Abstract
Introduction:
Triggering receptor expressed on myeloid cells-1 (TREM-1) has important implications in sepsis and inflammation and is a novel receptor for extracellular cold-inducible RNA-binding protein (eCIRP). We hypothesize that the inhibition of TREM-1 via its interaction with eCIRP by novel peptide inhibitor M3 or knockout gene will attenuate the inflammation and injury associated with severe hepatic ischemia/reperfusion (I/R).
Methods:
Wild-type (WT) C57BL/6 and TREM-1−/− mice underwent 60 min of 70% hepatic ischemia, with 24 h of reperfusion. Additionally, WT mice underwent hepatic I/R and were treated with M3 (10 mg/kg BW) or vehicle (normal saline) at the start of reperfusion. Blood and ischemic liver tissues were collected, and analysis was performed using enzymatic assays, ELISA, reverse-transcription quantitative PCR (RT-qPCR), and pathohistology techniques. For survival surgery, mice additionally underwent resection of non-ischemic lobes of the liver and survival was monitored for 10 days.
Results:
There was an increase in serum levels of tissue markers including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) as well as cytokine levels (IL-6) and histological scoring of H&E sections in WT I/R mice. These markers decreased substantially in TREM-1−/− mice. Additionally, neutrophil infiltration markers and markers of local inflammation (MPO, MIP-2, COX-2) were attenuated in TREM-1−/− mice. Similarly, we show a significant decrease in injury and inflammation markers with M3 treatment. Additionally, we demonstrate decreased apoptosis with TREM-1 inhibition. Finally, M3 treatment improved the survival rate from 42% to 75% after hepatic I/R.
Conclusion:
TREM-1 is an important eCIRP receptor in the inflammatory response of hepatic I/R, and deficiency of TREM-1 via knockout gene or peptide inhibition attenuated liver injury and inflammation, and improved survival. Inhibition of the TREM-1 and eCIRP interaction in hepatic I/R may have important therapeutic potential.
Keywords: Hepatic ischemia/reperfusion, TREM-1, eCIRP, Inflammation, Apoptosis
Introduction
Hepatic ischemia-reperfusion (I/R) injury is a serious disease process that is seen in different clinical settings including: circulatory shock, trauma, hepatic resection, and transplantation(1, 2). In fact, in transplantation, I/R injury is thought to be the cause of up to 10% of early organ failure and can help promote acute and chronic rejection of the transplanted liver(2). Additionally, hepatic I/R has been found to lead to multisystem organ failure, and in these patients, is a source of significant morbidity and mortality(2, 3). Hepatic I/R results in a complex cellular response involving all populations of cells within the liver. Ischemia causes direct damage to hepatocytes and other cells releasing damage-associated molecular patterns (DAMPs) and cellular contents(2, 4). During reperfusion, there is cell death and release of reactive oxygen species (ROS)(2, 5). Additionally, Kupffer cells are activated, causing the release of cytokines and chemokines(5). Neutrophil infiltration then causes the release of additional ROS and proteases, which contributes to a large amount of necrosis seen in hepatic tissue(2). As hepatic I/R is a sterile process, DAMPs are the main mediator of inflammation(6, 7). There have been numerous attempts and strategies to reduce the amount of inflammation and injury observed, however there remains a need for identifying novel receptors, DAMPs, and techniques to develop novel therapeutics to treat hepatic I/R(8).
Cold-inducible RNA-binding protein (CIRP) is a 172-amino acid nucleoprotein that belongs to a family of cold shock proteins(9, 10). CIRP has different functions intracellularly and extracellularly. Intracellular CIRP facilitates stabilizing mRNAs when the cell is under stressful conditions including hypothermia or hypoxia(10). Recently, our lab has discovered that extracellular CIRP (eCIRP) acts as a DAMP that promotes inflammation(9). We have discovered that eCIRP is released in sepsis and sterile inflammation (including hemorrhagic shock and I/R injury) and helps promote inflammation(9, 11, 12). This is done through eCIRP’s binding to the TLR4 and myeloid differentiation factor-2 (MD2) complex(9).
TLR4 is thus known to be the primary receptor for eCIRP, however, we recently discovered that the triggering receptor expressed on myeloid cells-1 (TREM-1) is a novel receptor for eCIRP(13). TREM-1 is an immunoglobulin superfamily receptor expressed on immune cells that can induce inflammation independently or synergistically with TLR4 and is implicated in sepsis and inflammatory disease(14–16). Our laboratory discovered that eCIRP is a novel ligand for TREM-1, and this interaction promotes inflammation by releasing cytokines and chemokines(13). Additionally, we developed a novel human eCIRP-derived ligand-dependent 7-amino acid peptide (RGFFRGG) called M3, which blocks the interaction between eCIRP and TREM-1, thus being a novel TREM-1 inhibitor. M3 administration was found to be protective in sepsis demonstrating less inflammation and improved survival(13). Therefore, we believe that inhibition of TREM-1 via the eCIRP-TREM-1 interaction could serve as a novel mechanism for therapeutics in the acute inflammatory response of hepatic I/R.
In this study, we hypothesize that TREM-1 is an important receptor in the inflammatory response and injury seen in hepatic I/R. We further hypothesize that inhibition of TREM-1 with our novel TREM-1 inhibitor M3 will attenuate the inflammation and injury seen in hepatic I/R. To investigate this, we established a mouse model of hepatic I/R and performed hepatic I/R in both WT and TREM-1−/− mice. We then measured organ damage, inflammation, neutrophil infiltration, and apoptosis. Finally, we studied the effects of M3 treatment on organ damage, inflammation, and survival.
Materials and Methods
Experimental animals
Adult male wild-type (WT) C57BL/6 mice (20–25g) were purchased from Charles River Laboratories (Wilmington, MA), and housed in a temperature-controlled room on a 12-h light-dark cycle and fed a standard mouse chow diet. Mice were acclimated to the environment for 5–7 days. The TREM-1−/− mouse, Trem1tm1(KOMP)Vlcg was generated by the trans-NIH Knockout Mouse Project (KOMP) and obtained from the KOMP Repository, University of California, Davis. Age-matched (8–12 weeks) healthy mice were used as controls in all experiments. Every attempt was made to limit the number of animals used. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institutes for Medical Research and were performed in accordance with the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals.
Animal model of hepatic I/R
Hepatic I/R was performed as described previously(12, 17). Animals underwent induction of anesthesia with 2–4% inhalation isoflurane, after which the ventral abdomen was shaved and disinfected by swabbing with Betadine alternated two times with 70% alcohol. The animals were placed on a heating pad connected to an indwelling rectal thermometer to maintain core body temperatures of 35 °C. A 3–4 cm midline incision was performed. The ligamentous attachments connecting the liver, diaphragm, and abdominal wall were divided. The hilum of the liver was exposed to locate the hepatic artery and portal vein. A vascular microclip was placed across the hilum containing the left and median lobes of the liver to produce 70% ischemia for 60 min. Ischemia was confirmed with the color change of the liver. Reperfusion was initiated with the release of the clamp. Upon closure of the abdomen, 0.5 mL bolus of normal saline and buprenorphine (0.05mg/kg) was given subcutaneously. Blood and liver tissues were collected 24 h after ischemia. A portion of the left lobe of the liver was preserved in 10% formalin for histopathological analysis, and the remaining was stored at −80°C for quantitative analysis. WT and TREM-1−/− mice were assigned to two groups randomly to create a total of 4 groups: WT sham (n=6), TREM-1−/− sham (n=5), WT I/R (n=8), and TREM-1−/− I/R (n=7). In a separate experiment, WT mice were assigned to three groups randomly: sham, vehicle, and M3 treatment (n=8/group).
Only male mice were used in this study as sex-based disparities in ischemia/reperfusion injury have been well established in rodent studies, and female hormones have been demonstrated to be protective in reducing inflammation(18, 19).
Survival study
Following the procedure of hepatic I/R, the remaining 30% of the nonischemic liver was resected with electrocautery at the start of reperfusion, as this model of hepatic I/R is nonlethal without resection(12, 20). After, vehicle (normal saline, n=19) or M3 (n=20) treatment was administered. Mice were checked two times daily for a total of 10 days.
Peptide
M3 (RGFFRGG)(13) was synthesized by GenScript USA Inc. (Piscataway, NJ; >95% purity) and provided as a lyophilized powder. The powder is then resuspended in ultrapure water. The desired concentration of the peptide is prepared in sterile normal saline and filter sterilized by 0.22 μm filter prior to use in mice.
In vivo administration of M3
At the start of reperfusion, mice abdomen were closed, and then vehicle (normal saline) or M3 peptide was injected intravenously via retroorbital injection at a dose of 10mg/kg using a 28G needle(13).
Measurement of organ injury markers
Whole blood samples were centrifuged at 3000 x g for 10 min to collect serum, which was then stored at −80°C prior to use. Serum levels of AST, ALT, and LDH were determined using specific colorimetric enzymatic assays (Pointe Scientific, Canton, MI) according to manufacturer’s instructions.
Cytokine measurements by enzyme-linked immunosorbent assay (ELISA)
Serum was analyzed by ELISA kits specific for IL-6 (BD Biosciences, San Jose, CA), and eCIRP (CUSABio Technology LLC) according to manufacturer’s instructions. For eCIRP, kit specific buffers were used.
Hepatic myeloperoxidase assessment
Ischemic liver tissue (100mg) was weighed and homogenized by sonication in 1 mL of potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide. Two freeze-thaw cycles were performed and then samples were centrifuged to collect the supernatant. The reaction was carried out in a 96-well plate by adding samples into phosphate buffer containing o-dianisidine hydrochloride and H2O2. Light absorbance was read at 460nm over a period of 5 min. MPO activity (1 unit was equal to the change in absorbance per min) was expressed as units per gram of tissue.
Measurement of cytokines by reverse transcription-quantitative (RT-qPCR) analysis
Total RNA was extracted from ischemic portions of the liver by TRIzol reagent (Invitrogen, Thermo Fisher Scientific Inc.) and was reverse-transcribed into cDNA with reverse transcriptase (Applied Biosystems, Thermo Fisher Scientific Inc.). PCR reactions were carried out in 20 μL of a final volume of 0.08 μM of each forward and reverse primer, cDNA, water, and SYBR Green master mix (Applied Biosystems, Thermo Fisher Scientific Inc.). Amplification and analysis were conducted in a Step One Plus real-time PCR machine (Applied Biosystems, Thermo Fisher Scientific Inc.). Mouse β-actin mRNA was used as an internal control for amplification, and relative gene expression levels were calculated using 2−ΔΔCt method. Relative expression of mRNA was expressed as a fold change in comparison with sham tissues. The sequence of primers for this study is listed as follows:
IL-6, 5’-CCGGAGAGGAGACTTCACAG-3’ (forward), and 5’-CAGAATTGCCATTGCACAAC-3’ (reverse);
MIP-2, 5’-CCCTGGTTCAGAAAATCATCCA-3’ (forward), and 5’-GCTCCTC-CTTTCCAGGTCAGT-3’ (reverse);
COX2, 5’CTCAGCCAGGCAGCAAATC-3’ (forward), and 5’-ACATTCCCCACGGTTTTGAC’-3’ (reverse);
TREM-1, 5’-CTACAACCCGATCCCTACCC-3’ (forward), and 5’-AAACCAGGCTCTTGCTGAGA-3’ (reverse);
β-actin, 5’-CGTGAAAAGATGACCCAGATCA-3’ (forward), and 3’-TGGTACGACCAGAGGCATACAG-3’ (reverse).
Histological evaluation of liver injury
Liver tissues were taken from the left lobe following 24 h reperfusion and stored in 10% formalin before being embedded in paraffin. Biopsies were then sectioned to 5 μm cuts and stained with hematoxylin-eosin. Liver parenchymal injury was assessed in a blinded fashion using semi-quantitative light microscopy evaluation. The histologic injury score for each sample was expressed as the sum of the individual scores given 3 different parameters based on the Suzuki criteria: congestion (None = 0, Minimal = 1, Mild = 2, Moderate = 3, Severe = 4), vacuolization (None = 0, Minimal = 1, Mild = 2, Moderate = 3, Severe = 4), and necrosis (None = 0, single cell necrosis = 1, <30% = 2, 30 – 60% = 3, >60% = 4)(17, 21). Scores for each finding ranged from 0 to 4, with a maximum possible score of 12.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
For TUNEL staining, fluorescence staining was performed using a commercially available In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN). The assay was conducted according to the manufacturer’s instructions. 4’, 6-diamidino-2-phenylindole (DAPI) was used as a nuclear counterstain.
Statistical analysis
Data represented in the figures are expressed as mean ± SEM and compared by using Student’s t test for two groups or one-way analysis of variance (ANOVA) using Student-Newman-Keuls (SNK) post hoc analysis for multiple groups. Survival rates were analyzed by the Kaplan-Meier estimator and compared using a log-rank test. Differences in values were considered significant if p ≤ 0.05. Data analysis was carried out using GraphPad Prism graphing and statistical software (GraphPad Software).
Results
TREM-1 gene expression and serum eCIRP are increased after hepatic I/R
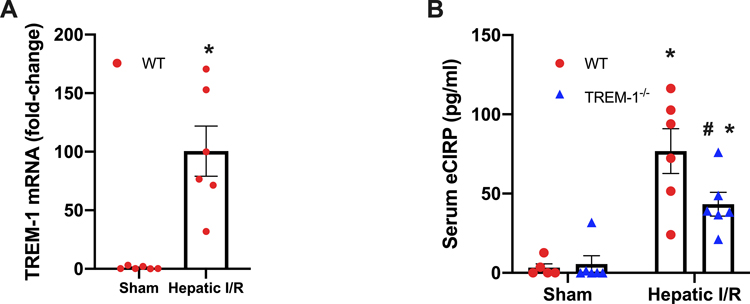
TREM-1 gene expression levels were found to be 100-fold higher in WT I/R mice compared to sham mice (Fig. 1A); a similar relationship is observed in sepsis(22). Additionally, serum eCIRP levels were increased by 24-fold after hepatic I/R. There was a reduction of 44% in TREM-1−/− mice compared to WT I/R mice (Fig. 1B). Together, these results point to the importance of the eCIRP-TREM-1 pathway in hepatic I/R.
Fig. 1. TREM-1 gene expression and serum eCIRP increase after hepatic I/R.
Blood and ischemic liver tissue was collected at 24 h reperfusion from WT sham, TREM-1−/− sham, WT I/R, and TREM-1−/− I/R groups. (A) TREM-1 mRNA was measured by RT-qPCR in WT sham and WT I/R groups (n=6/group). Levels were normalized to β-actin. (B) Serum eCIRP was measured by ELISA in all groups (n=5–6/group). Data expressed as means ± SE and compared by one-way ANOVA and SNK method, and students t-test for TREM-1 (*P ≤ 0.05 versus WT Sham; #P ≤ 0.05 vs WT I/R).
TREM-1−/− mice demonstrate decreased injury markers and tissue damage
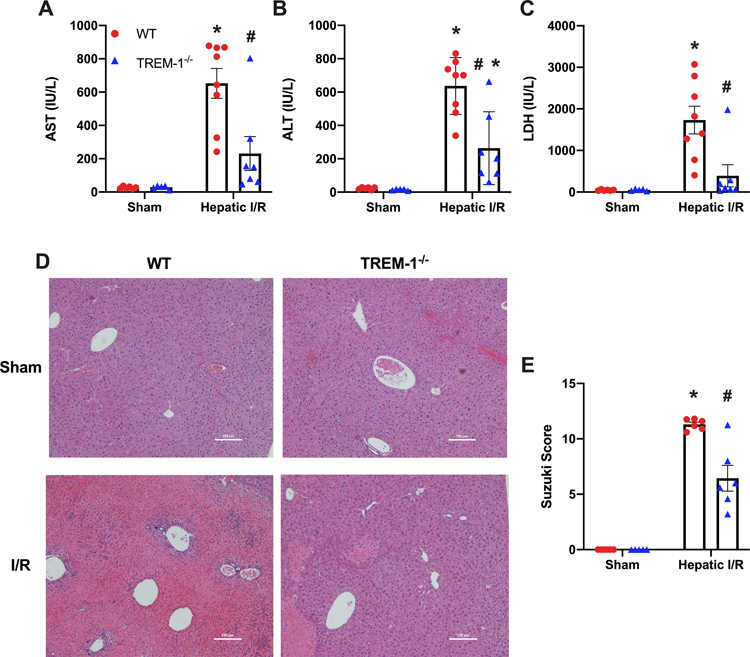
After hepatic I/R, there was an increase in AST, ALT, and LDH in WT mice by 25-fold, 29-fold, and 38-fold respectively versus WT sham mice. TREM-1−/− mice showed a decrease of 65%, 59%, and 77% respectively (Fig. 2 A–C). Histological evaluation of the H&E staining was measured using the Suzuki Scoring Criteria and included degree of necrosis, congestion, and vacuolization of the sections. There was significant necrosis and disruption in the normal architecture of the liver in the WT I/R group, which improves dramatically in TREM-1−/− mice. This is correlated with our histological scoring, where we observe a 43% decrease in the TREM-1−/− mice (Fig. 2 D–E).
Fig. 2. TREM-1−/− mice show improved systemic organ injury markers and decreased liver damage after hepatic I/R.
Blood and ischemic liver tissue was collected at 24 h reperfusion from WT sham, TREM-1−/− sham, WT I/R, and TREM-1−/− I/R groups. Serum (A) AST, (B) ALT, and (C) LDH were determined using specific colorimetric enzymatic assays (n = 5–8/group). (D), Representative images of hematoxylin & eosin stained liver tissue at 100x. Scale bar: 100 μm. (E), Extent of liver injury was graded using the Suzuki score by a blinded investigator as described in our methods (n=5–6/group). Data expressed as means ± SE and compared by one-way ANOVA and SNK method (*P ≤ 0.05 versus WT Sham; #P ≤ 0.05 vs WT I/R).
TREM-1−/− mice are protected from local and systemic inflammation
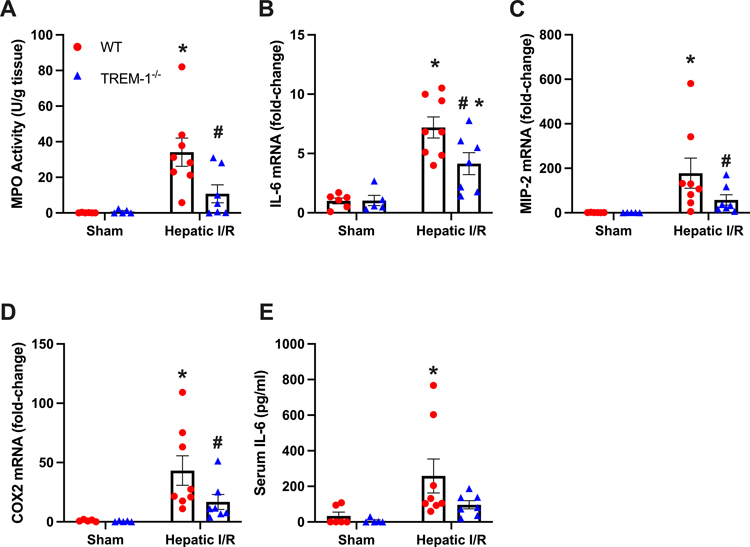
To determine the local inflammatory response in our model of hepatic I/R, inflammatory markers were measured in ischemic liver tissues. Myeloperoxidase (MPO) were checked to determine the degree of neutrophil infiltration in hepatic tissue and demonstrated a dramatic increase in WT mice and a decrease of 68% in TREM-1−/− mice (Fig. 3A). IL-6 mRNA levels were measured and increased by 7.2-fold in WT I/R mice. TREM-1−/− mice showed a reduction of 42% (Fig. 3B). Chemokine macrophage inflammatory protein-2 (MIP-2) and neutrophil inflammatory marker cyclooxygenase-2 (COX-2) mRNA were elevated by 178-fold and 43-fold respectively in WT mice. Decreases of 68% and 61% were observed in TREM-1−/− mice (Fig. 3 C–D). Additionally, serum levels of IL-6 increased by 8-fold in WT mice. TREM-1−/− mice demonstrated a decrease of 63% (Fig. 3E). Of note, the difference in IL-6 levels between WT and TREM-1−/− I/R groups was not statistically significant (p = 0.06), however there was a noticeable downtrend with TREM-1−/− mice. Taken together, we show that TREM-1−/− mice demonstrate lower levels of local and systemic inflammation, indicating a protective phenotype in hepatic I/R.
Fig. 3. TREM-1−/− mice show decreased liver and systemic inflammation.
(A) MPO activity in the ischemic liver tissue from different groups was determined spectrophotometrically. The mRNA levels of (B) IL-6, (C) MIP-2, and (D) COX-2 were measured by RT-qPCR. Levels were normalized to β-actin. The serum levels of (E) IL-6 were measured by ELISA (n = 5–8/group). Data expressed as means ± SE and compared by one-way ANOVA and SNK method, and students t-test for TREM-1 (*P ≤ 0.05 versus WT Sham; #P ≤ 0.05 vs WT I/R).
M3 treatment improves systemic injury markers and tissue damage
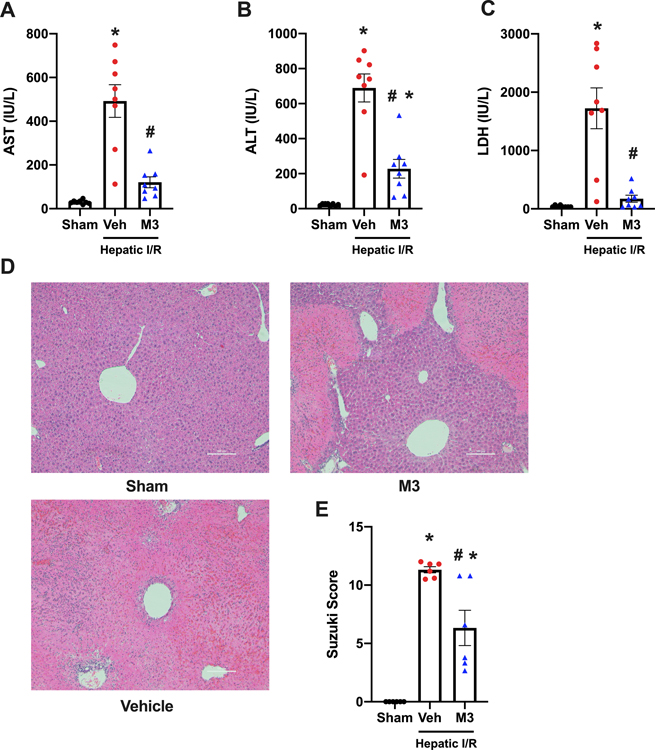
We then focused on the peptide M3, a novel eCIRP-TREM-1 inhibitor(13). AST, ALT, and LDH levels were increased by 16-fold, 30-fold, and 41-fold in vehicle mice respectively, relative to sham. M3 was protective, and these injury markers dropped by 75%, 67%, and 89% respectively (Fig. 4 A–C). Histology analysis was also performed to compare our vehicle and treatment models. Similar to before, significant necrosis and architectural destruction of the normal liver tissue was observed in our vehicle group, which improved with M3 treatment. Histological scoring of the sections showed a 44% reduction with M3 treatment compared to vehicle (Fig. 4 D–E). Taken together, M3 treatment prevents liver tissue damage in our model of hepatic I/R.
Fig. 4. M3 treatment attenuates systemic organ injury markers and decreases tissue damage after hepatic I/R.
Blood and ischemic liver tissue was collected at 24 h reperfusion from sham, vehicle (normal saline), and M3 groups. Serum (A) AST, (B) ALT, and (C) LDH were determined using specific colorimetric enzymatic assays (n = 8/group). (D), Representative images of hematoxylin & eosin stained lung tissue at 100x. Scale bar: 100 μm. (E), Extent of liver injury was graded using the Suzuki score by a blinded investigator as described in our methods (n = 6/group). Data expressed as means ± SE and compared by one-way ANOVA and SNK method (*P ≤ 0.05 versus Sham; #P ≤ 0.05 vs vehicle).
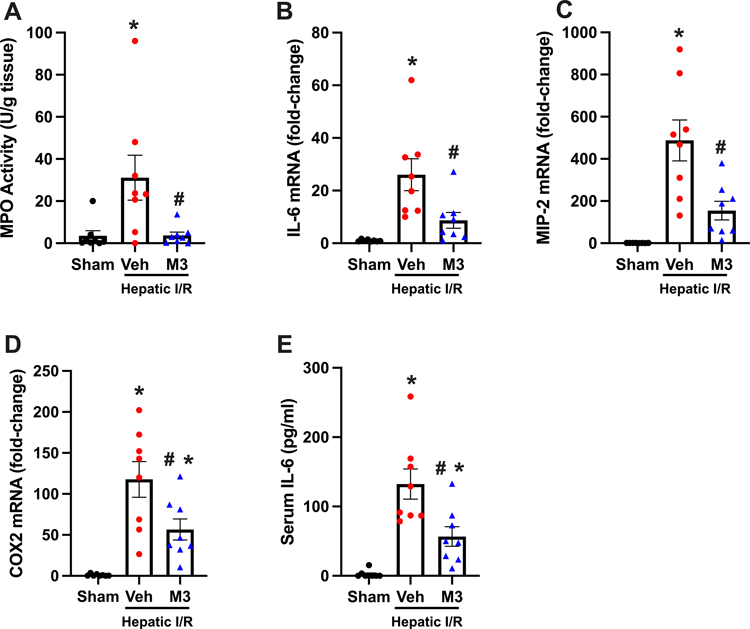
M3 treatment attenuates local and systemic inflammation
Ischemic liver tissues were then examined to determine the degree of local inflammation in our treatment model. First, neutrophil infiltration was assessed with (MPO) levels, and was increased by 9-fold in the vehicle group. A reduction of 89% was observed with M3 treatment (Fig. 5A). IL-6 mRNA was measured and showed a dramatic increase by 26-fold in the vehicle group. M3 treatment attenuated these levels by 67% (Fig. 5B). mRNA levels of MIP-2 and COX-2 were then measured and were increased by 488 and 118-fold respectively. M3 reduced these levels by 68% and 52% (Fig. 5 C–D). Additionally, serum levels of IL-6 increased in the vehicle group by 57-fold. M3 treatment showed a reduction of 57% (Fig. 5E). These results demonstrate a significant decrease in both local and systemic inflammation with M3 treatment.
Fig. 5. M3 treatment ameliorates local and systemic inflammation.
(A) MPO activity was determined spectrophotometrically. The mRNA levels of (B) IL-6, (C) MIP-2, and (D) COX-2 were measured by RT-qPCR. Levels were normalized to β-actin. The serum levels of (E) IL-6 were measured by ELISA (n = 8/group). Data expressed as means ± SE and compared by one-way ANOVA and SNK method (*P ≤ 0.05 versus sham; #P ≤ 0.05 vs vehicle).
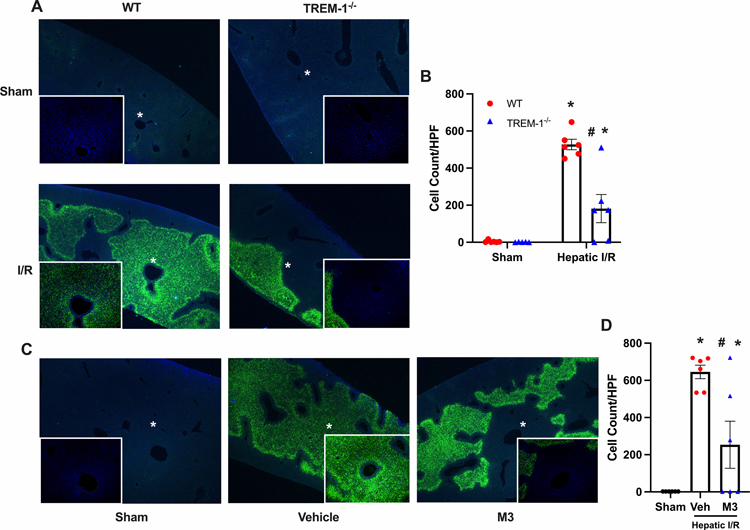
TREM-1−/− mice and M3 treatment demonstrate decreased apoptosis
Further, TUNEL staining was performed to determine the amount of apoptosis in both our TREM-1−/− and M3 treatment models. A significant increase in the number of TUNEL-positive nuclei was observed in the WT I/R group, which was mostly absent in the TREM-1−/− group. TUNEL-positive nuclei in each group were counted, and a decrease of 65% in TREM-1−/− mice was noted (Fig. 6 A–B). Similarly, for M3, there were a significant number of TUNEL-positive nuclei in the vehicle group, which was decreased by 61% with M3 treatment (Fig 6 C–D).
Fig. 6. TREM-1−/− mice and M3 treatment demonstrate decreased apoptosis.
Ischemic liver tissues were harvested at 24 h reperfusion and sections were subjected to TUNEL assay to detect DNA fragmentation. TUNEL staining (green fluorescent) and nuclear counterstaining (blue fluorescent) of liver sections are shown. Images are of 40x with 100x magnified images shown as insets for all groups, with * representing the magnified area. (A) Representative images of different groups are shown. (B) TUNEL positive cells were counted using ImageJ software. (C) Representative images of different groups are shown. (D) TUNEL positive cells were counted (n = 5–6/group). Data expressed as means ± SE and compared by one-way ANOVA and SNK method, (*P ≤ 0.05 versus sham; #P ≤ 0.05 vs vehicle).
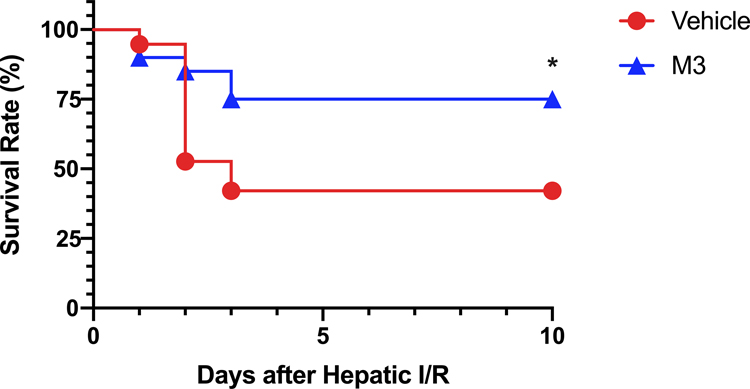
M3 treatment improves survival
Finally, to evaluate the potential survival benefit of M3, we used a model of total hepatic ischemia as previously described(12, 20). We observed that 42% of vehicle-treated animals survived for 10 days. With M3 treatment, we observed a significant increase to 75% survival after 10 days (Fig. 7). Therefore, M3 treatment improves survival in our model of total hepatic ischemia.
Fig. 7. M3 Improves survival in hepatic I/R.
Survival rates over a 10-day period following 70% hepatic I/R with resection of the remaining non-ischemic 30% liver tissue are demonstrated for vehicle (circle) and M3 treatment (triangle). Survival rates were analyzed by the Kaplan-Meier estimator using a log-rank test (n = 19–20/group). *P ≤ 0.05 vs vehicle.
Discussion
Hepatic I/R is a significant clinical problem that is characterized by both the initial insult, and injury from the robust inflammatory response that occurs during reperfusion(2). Therefore, in developing therapies, it is essential to target the inflammatory response. Further, it is a sterile process, thus, inflammation occurs due to cellular injury and DAMPs and not bacterial cell products(7). In this study, we focused on the relationship between the receptor TREM-1 and the DAMP, eCIRP. The purpose of our study was to determine whether TREM-1 plays a role in the inflammation observed in hepatic I/R and whether TREM-1 inhibition with our novel eCIRP/TREM-1 peptide inhibitor could provide therapeutic benefit. We demonstrated that TREM-1 gene expression is elevated in hepatic I/R, as is observed in sepsis(22). We also showed that serum eCIRP levels increase in hepatic I/R, confirming our previous findings(12). Interestingly, we demonstrated that eCIRP levels were decreased in TREM-1−/− mice compared to WT mice. As TREM-1 and eCIRP were both elevated, and promoted inflammation through their interaction, we believed the interaction between TREM-1 and eCIRP was a potential target for therapeutics in hepatic I/R. In both TREM-1−/− mice and M3 treatment groups, we demonstrated protection through decreased injury and inflammatory markers, improved histology scoring, and decreased apoptosis. Importantly, we showed improved survival after M3 administration.
TREM-1 is an innate immune receptor, expressed on macrophages and neutrophils and is important in generating inflammation in sepsis and tissue injury(13, 14, 16). In the liver under normal conditions, TREM-1 is expressed on Kupffer cells, and not on hepatocytes(23, 24). TREM-1 promotes inflammation by releasing cytokines and chemokines, via the activation of DNAX activating protein 12 (DAP12) and the spleen tyrosine kinase (Syk) (14, 25). Further, TREM-1 has an important relationship with TLR4, and together they can synergistically work to enhance inflammation(15, 22). TREM-1 has been studied in different chronic liver pathologies including liver fibrosis, hepatocellular carcinoma, and nonalcoholic fatty liver disease (NAFLD)(23, 24, 26, 27). The pro-inflammatory receptor has been found to promote these disease processes and inhibition was protective in fibrosis and in NAFLD. Our laboratory recently established that TREM-1 is a novel ligand for the DAMP eCIRP(13). In fact, eCIRP actually was found to have a higher affinity for TREM-1 (KD 11.7 × 10−8 M) than TLR4 (KD 6.17 × 10−7 M), which has been thought to be the primary receptor for eCIRP(9, 13). In vitro analysis showed that peritoneal macrophages isolated from TREM-1−/− mice stimulated with rmCIRP were found to produce lower levels of cytokines compared to WT macrophages(13). Further, TREM-1 inhibition with LP17 (a known peptide TREM-1 inhibitor) has been shown to be protective in sepsis, mesenteric I/R, and hemorrhagic shock(13, 28, 29). There is substantial supporting data that TREM-1 is a critical receptor in the generation of acute inflammation. Therefore, we believed we could apply our work to acute inflammation of the liver given the role TREM-1 plays in different liver pathologies.
Our studies in fact show that TREM-1−/− mice are protected in hepatic I/R. We demonstrate TREM-1−/− mice have lower levels of injury markers (AST, ALT, LDH), lower levels of cytokines/chemokines (IL-6, MIP-2), less neutrophil infiltration (MPO, COX-2), less apoptosis, and less tissue damage. Neutrophil infiltration is a key process that occurs as a result of the inflammation in hepatic I/R and leads to significant injury. Our results show that TREM-1 signaling is critical in this process as these markers are substantially lower in TREM-1−/− mice. It is important to mention that as the H&E staining mostly demonstrates necrosis in our model of hepatic I/R, we believe the primary process of cellular death in our model to be necrosis rather than apoptosis. However, there is a clear decrease in apoptosis, and this is likely a secondary protective benefit of TREM-1 inhibition.
Additionally, TREM-1 levels in ischemic liver tissue were dramatically elevated, which is likely related to TREM-1 upregulation on Kupffer cells, but also TREM-1 expression on infiltrating neutrophils and macrophages. We also show that eCIRP levels increase and believe this result plus elevated levels of TREM-1, contribute to the robust inflammatory response seen after hepatic I/R. Interestingly, we found that eCIRP levels were decreased in TREM-1−/− mice after hepatic I/R. This is a novel finding and is consistent with the lower injury and inflammatory markers seen in TREM-1−/− mice. eCIRP gene expression was not measured in our study as gene expression of CIRP does not differentiate between intracellular (iCIRP) and eCIRP. iCIRP primarily functions as an RNA chaperone and is upregulated during cellular stress including hypoxia and hypothermia(9, 30, 31). This however is not a reflection of eCIRP’s levels as a DAMP. The mechanism by which eCIRP released into the circulation during hepatic I/R and how this release is downregulated in TREM-1−/− mice remains to be determined.
To inhibit TREM-1, we target the eCIRP/TREM-1 interaction with a previously developed novel 7-amino acid peptide derived from human rmCIRP called M3(13). This study represents the first time our lab has used a peptide molecule as a therapeutic in hepatic I/R. Previously, we have studied the role of eCIRP in hepatic I/R using a neutralizing antibody to eCIRP, and showed protection in terms of inflammation and survival(12). M3 is a highly specific peptide that blocks the interaction between eCIRP and TREM-1, and inhibits TREM-1 signaling. M3 has been studied both in vitro and in vivo conditions prior to use in our study. In vitro analysis demonstrated that macrophages treated with rmCIRP and M3 produce lower levels of inflammatory cytokines than just rmCIRP alone(13). Further, in vivo analysis showed benefits of M3 in rmCIRP injected mice, CLP and LPS induced sepsis, hemorrhagic shock, and mesenteric I/R injury, lowering inflammatory and injury markers(13, 32, 33). Additionally, M3 was found to improve survival in sepsis.
Here we show that M3 therapy was effective in lowering injury/inflammatory markers, tissue damage, neutrophil infiltration, and cell death. Notably, we demonstrate improved survival in a model of total hepatic ischemia. It can be questioned that M3 treatment could further reduce injury or inflammation in TREM-1−/− mice and that how specific is M3 to TREM-1. Previous experiments in vitro, have shown that M3 is specific to TREM-1. To demonstrate the specificity of M3 to TREM-1, RAW 264.7 cells were plated on anti-TREM-1 antibody coated plate, an agonist known to induce production of TNF-α through TREM-1 cross-linking. M3, M3 scramble, and PBS were administered, and M3 demonstrated a significant reduction of TNF-α levels compared to M3 scramble and PBS groups(13). Additionally, a FRET assay demonstrated a disruption of the interaction of rmCIRP and TREM-1 in the presence of M3(13). This indicates M3s protective effects are specific to TREM-1. As TREM-1−/− already demonstrates substantial protection after hepatic I/R, it is very unlikely that M3 treatment would provide any additional significant benefit in TREM-1−/−. Similarly the perceived greater impact on liver I/R injury with M3 compared to TREM-1 deletion could simply be due to inter assay variation of the biochemical markers tested.
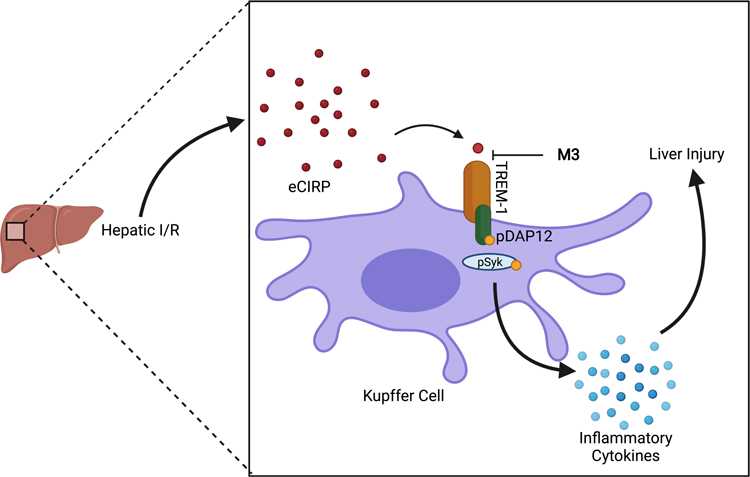
These results point to the importance of the eCIRP-TREM-1 axis in promoting injury and inflammation in hepatic I/R as described in our proposed schema (Fig 8). Kupffer cells are the resident macrophage of the liver and are the primary cell type that express TREM-1 in the liver. Therefore, Kupffer cells are a key cell in the primary eCIRP-TREM-1 response after hepatic I/R. Although neutrophil inflammation and injury in hepatic I/R is very crucial, these cells infiltrate after the primary response of Kupffer cells. However, neutrophils are not included in our schema as this schema represents the activation of the inflammatory response after hepatic I/R. TREM-1 inhibition via this mechanism should be considered as a viable target for therapeutics. Peptides as therapeutics present both strengths and weaknesses. Although peptide molecules are non-toxic and can be administrated easily, they have a very short half-life and are unstable after administration (34–36). Different dosing strategies or peptide modifications will likely be needed for it to be an effective therapeutic for M3 to be an effective therapeutic compound in clinical setting. Future preclinical time course and dose response studies should include both male and female mice to dissect the sex-based disparities in liver I/R.
Fig. 8. Proposed mechanism for the protective role of M3.
In our proposed schema, eCIRP levels increase in hepatic I/R. eCIRP binds to TREM-1, and through signaling of pDAP12 and pSyk, inflammatory cytokines are released and contribute to liver injury. M3 acts as an inhibitor of TREM-1 via blocking the interaction of eCIRP and TREM-1, and as a result, decreases the amount of liver damage seen in hepatic I/R (Created with BioRender.com).
It is known that TREM-1 has other ligands that are important in signaling, including HMGB-1(37), extracellular actin(38), and peptidoglycan recognition protein-1(39). We can speculate that other DAMPs mediate inflammation via TREM-1 in hepatic I/R, as there is a large amount of cellular damage(7, 40, 41). We show that M3 provides substantial benefits, however we still see an elevation in inflammation and mortality after M3 treatment. Although eCIRP plays an important role in the inflammatory response, it is not the only DAMP known to promote inflammation in hepatic I/R. For example, HMGB-1 is known to also play an important role in hepatic I/R, and use of a neutralizing antibody to HMGB-1 attenuated injury and inflammation(42). HMGB-1 is also implicated in TREM-1 signaling(37); it is likely that this interaction is also present. Additionally, TLR4 is also known to promote inflammation during hepatic I/R(43). It can be hypothesized that further blockade of TLR4, in addition to TREM-1, could contribute to more dampening of inflammation. However, it is also known that by blocking TREM-1, we also see an effect on TLR4. TREM-1 silencing demonstrates decreased gene expression of downstream signaling proteins of TLR4(44). Further investigation is needed to fully understand this process and regulation of TLR4 in the context of knockout gene or peptide inhibition of TREM-1. An additional study could be to look at peptide inhibition of both TREM-1 and TLR4 together, as our lab has developed a TLR4/MD2 inhibitor called C23(10).
We acknowledge that our study has limitations. For M3 experiments, one time point and one dose of M3 was used; we administered M3 at the start of reperfusion. M3 administration at different time points could elucidate the full clinical potential of the therapeutic benefits that M3 may have. Time points including pretreatment, posttreatment, and multiple doses will be interesting extensions to our study and potentially expand clinical relevance. The start of reperfusion is usually a defined time point during surgery, however in other clinical scenarios, this is not the case. During circulatory shock, there usually is not as well a defined time point when reperfusion occurs; thus, drug administration at different time points will elucidate the clinical potential of M3. Along with this, we currently do not know the half-life of M3 and have not used M3 at different doses; the current dose being used, 10 mg/kg, is what was effective during a sepsis model and did not demonstrate immunogenicity(13). More studies that investigate M3s properties will be needed.
In conclusion, we show that TREM-1 is an important mediator of inflammation in hepatic I/R via the DAMP eCIRP and that TREM-1−/− mice were protected. We further show therapeutic benefit in terms of injury, inflammation, and survival with our novel TREM-1 peptide inhibitor, M3, in our mouse model of hepatic I/R. These findings elucidate the importance of the eCIRP and TREM-1 pathway in hepatic I/R. Therefore, TREM-1 inhibition via the eCIRP-TREM-1 interaction should be considered a promising target for future therapies.
Acknowledgements
We thank Dr. Zhimin Wang and Dr. Fangming Zhang of the Center for Immunology and Inflammation, Feinstein Institutes for Medical Research for technical assistance.
Funding:
National Institutes of Health (NIH) grants R35GM118337 and R01HL076179 (to PW).
Footnotes
Conflict of Interest/Disclosure Statement
The authors have no conflicts of interest to disclose.
The manuscript is from the winner of the New Investigator Competition of the 44th Annual Conference on Shock, Timothy Borjas MD.
REFERENCES
- 1.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Piña E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res 147(1):153–159, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rampes S, Ma D: Hepatic ischemia-reperfusion injury in liver transplant setting. mechanisms and protective strategies. J Biomed Res 33(4):221–234, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nastos C, Kalimeris K, Papoutsidakis N, Tasoulis M-K, Lykoudis PM, Theodoraki K, Nastou D, Smyrniotis V, Arkadopoulos N. Global consequences of liver ischemia/reperfusion injury. Oxidative Medicine and Cellular Longevity 2014:906965, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan L-Y, Fu P-Y, Li P-D, Li Z-N, Liu H-Y, Xin M-G, Li W. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg 6(7):122–128, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konishi T, Lentsch AB. Hepatic ischemia/reperfusion: mechanisms of tissue injury, repair, and regeneration. Gene Expr 17(4):277–287, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nature Medicine 17(11):1391–1401, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Golen RF, Reiniers MJ, Olthof PB, van Gulik TM, Heger M. Sterile inflammation in hepatic ischemia/reperfusion injury: present concepts and potential therapeutics. Journal of Gastroenterology and Hepatology 28(3):394–400, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Chen J, Meng Y, Chen Z, Yang J. Novel targets for treating ischemia-reperfusion injury in the liver. Int J Mol Sci 19(5):1302, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiang X, Yang W-L, Wu R, Zhou M, Jacob A, Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med 19(11):1489–1495, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aziz M, Brenner M, Wang P. Extracellular CIRP (eCIRP) and inflammation. J Leukoc Biol 106(1):133–146, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cen C, Yang W-L, Yen H-T, Nicastro JM, Coppa GF, Wang P. Deficiency of cold-inducible ribonucleic acid-binding protein reduces renal injury after ischemia-reperfusion. Surgery 160(2):473–483, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godwin A, Yang W-L, Sharma A, Khader A, Wang Z, Zhang F, Nicastro J, Coppa GF, Wang P. Blocking cold-inducible RNA-binding protein protects liver from ischemia-reperfusion injury. Shock 43(1):24–30, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denning N-L, Aziz M, Murao A, Gurien SD, Ochani M, Prince JM, Wang P. Extracellular CIRP as an endogenous TREM-1 ligand to fuel inflammation in sepsis. JCI Insight 5(5):e134172, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol 164(10):4991, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Dower K, Ellis DK, Saraf K, Jelinsky SA, Lin L-L. Innate immune responses to TREM-1 activation: overlap, divergence, and positive and negative cross-talk with bacterial lipopolysaccharide. J Immunol 180(5):3520, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacology & Therapeutics 177:81–95, 2017. [DOI] [PubMed] [Google Scholar]
- 17.Khader A, Yang W-L, Godwin A, Prince JM, Nicastro JM, Coppa GF, Wang P. Sirtuin 1 stimulation attenuates ischemic liver injury and enhances mitochondrial recovery and autophagy. Crit Care Med 44(8):e651–e663, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crockett ET, Spielman W, Dowlatshahi S, He J. Sex differences in inflammatory cytokine production in hepatic ischemia-reperfusion. J Inflamm (Lond) 3:16–16, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lü P, Liu F, Wang C-Y, Chen D-D, Yao Z, Tian Y, Zhang J-H, Wu Y-H. Gender differences in hepatic ischemic reperfusion injury in rats are associated with endothelial cell nitric oxide synthase-derived nitric oxide. World J Gastroenterol 11(22):3441–3445, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda A, Jacob A, Wu R, Zhou M, Aziz M, Wang P. Milk fat globule--EGF factor VIII ameliorates liver injury after hepatic ischemia-reperfusion. J Surg Res 180(1):e37–e46, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behrends M, Martinez-Palli G, Niemann CU, Cohen S, Ramachandran R, Hirose R. Acute hyperglycemia worsens hepatic ischemia/reperfusion injury in rats. J Gastrointest Surg 14(3):528–535, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410(6832):1103–1107, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen-Lefebvre AT, Ajith A, Portik-Dobos V, Horuzsko DD, Arbab AS, Dzutsev A, Sadek R, Trinchieri G, Horuzsko A. The innate immune receptor TREM-1 promotes liver injury and fibrosis. J Clin Invest 128(11):4870–4883, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun H, Feng J, Tang L. Function of TREM1 and TREM2 in Liver-Related Diseases. Cells 9(12):2626, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arts RJW, Joosten LAB, van der Meer JWM, Netea MG. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. Journal of Leukocyte Biology 93(2):209–215, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Duan M, Wang Z-C, Wang X-Y, Shi J-Y, Yang L-X, Ding Z-B, Gao Q, Zhou J, Fan J. TREM-1, an inflammatory modulator, is expressed in hepatocellular carcinoma cells and significantly promotes tumor progression. Annals of Surgical Oncology 22(9):3121–3129, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Rao S, Huang J, Shen Z, Xiang C, Zhang M, Lu X. Inhibition of TREM-1 attenuates inflammation and lipid accumulation in diet-induced nonalcoholic fatty liver disease. J Cell Biochem 120(7):11867–11877, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibot S, Massin F, Alauzet C, Montemont C, Lozniewski A, Bollaert P-E, Levy B. Effects of the TREM-1 pathway modulation during mesenteric ischemia-reperfusion in rats. Critical Care Medicine 36(2), 2008. [DOI] [PubMed] [Google Scholar]
- 29.Gibot S, Massin F, Alauzet C, Derive M, Montemont C, Collin S, Fremont S, Levy B. Effects of the TREM-1 pathway modulation during hemorrhagic shock in rats. Shock 32(6), 2009. [DOI] [PubMed] [Google Scholar]
- 30.De Leeuw F, Zhang T, Wauquier C, Huez G, Kruys V, Gueydan C. The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor. Experimental Cell Research 313(20):4130–4144, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Zhong P, Huang H. Recent progress in the research of cold-inducible RNA-binding protein. Future Sci OA 3(4):FSO246–FSO246, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurien SD, Aziz M, Cagliani J, Denning N-L, Last J, Royster W, Coppa GF, Wang P. An extracellular cold-inducible RNA-binding protein-derived small peptide targeting triggering receptor expressed on myeloid cells-1 attenuates hemorrhagic shock. Journal of Trauma and Acute Care Surgery 88(6):809–815, 2020. [DOI] [PubMed] [Google Scholar]
- 33.Denning N-L, Aziz M, Ochani M, Prince JM, Wang P. Inhibition of a triggering receptor expressed on myeloid cells-1 (TREM-1) with an extracellular cold-inducible RNA-binding protein (eCIRP)-derived peptide protects mice from intestinal ischemia-reperfusion injury. Surgery 168(3):478–485, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haggag YA, Donia AA, Osman MA, El-Gizawy SA. Peptides as drug candidates: limitations and recent development perspectives. Biomed J 1:3, 2018. [Google Scholar]
- 35.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discovery Today 20(1):122–128, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Lau JL, Dunn MK. Therapeutic peptides: historical perspectives, current development trends, and future directions. Bioorganic & Medicinal Chemistry 26(10):2700–2707, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Li J, Salcedo R, Mivechi NF, Trinchieri G, Horuzsko A. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res 72(16):3977–3986, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu L, Han L, Xie C, Li W, Lin L, Pan S, Zhou Y, Li Z, Jin M, Zhang A. Identification of extracellular actin as a ligand for triggering receptor expressed on myeloid cells-1 signaling. Frontiers in Immunology 8:917, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Read CB, Kuijper JL, Hjorth SA, Heipel MD, Tang X, Fleetwood AJ, Dantzler JL, Grell SN, Kastrup J, Wang C, et al. Cutting Edge: identification of neutrophil PGLYRP1 as a ligand for TREM-1. J Immunol 194(4):1417–1421, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mihm S Danger-associated molecular patterns (DAMPs): molecular triggers for sterile inflammation in the liver. Int J Mol Sci 19(10):3104, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Golen RF, Reiniers MJ, Marsman G, Alles LK, van Rooyen DM, Petri B, Van der Mark VA, van Beek AA, Meijer B, Maas MA, et al. The damage-associated molecular pattern HMGB1 is released early after clinical hepatic ischemia/reperfusion. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1865(6):1192–1200, 2019. [DOI] [PubMed] [Google Scholar]
- 42.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 201(7):1135–1143, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol 175(11):7661, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Ornatowska M, Azim AC, Wang X, Christman JW, Xiao L, Joo M, Sadikot RT. Functional genomics of silencing TREM-1 on TLR4 signaling in macrophages. Am J Physiol Lung Cell Mol Physiol 293(6):L1377–L1384, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]