Abstract
Background
The worldwide spread of coronavirus disease 2019 (COVID-19) is still not under control and vaccination in Japan started in February 2021, albeit later than in Europe and the USA. The COVID-19 vaccination frequently leads to minor adverse reactions, which may be more intense after the second dose. The number of case reports of myocarditis following COVID-19 vaccination have been recently increased.
Case summary
We report a case of a 26-year-old healthy man who presented to our hospital with chest pain on 24 May 2021, 4 days after his second COVID-19 vaccination. The electrocardiogram showed ST elevation with upward concavity in I, II, aVL, aVF, V4 to V6, and small Q wave in II, III, aVF. Laboratory studies revealed elevation of troponin I, creatine kinase, C-reactive protein, and negative viral serologies. Acute aortic dissection and pulmonary thromboembolism were ruled out by contrast-enhanced thoracoabdominal computed tomography. An urgent coronary angiogram was performed because an acute coronary syndrome was suspected, but no significant stenosis was found. Cardiac magnetic resonance imaging demonstrated oedema and late gadolinium enhancement of the left ventricle in a mid-myocardial and epicardial distribution.
Discussion
Although the temporal association does not prove causation, the very short span between the second vaccination and the onset of myocarditis suggests that this acute myocarditis seemed to be an adverse reaction to COVID-19 vaccine. To the best of our knowledge, this is the first published case of acute myocarditis following COVID-19 vaccine in Asia.
Keywords: Myocarditis, COVID-19, Vaccination
Learning points.
As coronavirus disease 2019 (COVID-19) vaccination has increased, reports of adverse reactions have also increased.
We should be aware that acute myocarditis after administration of COVID-19 vaccine may also occur even in healthy young men without immune abnormalities.
Cardiac magnetic resonance imaging is useful in the diagnosis of acute myocarditis.
Introduction
The worldwide spread of coronavirus disease 2019 (COVID-19) is still not under control and vaccination in Japan started in February 2021, albeit later than in Europe and the USA. Adverse reactions to vaccination are usually not very significant. However, with the second dose of the COVID-19 vaccine, mild adverse reactions are often more severe and more serious adverse reactions have been reported.1 Very recently, several cases have been published of myocarditis associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines such as BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna).2,3 At time of writing, there are no published case reports of COVID-19 vaccine-related myocarditis in Asia.
Timeline
| Time | Event |
|---|---|
| 30 April | First dose of coronavirus disease 2019 (COVID-19) vaccine. |
| 21 May | Second dose of COVID-19 vaccine. |
| 22 May | Fever, headache, appetite loss, general malaise, and shoulder stiffness, which was treated with acetaminophen. |
| 23 May | Onset of chest pain and persistent fever, headache, and appetite loss. |
| 24 May | The patient visited outpatient clinic and was admitted to the high care unit; elevated markers of myocardial damage such as high-sensitivity troponin I, creatine kinase, C-reactive protein, and diffuse ST-segment elevation on electrocardiogram (ECG). An urgent coronary angiogram showed no significant stenosis. Anti-inflammatory drugs were started and the patient’s symptoms improved. |
| 25–26 May | The patient was haemodynamically stable and asymptomatic. A subsequent ECG showed partial resolution of the ST changes and a trend towards improvement. |
| 27 May | The patient was discharged in order to perform cardiac magnetic resonance imaging (MRI). |
| 31 May | The patient visited the outpatient clinic with mild general fatigue. Cardiac MRI showed myocardial late gadolinium enhancement with epicardial predominance in the antero-septal, inferior and lateral walls of the basal segment and apex, which were consistent with acute myocarditis. |
| 3 June | The patient recovered and returned to work. |
| 14 June | Despite no deterioration in cardiac function, the patient complained of appetite loss and fatigue on exertion, was diagnosed as post-vaccination syndrome and was absent from work for a month. |
| 12 July | The patient recovered to some extent and returned to work. |
| 29 July | The patient complained of general malaise and sleep disturbance, was diagnosed as depressive state and is currently on leave. |
Case presentation
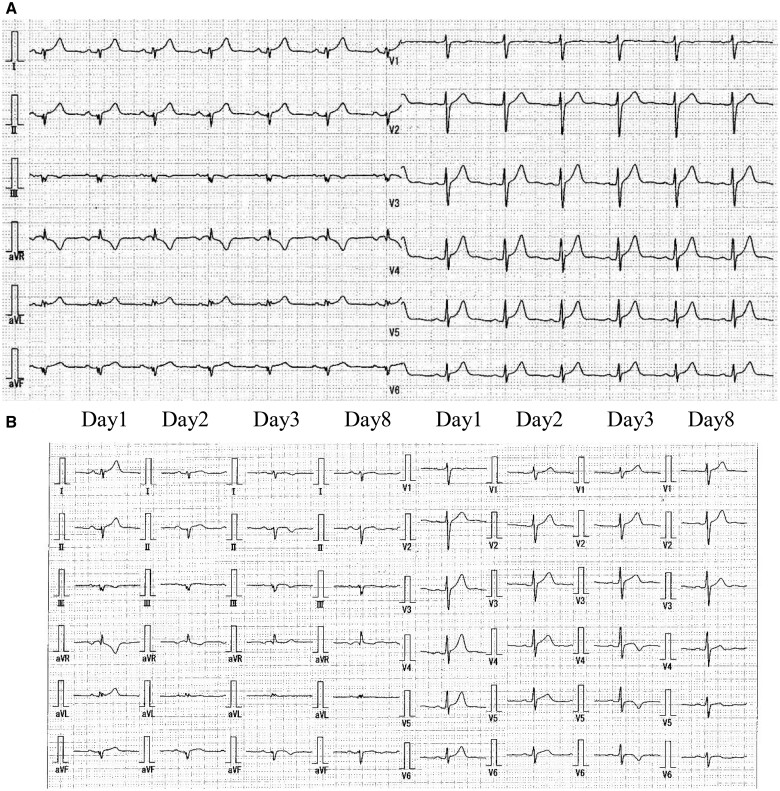
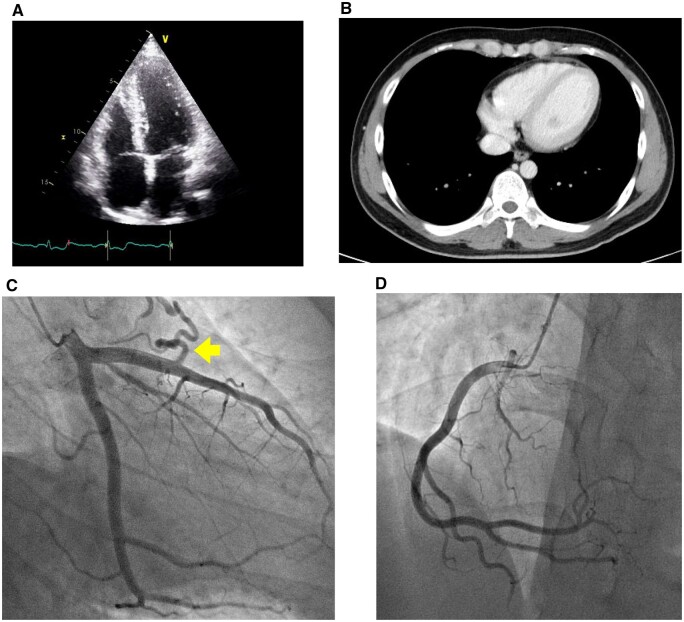
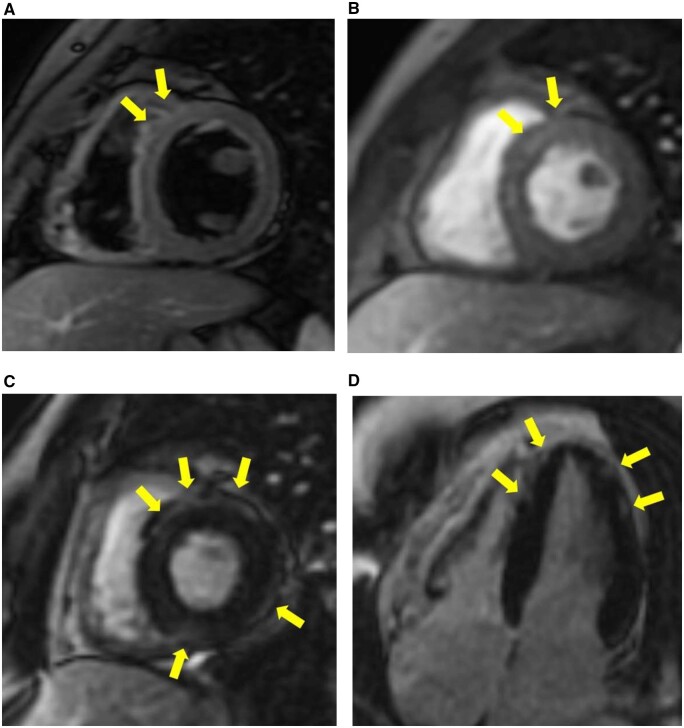
We present the case of a 26-year-old male clinical engineer working in our hospital who came to our hospital complaining of acute substernal chest pain on 21 May 2021, 4 days after his second dose of BNT162b2 vaccine. The patient was an Asians of Mongoloid descent and had no previous medical history. The patient gave informed consent for the write-up and publication of this clinical case. The electrocardiogram (ECG) on arrival showed small Q waves in II, III, aVF, ST elevation with upward concavity in I, II, aVL, aVF and V4–V6, and ST depression and deep negative T wave in aVR (Figure 1A). Laboratory studies revealed elevated markers of myocardial damage such as high-sensitivity troponin I [5362.4 pg/mL, referential range (RR) 0–26.2 pg/mL], creatine kinase (332 U/L, RR 59–248 U/L), C-reactive protein (7.57 mg/dL, RR 0–0.14 mg/dL), and a negative polymerase chain reaction test for COVID-19. The plasma electrolytes such as sodium and potassium were within normal limits. Viral studies including coxsackie group B viruses and echoviruses were all negative. Autoimmune studies such as antinuclear antibodies and thyroid hormones were within normal limits. There were no significant findings on chest X-ray. On admission, transthoracic echocardiography revealed good left ventricular function, no regional wall motion abnormalities, no significant valvular disease, and no pericardial effusion (Figure 2A), his left ventricular ejection fraction was 53%, within normal limits, and further improved to 65% during his hospitalization. Acute aortic dissection and pulmonary thromboembolism were ruled out by contrast-enhanced thoracoabdominal computed tomography (CT). Thoracic CT showed no pericardial effusion or ventricular wall thickening (Figure 2B). An urgent coronary angiogram was performed because an acute coronary syndrome was suspected, but no significant stenosis of the coronary arteries was found except for an incidental finding of coronary artery (left anterior descending artery) to pulmonary artery fistula (Figure 2C and D). The patient was admitted to the high care unit, although he remained haemodynamically stable and asymptomatic. Subsequent ECGs showed partial resolution of the ST changes and a trend towards improvement (Figure 1B). No endocardial biopsy was performed because of the patient’s low risk and good course. The patient was discharged after 4 days with resolution of symptoms and improvement in inflammatory response and myocardial injury markers (Table 1). Cardiac magnetic resonance imaging (MRI) examination, which was obtained on discharge 6 days after second dose of vaccination, showed maintained systolic function, increased myocardial and pericardial signal intensity on short T1 inversion recovery sequences and slight elevated signals of the myocardium on dynamic contrast-enhanced sequences, suggesting increased vascular flow. Fast imaging employing steady state acquisition (FIESTA) sequences, which were performed 15 min after intravenous administration of gadolinium, demonstrated sub-epicardial enhancement of the myocardium, which was considered consistent with acute myocarditis. T1-weighted (T1WI) sequences also showed late subepicardial enhancement in the anterior wall and interventricular septum near the apex (Figure 3). These findings were considered consistent with acute myocarditis. The patient was initially treated with oral acetaminophen, antitussives and no steroids, with progressive resolution of his symptoms. The patient has scheduled outpatient cardiology follow-up. On the 4th day after discharge, the patient presented to the outpatient clinic with only mild general malaise and returned to work 3 days later. However, despite no deterioration in cardiac function, the patient complained of appetite loss and fatigue on exertion, was diagnosed as post-vaccination syndrome and was absent from work for a month. The patient recovered to some extent and returned to work, but 2 weeks later, he complained of general malaise and sleep disturbance, was diagnosed with situational depression and is currently on leave to see a psychotherapist.
Figure 1.
(A) Electrocardiogram showing small Q waves in II, III, aVF, ST elevation with upward concavity in I, II, aVL, aVF and V4–V6, and ST depression and deep negative T-wave in aVR. (B) Serial changes in electrocardiogram showing negative and or flattened T waves in I, II, aVL, aVF, V4–V6.
Figure 2.
Echocardiogram and thoracic computed tomography showed no pericardial effusion or ventricular wall thickening (A and B). Coronary angiography demonstrated no significant stenosis of the coronary arteries except for a coronary artery (left anterior descending) to pulmonary artery fistula (arrow) (C and D).
Table 1.
Markers of myocardial injury and inflammatory response have improved within 1 week
| Day 1 | Day 2 | Day 3 | Day 4 | Day 8 | |
|---|---|---|---|---|---|
| CRP (mg/dL) (0–0.14) | 7.57 | 4.24 | 1.57 | 0.70 | 0.13 |
| Hs-Tn-I (pg/mL) (0–26.2) | 5362.4 | 4426.0 | 2768.1 | ||
| WBC (/μL) (3300–8600) | 8560 | 5440 | 4460 | 4320 | 5600 |
| Eosinophil (/μL) (2–4%) | 30 (0.4%) | 140 (2.6%) | 170 (3.8%) | 200 (4.6%) | 190 (3.4%) |
| CK (U/L) (59–248) | 332 | 315 | 96 | 55 | 39 |
| AST (U/L) (13–30) | 44 | 48 | 29 | 27 | 25 |
| ALT (U/L) (10–42) | 38 | 33 | 30 | 37 | 47 |
| LD (U/L) (124–222) | 226 | 219 | 226 | 200 | 191 |
| BNP (pg/mL) (0–18.4) | 20.2 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, brain natriuretic peptide; CK, creatine kinase; CRP, C-reactive protein; Hs-Tn-I, high-sensitivity troponin-I; LD, lactate dehydrogenase; WBC, white blood cell.
Figure 3.
Cardiac magnetic resonance images. Short T1 inversion recovery axis image acquired along the basal short-axis view demonstrates increased subepicardial and mesocardial signal intensity of the antero-septal myocardial segments (A). Dynamic contrast-enhanced sequence demonstrates slight elevated signals of the antero-septal myocardial segments (B). Fast imaging employing steady state acquisition sequences acquired along the basal short-axis view (C) and four-chamber view (D) demonstrate myocardial late gadolinium enhancement with epicardial predominance in the antero-septal, inferior and lateral walls of the basal segment and apex.
Discussion
In the present case, a healthy young adult male developed acute myocarditis after COVID-19 vaccination. The diagnosis of myocarditis was confirmed by cardiac MRI with late gadolinium enhancement sequence, although no endocardial biopsy was performed. Non-invasive imaging modalities such as MRI, CT, and echocardiogram are useful for diagnosis and are of benefit to patients. In addition, FIESTA with the superior temporal resolution is thought to provide better depiction of late enhancement effect in the myocardium than conventional T1WI. The temporal association between vaccination and the onset of symptoms, and the exclusion of other acute cardiac diseases, suggested that this acute myocarditis seemed to be an adverse reaction to the BNT162b2 vaccine. Although the temporal association does not prove causation, the very short span between the second vaccination and the onset of myocarditis suggests a possible relationship. Since April 2021, the number of cases of cardiac inflammation (myocarditis and pericarditis) after mRNA COVID-19 vaccination (Pfizer-BioNTech and Moderna) have been increasing in the USA.4 The Centers for Disease Control and Prevention (CDC) has announced that more than 1000 cases of myocarditis/pericarditis after mRNA vaccination have been reported to the Vaccine Adverse Event Reporting System (VAERS) on 25 June 2021. Myocarditis/pericarditis develops with sudden chest pain 1–5 days after vaccination, is more often in males under 30 years of age, and is more often after the second vaccination, so this case shows a typical pattern of onset. Although the onset of the disease may be mediated by the immune system, it is currently unclear what immune response to the mRNA vaccine leads to the development of myocarditis/pericarditis. A possible pathogenic mechanism is direct spike mediated toxicity. Myocarditis/pericarditis has also been reported as a complication of SARS-CoV-2 infection and may involve an immune response to spike proteins, but unfortunately, we did not perform quantitative SARS-CoV-2 antibody assays in this case. Another hypothesis is a delayed hypersensitivity reaction and eosinophilic myocarditis that develops directly after vaccination.5 As shown in Table 1, the changes in eosinophils in peripheral blood showed a low level of 0.4% (30 cells/μL) on Day 1, the day of admission, followed by a gradual increase and a mild increase to 4.6% (200 cells/μL) on Day 4. We speculate that an eosinophilic infiltration of the myocardial tissue may have occurred immediately after the second vaccination, but this cannot be proven as we have not performed a myocardial biopsy.
In Japan, priority vaccination of healthcare providers first started in February 2021, and vaccination at our hospital started in May. In June, vaccination of elderly people aged 75 years and over started on a priority basis, but vaccination of the general young population, especially those in their 20s, is still largely unavailable. Therefore, as vaccination spreads to the younger generation, there is a concern that cases of myocarditis and pericarditis may increase. The Ministry of Health, Labour and Welfare announced 12 cases of Myocarditis/pericarditis as of 2 July, and the Japanese Circulation Society issued a statement on ‘Acute myocarditis and acute pericarditis after vaccination with novel coronaviruses’ on 21 July. We healthcare providers have to pay attention to this issue as vaccination in Japan will progress from the elderly to the younger generation. However, it appears that there is a significantly higher risk of cardiac involvement from COVID-19 infection compared to COVID-19 vaccination.6 We therefore believe that COVID-19 vaccination plays an important role in herd immunity and overall provide more benefit than harm.
The patient was a medical professional and was well aware that myocarditis can be fatal in severe cases, which may have been traumatic and led to depressive state. It is a priority to provide psychological support for the post-traumatic stress disorder-like depression which can occur as an adverse complication of the vaccine.
In conclusion, to the best of our knowledge, this is the first published case of acute myocarditis as an adverse reaction to SARS-CoV-2 vaccine in Asia.
Lead author biography

During the year 1984, Dr Masato Ohnishi studied BSc in the Faculty of Science, Kobe University, Kobe, Japan. He graduated MD in the Faculty of Medicine, Shi ga University of Medical Science, Otsu, Japan in 1990. In 1999, he did PhD in Graduate School of Medicine, Shiga University of Medical Science, Otsu, Japan. He is an Assistant Professor in the Department of Cardiology, Shiga Universit y of Medical Science in 2001, Director of Cardiology, Kusatsu General Hospital in 2003, and an Associate Professor in the Department of Comprehensive Intern al Medicine, Shiga University of Medical Science, Director of Cardiovascular M edicine, National Hospital Organization Higashi-Ohmi General Medical Center in 2011. He is a Fellow of the Japanese Society of Internal Medicine and a Board Certified Member of the Japanese Circulation Society.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We thank Dr Hiroshi Sakai (Department of Cardiology, Shiga University of Medical Science, Otsu, Japan) for arranging the cardiac MRI and Dr Yukihiro Nagatani (Department of Radiology, Shiga University of Medical Science, Otsu, Japan) for reading the cardiac MRI.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
- 1. Center for Disease Control and Prevention. Possible side effects after getting a COVID-19 vaccine. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html (25 May 2021).
- 2. Bautista García J, Peña Ortega P, Bonilla Fernández JA, Cárdenes León A, Ramírez Burgos L, Caballero Dorta E.. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol (Engl Ed) 2021;74:812–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albert E, Aurigemma G, Saucedo J, Gerson DS.. Myocarditis following COVID-19 vaccination. Radiol Case Rep 2021;16:2142–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Center for Disease Control and Prevention. Myocarditis and pericarditis following mRNA COVID-19 vaccination. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html (25 May 2021).
- 5. Yamamoto H, Hashimoto T, Ohta-Ogo K, Ishibashi-Ueda H, Imanaka-Yoshida K, Hiroe M. et al. A case of biopsy-proven eosinophilic myocarditis related to tetanus toxoid immunization. Cardiovasc Pathol 2018;37:54–57. [DOI] [PubMed] [Google Scholar]
- 6. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S. et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.