A substantial proportion of patients with coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection might develop severe pneumonia and fibrotic lung damage at later stages after the infection. Efficient antiviral immunity and prevention of fibrosis progression are critical for COVID-19 control. Natural killer (NK) cells, whose targeting strategies have already provided therapeutic benefits in immunotherapy against tumors, are innate lymphocytes that also play an important role in antiviral immunity. In support of targeting NK cells in patients with COVID-19, Krämer et al. recently provided evidence that NK cells display anti-SARS-CoV-2 activity [1]. They showed that purified peripheral NK cells from healthy individuals reduce viral protein levels in cocultured SARS-CoV-2-infected cells. The effector cytokines IFN-γ and TNF-α produced by NK cells are essential for this process. In addition to antiviral activity, NK cells also function to limit tissue fibrosis [2]. Krämer et al. showed that IL-2-activated NK cells from control individuals reduce the expression of the profibrotic marker genes COL1A1 and ACTA2 in human lung fibroblasts, suggesting that NK cells might normally possess antifibrotic activity in the lung. Based on these data, the normal activity of NK cells might improve the control of COVID-19 by suppressing the virus and inhibiting fibrosis progression.
However, consistent with previous studies on COVID-19-associated NK cells [3–5], Krämer et al. showed that NK cells from patients with COVID-19 display a dysfunctional status similar to tumor-associated NK cells [6] (Fig. 1), which might compromise their anti-SARS-CoV-2 activity and potential antifibrotic activity. First, the absolute numbers of circulating NK cells are decreased in patients with COVID-19, especially in individuals with a severe disease. This decrease in NK cell numbers correlated with the intensity of inflammation and might result from virus-induced apoptosis since SARS-CoV-2 nucleocapsid protein increases the levels of active caspase-3 and CD95 in NK cells. Both the quantity and “quality” of COVID-19-associated NK cells are also compromised. Single-cell transcriptomic analysis indicated an altered NK cell composition with increased levels of the “inflamed CD56dim” subset (selected marker genes: IFI6, ISG15, IFI44L, XAF1, MX1, PRF1, GZMB, and FCGR3A) and the “proliferating CD56dim” subset (selected marker genes: MKI67, LGALS1, STMN1, TUBA1B, and HMGB2) in patients with a severe disease. These changes in the NK cell composition might underlie the compromised effector functions of COVID-19-associated NK cells in response to K562 cell stimulation, as evidenced by decreased production of IFN-γ and TNF-α, which was more pronounced in individuals with a severe disease. More importantly, the ability of COVID-19-associated NK cells to reduce viral protein levels in SARS-CoV-2-infected cells was significantly lower than that of control NK cells, possibly due to decreased production of IFN-γ and TNF-α by COVID-19-associated NK cells. In addition, NK cells from patients with severe COVID-19 displayed a similar gene expression signature to pulmonary NK cells from patients with lung fibrosis (upregulation of AREG, DUSP2, ZFP36L2, and TSC22D3), which was accompanied by a compromised ability to suppress the expression of the profibrotic marker genes COL1A1 and ACTA2 in human lung fibroblasts. Taken together, COVID-19-associated NK cells exhibit impairments in both their anti-SARS-CoV-2 activity and antifibrotic activity.
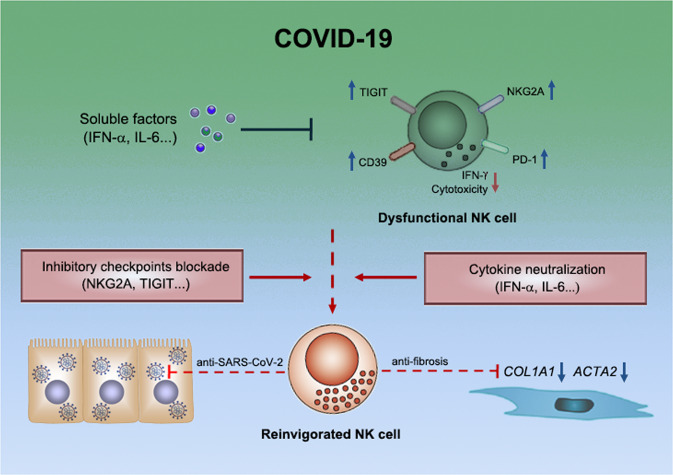
Fig. 1.
NK cell dysfunction in patients with COVID-19. NK cells normally possess antiviral and antifibrotic activities, which are impaired in patients with severe COVID-19, possibly by soluble factors such as IFN-α and/or by inhibitory checkpoint receptors such as NKG2A. Treatments targeting these factors/receptors might potentially reinvigorate NK cells for better control of the disease
Studies have indicated that a cytokine storm occurs in the pathogenesis of COVID-19 [7], which might result in the dysfunctional status of NK cells. According to Krämer et al., incubation of NK cells with plasma from patients with COVID-19, especially plasma from patients with severe disease, leads to impaired NK cell effector functions in response to K562 cell stimulation compared with an incubation with control plasma. These effects were observed using plasma from patients with severe COVID-19 as early as one week post-infection. Since cytokines are soluble factors in the plasma that most likely contribute to these effects, Krämer et al. examined cytokine levels in the plasma from patients with COVID-19 and showed that IFN-α, TNF-α, IFN-γ, IL-6, and IL-10 levels increased in the plasma during the early stage of severe COVID-19. While increased plasma levels of TNF-α, IFN-γ, IL-6, and IL-10 persisted in the second week after infection in patients with severe COVID-19, IFN-α levels decreased after the first week. Despite its temporal increase in early severe COVID-19, IFN-α is the only cytokine in the plasma at this early phase after infection that is significantly correlated with the severity of COVID-19. More importantly, the single-cell transcriptomic analysis indicated that the IFN-α response signature increased in patients from two cohorts who succumbed to SARS-CoV-2 infection. Krämer et al. found that IFN-α directly suppresses IFN-γ production by NK cells and that NK cells from patients with early severe COVID-19 were enriched in IFN-α response-related genes, suggesting that IFN-α derived from the plasma of patients with early severe COVID-19 might at least be one of the soluble factors that leads to the dysfunctional status described above. However, neutralization of single or multiple cytokines, including IFN-α, failed to significantly reverse NK cell dysfunction caused by an incubation with plasma from patients with severe COVID-19, possibly because the in vitro system might not fully recapitulate the physiological effects of cytokines on COVID-19-associated NK cells. On the other hand, data from Krämer et al. showed that plasma collected from patients with severe COVID-19 at 2 or 3 weeks post-infection, when IFN-α level gradually decreased to almost baseline, also significantly impaired IFN-γ and TNF-α production and degranulation by NK cells, indicating that factors other than IFN-α were present and suppressed NK cell function in the later phase. For example, the level of the proinflammatory cytokine IL-6 was increased in the plasma of patients with severe COVID-19 from the first week to the third week, which also suppresses IFN-γ production in NK cells in vitro and possibly affects the normal activity of NK cells in patients with severe disease.
In addition to cytokines, increasing evidence suggests that inhibitory checkpoint receptors on NK cells might contribute to the dysfunctional status of COVID-19-associated NK cells. For example, Demaria et al. detected higher levels of NKG2A, PD-1, and CD39 in COVID-19-associated NK cells from the peripheral blood and bronchoalveolar lavage fluid [8]. Blocking the interaction of NKG2A with its ligand with an anti-NKG2A mAb enhanced the cytotoxic activity of NK cells from patients with COVID-19 [8]. On the other hand, Krämer et al. detected increased TIGIT expression on NK cells in some patients with COVID-19. TIGIT+ NK cells produced more IFN-γ than TIGIT+ NK cells from patients with COVID-19. These studies indicated that the activity of NK cells in patients with COVID-19 is suppressed by inhibitory checkpoint receptors.
Research on NK cell dysfunction in tumors has significantly contributed to the development of NK-based tumor immunotherapy strategies. Targeting inhibitory checkpoint molecules in NK cells potently reverses the dysfunctional status of tumor-associated NK cells to better control tumors [6] in both preclinical studies and patients with tumors. Krämer et al. provided evidence that NK cells possess anti-SARS-CoV-2 and antifibrotic activities and that these activities are compromised in patients with COVID-19, suggesting that reversing the dysfunctional status of COVID-19-associated NK cells might facilitate viral control and tissue protection. Consistent with this finding, several ongoing clinical trials are testing the therapeutic benefit of NK cells against COVID-19 (ClinicalTrials.gov: NCT04797975, NCT04634370, and NCT04280224). Furthermore, Krämer et al. and other researchers also provided evidence that cytokines (such as IFN-α and IL-6) and inhibitory checkpoint receptors (such as NKG2A and TIGIT) might contribute to COVID-19-associated NK cell dysfunction, suggesting that these molecules potentially serve as targets to enhance the activity of NK cells in patients with this disease. However, very few reports regarding the application of NK-based immunotherapy in contexts other than in tumors are currently available, and thus the extent to which the current in vitro observations could be translated to the clinic is unclear. Therefore, more studies are required to investigate the therapeutic benefit of NK-based immunotherapy in patients with COVID-19-associated physiological conditions.
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFA0710802), the Natural Science Foundation of China (reference number 82071768), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDPB18).
Competing interests
The author declares no competing interests.
References
- 1.Krämer B, Knoll R, Bonaguro L, ToVinh M, Raabe J, Astaburuaga-García R, et al. Early IFN-alpha signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19. Immunity. 2021;54:2650–69. doi: 10.1016/j.immuni.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–52. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 3.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26:1070–6. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–5. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi J, Tian Z. NK cell dysfunction and checkpoint immunotherapy. Front Immunol. 2019;10:1999. doi: 10.3389/fimmu.2019.01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demaria O, Carvelli J, Batista L, Thibult ML, Morel A, André P, et al. Identification of druggable inhibitory immune checkpoints on Natural Killer cells in COVID-19. Cell Mol Immunol. 2020;17:995–7. doi: 10.1038/s41423-020-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]