Abstract
Background and Objectives
To perform the first screen of 44 amyotrophic lateral sclerosis (ALS) genes in a cohort of African genetic ancestry individuals with ALS using whole-genome sequencing (WGS) data.
Methods
One hundred three consecutive cases with probable/definite ALS (using the revised El Escorial criteria), and self-categorized as African genetic ancestry, underwent WGS using various Illumina platforms. As population controls, 238 samples from various African WGS data sets were included. Our analysis was restricted to 44 ALS genes, which were curated for rare sequence variants and classified according to the American College of Medical Genetics guidelines as likely benign, uncertain significance, likely pathogenic, or pathogenic variants.
Results
Thirteen percent of 103 ALS cases harbored pathogenic variants; 5 different SOD1 variants (N87S, G94D, I114T, L145S, and L145F) in 5 individuals (5%, 1 familial case), pathogenic C9orf72 repeat expansions in 7 individuals (7%, 1 familial case) and a likely pathogenic ANXA11 (G38R) variant in 1 individual. Thirty individuals (29%) harbored ≥1 variant of uncertain significance; 10 of these variants had limited pathogenic evidence, although this was insufficient to permit confident classification as pathogenic.
Discussion
Our findings show that known ALS genes can be expected to identify a genetic cause of disease in >11% of sporadic ALS cases of African genetic ancestry. Similar to European cohorts, the 2 most frequent genes harboring pathogenic variants in this population group are C9orf72 and SOD1.
Our understanding of the epidemiologic, clinical, and genetic aspects of amyotrophic lateral sclerosis (ALS) has increased substantially due to global research efforts. Because data originate largely from Europe, North America, and Asia, very little is known about ALS in individuals with African genetic ancestry.
The reported worldwide incidence of ALS (number of new cases per 100,000 person-years) is highest in Northern Europe (1.89) and lowest in South and East Asia (0.73–0.83), whereas robust estimates of incidence rates on the African continent are unknown.1 Reports from geographical areas with populations of different genetic ancestries, such as in the United States and a small study in South Africa,2 suggest that ALS incidence rates in individuals with African ancestry are the lowest worldwide (0.56). However, underascertainment of ALS attributed to limited access to health care services and an overall younger population in developing African countries may be contributing factors.
The genetic architecture of ALS is complex and includes highly penetrant pathogenic variants and lower penetrant risk alleles, which may be present at a low background frequency in the general population.3 The former category of variants explains a high proportion of ALS cases, which segregate in families (e.g., C9orf72 pathogenic repeat expansion and pathogenic variants in SOD1), whereas lower penetrant risk alleles/variants may act in combination together with age and environmental factors consistent with an oligogenic and/or multistep basis of disease.3
Although ALS is diagnosed by clinical means, increasingly genetic testing in the form of ALS gene panel screening using next-generation sequencing may be offered to patients with ALS in developed countries. A research discovery approach is to identify rare variants in established ALS genes based on reported variant frequencies in high-resolution population databases such as the Genome Aggregation Database (gnomAD) and then curate various evidence sources (ClinVar database and scientific literature) to classify them as benign or pathogenic according to the American College of Medical Genetics (ACMG) guidelines.4
Beyond the identification of known, highly penetrant pathogenic variants in causative ALS genes such as C9orf72, SOD1, FUS, and TARDBP, there is a need to systematically classify ALS-associated variants in other genes to better understand the pathogenetic mechanisms of ALS. This remains challenging due to the complex genetic architecture of ALS. First, it appears that the growing list of ALS genes (eFigure 1 and eMethods, links.lww.com/NXG/A509) seems to have unique mutational landscapes with some harboring clusters of missense variants in mutational hotspots corresponding to protein functional domains (e.g., FUS),5 whereas others harbor an excess of loss of function (LOF) variants either throughout the protein (e.g., NEK1)6 or in a specific region (e.g., KIF5A).7 Second, pathogenic variants in some ALS genes such as the C9orf72 expansion are found in cases of ALS, frontotemporal dementia (FTD), or a combination of both. ALS genes may be pleiotropic such as KIF5A, which also harbors variants implicated in neurologic conditions other than ALS. Lower penetrance risk alleles, which may also contribute to ALS susceptibility and/or have disease-modifying effects, may be missed by assuming that they are very rare. Determining the appropriate minor allele frequency (MAF) threshold for such variants remains a challenge. Considering these issues, it is clear that application of the ACMG guidelines4 (which were not designed for complex disorders) requires modification to adequately capture the nuances of variant classification in ALS. The dissection of the genetic underpinnings of ALS in Africans, where there is poor gene variant representation in public databases, adds an additional layer of complexity.
Apart from 2 small studies in South Africans with ALS investigating the C9orf72 pathogenic repeat expansion frequency8 and intermediate repeat expansion frequencies in candidate genes, which have been reported as risk alleles in Europeans,9 the genetic profile of patients with ALS with African genetic ancestry is unknown. Here, we characterized the genetic variation spectrum of African ancestry patients with ALS by screening ALS genes (based on European ancestry data) (eFigure 1 and eMethods, links.lww.com/NXG/A509) using whole-genome sequencing (WGS) data.
Methods
Patient Cohort
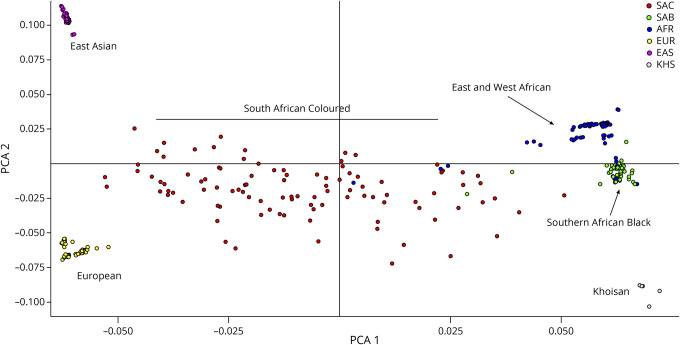
We included 103 consecutive African ancestry patients with ALS attending the ALS clinic at Groote Schuur Hospital in Cape Town, South Africa, between 2014 and 2019. All patients provided informed written consent to donate a blood sample to undergo WGS. Patients were diagnosed as clinically probable or definite ALS according to the revised El Escorial criteria10 and categorized according to their phenotype at presentation as ALS, ALS with FTD (ALS-FTD),11 or one of the subtypes: predominantly upper motor neuron (UMN)-ALS, predominantly lower motor neuron (LMN)-ALS (including flail arm/leg), and a young patient with progressive dysphagia and myopathy, dystrophic changes on muscle biopsy, who later manifested and died of ALS (labeled ALS-myopathy) (Table 1). Fifty-five (53%) patients were men, and the overall average age at disease onset was 52.5 years. Four (4%) patients had ≥1 first-degree relative with ALS (only probands included here). All participants were of African genetic ancestry and categorized themselves according to the South African racial census categories (statssa.gov.za) as either self-assigned South African Coloured (SAC, n = 76) or Black African (n = 27). SAC refers to South Africans, mainly living in the Western Cape province, who derive their ancestry largely from Khoisan (hunter-gatherers) and Black African populations (∼60%) with lesser contributions from Southeast Asians (∼20%) and Europeans (∼20%).12 The African genetic ancestry of Black African and SAC patients was verified by ancestry principal component and admixture analysis (Figure 1, eFigure 2, and eMethods, links.lww.com/NXG/A509).
Table 1.
Characteristics of South African Patients With ALS According to the African Ancestry Group
Figure 1. Ancestry Principal Component Analysis Plot of African Ancestry ALS Cases and Controls.
ALS = amyotrophic lateral sclerosis; AFR = East and West African (blue); EAS = East Asian (purple); EUR = European (yellow); KHS = Khoisan (gray); SAB = Southern African Black (green); SAC = South African Coloured (red).
Raw data from 238 samples from various WGS data sets were included in the analysis pipeline as non-ALS population controls (referred to as in-house controls, see eFigure 3 and eMethods, links.lww.com/NXG/A509). These included 138 samples of African genetic ancestry including those from South Africa as previously described (see eMethods). The STREGA reporting guidelines were used (goodreports.org/reporting-checklists/strega/) (eMethods).
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the University of Cape Town Research Ethics Committee. All patients provided informed written consent to donate a blood sample to undergo WGS.
Statistical Analysis
GraphPad Prism v9.1.0 (2016) was used to analyze the demographic and clinical data. Categorical variables were analyzed using the Fisher exact test, and for continuous variables, independent sample t tests were used for normally distributed data and Mann-Whitney or 1-way analysis of variance tests for skewed data.
Whole-Genome Sequencing
WGS (≥30× coverage) with read lengths 2 × 150 base pair (bp) in 103 ALS cases, and between 2 × 100 bp or 150 bp in 238 controls, was performed on different Illumina platforms (see eMethods, links.lww.com/NXG/A509). For consistency, the raw data (fastq files) from all patient and control samples (n = 341) were aligned to the NCBI GRCh38 reference genome (with alt contigs), and joint variant calling was performed according to the Genome Analysis Toolkit best practice guidelines on the Ilifu high-performance computing facility (ilifu.ac.za/) using the following pipeline (github.com/grbot/varcall). However, potential batch effects related to the use of different sequencing platforms cannot be excluded, and this is a limitation of this case-control study design.
Ancestry Principal Component Analysis
An ancestry principal component analysis (PCA) plot was constructed following single nucleotide variation (formerly single nucleotide polymorphism) pruning to extract the top 10 principal components of the variance-standardized relationship matrix (Figure 1 and eMethods, links.lww.com/NXG/A509).
Standard Variant Annotation and Filtering
To filter for rare variants, the VCF file was annotated with frequency information from gnomAD v3.1 genomes and we removed variants which were either absent or with MAF <1% in any of the 9 subpopulations in this database (Ashkenazi Jewish, South Asian, African, Other, Amish, European-Finnish, East Asian, European-non-Finnish, and Ad Mixed American). In addition, variants present in any in-house controls were removed (1/476 alleles ∼MAF 0.2%) (eFigure 3 and eMethods, links.lww.com/NXG/A509). The Ensembl variant effect predictor (VEP) was used to annotate variants with their sequence context information, and ClinVar annotations were added from the January 28, 2021, ClinVar release (eMethods).
Repeat Expansion Detection in C9orf72 and ATXN2 Genes
For the 46 participants in the Clinical Research in ALS and related disorders for Therapeutic Development (CReATe) Consortium's Phenotype, Genotype and Biomarker study, the repeat length of the pathogenic C9orf72 GGGGCC repeat expansion was determined by the 2-step repeat-primed PCR protocol and validated by Southern blot.13,14 For the remaining patients with ALS, the Illumina ExpansionHunter v4.0.2 tool was used, and C9orf72 repeat expansions in the pathogenic range were validated by repeat-primed PCR.8,9 Intermediate-length CAG repeat expansions in ATXN2 were determined by ExpansionHunter.
ALS-Associated Genes
Single nucleotide variants and insertions and deletions in 44 ALS genes (eFigure 1 and eMethods, links.lww.com/NXG/A509) with the following VEP variant consequences were selected for further analysis (n = 62): missense, inframe insertions and deletions, stop-gain, frameshift, start-lost, stop-lost, splice region, splice donor, and splice acceptor with the exception of C9orf72 and ATXN2 (see eMethods, describing gene selection).
Variant Classification According to ACMG Guidelines
We classified each short variant identified in 44 ALS genes as benign, likely benign (LB), uncertain significance (variants of uncertain significance, VUS), likely pathogenic (LP), or pathogenic by applying the ACMG/AMP guidelines for the interpretation of sequence variants4 (summarized in eTable 1, links.lww.com/NXG/A509). Variant classification in ALS is complicated by issues such as oligogenicity, genes that may act as risk factors, and reduced penetrance of pathogenic variants. In the absence of ALS-specific variant curation guidelines, we incorporated the ClinGen Sequence Variant Interpretation working group's general use recommendations, consensus decisions from the CReATe Genetics Working Group discussions, and recently proposed guidelines.15
For each variant remaining after filtering (n = 62), we retrieved its allele frequency (AF) from gnomAD databases (v2.1.1/n = 114,704 exomes; v3.1/n = 67,442 genomes) considering only samples with nonneurologic phenotypes (eTable 1, links.lww.com/NXG/A509). Variants were reported to be absent from gnomAD if sufficient coverage (>20×) of the variant site could be confirmed by examining the integrative genomics viewer read data plots in the gnomAD browser. A universal MAF filter for ALS variants (both dominant and recessive modes of inheritance) would theoretically need to be set at around 1% to retain the SOD1 D91A variant and pathogenic C9orf72 repeat expansions, both of which have risen to appreciable background frequencies in the general population in certain bottle-necked European ancestry populations (eFigure 3 and eMethods). In contrast, the maximum credible population AF for ALS variants has been calculated at 0.01% using an online calculator (cardiodb.org/allelefrequencyapp/), which considers ALS disease-specific factors such as prevalence, genetic and allelic heterogeneity, inheritance mode, and penetrance (eFigure 3 and eMethods).15 To enable the identification of variants with reduced penetrance in risk factor genes (which may have MAF >0.01%), we elected to use a MAF threshold of 0.1%16 for filtering. This threshold is appropriate for our African ancestry sample which is expected to have a low likelihood of harboring pathogenic founder variations and we confirmed that the SOD1 D91A variant was absent from our data set. We also genotyped the c9orf72 repeat expansion in each sample. Consequently, BS1 (strong benign evidence strength) was assigned where the gnomAD popmax filtering AF (or highest population AF if popmax unavailable) in gnomAD was >0.1%, whereas PM2 (supporting pathogenic evidence strength) was assigned for the remaining variants (frequency ≤0.1% in gnomAD). A subset of rare variants in gnomAD (PM2) exceeded 1 allele in the South African ALS sample (n = 7); for these variants, we also used MAF information from a small sample of matched South African ancestry controls to interpret the rarity of these variants (eMethods).
For functional effect prediction of missense variants, we used the REVEL metapredictor score17 assigning BP4 for variants with a score <0.5 (predicted benign effect) and PP3 for those with a score ≥0.5 (predicted deleterious effect), both at a supporting strength level. Additional detail pertaining to the application of other ACMG evidence codes is described in eMethods (links.lww.com/NXG/A509).
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Cohort Demographics and Phenotypes
The demographic and clinical characteristics of 103 South Africans with ALS reflect the ancestry composition of the Western Cape province of South Africa where this study is based (Table 1). The majority (74%) of participants had SAC genetic ancestry (n = 76), whereas the remainder had Black African genetic ancestry (n = 27). Classical ALS was the most common phenotype (72%), and the median disease duration of both UMN-ALS (78 months) and LMN-ALS cases (96 months) was significantly longer than classical ALS cases (25 months) (p < 0.0001).
An ancestry PCA plot representing the case and control samples included in this study (n = 341) showed that Black Southern Africans cluster separately from the East and West African controls in the 1000 Genomes data set (Figure 1). SAC patients and controls form a heterogeneous spread reflecting the genetic contributions from Khoisan, African, European, and Southeast Asian populations. The plot highlights the fact that the East and West African control samples in the 1000 Genomes and gnomAD data sets are not a suitable proxy for Southern Africans.
Genetic Findings
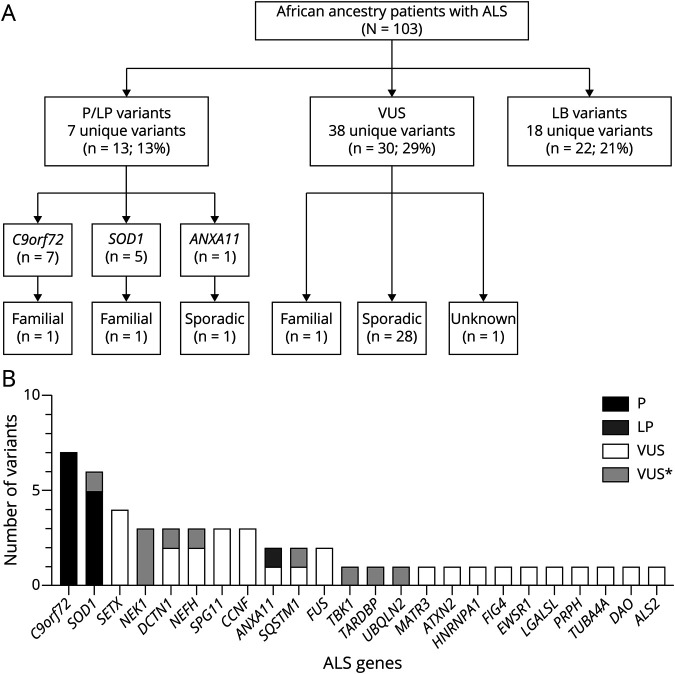
We identified pathogenic repeat expansions (GGGGCC repeat length >30) in the C9orf72 gene in 7 patients (4 SAC individuals were previously reported)8 (Figure 2A) and intermediate length repeat expansions in ATXN2 (27–33 CAG repeats, classified as VUS) in 1 patient (previously reported).9 In the other ALS genes (eFigure 1 and eMethods, links.lww.com/NXG/A509), 62 variants were identified, which were classified according to ACMG evidence codes (eTable 1).
Figure 2. Mutational Spectrum of South Africans With ALS.
(A) Schematic showing the numbers of likely pathogenic (LP) and pathogenic (P) variants, variants of uncertain significance (VUS), and likely benign (LB) variants identified in African ancestry patients with amyotrophic lateral sclerosis (ALS). C9orf72 refers to the pathogenic C9orf72 repeat expansion. (B) Graph showing gene counts of pathogenic (P) and variants of uncertain significance (VUS) in African ancestry patients with ALS. *Refers to prioritized VUS variants with a high likelihood of pathogenicity.
LB Variants
Eighteen of 62 variants (29%) were classified as LB (see eTable 1, links.lww.com/NXG/A509). Seven variants were found in >1 patient with ALS, and 4 are South African population-specific variations (MAF >0.5% in AWI-GEN control data set and rarity in gnomAD). Of the 18 LB variants, 15 have been cited in the ClinVar database with interpretations ranging from benign to VUS. Twenty-two (21%) of African ancestry patients with ALS had a LB variant identified (Figure 2A).
Variants of Uncertain Significance
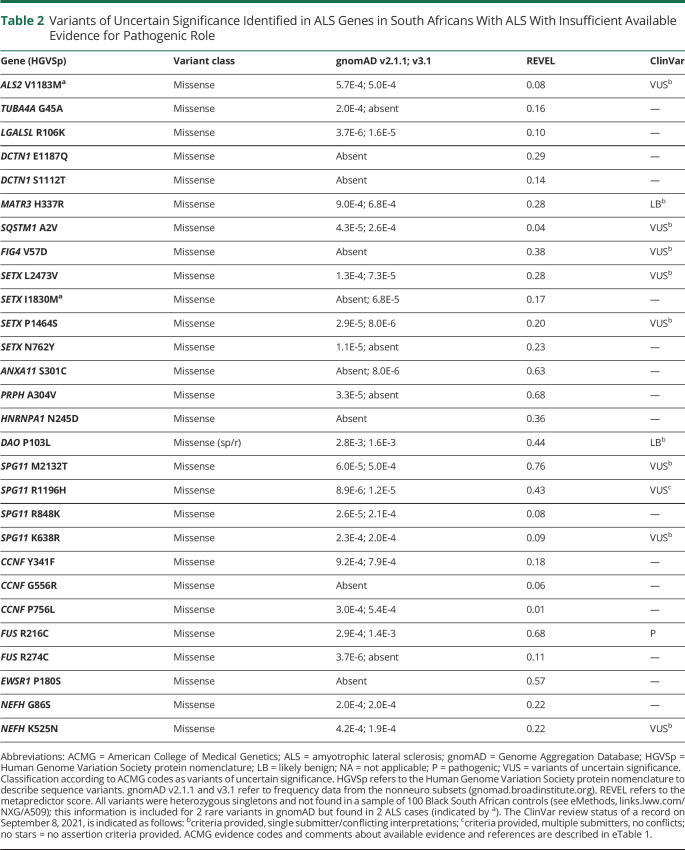
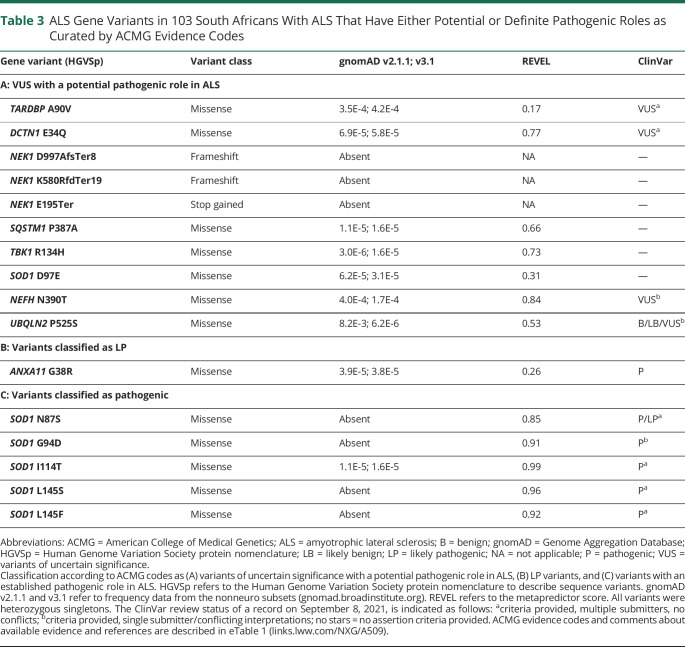
Thirty-eight of 62 variants (61%) were classified as VUS where the cumulative evidence for a benign or pathogenic classification could not be met; 16/38 VUS have been cited in ClinVar with interpretations ranging from benign to pathogenic (eTable 1, links.lww.com/NXG/A509). For each VUS, we comprehensively assessed the overall ACMG evidence codes; 28 variants had insufficient available evidence for a pathogenic role (Table 2), whereas 10 prioritized variants had pathogenic evidence, but this was insufficient to result in a confident pathogenic classification (Table 3 and Figure 2B).
Table 2.
Variants of Uncertain Significance Identified in ALS Genes in South Africans With ALS With Insufficient Available Evidence for Pathogenic Role
Table 3.
ALS Gene Variants in 103 South Africans With ALS That Have Either Potential or Definite Pathogenic Roles as Curated by ACMG Evidence Codes
For example, TARDBP A90V may represent an ALS risk allele with reduced penetrance, and experimental studies have shown altered protein function for this variant, though not to the same degree as established pathogenic TARDBP mutants.18 Another VUS, DCTN1 E34Q, is located in the CAP-gly domain of the dynactin protein together with all the reported pathogenic DCTN1 variants associated with Perry syndrome and distal hereditary motor neuropathy type 7B. However, functional studies of this variant have not shown a clear deleterious effect on dynactin protein function.19 Three different predicted LOF variants were identified in the NEK1 gene. Although interesting given the recently reported association of NEK1 LOF variants with ALS,6 the pathogenicity of these specific variants remains uncertain particularly because the background frequency of NEK1 LOF variants has not yet been established in African ancestry controls. The SQSTM1 P387A variant was highlighted as another amino acid change involving the same residue (P387L), which has been found in FTD cases,20 including segregation with disease in an FTD pedigree21 and incomplete penetrance in familial Paget disease,22 as well as 2 reports of impact on protein function.23,24 NEFH N390T has been described in a case of FTD.25 UBQLN2 P525S has been reported in FTD families with incomplete penetrance,26,27 but functional studies report conflicting results for this variant.28-30 TBK1 R134H has been reported in a patient with ALS,31 and functional studies suggest that this variant alters protein function.32 SOD1 D97E involves the same amino acid described in a recessive ALS family where affected individuals carry heterozygous SOD1 D97N and D91A (or D90A) variants.33
LP and Pathogenic Variants
The LP ANXA11 G38R variant (Table 3) has been reported in 5 cases with ALS,34-36 2 of which had prominent spasticity and associated cognitive impairment/FTD, similar to our case. Furthermore, 1 of these ALS cases showed typical ALS neuropathology in the spinal cord and TDP-43 pathology in the cortex.36 The benign REVEL score reported for this variant conflicts with both the neuropathologic evidence and the functional studies, which show altered protein function.34,36-38 Although gene curation is performed in an unbiased manner, the clinical milieu is considered in the final step; we found the SOD1 D97E and ANXA11 G38R variants in a single individual with prominent spasticity and increasing behavioral dysfunction for 3–4 years before presenting with ALS.
We identified 5 different pathogenic SOD1 variants (REVEL scores ≥0.85) in exons 4 (N87S, G94D, and I114T) and 5 (L145S and L145F) of the SOD1 protein (Table 3). The SOD1 L145F variant was in an LMN-ALS case with a family history and confirmed in 2 siblings (not included in the WGS analysis). The individual with SOD1 G94D also had predominantly LMN-ALS onset, whereas ALS presentations occurred in those with SOD1 I114T and L145S, and another with SOD1 N87S had early diaphragmatic involvement and died within 8 months.
In the 44 ALS genes examined in this study, 24 harbored pathogenic variants and/or VUS (Figure 2B) in African ancestry patients. Pathogenic and LP variants were identified in 13/103 (13%) individuals: pathogenic C9orf72 repeat expansions (7%), SOD1 (5%), and ANXA11 (1%), whereas 38 VUS were identified in 30/103 individuals (29%) (Figure 2A). All 11 SAC patients harboring pathogenic and LP variants were determined to have a proportion of African genetic ancestry by admixture analysis (eFigure 2 in eMethods, links.lww.com/NXG/A509). The C9orf72 and SOD1 carrier frequencies did not differ when patients with a family history of ALS or no available family history data were excluded (Figure 2A).
Discussion
In this report, we describe the genetic variation spectrum in a sample of patients with ALS from the Western Cape region of South Africa using a virtual ALS gene screen of WGS data. Although these patients had African ancestry, they comprised predominantly the SAC ancestry group (74%), whereas Black Africans represented 26% of our patients. The frequency of LP and pathogenic variants in this African ancestry ALS cohort without a family history of ALS (11/100; 11%) was lower than a similar-sized European ancestry cohort with sporadic ALS (15/93, 16%),16 although the 2 most frequent genes harboring pathogenic variants in both cohorts were C9orf72 and SOD1.
A pathogenic hexanucleotide repeat expansion (>30) in the noncoding region of the C9orf72 is the most common pathogenic genetic variant in ALS/FTD identified to date.13,14 This finding was replicated in our African ancestry cohort where we found a C9orf72 pathogenic expansion carrier frequency of 6.8% (7.9% in SAC and 3.7% in Black patients), which does not differ substantially from reported frequencies among sporadic ALS cases with European genetic ancestry (5.1%–7%).16 Pathogenic SOD1 variants the next most frequently identified in Europeans with ALS (1.2%),16 were found in 4% of African ancestry patients without a family history of ALS. We identified I114T (or I113T) in 1 SAC individual, which is the most common pathogenic SOD1 variant in patients with ALS in the United Kingdom,16 but did not observe D91A (the most common pathogenic variant worldwide) and A5V (the most common pathogenic variant in North America). Although the sample size is small, our findings suggest that pathogenic SOD1 variants are an important genetic cause of ALS in African ancestry populations, at least in Southern Africa.
Variant curation efforts in ALS are already challenging due to the complex genetic architecture of the disease while gene panel testing for ALS in African genomes may pose additional challenges due to ancestry-related bias. African genomes are highly diverse as a result of their ancient origins. A significant proportion of this variation is either novel (not represented in high-resolution population genetic databases such as gnomAD, which lack Southern African samples) or misclassified (as VUS or pathogenic variants in the ClinVar database when they in fact represent benign African-specific variations). For example, 33% (6/18) of the rare variants we curated as LB according to ACMG evidence codes were classified as VUS in the ClinVar database.
In this African ancestry ALS cohort, 29% harbored ≥1 VUS, which is comparable to reported frequencies in a British cohort of similar size (23%).16 The pathogenicity of the 10 variants identified in African ancestry samples, which we prioritized for future research (Table 2), could be questioned on the grounds of population AF (MAF >0.1%), reports of genotype-phenotype discordance within previously studied families (incomplete penetrance), and benign predictions by computational tools, which are in conflict with functional studies. However, it has been shown that the AF of pathogenic variants related to later adult-onset dominant diseases is higher compared with those related to earlier-onset dominant diseases as they are not subject to the same selective pressures.39 It is also recognized that intrafamilial genetic heterogeneity, shared de novo or epivariations and pleiotropy may explain the genotype-phenotype discordance, which is not infrequently observed in ALS families.40 Consequently, the task of variant curation in ALS should consider that the genetic basis of this disease exists on a spectrum from monogenic (excessively rare, highly penetrant variants) to oligogenic (higher frequency risk factor variants requiring one or more second hits, which may be genetic or environmental). It is therefore unlikely that universal application of filtering thresholds will be adequate to capture variants at both extremes of this genetic spectrum.
In conclusion, an ALS gene screen in African ancestry patients with ALS, without a family history of ALS, yielded a pathogenic variant (C9orf72, SOD1, or ANXA11) in 11% of cases. This highlights the proportion of Africans with ALS who would benefit from genetic testing and would be eligible for gene therapy trials, which are already underway for patients carrying pathogenic SOD1 and C9orf72 variants. Although this study highlights challenges for the clinical interpretation of sequence variants in patients with ALS of African genetic ancestry, ALS cohorts from Africa should be included in large-scale ALS gene discovery efforts.
Acknowledgment
The authors thank Dr. C. Albertyn for cognitive phenotyping of the ALS cases. The authors thank the Africa Wits-INDEPTH partnership for Genomics studies (AWI-Gen), a Human Heredity and Health in Africa (H3A) Consortium study, for providing variant frequency information for South African control subjects. The authors also thank the Southern African Human Genome Programme (SAHGP) participants. The South African WGS data set was generated by the national SAHGP initiative funded by the Department of Science and Technology of South Africa. The authors acknowledge the use of the Ilifu cloud computing facility (ilifu.ac.za), a partnership between the University of Cape Town, the University of the Western Cape, the University of Stellenbosch, Sol Plaatje University, the Cape Peninsula University of Technology, and the South African Radio Astronomy Observatory. The Ilifu facility is supported by contributions from the Inter-University Institute for Data Intensive Astronomy (IDIA—a partnership between the University of Cape Town, the University of Pretoria, and the University of the Western Cape), the Computational Biology division at UCT, and the Data Intensive Research Initiative of South Africa (DIRISA). JMH and AM receive funding from the National Research Foundation of South Africa. MN is the recipient of a CReATe scholar award and a Carnegie Developing Emerging Academic Leaders (DEAL) award. This publication was made possible (in part) by a grant from Carnegie Corporation of New York and a L'Oréal-UNESCO For Women in Science South African Young Talents Award. The statements made and views expressed are solely the responsibility of the authors.
Glossary
- ACMG
American College of Medical Genetics
- AF
allele frequency
- ALS
amyotrophic lateral sclerosis
- bp
base pair
- CReATe
Clinical Research in ALS and related disorders for Therapeutic Development
- FTD
frontotemporal dementia
- gnomAD
Genome Aggregation Database
- LB
likely benign
- LMN
lower motor neuron
- LOF
loss of function
- LP
likely pathogenic
- MAF
minor allele frequency
- PCA
principal component analysis
- SAC
South African Coloured
- UMN
upper motor neuron
- VEP
variant effect predictor
- VUS
variants of uncertain significance
- WGS
whole-genome sequencing
Appendix. Authors
Contributor Information
Melissa Nel, Email: melissa.nel@alumni.uct.ac.za.
Amokelani C. Mahungu, Email: mhnamo001@myuct.ac.za.
Nomakhosazana Monnakgotla, Email: mnnnom009@myuct.ac.za.
Gerrit R. Botha, Email: gerrit.botha@uct.ac.za.
Nicola J. Mulder, Email: nicola.mulder@uct.ac.za.
Gang Wu, Email: gang.wu@stjude.org.
Evadnie Rampersaud, Email: evadnie.rampersaud@stjude.org.
Marka van Blitterswijk, Email: vanblitterswijk.marka@mayo.edu.
Joanne Wuu, Email: jwuu@med.miami.edu.
Anne Cooley, Email: acooley@med.miami.edu.
Jason Myers, Email: jason.myers@stjude.org.
Rosa Rademakers, Email: rosa.rademakers@uantwerpen.vib.be.
J. Paul Taylor, Email: jpaul.taylor@stjude.org.
Michael Benatar, Email: mbenatar@med.miami.edu.
Study Funding
The authors acknowledge the support of Nicola Mulder's group who funded the whole-genome sequencing of 25 ALS cases (Human Genome Research Institute: U24HG006941). They thank the CReATe consortium and Paul Taylor's laboratory at St Jude's funded by Amyotrophic Lateral Sclerosis Association (ALSA) and St Jude American Lebanese Syrian Associated Charities (ALSAC). The Clinical Research in ALS and related disorders for Therapeutic Development (CReATe) Consortium (U54NS092091) is part of Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS). This consortium is funded through collaboration between NCATS and the NINDS.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NG for full disclosures.
References
- 1.Marin B, Boumé diene F, Logroscino G, et al. Variation in world wide incidence of amyotrophic lateral sclerosis: a meta-analysis. Int J Epidemiol. 2017;46(1):57-74. doi: 10.1093/ije/dyw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henning F, Heckmann JM, Naidu K, Vlok L, Cross HM, Marin B. Incidence of motor neuron disease/amyotrophic lateral sclerosis in South Africa: a 4-year prospective study. Eur J Neurol. 2021;28(1):81-89. doi: 10.1111/ene.14499. [DOI] [PubMed] [Google Scholar]
- 3.Shatunov A, Al-Chalabi A. The genetic architecture of ALS. Neurobiol Dis. 2021;147:105156. doi: 10.1016/j.nbd.2020.105156. [DOI] [PubMed] [Google Scholar]
- 4.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwiatkowski TJ, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205-1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 6.Kenna KP, Van Doormaal PTC, Dekker AM, et al. NEK1 variants confer susceptibility to amyotrophic lateral sclerosis. Nat Genet. 2016;48(9):1037-1042. doi: 10.1038/ng.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolas A, Kenna KP, Renton AE, et al. Genome-wide analyses identify KIF5A as a novel ALS gene. Neuron. 2018;97(6):1268-1283.e6. doi: 10.1016/j.neuron.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nel M, Agenbag GM, Henning F, Cross HM, Esterhuizen A, Heckmann JM. C9orf72 repeat expansions in South Africans with amyotrophic lateral sclerosis. J Neurol Sci. 2019;401:51-54. doi: 10.1016/j.jns.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nel M, Mavundla T, Gultig K, et al. Repeats expansions in ATXN2, NOP56, NIPA1 and ATXN1 are not associated with ALS in Africans. IBRO Neurosci Rep. 2021;10:130-135. doi: 10.1016/j.ibneur.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2000;1(5):293-299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 11.Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis—frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Front Degener. 2017;18(3-4):153-174. doi: 10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Wit E, Delport W, Rugamika CE, et al. Genome-wide analysis of the structure of the South African Coloured population in the Western Cape. Hum Genet. 2010;128(2):145-153. doi: 10.1007/s00439-010-0836-1. [DOI] [PubMed] [Google Scholar]
- 13.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245-256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257-268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lattante S, Marangi G, Doronzio PN, et al. High-throughput genetic testing in ALS: the challenging path of variant classification considering the ACMG guidelines. Genes (Basel). 2020;11(10):1123. doi: 10.3390/genes11101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepheard SR, Parker MD, Cooper-Knock J, et al. Value of systematic genetic screening of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2021;92(5):510-518. doi: 10.1136/jnnp-2020-325014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioannidis NM, Rothstein JH, Pejaver V, et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99(4):877-885. doi: 10.1016/j.ajhg.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wobst HJ, Wesolowski SS, Chadchankar J, et al. Cytoplasmic relocalization of TAR DNA-binding protein 43 is not sufficient to reproduce cellular pathologies associated with ALS in vitro. Front Mol Neurosci. 2017;10:46. doi: 10.3389/fnmol.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockmann M, Meyer-Ohlendorf M, Achberger K, et al. The dynactin p150 subunit: cell biology studies of sequence changes found in ALS/MND and Parkinsonian Syndromes. J Neural Transm. 2013;120(5):785-798. doi: 10.1007/s00702-012-0910-z. [DOI] [PubMed] [Google Scholar]
- 20.van der Zee J, Van Langenhove T, Kovacs GG, et al. Rare mutations in SQSTM1 modify susceptibility to frontotemporal lobar degeneration. Acta Neuropathol. 2014;128(3):397-410. doi: 10.1007/s00401-014-1298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Ber I. SQSTM1 mutations in French patients with frontotemporal dementia or frontotemporal dementia with amyotrophic lateral sclerosis. JAMA Neurol. 2013;70(11):1403-1410. doi: 10.1001/jamaneurol.2013.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson-Pais TL, Wisdom JH, Weldon KS, et al. Three novel mutations in SQSTM1 identified in familial Paget's disease of bone. J Bone Miner Res. 2003;18(10):1748-1753. doi: 10.1359/jbmr.2003.18.10.1748. [DOI] [PubMed] [Google Scholar]
- 23.Cavey JR, Ralston SH, Sheppard PW, et al. Loss of ubiquitin binding is a unifying mechanism by which mutations of SQSTM1 cause Paget's disease of bone. Calcif Tissue Int. 2006;78(5):271-277. doi: 10.1007/s00223-005-1299-6. [DOI] [PubMed] [Google Scholar]
- 24.Leach RJ, Singer FR, Ench Y, Wisdom JH, Pina DS, Johnson-Pais TL. Clinical and cellular phenotypes associated with sequestosome 1 (SQSTM1) mutations. J Bone Miner Res. 2006;21(S2):P45-P50. doi: 10.1359/jbmr.06s208. [DOI] [PubMed] [Google Scholar]
- 25.Blauwendraat C, Wilke C, Simón-Sánchez J, et al. The wide genetic landscape of clinical frontotemporal dementia: systematic combined sequencing of 121 consecutive subjects. Genet Med. 2018;20(2):240-249. doi: 10.1038/gim.2017.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng HX, Chen W, Hong ST, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477(7363):211-215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özoğuz A, Uyan Ö, Birdal G, et al. The distinct genetic pattern of ALS in Turkey and novel mutations. Neurobiol Aging. 2015;36(4):1764.e9-1764.e18. doi: 10.1016/j.neurobiolaging.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang L, Monteiro MJ. Defective proteasome delivery of polyubiquitinated proteins by ubiquilin-2 proteins containing ALS mutations. PLoS One. 2015;10(6):e0130162. doi: 10.1371/journal.pone.0130162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SH, Stiles SG, Feichtmeier JM, et al. Mutation-dependent aggregation and toxicity in a Drosophila model for UBQLN2-associated ALS. Hum Mol Genet. 2018;27(2):322-337. doi: 10.1093/hmg/ddx403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dao TP, Martyniak B, Canning AJ, et al. ALS-linked mutations affect UBQLN2 oligomerization and phase separation in a position- and amino acid-dependent manner. Structure. 2019;27(6):937-951.e5. doi: 10.1016/j.str.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cirulli ET, Lasseigne BN, Petrovski S, et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347(6229):1436-1441. doi: 10.1126/science.aaa3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye J, Cheung J, Gerbino V, et al. Effects of ALS-associated TANK binding kinase 1 mutations on protein-protein interactions and kinase activity. Proc Natl Acad Sci USA. 2019;116(49):24517-24526. doi: 10.1073/pnas.1915732116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hand CK, Mayeux-Portas V, Khoris J, et al. Compound heterozygous D90A and D96N SOD1 mutations in a recessive amyotrophic lateral sclerosis family. Ann Neurol. 2001;49(2):267-271. doi: . [DOI] [PubMed] [Google Scholar]
- 34.Smith BN, Topp SD, Fallini C, et al. Mutations in the vesicular trafficking protein annexin A11 are associated with amyotrophic lateral sclerosis. Sci Transl Med. 2017;9(388):eaad9157. doi: 10.1126/scitranslmed.aad9157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller K, Brenner D, Weydt P, et al. Comprehensive analysis of the mutation spectrum in 301 German ALS families. J Neurol Neurosurg Psychiatry. 2018;89(8):817-827. doi: 10.1136/jnnp-2017-317611. [DOI] [PubMed] [Google Scholar]
- 36.Teyssou E, Muratet F, Amador MDM, et al. Genetic screening of ANXA11 revealed novel mutations linked to amyotrophic lateral sclerosis. Neurobiol Aging. 2021;99:102.e11-102.e20. doi: 10.1016/j.neurobiolaging.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Liao D, Liao Q, Huang C, Bi F. Mutations of G38R and D40G cause amyotrophic lateral sclerosis by reducing Annexin A11 protein stability. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(6):577-582. doi: 10.11817/j.issn.1672-7347.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Nahm M, Lim SM, Kim YE, et al. ANXA11 mutations in ALS cause dysregulation of calcium homeostasis and stress granule dynamics. Sci Transl Med. 2020;12(566):eaax3993. doi: 10.1126/scitranslmed.aax3993. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Jin Y, Yin Y. Allele frequency of pathogenic variants related to adult-onset Mendelian diseases. Clin Genet. 2019;96(3):226-235. doi: 10.1111/cge.13579. [DOI] [PubMed] [Google Scholar]
- 40.Lowry JL, Ryan ÉB, Esengul YT, Siddique N, Siddique T. Intricacies of aetiology in intrafamilial degenerative disease. Brain Commun. 2020;2(2):fcaa120. doi: 10.1093/braincomms/fcaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.