Abstract
The endothelium acts as the barrier that prevents circulating lipids such as lipoproteins and fatty acids into the arterial wall; it also regulates normal functioning in the circulatory system by balancing vasodilation and vasoconstriction, modulating the several responses and signals. Plasma lipids can interact with endothelium via different mechanisms and produce different phenotypes. Increased plasma-free fatty acids (FFAs) levels are associated with the pathogenesis of atherosclerosis and cardiovascular diseases (CVD). Because of the multi-dimensional roles of plasma FFAs in mediating endothelial dysfunction, increased FFA level is now considered an essential link in the onset of endothelial dysfunction in CVD. FFA-mediated endothelial dysfunction involves several mechanisms, including dysregulated production of nitric oxide and cytokines, metaflammation, oxidative stress, inflammation, activation of the renin-angiotensin system, and apoptosis. Therefore, modulation of FFA-mediated pathways involved in endothelial dysfunction may prevent the complications associated with CVD risk. This review presents details as to how endothelium is affected by FFAs involving several metabolic pathways.
Keywords: Endothelial cells, Atherosclerosis, CVD, FFA, LCPUFAs, Metaflammation, Prostaglandins
Introduction
The tightly regulated vascular endothelium forms a vast interface between the flowing blood and neighboring tissues. The endothelium, which consists of a monolayer of endothelial cells, has a thickness of ≤ 1 μm and covers a total surface area of 4000 -7000 m2. Endothelial cells play various roles in the maintenance of vascularity of endothelium. Endothelial cells are responsible for a wide range of critical functions, including maintaining vascular tone, fluidity of blood, blood flow, and permeability of endothelium [1, 2]. In addition, the endothelium regulates normal functioning in the circulatory system by balancing vasodilation and vasoconstriction between thrombosis and hemostasis by modulating the several responses and signals [1, 3]. Thus, impairment of the endothelial functions plays deleterious roles in developing various disorders/diseases, including inflammatory angiitis syndrome, thrombotic embolism, disseminated intravascular coagulation disorder, neovascularization, tumor progression, and diabetic retinopathy [4].
The presence of atherosclerosis is a prevalent characteristic in cardiovascular disease (CVD), and that endothelial dysfunction is thought to be one of the early steps in the pathogenesis of atherosclerosis. Endothelial cells can affect various pathophysiological properties by producing different molecules [5]. Endothelium-secreted compounds such as angiotensin II (Ang II), endothelin-1 (ET-1), thromboxane(Tx) A2, and prostaglandin (PG) H2 involve in vasoconstriction, whereas nitric oxide (NO), prostacyclin (PGI2), bradykinin, and hyperpolarizing factor involve in vasodilation thus maintain a balance between the vasoconstriction and vasodilation [3]. Although dysregulated NO production is the main regulator of endothelium dysfunction, PGI2 and bradykinin also participate in regulating endothelium function. Therefore, the delicate balance between these endothelium-secreted molecules is critically required for the appropriate functioning of the endothelium [5]. Located at the interface between the circulation and the vessel wall, the endothelium reacts by synthesizing and releasing vasoactive substances with anti-thrombotic, vasodilating, and anti-atherogenic properties to maintain vascular homeostasis [6, 7].
The endothelium has a major function in regulating vascular tone, controlling blood flow, and blood fluidity and inflammatory responses [7, 8]. The inner layer of endothelium that comprises endothelial cells allows free blood flow and its cellular components. In addition, endothelial cells produce various factors regulating cellular adhesion, platelet aggregation response, smooth muscle cell proliferation, and inflammation [8, 9]. In addition, the endothelium exerts multiple actions on the expression of several pro-inflammatory genes, including those encoding adhesion molecules, chemokines, and other soluble cytokines of the endothelium [10–12].
Endothelial biology is affected by circulating lipids such as triglycerides-rich particles, chylomicron resident time, lipoproteins, and free fatty acids (FFAs) [13]. FFAs are released into the blood through the action of hormone-sensitive lipase on triacylglycerol stores in adipose tissue. Chylomicrons also contribute to plasma FFA levels, especially when high-fat diets are consumed. Increased plasma FFAs cause insulin resistance and endothelial dysfunction. The most observed lipid abnormalities in type 2 diabetes, metabolic syndrome, and CVD are hypertriglyceridemia, hyperlipoproteinemia, higher levels of FFAs, greater levels of small dense low-density lipoprotein (LDL) [14]. Several human epidemiologic and clinical intervention data suggested the association between circulating triacylglycerol levels and atherosclerosis [15, 16]. Plasma levels of FFAs, a well-established risk factor of CVD [17] are strongly associated with metabolic syndromes, obesity, and type 2 diabetes mellitus [18]. Several studies demonstrated that FFAs modulate the endothelial function and subsequent atherosclerosis processes. Elevated FFAs directly affect transcription factors of several genes involved in inflammation and oxidative stress in the endothelium.
FFA-induced endothelial dysfunction is mediated via several mechanisms, including impaired insulin signaling, dysregulation of NO synthesis, oxidative stress, inflammation, and the activation of the renin-angiotensin system (RAS) and apoptosis. Insulin resistance, oxidative stress, and inflammatory conditions are responsible for FFA-induced endothelium dysfunction [19–21]. Activation of endothelial cells by inflammation, proliferation, hypercoagulability, activation of vasoactive factors, enhanced apoptosis, increased vascular permeability, presence of free radicals is defined as endothelial dysfunction [22]. Because of the multifaceted roles of plasma FFAs in modulating endothelial function, elevated FFA level is now considered an essential link in the onset of endothelial dysfunction due to metabolic syndromes such as diabetes and obesity. Therefore, modulation of the signaling pathways involved in FFA-induced endothelial dysfunction may protect against endothelial dysfunction and subsequent CVD complications such as atherosclerosis.
Endothelial dysfunction is a significant feature in atherosclerosis, hypertension, diabetes, and cardiovascular disorders [7, 23]. Defective endothelium results in leukocyte adhesion, activation of platelets, pro-oxidation of mitogens, dysregulated synthesis of PGI2, NO, and endothelium-derived hyperpolarizing factor (EDHF), and other vasoconstriction factors such as Ang II and PGH2, resulting in atherosclerosis and thrombosis [24]. A balance between the levels of vasoconstrictors (TxA2, PGH2, ET-1) and vasodilators (NO, EDHF, PGI2) produced by the endothelium determines the vascular tone. The expression of adhesion molecules on endothelial cells also plays an important role in the atherosclerosis process. The adhesive molecules expressed on endothelial cells are selectins, intracellular adhesive molecules (ICAMs), and vascular adhesive molecules (VCAM-1).
This review aimed to describe the roles and fundamental mechanisms of FFA-mediated endothelial dysfunction. Moreover, how the endothelium participates in the uptake and metabolism of fatty acids and involves various biochemical pathways in health and disease is also discussed.
Effects of fatty acids on endothelial function
Fatty acids are taken up and metabolized by endothelial cells, depending on the chain length, number, and position of double bonds. Fatty acids are beta-oxidized into mitochondria to fuel the tricarboxylic acid cycle and produce ATPs. At the same time, excess intracellular fatty acids are stored as cytosolic lipid droplets [25]. Protection from endoplasmic reticulum stress from excess FFA is correlated with endothelial stored lipid droplets [25]. Fatty acids are also synthesized de novo by endothelial cells even though they can uptake FFAs [26, 27]. Fatty acids are transported to mitochondria by the rate-limiting enzyme carnitine palmitoyltransferase 1a, the key to fatty acid oxidation and endothelial permeability [28, 29].
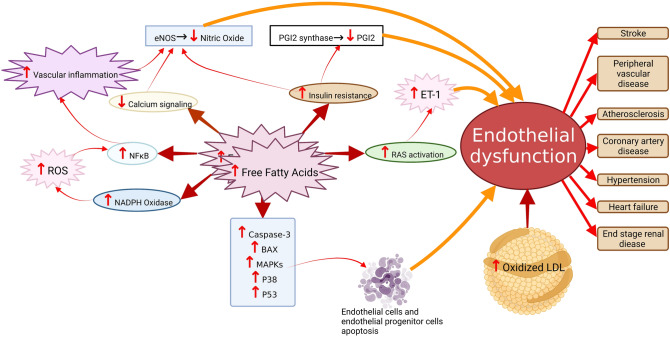
High levels of blood FFAs affect endothelium function in different ways. First, FFAs may contribute to inflammation resulting in increased permeability of the endothelium. Second, elevated blood levels of FFAs also downregulate insulin-mediated production of NO and reduce blood flow peripherally. FFAs mediate these effects via two different mechanisms by (a) decreasing tyrosine phosphorylation IRS-1/2 and preventing the PI3K/Akt pathway, which regulates insulin-stimulated glucose uptake and NO production by endothelial nitric oxide synthase (eNOS) [18]. Figure 1 describes the mechanism of FFA-induced endothelial dysfunction.
Fig. 1.
Mechanism of free fatty acids-induced endothelial dysfunction. Increased levels of free fatty acids reduce nitric oxide production directly or indirectly by mediating oxidative stress (ROS upregulation), upregulation of inflammatory signaling (NF-κB upregulation), and downregulation of insulin and calcium signaling. In addition, RAS activation by free fatty acids or oxidation of LDL can lead to vasoconstriction by ET-1 upregulation. Even apoptotic pathway activation by free fatty acids induces endothelial cells and endothelial progenitor cells apoptosis. Endothelial dysfunction ultimately leads to atherosclerosis events, coronary artery disease, hypertension, heart failure, peripheral vascular disease, stroke, and end-stage renal disease
In contrast, the endothelium-independent vasodilation remains unaffected [30], indicating that FFAs have specific inhibitory effects on NO synthesis by the endothelial cells. Infusion of FFA in insulin-sensitive human subjects was shown to significantly reduce NOS flux and impair shear stress-induced NO synthesis [31]. Palmitoylation of eNOS regulates the bioavailability of endothelial eNOS and thus NO release [32]. During atherosclerotic plaque formation, endothelial barrier, aberrant NO production, ROS production, and pro-inflammatory and pro-coagulative factors cause endothelial dysfunction, which enhances LDL transcytosis, upregulation of LDL oxidation, activation of blood platelet and leukocytes [33]. N-6 polyunsaturated fatty acids (PUFAs) may lower LDL cholesterol but increase LDL cholesterol susceptibility to oxidation and lower HDL cholesterol. Monounsaturated fatty acids are more beneficial than SFA and n-6 PUFAs in terms of their effects on LDL oxidizability [34]. They also prevented n-3 fatty acids from exerting their potential adverse effects of LDL oxidation. 15-Lipoxygenase is implicated in the in vivo oxidation of LDL and is a process thought to involved in initiation and progression of endothelium dysfunction and atherosclerosis.
More recently, inhibition of eNOS mRNA expression by FFAs was shown in rat aortic endothelial cells, thus lowering the eNOS activity and increased oxidative stress and inflammatory status [35]. FFAs downregulate insulin-mediated eNOS activity through upregulation of PTEN (phosphatase and tensin homolog) and simultaneous inhibition of Akt kinase [36]. The AMPK/PI3K/Akt/eNOS pathway in endothelium dysfunction was diminished with elevated levels of FFAs due to consuming a high-fat diet [37].
FFAs increase ROS levels in monocytes dose-dependently, leading to adhesion of the monocytes to the endothelial cells [38]—a critical mechanism in the development of atherosclerosis [39]. Furthermore, FFAs-induced increases in CVD risk factors, characterized by elevated endothelial markers, are also observed in healthy subjects [40]. Therefore, clinical and experimental studies exploit the protective effects of several dietary fatty acids/functional foods and drugs via suppression of inflammatory conditions and oxidative stress in FFA-induced endothelium dysfunction. As observed in human studies, calcium channel blockers such as dihydropyridine and amlodipine prevent FFAs-induced endothelium dysfunction, leucocyte activation, and oxidative stress. The protective effects of these drugs are mediated by the suppression of NF-κB p65 phosphorylation [41]. Moreover, these compounds involve in the nuclear localization of Nrf2 and reduce oxidative stress and enhance the expression of the cytoprotective genes in endothelial cells.
The RAS is one of the critical determinants of arterial blood pressure, and Ang II is a potent vasoconstrictor. The endothelial cell membrane expresses the angiotensin-converting enzyme (ACE) that produces Ang II. Ang II induces vasoconstriction by stimulation of endothelium and exhaustion of NO. Therefore, inhibition of ACE stimulates vasodilation via the NO pathway [42]. While Ang II increases FFAs levels via downregulating the fatty acid oxidation [43], on the other hand, FFAs also activate the RAS system [44]. ACE−/− mice had increased expression of genes responsible for lipolysis and oxidation of fatty acids [45], explaining the possible interplay between RAS and FFAs. By activating leukocytes, thus FFAs contribute to the adhesion of leukocytes to the endothelium in an Ang II-dependent manner, leading to the initiation of endothelium dysfunction [46]. Consequently, inhibition of the RAS prevents the FFA-induced endothelium dysfunction [47]. A single dose of either losartan (an Ang II receptor antagonist) or perindopril (an ACE inhibitor) completely prevented the FFAs-induced dysregulation of endothelium-dependent vasodilation, suggesting the blockade of RAS as an effective treatment for FFAs-induced endothelium dysfunction [47].
The risk of CVD is associated with saturated fatty acids (SFAs). In contrast, unsaturated fatty acids are unlikely involved in CVDs, and instead, these fatty acids are mostly found beneficial in CVD. SFAs increase blood LDL levels, a significant risk factor for CVDs [48]. Studies have demonstrated that long-chain saturated fatty acids(c > 14) (LCFAs) impart a greater risk for CVD than by the medium-chain fatty acids(c8-c12) (MCFAs) [48]. The most common saturated LCFAs present in the western diet are myristic acid,14:0 (MA), palmitic acid, 16:0 (PA), and stearic acid,18:0 (SA). While SFAs have been shown to increase total cholesterol and LDL cholesterol, there is also an increase in high-density lipoprotein (HDL) cholesterol [49]. CVD risk associated with SFAs varies from no association to a significantly significant risk [50]. SFAs, depending on chain length, have diverse effects on cholesterol metabolism in the body. For example, stearic acid, 18:0 (SA), is considered neutral on cholesterol metabolism compared with the other long-chain SFAs due to its high conversion rate to oleic acid 18:1n-9 (OA) by Δ9-desaturase enzyme. Long-chain SFAs, the health impact of stearic acid has no harmful effect on CVD risk. SFAs exert their atherogenic and thrombogenic influence through increased production of VLDL particles and ApoA1, decreasing LDL receptors-specific activity and increased platelet aggregation [51]. Unlikely other SFAs, SA showed no atherogenic or thrombogenic effect since, after absorption, it is desaturated to monounsaturated fatty acid, OA, which is associated with a beneficial effect on cardiovascular health. OA is incorporated into phospholipids rather than into triglycerides and cholesterol esters. OA exerts significant beneficial effects on atherosclerosis and thrombosis [52–54].
Various studies have concluded that PA substantially contributes to the development of atherosclerosis [55]. Both in vivo and in vitro studies have demonstrated the mechanisms by which PA contributes to the pathogenesis of CVDs. PA promotes inflammatory responses and cellular senescence in cardiac fibroblasts. PA achieves senescence in these cells by activating toll-like receptor 4 (TLR4) and NLRP3 inflammasome, increasing mitochondrial ROS levels [56]. PA also induces apoptosis of the vascular smooth muscle cells by inducing the TLR4 pathway and generating ROS [57]. Both PA and SA downregulated eNOS in porcine aortic endothelial cells. Although PUFAs have a protective role against endothelium dysfunction [58], their elevated FFAs adversely affect the endothelium by decreasing NO release and increasing ET-1 levels. Linoleic acid,18:2n-6 (LA) negatively regulates eNOS phosphorylation and affects the intracellular NO levels in ECV304 cells [59].
Many clinical studies showed protective effects of OA on flow-mediated dilation and other endothelial markers; however, they did not focus on FFA-induced endothelium dysfunction [60–62]. Studies demonstrated that n-3 long-chain polyunsaturated fatty acids (LCPUFAs) such as docosahexaenoic acid, 22:6n-3 (DHA), eicosapentaenoic acid,20:5n-3 (EPA), and polyphenols were beneficial in FFA-induced endothelium dysfunction [59, 63–65]. The mitochondria-related AMPK/eNOS pathway alleviates endothelium dysfunction and atherosclerosis in mice fed with a high-fat diet [66]. L-carnitine, an essential factor for fatty acid transport/oxidation in the mitochondria, attenuates FFA-induced obesity-related endothelium dysfunction in human subjects [67].
Moreover, SA showed a similar reducing impact on platelet aggregation and coagulation factors as the OA and LA [68]. Besides, a high-fat diet, a significant source of SFAs in blood levels of FFAs, induces oxidative stress in endothelium [69]. Elevated FFAs induce expression of NADPH and mediates oxidative stress in rats with characteristics of both obesity and type 2 diabetes mellitus [70]. FFAs-induced endoplasmic reticulum stress was observed in endothelial cells isolated from healthy human subjects [71]. Infusion of intralipid in healthy subjects increased FFAs by a 4.2-fold increase, and that was associated with reducing the hyperemic response in the leg without a change in flow-mediated dilation of the brachial artery. The mRNA levels of the genes ATF6 and IRE1, responsible for early adaptive responses to endothelium reticulum stress, increased in endothelial cells.
Trans fatty acids (TFAs), obtained from partial hydrogenation of plant oils, have the highest atherogenic activity. Thus industrial foods—cakes, cookies, and crackers often contain a high content of TFAs. TFAs have one double bond in which the hydrogens are on the opposite side to one another resulting in physiochemical properties close to those of SFAs. Main TFAs are elaidic acid, 18:1n-9t, and trans-vaccenic acid, 18:1n-7t. TFAs are associated with a higher risk of CVD and type 2 diabetes mellitus [72]. Consumption of TFAs increased plasma LDL cholesterol, lowered HDL cholesterol, and increased lipoprotein (a) and plasma triglyceride levels [73] and smaller flow-mediated vasodilation [74]. TFAs can influence the thrombotic state through the eicosanoid synthesis pathway [72]. TFAs also adversely affect endothelial function, which partly explains their association with CVD risk [75]. Intake TFAs also induced inflammation and endothelial dysfunction, as evidenced by increased plasma levels of CRP, IL-6, soluble tumor necrosis factor receptor (TNF-2), E-selectin, and soluble intercellular and vascular cell adhesion molecules (sICAM-1 and sVCAM-1) in apparently healthy women [75]. TFAs also activate the NF-κB pathway that increases endothelial superoxide production and reduces NO synthesis [76]. FFAs-induced endothelium dysfunction is mediated via the activation of NF-κB [77]. Thus, the NF-κB pathway is a significant player mediating the harmful effects of TFAs on human coronary artery endothelial cells [78].
FFAs-induced NLRP3 inflammasome also increases endothelial permeability. PA activates the NLRP3 inflammasome with a resulting decrease in endothelial tight junction proteins—zonula occludens-1 and -2 in microvascular endothelial cells. FFAs also increases the synthesis of high-mobility group box 1 (HMGB1) protein, which might explain the early onset of endothelial injury during obesity by FFAs.
Endothelial progenitor cells (EPCs) participate in endothelial recovery following arterial injury and oxidative stress-induced damages [79]. Dysfunctional EPCs are involved in the pathogenesis of CVD [80]. PA contributes to the apoptosis of EPCs mediated via the p38 and JNK/MAPKs pathways [81]. PA has harmful impacts on EPCs in metabolic syndrome patients via regulating long non-coding RNA (LncRNA) maternally expressed gene 3 (MEG3) [81]. These data raise interest for further studies on the pathophysiological roles of MEG3 in both EPCs and endothelial cells.
Metabolism of n-3 and n-6 long-chain polyunsaturated fatty acids and their impacts on endothelium
Dietary polyunsaturated fatty acids (PUFAs) commonly consumed by humans encompass two major groups: the n-3 and n-6 families of fatty acids. Linoleic acid, 18:2n-6 (LA) and alpha-linolenic acid,18:3n-3 (ALA) are the dietary essential fatty acids (EFAs) [82]. LA and ALA are not interchangeable but can be further elongated and desaturated by the same enzyme systems to produce n-6 and n-3 LCPUFAs in the body. Some common n-3 PUFAs include ALA, EPA and DHA, and common n-6 ones include LA and arachidonic acid,20:4n-6 (ARA). LCPUFAs are the precursors for eicosanoid biosynthesis and various signaling compounds with relevant roles in human health and disease. Whereas dietary LA and ALA are primarily from vegetable oils, preformed LCPUFAs may also be consumed in animal-origin foods. The importance of LCPUFAs has been related to their structural action, their specific interaction with membrane proteins, and their ability to serve as precursors of second messengers. LCPUFAs (20 carbon) are substrates for cyclooxygenase (prostaglandin-endoperoxide synthase) and lipoxygenases and produce various compounds collectively called eicosanoids. These compounds have diverse biological functions in cell growth and development, inflammation, and the cardiovascular system. The biological response elicited after eicosanoid release is dependent on the net balance of eicosanoids derived from n-6 and n-3 LCPUFAs. ARA is the most predominant precursor fatty acid of the highly biologically active eicosanoids of the 2 series in the Western diet. Also, the endogenous formation of cyclooxygenase and non-cyclooxygenase metabolites of fatty acids has been implicated in gene expression. Because each type of EFA can interfere with the other's metabolism, an excess of n-6 fatty acids will reduce the metabolism of ALA, possibly leading to a deficit of n-3 LCPUFA metabolites. Therefore, a proper balance between the n-6 and n-3 fatty acids in the diet is essential to maintain optimum health.
Endothelial cells metabolize ARA in three different ways, cyclooxygenase (COX), lipoxygenase and, cytochrome P450 system. The primary metabolites of the COX pathway are PGI2 with lesser amounts of PGE2, TxA2, and 12-hydroxyheptadecatrienoic acid [83]. 12- and 15-hydroxyeicosatetraenoic acids are the primary lipoxygenase metabolites. Endothelial cells synthesize the four regioisomeric epoxyeicosatrienoic acids (EETs), 14,15-, 11,12-, 8,9-, and 5,6-EETs, with 14,15- and 11,12-EETs the significant metabolites.EETs are cytochrome P450 epoxygenase metabolites of ARA. PGE2 and TxA2 play an essential role in maintaining vascular homeostasis [83]. PGI2 is a vasodilator and a platelet aggregation inhibitor, whereas TxA2 is a vasoconstrictor and activator/aggregator of blood platelets [84, 85]. PGI2 has vasodilating properties, inhibits platelet aggregation by elevating intracellular levels of cAMP [86]. At the same time, vasorelaxation and platelet inhibitory actions of NO are mediated predominately by activating intracellular guanylyl cyclase, leading to cGMP formation [8]. Thus, PGI2 and TxA2 play a critical role in maintaining vascular homeostasis. PGI2 is a vasodilator and an inhibitor of platelet aggregation, whereas TxA2 is a vasoconstrictor and a promoter of platelet aggregation [86, 87]. Therefore, an imbalance in PGI2 or TxA2 production is implicated in the pathophysiology of many thrombotic and cardiovascular disorders [83, 87]. The protective effect of PGI2 against the development of CVD is mediated through the inhibition of various cellular processes, including inhibition of platelet activation, leukocyte adhesion to endothelium. Prostaglandins also enhance endothelial cell survival through the upregulation of the anti-apoptotic protein B cell lymphoma (Bcl)-2 [88] and the activation of phosphatidylinositol (PI)3-kinase (K)-Akt pathway [89].
N-6 LCPUFAs mediate acute inflammatory responses by synthesizing pro-inflammatory eicosanoids [90], whereas n-3 LCPUFAs produce anti-inflammatory or neutral eicosanoids. ARA-derived eicosanoids control cellular membrane lipid composition, inflammation, coagulation, and vascular homeostasis [91, 92]. Also, ARA-induced synthesized cytokines and adipokines play a vital role in metabolism and inflammation [93]. LA induces inflammation by increasing the levels of TNF-α, MCP-1, VCAM-1, and ICAM-1 through the activation of NF-κB and activator protein 1 (AP-1) [94], and it affects the release of NO [95].
Circulating blood platelets do not attach to the negatively charged surface of the endothelium. However, the activated platelets can bind GpIbα to either P-selectin or VWF on the surface of the endothelium, and indirectly via a fibrin bridge that joins GpIIb/IIIa and ICAM-1. Whereas NO decreases the intracellular level of Ca2+, the transformation of GPIIb/IIIa platelet receptor and suppresses the integrin's binding to fibrinogen [96, 97]. The ecto-ADPase (CD-39), on the surface of endothelial cells, hydrolyzes both ATP and ADP to generate AMP, thus decreases platelet aggregation/activation [86, 96]. TxA2, produced by the endothelium from ARA, aggregates platelets and expresses adhesive co-factors for platelets such as vWF, fibronectin, and thrombospondin procoagulant factors such as factor V [84, 98].
Endothelial cells actively prevent thrombus formation by suppressing platelet adhesion and activation [99]. The vasoprotective function of endothelial cells is also associated, among others, with biosynthesis and release of NO, PGI2, PGE2, and tissue plasminogen activator (tPA). Platelet activation is counteracted by PGI2 and PGE2, produced from ARA by the endothelium after activation by various vasoactive agents, including thrombin. PGI2 is produced by the endothelium of large vessels, while the endothelial cells from smaller vessels produce PGE2 [100]. The effect of PGI2 is enhanced by NO produced by endothelial nitric oxide synthase (eNOS). The endogenous fibrinolytic system is responsible for the dissolution of the thrombus. It is regulated by the endothelium-derived profibrinolytic factor, tissue plasminogen activator (tPA), and its inhibitor, plasminogen activator inhibitor type-1 (PAI-1) [101]. Fatty acids, depending on their structure, regulate PAI-1 and tPA activity. However, the definitive conclusions are yet to be reached [102–104]. These endothelium-derived compounds can inhibit activation of platelets and leukocytes, promote fibrinolysis, maintain tissue perfusion and protect the vascular wall against acute damage and chronic remodeling. Endothelial dysfunction is associated not only with suppression in the release of these compounds but also with the secretion of deleterious compounds such as PGH2, PGG2, superoxide anion (O2-, peroxynitrite (ONOO-), and plasminogen activator inhibitor (PAI-1).
Several epidemiological, experimental, and clinical studies indicate that n-3 fatty acids can decrease CVD risk via several mechanisms, including improving vascular function. Incorporation in cell membranes, n-3 PUFAs precisely activate cardiovascular protective signaling pathways [105], increase NO production [106], reduce oxidative stress [107] and inflammation [12]. N-3 fatty acids also increase eNOS expression in the endothelium via several pathways such as phosphorylation of AMPK [63] and increased expression of eNOS mRNA [108]; stimulation of SIRT-1 and heat-shock protein 90 protein [109, 110]; and finally, eNOS translocation from caveolae to the cytosol [111]. N-3 PUFAs can decrease the expression of these adhesive factors of endothelial cells and thus reduce the activation of the endothelium.
The vasoprotective properties of n-3 fatty acids include decreased arterial plaque formation, anti-inflammatory activity, improved endothelial-dependent vasodilation, lowered arterial blood pressure, and increased antioxidant activity. N-3 fatty acids reduce arterial stiffness and blood pressure via eNOS expression.[112, 113]. Monocyte-endothelium interaction is essential in many acute and chronic inflammatory diseases. Both EPA and DHA significantly reduce PAF synthesis, monocyte rolling, and adherence, whereas endothelial adhesion molecules expression was unaltered.
Both EPA and DHA compete with ARA as substrates to form pro-inflammatory mediators, such as 4 series of leukotrienes, 2 series of prostaglandins, and cytokines. In addition to competitive inhibition of the n -6 fatty acid pathway, n-3 fatty acids also inhibit the production of C reactive protein, TNFα, matrix metalloproteinase (MPP)-2 and MPP-9, and tissue inhibitors of MPP [114–116]. Furthermore, N-3 fatty acids inhibit COX-2 expression as therapeutic potential as COX-2 overexpression is involved in different inflammatory/degenerative diseases apart from atherosclerosis [112].
Endothelial cells express ICAM-1, VCAM-1, E-selectin, and P-selectin involved in leukocyte recruitment and platelet adhesion during thrombosis and inflammation and contribute to early phases of atherosclerosis. Cytokine-induced endothelial activation involves increased expression of genes for ICAM-1, VCAM-1, and E-selectin, whereas n-3 PUFAs suppress the synthesis of inflammatory cytokines that activate the endothelium. Thus, n-3 PUFAs decrease atherosclerosis and inflammation by reducing the expression of adhesion and migration of monocytes to the endothelium.
N-3 LCPUFAs supplementation replaces ARA content in plasma membrane phospholipids to improve endothelium vasodilating effects [117]. Thus, n-3 PUFAs can modify n-6 eicosanoids production to favor vasodilation and anti-thrombotic actions. N-3 PUFAs increase endothelium-dependent relaxation by increased release of NO. NO inhibits platelet aggregation and adhesion, leukocyte adhesion, and smooth muscle cell proliferation. The protective role of n-3 LCPUFAs has been shown by reducing FFA-induced endothelium dysfunction via the AMPK/PI3K/Akt/eNOS pathway. EPA has a protective role against PA-induced endothelium dysfunction mediated via activation of the AMPK/eNOS pathway. The EPA also inhibited PA-induced apoptosis of endothelial cells and activated apoptosis-related proteins, such as caspase-3, p53, and Bax [63]. N-3 PUFAs can protect endothelium by reducing platelet TxA2 synthesis, plasminogen activator inhibitor-1 activity, COX 1 or 2 productions, platelet activation, and adhesion to endothelium [118–121]. Moreover, n-3 PUFAs interfere with the vitamin K-dependent carboxylation of coagulation factors II, VII, IX, and X) [122, 123].
Dietary n-3 PUFAs reduce plasma triglycerides and very-low-density lipoproteins (VLDL) [124–126] and thus protect the endothelium from lipid stress. Inhibition of 1,2 diglyceride acyltransferase or sterol regulatory element-binding protein 1c by n-3 PUFA reduces hepatic triglyceride synthesis [127–129]. Also, n-3 PUFA activates peroxisome proliferator-activated receptor alpha (PPARα) to favor catabolism of circulating triglycerides and VLDL by promoting fatty acid beta-oxidation [127, 130]. N-3 PUFA induced non-proteasomal degradation of apolipoprotein B reduces naïve hepatic VLDL secretion [131–133]. Several studies found that n-3 PUFAs increase HDL levels despite fluctuating effects on LDL levels, possibly by decreasing cholesteryl ester transfer protein activity [134–138].
As an independent risk factor of CVDs, hypertension can be favorably regulated by n-3 PUFAs supplementation [139, 140]. N-3 PUFAs mediated blood pressure regulation by producing vasodilator prostaglandins, activating eNOS, incrementing renin release from the kidney, suppressing ACE activity, and activating large-conductance Ca2+-activated K+ channels has been demonstrated [141–144]. In addition, studies found that reduced n-3 PUFAs on red blood cells induce vasoconstriction and increased production of pro-inflammatory eicosanoids [145, 146].
Fatty acid transport across the endothelium
Fatty acids pass through the endothelial cell membrane to enable tissue uptake in muscle and likely allow fatty acids to penetrate the arterial wall. The trans-endothelial crossing could take place through movement around or between endothelial cells. Large topical concentrations of FFAs disrupt the endothelial barrier, as does active lipolysis, which can also upregulate LDL passage into the artery [147]. Fatty acid uptake by endothelial cells is not entirely understood clearly; it might involve both protein-mediated uptake and non-specific uptake [148], which might occur in the presence of high topical fatty acid concentrations, e.g., those that occur during chylomicron but not VLDL lipolysis [149]. Fatty acid metabolism by vascular endothelial cells occurs both under basal conditions and following endothelial cell stimulation or injury.
Endothelial cell reigns in the metabolic homeostasis of the various tissues via their growth and function. Endothelial cell activates in response to vessel wall permeability and interaction with multiple molecules of blood. However, glucose is the primary source of endothelial energy, fatty acid uptake, transport, and metabolic pathways on endothelial cells have become an arena of interest for many years [150]. As the metabolic gatekeeper, endothelial cells regulate fatty acid transcytosis, and dysregulated endothelial fatty acid transport can pronounce insulin resistance, which plays a pivotal role in various pathological processes [151].
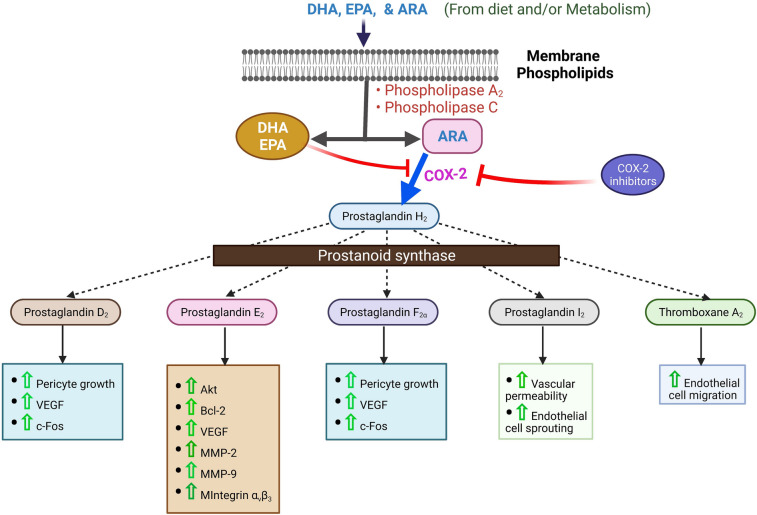
Endothelial fatty acid transport can be either passively by crossing paracellularly through intercellular space between neighboring endothelial cells or actively, where fatty acids cross the endothelial layer by different transporters such as plasma membrane fatty acid-binding protein (FABPpm), fatty acid transport proteins (FATPs), cluster of differentiation 36 (CD36), or fatty acid translocase (FAT), and cytoplasmic fatty acid-binding proteins (FABPs) [152]. Unlike fenestrated hepatic endothelial lining, continuous and non-fenestrated cardiac endothelial lining signifies the importance of the endothelial fatty acid transport system. Unlike medium and short-chained fatty acids, generally consumed long dietary chain (c ≥ 14 carbons) fatty acid uptake in endothelial cells is upregulated by synergistic co-expression of FATP3 and FATP4. Secretion of vascular endothelial growth factor B by cardiomyocytes or production of 3-hydroxy-isobutyrate by skeletal muscle regulates the expression of FATP3 and FATP4 in neighboring endothelial cells [27, 153]. FATP4 and FATP5 are mainly expressed by capillaries and venules of the heart, skeletal muscle, and adipose tissue [154]. Apelin signaling within endothelial cells inhibits FATP4 expression by activating transcription factor FOXO1, which may stabilize VE-cadherin-mediated endothelial cell junctions [155, 156]. In cardiac and skeletal muscle, deficiency of fatty acid uptake is compensated by glucose uptake for energy production [154]. Figure 2 describes the prostanoid-induced angiogenic role of COX-2 in endothelial cells.
Fig. 2.
Rate limiting the prostanoid-induced inflammatory angiogenic role of COX-2 on endothelial cell. N-3 or N-6 LCPUFA6 (DHA, EPA and ARA) from dietary or metabolism are incorporated into cell membrane phospholipids. DHA, EPA, or ARA can be liberated by phospholipase A2 or phospholipase C. COX-2 then converts ARA to prostaglandin H2, which can be inhibited by the liberated DHA, EPA, or COX-2 inhibitors. Prostaglandin H2 converts into prostaglandin D2, prostaglandin E2, prostaglandin F2α, prostaglandin I2, and thromboxane A2 by prostanoid synthase to promote-specific angiogenic steps and mediators
FAT/CD36 facilitates fatty acid transport across the endothelial layer and oxidation in the heart, skeletal muscle, and adipose tissue [157, 158]. Silencing FAT/CD36 or lipoprotein lipase downregulates peroxisome proliferator-activated receptor alpha mediated cardiomyocyte lipid deposition, ultimately resulting in lipotoxic cardiomyopathy [159]. Surprisingly the opposite effect of FAT/CD36 is observed in the liver, which may be due to the high permeability of hepatic endothelial cells comparatively [160]. As the principal regulator of fat metabolism, peroxisome proliferator-activated receptor-gamma ((PPARγ) maintains different fatty acid transport proteins expression, including FAT/CD36 in nonfenestrated endothelium [161]. Ultimately fatty acid transport, storage, and release in heart, skeletal muscle, and adipose tissues solely depend on PPARγ [162].
Surprisingly, when fatty acid level crosses the metabolic limit, FAT/CD36 becomes dysfunctional and consistently stays at the plasmalemma [163]. Although n-3 PUFAs downregulate the ongoing relocation of FAT/CD36 on sarcolemma and prevents insulin resistance [164]. Different factors control post-translational modification of FAT/CD36, such as glycosylation, ubiquitination, acetylation, palmitoylation, etc. [165]. A better understanding of these processes will help us to understand FAT/CD36 trafficking in the cells.
Overexpression of FAT/CD36 upregulates fatty acid oxidation around three-fold [166]. FAT/CD36 works as the ferry for disposing fatty acids into mitochondria. Mitochondrial co-localization of FAT/CD36 is proportionate to the amount of fatty acid oxidation [167]. FAT/CD36 can solely upregulate fatty acid esterification and/or lipoprotein packaging [168]. FAT/CD36 seems to be interrelated with AMPK. FAT/CD36 -induced AMPK increases fatty acid oxidation, while AMPK induces FAT/CD36 on the cell membrane [163, 169, 170]. FAT/CD36 inhibits AMPK by allowing tyrosine kinase Fyn to block AMPK-kinase LKB1 reversely without fatty acid. These coordinated functions can be dysregulated by interrupted AMPK signaling due to FAT/CD36 deletion [171–173].
Also, FAT/CD36 regulates cytosolic Ca2+ and Ca2+-dependent phospholipase A2 activation [174]. Either interruption of Sarco/endoplasmic reticulum calcium ATPase (SERCA) or following reduction of Ca2+, endoplasmic reticulum Ca2+ sensor Stromal interaction molecule 1 stimulates membrane store-operated Ca2+ channels (Orai protein multimers) to maintain cytosolic Ca2+ [175]. FAT/CD36 regulates store-operated Ca2+ channels, which are vital for maintaining myocardial health. FAT/CD36 knockdown interrupts cytosolic Ca2+ clearance, which upregulates SERCA2 as compensation. Conduction anomalies, e.g., bradycardia, atrioventricular blockage, are obvious following FAT/CD36 knock-down, while tachyarrhythmogenic effects are caused by FAT/CD36 overexpression [173, 176]. Although FAT/CD36 overexpressed heart reduces infarction size following infarction [176]. Myocardial remodeling is influenced by FAT/CD36 mediated Ca2+-dependent phospholipase A2 activation [174].
Angiogenic regulator Notch has also shown a predominant role in FABP4, FABP5, lipoprotein lipase, FAT/CD36, and angiopoietin-like 4 (ANGPTL4) expression [177]. Ultimately, endothelial Notch signaling interrupts insulin sensitivity and glucose tolerance [178]. Even current finding has shown the pivotal role of another angiogenic regulator, angiopoietin 2 (ANGPT2), in modulating FAT/CD36 and FATP3 expression through integrin α5β1 signaling and key to insulin resistance [179]. All these findings demand to investigate more precisely the relationship between angiogenesis and fatty acid transport.
It is noteworthy that fatty acid transport and intravascular lipoproteins transport occur on the apical endothelial surface. For example, approximately 30% of LDL is metabolized by fenestrated endothelium, but LDL receptors and CD36 are crucial for LDL internalization [180, 181]. On the other hand, high-density lipoprotein is known as good lipoprotein transcytoses by several transporters, such as ATP binding cassette transporter G1, A1, and scavenger receptor class B type I. But it’s still obscured if triglyceride can cross the endothelium or not. Again glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1(GPIHBP1)-dependent lipoprotein lipase is transported from adipocytes or myocytes to endothelium for hydrolysis of triglyceride and lipolysis of chylomicrons [182, 183].
Cytoplasmic FABPs are highly expressed in metabolically active tissues [184]. FABPs are responsible for metabolic homeostasis and different lipid-mediated biological processes in various tissues despite the similarities in their tertiary structures and ligand affinity [152, 185]. Metabolic disorders play a crucial role in CVDs, abnormal lipid signaling, high lipids storage, trafficking, and signaling capacity of macrophages and adipocytes. Henceforth, the contribution of FABPs in lipid-related physiology and cardiovascular impacts is significant [184, 186]. However, the biological activities of FABPs remain a mystery [152]. Different studies have found that endothelial-FABP4 expression is upregulated in various organs [187–189]. The pro-angiogenic role of endothelial-FABP4 is induced by promoting cell proliferation, survival, and migration [188–190]. FABP4 also regulates the functions of various mitogenic pathways, such as stem cell factor/c-kit signaling, which plays a vital role in its pro-angiogenic activity. In turn, FABP4 expression is controlled by vascular endothelial growth factor-A (VEGF-A) and mammalian endothelial target of rapamycin complex 1 [188, 189]. FABP4 also determines the inflammatory function of endothelial cells by regulating the gene expression that plays crucial roles in endothelial cell activation, including endothelial eNOS and intercellular adhesion molecule 1.
FABP5 has a 55% amino acid sequence homology with FABP4 and primarily microvascular expression pattern in endothelial cells [154, 191]. Although, the FABP5 function in endothelial cells remains unclear. The current research has found the vital role of FABP4 and FABP5 in fatty acid uptake in the heart and skeletal muscle endothelial cells [154]. In animal models, combined deficiency of FABP4 and FABP5 ensures more excellent defence against insulin resistance, obesity, and atherosclerosis than mice deficient for either FABP4 or FABP5 [192, 193]. Along with LCFAs, FABP5 binds with PPARd and retinoic acid; upon binding these ligands, it mobilizes to the nucleus to induce PPARd and regulates cell growth and development. Regulation of endothelial cells homeostasis and vascular integrity is vital to organ physiology and tissue repair, regeneration, and tumor growth. Based on the additive functions of FABP4 and FABP5 in other cell types and their co-expression in microvascular endothelial cells, FABP5 played a role in regulating angiogenesis-related endothelial cell functions, including proliferation, migration, and survival. As a downstream inducer of FABP5-related effects in endothelial cells, the critical role of PPARδ was demonstrated.
The expression of FABP3 and FABP5 has been demonstrated in the microvascular of cardiac tissues and skeletal muscles. Also, FABP4 and FABP5 expressions were figured out in the microvessels of other organs with higher fatty acids metabolic activity, such as hepatic and adipose tissues [194, 195]. Endothelial cells can regulate the expression of FABP5 depending on tissue types and microvasculature [191]. Further investigations are needed to figure out the exact role of FABPs in lipid metabolism.
Roles of fatty acid metabolism in endothelial cells on angiogenesis
The metabolism of endothelial cells has only recently been recognized as a driving force of angiogenesis. Metabolic pathways, such as glycolysis, fatty acid oxidation, and glutamine metabolism, have distinct, essential roles during vessel formation. Moreover, endothelial cell metabolism is markedly perturbed in pathologies such as cancer and diabetes. For instance, because tumor endothelial cells increase glycolysis, lowering hyper glycolysis in tumor endothelial cells induces therapeutic benefits in preclinical tumor models. Expanding our knowledge of how endothelial cells alter their metabolism in disease could pave the way for novel therapeutic opportunities. The most recent data describe the association of endothelial cell metabolism in health and disease, emphasizing the changes in metabolism in the tumor endothelium.
The endothelial lining on the vessel wall is crucial for nutrients and oxygen supply to the tissue. Reduced nutrients availability or oxygen tension stimulates activation of quiescent endothelial cells for angiogenesis (de novo blood vessel formation from pre-existing blood vessels) [196, 197]. Angiogenic sprouting involves interaction between endothelial cells and their metabolic microenvironment. First, proangiogenic proteins are secreted from the oxygen and nutrition demanding tissues to activate endothelial cells. Activated endothelial cells extend filopodia (known as tip cells) towards angiogenic stimuli [198]. Migratory tip cells are followed by proliferating endothelial cells (known as stalk cells) to elongate the sprouting process [199]. Endothelial cells continuously compete to take the lead as tip cells [200]. Ultimately tip cells communicate with tip cells of adjunct sprouts to form de novo vascular circuits throughout the process until meeting oxygen and nutritional demands. Then proangiogenic factors downregulate, basement matrix establishes, pericytes recruit to onset perfusion, and endothelial cells become quiescent again. These endothelial cells of de novo blood vessels are defined as phalanx cells [201].
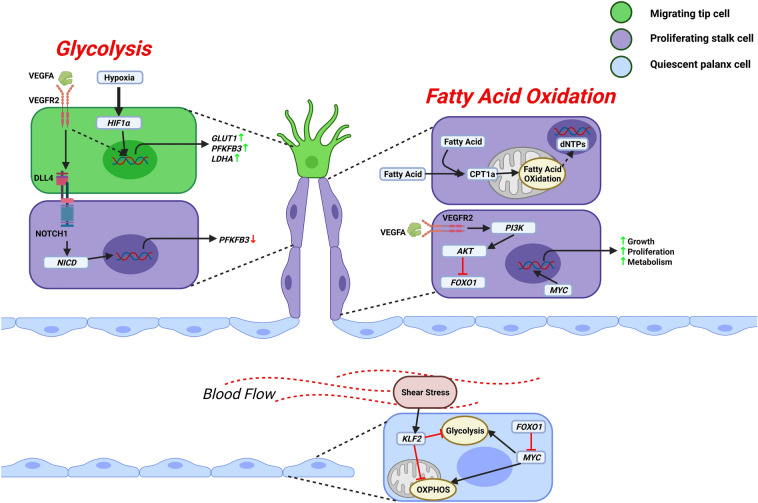
Angiogenesis requires coordinated not only endothelial morphogenic behavior but also metabolic activities. The metabolic activities of endothelial cells are different from other differentiating cells. Rapid energy production is needed for tip cell migration and navigation, while proliferative stalk cells need to produce cellular components [202]. Like cancer cells, around 85% energy production of endothelial cells depends on the aerobic glycolytic pathway [203–205]. Accelerated glycolysis by VEGFA increases endothelial glucose uptake and upregulation of glycolysis activators. Moreover, VEGF induces endothelial cell proliferation through the upregulation of FABP4 [190]. Figure 3 describes the regulation of endothelial metabolism by different factors. Also, VEGFR2 and NOTCH1 activities rely on glycolysis [206, 207]. But hypoxia-induced HIF1α can also activate glycolytic genes, e.g., SLC2A1, LDHA, PFKFB3 [208–210]. PFKFB3 driven glycolysis is vital for tip cell migration and stalk cell proliferation [205]. Although, the rate of glycolysis varies on endothelial subtypes. Microvascular endothelial cells are known to be more glycolytic and proliferative [205, 211]. Despite generating very little ATP, endothelial cells are relied on less efficient glycolysis due to higher endothelial cell glucose uptake capability and faster ATP production [212, 213]. Endothelial cells use stored glycogen for energy production in vascular tissues [214, 215].
Fig. 3.
Regulation of endothelial metabolism. VEGFA induces LDHA, PFKFB3, GLUT1, etc., through the VEGFR2 mediated glycolysis pathway to support tip cell migration. Hypoxia can also promote glycolysis by inducing HIF1α. Glycolysis also supports stalk cell proliferation by downregulating DLL4-NOTCH1 signalling-dependent PFKFB3 gene expression. Furthermore, even VEGFA blocks growth-inhibiting transcription factor FOXO1 via VEGFR2 mediated PI3K/AKT pathway to support the proliferation of stalk cells. Also, growth-enhancing transcription factor MYC promotes growth, anabolic metabolism, and proliferation of stalk cells. Along with glucose, proliferating stalk cells consume fatty acids, contributing to nucleotide synthesis for cellular proliferation. But in quiescent phalanx cells, FOXO1 represses MYC signaling to reduce glycolysis and mitochondrial metabolism. In addition, turbulent blood flow-induced shear stress-mediated activation of transcription factor KLF2 leads to reduced metabolic rate
Although endothelial cells are not dependent on mitochondria for energy production, mitochondria act as biosynthetic hub endothelial growth and proliferation. Fatty acid oxidation sustains vessel growth. Stalk cells rely on fatty acid oxidation for their normal functions. Beta oxidation of fatty acids supports the DNA replication process. Shuttled long-chain fatty acids into mitochondria are metabolized to acetyl-CoA, used for the citric acid cycle. The use of fatty acid-derived carbon for nucleotide biosynthesis differentiates endothelial cells from glucose and glutamine-dependent proliferating cells [28, 216]. Maybe during a nutrition-deprived state, endothelial cells catabolize fatty acids from stored lipids. Growth and division, the development of new membrane, the generation of signaling molecules, and modulating cellular signaling, endothelial cells are solely dependent on fatty acids and other lipids [217]. To improve the intracellular lipid storage, oxaloacetate and acetyl-CoA genesis occur into the cytosol from the citric acid cycle derived citrate. Fatty acid synthase enzyme converts acetyl-CoA to fatty acids, which is crucial for angiogenesis and vascular homeostasis [26, 218].
Though, mitochondrial fatty acid biosynthesis diminishes mitochondrial integrity due to exhaust the citric acid cycle intermediates. Anaplerosis (the replenishing process of the citric acid cycle intermediates) can only prevent mitochondrial disintegration and cell death [212]. As an essential nitrogen source for nucleotide biosynthesis, endocytosed non-essential amino acid glutamine converts to glutamate and α-ketoglutarate to contribute to anaplerosis [219]. Glutaminase is the rate-limiting enzyme for the conversion of glutamine. Upregulation of glutaminase activity is apparent during angiogenesis [220]. Besides anaplerosis, glutamine can be used for energy production, biosynthesis of glutathione, and reductive carboxylation for lipid production [219, 221]. Prostanoids mediate angiogenesis through multiple mechanisms, including the induction of VEGF production [222], the stimulation of endothelial cell sprouting, migration, and tube formation [223–225].
Although extracellular-regulated kinase phosphorylated, MYC (V-Myc avian myelocytomatosis viral oncogene homolog) regulates endothelial metabolism and growth factor signaling for cell growth, proliferation, and anabolic metabolism [226, 227]. In addition, accumulated MYC increases glycolysis, mitochondrial function, and cell cycle progression [228]. Many studies focused on angiogenic endothelial cells; however, the metabolic role of quiescent endothelial cells is critical for normal vascular function. Transcription factor FOXO1 drives endothelial quiescence by reducing overall metabolic activity. In addition, FOXO1 suppresses MYC activity [226].
Interestingly, quiescent endothelial cells consistently reduce glucose uptake or glycolysis and increase fatty acid uptake [29]. The change in nutrient utilization or slower metabolic rate in quiescent endothelial cells needs to elucidate. Reduced metabolic activity helps stabilize vessels and ensures efficient nutrient and O2 delivery to perivascular tissues. Also, endothelial cells protect themselves from mitochondrial-derived ROS-mediated damage due to high oxygen levels in the bloodstream. So, it is convincible that the reduced metabolic rate of quiescent endothelial cells is a primary adaptive response.
Endothelial cells respond to changes in environmental conditions, e.g., hypoxia, glucose deprivation, and blood flow-mediated shear stress activates AMPK to promote catabolic pathways in endothelial cells by fatty acid oxidation to produce energy [169, 229, 230]. Besides, AMPK inhibits metabolic sensor mammalian targets of rapamycin complex 1 to promote endothelial cell migration and angiogenesis [231, 232]. In addition, another angiogenic regulator, sirtuin 1, also senses endothelial metabolic state [233].
Metaflammation and ectopic fat deposition: effects on cardiovascular disease
Metabolic stress-induced chronic low-grade sterile inflammation due to innate immune response has been termed “metaflammation” [234]. Metaflammation is the critical driver of various chronic diseases, e.g., CVDs, diabetes, nonalcoholic steatohepatitis. In the body, inflammation is tightly controlled by distinct signaling cascades [235]. Pro-inflammatory mediators activate the innate immune system via various pattern recognition receptors [236, 237]. Most studies have focused on damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns to distort the immune system. DAMPs have been found to link with the pathogenesis of chronic metabolic diseases or disorders [238]. But DAMPs are quite varied in structure and origin, and the list of DAMPs is enriching rapidly. Pattern recognition receptors sense free fatty acids, oxidized LDL, cholesterol crystals, glucose, advanced glycation end products. To delineate the inflammatory stimuli due to metabolic stress from other damage-associated immune responses, Wang et al. have used the fresh term “metabolic-associated molecular patterns” [239]. Molecules derived due to persistent excessive FFAs and glucose in plasma are considered to become metabolic-associated molecular patterns or so-called “DAMPs” for innate immune response mediated metaflammation [240, 241].
Pancreatic beta-cell dysfunction, metabolic syndrome, and insulin resistance are correlated with elevated plasma-FFAs [242–244]. Toll-like receptor (TLR)-2 or TLR-4 mediated production of interleukin-1β, tumor necrosis factor-α, macrophage inflammatory protein-1α, chemokine (C–C motif) ligand 2, and chemokine (C–C motif) ligand 4 are triggered by upregulated plasma saturated fatty acids [245–250].
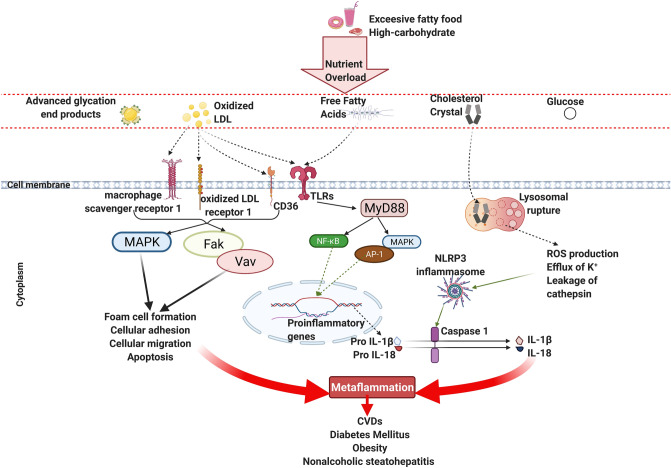
Also, oxLDL induces pattern recognition receptors mediated chronic inflammation, which leads to various metabolic diseases or disorders, e.g., atherosclerosis, heart disease, hypertension, obesity [251–254]. 80% oxLDL is taken up by transmembrane scavenger receptors (macrophage scavenger receptor 1, CD36, and lectin-type ox LDL receptor 1) to activate different intracellular signaling pathways, e.g., NF-κB and MAPK [255–257]. Figure 4 shows the metabolic-associated molecular patterns that induce metaflammation. Even oxLDL can form immune complexes with antibodies to activate NLPR3 inflammasome, extracellular signal-regulated kinase 1/2, phospholipase C gamma, c-Jun N-terminal kinase, paxillin, spleen tyrosine kinase, and cell division control protein 42 homolog signaling by Fc gamma receptor, CD36, and/or TLR-4 [258, 259]. Like oxLDL, oxidized phospholipids in platelet induce CD36/TLR- 2/TLR-6 complex-mediated MyD88 signaling in the progression of atherothrombosis [260].
Fig. 4.
Metabolic-associated molecular patterns induce metaflammation. High glucose/fat causes an overload of nutrient metabolites, e.g., glucose, advanced glycation end products, free fatty acids, oxidized LDL, and cholesterol crystal. MAPK and canonical NF-κB pathway-mediated transcription of proinflammatory genes are driven by free fatty acids and/or oxidized LDL-induced TLRs. Transcription factors AP-1 and NF-κB are responsible for inciting the proinflammatory genes. Besides, scavenger receptors take oxidized LDL to form foam cells. Cholesterol crystals can damage the lysosome, which leads to ROS production, K+ efflux, and cathepsin leakage. These stimulatory factors activate the NLRP3 inflammasome and result in maturation and secretion of IL-1β and IL-18, thereby instigating metaflammation
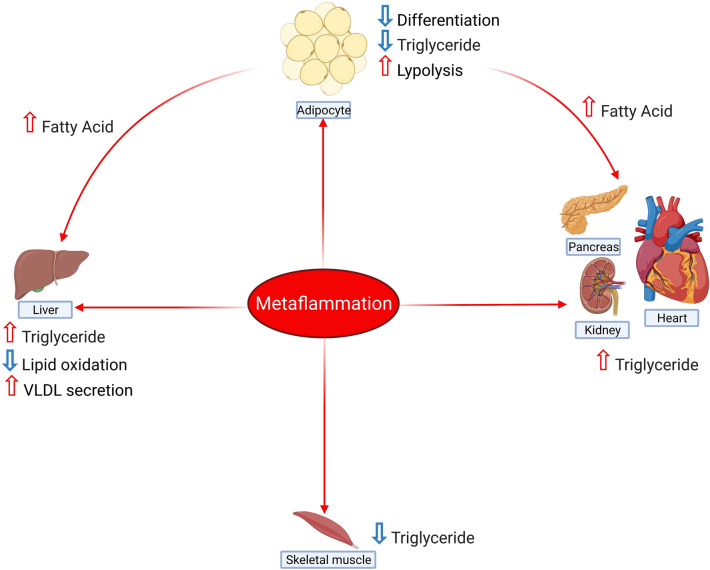
Inflammation provokes lipolysis in adipocytes and increases serum FFAs [261, 262]. Similar to adipose tissue, inflammation negatively regulates lipid deposition in skeletal muscle [263]. However, TNFα doesn’t affect fatty acid oxidation but upregulates fatty acid incorporation with diacylglycerol [264–266]. Figure 5 summarises the effects of metaflammation in different organs.
Fig. 5.
Effect of metaflammation on different organs. Metaflammation-induced cytokines to increase lipid deposition in the liver, heart, kidney, and pancreas, while lipolysis and fatty acid production are evident in adipose tissue. Similarly, triglyceride content reduces in skeletal muscle following chronic low-grade inflammation
As the prime metabolic organ, de novo lipogenesis (DNL), an influx of FFAs from adipose tissue due to lipolysis, fatty acid oxidation, and VLDL secretion maintain hepatic lipid homeostasis [267]. In addition, inflammation is associated with overexpression of FAT/CD36 and FABPs in the liver [268–270]. Systemic inflammation also increases hepatic de novo lipid synthesis by upregulation of several genes such as sterol regulatory element-binding protein 1c (SREBP-1c), FAS, ACC, and SCD-1, which can be ameliorated by sirtuin1 [271, 272]. More research is required to understand the mechanism of inflammation and its effects on non-adipose tissues.
Conclusions
The endothelium transcends key regulators to the function of every organ system. Endothelium dysfunction can lead to many diseases. Elevated levels of FFAs due to the result of metabolic defects contribute to endothelium dysfunction and subsequently lead to many diseases such as CVD. Dysfunction endothelium develops decreased NO production, increased cytokine production, impaired vasodilation, hyperactivity of platelets, and increased oxidative stress and inflammation. Consuming foods rich in n-3 fatty acids can lower plasma FFAs and other lipids with associated inflammatory cytokines, oxidative stress and may protect the endothelium. However, it would require a better understanding of FFAs and their transport and actions in endothelium and identify some better possible targets that could be used to develop better therapeutic approaches to intervene in the early events endothelium dysfunction.
Acknowledgements
This work is supported in part by grants from Throne Holst Foundation and the Faculty of Medicine, University Oslo, Norway.
Abbreviations
- ACE
Angiotensin-converting enzyme
- ANGPT2
Angiopoietin 2
- ANGPTL4
Angiopoietin-like 4
- COX
Cyclooxygenase
- CVD
Cardiovascular disease
- DAMP
Damage-associated molecular pattern
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- ET-1
Endothelin-1
- EPC
Endothelial progenitor cell
- FFA
Free fatty acid
- FABP
Fatty acid-binding protein
- CD36
Cluster of differentiation 36
- FATP
Fatty acid transport proteins
- LCFA
Long-chain saturated fatty acid
- LCPUFA
Long-chain polyunsaturated fatty acid
- LDL
Low-density lipoprotein
- MYC
V-Myc avian myelocytomatosis viral oncogene homolog
- NO
Nitric oxide
- PPARα
Peroxisome proliferator-activated receptor alpha
- PPARγ
Peroxisome proliferator-activated receptor-gamma
- PG
Prostaglandin
- PUFA
Polyunsaturated fatty acid
- RAS
Renin-angiotensin system
- ROS
Reactive oxygen species
- SREBP-1c
Sterol regulatory element-binding protein 1c
- SERCA
Sarco/endoplasmic reticulum calcium ATPase
- Tx
Thromboxane
- TNFα
Tumor necrotic factor α
- TLR
Toll-like receptor
Authors' contributions
Both the authors, RM and AKDR, conceived and wrote the review and the figures.
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital). Not Applicable.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chlopicki S. Perspectives in pharmacology of endothelium: from bench to bedside. Pharmacol Rep. 2015;67:vi–ix. doi: 10.1016/j.pharep.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31:S221–S230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- 3.Lai WK, Kan MY. Homocysteine-induced endothelial dysfunction. Ann Nutr Metab. 2015;67:1–12. doi: 10.1159/000437098. [DOI] [PubMed] [Google Scholar]
- 4.Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20:II-3–II-10. doi: 10.1002/j.1932-8737.1997.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe EA. Cell biology of endothelial cells. Hum Pathol. 1987;18:234–239. doi: 10.1016/s0046-8177(87)80005-9. [DOI] [PubMed] [Google Scholar]
- 6.De Caterina R. Endothelial dysfunctions: common denominators in vascular disease. Curr Opin Clin Nutr Metab Care. 2000;3:453–467. doi: 10.1097/00075197-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 8.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006 doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- 9.Munro JM, Cotran RS. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988;58:249–261. [PubMed] [Google Scholar]
- 10.Jude S, Roger S, Martel E, Besson P, Richard S, Bougnoux P, Champeroux P, Le Guennec JY. Dietary long-chain omega-3 fatty acids of marine origin: a comparison of their protective effects on coronary heart disease and breast cancers. Prog Biophys Mol Biol. 2006;90:299–325. doi: 10.1016/j.pbiomolbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Egert S, Stehle P. Impact of n-3 fatty acids on endothelial function: results from human interventions studies. Curr Opin Clin Nutr Metab Care. 2011;14:121–131. doi: 10.1097/MCO.0b013e3283439622. [DOI] [PubMed] [Google Scholar]
- 12.Hung AM, Booker C, Ellis CD, Siew ED, Graves AJ, Shintani A, Abumrad NN, Himmelfarb J, Ikizler TA. Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol Dial Transplant. 2015;30:266–274. doi: 10.1093/ndt/gfu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg IJ, Bornfeldt KE. Lipids and the endothelium: bidirectional interactions. Curr Atheroscler Rep. 2013;15:365. doi: 10.1007/s11883-013-0365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsberg HN. Lipoprotein physiology in nondiabetic and diabetic states. Relationship to atherogenesis. Diabetes Care. 1991;14:839–855. doi: 10.2337/diacare.14.9.839. [DOI] [PubMed] [Google Scholar]
- 15.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S, American Heart Association Clinical Lipidology T, Prevention Committee of the Council on Nutrition PA, Metabolism, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular N and Council on the Kidney in Cardiovascular D (2011) Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123:2292–333. 10.1161/CIR.0b013e3182160726 [DOI] [PubMed]
- 17.Egan BM, Greene EL, Goodfriend TL. Nonesterified fatty acids in blood pressure control and cardiovascular complications. Curr Hypertens Rep. 2001;3:107–116. doi: 10.1007/s11906-001-0021-y. [DOI] [PubMed] [Google Scholar]
- 18.Boden G (2008) Obesity and free fatty acids. Endocrinol Metab Clin N Am 37:635–646, viii–ix. 10.1016/j.ecl.2008.06.007 [DOI] [PMC free article] [PubMed]
- 19.Iantorno M, Campia U, Di Daniele N, Nistico S, Forleo GB, Cardillo C, Tesauro M. Obesity, inflammation and endothelial dysfunction. J Biol Regul Homeost Agents. 2014;28:169–176. [PubMed] [Google Scholar]
- 20.Virdis A. Endothelial dysfunction in obesity: role of inflammation. High Blood Press Cardiovasc Prev. 2016;23:83–85. doi: 10.1007/s40292-016-0133-8. [DOI] [PubMed] [Google Scholar]
- 21.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- 23.Guajardo I, Ayer A, Johnson AD, Ganz P, Mills C, Donovan C, Scherzer R, Shah SJ, Peralta CA, Dubin RF. Sex differences in vascular dysfunction and cardiovascular outcomes: the cardiac, endothelial function, and arterial stiffness in ESRD (CERES) study. Hemodial Int. 2018;22:93–102. doi: 10.1111/hdi.12544. [DOI] [PubMed] [Google Scholar]
- 24.Sena CM, Pereira AM, Seica R. Endothelial dysfunction—a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Kuo A, Lee MY, Sessa WC. Lipid droplet biogenesis and function in the endothelium. Circ Res. 2017;120:1289–1297. doi: 10.1161/CIRCRESAHA.116.310498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X, Schneider JG, Shenouda SM, Lee A, Towler DA, Chakravarthy MV, Vita JA, Semenkovich CF. De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation. J Biol Chem. 2011;286:2933–2945. doi: 10.1074/jbc.M110.193037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, Klar J, Genove G, Pietras K, Stone-Elander S, Claesson-Welsh L, Yla-Herttuala S, Lindahl P, Eriksson U. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 28.Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, Heggermont W, Godde L, Vinckier S, Van Veldhoven PP, Eelen G, Schoonjans L, Gerhardt H, Dewerchin M, Baes M, De Bock K, Ghesquiere B, Lunt SY, Fendt SM, Carmeliet P. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patella F, Schug ZT, Persi E, Neilson LJ, Erami Z, Avanzato D, Maione F, Hernandez-Fernaud JR, Mackay G, Zheng L, Reid S, Frezza C, Giraudo E, Fiorio Pla A, Anderson K, Ruppin E, Gottlieb E, Zanivan S. Proteomics-based metabolic modeling reveals that fatty acid oxidation (FAO) controls endothelial cell (EC) permeability. Mol Cell Proteomics. 2015;14:621–634. doi: 10.1074/mcp.M114.045575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231–1238. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- 32.Pircher A, Treps L, Bodrug N, Carmeliet P. Endothelial cell metabolism: a novel player in atherosclerosis? Basic principles and therapeutic opportunities. Atherosclerosis. 2016;253:247–257. doi: 10.1016/j.atherosclerosis.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 34.Kratz M, Cullen P, Kannenberg F, Kassner A, Fobker M, Abuja PM, Assmann G, Wahrburg U. Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur J Clin Nutr. 2002;56:72–81. doi: 10.1038/sj.ejcn.1601288. [DOI] [PubMed] [Google Scholar]
- 35.Yu YR, Li HL, Zhang XX. Effects of free fatty acids on nitric oxide synthase activity and mRNA expression in endothelial cell of SD rat aorta. Sichuan Da Xue Xue Bao Yi Xue Ban. 2008;39:193–196. [PubMed] [Google Scholar]
- 36.Wang XL, Zhang L, Youker K, Zhang MX, Wang J, LeMaire SA, Coselli JS, Shen YH. Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes. 2006;55:2301–2310. doi: 10.2337/db05-1574. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Prieto CF, Hernandez-Nuno F, Rio DD, Ruiz-Hurtado G, Aranguez I, Ruiz-Gayo M, Somoza B, Fernandez-Alfonso MS. High-fat diet induces endothelial dysfunction through a down-regulation of the endothelial AMPK-PI3K-Akt-eNOS pathway. Mol Nutr Food Res. 2015;59:520–532. doi: 10.1002/mnfr.201400539. [DOI] [PubMed] [Google Scholar]
- 38.Zhang WY, Schwartz E, Wang Y, Attrep J, Li Z, Reaven P. Elevated concentrations of nonesterified fatty acids increase monocyte expression of CD11b and adhesion to endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:514–519. doi: 10.1161/01.ATV.0000200226.53994.09. [DOI] [PubMed] [Google Scholar]
- 39.Esenabhalu VE, Schaeffer G, Graier WF. Free fatty acid overload attenuates Ca2+ signaling and NO production in endothelial cells. Antioxid Redox Signal. 2003;5:147–153. doi: 10.1089/152308603764816505. [DOI] [PubMed] [Google Scholar]
- 40.Mathew M, Tay E, Cusi K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol. 2010;9:9. doi: 10.1186/1475-2840-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasu T, Kobayashi M, Mutoh A, Yamakawa K, Momomura S, Ueda S. Dihydropyridine calcium channel blockers inhibit non-esterified-fatty-acid-induced endothelial and rheological dysfunction. Clin Sci (Lond) 2013;125:247–255. doi: 10.1042/CS20120311. [DOI] [PubMed] [Google Scholar]
- 42.Luscher TF. Endothelial dysfunction: the role and impact of the renin-angiotensin system. Heart. 2000;84(Suppl 1):i20–i22. doi: 10.1136/heart.84.suppl_1.i20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellieux C, Montessuit C, Papageorgiou I, Lerch R. Angiotensin II downregulates the fatty acid oxidation pathway in adult rat cardiomyocytes via release of tumour necrosis factor-alpha. Cardiovasc Res. 2009;82:341–350. doi: 10.1093/cvr/cvp004. [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Luo J, Ruan Y, Xiu L, Fang B, Zhang H, Wang M, Chen H. Free fatty acids activate renin-angiotensin system in 3T3-L1 adipocytes through nuclear factor-kappa B pathway. J Diabetes Res. 2016;2016:1587594. doi: 10.1155/2016/1587594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jayasooriya AP, Mathai ML, Walker LL, Begg DP, Denton DA, Cameron-Smith D, Egan GF, McKinley MJ, Rodger PD, Sinclair AJ, Wark JD, Weisinger HS, Jois M, Weisinger RS. Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc Natl Acad Sci USA. 2008;105:6531–6536. doi: 10.1073/pnas.0802690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azekoshi Y, Yasu T, Watanabe S, Tagawa T, Abe S, Yamakawa K, Uehara Y, Momomura S, Urata H, Ueda S. Free fatty acid causes leukocyte activation and resultant endothelial dysfunction through enhanced angiotensin II production in mononuclear and polymorphonuclear cells. Hypertension. 2010;56:136–142. doi: 10.1161/HYPERTENSIONAHA.110.153056. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe S, Tagawa T, Yamakawa K, Shimabukuro M, Ueda S. Inhibition of the renin-angiotensin system prevents free fatty acid-induced acute endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2005;25:2376–2380. doi: 10.1161/01.ATV.0000187465.55507.85. [DOI] [PubMed] [Google Scholar]
- 48.Briggs MA, Petersen KS, Kris-Etherton PM. Saturated fatty acids and cardiovascular disease: replacements for saturated fat to reduce cardiovascular risk. Healthcare (Basel) 2017 doi: 10.3390/healthcare5020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 50.Houston M. The relationship of saturated fats and coronary heart disease: fa(c)t or fiction? A commentary. Ther Adv Cardiovasc Dis. 2018;12:33–37. doi: 10.1177/1753944717742549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter JE, Zhang J, Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr. 2010;91:46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- 52.Tholstrup T, Marckmann P, Hermansen J, Holmer G, Sandstrom B. Effect of modified dairy fat on fasting and postprandial haemostatic variables in healthy young men. Br J Nutr. 1999;82:105–113. doi: 10.1017/S0007114599001257. [DOI] [PubMed] [Google Scholar]
- 53.Hunter KA, Crosbie LC, Weir A, Miller GJ, Dutta-Roy AK. A residential study comparing the effects of diets rich in stearic acid, oleic acid, and linoleic acid on fasting blood lipids, hemostatic variables and platelets in young healthy men. J Nutr Biochem. 2000;11:408–416. doi: 10.1016/s0955-2863(00)00097-8. [DOI] [PubMed] [Google Scholar]
- 54.Duttaroy AK. Postprandial activation of hemostatic factors: role of dietary fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2005;72:381–391. doi: 10.1016/j.plefa.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Chen X, Liu L, Palacios G, Gao J, Zhang N, Li G, Lu J, Song T, Zhang Y, Lv H. Plasma metabolomics reveals biomarkers of the atherosclerosis. J Sep Sci. 2010;33:2776–2783. doi: 10.1002/jssc.201000395. [DOI] [PubMed] [Google Scholar]
- 56.Sokolova M, Vinge LE, Alfsnes K, Olsen MB, Eide L, Kaasboll OJ, Attramadal H, Torp MK, Fosshaug LE, Rashidi A, Lien E, Finsen AV, Sandanger O, Aukrust P, Ranheim T, Yndestad A. Palmitate promotes inflammatory responses and cellular senescence in cardiac fibroblasts. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:234–245. doi: 10.1016/j.bbalip.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Xia G, Zhang Y, Liu J, Liu X, Li W, Lv Y, Wei S, Liu J, Quan J. Palmitate induces VSMC apoptosis via toll like receptor (TLR)4/ROS/p53 pathway. Atherosclerosis. 2017;263:74–81. doi: 10.1016/j.atherosclerosis.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Das UN. Long-chain polyunsaturated fatty acids interact with nitric oxide, superoxide anion, and transforming growth factor-beta to prevent human essential hypertension. Eur J Clin Nutr. 2004;58:195–203. doi: 10.1038/sj.ejcn.1601766. [DOI] [PubMed] [Google Scholar]
- 59.Storniolo CE, Rosello-Catafau J, Pinto X, Mitjavila MT, Moreno JJ. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol. 2014;2:971–977. doi: 10.1016/j.redox.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stirban A, Nandrean S, Gotting C, Tamler R, Pop A, Negrean M, Gawlowski T, Stratmann B, Tschoepe D. Effects of n-3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. Am J Clin Nutr. 2010;91:808–813. doi: 10.3945/ajcn.2009.28374. [DOI] [PubMed] [Google Scholar]
- 61.Sawada T, Tsubata H, Hashimoto N, Takabe M, Miyata T, Aoki K, Yamashita S, Oishi S, Osue T, Yokoi K, Tsukishiro Y, Onishi T, Shimane A, Taniguchi Y, Yasaka Y, Ohara T, Kawai H, Yokoyama M. Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc Diabetol. 2016;15:121. doi: 10.1186/s12933-016-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwingshackl L, Christoph M, Hoffmann G. Effects of olive oil on markers of inflammation and endothelial function-A systematic review and meta-analysis. Nutrients. 2015;7:7651–7675. doi: 10.3390/nu7095356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee CH, Lee SD, Ou HC, Lai SC, Cheng YJ. Eicosapentaenoic acid protects against palmitic acid-induced endothelial dysfunction via activation of the AMPK/eNOS pathway. Int J Mol Sci. 2014;15:10334–10349. doi: 10.3390/ijms150610334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamagata K. Docosahexaenoic acid regulates vascular endothelial cell function and prevents cardiovascular disease. Lipids Health Dis. 2017;16:118. doi: 10.1186/s12944-017-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamagata K. Prevention of endothelial dysfunction and cardiovascular disease by n-3 fatty acids-inhibiting action on oxidative stress and inflammation. Curr Pharm Des. 2020;26:3652–3666. doi: 10.2174/1381612826666200403121952. [DOI] [PubMed] [Google Scholar]
- 66.Xing SS, Yang XY, Zheng T, Li WJ, Wu D, Chi JY, Bian F, Bai XL, Wu GJ, Zhang YZ, Zhang CT, Zhang YH, Li YS, Jin S. Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway. Vascul Pharmacol. 2015;72:141–152. doi: 10.1016/j.vph.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Shankar SS, Mirzamohammadi B, Walsh JP, Steinberg HO. L-carnitine may attenuate free fatty acid-induced endothelial dysfunction. Ann N Y Acad Sci. 2004;1033:189–197. doi: 10.1196/annals.1320.018. [DOI] [PubMed] [Google Scholar]
- 68.Thijssen MA, Hornstra G, Mensink RP. Stearic, oleic, and linoleic acids have comparable effects on markers of thrombotic tendency in healthy human subjects. J Nutr. 2005;135:2805–2811. doi: 10.1093/jn/135.12.2805. [DOI] [PubMed] [Google Scholar]
- 69.Williams MJ, Sutherland WH, McCormick MP, de Jong SA, Walker RJ, Wilkins GT. Impaired endothelial function following a meal rich in used cooking fat. J Am Coll Cardiol. 1999;33:1050–1055. doi: 10.1016/s0735-1097(98)00681-0. [DOI] [PubMed] [Google Scholar]
- 70.Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology. 2007;148:160–165. doi: 10.1210/en.2006-1132. [DOI] [PubMed] [Google Scholar]
- 71.Tampakakis E, Tabit CE, Holbrook M, Linder EA, Berk BD, Frame AA, Breton-Romero R, Fetterman JL, Gokce N, Vita JA, Hamburg NM. Intravenous lipid infusion induces endoplasmic reticulum stress in endothelial cells and blood mononuclear cells of healthy adults. J Am Heart Assoc. 2016 doi: 10.1161/JAHA.115.002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001;20:5–19. doi: 10.1080/07315724.2001.10719008. [DOI] [PubMed] [Google Scholar]
- 73.Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Giampaoli S, Jansen A, et al. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med. 1995;24:308–315. doi: 10.1006/pmed.1995.1049. [DOI] [PubMed] [Google Scholar]
- 74.de Roos NM, Bots ML, Katan MB. Replacement of dietary saturated fatty acids by trans fatty acids lowers serum HDL cholesterol and impairs endothelial function in healthy men and women. Arterioscler Thromb Vasc Biol. 2001;21:1233–1237. doi: 10.1161/hq0701.092161. [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135:562–566. doi: 10.1093/jn/135.3.562. [DOI] [PubMed] [Google Scholar]
- 76.Iwata NG, Pham M, Rizzo NO, Cheng AM, Maloney E, Kim F. Trans fatty acids induce vascular inflammation and reduce vascular nitric oxide production in endothelial cells. PLoS ONE. 2011;6:e29600. doi: 10.1371/journal.pone.0029600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H, Li H, Bao Y, Zhang X, Yu Y. Free fatty acids induce endothelial dysfunction and activate protein kinase C and nuclear factor-kappaB pathway in rat aorta. Int J Cardiol. 2011;152:218–224. doi: 10.1016/j.ijcard.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 78.Staiger K, Staiger H, Weigert C, Haas C, Haring HU, Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 2006;55:3121–3126. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 79.Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, Doerries C, Horvath T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 80.Werner N, Nickenig G. Influence of cardiovascular risk factors on endothelial progenitor cells: limitations for therapy? Arterioscler Thromb Vasc Biol. 2006;26:257–266. doi: 10.1161/01.ATV.0000198239.41189.5d. [DOI] [PubMed] [Google Scholar]
- 81.Jiang H, Liang C, Liu X, Jiang Q, He Z, Wu J, Pan X, Ren Y, Fan M, Li M, Wu Z. Palmitic acid promotes endothelial progenitor cells apoptosis via p38 and JNK mitogen-activated protein kinase pathways. Atherosclerosis. 2010;210:71–77. doi: 10.1016/j.atherosclerosis.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 82.Lands B (2015) Fatty acids: essential fatty acids. Encyclopedia of food and health
- 83.Moncada S. Prostacyclin and arterial wall biology. Arteriosclerosis. 1982;2:193–207. doi: 10.1161/01.atv.2.3.193. [DOI] [PubMed] [Google Scholar]
- 84.Dutta-Roy AK, Ray TK, Sinha AK. Prostacyclin stimulation of the activation of blood coagulation factor X by platelets. Science. 1986;231:385–388. doi: 10.1126/science.3001935. [DOI] [PubMed] [Google Scholar]
- 85.Dutta-Roy AK, Gordon MJ, Campbell FM, Crosbie LC. Arachidonic acid uptake by human platelets is mediated by CD36. Platelets. 1996;7:291–295. doi: 10.3109/09537109609023591. [DOI] [PubMed] [Google Scholar]
- 86.Dutta-Roy AK, Kahn NN, Sinha AK. Prostaglandin E1: the endogenous physiological regulator of platelet mediated blood coagulation. Prostaglandins Leukot Essent Fatty Acids. 1989;35:189–195. doi: 10.1016/0952-3278(89)90002-1. [DOI] [PubMed] [Google Scholar]
- 87.Oates JA, FitzGerald GA, Branch RA, Jackson EK, Knapp HR, Roberts LJ., 2nd Clinical implications of prostaglandin and thromboxane A2 formation (2) N Engl J Med. 1988;319:761–767. doi: 10.1056/NEJM198809223191206. [DOI] [PubMed] [Google Scholar]
- 88.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 89.Arico S, Pattingre S, Bauvy C, Gane P, Barbat A, Codogno P, Ogier-Denis E. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J Biol Chem. 2002;277:27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 90.Yui K, Imataka G, Nakamura H, Ohara N, Naito Y. Eicosanoids derived from arachidonic acid and their family prostaglandins and cyclooxygenase in psychiatric disorders. Curr Neuropharmacol. 2015;13:776–785. doi: 10.2174/1570159x13666151102103305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glatzel DK, Koeberle A, Pein H, Loser K, Stark A, Keksel N, Werz O, Muller R, Bischoff I, Furst R. Acetyl-CoA carboxylase 1 regulates endothelial cell migration by shifting the phospholipid composition. J Lipid Res. 2018;59:298–311. doi: 10.1194/jlr.M080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mitchell JA, Ahmetaj-Shala B, Kirkby NS, Wright WR, Mackenzie LS, Reed DM, Mohamed N. Role of prostacyclin in pulmonary hypertension. Glob Cardiol Sci Pract. 2014;2014:382–393. doi: 10.5339/gcsp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toborek M, Lee YW, Garrido R, Kaiser S, Hennig B. Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cells. Am J Clin Nutr. 2002;75:119–125. doi: 10.1093/ajcn/75.1.119. [DOI] [PubMed] [Google Scholar]
- 95.Kuroda R, Hirata K, Kawashima S, Yokoyama M. Unsaturated free fatty acids inhibit Ca2+ mobilization and NO release in endothelial cells. Kobe J Med Sci. 2001;47:211–219. [PubMed] [Google Scholar]
- 96.Jin RC, Voetsch B, Loscalzo J. Endogenous mechanisms of inhibition of platelet function. Microcirculation. 2005;12:247–258. doi: 10.1080/10739680590925493. [DOI] [PubMed] [Google Scholar]
- 97.Moncada S. Adventures in vascular biology: a tale of two mediators. Philos Trans R Soc Lond B. 2006;361:735–759. doi: 10.1098/rstb.2005.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.FitzGerald GA. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am J Cardiol. 1991;68:11B–15B. doi: 10.1016/0002-9149(91)90379-y. [DOI] [PubMed] [Google Scholar]
- 99.Sixma JJ, van Zanten GH, Huizinga EG, van der Plas RM, Verkley M, Wu YP, Gros P, de Groot PG. Platelet adhesion to collagen: an update. Thromb Haemost. 1997;78:434–438. doi: 10.1055/s-0038-1657565. [DOI] [PubMed] [Google Scholar]
- 100.Gerritsen ME. Functional heterogeneity of vascular endothelial cells. Biochem Pharmacol. 1987;36:2701–2711. doi: 10.1016/0006-2952(87)90252-8. [DOI] [PubMed] [Google Scholar]
- 101.Oliver JJ, Webb DJ, Newby DE. Stimulated tissue plasminogen activator release as a marker of endothelial function in humans. Arterioscler Thromb Vasc Biol. 2005;25:2470–2479. doi: 10.1161/01.ATV.0000189309.05924.88. [DOI] [PubMed] [Google Scholar]
- 102.Byberg L, Smedman A, Vessby B, Lithell H. Plasminogen activator inhibitor-1 and relations to fatty acid composition in the diet and in serum cholesterol esters. Arterioscler Thromb Vasc Biol. 2001;21:2086–2092. doi: 10.1161/hq1201.100224. [DOI] [PubMed] [Google Scholar]
- 103.Hunter KA, Crosbie LC, Horgan GW, Miller GJ, Dutta-Roy AK. Effect of diets rich in oleic acid, stearic acid and linoleic acid on postprandial haemostatic factors in young healthy men. Br J Nutr. 2001;86:207–215. [PubMed] [Google Scholar]
- 104.Hunter KA, Crosbie L, Weir A, Miller GJ, DuttaRoy AK. The effects of physiological meals containing structured triglycerides with different fatty acid compositions on postprandial haemostasis. Atherosclerosis. 1997;134:191–191. doi: 10.1016/s0021-9150(97)88995-2. [DOI] [Google Scholar]
- 105.Mason RP, Jacob RF, Shrivastava S, Sherratt SCR, Chattopadhyay A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim Biophys Acta. 2016;1858:3131–3140. doi: 10.1016/j.bbamem.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Zhang W, Fu F, Tie R, Liang X, Tian F, Xing W, Li J, Ji L, Xing J, Sun X, Zhang H. Alpha-linolenic acid intake prevents endothelial dysfunction in high-fat diet-fed streptozotocin rats and underlying mechanisms. Vasa. 2013;42:421–428. doi: 10.1024/0301-1526/a000311. [DOI] [PubMed] [Google Scholar]
- 107.Gortan Cappellari G, Losurdo P, Mazzucco S, Panizon E, Jevnicar M, Macaluso L, Fabris B, Barazzoni R, Biolo G, Carretta R, Zanetti M. Treatment with n-3 polyunsaturated fatty acids reverses endothelial dysfunction and oxidative stress in experimental menopause. J Nutr Biochem. 2013;24:371–379. doi: 10.1016/j.jnutbio.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 108.Lopez D, Orta X, Casos K, Saiz MP, Puig-Parellada P, Farriol M, Mitjavila MT. Upregulation of endothelial nitric oxide synthase in rat aorta after ingestion of fish oil-rich diet. Am J Physiol Heart Circ Physiol. 2004;287:H567–H572. doi: 10.1152/ajpheart.01145.2003. [DOI] [PubMed] [Google Scholar]
- 109.Jung SB, Kwon SK, Kwon M, Nagar H, Jeon BH, Irani K, Yoon SH, Kim CS. Docosahexaenoic acid improves vascular function via up-regulation of SIRT1 expression in endothelial cells. Biochem Biophys Res Commun. 2013;437:114–119. doi: 10.1016/j.bbrc.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 110.Stebbins CL, Stice JP, Hart CM, Mbai FN, Knowlton AA. Effects of dietary decosahexaenoic acid (DHA) on eNOS in human coronary artery endothelial cells. J Cardiovasc Pharmacol Ther. 2008;13:261–268. doi: 10.1177/1074248408322470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Omura M, Kobayashi S, Mizukami Y, Mogami K, Todoroki-Ikeda N, Miyake T, Matsuzaki M. Eicosapentaenoic acid (EPA) induces Ca(2+)-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett. 2001;487:361–366. doi: 10.1016/s0014-5793(00)02351-6. [DOI] [PubMed] [Google Scholar]
- 112.Zanetti M, Grillo A, Losurdo P, Panizon E, Mearelli F, Cattin L, Barazzoni R, Carretta R. Omega-3 polyunsaturated fatty acids: structural and functional effects on the vascular wall. Biomed Res Int. 2015;2015:791978. doi: 10.1155/2015/791978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barden AE, Burke V, Mas E, Beilin LJ, Puddey IB, Watts GF, Irish AB, Mori TA. n-3 Fatty acids reduce plasma 20-hydroxyeicosatetraenoic acid and blood pressure in patients with chronic kidney disease. J Hypertens. 2015;33:1947–1953. doi: 10.1097/HJH.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 114.He K, Liu K, Daviglus ML, Jenny NS, Mayer-Davis E, Jiang R, Steffen L, Siscovick D, Tsai M, Herrington D. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2009;103:1238–1243. doi: 10.1016/j.amjcard.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poudyal H, Panchal SK, Diwan V, Brown L. Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog Lipid Res. 2011;50:372–387. doi: 10.1016/j.plipres.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 116.Bloomer RJ, Larson DE, Fisher-Wellman KH, Galpin AJ, Schilling BK. Effect of eicosapentaenoic and docosahexaenoic acid on resting and exercise-induced inflammatory and oxidative stress biomarkers: a randomized, placebo controlled, cross-over study. Lipids Health Dis. 2009;8:36. doi: 10.1186/1476-511X-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walker CG, West AL, Browning LM, Madden J, Gambell JM, Jebb SA, Calder PC. The pattern of fatty acids displaced by EPA and DHA following 12 months supplementation varies between blood cell and plasma fractions. Nutrients. 2015;7:6281–6293. doi: 10.3390/nu7085285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lagarde M, Calzada C, Guichardant M, Vericel E. In vitro and in vivo bimodal effects of docosahexaenoic acid supplements on redox status and platelet function. Prostaglandins Leukot Essent Fatty Acids. 2016;108:1–4. doi: 10.1016/j.plefa.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 119.Phang M, Lincz LF, Garg ML. Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women. J Nutr. 2013;143:457–463. doi: 10.3945/jn.112.171249. [DOI] [PubMed] [Google Scholar]
- 120.Liu M, Boussetta T, Makni-Maalej K, Fay M, Driss F, El-Benna J, Lagarde M, Guichardant M. Protectin DX, a double lipoxygenase product of DHA, inhibits both ROS production in human neutrophils and cyclooxygenase activities. Lipids. 2014;49:49–57. doi: 10.1007/s11745-013-3863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lagarde M, Calzada C, Guichardant M, Vericel E. Dose-effect and metabolism of docosahexaenoic acid: pathophysiological relevance in blood platelets. Prostaglandins Leukot Essent Fatty Acids. 2013;88:49–52. doi: 10.1016/j.plefa.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 122.Leray C, Wiesel ML, Freund M, Cazenave JP, Gachet C. Long-chain n-3 fatty acids specifically affect rat coagulation factors dependent on vitamin K: relation to peroxidative stress. Arterioscler Thromb Vasc Biol. 2001;21:459–465. doi: 10.1161/01.atv.21.3.459. [DOI] [PubMed] [Google Scholar]
- 123.Vanschoonbeek K, Wouters K, van der Meijden PE, van Gorp PJ, Feijge MA, Herfs M, Schurgers LJ, Hofker MH, de Maat MP, Heemskerk JW. Anticoagulant effect of dietary fish oil in hyperlipidemia: a study of hepatic gene expression in APOE2 knock-in mice. Arterioscler Thromb Vasc Biol. 2008;28:2023–2029. doi: 10.1161/ATVBAHA.107.156992. [DOI] [PubMed] [Google Scholar]
- 124.Bang HO, Dyerberg J. Plasma lipids and lipoproteins in Greenlandic west coast Eskimos. Acta Med Scand. 1972;192:85–94. doi: 10.1111/j.0954-6820.1972.tb04782.x. [DOI] [PubMed] [Google Scholar]
- 125.Dyerberg J, Bang HO, Hjorne N. Plasma cholesterol concentration in Caucasian Danes and Greenland West-coast Eskimos. Dan Med Bull. 1977;24:52–55. [PubMed] [Google Scholar]
- 126.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 127.Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17:387–393. doi: 10.1097/01.mol.0000236363.63840.16. [DOI] [PubMed] [Google Scholar]
- 128.Landa V, Zidek V, Mlejnek P, Simakova M, Silhavy J, Trnovska J, Kazdova L, Pravenec M. Sterol regulatory element binding protein 2 overexpression is associated with reduced adipogenesis and ectopic fat accumulation in transgenic spontaneously hypertensive rats. Physiol Res. 2014;63:587–590. doi: 10.33549/physiolres.932751. [DOI] [PubMed] [Google Scholar]
- 129.Shao W, Espenshade PJ. Expanding roles for SREBP in metabolism. Cell Metab. 2012;16:414–419. doi: 10.1016/j.cmet.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walczak R, Tontonoz P. PPARadigms and PPARadoxes: expanding roles for PPARgamma in the control of lipid metabolism. J Lipid Res. 2002;43:177–186. doi: 10.1016/S0022-2275(20)30159-0. [DOI] [PubMed] [Google Scholar]
- 131.Packard CJ, Demant T, Stewart JP, Bedford D, Caslake MJ, Schwertfeger G, Bedynek A, Shepherd J, Seidel D. Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J Lipid Res. 2000;41:305–318. doi: 10.1016/S0022-2275(20)32065-4. [DOI] [PubMed] [Google Scholar]
- 132.Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J Biol Chem. 2001;276:27855–27863. doi: 10.1074/jbc.M008885200. [DOI] [PubMed] [Google Scholar]
- 133.Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J Clin Invest. 2004;113:1277–1287. doi: 10.1172/JCI19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jacobson TA, Glickstein SB, Rowe JD, Soni PN. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol. 2012;6:5–18. doi: 10.1016/j.jacl.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 135.Raposo HF, Patricio PR, Simoes MC, Oliveira HC. Fibrates and fish oil, but not corn oil, up-regulate the expression of the cholesteryl ester transfer protein (CETP) gene. J Nutr Biochem. 2014;25:669–674. doi: 10.1016/j.jnutbio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 136.Chan DC, Watts GF, Nguyen MN, Barrett PH. Factorial study of the effect of n-3 fatty acid supplementation and atorvastatin on the kinetics of HDL apolipoproteins A-I and A-II in men with abdominal obesity. Am J Clin Nutr. 2006;84:37–43. doi: 10.1093/ajcn/84.1.37. [DOI] [PubMed] [Google Scholar]
- 137.Fialkow J. Omega-3 fatty acid formulations in cardiovascular disease: dietary supplements are not substitutes for prescription products. Am J Cardiovasc Drugs. 2016;16:229–239. doi: 10.1007/s40256-016-0170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mason RP, Sherratt SC, Jacob RF. Eicosapentaenoic acid inhibits oxidation of ApoB-containing lipoprotein particles of different size in vitro when administered alone or in combination with atorvastatin active metabolite compared with other triglyceride-lowering agents. J Cardiovasc Pharmacol. 2016;68:33–40. doi: 10.1097/FJC.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McInnes GT. Lowering blood pressure for cardiovascular risk reduction. J Hypertens Suppl. 2005;23:S3–8. doi: 10.1097/01.hjh.0000165622.34192.fd. [DOI] [PubMed] [Google Scholar]
- 140.Huang T, Shou T, Cai N, Wahlqvist ML, Li D. Associations of plasma n-3 polyunsaturated fatty acids with blood pressure and cardiovascular risk factors among Chinese. Int J Food Sci Nutr. 2012;63:667–673. doi: 10.3109/09637486.2011.652076. [DOI] [PubMed] [Google Scholar]
- 141.Rasmussen BM, Vessby B, Uusitupa M, Berglund L, Pedersen E, Riccardi G, Rivellese AA, Tapsell L, Hermansen K, Group KS Effects of dietary saturated, monounsaturated, and n-3 fatty acids on blood pressure in healthy subjects. Am J Clin Nutr. 2006;83:221–6. doi: 10.1093/ajcn/83.2.221. [DOI] [PubMed] [Google Scholar]
- 142.Cabo J, Alonso R, Mata P. Omega-3 fatty acids and blood pressure. Br J Nutr. 2012;107(Suppl 2):S195–200. doi: 10.1017/S0007114512001584. [DOI] [PubMed] [Google Scholar]
- 143.Agbor LN, Wiest EF, Rothe M, Schunck WH, Walker MK. Role of CYP1A1 in modulating the vascular and blood pressure benefits of omega-3 polyunsaturated fatty acids. J Pharmacol Exp Ther. 2014;351:688–698. doi: 10.1124/jpet.114.219535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hoshi T, Wissuwa B, Tian Y, Tajima N, Xu R, Bauer M, Heinemann SH, Hou S. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca(2)(+)-dependent K(+) channels. Proc Natl Acad Sci USA. 2013;110:4816–4821. doi: 10.1073/pnas.1221997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Novgorodtseva TP, Kantur TA, Karaman YK, Antonyuk MV, Zhukova NV. Modification of fatty acids composition in erythrocytes lipids in arterial hypertension associated with dyslipidemia. Lipids Health Dis. 2011;10:18. doi: 10.1186/1476-511X-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005;82:1178–1184. doi: 10.1093/ajcn/82.6.1178. [DOI] [PubMed] [Google Scholar]
- 147.Eiselein L, Wilson DW, Lame MW, Rutledge JC. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am J Physiol Heart Circ Physiol. 2007;292:H2745–H2753. doi: 10.1152/ajpheart.00686.2006. [DOI] [PubMed] [Google Scholar]
- 148.Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta. 2012;1821:852–857. doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bharadwaj KG, Hiyama Y, Hu Y, Huggins LA, Ramakrishnan R, Abumrad NA, Shulman GI, Blaner WS, Goldberg IJ. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem. 2010;285:37976–37986. doi: 10.1074/jbc.M110.174458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Theodorou K, Boon RA. Endothelial cell metabolism in atherosclerosis. Front Cell Dev Biol. 2018;6:82. doi: 10.3389/fcell.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pi X, Xie L, Patterson C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ Res. 2018;123:477–494. doi: 10.1161/CIRCRESAHA.118.313237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Duttaroy AK. Transport of fatty acids across the human placenta: a review. Prog Lipid Res. 2009;48:52–61. doi: 10.1016/j.plipres.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 153.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Iso T, Maeda K, Hanaoka H, Suga T, Goto K, Syamsunarno MR, Hishiki T, Nagahata Y, Matsui H, Arai M, Yamaguchi A, Abumrad NA, Sano M, Suematsu M, Endo K, Hotamisligil GS, Kurabayashi M. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler Thromb Vasc Biol. 2013;33:2549–2557. doi: 10.1161/ATVBAHA.113.301588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sawane M, Kajiya K, Kidoya H, Takagi M, Muramatsu F, Takakura N. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes. 2013;62:1970–1980. doi: 10.2337/db12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hwangbo C, Wu J, Papangeli I, Adachi T, Sharma B, Park S, Zhao L, Ju H, Go GW, Cui G, Inayathullah M, Job JK, Rajadas J, Kwei SL, Li MO, Morrison AR, Quertermous T, Mani A, Red-Horse K, Chun HJ. Endothelial APLNR regulates tissue fatty acid uptake and is essential for apelin's glucose-lowering effects. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aad4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Harmon CM, Abumrad NA. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J Membr Biol. 1993;133:43–49. doi: 10.1007/BF00231876. [DOI] [PubMed] [Google Scholar]
- 158.Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 159.Duncan JG, Bharadwaj KG, Fong JL, Mitra R, Sambandam N, Courtois MR, Lavine KJ, Goldberg IJ, Kelly DP. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-alpha transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-alpha activators. Circulation. 2010;121:426–435. doi: 10.1161/CIRCULATIONAHA.109.888735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Goudriaan JR, Dahlmans VE, Teusink B, Ouwens DM, Febbraio M, Maassen JA, Romijn JA, Havekes LM, Voshol PJ. CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J Lipid Res. 2003;44:2270–2277. doi: 10.1194/jlr.M300143-JLR200. [DOI] [PubMed] [Google Scholar]
- 161.Goto K, Iso T, Hanaoka H, Yamaguchi A, Suga T, Hattori A, Irie Y, Shinagawa Y, Matsui H, Syamsunarno MR, Matsui M, Haque A, Arai M, Kunimoto F, Yokoyama T, Endo K, Gonzalez FJ, Kurabayashi M. Peroxisome proliferator-activated receptor-gamma in capillary endothelia promotes fatty acid uptake by heart during long-term fasting. J Am Heart Assoc. 2013;2:e004861. doi: 10.1161/JAHA.112.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 163.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 164.Franekova V, Angin Y, Hoebers NT, Coumans WA, Simons PJ, Glatz JF, Luiken JJ, Larsen TS. Marine omega-3 fatty acids prevent myocardial insulin resistance and metabolic remodeling as induced experimentally by high insulin exposure. Am J Physiol Cell Physiol. 2015;308:C297–307. doi: 10.1152/ajpcell.00073.2014. [DOI] [PubMed] [Google Scholar]
- 165.Pepino MY, Kuda O, Samovski D, Abumrad NA. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr. 2014;34:281–303. doi: 10.1146/annurev-nutr-071812-161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K, Cameron R, Abumrad NA. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem. 1999;274:26761–26766. doi: 10.1074/jbc.274.38.26761. [DOI] [PubMed] [Google Scholar]
- 167.Bezaire V, Bruce CR, Heigenhauser GJ, Tandon NN, Glatz JF, Luiken JJ, Bonen A, Spriet LL. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: essential role in fatty acid oxidation. Am J Physiol Endocrinol Metab. 2006;290:E509–E515. doi: 10.1152/ajpendo.00312.2005. [DOI] [PubMed] [Google Scholar]
- 168.Xu S, Jay A, Brunaldi K, Huang N, Hamilton JA. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry. 2013;52:7254–7261. doi: 10.1021/bi400914c. [DOI] [PubMed] [Google Scholar]
- 169.Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Samovski D, Sun J, Pietka T, Gross RW, Eckel RH, Su X, Stahl PD, Abumrad NA. Regulation of AMPK activation by CD36 links fatty acid uptake to beta-oxidation. Diabetes. 2015;64:353–359. doi: 10.2337/db14-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nahle Z, Hsieh M, Pietka T, Coburn CT, Grimaldi PA, Zhang MQ, Das D, Abumrad NA. CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta-mediated adaptation to metabolic stress. J Biol Chem. 2008;283:14317–14326. doi: 10.1074/jbc.M706478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pietka TA, Sulkin MS, Kuda O, Wang W, Zhou D, Yamada KA, Yang K, Su X, Gross RW, Nerbonne JM, Efimov IR, Abumrad NA. CD36 protein influences myocardial Ca2+ homeostasis and phospholipid metabolism: conduction anomalies in CD36-deficient mice during fasting. J Biol Chem. 2012;287:38901–38912. doi: 10.1074/jbc.M112.413609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kuda O, Jenkins CM, Skinner JR, Moon SH, Su X, Gross RW, Abumrad NA. CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J Biol Chem. 2011;286:17785–17795. doi: 10.1074/jbc.M111.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Neckar J, Silhavy J, Zidek V, Landa V, Mlejnek P, Simakova M, Seidman JG, Seidman C, Kazdova L, Klevstig M, Novak F, Vecka M, Papousek F, Houstek J, Drahota Z, Kurtz TW, Kolar F, Pravenec M. CD36 overexpression predisposes to arrhythmias but reduces infarct size in spontaneously hypertensive rats: gene expression profile analysis. Physiol Genomics. 2012;44:173–182. doi: 10.1152/physiolgenomics.00083.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jabs M, Rose AJ, Lehmann LH, Taylor J, Moll I, Sijmonsma TP, Herberich SE, Sauer SW, Poschet G, Federico G, Mogler C, Weis EM, Augustin HG, Yan M, Gretz N, Schmid RM, Adams RH, Grone HJ, Hell R, Okun JG, Backs J, Nawroth PP, Herzig S, Fischer A. Inhibition of endothelial notch signaling impairs fatty acid transport and leads to metabolic and vascular remodeling of the adult heart. Circulation. 2018;137:2592–2608. doi: 10.1161/CIRCULATIONAHA.117.029733. [DOI] [PubMed] [Google Scholar]
- 178.Hasan SS, Jabs M, Taylor J, Wiedmann L, Leibing T, Nordstrom V, Federico G, Roma LP, Carlein C, Wolff G, Ekim-Ustunel B, Brune M, Moll I, Tetzlaff F, Grone HJ, Fleming T, Geraud C, Herzig S, Nawroth PP, Fischer A. Endothelial Notch signaling controls insulin transport in muscle. EMBO Mol Med. 2020;12:e09271. doi: 10.15252/emmm.201809271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bae H, Hong KY, Lee CK, Jang C, Lee SJ, Choe K, Offermanns S, He Y, Lee HJ, Koh GY. Angiopoietin-2-integrin alpha5beta1 signaling enhances vascular fatty acid transport and prevents ectopic lipid-induced insulin resistance. Nat Commun. 2020;11:2980. doi: 10.1038/s41467-020-16795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Molino Y, David M, Varini K, Jabes F, Gaudin N, Fortoul A, Bakloul K, Masse M, Bernard A, Drobecq L, Lecorche P, Temsamani J, Jacquot G, Khrestchatisky M. Use of LDL receptor-targeting peptide vectors for in vitro and in vivo cargo transport across the blood-brain barrier. FASEB J. 2017;31:1807–1827. doi: 10.1096/fj.201600827R. [DOI] [PubMed] [Google Scholar]
- 181.Mao H, Lockyer P, Li L, Ballantyne CM, Patterson C, Xie L, Pi X. Endothelial LRP1 regulates metabolic responses by acting as a co-activator of PPARgamma. Nat Commun. 2017;8:14960. doi: 10.1038/ncomms14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Davies BS, Goulbourne CN, Barnes RH, 2nd, Turlo KA, Gin P, Vaughan S, Vaux DJ, Bensadoun A, Beigneux AP, Fong LG, Young SG. Assessing mechanisms of GPIHBP1 and lipoprotein lipase movement across endothelial cells. J Lipid Res. 2012;53:2690–2697. doi: 10.1194/jlr.M031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Beigneux AP, Miyashita K, Ploug M, Blom DJ, Ai M, Linton MF, Khovidhunkit W, Dufour R, Garg A, McMahon MA, Pullinger CR, Sandoval NP, Hu X, Allan CM, Larsson M, Machida T, Murakami M, Reue K, Tontonoz P, Goldberg IJ, Moulin P, Charriere S, Fong LG, Nakajima K, Young SG. Autoantibodies against GPIHBP1 as a Cause of Hypertriglyceridemia. N Engl J Med. 2017;376:1647–1658. doi: 10.1056/NEJMoa1611930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest. 2008;118:2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dutta-Roy AK, Gopalswamy N, Trulzsch DV. Prostaglandin E1 binds to Z protein of rat liver. Eur J Biochem. 1987;162:615–619. doi: 10.1111/j.1432-1033.1987.tb10683.x. [DOI] [PubMed] [Google Scholar]
- 186.Dutta-Roy AK. Cellular uptake of long-chain fatty acids: role of membrane-associated fatty-acid-binding/transport proteins. Cell Mol Life Sci. 2000;57:1360–1372. doi: 10.1007/pl00000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cataltepe O, Arikan MC, Ghelfi E, Karaaslan C, Ozsurekci Y, Dresser K, Li Y, Smith TW, Cataltepe S. Fatty acid binding protein 4 is expressed in distinct endothelial and non-endothelial cell populations in glioblastoma. Neuropathol Appl Neurobiol. 2012;38:400–410. doi: 10.1111/j.1365-2990.2011.01237.x. [DOI] [PubMed] [Google Scholar]
- 188.Elmasri H, Ghelfi E, Yu CW, Traphagen S, Cernadas M, Cao H, Shi GP, Plutzky J, Sahin M, Hotamisligil G, Cataltepe S. Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: role of stem cell factor/c-kit pathway. Angiogenesis. 2012;15:457–468. doi: 10.1007/s10456-012-9274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Elmasri H, Karaaslan C, Teper Y, Ghelfi E, Weng M, Ince TA, Kozakewich H, Bischoff J, Cataltepe S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23:3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ghelfi E, Yu CW, Elmasri H, Terwelp M, Lee CG, Bhandari V, Comhair SA, Erzurum SC, Hotamisligil GS, Elias JA, Cataltepe S. Fatty acid binding protein 4 regulates VEGF-induced airway angiogenesis and inflammation in a transgenic mouse model: implications for asthma. Am J Pathol. 2013;182:1425–1433. doi: 10.1016/j.ajpath.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Masouye I, Hagens G, Van Kuppevelt TH, Madsen P, Saurat JH, Veerkamp JH, Pepper MS, Siegenthaler G. Endothelial cells of the human microvasculature express epidermal fatty acid-binding protein. Circ Res. 1997;81:297–303. doi: 10.1161/01.RES.81.3.297. [DOI] [PubMed] [Google Scholar]
- 192.Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, Minokoshi Y, Kahn BB, Parker RA, Hotamisligil GS. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–119. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 193.Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110:1492–1498. doi: 10.1161/01.CIR.0000141735.13202.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Antohe F, Popov D, Radulescu L, Simionescu N, Borchers T, Spener F, Simionescu M. Heart microvessels and aortic endothelial cells express the 15 kDa heart-type fatty acid-binding proteins. Eur J Cell Biol. 1998;76:102–109. doi: 10.1016/S0171-9335(98)80022-8. [DOI] [PubMed] [Google Scholar]
- 195.Yu CW, Liang X, Lipsky S, Karaaslan C, Kozakewich H, Hotamisligil GS, Bischoff J, Cataltepe S. Dual role of fatty acid-binding protein 5 on endothelial cell fate: a potential link between lipid metabolism and angiogenic responses. Angiogenesis. 2016;19:95–106. doi: 10.1007/s10456-015-9491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 197.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 198.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. 2011;138:4569–4583. doi: 10.1242/dev.062323. [DOI] [PubMed] [Google Scholar]
- 200.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 201.Mazzone M, Dettori D, de Oliveira RL, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, de Almodovar CR, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vandekeere S, Dewerchin M, Carmeliet P. Angiogenesis revisited: an overlooked role of endothelial cell metabolism in vessel sprouting. Microcirculation. 2015;22:509–517. doi: 10.1111/micc.12229. [DOI] [PubMed] [Google Scholar]
- 203.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Culic O, Gruwel ML, Schrader J. Energy turnover of vascular endothelial cells. Am J Physiol. 1997;273:C205–C213. doi: 10.1152/ajpcell.1997.273.1.C205. [DOI] [PubMed] [Google Scholar]
- 205.De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 206.Markowska AI, Jefferies KC, Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem. 2011;286:29913–29921. doi: 10.1074/jbc.M111.226423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 208.Ebert BL, Gleadle JM, O'Rourke JF, Bartlett SM, Poulton J, Ratcliffe PJ. Isoenzyme-specific regulation of genes involved in energy metabolism by hypoxia: similarities with the regulation of erythropoietin. Biochem J. 1996;313(Pt 3):809–814. doi: 10.1042/bj3130809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Obach M, Navarro-Sabate A, Caro J, Kong X, Duran J, Gomez M, Perales JC, Ventura F, Rosa JL, Bartrons R. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem. 2004;279:53562–53570. doi: 10.1074/jbc.M406096200. [DOI] [PubMed] [Google Scholar]
- 210.Fukasawa M, Tsuchiya T, Takayama E, Shinomiya N, Uyeda K, Sakakibara R, Seki S. Identification and characterization of the hypoxia-responsive element of the human placental 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. J Biochem. 2004;136:273–277. doi: 10.1093/jb/mvh137. [DOI] [PubMed] [Google Scholar]
- 211.Parra-Bonilla G, Alvarez DF, Al-Mehdi AB, Alexeyev M, Stevens T. Critical role for lactate dehydrogenase A in aerobic glycolysis that sustains pulmonary microvascular endothelial cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L513–L522. doi: 10.1152/ajplung.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 213.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Amemiya T. Glycogen metabolism in the capillary endothelium. Electron histochemical study of glycogen synthetase and phosphorylase in the pecten capillary of the chick. Acta Histochem. 1983;73:93–96. doi: 10.1016/S0065-1281(83)80080-4. [DOI] [PubMed] [Google Scholar]
- 215.Vizan P, Sanchez-Tena S, Alcarraz-Vizan G, Soler M, Messeguer R, Pujol MD, Lee WN, Cascante M. Characterization of the metabolic changes underlying growth factor angiogenic activation: identification of new potential therapeutic targets. Carcinogenesis. 2009;30:946–952. doi: 10.1093/carcin/bgp083. [DOI] [PubMed] [Google Scholar]
- 216.Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, Ogrodzinski M, Hecht V, Xu K, Acevedo PN, Hollern DP, Bellinger G, Dayton TL, Christen S, Elia I, Dinh AT, Stephanopoulos G, Manalis SR, Yaffe MB, Andrechek ER, Fendt SM, Vander Heiden MG. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol Cell. 2015;57:95–107. doi: 10.1016/j.molcel.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schug ZT, Frezza C, Galbraith LC, Gottlieb E. The music of lipids: how lipid composition orchestrates cellular behaviour. Acta Oncol. 2012;51:301–310. doi: 10.3109/0284186X.2011.643823. [DOI] [PubMed] [Google Scholar]
- 218.Browne CD, Hindmarsh EJ, Smith JW. Inhibition of endothelial cell proliferation and angiogenesis by orlistat, a fatty acid synthase inhibitor. FASEB J. 2006;20:2027–2035. doi: 10.1096/fj.05-5404com. [DOI] [PubMed] [Google Scholar]
- 219.DeBerardinis RJ, Cheng T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood. 2010;115:5102–5110. doi: 10.1182/blood-2009-07-230284. [DOI] [PubMed] [Google Scholar]
- 221.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF, Levine AC. Prostaglandin E2 induces hypoxia-inducible factor-1alpha stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem. 2002;277:50081–50086. doi: 10.1074/jbc.M201095200. [DOI] [PubMed] [Google Scholar]
- 223.Hahn GL, Polgar PR. Prostaglandin production in phenotypically distinct cultured bovine pulmonary artery endothelium. Atherosclerosis. 1984;51:143–150. doi: 10.1016/0021-9150(84)90150-3. [DOI] [PubMed] [Google Scholar]
- 224.Kuwashima L, Graeber J, Glaser BM. Stimulation of endothelial cell prostacyclin release by retina-derived factors. Invest Ophthalmol Vis Sci. 1988;29:1213–1220. [PubMed] [Google Scholar]
- 225.Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner JM. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- 226.Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger T, Braun T, Fruttiger M, Rajewsky K, Keller C, Bruning JC, Gerhardt H, Carmeliet P, Potente M. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529:216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV. MYC and metabolism on the path to cancer. Semin Cell Dev Biol. 2015;43:11–21. doi: 10.1016/j.semcdb.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 230.Dagher Z, Ruderman N, Tornheim K, Ido Y. Acute regulation of fatty acid oxidation and amp-activated protein kinase in human umbilical vein endothelial cells. Circ Res. 2001;88:1276–1282. doi: 10.1161/hh1201.092998. [DOI] [PubMed] [Google Scholar]
- 231.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 232.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Antonelli M, Kushner I. It's time to redefine inflammation. FASEB J. 2017;31:1787–1791. doi: 10.1096/fj.201601326R. [DOI] [PubMed] [Google Scholar]
- 235.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 237.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 238.Rozenfeld R, Devi LA. Receptor heteromerization and drug discovery. Trends Pharmacol Sci. 2010;31:124–130. doi: 10.1016/j.tips.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang X, Wang Y, Antony V, Sun H, Liang G. Metabolism-associated molecular patterns (MAMPs) Trends Endocrinol Metab. 2020;31:712–724. doi: 10.1016/j.tem.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 240.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lyons CL, Kennedy EB, Roche HM. Metabolic inflammation-differential modulation by dietary constituents. Nutrients. 2016 doi: 10.3390/nu8050247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–533. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 243.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 244.Wang Y, Qian Y, Fang Q, Zhong P, Li W, Wang L, Fu W, Zhang Y, Xu Z, Li X, Liang G. Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat Commun. 2017;8:13997. doi: 10.1038/ncomms13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 246.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 247.Ahmad R, Akhter N, Al-Roub A, Kochumon S, Wilson A, Thomas R, Ali S, Tuomilehto J, Sindhu S. MIP-1alpha induction by palmitate in the human monocytic cells implicates TLR4 signaling mechanism. Cell Physiol Biochem. 2019;52:212–224. doi: 10.33594/000000015. [DOI] [PubMed] [Google Scholar]
- 248.Ahmad R, Al-Roub A, Kochumon S, Akther N, Thomas R, Kumari M, Koshy MS, Tiss A, Hannun YA, Tuomilehto J, Sindhu S, Rosen ED. The synergy between palmitate and TNF-alpha for CCL2 production is dependent on the TRIF/IRF3 pathway: implications for metabolic inflammation. j Immunol. 2018;200:3599–3611. doi: 10.4049/jimmunol.1701552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kochumon S, Wilson A, Chandy B, Shenouda S, Tuomilehto J, Sindhu S, Ahmad R. Palmitate activates CCL4 expression in human monocytic cells via TLR4/MyD88 dependent activation of NF-kappaB/MAPK/PI3K signaling systems. Cell Physiol Biochem. 2018;46:953–964. doi: 10.1159/000488824. [DOI] [PubMed] [Google Scholar]
- 250.Yang W, Cao M, Mao X, Wei X, Li X, Chen G, Zhang J, Wang Z, Shi J, Huang H, Yao X, Liu C. Alternate-day fasting protects the livers of mice against high-fat diet-induced inflammation associated with the suppression of Toll-like receptor 4/nuclear factor kappaB signaling. Nutr Res. 2016;36:586–593. doi: 10.1016/j.nutres.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 251.Moyer BJ, Rojas IY, Kerley-Hamilton JS, Hazlett HF, Nemani KV, Trask HW, West RJ, Lupien LE, Collins AJ, Ringelberg CS, Gimi B, Kinlaw WB, 3rd, Tomlinson CR. Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. Model for AHR activation by kynurenine via oxidized-LDL, TLR2/4, TGFbeta, and IDO1. Toxicol Appl Pharmacol. 2016;300:13–24. doi: 10.1016/j.taap.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Catar RA, Muller G, Heidler J, Schmitz G, Bornstein SR, Morawietz H. Low-density lipoproteins induce the renin-angiotensin system and their receptors in human endothelial cells. Horm Metab Res. 2007;39:801–805. doi: 10.1055/s-2007-991158. [DOI] [PubMed] [Google Scholar]
- 253.Kita T, Kume N, Yokode M, Ishii K, Arai H, Horiuchi H, Moriwaki H, Minami M, Kataoka H, Wakatsuki Y. Oxidized-LDL and atherosclerosis. Role of LOX-1. Ann N Y Acad Sci. 2000;902:95–100. doi: 10.1111/j.1749-6632.2000.tb06304.x. [DOI] [PubMed] [Google Scholar]
- 254.Koenig W, Karakas M, Zierer A, Herder C, Baumert J, Meisinger C, Thorand B. Oxidized LDL and the risk of coronary heart disease: results from the MONICA/KORA Augsburg Study. Clin Chem. 2011;57:1196–1200. doi: 10.1373/clinchem.2011.165134. [DOI] [PubMed] [Google Scholar]
- 255.Zani IA, Stephen SL, Mughal NA, Russell D, Homer-Vanniasinkam S, Wheatcroft SB, Ponnambalam S. Scavenger receptor structure and function in health and disease. Cells. 2015;4:178–201. doi: 10.3390/cells4020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Vohra RS, Murphy JE, Walker JH, Ponnambalam S, Homer-Vanniasinkam S. Atherosclerosis and the Lectin-like OXidized low-density lipoprotein scavenger receptor. Trends Cardiovasc Med. 2006;16:60–64. doi: 10.1016/j.tcm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 257.Chen M, Qiu H, Lin X, Nam D, Ogbu-Nwobodo L, Archibald H, Joslin A, Wun T, Sawamura T, Green R. Lectin-like oxidized low-density lipoprotein receptor (LOX-1) in sickle cell disease vasculopathy. Blood Cells Mol Dis. 2016;60:44–48. doi: 10.1016/j.bcmd.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Rhoads JP, Lukens JR, Wilhelm AJ, Moore JL, Mendez-Fernandez Y, Kanneganti TD, Major AS. Oxidized low-density lipoprotein immune complex priming of the Nlrp3 inflammasome involves TLR and FcgammaR cooperation and is dependent on CARD9. J Immunol. 2017;198:2105–2114. doi: 10.4049/jimmunol.1601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Choi SH, Yin H, Ravandi A, Armando A, Dumlao D, Kim J, Almazan F, Taylor AM, McNamara CA, Tsimikas S, Dennis EA, Witztum JL, Miller YI. Polyoxygenated cholesterol ester hydroperoxide activates TLR4 and SYK dependent signaling in macrophages. PLoS ONE. 2013;8:e83145. doi: 10.1371/journal.pone.0083145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Biswas S, Zimman A, Gao D, Byzova TV, Podrez EA. TLR2 plays a key role in platelet hyperreactivity and accelerated thrombosis associated with hyperlipidemia. Circ Res. 2017;121:951–962. doi: 10.1161/CIRCRESAHA.117.311069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kim JY, Tillison K, Lee JH, Rearick DA, Smas CM. The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNF-alpha in 3T3-L1 adipocytes and is a target for transactivation by PPARgamma. Am J Physiol Endocrinol Metab. 2006;291:E115–E127. doi: 10.1152/ajpendo.00317.2005. [DOI] [PubMed] [Google Scholar]
- 262.Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M. Isoproterenol, TNFalpha, and insulin downregulate adipose triglyceride lipase in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2005;240:43–49. doi: 10.1016/j.mce.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 263.Liu L, Mei M, Yang S, Li Q. Roles of chronic low-grade inflammation in the development of ectopic fat deposition. Mediators Inflamm. 2014;2014:418185. doi: 10.1155/2014/418185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol. 2006;20:3364–3375. doi: 10.1210/me.2005-0490. [DOI] [PubMed] [Google Scholar]
- 265.Bruce CR, Dyck DJ. Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab. 2004;287:E616–E621. doi: 10.1152/ajpendo.00150.2004. [DOI] [PubMed] [Google Scholar]
- 266.Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 267.Nguyen P, Leray V, Diez M, Serisier S, Le Bloc'h J, Siliart B, Dumon H. Liver lipid metabolism. J Anim Physiol Anim Nutr (Berl) 2008;92:272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 268.Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T, Laitinen S, Kolak M, Fisher RM, Hamsten A, Auvinen P, Yki-Jarvinen H. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–G1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 269.Basar O, Akbal E, Koklu S, Tuna Y, Kocak E, Basar N, Tok D, Erbis H, Senes M. Increased H-FABP concentrations in nonalcoholic fatty liver disease. Possible marker for subclinical myocardial damage and subclinical atherosclerosis. Herz. 2013;38:417–422. doi: 10.1007/s00059-012-3714-x. [DOI] [PubMed] [Google Scholar]
- 270.Vida M, Serrano A, Romero-Cuevas M, Pavon FJ, Gonzalez-Rodriguez A, Gavito AL, Cuesta AL, Valverde AM, Rodriguez de Fonseca F, Baixeras E. IL-6 cooperates with peroxisome proliferator-activated receptor-alpha-ligands to induce liver fatty acid binding protein (LFABP) up-regulation. Liver Int. 2013;33:1019–1028. doi: 10.1111/liv.12156. [DOI] [PubMed] [Google Scholar]
- 271.Mei M, Zhao L, Li Q, Chen Y, Huang A, Varghese Z, Moorhead JF, Zhang S, Powis SH, Li Q, Ruan XZ. Inflammatory stress exacerbates ectopic lipid deposition in C57BL/6J mice. Lipids Health Dis. 2011;10:110. doi: 10.1186/1476-511X-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yamazaki Y, Usui I, Kanatani Y, Matsuya Y, Tsuneyama K, Fujisaka S, Bukhari A, Suzuki H, Senda S, Imanishi S, Hirata K, Ishiki M, Hayashi R, Urakaze M, Nemoto H, Kobayashi M, Tobe K. Treatment with SRT1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in MSG mice. Am J Physiol Endocrinol Metab. 2009;297:E1179–E1186. doi: 10.1152/ajpendo.90997.2008. [DOI] [PubMed] [Google Scholar]