Abstract
As swelling occurs, CSF is preferentially displaced from the ischemic hemisphere. The ratio of CSF volume in the stroke-affected hemisphere to that in the contralateral hemisphere may quantify the progression of cerebral edema. We automatically segmented CSF from 1,875 routine CTs performed within 96 hours of stroke onset in 924 participants of a stroke cohort study. In 737 subjects with follow-up imaging beyond 24-hours, edema severity was classified as affecting less than one-third of the hemisphere (CED-1), large hemispheric infarction (LHI, over one-third the hemisphere), without midline shift (CED-2) or with midline shift (CED-3). Malignant edema was LHI resulting in deterioration, requiring osmotic therapy, surgery, or resulting in death. Hemispheric CSF ratio was lower on baseline CT in those with LHI (0.91 vs. 0.97, p < 0.0001) and decreased more rapidly in those with LHI who developed midline shift (0.01 per hour for CED-3 vs. 0.004/hour CED-2). The ratio at 24-hours was lower in those with midline shift (0.41, IQR 0.30–0.57 vs. 0.66, 0.56–0.81 for CED-2). A ratio below 0.50 provided 90% sensitivity, 82% specificity for predicting malignant edema among those with LHI (AUC 0.91, 0.85–0.96). This suggests that the hemispheric CSF ratio may provide an accessible early biomarker of edema severity.
Keywords: Stroke, brain edema, CT, imaging, cerebrospinal fluid
Introduction
Cerebral edema, the pathologic accumulation of brain water, begins around the region of ischemic injury within the first hours after a stroke. 1 However, it usually manifests clinically only after 24–48 hours, when it can precipitate neurologic deterioration and death. 2 Directly interrogating the evolution of early post-stroke edema has been hampered by lack of sensitive and accessible biomarkers to capture this dynamic process. Imaging-based measures of lesion volume do not distinguish the volume of infarcted tissue from amount of surrounding edema, providing a crude estimate of injured brain that only roughly correlates with risk of deterioration. 3 Although midline shift is a more direct consequence of severe edema, its onset is delayed and many stroke patients develop meaningful volumes of edema that never result in midline shift but still impair recovery. 4
Displacement of CSF from the extra-parenchymal spaces of the affected hemisphere is the earliest manifestation of edema, occurring within hours and providing a compensatory reserve to attenuate rises in compartmental pressure that result in midline shift. 5 Once this reserve of CSF in the sulci, cisterns, and ventricles is exhausted, cerebral herniation with neurologic deterioration will rapidly ensue. 6 Volumetric assessment of CSF displacement provides a quantitative surrogate for edema severity that is measurable from routine CT scans within the first 24-hours after stroke, often before infarct-related hypodensity and midline shift are apparent on CT.7,8 However, quantifying ΔCSF requires comparison of baseline and follow-up imaging and only captures global edema, obscuring prominent early compartmental shifts. We propose that the ratio of CSF volume in the lesional hemispheric to the contralateral unaffected hemisphere may provide a powerful dynamic biomarker of edema that can be measured from routine post-stroke CTs. We evaluate whether this hemispheric CSF ratio captures variability in early edema progression across the spectrum of stroke size and severity and distinguishes those destined for midline shift and malignant edema.
Methods
Study participants, protocol approvals, and consents
We selected subjects from three sites participating in an international prospective cohort study (Genetics of Early Neurological Instability after Ischemic Stroke, GENISIS) that enrolled patients with acute ischemic stroke presenting within six hours of onset between 2008 and 2017. We excluded subjects if onset time was unknown, those without any follow-up imaging within 96 hours of stroke onset, those with stroke in the brainstem or cerebellum, if subacute stroke was already visible on admission CT, or if final hospital diagnosis was not a stroke. We excluded CTs performed more than 96 hours after stroke onset or after surgical intervention. The parent cohort study was approved by each participating institution’s ethical review board and the retrospective imaging study was approved by the Washington University in St. Louis Human Research Protection Office. All participants provided written informed consent.
Image analysis
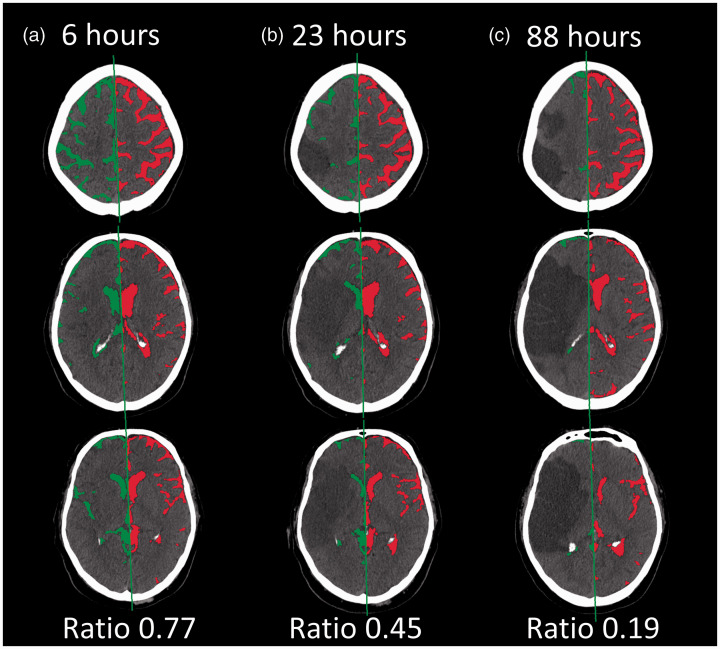
All available CT imaging was transferred to a central imaging repository for independent analysis. 9 Images were processed following an established workflow, blind to clinical data and outcomes. 10 This included automated brain extraction followed by registration of each image to a CT-specific atlas template and co-registration of each serial CT for a given patient to their baseline CT to ensure consistency of cranial volumes. 11 CSF in the ventricles, sulci, and cisterns was then automatically segmented from each CT (within the co-registered region-of-interest) using a deep learning algorithm. 12 In order to separate CSF from each hemisphere, the midline of each slice was automatically delineated by transforming the geometric midline of the atlas onto each participant’s CT. Accuracy of this approach was evaluated in comparison to midlines manually drawn on 636 slices from a subset of 30 stroke CTs. The error (estimated by the root mean square deviation of automated vs. manual lines, compared at both anterior and posterior points where midline crosses the skull) was median of 2-mm (IQR 1.4-mm) and the automated lines crossed within 2.5-mm of the manually drawn midline in 92% of cases. In addition, we manually reviewed all the segmentation and midline results (Figure 1), excluding cases where registration or CSF segmentation failed, midline delineation was inaccurate, or if CT exhibited enlarging hydrocephalus (i.e. where contralateral CSF volume would be artificially inflated). As stroke side was not known in all cases, the hemispheric CSF ratio was calculated as the smaller hemispheric CSF volume divided by the larger volume, assuming that the affected hemisphere would exhibit greater reductions in CSF volume. However, we performed sensitivity analysis in those where stroke side was known (based on visible infarct on follow-up CT). In these, we recalculated the CSF ratio for all CTs from those subjects, using the affected hemisphere as the numerator.
Figure 1.
Example of automated CSF segmentation and calculation of hemispheric CSF ratio on serial CTs after large hemispheric stroke. A patient with large hemispheric infarction who had CTs performed at: (a) baseline (6-hours after stroke onset); (b) at 23-hours; and (c) again at 88-hours (when 5-mm of midline shift had developed). The figure shows CSF segmentation with midline delineated on each slice (three slices shown at each time point). The two hemispheres (right, ipsilateral to stroke, in green, left in right) are separated and the hemispheric ratio shown below each.
ASPECT scores were manually ascertained on baseline CT by a single trained investigator. 13 ASPECTS of seven or below was considered low, as this has been demonstrated to predict higher risk of malignant edema. 14 Regions of visible acute infarct-related hypodensity were manually outlined on follow-up CTs and midline shift was measured (when present) at the level of the septum pellucidum. The severity of edema was categorized using an established classification scheme, in those with follow-up CT available beyond 24-hours: 15 CED-0 – no infarct seen; CED-1 – focal swelling involving less than one-third of hemisphere; CED-2 –swelling occupying over one-third of hemisphere but without midline shift; CED-3 – large hemispheric infarction with midline shift. This approach has been used to classify large stroke cohorts by edema severity. 16
The study also collected clinical variables such as age and NIHSS (obtained both within six hours of stroke onset and at 24-hours), treatment with tPA, glucose on admission, and TOAST stroke etiologic classification. 17 We categorized baseline NIHSS by severity: mild (0–5), moderate (6–12), severe (13 and above). All participants were followed until hospital discharge for clinical deterioration and death. Malignant edema was defined as cases with midline shift that resulted in deterioration that required osmotic therapy, surgical intervention, or resulted in death.
Statistical analysis
Measurements of hemispheric ratio, as well as other variables not meeting assumptions for normal distributions, are presented as medians with interquartile range (IQR). The ratios were compared between groups using Kruskal-Wallis analysis of variance tests. Post-hoc comparisons of groups was accomplished using pairwise Mann-Whitney U-tests with adjustment for multiple comparisons using the Benjamini & Hochberg method. 18 All tests were two-sided. Visual analysis of individual patient trajectories for the CSF ratio suggested a relatively stable linear change over the first 96 hours (shown in Supplemental Figure 1). Therefore, we employed random coefficients linear mixed-effects models to provide individual rates of change in CSF ratio for all patients and then estimate averages across groups, specifically within each NIHSS and CED group; age and NIHSS (for the CED model) were employed as covariates. We also selected the scan performed closest to 24-hours after stroke in each subject (if available) for cross-sectional analysis. We performed ROC analyses to calculate area-under-curve (AUC) and Youden’s method to find the optimal threshold to predict malignant edema. Bootstrapping was used to compare AUC between predictors. Multivariate logistic regression was employed to obtain adjusted odds ratio (OR) for risk of malignant edema. Analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria) and mixed models in SAS. Data supporting this analysis are available upon reasonable request to the corresponding author.
Results
Stroke cohort and CT imaging description
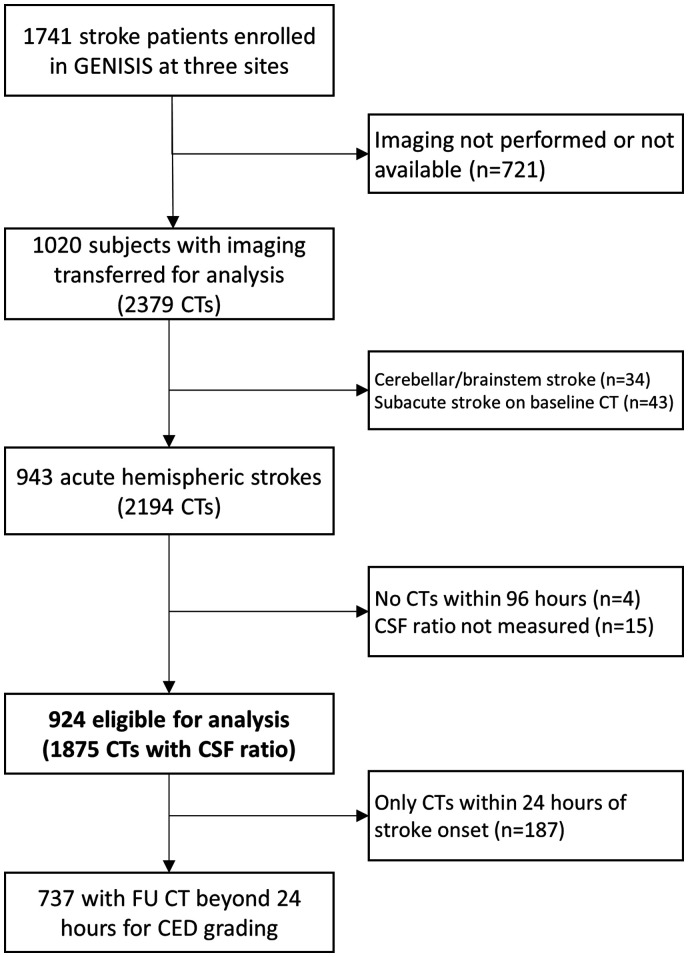
A total of 1741 participants with acute stroke were enrolled at three study sites. We obtained 2,379 CT scans on 1020 study participants. After exclusion of ineligible subjects and scans, there were 924 subjects with acute hemispheric strokes with follow-up CT imaging within 96 hours of onset (Figure 2). Participants excluded from analysis had lower baseline stroke severity (NIHSS of 5 vs. 7, p < 0.0001) and only six (1%) developed clinical signs of cerebral edema (full comparison of eligible versus ineligible cohorts is provided in Supplemental Table 1). Scan co-registration failed in 24 and CSF segmentation failed in only 19 (1%) of scans. Algorithm failure occurred primarily in the presence of significant artifact obscuring CSF visualization or when a scan (usually head combined with neck and/or chest) failed co-registration to the baseline scan. Midline delineation was inaccurate, on visual inspection of hemispheric CSF results, in only five scans. Twenty-eight scans were excluded because they were obtained after hemicraniectomy and twelve scans were excluded due to contralateral hydrocephalus. This left 1,875 measurements of the hemispheric CSF ratio within 96 hours of stroke onset for analysis.
Figure 2.
Flow of subjects and scans analyzed.
In this cohort, the median NIHSS was seven (IQR 4-14) with 349 (38%) having mild strokes, 302 (33%) having moderately severe strokes and 270 (29%) having severe strokes. Although two-thirds of this acute stroke cohort received thrombolytic therapy, given the time period of study, thrombectomy was relatively rarely performed. Similarly, angiographic data on vessel occlusions was lacking in the majority of cases. CED grading could be assigned in 737 with imaging beyond 24-hours: CED-3 in 101 (14%), CED-2 in 72 (10%), for a total of 173 (23%) having large hemispheric infarction (LHI). Four subjects had additional areas of infarction in the contralateral hemisphere but in all four these regions were small without edema. Characteristics of the study population, classified by edema severity, are shown in Table 1. Although NIHSS was lower in those who did not develop LHI (CED 0–1: 6, IQR 4–11), those with LHI who developed midline shift had similar baseline severity to those who did not develop midline shift (NIHSS 17 for CED-3 vs. 16 for CED-2). Malignant edema developed in 55 (6%), including 25 who died or required decompressive hemicraniectomy.
Table 1.
Description of the study cohort, divided by edema (CED) severity groups.
| Total (n = 924) | CED-3 (n = 101) | CED-2 (n = 72) | CED-1 (n = 287) | CED-0 (n = 277) | |
|---|---|---|---|---|---|
| Age, years, median [IQR] | 69 [59–79] | 71 [58–78.5] | 74 [64–83] | 69 [60–78] | 69 [61–79) |
| Sex, male, n (%) | 518 (56%) | 53 (53%) | 30 (41%) | 159 (55%) | 166 (60%) |
| Race, nonwhite, n (%) | 97 (11%) | 19 (19%) | 8 (11%) | 27 (9%) | 17 (6%) |
| Baseline NIHSS, median [IQR] | 7 [4–14] | 17 [14–21] | 16 [11–19] | 7 [4–12] | 6 [3–11] |
| Acute therapies | |||||
| tPA treatment, n (%) | 614 (66%) | 69 (69%) | 48 (67%) | 197 (69%) | 209 (75%) |
| Thrombectomy, n (%) | 132 (14%) | 25 (25%) | 18 (25%) | 37 (13%) | 41 (15%) |
| Both tPA and thrombectomy, n (%) | 101 (11%) | 19 (19%) | 10 (14%) | 28 (10%) | 32 (12%) |
| Diabetes mellitus, n (%) | 204 (22%) | 24 (24%) | 24 (33%) | 68 (24%) | 58 (21%) |
| Glucose, mg/dl, median [IQR] | 121 [105–146] | 130 [115–158] | 130 [110–170] | 122 [108–147] | 115 [102–133] |
| Systolic BP, mm Hg, median [IQR] | 155 [138–170] | 151 [130–167] | 148 [135–170] | 158 [140–170] | 154 [139–170] |
| Time to baseline CT, h, median [IQR] | 1.9 [1.1–3.5] | 1.5 [1.0–3.7] | 1.9 [1.1–4.9] | 1.8 [1.1–3.3] | 1.9 [1.1–3.6] |
| Baseline ASPECTS, median [IQR] | 10 [9–10] | 8 [6–9] | 9 [6–10] | 9 [8–10] | 10 [10–10] |
| Baseline CSF volume, ml, median [IQR] | 151 [106–202] | 133 [93–171] | 150 [104–199] | 153 [112–205] | 166 [122–213] |
| Baseline hemispheric CSF ratio, median [IQR] | 0.93 [0.87–0.97] | 0.88 [0.81–0.94] | 0.91 [0.83–0.95] | 0.93 [0.88–0.97] | 0.94 [0.90–0.97] |
| 24-hour NIHSS, median [IQR] | 4 [2–11] | 16 [12–22] | 13 [5–18] | 4 [2–9] | 3 [1–6] |
| Time to FU CT, h, media [IQR] | 26 [23–28] | 24 [20–27] | 25 [19–29] | 26 [24–28] | 26 [24–29] |
| TOAST stroke etiology, n (%) | |||||
| Large artery atherosclerosis | 146 (16%) | 18 (18%) | 12 (17%) | 48 (17%) | 48 (17%) |
| Cardioembolism | 363 (39%) | 47 (47%) | 37 (51%) | 130 (45%) | 92 (33%) |
| Small vessel disease | 68 (7%) | 0 | 0 | 14 (5%) | 22 (8%) |
| Other pathogenesis | 37 (4%) | 5 (5%) | 2 (3%) | 12 (4%) | 7 (3%) |
| Undetermined | 310 (34%) | 31 (30%) | 21 (29%) | 83 (29%) | 108 (39%) |
| Peak infarct volume, ml, median [IQR] | 0 [0–28] | 207 [101–294] | 100 [64–138] | 11 [2–29] | 0 [0–0] |
| Peak midline shift, ml, median [IQR] | 0 [0–0] | 5 [3.1–8.9] | 0 [0–0] | 0 [0–0] | 0 [0–0] |
| Inpatient mortality, n (%) | 37 (4%) | 19 (19%) | 6 (8%) | 3 (1%) | 5 (2%) |
ASPECTS: Alberta Stroke Program Early CT Score; BP: blood pressure; FU: follow-up (closest to 24-hours).
Baseline imaging analysis
Baseline CT was available for 888 subjects at a median of 1 hour 51 minutes after stroke onset (IQR 1–3.5 hours). Median ASPECTS was 10 (IQR 9–10) but 94 (11%) had a score of seven or below. The median hemispheric CSF ratio was 0.93 at baseline (IQR 0.87–0.97) but was lower in those with low ASPECTS (0.88 vs. 0.92 for ASPECTS 8–9 and 0.93 for ASPECTS of 10, p = 0.0001). Presence of old strokes (in 48 cases, 5%) with areas of encephalomalacia lowered the CSF ratio on baseline CT (0.88 vs. 0.93, p = 0.002). It was also lower at baseline in those who developed large hemispheric infarction (0.89 vs. 0.93, p < 0.0001). Excluding those with old strokes, the measurement of hemispheric ratio from baseline CT could predict large infarction with 74% specificity but only 51% sensitivity (at a threshold below 0.89), for AUC of 0.65 (95% CI 0.60–0.70). Furthermore, the baseline CSF ratio was an independent predictor of developing large cerebral infarction with midline shift at this early time point, adjusting for age, baseline NIHSS, tPA treatment, and total CSF volume (OR 1.66 per 10% reduction in ratio, 95% CI 1.22–2.28, p = 0.001).
The baseline ratio, recalculated based on known stroke side (in the subset of 461 with infarcts visible on repeat imaging), was a median of 0.95 (IQR 0.87–1.02) and was strongly correlated with the ratio based on lower hemispheric volume (r = 0.93). It similarly differed by edema severity: 0.97 in CED 0–1, 0.92 CED-2, and 0.89 for CED-3 (p < 0.0001). It was lower in those who went on to develop LHI (0.91 vs. 0.97, p < 0.0001) but did not differ between CED-2 and 3 groups (p = 0.39). Given the comparable findings, we analyzed the longitudinal data with the crude ratio, as it was available for all subjects regardless of known stroke side.
Trajectory of hemispheric CSF ratio
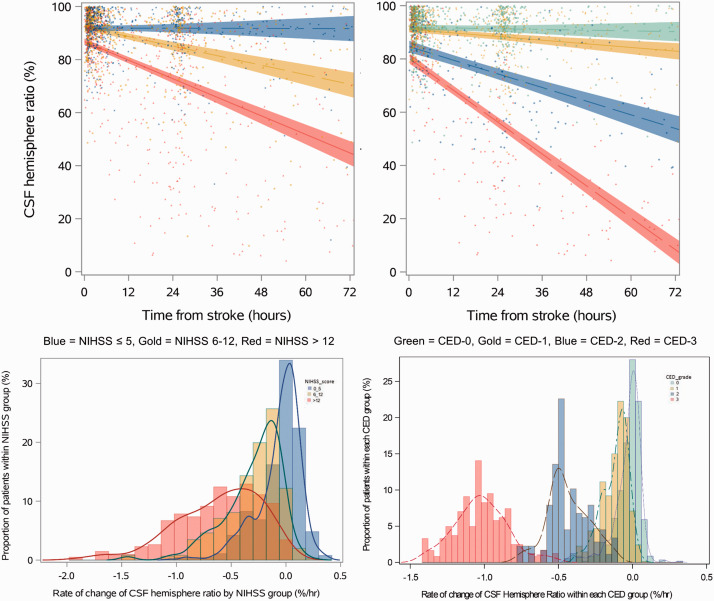
A linear mixed-effects model was used to characterize the change in hemispheric ratio over time. The baseline ratio was associated with age (β + 0.0015 per year, p < 0.0001) and baseline NIHSS (β −0.0023, p < 0.0001), as well as CED grade. Those in the CED-2 group had lower ratio (β −0.057, p < 0.001) as did those in the CED-3 group (β −0.087, p < 0.001 vs. CED-1, p = 0.045 relative to CED-2). The ratio decreased significantly (Figure 3), by an estimated 0.01 per hour in those with CED-3, but was significantly less for CED-2 (0.004/hour) and CED-1 (0.001/hour) and there was no change over time for those without visible infarction (all comparisons, p < 0.001). We also demonstrated distinct trajectories of edema grouping by baseline stroke severity. Those with mild strokes (NIHSS 0–5) had no reduction in CSF ratio over time, while those with moderately severe strokes (NIHSS 6–12) had a gradual downward trajectory (0.003/hour) while those with NIHSS of 13 or above had a mean slope of 0.006 per hour (p < 0.001 compared with both other groups). The most severe strokes also had a lower ratio at baseline by 0.05 (p < 0.001). There was variability with a broad distribution for individual subjects’ trajectories within each strata (see bottom panel of Figure 3). The hemispheric ratio was strongly correlated to measurable infarct volume (r = 0.87, p < 0.00001). However, the CSF ratio was reduced in those developing large infarcts even before infarct-related hypodensity was visible (median 0.72 for CED-3 and 0.81 for CED-2) on follow-up scans performed within twenty-four hours of stroke onset with no visible hypodensity.
Figure 3.
Hemispheric CSF ratio over time, modeled by NIHSS (left) and CED (right) strata. Hemispheric CSF ratio modeled over time in 924 stroke patients, divided by baseline stroke severity (left) and in 738 with follow-up CTs divided by CED grades (right). NIHSS strata are mild (0-5) in blue, moderate (6-12) in gold, and severe (> 12) in red. Edema severity groups are CED-0 (green), CED-1 (gold), CED-2 (blue), and CED-3 (red). The top panel shows group slopes using linear mixed models. The bottom panel shows histograms comparing the estimated slopes of individual subjects within each strata.
Hemispheric CSF ratio at 24-hours
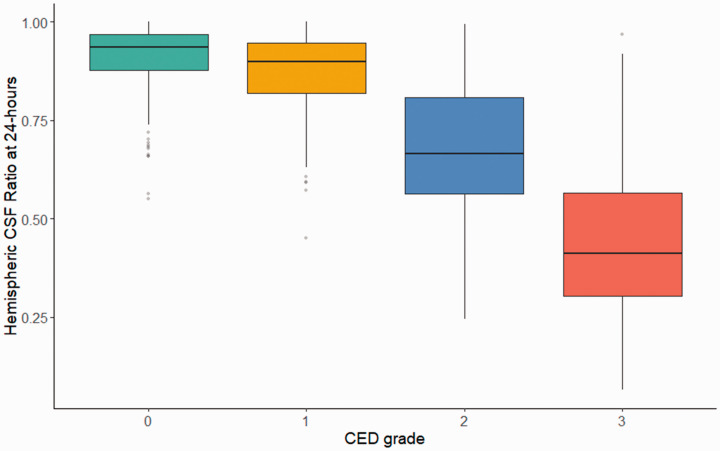
The hemispheric ratio on CT closest to 24-hours was available for 553 stroke patients with imaging between 12–48 hours. Median time from stroke onset to CT was 26 hours (IQR 23–28). The measured CSF ratio at 24-hours was progressively lower as severity of edema increased (Figure 4), even though scans for the higher CED groups were performed on average one hour earlier (24.8 vs. 26.1 hours, p = 0.001). In the most severe edema group (CED-3), midline shift was still minimal (median 3.1-mm, IQR 1.8–4.3) but the CSF volume in the stroke hemisphere was already reduced to less than half that in the contralateral hemisphere (median ratio 0.41, IQR 0.30–0.57). The CSF ratio at 24-hours was strongly correlated with peak midline shift (r = −0.72, p < 0.0001). In multivariable analysis of baseline variables, lower CSF ratio at 24-hours was associated with higher NIHSS (β −0.01 per point), lower ASPECTS (β −0.19 for scores below 8) as well as lower baseline CSF volume (β −0.01 per 10-ml, all p < 0.0001) and age (β −0.018 per 10-years, p = 0.009), but not with sex, glucose, tPA, or thrombectomy, adjusting for time between scans and baseline ratio (model R2 of 0.47).
Figure 4.
Hemispheric CSF ratio at 24-hours by edema severity. The hemispheric CSF ratio on the CT scan closest to 24-hours in 553 stroke patients, grouped by edema severity. The median and interquartile range are 0.41 (0.30–0.57) for CED-3 (red), 0.66 (0.56–0.81) for CED-2 (gold), 0.90 (0.82–0.95) for CED-1 (blue), and 0.93 (0.88–0.97) for CED-0 (green), p<0.0001 for all comparisons.
Prediction of malignant edema
The risk of malignant edema was 34% in those with high baseline NIHSS and LHI. A hemispheric ratio below 0.50 had 90% sensitivity and 82% specificity (AUC 0.91, 95% CI 0.85–0.96) to identify which of these patients would develop malignant edema. This compared favorably to infarct-related hypodensity, where an optimal threshold of 150-ml provided only 67% sensitivity to predict malignant edema with 88% specificity (AUC 0.78, 0.68–0.88, p = 0.017 for comparison). The hemispheric ratio at 24-hours was independently associated with risk of malignant edema, adjusting for NIHSS and infarct volume (OR 2.98 per 10% reduction, 95% CI 2.05–4.68, p < 0.0001).
Discussion
As cerebral edema develops in and around an evolving hemispheric infarction, CSF is preferentially displaced from the ipsilateral hemisphere. Effacement of sulci in the region of a stroke is well established as one of the earliest manifestations of stroke-related edema, observed qualitatively on brain imaging performed even in the first few hours after a stroke. In this study, we quantified early post-stroke edema by measuring the ratio of CSF volume between the two hemispheres in a large cohort of hemispheric stroke patients with a spectrum of severities at serial time points over the first four days after stroke.
We confirmed that the hemispheric CSF ratio provides an early indicator of edema, even on baseline CT within a few hours of stroke onset. We found that those with lower ASPECTS, an established measure of early stroke-related hypodensity, had lower CSF ratio on baseline CT. Both imaging metrics are capturing aspects of early infarction and edema. However, the hemispheric CSF ratio can be automatically and objectively quantified, while ASPECTS rating requires training and suffers only moderate reproducibility. 19 The CSF ratio is capturing the reciprocal of hemispheric volume increase, an accepted measure of post-stroke edema that is difficult to measure from CT but has been correlated with edema using MRI. 20 We demonstrated that the baseline CSF ratio was significantly lower in those who would develop large infarction and could predict with moderate accuracy the development of hemispheric edema, even accounting for stroke severity and other baseline variables.
Mapping the evolution of this biomarker using serial CTs suggests that edema progresses linearly over the first 72 hours after stroke. We demonstrated distinct trajectories of edema progression based on admission stroke severity as well as the ultimate extent of edema formation. A separation in slopes was clearly visible within the first 12–24 hours. There was little longitudinal change in the hemispheric ratio in those who developed minimal edema while those ultimately developing large strokes with midline shift had the steepest decline, even compared to those with similar baseline stroke severities who did not develop midline shift.
This ratio of volumes between the hemispheres may be capturing an aspect of the critical reserve that CSF affords for the brain volume increase from edema. When a majority of the CSF in the ipsilateral hemisphere has been displaced, the ratio will become critically reduced, and midline shift will develop. Unlike midline shift however, this CSF biomarker captures the early stages of edema formation and the entire spectrum of severities, from relatively mild to the most malignant. This makes it an especially appealing quantitative endophenotype for studies that seek to understand the underlying clinical and biologic factors mediating edema formation. 21 For example, it could be applied to quantify differences in edema formation in relation to collateral circulation, reperfusion, and other important interventions.22,23
This imaging phenotype shares similarities to another recently proposed edema biomarker: the ratio of edema-related hypodensity to the density of the contralateral hemisphere (the net water uptake, NWU). 24 NWU can also be measured from both baseline CTs and later CTs with clear hypodensity and midline shift. 25 NWU increases even within the first few hours of stroke and does so faster in those with poor collaterals and lower ASPECTS. 23 However, calculating NWU generally requires either the region of infarction to be visible or CT perfusion imaging to provide an outline of the infarct core. The hemispheric CSF ratio can be calculated from any clinical CT, even before the infarct is visible, making it more broadly applicable to early time points and the spectrum of stroke severities. Another significant advance in this study was the implementation of an automated image-processing pipeline that allowed the extraction of hemispheric CSF volumes from almost two thousand routine stroke CT scans. In contrast, NWU measurement requires manual delineation of infarcted region and mirroring that region to the contralateral hemisphere.
Studies have suggested that imaging features, such as midline shift and ventricular compression, can inform on the risk of malignant edema. 26 We have previously shown that global ΔCSF is greater in those with more severe edema. 8 An advantage of the CSF ratio over ΔCSF is that it can be assessed on a single follow-up CT, without comparison to baseline imaging. Assessment of the hemispheric CSF ratio from CT at 24-hours appears to provide a powerful predictive biomarker for malignant edema, even outperforming assessment of infarct volume. By 24-hours, those with malignant edema often had significant hemispheric asymmetry, with a CSF ratio at or below 0.50 providing high predictive value even among the subgroup with large hemispheric infarction. We propose that measuring the hemispheric CSF ratio even before 24-hours could predict those at risk for a malignant trajectory before substantial midline shift and clinical deterioration occur. Application of CSF ratio for early warning of edema is plausible because it is precisely when capacity for ipsilateral CSF compensation is exhausted that compartmental pressure rises and midline shift precipitously develops. 27 Although further validation studies are needed, it appears to be a promising imaging biomarker to predict which patients require closer monitoring and possibly medical and/or surgical interventions prior to herniation.
While providing promising preliminary evaluation of a novel imaging biomarker, this study has several limitations. We analyzed a large cohort of diverse stroke patients but were restricted to what imaging was performed as part of routine care; not all patients had serial imaging and this might have introduced some bias toward those with larger strokes and more edema to have follow-up imaging. We also did not have data on vessel occlusion status or reperfusion in this cohort. Additional studies, ideally with prospective imaging, should evaluate how this biomarker performs in cohorts with severe strokes and/or proximal occlusions, where the risk of edema is greatest. This would also allow the impact of reperfusion status on edema development to be more completely evaluated. 22
A hemispheric approach to evaluating post-stroke edema is likely to fail in a few scenarios. One is when strokes affect both hemispheres, causing bilateral edema. This was rare in our cohort, but, if it occurred, could lead to relatively preserved CSF symmetry despite a reduction in CSF in both hemispheres; in such cases, measuring the total change in CSF over time would better capture this. A second confounder is the presence of old infarcts with encephalomalacia, which could cause baseline CSF asymmetry (i.e. altered CSF ratio due to more preexisting CSF in one hemisphere), as noted in several cases in our study. We propose that identification of such cases may be aided by noting unexpectedly low baseline CSF ratio, confirmed by visual inspection of hemispheric CSF results. In such cases, we propose that only relative changes in the ratio be used (i.e. if edema were to develop, the ratio would still go up or down) but that absolute thresholds cannot be applied in those with old hemispheric infarcts. In addition, CSF volumes cannot be accurately measured from CTs with significant artifact and these need to be excluded. Finally, in the presence of midline shift, some parts of the shifted ipsilateral ventricle may be falsely classified within the contralateral hemisphere using our automated midline technique; this would bias toward somewhat lower ratio in those with edema and shift, accentuating actual differences. We did not find this introduced large errors in our cohort, which focused on scans within the first 24–48 hours, when little midline shift has developed. However, it could hinder accurate estimation of the hemispheric ratio as later time points or in cases where shift is severe.
We are now refining our algorithms so that extraction of hemispheric CSF volumes is seamless and scalable, from image input to volumetric output. We are integrating all processing steps into an integrated pipeline that can assess scan quality (excluding those with artifacts), perform segmentation, and provide results within minutes. We also intend to integrate advanced registration and deep learning approaches to increase the accuracy of delineating the brain midline, especially when it is shifted. Finally, the ratio assumes that the contralateral hemisphere is a static comparator, but it is possible that some CSF displacement occurs there after large strokes. Experimental studies have suggested that this does occur but less noticeably and later. 28 We intend to study the kinetics of these individual CSF compartments in future studies of edema.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211018210 for Hemispheric CSF volume ratio quantifies progression and severity of cerebral edema after acute hemispheric stroke by Rajat Dhar, Ali Hamzehloo, Atul Kumar, Yasheng Chen, June He, Laura Heitsch, Agnieszka Slowik, Daniel Strbian and Jin-Moo Lee in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X211018210 for Hemispheric CSF volume ratio quantifies progression and severity of cerebral edema after acute hemispheric stroke by Rajat Dhar, Ali Hamzehloo, Atul Kumar, Yasheng Chen, June He, Laura Heitsch, Agnieszka Slowik, Daniel Strbian and Jin-Moo Lee in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JML received funding from NIH (R01NS085419, U24NS107230); RD received funding from NIH (K23NS099440); LH received funding from NIH K23NS099487
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: Study concept and design: RD, JML. Acquisition, analysis, and interpretation of data: RD, AH, AK, YC, JH, LH, AS, DS, JML. Drafting of the manuscript: RD. Critical revision of the manuscript for important intellectual content: AH, AK, YC, JH, LH, AS, DS, JML. Study supervision: JML.
ORCID iD: Rajat Dhar https://orcid.org/0000-0002-5167-5097
Supplemental material: Supplemental material for this article is available online.
References
- 1.Simard JM, Kent TA, Chen M, et al. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol 2007; 6: 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacke W, Schwab S, Horn M, et al. Malignant middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 1996; 53: 309–315. [DOI] [PubMed] [Google Scholar]
- 3.Park J, Goh D-H, Sung J-K, et al. Timely assessment of infarct volume and brain atrophy in acute hemispheric infarction for early surgical decompression: strict cutoff criteria with high specificity. Acta Neurochir 2012; 154: 79–85. [DOI] [PubMed] [Google Scholar]
- 4.Battey TW, Karki M, Singhal AB, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke 2014; 45: 3643–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guluma KZ, Oh H, Yu S-W, et al. Effect of endovascular hypothermia on acute ischemic edema: morphometric analysis of the ICTuS trial. Neurocrit Care 2008; 8: 42–47. [DOI] [PubMed] [Google Scholar]
- 6.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med 1986; 314: 953–958. [DOI] [PubMed] [Google Scholar]
- 7.Dhar R, Yuan K, Kulik T, et al. CSF volumetric analysis for quantification of cerebral edema after hemispheric infarction. Neurocrit Care 2016; 24: 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhar R, Chen Y, Hamzehloo A, et al. Reduction in cerebrospinal fluid volume as an early quantitative biomarker of cerebral edema after ischemic stroke. Stroke 2020; 51: 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foroushani HM, Dhar R, Chen Y, et al. Stroke neuroimaging phenotype repository. In: (eds) Medical imaging 2020: imaging informatics for healthcare, research, and applications. Vol. 11318. Houston, TX: International Society for Optics and Photonics, 2020, p.113180B. [Google Scholar]
- 10.Dhar R, Chen Y, An H, et al. Application of machine learning to automated analysis of cerebral edema in large cohorts of ischemic stroke patients. Front Neurol 2018; 9: 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rorden C, Bonilha L, Fridriksson J, et al. Age-specific CT and MRI templates for spatial normalization. Neuroimage 2012; 61: 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Dhar R, Heitsch L, et al. Automated quantification of cerebral edema following hemispheric infarction: application of a machine-learning algorithm to evaluate CSF shifts on serial head CTs. Neuroimage Clin 2016; 12: 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta stroke program early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001; 22: 1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 14.MacCallum C, Churilov L, Mitchell P, et al. Low Alberta stroke program early CT score (ASPECTS) associated with malignant Middle cerebral artery infarction. Cerebrovasc Dis 2014; 38: 39–45. [DOI] [PubMed] [Google Scholar]
- 15.Strbian D, Meretoja A, Putaala J, et al. Cerebral edema in acute ischemic stroke patients treated with intravenous thrombolysis. Int J Stroke 2013; 8: 529–534. [DOI] [PubMed] [Google Scholar]
- 16.Thoren M, Azevedo E, Dawson J, et al. Predictors for cerebral edema in acute ischemic stroke treated with intravenous thrombolysis. Stroke 2017; 48: 2464–2471. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP, Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate – a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57: 289–300. [Google Scholar]
- 19.Naylor J, Churilov L, Rane N, et al. Reliability and utility of the Alberta stroke program early computed tomography score in hyperacute stroke. J Stroke Cerebrovasc Dis 2017; 26: 2547–2552. [DOI] [PubMed] [Google Scholar]
- 20.Yoo AJ, Sheth KN, Kimberly WT, et al. Validating imaging biomarkers of cerebral edema in patients with severe ischemic stroke. J Stroke Cerebrovasc Dis 2013; 22: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jian X, Fornage M. Imaging endophenotypes of stroke as a target for genetic studies. Stroke 2018; 49: 1557–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irvine HJ, Ostwaldt AC, Bevers MB, et al. Reperfusion after ischemic stroke is associated with reduced brain edema. J Cereb Blood Flow Metab 2018; 38: 1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broocks G, Kemmling A, Meyer L, et al. Computed tomography angiography collateral profile is directly linked to early edema progression rate in acute ischemic stroke. Stroke 2019; 50: 3424–3430. [DOI] [PubMed] [Google Scholar]
- 24.Broocks G, Flottmann F, Scheibel A, et al. Quantitative lesion water uptake in acute stroke computed tomography is a predictor of malignant infarction. Stroke 2018; 49: 1906–1912. [DOI] [PubMed] [Google Scholar]
- 25.Vorasayan P, Bevers MB, Beslow LA, et al. Intravenous glibenclamide reduces lesional water uptake in large hemispheric infarction. Stroke 2019; 50: 3021–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijdicks EF, Diringer MN. Middle cerebral artery territory infarction and early brain swelling: progression and effect of age on outcome. Mayo Clin Proc 1998; 73: 829–836. [DOI] [PubMed] [Google Scholar]
- 27.Gerriets T, Stolz E, Konig S, et al. Sonographic monitoring of midline shift in space-occupying stroke: an early outcome predictor. Stroke 2001; 32: 442–447. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien MD, Waltz AG, Jordan MM. Ischemic cerebral edema. Distribution of water in brains of cats after occlusion of the middle cerebral artery. Arch Neurol 1974; 30: 456–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211018210 for Hemispheric CSF volume ratio quantifies progression and severity of cerebral edema after acute hemispheric stroke by Rajat Dhar, Ali Hamzehloo, Atul Kumar, Yasheng Chen, June He, Laura Heitsch, Agnieszka Slowik, Daniel Strbian and Jin-Moo Lee in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X211018210 for Hemispheric CSF volume ratio quantifies progression and severity of cerebral edema after acute hemispheric stroke by Rajat Dhar, Ali Hamzehloo, Atul Kumar, Yasheng Chen, June He, Laura Heitsch, Agnieszka Slowik, Daniel Strbian and Jin-Moo Lee in Journal of Cerebral Blood Flow & Metabolism