ABSTRACT
Background
Whether hyperkalaemia in CKD is chronic or transient, and whether this has different outcome implications, is not known.
Methods
This was an observational study of adults with CKD G3–5 from Stockholm, Sweden 2006–11. We examined individual trajectories of potassium from all measurements obtained through routine outpatient care. For each month of follow-up, we created a rolling assessment of the proportion of time in which potassium was abnormal during the previous 12 months. We defined patterns of hyperkalaemia as transient (≤50% of time during the previous year with potassium >5.0 mmol/L) and chronic (>50% of time with potassium >5.0 mmol/L), and examined whether previous hyperkalaemia pattern offers additional predictive value beyond that provided by the most recent (current) potassium value.
Results
We included 36 511 participants (56% women) with CKD G3–5 and median estimated glomerular filtration rate 46 mL/min/1.73 m2. Transient and chronic hyperkalaemia, respectively, were observed in 15% and 4% of patients with CKD G3a, and in 50% and 17% of patients with CKD G5. In fully adjusted models, transient (hazard ratio 1.36, 95% confidence interval 1.29–1.46) or chronic (1.16, 1.04–1.32) hyperkalaemia patterns, but not current hyperkalaemia, were associated with major adverse cardiovascular events (MACE), compared with normokalaemia. Transient hyperkalaemia (1.43, 1.35–1.52) and current potassium values, but not chronic hyperkalaemia, were associated with the risk of death.
Conclusions
Between 4% and 17% of patients with CKD G3–5 develop chronic hyperkalaemia. In general, hyperkalaemia predicted MACE and death; however, the lack of effect of current potassium on MACE when adjusted for the previous pattern, and the stronger effects on death than on MACE, lead us to question whether hyperkalaemia is causal in these relationships.
Keywords: cardiovascular, CKD, epidemiology, hyperkalaemia, survival analysis
INTRODUCTION
Patients with chronic kidney disease (CKD) have multiple risk factors, comorbidities and medications that increase circulating levels of potassium and contribute to risk of hyperkalaemia [1, 2]. Hyperkalaemia in CKD is often described as chronic, but previous studies have based their conclusions on a single potassium measurement [1, 2]. A single potassium assessment importantly predicts the risk of major adverse cardiovascular events (MACE), cardiac arrest and death [3, 4]. However, whether hyperkalaemia in patients with CKD is chronic, and whether this distinction is of clinical relevance, are poorly understood.
Although repetitive consecutive measurements facilitate determination of whether hyperkalaemia is a chronic or transient event [5, 6], there is no consensus on the number of tests required to characterize or define chronic hyperkalaemia. Some studies have attempted to evaluate this issue; one study reported increased risk of death for heart failure patients with chronic or transient hyperkalaemia during a 1-year period based on the number of routinely measured potassium tests above a certain threshold [7]. A limitation of such a definition is that the frequency of testing is influenced by disease severity, and acute hyperkalaemia may prompt intense testing within a short period of time, which differs from persistently elevated potassium over longer periods. Other studies define chronic states by the concordance between two potassium measurements 1 year apart [8], which may introduce bias because it requires the patients to survive that period to be eligible for inclusion.
Evaluating outcomes associated with chronic hyperkalaemia patterns answers a different question than studies evaluating potassium trajectories [8, 9]. Because the majority of hyper- or hypokalaemia events are likely transient, the potassium slopes are typically fairly flat even in advanced CKD [10], and may not be informative. We here tried to characterize the epidemiology of chronic and transient hyperkalaemia in patients with CKD G3–5 not on dialysis (G3–5ND) and to explore the association between patterns of hyper- and hypokalaemia and adverse health outcomes, namely MACE and death.
MATERIALS AND METHODS
Data sources
This study utilizes the Stockholm CREAtinine Measurements (SCREAM) project [11], which includes all residents in the region of Stockholm (Sweden) undertaking at least one measurement of plasma creatinine in connection with a healthcare encounter during 2006–11. Using each citizen’s unique personal identity number, SCREAM laboratory data were linked to other nationwide government-run registries that collect complete information on drugs dispensed at Swedish pharmacies [12]; outpatient specialist consultations and hospitalizations [13]; and date and causes of death [14]. The study was approved by the regional ethical review boards and informed consent was waived.
Patient selection and study design
We included participants aged ≥18 years who had an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 and at least one plasma/serum potassium measured in outpatient routine care. The index date was the earliest date on which these criteria were met. The diagnosis of CKD was defined by at least two consecutive outpatient creatinine measurements (>3 months and ˂1 year apart) indicating an eGFR <60 mL/min/1.73 m2. In order to reduce misclassification bias, patients in whom ≥10% of subsequent eGFR measurements were ≥60 mL/min/1.73 m2 were excluded. Categories of eGFR were defined by KDIGO criteria [15]: G3a = eGFR <60–45; G3b = GFR <45–30; G4 = eGFR <30–15; and G5 non-dialysis = eGFR <15 mL/min/1.73 m2. Patients with a history of kidney transplant or undergoing dialysis at baseline (i.e. first encountered outpatient potassium test) were excluded.
Study exposure: patterns of dyskalaemia
After study inclusion, we extracted information on all subsequent measurements of potassium and interpolated a linear rate of change of potassium (see Supplementary data, Figure S1) to model the trajectory of potassium from inclusion until death, kidney replacement therapy (KRT) or end of follow-up (December 2011) on monthly intervals, analogous to time-in-therapeutic-range methods used for international normalized ratio monitoring [16]. At each month, we evaluated the pattern of dyskalaemia based on the proportion of time spent with abnormal potassium values during the preceding 12 months, as follows: normokalaemia: all time spent between 3.5 and 5 mmol/L; transient hyperkalaemia: >0 but ≤50% of time spent with potassium >5.0 mmol/L; chronic hyperkalaemia: >50% of time spent with potassium >5.0 mmol/L. For completeness, we also defined transient hypokalaemia: >0 but ≤50% of time with potassium <3.5 mmol/L; and chronic hypokalaemia: >50% of time with potassium <3.5 mmol/L. Whenever the rolling assessment would fit more than one category definition we classified that person month as ‘undetermined’ and treated it as a separate category in the model, thus making our classification mutually exclusive.
Study outcomes: MACE and death
The primary outcome was the incidence of MACE, defined as the composite of non-fatal stroke, heart failure, myocardial infarction or death attributed to cardiovascular causes (Supplementary data, Table S1). The secondary outcome was all-cause death, because potassium disturbances are thought to affect specifically the cardiovascular system. Patients were followed until event or censoring (i.e. end of follow-up, migration outside Stockholm region, non-cardiovascular death—when applicable—or KRT). There was no loss to follow-up.
Study covariates
Study covariates included age, sex, laboratory measurements, comorbidities and medications, which were updated at each month. Laboratory measurements were potassium and creatinine tests obtained during outpatient care; serum/plasma potassium was assessed by potentiometric titration [17, 18]. Plasma potassium was the most frequent form of measurement, accounting for 91% of all measurements in the system; in our data, the average plasma value was 0.1 mmol/L lower than the average serum value. Serum and plasma creatinine was measured by the enzymatic or Jaffe method, and eGFR was calculated using the CKD Epidemiology Collaboration equation [19]. eGFR was updated at each potassium measurement. Hence, a patient may contribute to different CKD stages as he/she progresses in the disease. Comorbid conditions (Supplementary data, Table S2) were defined using diagnostic codes [20]. Medications (Supplementary data, Table S3) were assumed concomitant if there was a pharmacy dispensation at the time of or within the previous 6 months from each time interval, except for sodium polystyrene sulphonate (SPS). Given the SPS presentation as a 500 g package, and its commonly off-label use in Sweden at low dosages to prevent hyperkalaemia [21], we defined concomitant use with a 1-year window prior. Calcium polystyrene sulphonate, novel potassium binders and sodium–glucose cotransporter 2 (SGLT2) inhibitors were not available in the dataset.
Statistical analysis
Baseline characteristics were described as median and interquartile range (IQR) or counts with proportions. First, we evaluated the proportion of person-months occurring in each dyskalaemia pattern, and the proportion of unique individuals meeting each pattern definition. Secondly, we assessed clinical characteristics associated with each pattern through logistic regression models with clustered robust standard errors for repeated measures, setting normokalaemia as the reference level. Results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). Thirdly, we investigated the association between dyskalaemia patterns and study outcomes using time-dependent Cox models. We evaluated whether the current potassium level (i.e. the potassium encountered at each month interval) affected the association between dyskalaemia patterns and outcomes by fitting and comparing models with one or both of these measures. Since dyskalaemia patterns are based on the preceding 12 months, this analysis aims to distinguish the acute from the chronic effects of dyskalaemia. Because stroke is not pathophysiologically a consequence of hyperkalaemia, we repeated our MACE analyses excluding the component of stroke. Fourthly, consistency of results for our primary study outcome was explored through stratified analyses by age, sex, CKD G category, heart failure and use of renin–angiotensin–aldosterone system inhibitors (RAASi). Finally, we repeated the main analyses stratified by sex. All analyses were performed using R version 3.4.3 software (The R Project for Statistical Computing, Vienna, Austria).
RESULTS
Patients’ characteristics and patterns of dyskalaemia
After applying inclusion and exclusion criteria, 36 511 individuals with confirmed CKD G3–5ND were included in the study (Supplementary data, Figure S2), contributing 1 312 841 person-months of observation. At inclusion, the median age was 81 years and 56% were women (Table 1). The majority of patients had CKD G3 (87%). The most common comorbidity was hypertension (65%), followed by history of heart failure (32%) and diabetes (25%). A high proportion of individuals were receiving β-blockers (51%), diuretics (49%) angiotensin-converting enzyme inhibitor (ACEi; 32%) and angiotensin II receptor blockers (ARBs; 23%). At inclusion, compared with women, men were younger (median age 79 versus 83 years old), had more commonly a history of myocardial infarction (25% versus 16%) and diabetes (28% versus 22%), and had a higher proportion of individuals treated with ACEi (37% versus 27%) and SPS (1.5% versus 0.4%) (Supplementary data, Table S4).
Table 1.
Characteristics of patients with CKD G3–5 at study inclusion
| Characteristic | |
|---|---|
| Number of individuals | 36 511 |
| Age (years) | 81 (74, 87) |
| Women | 20 560 (56) |
| eGFR (mL/min/1.73 m2) | 46 (37, 58) |
| eGFR category | |
| <60–45 mL/min/1.73 m2 | 19 257 (53) |
| <45–30 mL/min/1.73 m2 | 12 392 (34) |
| <30–15 mL/min/1.73 m2 | 4099 (11) |
| <15 mL/min/1.73 m2 | 763 (2) |
| Comorbidities | |
| Hypertension | 23 738 (65) |
| Myocardial infarction | 7290 (20) |
| Heart failure | 11 735 (32) |
| Peripheral vascular disease | 4052 (11) |
| Cerebrovascular disease | 7362 (21) |
| Diabetes mellitus | 9065 (25) |
| Medications | |
| ACEi | 11 521 (32) |
| ARBs | 8375 (23) |
| MRAs | 4661 (13) |
| β-blockers | 18 680 (51) |
| Potassium sparing diuretics | 591 (2) |
| Thiazide-loop diuretics | 18 066 (49) |
| SPS | 331 (1) |
Characteristics are presented as median and IQR or counts and proportion.
MRAs, mineralocorticoid receptor antagonists.
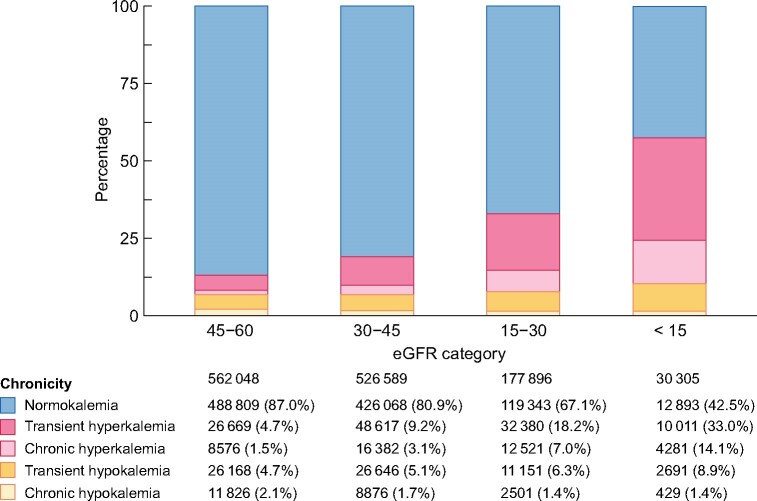
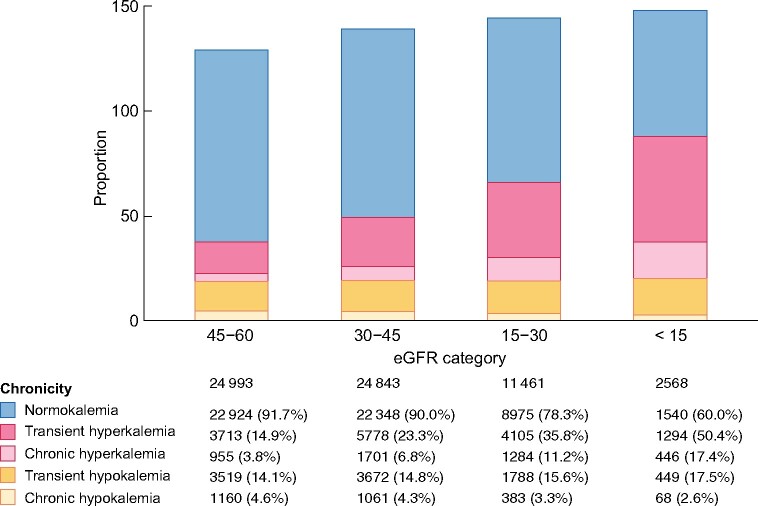
We identified 601 774 potassium measurements [median 14 (IQR 9–23) measurements per patient] performed during outpatient consultations, and used them to create individual potassium trajectories through linear interpolation. We observed that the majority of person-months were preceded by a pattern of normokalaemia (Figure 1), but this ranged from 87% of person-months with CKD G3a to 43% with CKD G5. The proportion of person-months preceded by transient and chronic hyperkalaemia increased with more severe CKD G categories, from 5% and 2% in CKD G3a, to 33% and 14% in CKD G5ND (transient and chronic hyperkalaemia patterns, respectively). The proportion of person-months preceded by a state of transient hypokalaemia also increased with more severe CKD G categories, but chronic hypokalaemia was rare and lower in frequency with lower eGFR. Figure 2 shows similar trends when plotting the proportion of unique individuals within each CKD G category: 50% of patients with CKD G5 had transient hyperkalaemia at least once during observation, compared with 15% of patients with CKD G3a; likewise, 17% of patients with CKD G5ND had chronic hyperkalaemia at least once during the study period, compared with 4% of patients with CKD G3a.
FIGURE 1:
Distribution of person-months (n = 1 296 838) preceded by different dyskalaemia patterns across eGFR categories. At each month, the patterns of dyskalaemia were defined from the potassium levels of the preceding 12 months. Results are based on rolling assessments, so that participants contribute more than once to the analysis. Normokalaemia: all time spent between 3.5 and 5 mmol/L; transient hyperkalaemia: >0 but ≤50% of time spent with potassium levels >5.0 mmol/L; chronic hyperkalaemia: >50% of time spent with potassium levels >5.0 mmol/L; transient hypokalaemia: >0 but ≤50% of time with potassium levels ˂3.5 mmol/L; and chronic hypokalaemia: >50% of time with potassium levels ˂3.5 mmol/L.
FIGURE 2:
Frequency of dyskalaemia patterns among unique individuals of different eGFR categories. Unique individuals are considered in each CKD G category, but one individual can contribute to different CKD G categories during follow-up as he/she progresses in the disease. For that reason, the frequency exceeds 100%. The patterns of dyskalaemia were defined from the potassium levels of the preceding 12 months; normokalaemia: all time spent between 3.5 and 5 mmol/L; transient hyperkalaemia: >0 but ≤50% of time spent with potassium levels >5.0 mmol/L; chronic hyperkalaemia: >50% of time spent with potassium levels >5.0 mmol/L; transient hypokalaemia: >0 but ≤50% of time with potassium levels ˂3.5 mmol/L; and chronic hypokalaemia: >50% of time with potassium levels ˂3.5 mmol/L.
Compared with women, men tend to have a higher percentage of person-months with transient or chronic hyperkalaemia, throughout all eGFR categories (Supplementary data, Figure S3). Conversely, men have a lower percentage of person-months spent with hypokalaemia. Similar results are also observed when unique individuals are considered. Men tend to be more likely to experience hyperkalaemia episodes (both chronic and transient) at least once, while women have a higher proportion of occurrences of hypokalaemia episodes (Supplementary data, Figure S4).
Clinical characteristics associated with patterns of dyskalaemia
Baseline predictors of chronic or transient hyperkalaemia were similar (Table 2): younger age, male sex, lower CKD eGFR category, presence of diabetes, heart failure and peripheral vascular disease, and concomitant use of RAASi or SPS. While the use of thiazide/loop diuretics was associated with a modestly higher risk of transient hyperkalaemia, it was associated with a lower risk of chronic hyperkalaemia.
Table 2.
Logistic regression models predicting patterns of dyskalaemia (normokalaemia as referent category)
| Transient hyperkalaemia | Chronic hyperkalaemia | Transient hypokalaemia | Chronic hypokalaemia | |
|---|---|---|---|---|
| Demographics | ||||
| Age (per 10 years older) | 0.84 (0.82–0.86) | 0.86 (0.83–0.90) | 0.77 (0.75–0.80) | 0.78 (0.73–0.83) |
| Women | 0.72 (0.68–0.75) | 0.67 (0.61–0.74) | 1.40 (1.31–1.50) | 1.44 (1.26–1.64) |
| CKD G3a | Reference | Reference | Reference | Reference |
| CKD G3b | 1.93 (1.84–2.02) | 2.13 (1.93–2.36) | 1.06 (1.00–1.11) | 0.84 (0.75–0.93) |
| CKD G4 | 3.96 (3.72–4.21) | 4.93 (4.34–5.59) | 1.36 (1.26–1.47) | 0.77 (0.65–0.92) |
| CKD G5 | 8.05 (7.20–9.00) | 8.74 (7.16–10.66) | 2.69 (2.33–3.12) | 1.05 (0.73–1.49) |
| Comorbidities | ||||
| Diabetes | 1.44 (1.36–1.52) | 1.60 (1.44–1.77) | 0.84 (0.78–0.90) | 0.74 (0.64–0.85) |
| Hypertension | 0.93 (0.88–0.99) | 0.90 (0.80–1.01) | 1.45 (1.35–1.56) | 1.90 (1.64–2.21) |
| Myocardial infarction | 1.03 (0.96–1.09) | 1.03 (0.92–1.16) | 0.88 (0.81–0.95) | 0.79 (0.67–0.93) |
| Heart failure | 1.33 (1.25–1.41) | 1.14 (1.01–1.28) | 1.27 (1.18–1.36) | 0.96 (0.83–1.11) |
| Peripheral vascular disease | 1.16 (1.09–1.25) | 1.21 (1.07–1.38) | 1.06 (0.97–1.16) | 1.08 (0.91–1.29) |
| Cerebrovascular disease | 1.01 (0.95–1.07) | 0.89 (0.79–1.00) | 1.12 (1.04–1.20) | 1.06 (0.92–1.22) |
| Medications | ||||
| ACEi/ARBs | 1.55 (1.48–1.63) | 1.66 (1.50–1.84) | 0.55 (0.52–0.59) | 0.40 (0.36–0.45) |
| MRAs | 1.76 (1.66–1.87) | 1.26 (1.10–1.45) | 1.09 (1.01–1.18) | 0.72 (0.61–0.86) |
| β-blockers | 1.05 (1.00–1.10) | 0.94 (0.85–1.03) | 0.90 (0.85–0.95) | 0.84 (0.75–0.94) |
| Potassium-sparing diuretics | 0.93 (0.76–1.13) | 0.62 (0.39–0.98) | 1.09 (0.89–1.35) | 1.04 (0.70–1.55) |
| Thiazide/loop diuretics | 1.08 (1.03–1.14) | 0.81 (0.73–0.90) | 2.27 (2.13–2.42) | 2.03 (1.79–2.31) |
| SPS | 8.41 (7.12–9.93) | 12.66 (10.28–15.6) | 0.99 (0.73–1.36) | 0.33 (0.11–0.99) |
The results are presented as OR and 95% CIs.
MRAs, mineralocorticoid receptor antagonists.
Predictors of transient and chronic hypokalaemia were generally the opposite to the hyperkalaemia models (Table 2): female sex, absence of diabetes or myocardial infarction, absence of RAASi medication or β-blockers or use of thiazide diuretics. In addition, the odds of transient hypokalaemia also increased across worse CKD G categories (lower GFR), presence of heart failure and cerebrovascular disease. The association between predictors and patterns of dyskalaemia was similar in sex-stratified analyses (Supplementary data, Tables S5–S6).
Association between dyskalaemia patterns and adverse events
During a median follow-up time of 3 years (IQR 1.3–4.6), 13 104 (36%) individuals experienced MACE. Compared with normokalaemia patterns (Table 3A), patients with transient or chronic hyperkalaemia were at higher risk of MACE, with the magnitude of this risk being higher for transient [hazard ratio (HR) 1.36, 95% CI 1.29–1.44] than for chronic hyperkalaemia (HR 1.16, 95% CI 1.05–1.28) states. A state of transient hypokalaemia (HR 1.44, 95% CI 1.34–1.54) also was associated with increased risk of MACE, but no association was observed for chronic hypokalaemia (HR 1.13, 95% CI 0.99–1.30). The pattern of dyskalaemia, however, does not always correspond by a similar level of the most recent potassium (Supplementary data, Table S7). As expected, the current levels of potassium tend to be more often outside normal ranges during periods in which chronic episodes of dyskalaemia are detected compared with transient episodes. When included in the model current (most recent) single potassium values >5.0 or <3.5 mmol/L were also associated with increased risk of MACE. When both dyskalaemia patterns (i.e. based on the potassium trajectory in the preceding 12 months) and current potassium values (i.e. based on the potassium value at each month) were included in the same model, patterns of dyskalaemia and potassium <3.0 mmol/L were associated with the risk of MACE. Similar findings were observed when excluding stroke events from the composite MACE (Supplementary data, Table S8).
Table 3.
Association between patterns of dyskalaemia within the preceding 12 months (Model 1), current potassium value (in mmol/L, Model 2) or both in the same model (Model 3), with the risk of MACE (A) or death (B)
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| (A) Risk of MACE | |||
| Patterns of dyskalaemia | |||
| Transient hyperkalaemia | 1.36 (1.29–1.44) | – | 1.37 (1.29–1.46) |
| Chronic hyperkalaemia | 1.16 (1.05–1.28) | – | 1.17 (1.04–1.32) |
| Normokalaemia | Reference | – | Reference |
| Transient hypokalaemia | 1.44 (1.34–1.54) | – | 1.39 (1.29–1.50) |
| Chronic hypokalaemia | 1.13 (0.99–1.30) | – | 1.05 (0.89–1.23) |
| Current potassium value | |||
| >5.5 mmol/L | – | 1.38 (1.15–1.66) | 1.17 (0.96–1.42) |
| >5.0–5.5 mmol/L | – | 1.15 (1.06–1.25) | 0.95 (0.86–1.05) |
| 3.5–5.0 mmol/L | – | Reference | Reference |
| <3.5–3.0 mmol/L | – | 1.31 (1.17–1.46) | 1.10 (0.96–1.25) |
| <3.0 mmol/L | – | 2.36 (1.58–3.51) | 2.02 (1.35–3.02) |
| Likelihood ratio testa | <0.001 | <0.001 | – |
| (B) Risk of death | |||
| Patterns of dyskalaemia | |||
| Transient hyperkalaemia | 1.51 (1.43–1.59) | – | 1.43 (1.35–1.52) |
| Chronic hyperkalaemia | 1.24 (1.13–1.37) | – | 1.07 (0.95–1.20) |
| Normokalaemia | Reference | – | Reference |
| Transient hypokalaemia | 1.88 (1.77–2.00) | – | 1.64 (1.53–1.76) |
| Chronic hypokalaemia | 1.47 (1.31–1.65) | – | 1.07 (0.93–1.23) |
| Current potassium value | |||
| >5.5 mmol/L | – | 2.09 (1.91–2.28) | 1.58 (1.42–1.76) |
| >5.0–5.5 mmol/L | – | 1.33 (1.22–1.44) | 1.13 (1.03–1.25) |
| 3.5–5.0 mmo/L | – | Reference | Reference |
| <3.5–3.0 mmol/L | – | 2.34 (2.03–2.71) | 2.09 (1.79–2.44) |
| <3.0 mmol/L | – | 5.93 (4.50–7.82) | 4.69 (3.61–6.08) |
| Likelihood ratio testa | <0.001 | <0.001 | – |
The likelihood ratio test compares the model fit against Model 3. Model 3 offers a better fit than Models 1 and 2. Statistically significant HRs are marked in bold. Models are adjusted for: age, sex, eGFR, comorbidities (diabetes mellitus, hypertension, heart failure, myocardial infarction, peripheral vascular disease and cerebrovascular disease) and medications (SPS, RAASi, β-blockers, potassium-sparing diuretics and thiazide/loop diuretics).
A total of 13 570 deaths were registered (Table 3B). Again, both patterns of dyskalaemia and updated monthly potassium levels were strongly associated with the risk of death when modelled separately. When modelled together, abnormal current potassium values remained strongly associated with the risk of death, together with patterns of transient hypo- and hyperkalaemia.
The risk of MACE and death associated with dyskalaemia patterns and current potassium values were similar in sex-stratified analyses (Supplementary data, Tables S9 and S10).
Stratified analysis suggests that the risk of MACE associated with dyskalaemia patterns is higher in less severe CKD G categories (Figure 3), with similar results observed among men and women (Supplementary data, Figures S5 and S6). Transient dyskalaemias had a stronger association with the risk of MACE in patients with RAASi compared with without, but the risk magnitude was high in both (Supplementary data, Figure S7). No suggestion of heterogeneity was observed across age, sex and heart failure strata (Supplementary data, Figures S8 and S10).
FIGURE 3:
Association between patterns of dyskalaemia and MACE by CKD G category. Model adjusted for age, sex, eGFR, comorbidities (diabetes mellitus, hypertension, heart failure, myocardial infarction, peripheral vascular disease and cerebrovascular disease) and use medications (SPS, RAASi, β-blockers, potassium-sparing diuretics and thiazide/loop diuretics). Normokalaemia was the referent category. P int., P-value for interaction.
DISCUSSION
This observational study of people with CKD G3–5ND found that abnormalities in potassium homoeostasis, especially hyperkalaemia, are increasingly more prevalent with lower kidney function, in keeping with previous studies [1, 2]. Our novel findings were firstly, that though the majority of hyperkalaemia events were transient, between 11% and 17% of patients with G4–5 experience chronic hyperkalaemia (>6 months in a 12-month period with potassium >5.0 mmol/L) at least once. Secondly, we found that separately, previous patterns of dyskalaemia and current potassium values were both associated with the risk of MACE and death. When both metrics are introduced in the same model, patterns of hyperkalaemia (transient and chronic), but not current hyperkalaemia, remained associated with the risk of MACE. Likewise, the transient hyperkalaemia pattern and current hyperkalaemia, but not the chronic hyperkalaemia pattern, were associated with the risk of death.
Strengths of our analysis include the complete regional capture of patients undergoing creatinine and potassium testing from all types of healthcare, which minimizes biases. The identification of the presence and G category of CKD by eGFR is also a strength, given poor awareness and underutilization of International Classification of Diseases codes with inability to distinguish disease severity reliably [22, 23]. Our design of continuous rolling assessments overcomes previous limitations in the literature imposed by the frequency of testing or the need to survive a certain time to be eligible for inclusion (survivor bias).
As expected from the role of the kidney in eliminating dietary potassium [1], the occurrence and recurrence [2, 3, 24, 25] of hyperkalaemia are prevalent in our data. In a private US health system, potassium was >5.0 mmol/L in 13% of observation time for patients with CKD G3a, and this figure rose to 32% for those with CKD G4–5 [5]. However, the authors did not evaluate whether these potassium elevations were persistent over time (i.e. chronic). In people with CKD, serum potassium appeared to decrease more slowly and tended not to reach pre-hyperkalaemia concentrations after a recurrent hyperkalaemia, compared with people with a single hyperkalaemia event [26]. Our observations are in line with this evidence, using new methods and suggesting new definitions to demonstrate that chronic hyperkalaemia is prevalent in patients with CKD, and that transient hyperkalaemia, is more prevalent. We are not aware of previous studies that have evaluated this, and we recognize that there is no consensus on how to define chronic elevations in routine clinical practice. We offer these tentative definitions in the hope that they will be useful to others and that between-study comparisons will be possible. We think our definition based on >50% of person time during the previous year is conservative.
In our study, transient hypokalaemia patterns were also more frequent with lower eGFR. This agrees with a previous report [5] and possibly results from intercurrent events, including diuretic use. For this reason, we will focus our discussion on findings pertaining to hyperkalaemia.
Beyond GFR, our multivariable risk analyses confirm male sex, diabetes and vascular disease as potential risk factors for both transient and chronic hyperkalaemia patterns. RAASi use increases the risk of hyperkalaemia, expected to be a direct effect [27], while the association we observe with SPS is likely confounding by indication (i.e. the hyperkalaemia is the reason for the SPS prescribing, rather than the SPS is a risk factor for hyperkalaemia) [21, 28].
We found that the previous pattern of hyperkalaemia over a year period, whether chronic or transient, was associated with the risk of MACE, even after adjustment for the current potassium, suggesting that there is additional information in the previous potassium pattern beyond potassium measured at a single time point. We also found that previous patterns of transient hypo- and hyperkalaemia, as well as single abnormal potassium values (both in the hypo- or hyperkalaemia range), were all associated with the risk of death. Matsushita et al. [7] evaluated patterns of hyperkalaemia in 118 477 US veterans with incident heart failure based on the number of elevated serum potassium levels within the first year of observation. They observed that patterns of persistent, transient and intermittent hyperkalaemia predicted the subsequent risk of death compared with patterns of normokalaemia, but whether this was independent of the current potassium or not was not explored. Provenzano et al. [8], evaluated the persistence of hyperkalaemia based on two measurements 1 year apart among 2443 patients with CKD G1–5ND, and reported a graded association (lowest for hyperkalaemia with no visit, and increasing with hyperkalaemia at Visit 1 only, Visit 2 only or both) for risk of end-stage kidney disease, but no association with risk of death. Our results thus agree with and expand this evidence.
From a pathophysiologic perspective, individual hypo- and hyperkalaemia events (reflected by analysis of current potassium) may be more important than the average trajectory of all potassium values, and our MACE risk analysis also shows that the magnitude of predicted risk for transient hypo- and hyperkalaemia appears greater than that for a chronic state. It is possible that transient patterns signify general potassium instability or increased potassium variability. The situation may be similar to the classic association between within-person variability of haemoglobin and the risk of death [29]. However, further research into the variability of haemoglobin suggested that the variability and risk were attributable, to a large extent, to underlying disease severity [30]. When considering whether the relationship between potassium values and outcomes might be causal, we can apply the ideas of Bradford Hill [31]; though the mechanism would be postulated to be through cardiac events, the associations we observed are more consistent and larger in magnitude for the outcome death than for MACE, suggesting low specificity. The lack of additional prognostic value of current potassium once the previous potassium pattern is in the model may suggest that the effect seen is not fully mediated by potassium, since if it was, one would expect the most recent value to have the greatest effect on outcomes. Taken together, our findings do not offer strong support to the idea of a causal association between hyperkalaemias and outcomes. This is not to doubt that very high potassium levels are associated with arrhythmia and death [32], but rather to consider that we do not know at what threshold this effect begins and the strength of that relationship at different levels of hyperkalaemia.
We chose hyperkalaemia thresholds of >5.0 and >5.5 in parallel with other work, and to maximize power, but we acknowledge that it is unlikely that narrowly exceeding these thresholds is directly associated with risk—whether of arrhythmia or arrhythmia-mediated events such as MACE or death. Rather, they reflect markers of the risk of more extreme future excursions.
We observed an interaction between CKD severity and hyperkalaemia patterns: the association of hyperkalaemia with MACE is ‘less strong’ at lower eGFR (Figure 3). This may be explained by reduced power at lower eGFR levels, but our observations are internally consistent and consistent with others [33–35]. It is possible that this has true biological meaning, e.g. that tolerance and electrophysiological adaptation is responsible. However, we and others [1] have not been able to identify any direct evidence in support of this hypothesis. We judge it more likely that the mitigation of risk associated with hyperkalaemia in persons with G4 and G5 is owing to uncontrolled confounding. A given physiological disturbance is more likely to cause hyperkalaemia in someone with CKD G4–5 than someone with more normal kidney function. Many physiological disturbances are subclinical and may not be captured in administrative data such as ours. In patients with advanced CKD, smaller perturbations in physiology lead to hyperkalaemia and the magnitude of the subsequent risk is more related to the magnitude of the perturbation than causally related to the hyperkalaemia.
Sweden has universal healthcare access and Stockholm, a sole healthcare provider. Our exposure is dependent on potassium testing, but we ascertain it based on all potassium measurements performed in Stockholm healthcare. This being said, we recognized it is likely that patients may have suffered undetected hypo- and hyperkalaemia episodes. Other limitations in the interpretation of our study results are its retrospective nature and the lack of electrocardiography data to associate potassium levels with cardiac rhythm disturbances. Data reflect routine care in the region of Stockholm during 2006–11 in a predominantly Caucasian population, and findings may not necessarily generalize to other ethnicities, periods or settings. We have no data on dietary potassium intake. Additionally, we could not evaluate the effects of SGLT2 inhibitors and novel potassium binders since they were not available in the observed period. As with any observational research, residual confounding due to unmeasured or undetected factors may affect our findings.
To conclude, we observed associations between patterns of hyper- and hypokalaemia and outcomes in a large cohort of patients with CKD G3–5. These associations tended to be stronger and more consistent for death than for MACE. This is counter to the theoretical model which suggests that hyperkalaemia leads to death mediated by MACE, and demonstrates a lack of specificity [31]. It is possible that the observed risk of death may be the result of residual confounding by severity of a physiological disturbance (which we did not assess) that led to the hyperkalaemia, rather than harm resulting directly from hyperkalaemia. As a clinical application, because any potassium abnormality here modelled predicted worse outcomes, our results support the value of potassium monitoring and evaluation of potassium trends in clinical practice. However, they are also consistent with the idea that dyskalaemias are a biomarker of underlying illness or physiologic disturbance, rather than a causal risk factor.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the anonymous reviewers and editors of this manuscript for several ideas that enriched the manuscript.
FUNDING
This study was supported by an unconditional grant from Vifor Pharma to Karolinska Institute. In addition, we acknowledge grant support from the Swedish Research Council (2019-01059) and the Swedish Heart and Lung Foundation.
CONFLICT OF INTEREST STATEMENT
C.M.C. reports consultation, advisory board membership or research funding from Sanofi, Pfizer, Leo Pharma, Astellas, Janssen, Amgen, Boehringer-Ingelheim and Baxter. M.E. reports speaker or advisory board fees from AstraZeneca, Astellas Pharma and Vifor Pharma. T.P. is a Vifor Pharma employee. J.J.C. reports funding from Astellas and AstraZeneca outside the submitted work and speaker or advisory board fees from Baxter, AstraZeneca and Astellas Pharma. J.F.L., M.T. and A.S. have no conflicts of interest to report.
DATA AVAILABILITY STATEMENT
Data is available for collaborative research upon reasonable request to the principal investigator (juan.jesus.carrero@ki.se)
Contributor Information
Marco Trevisan, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Catherine M Clase, Department of Medicine and Health Research Methods, Evidence and Impact, McMaster University, ON, Hamilton, Canada.
Marie Evans, Department of Clinical Science Intervention and Technology, Karolinska University Hospital Huddinge, Stockholm, Sweden.
Tamara Popov, Medical Affairs, Vifor Pharma Group, Glattbrugg, Switzerland.
Jonas F Ludvigsson, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Department of Pediatrics, Örebro University Hospital, Örebro, Sweden.
Arvid Sjölander, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Juan Jesus Carrero, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
REFERENCES
- 1. Clase CM, Carrero JJ, Ellison DH et al. ; Conference Participants. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2020; 97: 42–61 [DOI] [PubMed] [Google Scholar]
- 2. Palmer BF, Carrero JJ, Clegg DJ et al. Clinical management of hyperkalemia. Mayo Clin Proc 2021; 96: 744–762 [DOI] [PubMed] [Google Scholar]
- 3. Kovesdy CP, Matsushita K, Sang Y et al. ; CKD Prognosis Consortium. Serum potassium and adverse outcomes across the range of kidney function: a CKD prognosis consortium meta-analysis. Eur Heart J 2018; 39: 1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gasparini A, Evans M, Barany P et al. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial Transplant 2019; 34: 1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo J, Brunelli SM, Jensen DE et al. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol 2016; 11: 90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Kidney Foundation. Best Practices in Managing Hyperkalemia in Chronic Kidney Disease. https://www.kidney.org/sites/default/files/02-10-7259_DBH_Best-Practices-in-Managing-Hyperkalemia-in-CKD.pdf (21 January 2021, date last accessed)
- 7. Matsushita K, Sang Y, Yang C et al. Dyskalemia, its patterns, and prognosis among patients with incident heart failure: a nationwide study of US veterans. PLoS One 2019; 14: e0219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Provenzano M, Minutolo R, Chiodini P et al. Competing-risk analysis of death and end stage kidney disease by hyperkalaemia status in non-dialysis chronic kidney disease patients receiving stable nephrology care. J Clin Med 2018; 7: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Núñez J, Bayés-Genís A, Zannad F et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation 2018; 137: 1320–1330 [DOI] [PubMed] [Google Scholar]
- 10. Dashputre AA, Sumida K, Potukuchi PK et al. Potassium Trajectories prior to Dialysis and Mortality following Dialysis Initiation in Patients with Advanced CKD. Nephron 2021; 145: 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Runesson B, Gasparini A, Qureshi AR et al. The Stockholm CREAtinine Measurements (SCREAM) project: protocol overview and regional representativeness. Clin Kidney J 2016; 9: 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wettermark B, Hammar N, Fored CM et al. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16: 726–735 [DOI] [PubMed] [Google Scholar]
- 13. Ludvigsson JF, Almqvist C, Bonamy AK et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016; 31: 125–136 [DOI] [PubMed] [Google Scholar]
- 14. Brooke HL, Talback M, Hornblad J et al. The Swedish cause of death register. Eur J Epidemiol 2017; 32: 765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chapter 1: Definition and classification of CKD. Kidney Int Suppl 2013; 3: 19–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosendaal FR, Cannegieter SC, van der Meer FJ et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993; 69: 236–239 [PubMed] [Google Scholar]
- 17. Rustad P, Felding P, Franzson L et al. The Nordic Reference Interval Project 2000: recommended reference intervals for 25 common biochemical properties. Scand J Clin Lab Invest 2004; 64: 271–284 [DOI] [PubMed] [Google Scholar]
- 18. Nilsson E, Gasparini A, Arnlov J et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol 2017; 245: 277–284 [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). ; A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ludvigsson JF, Andersson E, Ekbom A et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laureati P, Xu Y, Trevisan M et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant 2019; 35: 1518–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gasparini A, Evans M, Coresh J et al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 2016; 31: 2086–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plantinga LC, Boulware LE, Coresh J et al. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med 2008; 168: 2268–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomsen RW, Nicolaisen SK, Hasvold P et al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes-a Danish population-based cohort study. Nephrol Dial Transplant 2018; 33: 1610–1620 [DOI] [PubMed] [Google Scholar]
- 25. Gasparini A, Evans M, Barany P et al. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial Transplant 2018; 34: 1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adelborg K, Nicolaisen SK, Hasvold P et al. Predictors for repeated hyperkalemia and potassium trajectories in high-risk patients — a population-based cohort study. PLoS One 2019; 14: e0218739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heerspink HJ, Gao P, de Zeeuw D et al. The effect of ramipril and telmisartan on serum potassium and its association with cardiovascular and renal events: results from the ONTARGET trial. Eur J Prev Cardiol 2014; 21: 299–309 [DOI] [PubMed] [Google Scholar]
- 28. Noel JA, Bota SE, Petrcich W et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med 2019; 179: 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang W, Israni RK, Brunelli SM et al. Hemoglobin variability and mortality in ESRD. J Am Soc Nephrol 2007; 18: 3164–3170 [DOI] [PubMed] [Google Scholar]
- 30. Weinhandl ED, Peng Y, Gilbertson DT et al. Hemoglobin variability and mortality: confounding by disease severity. Am J Kidney Dis 2011; 57: 255–265 [DOI] [PubMed] [Google Scholar]
- 31. Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965; 58: 295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thuillier J, Hazard J. Correction of electrocardiographic signs of hyperpotassemia by calcium chloride. C R Seances Soc Biol Fil 1952; 146: 840–843 [PubMed] [Google Scholar]
- 33. Einhorn LM, Zhan M, Hsu VD et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169: 1156–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Korgaonkar S, Tilea A, Gillespie BW et al. Serum potassium and outcomes in CKD: insights from the RRI-CKD cohort study. Clin J Am Soc Nephrol 2010; 5: 762–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakhoul GN, Huang H, Arrigain S et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41: 456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available for collaborative research upon reasonable request to the principal investigator (juan.jesus.carrero@ki.se)