Abstract
Background
Vaccination is the most important measure to control the coronavirus disease 2019 (COVID-19) pandemic. Myocarditis has been reported as a rare adverse reaction to COVID-19 vaccines. The clinical presentation of myocarditis in such cases can range from mild general symptoms to acute heart failure.
Case summary
We report the cases of two young men who presented with chest pain and dyspnoea following the administration of the mRNA COVID-19 vaccine. Cardiac investigations revealed findings typical of acute myocarditis.
Discussion
Myocarditis is a rare complication following mRNA COVID-19 vaccination. In this case series, the temporal proximity of the development of acute myocarditis and the administration of the mRNA COVID-19 vaccine was acknowledged. In the absence of other causative factors, myocarditis in these patients potentially occurred due to an adverse reaction to the mRNA COVID-19 vaccine. However, a causal relationship remains speculative. Clinical suspicion of myocarditis should be high if patients present with chest pain or dyspnoea after receiving COVID-19 vaccination.
Keywords: COVID-19, Vaccine, Myocarditis, Adverse reaction, Case report
Learning points.
Myocarditis may be a complication of vaccination.
Clinical suspicion of myocarditis should be high if patients present with cardiovascular symptoms after administration of coronavirus disease 2019 vaccination.
Introduction
Vaccination is an essential means to control the coronavirus disease 2019 (COVID-19) pandemic. Myocarditis has been reported as a rare adverse reaction to COVID-19 vaccines. Recent reports have identified mRNA COVID-19 vaccine administration in young healthy men as a precursor of acute myocarditis.1–5 This is particularly important as the European Union and US governments are expanding their vaccination programmes and are administering large quantities of recently approved mRNA COVID-19 vaccines.6
Here, we report the cases of two young men who developed acute myocarditis shortly after receiving the 2nd dose of the COVID-19 vaccine ‘Moderna’. Both were hospitalized at our institution, a general hospital in an urban area in Germany, in June 2021.
Timeline
| Case 1 | |
| Day | |
| 0 | First dose of coronavirus disease 2019 (COVID-19) Vaccine Moderna. Patient remained without symptoms |
| 36 | Second dose of COVID-19 Vaccine Moderna |
| 37 | General malaise and fatigue, later chest discomfort |
| 41 | Hospital admission |
| 41–44 | Intermediate care unit |
| No heart rhythm anomalies | |
| Steady resolution of symptoms | |
| 44 | No symptoms |
| Transfer to general ward | |
| Cardiac magnetic resonance imaging (MRI) confirms myocarditis | |
| 45 and 46 | Resolution of wall motion abnormalities and normalization of troponin-T |
| [Admission of Case 2] | |
| 47 | Discharge of Patient 1 |
| Case 2 | |
| Day | |
| 0 | First dose of COVID-19 Vaccine Moderna. Patient remained without symptoms |
| 47 | Second dose of COVID-19 Vaccine Moderna |
| 48 | Arthralgia and general malaise, fever |
| 49 | Epigastric pain |
| 50 | Transfer from tertiary centre and hospital admission |
| 51 | Coronary angiography, coronary artery disease ruled out |
| 50–54 | Intermediate care unit |
| Heart failure therapy | |
| No heart rhythm anomalies | |
| Slow resolution of symptoms | |
| 53 | Cardiac MRI confirms myocarditis |
| 54 | No epigastric pain |
| Decrease of troponin-T | |
| Transfer to general ward | |
| 56 | No symptoms |
| Resolution of wall motion abnormalities | |
| Discharge of Patient 2 | |
Case presentation
Patient 1
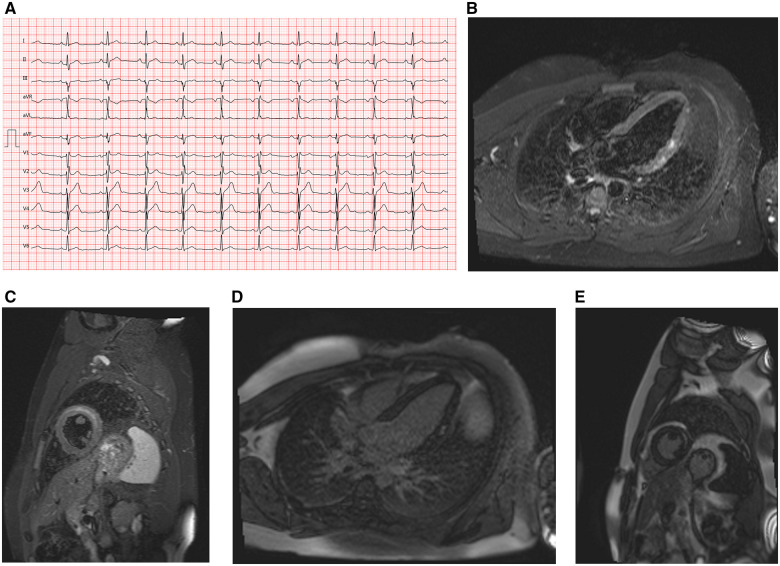
A 23-year-old male firefighter presented to the emergency department of our community hospital. He had received the first dose of the COVID-19 vaccine ‘Moderna’ (100 µg messenger-RNA) 6 weeks prior and the 2nd dose 5 days prior. Four days before admission, he experienced general malaise and fatigue. On the day of admission, his family physician hospitalized him because of a high troponin level in an ambulatory blood sample. In the emergency department, he experienced mild retrosternal pain, which had been of stronger intensity the day before. He was not taking any medications and had no relevant medical history. On initial evaluation, his blood pressure was 176/95 mmHg, heart rate was 96 b.p.m., and temperature was 37.2°C. Upon cardiac auscultation, there were no audible murmurs, and his lungs were clear. No oedema and no signs of congestion were noted. His other physical parameters were normal. Laboratory data revealed elevated levels of high-sensitivity cardiac troponin-T (1086 pg/mL; normal: <14 pg/mL), creatine kinase-MB (27 U/L; normal: <24 U/L), C-reactive protein (19.8 mg/L; normal: 0–5 mg/L), and lactate dehydrogenase (349 U/L; normal: 135–225 U/L). His white blood cell count was normal, and eosinophilia was absent. His nasopharyngeal swab sample tested negative for severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) at admission. An electrocardiogram revealed a sinus rhythm, normal QRS axis and a QRS complex of 102 ms duration with an rSr′ configuration in V1–V3 and a slight notch in aVL (Figure 1A). An echocardiogram revealed normal left ventricular systolic function, with a left ventricular (LV) ejection fraction (LVEF) of 55% (Video 1). No pericardial effusion was observed. His valve function was normal. We did not consider invasive angiography necessary because of the low probability of coronary artery disease in a 23-year-old patient. Additionally, his clinical presentation was not suggestive of coronary malformation. In the cardiac magnetic resonance imaging (MRI) performed 72 h after admission, T2-weighted short-tau inversion recovery (T2 STIR) sequences revealed oedema of the anterolateral myocardial segments. Cine sequences revealed normal systolic function with hypokinesis of the anterolateral myocardial segments (Figure 1B and C). The pericardial thickness was normal. Streaky late gadolinium enhancement (LGE) of the lateral left ventricular wall in a non-ischaemic distribution pattern was noted (Figure 1D and E), suggesting a diagnosis of myocarditis according to the updated Lake Louise criteria.7
Figure 1.
(A) Case 1 electrocardiogram on presentation showing sinus rhythm, rSr′ configuration in V1–V3, and a slight notch in aVL. (B) Case 1 cardiac magnetic resonance imaging, T2-weighted stir sequence, four-chamber view, showing an increased subepicardial and intramyocardial signal intensity of the basal and mid anterolateral segment. (C) Case 1 cardiac magnetic resonance imaging, T2-weighted stir sequence, midventricular short-axis view, showing an increased intramyocardial signal intensity of the midventricular anterolateral segment. (D) Case 1 cardiac magnetic resonance imaging, inverse-recovery late gadolinium enhancement sequence, four-chamber view, showing focal late gadolinium enhancement of the mid anterolateral segment. (E) Case 1 cardiac magnetic resonance imaging, inverse-recovery late gadolinium enhancement sequence, midventricular short-axis view, showing late gadolinium enhancement with a subepicardial distribution of the mid anterolateral segment.
In the diagnostic workup, there were no signs of an autoimmune or infectious cause of myocarditis. Serology for cardiotropic viruses was not performed according to local standard operating procedures, in line with the European Society of Cardiology recommendations.8 Serology for human immunodeficiency virus or hepatitis C virus was not performed as there was no specific clinical suspicion. The patient’s history and clinical exam did not provide specific indications that led us to suspect autoimmune disorders. The patient tested negative for cardiac autoantibodies. His thyroid function tests were normal, suggesting that thyrotoxicosis did not cause immune-mediated myocarditis. There was no family history of genetic or autoimmune diseases. A bacterial infectious cause of myocarditis was improbable as there were no predisposing comorbidities and rapid recovery was achieved without antibiotic therapy.
A quantitative SARS-CoV-2 antibody assay (SARS-CoV-2 TrimericS IgG Assay, DiaSorin, Saluggia, Italy) was performed and revealed an anti-spike post-vaccination titre of >2080 BAU/mL (normal: <33.8 BAU/mL). Coronavirus disease 2019 polymerase chain reaction (PCR) testing was negative.
The patient was monitored for 4 days with no relevant heart rhythm anomalies. Repeat echocardiography revealed no wall motion abnormalities. He showed total symptom resolution without any specific medical therapy. His high-sensitivity cardiac-specific troponin-T levels decreased progressively until normalization, and he was discharged with a recommendation to avoid exercise for the next 6 months. A follow-up medical appointment with an ambulatory care cardiologist was scheduled.
Patient 2
A 20-year-old male firefighter was transferred by a tertiary hospital to the emergency department of our hospital because of a presumed diagnosis of ST-segment elevation myocardial infarction.
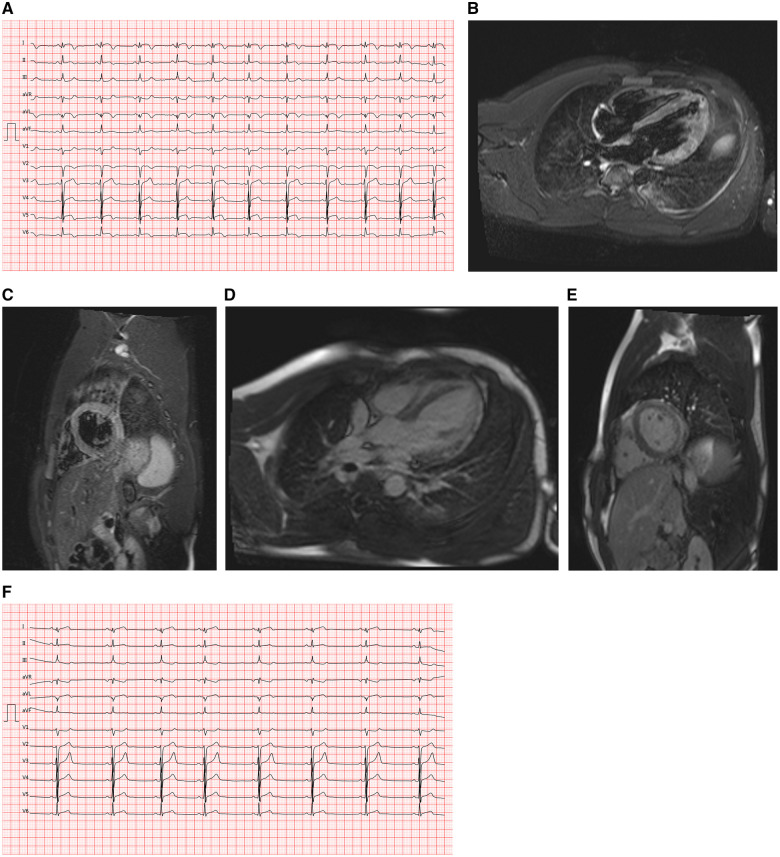
He had received the 1st dose of the COVID-19 vaccine ‘Moderna’ (100 µg messenger-RNA) 7 weeks prior and the 2nd dose 3 days prior. He complained of epigastric pain at admission. Two days earlier, he had experienced severe general symptoms with arthralgia and general malaise and had a temperature of 38°C. The epigastric pain had started the next day. He had a history of attention-deficit/hyperactivity disorder and was on methylphenidate 40 mg q.d. since 8 years of age. There were no other comorbidities. On initial evaluation, his blood pressure was 120/70 mmHg, heart rate was 87 b.p.m. and temperature was 37.1°C. Upon cardiac auscultation, there were no audible murmurs, and his lungs were clear. No oedema was noted. He experienced slight dysphasia after the referring physician had administered morphine. Additionally, he had received heparin 5000 IU and acetylsalicylic acid 250 mg I.V. The other physical parameters were normal. Initial laboratory data revealed elevated levels of high-sensitivity cardiac troponin-T (606 pg/mL; normal: <14 pg/mL), creatine kinase-MB (68 U/L; normal: <24 U/L), C-reactive protein (44.2 mg/L; normal: 0–5 mg/L), and lactate dehydrogenase (508 U/L; normal: 135–225 U/L). His white blood cell count was elevated (17.4 G/L; normal: 3.9–10.2 G/L), and eosinophilia was absent. An electrocardiogram revealed a sinus rhythm, normal QRS axis and ST-elevation in I, aVL, V4–V6, ST-depression in V1, and aVR (Figure 2A). Coronary angiography revealed normal coronary arteries. Chest X-ray revealed signs of pulmonary congestion. The echocardiogram revealed a moderately reduced systolic LV function with diffuse hypokinesis and an LVEF of 40% (Video 2). No pericardial effusion was noted, and his valve function was normal. Cardiac MRI performed 72 h after admission revealed a moderately reduced systolic LV function. T2-STIR sequences showed myocardial oedema in the lateral and anterior LV wall (Figure 2B and C). There was thickening and enhancement of the pericardium but no pericardial effusion. A patchy non-ischaemic pattern of myocardial LGE was noted, predominantly in the lateral wall (Figure 2D and E), suggesting a diagnosis of myocarditis.7
Figure 2.
(A) Case 2 electrocardiogram on presentation, showing ST-elevation in I, aVL, V4–V6, ST-depression in V1 and aVR. (B) Case 2 cardiac magnetic resonance imaging, T2-weighted stir sequence, four-chamber view, showing diffuse increased signal intensity in the anterolateral and apical segments. (C) Case 2 cardiac magnetic resonance imaging, T2-weighted stir sequence, midventricular short-axis view, showing an increased signal intensity in the mid inferolateral and anterolateral segments. (D) Case 2 cardiac magnetic resonance imaging, inverse-recovery late gadolinium enhancement sequence, four-chamber view, showing subepicardial late gadolinium enhancement of the anterolateral and apical segments. (E) Case 2 cardiac magnetic resonance imaging, inverse-recovery late gadolinium enhancement sequence, midventricular short-axis view, showing subepicardial late gadolinium enhancement of the mid inferolateral and anterolateral segments. (F) Case 2 electrocardiogram at Day 7, showing sinus rhythm, biphasic T waves in I, aVL, and ST-depression in III, aVR, and V1.
There were no indications of an immune-mediated or infectious cause of myocarditis. Serology for cardiotropic viruses was not performed. Without a clinical suspicion for a specific infection, serology for human immunodeficiency virus or hepatitis C virus was not performed. There were no specific indications that led us to examine for autoimmune disorders. Cardiac autoantibodies tested negative. There were no signs suggestive of thyrotoxicosis-mediated myocarditis. There was no family history of genetic or autoimmune diseases. Bacterial myocarditis was improbable as there were no predisposing comorbidities and rapid recovery was achieved without antibiotic therapy. Additionally, procalcitonin tested negative.
A quantitative SARS-CoV-2 antibody assay (SARS-CoV-2 TrimericS IgG Assay, DiaSorin) was performed and revealed an anti-spike post-vaccination titre of >2080 BAU/mL (normal: <33.8 BAU/mL). Coronavirus disease 2019 PCR testing was negative.
Monitoring for 5 days revealed no relevant heart rhythm anomalies. Repeat echocardiography revealed resolution of the wall motion abnormalities. The patient was treated with heart failure therapy according to the European Society of Cardiology guidelines,9 including I.V. loop diuretics, beta-blockers, mineralocorticoid receptor antagonists, and an angiotensin-converting enzyme inhibitor. Colchicine was administered for suspicion of pericarditis. He showed symptom resolution. Follow-up electrocardiogram revealed regression of the ST-segment elevation and showed sinus rhythm, biphasic T waves in I, aVL and ST-depression in III, aVR, V1 (Figure 2F). High-sensitivity cardiac-specific troponin-T and C-reactive protein levels decreased progressively, and he was discharged with heart failure therapy according to the European Society of Cardiology guidelines. Intravenous loop diuretics were discontinued, and the same drugs were administered orally. With the amelioration of systolic LV function, mineralocorticoid receptor antagonists were discontinued. We recommended avoidance of exercise for the next 6 months and discontinuation of methylphenidate. A follow-up medical appointment with an ambulatory care cardiologist was scheduled.
We reported both cases as suspected adverse drug reactions to the German Federal Institute for Drugs and Medical Devices. Both patients worked as firefighters, although at different fire stations. Both reported that several hundreds of their co-workers had been vaccinated as well. None of their vaccinated co-workers reported similar complaints.
Discussion
We report two cases of myocarditis following the administration of an mRNA COVID-19 vaccine. Considering the increasing number of reports regarding the development of myocarditis after mRNA COVID-19 vaccination,1–5 awareness of this adverse drug reaction is important, especially considering the ongoing worldwide vaccination campaign. We did not find evidence of any causative factors other than the administration of the COVID-19 Vaccine ‘Moderna’, although alternative causes were not comprehensively excluded. Symptom onset occurred after the administration of the 2nd dose in both cases. Both patients tested negative for cardiac autoantibodies; however, this excludes neither autoimmune myocarditis nor autoantibody-negative autoimmune diseases. Especially in the 2nd case, we could not rule out the fact that the attention-deficit/hyperactivity disorder therapy with methylphenidate might have aggravated or even caused heart failure because of its sympathomimetic properties.10 In this patient, we did not consider the ST-elevations as a sign of acute ischaemia. Therefore, coronary angiography was performed the next morning and not immediately. Although pericardial inflammation might have caused the electrocardiographic changes, those too can be explained in light of the myocardial inflammation. We found neither pericardial effusion nor pericardial friction rubs. Hence, a diagnosis of myopericarditis seemed less probable. Nevertheless, we continued colchicine therapy.
In Case 1, there was a discrepancy in the findings between cardiac MRI and echocardiography. Regional wall motion abnormalities on cardiac MRI were probably evident because of better endocardial definition, whereas no wall motion abnormalities were seen on echocardiography.
The quantitative SARS-CoV-2 antibody assay revealed high titres in both patients. Thus, the vaccination of the patients could be considered effective.11
Recently published cases of myocarditis after COVID-19 vaccination reported similar clinical courses. There was a predominance of male patients. Almost all had received mRNA vaccines. Symptom onset began shortly after the administration of the 2nd dose, and the resolution of symptoms was rapid.1–5 Previous reports described normal LV function in a majority of patients.1–5 In contrast, we noted reduced systolic LV function in one of the two patients. As we reported only two patients, this might be a coincidence. On comparing our patients with previously reported cases, we noted remarkable similarities in terms of patient characteristics, clinical course, vaccine type, and time of symptom onset (Table 1). A final diagnosis of COVID-19 vaccine-induced myocarditis might be supported by these findings.
Table 1.
| Case 1 | Case 2 | Rosner et al., 7 patients 1 | Montgomery et al., 23 patients 4 | |
|---|---|---|---|---|
| Age (years) | 23 | 20 | Mean 27 | Mean 25 |
| Sex | Male | Male | All male | All male |
| Ethnicity | White | White | 6 white, 1 Hispanic | — |
| Vaccine type | mRNA COVID-19 vaccine Moderna | mRNA COVID-19 vaccine Moderna |
|
|
| Vaccine dose | 2nd | 2nd |
|
|
| Days to presentation | 5 | 3 | Mean 4 | — |
| Days to symptom onset | 1 | 1 | — | Mean 2 |
| Prior COVID-19 infection | 0 | 0 | 1 of 7 | 3 of 23 |
COVID-19, coronavirus disease 2019.
Myocarditis is a rare complication following the administration of the mRNA COVID-19 vaccine.1–5 It is also a rare complication of vaccination in general.12 In this case series, the temporal proximity of the development of acute myocarditis and the administration of the mRNA COVID-19 vaccine ‘Moderna’ was recognized. It therefore seems possible that myocarditis occurred due to an adverse reaction to the vaccine. However, a causal relationship remains speculative. Despite various presumed mechanisms underlying the development of COVID-19 vaccine-induced myocarditis, a substantial pathophysiological explanation for the myocardial inflammation caused by the mRNA COVID-19 vaccine has not yet been provided.13–15 Still, myocarditis occurring after exposure to either a prior vaccine dose or previous SARS-CoV-2 infection followed by rapid recovery supports a plausible mechanism of a drug-induced hypersensitivity reaction.4,7
At all events, the temporal proximity of acute myocarditis after the administration of mRNA COVID-19 vaccines in two young healthy males in the setting of a community hospital might lead one to speculate that myocarditis after COVID-19 vaccination is not as rare as previously reported. To date, in Germany, 1243 cases of adverse events of myocarditis/pericarditis after COVID-19 vaccination have been reported. Considering all types of COVID-19 vaccines, 107.8 million vaccine doses were administered in Germany until the end of September 2021.16 The European Medicines Agency informs of a possible link between myocarditis and COVID-19 mRNA vaccines.17 In the USA and Germany, COVID-19 mRNA vaccines are recommended for persons aged ≥12 years.18,19
Both cases of myocarditis occurred only after the 2nd vaccine dose. Investigation of the safety and efficacy of the administration of only a single dose of mRNA COVID-19 vaccine may be considered in adolescents and young adults.20
Because of the novelty of mRNA vaccine technology, further investigations are warranted, especially an elucidation of the presumed mechanism of myocarditis in the context of COVID-19 vaccination. The clinical suspicion of myocarditis should be high in patients who present with chest pain or dyspnoea after receiving COVID-19 vaccination.
Conclusion
In this case series, we describe two cases of myocarditis in previously healthy patients after mRNA COVID-19 immunization. We reported these as potential vaccine-related adverse events. The patients’ clinical course and temporal relation suggested the possibility of a COVID-19 vaccine-associated inflammation of the myocardium.
Lead author biography

Dr Christopher Paul Bengel finished his medical studies at the Johannes Gutenberg-University Mainz (Germany). He was trained in cardiology at the Bad Soden Hospital, Germany. Currently, he is working as a senior physician at the Kliniken Frankfurt-Main-Taunus/ Bad Soden Hospital. His main field is interventional cardiology.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case series including images and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest: C.P.B. declares stock ownership of Moderna, Inc. R.K. has no conflict of interest to declare.
Lead author photography courtesy of Clinics of the Main Taunus District GmbH.
Funding: none declared.
Supplementary Material
References
- 1.Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S. et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation 2021;144:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muthukumar A, Narasimhan M, Li QZ, Mahimainathan L, Hitto I, Fuda F. et al. In depth evaluation of a case of presumed myocarditis following the second dose of COVID-19 mRNA vaccine. Circulation 2021;144:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Angelo T, Cattafi A, Carerj ML, Booz C, Ascenti G, Cicero G. et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol 2021;37:1665–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L. et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 2021;6:e212833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS. et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics 2021;148:e2021052478. [DOI] [PubMed] [Google Scholar]
- 6.Guarascio F, Richardson A.. 2021. EU takes up option to buy 150 million more Moderna COVID-19 shots. https://www.reuters.com/world/europe/eu-takes-up-option-buy-150-million-more-moderna-covid-19-vaccines-2021-06-22/ (29 June 2021).
- 7.Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M. et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail 2020;13:e007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB. et al. ; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 9.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M. et al. ; ESC Scientific Document Group. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 10.Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG. et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA 2011;306:2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–1211. [DOI] [PubMed] [Google Scholar]
- 12.Engler RJM, Nelson MR, Collins LC, Spooner C, Hemann BA, Gibbs BT. et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One 2015;10:e0118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segal Y, Shoenfeld Y.. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol 2018;15:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozkurt B, Kamat I, Hotez PJ.. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra DP, Agarwal V, Gasparyan AY, Zimba O.. Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol 2020;39:2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.German Federal Institute for Vaccines and Biomedicines. Reports on suspected cases of adverse effects and vaccination complications following a vaccination for the protection against COVID-19 (reporting period 27 December 2020 - 30 September 2021) (German only). 2021. https://www.pei.de/EN/newsroom/dossier/coronavirus/coronavirus-content.html;jsessionid=85EE495A111741EDA947575B1E7E24E4.intranet212?cms_pos=6 (7 December 2021).
- 17.European Medicines Agency. Comirnaty and Spikevax: possible link to very rare cases of myocarditis and pericarditis. 2021. https://www.ema.europa.eu/en/news/comirnaty-spikevax-possible-link-very-rare-cases-myocarditis-pericarditis (20 August 2021).
- 18.Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices (ACIP). 23-25 June, 2021 Meeting Coronavirus Disease. 2019 (COVID-19) vaccines. 2021. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-06.html (29 June 2021).
- 19.Robert Koch Institute, Standing Committee on Vaccination. STIKO communication on the update of the COVID-19 vaccination recommendation for children and adolescents. Ger Only. https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/PM_2021-08-16.html (20 August 2021).
- 20.Saadat S, Rikhtegaran Tehrani Z, Logue J, Newman M, Frieman MB, Harris AD. et al. Binding and neutralization antibody titres after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA 2021;325:1467–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.