Abstract
Purpose:
To compare the rate of postoperative endophthalmitis after immediately sequential bilateral cataract surgery (ISBCS) versus delayed sequential bilateral cataract surgery (DSBCS) using the American Academy of Ophthalmology Intelligent Research in Sight (IRIS) Registry database.
Design:
Retrospective cohort study.
Participants:
Patients in the IRIS Registry who underwent cataract surgery from 2013 through 2018.
Methods:
Patients who underwent cataract surgery were divided into 2 groups: (1) ISBCS and (2) DSBCS (second-eye surgery ≥1 day after the first-eye surgery) or unilateral surgery. Postoperative endophthalmitis was defined as endophthalmitis occurring within 4 weeks of surgery by International Classification of Diseases (ICD) code and ICD code with additional clinical criteria.
Main Outcome Measures:
Rate of postoperative endophthalmitis.
Results:
Of 5 573 639 IRIS Registry patients who underwent cataract extraction, 165 609 underwent ISBCS, and 5 408 030 underwent DSBCS or unilateral surgery (3 695 440 DSBCS, 1 712 590 unilateral surgery only). A total of 3102 participants (0.056%) met study criteria of postoperative endophthalmitis with supporting clinical findings. The rates of endophthalmitis in either surgery eye between the 2 surgery groups were similar (0.059% in the ISBCS group vs. 0.056% in the DSBCS or unilateral group; P = 0.53). Although the incidence of endophthalmitis was slightly higher in the ISBCS group compared with the DSBCS or unilateral group, the odds ratio did not reach statistical significance (1.08; 95% confidence interval, 0.87–1.31; P = 0.47) after adjusting for age, sex, race, insurance status, and comorbid eye disease. Seven cases of bilateral endophthalmitis with supporting clinical data in the DSBCS group and no cases in the ISBCS group were identified.
Conclusions:
Risk of postoperative endophthalmitis was not statistically significantly different between patients who underwent ISBCS and DSBCS or unilateral cataract surgery.
Keywords: Complications, COVID-19, Delayed sequential bilateral cataract surgery (DSBCS), Immediately sequential bilateral cataract surgery (ISBCS), Intelligent Research in Sight Registry (IRIS® Registry), Outcomes, Postoperative endophthalmitis, Same-day bilateral cataract surgery, SARS-CoV-2, Simultaneous bilateral cataract surgery
Cataract surgery is the most commonly performed intraocular ophthalmic surgery in the United States, with more than 2 million surgeries annually.1 Most cataract surgeries are considered elective, with both ophthalmologists and patients usually deciding the optimal timing of cataract surgeries. In the United States, most cataract surgeries are performed on separate days (delayed sequential bilateral cataract surgery [DSBCS]), but same-day surgery, or immediately sequential bilateral cataract surgery (ISBCS), is the preferred approach in many other countries, where it has been performed for many years without apparent increased risk.2
Immediately sequential bilateral cataract surgery offers potential advantages over DSBCS, including immediate vision improvement, fewer follow-up visits, and reduced costs for the patient and system.3 However, concerns associated with ISBCS include the inability to adjust the power of the second eye intraocular lens based on the refractive outcome of the first eye4 or to address dysphotopsia or other optical symptoms by trying a different intraocular lens type or surgical approach in the second eye and the feared risk of either unilateral or bilateral endophthalmitis.5 Although ISBCS is performed with strict aseptic separation between procedures, which includes newly prepping and draping the patient, changing all personal protective equipment, preparing a new sterile field, and obtaining new instruments before the second procedure,6,7 the risk of bilateral endophthalmitis remains. However, only a few cases of bilateral simultaneous postoperative endophthalmitis (BSPOE) after ISBCS have been reported in the literature, and most were associated with failure to achieve proper aseptic separation between 2 eyes.8 Other theoretical concerns with ISBCS are the risk of bilateral toxic anterior segment syndrome and increased risk for incorrect intraocular lens placement through fellow-eye confusion.
The coronavirus disease 2019 (COVID-19) pandemic has resulted in unprecedented disruption in medical care, particularly in the field of ophthalmology. Two recent editorials published in Ophthalmology highlight the risks and benefits of ISBCS versus DSBCS in light of the COVID-19 pandemic.9,10 Ahmed et al9 argue that ISBCS reduces risk of COVID-19 exposure. Considering all risks associated with bilateral surgery, including complications such as endophthalmitis, risks related to anesthesia, and now COVID-19, they argue that ISBCS is safer than DSBCS. In contrast, according to Masket,10 cost savings are the main benefit of ISBCS, and COVID-19 considerations are less relevant because cataract surgery primarily is elective. Masket agrees that the risk of bilateral endophthalmitis is very small but points out that cases may be underreported in the literature; thus, all precautions are warranted when blindness is a potential outcome.
Our recent analyses of the refractive outcomes of ISBCS versus DSBCS using the American Academy of Ophthalmology Intelligent Research in Sight (IRIS) Registry revealed nonclinically significant difference in visual outcome between 2 groups unless patients have multiple risk factors for worse outcome.11 In this study, we evaluated the rate of postoperative endophthalmitis after cataract surgery in the IRIS Registry to determine if there was any difference in risk of endophthalmitis between ISBCS and DSBCS approaches in this large dataset with nearly 60 million unique patients and 16 000 clinicians reporting from across the United States.12 The baseline rate of postoperative endophthalmitis is rare regardless of surgical approaches. Given that the number of ISBCS procedures is relatively small in literature, the evaluation of endophthalmitis rate between 2 surgical approaches is difficult with smaller datasets. Thus, we took advantage of the unprecedented size of cataract surgery records within the IRIS Registry to evaluate this challenging question.
Methods
This study was conducted in accordance with the tenets of the Declaration of Helsinki. Given the use of de-identified patient data, the review was exempted from the University of Washington Institutional Review Board. The methods of data collection and aggregation of the IRIS Registry database have been described previously.13,14 Version 2020_07_28 of the IRIS Registry, which was last modified on October 23, 2020, was used for this study.
Study Patient Population
Patients in the IRIS Registry with a history of first cataract surgery from 2013 through 2018 were included in the study after reviewing the entire IRIS Registry dataset for patients who underwent cataract surgery in at least 1 eye. Cataract surgery procedure was defined using Current Procedural Terminology codes 66982 and 66984. Immediately sequential bilateral cataract surgery was defined as both eye cataract extraction procedures occurring on the same date. For the patients who underwent ISBCS, we randomly chose either the left or the right eye to be coded as the first surgery eye. Delayed sequential bilateral cataract surgery was defined by the second eye surgery occurring 1 day or more after the first-eye surgery.
The primary outcome was the rate of endophthalmitis within 4 weeks of cataract surgery in at least 1 eye. We included all patients who underwent unilateral cataract surgery with the DSBCS group because a significant proportion of patients who experienced postoperative endophthalmitis after the first surgery did not proceed with the second eye; thus, excluding unilateral cases would result in bias toward a lower endophthalmitis rate in the DSBCS group. In our cohort, 68% of people who had no endophthalmitis diagnosis after the first surgery went on to undergo cataract surgery in their other eye. In comparison, only 33% of patients who had an endophthalmitis diagnosis after the first-eye surgery underwent a cataract surgery in the other eye. Additional secondary analyses were performed with unilateral cases excluded comparing only the ISBCS and DSBCS groups.
Postoperative endophthalmitis was determined in 2 ways: (1) any endophthalmitis diagnosis code based on International Classification of Diseases (ICD), Ninth Revision, Clinical Modification, and ICD, Tenth Revision, Clinical Modification, codes (098.42, 360.0*, 360.1*, H44.0*, and H44.1*) within 4 weeks of surgery and (2) a diagnosis code with supporting clinical findings, which were identified programmatically. Supporting clinical findings were defined by the endophthalmitis code occurring with either (1) a best-corrected visual acuity drop to 20/200 or worse or (2) an intraocular tap or injection procedure, or both, on or up to 3 days after the day of endophthalmitis diagnosis in the same eye within 4 weeks of surgery. Analysis with this stricter definition of postoperative endophthalmitis was included to account for the possibility of miscoding or lack of laterality information or that a prior endophthalmitis diagnosis might have been carried forward through patient’s records (i.e., the patient did not actually have an active case after cataract surgery). Because bilateral endophthalmitis is rare, we performed a manual check on all suspected bilateral endophthalmitis cases identified within 4 weeks of cataract surgery. Two board-certified ophthalmologists (C.S.L., A.Y.L.) reviewed all available clinical data including visual acuity, intraocular pressure, visit dates, and procedure and diagnosis codes for each eye to confirm both eyes had supporting evidence of endophthalmitis. In addition, to address potential biases in choosing different periods to define endophthalmitis, we performed secondary analyses of postoperative endophthalmitis occurring 2 and 6 weeks after cataract surgery. Supporting clinical evidence for bilateral endophthalmitis in the 2- and 6-week group analyses was evaluated with the same aforementioned criteria.
The following demographic and clinical variables were extracted: age at surgery, sex, race, insurance, history of comorbid ophthalmic disease diagnoses based on ICD, Ninth and Tenth Revisions, Clinical Modification, codes (age-related macular degeneration [AMD], diabetic retinopathy [DR], and glaucoma) in the year leading up to surgery (Table S1, available at www.aaojournal.org), laterality of first surgery, and number of days between bilateral cataract surgeries.
If patients had more than 1 form of insurance, a hierarchical heuristic was used to prioritize insurers in the following order: commercial, Medicare or Medicare Advantage, Medicaid, or other. Date of birth is redacted in the IRIS Registry for patients 87 years of age old or older at the time of data release, and as such, anyone older than 86 years does not have an age at cataract surgery and is grouped with the individuals in the ninth decade of life.
Statistical Analysis
Baseline demographic features and the outcome measures were summarized by surgery group (ISBCS vs. DSBCS or unilateral), and counts and percentages were reported. The primary outcome of interest was development of postoperative endophthalmitis within 4 weeks of cataract surgery. The number and proportion of patients developing endophthalmitis was reported with Clopper–Pearson confidence intervals (CI) overall and for the 2 surgery groups. Fisher exact tests were performed to determine whether the proportion of patients developing endophthalmitis in either eye in the ISBCS group and the DSBCS or unilateral group were significantly different.
Multivariable logistic regression models were run to estimate odds ratios (OR) for postoperative endophthalmitis developing in the ISBCS group relative to the DSBCS or unilateral group in at least 1 eye, adjusting for decade of life, race, insurance type, and history of AMD, DR, or glaucoma. One model was run for each criterion for postoperative endophthalmitis. Patients missing age (decade of life) or sex were excluded from regression models. For patients in whom postoperative endophthalmitis developed, AMD, DR, and glaucoma status from the affected eye was used. For patients with no postoperative endophthalmitis, the patient was considered to have a history of AMD, DR, or glaucoma if they had a diagnosis code in either eye before surgery.
Secondary analyses were performed to evaluate potential biases in choosing different surgical eye of interest, comparator groups, and follow-up periods. Modeling was repeated for OR of endophthalmitis developing in the first-surgery eye. Further modeling was performed comparing DSBCS alone with ISBCS with odds of endophthalmitis developing in either eye and the second surgery eye as the outcome. All analyses were repeated for endophthalmitis cases within postoperative periods of 2 and 6 weeks. In the cases in which endophthalmitis risk in the first or second eye was examined, AMD, DR, and glaucoma history from the surgery eye was used. Logistic regression models were also fit for odds of confirmed endophthalmitis separately for the ISBCS and DSBCS or unilateral surgery groups to assess the impacts of covariates on endophthalmitis within each group. Ninety-five percent CIs for the OR were computed using the score method. All analyses were performed with R software (R Foundation for Statistical Computing).
Results
Patient Demographic and Baseline Clinical Characteristics
The IRIS Registry database documented 5 573 639 patients who underwent cataract surgery between 2013 and 2018. A total of 165 609 of these patients (3%) underwent ISBCS, and 5 408 030 patients (97%) underwent DSBCS (n = 3 695 440 [66%]) or unilateral cataract surgery (n = 1 712 590 [31%]; Fig 1). Patient demographic factors are reported in Table 1. Most patients in both groups were female (59% and 58%) and White (74% and 73%). Rates of any prior AMD (3% vs. 2.8%), DR (1.5% vs. 1.7%), and glaucoma (4.6% vs. 4.4%) in the year before surgery were similar between the 2 groups.
Figure 1.
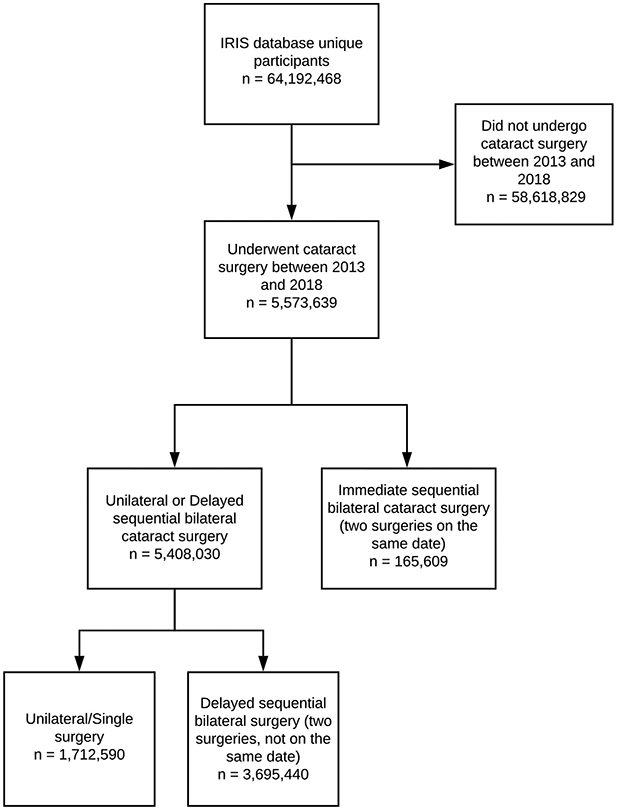
Flow diagram of study participants.
Table 1.
Demographic and Baseline Clinical Factors of Study Populations by Surgery Group
| Unilateral (n = 1 712 590) |
Delayed Sequential Bilateral Cataract Surgery (n = 3 695 440) |
Delayed Sequential Bilateral Cataract Surgery and Unilateral Surgery (n = 5 408 030) |
Immediate Sequential Bilateral Cataract Surgery (n = 165 609) |
Total (n = 5 573 639) |
|
|---|---|---|---|---|---|
| Decade of life at first surgery (yrs) | |||||
| 0–9 | 1133 (< 0.1) | 689 (< 0.1) | 1822 (< 0.1) | 101 (< 0.1) | 1923 (< 0.1) |
| 10–19 | 2097 (0.1) | 1271 (< 0.1) | 3368 (< 0.1) | 166 (0.1) | 3534 (< 0.1) |
| 20–29 | 4760 (0.3) | 3008 (< 0.1) | 7768 (0.1) | 334 (0.2) | 8102 (0.1) |
| 30–39 | 12 196 (0.7) | 8604 (0.2) | 20 800 (0.4) | 839 (0.5) | 21 639 (0.4) |
| 40–49 | 46 164 (2.7) | 46 021 (1.2) | 92 185 (1.7) | 3078 (1.9) | 95 263 (1.7) |
| 50–59 | 190 144 (11) | 282 911 (7.7) | 473 055 (8.7) | 15 371 (9.3) | 488 426 (8.8) |
| 60–69 | 519 982 (30) | 1 186 682 (32) | 1 706 664 (32) | 53 232 (32) | 1 759 896 (32) |
| 70–79 | 615 410 (36) | 1 589 310 (43) | 2 204 720 (41) | 67 046 (40) | 2 271 766 (41) |
| 80+ | 320 321 (19) | 576 623 (16) | 896 944 (17) | 25 398 (15) | 922 342 (17) |
| Missing age | 383 | 321 | 704 | 44 | 748 |
| Gender | |||||
| Female | 905 774 (53) | 2 225 135 (60) | 3 130 909 (58) | 97 351 (59) | 3 228 260 (58) |
| Male | 800 072 (47) | 1 459 533 (39) | 2 259 605 (42) | 67 790 (41) | 2 327 395 (42) |
| Not reported | 6744 (0.4) | 10 772 (0.3) | 17 516 (0.3) | 468 (0.3) | 17 984 (0.3) |
| Race | |||||
| White | 1 207 426 (71) | 2 816 202 (76) | 4 023 628 (74) | 120 224 (73) | 4 143 852 (74) |
| Black or African American | 139 019 (8.1) | 211 521 (5.7) | 350 540 (6.5) | 11 860 (7.2) | 362 400 (6.5) |
| Asian | 48 297 (2.8) | 73 758 (2.0) | 122 055 (2.3) | 3774 (2.3) | 125 829 (2.3) |
| Other | 19 544 (1.1) | 31 870 (0.9) | 51 414 (1.0) | 2054 (1.2) | 53 468 (1.0) |
| Unknown | 298 304 (17) | 562 089 (15) | 860 393 (16) | 27 697 (17) | 888 090 (16) |
| Insurance | |||||
| Medicare | 431 429 (25) | 1 108 236 (30) | 1 539 665 (28) | 50 655 (31) | 1 590 320 (29) |
| Medicaid | 23 501 (1.4) | 42 490 (1.1) | 65 991 (1.2) | 5267 (3.2) | 71 258 (1.3) |
| Commercial | 928 930 (54) | 2 161 988 (59) | 3 090 918 (57) | 87 356 (53) | 3 178 274 (57) |
| Other | 36 090 (2.1) | 62 335 (1.7) | 98 425 (1.8) | 4915 (3.0) | 103 340 (1.9) |
| Unknown | 292 640 (17) | 320 391 (8.7) | 613 031 (11) | 17 416 (11) | 630 447 (11) |
| Prior eye disease | |||||
| First surgery eye | |||||
| AMD | 46 985 (2.7) | 73 777 (2.0) | 120 762 (2.2) | 3208 (1.9) | 123 970 (2.2) |
| DR | 27 983 (1.6) | 35 539 (1.0) | 63 522 (1.2) | 2040 (1.2) | 65 562 (1.2) |
| Glaucoma | 88 323 (5.2) | 109 414 (3.0) | 197 737 (3.7) | 4928 (3.0) | 202 665 (3.6) |
| Second surgery eye* | |||||
| AMD | — | 79 145 (2.1) | 79 145 (2.1) | 3147 (1.9) | 82 292 (2.1) |
| DR | — | 40 264 (1.1) | 40 264 (1.1) | 2031 (1.2) | 42 295 (1.1) |
| Glaucoma | — | 121 037 (3.3) | 121 037 (3.3) | 4913 (3.0) | 125 950 (3.3) |
| Either eye | |||||
| AMD | 46 985 (2.7) | 117 925 (3.2) | 164 910 (3.0) | 4711 (2.8) | 169 621 (3.0) |
| DR | 27 983 (1.6) | 54 432 (1.5) | 82 415 (1.5) | 2755 (1.7) | 85 170 (1.5) |
| Glaucoma | 88 323 (5.2) | 158 059 (4.3) | 246 382 (4.6) | 7283 (4.4) | 253 665 (4.6) |
AMD = age-related macular degeneration; DR = diabetic retinopathy; — = not applicable.
Data are presented as no. (%).
Percentages taken from the number of participants who underwent bilateral cataract surgery (n = 3 695 440, n = 165 609, and n = 3 861 049, respectively).
Postoperative Endophthalmitis
Overall, 3703 patients (0.066%) had a diagnosis of endophthalmitis within 4 weeks of cataract surgery, and of these, 3102 patients (0.056%) had a diagnosis of endophthalmitis with supporting clinical results. Rates of endophthalmitis within 4 weeks of surgery were similar between the 2 surgery groups, with 116 cases (0.070%; 95% CI, 0.058%–0.084%) in the group of patients who underwent ISBCS and 3587 cases (0.066%; 95% CI, 0.064%–0.069%) in the group of patients who underwent either DSBCS or unilateral cataract surgery (P = 0.56). For endophthalmitis diagnosis with supporting clinical findings, 98 cases (0.059%; 95% CI, 0.048%–0.072%) were identified in the ISBCS group, and 3004 cases (0.056%; 95% CI, 0.054%–0.058%) were identified in the DSBCS or unilateral group (P = 0.53). After expert review of examination findings, intraocular pressure results, diagnosis records, and procedure records by an established criteria mentioned in “Methods,” a total of 7 patients (0.00013%) were found to have had bilateral endophthalmitis within 4 weeks of cataract surgery, all of whom had undergone DSBCS. None of 4 patients with bilateral endophthalmitis identified by diagnosis in the ISBCS group (3 during the 4-week period and 1 during the 6-week period of follow-up) were found to have clinical findings that met our confirmation criteria, which was upheld on manual review (Table 2; Table S2, Table S3 and Supplemental Appendix, available at www.aaojournal.org).
Table 2.
Cases of Postoperative Endophthalmitis within 4 Weeks after Cataract Surgery Diagnosed with or without Supporting Clinical Findings
| Delayed Sequential Bilateral Cataract Surgery and Unilateral Surgery |
||||||
|---|---|---|---|---|---|---|
| Diagnosis | Eye |
Unilateral Surgery (n = 1 712 590) |
Delayed Sequential Bilateral Cataract Surgery (n = 3 695 440) |
Total (n = 5 408 030) |
Immediate Sequential Bilateral Cataract Surgery (n = 165 609) |
Total (n = 5 573 639) |
| Endophthalmitis diagnosis | Either eye* | 1311 (0.077) | 2276 (0.062) | 3587 (0.066) | 116 (0.070) | 3703 (0.066) |
| First eye | 1311 (0.077) | 690 (0.019) | 2001 (0.037) | 55 (0.033) | 2056 (0.037) | |
| Second eye† | — | 1623 (0.044) | 1623 (0.044) | 64 (0.039) | 1687 (0.044) | |
| Both eyes† | — | 37 (0.001) | 37 (0.001) | 3 (0.002) | 40 (0.001) | |
| Diagnosis with supporting clinical findings | Either eye* | 1118 (0.065) | 1886 (0.051) | 3004 (0.056) | 98 (0.059) | 3102 (0.056) |
| First eye | 1118 (0.065) | 524 (0.014) | 1642 (0.030) | 44 (0.027) | 1686 (0.030) | |
| Second eye† | — | 1369 (0.037) | 1369 (0.037) | 54 (0.033) | 1423 (0.037) | |
| Both eyes† | — | 7 (0.0002) | 7 (0.0001) | 0 (0.0) | 7 (0.0001) | |
— = not applicable.
Data are presented as no. (%).
Patients demonstrated postoperative endophthalmitis in either the first or second surgery eye, or both.
Percentages taken from the number of participants who underwent bilateral cataract surgery (n = 3 695 440, n = 165 609, and n = 3 861 049).
Secondary analyses failed to show a statistically significant difference in the rate of endophthalmitis between different groups. The OR of any diagnosis of postoperative endophthalmitis in either eye adjusted for decade of life, sex, race, and insurance type was 1.07 (95% CI, 0.88–1.08) for the ISBCS group compared with the DSBCS or unilateral surgery group; however, this was not statistically significant (P = 0.49). Results were similar in the subset of patients with supporting clinical findings with the OR of 1.08 (95% CI, 0.87–1.31), which also was not statistically significant (P = 0.47; Table 3). The OR of a diagnosis of postoperative endophthalmitis in the first surgery eye adjusted for decade of life, sex, race, and insurance type was 0.91 (95% CI, 0.68–1.18) for the ISBCS group compared with the DSBCS or unilateral surgery group; however, this was not statistically significant (P = 0.48). Results were similar in the subset of these patients with supporting clinical findings with the OR of 0.88 (95% CI, 0.64–1.17), which was not statistically significant (P = 0.40; Table 3).
Table 3.
Comparison of Odds Ratios for Risk of Postoperative Endophthalmitis between the Immediate Sequential Bilateral Cataract Surgery and Delayed Sequential Bilateral Cataract Surgery or Unilateral Surgery Groups
| Duration of Postoperative Follow-up for Endophthalmitis Diagnosis (wks) |
Diagnosis | Eye | Odds Ratio* (95% Confidence Interval) and P Value |
|---|---|---|---|
| 2 | Endophthalmitis diagnosis | Either eye† | 0.81 (0.64–1.01), 0.08 |
| First eye | 0.78 (0.56–1.06), 0.14 | ||
| Diagnosis with supporting clinical findings | Either eye† | 0.79 (0.61–1.01), 0.07 | |
| First eye | 0.74 (0.50–1.04), 0.10 | ||
| 4 | Endophthalmitis diagnosis | Either eye† | 1.07 (0.88–1.28), 0.49 |
| First eye | 0.91 (0.68–1.18), 0.48 | ||
| Diagnosis with supporting clinical findings | Either eye† | 1.08 (0.87–1.31), 0.47 | |
| First eye | 0.88 (0.64–1.17), 0.40 | ||
| 6 | Endophthalmitis diagnosis | Either eye† | 1.11 (0.93–1.32), 0.34 |
| First eye | 0.88 (0.67–1.13), 0.25 | ||
| Diagnosis with supporting clinical findings | Either eye† | 1.11 (0.91–1.33), 0.31 | |
| First eye | 0.84 (0.62–1.12), 0.25 |
For endophthalmitis developing after immediately sequential bilateral cataract surgery relative to delayed sequential bilateral cataract surgery or unilateral surgery based on logistic regression model adjusted for decade of life, sex, race, insurance, and history of age-related macular degeneration, diabetic retinopathy, and glaucoma.
Patients demonstrated postoperative endophthalmitis in either the first or second surgery eye, or both.
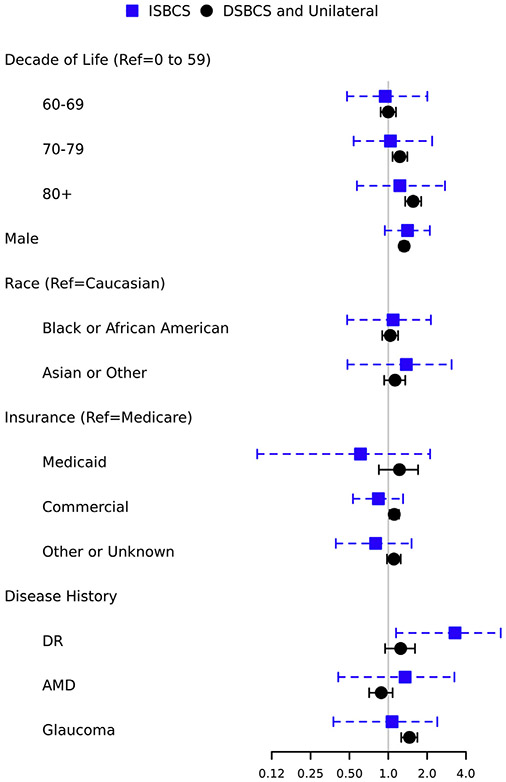
Figure 2 summarizes OR and 95% CIs associated with each covariate for development of endophthalmitis by surgery group. For the DSBCS or unilateral group, endophthalmitis odds were lowest for those 69 years of age and younger, with OR increasing for those 70 to 79 years of age (OR, 1.23; 95% CI, 1.08–1.40) and highest for those 80 years of age and older (OR, 1.56; 95% CI, 1.35–1.80) relative to those 0 to 59 years of age. Men showed higher odds of endophthalmitis developing relative to women (OR, 1.33; 95% CI, 1.24–1.43). No significant differences were found in OR for endophthalmitis based on race. Patients with commercial insurance showed a higher OR of endophthalmitis relative to those with Medicare (OR, 1.11; 95% CI, 1.02–1.21), and patients with prior history of glaucoma showed a higher odds of endophthalmitis (OR, 1.46; 95% CI, 1.26–1.68) relative to those without prior glaucoma.
Figure 2.
Forest plot showing risk of endophthalmitis associated with covariates for immediately sequential bilateral cataract surgery group (ISBCS; n = 165 609) versus delayed sequential bilateral cataract surgery (DSBCS) or unilateral surgery (n = 5 408 030). AMD = age-related macular degeneration; DR = diabetic retinopathy.
Secondary Analyses
No significant differences in estimated odds of endophthalmitis developing after cataract surgery within 2 or 6 weeks were found between the ISBCS group and the combined DSBCS or unilateral group. Endophthalmitis rates based on diagnosis with supporting clinical findings in either eye in the ISBCS group were 0.037% at 2 weeks and 0.065% at 6 weeks, and in the combined DSBCS or unilateral group, they were 0.047% at 2 weeks (OR, 0.79; 95% CI, 0.61–1.01) and 0.060% at 6 weeks (OR, 1.11; 95% CI, 0.91–1.33). Endophthalmitis rates by diagnosis with supporting clinical findings in the first-surgery eye in the ISBCS group were 0.019% at 2 weeks and 0.028% at 6 weeks, and the rates in the combined DSBCS or unilateral group were 0.025% (OR, 0.74; 95% CI, 0.50–1.04) at 2 weeks and 0.033% at 6 weeks (OR, 0.84; 95% CI, 0.62–1.12), respectively (Table 3).
When comparing the ISBCS group with the DSBCS patients only, the odds of endophthalmitis developing by diagnosis with supporting clinical findings in the second eye were slightly lower at 2 weeks (OR, 0.59; 95% 0.40–0.83) but similar when looking at endophthalmitis within 4 weeks (OR, 0.91; 95% CI, 0.68–1.18) and 6 weeks (OR, 0.99; 95% CI, 0.76–1.26) of surgery (Table S4, available at www.aaojournal.org).
Discussion
In analyses of 5 408 030 DSBCS or unilateral cataract surgeries and 165 609 ISBCS included in the IRIS Registry from 2013 through 2018, no significant difference in the odds of postoperative endophthalmitis between the ISBCS and DSBCS or unilateral groups were found after controlling for age, sex, race, insurance status, and comorbid eye diseases. Bilateral endophthalmitis was extremely rare in general, and all 7 cases of bilateral postoperative endophthalmitis (BPOE), confirmed by clinical review, occurred in the DSBCS group.
Endophthalmitis is one of the most serious complications associated with cataract surgery and is rare in the United States. Previous smaller studies in the United States have reported rates of postoperative endophthalmitis ranging from 0.04% to 0.215%.15-18 The difference in the observed postoperative period after cataract surgery may have contributed to the varying rates. For example, researchers at the Shandong Eye Institute defined the study period as 1 to 37 days and found an endophthalmitis rate of 0.13%, which was much higher than our rate of approximately 0.05% over 6 years.19 Another recent retrospective study of more than 8 million eyes in the IRIS Registry undergoing unilateral cataract surgery between 2013 and 2017 estimated an overall incidence of endophthalmitis occurring within 30 days after surgery to be 0.04% based on the ICD, Ninth and Tenth Revisions, Clinical Modification, diagnosis codes, the Current Procedural Terminology codes for intravitreal antibiotics, or both.20 Unlike our study, Pershing et al20 excluded patients undergoing ISBCS but included patients who underwent cataract surgery combined with other ocular procedures and found a higher rate of endophthalmitis in this group. Using the most recent version of the IRIS Registry, we found a slightly higher overall rate of endophthalmitis (0.056%) when we restricted our endophthalmitis definition within 4 weeks after cataract surgery using a stricter criterion (ICD code diagnosis combined with clinical results in patients undergoing unilateral surgery, DSBCS, and ISBCS). When we extended the postoperative period in question after cataract surgery, the overall rate of endophthalmitis increased slightly (0.060% at 6 weeks).
Previous studies also compared the rates of postoperative endophthalmitis between ISBCS and DSBCS. However, all were limited by relatively small sample sizes ranging from 980 to 125 188 eyes.8,21,22 Two smaller randomized control trials in Europe analyzing visual acuity outcomes found no cases of postoperative endophthalmitis in either ISBCS patients (480–834 eyes) or DSBCS patients.21,22 A larger cohort study involving 125 188 eyes (ISBCS, n = 95 606 eyes; DSBCS, n = 29 582 eyes) in 10 countries found the incidence of postoperative endophthalmitis after ISBCS to be 0.017% overall and a reduced incidence of 0.00696% with intracameral antibiotics.8 These endophthalmitis rates were based on self-reported data from members of the International Society of Bilateral Cataract Surgeons; thus, the true rates may have been different. In comparison, our study relied on anonymized electronic health record data information from more than 16 000 clinicians in the IRIS Registry.12 We also performed an analysis using a stricter definition for endophthalmitis that assessed for clinical supporting evidence, including antibiotic injection codes and visual acuity measurements, and found no statistically significant difference in the rate of endophthalmitis between ISBCS and DSBCS with or without unilateral surgery groups.
One of the primary concerns with performing ISBCS is the risk of BSPOE,23 which could result in profound blindness in both eyes, although the risk of bilateral endophthalmitis exists with either ISBCS or DSBCS. The existing literature shows the risk of BSPOE after ISBCS to be extremely small.23,24 Only 5 cases of BSPOE after ISBCS have been reported to the best of our knowledge, making the overall rate prediction difficult.25-29 In addition, the first 4 cases were believed to be the result of a breach in the aseptic protocol published by the International Society of Bilateral Cataract Surgeons.23,24,30 Operating room protocol preparation and instrumentation protocol were unknown for the fifth and most recent case.29 Moreover, no cases of bilateral endophthalmitis were reported among studies comparing outcomes between ISBCS and DSBCS.8,21,22,31 The lack of bilateral endophthalmitis in the ISBCS group in our study suggests that an aseptic protocol between the 2 surgery eyes was likely followed strictly in the IRIS Registry population. The absence of BPOE cases in the DSBCS group in previous literature could have been the result of having a smaller sample size overall, because we found several BPOE cases in the DSBCS group. Nevertheless, immediately sequential bilateral surgery in other specialties such as orthopedics have been performed routinely and continue to be the standard or a viable choice.32-34
Endophthalmitis is a rare surgical occurrence, and BPOE is extremely rare.15-18,20 When evaluating studies in this topic, it is critical to consider whether the sample size was sufficient to have observed any cases of endophthalmitis. To our best knowledge, our analysis is the largest and most current analysis of endophthalmitis risk in both ISBCS and DSBCS populations in the United States or in the existing literature, with 3.5 times more ISBCS patients than other similar studies.8,21,22,31 However, based on the rate of unilateral endophthalmitis detected in the ISBCS group (0.070%) and assuming that endophthalmitis risk is independent between the 2 eyes, we would need roughly 2 million patients (more than 10 times the number of patients that we included in this group) before we would expect to see any bilateral endophthalmitis. Thus, even a larger amount of data from diverse populations is needed to confirm this rate. It is noteworthy that the only other study in the United States to evaluate the endophthalmitis risk in the ISBCS versus DSBCS groups was restricted to patients from Northern California from 2013 through 2015.31 Thus, the current study results using the IRIS Registry, which come from a wide distribution of geographic locations throughout the entire United States, may be more generalizable.
It is important to note that we included unilateral cases in the DSBCS group in our primary analysis because patients who had endophthalmitis after the first cataract surgery were less likely to have proceeded to a second surgery. By excluding patients who underwent surgery in only 1 eye, we may have missed a small number of patients who did not undergo second-eye cataract surgery because of endophthalmitis after the first surgery, thus biasing our results toward less endophthalmitis in the DSBCS group and making the odds of any endophthalmitis developing in the ISBCS group seem deceptively higher in comparison. It is also possible that these patients could have undergone 2 surgeries, with only 1 surgery reported in the IRIS Registry. However, at the same time, our inclusion of unilateral cases with DSBCS also could have biased the results toward a higher endophthalmitis rate in this group, because the sample of patients who undergo unilateral surgery may overrepresent those in whom endophthalmitis developed after the first-eye surgery, may have more underlying systemic or ocular comorbidities that might increase odds for endophthalmitis, or both.
We did not find a significant difference in OR for endophthalmitis between the ISBCS and DSBCS or unilateral groups in our study. We performed multiple secondary analyses such as evaluating the risks of endophthalmitis in either eye or specifically in the first eye, excluding all unilateral cases from the DSBCS or unilateral group, comparing the first-eye rates of endophthalmitis in the combined group with the ISBCS group, and repeating all analyses for 2 and 6 weeks of postoperative follow-up to address any potential biases. Nevertheless, results were consistent for all analytic approaches, that is, none of these analyses revealed a significant difference in the rate of endophthalmitis between ISBCS and DSBCS with or without unilateral surgery groups.
The COVID-19 pandemic has presented many challenges to outpatient elective surgeries. Most notable is the fear of COVID-19 transmission in a medical setting, because hospitals are thought to be possible hot zones of potential COVID-19 transmission.35,36 As a result, patients are less likely to elect to undergo cataract surgery because of concerns of COVID-19 exposure in hospital or during travel to the hospital.37 In addition to decreased patient burden and recovery time,38 proponents of ISBCS9 also have highlighted the overall reduced COVID-19 exposure risk with ISBCS because of the decrease of patient visits and contact exposures by half, which is especially significant for the elderly population, who are at higher risk of death resulting from COVID-19.9,39
Several limitations exist in our study. First, we can measure only associations of various exposure and not causality. Second, we used ICD and Current Procedural Terminology codes and electronic health records to obtain patient information, which may have included misclassified or missing data. Coding error in particular would have resulted in a higher rate of endophthalmitis. However, we had strict criteria for defining endophthalmitis using the available clinical data such as 20/200 vision or worse, thus missing less severe cases and underrepresenting the true rate. Therefore, we believe that the true rate of endophthalmitis will be close to these 2 estimates of the endophthalmitis rate. Third, we did not have access to operative complications, and the cases that resulted in complications likely had a higher rate of endophthalmitis. Fourth, we included only patients who had undergone the first surgery between 2013 and 2018 after evaluating the records of patients who underwent cataract surgery in the entire dataset, including the years before 2013. However, it is possible that only 1 surgery that a patient underwent may have been reported in the IRIS Registry or that a patient underwent the second surgery after 2018, such that only 1 surgery would have been included in our dataset. Therefore, we focused on the patient-level data and analyzed primarily the rate of endophthalmitis in either eye. Fifth, we cannot account for many ocular or systemic factors that would contraindicate ISBCS in our dataset. However, ruling out these factors likely would occur by any clinicians before balancing the risks of endophthalmitis, and this may not have been a critical consideration because the number of DSBCS cases are overwhelmingly higher than that of ISBCS in the United States. A future randomized clinical trial likely would not be possible given the power needed to study this question, forcing us to rely on a large retrospective data analysis with important caveats. Therefore, despite these limitations, we believe our results are still likely applicable to routine clinical settings. Finally, our dataset may not represent other patient populations in the United States such as patients within the Veterans Affairs health care system or at other sites not participating in the IRIS Registry.
Based on data of over 5 million patients who underwent cataract surgery in a routine clinical setting, we found no statistically significant difference in the rates of postoperative endophthalmitis for patients who underwent ISBCS and those who had DSBCS or unilateral cataract surgery. Bilateral postoperative endophthalmitis is extremely rare, and it is possible that surgery center–related confounders may have a greater influence than surgical timing. Additionally, the lack of BSPOE in the ISBCS group may be the result of its significantly smaller sample size compared with the DSBCS and unilateral group. As ISBCS increases in popularity, expansion of the present study will be valuable to confirm our findings, and we hope to see progressive decline in endophthalmitis frequencies in all groups. In conclusion, our results suggest that the risk of postoperative endophthalmitis is similarly low for ISBCS and DSBCS. We were unable to validate it as a significant factor for patients choosing between these 2 approaches.
Supplementary Material
Acknowledgments
Supported by the National Eye Institute, National Institutes of Health (NIH), Bethesda, Maryland (grant no.: K23EY029246); the National Institute on Aging, NIH (grant no.: R01AG060942); the Latham Vision Research Innovation Award, Seattle, Washington; and Research to Prevent Blindness, Inc., New York, New York (unrestricted grant). The funding organizations had no role in the design or conduct of this research.
Abbreviations and Acronyms:
- AMD
age-related macular degeneration
- BPOE
bilateral postoperative endophthalmitis
- BSPOE
bilateral simultaneous postoperative endophthalmitis
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- DR
diabetic retinopathy
- DSBCS
delayed sequential bilateral cataract surgery
- ICD
International Classification of Diseases
- IRIS
Intelligent Research in Sight
- ISBCS
immediately sequential bilateral cataract surgery
- OR
odds ratio
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form. The author(s) have made the following disclosure(s): S.P.: Consultant – Acumen LLC, Verana Health
A.Y.L.: Consultant – Genentech/Roche, Gyroscope, Johnson & Johnson; Financial support – Lowy Medical Research Institute, Santen, Carl Zeiss Meditec; Lecturer – Topcon
Emily Chew, Joan Miller, and Russell Van Gelder, members of the editorial board of this journal, were recused from the peer-review process of this article and had no access to information regarding its peer-review.
IRIS Registry Analytic Center Consortium Study Group members: Flora Lum, MD (American Academy of Ophthalmology, San Francisco, CA), Emily Chew, MD (National Eye Institute, Bethesda, MD), Julia A. Haller, MD (Wills Eye Hospital, Philadelphia, PA), Alice C. Lorch, MD, MPH (Department of Ophthalmology, Mass Eye and Ear, Harvard Medical School, Boston, MA), Joan W. Miller, MD (Department of Ophthalmology, Mass Eye and Ear, Harvard Medical School, Boston, MA).
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at the University of Washington exempted this review, given the use of de-identified patient data. All research adhered to the tenets of the Declaration of Helsinki.
No animal subjects were included in this study.
Supplemental material available at www.aaojournal.org.
Presented at: Association for Research in Vision and Ophthalmology Annual Meeting, May 2021 (Virtual).
References
- 1.Cataract surgical rates. Community Eye Health. 2017;30:88–89. [PMC free article] [PubMed] [Google Scholar]
- 2.Grzybowski A, Wasinska-Borowiec W, Claoué C. Pros and cons of immediately sequential bilateral cataract surgery (ISBCS). Saudi J Ophthalmol. 2016;30:244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lansingh VC, Eckert KA, Strauss G. Benefits and risks of immediately sequential bilateral cataract surgery: a literature review. Clin Exp Ophthalmol. 2015;43:666–672. [DOI] [PubMed] [Google Scholar]
- 4.Jivrajka RV, Shammas MC, Shammas HJ. Improving the second-eye refractive error in patients undergoing bilateral sequential cataract surgery. Ophthalmology. 2012;119:1097–1101. [DOI] [PubMed] [Google Scholar]
- 5.Cao H, Zhang L, Li L, Lo S. Risk factors for acute endophthalmitis following cataract surgery: a systematic review and meta-analysis. PLoS One. 2013;8:e71731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson B Simultaneous bilateral cataract surgery: pro. Can J Ophthalmol. 2010;45:572–574. [DOI] [PubMed] [Google Scholar]
- 7.Arshinoff SA. Need for strict aseptic separation of the 2 procedures in simultaneous bilateral cataract surgery. J Cataract Refract Surg. 2006;32:376–377. [DOI] [PubMed] [Google Scholar]
- 8.Arshinoff SA, Bastianelli PA. Incidence of postoperative endophthalmitis after immediate sequential bilateral cataract surgery. J Cataract Refract Surg. 2011;37:2105–2114. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed IIK, Hill WE, Arshinoff SA. Bilateral same-day cataract surgery: an idea whose time has come #COVID-19. Ophthalmology. 2021;128:13–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masket S Same day bilateral cataract surgery: who benefits? Ophthalmology. 2021;128:11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owen JP, Blazes M, Lacy M, et al. Refractive outcomes after immediate sequential vs delayed sequential bilateral cataract surgery. JAMA Ophthalmol. 2021. Jul 1. 10.1001/jamaophthalmol.2021.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Academy of Ophthalmology. IRIS® Registry data analysis. Available at: https://www.aao.org/iris-registry/data-analysis/requirements; 2017. Accessed May 3, 2021.
- 13.Chiang MF, Sommer A, Rich WL, et al. The 2016 American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight) database: characteristics and methods. Ophthalmology. 2018;125:1143–1148. [DOI] [PubMed] [Google Scholar]
- 14.Lee CS, Owen JP, Yanagihara RT, et al. Smoking is associated with higher intraocular pressure regardless of glaucoma: a retrospective study of 12.5 million patients using the Intelligent Research in Sight (IRIS®) Registry. Ophthalmol Glaucoma. 2020;3:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen MK, Fiscella RG, Moshirfar M, Mooney B. Third- and fourth-generation fluoroquinolones: retrospective comparison of endophthalmitis after cataract surgery performed over 10 years. J Cataract Refract Surg. 2008;34: 1460–1467. [DOI] [PubMed] [Google Scholar]
- 16.Miller JJ, Scott IU, Flynn HW Jr, et al. Acute-onset endophthalmitis after cataract surgery (2000–2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005;139:983–987. [DOI] [PubMed] [Google Scholar]
- 17.West ES, Behrens A, McDonnell PJ, et al. The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112:1388–1394. [DOI] [PubMed] [Google Scholar]
- 18.Schmier JK, Hulme-Lowe CK, Covert DW, Lau EC. An updated estimate of costs of endophthalmitis following cataract surgery among Medicare patients: 2010–2014. Clin Ophthalmol. 2016;10:2121–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Zhan Y, Xie L. Clinical observations of acute-onset endophthalmitis after clear corneal phacoemulsification. Zhonghua Yan Ke Za Zhi. 2015;51:918–923. [PubMed] [Google Scholar]
- 20.Pershing S, Lum F, Hsu S, et al. Endophthalmitis after cataract surgery in the United States: a report from the Intelligent Research in Sight Registry, 2013–2017. Ophthalmology. 2020;127:151–158. [DOI] [PubMed] [Google Scholar]
- 21.Sarikkola A-U, Uusitalo RJ, Hellstedt T, et al. Simultaneous bilateral versus sequential bilateral cataract surgery: Helsinki Simultaneous Bilateral Cataract Surgery Study Report 1. J Cataract Refract Surg. 2011;37:992–1002. [DOI] [PubMed] [Google Scholar]
- 22.Serrano-Aguilar P, Ramallo-Fariña Y, Cabrera-Hernández JM, et al. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refract Surg. 2012;38:1734–1742. [DOI] [PubMed] [Google Scholar]
- 23.Li O, Kapetanakis V, Claoue C. Simultaneous bilateral endophthalmitis after immediate sequential bilateral cataract surgery: what’s the risk of functional blindness? Am J Ophthalmol. 2014;157:749–751.e1. [DOI] [PubMed] [Google Scholar]
- 24.Singh R, Dohlman TH, Sun G. Immediately sequential bilateral cataract surgery: advantages and disadvantages. Curr Opin Ophthalmol. 2017;28:81–86. [DOI] [PubMed] [Google Scholar]
- 25.Benezra D, Chirambo MC. Bilateral versus unilateral cataract extraction: advantages and complications. Br J Ophthalmol. 1978;62:770–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozdek SC, Onaran Z, Gürelik G, et al. Bilateral endophthalmitis after simultaneous bilateral cataract surgery. J Cataract Refract Surg. 2005;31:1261–1262. [DOI] [PubMed] [Google Scholar]
- 27.Kashkouli MB, Salimi S, Aghaee H, Naseripour M. Bilateral Pseudomonas aeruginosa endophthalmitis following bilateral simultaneous cataract surgery. Indian J Ophthalmol. 2007;55:374–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puvanachandra N, Humphry RC. Bilateral endophthalmitis after bilateral sequential phacoemulsification. J Cataract Refract Surg. 2008;34:1036–1037. [DOI] [PubMed] [Google Scholar]
- 29.Callaway NF, Ji MH, Mahajan VB, Moshfeghi DM. Bilateral endophthalmitis after immediately sequential bilateral cataract surgery. Ophthalmol Retina. 2019;3:618–619. [DOI] [PubMed] [Google Scholar]
- 30.Arshinoff S Bilateral endophthalmitis after simultaneous bilateral cataract surgery. J Cataract Refract Surg. 2008;34: 2006–2008. author reply 2008. [DOI] [PubMed] [Google Scholar]
- 31.Herrinton LJ, Liu L, Alexeeff S, et al. Immediate sequential vs. delayed sequential bilateral cataract surgery: retrospective comparison of postoperative visual outcomes. Ophthalmology. 2017;124:1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo S, Shao H, Huang Y, et al. Retrospective cohort study comparing complications, readmission, transfusion, and length of stay of patients undergoing simultaneous and staged bilateral total hip arthroplasty. Orthop Surg. 2020;12:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson SS, Kahlenberg CA, Blevins JL, et al. Complications associated with staged versus simultaneous bilateral total knee arthroplasty: an analysis of 7747 patients. Knee. 2019;26:1096–1101. [DOI] [PubMed] [Google Scholar]
- 34.Yoon H-S, Han C-D, Yang I-H. Comparison of simultaneous bilateral and staged bilateral total knee arthroplasty in terms of perioperative complications. J Arthroplasty. 2010;25:179–185. [DOI] [PubMed] [Google Scholar]
- 35.Al-Balas M, Al-Balas HI, Al-Balas H. Surgery during the COVID-19 pandemic: a comprehensive overview and perioperative care. Am J Surg. 2020;219:903–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Q, Gao Y, Wang X, et al. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naderi K, Maubon L, Jameel A, et al. Attitudes to cataract surgery during the COVID-19 pandemic: a patient survey. Eye. 2020;34:2161–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rush SW, Gerald AE, Smith JC, et al. Prospective analysis of outcomes and economic factors of same-day bilateral cataract surgery in the United States. J Cataract Refract Surg. 2015;41:732–739. [DOI] [PubMed] [Google Scholar]
- 39.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.