Highlights
-
•
Blended grains from varieties of emmer, spelt and bread wheat were compared.
-
•
Wholemeal flours were processed using sourdough and yeast systems.
-
•
Flours, doughs and breads were analysed to determine fibre and metabolites.
-
•
Small differences were observed between the products from the three types of wheat.
-
•
The greatest differences were between the between sourdough and yeast breads.
Keywords: Wheat, Sourdough, Breadmaking, Ancient wheats, Dietary fibre
Abstract
Wholemeal flours from blends of bread wheat, emmer and spelt were processed into bread using yeast-based and sourdough fermentation. The bread wheat flour contained significantly higher concentrations of total dietary fibre and fructans than the spelt and emmer flours, the latter having the lowest contents. Breadmaking using sourdough and yeast systems resulted in changes in composition from flour to dough to bread including increases in organic acids and mannitol in the sourdough system and increases in amino acids and sugars (released by hydrolysis of proteins and starch, respectively) in both processing systems. The concentrations of fructans and raffinose (the major endogenous FODMAPs) were reduced by yeast and sourdough fermentation, with yeast having the greater effect. Both systems resulted in greater increases in sugars and glycerol in emmer than in bread wheat and spelt, but the significance of these differences for human health has not been established.
1. Introduction
Wheat is a major staple crop in much of the world contributing between about 20% and 50% of the total intake of calories. About 95% of the wheat grown globally is modern hexaploid bread wheat (Triticum aestivum L subsp. aestivum, genome constitution ABD) with most of the remaining 5% being tetraploid pasta wheat (Triticum turgidum L. subsp. durum, AB genomes). However, both of these types of wheat are highly diverse, with well over 25,000 genotypes of bread wheat being represented in germplasm collections. Both bread and durum wheats are “free threshing”, meaning that the hulls are readily separated from the grain during harvest. However, small amounts of older types of hulled wheats are still grown, either for the production of traditional foods or because of suggested health benefits. These are diploid einkorn (T. monococcum, L., A genome), tetraploid emmer (T. turgidum L. subsp. dicoccum Thell., AB genomes) and hexaploid spelt (T. aestivum L. subsp. spelta Thell, ABD genomes). These are often referred to as “ancient wheats” and are widely assumed to differ significantly from modern wheats in their contents of bioactive components. Einkorn is a distinct species while emmer and spelt are subspecies (sometimes also called varieties) of T. turgidum (which also includes pasta wheat) and of T. aestivum (which also includes bread wheat), respectively.
Variation in the compositions of wheat-based foods results from three factors and the interactions of these. Firstly, genetic variation (G) in the compositions of different species, subspecies and genotypes (Ziegler et al., 2016a, Longin et al., 2016). Secondly, the compositions of all types of wheat are strongly influenced by the environment (E), and how this interacts with individual genotypes (G × E). Thirdly, the chemical compositions of wheat-based foods are highly affected by the processing system, which in the case of bread (the most widely consumed wheat-based food) comprises two stages: milling of the grain to give flour followed by mixing, fermentation and baking. Milling is required to separate the major grain tissues, bran, germ and starchy endosperm, and reduce the latter to fine white flour. Although white flour is most widely used for breadmaking, it may also be recombined with the other grain fractions to reconstitute wholemeal flour.
Fermentation is used to produce leavened breads and most modern breadmaking processes use yeast and short fermentation times. However, sourdough systems are also used and have become increasingly popular for artisan breads. Sourdough systems exploit unique mixtures of microbiota (bacteria and yeasts) that are naturally present in the flour and in the environment that the flour and dough are exposed to during bread making. Consequently, although all sourdough systems have high concentrations of lactic acid-forming bacteria (Lactobacillus species) (LAB), there is wide diversity in their compositions of microbiota (with numerous strains being present in the same dough) and hence their production of enzymes and synthesis of metabolites. As a result, the effects of sourdough fermentation on the degradation of carbohydrates and proteins and the synthesis of vitamins, amino acids and other metabolites are also specific for each kind of sourdough (Arora et al., 2021). In large scale commercial bread making, sourdough may also be combined with yeast fermentation to reduce the fermentation time, increase dough volume and stabilise the product quality.
The relative contributions of these three factors, genotype, growth conditions and processing system, on the compositions of bread and other wheat-based foods are difficult to quantify, which has contributed to widely accepted but largely unsubstantiated assumptions about the relative qualities and benefits of sourdough and yeast breads made from ancient and modern wheats. Furthermore, concerns have been expressed about adverse impacts of modern yeast-based systems (with short time fermentation times and the use of ‘improvers’) and ancient grains suggested to be less likely to trigger gluten-related sensitivities. Although this has not been substantiated (Ribeiro et al., 2016) it has contributed to increased consumer interest in ancient grains and artisanal breadmaking systems (notably sourdough).
Sourdough fermentation has also been suggested to result in other beneficial effects, such as a pH-induced reduction in binding of minerals to phytate leading to increased bioaccessibility (Rodriguez-Ramiro et al., 2017). Targeted modification of the fermentation conditions, to increase beneficial effects, can be made by the inclusion of isolated microbiota with specific degradative or synthetic capacities including the degradation of components that are associated with adverse reactions in small percentages of the population, for example gluten, α-amylase/trypsin inhibitors and FODMAPs (Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols) (Huang et al., 2020, Arora et al., 2021).
FODMAPs are not absorbed but fermented in the colon to produce short chain fatty acids and gas. The gas produced may lead to discomfort in susceptible individuals, notably those suffering from irritable bowel syndrome (IBS) (Barrett and Gibson, 2012) and the use of a low FODMAP diet has proved to be beneficial for reducing intestinal distress symptoms in this condition (Marsh, Eslick et al,. 2016). The concentrations of FODMAPs in bread products may differ according to grain type and processing methods and reductions in the content of FODMAPs over 50% have been reported when using yeast and over 80% when using sourdough systems, the major factors being fermentation time and the specific strains of yeast and/or sourdough bacilli used (Struyf et al., 2017, Longin et al., 2020).
A number of studies have compared the effects of “ancient” and modern wheats and of sourdough and yeast systems on digestion and adverse reactions in the human gastro-intestinal tract but these have often used experimental material which is poorly described and characterised (as discussed by Shewry, 2018). We have therefore carried out detailed analyses of wholemeal flours, doughs and breads made from mixed grists of three (emmer) and five (spelt and bread wheat) commercially available grain samples using well defined yeast-based and sourdough systems. This has allowed us to explore the hypothesis that “differences in the composition of breads made from modern and ancient wheats are due more to the processing system used than to intrinsic differences in grain composition”. The data obtained will also underpin the analysis of associations with adverse reactions in susceptible individuals after the consumption of the different types of wheats and breads.
2. Materials and methods
2.1. Grain samples
Commercial samples of 5 cultivars of bread wheat (cvs. Reform, Cato, Bernstein, Kometus, Akteur) and spelt (cvs. Oberkulmer Rotkorn, Zollernspelt, Altergauer, Baulander Spelz, Franckenkorn) were provided by Graanhandel Gebroeders Dyke, (Tholen, Netherlands) who obtained them from Ir. Bernd Geldner (Saatgut Freudenberger, Krefeld, Germany) and Dresdner Mühle (Dresden, Germany). Commercial samples of three emmer cultivars (Ramses, Roten Heidfelder, Späths Albjuwel) were obtained from I.G. Pflanzenzucht Oberlimpurg GmbH (Schwäbisch Hall, Germany) and Südwestdeutsche Saatzucht GmbH&Co.KG (Rheinfeld, Germany). All were grown using conventional (ie. not organic) systems, but no further details of their preharvest agronomy were available.
2.2. Dehulling, blending and milling
Spelt and emmer seeds were dehulled by Het Geweide Hof (Garmerwolde, Groningen, NL). Blends comprising equal proportions of the five bread wheat and spelt and three emmer cultivars were milled by a certified miller Jan Pot (Mulder Pot, NL) which provided 10 fractions. The mill was cleaned thoroughly between blends. The particle size resulting from the milling ranged from 0.16 mm to 0.125 mm, with some bran particles of 1–3 mm.
The reconstituted wholemeal flours comprised approx. 98.5% of the wholegrains and were stored at −18 °C and re-milled in a ball mill for biochemical analysis.
2.3. Breadmaking
Full recipes and breadmaking conditions for the breads are given in Supporting Material Table S1. The recipes were the same for the three types of wheat except for water which was adjusted based on the water absorption of the flour determined by Farinograph. The amount of yeast was also increased to 0.8% dough wt for the emmer bread, compared to 0.6% dough wt for the bread wheat and spelt recipes, in order to produce an acceptable loaf. The commercial sourdough starter culture ‘Maillander Le Chef’ (Böcker, Germany) was used to prepare starter doughs in three steps (Supporting Material Table S2). Baking was carried out in a Wachtel INFRA CE 408/41 H oven at 235 °C upper heat input/255 °C lower heat input for a total of 37 mins for yeast breads and for sourdough breads 235 °C upper heat input/255 °C lower heat input for 10 min followed by 225 °C upper heat input 235 °C lower heat input for 27 min (total time 37 min). Examples of baked breads are shown in Supporting Material Fig. S1. Doughs and breads were prepared in triplicate and replicate samples of the dough after proofing and the bread crumb were freeze dried, milled in a ball mill and stored at −20 °C.
2.4. Determination of dietary fibre (DF) and total dietary fibre (TDF)
Dietary fibre (DF) was determined by the Uppsala method (Theander et al., 1995), which combines the amounts of sugars released by acid hydrolysis of cell wall polysaccharides, the arabinogalactan part of arabinogalactan peptide (AGP) and resistant starch, uronic acid derived from hydrolysis of glucuronoarabinoxylans (complex arabinoxylans present in the bran) and Klason lignin. Total dietary fibre (TDF) was the sum of DF from the Uppsala method and fructans.
2.5. Determination of arabinoxylan and β-glucan by enzyme digestion and HPAEC
For enzyme fingerprinting (Lovegrove et al., 2013) flour was digested using a mixture of endoxylanase and lichenase (β-glucanase) to release arabinoxylan oligosaccharides (AXOS) and gluco-oligosaccharides (GOS) comprising 3 and 4 residues (G3, G4), respectively. These were separated high performance anion-exchange chromatography (HPAEC) using a Carbopac PA-1 (Dionex) column with dimensions 2 mm × 250 mm and a flow rate of 0.25 ml/min as described (dx.https://doi.org/10.17504/protocols.io.babriam6). At least two technical replicates of each biological replicate were analysed. The areas under the G3 and G4 GOS peaks were combined to give total β-glucan (expressed in arbitrary units).
2.6. Determination of water-extractable and total polysaccharides as monosaccharides after hydrolysis
For water-extractable (WE) polysaccharides, triplicate 5 mg samples were resuspended in 1 ml of reverse osmosis (RO) water, vortexed and incubated on a spiral mixer for 30 min at 25 °C. The suspension was centrifuged at 2500×g for 10 min and the supernatant collected. 500 µl aliquots were removed into fresh tubes and freeze dried overnight. For total polysaccharides, 5 mg of flour was resuspended in 10 ml RO water, vortexed and homogenised in a glass/Teflon homogeniser. 200 μl aliquots were removed into fresh tubes and freeze-dried overnight. WE and total polysaccharides were determined as monosaccharides after hydrolysis. Briefly, 400 µl of freshly prepared 2 M trifluoroacetic acid (TFA) was added to the dried samples and heated to 120 °C for 1 h. Samples were vacuum dried and the pellets washed in 500 µl RO water and vacuum dried again. Pellets were dissolved in 500 µl of Milli-Q water and stored at −20 °C until analysis. Monosaccharides were determined using HPAEC using a Dionex ICS-5000+ with eluent generator (Thermo Scientific, US) and a CarboPac PA-20 column (guard column: 3x30 mm, analytical column: 3 × 150 mm; Thermo Scientific, US). Twenty-five µL of diluted and filtered sample was injected onto the column with run conditions as described in (Kosik et al., 2020). Calibration curves were generated with authentic standards of fucose, rhamnose, arabinose, galactose, glucose, xylose and mannose prepared following TFA treatment as described above. Chromeleon analytical software (version 7.2SR4; Thermo Scientific, US) was used for peak marking and quantification.
2.7. Analysis of fructans
Fructans were determined by high performance liquid chromatography (HPLC) (Verspreet et al., 2013). Triplicate 10 mg samples of flour were weighed into 2 ml Eppendorf tubes and 1 ml deionised water added and vortexed thoroughly. Tube lids were clamped and placed in a Thermomixer (set at 300 rpm) at 80 °C for 60 mins. Following cooling to room temperature samples were centrifuged at 9000×g for 10 mins. 50 μl aliquots of supernatants from each replicate were transferred to fresh tubes labelled ‘A’ and ‘B’. Tubes labelled ‘B’ were hydrolysed with HCl at a final concentration of 60 mM for 90 mins at 70 °C in a Thermomixer at 300 rpm. Two μl of 1 M sodium carbonate was then added, vortexed and the sample diluted to a final volume of 1 ml in deionised water. To tube ‘A’ 950 μl of deionised water was added and the sample vortexed. All samples were filtered (0.22um) and 25 μl injected into a CarboPac PA 100 guard column linked to a CarboPac PA 100 analytical column and fructose and glucose and melibiose analysed on an Dionex ICS-3000 (Thermo Scientific Ltd) with a PAD Detector using chromatography conditions as described in Verspreet et al. (2013). Corrections were made for fructose and glucose in the unhydrolyzed sample and for raffinose by measurement of melibiose in the hydrolysed sample (raffinose is a trisaccharide made up of galactose, glucose and fructose which is split into fructose and melibiose (galactose + glucose) under mild acid conditions, as described in Verspreet et al., 2013). Calibration curves generated using authentic standards were used for quantification using Thermo Scientific Chromeleon software. The fructan degree of polymerisation (DP) was determined as described by Huynh et al., (2008).
2.8. Determination of polar metabolites by 1H NMR spectroscopy
30 mg samples of wholemeal flour were extracted at 50 °C using D2O:CD3OD (80:20) containing 0.05% d4–trimethylsilylpropionate (TSP) (1 ml) as internal standard (Shewry et al., 2017). 1H NMR spectra were acquired at 300 °K using an Avance Neo Spectrometer (Bruker Biospin, Coventry, UK) operating at 600.0528 MHz, with a cryoplatform and a 5 mm triple resonance inverse probe. Spectra were acquired using 16 scans of 65,536 data points with a spectral width of 7143 Hz. A water suppression pulse sequence (zgpr) was utilised with a 90° pulse and a 5 s relaxation delay. Spectra were Fourier-transformed using an exponential window with line broadening of 0.5 Hz. Phasing and baseline correction were carried out in automation. Spectra were automatically reduced, using Amix (Analysis of MIXtures software, BrukerBiospin), to ASCII files containing integrated regions of equal width (0.01 ppm) and spectral intensities scaled to the d4-TSP region (δ0.05 to –0.05). Signal intensities for spectral regions for 29 major metabolites were extracted by comparison to spectra of known standards run under the same condition.
2.9. Determination of polar metabolites by gas chromatography mass spectrometry (GC–MS)
20 mg samples were extracted as described by Lisec et al. (2006) and (Carreno-Quintero et al., 2012) with 1.4 ml of 80 % (v/v) methanol (pre-cooled at −20 °C) containing 55 µg/ml of ribitol (Sigma Aldritch) as internal standard. After incubation at 70 °C for 10 min in a thermomixer (Eppendorf) at 950 rpm and centrifugation at 14,000g (Eppendorf Centrifuge), 500 µl of the supernatants was mixed with 375 µl of chloroform (pre-cooled at −20 °C) and 750 µl dH2O (pre-cooled at 4 °C). After mixing and centrifugation, aliquots of 50 µl and 400 μl of the upper (polar) phase were transferred to a glass insert placed in a 2 ml vial (Grace Davidson). Samples were dried overnight (16 h) by vacuum centrifugation (Savant®, SPD121P, Thermo Scientific) at room temperature and the vials were closed under an argon atmosphere using magnetic crimp caps. The dried samples were derivatized online as described by Carreno-Quintero et al. (2012). First, 12.5 µl of O-methylhydroxylamine hydrochloride (20 mg/mL pyridine; Sigma Aldrich, cat. no. 593-56-6) was added and incubated for for 30 min at 40 °C with agitation. The samples were then derivatized by adding 17.5 µl of N-methyl-N-(trimethylsilyl)trifluoroacetamide) (MSTFA reagent) (Sigma Aldritch) and incubated for 60 min at 40 °C. Five microliters of an alkane series (C10-C32) was added to each sample prior to injection using a TriPlusRSH autosampling/injection robot (Thermo Scientific) and analysed using a GC–MS system consisting of a Trace 1300 gas chromatograph (Thermo Scientific) coupled to a TSQ8000 DUO-series triple quadrupole mass spectrometer (Thermo Scientific). One microliter of sample was introduced to the PTV injector at 70 °C using a split flow of 19 ml/min and the injector temperature rapidly raised to 240 °C. Chromatographic separation was on a VF-5 ms capillary column (Varian; 30 m × 0.25 mm i.d. × 0.25 µm film thickness) including a 10-m guardian column with helium as carrier gas at a column flow rate of 1 ml/min. The GC oven was run with the following gradient: 70 °C for 2 min, 10 °C/minute increase to 310 °C with a 10 min hold. The column effluent was ionized by electron impact at 70 eV. The mass spectrometer was used in full scan mode with a scanning range of m/z 50–600. A solvent delay of 420 s was set.
2.10. Data processing and multivariate statistical analysis of GC–MS data
Unbiased picking of mass peaks and alignment of the raw data sets from GC–MS were carried out separately for each sample type using MetAlign software (Lommen, 2009). Mass features present in < 3 samples were filtered out and non-detects were replaced by a random value of 45 % − 55 % of the local noise as calculated by MetAlign, using an in-house script called MetAlign Output Transformer – METOT (Houshyani et al., 2012). The remaining mass peaks, including molecular ions, fragments and their natural isotopes, were then clustered using MSClust software into so-called reconstructed metabolites (Tikunov et al., 2012), according to their retention times and peak intensity patterns across samples. For annotation of metabolites, the mass spectra were compared with available EI-spectral libraries, including the NIST2017 and the Golm spectral database (http://gmd.mpimp-golm.mpg.de/), as well as an in-house library of derivatized authentic standards characterized in previous experiments. In addition, retention indices (RI) were calculated based on the alkane series (C10-C32) and calculated RIs were compared with published RIs. Annotations were manually evaluated and only confident matches were retained. Annotation of compounds followed the rules of Sumner et al. (2007). The relative intensities of the reconstructed metabolites were used for statistical analyses.
2.11. Statistical analysis
Principal Component Analysis (PCA) was performed in SIMCA15.0.2 (Sartorius Stedim Data Analytics AB, Sweden). Boxplots were created in R (Version 4.0.2) using the ggplot2 package. The means and standard errors are given in Supporting Material Tables S3, S4 and S5.
ANOVA analysis was performed in Genstat 20. The block structure used was flour/sample/subsample/pseudo to account for the nested sampling structure where six separate samples of each of the three flour mixes were used for the doughs (3 sourdough, 3 yeast), subsamples were taken from each sample (dough) at each of the different stages (dough and bread) and 'pseudo'-replicates were taken from each of the flours before processing into dough and bread).
The treatment structure used was type*(processed/(stage*dough)) where type indicates the three grain types, processed indicates whether the sample is from processed (i.e. dough or bread) or unprocessed flour, stage further distinguishes the samples as either dough or bread and dough indicates the type of dough (sourdough or yeast). This results in the following tests:
-
1.
Grain type: This is to determine overall differences between the three grain types (emmer, spelt and bread wheat) (across all stages). However, because only single flour blends of each wheat type were used, with no independent replicate, the significance of differences between the three blends could not be tested.
-
2.
Processed: are there differences between the compositions of flour and processed products (dough and bread)?
-
3.
Type.processed: are the effects of processing on composition (the differences between flour vs dough and bread) different for the 3 types of wheat?
-
4.
Processed.dough: are there differences between the compositions of sourdough and yeast dough across all wheat types
-
5.
Type.processed.dough: are the differences between the compositions of the sourdough and yeast dough similar or different for the 3 wheat types?
-
6.
Processed.Stage: are there differences in composition between dough and bread?
-
7.
Type.processed.stage: are the differences in composition between dough and bread similar or different for the 3 wheat types?
-
8.
Processed.stage.dough: are the changes in dough and bread composition influenced differently by sourdough and yeast processing. i.e is there an interaction between stage and dough?
-
9.
Type.processed.stage.dough: is the interaction between stage and dough different for the 3 wheat types?
Some variables required transformation (square root or Loge) to meet the assumptions of the analysis. Where this is the case it has been indicated in the tables.
3. Results and discussion
Emmer, spelt and bread wheat were sourced from commercial grain merchants as described above. To allow for variation in composition due to genotype and environment, and obtain a market representative sample, grains of 5 cultivars each of bread wheat and spelt and 3 cultivars of emmer (because more were not available commercially) were blended in equal weights for milling and baking. This blending reflects normal commercial practice, with samples being blended to give mixed grists.
3.1. Effects of fermentation and baking on composition
The emmer, spelt and bread wheat flours were used to prepare doughs and breads using sourdough and yeast-based systems. Because only single flour blends of each wheat species (called types below) were used it was not possible to calculate the statistical significance of differences in composition. However, the analysis of three replicate samples of doughs and breads allowed the use of ANOVA to determine the significance of differences in compositions of doughs and breads, and to determine whether these were significantly different between the sourdough and yeast systems and between the three types of wheat (types).
Tables of means for all components determined are given in Supporting Material Tables S3B and S4B. The p values of all ANOVA analyses are given in Supporting Material Tables S3A and S4A and for selected components and groups of components are given in Table 1. Significant effects of processing, and differences between the doughs and breads made using the sourdough and yeast systems, were observed for most metabolites (amino acids, organic acids, sugars) and FODMAPs (raffinose, mannitol and fructans), but the differences between the products made from the three wheat types were less pronounced. Similarly, the differences in the contents of fibre components were less and differed between individual components.
Table 1.
p values from ANOVA of contents of groups of components in flour, dough and bread of the three types of wheat.
| processed (flours v products) | type. processed (are the effects of processing the same for three wheat types) | processed.dough (sourdough v yeast dough) | type.processed.dough (are the differences between sourdough and yeast dough the same for the three wheat types) | processed.stage (dough v bread) | type.processed.stage (are the differences between dough and bread the same for three wheat types) | processed.stage.dough (are the differences between dough and bread the same for the sourdough and yeast dough) | type.processed.stage.dough (are the differences resulting from yeast and sourdough fermention the same for the three wheat types) | |
|---|---|---|---|---|---|---|---|---|
| Metabolites | ||||||||
| Amino acids | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.360 | 0.025 | 0.790 |
| Sqrt total organic acids | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | 0.101 | <0.001 | 0.214 |
| Sqrt Lactic acid | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| Loge Sugars | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.035 | <0.001 | 0.283 |
| Methyl donors | <0.001 | 0.716 | <0.001 | 0.119 | 0.068 | 0.778 | 0.014 | 0.970 |
| Glycerol | 0.01 | <0.001 | 0.17 | 0.065 | <0.001 | 0.445 | <0.001 | 0.889 |
| FODMAPs | ||||||||
| Raffinose | <0.001 | <0.001 | <0.001 | 0.041 | <0.001 | 0.722 | 0.193 | 0.006 |
| sqrt Mannitol | <0.001 | <0.001 | <0.001 | 0.064 | <0.001 | 0.588 | <0.001 | 0.066 |
| Sqrt Fructan content | <0.001 | 0.001 | <0.001 | 0.227 | <0.001 | 0.117 | 0.012 | 0.411 |
| Loge Fructan degree of polymerisation | 0.123 | 0.028 | 0.570 | 0.010 | 0.532 | 0.687 | 0.021 | 0.735 |
| Fibre components | ||||||||
| Total dietary fibre | 0.006 | 0.498 | 0.659 | 0.846 | 0.009 | 0.046 | 0.239 | 0.939 |
| Klason lignin | 0.122 | 0.410 | <0.001 | 0.312 | 0.279 | 0.238 | 0.018 | 0.197 |
| Sqrt glucose (from cellulose and β-glucan) | 0.055 | 0.916 | <0.001 | 0.330 | <0.001 | 0.440 | 0.466 | 0.398 |
| Loge arabinoxylan | 0.019 | 0.598 | 0.228 | 0.522 | 0.002 | 0.015 | 0.444 | 0.570 |
| Loge ratio WE-xylose:TOT-xylose | <0.001 | 0.247 | <0.001 | 0.803 | 0.365 | 0.491 | 0.238 | 0.174 |
| Loge TOT β-glucan | <0.001 | 0.514 | 0.002 | 0.796 | <0.001 | 0.003 | 0.006 | 0.142 |
| AGP (arabinose equivalents) | <0.001 | 0.536 | 0.040 | 0.052 | 0.364 | 0.330 | 0.002 | 0.066 |
Statistically significant values (p < 0.05) are in bold. Some variables required transformation, square root (Sqrt) or Loge, to meet the assumptions of the analysis.
3.2. Soluble metabolites
NMR spectroscopy was used to quantify a number of polar metabolites which were classified for statistical analysis into the following groups: amino acids (tryptophan, tyrosine, phenylalanine, alanine, asparagine, aspartic acid, γ-amino butyric acid (GABA), glutamic acid, glutamine, methionine, leucine, isoleucine, valine), organic acids (fumarate, lactate, succinate, malate), non-fermented sugars (glucose, arabinose, fructose, sucrose, maltose and trehalose), FODMAPs (raffinose, mannitol), methyl donors (choline and betaine) and glycerol (a polyol) (Table 1). Adenosine and trigonelline were also determined but are not discussed further because they are minor components and showed no biologically significant differences between samples (Supporting Material Table S3). Data for acetate and ethanol are not presented because of potential losses from the samples due to volatility.
More detailed analyses of organic acids and sugar alcohols were carried out by GC–MS, which identified minor components not detected by NMR spectroscopy. However, because the data from GC–MS are semi-quantitative it is not possible to combine them with the NMR datasets and they are not included in the combined values in Table 1 and Fig. 1 but are included in Supporting Material Table S3 and Supporting Material Figs. S3 and S6.
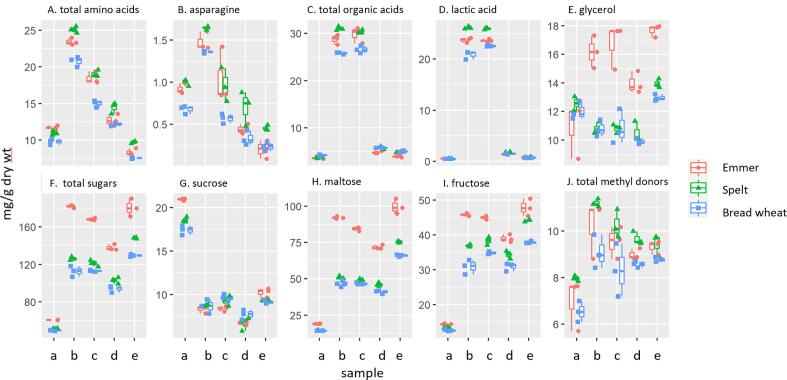
Fig. 1.
The contents of groups of polar metabolites and selected individual metabolites in the fours, doughs and breads determined by NMR spectroscopy. Samples are: a, wholemeal flour; b, sourdough dough; c, sourdough bread; d, yeast dough; e, yeast bread. The points are the values from three replicate analyses as described in the text.
3.3. Amino acids
The total content of the 13 amino acids determined by NMR was slightly higher in emmer and lower in bread wheat than in spelt. The contents increased in the doughs and then decreased in the breads and were higher in the sourdoughs and sourdough breads (called sourdough products) than in the yeast doughs and breads (yeast products), with the concentrations in the yeast breads being lower than those in the flours (Fig. 1). The changes during processing did not differ significantly between the three types of wheat and the differences due to processing were much larger than between the different types of wheat (Table 1).
The major amino acid in all of the samples was glutamate, which accounted for a third or more of the total fraction, followed by leucine, aspartate, asparagine, GABA, methionine and glutamine. These amino acids, together with alanine, isoleucine, leucine, and valine, showed broadly similar changes in concentration to the total free amino acid content. However, some differences were observed. Notably, the concentration of asparagine was higher in sourdough than in flour, similar in flour and sourdough bread, but lower in both yeast products than in flour (Fig. 1, Supporting Material Fig. S2 and Table S3). The concentration of the three aromatic amino acids, (phenylalanine, tyrosine and tryptophan) also showed broadly similar trends to the total amino acids, but the concentrations varied more between wheat types and products with higher experimental errors.
These analyses represent the free amino acids that are present in the undigested food. Larger amounts of amino acids will be released by hydrolysis of proteins and will be available for immediate absorption during human digestion.
The observed increases in the concentration of free amino acids in the doughs result from proteolysis during fermentation and the subsequent decreases in breads to their degradation or modification during baking. Modifications during baking include Maillard reactions with sugars, which in the case of asparagine lead to the formation of acrylamide, a neurotoxin and potential carcinogen (Mottram et al., 2002). The asparagine concentration is usually the limiting factor for acrylamide formation in cereal processing (Curtis and Halford, 2016). We did not measure acrylamide in the breads, but the observed concentrations of asparagine in the doughs indicate that the potential for acrylamide formation is higher after sourdough fermentation than after yeast fermentation.
The concentration of GABA was slightly higher in the yeast breads than in the flours and higher in sourdough and sourdough bread compared to yeast dough and bread. GABA is a non-proteinogenic amino acid that acts as an inhibitor of neurotransmitters and has been suggested to have anti-hypertension, antioxidant, anti-inflammation, anti-microbial, anti-allergy, and intestinal protection properties (Ngo and Vo 2019). The higher contents in the products may therefore be beneficial for human health. GABA is widely present in microorganisms, plants and vertebrates. GABA is also produced by specific lactobacilli (Cui et al 2020) and increased concentrations in sourdough breads have been noted previously (Venturi et al., 2019).
3.4. Organic acids
The total contents of organic acids (based on the sum of fumaric, lactic, malic and succinic acids) determined by NMR were similar in the three flours and increased only in the sourdoughs and sourdough breads (Fig. 1, Table 1). However, this increase largely resulted from lactic acid (Supporting Material Table S3 and Fig. S3) which is therefore included in Fig. 1 and Table 1. Succinate showed a similar pattern to lactate.
Unlike amino acids, the concentration of lactic acid did not decrease during baking of the sourdoughs. The concentration was highest in spelt and lowest in bread wheat. Only small increases were observed in the bread wheat doughs with the concentrations decreasing after baking. A total of 12 organic acids (including the four above) were determined in the flours and breads by GC–MS (Supplementary Fig. S3).
3.5. Sugars and glycerol
The monosaccharides glucose, arabinose and fructose and the disaccharides maltose, trehalose and sucrose are all readily absorbed in the small intestine. Their concentrations determined by NMR are therefore combined in Table 1 and Fig. 1. The total concentrations of these sugars are higher in yeast and sourdough products than in flours, and higher in yeast bread than in yeast dough (but not in sourdough bread compared to sourdough). The concentrations are also higher in emmer than in bread wheat or spelt, with the difference being greater in the products than in the flours.
Analysis of the individual sugars shows that maltose is the major component, followed by fructose, glucose (α- and β-forms) and sucrose, with only small amounts of trehalose and arabinose (Supporting Material Fig. S4). Of the individual sugars, only sucrose was present in higher concentrations in the flours than in the doughs and breads. However, the concentrations of the other sugars varied between wheat types and products. In particular, the emmer products had substantially higher concentrations of fructose and maltose (the two major sugars) than the products from bread wheat and spelt.
The decrease in sucrose in the doughs and breads is presumably due to its use as an energy source by yeast and lactobacilli. Maltose is the first product released by the digestion of starch by amylase and is further digested to release glucose, while fructose is released by digestion of fructans (see below). The differences in the concentrations of these three sugars (maltose, glucose, fructose) presumably result from differences in these three enzyme activities. However, because all of these sugars are readily absorbed the total contents have greater physiological relevance than the individual amounts.
Glycerol is a polyol, most of which is derived from the hydrolysis of triacylglycerols (storage lipids). Although the contents were similar in the three flours, the amount was higher in the doughs and breads from emmer than from the other cereals (Fig. 1). It was also higher in the yeast breads, but not in the doughs, from bread wheat and spelt: the reason for this not known.
Glycerol is readily absorbed in the small intestinal epithelium where it is used together with absorbed fatty acids in a re-esterification process to produce triacylglycerols.
3.6. Methyl donors
Betaine and choline are biosynthetically related components which are considered to be beneficial for cardio-vascular health, by acting as methyl donors in the homocysteine cycle. They are therefore combined in Fig. 1 but shown separately in Supplementary Fig. S5. The concentrations of methyl donors are increased in all dough and bread products compared to flour. Glycine betaine and choline sulphate display higher concentrations in spelt and emmer products compared to wheat.
3.7. Fodmaps
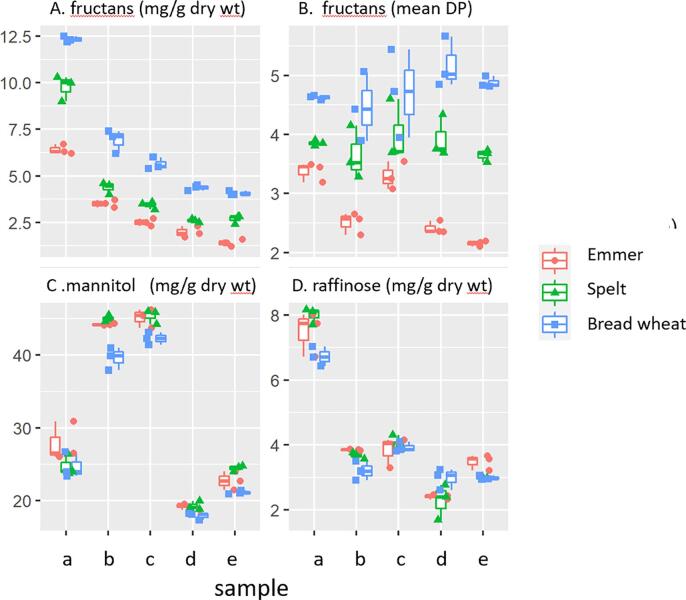
The major FODMAPs in wheat are fructans and the total concentration and the average degree of polymerisation (DP, number of sugar residues per molecule) are presented in Table 1 and Fig. 2. Both the concentrations and DP of fructans were highest in bread wheat and lowest in emmer. The total concentrations of fructans decreased significantly in all three cereals during processing, but the decreases were greater in the yeast than in the sourdough system with all three cereals. The fructan DP was similar in the flours and products of spelt and bread wheat, but the sourdough and yeast products of emmer had substantially lower DPs, indicative of more extensive degradation. This could be due to the use of a slightly higher concentration of yeast for the emmer bread (0.8% compared to 0.6%) in order to ensure an acceptable product.
Fig. 2.
The contents of FODMAPs (fructans, mannitol and raffinose) and the average degree of polymerization (DP) of fructans in the flours, doughs and breads. Samples are: a, wholemeal flour; b, sourdough dough; c, sourdough bread; d, yeast dough; e, yeast bread. The points are the values from three replicate analyses as described in the text.
The second major FODMAP in wheat is the trisaccharide raffinose (galactose, glucose, fructose). The concentrations of raffinose were similar in the three flours and decreased significantly on processing, with the decreases also being greater in the yeast system than in the sourdough system (Table 1, Fig. 2).
Mannitol is a fermentable sugar alcohol which is present in small amounts in wheat grain. The concentration was increased in the sourdough system, particularly with the emmer and spelt flours (Fig. 1, Table 1), but decreased in the yeast doughs and breads. It has been reported that mannitol is synthesised in sourdough from fructose released from the hydrolysis of fructans, catalysed by enzymes released by lactobacilli (Loponne and Gânzle, 2018).
Small amounts of other sugar alcohols were also present and determined by GC–MS (Supporting Material Fig. S6). Arabitol, lactitol and melibiose/gentiobiose were higher in both yeast and sourdough products compared to flours, threitol in sourdough products only and sorbitol showed no differences between the flours and products.
Wheat also contains other fermentable soluble fibre components, notably arabinoxylan (AX) and β-glucan, which are discussed below.
3.8. Multivariate analysis of metabolites
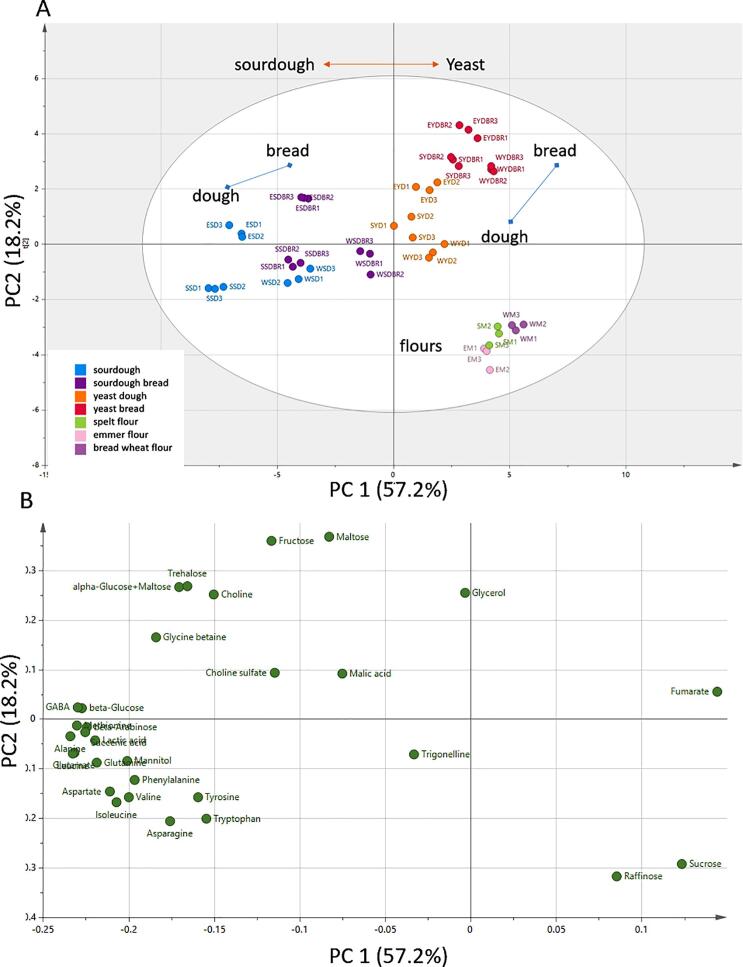
Multivariate analysis of the metabolites determined by NMR spectroscopy was carried out by principal component analysis (PCA), where the first 4 PCs (principal components) accounted for 90% of the total variation. A plot of principal components (PCs) 1 and 2, which corresponded to 57.2% and 18.2% of the total variation, respectively, showed clear groupings of the flours, doughs and breads (Fig. 3A), with the only overlap being between the spelt sourdough bread and wheat sourdough. The scores plot (Fig. 3B) shows the major components responsible for the separations, notably higher levels of sucrose and raffinose in the flours and of mannitol, amino acids and organic acids in the sourdough products.
Fig. 3.
Principal component analysis (PCA) of polar metabolites determined by NMR spectroscopy. A, scores plot, B, loadings plot.
3.9. Fibre components
Dietary fibre is defined as comprising “carbohydrate oligomers and polymers that are not digested and absorbed in the small intestine and are partially or completely fermented in the colon. It also includes associated components such as lignin” (Jones, 2014). Wholegrain wheat contains 11.5% to 15% total dietary fibre (TDF), comprising lignin, cellulose, arabinoxylan, (1 → 3,1 → 4)-β-d-glucans (β-glucan, also called mixed linkage glucan), fructans, arabinogalactan peptide, and minor cell wall polysaccharides. Starch may be converted during food processing into retrograded type 3 resistant starch (RS) which forms part of the dietary fibre fraction.
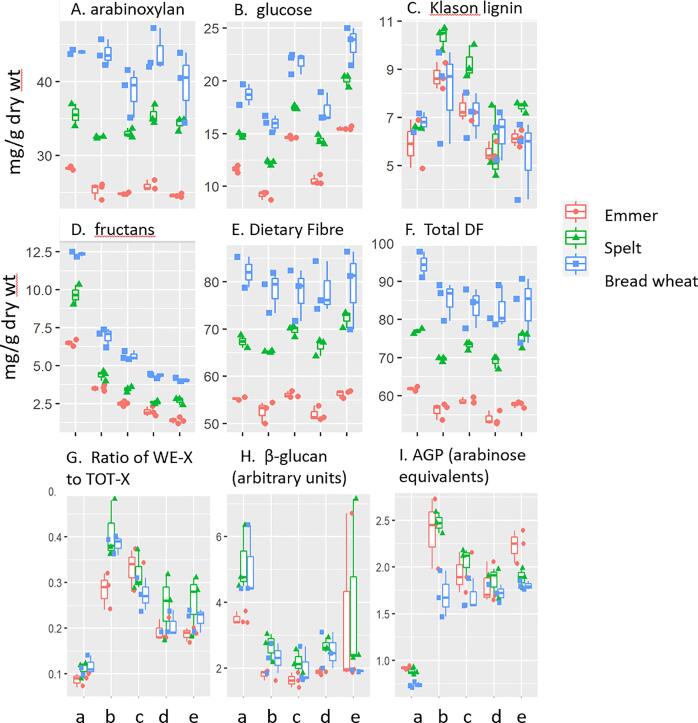
Fig. 4 summarise the analyses of the major DF components, with individual components determined by the Uppsala method shown in Supporting Material Fig. S7.
Fig. 4.
Contents of dietary fibre components in the flours, doughs and breads. A-E, Major groups determined using the Uppsala method. Glucose includes resistant starch, β-glucan and cellulose. Dietary fibre (DF) is determined by the Uppsala method and total dietary fibre (TDF) is DF plus fructans. Arabinoxylan is calculated from arabinose, xylose and galactose residue values assuming that the arabinose to xylose ratio in arabinogalactan peptide is 0.69. F, The ratio of water-extractable xylose (WE-X) to total xylose (TOT-X) in polysaccharides is determined after acid hydrolysis and provides a measure of arabinoxylan. G, the amount of β-glucan measured as arbitrary units determined by HP-AEC. H, arabinogalactan peptide (AGP) measured as arabinose equivalents (mg/g dry wt) by 1H NMR spectroscopy. Samples are: a, wholemeal flour; b, sourdough dough; c, sourdough bread; d, yeast dough; e, yeast bread.
The flour samples differed substantially in TDF, from 6.2% in emmer to 7.7% in spelt and 9.4% in bread wheat (Fig. 4F). These differences were also reflected in the products, all of which had lower contents of TDF than the flours. The breads produced from emmer and spelt had higher TDF contents than the corresponding doughs which may result from the formation in bread of type 3 resistant starch (RS), which contributes to glucose determined using the Uppsala method. Glucose (derived from RS, cellulose and β-glucan) was also higher all of the breads and the fact that this was not reflected in the TDF content of the bread wheat products may result from experimental errors (which were higher for the bread wheat products) Fig. 4B).
Lignin is highly resistant to chemical treatments and “Klason lignin” is determined as the residue after hydrolysis of polysaccharides and proteins. The fraction would therefore contain other components with similar resistance to hydrolysis (such as tannins which are minor components in whole grain). The concentrations of “Klason lignin” were broadly similar in the flours and yeast products but higher in the sourdough products, particularly those made from spelt (Fig. 4C). It is therefore likely that these increases resulted from other resistant components either present in or secreted by the sourdough microorganisms.
Arabinoxylan is the major cell wall polysaccharide in wheat, accounting for about half of the TDF in whole grain (Andersson et al., 2013). AX occurs in soluble (water-extractable, WE) and insoluble forms, which differ in their behaviour during processing and in the human gastro-intestinal (GI) tract (Courtin and Delcour, 2002, Gill et al., 2021). The concentration of AX was calculated by combining the concentrations of arabinose and xylose, after adjusting the former for arabinose present in AGP (Andersson et al., 2013).
The AX concentrations in flour showed similar differences to TDF, being substantially higher in bread wheat and lower in emmer (Fig. 4A). The concentration decreased in the wheat breads but not in the doughs, with no differences between the sourdough and yeast systems. By contrast, the total concentration of AX was lower in all products made from emmer and in the sourdough products only from spelt.
The proportion of WE-AX was determined by comparing the contents of xylose (which is only present in significant quantity in AX in wheat grain) in WE and total (TOT) samples. The ratio of WE:TOT-AX was slightly higher in bread wheat and spelt than in emmer (Fig. 4G) and increased in all products, particularly in the sourdough and bread. This increase could result from the action of endoxylanase enzymes present in the grain samples, or from release of AX polymers from the cell wall matrix.
β-glucan is a polymer of glucose and therefore contributes to the total glucose determined by the Uppsala method. In order to provide a separate measurement, it was also determined as a separate component by enzyme fingerprinting (which is discussed in further detail below). This determines the amounts in arbitrary units which cannot be directly compared with the glucose determined by the Uppsala method. The fingerprinting data (Fig. 4H) showed higher concentrations of β-glucan in bread and spelt wheats than in emmer, with the concentrations decreasing in the doughs and breads made thereof.
In addition to AX and β-glucan, wheat grain also contain small amounts of other cell wall polysaccharides (pectins, xyloglucan, callose, glucomannan) (Palmer et al, 2015), although only glucomannan is present in sufficient amounts to be determined by direct biochemical analysis. There was a clear increase in the concentration of mannose released by hydrolysis in the yeast doughs and breads (Supplementary Fig. S7) which may have been derived from mannoproteins in the yeast cell walls.
The final fibre component determined was AGP, which consists of a short (15 residue amino acid) peptide with three hydroxyproline residues which are o-glycosylated with branched arabinogalactan chains. The basic structure of the AG moiety has been determined (Tryfona et al., 2010) but the precise structures of the individual chains are not known and are likely to vary. NMR spectroscopy was used to determine the abundances of terminal arabinose and galactose residues in AGP, allowing it to be quantified as “arabinose equivalents” and “galactose equivalents” with structural variation determined as the ratio of these two measurements.
The individual flour samples did not differ significantly in the amounts of AGP expressed as arabinose equivalents (Fig. 4I) or galactose equivalents (Supporting Material Fig. S8), or the ratio of these residues (Supporting Material Fig. S8) but the concentrations were significantly higher in all of the products (doughs and breads).
3.10. AX and β-glucan structure
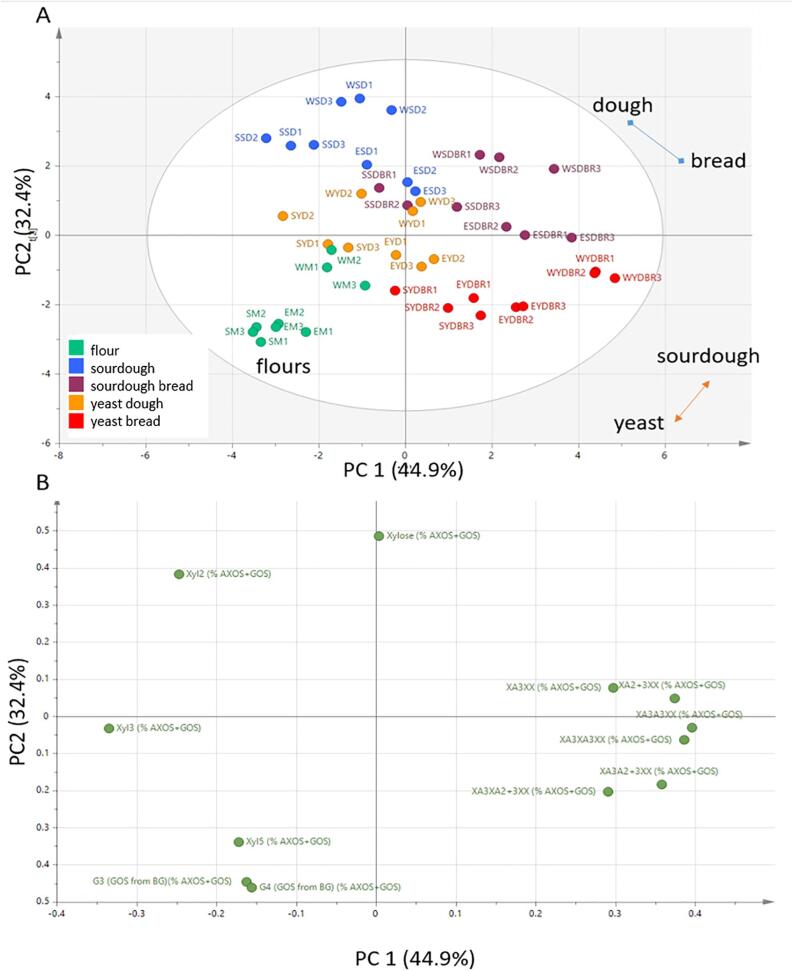
Variation in the structures of AX and β-glucan was determined by “enzyme fingerprinting”, using a mixture of endoxylanase and lichenase (β-glucanase). Endoxylanases release arabinoxylan oligosaccharides (AXOS) which differ in their size and structure, including the proportions and distribution of xylose residues substituted with one or two arabinose residues. Lichenase releases gluco-oligosaccharides (GOS), mostly the trisaccharide (G3) and tetrasaccharide (G4), which reflects the ratio of β(1–3) to β(1–4) bonds. The proportions of the individual AXOS and GOS in the flours and products are shown in Supporting Material Fig. S9 and statistical analyses in Supporting Material Table S1.
PCA analysis showed that only three PCs accounted for 90% of the total variation. A plot of PCs 1 (44.9% of total variation) and 2 (34.4% of total variation) (Fig. 5A) showed broad separation of the flours and products, with the flours being characterised by higher proportions of GOS and X5 (5 xyloses), the sourdoughs higher proportions of X (xylose) and X2 (xylobiose), and the yeast and sourdough breads higher proportions of substituted AXOS (Fig. 5B).
Fig. 5.
Principal component analysis (PCA) of the proportions (% total) of oligosaccharides released by enzyme digestion of arabinoxylan (AXOS) and β-glucan (GOS), showing variation in the structures of these two major fibre components. A, scores plot, B, loadings plot.
4. General discussion
We have compared metabolites and fibre components in wholemeal flour, dough and bread of emmer, spelt and bread wheat, using two fermentation systems. The flour samples were milled from mixed grists of commercially sourced samples of five bread wheat, five spelt and three emmer cultivars, and hence should represent the types of wheat available to processors in Western Europe. However, they were not grown under defined conditions and it is not possible to discriminate between the effects of genotype and environment on composition. Hence, the differences in composition cannot be assumed to apply to the three types of wheat in general. Nevertheless, some clear differences were apparent.
Most notably, bread wheat contained a higher concentration of TDF than spelt which was in turn higher than in emmer. These differences resulted mainly from differences in the content of arabinoxylan, the major cell wall polysaccharide and DF component of wheat grain. These differences are consistent with a meta-analysis of DF in wheats, which showed that bread wheat, in contrast to what is often suggested, consistently had higher concentrations of TDF than ancient wheats including emmer and spelt (Shewry and Hey, 2015). The same meta-analysis showed similar contents of β-glucan in bread wheat and spelt, with agrees with the present study (although emmer had a lower content).
The bread wheat flour studied here contained a substantially higher content of fructans than the spelt and emmer flours, the latter having the lowest content. These differences are greater than those reported by Ziegler et al (2016b), who showed that the concentrations of fructans in bread wheat and spelt overlapped, with the mean concentration being higher in bread wheat, and that both were significantly higher than in emmer. Similar results were reported for 29 spelts and 11 bread wheats by Escarnot et al (2015). By contrast, Longin et al (2020) showed that 21 varieties of bread wheat and 9 of emmer had similar contents of fructans while the content in 9 varieties of spelt was significantly higher.
Processing of the three types of wheat using sourdough and yeast systems for breadmaking showed broadly similar changes (from flour to dough to bread). These are described in detail above and included previously described increases in organic acids and mannitol in the sourdough system and increases in amino acids and sugars (glucose, fructose and maltose) released by hydrolysis of proteins and starch in both processing systems. However, several aspects are worthy of further discussion.
The effects of processing on FODMAP content is of interest in relation to developing diets for IBS and fermentation has been widely proposed as a strategy for reducing FODMAP concentrations. The concentrations of fructans and raffinose (the major endogenous FODMAPs) were reduced by yeast and sourdough fermentation, with yeast having the greater effect. However, the relative effects of the two systems cannot be extrapolated to other sourdough systems which vary widely in their microbial composition and effectiveness at degrading FODMAPs (Loponen and Gänzle, 2018). In addition, sourdough fermentation results in the synthesis of mannitol (a fermentable polyol) from fructose (Wisselink et al., 2002). Mannitol is a recognised component of sourdoughs and the concentration in breads can be minimised by controlling the raw material, the use of mannitol-fermenting lactobacilli and the fermentation time (reviewed by Loponen and Gänzle, 2018).
Although the three types of wheat were broadly similar in the effects of processing on composition, emmer showed some differences from bread wheat and spelt. In particular, both fermentation systems resulted in greater increases in sugars (excepting sucrose which decreased in all cereals) and glycerol in the emmer than in bread and spelt wheats: we do not know the reason for this.
Glycerol is readily absorbed in the small intestinal epithelium where it is partly used together with absorbed fatty acids in a re-esterification process to produce triacylglycerols. Remaining non-esterified glycerol will enter the circulation, most of which will be taken up by muscle to serve re-esterification and triacylglycerol storage.
Finally, our detailed analysis of DF indicated the presence of additional components derived from or secreted by the yeast and bacteria used for fermentation. There was an increase in unhydrolyzed components determined as Klason lignin in the sourdough system but not in the yeast system. This is perhaps surprising because the cell walls of yeast contain chitin (Lipke and Ovalle, 1998) which would be present in the Klason lignin fraction. By contrast, neither chitin nor other non-hydrolysable products are present in the cell walls of lactobacilli. Similarly, the concentration of arabinogalactan measured by NMR spectroscopy increased in all products, in some cases by more than two-fold. These increases are much greater than those reported by Loosveld et al (1988), who showed that the amount of “water-extractable” AGP increased by about 10% during breadmaking using a yeast system and noted that “as it is generally assumed that wheat flour does not contain water-unextractable AGP the increased solubility should be ascribed to physical phenomena”.
The important question is, of course, whether the differences in composition (between wheat types and products) reported here have implications for human health and adverse reactions to wheat. Most of the metabolites studied are present at low concentrations and the differences are likely to have little or no impact. However, differences in the concentrations of two groups of components may have significant effects: total dietary fibre (determined mainly by arabinoxylan concentration) and fructans, with both being present at significantly higher concentrations in bread wheat and lower concentrations in emmer. Furthermore, the relative differences in fructan concentration of the grains were retained in the doughs and breads, although the concentrations were significantly reduced by fermentation.
It can be concluded from these studies that the differences in the compositions of wholemeal flours of the three types of wheat are much smaller than the changes in composition which occur during breadmaking, and the differences between the compositions of doughs and breads made using the yeast and sourdough systems. The changes in composition during processing are essentially similar for the three types of wheat with only small differences between the compositions of the resulting products.
CRediT authorship contribution statement
Peter R. Shewry: Conceptualization, Supervision, Writing – original draft, Writing – review & editing, Project administration. Antoine H.P. America: Investigation, Formal analysis, Supervision, Writing – original draft, Writing – review & editing, Project administration. Alison Lovegrove: Investigation, Formal analysis, Supervision, Writing – review & editing. Abigail J. Wood: Formal analysis. Amy Plummer: Formal analysis. Jessica Evans: Formal analysis, Writing – original draft, Writing – review & editing. Hetty C. van den Broeck: Formal analysis. Luud Gilissen: Conceptualization, Writing – review & editing, Project administration. Roland Mumm: Formal analysis. Jane L. Ward: Investigation, Supervision, Writing – review & editing. Zsuzsan Proos: Investigation, Formal analysis, Supervision. Petra Kuiper: Formal analysis. C. Friedrich H. Longin: Supervision, Writing – review & editing. Annica A.M. Andersson: Formal analysis, Writing – review & editing. Jan Philip van Straaten: Conceptualization, Supervision, Writing – review & editing, Project administration. Daisy Jonkers: Conceptualization, Supervision, Project administration. Fred Brouns: Conceptualization, Supervision, Writing – original draft, Writing – review & editing, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The work forms part of the “Well on Wheat?” project which is financed by a grant of the Dutch Government –“TKI- Top Knowledge Institute” (TKI1601P01) and a wide range of additional partners* from the Agri-Food chain who donated unrestricted research grants. *These partners are: AB-Mauri bakery Ingredients, Made, Netherlands; Borgesius Holding BV-Albert Heijn, Stadskanaal Netherlands; CSM innovation Bakery Center, Bingen, Germany; CYMMIT, Texcoco, Mexico; DSM Food Specialties, Delft, Netherlands; Fazer Bakeries Oy, Helsinki, Finland; Health Grain Forum, Vienna, Austria; ICC- Intl. Vienna, Austria; IWGA, Kansas 66210, USA; Lantmännen EK, Stockholm, Sweden; Mondelez, Saclay, France; Nederlands Bakkerij Centrum, Wageningen, Netherlands; Baking Industry Research Trust, Wellington, New Zealand; Nutrition et Sante, Revel, France; Puratos BV, Groot Bijgaarden, Belgium; Rademaker BV-Bakery equipments, Culemborg, Netherlands; Sonneveld Group BV, Papendrecht, Netherlands; Zeelandia- HJ Doeleman BV, Zierikzee, Netherlands, The project is coordinated and executed by an academic research consortium team (ARCT). Research specialists from funding partners, represented in a Research Steering Team (RST), are able to offer advice but decisions on design, execution and data interpretation and scientific publications are exclusively taken by ARCT.
We thank Alice Bellisai and Gianluca Ruvo (Rothamsted Research) for the sample extraction and metabolite data collection. Rothamsted Research receives strategic funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and the work forms part of the Designing Future Wheat strategic programme (BB/P016855/1).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.foodchem.2021.131710.
Contributor Information
Peter R. Shewry, Email: peter.shewry@rothamsted.ac.uk.
Antoine H.P. America, Email: twan.america@wur.nl.
Alison Lovegrove, Email: alison.lovegrove@rothamsted.ac.uk.
Amy Plummer, Email: amy.plummer@live.co.uk.
Jessica Evans, Email: jess.evans@rothamsted.ac.uk.
Hetty C. van den Broeck, Email: hetty.busink-vandenbroeck@wur.nl.
Luud Gilissen, Email: luud.gilissed@wurl.nl.
Roland Mumm, Email: roland.mumm@wur.nl.
Jane L. Ward, Email: jane.ward@rothamsted.ac.uk.
Zsuzsan Proos, Email: zsuzsan@futurefoodnext.nl.
Petra Kuiper, Email: petra.kuiper@petron.eu.
C. Friedrich H. Longin, Email: friedrich.longin@uni-hohenheim.de.
Annica A.M. Andersson, Email: annica.andersson@slu.se.
Daisy Jonkers, Email: d.jonkers@maastrichtuniversity.nl.
Fred Brouns, Email: fred.brouns@maastrichtuniversity.nl.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Andersson A.A.M., Andersson R., Piironen V., Lampi A.-M., Nyström L., Boros D.…Åman P. Contents of dietary fibre components and their relation to associated bioactive components in whole grain wheat samples from the HEALTHGRAIN diversity screen. Food Chemistry. 2013;136:1243–1248. doi: 10.1016/j.foodchem.2012.09.074. [DOI] [PubMed] [Google Scholar]
- Arora K., Ameur H., Polo A., Di Cagno R., Rizzello C.G., Gobbetti M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends in Food Science & Technology. 2021;108:71–83. doi: 10.1016/j.tifs.2020.12.008. [DOI] [Google Scholar]
- Barrett J.S., Gibson P.R. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals. Therapeutic Advances in Gastroenterology. 2012;5:261–268. doi: 10.1177/1756283X11436241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno-Quintero N., Acharjee A., Maliepaard C., Bachem C.W.B., Mumm R., Bouwmeester H.…Keurentjes J.J.B. Untargeted metabolic quantitative trait loci analyses reveal a relationship between primary metabolism and potato tuber quality. Plant Physiology. 2012;158:1306–1318. doi: 10.1104/pp.111.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin C.M., Delcour J.A. Arabinoxylans and endoxylanases in wheat flour breadmaking. Journal of Cereal Science. 2002;35:225–243. doi: 10.1006/jcrs.2001.0433. [DOI] [Google Scholar]
- Cui Y., Miao K., Niyaphorn S., Qu X. Production of gamma-aminobutyric acid from lactic acid bacteria: a systematic review. International Journal of Molecular Sciences. 2020;21:995. doi: 10.3390/ijms21030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis T.Y., Halford N.G. Reducing the acrylamide-forming potential of wheat. Food and Energy Security. 2016;5:153–164. doi: 10.1002/fes3.85. [DOI] [Google Scholar]
- Escarnot E., Dornez E., Verspreet J., Agneessens R., Courtin C.M. Quantification and visualization of dietary fibre components in spelt and wheat kernels. Journal of Cereal Science. 2015;62:124–133. doi: 10.1016/j.jcs.2015.01.003. [DOI] [Google Scholar]
- Gill, S.K., Rossi, M., Bajka, B., & Whelan, K. (2021). Dietary fibre in gastrointestinal health and disease. Nature Reviews Gastroenterology and Hepatology, 18, 101-116. https://doi.org/ 10.1038/s41575-020-00375-4. [DOI] [PubMed]
- Houshyani B., Kabouw P., Muth D., de Vos R.C.H., Bino R.J., Bouwmeester H.J. Characterization of the natural variation in Arabidopsis thaliana metabolome by the analysis of metabolic distance. Metabolomics. 2012;8:131–145. doi: 10.1007/s11306-011-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Schuppan D., Rojas Tovar L.E., Zevallos V.F., Loponen J., Gänzle M. Sourdough Fermentation degrades wheat alpha-amylase/trypsin inhibitor (ATI) and reduces pro-inflammatory activity. Foods. 2020;9:943. doi: 10.3390/foods9070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh B.-L., Palmer L., Mather D.E., Wallwork H., Graham R.D., Welch R.M., Stangoulis J.C.R. Genotypic variation in wheat grain fructan content revealed by a simplified HPLC method. Journal of Cereal Science. 2008;48:369–378. doi: 10.1016/j.jcs.2007.10.004. [DOI] [Google Scholar]
- Jones, J. M. (2014). CODEX-aligned dietary fibre definitions help to bridge the “fibre gap”. Nutrition Journal, 13, 34. https://doi.org/www.nutritionj.com/content/13/1/34. [DOI] [PMC free article] [PubMed]
- Kosik O., Romero M.V., Bandonill E.H., Abilgos-Ramos R.G., Sreenivasulu N., Shewry P., Lovegrove A. Diversity of content and composition of cell wall-derived dietary fibre in polished rice. Journal of Cereal Science. 2020;96 doi: 10.1016/j.jcs.2020.103122. [DOI] [Google Scholar]
- Lipke P.N., Ovalle R. Cell wall architecture in yeast: new structure and new challenges. Journal of Bacteriology. 1998;180:3735–3740. doi: 10.1128/JB.180.15.3735-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisec J., Schauer N., Kopka J., Willmitzer L., Fernie A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nature Protocols. 2006;1:387–396. doi: 10.1038/nprot.2006.59. [DOI] [PubMed] [Google Scholar]
- Lommen A. Metalign: Interface-driven, versatile metabolomics tool for hyphenated full-scan mass spectrometry data preprocessing. Analytical Chemistry. 2009;81:3079–3086. doi: 10.1021/ac900036d. [DOI] [PubMed] [Google Scholar]
- Longin C.F.H., Ziegler J., Schweiggert R., Koehler P., Carle R., Würschum T. Comparative study of hulled (einkorn, emmer and spelt) and naked wheats (durum and bread wheat): Agronomic performance and quality traits. Crop Science. 2016;56:302–311. doi: 10.2135/cropsci2015.04.0242. [DOI] [Google Scholar]
- Longin C.F.H., Beck H., Gütler A., Gütler H., Heilig W., Zimmermann J.…Würschum T. Influence of wheat variety and dough preparation on FODMAP content in yeast-leavened wheat breads. Journal of Cereal Science. 2020;95 doi: 10.1016/j.jcs.2020.103021. [DOI] [Google Scholar]
- Loosveld A.-M.-A., Maes C., Grobet P.J., Delcour J.A. Quantitative and qualitative study of arabinogalactan-peptide during bread making. Journal of Agricultural and Food Chemistry. 1998;46:5026–5030. doi: 10.1021/jf9805350. [DOI] [Google Scholar]
- Loponen J., Gänzle M.G. Use of sourdough in low FODMAP baking. Foods. 2018;7:96. doi: 10.3390/foods7070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovegrove A., Wilkinson M.D., Freeman J., Pellny T., Tosi P., Saulnier L.…Mitchell R.A.C. RNA interference suppression of genes in glycosyl transferase families 43 and 47 in wheat starchy endosperm causes large decreases in arabinoxylan content. Plant Physiology. 2013;163:95–107. doi: 10.1104/pp.113.222653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A., Eslick E.M., Eslick G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. European Journal of Nutrition. 2016;55:897–906. doi: 10.1007/s00394-015-0922-1. [DOI] [PubMed] [Google Scholar]
- Mottram D.S., Wedzicha B.L., Dodson A.T. Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- Ngo D.-H., Vo T.S. An updated review on pharmaceutical properties of gamma-Aminobutyric Acid. Molecules. 2019;24(15):2678. doi: 10.3390/molecules24152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, R., Shewry, P.R., Knox, P., & Tosi, P. (2015). Comparative in situ analyses of cell wall matrix polysaccharide dynamics in developing rice and wheat grain. Planta, 241, 669-685.https://doi.org/10.1007/s00425-014-2201-4. [DOI] [PMC free article] [PubMed]
- Ribeiro M., Martinez-Quijano M., Nunes F.M., Carillo J.M., Branlard G., Igrejas G. New insights into wheat toxicity: Breeding did not seem to contribute to a prevalence of potential coeliac disease’s immunostimulatory epitopes. Food Chemistry. 2016;213:8–18. doi: 10.1016/j.foodchem.2016.06.043. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ramiro I., Brearley C.A., Bruggaber S.F.A., Perfecto A., Shewry P.R., Fairweather-Tait S. Assessment of iron bioavailability from different bread making processes using an in vitro intestinal cell model. Food Chemistry. 2017;228:91–98. doi: 10.1016/j.foodchem.2017.01.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewry P.R. Do ancient types of wheat have health benefits compared with modern bread wheat? Journal of Cereal Science. 2018;79:469e476. doi: 10.1016/j.jcs.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewry, P.R., Corol, D., Jones, H.D., Beale, M.H., & Ward, J.L. (2017). Defining genetic and chemical diversity in wheat grain by 1H-NMR spectroscopy of polar metabolites. Molecular Nutrition and Food Research, 61, 1600807. https://doi.org/ 10.1002/mnfr.201600807. [DOI] [PMC free article] [PubMed]
- Shewry P.R., Hey S. Do “ancient” wheat species differ from modern bread wheat in their contents of bioactive components? Journal of Cereal Science. 2015;65:246–253. doi: 10.1016/j.jcs.2015.07.014. [DOI] [Google Scholar]
- Struyf N., Laurent J., Verspreet J., Verstrepen K.J., Courtin C.M. Saccharomyces cerevisiae and Kluyveromyces marxianus co-cultures allow reduction of fermentable oligo-, di-, and monosaccharides and polyols levels in whole wheat bread. Journal of Agricultural and Food Chemistry. 2017;65:8704–8713. doi: 10.1021/acs.jafc.7b02793. [DOI] [PubMed] [Google Scholar]
- Sumner L.W., Amberg A., Barrett D., Beale M.H., Beger R., Daykin C.A.…Viant M.R. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI) Metabolomics. 2007;3:211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theander O., Åman P., Westerlund E., Andersson R., Pettersson D. Total dietary fibre determined as neutral sugar residues, uronic acid residues, and Klason Lignin (The Uppsala Method): Collaborative study. Journal of AOAC International. 1995;78:1030–1044. doi: 10.1093/jaoac/78.4.1030. [DOI] [PubMed] [Google Scholar]
- Tikunov Y.M., Laptenok S., Hall R.D., Bovy A., de Vos R.C.H. MSClust: A tool for unsupervised mass spectra extraction of chromatography-mass spectrometry ion-wise aligned data. Metabolomics. 2012;8:714–718. doi: 10.1007/s11306-011-0368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryfona T., Liang H-C., Kotake T., Saneko S., Marsh J., Lovegrove A.…Dupree P., et al. Carbohydrate structural analysis of wheat flour arabinogalactan protein. Carbohydrate Research. 2010:2648–2656. doi: 10.1016/j.carres.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Venturi M., Galli V., Pini N., Guerrini L. Use of selected lactobacilli to increase gamma-aminobutyric acid content in sourdough bread enriched in amaranth flour. Foods. 2019;8 doi: 10.3390/foods8060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspreet J., Hemdane S., Dornez E., Cuyvers S., Pollet A., Delcour J.A. Analysis of storage and structural carbohydrates in developing wheat (Triticum. aestivum L.) grains using quantitative qnalysis and microscopy. Journal of Agricultural and Food Chemistry. 2013;61:9251–9259. doi: 10.1021/jf402796u. [DOI] [PubMed] [Google Scholar]
- Wisselink H.W., Weusthuis R.A., Eggink G., Hugenholz J., Grobben G.J. Mannitol production by lactobacteria: a reviewnnitol production by lactobacteria: a review. International Dairy Journal. 2002;12(151–161) doi: 10.1016/S0958-6946(01)00153-4. [DOI] [Google Scholar]
- Ziegler, J.U., R.M. Schweiggert, R.M., Würschum, T., Longin, C.F., & Carle, R. (2016). Lipophilic antioxidants in wheat (Triticum spp.): A target for breeding new varieties for future functional cereal products. Journal of Functional Foods, 20, 594-605. 10.1016/j.jff.2015.11.022.
- Ziegler J.U., Steiner D., Longin C.F.H., Würschum T., Schweiggert R.M., Carle R. Wheat and the irritable bowel syndrome – FODMAP levels of modern and ancient species and their retention during bread making. Journal of Functional Foods. 2016;25:257–266. doi: 10.1016/j.jff.2016.05.019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.