Abstract
Mild traumatic brain injury (mTBI), often referred to as concussion, is a major cause of morbidity and mortality worldwide. Sleep disturbances are common after mTBI. Moreover, subjects who develop subjective sleep complaints after mTBI also report more severe somatic, mental health, and cognitive impairment and take longer to recover from mTBI sequelae. Despite many previous studies addressing the role of sleep in post-mTBI morbidity, the mechanisms linking sleep to recovery after mTBI remain poorly understood. The glymphatic system is a brain-wide network that supports fluid movement through the cerebral parenchyma and the clearance of interstitial solutes and wastes from the brain. Notably, the glymphatic system is active primarily during sleep. Clearance of cellular byproducts related to somatic, mental health, and neurodegenerative processes (e.g., amyloid-β and tau, among others) depends in part on intact glymphatic function, which becomes impaired after mTBI. In this viewpoint, we review the current knowledge regarding the association between sleep disturbances and post-mTBI symptoms. We also discuss the role of glymphatic dysfunction as a potential link between mTBI, sleep disruption, and post-traumatic morbidity. We outline a model where glymphatic dysfunction and sleep disruption caused by mTBI may have an additive effect on waste clearance, leading to cerebral dysfunction and impaired recovery. Finally, we review the novel techniques being developed to examine glymphatic function in humans and explore potential interventions to alter glymphatic exchange that may offer a novel therapeutic approach to those suffering from poor sleep and prolonged symptoms after mTBI.
Keywords: Glymphatic system, sleep, traumatic brain injury, perivascular spaces, Virchow Robin spaces
Introduction
Traumatic brain injury (TBI) is defined as an alteration in brain function or other evidence of brain pathology caused by an external force (1). It is estimated that 3.5 million TBI cases occur in the United States per year (2). TBI is classified as mild, moderate, or severe based on five clinical signs (Supplementary Table 1): a period of decreased consciousness, post-traumatic amnesia, alteration in mental state (i.e., confusion, disorientation, slowed thinking), neurological deficits (i.e., weakness, imbalance, change in vision, weakness, sensory loss, aphasia), or an intracranial lesion (3). Mild TBI (mTBI), often referred to as concussion, represents greater than 80% of all TBI (4). The Veterans Affairs/Department of Defense Clinical Practice Guideline for Management of Concussion defines mTBI is defined as any TBI with loss of consciousness of fewer than 30 minutes, Glasgow Coma Score between 13–15, and normal neuroimaging (3).
Sleep disturbances are commonly reported after mTBI (5). Post-mTBI sleep disturbances impair the recovery process and are associated with persistent symptoms such as memory problems, mood disturbances, anxiety, headaches, and poor quality of life (6). Moreover, post-mTBI symptoms such as headaches, depression, somatic pain, and post-traumatic stress disorder (PTSD) can further disrupt sleep, creating a vicious cycle. The mechanisms underlying this bi-directional relationship remain largely unknown.
The glymphatic system is a network of perivascular pathways along which cerebrospinal fluid (CSF) and brain interstitial fluid exchange (7, 8). Functionally connected to the recently-characterized meningeal lymphatic vascular drainage via the cisternal CSF compartment (9), glymphatic exchange along perivascular pathways plays a role in eliminating waste generated by the brain (8). Importantly, in rodents, glymphatic clearance increases during sleep and becomes impaired after moderate TBI (10, 11). Both in animal models and humans, the aberrant accumulation of cerebral waste, such as amyloid-β and tau, has been linked with neurodegeneration and cerebral dysfunction (12). Glymphatic dysfunction thus offers a potential biological mechanism for the observed association between sleep disturbances and persistence of post-mTBI symptoms.
This review discusses recent advances in our understanding of glymphatic biology and its modulation by mTBI and sleep. We also outline a potential mechanistic link between sleep disturbances, glymphatic dysfunction, and persistent symptoms following mTBI. This review focuses on mTBI as it represents the most prevalent presentation of TBI. However, we also discuss more severe forms of TBI when information specific to mTBI is missing.
SLEEP DISTURBANCES POST-mTBI
Clinical manifestations
Disrupted sleep is observed in 30–70% of patients with TBI of all severities, and it is more frequent after mTBI (13–15). Insomnia and excessive daytime sleepiness are observed in 30–60% of individuals with TBI of all severities (15–17). Hypersomnia has been reported in approximately 30% of subjects with TBI of all severities (18). Other sleep disturbances such as sleep-related breathing disorders, circadian rhythm sleep disturbances, and parasomnias/movement disorders have also been described following TBI, regardless of severity (19–22). Patients with mTBI also show altered sleep architecture, with significantly higher periods of non-rapid eye movement (NREM) and decreased REM periods (23).
Basic mechanisms underlying sleep disturbances post-mTBI
mTBI causes a ‘neurometabolic’ cascade in animals and humans. This cascade is characterized by the release of excitotoxic molecules, increased intracellular calcium, microtubular disruption, and the release of intracellular amyloid-β and tau (24). In addition, mTBI causes impaired neurotransmitter function (25–27), alterations in cerebral autoregulation (28), disruption of the blood-brain barrier, and neuroinflammation (29). These changes can affect several areas involved in sleep initiation and maintenance, resulting in dysregulation of sleep after injury.
Hypothalamic dysregulation.
Orexin – a neuropeptide produced by the posterior lateral hypothalamus – is involved in maintaining wakefulness. Orexin-producing neurons have extensive projections to wake-promoting neuroanatomical structures. These include cholinergic and monoaminergic neurons located in other hypothalamic nuclei, the limbic system, thalamus, cortex, and spinal cord. Circadian levels of orexin correlate with wakefulness cycles (30). In animals, mTBI induces disruptions in orexin levels and orexin neuronal activity (31, 32).
Pineal gland, hypothalamus, and brainstem dysregulation.
Circadian release of melatonin by the pineal gland plays an essential role in sleep-wake cycle regulation (33). Low levels of evening melatonin are observed in humans after severe TBI (34, 35). However, melatonin supplementation did not improve sleep in these subjects (36). Damage to other sleep-promoting structures such as galanin and γ-aminobutyric acid (GABA) producing neurons in the ventrolateral preoptic nuclei and histamine-producing neurons in the mammillary bodies may play a role in sleep dysregulation post-mTBI (6). Alternatively, mTBI could induce damage to wake-inducing brainstem structures such as noradrenergic neurons in the locus coeruleus and serotonin-secreting neurons in the dorsal raphe nuclei of the midbrain.
Cerebral cortex.
In animals, extracellular glutamate in the prefrontal cortex increases during wakefulness and decreases during sleep (37). Animal models of mTBI-induced sleep disruption have shown decreased presynaptic glutamate within nerve terminals contacting orexin neurons in the hypothalamus seven days after injury (38). Dietary supplementation with branched-chain amino acids (BCAA), a precursor to de novo glutamate synthesis, restores glutamate levels (38). In these animals, BCAA supplementation also restored the sleep-wake cycle (31). These findings suggest a link between post-mTBI imbalances in glutamate and mTBI-induced sleep disruption.
Relationship between post-mTBI sleep disturbances and chronic neurobehavioral symptoms
One of the main functions of sleep is posited to help restore cellular and neuronal processes. Even in the absence of injury, there is a clear relationship between lack of sleep and worsening psychomotor function, impaired judgment, and somatic disturbances such as immune dysregulation and impaired cardiovascular function (39–41). Importantly, there is a well-documented bidirectional relationship between poor sleep and mental health problems, including depression (42, 43) and anxiety (44). This relationship has also been observed in subjects with mTBI (41). Thus, sleep disturbances post-mTBI likely have outsized effects on mood regulation. In a study of 101 adults with closed-head injury, including mTBI, sleep disturbances in the acute period were associated with increased symptoms of depression, anxiety, and apathy at 12 months post-injury (45). Accordingly, treating post-mTBI sleep disturbances may have the potential to improve mental health (46).
Although the exact mechanism by which mTBI affects sleep is still unknown, there is a clear association between the presence of sleep disturbances and impaired recovery after mTBI. Mechanistically, post-mTBI sleep disturbances have been linked to impairment in brain homeostatic processes such as glutamate metabolism (47), maintenance of cerebral temperature (48), and maintenance of cerebral glycogen storage (49). In addition, post-mTBI sleep deprivation can also cause a release of pro-inflammatory cytokines such as interleukin-1β, interleukin-6, and tumor necrosis factor-α (50, 51). Neuroinflammation can lead to impairment of neuronal function and delayed recovery. Interestingly, animal models of mTBI suggest that pre-injury sleep deprivation may have beneficial effects (52). The physiological mechanisms underlying this association are still unknown. However, it has been postulated that sleep deprivation preceding injury generates a form of “ischemic preconditioning,” effectively reducing the impact of mTBI on brain function (53).
The relationship between sleep and post-mTBI symptoms is not linear. Worse post-mTBI symptoms are reported by those who experience too little (insomnia) or too much (increased daytime sleepiness) sleep (54). A possible explanation for this is that despite the increased sleep time, sleep architecture is disrupted in those with daytime sleepiness, thus impairing the restorative processes during sleep. Clinically, mTBI is usually followed by a period of hypersomnolence lasting approximately two weeks (55, 56). In most patients, as sleeping patterns improve, so do other post-mTBI symptoms. Those in whom sleep is not restored tend to have more protracted symptoms, including cognitive impairment, fatigue, anxiety, mood dysregulation, pain, and PTSD (6). It has been postulated that post-mTBI sleep disturbances simply reflect other symptoms such as mood disturbances or PTSD. However, studies suggest that sleep disturbances constitute an independent risk factor for poor recovery. In a study of 374 adults with mTBI, persistent sleep disturbances were significant predictors of functional and social outcomes, independently of psychological distress (57). In a study of 92 workers with mTBI, insomnia six months post-injury was a significant determinant of disability level, independent of depression, anxiety, or pain (58). Last, adolescents with sports-related mTBI who reported sleep disruption also reported more severe symptoms during recovery (59).
GLYMPHATIC IMPAIRMENT POST-mTBI
Introduction to the glymphatic system
The glymphatic system is a brain-wide network of perivascular spaces that supports fluid movement into and through the brain parenchyma and interstitial solutes’ clearance from the brain (7, 8). This perivascular fluid exchange is dependent upon the astroglial water channel aquaporin-4 (AQP4) (8, 60) and is functionally coupled to solute clearance via the meningeal lymphatic vasculature (9). Initially characterized in rodents, key features of the glymphatic system have been validated in humans. These include the occurrence of peri-arterial CSF contrast influx (61, 62), the demonstration that intrathecal contrast distribution into human brain tissue includes a convective component (63), and the more rapid clearance of interstitial solutes during sleep (64). However, a few important differences between rodent and human glimphatics have arisen. Dynamic contrast-enhanced (DCE)-MRI studies in rodents suggest that intrathecal contrast uptake peaks within 3 hours of administration, whereas analogous studies in humans demonstrate peak contrast uptake at least 24 hours after injection. On the other hand, the human brain is also predicted to be more dependent upon the convective CSF-interstitial fluid exchange of the glymphatic system, given the impact that distance has on the efficacy of diffusive (as opposed to convective) transport of solutes.
The confluence of four observations supports a role for glymphatic dysfunction as a novel mechanistic link between sleep disturbances and poor outcomes after TBI of all severities, including mTBI. First, repeated TBI, even in its milder forms, confers a risk of neurological dysfunction and neurodegeneration, as seen in histopathological studies of subjects with chronic traumatic encephalopathy (CTE) (65–67). Second, sleep dysfunction constitutes an independent risk factor for poor neurologic outcomes after mTBI, while sleep disruption in the middle to late life is associated with increased amyloid and tau pathology measures. Third, fluid movement through the glymphatic system is active primarily during sleep and is impaired by sleep deprivation (11, 60). Lastly, in rodents, glymphatic exchange is impaired after moderate TBI, leading to decreased clearance of cell byproducts such as amyloid-β and tau (10).
Role of the perivascular space in the exchange between cerebrospinal fluid and interstitial cerebral fluid.
According to the classic model, CSF is produced by the choroid plexi and circulates to the subarachnoid space. From there, CSF is reabsorbed into the venous sinuses by the arachnoid granulations. Recent evidence, however, suggests that CSF travels into the brain parenchyma along the penetrating arteries (7, 8, 62). This CSF influx occurs along the perivascular spaces, also known as the Virchow Robin spaces (Figure 1). Similarly, fluid and solute movement through brain tissue occurs primarily along privileged anatomical pathways, including perivascular spaces and white matter tracks, eventually returning to cisternal CSF compartments housing dural sinuses. Fluid movement along perivascular spaces through brain tissue is driven by arterial pulsatility (68), and it is facilitated by the astrocytic endfeet water channel AQP4 (8, 69).
Figure 1.
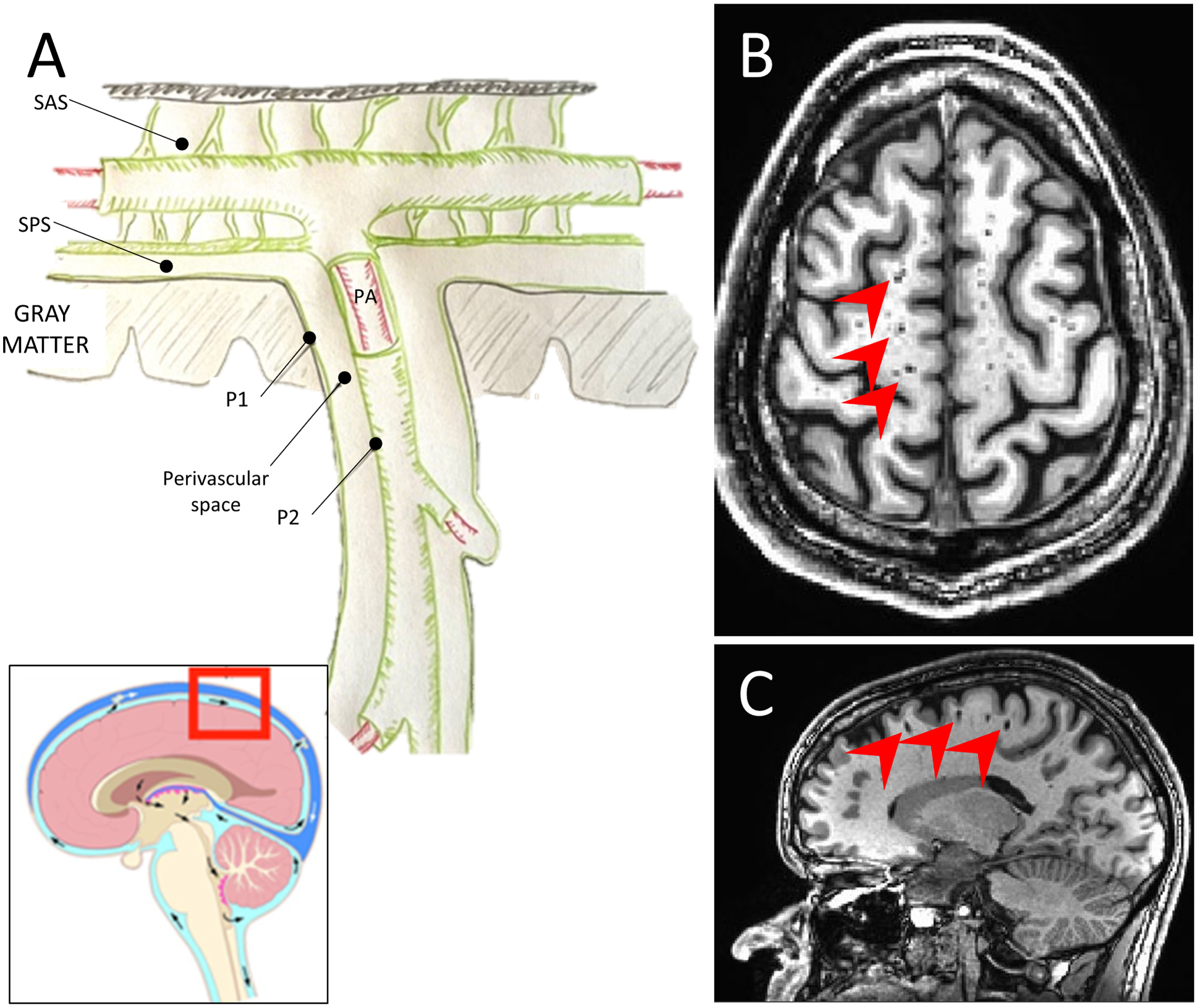
Schematic representation of the perivascular space (A). The pia mater separates the subarachnoid space (SAS) from the subpial space (SPS). The perivascular space (PVS) surrounds penetrating arteries (PA) as they enter the brain parenchyma. The PVS is lined on the inside by a layer of pia mater surrounding the artery (P1) and on the outside by a layer of pia matter in contact with the brain parenchyma (P2). T1-weighted MRI, axial (B), and sagittal (C) views, showing MRI-visible PVS (red arrowheads).
Glymphatic function is modulated by sleep and impaired post-TBI
Recent evidence shows that glymphatic exchange is significantly increased during sleep, and it is suppressed during wakefulness. In a foundational study using two-photon microscopy in mice, Xie et al. observed a significant increase in interstitial volume and CSF flow during anesthesia or naturally occurring sleep, compared to wakefulness (11). These observations suggest that sleep plays a vital role in removing neurotoxic waste produced during wakefulness via the glymphatic system.
In addition to being modulated by sleep, studies in animal models have demonstrated that glymphatic clearance is impaired after TBI. In rodents, moderate TBI results in loss of perivascular AQP4 polarity and glymphatic flow impairment (10). This impairment is characterized by markedly decreased CSF flow into the brain parenchyma and clearance of interstitial solutes and cell byproducts such as tau, starting 1-hour post-injury and persisting for up to 28 days (10, 70). Additional data in rodents show that, in addition to tau, other TBI biomarkers such as glial fibrillary acidic protein, S100B, and neuron-specific enolase are also cleared from the brain parenchyma through the glymphatic system. Genetic deletion of AQP4 results in decreased plasma levels of these biomarkers following TBI (71).
Glymphatic disruption, sleep disturbances, and persistent neurobehavioral symptoms post-mTBI
Glymphatic dysfunction may link mTBI and neurobehavioral symptoms by two distinct mechanisms. First, loss of perivascular AQP4 in the post-mTBI brain may directly slow cellular waste clearance from the interstitial space. Second, mTBI may indirectly reduce sleep-active glymphatic function by disrupting sleep phases in which glymphatic exchange is most active. Thus, by reducing both the opportunity for sleep-active glymphatic exchange and by reducing the efficacy of glymphatic function that does occur, mTBI may promote the accumulation of cellular waste, neuropeptides, and inflammatory mediators in the interstitial compartment, promoting neuronal dysfunction and propagation/persistence of symptoms (Figure 2). In order to illustrate our point, we will draw from examples of two clinical entities: post-concussive headaches (PCH) and post-traumatic dementia (including Alzheimer’s disease and chronic traumatic encephalopathy or CTE). PCH may develop after a single episode of mTBI, whereas post-traumatic dementia and CTE are associated with repeated exposure to mTBI. Both these entities are closely related to and have implications for other potential processes that may lead to mTBI-associated changes in mental health. Chronic migraines have been associated with depression, anxiety, post-traumatic stress disorder, panic disorder, and bipolar disorder (72, 73). Individuals with dementia experience higher rates of depression, anxiety, and post-traumatic stress disorder (74). Chronic traumatic encephalopathy (CTE), in particular, is characterized by early and profound changes in mental health, including mood swings, anxiety, impulse control, and depression; these symptoms can occur well before cognitive symptoms (75).
Figure 2.
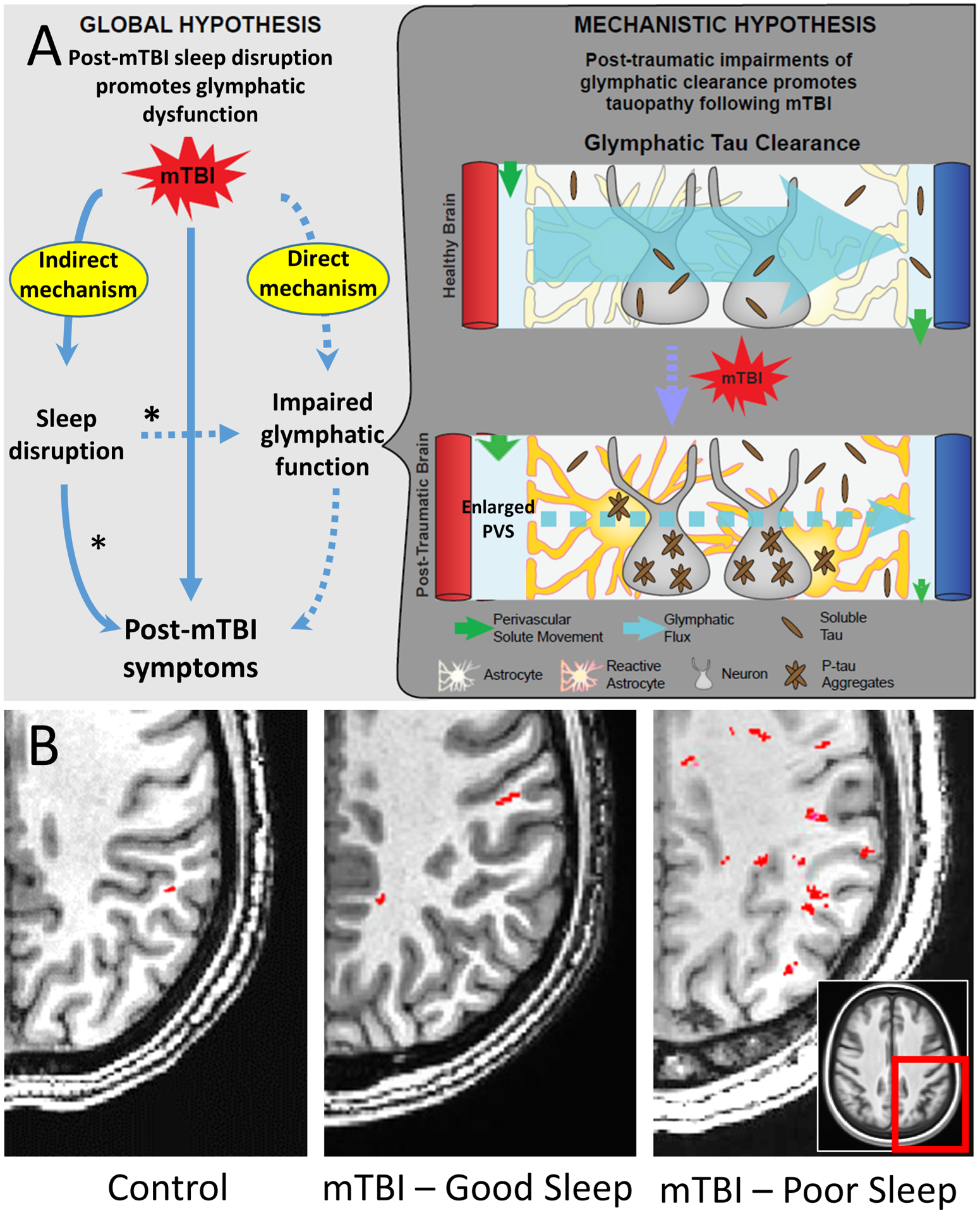
Top panel (A): a proposed mechanism integrating glymphatic dysfunction, sleep disruption, and post-mTBI symptoms. The asterisks (*) indicate potential mechanisms by which improving sleep may improve recovery after mTBI. Bottom panel (B): Effect of mTBI and poor sleep on perivascular space burden. Axial T1-weighted image slices, one cm-thick, through the posterior quadrant of the centrum semiovale (box) in a control subject with no history of mTBI and good seep, a subject with mTBI and good sleep, and a subject with mTBI and poor sleep. Perivascular spaces (PVS, in red) were identified with a semi-automated detection algorithm (100, 120, 121). Note the increased number of PVS in the posterior region in subject C.
mTBI: mild traumatic brain injury.
[Adapted from Piantino et al., 2021 J Neurotrauma (110)]
Glymphatic dysfunction and risk of PCH.
Headaches, particularly of the migrainous type, are common in the acute phase following mTBI and persist for at least one year after injury in 18–58% of mTBI patients (76). Even in the absence of TBI, sleep disturbances and headaches still share close clinical associations. Sleep disturbances are common headache triggers (77, 78). Conversely, sleep is a widely reported migraine abortive. This relationship between sleep disruption and headaches is observed in subjects following mTBI as well. However, whether sleep disturbances following mTBI contribute directly to PCH development or whether these two processes result from a common upstream mechanistic driver dysfunction is unknown. The release of vasoactive neuropeptides, including calcitonin gene-related peptide (CGRP), by perivascular trigeminal afferents and central efferent projections, is proposed to underlie the vasodilation and pain associated with migraine (79). The trigeminal afferents innervating the leptomeningeal vasculature are located within the perivascular spaces comprising the glymphatic pathway. Cortical spreading depolarization, the proposed neurophysiological basis for migraine aura, occurs following mTBI and causes CGRP release. Once released, the route along which CGRP is cleared from the perivascular compartment to the blood has not yet been defined. Presumably unable to cross the blood-brain barrier, the clearance of CGRP and other trigeminal neuropeptides from these perivascular compartments are likely dependent upon glymphatic exchange. Glymphatic impairment caused directly by mTBI and indirectly by post-mTBI sleep disturbances may impair CGRP clearance, propagating PCH.
Glymphatic dysfunction and post-mTBI vulnerability to neurodegenerative disease.
Multiple studies have linked severe TBI to neurodegenerative diseases, including Alzheimer’s disease (80, 81). Currently, mTBI is a less established predictor of cognitive decline and neurodegeneration than severe TBI (82). Studies linking mTBI to neurodegenerative processes are limited by referral bias, challenges quantifying single versus repeated mTBI exposure, and potential confounding (83). Despite these limitations, evidence suggests a link between mTBI exposure, cognitive decline, and neurodegeneration (84–86). Recent studies have linked sleep disturbances with Alzheimer’s disease, even in the absence of mTBI (87–90). However, the mechanisms underlying these associations remain unknown. Both Alzheimer’s disease and CTE are linked to abnormal deposition of cellular byproducts in the brain. In Alzheimer’s disease, there is an abnormal deposition of amyloid-β in the cortex (91). In CTE, the pathognomonic feature is perivascular deposit of tau, starting in the cortical surface of the temporal lobes and extending to the rest of the cortex (65–67). mTBI results in axonal damage and release of intracellular amyloid-β and tau present in microtubules (24). It has been postulated that excess interstitial tau leads to cellular uptake and formation of fibrillary aggregates, resulting in intracellular tangles (92). In rodents, moderate TBI caused impaired clearance of both amyloid-β and tau by the glymphatic system (10). Sleep disturbances may, in turn, aggravate this impairment, promoting the aggregation of these proteins and the ensuing neuronal dysfunction. Thus it is plausible that a cumulative effect of mTBI and poor sleep could increase the risk of future neurodegenerative diseases. Supporting this hypothesis, recent studies in humans with mTBI show that poor sleepers have elevated serum levels of neurofilament light, a marker of neurodegeneration, and lower cognitive function compared to mTBI good sleepers and controls (93).
Measurement of glymphatic function in humans
Glymphatic function in rodents is evaluated using dynamic imaging of contrast exchange from the CSF to the brain interstitial compartment, including by DCE-MRI following intrathecal gadolinium-based contrast agent injection (7, 8). Measuring glymphatic function in humans has been more challenging. Recent techniques to assess human glymphatic function include the use of contrast agents to trace CSF flow, the measurement of perivascular spaces, and CSF flow measurements with functional MRI.
Measurements of glymphatic flow with gadolinium-based contrast agents.
A series of seminal studies by Ringstad, Eide, and colleagues have shown that in healthy individuals, a gadolinium-based CSF tracer injected intrathecally penetrates the brain parenchyma from the subarachnoid space to the deep brain regions and drains into the cervical lymph nodes (62, 94, 95). The tracer’s clearance is delayed in certain disorders associated with glymphatic dysfunction, such as idiopathic normal pressure hydrocephalus (62). These techniques, however, are invasive and not feasible for widespread clinical use. Using intravenous gadolinium-based contrast, investigators have shown time-dependent enhancement of the basal ganglia perivascular spaces (96), subarachnoid peri-arterial spaces, and meningeal lymphatic vessels (97). An additional study found time-dependent gadolinium clearance in subjects with white matter hyperintensities (found in cerebral small vessel disease) which suggest impaired glymphatic clearance (98). Although the leakage of contrast into the perivascular space in subjects with an intact blood-brain barrier is minimal, the use of intravenous contrast agents is less invasive and represents a promising method for studying glymphatic flow in healthy and diseased subjects.
Visualization of perivascular spaces and CSF flow.
Previously considered a normal neuroradiologic variant (99), MRI-visible perivascular spaces (PVS) are seen across the lifespan (100–102) and are increasingly considered a putative marker of glymphatic pathway impairment. Higher PVS burden is seen in conditions associated with glymphatic function impairment, such as Alzheimer’s, cerebral small vessel disease, cerebral amyloid angiopathy, and multiple sclerosis (103–106). Dilation of these spaces is observed in patients with mTBI (107, 108). In a study of 38 veterans, Opel et al. found a relation between PVS burden and decreased total sleep time, but only in those with a history of mTBI (109). In a more recent study of 56 Iraq/Afghanistan veterans, Piantino et al. found a correlation between the number of mTBI sustained in the military and PVS burden (110). Moreover, PVS burden was further increased in those who, in addition to mTBI, also reported poor sleep (Figure 2). These findings suggest an interaction between mTBI and poor sleep on PVS burden. It has been hypothesized that translocation of AQP4 following mTBI leads to impaired CSF flow out of the PVS, thus causing a volume expansion (110). In addition to structural measurements of PVS, other methods to assess glymphatic flow have been proposed. Using simultaneous measurements of blood oxygen level-dependent (BOLD) functional MRI, electroencephalography (EEG), and CSF flow in healthy individuals, Fultz et al. observed that during sleep, the brain exhibits waves of CSF flow on a macroscopic scale, which are coupled with hemodynamic and neural activity (111). Although a direct correlation between these CSF waves and glymphatic flow has not been established, these intriguing findings offer a glimpse at the interaction between sleep, neural activity, CSF flow, and cerebral hemodynamics.
Targeting glymphatics
The evidence suggesting impairment of glymphatic flow as an important factor in post-mTBI morbidity has led to the hypothesis that increasing glymphatic function, either pharmacologically or otherwise, could lead to clinical improvement of post-mTBI neurobehavioral symptoms.
Sleep-wake regulation of glymphatic exchange is mediated in part through central noradrenergic tone. In rodents, dexmedetomidine and xylazine (alpha-2 noradrenergic autoreceptor agonists) cause a reduction in central noradrenergic output and increase glymphatic flow (112, 113). In a study of 63 Veterans with a history of mTBI and post-traumatic headaches, Ruff and colleagues reported that a combined treatment approach including sleep hygiene and the centrally active α1 adrenergic antagonist prazosin markedly reduced both headache pain intensity and frequency, both after the 9-week treatment period and six months following initiation of treatment (114). Prazosin has also been used to treat sleep disturbances and nightmares associated with PTSD, but its treatment efficacy is still unclear (115).
Other non-pharmacological treatments have been proposed, including adjusting body posture (116) and modulation of respiration and other integrative health approaches (117), although these approaches are still in the early validation stages.
Challenges and future directions
Studies using DCE-MRI in the human brain have begun to corroborate animal data. However, rodent and human glymphatics may differ, and key preclinical findings still need to be replicated in human populations. First, we must define whether glymphatic function is more rapid in sleeping than the waking human brain and whether glymphatic function is slowed in humans following mTBI. Similarly, although glymphatic dysfunction promotes the development of amyloid-β and tau pathology in both the aging and the post-traumatic rodent brain, we must determine whether glymphatic dysfunction promotes the pathology underlying neurodegenerative conditions such as Alzheimer’s or CTE in human populations. Furthermore, we must investigate whether glymphatic dysfunction represents a mechanism underlying other common sequelae of mTBI, such as headaches and depression.
Central to all of these issues is developing non-invasive approaches to assess glymphatic pathway function in humans. DCE-MRI requires the injection of intrathecal contrasts, and therefore it is not feasible for large studies. In addition, intravenous contrast requires blood-brain barrier breakdown to access the brain parenchyma (97), and therefore it may also have limited use. Recently, several contrast-free MRI sequences have been used to capture different elements of glymphatic biology, including trans-endothelial water exchange, perivascular diffusion, and MRI encephalography (118). However, none of these non-invasive approaches have been validated against the gold-standard DCE-MRI approach to measuring perivascular CSF-interstitial fluid exchange. Despite promising preliminary data, the role of MRI-visible perivascular spaces as markers of glymphatic function remains hypothetical. Studies comparing PVS morphology to intrathecal contrast clearance (through DCE-MRI) in different disease stages may provide crucial validation of PVS as a non-invasive, putative marker of glymphatic dysfunction.
The majority of TBI in humans is mild. However, preclinical experiments of post-injury glymphatic dysfunction utilized a moderate TBI model. Replicating these findings in mTBI models may help move the field forward. Last, preclinical models may offer an alternative way to study the role of PVS in glymphatic function. Using two-photon microscopy in a mouse or migraine, Schain et al. have demonstrated rapid and nearly complete PVS closure minutes following cortical spreading depolarization, an electrical phenomenon observed during migraine auras (119). These findings suggest that PVS morphology is dynamic and that two-photon microscopy can be utilized in other preclinical disease models such as mTBI.
Conclusion
In conclusion, sleep disturbances are prevalent following mTBI and directly exacerbate neurobehavioral symptoms, including mood, anxiety, and chronic headaches. We propose a mechanistic model in which impairment of perivascular glymphatic exchange explains the bi-directional relationships between mTBI, sleep, and post-mTBI symptoms. Although evidence supporting this model is emerging, this remains a theoretical framework. The validation of non-invasive measurement of glymphatic function will allow us to delineate the role of glymphatic impairment in post-mTBI morbidity. In turn, modulation of sleep-active glymphatic exchange may emerge as a valuable therapeutic target for preventing and treating post-traumatic dysfunction.
Supplementary Material
Acknowledgments
These studies were supported by funding from NIH NHLBI K23HL150217-01 to J.A.P.; NIH NIA AG054456, AG066509, and NIH NINDS 089709 to J.J.I.; and VA CX002022, DoD PT160162, and Center for Neuroscience & Regenerative Medicine Award to M.M.L.The authors would like to thank members of the DISECT-TBI (Defining Imaging, Sleep, and Exchange of CSF in TBI) Consortium for their collaborations and input on TBI, biomarkers, and sleep, including Drs. Jessica Gill, Christine MacDonald, Swati Rane Levendowsky, Jonathan Elliott, Sara Mithani, Vivian Guedes, Nadir Balba and Molly Braun. We would also like to thank Drs. Lisa Silbert and Randy Woltjer at OHSU for scientific discussions about perivascular spaces. Finally, we would like to express our sincere gratitude for the participants in our research studies.
This material is the result of work supported with resources and the use of facilities at the VA Puget Sound Health Care System and the VA Portland Health Care System. Interpretations and conclusions are those of the authors and do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Study support:
Juan A. Piantino: National Heart, Lung, and Blood Institute (NHLBI) K23HL150217-01.
Jeffrey J. Iliff: NIA, NINDS (AG054456, NS089709, AG066509)
Miranda M. Lim: VA CSRD Merit #I01 CX002022, Center for Neuroscience & Regenerative Medicine Award, DoD Award #PT160162
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosure statement:
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Menon DK, Schwab K, Wright DW, Maas AI (2010): Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 91:1637–1640. [DOI] [PubMed] [Google Scholar]
- 2.Coronado VG, McGuire LC, Sarmiento K, Bell J, Lionbarger MR, Jones CD, et al. (2012): Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995–2009. Journal of safety research. 43:299–307. [DOI] [PubMed] [Google Scholar]
- 3.(2009): VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. Journal of rehabilitation research and development. 46:Cp1–68. [PubMed] [Google Scholar]
- 4.Faul MXL, Wald MM, Coronado V (2010): Traumatic brain injury in the United States. In: Centers for Disease Control and Prevention NCfIPaC, editor. Atlanta, GA. [Google Scholar]
- 5.Clinchot DM, Bogner J, Mysiw WJ, Fugate L, Corrigan J (1998): Defining sleep disturbance after brain injury. American journal of physical medicine & rehabilitation. 77:291–295. [DOI] [PubMed] [Google Scholar]
- 6.Wickwire EM, Williams SG, Roth T, Capaldi VF, Jaffe M, Moline M, et al. (2016): Sleep, Sleep Disorders, and Mild Traumatic Brain Injury. What We Know and What We Need to Know: Findings from a National Working Group. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 13:403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. (2013): Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 123:1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. (2012): A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science translational medicine. 4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J (2017): Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. The Journal of Clinical Investigation. 127:3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. (2014): Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 34:16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. (2013): Sleep drives metabolite clearance from the adult brain. Science. 342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullan MJ, Asken BM, Jaffee MS, DeKosky ST, Bauer RM (2018): Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neuroscience and biobehavioral reviews. 84:316–324. [DOI] [PubMed] [Google Scholar]
- 13.Collen J, Orr N, Lettieri CJ, Carter K, Holley AB (2012): Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 142:622–630. [DOI] [PubMed] [Google Scholar]
- 14.Kempf J, Werth E, Kaiser PR, Bassetti CL, Baumann CR (2010): Sleep-wake disturbances 3 years after traumatic brain injury. J Neurol Neurosurg Psychiatry. 81:1402–1405. [DOI] [PubMed] [Google Scholar]
- 15.Ouellet MC, Beaulieu-Bonneau S, Morin CM (2015): Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 14:746–757. [DOI] [PubMed] [Google Scholar]
- 16.Ouellet MC, Beaulieu-Bonneau S, Morin CM (2006): Insomnia in patients with traumatic brain injury: frequency, characteristics, and risk factors. J Head Trauma Rehabil. 21:199–212. [DOI] [PubMed] [Google Scholar]
- 17.Imbach LL, Valko PO, Li T, Maric A, Symeonidou ER, Stover JF, et al. (2015): Increased sleep need and daytime sleepiness 6 months after traumatic brain injury: a prospective controlled clinical trial. Brain : a journal of neurology. 138:726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathias JL, Alvaro PK (2012): Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep Med. 13:898–905. [DOI] [PubMed] [Google Scholar]
- 19.Webster JB, Bell KR, Hussey JD, Natale TK, Lakshminarayan S (2001): Sleep apnea in adults with traumatic brain injury: a preliminary investigation. Arch Phys Med Rehabil. 82:316–321. [DOI] [PubMed] [Google Scholar]
- 20.Ayalon L, Borodkin K, Dishon L, Kanety H, Dagan Y (2007): Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology. 68:1136–1140. [DOI] [PubMed] [Google Scholar]
- 21.Pillar G, Averbooch E, Katz N, Peled N, Kaufman Y, Shahar E (2003): Prevalence and risk of sleep disturbances in adolescents after minor head injury. Pediatr Neurol. 29:131–135. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman Y, Tzischinsky O, Epstein R, Etzioni A, Lavie P, Pillar G (2001): Long-term sleep disturbances in adolescents after minor head injury. Pediatr Neurol. 24:129–134. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber S, Barkai G, Gur-Hartman T, Peles E, Tov N, Dolberg OT, et al. (2008): Long-lasting sleep patterns of adult patients with minor traumatic brain injury (mTBI) and non-mTBI subjects. Sleep Med. 9:481–487. [DOI] [PubMed] [Google Scholar]
- 24.Giza CC, Hovda DA (2014): The new neurometabolic cascade of concussion. Neurosurgery. 75 Suppl 4:S24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valko PO, Gavrilov YV, Yamamoto M, Finn K, Reddy H, Haybaeck J, et al. (2015): Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Annals of neurology. 77:177–182. [DOI] [PubMed] [Google Scholar]
- 26.Baumann CR, Stocker R, Imhof HG, Trentz O, Hersberger M, Mignot E, et al. (2005): Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 65:147–149. [DOI] [PubMed] [Google Scholar]
- 27.Rye DB, Bliwise DL, Parker K, Trotti LM, Saini P, Fairley J, et al. (2012): Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Science translational medicine. 4:161ra151. [DOI] [PubMed] [Google Scholar]
- 28.Junger EC, Newell DW, Grant GA, Avellino AM, Ghatan S, Douville CM, et al. (1997): Cerebral autoregulation following minor head injury. J Neurosurg. 86:425–432. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Loane DJ (2012): Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain, behavior, and immunity. 26:1191–1201. [DOI] [PubMed] [Google Scholar]
- 30.Piper DC, Upton N, Smith MI, Hunter AJ (2000): The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. The European journal of neuroscience. 12:726–730. [DOI] [PubMed] [Google Scholar]
- 31.Lim MM, Elkind J, Xiong G, Galante R, Zhu J, Zhang L, et al. (2013): Dietary therapy mitigates persistent wake deficits caused by mild traumatic brain injury. Science translational medicine. 5:215ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somach RL MM; Cohen AS (2021): Altered Orexin Function Following Mild Traumatic Brain Injury. Society for Neuroscience. Chicago, IL. [Google Scholar]
- 33.Redman J, Armstrong S, Ng KT (1983): Free-running activity rhythms in the rat: entrainment by melatonin. Science. 219:1089–1091. [DOI] [PubMed] [Google Scholar]
- 34.Shekleton JA, Parcell DL, Redman JR, Phipps-Nelson J, Ponsford JL, Rajaratnam SM (2010): Sleep disturbance and melatonin levels following traumatic brain injury. Neurology. 74:1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grima NA, Ponsford JL, St Hilaire MA, Mansfield D, Rajaratnam SM (2016): Circadian Melatonin Rhythm Following Traumatic Brain Injury. Neurorehabilitation and neural repair. 30:972–977. [DOI] [PubMed] [Google Scholar]
- 36.Kemp S, Biswas R, Neumann V, Coughlan A (2004): The value of melatonin for sleep disorders occurring post-head injury: a pilot RCT. Brain Inj. 18:911–919. [DOI] [PubMed] [Google Scholar]
- 37.Naylor E, Aillon DV, Gabbert S, Harmon H, Johnson DA, Wilson GS, et al. (2011): Simultaneous real-time measurement of EEG/EMG and L-glutamate in mice: A biosensor study of neuronal activity during sleep. Journal of electroanalytical chemistry (Lausanne, Switzerland). 656:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott JE, De Luche SE, Churchill MJ, Moore C, Cohen AS, Meshul CK, et al. (2018): Dietary therapy restores glutamatergic input to orexin/hypocretin neurons after traumatic brain injury in mice. Sleep. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dongen HP, Maislin G, Mullington JM, Dinges DF (2003): The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 26:117–126. [DOI] [PubMed] [Google Scholar]
- 40.Banks S, Dinges DF (2007): Behavioral and physiological consequences of sleep restriction. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 41.Duffield TC, Lim MM, Novak M, Lin A, Luther M, Williams CN, et al. (2021): The relationship between depressive symptoms, somatic complaints, and concussion history with poor sleep in collegiate athletes. Sleep health. 7:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Callaghan VS, Couvy-Duchesne B, Strike LT, McMahon KL, Byrne EM, Wright MJ (2021): A meta-analysis of the relationship between subjective sleep and depressive symptoms in adolescence. Sleep Med. 79:134–144. [DOI] [PubMed] [Google Scholar]
- 43.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. (2011): Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of affective disorders. 135:10–19. [DOI] [PubMed] [Google Scholar]
- 44.Soehner AM, Harvey AG (2012): Prevalence and Functional Consequences of Severe Insomnia Symptoms in Mood and Anxiety Disorders: Results from a Nationally Representative Sample. Sleep. 35:1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao V, McCann U, Han D, Bergey A, Smith MT (2014): Does acute TBI-related sleep disturbance predict subsequent neuropsychiatric disturbances? Brain Inj. 28:20–26. [DOI] [PubMed] [Google Scholar]
- 46.Killgore WDS, Vanuk JR, Shane BR, Weber M, Bajaj S (2020): A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiology of disease. 134:104679. [DOI] [PubMed] [Google Scholar]
- 47.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G (2009): Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 29:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franken P, Dijk DJ, Tobler I, Borbely AA (1991): Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 261:R198–208. [DOI] [PubMed] [Google Scholar]
- 49.Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD (2002): Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 22:5581–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, et al. (2001): Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 107:165–170. [DOI] [PubMed] [Google Scholar]
- 51.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. (2004): Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 89:2119–2126. [DOI] [PubMed] [Google Scholar]
- 52.Hsu JC, Lee YS, Chang CN, Ling EA, Lan CT (2003): Sleep deprivation prior to transient global cerebral ischemia attenuates glial reaction in the rat hippocampal formation. Brain research. 984:170–181. [DOI] [PubMed] [Google Scholar]
- 53.Dirnagl U, Becker K, Meisel A (2009): Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 8:398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiseman-Hakes C, Gosselin N, Sharma B, Langer L, Gagnon I (2019): A Longitudinal Investigation of Sleep and Daytime Wakefulness in Children and Youth With Concussion. ASN neuro. 11:1759091418822405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Billiard M, Podesta C (2013): Recurrent hypersomnia following traumatic brain injury. Sleep Med. 14:462–465. [DOI] [PubMed] [Google Scholar]
- 56.Sommerauer M, Valko PO, Werth E, Baumann CR (2013): Excessive sleep need following traumatic brain injury: a case-control study of 36 patients. J Sleep Res. 22:634–639. [DOI] [PubMed] [Google Scholar]
- 57.Chan LG, Feinstein A (2015): Persistent Sleep Disturbances Independently Predict Poorer Functional and Social Outcomes 1 Year After Mild Traumatic Brain Injury. J Head Trauma Rehabil. 30:E67–75. [DOI] [PubMed] [Google Scholar]
- 58.Mollayeva T, Pratt B, Mollayeva S, Shapiro CM, Cassidy JD, Colantonio A (2016): The relationship between insomnia and disability in workers with mild traumatic brain injury/concussion: Insomnia and disability in chronic mild traumatic brain injury. Sleep Med. 20:157–166. [DOI] [PubMed] [Google Scholar]
- 59.Kostyun RO, Milewski MD, Hafeez I (2015): Sleep disturbance and neurocognitive function during the recovery from a sport-related concussion in adolescents. The American journal of sports medicine. 43:633–640. [DOI] [PubMed] [Google Scholar]
- 60.Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, et al. (2018): Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eide PK, Ringstad G (2015): MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta radiologica open. 4:2058460115609635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ringstad G, Vatnehol SAS, Eide PK (2017): Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain : a journal of neurology. 140:2691–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valnes LM, Mitusch SK, Ringstad G, Eide PK, Funke SW, Mardal KA (2020): Apparent diffusion coefficient estimates based on 24 hours tracer movement support glymphatic transport in human cerebral cortex. Scientific reports. 10:9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eide PK, Vinje V, Pripp AH, Mardal KA, Ringstad G (2021): Sleep deprivation impairs molecular clearance from the human brain. Brain : a journal of neurology. 144:863–874. [DOI] [PubMed] [Google Scholar]
- 65.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. (2009): Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. Journal of neuropathology and experimental neurology. 68:709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKee AC, Stein TD, Kiernan PT, Alvarez VE (2015): The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 25:350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, et al. (2013): The spectrum of disease in chronic traumatic encephalopathy. Brain : a journal of neurology. 136:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, et al. (2018): Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nature communications. 9:4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iliff JJ, Nedergaard M (2013): Is there a cerebral lymphatic system? Stroke; a journal of cerebral circulation. 44:S93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L, Chopp M, Ding G, Davoodi-Bojd E, Zhang L, Li Q, et al. (2020): MRI detection of impairment of glymphatic function in rat after mild traumatic brain injury. Brain research. 1747:147062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, et al. (2015): Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 35:518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burch RC, Buse DC, Lipton RB (2019): Migraine: Epidemiology, Burden, and Comorbidity. Neurologic Clinics. 37:631–649. [DOI] [PubMed] [Google Scholar]
- 73.Minen MT, Begasse De Dhaem O, Kroon Van Diest A, Powers S, Schwedt TJ, Lipton R, et al. (2016): Migraine and its psychiatric comorbidities. Journal of Neurology, Neurosurgery & Psychiatry. 87:741–749. [DOI] [PubMed] [Google Scholar]
- 74.Kuring JK, Mathias JL, Ward L (2018): Prevalence of Depression, Anxiety and PTSD in People with Dementia: a Systematic Review and Meta-Analysis. Neuropsychology Review. 28:393–416. [DOI] [PubMed] [Google Scholar]
- 75.Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR, et al. (2017): Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. Jama. 318:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piantino J, Lim MM, Newgard CD, Iliff J (2019): Linking Traumatic Brain Injury, Sleep Disruption and Post-Traumatic Headache: a Potential Role for Glymphatic Pathway Dysfunction. Current pain and headache reports. 23:62. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, Huang Q, Li N, Tan G, Chen L, Zhou J (2013): Triggers of migraine and tension-type headache in China: a clinic-based survey. Eur J Neurol. 20:689–696. [DOI] [PubMed] [Google Scholar]
- 78.Boardman HF, Thomas E, Millson DS, Croft PR (2005): Psychological, sleep, lifestyle, and comorbid associations with headache. Headache. 45:657–669. [DOI] [PubMed] [Google Scholar]
- 79.Edvinsson L (2017): The Trigeminovascular Pathway: Role of CGRP and CGRP Receptors in Migraine. Headache. 57 Suppl 2:47–55. [DOI] [PubMed] [Google Scholar]
- 80.Mortimer JA, Van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, et al. (1991): Head Trauma as a Risk Factor for Alzheimer’s Disease: A Collaborative Re-Analysis of Case-Control Studies. International Journal of Epidemiology. 20:S28–S35. [DOI] [PubMed] [Google Scholar]
- 81.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A (2003): Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 74:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Markovic SJ, Fitzgerald M, Peiffer JJ, Scott BR, Rainey-Smith SR, Sohrabi HR, et al. (2021): The impact of exercise, sleep, and diet on neurocognitive recovery from mild traumatic brain injury in older adults: A narrative review. Ageing research reviews. 68:101322. [DOI] [PubMed] [Google Scholar]
- 83.Gardner RC, Yaffe K (2015): Epidemiology of mild traumatic brain injury and neurodegenerative disease. Molecular and cellular neurosciences. 66:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J (2012): Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 11:1103–1112. [DOI] [PubMed] [Google Scholar]
- 85.De Beaumont L, Théoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, et al. (2009): Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain : a journal of neurology. 132:695–708. [DOI] [PubMed] [Google Scholar]
- 86.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, et al. (2005): Association between Recurrent Concussion and Late-Life Cognitive Impairment in Retired Professional Football Players. Neurosurgery. 57:719–726. [DOI] [PubMed] [Google Scholar]
- 87.Beydoun HA, Beydoun MA, Weiss J, Hossain S, Huang S, Alemu BT, et al. (2021): Insomnia as a predictor of diagnosed memory problems: 2006–2016 Health and Retirement Study. Sleep Med. 80:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robbins R, Quan SF, Weaver MD, Bormes G, Barger LK, Czeisler CA (2021): Examining sleep deficiency and disturbance and their risk for incident dementia and all-cause mortality in older adults across 5 years in the United States. Aging. 13:3254–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. (2009): Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 326:1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lim MM, Gerstner JR, Holtzman DM (2014): The sleep-wake cycle and Alzheimer’s disease: what do we know? Neurodegenerative disease management. 4:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thal DR, Walter J, Saido TC, Fändrich M (2015): Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer’s disease. Acta neuropathologica. 129:167–182. [DOI] [PubMed] [Google Scholar]
- 92.Guo JL, Lee VM (2011): Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. The Journal of biological chemistry. 286:15317–15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Werner JK, Shahim P, Pucci JU, Chen L, Raiciulescu S, Gill JM, et al. (2020): Poor sleep correlates with biomarkers of neurodegeneration in mild traumatic brain injury patients: a CENC Study. Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SS, Emblem KE, et al. (2018): Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI insight. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eide PK, Vatnehol SAS, Emblem KE, Ringstad G (2018): Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Scientific reports. 8:7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Naganawa S, Nakane T, Kawai H, Taoka T (2017): Gd-based Contrast Enhancement of the Perivascular Spaces in the Basal Ganglia. Magnetic resonance in medical sciences : MRMS : an official journal of Japan Society of Magnetic Resonance in Medicine. 16:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu CH, Lirng JF, Ling YH, Wang YF, Wu HM, Fuh JL, et al. (2021): Non-invasive Characterization of Human Glymphatics and Meningeal Lymphatics in an in vivo Model of Blood-Brain Barrier Leakage. Annals of neurology. 89:111–124. [DOI] [PubMed] [Google Scholar]
- 98.Deike-Hofmann K, Reuter J, Haase R, Paech D, Gnirs R, Bickelhaupt S, et al. (2019): Glymphatic Pathway of Gadolinium-Based Contrast Agents Through the Brain: Overlooked and Misinterpreted. Investigative Radiology. 54:229–237. [DOI] [PubMed] [Google Scholar]
- 99.Groeschel S, Chong WK, Surtees R, Hanefeld F (2006): Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology. 48:745–754. [DOI] [PubMed] [Google Scholar]
- 100.Piantino J, Boespflug EL, Schwartz DL, Luther M, Morales AM, Lin A, et al. (2020): Characterization of MR Imaging-Visible Perivascular Spaces in the White Matter of Healthy Adolescents at 3T. AJNR Am J Neuroradiol. 41:2139–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rollins NK, Deline C, Morriss MC (1993): Prevalence and clinical significance of dilated Virchow-Robin spaces in childhood. Radiology. 189:53–57. [DOI] [PubMed] [Google Scholar]
- 102.Barisano G, Sheikh-Bahaei N, Law M, Toga AW, Sepehrband F (2020): Body mass index, time of day, and genetics affect perivascular spaces in the white matter. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism.271678×20972856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramirez J, Berezuk C, McNeely AA, Scott CJ, Gao F, Black SE (2015): Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer’s disease patients and normal elderly from the Sunnybrook Dementia Study. J Alzheimers Dis. 43:415–424. [DOI] [PubMed] [Google Scholar]
- 104.Potter GM, Chappell FM, Morris Z, Wardlaw JM (2015): Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 39:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A (2005): Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 26:1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 106.Charidimou A, Jaunmuktane Z, Baron JC, Burnell M, Varlet P, Peeters A, et al. (2014): White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology. 82:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inglese M, Grossman RI, Diller L, Babb JS, Gonen O, Silver JM, et al. (2006): Clinical significance of dilated Virchow-Robin spaces in mild traumatic brain injury. Brain Inj. 20:15–21. [DOI] [PubMed] [Google Scholar]
- 108.Orrison WW, Hanson EH, Alamo T, Watson D, Sharma M, Perkins TG, et al. (2009): Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J Neurotrauma. 26:689–701. [DOI] [PubMed] [Google Scholar]
- 109.Opel RA, Christy A, Boespflug EL, Weymann KB, Case B, Pollock JM, et al. (2018): Effects of traumatic brain injury on sleep and enlarged perivascular spaces. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism.271678X18791632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piantino J, Schwartz DL, Luther M, Newgard CD, Silbert L, Raskind M, et al. (2021): Link between mild traumatic brain injury, poor sleep, and MRI-visible perivascular spaces in Veterans. J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, et al. (2019): Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 366:628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, et al. (2017): Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology. 127:976–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, et al. (2019): Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Science advances. 5:eaav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ruff RL, Ruff SS, Wang XF (2009): Improving sleep: initial headache treatment in OIF/OEF veterans with blast-induced mild traumatic brain injury. Journal of rehabilitation research and development. 46:1071–1084. [DOI] [PubMed] [Google Scholar]
- 115.Raskind MA, Peskind ER, Chow B, Harris C, Davis-Karim A, Holmes HA, et al. (2018): Trial of Prazosin for Post-Traumatic Stress Disorder in Military Veterans. N Engl J Med. 378:507–517. [DOI] [PubMed] [Google Scholar]
- 116.Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, et al. (2015): The Effect of Body Posture on Brain Glymphatic Transport. J Neurosci. 35:11034–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spector R, Robert Snodgrass S, Johanson CE (2015): A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol. 273:57–68. [DOI] [PubMed] [Google Scholar]
- 118.Hennig J, Kiviniemi V, Riemenschneider B, Barghoorn A, Akin B, Wang F, et al. (2021): 15 Years MR-encephalography. Magma (New York, NY). 34:85–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schain AJ, Melo-Carrillo A, Strassman AM, Burstein R (2017): Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J Neurosci. 37:2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boespflug EL, Schwartz DL, Lahna D, Pollock J, Iliff JJ, Kaye JA, et al. (2018): MR Imaging-based Multimodal Autoidentification of Perivascular Spaces (mMAPS): Automated Morphologic Segmentation of Enlarged Perivascular Spaces at Clinical Field Strength. Radiology. 286:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schwartz DL, Boespflug EL, Lahna DL, Pollock J, Roese NE, Silbert LC (2019): Autoidentification of perivascular spaces in white matter using clinical field strength T1 and FLAIR MR imaging. NeuroImage.116126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


