Abstract
Accumulating evidence suggests that the influence on developmental traits might have long-term effects on aging and health later in life. Metformin is a widely used drug for treating type 2 diabetes and is also used for delaying sexual maturation in girls with precocious puberty. The current report focuses on investigating the effects of metformin on development and metabolic traits. Heterogeneous mice (UM-HET3) were treated with i.p. metformin between the ages of 15 and 56 days. Our results show that body weight and food consumption were increased in both sexes, and sexual maturation was delayed in females. Tail length and circulating insulin-like growth factor 1 (IGF1) levels were significantly increased in both sexes. No significant difference was found in insulin tolerance test, but glucose tolerance was significantly reduced in the males. Circulating adiponectin and insulin levels were altered by metformin treatment in a sex-specific manner. Analysis of quantitative insulin sensitivity check index (QUICKI) suggests that metformin treatment increased insulin sensitivity in female pups, but had opposite effect in male pups. This study revealed that early life metformin treatment alters development and metabolism of mice in both sex-specific and non-specific manners. These effects of metformin may have long-term impacts on aging-related traits.
Keywords: GnRH, GHRH, sex maturation, metabolism, body size
Introduction
Metformin, a biguanide, has been used for decades for the treatment of type 2 diabetes (T2D) and metabolic syndrome. It has an excellent safety record in both children and adults. Over the past decades, accumulating evidence suggests that metformin has profound beneficial effects on human healthspan, e.g. reducing the incidence of T2D [1], reducing the risks of cardiovascular diseases [2], decreasing overall cancer incidence and cancer mortality [3], and improving cognitive function of T2DM patients [4]. The underlying mechanisms have been investigated intensively. Metformin enters cells through the organic cation transporters [5] and inhibits the Complex I within the mitochondrion, reducing the efficiency of the electron transport chain (ETC), resulting in a reduction in ATP generation. Meantime, metformin also inhibits AMP deaminase and, therefore, increases the AMP level. The increased AMP: ATP ratio activates the AMP-activated protein kinase (AMPK), a serine/threonine kinase that has a wide range of intracellular effects, leading to the activation of catabolic pathways and inhibition of anabolic pathways. The AMPK pathway interacts with a variety of metabolic- and inflammation-related pathways, including mTORC1, PGC1-α, Insulin-IGF1, as well as SIRT1 and NF-κB [6]. The inhibition of mitochondrial ETC also leads to AMPK-independent effects by reducing reactive oxygen species (ROS) and advanced glycation end-products (AGEs), thereby reducing DNA and macromolecular damage [7]. Extracellularly, metformin modulates the gut microbiota, further improving metabolism and reducing inflammation [8]. These studies make metformin a promising geroprotective drug that may slow the aging process and reduce aging-related diseases. Currently, metformin is being tested in a clinical trial to examine its potential for extending human healthspan [9].
The effect of metformin on rodent longevity has been tested in several studies. However, the reported effects have not been consistent. A study by the NIA Interventions Testing Program (ITP), initiating metformin treatment in mice at nine months of age, showed no significant lifespan alteration in female mice at all three participating sites. In the male mice, two of the three sites found >10% increases of the median lifespan, one site found the median lifespan reduced by 1%, and the pooled data did not show significant difference (https://phenome.jax.org/itp/surv/Met/C2011). However, the same metformin dosage, starting at the age of 12 months, significantly and suggestively extended lifespan in C57BL/6 males and B6C3F1 males respectively [10]. These results suggest that the effect of metformin on lifespan may be dependent on genetic background and sex. Although its effect on lifespan of mice is still debatable, clinical usage has found that metformin can prevent precocious puberty in girls [10–12]. It has been reported that delayed pubertal onset is associated with reduced risks of adult obesity and cardiovascular disease in human [13–19]. Importantly, across mouse inbred strains and in the human population, delayed sexual maturation is associated with extended lifespan [20, 21]. These results inspired us to investigate the ability of metformin treatment, given in early life, to alter metabolism and development, and to lay groundwork for investigating its effects on health conditions in late life and on lifespan.
Materials and methods
Animals:
UM-HET3 mice were generated by breeding female CB6F1/J (#100007) with male C3D2F1/J (#100004) mice. Ten breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, Maine 04609). Ten litters, with a total of 60 female and 56 male pups, were randomly assigned to intraperitoneal injections (i.p.) metformin treatment or saline control groups. Sample sizes of each group can be found in Fig. 2. Mice were housed in environmentally controlled rooms maintained on a 12:12-h light: dark cycle at 22 ± 1°C with 35% to 50% relative humidity. Access to food (Cat. #: 5001, LabDiet) and sterilized water was ad libitum. Animal care and handling were conducted in accordance with NIH guidelines and the policies of the Laboratory Animal Care and Use Committee at Southern Illinois University School of Medicine, Springfield, IL.
Figure 2. Metformin delays onset of puberty of female pups.
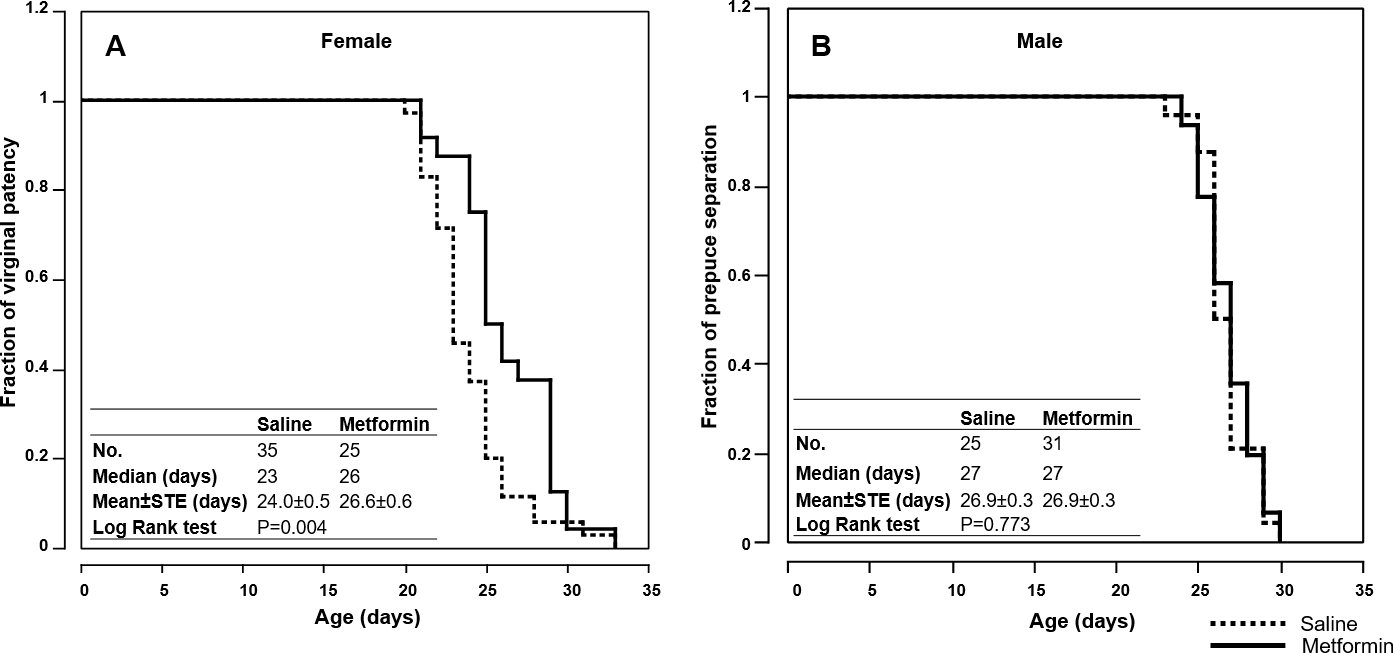
(A) Fraction of females with vaginal patency. (B) Fraction of males with prepuce separation. Embedded tables show sample size, medium and mean of ages at sexual maturation. The significance of the difference between the curves of i.p. metformin and saline groups was determined by log rank test.
The UM-HET3 is a heterogeneous population, which limits the occurrence of strain-specific effects, thus providing a far more realistic model of human populations. Moreover, although UM-HET3 mice are individually different, as a population, they share the same genetic pool, which makes the study results repeatable [22]. The UM-HET3 mice were first developed by the Geriatrics Center’s Core Facility for Aged Rodents program and have been widely used in NIA sponsored studies, such as the Interventions Testing Program (https://www.nia.nih.gov/research/dab/interventions-testing-program-itp).
Metformin treatment:
From day 15 to day 56, pups were given 200mg/kg metformin (Cat. #151691, MP Biomedicals, Ohio, 44139) or same volume of saline daily via i.p. injection. In humans, to prevent T2D, the dosage is 850mg/day, according to FDA guidelines (https://www.fda.gov/media/72309/). The equivalent dose in mice is approximately 170mg/kg/day, based on body surface area. The maximum safe dosage for treating T2D in human children is 2000mg/day, which equals 400mg/kg/day. Based on these data, we determined to use 200 mg/kg/day to treat the pre-mature mouse pups. According to previous published research in mice, 200mg/kg i.p. may yield a serum concentration between 120 uM ~ 160 uM [23, 24].
Although in most of the clinical studies and experiments using adult rodents, metformin was orally administered, we treated the pups via i.p. injection to ensure the accuracy of the dosage and minimize invasiveness. If metformin were mixed in the food, most of the metformin consumed by the pups would come from the dam’s milk. It would be impossible to ensure the pups received the correct dose of metformin. Variation in milk intake by the pups may further complicate the study, because developmental traits, such as body weight, could be affected by the volume of milk consumed. Gavage feeding could cause serious intra-esophageal irritation or injury to pups, because the upper gastrointestinal tract of a pre-weaned mouse pup is fragile [25]. Notably, metformin may exert its geroprotective effect by impacting the microbiome [26], which means there could be a difference in regulation by metformin depending on oral feeding or i.p. injection of the animals. This difference in administration of metformin needs to be considered when comparing the results of this report with other studies.
Development-related traits:
Body weight was determined every day. Food consumption was measured from day 22, on which pups were weaned. Tail length was measured at day 70. Female and male pups were examined daily for VP and PS respectively, starting at postnatal day 18 and ending at day 34, when VP or PS was observed in all mice.
GTT and ITT
At the age of eight weeks, GTT was performed after 16 hours fasting. ITT was performed one week later without fasting. Glucose (1g/kg) or insulin (1IU/kg, Sigma, St. Louis, MO, USA) was injected i.p. and blood glucose was determined from the tail vein at 0, 15, 30, 45, 60, 120 min using AgaMatrix Wavesense Presto Blood Glucose Monitoring System (AgaMatrix, New Hampshire, 03079). The GTT and ITT data are presented as a percentage of baseline glucose and area under the curve.
Hormone levels
At the age of 12 weeks, non-fasting and 16 hours fasting blood samples were drawn from the submandibular vein. Plasma IGF1, adiponectin, and insulin were determined by ELISA kits (Cat. #: 80574, 80569, 90080, Crystal Chem, Elk Grove Village, IL, 60007, USA). Final concentration was determined by measuring the absorbance at 450 nm subtracted from 630 nm. Fasting glucose was determined using AgaMatrix as described above. According to the fasting insulin and glucose concentration, the QUICKI was calculated using the following formula: QUICKI = 1/[log(fasting insulin in mU/L) + log(fasting glucose in mg/dL)].
Data analysis
The curves of the age of VP of females and PS of males were drawn by Kaplan Meier method and compared by using log rank test. The effects of metformin on body weight and food consumption were compared by pairwise t test. The significance of the differences in tail length, tail length ADM, GTT relative AUC, ITT relative AUC, and QUICKI were tested by two-way ANOVA. Glucose and hormone levels were examined by three-way ANOVA. The significance of the mean values between two groups was examined by the t test. All the statistical analyses were performed in in JMP (JMP 10.0, SAS Institute). A P value < 0.05 was considered as statistically significant.
Results
Metformin increases body weight and food consumption of female and male UM-HET3 pups.
In the current study, starting on day 15, pups were given i.p. injection of 200 mg/kg metformin or saline daily through day 56. Body weight was determined every day, and food consumption was measured from day 22, when the pups were weaned. After day 46, mice were subjected to metabolism-related studies, including GTT, ITT, and hormone assays (Fig. 1A). To determine if i.p. metformin treatment altered body weight, we calculated the average body weight of each group on every day, from day 15 to day 56. Comparing body weight between the i.p. metformin-treated and the saline groups, the pairwise t test showed the difference was significant (P=0.001 and <0.0001, for female and male respectively, Fig. 1B&C). At day 46, before the study of glucose homeostasis, i.p. metformin-treated females and males have 5.0% and 4.5% increases in body weight compared to the control mice (17.80±0.18 vs. 16.95±0.21, 22.65±0.33 vs. 21.67±0.27, female and male respectively; P<0.05 of both sexes, t test). Consistent with the increased body weight, i.p. metformin-treated pups consumed more food than control pups of the same sex. As shown in Fig. 1D&E, the food consumption curves of i.p. metformin-treated female and male pups were consistently above the curves of control pups. To test for statistical significance of the difference, we calculated the average food consumption of each group on every day, from day 22 to day 46. The pairwise t test showed the difference was significant (P=0.001 and <0.0001, female and male respectively). It should be noted that another group of UM-HET3 mice that are under the same housing conditions, provided with same food, but without any treatment, had no significant difference in body weight, compared with the control group of this study (data not shown).
Figure 1. Metformin increases body weight and food consumption of female and male pups.
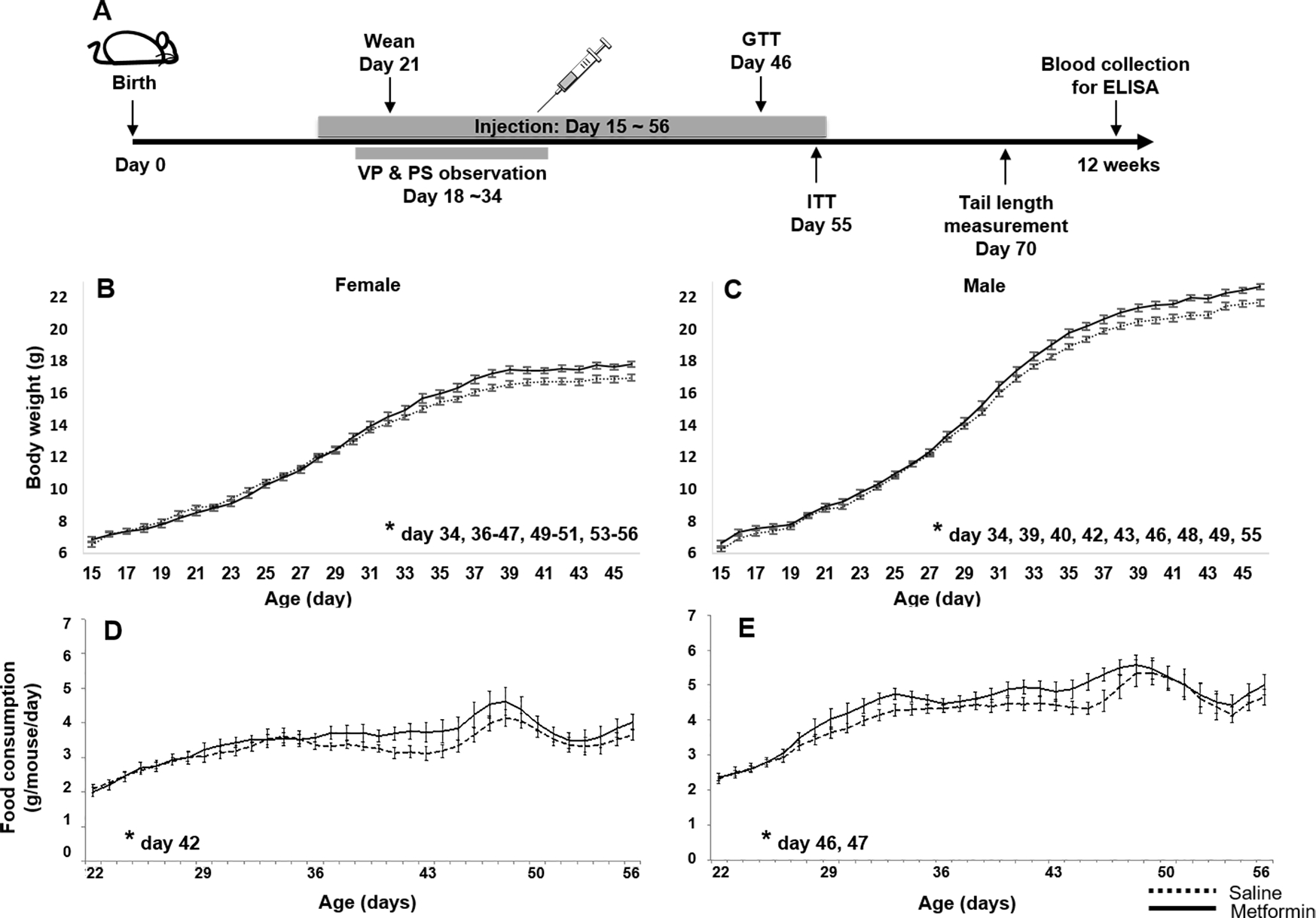
(A) Experiment scheme. VP: vaginal patency; PS: prepuce separation. (B) Female and (C) Male body weight. (D) Female and (E) male food consumption (three days rolling average). n>=25, * p<0.05, t test between i.p. metformin-treated and control group on specific ages.
Metformin delays onset of puberty of female pups.
Sexual maturation is a milestone of development in young animals. To evaluate the effect of i.p. metformin on sexual maturation, we monitored vaginal patency (VP) of female mice and prepuce separation (PS, also called balano-preputial separation) in male mice. As shown in Fig. 2A, the median age of VP was delayed 13% in the i.p. metformin-treated females compared to the controls (26 vs. 23 days). Log rank test shows the delay is significant (P=0.004). However, no significant difference in PS was found in males (Fig. 2B).
Metformin increases tail length and reduces the variation of tail length.
To determine whether changes in body weight were accompanied by changes in skeletal growth, we measured the tail length. Interestingly, ANOVA showed that sex and i.p. metformin treatment had significant effect on tail length at day 70 in both sexes (P<0.001), corresponding with the gain of body weight. Further analysis found that in both sexes, i.p. metformin treatment significantly increases the tail length (P<0.05, t test; Fig. 3A, S. Table 1). Surprisingly, despite the increase of tail length, the variation of tail length, referred as absolute difference to median (ADM=|individual – median|/median), decreased in the i.p. metformin-treated mice (Fig. 3B). ANOVA shows the difference in the variation of tail length between treatment and control groups is significant (P=0.003, S. Table 1). t test shows that in females the difference is significant (P=0.019). In males, the difference is suggestive (P=0.081).
Figure 3. Metformin increases tail length and non-fasting IGF1 but reduces the variations.
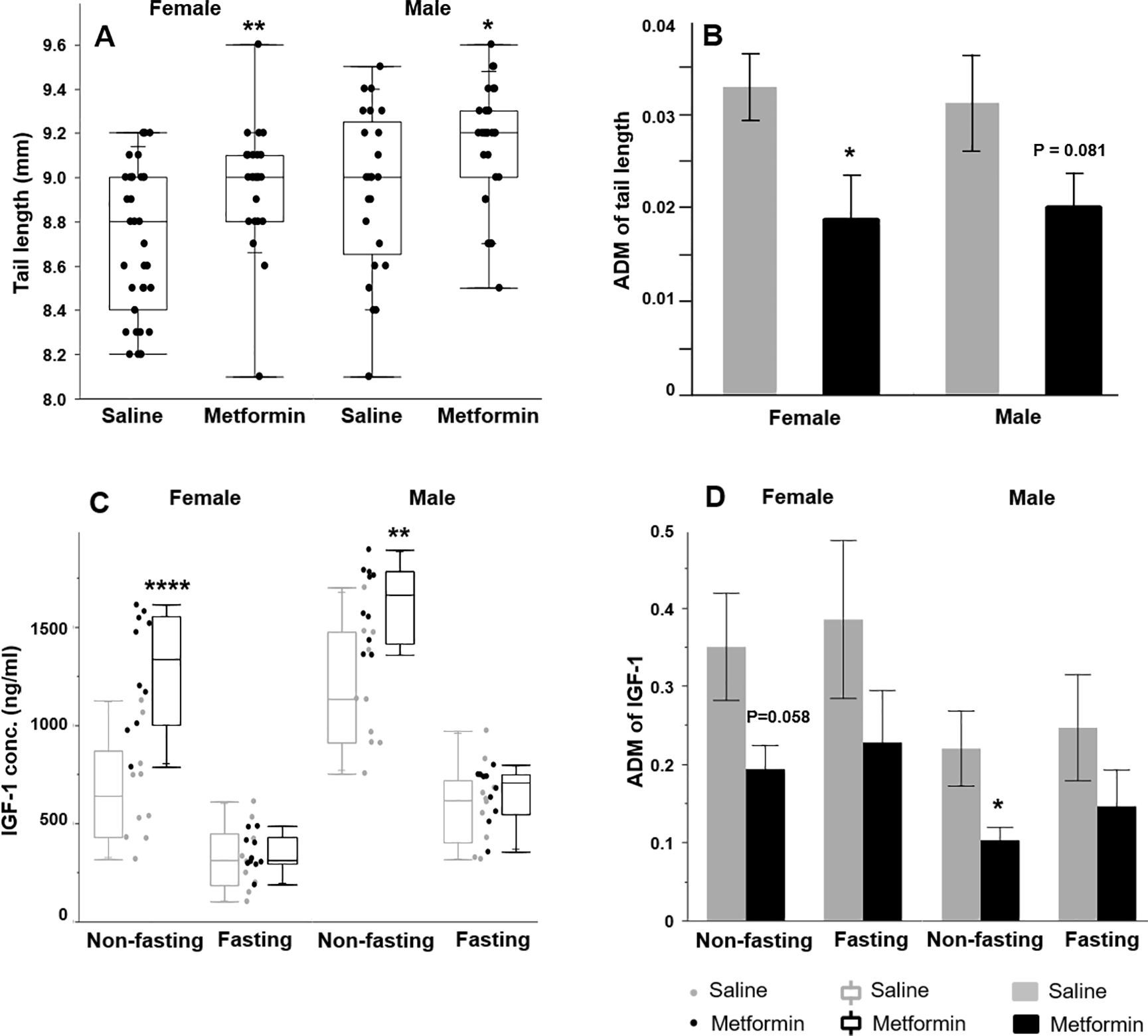
(A) Female and male tail length and (B) tail length variation (n>=25). (C) Circulating IGF1 concentration and (D) variation in non-fasting and fasting pups (n>=9). ADM: |individual data – median |/median. * p<0.05, ** p<0.01, *** p<0.0001, t test between i.p. metformin-treated and control group of the same sex. In the box plots (A & C), the bottom and top lines of the box represent the minimum and maximum data. The box represents two inner quartiles where 50% of the data resides, and it ranges from the first quartile to the third quartile. The horizontal line in the box represents the median of the data.
Table 1.
ANOVA of developmental and metabòlic traits.
| Source | Nparm | DF | Sum of Squares | F ratio | Prob > F | |
|---|---|---|---|---|---|---|
|
| ||||||
| Age | 1 | 1 | 3862.45 | 1335.83 | <0.0001 | |
| Body weight | Sex | 1 | 1 | 349.98 | 121.04 | <0.0001 |
| Treatment | 1 | 1 | 11.41 | 3.94 | 0.049 | |
| Treatment*Sex | 1 | 1 | 0.58 | 0.20 | 0.654 | |
| Age | 1 | 1 | 45.75 | 178.03 | <0.0001 | |
| Sex | 1 | 1 | 26.86 | 104.50 | <0.0001 | |
| Food consumption | Treatment | 1 | 1 | 2.32 | 9.04 | 0.003 |
| Treatment*Sex | 1 | 1 | 0.01 | 0.05 | 0.818 | |
| Sex | 1 | 1 | 1.27 | 13.44 | <0.001 | |
| Tail length (70 days) | Treatment | 1 | 1 | 1.47 | 15.54 | <0.001 |
| Treatment*Sex | 1 | 1 | 0.03 | 0.36 | 0.551 | |
| Sex | 1 | 1 | 0.00 | 0.00 | 0.966 | |
| Tail length ADM | Treatment | 1 | 1 | 0.00 | 9.17 | 0.003 |
| Treatment*Sex | 1 | 1 | 0.00 | 0.13 | 0.720 | |
| Sex | 1 | 1 | 2552979.97 | 51.70 | <0.0001 | |
| Treatment | 1 | 1 | 1600761.36 | 32.42 | <0.0001 | |
| Fasting | 1 | 1 | 10131049.51 | 205.18 | <0.0001 | |
| IGF1 conc. (ng/mI) | Treatment*Sex | 1 | 1 | 27795.97 | 0.56 | 0.456 |
| Fasting*Sex | 1 | 1 | 91706.42 | 1.86 | 0.177 | |
| Fasting*Treatment | 1 | 1 | 1192233.28 | 24.15 | <0.0001 | |
| Fasting*Treatment*Sex | 1 | 1 | 48797.32 | 0.99 | 0.324 | |
| Sex | 1 | 1 | 0.24 | 6.58 | 0.012 | |
| Treatment | 1 | 1 | 0.35 | 9.56 | 0.003 | |
| Fasting | 1 | 1 | 0.02 | 0.65 | 0.422 | |
| IGF1 variation | Treatment*Sex | 1 | 1 | 0.01 | 0.31 | 0.579 |
| Fasting*Sex | 1 | 1 | 0.00 | 0.00 | 0.997 | |
| Fasting*Treatment | 1 | 1 | 0.00 | 0.01 | 0.928 | |
| Fasting*Treatment*Sex | 1 | 1 | 0.00 | 0.01 | 0.920 | |
| Sex | 1 | 1 | 11316.80 | 11.45 | 0.001 | |
| GTT basal glucose | Treatment | 1 | 1 | 19211.00 | 19.43 | <0.0001 |
| Treatment*Sex | 1 | 1 | 4210.76 | 4.26 | 0.041 | |
| Sex | 1 | 1 | 64316.63 | 42.11 | <.0001 | |
| GTT AUC | Treatment | 1 | 1 | 17732.97 | 11.61 | <.0001 |
| Treatment*Sex | 1 | 1 | 2859.27 | 1.87 | 0.174 | |
| Sex | 1 | 1 | 33028.35 | 60.06 | <0.0001 | |
| ITT basal glucose | Treatment | 1 | 1 | 797.71 | 1.45 | 0.231 |
| Treatment*Sex | 1 | 1 | 0.04 | 0.00 | 0.993 | |
| Sex | 1 | 1 | 112062.23 | 112.27 | <.0001 | |
| ITT AUC | Treatment | 1 | 1 | 570.57 | 0.57 | 0.451 |
| Treatment*Sex | 1 | 1 | 4.90 | 0.00 | 0.944 | |
| Sex | 1 | 1 | 0.69 | 20.31 | <0001 | |
| Treatment | 1 | 1 | 0.04 | 1.30 | 0.259 | |
| Fasting | 1 | 1 | 0.71 | 20.63 | <0001 | |
| Insulin level | Treatment*Sex | 1 | 1 | 0.17 | 4.92 | 0.029 |
| Fasting*Sex | 1 | 1 | 0.36 | 10.46 | 0.002 | |
| Fasting*Treatment | 1 | 1 | 0.01 | 0.44 | 0.511 | |
| Fasting*Treatment*Sex | 1 | 1 | 0.06 | 1.74 | 0.191 | |
| Sex | 1 | 1 | 0.01 | 10.95 | 0.002 | |
| QUICKI | Treatment | 1 | 1 | 0.00 | 0.05 | 0.825 |
| Treatment*Sex | 1 | 1 | 0.01 | 10.01 | 0.003 | |
| Sex | 1 | 1 | 4.87 | 0.98 | 0.326 | |
| Treatment | 1 | 1 | 4.74 | 0.95 | 0.333 | |
| Fasting | 1 | 1 | 338.87 | 68.00 | <0.0001 | |
| Adiponectin level | Treatment*Sex | 1 | 1 | 60.92 | 12.22 | 0.001 |
| Fasting*Sex | 1 | 1 | 0.16 | 0.03 | 0.856 | |
| Fasting*Treatment | 1 | 1 | 1.31 | 0.26 | 0.610 | |
| Fasting*Treatment*Sex | 1 | 1 | 31.32 | 6.28 | 0.014 | |
Metformin increases the levels and reduces the variation of circulating IGF1 in non-fasting pups.
IGF1 is a key regulator of sexual maturation and body size [27, 28]. Because of the significant alterations in sexual maturation, body weight, and tail length by i.p. metformin treatment, we measured circulating IGF1 levels (at the age of 82 days). ANOVA showed that sex, metformin treatment, and fasting, as well as the interaction between fasting and metformin treatment, had significant effects on IGF1 levels (P<0.0001, S. Table 1). Further comparison between both sexes of the i.p. metformin and saline groups, with or without fasting, showed no significant difference in IGF1 levels in the fasting mice. However, in the non-fasting mice, i.p. metformin significantly increased IGF1 levels in both sexes (P<0.05, t test; Fig. 3C). Correlation analysis found that there is a significant correlation between non-fasting IGF1 and tail length (Pearson correlation test, R2 = 0.41, P<0.0001). Mice with higher IGF1 have longer tails. No significant correlation between tail length and the fasting IGF1 level was found. Similar to the variation of tail length, IGF1 ADM was also reduced in i.p. metformin-treated mice (Fig. 3D, S. Table 1, ANOVA, P=0.003). While values of the standard deviations or standard errors of the mean are routinely reported, the distribution of individual data is rarely analyzed or discussed. In fact, in aging research, the variations of aging related parameters including IGF1 level and lifespan in a population are related to the resilience and robustness of resisting the impacts of aging [29, 30]. Because of the important role of IGF1 in regulating aging [31] and the close relationship of body size with longevity [32], it will be interesting to test whether the variation of lifespan can be also reduced by the early-lifespan treatment of i.p. metformin, which reduced the variations in tail length and non-fasting IGF1 level.
Metformin effects on glucose and insulin tolerance.
In the clinical setting, metformin is widely used to treat type 2 diabetes, primarily by improving insulin sensitivity. In this study, after 32 days of treatment, we examined the effects of i.p. metformin on glucose (day 47 to day 49) and insulin (day 54 to day 56) tolerance in mice. In the GTT assay, the basal level of glucose, measured after 16 hours of fasting (6:00pm – 10:00am), was elevated in both sexes of i.p. metformin-treated mice (P=0.065 and <0.001, t test, female and male respectively, Fig. 4A, B). At 120 minutes after glucose injection, the glucose level was significantly higher in the i.p. metformin treated females than that of the control females (t test, P<0.05, Fig. 4A). ANOVA showed that sex and the treatment significantly altered the area under curve (AUC, P<0.001, S. Table 1). Further analysis found that AUC of GTT was significantly greater in i.p. metformin-treated males than that of control males, indicating impaired glucose tolerance. In females, no significant difference in AUC of GTT was found (Fig. 4C). In the ITT, no significant difference was found between i.p. metformin-treated and control groups of both sexes in the levels of glucose or AUC (Fig. 4D–F, S. table 1).
Figure 4: Metformin improves glucose tolerance but not insulin tolerance.
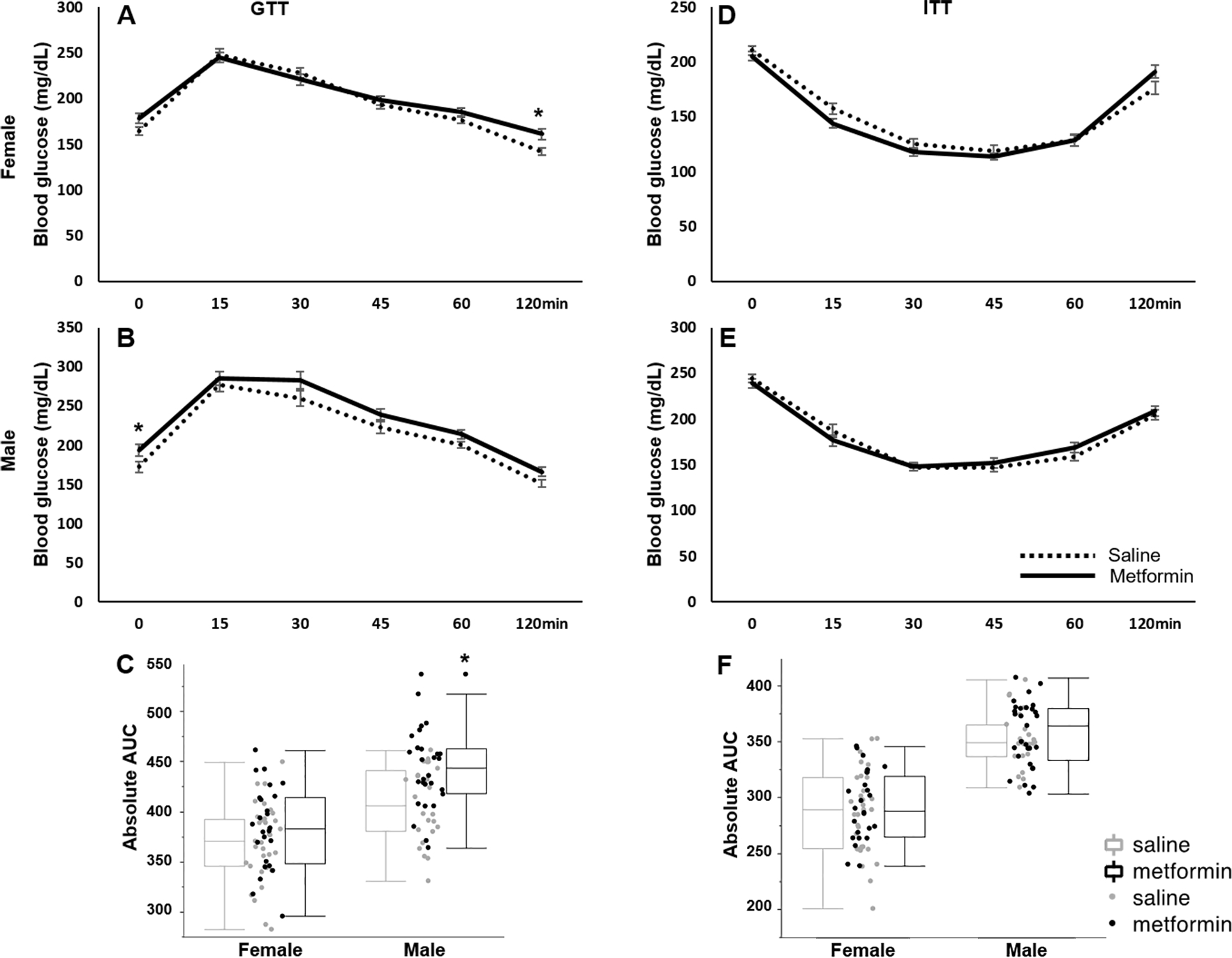
(A) Female and (B) male GTT test. (C) AUC of GTT in female and male. (D) Female and (E) male ITT. (F) AUC of ITT in female and male. n>=25. AUC: area under curve. * p<0.05, t test between i.p. metformin-treated and control group of the same sex.
Metformin alters adiponectin levels, insulin levels, and insulin sensitivity in a sex-specific manner.
ANOVA indicated that fasting increased adiponectin significantly (P<0.0001, S. Table 1). In females, both fasting and non-fasting adiponectin levels were significantly increased in the i.p. metformin-treated group compared to the control group (P<0.05, t test, Fig. 5A). Surprisingly, in males, adiponectin remained unchanged in non-fasting status, while the i.p. metformin-treated group had suggestively reduced fasting adiponectin (P=0.095, Fig. 5A). ANOVA revealed a significant interaction between treatment and sex (p=0.001), indicating that metformin has a sex-specific effect on adiponectin levels. The difference found between sexes in adiponectin may suggest the existence of sex differences in adiposity; however it needs further investigation.
Figure 5: Metformin differently alters adiponectin, insulin levels and quantitative insulin check index (QUICKI) in female and male pups.
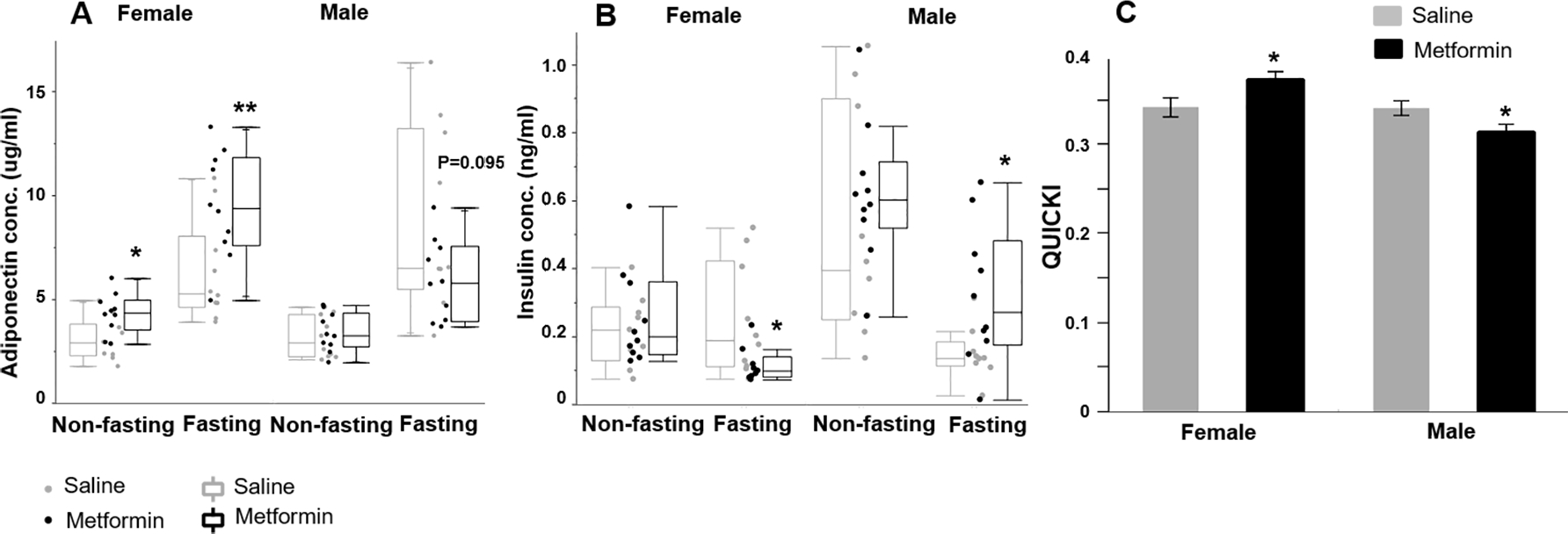
(A) Circulating adiponectin and (B) Insulin level under non-fasting and fasting conditions. (C) QUICKI in female and male. n>=9. *p<0.05, **p<0.01, t test between metformin-treated and control group of the same sex.
The sex-specific effect of metformin was also found in the circulating insulin levels. Under fasting and non-fasting conditions, i.p. metformin-treated females had lower levels of insulin than the control females. Under fasting conditions, the difference was significant (P=0.038, t test). Unexpectedly, the opposite was found in males. Under fasting conditions, i.p. metformin-treated males had significantly higher insulin levels (P=0.031, t test, Fig. 5B). ANOVA indicated the interactive effect of sex and metformin treatment on insulin is significant (P=0.026, S. Table 1).
Based on fasting glucose and insulin levels, we calculated insulin sensitivity index by using QUICKI. At the adolescent stage, QUICKI- and clamp-measured insulin sensitivity are effective in humans and are highly correlated to most of the anthropometric and biochemical indices [33]. In neonatal mice, previous studies [34] showed serum glucose correlated positively with insulin levels and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), and negatively with Homeostatic Model Assessment for Insulin Sensitivity (HOMA-S) and QUICKI. These results indicate that QUICKI and similar parameters can be effective markers for measuring insulin sensitivity at various ages. As shown in Fig. 5C, i.p. metformin-treated females exhibited significantly higher QUICKI, and i.p. metformin-treated males showed the opposite result (P=0.041 and 0.030, female and male respectively, t test). Consistent with circulating insulin levels, ANOVA showed that the interaction between treatment and sex significantly influenced insulin sensitivity, indicating that metformin has sex-specific effects on insulin levels and insulin sensitivity (P=0.003, S. Table 1).
Discussion
Metformin treatment at an early age may regulate body weight and food consumption differently from the treatment in adults.
In most studies, including human and rodents, metformin treatment reduces body weight [35–37], consistent with its major biological function, the activation of the catabolic pathway and inhibition of the anabolic pathway. It has been reported that more than 60% of metformin’s anti-diabetic effect is attributable to its ability of lowering body weight in a sustained manner [38]. A recent study showed that in wild-type mice, oral metformin increased the circulating level of the peptide hormone growth/differentiation factor 15 (GDF15), with GDF15 expression increasing predominantly in the distal intestine and the kidney. Suppressing GDF15 or its receptor GDNF family receptor α-like (GFRAL) diminished the beneficial effects on body weight in mice fed with high fat diet [38]. The reduction of body weight by metformin may also be related to its appetite suppressant action, which is positively associated with the dosage [39]. A previous study found that metformin can cross the blood-brain-barrier and may exert its anorexic effect by the inhibition of NPY and AgRP gene expression through the STAT3 signaling pathway in the hypothalamus [40].
Most of the subjects in the aforementioned research are adults. When focusing on prepubescent subjects, the effect of metformin on body weight and food intake becomes vague. Treating neonatal 129/Sv pups with metformin at ages of 3, 5, and, 7 days, Anisimov VN et al. reported that males consumed less food and their body weight was decreased as compared with control mice over their entire lifespan. However, females consumed more food and were heavier than controls [41]. A short-term treatment with metformin in C57BL/6 pups from age of day 7 to day 14 did not change the body weight significantly [42]. In humans, a meta-analysis of 28 studies (n=3,976) of patients with gestational diabetes mellitus found that following intrauterine exposure to metformin treatment, their neonates were significantly smaller than neonates whose mothers were treated with insulin during pregnancy. However, metformin-exposed children appear to experience accelerated postnatal growth, resulting in heavier infants and higher BMI by mid-childhood (5–9 years old), compared to children whose mothers were treated with insulin [43]. In our study, we continuously treated UM-HET3 pups via i.p. injection for 42 days, day 15 through day 56, and found consistently increased food consumption and body weight in both sexes. Further study of the orexigenic genes, NPY and AgRP, expressed in the hypothalamus, and the circulating peptide hormone GDF15 and its expression, may reveal the mechanism underlying the increased food intake and body weight observed in this study.
Metformin treatment at an early age may regulate metabolism sex-specifically.
Surprisingly, our study showed that the fasting glucose levels were increased in the i.p. metformin-treated mice, and the glucose tolerance and insulin sensitivity were impaired in the i.p. metformin-treated males assessed by the GTT and QUICKI analyses respectively. However, this is consistent with previous studies suggesting that due to rapid growth and high growth hormone levels, juvenile mice may show insulin resistance traits that antagonize metformin action [44, 45]. The underlying mechanism may be also related to the major molecular function, the activation AMPK, which plays an important role in maintaining glucose homeostasis [46]. It was reported that the AMPK activity is essential for increasing the glucose level induced by exercise via the upregulation of glycogenolytic flux [46]. Therefore, it is reasonable to hypothesize that fasting may increase the activity of foraging, leading to the increase of glycogenolytic flux, which is higher in the metformin-treated animals because of the upregulated AMPK activity.
Interestingly, the differences in insulin sensitivity between sexes correlate with the difference in the levels of adiponectin. Metformin significantly increased the levels of adiponectin in females under non-fasting and fasting conditions. However, in males, metformin did not alter the level of adiponectin under the non-fasting condition, but significantly reduced the level under the fasting condition. Adiponectin is a proven insulin sensitizer [47], and these results emphasize the importance of adiponectin in glucose homeostasis during development.
Metformin treatment at an early age delays female sexual maturation and may regulate longevity.
A major finding in this study is that i.p. metformin treatment could significantly delay the age of VP, a biomarker of female sexual maturation. This result is consistent with the clinical report that metformin treatment delays the rapid progression of puberty and postpones the onset of menarche in girls with precocious puberty [12]. Sexual maturation is under control of the hypothalamus-pituitary-gonadal (HPG) axis, which can be modulated by metformin [48]. In primary rat pituitary cells, metformin reduced luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion induced by gonadotropin-releasing hormone (GnRH) [49]. Furthermore, metformin may suppress the activity of aromatase, leading to the reduced conversion of androgens to estrogens [50]. Identifying the mechanisms of delayed age of VP in female pups in this study may provide a model for further investigating and developing novel therapeutic methods for treating precocious puberty.
Accumulating evidence suggests that female reproduction and aging might be co-regulated [20, 27, 51, 52]. Indeed, interventions before and after puberty may have different effects on aging and longevity. For example, it has been reported that treating adult dwarf mice, which have impaired somatotrope and gonadotrope axes, with growth hormone (GH) increased body size and fertility, but did not diminish lifespan or lower the resistance of dwarf mice to cataracts and kidney disease. However, treating pups of dwarf mice with GH before the onset of puberty significantly reduced their longevity [53–55]. These studies indicate that early-life interventions can impact aging.
A potential mechanism of the co-regulation of reproduction and aging by metformin may be related to its molecular mechanism of upregulating AMPK activity [56]. Mammalian reproduction is an energy-consuming process that occurs when there is adequate nutrition [57]. AMPK is a sensor of nutrient status and it is activated by the decrease of ATP/AMP ratio or starvation. Activated AMPK acts to switch off ATP-consuming pathways, such as protein synthesis, lipogenesis, and gluconeogenesis, and turn on ATP-generating pathways such as fatty acid oxidation, glycolysis, and autophagy [58]. On the molecular level, activated AMPK inhibits mammalian target of rapamycin (mTOR) by directly phosphorylating the tumor suppressor tuberous sclerosis complex 2 (TSC2) and regulatory-associated protein of mTOR (RAPTOR) [59]. Elevating mTOR signaling can significantly accelerate female sexual maturation and enhance female reproduction. Suppressing mTOR has been shown to suppress female reproduction, but improve successful aging and extend healthspan [60]. Therefore, the interaction of metformin with mTOR signaling might constitute the molecular mechanism of co-regulating sexual maturation and longevity.
Delayed female sexual maturation not only associates with extended longevity, it may also be related to the sex disparity in longevity. Comparisons of the lifespans across mouse inbred strains revealed that the disparity between female and male animals from the same strain may be related to strain-specific age of female sexual maturation. An accelerated age of vaginal patency is associated with greater sexual disparity in lifespan. Importantly, gene mutations that alter the age of VP also alter the difference in lifespan between sexes [61]. Therefore, it will be of great interest to test if early-life treatment with metformin, which alters sexual maturation, would also exert long-term effects on lifespan and the sex inequality in lifespan.
Notably, puberty onset, measured by VO and PS, was delayed only in females, with no significant change in males. Similar results also have been reported in the diet restriction study, which significantly extended longevity in both sexes, but sexual maturation was delayed only in female animals [62–64]. These results indicate that during development, the regulatory mechanisms of the gonadotropic axis may be different between the sexes. Further studies designed to test the effects of metformin and diet restriction during development on the gonadotropic axis will be necessary to reveal the underlying mechanisms.
Metformin treatment at an early age upregulates the IGF1 and may regulate the traits of lifespan.
IGF1, an effector of the hypothalamus-pituitary-somatotropic (HPS) axis, plays important roles in regulating sexual maturation, body growth and body size, as well as aging and lifespan. Clinical data suggests that the effects of metformin treatment on IGF1 are altered by multiple factors, including age, duration, and dosage of treatment [65]. In the current study, we found that non-fasting IGF1 levels were significantly elevated by i.p. metformin treatment in both sexes. Interestingly, a previous study in patients with PCOS showed that metformin treatment significantly increased the responsiveness of GH to growth hormone-releasing hormone (GHRH) [66]. The underlying mechanism may be related to the regulatory effects of metformin on activities of CREB (cAMP-response element binding protein) and CREB binding protein (CBP) [67, 68]. These studies suggest that metformin may upregulate IGF1 levels by increasing GH secretion from the pituitary under non-fasting conditions, although the mechanism of increased responsiveness of IGF1 to GH in the liver cannot be excluded.
Many studies have reported that reducing GH/IGF1 signaling correlates with extended longevity in a variety of species [69–72]. However, IGF1 at old age has many positive effects, including protecting neurons from degenerative diseases and increasing insulin sensitivity; therefore, it has been suggested that increased IGF1 at old age may extend lifespan [31, 73, 74]. Importantly, non-fasting IGF1 levels at young age (6 months) associated with median lifespan in a sex non-specific manner [20], but significantly associated with the variation of lifespan in a sex-specific manner [61]. In female mice, strains with higher IGF1 levels have reduced variation in lifespan. In males the opposite is true, higher IGF1 is associated with increased variation. Further analyses found that the reduced lifespan variation in female mice with higher IGF1 levels may be due to the significantly reduced risk of early death (death before 180 days) and also reduced maximum lifespan. Surprisingly, the increased variation of lifespan in male mice with higher IGF1 levels may be due to the extended maximum lifespan [61]. Previous studies have shown that metformin has a clear sex-specific effect of extending longevity in C57BL/6J and 129/Sv males [10, 41]. A similar result was also found in an ITP study using UM-HET3 mice (https://phenome.jax.org/itp/surv/Met/C2011). It will be particularly interesting to investigate whether the sex-specific extension in lifespan observed in the metformin-treated male mice is due to increased IGF1 levels, and whether the early-life treatment of metformin will have sex-specific effects on lifespan traits, such as risk of early death and variation of lifespan.
Metformin treatment at an early age may decouple the connection between gonadotropic and somatotropic axes.
An interesting finding in this study is that metformin treatment not only delayed age of female sexual maturation, but also significantly increased IGF1, body weight, and tail length. This is consistent with previous studies in girls with precocious puberty, in which metformin treatment significantly delayed the onset of puberty, but augmented postmenarcheal height [75]. These results suggest that metformin treatment may suppress the activity of the HPG axis, but upregulate the activity of the HPS axis. This is contradictory to previous findings in calorie restricted and GH/IGF1-inhibited animals in which delayed sexual maturation was associated with reduced IGF1 and body weight [69]. Indeed, the HPG and HPS axes are co-regulated tightly, and there are complicated interactions between them. For example, signaling of the somatotropic hormones, GH and IGF1, can significantly regulate the development of the mammary gland, ovary, and uterus, not only through endocrine effects, but also via paracrine effects [76–80]. On the other hand, the hormones of the gonadotropic axis, including estradiol and testosterone, have a significant impact on the function of liver, the major producer of circulating IGF1 [81, 82]. In aging research, the parallel regulation of gonadotropic and somatotropic axes has been exemplified by many genetically modified animal models, e.g. dwarf mice, in which pituitary development is suppressed by genetic mutations [83], and our previously reported nuclear receptor interacting protein 1 knockout model [27, 84], as well as dietary restriction in a variety of species [52, 85, 86]. In these models, longevity extension is accompanied with reduced fecundity and body size, suggesting the suppressed activities of both gonadotropic and somatotropic axes. As mentioned earlier, the delayed female sexual maturation found in animal and clinical studies might be related to the effects of metformin on the response of gonadotrophs to GnRH [49], while the elevated IGF1 levels and increased body size might be related to the effects of metformin on the response of somatotrophs to GHRH [87]. Taken together, the specific effects of metformin on body growth and sexual maturation suggest that metformin regulates gonadotrophins and somatotrophins separately.
Conclusion:
In this study, i.p. metformin treatment at an early age significantly increased body size in both sexes and delayed sexual maturation in female mice. Some of the short-term effects on metabolic traits, including the circulating adiponectin, insulin, and IGF1 levels, as well as the insulin sensitivity measured by QUICKI, by i.p. metformin treatment were sex-specific, while others were not. The long-term effects of metformin treatment at an early age on metabolism and aging need to be further investigated.
Highlights:
Post-natal development affects health condition in adults.
Metformin is a drug for type 2 diabetes, also delaying sexual maturation in girls.
Metformin alters early life metabolisms in sex-specific and non-specific manners.
Metformin regulates development traits in sex-specific and non-specific manners.
Acknowledgement:
Lisa Hensley kindly edited the manuscript. Division of Laboratory Animal Medicine of Southern Illinois University School of Medicine provides excellent environment for animal research. This study is supported by William E. McElroy Charitable Foundation, NIA R21AG062985, American Diabetes Association 1–19-IBS-126, to AB, and SIUSOM RSG to RY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diabetes Prevention Program Research G, HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: a randomized clinical trial. Diabetes Care, 2015. 38(1): p. 51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg R, et al. , Lifestyle and metformin treatment favorably influence lipoprotein subfraction distribution in the Diabetes Prevention Program. J Clin Endocrinol Metab, 2013. 98(10): p. 3989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandini S, et al. , Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila), 2014. 7(9): p. 867–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng C, et al. , Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol A Biol Sci Med Sci, 2014. 69(10): p. 1299–305. [DOI] [PubMed] [Google Scholar]
- 5.Markowicz-Piasecka M, et al. , Is Metformin a Perfect Drug? Updates in Pharmacokinetics and Pharmacodynamics. Curr Pharm Des, 2017. 23(17): p. 2532–2550. [DOI] [PubMed] [Google Scholar]
- 6.Foretz M, Guigas B, and Viollet B, Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol, 2019. 15(10): p. 569–589. [DOI] [PubMed] [Google Scholar]
- 7.Phimphilai M, Pothacharoen P, and Kongtawelert P, Age-Influenced Receptors of Advanced Glycation End Product Overexpression Associated With Osteogenic Differentiation Impairment in Patients With Type 2 Diabetes. Front Endocrinol (Lausanne), 2021. 12: p. 726182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallianou NG, Stratigou T, and Tsagarakis S, Metformin and gut microbiota: their interactions and their impact on diabetes. Hormones (Athens), 2019. 18(2): p. 141–144. [DOI] [PubMed] [Google Scholar]
- 9.Barzilai N, et al. , Metformin as a Tool to Target Aging. Cell Metab, 2016. 23(6): p. 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Montalvo A, et al. , Metformin improves healthspan and lifespan in mice. Nat Commun, 2013. 4: p. 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dilman VM, Ageing, metabolic immunodepression and carcinogenesis. Mech Ageing Dev, 1978. 8(3): p. 153–73. [DOI] [PubMed] [Google Scholar]
- 12.Ibanez L, et al. , Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab, 2006. 91(8): p. 2888–91. [DOI] [PubMed] [Google Scholar]
- 13.Zheng T, et al. , Effects of Environmental Exposures on Fetal and Childhood Growth Trajectories. Ann Glob Health, 2016. 82(1): p. 41–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langley-Evans SC, Nutrition in early life and the programming of adult disease: a review. J Hum Nutr Diet, 2015. 28 Suppl 1: p. 1–14. [DOI] [PubMed] [Google Scholar]
- 15.McMullen S and Mostyn A, Animal models for the study of the developmental origins of health and disease. Proc Nutr Soc, 2009. 68(3): p. 306–20. [DOI] [PubMed] [Google Scholar]
- 16.Dekker MC, et al. , Developmental trajectories of depressive symptoms from early childhood to late adolescence: gender differences and adult outcome. J Child Psychol Psychiatry, 2007. 48(7): p. 657–66. [DOI] [PubMed] [Google Scholar]
- 17.Monaghan P, Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc Lond B Biol Sci, 2008. 363(1497): p. 1635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Power C, Lake JK, and Cole TJ, Measurement and long-term health risks of child and adolescent fatness. Int J Obes Relat Metab Disord, 1997. 21(7): p. 507–26. [DOI] [PubMed] [Google Scholar]
- 19.Widen E, et al. , Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care, 2012. 35(4): p. 850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan R, et al. , Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell, 2009. 8(3): p. 277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shadyab AH, et al. , Ages at menarche and menopause and reproductive lifespan as predictors of exceptional longevity in women: the Women’s Health Initiative. Menopause, 2017. 24(1): p. 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller RA and Chrisp C, Lifelong treatment with oral DHEA sulfate does not preserve immune function, prevent disease, or improve survival in genetically heterogeneous mice. J Am Geriatr Soc, 1999. 47(8): p. 960–6. [DOI] [PubMed] [Google Scholar]
- 23.LaMoia TE and Shulman GI, Cellular and Molecular Mechanisms of Metformin Action. Endocr Rev, 2021. 42(1): p. 77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iversen AB, et al. , Results from C-11-metformin-PET scans, tissue analysis and cellular drug-sensitivity assays questions the view that biguanides affects tumor respiration directly. Scientific Reports, 2017. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis F, et al. , Probiotic Studies in Neonatal Mice Using Gavage. Jove-Journal of Visualized Experiments, 2019(143). [DOI] [PubMed] [Google Scholar]
- 26.Induri SNR, et al. , The Gut Microbiome, Metformin, and Aging. Annu Rev Pharmacol Toxicol, 2021. [DOI] [PubMed] [Google Scholar]
- 27.Yuan R, et al. , Genetic coregulation of age of female sexual maturation and lifespan through circulating IGF1 among inbred mouse strains. Proc Natl Acad Sci U S A, 2012. 109(21): p. 8224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauras N, et al. , Sex steroids, growth hormone, insulin-like growth factor-1: neuroendocrine and metabolic regulation in puberty. Horm Res, 1996. 45(1–2): p. 74–80. [DOI] [PubMed] [Google Scholar]
- 29.Liao M, et al. , Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med, 2020. 26(6): p. 842–844. [DOI] [PubMed] [Google Scholar]
- 30.Bartke A, Evans TR, and Musters CJM, Anti-aging interventions affect lifespan variability in sex, strain, diet and drug dependent fashion. Aging (Albany NY), 2019. 11(12): p. 4066–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashpole NM, et al. , IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience, 2017. 39(2): p. 129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller RA, et al. , Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell, 2002. 1(1): p. 22–9. [DOI] [PubMed] [Google Scholar]
- 33.Mirzaalian Y, et al. , The association of quantitative insulin sensitivity indices (HOMA-IR and QUICKI) with anthropometric and cardiometabolic indicators in adolescents. Arch Med Sci Atheroscler Dis, 2019. 4: p. e32–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naseh A, et al. , Associations between anthropometric characteristics and insulin markers in mothers and their neonates and with neonate’s birth weight: An observational cohort study. Turkish Journal of Pediatrics, 2017. 59(6): p. 625–635. [DOI] [PubMed] [Google Scholar]
- 35.Li P, et al. , Immunoregulatory Effect of Acanthopanax trifoliatus (L.) Merr. Polysaccharide on T1DM Mice. Drug Des Devel Ther, 2021. 15: p. 2629–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samadi-Noshahr Z, et al. , The hepatoprotective effects of fennel seeds extract and trans-Anethole in streptozotocin-induced liver injury in rats. Food Sci Nutr, 2021. 9(2): p. 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patil JS, et al. , Effect of Glucose Tolerance Factor (GTF) on Lipid Profile, Blood Glucose Levels, and Food Intake in Streptozotocin-Induced Diabetes in Rats. Maedica (Bucur), 2020. 15(2): p. 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lachin JM, et al. , Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes, 2007. 56(4): p. 1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee A and Morley JE, Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res, 1998. 6(1): p. 47–53. [DOI] [PubMed] [Google Scholar]
- 40.Lv WS, et al. , The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res, 2012. 1444: p. 11–9. [DOI] [PubMed] [Google Scholar]
- 41.Anisimov VN, et al. , Sex differences in aging, life span and spontaneous tumorigenesis in 129/Sv mice neonatally exposed to metformin. Cell Cycle, 2015. 14(1): p. 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Cai Y, and Fan X, Metformin Administration During Early Postnatal Life Rescues Autistic-Like Behaviors in the BTBR T+ Itpr3tf/J Mouse Model of Autism. Front Behav Neurosci, 2018. 12: p. 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarry-Adkins JL, Aiken CE, and Ozanne SE, Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis. PLoS Med, 2019. 16(8): p. e1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelsey MM and Zeitler PS, Insulin Resistance of Puberty. Curr Diab Rep, 2016. 16(7): p. 64. [DOI] [PubMed] [Google Scholar]
- 45.Teixeira PDS, Tavares MR, and Jose D, Temporal characterization of the insulin resistance during puberty in mice. Endocr Regul, 2021. 55(1): p. 1–4. [DOI] [PubMed] [Google Scholar]
- 46.Hughey CC, et al. , Loss of hepatic AMP-activated protein kinase impedes the rate of glycogenolysis but not gluconeogenic fluxes in exercising mice. J Biol Chem, 2017. 292(49): p. 20125–20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tishinsky JM, Robinson LE, and Dyck DJ, Insulin-sensitizing properties of adiponectin. Biochimie, 2012. 94(10): p. 2131–6. [DOI] [PubMed] [Google Scholar]
- 48.Krysiak R, Szkrobka W, and Okopien B, The Effect of Metformin on Serum Gonadotropin Levels in Postmenopausal Women with Diabetes and Prediabetes: A Pilot Study. Exp Clin Endocrinol Diabetes, 2018. 126(10): p. 645–650. [DOI] [PubMed] [Google Scholar]
- 49.Tosca L, et al. , Metformin decreases GnRH- and activin-induced gonadotropin secretion in rat pituitary cells: potential involvement of adenosine 5’ monophosphate-activated protein kinase (PRKA). Biol Reprod, 2011. 84(2): p. 351–62. [DOI] [PubMed] [Google Scholar]
- 50.Rice S, et al. , Dual effect of metformin on growth inhibition and oestradiol production in breast cancer cells. Int J Mol Med, 2015. 35(4): p. 1088–94. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, et al. , Deletion of Nrip1 Extends Female Mice Longevity, Increases Autophagy, and Delays Cell Senescence. J Gerontol A Biol Sci Med Sci, 2018. 73(7): p. 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moatt JP, et al. , The effect of dietary restriction on reproduction: a meta-analytic perspective. BMC Evol Biol, 2016. 16(1): p. 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vergara M, et al. , Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci, 2004. 59(12): p. 1244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panici JA, et al. , Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J, 2010. 24(12): p. 5073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun LY, et al. , Longevity is impacted by growth hormone action during early postnatal period. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulkarni AS, Gubbi S, and Barzilai N, Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab, 2020. 32(1): p. 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dupont J, et al. , Nutritional signals and reproduction. Mol Cell Endocrinol, 2014. 382(1): p. 527–537. [DOI] [PubMed] [Google Scholar]
- 58.Hardie DG, AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol, 2007. 8(10): p. 774–85. [DOI] [PubMed] [Google Scholar]
- 59.Shaw RJ, LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf), 2009. 196(1): p. 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo Z and Yu Q, Role of mTOR Signaling in Female Reproduction. Front Endocrinol (Lausanne), 2019. 10: p. 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan R, et al. , Genetic differences and longevity-related phenotypes influence lifespan and lifespan variation in a sex-specific manner in mice. Aging Cell, 2020. 19(11): p. e13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merry BJ and Holehan AM, Onset of puberty and duration of fertility in rats fed a restricted diet. J Reprod Fertil, 1979. 57(2): p. 253–9. [DOI] [PubMed] [Google Scholar]
- 63.Hamilton GD and Bronson FH, Food restriction and reproductive development in wild house mice. Biol Reprod, 1985. 32(4): p. 773–8. [DOI] [PubMed] [Google Scholar]
- 64.Glass AR, Herbert DC, and Anderson J, Fertility onset, spermatogenesis, and pubertal development in male rats: effect of graded underfeeding. Pediatr Res, 1986. 20(11): p. 1161–7. [DOI] [PubMed] [Google Scholar]
- 65.Yang X, et al. , The influence of metformin on IGF-1 levels in humans: A systematic review and meta-analysis. Pharmacol Res, 2020. 151: p. 104588. [DOI] [PubMed] [Google Scholar]
- 66.Guido M, et al. , Effect of metformin on the growth hormone response to growth hormone-releasing hormone in obese women with polycystic ovary syndrome. Fertil Steril, 2005. 84(5): p. 1470–6. [DOI] [PubMed] [Google Scholar]
- 67.Cohen LE, et al. , CREB-independent regulation by CBP is a novel mechanism of human growth hormone gene expression. J Clin Invest, 1999. 104(8): p. 1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faggi L, Giustina A, and Tulipano G, Effects of metformin on cell growth and AMPK activity in pituitary adenoma cell cultures, focusing on the interaction with adenylyl cyclase activating signals. Mol Cell Endocrinol, 2018. 470: p. 60–74. [DOI] [PubMed] [Google Scholar]
- 69.Vitale G, et al. , ROLE of IGF-1 System in the Modulation of Longevity: Controversies and New Insights From a Centenarians’ Perspective. Front Endocrinol (Lausanne), 2019. 10: p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berryman DE, et al. , Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res, 2008. 18(6): p. 455–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartke A, et al. , Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology, 2003. 4(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 72.Bokov AF, et al. , Does reduced IGF-1R signaling in Igf1r+/− mice alter aging? PLoS One, 2011. 6(11): p. e26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharples AP, et al. , Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell, 2015. 14(4): p. 511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wrigley S, Arafa D, and Tropea D, Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front Cell Neurosci, 2017. 11: p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ibanez L, et al. , Early metformin therapy to delay menarche and augment height in girls with precocious pubarche. Fertil Steril, 2011. 95(2): p. 727–30. [DOI] [PubMed] [Google Scholar]
- 76.Izadyar F, et al. , Messenger RNA expression and protein localization of growth hormone in bovine ovarian tissue and in cumulus oocyte complexes (COCs) during in vitro maturation. Mol Reprod Dev, 1999. 53(4): p. 398–406. [DOI] [PubMed] [Google Scholar]
- 77.Modina S, et al. , Relationship between growth hormone concentrations in bovine oocytes and follicular fluid and oocyte developmental competence. Eur J Histochem, 2007. 51(3): p. 173–80. [PubMed] [Google Scholar]
- 78.Izadyar F, et al. , Stimulatory effect of growth hormone on in vitro maturation of bovine oocytes is exerted through cumulus cells and not mediated by IGF-I. Mol Reprod Dev, 1997. 47(2): p. 175–80. [DOI] [PubMed] [Google Scholar]
- 79.Kooistra HS, et al. , Progestin-induced growth hormone (GH) production in the treatment of dogs with congenital GH deficiency. Domest Anim Endocrinol, 1998. 15(2): p. 93–102. [DOI] [PubMed] [Google Scholar]
- 80.Gregoraszczuk EL, et al. , Progesterone-induced secretion of growth hormone, insulin-like growth factor I and prolactin by human breast cancer explants. Gynecol Endocrinol, 2001. 15(4): p. 251–8. [DOI] [PubMed] [Google Scholar]
- 81.Sidhom S, et al. , 17alpha-Estradiol Modulates IGF1 and Hepatic Gene Expression in a Sex-Specific Manner. J Gerontol A Biol Sci Med Sci, 2021. 76(5): p. 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adamek A and Kasprzak A, Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int J Mol Sci, 2018. 19(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bartke A, Growth Hormone and Aging: Updated Review. World J Mens Health, 2019. 37(1): p. 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, et al. , Deletion of Nrip1 extends female mice longevity, increases autophagy, and delays cell senescence. J Gerontol A Biol Sci Med Sci, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu A, et al. , Effects of dietary restriction on growth, neurobehavior, and reproduction in developing Kunmin mice. Toxicol Sci, 2002. 70(2): p. 238–44. [DOI] [PubMed] [Google Scholar]
- 86.Ingram DK and de Cabo R, Calorie restriction in rodents: Caveats to consider. Ageing Res Rev, 2017. 39: p. 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anisimov VN and Bartke A, The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol, 2013. 87(3): p. 201–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


