Abstract
Background
Thromboxane A2 (TXA2) is a platelet- and cyclooxygenase-derived eicosanoid that has been linked to metastasis. We investigated the role of TXA2 in the development of lethal prostate cancer in African American (AA) and European American (EA) men.
Methods
We measured urinary 11-dehydrothromboxane B2 (TXB2), a stable metabolite of TXA2, with mass spectrometry. Samples were obtained from 977 cases and 1022 controls at time of recruitment. We applied multivariable logistic and Cox regression modeling to examine associations of TXB2 with prostate cancer and patient survival. The median survival follow-up was 8.4 years, with 246 deaths among cases. Aspirin use was assessed with a questionnaire. Race was self-reported.
Results
Urinary TXB2 was inversely associated with aspirin use. High (>median) TXB2 was associated with prostate cancer in AA (adjusted odds ratio [OR] = 1.50, 95% confidence interval [CI] = 1.13 to 2.00) but not EA men (OR = 1.07, 95% CI = 0.82 to 1.40), suggesting upregulated TXA2 synthesis in AA men with prostate cancer. High TXB2 was positively associated with metastatic prostate cancer (OR = 2.60, 95% CI = 1.08 to 6.28) compared with low (≤median) TXB2. Furthermore, high TXB2 was also associated with all-cause (adjusted hazard ratio = 1.59, 95% CI = 1.06 to 2.40) and prostate cancer-specific mortality (hazard ratio = 4.74, 95% CI = 1.62 to 13.88) in AA men only.
Conclusions
We report a distinct association of TXB2 with prostate cancer outcomes in AA men. In this high-risk group of men, upregulation of TXA2 synthesis may promote metastasis and lethal disease. Our observation identifies a potential benefit of aspirin in preventing lethal prostate cancer through inhibition of TXA2 synthesis.
Systemic low-grade inflammation and an inflammatory tumor microenvironment are candidate risk factors for prostate cancer that promote aggressive disease (1-3). Prostate tumors in African American (AA) men harbor a distinct immune and inflammation signature consistent with a unique immunobiology and the activation of inflammation pathways (4-6). These observations, coupled with our previous findings that regular use of aspirin is associated with decreased odds of lethal prostate cancer in AA men (7,8), suggest that low-grade chronic inflammation might be a driver of adverse outcomes in AA prostate cancer patients.
The preventative benefits of aspirin have been attributed to inhibition of the arachidonic acid signaling pathway (9,10). Arachidonic acid is broken down to eicosanoids by the cyclooxygenase (COX) 1 and 2 enzymes. These enzymes and subsequent production of these eicosanoids are important for normal physiological processes, including the modulation of immune responses and regulation of blood clotting. However, as a promoter of inflammatory responses, eicosanoid synthesis is commonly upregulated in cancer and may contribute to cancer progression (11).
Thromboxane A2 (TXA2), an eicosanoid produced primarily via COX1 in activated platelets, orchestrates platelet aggregation. TXA2, activated and elevated at times of inflammation, contributes to carcinogenesis through roles in vasoconstriction, endothelial adhesion (12), cell motility (13), and cell proliferation (14). Importantly, platelet-derived TXA2 is pro-metastatic (15). Aspirin may reduce metastatic cancer and inhibit the pro-metastatic effects of platelet-derived COX1 or TXA2, as shown in an animal model of lung metastasis (15-17).
There have been no studies investigating the relationship of TXA2 or TXB2 formation with adverse prostate cancer outcomes. Hence, we assessed the role of TXA2 levels in the development of lethal prostate cancer in a diverse study population .
Methods
Study Population
The National Cancer Institute (NCI)-Maryland prostate cancer case-control study has been described (7,18). The study was initiated to test the primary hypothesis that environmental exposures and ancestry-related factors contribute to the excessive prostate cancer burden among AA men. Before the interview, all individuals signed informed consent for participation. All study forms and procedures were approved by the NCI (protocol # 05-C-N021) and the University of Maryland (protocol #0298229) institutional review boards. Research followed the ethical guidelines set by the Declaration of Helsinki. Cases were recruited at the Baltimore Veterans Affairs Medical Center and the University of Maryland Medical Center through arrangements with physicians. Controls were identified through the Maryland Department of Motor Vehicle Administration database and were frequency matched to cases on age and race. This article follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for the reporting of observational studies. See the Supplementary Methods (available online) for exclusion and inclusion criteria, questionnaire, and biospecimen information.
Laboratory Assay for Urinary TXB2 Measurement
Because TXA2 is unstable, its primary, more stable metabolite, urinary 11-dehydrothromboxane B2 (TXB2), was measured by the Eicosanoid Core Laboratory at Vanderbilt University Medical Center (Nashville, TN). See the Supplementary Methods (available online) for more details about this assay and its quality control performance. It has previously been shown that urinary TXB2 correlates well with platelet-derived TXA2 synthesis (19).
Assessment of Aspirin Use
Our survey evaluated aspirin use with the following question: “Have you taken aspirin or aspirin-containing compounds (such as Bufferin, Anacin, Ascriptin, Excederin) regularly—at least 1 pill per week for 2 months during the past 5 years?” Responses were no, yes, or do not know.
Statistical Analysis
Data analysis was performed using the Stata/SE 16.0 statistical software package (StataCorp). All statistical tests were 2-sided. An association was considered statistically significant with P less than .05. For analysis, we assessed TXB2 as either a continuous measure or assigned TXB2 values to quartiles (Q1-Q4, Q1 being the lowest, Q4 being the highest) and median (≤median or >median) with cutoff points determined using the distribution of TXB2 values among all controls. TXB2 data analyzed as a continuous measure were log2 transformed. The nonparametric Kruskal-Wallis or the Mann-Whitney test was used to determine differences in TXB2 levels across groups. Furthermore, cases were assigned to risk groups according to National Comprehensive Cancer Network (NCCN) Risk Score classification, which stratifies patients into pretreatment recurrence risk groups according to the clinical tumor stage, biopsy Gleason score, and serum prostate-specific antigen level (20). We condensed these risk groups into 4 categories (low, intermediate, high or very high, and regional or metastatic ).
Unconditional logistic regression models were used to calculate adjusted odds ratios (OR) and 95% confidence intervals (CI) to assess the association of TXB2 with use of aspirin, case status, or the NCCN risk score. We adjusted for the following potential confounding factors: age at study entry, body mass index, diabetes, aspirin use, education, family history of prostate cancer, self-reported race, smoking history, treatment, disease stage, and Gleason score (see the Supplementary Methods, available online, for more information). To test for a statistical interaction between aspirin use and TXB2 levels, we applied the multivariable logistic regression model with and without the interaction term and examined statistical significance with the likelihood ratio test. A P less than .05 was considered as statistical evidence for effect modification.
We applied the Cox regression model to estimate adjusted hazard ratios (HR) and 95% CI for all-cause mortality and prostate cancer-specific mortality in cases. Median survival follow-up was 8.4 years. In the analysis of all-cause mortality, median follow-up time to death from any cause was 4.52 years for AA men and 5.99 years for EA men. In the analysis of prostate cancer-specific survival, median follow-up time to death from prostate cancer was 2.75 years for AA men and 7.7 years for EA men. We adjusted for potential confounding factors (defined in the Supplementary Methods, available online). We calculated survival for cases and controls from date of diagnosis to either date of death or to the censor date of December 31, 2018. We confirmed nonviolation of the proportionality assumption based on the goodness-of-fit test using Schoenfeld residuals. In a sensitivity analysis, the Fine-Gray competing risk model was used to estimate whether the association of TXB2 with prostate cancer survival was influenced by other causes of death. For survival analysis with the Kaplan-Meier method, the log-rank test was used to examine differences in all-cause and prostate cancer–specific mortality according to TXB2 levels.
Results
Clinical and Demographic Characteristics of Participants in the NCI-Maryland (NCI-MD) Prostate Cancer Case-Control Study
Demographic characteristics of the enrolled participants are shown in Supplementary Table 1 (available online) with the disease characteristics of the cases. The study enrolled 977 cases (490 AA and 487 EA) and 1022 population controls (479 AA and 543 EA) from the greater Baltimore area in Maryland. Race or ethnicity was self-reported as part of the eligibility screener and with the survey. The distributions of age and body mass index were very similar in cases and controls. Controls had higher levels of education, with 24.9% reporting a graduate school qualification compared with 14.6% of cases. Cases had a higher proportion of current smokers at 24.8% compared with 14.5% of controls .
Urinary TXB2 Levels Among Cases and Controls and Their Association With Aspirin Use
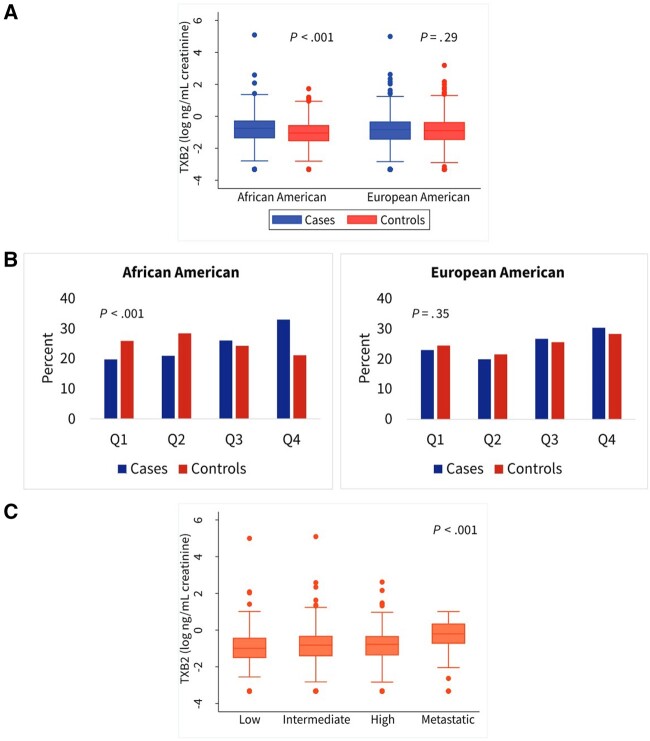
Urinary TXB2 was statistically significantly upregulated in AA men with prostate cancer (Figure 1, A). Among controls, EA men tended to have higher levels than AA men (P = .03). With TXB2 data distributed into quartiles according to control values and stratified by race, a higher proportion of AA cases presented with the most elevated (Q4) TXB2 levels compared with controls. There was a statistically significant difference in Q4 TXB2 levels between cases and controls for AA men (P < .001) but not for EA men (P = .35) (Figure 1, B).
Figure 1.
Urinary 11-dehydrothromboxane B2 (TXB2) levels in African American and European American men and their association with prostate cancer and National Comprehensive Cancer Network (NCCN) risk scores. A) Urinary TXB2 levels in cases and controls shown as a continuous measure, stratified by race. B) Bar charts show distribution of cases and controls stratified into African American and European American men across TXB2 quartiles (Q). Q cutoff points originate from the combined control groups and are 0.35, 0.505, and 0.738 ng TXB2/mg creatinine. C) Shown are urinary TXB2 levels across 4 NCCN risk score categories. Two-sided Mann-Whitney (A), χ2 (B), and Kruskal-Wallis (C) tests were applied for statistical significance testing. The error bars in (A) and (C) represent the 95% confidence interval.
Next, we investigated whether intake of the most common antiinflammatory drug, aspirin, would have the anticipated inhibitory effect on urinary TXB2. Data on aspirin use was available for 99.1% of our cases (968 of 977), with 49.6% reporting regular aspirin use, which is generally consistent with aspirin use in this age group (21). However, broken down by self-reported race, 43.1% of AA men reported to have taken aspirin compared with 56.9% of EA men. Aspirin use was associated with decreased TXB2 in both cases and controls (Table 1). For cases who used aspirin, the adjusted odds of having high urinary TXB2 levels (Q4) were statistically significantly reduced compared with nonusers (OR = 0.27, 95% CI = 0.18 to 0.41) (Table 1), and this observation remained when data were stratified by race (AA men: OR = 0.20, 95% CI = 0.11 to 0.36; EA men: OR = 0.39, 95% CI = 0.21 to 0.70), suggesting strong inhibition of TXB2 formation by aspirin in men with prostate cancer. There was also a strong inhibition of TXB2 by aspirin use in controls (OR = 0.21, 95% CI = 0.14 to 0.32) (Table 1). This was consistent for both AA and EA controls. These findings agree with previous work showing inhibition of TXA2 by aspirin through inhibition of the COX1 and TXA2 pathway (15). Notably, however, the aspirin inhibitory effect tended to be more robust among both AA cases and controls than EA cases and controls.
Table 1.
Inverse association of regular aspirin use with urinary TXB2 levels in cases and controls
| TXB2 | Aspirin use |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All |
African American |
European American |
|||||||
| No, No. (%) | Yes, No. (%) | OR (95% CI) | No, No. (%) | Yes, No. (%) | OR (95% CI) | No, No. (%) | Yes, No. (%) | OR (95% CI) | |
| Cases | |||||||||
| Q1a | 72 (15.1) | 132 (27.9) | Refb | 35 (12.9) | 60 (29.6) | Refb | 37 (18.0) | 72 (26.7) | Refb |
| Q2 | 85 (17.9) | 107 (22.6) | 0.72 (0.46 to 1.14)b | 52 (19.2) | 46 (22.7) | 0.47 (0.24 to 0.83)b | 33 (16.1) | 61 (22.6) | 1.16 (0.58 to 2.30)b |
| Q3 | 128 (26.9) | 123 (26.0) | 0.47 (0.31 to 0.73)b | 74 (27.3) | 51 (25.1) | 0.31 (0.17 to 0.56)b | 54 (26.3) | 72 (26.7) | 0.70 (0.37 to 1.31)b |
| Q4 | 191 (40.1) | 111 (23.5) | 0.27 (0.18 to 0.41)b | 110 (40.6) | 46 (22.7) | 0.20 (0.11 to 0.36)b | 81 (39.5) | 65 (24.1) | 0.39 (0.21 to 0.70)b |
| Ptrend | <.001 | <.001 | <.001 | ||||||
| Controls | |||||||||
| Q1 | 71 (16.4) | 180 (31.8) | Refc | 38 (16.6) | 84 (35.4) | Refc | 33 (16.3) | 96 (29.1) | Refc |
| Q2 | 86 (19.9) | 163 (21.8) | 0.69 (0.46 to 1.04)c | 57 (24.9) | 75 (31.7) | 0.48 (0.27 to .84)c | 29 (14.3) | 88 (26.7) | 0.91 (0.49 to 1.68)c |
| Q3 | 129 (29.9) | 121 (21.3) | 0.31 (0.21 to 0.46)c | 66 (28.8) | 47 (19.8) | 0.27 (0.15 to 0.48)c | 63 (31.0) | 74 (22.4) | 0.32 (0.18 to 0.56)c |
| Q4 | 146 (33.8) | 103 (18.2) | 0.21 (0.14 to 0.32)c | 68 (29.7) | 31 (13.1) | 0.14 (0.08 to 0.27)c | 78 (38.4) | 72 (21.8) | 0.27 (0.15 to 0.47)c |
| Ptrend | <.001 | <.001 | <.001 | ||||||
Quartile (Q) cutoff points are 0.35, 0.505, and 0.738 ng TXB2/mg creatinine. CI = confidence interval; OR = odds ratio; Ref = reference.
Logistic regression analysis adjusted for age at study entry, body mass index (kg/m2), diabetes (yes or no), education (high school or less, some college, college, professional school), family history of prostate cancer (first-degree relatives, yes or no), self-reported race (not included in stratified analysis), disease stage (1 = stage I, 2 = stage IIA and IIB, 3 = stage III, 4 = stage IV), Gleason score (0 = Gleason ≤7 and 1 = Gleason >7), smoking history (never, former, current), and treatment (0 = none, 1 = surgery, 2 = radiation, 3 = hormone, 4 = combination).
Logistic regression analysis adjusted for age at study entry, BMI (kg/m2), diabetes (yes or no), education (high school or less, some college, college, professional school), family history of prostate cancer (first-degree relatives, yes or no), self-reported race (not included in stratified analysis), and smoking history (never, former, current).
TXB2 and Aggressive Prostate Cancer
We used unconditional logistic regression to determine the odds of having prostate cancer when TXB2 is elevated. The adjusted OR for prostate cancer was 1.24 (95% CI = 1.02 to 1.50) for men with high (>median) TXB2 compared with low TXB2 (≤median) (Table 2), pointing to a moderate but statistically significant association with the disease. To further understand the importance of this finding for AA men, we stratified the analysis by race. In this analysis, a statistically significant association between high TXB2 and having prostate cancer was observed only among AA men (OR = 1.50, 95% CI = 1.13 to 2.00) but not EA men (OR = 1.07, 95% CI = 0.82 to 1.40). We previously reported that aspirin use associates with a reduced risk of developing advanced prostate cancer among AA in our study cohort (7). Given this observation, we conducted a mediation analysis to estimate direct effects of aspirin use on prostate cancer risk and indirect effects via TXB2. From this analysis, we estimate that the indirect effect of TXB2 as a mediator of prostate cancer risk accounts for almost one-half of the protective effect of aspirin use on prostate cancer risk in AA men (Supplementary Table 2, available online).
Table 2.
Association of urinary TXB2 levels with prostate cancer
| TXB2 | Odds of case status |
||
|---|---|---|---|
| Control, No. (%) | Case, No. (%) | OR (95% CI)a | |
| All cases | |||
| ≤Medianb | 500 (50.0) | 397 (41.8) | Ref |
| >Median | 499 (50.0) | 554 (58.2) | 1.24 (1.02 to 1.50) |
| African American | |||
| ≤Median | 254 (54.5) | 193 (40.6) | Ref |
| >Median | 212 (45.5) | 282 (59.4) | 1.50 (1.13 to 2.00) |
| European American | |||
| ≤Median | 246 (46.2) | 204 (42.9) | Ref |
| >Median | 287 (53.8) | 272 (57.1) | 1.07 (0.82 to 1.40) |
Unconditional logistic regression adjusted for age at study entry, body mass index (kg/m2), diabetes (no or yes), aspirin (no or yes), education (high school or less, some college, college, professional school), family history of prostate cancer (first-degree relatives, yes or no), self-reported race (not included in stratified analysis), and smoking history (never, former, current). CI = confidence interval; OR = odds ratio; Ref = reference.
Median cutoff point is 0.505 ng TXB2/mg creatinine.
We further investigated the association between TXB2, aggressive disease, and metastasis, and assigned men with prostate cancer into NCCN risk groups as described under the Methods and shown in Supplementary Table 3 (available online). In the unadjusted analysis, the highest median level of TXB2 associated with prostate cancer patients who had developed regional and distant metastatic disease (Figure 1, C). This association remained statistically significant using an adjusted logistic regression model. High levels of TXB2 associated with prostate cancer only among cases who were in the risk group for regional and distant metastatic disease (Table 3) (OR = 2.60, 95% CI = 1.08 to 6.28, >median vs ≤median). We did not find an association of TXB2 with localized disease, consistent with the role of TXA2 or TXB2 signaling in metastasis (15). These observations suggest a distinct relationship between high TXB2 levels and lethal prostate cancer.
Table 3.
Association of high urinary TXB2 with NCCN Risk Score for metastatic prostate cancer
| NCCN risk score | OR (95% CI)a | P b |
|---|---|---|
| Low | Ref | |
| Intermediate | 1.49 (0.98 to 2.26) | .06 |
| High or very high | 1.34 (0.80 to 2.26) | .27 |
| Regional or metastatic | 2.60 (1.08 to 6.28) | .03 |
Unconditional logistic regression adjusted for age at study entry, body mass index (kg/m2), diabetes (no or yes), aspirin (no or yes), education (high school or less, some college, college, professional school), family history of prostate cancer (first-degree relatives, yes or no), self-reported race, smoking history (never, former, current), and treatment (0 = none, 1 = surgery, 2 = radiation, 3 = hormone, 4 = combination). CI = confidence interval; NCCN = National Comprehensive Cancer Network; OR = odds ratio; Ref = reference.
P value was calculated using 2-sided Wald statistical test.
Association of High TXB2 With Survival and the Effect of Aspirin Use
To build on our findings that high TXB2 is associated with prostate cancer among AA men, we next examined if there is an association between high levels of TXB2 and survival outcomes in our population. As of the end of 2018, there have been 246 deaths in our case population, of whom 47.6% had a cancer diagnosis as the recorded primary cause of death, and 26.8% of all deaths (n = 66) were directly attributed to prostate cancer. In agreement with the literature, AA men in the NCI-MD study were more likely to die after a prostate cancer diagnosis than EA men (all-cause mortality, adjusted HR = 1.59, 95% CI = 1.20 to 2.10) (Supplementary Table 4, available online). Moreover, a higher proportion of them died because of prostate cancer (prostate cancer-specific mortality, adjusted HR = 1.71, 95% CI = 0.97 to 3.01) (Supplementary Table 5, available online), corroborating that AA patients are at an increased risk of lethal prostate cancer (22).
To examine the role of TXA2 in this survival disparity, we investigated the relationship of urinary TXB2 with vital status and survival among the men in our study. An initial analysis showed that a greater proportion of the all-cause mortality among cases associated with high TXB2 (>median) (Supplementary Table 6, available online). This relationship was most pronounced in AA men, where 70.6% of the case deaths associated with high TXB2 compared with 57.3% of EA cases, reaching statistical significance only among the AA cases. Additionally, we did not find a relationship between TXB2 levels and vital status in the controls (Supplementary Table 6, available online). We then investigated whether high TXB2 was associated with survival outcomes in our case population. In the Kaplan-Meier (Supplementary Figure 1, available online) and multivariable-adjusted Cox regression survival analyses (Table 4), there was a marginal but positive association between high TXB2 levels and an increased all-cause mortality after a prostate cancer diagnosis (HR = 1.33, 95% CI = 0.99 to 1.79). However, when the data were stratified by self-reported race, high TXB2 was associated with an elevated all-cause mortality in AA men (HR = 1.59, 95% CI = 1.06 to 2.40) but not in EA men (HR = 1.11, 95% CI = 0.70 to 1.76). Associations were observed with both dichotomized and continuous TXB2 level data in the survival analysis, although the continuous data additionally supported an association among EA men (Table 4). Lastly, this relationship of urinary TXB2 with all-cause mortality was uniquely observed among men with prostate cancer but not in our control population (Supplementary Table 7, available online).
Table 4.
Association of urinary TXB2 levels with all-cause mortality among prostate cancer patients
| TXB2 | All cases |
African American |
European American |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Alive, No. (%) | Dead, No. (%) | HR (95% CI)a | Alive, No. (%) | Dead, No. (%) | HR (95% CI)a | Alive, No. (%) | Dead, No. (%) | HR (95% CI)a | |
| ≤Medianb | 313 (43.6) | 82 (33.2) | Ref | 153 (44.3) | 39 (28.7) | Ref | 160 (42.9) | 43 (39.6) | Ref |
| >Median | 396 (56.4) | 158 (66.8) | 1.33 (0.99 to 1.79) | 186 (55.7) | 95 (71.3) | 1.59 (1.06 to 2.40) | 210 (57.1) | 63 (60.4) | 1.11 (0.70 to 1.76) |
| Continuousc | — | — | 1.24 (1.09 to 1.41) | — | — | 1.31 (1.10 to 1.56) | — | — | 1.21 (1.01 to 1.45) |
Unconditional Cox regression adjusted for age at study entry, body mass index (kg/m2), diabetes (no or yes), aspirin (no or yes), education (high school or less, some college, college, professional school), family history of prostate cancer (first-degree relatives, yes or no), self-reported race (not included in stratified analysis), smoking history (never, former, current), treatment (0 = none, 1 = surg, 2 = radiation, 3 = hormone, 4 = combination), disease stage (1= stage I, 2 = stage IIA and IIB, 3 = stage III, 4 = stage IV), and Gleason score (0 = Gleason ≤7 and 1 = Gleason >7). CI = confidence interval; OR= odds ratio; Ref = reference.
Median cutoff point is 0.505 ng TXB2/mg creatinine.
TXB2 as a continuous, log2-transformed variable.
Although all-cause mortality is an important outcome measure for prostate cancer patients with advanced disease, prostate cancer-specific survival is the most rigorous outcome determinant for prostate cancer patients. We found that high TXB2 was associated with lethal prostate cancer in AA men (HR = 4.74, 95% CI = 1.62 to 13.88 with data dichotomized at the median) but not in EA men (HR = 1.12, 95% CI = 0.34 to 3.66) (Table 5; Supplementary Figures 2 and 3, available online). Similar relationships were observed when we used continuous TXB2 data in the survival analysis. Additional Fine-Gray competing risk analysis showed that the association of TXB2 with lethal prostate cancer was largely independent of competing causes of death (subdistribution HR = 3.83, 95% CI = 1.29 to 11.35) (Table 5).
Table 5.
Association of urinary TXB2 levels with prostate cancer–specific mortality
| TXB2 | All cases |
African American |
European American |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Death from PC, No. (%) | Death from other cause, No. (%) | HRa (95% CI) | SHRa (95% CI) | Death from PC, No. (%) | Death from other cause, No. (%) | HRa (95% CI) | SHRa (95% CI) | Death from PC, No. (%) | Death from other cause, No. (%) | HRa (95% CI) | SHRa (95% CI) | |
| ≤Medianb | 20 (30.8) | 62 (35.4) | Ref | Ref | 7 (17.5) | 32 (34.1) | Ref | Ref | 13 (52.0) | 30 (37.1) | Ref | Ref |
| >Median | 45 (69.2) | 113 (64.6) | 1.69 (0.88 to 3.24) | 1.58 (0.82 to 3.05) | 33 (82.5) | 62 (65.9) | 4.74 (1.62 to 13.88) | 3.83 (1.29 to 11.35) | 12 (48.0) | 51 (62.9) | 1.12 (0.34 to 3.66) | 0.84 (0.26 to 2.74) |
| Continuousc | — | — | 1.33 (1.02 to 1.75) | — | — | — | 1.59 (1.07 to 2.36) | — | — | — | 1.35 (0.90 to 2.01) | — |
Cox regression model and Fine and Gray competing risks regression models adjusted for age at study entry, BMI (kg/m2), diabetes (no or yes), aspirin (no or yes), education (high school or less, some college, college, professional school), family history of prostate cancer (first-degree relatives, yes or no), self-reported race (not included in stratified analysis), smoking history (never, former, current), treatment (0 = none, 1 = surgery, 2 = radiation, 3 = hormone, 4 = combination), disease stage (1 = stage I, 2 = stage IIA and IIB, 3 = stage III, 4 = stage IV), and Gleason score (0 = Gleason ≤7 and 1 = Gleason >7). CI = confidence interval; HR = hazard ratio; OR= odds ratio; Ref = reference; SHR = subdistribution hazard ratio.
Median cutoff point is 0.505 ng TXB2/mg creatinine.
TXB2 as a continuous, log2-transformed variable.
As a final point, we explored the possibility of an interaction between aspirin use and TXB2 on survival. Although a statistically significant interaction was not found in the analysis of all-cause and prostate cancer-specific survival, stratification of cases by aspirin use status (yes or no) revealed disparate outcomes. The association between high TXB2 and all-cause mortality remained statistically significant only in AA men who did not use aspirin (Supplementary Table 8, available online) but not in aspirin users. Our observations suggest that aspirin may reduce all-cause mortality by decreasing TXA2 levels in AA patients with an otherwise upregulated pro-metastatic COX1 and TXA2 pathway .
Discussion
Determining which prostate cancers will develop into lethal disease is an unmet clinical need. The choice is currently between active surveillance or immediate intervention with surgery or radiation, which can lead to complications. Although AA men are at increased risk of aggressive disease, consensus has not been reached on the appropriateness of active surveillance (23,24). Identifying novel markers of aggressive disease and targets for therapy would therefore be important for men of African descent who experience a disproportionately high burden of prostate cancer lethality (22,25).
Here, we report a distinct association of upregulated TXB2 with lethal prostate cancer in AA men. Furthermore, because TXA2 and TXB2 formation are inhibited through aspirin use, as shown by our and other data, inhibitors of the COX1 and TXA2 signaling pathway, like aspirin, might be used to reduce the excess prostate cancer mortality among AA patients. Our findings further corroborate the hypothesis that a systemic low-grade inflammation is prevalent in AA men with prostate cancer, and these elevated inflammatory processes are contributing to adverse outcomes for this population.
A reduction of metastasis due to aspirin use has been established in both murine models and clinical studies, but until recently the molecular mechanism remained elusive (16,17,26). Lucotti et al. (15) used a murine model of lung cancer to demonstrate suppression of an early metastatic niche in the vasculature via inhibition of the COX1 and TXA2 pathway. Aspirin inhibited this metastatic process. Although very intriguing, similar experimental data for prostate cancer and bone metastasis have not been reported. Nevertheless, our study further supports the observations from the lung cancer model, providing epidemiological evidence that high TXA2 levels may increase the odds of developing metastatic prostate cancer in AA men.
Aspirin use has been associated with a decreased prostate cancer–specific mortality (8,27). The mechanism for this association has yet to be established; however, our findings suggest involvement of arachidonic acid signaling, specifically inhibition of TXA2, in AA men. We did not find an association between TXB2 levels and prostate cancer–specific mortality among EA men in our study. The reasons for this are unclear and will have to be investigated in future studies. Consistent with other studies, AA men in the NCI-MD study were less likely to report taking aspirin regularly compared with EA men (27). This is important in the context of our findings, where high TXB2 associated with increased risk of mortality for AA men could possibly be mitigated by aspirin use. Through platelet aggregation, TXA2 is a mediator of cardiovascular disease (CVD). Irreversible inhibition of platelet COX1-derived TXA2 with low-dose aspirin affords protection against vascular thrombotic events (28). This established link between TXA2 and CVD is relevant to this study, because use of low-dose aspirin may afford protection from both CVD events and lethal prostate cancer in AA men.
Our study has limitations. Firstly, the case-control study design is retrospective, so it remains unknown if elevated TXA2 is a risk factor for prostate cancer development. Secondly, the question remains as to why AA men with prostate cancer have higher TXB2 levels than EA men. Current lines of enquiry include whether increased arachidonic acid signaling may contribute to higher incidence of CVD comorbidities in AA men (29). Higher dietary ingestion of arachidonic acid or linoleic acid may also increase levels of TXB2. Thirdly, aspirin use data were self-reported. However, our findings that aspirin use may reduce TXA2 or TXB2 levels is very much in agreement with the published literature providing strong support of the correctness of the self-reported aspirin use data. Lack of dose information for aspirin use prevents us from making any conclusions as to what dose is required to inhibit TXA2 formation. However, it is known that low-dose aspirin inhibits COX1, and the antimetastatic effects of aspirin are attributed to both low and high doses (15,16). Lastly, in our analysis of the relationship of TXB2 with metastatic disease, we could not further stratify by race because too few men presented with metastatic prostate cancer in our study population (AA = 30, EA = 22) at the time of enrollment.
In conclusion, this study identifies a novel association between high urinary TXB2 and aggressive prostate cancer as well as adverse survival outcomes for AA men. These observations need further validation, but they are consistent with our previous findings of a prevalent immune-inflammation signature and an inverse association of aspirin use with lethal prostate cancer in these patients (6–8). Our study highlights the potential benefit of aspirin for prevention of lethal prostate cancer in this high-risk group of men through inhibition of TXA2 synthesis.
Funding
This research was supported by the DoD award W81XWH1810588 (to S.A. and C.Y.) and the Intramural Research Program of the NIH, National Cancer Institute (NCI), Center for Cancer Research and Division of Cancer Epidemiology and Genetics; Maeve Kiely is supported by the NCI Cancer Prevention Fellowship program.
Notes
Role of the funders: No sponsor had any role in the study design, data collection, analysis, interpretation, the writing, and decision to submit the manuscript.
Disclosures: None of the contributing authors have any conflicts of interest or financial ties to disclose.
Author contributions: MK: Conceptualization; Investigation: Data curation; Methodology; Formal Analysis; Writing—original draft; Writing—review and editing, GM: Methodology, TZM: Conceptualization; Writing—review and editing, TD: Data curation; Project administration, WT: Supervision; Writing—review and editing, CJS: Writing—review and editing, FB: Project administration, CL: Methodology; Writing—review and editing, CY: Writing—review and editing, MBC: Methodology; Writing—review and editing, SA: Conceptualization; Methodology; Funding acquisition; Resources; Writing—review and editing; Supervision.
Acknowledgements: We would like to thank personnel at the University of Maryland and the Baltimore Veterans Administration Hospital for their contributions with the recruitment of participants. We would also like to thank the participants who contributed their time and biospecimens to make the NCI-Maryland study possible.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Gurel B, Lucia MS, Thompson IM, et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2014;23(5):847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klink JC, Bañez LL, Gerber L, et al. Intratumoral inflammation is associated with more aggressive prostate cancer. World J Urol. 2013;31(6):1497–1503. [DOI] [PubMed] [Google Scholar]
- 3. Sfanos KS, Yegnasubramanian S, Nelson WG, et al. The inflammatory microenvironment and microbiome in prostate cancer development. Nat Rev Urol. 2018;15(1):11–24. [DOI] [PubMed] [Google Scholar]
- 4. Wallace TA, Prueitt RL, Yi M, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68(3):927–936. [DOI] [PubMed] [Google Scholar]
- 5. Minas TZ, Kiely M, Ajao A, et al. An overview of cancer health disparities: new approaches and insights and why they matter. Carcinogenesis. 2021;42(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang W, Wallace TA, Yi M, et al. IFNL4-ΔG allele is associated with an interferon signature in tumors and survival of African-American men with prostate cancer. Clin Cancer Res. 2018;24(21):5471–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith CJ, Dorsey TH, Tang W, et al. Aspirin use reduces the risk of aggressive prostate cancer and disease recurrence in African-American men. Cancer Epidemiol Biomarkers Prev. 2017;26(6):845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang W, Fowke JH, Hurwitz LM, et al. Aspirin use and prostate cancer among African American men in the Southern Community Cohort Study. Cancer Epidemiol Biomarkers Prev. 2021;30(3):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan AT, Ogino S, Fuchs CS.. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–2142. [DOI] [PubMed] [Google Scholar]
- 10. Spite M, Serhan CN.. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107(10):1170–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pannunzio A, Coluccia M.. Cyclooxygenase-1 (COX-1) and COX-1 inhibitors in cancer: a review of oncology and medicinal chemistry literature. Pharmaceuticals. 2018;11(4):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashton AW, Ware JA.. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res. 2004;95(4):372–379. [DOI] [PubMed] [Google Scholar]
- 13. Nie D, Guo Y, Yang D, et al. Thromboxane A2 receptors in prostate carcinoma: expression and its role in regulating cell motility via small GTPase Rho. Cancer Res. 2008;68(1):115–121. [DOI] [PubMed] [Google Scholar]
- 14. Sakai H, Suzuki T, Takahashi Y, et al. Upregulation of thromboxane synthase in human colorectal carcinoma and the cancer cell proliferation by thromboxane A2. FEBS Lett. 2006;580(14):3368–3374. [DOI] [PubMed] [Google Scholar]
- 15. Lucotti S, Cerutti C, Soyer M, et al. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A2. J Clin Invest. 2019;129(5):1845–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379(9826):1591–1601. [DOI] [PubMed] [Google Scholar]
- 17. Algra AM, Rothwell PM.. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13(5):518–527. [DOI] [PubMed] [Google Scholar]
- 18. Minas TZ, Tang W, Smith CJ, et al. IFNL4-DeltaG is associated with prostate cancer among men at increased risk of sexually transmitted infections. Commun Biol. 2018;1(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catella F, Healy D, Lawson JA, et al. 11-Dehydrothromboxane B2: a quantitative index of thromboxane A2 formation in the human circulation. Proc Natl Acad Sci USA. 1986;83(16):5861–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Network NCC. The NCCN Clinical Practice Guidelines in Oncology for Prostate Cancer, V4.2019. https://www.nccn.org/professionals/physician_gls/default.aspx#prostate. Accessed November 16, 2020.
- 21. Williams CD, Chan AT, Elman MR, et al. Aspirin use among adults in the US: results of a national survey. Am J Prev Med. 2015;48(5):501–508. [DOI] [PubMed] [Google Scholar]
- 22. Butler EN, Kelly SP, Coupland VH, et al. Fatal prostate cancer incidence trends in the United States and England by race, stage, and treatment. Br J Cancer. 2020;123(3):487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schenk JM, Newcomb LF, Zheng Y, et al. African American race is not associated with risk of reclassification during active surveillance: results from the canary prostate cancer active surveillance study. J Urol. 2020;203(4):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deka R, Courtney PT, Parsons JK, et al. Association between African American race and clinical outcomes in men treated for low-risk prostate cancer with active surveillance. JAMA. 2020;324(17):1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rebbeck TR, Devesa SS, Chang B-L, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of African descent. Prostate Cancer. 2013;2013:560857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guillem-Llobat P, Dovizio M, Bruno A, et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget. 2016;7(22):32462–32477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hurwitz LM, Joshu CE, Barber JR, et al. Aspirin and non-aspirin NSAID use and prostate cancer incidence, mortality, and case fatality in the atherosclerosis risk in communities study. Cancer Epidemiol Biomarkers Prev. 2019;28(3):563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patrono C, García Rodríguez LA, Landolfi R, et al. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373–2383. [DOI] [PubMed] [Google Scholar]
- 29. Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69(4):619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.