulin sensitivity, such as low triglycerides and high HDL cholesterol, are associated with relatively low CVD risk (39–41). In a prospective cohort study, insulin-resistant dyslipoproteinemia compared with LDL cholesterol was a stronger biomarker risk factor for early-onset coronary heart disease in women (13). According to a modeling study, insulin resistance compared with LDL cholesterol could account for a greater proportion of coronary artery disease risk in adults aged 20–30 y (42) (reflecting a range included in our trial). Moreover, in the pharmacological management of risk factors, drugs for elevated LDL cholesterol (were it to occur on a low-carbohydrate diet) are generally more effective and better tolerated than drugs for metabolic syndrome components (were they to occur on a high-carbohydrate diet). A Mediterranean-style low-carbohydrate diet, with an emphasis on unsaturated fats, provides a nonpharmacological option to target both insulin-resistant dyslipoproteinemia and elevated LDL cholesterol (43).
Our study could also have special public health relevance during the Coronavirus Disease 2019 (COVID-19) pandemic. Obesity is among the most important risk factors for disease susceptibility and severity (44–46), perhaps second only to advanced age. Insulin resistance might mediate this relation, in part, through effects on numerous metabolic, immunological, and inflammatory pathways (47, 48). Thus, carbohydrate restriction could play an ancillary role, in addition to vaccination, in promoting metabolic health and resistance to COVID-19 morbidity and mortality.
Strengths of this trial include use of a feeding protocol to enhance dietary adherence and differentiation between groups; inclusion of diets differing substantially in carbohydrate, but without extreme restriction of any macronutrient, potentially enhancing clinical practicality; high participant retention rate, reducing bias from missing data; large sample size for a feeding study, providing comparatively strong power; long duration, exceeding the time thought necessary to reach a steady state for LDL cholesterol following weight loss (38); and control for dietary protein and body weight. Another notable design feature was a run-in phase designed to achieve a clinically relevant, but not unrealistic, amount of weight loss, considering that weight reduction is the first-line approach for CVD risk reduction in individuals with overweight or obesity.
The main limitation is generalizability. Our cohort comprised young to middle-aged, relatively healthy adults with low LDL cholesterol. We do not know how our findings would apply to other populations, especially older, higher-risk groups, or individuals consuming more restrictive diets (e.g., a ketogenic diet, with carbohydrate <10% of total energy). Furthermore, even within the macronutrient targets in our study, diets can differ in myriad ways, such as the ratio of saturated to unsaturated fatty acids, amounts of MUFAs and PUFAs, fiber type and amount, food processing, glycemic index, and micronutrient content. Therefore, the effects observed here might not occur on all diets with similar macronutrients. However, we aimed to employ healthful, palatable, and pragmatic representations of each diet type, with relevance to clinical translation. Another limitation is risk of false discovery. However, for the key findings involving LPIR and Lp(a), the low- compared with high-carbohydrate diet comparisons would remain statistically significant after conservative Bonferroni adjustment for all 20 outcomes considered in this study. Outcomes with less robust P values (notably adiponectin, HDL cholesterol, and large HDL-P) should be interpreted cautiously, although consistent data from prior studies can enhance confidence in the findings for HDL cholesterol and HDL-P (28, 29, 33). In contrast, the informative negative outcomes involving LDL cholesterol and LDL-P are clearly nonsignificant, although we lack power to rule out a small diet effect.
In conclusion, we found that carbohydrate restriction had benefits for insulin-resistant dyslipoproteinemia and Lp(a), without adverse effects on LDL cholesterol or inflammation. This finding, together with preliminary data on body weight, glycemia, and other cardiometabolic risk factors, suggests that low-carbohydrate diets can have novel benefits for preventing both diabetes and CVD in an era with highly prevalent obesity and insulin resistance. Multicentered trials powered for hard outcomes, comparable to the Women's Health Initiative clinical trial and Look Ahead Study, which utilized low-fat diets, are needed to test this possibility.
Supplementary Material
Acknowledgments
We thank the study participants for their time and commitment to advancing science. We also acknowledge Gloria Klein (Study Director, oversight of study operations), Patricia Luoto (Study Director, Framingham State University), Lisa Bielak (Nutrition Research Manager, implementation of dietary intervention), and Shui Yu (Biostatistician, replication of data analysis).
The authors’ responsibilities were as follows—CBE, DSL: principal investigators of the parent trial and participated in all aspects of design, conduct, and manuscript preparation for the current study; AK, AJ: participated in design and data acquisition, and revised the manuscript; JMWW: participated in conduct of the parent trial and revised the manuscript; KFG, CM: conducted the statistical analyses and revised the manuscript; SM: helped design the study and interpret the data and revised the manuscript; and all authors: read and approved the final manuscript. SM has served as a consultant to Pfizer and Quest Diagnostics for work unrelated to the current study, and has a patent regarding the use of GlycA, an NMR-measured biomarker, in relation to colorectal cancer risk. DSL reported receiving royalties from books on nutrition and obesity that recommend a carbohydrate-modified diet; and his spouse owns a nutrition education and consulting business. All other authors report no conflicts of interest.
Notes
This work was supported by grants from Nutrition Science Initiative (made possible by gifts from Arnold Ventures and Robert Lloyd Corkin Charitable Foundation), New Balance Foundation, Many Voices Foundation, and Blue Cross Blue Shield. DSL was supported by a mid-career mentoring award from the National Institute of Diabetes and Digestive and Kidney Diseases (K24DK082730). SM was supported by the National Heart, Lung, and Blood Institute (K24HL136852). Nutrition Science Initiative monitored study progress and was given an opportunity to comment on the manuscript. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; approval of the manuscript; and decision to submit the manuscript for publication. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the study sponsors.
DSL is an Associate Editor of The American Journal of Clinical Nutrition and was not involved in the editorial evaluation of this manuscript.
Supplemental Methods, Supplemental Material, Supplemental Tables 1–6, and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: Carb, carbohydrate; CVD, cardiovascular disease; HDL-P, high-density lipoprotein particle; HMW, high molecular weight; hsCRP, high-sensitivity C-reactive protein; LPIR, lipoprotein insulin resistance; Lp(a), lipoprotein(a); LDL-P, low-density lipoprotein particle; TRL-P, triglyceride-rich lipoprotein particle.
Contributor Information
Cara B Ebbeling, New Balance Foundation Obesity Prevention Center, Boston Children's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Amy Knapp, Department of Biology, Framingham State University, Framingham, MA, USA.
Ann Johnson, Department of Food and Nutrition, Framingham State University, Framingham, MA, USA.
Julia M W Wong, New Balance Foundation Obesity Prevention Center, Boston Children's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Kimberly F Greco, Institutional Centers for Clinical and Translational Research, Boston Children's Hospital, Boston, MA, USA.
Clement Ma, Harvard Medical School, Boston, MA, USA; Dana-Farber/Boston Children's Cancer and Blood Disorders Center, Boston, MA, USA.
Samia Mora, Harvard Medical School, Boston, MA, USA; Center for Lipid Metabolomics, Divisions of Preventive and Cardiovascular Medicine, Brigham and Women's Hospital, Boston, MA, USA.
David S Ludwig, New Balance Foundation Obesity Prevention Center, Boston Children's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Data Availability
The full trial protocol and the database are publicly available on Open Science Framework (https://osf.io/rvbuy/).
References
- 1. Jahns L, Davis-Shaw W, Lichtenstein AH, Murphy SP, Conrad Z, Nielsen F. The history and future of dietary guidance in America. Adv Nutr. 2018;9(2):136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kris-Etherton PM, Krauss RM. Public health guidelines should recommend reducing saturated fat consumption as much as possible: YES. Am J Clin Nutr. 2020;112(1):13–18. [DOI] [PubMed] [Google Scholar]
- 3. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JGet al. . Dietary fats and cardiovascular disease: a Presidential Advisory from the American Heart Association. Circulation. 2017;136(3):e1–e23. [DOI] [PubMed] [Google Scholar]
- 4. Jakobsen MU, Dethlefsen C, Joensen AM, Stegger J, Tjonneland A, Schmidt EB, Overvad K. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr. 2010;91(6):1764–8. [DOI] [PubMed] [Google Scholar]
- 5. Reaven GM. Do high carbohydrate diets prevent the development or attenuate the manifestations (or both) of syndrome X? A viewpoint strongly against. Curr Opin Lipidol. 1997;8(1):23–7. [DOI] [PubMed] [Google Scholar]
- 6. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91(3):502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, Mitri J, Pereira RF, Rawlings K, Robinson Set al. . Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, Volek JS, Phinney SD, McCarter JP. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. 2019;10:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saslow LR, Daubenmier JJ, Moskowitz JT, Kim S, Murphy EJ, Phinney SD, Ploutz-Snyder R, Goldman V, Cox RM, Mason AEet al. . Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr Diabetes. 2017;7(12):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harada PHN, Demler OV, Dugani SB, Akinkuolie AO, Moorthy MV, Ridker PM, Cook NR, Pradhan AD, Mora S. Lipoprotein insulin resistance score and risk of incident diabetes during extended follow-up of 20 years: the Women's Health Study. J Clin Lipidol. 2017;11(5):1257–67.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mozaffarian D. The great fat debate: taking the focus off of saturated fat. J Am Diet Assoc. 2011;111(5):665–6. [DOI] [PubMed] [Google Scholar]
- 12. Dugani SB, Akinkuolie AO, Paynter N, Glynn RJ, Ridker PM, Mora S. Association of lipoproteins, insulin resistance, and rosuvastatin with incident type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1(2):136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dugani SB, Moorthy MV, Li C, Demler OV, Alsheikh-Ali AA, Ridker PM, Glynn RJ, Mora S. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol. 2021;6(4):437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pagidipati NJ, Pencina M, Sniderman AD. The enigma of glucose and lipid metabolism. JAMA Cardiol. 2016;1(2):145–6. [DOI] [PubMed] [Google Scholar]
- 15. Shalaurova I, Connelly MA, Garvey WT, Otvos JD. Lipoprotein insulin resistance index: a lipoprotein particle-derived measure of insulin resistance. Metab Syndr Relat Disord. 2014;12(8):422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ebbeling CB, Klein GL, Luoto PK, Wong JMW, Bielak L, Eddy RG, Steltz SK, Devlin C, Sandman M, Hron Bet al. . A randomized study of dietary composition during weight-loss maintenance: rationale, study design, intervention, and assessment. Contemp Clin Trials. 2018;65:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong JM, Bielak L, Eddy RG, Stone L, Lakin PR, Sandman M, Devlin C, Seger-Shippee L, Wiroll D, Luoto PKet al. . An academia-industry partnership for planning and executing a community-based feeding study. Curr Dev Nutr. 2018;2(9):nzy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak L, Steltz SK, Luoto PK, Wolfe RR, Wong WW, Ludwig DS. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aday AW, Lawler PR, Cook NR, Ridker PM, Mora S, Pradhan AD. Lipoprotein particle profiles, standard lipids, and peripheral artery disease incidence. Circulation. 2018;138(21):2330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flores-Guerrero JL, Connelly MA, Shalaurova I, Gruppen EG, Kieneker LM, Dullaart RPF, Bakker SJL. Lipoprotein insulin resistance index, a high-throughput measure of insulin resistance, is associated with incident type II diabetes mellitus in the Prevention of Renal and Vascular End-Stage Disease study. J Clin Lipidol. 2019;13(1):129–37.e1. [DOI] [PubMed] [Google Scholar]
- 21. Marcovina SM, Albers JJ, Scanu AM, Kennedy H, Giaculli F, Berg K, Couderc R, Dati F, Rifai N, Sakurabayashi Iet al. . Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a). Clin Chem. 2000;46(12):1956–67. [PubMed] [Google Scholar]
- 22. Ludwig DS. The ketogenic diet: evidence for optimism but high-quality research needed. J Nutr. 2020;150(6):1354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, Willett WC, Hu FB. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016;176(8):1134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. Int J Mol Sci. 2019;20(5):1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, Orringer CE. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374–92. [DOI] [PubMed] [Google Scholar]
- 26. Enkhmaa B, Petersen KS, Kris-Etherton PM, Berglund L. Diet and Lp(a): does dietary change modify residual cardiovascular risk conferred by Lp(a)?. Nutrients. 2020;12(7):2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ginsberg HN, Kris-Etherton P, Dennis B, Elmer PJ, Ershow A, Lefevre M, Pearson T, Roheim P, Ramakrishnan R, Reed Ret al. . Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the DELTA Study, protocol 1. Arterioscler Thromb Vasc Biol. 1998;18(3):441–9. [DOI] [PubMed] [Google Scholar]
- 28. Dong T, Guo M, Zhang P, Sun G, Chen B. The effects of low-carbohydrate diets on cardiovascular risk factors: a meta-analysis. PLoS One. 2020;15(1):e0225348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huntriss R, Campbell M, Bedwell C. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr. 2018;72(3):311–25. [DOI] [PubMed] [Google Scholar]
- 30. Hyde PN, Sapper TN, Crabtree CD, LaFountain RA, Bowling ML, Buga A, Fell B, McSwiney FT, Dickerson RM, Miller VJet al. . Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight. 2019;4(12):e128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skytte MJ, Samkani A, Petersen AD, Thomsen MN, Astrup A, Chabanova E, Frystyk J, Holst JJ, Thomsen HS, Madsbad Set al. . A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia. 2019;62(11):2066–78. [DOI] [PubMed] [Google Scholar]
- 32. Jenkins DJA, Dehghan M, Mente A, Bangdiwala SI, Rangarajan S, Srichaikul K, Mohan V, Avezum A, Díaz R, Rosengren Aet al. . Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. 2021;384(14):1312–22. [DOI] [PubMed] [Google Scholar]
- 33. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. a meta-analysis. PLoS One. 2015;10(10):e0139817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwingshackl L, Hoffmann G. Comparison of effects of long-term low-fat vs high-fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta-analysis. J Acad Nutr Diet. 2013;113(12):1640–61. [DOI] [PubMed] [Google Scholar]
- 35. Gjuladin-Hellon T, Davies IG, Penson P, Amiri Baghbadorani R. Effects of carbohydrate-restricted diets on low-density lipoprotein cholesterol levels in overweight and obese adults: a systematic review and meta-analysis. Nutr Rev. 2019;77(3):161–80. [DOI] [PubMed] [Google Scholar]
- 36. Shih CW, Hauser ME, Aronica L, Rigdon J, Gardner CD. Changes in blood lipid concentrations associated with changes in intake of dietary saturated fat in the context of a healthy low-carbohydrate weight-loss diet: a secondary analysis of the Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) trial. Am J Clin Nutr. 2019;109(2):433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldberg IJ, Ibrahim N, Bredefeld C, Foo S, Lim V, Gutman D, Huggins LA, Hegele RA. Ketogenic diets, not for everyone. J Clin Lipidol. 2021;15:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Phinney SD, Tang AB, Waggoner CR, Tezanos-Pinto RG, Davis PA. The transient hypercholesterolemia of major weight loss. Am J Clin Nutr. 1991;53(6):1404–10. [DOI] [PubMed] [Google Scholar]
- 39. Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104(25):3046–51. [DOI] [PubMed] [Google Scholar]
- 40. Bartlett J, Predazzi IM, Williams SM, Bush WS, Kim Y, Havas S, Toth PP, Fazio S, Miller M. Is isolated low high-density lipoprotein cholesterol a cardiovascular disease risk factor? New insights from the Framingham Offspring Study. Circulation. 2016;9(3):206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Low triglycerides-high high-density lipoprotein cholesterol and risk of ischemic heart disease. Arch Intern Med. 2001;161(3):361–6. [DOI] [PubMed] [Google Scholar]
- 42. Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care. 2009;32(2):361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Falkenhain K, Locke SR, Lowe DA, Reitsma NJ, Lee T, Singer J, Weiss EJ, Little JP. Keyto app and device versus WW app on weight loss and metabolic risk in adults with overweight or obesity: a randomized trial. Obesity. [Internet]2021. doi:10.1002/oby.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoong CWS, Hussain I, Aravamudan VM, Phyu EE, Lin JHX, Koh H. Obesity is associated with poor Covid-19 outcomes: a systematic review and meta-analysis. Horm Metab Res. 2021;53(2):85–93. [DOI] [PubMed] [Google Scholar]
- 45. Malik P, Patel U, Patel K, Martin M, Shah C, Mehta D, Malik FA, Sharma A. Obesity a predictor of outcomes of COVID-19 hospitalized patients—a systematic review and meta-analysis. J Med Virol. 2021;93(2):1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93(1):257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bansal R, Gubbi S, Muniyappa R. Metabolic syndrome and COVID 19: endocrine-immune-vascular interactions shapes clinical course. Endocrinology. 2020;161(10):bqaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Santos A, Magro DO, Evangelista-Poderoso R, Saad MJA. Diabetes, obesity, and insulin resistance in COVID-19: molecular interrelationship and therapeutic implications. Diabetol Metab Syndr. 2021;13(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full trial protocol and the database are publicly available on Open Science Framework (https://osf.io/rvbuy/).