Abstract
Background
Paediatric flat feet are a common presentation in primary care; reported prevalence approximates 15%. A minority of flat feet can hurt and limit gait. There is no optimal strategy, nor consensus, for using foot orthoses (FOs) to treat paediatric flat feet.
Objectives
To assess the benefits and harms of foot orthoses for treating paediatric flat feet.
Search methods
We searched CENTRAL, MEDLINE, and Embase to 01 September 2021, and two clinical trials registers on 07 August 2020.
Selection criteria
We identified all randomised controlled trials (RCTs) of FOs as an intervention for paediatric flat feet. The outcomes included in this review were pain, function, quality of life, treatment success, and adverse events. Intended comparisons were: any FOs versus sham, any FOs versus shoes, customised FOs (CFOs) versus prefabricated FOs (PFOs).
Data collection and analysis
We followed standard methods recommended by Cochrane.
Main results
We included 16 trials with 1058 children, aged 11 months to 19 years, with flexible flat feet. Distinct flat foot presentations included asymptomatic, juvenile idiopathic arthritis (JIA), symptomatic and developmental co‐ordination disorder (DCD). The trial interventions were FOs, footwear, foot and rehabilitative exercises, and neuromuscular electrical stimulation (NMES). Due to heterogeneity, we did not pool the data. Most trials had potential for selection, performance, detection, and selective reporting bias. No trial blinded participants. We present the results separately for asymptomatic (healthy children) and symptomatic (children with JIA) flat feet.
The certainty of evidence was very low to low, downgraded for bias, imprecision, and indirectness.
Three comparisons were evaluated across trials: CFO versus shoes; PFO versus shoes; CFO versus PFO.
Asymptomatic flat feet
1. CFOs versus shoes (1 trial, 106 participants): low‐quality evidence showed that CFOs result in little or no difference in the proportion without pain (10‐point visual analogue scale (VAS)) at one year (risk ratio (RR) 0.85, 95% confidence interval (CI) 0.67 to 1.07); absolute decrease (11.8%, 95% CI 4.7% fewer to 15.8% more); or on withdrawals due to adverse events (RR 1.05, 95% CI 0.94 to 1.19); absolute effect (3.4% more, 95% CI 4.1% fewer to 13.1% more).
2. PFOs versus shoes (1 trial, 106 participants): low to very‐low quality evidence showed that PFOs result in little or no difference in the proportion without pain (10‐point VAS) at one year (RR 0.94, 95% CI 0.76 to 1.16); absolute effect (4.7% fewer, 95% CI 18.9% fewer to 12.6% more); or on withdrawals due to adverse events (RR 0.99, 95% CI 0.79 to 1.23).
3. CFOs versus PFOs (1 trial, 108 participants): low‐quality evidence found no difference in the proportion without pain at one year (RR 0.93, 95% CI 0.73 to 1.18); absolute effect (7.4% fewer, 95% CI 22.2% fewer to 11.1% more); or on withdrawal due to adverse events (RR 1.00, 95% CI 0.90 to 1.12).
Function and quality of life (QoL) were not assessed.
Symptomatic (JIA) flat feet
1. CFOs versus shoes (1 trial, 28 participants, 3‐month follow‐up): very low‐quality evidence showed little or no difference in pain (0 to 10 scale, 0 no pain) between groups (MD ‐1.5, 95% CI ‐2.78 to ‐0.22). Low‐quality evidence showed improvements in function with CFOs (Foot Function Index ‐ FFI disability, 0 to 100, 0 best function; MD ‐18.55, 95% CI ‐34.42 to ‐2.68), child‐rated QoL (PedsQL, 0 to 100, 100 best quality; MD 12.1, 95% CI ‐1.6 to 25.8) and parent‐rated QoL (PedsQL MD 9, 95% CI ‐4.1 to 22.1) and little or no difference between groups in treatment success (timed walking; MD ‐1.33 seconds, 95% CI ‐2.77 to 0.11), or withdrawals due to adverse events (RR 0.58, 95% CI 0.11 to 2.94); absolute difference (9.7% fewer, 20.5 % fewer to 44.8% more).
2. PFOs versus shoes (1 trial, 25 participants, 3‐month follow‐up): very low‐quality evidence showed little or no difference in pain between groups (MD 0.02, 95% CI ‐1.94 to 1.98). Low‐quality evidence showed no difference between groups in function (FFI‐disability MD ‐4.17, 95% CI ‐24.4 to 16.06), child‐rated QoL (PedsQL MD ‐3.84, 95% CI ‐19 to 11.33), or parent‐rated QoL (PedsQL MD ‐0.64, 95% CI ‐13.22 to 11.94).
3. CFOs versus PFsO (2 trials, 87 participants): low‐quality evidence showed little or no difference between groups in pain (0 to scale, 0 no pain) at 3 months (MD ‐1.48, 95% CI ‐3.23 to 0.26), function (FFI‐disability MD ‐7.28, 95% CI ‐15.47 to 0.92), child‐rated QoL (PedsQL MD 8.6, 95% CI ‐3.9 to 21.2), or parent‐rated QoL (PedsQL MD 2.9, 95% CI ‐11 to 16.8).
Authors' conclusions
Low to very low‐certainty evidence shows that the effect of CFOs (high cost) or PFOs (low cost) versus shoes, and CFOs versus PFOs on pain, function and HRQoL is uncertain. This is pertinent for clinical practice, given the economic disparity between CFOs and PFOs. FOs may improve pain and function, versus shoes in children with JIA, with minimal delineation between costly CFOs and generic PFOs.
This review updates that from 2010, confirming that in the absence of pain, the use of high‐cost CFOs for healthy children with flexible flat feet has no supporting evidence, and draws very limited conclusions about FOs for treating paediatric flat feet.
The availability of normative and prospective foot development data, dismisses most flat foot concerns, and negates continued attention to this topic. Attention should be re‐directed to relevant paediatric foot conditions, which cause pain, limit function, or reduce quality of life. The agenda for researching asymptomatic flat feet in healthy children must be relegated to history, and replaced by a targeted research rationale, addressing children with indisputable foot pathology from discrete diagnoses, namely JIA, cerebral palsy, congenital talipes equino varus, trisomy 21 and Charcot Marie Tooth. Whether research resources should continue to be wasted on studying flat feet in healthy children that do not hurt, is questionable. Future updates of this review will address only relevant paediatric foot conditions.
Plain language summary
Foot orthoses for treating flat feet in children
Review question
What are the benefits and harms of using foot orthoses (shoe inserts) to treat flat feet in children?
Background
Children with flat feet have a lower foot arch. When the child is standing, the foot arch looks flat against the floor, and may roll inwards, and even touch the floor. Sometimes, flat feet can cause pain, or change the way a child walks.
There are many types of non‐surgical treatments for flat feet, but unless painful, most children do not need any treatment.
Foot orthoses (FOs) or shoe inserts, muscle stretching, footwear selection, physical activity modification, and reducing body weight, may be part of an overall foot and activity management. The short‐term use of medication for pain and inflammation may be prescribed.
Study characteristics
This Cochrane Review is current to September 2021. There are 16 studies (1058 children, aged 11 months to 19 years) including three groups ‐ healthy children with painless flat feet; children with arthritis and painful flat feet; others (developmental coordination disorder; painful flat feet). The studies were conducted across the USA, Australia, India, Iran, Turkey, UK, and Republic of Korea. We found information about footwear, exercises, and different types of foot orthoses.
Results:
Comparing custom foot orthoses (CFOs) to shoes in painless flat feet:
Proportion without pain (1 trial, 106 children) at 12 months:
12% fewer children with CFOs were without pain (ranging from 26% fewer to 5.5% more)
67 out of 100 children were without pain with CFOs compared to 79 children out of 100 with shoes.
Withdrawal due to side effects (3 trials, 211 children):
3% more children with CFOs withdrew from treatment due to side effects (ranging from 4% fewer to 13% more) .
72 out of 100 children withdrew from treatment with CFOs compared with 69 children out of 100 with shoes.
Comparing prefabricated foot orthoses (PFOs) to shoes in painless flat feet
Proportion without pain (1 trial, 106 children) at 12 months:
5% fewer children with PFOs were without pain (ranging from 18.9% fewer to 12.6% more)
74 out of 100 children were without pain with PFOs compared to 79 out of 100 children with shoes.
Withdrawal due to side effects (4 trials, 338 children):
0.7% fewer children with PFOs withdrew from treatment due to side effects (ranging from 15.2% fewer to 16.6% more).
71 out of 100 children withdrew from treatment with PFOs compared with 72 out of 100 children with shoes.
Comparing CFOs to PFOs in painless flat feet
Proportion without pain (1 trial, 106 children) at 12 months:
7% fewer children with CFOs were without pain (ranging from 22.2% fewer to 11.1% more)
68 out of 100 children were without pain with CFOs compared to 74 out of 100 children with PFOs.
Withdrawal due to side effects (1 trial, 118 children):
0% fewer children withdrew from treatment with CFOs due to side effects (ranging from 9.2% fewer to 11% more).
91 out of 100 children withdrew from treatment with PFOs compared with 91 out of 100 children with PFOs.
Function, quality of life, treatment success and side effects were not reported in these trials
Quality of the evidence
In healthy children with painless flat feet, low to very low‐quality evidence shows that compared to shoes, CFOs and PFOs result in no difference in the proportion without pain or withdrawal due to side effects from treatment. The quality of the evidence is very low to low, weakening conclusions. We downgraded the certainty of the evidence because the trials were poorly conducted and there were not enough data.
Summary of findings
Background
Description of the condition
Despite decades of attention and scrutiny (Aharonson 1992; Brooks 1991; Mereday 1972; Staheli 1987), the paediatric flat foot remains a quandry for clinicians, researchers, and parents alike. It is established that some flat feet are associated with pain (Rome 2010), but not all flat feet are painful or debilitating. Debate about pre‐emptive treatment for flat feet in children has been misguided (Bresnahan 2009; D'Amico 2009; Evans 2008;Harris 2010). Hence, it is important to clarify whether any form of treatment is indicated, for children with flat feet, which are not painful. Prevalence estimates for paediatric flatfoot vary broadly. It has been reported as 44% in children aged three to six years, and 24% in children aged six years or older (Pfeiffer 2006); 70% in children aged three to four years, and 40% by five to eight years (Daneshmandi 2011); 23.5% in seven to 14 year olds (Yoosefinejad 2014); and between 2.2% and 12.3% in children aged four to 13 years (Garcia‐Rodriguez 1999).
In a study of 835 school children in Austria, Pfeiffer 2006 reported that 10% of children with flat feet were wearing foot orthoses, whilst only 1% were deemed pathological, indicating a marked over use of foot orthoses. Yan 2013 reported flatfoot in 90% of 100 normal Chinese children in Beijing, aged less than two years, and just 4% at age 10 years. Whilst different methods of assessment were used, this trend crosses both ethnicity and age groups, and is now further reinforced by both normative and prospective findings (Gijon‐Nogueron 2019; Martinez‐Nova 2018).
What has often failed to be appreciated, is the developing morphology of the paediatric foot structure, i.e. from flat to less flat across the first decade of life, with some variation (Bresnahan 2009; Evans 2008; Wenger 1989). The definition for flatfoot, whilst not universal, does find agreement across authors on the position of the heel (everted – valgoid), and the medial longitudinal foot arch (flat – convex (Capello 1998; Evans 2008; Staheli 1987; Wenger 1989). What is universal, and reasonable, is concern about pain and functional limitation that may occur with some children who have flat feet, to potentially diminish mobility, independence, and quality of life.
Markers of benign versus pathological paediatric flat feet have been identified, and assist with predicting the later symptomatic cases in older children (Evans 2021). The three markers are: (1) valgus heel, seen clinically as a greater than 10 degree resting calcaneal stance position (RCSP (Kerr 2015)); (2) talo‐navicular joint coverage angle, seen clinically (on x‐ray) as a greater than 35 degree medial talar head exposure (Moraleda 2011; Yan 2013); and (3) ankle range reduction or 'equinus', seen clinically as a less than 30 degree weight‐bearing lunge (Kim 2017).
Recent normative data, based on over 3000 healthy children, has shown that paediatric foot posture has a wide normal range across childhood, with the average Foot Posture Index (FPI) equal to +4(3), such that FPI scores within the range of +1 to +7 (1 standard deviation) include 68% of children (Gijon‐Nogueron 2019). Further, prospective data from over 1000 healthy children followed for three years, showed that at each age point, foot posture 'centralised'. This means that there were fewer pronated (flat) and highly pronated (flatter) foot types as age increased, with slight increases in supinated foot types. The greatest prospective shift was the increase of normal foot types as age increased (Martinez‐Nova 2018).
Flat feet are also commonly seen in children with diagnoses associated with indisputable risk of foot pathology, e.g. juvenile idiopathic arthritis (JIA), with a prevalence of approximately 1:5000 births; cerebral palsy, with a prevalence of approximately 1:400 births; and trisomy 21/Down syndrome, with a prevalence of approximately 1:900 births. However, the most significant paediatric foot pathologies are not actually flat feet, but feet with a high arch (cavus morphology), as affect children with congenital talipes equino varus (CTEV – prevalence approximates 1:1000 births), heritable motor neuropathies such as Charcot Marie Tooth (CMT – prevalence approximates 1:3300 births), infections such as Poliomyelitis.
Description of the intervention
Treatment options for paediatric flatfoot vary from non‐surgical to surgical approaches (Klaue 1997). The latter are rare, and are usually pursued only after failure of non‐invasive management, or for rigid flatfoot presentations (Bauer 2015). Non‐surgical interventions include advice, FOs (foot orthoses), stretching or strengthening exercises, footwear type and modifications, and less commonly, neuromuscular electrical stimulation (NMES), serial casting, weight reduction, analgesic and anti‐inflammatory medications (Blitz 2010).
Whilst FOs, per se, include a range of physical appliances, there are important distinctions between custom or bespoke FOs (CFOs), prefabricated FOs (PFOs), and customised PFOs (CPFOs).
How the intervention might work
The basic premise for foot orthoses as a treatment for paediatric flatfoot, is to promote a stable foot posture that allows an efficient gait function. By distributing forces and loads across the weight‐bearing, body‐carrying foot, joint ranges can be used effectively for gait function, without harmful stresses and strains. Paired with footwear, which is known to influence both foot stability and gait discretely, FOs are the treatment mainstay for paediatric flatfoot (Wegener 2011).
Other treatment options, such as stretching (e.g. calf musculature for ankle dorsiflexion range), specific muscle strengthening, and core muscle strengthening, have often been regarded as ‘adjunctive’ to FOs. Footwear acts as supplementary 'whole child' treatment directives in children with muscular hypotonia who also require strength and balance physical therapy (Rigoldi 2012; Weber 2014).
Footwear is frequently overlooked, and yet influences FOs as the basic ‘housing’ structure. All footwear, but especially athletic footwear, that is constructed as a structure external to the foot in gait, will influence foot functioning. Cursorily, athletic footwear is intentionally designed to stabilise the flat foot, co‐ordinate with the rectus foot, and cushion the high arch foot (Evans 2015). Hence the use of any FOs must only be subsequent to evaluation of footwear effects. This applies not only to the paediatric flat foot, but to all clinical gait evaluation, and always prior to any consideration of FOs use.
Why it is important to do this review
Most cases of paediatric flat feet fall within the range of normal findings (Gijon‐Nogueron 2019), yet there is a lack of confidence in both primary and specialist care (Carli 2012). Further, children's flat feet improve as they grow during their first 10 years (Gijon‐Nogueron 2019; Martinez‐Nova 2018).
There is clear need for accurate guidance from robust scientific evidence In this era of over‐diagnosis of disease (Moynihan 2012; Moynihan 2014; Moynihan 2017), overmedicalisation of regular range phenomena, and unnecessary treatment of normal variation (Evans 2017; Evans 2021). The cost to public health systems of unnecessary consultations, and unnecessary treatment is not insubstantial. Screening children for flat feet is unfounded, and both logically and economically refuted (Evans 2012; Wilson 1968). Most paediatric flatfoot presentations reveal flexible feet that are pain‐free. However, a flat foot that is either painful or rigid is not a normal finding, and requires both diagnosis and effective treatment. Specific subgroups of children have conditions known to predispose them to foot pain, such as JIA, increased joint laxity (e.g. Ehlers‐Danlos, Down, or Marfan syndromes), and tarsal coalitions. These may present overtly or covertly, and should be part of a differential diagnosis for painful paediatric flatfoot (Evans 2009; Evans 2021).
This review is an update of an earlier Cochrane Review, which found limited evidence from three trials on the use of non‐surgical interventions for the treatment of paediatric flatfoot (New Reference). Considering that a burden of paediatric flat feet is not universally demonstrable, and early identification has not been found to be beneficial, this review aims to provide answers as to whether a continued concern regarding flat feet in healthy children is necessary (Evans 2012).
Objectives
To assess the efficacy (benefits and harms) of foot orthoses as treatment for paediatric flat feet versus no treatment or other treatment.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and pseudo‐randomised controlled clinical trials (CCTs; using methods of allocating participants to a treatment that are not strictly random, for example date of birth, hospital record number, or alternation).
Types of participants
Since there is no universally accepted definition for paediatric flatfoot, flat feet or flatfoot are the terms used to describe a recognisable clinical foot morphology that involves several adjacent joints of the foot (Harris 2004). We included trials involving children younger than 16 years of age with a diagnosis of flat feet. Studies of various soft tissue diseases, and pain due to tendinitis, were eligible for inclusion, provided that the flat foot pain results were presented separately. Studies in which participants had heel pain, stress fractures of the metatarsals, ankle fractures, rheumatoid foot pathology, diabetic foot, or neuromuscular conditions were also eligible for inclusion, provided all children, or an identified subgroup of children, had flat feet.
We included trials that included children with asymptomatic flat feet, juvenile idiopathic arthritis (JIA; where flatfoot is a common clinical feature (Henry 2008)), or other clinical concerns.
Types of interventions
Interventions included rigid, semi‐rigid, or soft foot orthoses (FO), corrective footwear; strengthening exercises, stretching exercises, activity modification; manipulation; serial casting; weight reduction; anti‐inflammatory medication; anti‐pronatory strapping; neuromuscular electrical stimulation (NMES); and educational advice to children or their parents or guardians, and compared FOs versus sham or no intervention (control), or other non‐surgical interventions for paediatric flat feet.
Comparison were made with other interventions, and with no treatment (with deference to usual footwear in some trials). Epidemiological data regarding normal foot posture across childhood has provided a context for better clinical appreciation of normal variation, thus discouraging narrow intervention criteria (Gijon‐Nogueron 2019).
We excluded studies involving surgical interventions.
Types of outcome measures
Major outcomes
The following major outcomes will frame this review and are reported in the summary of findings tables: Table 2; Table 3; Table 4; Table 5; Table 6.
Summary of findings 2. Prefabricated foot orthoses compared to shoes in children with asymptomatic flat feet.
| Prefabricated foot orthosescompared to shoes in children with asymptomatic flat feet | ||||||
| Patient or population: children with asymptomatic flat feet Setting: outpatient hospital clinic Intervention: prefabricated foot orthoses (PFO) Comparison: shoes | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With shoes (N = 52) | With PFOs (N = 54) | Difference (absolute) | ||||
|
Pain
(measured as proportion with pain) follow‐up: 12 months № of participants: 106 (1 RCT) |
RR 0.94 (0.76 to 1.16) | 78.8% | 74.1% (59.9 to 91.5) | 4.7% fewer (18.9% fewer to 12.6% more) | ⊕⊕⊝⊝ Lowa,b | PFOs likely result in little to no difference in the proportion of children reporting pain, absolute reduction 4.7% (18.9% fewer to 12.6% more) |
| Function or disability | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Treatment success | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
|
Withdrawal due to adverse events follow‐up: 12 months № of participants: 338 (4 RCTs) |
RR 0.99 (0.79 to 1.23) | 72.3% | 71.6% (57.1% to 88.9%) | 0.7% fewer (15.2% fewer to 16.6% more) | ⊕⊝⊝⊝ Very lowa,b,c | We are uncertain of the effects of PFOs on withdrawal due to adverse events. Absolute reduction 0.7% (15.2 fewer to 16.6 more) |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Serious adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded for bias, (performance, attrition, other bias), participants, parents, and examiners not blinded; pain only assessed post hoc, as subgroup analysis; high attrition in some trials (notably Gould 1989) bDowngraded for imprecision; wide 95% CI for intervention cDowngraded for indirectness; variably aged participant samples between studies
Summary of findings 3. Custom foot orthoses compared to prefabricated foot orthoses for children with asymptomatic flat feet.
| Custom foot orthoses compared to prefabricated foot orthoses for children with asymptomatic flat feet | ||||||
| Patient or population: children with asymptomatic flat feet Setting: outpatient clinics Intervention: customised foot orthoses (CFO) Comparison: prefabricated foot orthoses (PFO) | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With PFOs (N = 54) | With CFOs (N = 54) | Difference (absolute) | ||||
|
Pain
(measured as proportion with pain) follow‐up: 12 months № of participants: 108 (1 RCT) |
RR 0.93 (0.73 to 1.18) | 74% | 68% (51.9% to 85.2%) | 7.4% fewer (22.2% fewer to 11.1% more) | ⊕⊕⊝⊝ Lowa,b | CFOs likely results in little to no difference in the proportion of children reporting pain. Absolute reduction 7.4% (22.2 % fewer to 11.1 % more) |
| Function or disability | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Treatment success | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
|
Withdrawal due to adverse events
follow up: 12 months № of participants: 118 (1 RCT) |
RR 1.00 (0.90 to 1.12) | 91.5% | 91.5% (82.4% to 100%) | 0.0% fewer (9.2% fewer to 11% more) | ⊕⊕⊝⊝ Lowa,b | The evidence suggests that CFOs do not increase or reduce withdrawal due to adverse events. |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Serious adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded for bias, (performance, other bias), participants, parents, and examiners not blinded; pain only assessed post hoc, as subgroup analysis bDowngraded for imprecision; wide 95% CI for CFO as intervention
Summary of findings 4. Custom foot orthoses compared to shoes in children with juvenile idiopathic arthritis and flat feet.
| Custom foot orthoses compared to shoes in children with juvenile idiopathic arthritis andflat feet | ||||||
| Patient or population: children with juvenile idiopathic arthritis (JIA) and flat feet Setting: outpatient rheumatology clinics Intervention: custom foot orthoses (CFO) Comparison: shoes | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With shoes (N = 13) | With CFOs (N = 15) | Difference | ||||
|
Pain
(measured on 0 to 10‐point VAS; lower = less pain) follow‐up: 3 months № of participants: 28 (1 RCT) |
The mean pain with shoes was 2.82 points | The mean pain with CFOs was 1.32 points | MD 1.5 points lower (2.78 points lower to 0.22 points lower) | ⊕⊝⊝⊝ Very lowa,b,c | CFOs likely results in little to no difference in pain. | |
|
Function or disability (measured on 0 to 100‐point FFI; 0 = no disability) follow‐up: 3 months № of participants: 28 (1 RCT) |
The mean FFI score with shoes was 34.15 points | The mean FFI score with CFOs was 15.6 points | MD 18.55 points lower (34.42 points lower to 2.68 points lower) | ⊕⊕⊝⊝ Lowa,b | CFOs may result in a clinically important improvement in function or disability. | |
|
Quality of life (child‐rated) (measured on 0 to 100‐point PedsQL; higher score = better QoL) follow‐up: 3 months № of participants: 25 (1 RCT) |
The mean child‐rated PedQL score with shoes was 59.78 points | The mean child‐rated PedQL score with CFOs was 47.68 points | MD 12.1 points higher (1.6 points lower to 25.8 points higher) | ⊕⊕⊝⊝ Lowa,c | CFOs may result in a clinically important improvement in child‐rated QoL. | |
|
Quality of life (parent‐rated) (measured on 0 to 100‐point PedsQL; higher score = better QoL) follow up: 3 months № of participants: 26 (1 RCT) |
The mean parent‐rated PedQL score with shoes was 55.95 points | The mean parent‐rated PedQL score with CFOs was 46.94 points | MD 9.01 points higher (4.08 points lower to 22.1 points higher) | ⊕⊕⊝⊝ Lowa,c | CFOs may result in a clinically important improvement in parent‐rated QoL. | |
|
Treatment success (measured on the 50FTW (seconds)) follow‐up: 3 months № of participants: 28 (1 RCT) |
The mean time for the 50FTW with shoes was 8.36 seconds | The mean time for the 50FTW with CFOs was 7.03 seconds | MD 1.33 seconds less (2.77 seconds less to 0.11 seconds more) | ⊕⊕⊝⊝ Lowa,c | CFOs likely result in little to no difference in timed walking. | |
|
Withdrawal due to adverse events
follow‐up: № of participants: 28 (1 study) |
RR 0.58 (0.11 to 2.94) | 23.1% | 13.4% (2.5% to 67.8%) | absolute difference 9.7% fewer (20.5% fewer to 44.8% more) |
⊕⊕⊝⊝ Lowa,c | CFOs likely result in little to no difference in withdrawals due to adverse events. Absolute reduction 9.7% (20.5 % fewer to 44.8% more) |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Serious adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; FFI: Foot Function Index; 50FTW: 50‐Foot Timed Walk; MD: mean difference; PedsQL: Pediatric quality of life inventory; RR: Risk ratio; VAS: visual analogue scale; QoL: quality of life | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded for bias; single blinded, children and their parents knew which treatment they had, which may have affected the assessment of pain bDowngraded for indirectness; only short‐term outcomes (3 months); FFI not validated in children; PedsQL has no foot‐related data cDowngraded for imprecision; small sample size and wide CI including both an increase and decrease in the effect estimate
Summary of findings 5. Prefabricated foot orthoses compared to shoes in children with juvenile idiopathic arthritis and flat feet.
| Prefabricated foot orthoses compared to shoes in children with juvenile idiopathic arthritis andflat feet | ||||||
| Patient or population: children with juvenile idiopathic arthritis and flat feet Setting: outpatient rheumatology clinics Intervention: prefabricated foot orthoses (PFO) Comparison: shoes | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With shoes (N = 12) | With PFOs (N = 12) | Difference | ||||
|
Pain
(measured on 0 to 10‐point VAS; lower = less pain) follow‐up: 3 months № of participants: 25 (1 RCT) |
The mean pain with shoes was 2.82 points | The mean pain with PFOs was 2.84 points | MD 0.02 points higher (1.94 points lower to 1.98 points higher) | ⊕⊝⊝⊝ Very lowa,b,c | PFOs likely result in little to no difference in pain. | |
|
Function or disability (measured on 0 to 100‐point FFI; 0 = no disability) follow‐up: 3 months № of participants: 25 (1 RCT) |
The mean FFI score with shoes was 34.15 points | The mean FFI score with PFOs was 38.32 points | MD 4.17 points lower (24.4 points lower to 16.06 points higher) | ⊕⊕⊝⊝ Lowa,c | PFOs likely result in little to no difference in function or disability. | |
|
Quality of life (child‐rated) (measured on 0 to 100‐point PedsQL; higher score = better QoL) follow up: 3 months № of participants: 22 (1 RCT) |
The mean child‐rated PedQL score with shoes was 59.78 points | The mean child‐rated PedQL score with PFOs was 37.99 points | MD 3.84 points on PedsQL lower (19.01 lower to 11.33 higher) | ⊕⊕⊝⊝ LOW 1 3 | PFOs likely results in little to no difference in child‐rated QoL. | |
|
Quality of life (parent‐rated) (measured on 0 to 100‐point PedsQL; higher score = better QoL) follow‐up: 3 months № of participants: 22 (1 RCT) |
The mean parent‐rated PedQL score with shoes was 55.95 points | The mean parent‐rated PedQL score with PFOs was 56.59 points | MD 0.64 points lower (13.22 points lower to 11.94 points higher) | ⊕⊕⊝⊝ Lowa,c | PFOs likely results in little to no difference in parent‐rated QoL. | |
|
Treatment success (measured on the 50FWT (seconds)) follow‐up: 3 months № of participants: 25 (1 RCT) |
The mean time for the 50FWT with shoes was 8.36 seconds | The mean time for the 50FWT with PFOs was 7.98 seconds | MD 0.38 seconds lower (1.9 seconds lower to 1.14 seconds higher) | ⊕⊕⊝⊝ Lowa,c | PFOs likely results in little to no difference in timed walking. | |
|
Withdrawal due to adverse events
follow‐up: № of participants: 25 (1 study) |
RR 0.72 (0.14 to 3.61) | 23.1% | 16.6% (3.2% to 83.3%) | absolute difference 6.5% less (19.8% less to 60.2 % more) |
‐ | PFOs likely results in little to no difference in withdrawals due to adverse events. Absolute reduction 6.5% (19.8% fewer to 60.2% more) |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Serious adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; FFI: Foot Function Index; 50FWT: 50‐Foot Timed Walk; MD: mean difference; PedsQL: Pediatric quality of life inventory; RR: Risk ratio; VAS: visual analogue scale; QoL: quality of life | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded for bias; single blinded; children and their parents knew which treatment they had, which may have affected their assessment of pain bDowngraded for indirectness; only short‐term outcomes (3 months); FFI not validated in children; PedsQL had no foot‐related data cDowngraded for imprecision; small sample size
Summary of findings 6. Custom foot orthoses compared to prefabricated foot orthoses in children with juvenile idiopathic arthritis and flat feet.
| Custom foot orthoses compared to prefabricated foot orthoses in children with juvenile idiopathic arthritis andflat feet | ||||||
| Patient or population: children with juvenile idiopathic arthritis and flat feet Setting: outpatient rheumatology clinics Intervention: custom foot orthoses (CFO) Comparison: prefabricated foot orthoses (PFO) | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With PFOs (N = 41) | With CFOs (N = 46) | Difference | ||||
|
Pain
(measured on 0 to 10‐point VAS; lower = less pain) follow‐up: 3 months to 6 months № of participants: 87 (2 RCTs) |
The mean pain with PFOs was 3.22 points | The mean pain with CFOs was 1.74 points | MD 1.48 points lower (3.23 points lower to 0.26 points higher) | ⊕⊕⊝⊝ Lowa,b | CFOs may result in little to no difference in pain. | |
|
Function or disability (measured on 0 to 100‐point FFI; 0 = no disability) follow‐up: 3 months № of participants: 27 (1 RCT) |
The mean FFI score with PFOs was 29.9 points | The mean FFI score with CFOs was 15.6 points | MD 14.38 points lower (30.22 points lower to 1.46 points higher) | ⊕⊕⊝⊝ Lowa,b | CFOs may result in little to no difference in function. | |
|
Quality of life (child‐rated) (measured on 0 to 100‐point PedsQL; higher score = better QoL) follow‐up: 3 months to 6 months № of participants: 83 (2 RCTs) |
The mean child‐rated PedQL score with PFOs was 55.94 points | The mean child‐rated PedQL score with CFOs was 64.58 points | MD 8.64 points higher (3.9 points lower to 21.18 points higher) | ⊕⊕⊝⊝ Lowa,b | CFOs may result in a small improvement in child‐rated QoL. | |
|
Quality of life (parent‐rated) (measured on 0 to 100‐point PedsQL; higher score = better QoL) follow up: 3 months to 6 months № of participants: 84 (2 RCTs) |
The mean parent‐rated PedQL score with PFOs was 55.31 points | The mean parent‐rated PedQL score with CFOs was 58.25 points | MD 2.94 points higher (11 points lower to 16.88 points higher) | ⊕⊕⊝⊝ Lowa,b | CFOs may result in little to no difference in parent‐rated QoL. | |
|
Treatment success (measured on the 50FWT (seconds)) follow‐up: 3 months № of participants: 27 (1 RCT) |
The mean time for the 50FWT with PFOs was 7.98 seconds | The mean time for the 50FWT with CFOs was 7.03 seconds | MD 0.95 seconds lower (1.88 seconds lower to 0.02 seconds lower) | ⊕⊕⊝⊝ Lowa,b | CFOs may result in little to no difference in timed walking | |
|
Withdrawal due to adverse events Follow‐up: № of participants: 87 (2 RCTs) |
RR 0.80 (0.13 to 4.87) | 4.9% | 3.9% (0.6% to 23.8%) | 1.0% fewer (4.2% fewer to 18.9% more) | ⊕⊕⊝⊝ Lowa,b | CFOs may result in little difference in withdrawals due to adverse events. |
| Adverse effects | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Serious adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; FFI: Foot Function Index; 50FWT: 50‐Foot Timed Walk; MD: mean difference; PedsQL: Pediatric quality of life inventory; RR: Risk ratio; VAS: visual analogue scale; QoL: quality of life | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded for bias; single blinded; children and their parents knew which treatment they had, which may have affected the assessment of pain bDowngraded for imprecision due to wide 95% CIs
Pain was considered as proportion and group means, with most interest on change from baseline to intervention, and comparative intervention effects. Pain was stipulated as being reported by the child, parent, or carer. This outcome was only applicable in studies involving symptomatic participants.
Function or disability indices of the foot
Quality of life measures
Treatment success (e.g. measured by a participant or proxy‐reported global impression of clinical change, as being very much improved, improved, or similar). The parameters of treatment success could include score changes, functional change, with changes measured as means, proportions, and variance.
Proportion of withdrawals for each trial group, both intervention and control
Proportion with adverse events, as reported
Proportion with serious adverse events, as reported
Minor outcomes
Goniometric measurements, x‐rays, or those that were collated in a gait laboratory (both kinetic and kinematic data).
Search methods for identification of studies
Electronic searches
We searched:
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 9) in the Cochrane Library (searched 1 September 2021; see Appendix 1);
MEDLINE Ovid (July 2009 to 1 September 2021; see Appendix 2)
Embase via Ovid (July 2009 to 1 September 2021; see Appendix 3)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialearch; searched 7 August 2020);
US National 7 August of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 7 August 2020).
We started the search in 2009 to allow overlap with the search from Evans 2010.
In MEDLINE, we combined subject specific search terms and free text words with the optimum search strategy for randomised trials described by Lefebvre 2008. We adapted the search strategy for the other databases
Searching other resources
We complemented the electronic search by checking reference lists of relevant articles for additional studies reported in published papers, scientific meetings, and personal communication.
Data collection and analysis
Selection of studies
One of the review authors (KR) assisted by the Cochrane Musculoskeletal Group's Information Specialist at the editorial base, carried out the initial searches. Pairs of review authors (FH and AE; KR and FH; KR and AE) independently assessed potentially eligible trials for inclusion; they resolved any disagreement through discussion. Titles of journals and names of authors or supporting institutions were not masked at any stage.
We conducted this systematic review in accordance with the PRISMA statement guidelines (Moher 2015).
Data extraction and management
Three authors (AE, KR, FH) independently extracted data using a pre‐piloted form. They resolved any disagreement through discussion, using electronic communications techniques.
We extracted the following study characteristics:
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study
Participants: N, mean age, age range, sex, disease duration (JIA), severity of condition, diagnostic criteria, important baseline data, inclusion criteria, and exclusion criteria
Interventions: intervention, comparison, concomitant medications, and excluded medications
Outcomes: primary and secondary outcomes specified and collected, and time points reported
Characteristics of the design of the trial as outlined below in the Assessment of risk of bias in included studies section.
Notes: funding sources for trials, and notable declarations of interest from authors.
When a trial included more than one measure for an outcome, we adopted a pre‐specified hierarchy, as follows: pain measures, gait and function, health‐related quality of life (HRQoL; 1. child, 2. parent proxy), with precedence given to validated outcome measures where multiple measures were available in a trial ‐ as reflects clinical and parent concerns and clinical care priorities.
Our decision for selecting data to extract, included:
if both final values and change from baseline values were reported for the same outcome, we extracted final values;
if both unadjusted and adjusted values for the same outcome were reported, we extracted unadjusted values
if data were analysed on the basis of intention‐to‐treat (ITT), we extracted the sample treated with custom foot orthoses (CFO; this did not differ for outcomes assessing benefits or harms);
If multiple time points were reported, we extracted final time points
Main comparisons
Note that in this review there are multiple populations and comparisons, and we have stated these in order of clinical importance:
Custom foot orthoses (CFO) versus shoes (CFOs are the most expensive FOs, and shoes are known to alter gait and foot mobility, as reported in the systematic review from Wegener 2011)
Prefabricated foot orthoses (PFO) versus shoes (children with JIA have indisputable disease, and frequently present with debilitating foot pain (Fellas 2017a))
CFO versus PFO (both are common interventions and comprise usual care for common concerns about flatfoot in children (Pfeiffer 2006). However, the cost ratio approximates 4:1 (CFO:PFO), and the justification for CFO use has been both questioned (Evans 2008), and defended (Bresnahan 2009; D'Amico 2009).
Assessment of risk of bias in included studies
Two review authors (FH, AE) independently assessed the risk of bias of each included trial against key criteria: random sequence generation; allocation concealment; blinding of participants, personnel; blinding of outcome assessment ‐ separately for subjective self‐reported outcomes, such as pain and function, and objective outcomes, such as adverse events; incomplete outcome data; selective outcome reporting; and other sources of bias (e.g. follow‐up times, participant maturation). This is in accordance with methods for RoB 1 recommended by The Cochrane Collaboration (Higgins 2017). Review authors resolved disagreements by consensus.
We classified each potential source of bias as high, low, or unclear risk, and provided a quote from the study report, together with a justification for our judgement in the risk of bias table. When information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table. We presented the figures generated by RoB 1 to provide summary assessments of the risk of bias.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RR) with 95% confidence intervals (CI). We calculated mean differences (MD) and 95% CI for continuous outcomes measured on the same scale, and standardised mean difference (SMD), if different scales were used to measure an outcome, and 95% CIs. We entered data presented as a scale with a consistent direction of effect across studies. SMDs were back‐translated to a typical scale (e.g. 0 to 10 for pain) by multiplying the SMD by a typical among‐person standard deviation (e.g. the standard deviation of the control group at baseline from the most representative trial; as per Chapter 6 of the Cochrane Handbook (Higgins 2020b)).
In the 'Comments' column of the summary of findings table, we reported the absolute percent difference, the number needed to treat number for an additional beneficial outcome (NNTB), and the number needed to treat for an additional harmful outcome (NNTH). We calculated the absolute percent change from the difference in the risks between the intervention and control group using GRADEpro GDT, and expressed as a percentage (GRADEpro GDT). The NNTB or NNTH is only provided for dichotomous outcomes that show a clinically significant difference). We calculated the NNTB or NNTH from the control group event rate and the relative risk, using the Visual Rx NNT calculator (Cates 2016). The minimal clinically important differences (MCID) were 0.9 points for pain, measured on a 10‐point visual analogue scale (VAS); and 7 points for disability, measured on the 100‐point disability subscale of the Foot Function Index (FFI), as calculated by Landorf 2008.
Unit of analysis issues
When multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. CFOs versus shoes, and CFOs versus sham orthoses) were combined in the same meta‐analysis, we halved the control group to avoid double‐counting. We clarified the presence of more than two intervention groups in the Characteristics of included studies table.
Dealing with missing data
We contacted investigators to verify key study characteristics and request missing numerical outcome data, when indicated (e.g. when a study was identified as abstract only, or when data were not available for all participants). Any assumptions and imputations used to handle missing data were reported explicitly in the Characteristics of included studies table, and the effect of assumptions or imputations was explored with sensitivity analyses.
For dichotomous outcomes (e.g. number of withdrawals due to adverse events), we calculated the withdrawal rate using the number of participants randomised to the group as the denominator.
For continuous outcomes (e.g. mean pain), we calculated the MD or SMD based on the number of participants analysed at that time point. If the number of participants analysed was not presented for each time point, we used the number of participants randomised to each group at baseline.
When possible, we computed missing standard deviations from other statistics, such as standard errors, confidence intervals, or P values, according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020). If we were unable to calculate standard deviations from the available data, we imputed them (e.g. from other studies in the meta‐analysis).
Assessment of heterogeneity
We assessed clinical and methodological diversity of participants, interventions, outcomes, and study characteristics for the included studies, to determine whether a meta‐analysis was appropriate. We assessed statistical heterogeneity by visually inspecting forest plots for obvious differences in result between the studies, and using the I² statistic. As recommended in the Cochrane Handbook for Systematic Reviews of Interventions, the interpretation of an I² value of 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% may represent considerable heterogeneity (Deeks 2020). If we identified substantial heterogeneity, we reported it. We planned to investigate possible causes with subgroup analyses, had data permitted.
Assessment of reporting biases
Had we been able to pool more than 10 trials, we planned to undertake formal statistical tests to investigate funnel plot asymmetry, and follow the recommendations in Section 13 of the Cochrane Handbook for Systematic Reviews of Interventions (Page 2020).
To assess outcome reporting bias, we checked trial protocols against published reports. For studies published after 1 July 2005, we screened the International Clinical Trials Registry Platform of the World Health Organisation for the trial protocol (apps.who.int/trialearch). We evaluated whether selective reporting of outcomes was present.
Data synthesis
We pooled data using a random‐effects model across studies for outcomes with common interventions and comparators, for participants with similar flatfoot conditions (i.e. JIA, pain, asymptomatic).
Subgroup analysis and investigation of heterogeneity
We did not plan any subgroup analyses.
Sensitivity analysis
Had there been sufficient data, we planned to examine the potential effect on results for pain and function of selection bias by restricting the analysis to studies at low risk of selection bias (adequate allocation concealment); detection bias by restricting the analysis to studies with low risk of detection bias (adequate blinding of outcome assessor ‐ the participants for these outcomes) and those with imputed missing data.
Interpreting results and reaching conclusions
We followed the guidelines provided in Chapter 15 of the Cochrane Handbook for Systematic Reviews of Interventions when interpreting results, and we were aware of distinguishing lack of evidence of effect from lack of effect (Schunemann 2020b). We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice, and our implications for research suggested priorities for future research and outlined remaining uncertainties in this area.
Summary of findings and assessment of the certainty of the evidence
GRADE and Summary of findings tables
We collated seven summary of findings (SoF) tables for the following comparisons:
custom foot orthoses (CFO) compared to shoes in asymptomatic flat feet (Table 1);
prefabricated foot orthoses (PFO) compared to shoes in asymptomatic flat feet (Table 2);
CFOs compared to PFOs in asymptomatic flat feet (Table 3);
CFOs compared to shoes in children with juvenile idiopathic arthritis (JIA; Table 4);
PFOs compared to shoes in children with JIA (Table 5);
CFOs compared to PFOs in children with JIA (Table 6);
PFOs compared to shoes in symptomatic flat feet (Table 7).
Summary of findings 1. Customised foot orthoses compared to shoes in children with asymptomatic flat feet.
| Customised foot orthosescompared to shoes in children with asymptomatic flat feet | ||||||
| Patient or population: children with asymptomatic flat feet Setting: outpatient hospital clinic Intervention: customised foot orthoses (CFO) Comparison: shoes | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With shoes (N = 52) | With CFOs (N = 54) | Difference (absolute) | ||||
|
Pain (measured as proportion with pain) follow‐up: 12 months № of participants: 106 (1 RCT) |
RR 0.85 (0.67 to 1.07) | 78.8% | 67% (52.8% to 84.4%) | 11.8% fewer (4.7% fewer to 15.8% more) | ⊕⊕⊝⊝ Lowa,b | CFOs may result in little to no difference in the proportion of children reporting pain (absolute reduction of 11.8% (4.7% fewer to 15.8% more)) |
| Function or disability | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Treatment success | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
|
Withdrawal due to adverse events follow‐up: 3 months to 4 months № of participants: 211 (3 RCTs) |
RR 1.05 (0.94 to 1.19) | 68.9% | 72.3% (64.7% to 82%) | 3.4% more (4.1% fewer to 13.1% more) | ⊕⊕⊝⊝ Lowa,b | The evidence suggests that CFOs result in little to no difference in withdrawal due to adverse events (absolute effect 3.4% more (4.1 % fewer to 13.1 % more)) |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Serious adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded for bias (participants, parents, and examiners were aware of treatment, which may have impacted self‐reported outcomes; subgroup analysis of those with pain was conducted (post hoc)) bDowngraded for imprecision due to wide confidence intervals including both an increase and decrease in the effect estimate
Summary of findings 7. Prefabricated foot orthoses compared to shoes in children with symptomatic flat feet.
| Prefabricated foot orthoses compared to shoes in children with symptomatic flat feet | ||||||
| Patient or population: children with symptomatic flat feet Setting: outpatient hospital clinic Intervention: prefabricated foot orthoses (PFO) Comparison: shoes | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With shoes (N = 26) | With PFOs (N = 26) | Difference | ||||
| Pain | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
|
Function or disability (global function assessed with 0 to 100‐point PODCI; higher scores = better functioning) follow‐up: mean 12 weeks № of participants: 52 (1 RCT) |
The mean PODCI score with shoes was 0.7 points | The mean PODCI score with PFOs was 3.7 points | MD 3 points higher (2.28 points higher to 3.72 points higher) | ⊕⊕⊝⊝ Lowa.b | The evidence suggests that PFOs results in little to no difference in function | |
|
Quality of life (measured on 0 to 100‐point PedsQL; higher score = better QoL) follow‐up: mean 12 weeks № of participants: 52 (1 RCT) |
The mean PedQL score with shoes was ‐1.1 points | The mean PedQL score with PFOs was 2.9 points | MD 1.8 points higher (1.07 points higher to 2.53 points higher) | ⊕⊕⊝⊝ Lowa,b | The evidence suggests that PFOs results in little to no difference in quality of life | |
| Treatment success | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Withdrawal due to adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Adverse effects | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| Serious adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PODCI: Pediatrics Outcomes Data Collection Instrument; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded for bias (participants and parents aware of treatment received; selective reporting of outcomes, as the published study included more outcomes than were listed in the trial registry (ClinicalTrials.gov NCT02414087)) bDowngraded for imprecision due to small sample size, small effects across scaled outcome measures
Summary of finding tables provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the major outcomes (Types of outcome measures), as recommended Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2020a).
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence comprised of the studies that contributed data to the meta‐analyses for the prespecified major outcomes. We reported the quality of evidence as high, moderate, low, or very low. We used GRADEpro GDT software to prepare and present the SoF tables (GRADEpro GDT). We justified all decisions to downgrade the quality of the evidence using footnotes. We provided the NNTB or NNTH and absolute percent change in the 'Comments' column of the SoF table, as described in the 'Measures of treatment effect' section above.
Results
Description of studies
Results of the search
Our updated search retrieved 422 records (66 from CENTRAL, 252 from MEDLINE, and 104 from Embase). Our search of WHO ICTRP retrieved 38 citations; ClinicalTrials.gov retrieved 47 citations, giving a total of 507 records. We removed 28 duplicates, and screened 452 records. We excluded 407 records, and retrieved 45 for full‐text review for possible inclusion. Of the 45 full‐text articles, we excluded a further 13 (Figure 1) (Benedetti 2011; Ford 2017; Hill 2020; Hurd 2010; MacKenzie 2012; Mosca 2010; Okamura 2020; Perhamre 2011; Perhamre 2012; Pothrat 2013; Riccio 2009; Uden 2017; Yung 2011) because of wrong study design and wrong population; 5 were trials already included (Ahn 2017; Kanatli 2016; Wenger 1989; Whitford 2007; Pandey 2013), Additional details of these excluded studies are reported in the Characteristics of excluded studies Table. Twelve ongoing studies were identified, and hence included in the table of Characteristics of ongoing studies. We contacted investigators of two RCTs in order to verify key study characteristics and obtain missing numerical outcome data (Pandey 2013; Sinha 2013). Both authors were contacted via email, and unfortunately this met with no success in gaining the required data (answers to queries, missing data, clarification of randomisation, and SDs). These studies were included in the table of Characteristics of studies awaiting classification.
1.
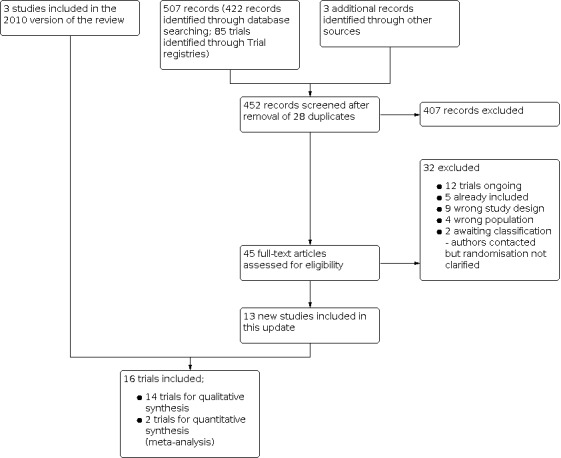
Study flow diagram for the trial search
Thus, 13 studies met the final eligibility criteria for inclusion in this review update (Abd‐Elmonem 2021; Aboutorabi 2013; Ahn 2017; Asgaonkar 2012; Bok 2016; Coda 2014; Gould 1989; Hsieh 2018; Jafarnezhadgero 2018; Kanatli 2016; Khamooshi 2017; Morrison 2013; Solanki 2020). However, two studies established only immediate effects of interventions, with no follow up data, and hence were regarded for description, but not for analysis (Aboutorabi 2013; Bok 2016). Gould 1989 was not included in the 2010 review, due to absence of a discrete control group, which was a criterion at that time (Rome 2010). Three studies were included from the previous version of the review (Powell 2005; Wenger 1989; Whitford 2007). Hence, a total of 16 studies were included in this review update.
Included studies
We included a total of 16 randomised controlled trials (RCT), details of which are included in the Characteristics of included studies tables and Table 8; Table 9; and Table 10.
1. Study characteristics of the 16 included trials.
| Study Country | Follow‐up time | Baseline sample size | Age (SD) | Final sample size (% of baseline) | Intervention | Outcome measures | |
| Flexible asymptomatic flat feet in healthy children (9 studies) | |||||||
| Wenger 1989 USA | 3 years | 131 | 1 to 6 years | 98 (75%) | Shoe: N = 28
Heel cup: N = 27 UCBL: N = 22 Control: N = 21 |
X‐ray Clinical photos | |
| Gould 1989 USA | 5 years | 125 | 11 to 14 months | 52 (42%) | SL shoe: N = 25 SL shoe/ cookie: N = 10 Ortho shoes: N = 7 Ortho/mla: N = 10 | X‐ray Pedotopography Clinical assess ment | |
| Whitford 2007 Australia | 1 year | 178 | 7 to 11 years | 160 (90%) | CFO: N = 59 FO: N = 59 Control: N = 60 | Pain SPPC Motor skills VO² max | |
| Asgaonkar 2012 India | 1 year | 80 | 5 to 15 years | 60 (75%) | Valgus insole: N = 30 Control: N = 30 | Pain (VAS) Physical cost (HR) Gait (step parameters) | |
| Kanatli 2016 Turkey | 2 to 5 years | 45 | 17 to 72 months (average 39.5 months) | 45 (100%) | Orthotic shoes: N = 21 Control: N = 24 | X‐ray Laxity AI | |
| Ahn 2017 Korea | 1 year | 40 | 10.14 years (4.99) | 40 (100%) | TCFO: N = 20 RFO: N = 20 | X‐ray RCSP | |
| Khamooshi 2017 Iran | 8 weeks | 60 | 9 to 13 years | 60 (100%) | Foot exercises: N = 20
Foot/core exercises: N = 20 Control: N = 20 |
Pedoscope Staheli AI ND Tiptoe/mla | |
| Jafarnezhadgero 2018 Iran | 4 months | 30 | 8 to 12 years | 30 | CFO: N = 15 Sham insole: N = 15 |
Gait kinematic Kinetic parameters | |
| Solanki 2020 India | 4 weeks | 44 | approximately 13 to 14 years | 44 | Conventional exercises + Faradic foot bath + rigid taping: N = 22 Conventional exercises + Faradic foot bath + sham tape: N = 22 | SEBT VJH IAT |
|
| Abd‐Elmonem 2021 Egypt | 4 months | 72 | 7 to 12 years | 66 | Corrective exercises + NMES: N = 36 Corrective exercises + sham NM ES: N = 36 | Staheli AI ND x‐ray |
|
| Children with juvenile idiopathic arthritis and foot pain (2 studies) | |||||||
| Powell 2005 USA | 3 months | 48 | 5 to 19 years | 40 (83%) | CFO: N = 15 Neoprene inserts: N = 12 Sports shoe: N = 13 | Pain (VAS) PedsQL Timed walk FFI |
|
| Coda 2014 UK | 0, 3, 6 months | 60 | 10 to 11 years (3.5) | 60 (100%) | CPFO: N = 31 PFO: N = 29 | VAS PedsQL | |
| Flexible flat feet in children with foot pain (1 study) | |||||||
| Hsieh 2018 Taiwan | 12 weeks | 52 | 6 to 7 years | 50 | PFO: N = 24 Control: N = 26 | Physical activity Function (PODCI) Psychometric (PODCI, HRQoL) |
|
| Flexible flat feet in children with foot pain (immediate effects only; 1 study) | |||||||
| Bok 2016 South Korea | immediate | 21 | 8 to 13 years (average 9.9 years) | 21 (100%) | 0° inverted CFO/15° inverted CFO/30° inverted CFO: N = not specified Shoes only (usual): N = not specified |
Pedar ‐ peak pressure, max. force, contact area | |
| Flexible flat feet in children without foot pain (immediate effects only: 1 study) | |||||||
| Aboutorabi 2013 Iran | immediate | 50 (30 flat feet: 20 controls) | 7.76 years (1.4) | 50 (100%) | Shoes + CFO/ Medical shoes/Barefoot: N = 30 Control (no flat feet): N = 20 | Gait ‐ Step – length, width, symmetry Velocity CoP | |
| Flexible flat feet in children with developmental co‐ordination disorder (1 study) | |||||||
| Morrison 2013 UK | 7 weeks | 22 | 6 to 11 years | 14 (64%) | CFO: N = 9 Control: N = 5 | 6‐minute walk Gait rite | |
|
Abbreviations: ADRs: adverse reactions; AI: arch index; CFO: customised/bespoke foot orthoses; CoP: centre of pressure; FF: flat feet; FO: foot orthoses; HR: heart rate; HRQoL: health‐related quality of life IAT: Illinois Agility test; JIA: juvenile idiopathic arthritis; ND: navicular drop; NMES: neuromuscular electrical stimulation NS: not significant; PedsQL: Pediatric quality of life inventory; PFO: prefabricated foot orthoses; PODCI: Paediatric outcome data collection instrument RCSP: resting calcaneal stance position; RFO: rigid FO; SEBT: start excursion balance test; SL: straight last (shoe); SPPC: self perception profile; TCFO: talus control FO; UCBL: University of California Biomechanics Laboratory heel cup orthosis; VAS: visual analogue score; VJH: vertical jump height. |
|||||||
Prefabricated foot orthoses definition
A prefabricated foot orthosis is an in‐shoe medical device that is not made from an individual scan, cast, or mould of the foot. This generic device is intended to alter the magnitudes and temporal patterns of the reaction acting on the plantar aspect of the foot and normalise foot and lower extremity function; decreasing abnormal loading forces on the structural components of the foot and lower extremity during weight‐bearing and related activity.
Customised prefabricated foot orthoses definition
A modified version of a basic generic device, which is initially mass produced, and then specifically modified for the foot and gait requirements of an individual child. The modifications are usually added by the treating clinician, and may include: additional arch fill, varus or valgus wedges, and topcovers.
Custom foot orthoses definition
A bespoke foot orthosis is an individually customised in‐shoe medical device that is made from an individual scan, cast, or mould of the foot. The design is prescribed by a qualified healthcare professional to alter the magnitudes and temporal patterns of the reaction forces acting on the plantar aspect of the foot, in order to allow more normal foot and lower extremity function, and to decrease pathologic loading forces on the structural components of the foot and lower extremity during weight‐bearing and related activity.
2. Outcome matrix per trial group comparison.
| Diagnosis | Pain | Function | HRQoL | Treatment success | Withdrawals | Adverse events | Serious adverse events |
| 1. CFO versus shoes | |||||||
| asymptomatic flat feet |
Whitford 2007 – post hoc subgroup (% pain) | Whitford 2007 – VO² max, motor skills | NR | NR |
Wenger 1989; Whitford 2007 |
NR | NR |
| JIA | Powell 2005 – VAS | Powell 2005 – timed walk | Powell 2005 – FFI | Powell 2005 | Powell 2005 | Powell 2005 – none | NR |
| DCD | NR | Morrison 2013 – 6MWT | NR | Morrison 2013 | Morrison 2013 | NR | NR |
| 2. PFO versus shoes | |||||||
| asymptomatic flat feet |
Whitford 2007 post hoc subgroup (% pain) Asgaonkar 2012 – VAS |
Whitford 2007 – VO² max, motor skills Asgaonkar 2012 – HR, gait |
NR | Asgaonkar 2012; Gould 1989; |
Asgaonkar 2012; Gould 1989; Wenger 1989; Whitford 2007 |
NR | NR |
| symptomatic flat feet |
NR | Hsieh 2018 | Hsieh 2018 – PODCI | Hsieh 2018 | Hsieh 2018 | NR | NR |
| JIA | Powell 2005 – VAS | Powell 2005 – timed walk | Powell 2005 – FFI | Powell 2005 | Powell 2005 | Powell 2005 – none | NR |
| 3. CFO versus PFO | |||||||
| asymptomatic flat feet |
Whitford 2007 – post hoc subgroup (% pain) | Whitford 2007 – VO² max, motor skills | NR | NR |
Wenger 1989; Whitford 2007 |
NR | NR |
| JIA | Coda 2014; Powell 2005 – VAS | Powell 2005 – timed walk | Coda 2014; Powell 2005 – PedsQL | Coda 2014; Powell 2005; | Coda 2014; Powell 2005; |
Coda 2014 – NR Powell 2005 – none |
NR |
6MWT: 6‐minute walk test; CFO: custom foot orthoses; DCD: developmental co‐ordination disorder; HRQoL: health‐related quality of life; JIA: juvenile idiopathic arthritis; HR: heart rate; NR: not reported; PedsQL: Pediatric quality of life inventory; PFO: prefabricated foot orthoses; PODCI: Paediatric outcome data collection instrument; FFI: Foot Function index; VAS: visual analogue scale
3. Shoes used within the trials.
| Study ID | Control shoe | Comparator shoes |
| Asymptomatic flat feet | ||
| Wenger 1989 | usual shoes | corrective shoes, usual shoes + Helfet heel cups, usual shoes + UCBL CFO |
| Gould 1989 | straight last shoes | ‐ straight last shoes plus longitudinal arch cookies ‐ orthopaedic shoes with long counters, solid shanks, Thomas heels, and 0.312 cm inside heel wedges ‐ orthopaedic shoes with long counters, solid shanks, Thomas heels, and 0.312 cm inside heel wedges, with supplemental thin longitudinal arch support |
| Whitford 2007 | usual shoes | none (PFO, CFO) |
| Asgaonkar 2012 | usual shoes | none (valgus insole) |
| Kanatli 2016 | usual shoes | corrective shoes, i.e. custom‐made orthopaedic shoes that had 0.5 to 0.9cm longitudinal arch support and 3 to 4 mm heel wedges |
| Ahn 2017 | usual shoes | none (2 CFO types) |
| Khamooshi 2017 | usual shoes | none (foot, core exercises) |
| Jafarnezhadgero 2018 | New Balance 759 (trainers) | New Balance 759 (trainers) |
| Solanki 2020 | not stated | not stated |
| Abd‐Elmonem 2021 | not stated | not stated |
| Symptomatic flat feet | ||
| Hsieh 2018 | usual shoes (encouraged to wear at least 5 hours daily) | usual shoes (encouraged to wear at least 5 hours daily) |
| JIA | ||
| Powell 2005 | new supportive athletic shoes with a medial longitudinal arch support and shock absorbing soles (cross‐training type shoes) | all children, regardless of intervention, received new athletic shoes at beginning of the study |
| Coda 2014 | usual shoes | none (PFO, CPFO) |
| DCD | ||
| Morrison 2013 | usual shoes | none (CFO) |
| Immediate effects studies | ||
| Bok 2016 | usual shoes | none (3 inverted CFOs) |
| Aboutorabi 2013 | no shoes (bare feet) | medical shoes, regular shoes (with FO) |
CFO:customised foot orthoses, CPFO: customised prefabricated foot orthoses; DCD: developmental co‐ordination disorder; FO: foot orthoses; JIA: juvenile idiopathic arthritis; PFO: prefabricated foot orthoses; UCBL: University of California Biomechanics Laboratory heel cup orthosis
Design
Ten of the 16 randomised RCTs included a no treatment control group (Abd‐Elmonem 2021; Asgaonkar 2012; Hsieh 2018; Jafarnezhadgero 2018; Kanatli 2016; Khamooshi 2017; Morrison 2013; Solanki 2020; Wenger 1989; Whitford 2007). The studies were conducted in nine countries (Australia, USA, UK, Iran, Egypt, Turkey, Republic of Korea, India, Taiwan) and were English language publications. The duration of trials ranged from four weeks (Solanki 2020), to five years (Gould 1989). The trials were parallel designs; seven had multiple arms (Aboutorabi 2013; Bok 2016; Gould 1989; Khamooshi 2017; Powell 2005; Wenger 1989; Whitford 2007), seven had a single intervention versus control group (Abd‐Elmonem 2021; Asgaonkar 2012; Hsieh 2018; Jafarnezhadgero 2018; Kanatli 2016; Morrison 2013; Solanki 2020), and two trials comparing two single interventions (Ahn 2017; Coda 2014).
Most studies only included data measured at baseline and final time points; four reported multiple time points (Gould 1989; Solanki 2020; Wenger 1989; Whitford 2007). Two studies investigated immediate effects only and provided no follow‐up data; we included them in the review, but not in the analyses (Aboutorabi 2013; Bok 2016).
Participants
The 16 studies included a total of 1058 children who completed the trials, and were aged from 11 months to 19 years, were included in the review. The inclusion and exclusion criteria used in all studies were clearly, if variably, defined. The majority of trials were in healthy children with flexible flat feet, with a range of inclusion criteria, and age groups. The inclusion criteria for trials of children with juvenile idiopathic arthritis (JIA) were: lower limb involvement, failed treatment with foot orthoses, ability to walk 15 metres, disease modifying anti‐rheumatic drugs (DMARDs) for six months or more (Coda 2014); foot pain for > 1 month but < 2 years (Powell 2005). One trial with 22 boys included those with a diagnosis of developmental co‐ordination disorder (DCD), and a Foot Posture Index (FPI) > +4 (Morrison 2013). Four trials recruited children with symptomatic flat feet (Bok 2016; Coda 2014; Hsieh 2018; Powell 2005).
Two groups, defined only by diagnosis, included:
1. Asymptomatic flat feet in healthy children
Ten trials (N = 805) assessed the effect of a number of comparisons on healthy children with asymptomatic, flexible flat feet. They collected data at baseline, and a number of defined time points. Whilst exhibiting obvious diversity, these trials represented the most children, a range of intervention modalities, common clinical practice presentations, and thus, the bulk of potentially relevant evidence (Abd‐Elmonem 2021; Ahn 2017; Asgaonkar 2012; Gould 1989; Jafarnezhadgero 2018; Kanatli 2016; Khamooshi 2017; Solanki 2020; Wenger 1989; Whitford 2007).
Wenger 1989 evaluated 131 children, aged 1 to 6 years, with clinically diagnosed pes planus and no pain. Bilateral pes planus was diagnosed by visual observation of the valgus position of the heel, and the low appearance of the arch upon weight bearing.
Gould 1989 assessed 125 normal toddlers (beginner walkers) aged 11‐14 months. Gender of children was not mentioned until the 5 year‐old results, in 25/50 Group 1 participants (15M, 10F).
Whitford 2007 evaluated 178 children, aged 7 to 11 years, with clinically diagnosed pes planus and no pain. Bilateral pes planus was diagnosed by the assessment of calcaneal eversion in RCSP, and by the navicular drop test. The navicular drop test measures the extent of excessive foot pronation in static stance.
Asgaonkar 2012 recruited 80 children with flat feet, aged between five and 15 years. It is not especially clear, but appears that 139/894 of the children initially screened had flat feet, from which a random 80/139 were enrolled in the study. Uneven groups of 45 treatment and 35 controls were formed at random. Flat foot assessment was based on inked footprints, and the ratio of midfoot: foot width.
Jafarnezhadgero 2018 recruited 30 boys, aged 8 to 12 years. The boys were randomised into two same‐size groups (N=15). Flat foot was assessed using navicular drop, arch height index, and resting calcaneal stance position.
Kanatli 2016 evaluated 45 children (33 boys, 12 girls) with mean age of 39.5 months (17‐72 months), with diagnosis of moderate flexible flat foot.
Khamooshi 2017 assessed 60 female aged 9 to13 year old, with good general health and flat feet.
Ahn 2017 investigated 40 children who were aged 10 years (4.5), with flexible flat feet.
Solanki 2020 investigated 44 children (sex unspecified) with mean age indicated as "students from 8th to 9th standard" (estimated age of 12 to 14 years), with clinically assessed 'hyperpronated' (flat) feet, as diagnosed by 'too many toes sign', navicular height, calcaneal angle.
Abd‐Elmonem 2021 evaluated 72 typically developing children aged from 7 to 12 years (31 boys, 35 girls at completion) with asymptomatic flexible flat feet, as a clinically diagnosed.
2. Children with JIA, and other clinical concerns
Two studies (N = 108) included children diagnosed with JIA, flat feet, and foot pain (Coda 2014; Powell 2005). Powell 2005 evaluated 48 children, aged 5 to19 years, diagnosed with JIA with pes planus and foot pain. Coda 2014 recruited 60 children with JIA, aged 10.64 (3.84) years, controls: 11.17 (3.51) years (controls: 6M: 23F; treatment group 9M: 22F).
Painful flatfoot:Hsieh 2018 evaluated 52 six‐year‐old children (28 males and 24 females) with symptomatic flexible flat feet.
Morrison 2013 investigated 22 boys with a diagnosis of DCD, who had flexible flat feet, a FPI > +4, and were aged 6 to 11 years.
Immediate effects only, were reported for two studies (Bok 2016; Aboutorabi 2013). Bok 2016 examined 21 children with flexible flat feet and foot pain, aged 8 to 13 years. Aboutorabi 2013 assessed 30 children with asymptomatic flexible flat feet, and a matched control group (N=20), with mean age 7.76 years. The two immediate effects trials, with data derived only at baseline time points were not included in analyses.
Overall, four RCTs were deemed too anomalous for inclusion in the main sub‐groups (Hsieh 2018; Morrison 2013; Bok 2016; Aboutorabi 2013)
Interventions
The 16 included studies included a range of interventions: custom foot orthoses (CFO), prefabricated foot orthoses (PFO), CPFOs (customised PFOs), valgus insoles, inverted FOs, heel cups, University of California Biomechanics Laboratory (UCBL) supports, orthopaedic shoes (straight last, straight last plus cookie, with medial arch strengthening), foot exercises, foot plus core exercises, anti‐pronation taping, neuromuscular electrical stimulation (NMES). Definitions of foot orthoses types, i.e. CFOs, PFOs, and CPFOs can be found in Table 8.
Reporting of intervention according to groups
1. Asymptomatic flat feet in healthy children
Wenger 1989 randomised each child into one of four groups: (1) orthopaedic shoes with no corrective features, the shoes were normal in contour and contained a steel shank; (2) shoes with a Thomas heel, a long medial counter and a navicular pad; (3) shoes with a Thomas heel, a long medial counter and a heel cup; (4) shoes and customised UCBL plastic foot orthoses. The trial ran for 3 years.
Gould 1989 randomised the children across four treatment groups: (Gp 1) straight last shoes (Gp 2) Gp 1 shoes plus long arch cookies, (Gp 3) orthopaedic shoes with long counters, solid shanks, Thomas heels, 0.3cm inside heel wedges, (Gp 4) Gp 3 shoes plus thin longitudinal arch support. The trial ran for 4 years.
Whitford 2007 randomised each child into one of three groups: (1) CFOs made from a rigid thermoplastic material with a vinyl cover; (2) PFOs made from a semi‐rigid thermoplastic material with a standard intrinsic heel postings of 4° and a 5mm metatarsal rise; (3) no treatment. All children with shortened ankle joint plantar flexors were taught how to conduct calf muscle stretches at home and the researchers discussed suitable shoes with the parents. The trial ran for 12 months.
In Asgaonkar 2012 the intervention group wore valgus PFO or insoles for 1 year, and the control group received no treatment.
Jafarnezhadgero 2018 randomised each child to either CFO treatment or sham insoles for a 4 month trial. All participants wore the same shoes from trial commencement.
Kanatli 2016 compared custom made orthopaedic shoes (N=21), with a control group using the outcomes of Joint laxity, AI, x‐ray angles over 34.6 +/‐ 10.9 months (2‐5 years). Shoes were renewed every 6 months in the treatment group, when x‐rays were also taken.
Ahn 2017 compared two CFO designs (N=40), Over 1 year, in children of mean age 10.14 (4.99) years.
Khamooshi 2017 compared foot exercises versus foot and core exercises with an untreated control group, in an 8 week trial for girls (N=60) aged 9 to 13 years.
Solanki 2020 randomised each child (N=44) to either rigid taping and conventional therapy (strengthening exercises, Faradic foot bath), versus sham taping and same conventional therapy, in a 4 week trial for children aged approximately 12 to 14 years, of unspecified sex.Solanki 2020
Abd‐Elmonem 2021 compared corrective (foot strength) exercises and NMES, versus corrective (foot strength) exercises and sham NMES (0 current), in a 4 month trial, for 72 children, aged 7 to 12 years (31 boys, 35 girls at completion).
2. Children diagnosed with JIA, flat feet and foot pain, or other clinical concerns
Powell 2005 randomised each child into one of three intervention groups: (1) custom‐made semi‐rigid orthotics made of metal‐particle reinforced plastic with shock‐absorbing posts; (2) prefabricated shoe inserts made from flat neoprene; and (3) new supportive athletic shoes with a medial longitudinal arch and shock‐absorbing insoles. All children received new athletic shoes at the beginning of the study. This trial ran for 3 months.
Coda 2014 randomised each child into two groups, with CPFOs versus control PFOs over a 6 month period.
Painful flexible flat foot: one study evaluated young children with painful flexible flat foot, N=52 (Hsieh 2018) aged 6 years, and compared customised PFOs (N=26), with a control group using the outcomes of physical activity, function and quality of life over 12 weeks.
Developmental coordination disorder (DCD): one study assessed boys diagnosed with DCD (Morrison 2013). All participants completed a 7 week rehabilitation programme, with a treatment group using CFOs from the start (N=9, mean age 8 years) and a control group who received CFOs at completion of the 7 week trial (N=5, mean age 6.5 years).
Two studies assessed only immediate effects of shoes and FOs (Aboutorabi 2013, Bok 2016), and no follow up data. Bok 2016 randomised children with painful flat foot in to one of four groups: (1) Sport shoes only (2) CFOs with no inverted angle (3) CFOs with a 15° inverted angle (4) CFOs with a 30° inverted angle. The other study randomised 30 children with asymptomatic flat foot, into 2 groups medical shoe versus a regular shoe with PFOs (Aboutorabi 2013).
Footwear advice or provision varied across trials. Six trials supplied the footwear, which was also analysed as an intervention (Powell 2005; Wenger 1989, Gould 1989; Kanatli 2016, Aboutorabi 2013, Jafarnezhadgero 2018). The supplied footwear varied from athletic footwear (Powell 2005, Jafarnezhadgero 2018) to medical/orthopaedic footwear (ankle‐high boots). Footwear was advised in three studies (Bok 2016, Morrison 2013, Whitford 2007), and otherwise footwear was specified, and so presumably the participant's usual shoes were used. Wenger 1989 provided a pedorthotist for all follow‐up visits to ensure the corrective shoes were fitted according to the standards and specifications of the Prescription Footwear Association.
Both Coda 2014 and Jafarnezhadgero 2018 utilised sham foot orthoses for the control groups.
Whitford 2007 prescribed calf muscle stretches to all children who required stretching, irrespective of the study group, and a seven week rehabilitation programme was directed for both intervention and control groups in boys with DCD (Morrison 2013).
Outcomes
Overall, the 16 included trials used a wide range of outcome measures, with the majority measuring pain, function, health‐related quality of life and foot X‐rays; see Table 9; Table 11; and Table 12.
4. Prefabricated foot orthoses versus control on function and pain outcomes at 12 months.
| Outcome Measure | No of participants | Prefabricated orthoses | Controls | P value | Effect size |
| Physical cost (mean (SD)) ‐ Whitford 2007 (VO² max) ‐ Asgaonkar 2012 (HR) |
Whitford 2007 = 160 Asgaonkar 2012 = 60 |
45.10 (4.88) 0.20 (0.06) |
44.95 (3.81) 0.26 (0.12) |
P = 0.899 P = 0.0264 |
MD 0.15, 95% CI ‐1.51 to 1.81 MD ‐0.06, 95% CI ‐0.11 to ‐0.01 |
| Pain (mean (SD)) ‐ Asgaonkar 2012 (VAS, mean (SD)) |
60 | 0.64 (1.09) | 4.33 (2.58) | P < 0.0001 | MD ‐3.69, 95% CI ‐4.60 to ‐2.78 |
| Pain (numbers (%)) ‐ Whitford 2007 (% without pain) |
160 | 36/54 (67%) | 41/52 (79%) | P = 0.56 | RR 0.85, 95% CI 0.67 to 1.07 |
Asgaonkar 2012 reported improvement in both pain and physical cost of children treated with prefabricated orthoses at 12 months versus the control group
Whitford 2007 found no difference between groups
CI: confidence interval; MD: mean difference; RR: risk ratio
5. Shoes versus control on x‐ray outcomes at 3 years.
| Outcome Measure | No of participants | Corrective shoes | Controls | P value | Effect size |
| Talo‐horizontal x‐ray change (mean (SD)) ‐ Wenger 1989 ‐ Kanatli 2016 |
98 45 |
6.47 (0.59) 0.17 |
5.48 (0.71) 0.13 |
P > 0.4 P = 0.19 |
‐0.16 (‐0.44 ‐ 0.16) |
| Talo‐1st metatarsal x‐ray change (mean (SD)) ‐ Wenger 1989 ‐ Kanatli 2016 |
98 45 |
6.80 (0.7) 0.45 |
5.78 (0.83) 0.46 |
P > 0.5 P = 0.72 |
‐0.50 (‐1 ‐ (‐0.02)) |
| Talocalcaneal (AP) x‐ray change (mean (SD)) ‐ Wenger 1989 ‐ Kanatli 2016 |
98 45 |
7.36 (0.78) 0.13 |
4.50 (0.91) 0.23 |
P > 0.5 P = 0.09 |
‐0.12 (‐0.05 ‐ 0.20) |
Wenger showed positive correlation between all radiographic parameters between initial and changed angles over three years (P < 0.001).
Both studies showed that the measured change in x‐ray angles was the same between treatment (shoes) and control groups after three years.
Trial durations and follow‐up ranged from immediate (Aboutorabi 2013; Bok 2016), to five years (Gould 1989; Kanatli 2016). Follow‐up ranged from four weeks to three years (approximately a two‐year average) in the majority of trials in healthy children with flexible flat feet; three to six months in the JIA studies; and over seven weeks in the DCD study.
Major outcomes
Pain
Five studies measured pain on VAS (Asgaonkar 2012; Coda 2014; Powell 2005; Whitford 2007).
Studies which measured pain using a continuous outcome measure (VAS), reported the proportions of participants with or without pain at follow up (Coda 2014, Powell 2005). These studies were specific to children with JIA.
Wenger 1989 stated that parents reported a reduction in pain symptoms in children with flat feet across the four groups (corrective shoes, heel cup, UCBL inserts, and the control group) but no data were provided.
Hsieh 2018 measured pain using parented reported Paediatric outcome data collection instrument (PODCI), in children recruited with painful flat feet. PODCI (The Pediatric Outcomes Data Collection Instrument, Daltroy 1998) measures capability primarily, and includes a paediatric version to be completed by a parent and an adolescent version that can be completed by the parent, child, or both (Klepper 2011).
Whitford 2007 made similar report, with pain at baseline and trial completion, reported as participant percentages. This was a sub‐group, as participant recruitment targeted asymptomatic, healthy children.
Whitford 2007 measured mean pain as a continuous outcome with VAS, but only reported the proportion with pain and the proportion with no pain (introducing a possible reporting bias). We extracted proportion with no pain, from the percentage data reported.
Hsieh 2018 did not directly measure pain, but pain/comfort are a psychometric property of the PODCI outcome measure used (Daltroy 1998).
Function
Overall, seven studies measured function (Aboutorabi 2013; Bok 2016; Hsieh 2018; Morrison 2013; Powell 2005; Solanki 2020; Whitford 2007). Four studies directly measured function (Powell 2005, Whitford 2007, Hsieh 2018; Solanki 2020). Five studies used gait as a measure of function (Aboutorabi 2013; Asgaonkar 2012; Bok 2016; Khamooshi 2017; Morrison 2013).
Powell 2005 measured function on the Foot Function index (FFI; Budiman 1991). The FFI was developed to measure the impact of foot pathology on function in terms of pain, disability and activity restriction.
Four studies measured timed walking (Powell 2005, Morrison 2013, Aboutorabi 2013, Hsieh 2018), using different methods, i.e. the six minute walk test (Morrison 2013), timed up and go test (Hsieh 2018), 50 feet walk (Powell 2005), step velocity (Aboutorabi 2013). The six minute walk test (6MWT) is validated (New Reference), with reference data for both healthy and disease paediatric status.
One study (Solanki 2020) assessed balance and agility, using vertical jump height (VJH) Montalvo 2021, single excursion balance test (SEBT) Gribble, 2012, Illinois agility test (IAT)Kutlu 2018.
Health‐related quality of life
Four studies investigated HRQoL (Coda 2014; Hsieh 2018; Powell 2005; Whitford 2007) .
Three studies measured health‐related quality of life using the PedsQL which is a well validated measure of many aspects of HRQoL (Coda 2014; Hsieh 2018; Powell 2005), with multiple domains, and reference disease data (Varni 1996; Varni 2002). Whitford 2007 used as proxy, the Self Perception Profile for Children (with six subscales for scholastic competence, social acceptance, behavioural conduct, physical appearance, athletic competence, global self‐worth).
Treatment success
No studies stipulated criteria for treatment success. However, four studies used gait parameters (Asgaonkar 2012; Gould 1989; Jafarnezhadgero 2018; Powell 2005).
Proportion of withdrawals due to adverse events
Five studies reported withdrawals due to adverse events (Wenger 1989; Whitford 2007Asgaonkar 2012; Gould 1989; Powell 2005).
Proportion with adverse events
None if the included studies reported the proportion of children with adverse events, or serious adverse events.
Proportion with serious adverse events
Not reported in any of the included studies.
Minor outcomes
Five studies used radiographic imaging (Abd‐Elmonem 2021; Ahn 2017; Gould 1989; Kanatli 2016; Whitford 2007).
Six studies measured gait assessment (Aboutorabi 2013; Asgaonkar 2012; Hsieh 2018; Jafarnezhadgero 2018; Morrison 2013; Powell 2005).
Two studies used plantar pressure measures (Bok 2016; Morrison 2013). Three studies used footprint measures (Gould 1989; Kanatli 2016; Khamooshi 2017).
Single studies used clinical photos (Wenger 1989), centre of pressure analysis (Aboutorabi 2013), joint range laxity (Kanatli 2016), and resting calcaneal stance position (RCSP (Ahn 2017)).
Two studies assessed physical expenditure (Asgaonkar 2012; Whitford 2007).
Two studies assessed clinical impressions (Wenger 1989; Whitford 2007).
Three studies recorded parent feedback (Wenger 1989, Coda 2014, Hsieh 2018).
Table 8 collates description of the 16 included trials.
Table 9 is the trial group comparison matrix.
Excluded studies
We excluded thirteen studies after full‐text screening, either because of wrong study design or wrong population. Nine studies were non‐randomised clinical trials (Benedetti 2011; Ford 2017; Hill 2020; Hurd 2010; MacKenzie 2012; Mosca 2010; Pothrat 2013; Riccio 2009; Uden 2017). Four studies did not include children (Yung 2011), did not address flat feet (Perhamre 2011; Perhamre 2012), or involved adult participants (Okamura 2020).
Additional details of the excluded studies are reported in the Characteristics of excluded studies table.
Ongoing studies
We identified 12 ongoing studies. Details are included in the table of Characteristics of ongoing studies.
Studies awaiting classification
We contacted investigators of two studies to verify key study characteristics and obtain missing numerical outcome data (Pandey 2013; Sinha 2013). We contacted both authors via email; but did not obtain the required data (answers to queries, missing data, clarification of randomisation, and standard deviations). Details of both studies are included in the table of Characteristics of studies awaiting classification.
Risk of bias in included studies
The summary of risk of bias is presented in Figure 2. We did not judge any of the 16 included trials at low risk of bias across all domains. Most trials were at risk of selection, performance, detection, and selective reporting biases.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
All trials reported that participants were randomised to an intervention, but only seven trials adequately described the method used to generate the random sequence (Abd‐Elmonem 2021; Coda 2014; Hsieh 2018; Powell 2005; Solanki 2020; Wenger 1989; Whitford 2007) and are at low risk of selection bias for random sequence generation.
Three trials did not describe if allocation of treatment was concealed (Coda 2014; Wenger 1989; Whitford 2007) and are at unclear risk of bias. Only five trials adequately reported allocation concealment (Abd‐Elmonem 2021; Hsieh 2018; Morrison 2013; Powell 2005; Solanki 2020) and are judged at low risk of selection bias for allocation concealment. We judged one trial at high risk of bias for both random sequence generation and allocation concealment (Kanatli 2016).
Blinding
We detected performance bias in most trials. We judged 11 out of the included 16 trials at high risk of performance bias (Aboutorabi 2013; Ahn 2017; Asgaonkar 2012; Coda 2014; Gould 1989; Hsieh 2018; Kanatli 2016; Morrison 2013; Powell 2005; Wenger 1989; Whitford 2007). We only judged two trials at low risk of performance bias (Jafarnezhadgero 2018; Solanki 2020). Due to the nature of the interventions, the participants could not be blinded.
We judged detection bias for self‐reporting outcomes at low risk in four trials (Abd‐Elmonem 2021; Coda 2014; Solanki 2020; Wenger 1989), and low risk for objective outcome assessment in four (Abd‐Elmonem 2021; Jafarnezhadgero 2018; Solanki 2020; Whitford 2007). We judged detection bias for self‐reporting outcomes at high risk in seven trials (Aboutorabi 2013; Ahn 2017; Asgaonkar 2012; Hsieh 2018; Jafarnezhadgero 2018; Powell 2005; Whitford 2007), and high risk for objective assessment in four (Asgaonkar 2012; Hsieh 2018; Morrison 2013; Powell 2005).
Incomplete outcome data
We judged seven trials at low risk of attrition bias (Abd‐Elmonem 2021; Coda 2014; Hsieh 2018; Jafarnezhadgero 2018; Kanatli 2016; Solanki 2020; Whitford 2007), five of which reported the reasons participants withdrew from the study (Abd‐Elmonem 2021; Coda 2014; Hsieh 2018; Kanatli 2016; Whitford 2007); two of which had no withdrawals (Jafarnezhadgero 2018; Solanki 2020). We assessed one trial at high risk of attrition bias due to an imbalance in the number of dropouts per group, and lack of reasons for withdrawal (Asgaonkar 2012). Apart from this, we judged that three studies were likely to have biased results due to participant withdrawals (Gould 1989; Morrison 2013; Wenger 1989), and it is largely unclear if the non‐completing participants biased the results, as the study authors did not report to which treatment groups these participants were randomised. Gould 1989's attrition rate was unbalanced, with group 1‐ 25/50; group 2‐ 10/25; group 3‐ 7/25 ; group 4‐ 10/25 finishing the study. In total, 52/125 (42%) children completed the four year study. Likewise, considerable attrition affected Wenger 1989 98/131 (75%) completions, and Morrison 2013 14/21 (68%) completions, reasons for attrition were not provided. Five studies provided insufficient information to permit a judgement of low risk or high risk of attrition bias, hence the risk of attrition bias was unclear (Aboutorabi 2013; Ahn 2017; Bok 2016; Khamooshi 2017; Powell 2005)
Selective reporting
We judged two trials at high risk of selective reporting bias, because of a discrepancy between the trial register and published outcomes, and the lack of follow‐up data for outcomes measured at baseline (Hsieh 2018; Kanatli 2016). Hsieh 2018 provided more outcomes in the published paper than were listed in the trial registry, and Kanatli 2016 only provided baseline data for Arch index scores, no follow‐up data.
We judged four studies at unclear risk of reporting bias, because of lack of gender‐related data, and lack of follow‐up data for outcomes measured at baseline (Bok 2016; Gould 1989; Morrison 2013; Wenger 1989). Gould 1989 stated that other lower extremity measurements (femoral, tibial torsions and knee varus/valgus) were to be subsequently reported, adding that knee valgus at age 5 years was "striking" (92.3%, of the 52/125 (42%) children who completed the 5 year trial) but without relationship to sex. In Wenger 1989 heel cord tightness and foot progression angles were only reported at baseline. Morrison 2013 assessed FPI‐6 and the LLAS were used for patient selection at baseline, but not further reported. In an immediate effects trial, without follow up, Bok 2016 selected children with painful flat feet, but then omitted pain as an outcome.
We judged eight studies at unclear risk of reporting bias as there was insufficient information to permit a judgement of low risk or high risk (Aboutorabi 2013; Ahn 2017; Asgaonkar 2012; Coda 2014; Jafarnezhadgero 2018; Powell 2005; Solanki 2020; Whitford 2007). In one study, outcome data were not clearly reported (Khamooshi 2017).
Other potential sources of bias
Five trials were at low risk of other bias. We assessed that four of them had no source of other bias (Abd‐Elmonem 2021; Hsieh 2018; Jafarnezhadgero 2018; Solanki 2020); the other one declared the source of funding, as well as independence from the funder for research design, conduct, and reporting (Morrison 2013). We judged all other trials at unclear risk of other bias due to insufficient information on funding sources.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
The effects of interventions are discussed by diagnostic population groups, then comparisons. Due to the diversity of trials and the differences in the interventions and outcomes reported, we were only able to pool data for JIA in meta‐analyses. Results of all other studies are reported separately.
1. Asymptomatic flat feet
Effects of interventions were assessed in 10 studies (Abd‐Elmonem 2021; Ahn 2017; Asgaonkar 2012; Gould 1989; Jafarnezhadgero 2018; Kanatli 2016; Khamooshi 2017; Solanki 2020; Wenger 1989; Whitford 2007). Results are presented in Table 1.
Custom foot orthoses (CFOs) compared to shoes
Studies reported findings at baseline and three months (Wenger 1989), at baseline, 3 months and 12 months (Whitford 2007). Jafarnezhadgero 2018 reported CFOs versus sham FOs (both feet were fitted with the same sports shoes) at baseline and after 4 months. Aboutorabi 2013 reported an immediate effect, but given the absence of follow‐up, we did not include the data in analyses.
Outcomes
Pain – CFOs may result in little to no difference in the proportion of children without pain (risk ratio (RR) 0.85, 95% confidence interval (CI) 0.67 to 1.07; 1 study, 106 participants; low‐certainty evidence; Analysis 1.1). There was an absolute reduction of 11.8% (4.7% fewer to 15.8% more); we downgraded for bias and imprecision.
Withdrawal due to adverse events – CFOs result in little to no difference in withdrawal due to adverse events (RR 1.05, 95% CI 0.94 to 1.19; 3 studies, 211 participants; low‐certainty evidence; Analysis 1.2). The absolute effect was 3.4% more (4.1 % fewer to 13.1 % more); we downgraded for bias and imprecision.
Function, quality of life, treatment success, and adverse events were not reported.
1.1. Analysis.

Comparison 1: Custom foot orthoses (CFOs) versus shoes for asymptomatic flat feet, Outcome 1: Proportion without pain
1.2. Analysis.

Comparison 1: Custom foot orthoses (CFOs) versus shoes for asymptomatic flat feet, Outcome 2: Withdrawal due to adverse events
Prefabricated foot orthoses (PFOs) compared to shoes
Four studies reported findings at three months (Wenger 1989), at 3 months and 12 months (Whitford 2007), at two years, three years, and five years of age (Gould 1989), and at one year (Asgaonkar 2012). Results are presented in Table 2.
Outcomes
Pain – PFOs likely result in little to no difference in the proportion of children without pain (RR 0.94, 95% CI 0.76 to 1.16; 1 study, 106 participants; low‐certainty evidence; Analysis 2.1). The absolute reduction was 4.7% (18.9% fewer to 12.6 % more); we downgraded for bias and imprecision.
Withdrawals due to adverse events – We are uncertain of the effects of PFOs on withdrawal due to adverse events (RR 0.99, 95% CI 0.79 to 1.23; 4 studies, 338 participants; very low‐certainty evidence; Analysis 2.2). The absolute reduction was 0.7% (15.2% fewer to 16.6% more); we downgraded for bias, imprecision, and indirectness due to variably aged participant samples between studies.
Function, quality of life, treatment success, and adverse events were not reported.
2.1. Analysis.

Comparison 2: Prefabricated foot orthoses (PFOs) versus shoes in asymptomatic flat feet, Outcome 1: Proportion without pain
2.2. Analysis.

Comparison 2: Prefabricated foot orthoses (PFOs) versus shoes in asymptomatic flat feet, Outcome 2: Withdrawal due to adverse events
CFOs compared to PFOs
Studies reported findings at three months (Wenger 1989), and at 3 months and 12 months (Whitford 2007). Results are presented in Table 3.
Outcomes
Pain – CFOs likely result in little to no difference in pain (RR 0.93, 95% CI 0.73 to 1.18; 1 study, 108 participants; low‐certainty evidence; Analysis 3.1). The absolute reduction was 7.4% (22.2% fewer to 11.1% more), we downgraded for bias and imprecision.
Proportion of withdrawals – CFOs result in no difference in withdrawals due to adverse events (RR 1.00, 95% CI 0.90 to 1.12; 1 study, 118 participants; low‐certainty evidence; Analysis 3.2). We downgraded for bias and imprecision.
Function, quality of life, treatment success, and adverse events were not reported.
3.1. Analysis.

Comparison 3: CFOs versus PFOs in asymptomatic flat feet, Outcome 1: Pain
3.2. Analysis.

Comparison 3: CFOs versus PFOs in asymptomatic flat feet, Outcome 2: Withdrawal due to adverse events
Abd‐Elmonem 2021 compared NMES and foot strengthening exercises with sham NMES and foot strengthening exercises, which did not comply with the comparison of interventions in this review.
Aboutorabi 2013 assessed immediate effects in both healthy children (N = 20) and those with flat feet (N = 30). The study reported that neither medical shoes nor PFOs showed significant gait effects in healthy children, however, the children with flat feet showed reduced centre of pressure (CoP) displacement with medical shoes (P < 0.05), and PFOs (not significant).
Ahn 2017 compared two types of CFO designs, which did not comply with the comparison of interventions in this review.
Kanatli 2016 compared an orthopaedic shoe with a control group, which did not comply with the comparison of interventions in this review.
Solanki 2020 compared anti‐pronation and conventional treatment (foot strengthening exercises, Faradic foot baths), with sham taping and conventional treatment, which did not comply with the comparison of interventions in this review.
2. Flat feet in juvenile idiopathic arthritis (JIA), or other clinical concerns
Two studies assessed these populations; Coda 2014 followed up at three months and six months; Powell 2005 followed up at three months.
CFO compared to shoes
Results are presented in Table 4; one trial examined this comparison (Powell 2005).
Outcomes
Pain – CFOs likely result in little or no difference in pain (0 to 10 VAS, 0 = no pain) compared to shoes (MD ‐1.50, 95% CI ‐2.78 to ‐0.22; 1 study, 28 participants; very low‐certainty evidence; Analysis 4.1). We downgraded for bias, imprecision, and indirectness; 1 study, 28 participants).
Function or disability – CFOs may result in a clinically important improvement in function or disability compared with shoes, measured with the FFI (MD ‐18.55, 95% CI ‐34.42 to ‐2.68; 1 study, 28 participants; low‐certainty evidence; Analysis 4.2; Figure 3); we downgraded for bias and imprecision.
Quality of life (QoL) – CFOs may result in a clinically important improvement in child‐rated QoL on the PedsQL (MD 12.10, 95% CI ‐1.60 to 25.80; 1 study, 25 participants; low‐certainty evidence; Analysis 4.3; Figure 4). CFOs may result in a clinically important improvement in parent‐rated QoL on the PedsQL (MD 9.01, 95% CI ‐4.08 to 22.10; 1 study, 26 participants; low‐certainty evidence; Analysis 4.3; Figure 4); we downgraded for bias and imprecision as the 95%CIs include both an improvement and no improvement, the trend towards significance in the CFO group, with the 5‐point minimally important clinical difference doubled ‐ PedsQL/Child 12‐point increase with CFO versus shoes, PedsQL/Parents 9‐point increase with CFO versus shoes low‐certainty evidence downgraded for bias and imprecision.
Treatment success – CFOs likely result in little to no difference in timed walking on the 50‐foot timed walk (50FTW; MD ‐1.33, 95% CI ‐2.77 to 0.11; 1 study, 28 participants; low‐certainty evidence; Analysis 4.4); we downgraded for bias and imprecision.
Withdrawals due to adverse events – CFOs likely result in little to no difference in withdrawals due to adverse events (RR 0.58, 95% CI 0.11 to 2.94; 1 study, 28 participants; low‐certainty evidence; Analysis 4.5; absolute reduction 9.7% (20.5% fewer to 44.8% more); we downgraded for bias and imprecision.
Proportion with adverse events – none reported
Proportion with serious adverse events – none reported
4.1. Analysis.

Comparison 4: CFOs versus shoes in juvenile idiopathic arthritis (JIA), Outcome 1: Pain
4.2. Analysis.

Comparison 4: CFOs versus shoes in juvenile idiopathic arthritis (JIA), Outcome 2: Function
3.

Forest plot of comparison: 4 CFOs versus shoes in JIA, outcome: 4.2 Function
4.3. Analysis.

Comparison 4: CFOs versus shoes in juvenile idiopathic arthritis (JIA), Outcome 3: Quality of life
4.

Forest plot of comparison: 4 CFOs versus shoes in JIA, outcome: 4.3 Quality of life
4.4. Analysis.
Comparison 4: CFOs versus shoes in juvenile idiopathic arthritis (JIA), Outcome 4: Treatment success (gait parameters)
4.5. Analysis.

Comparison 4: CFOs versus shoes in juvenile idiopathic arthritis (JIA), Outcome 5: Withdrawal due to adverse events
PFO compared to shoes
Results are summarised in Table 5; one trial examined this comparison (Powell 2005).
Outcomes
Pain – PFOs likely result in little to no difference in pain (MD 0.02, 95% CI ‐1.94 to 1.98; 1 study, 28 participants; very low‐certainty evidence; Analysis 5.1); we downgraded for bias, imprecision, and indirectness.
Function or disability – PFOs likely results in little to no difference in function (MD ‐4.17, 95% CI ‐24.40 to 16.06) or activity limitation between groups (MD ‐7.96, 95% CI ‐26.79 to 10.87; 1 study, 25 participants; low‐certainty evidence; Analysis 5.2); we downgraded for bias and imprecision. such that the PFO group activity was less limited at 3‐months, versus the shoe group, low‐certainty evidence downgraded for bias and imprecision. Powell 2005 also reported timed walking, which showed the PFO group slower than the shoe group over a distance of 50 feet, at 3‐month follow up MD ‐0.38 (95% CI ‐1.90, 1.14).
Quality of life – PFOs likely results in little to no difference in child‐rated QoL on the PedQL (MD ‐3.84, 95% CI ‐19.01 to 11.33; 1 study, 22 participants), indicating less pain in the PFO group low‐certainty evidence downgraded for bias and imprecision. PFOs likely results in no difference in or parent‐rated QoL on the PedQL (MD ‐0.64, 95% CI ‐13.22 to 11.94; 1 study, 22 participants; low‐certainty evidence; Analysis 5.3); we downgraded for bias and imprecision. Overall, Powell 2005 reported no significant improvement in QoL for either the PFO or the shoe group.
Treatment success – PFOs likely result in little to no difference in timed walking on the 50FTW (MD ‐0.38 seconds, 95% CI ‐1.9 to 1.14; 1 study, 25 participants; low‐certainty evidence; Analysis 5.4); we downgraded for bias and imprecision.
Withdrawal due to adverse events – PFOs likely results in little to no difference in withdrawals due to adverse events (RR 0.72, 95% CI 0.14 to 3.61; 1 study, 25 participants; low‐certainty evidence; Analysis 5.5); absolute reduction 6.5% (19.8% fewer to 60.2% more); we downgraded for bias and imprecision.
Proportion with adverse events – none reported
Proportion with serious adverse events – none reported
5.1. Analysis.

Comparison 5: PFOs versus shoes in JIA, Outcome 1: Pain
5.2. Analysis.

Comparison 5: PFOs versus shoes in JIA, Outcome 2: Function
5.3. Analysis.

Comparison 5: PFOs versus shoes in JIA, Outcome 3: Quality of life
5.4. Analysis.

Comparison 5: PFOs versus shoes in JIA, Outcome 4: Treatment success (Timed walking)
5.5. Analysis.

Comparison 5: PFOs versus shoes in JIA, Outcome 5: Withdrawal due to adverse events
CFO compared to PFO
Results are summarised in Table 6; we pooled data from two studies (Coda 2014; Powell 2005).
Outcomes
Pain – CFOs may result in little to no difference in pain (MD ‐1.48, 95% CI ‐3.23 to 0.26; 2 studies, 87 participants; low‐certainty evidence; Analysis 6.1;Figure 5); we downgraded for bias and imprecision. Sensitivity analysis, performed by excluding the study that did not blind participants (Powell 2005), was not significant due to overlapping confidence intervals (MD ‐2.88; 95%CI ‐15.70 to 9.94).
Function or disability – CFOs may result in little to no difference in function, on the FFI (MD ‐14.38, 95% CI ‐30.22 to 1.46; 1 study, 27 participants; low‐certainty evidence; Analysis 6.2); we downgraded for bias and imprecision.
Quality of life – CFOs may result in little to no difference in child‐rated QoL, using the PedsQL (MD 8.64, 95% CI ‐3.90 to 21.18; 1 study, 83 participants) or parent‐rated QoL, using the PedsQL (MD 2.94, 95% CI ‐11.00 to 16.88; 1 study, 84 participants; low quality evidence; Analysis 6.3; Figure 6); we downgraded for bias and imprecision. QoL was also reported at six months by Coda 2014; child‐rated PedsQL scores were 89.67 (17.92) with custom‐prefabricated foot orthoses (CPFOs) and 83.63 (27.14) with PFOs; parent‐rated PedQL scores were 83.70 (31.5) for CPFOs and 84.47 (35.58) for PFOs, indicating marginally improved in QoL at six months rated by children, but not by parents, low‐certainty evidence, downgraded for bias and imprecision.
Treatment success – CFOs may result in little difference in timed walking at 3 months, assessed with the 50FTW (MD ‐0.95 seconds, 95% CI ‐1.88 to ‐0.02; 1 study, 27 participants; low‐certainty evidence; Analysis 6.4); we downgraded for bias and imprecision.
Withdrawals due to adverse events – CFOs may result in little difference in withdrawals due to adverse events (RR 0.80, 95% CI 0.13 to 4.87; 2 studies, 87 participants; low‐certainty evidence; Analysis 6.5); we downgraded for bias and imprecision.
Proportion with adverse events – none reported
Proportion with serious adverse events – none reported
6.1. Analysis.

Comparison 6: CFOs versus PFOs in JIA, Outcome 1: Pain
5.

Forest plot of comparison: 6 CFOs versus PFOs in JIA, outcome: 6.1 Pain
6.2. Analysis.

Comparison 6: CFOs versus PFOs in JIA, Outcome 2: Function
6.3. Analysis.

Comparison 6: CFOs versus PFOs in JIA, Outcome 3: Quality of life
6.

Forest plot of comparison: 6 CFOs versus PFOs in JIA, outcome: 6.3 Quality of life
6.4. Analysis.

Comparison 6: CFOs versus PFOs in JIA, Outcome 4: Treatment success (timed walking)
6.5. Analysis.

Comparison 6: CFOs versus PFOs in JIA, Outcome 5: Withdrawal due to adverse events
3. Painful (symptomatic) flat feet
Assessed in two studies; Hsieh 2018 compared customised PFOs (adapted by heat gun moulding) versus no treatment and followed up at 12 weeks). Results are presented in Table 7. Bok 2016 outfitted all children with four sets of shoes with different degrees of orthotic, and measured immediately. Results are presented below.
PFO compared to shoes
Outcomes
Pain – not reported
Function or disability – PFOs result in little to no difference in function, assessed with PODCI (global function MD 3.00, 95% CI 2.28 to 3.72; 1 study, 50 participants; low‐certainty evidence; Analysis 7.1); we downgraded for bias and imprecision.
Quality of life – PFOs result in little to no difference in QoL, assessed with the PedsQL (total score MD 1.80, 95% CI 1.07 to 2.53; 1 study, 50 participants; low‐certainty evidence; Analysis 7.2); we downgraded for bias and imprecision.
Treatment success – not reported
Proportion of withdrawals – not reported
Proportion with adverse events – none reported
Proportion with serious adverse events – none reported
7.1. Analysis.

Comparison 7: PFOs versus shoes in symptomatic flat feet, Outcome 1: Function
7.2. Analysis.

Comparison 7: PFOs versus shoes in symptomatic flat feet, Outcome 2: Quality of life
Bok 2016 (21 children) reported the immediate effects of treatment, three designs of CFOs, inverted at 0, 10, and 30 degrees, compared to shoes with no orthotics. Foot function was evaluated as peak pressure, maximum force, and contact area, using the Pedar in‐shoe apparatus, in six foot regions (or 'masks'): medial forefoot (MF), central forefoot (CF), lateral forefoot (LF), medial midfoot (MM), lateral midfoot (LM), and rearfoot (RF).
Function or disability – CFOs with 0, 15, or 30 degrees inversion reduced MF and RF peak pressure (P < 0.001); increased maximum mid‐foot plantar pressure (especially 30 degree inversion); and increased the contact area at the MM and RF.
None of the other outcomes were reported.
4. Developmental co‐ordination disorder (DCD)
One study of 22 British boys with DCD examined the effects of foot orthoses (Morrison 2013).
CFO compared to shoes
Outcomes
Function or disability – the six‐minute walk test (P = 0.43), and spatio‐temporal parameters (Gaitrite™ system) did not differ between the CFO (N = 9) and the control (shoe) groups (N = 5) following the seven‐week rehabilitation programme. The CFO group walked a median of four metres further, and the control group walked a median of 15 metres further (1 study, 14 participants; Analysis 8.1).
Treatment success – no differences were found between groups for the 6MWT (P = 0.43), cadence (P = 0.019), or double‐support (P = 0.042), following the seven‐week rehabilitation programme (1 study, 14 participants; Analysis 8.1).
Withdrawals due to adverse events – data for proportion of children who withdrew due to adverse events were not specified.
Pain, quality of life, and adverse events – not reported
8.1. Analysis.
Comparison 8: CFOs versus shoes in DCD flat feet, Outcome 1: Gait parameters
| Gait parameters | ||||
| Study | Median | IQR | significance | group (N) |
| six minute walk test (6MWT; m) | ||||
| Morrison 2013 | 351 | 312.5 to 433.25 | P = 0.43 | CFO (9) |
| 390 | 375.5 to 437 | shoes (5) | ||
| cadence (steps/min.) | ||||
| Morrison 2013 | 114.7 | 108.6 to 124.9 | P = 0.019 | CFO (9) |
| 131.9 | 123.8 to 145 | shoes (5) | ||
| double support (%) | ||||
| Morrison 2013 | 25 | 21.7 to 26.9 | P = 0.042 | CFO (9) |
| 21.5 | 19.3 to 22.3 | shoes (5) | ||
| stride (m) | ||||
| Morrison 2013 | 107.5 | 102.2 to 122.3 | P = 0.23 | CFO (9) |
| 102 | 95.2 to 111.9 | shoes (5) | ||
Discussion
Summary of main results
The 16 randomised controlled trials (RCT) included in this updated Cochrane Review signify more than a five‐fold increase in available evidence from clinical trials since the previous review in 2010, which included just three RCTs. However, whilst there is more literature to peruse and critique, there continues to be heterogeneity, which limits data pooling and precludes meta‐analysis of all studies. Two of the 16 trials provided only immediate effects data, and hence, we only included their description in this review, rather than including their data in any analyses.
There are two discrete groups of RCTs investigating foot orthoses to treat the typical paediatric flat foot: (1) those addressing healthy children with asymptomatic flat feet: 10 trials (Abd‐Elmonem 2021; Ahn 2017; Asgaonkar 2012; Gould 1989; Jafarnezhadgero 2018; Kanatli 2016; Khamooshi 2017; Solanki 2020; Wenger 1989; Whitford 2007), and (2) those addressing flat feet in children with juvenile idiopathic arthritis (JIA): 2 trials (Powell 2005, Coda 2014); and other clinical concerns: painful flat feet: 1 trial (Hsieh 2018); developmental co‐ordination disorder (DCD): 1 trial (Morrison 2013); immediate intervention effects only: 2 trials (Aboutorabi 2013, Bok 2016).
Asymptomatic flexible flat feet in healthy children
Firstly, and most prodigiously, 10 Ten RCTs addressed the most commonly presenting paediatric foot concern of asymptomatic flat feet; interventions included shoes, foot orthoses (FOs), and exercises. Five of these 10 RCTs included a non‐intervention control group (Asgaonkar 2012; Kanatli 2016; Khamooshi 2017; Wenger 1989; Whitford 2007), three trials included a sham control group (Abd‐Elmonem 2021; Jafarnezhadgero 2018; Solanki 2020), and two trials intervention comparisons alone (Ahn 2017; Gould 1989). Whilst all studies address the typical presentation of flexible and asymptomatic flat feet in healthy children, there is considerable heterogeneity within the 805 initially enrolled children: age ranged from 11 months to 15 years. Initial sample sizes ranged from 30 to178, with seven trials enrolling less than 100 participants (Ahn 2017; Asgaonkar 2012; Jafarnezhadgero 2018; Kanatli 2016; Khamooshi 2017; Solanki 2020; Abd‐Elmonem 2021). One trial included girls only (N=60) (Khamooshi 2017). One trial included boys only (N=30) (Jafarnezhadgero 2018). The period of follow up ranged from 4 weeks to 5 years and study retention ranged from 42% to 100% (655/805 completions, average 81%). Interventions tested varied across the 10 studies comprising foot orthoses (heel cups, UCBL orthoses, CFOs, PFOs, CPFOs, valgus insoles, TCFOs, RFOs), shoes (straight last, straight last with cookie, orthopaedic, orthopaedic with arch support, usual footwear, supplied trial footwear, medical shoes), taping, NMES, and exercises (foot exercises, foot and core exercises). The outcome measures varied across all studies: x‐rays (5), pedoscope/footprints (3), pain (2), physical cost (2), self‐perception (1), motor skills (2), static measures (3), gait (1), joint laxity (1), kinematic and kinetic gait analysis (1). The included studies were conducted across 30 years, between 1989 and 2021. The most recent trials (Solanki 2020; Abd‐Elmonem 2021) discontinued the demonstrably fruitless investigation of foot orthoses for asymptomatic healthy children with flat feet, and implemented foot strength as the focus of intervention.
Flat feet in children with JIA, or other clinical concerns
Two RCTs addressed children with JIA (Powell 2005; Coda 2014), and provided data in a matter to enable us to pool them in meta‐analysis (Fellas 2017b). The small sample sizes of the studies made inference insufficient to guide practice, i.e. in all group comparisons, the mean difference was larger than the minimal clinically important difference (MCID) of 8 mm on a 100 mm visual analogue scale (VAS) for pain at six months, within large confidence intervals (CI). Overall results were inconclusive to support the use of FOs in JIA foot and ankle pain.
Other clinical concerns of flat feet in children with foot pain, DCD, and trials with only immediate effects comprise the remaining four trials that addressed either immediate intervention effects with no follow‐up data, in small samples with and without foot pain (Aboutorabi 2013; Bok 2016); a small sample with one‐third attrition over seven weeks in British boys identified as having a DCD (Morrison 2013); or a small sample of healthy children with painful flat feet (non‐comparable study groups at baseline) over 12 weeks (Hsieh 2018). Separately, or combined, these four trials offer almost nothing, and at most, a possible trend. The inclusion of a trial addressing children with flat feet and foot pain whilst non‐controversial, is also limited given the control group (only) received analgesics (Hsieh 2018).
Overall completeness and applicability of evidence
As a consequence of data heterogeneity, we were unable to conduct meta‐analyses for the studies addressing asymptomatic flat foot presentation in healthy children. The JIA meta‐analysis, with two studies, presents limited findings due to small samples. All studies were single‐blind trials, with the investigators being aware of the type of intervention received, which may have resulted in performance and detection (assessor) bias. Blinded healthcare providers may also differ from non‐blinded ones in their degree of attention to the children, or in their use of alternative forms of care. Time frames varied across the trials, ranging from four weeks to five years, making comparisons difficult. The age ranges differed across studies, and therefore, it is difficult to generalise about foot orthoses as a treatment for paediatric flat feet in children, aged 11 months to 16 years. There was limited technical information about the manufacturing process and prescription of the varying types of FOs used in the trials. Three studies recruited children of single sex (boys only (Jafarnezhadgero 2018; Morrison 2013); girls only (Khamooshi 2017)), hence, it is unclear as to whether results apply to both sexes. Twelve studies recruited both sexes. One study did not specify sex (Solanki 2020). The shoes used across the studies varied, the effects of which are unclear; see Table 10. However, the use of usual footwear favours external validity, and has wider community health relevance. Adverse effects were reported in one of the 16 trials, with no adverse effects from wearing FOs and shoes (Powell 2005). However, with only one trial reporting on this variable, and given the many limitations of the data available, caution must be noted. Gait analysis was included in four studies (Asgaonkar 2012; Bok 2016; Jafarnezhadgero 2018; Morrison 2013), but two studies only assessed the immediate effects of the intervention (Aboutorabi 2013; Bok 2016). More recent trials addressed foot strength, but provided no useful clinical information, given the small samples and short trial durations in healthy, asymptomatic participants (Abd‐Elmonem 2021; Solanki 2020).
The main outcome measures reported in the 16 trials, included pain, function, and quality of life. Three studies investigated the primary outcome specified by this review (pain reduction), but differentially over three months and 12 months, in children with JIA (Coda 2014; Hsieh 2018; Powell 2005). It is unclear if the Varni‐Thompson Paediatric Pain questionnaire (Varni 2002), used by Powell 2005 related to symptoms in the children's feet and lower extremities only, or if whole body pain was included. One trial conducted a post hoc subgroup analysis of pain reduction for those children who reported lower‐limb pain at baseline, using a 0 to 10 VAS (Whitford 2007). However, the study design did not have an a priori hypothesis to specifically test the effects of CFOs or PFOs on the treatment of painful flat feet. Asgaonkar 2012 measured pain using a 0 to 10 VAS, and Hsieh 2018 assessed pain using the PODCI (Daltroy 1998). Three studies directly investigated function as an outcome (Hsieh 2018; Powell 2005; Whitford 2007), whilst a further five studies afforded deference to gait as function (Aboutorabi 2013; Asgaonkar 2012; Bok 2016; Khamooshi 2017; Morrison 2013). The intervention effects after seven weeks in children with DCD (Morrison 2013), after 8 weeks in children with JIA (Powell 2005), after 12 weeks in children with painful flat feet (Hsieh 2018), and after 1 year in children with asymptomatic flat feet (Whitford 2007) were unsurprisingly, variable. Given the especially disparate clinical presentations, range of follow‐up periods, and small sample sizes of these studies, only the substantial trial by Whitford 2007 (N = 178), warrants further comment. Whitford 2007assessed both motor proficiency and exercise efficiency, and found no difference between the two intervention groups (CFO and PFO) versus the control group after 12 months.
Four studies investigated quality of life in children with JIA and foot pain (Coda 2014; Powell 2005), in children with asymptomatic flat feet (Whitford 2007), and in children with painful flat feet (Hsieh 2018). radiographic outcomes (x‐rays) were assessed in five studies in children with pain‐free flat feet (Abd‐Elmonem 2021; Ahn 2017; Gould 1989; Kanatli 2016; Whitford 2007), with follow‐up ranging from four months to five years (Table 12). Two trials involved very young children (11 to 14 months (Gould 1989), and 17 to 72 months (Kanatli 2016), which captured the expectedly more pronounced, and normal developmental flat foot posture and indistinct foot skeletal morphology, given the physiologic age (Evans 2012; Gijon‐Nogueron 2019; Martinez‐Nova 2018; Pfeiffer 2006). Unsurprisingly, the studies without control groups reported 'improvement' of one intervention group over another, with no meaningful comparison from baseline and differing sample ages and sizes (Ahn 2017; Gould 1989). Two studies with control groups found no difference in x‐ray findings between groups (Kanatli 2016; Whitford 2007), however, Abd‐Elmonem 2021 reported significant improvement in x‐ray parameters after four months of NMES versus sham‐NMES (both treatment and control groups also improved from receiving foot strength exercises).
The intervention effects after seven weeks in children with DCD (Morrison 2013), after 8 weeks in children with JIA (Powell 2005), after 12 weeks in children with painful flat feet (Hsieh 2018), and after 1 year in children with asymptomatic flat feet (Whitford 2007) were unsurprisingly, variable. Given the especially disparate clinical presentations, range of follow‐up periods, and small sample sizes of these studies, only the substantial trial by Whitford 2007 (N = 178), warrants further comment. Whitford 2007 assessed both motor proficiency and exercise efficiency, and found no difference between the two intervention groups (CFO and PFO) versus the control group after 12 months. Two studies with control groups found no difference in x‐ray findings between groups (Kanatli 2016; Whitford 2007), however, Abd‐Elmonem 2021 reported significant improvement in x‐ray parameters after four months of NMES versus sham‐NMES (both treatment and control groups also improved from receiving foot strength exercises).
Quality of the evidence
We assessed the quality evidence using GRADE for the major outcomes. We rated the evidence at low or very low certainty. No study returned high quality evidence for any outcome. Two studies were found to be of moderate quality evidence (Whitford 2007, Coda 2014). The remainder of the studies were rated as low and very low quality of evidence across all outcomes. We found 12 unpublished trials (Ongoing studies), which includes seven small samples of healthy children, 2 samples of children with JIA, 1 sample of children with neuromuscular disorders, and one large sample (N=1055) registered as two trials (Characteristics of ongoing studies).
For the major outcomes in asymptomatic feet, evidence for pain, function, quality of life and withdrawals due to adverse events was downgraded to low‐certainty due to possible selection, performance and detection biases, as well as imprecision due to data from mostly single studies. For the major outcomes in symptomatic feet in children with JIA, evidence for pain was downgraded to very low‐certainty due to detection bias as children and their parents knew which treatment they had, which may have affected their assessment of pain; as well as due to imprecision and indirectness. Evidence was mostly of low‐certainty for function, treatment success, quality of life and withdrawal due to adverse events in children with JIA, downgraded for bias and imprecision.
The GRADE findings are reported in the SoF tables Pain reduction was assessed in five trials (Asgaonkar 2012; Coda 2014; Powell 2005; Wenger 1989; Whitford 2007), improvement in gait and function was assessed in six trials (Aboutorabi 2013; Bok 2016; Khamooshi 2017; Morrison 2013; Powell 2005; Whitford 2007; Solanki 2020), and QoL in three trials (Coda 2014; Powell 2005; Whitford 2007). The findings from four trials including x‐ray imaging (Kanatli 2016; Ahn 2017; Gould 1989; Wenger 1989), are included in the recent systematic review of specifically x‐ray findings (Choia 2020), which similarly found low level certainty from the evidence.
Due to the poor methodological quality of the trials, and heterogeneity of the studies within this review, definite conclusions could not be made. Further, the necessity of further attention to healthy children with flexible, pain‐free flat feet is now demonstrably unfounded (Kanatli 2016, Martinez‐Nova 2018).
Potential biases in the review process
Two authors were involved in the previous systematic review in 2010 (Rome 2010). AE has several publications in this field. AE and KR, are co‐authors on an earlier literature review (MacKenzie 2012). However, all aspects of this review have been carried out with author independence, decisions reached by consensus, and reviewed by the Cochrane Musculoskeletal Group. We followed the Cochrane methodology to reduce sources of potential bias in the review process.
Agreements and disagreements with other studies or reviews
This updated Cochrane Review expanded the previous findings of Rome 2010, which stated that low quality evidence negated conclusive evidence on the benefits of non‐surgical interventions for flat feet. These findings are largely in agreement with the conclusions of a prior critical review (MacKenzie 2012).
Low quality evidence from studies addressing the asymptomatic flexible paediatric flat foot have occupied too much of the medical literature, and for too long (Evans 2021). Another systematic review, which included studies from lower levels of the evidence hierarchy, concluded: "FOs show potential as a treatment method for children with flexible pes planus" despite the poor methodological quality of studies (Dars 2018). A more recent systematic review evaluated the long‐term structural effect of orthoses for paediatric flexible flatfoot and did not support its use on the medial longitudinal arch, determined by radiographic imaging (Choia 2020). Further, it was revealed that flat feet in young children improved with growth, regardless of the type of footwear used. This is now a repeated theme across the literature (Gijon‐Nogueron 2019; Kanatli 2016; Martinez‐Nova 2018; Pfeiffer 2006; Wenger 1989; Whitford 2007), and there is no strong evidence that the long‐term use of foot orthoses improved the structure of flat feet in children (Choia 2020).
Hence, a decade later, more trials have contributed only low level evidence. It is concerning that this situation is continued in the majority of currently registered trials. We identified several ongoing trials, yet to be completed and published (ACTRN12616001082493; CTRI/2018/07/014989; CTRI/2019/08/020925; IRCT2017082235517N1; ISRCTN14602568; ISRCTN49672274; KCT0001717; NCT02414087; NCT02633566; NCT03151538; NCT04104555; NCT04410926). Concerningly, most of these trials are designed similarly to those we included in this review, i.e. most address the use of foot orthoses as an intervention for asymptomatic paediatric flat feet, in small samples of young and healthy children.
Authors' conclusions
Implications for practice.
We conclude that there is no evidence to support the efficacy of foot orthoses (FO) for children with asymptomatic (painless) flat feet. The evidence from randomised controlled trials (RCT) is thwarted by biases affecting the study quality, diverse participants, varied clinical presentations, ranging interventions, disparate outcome measures, small sample sizes, high attrition, and lack of follow‐up.
Further, the evidence across four decades remains very limited, with ill‐defined conclusions about foot orthoses for treating paediatric flat feet. Very‐low to low‐certainty evidence from two studies evaluating the effectiveness of FOs for foot and ankle pain in children with juvenile idiopathic arthritis (JIA) found inconclusive evidence on the benefits of foot orthoses on pain, child‐ and parent‐rated quality of life, quality of life, or withdrawals due to adverse events.
Whilst less frequent, children with painful flat feet are directed to use low‐cost PFOs with well‐selected footwear. Footwear, a given influence for FOs, is demonstrated to alter gait and foot mobility, and hence requires regard in both clinical and research settings. Available normative foot posture data, triage principles, and wider diagnoses must be considered before any intervention occurs.
Given the lack of difference between the low cost PFOs and the expensive CFOs, and the benefits of early treatment, clinicians may consider immediate use of PFOs for JIA foot and ankle pain. JIA is indisputably consequential, and many children experience disabling foot and lower limb pathology despite best available pharmaceutical measures.
It is hoped that this review will inform health professionals, researchers, parents, and children alike, so that concerns about paediatric flat foot presentations are triaged in accordance with best available evidence.
Implications for research.
This review identified low to very low‐certainty evidence that the effect of foot orthoses on pain, function, and quality of life in children with asymptomatic (painless) flat feet is uncertain.
Recently, the normal, age foot posture in children was published, and shows that children are expected to have varying flat feet as a part of normal growth (without foot pain, left and right feet looking similar and flat feet should reduce with age ‐ angelaevanspodiatrists.com.au/evidence-essentials-blog-8-june-2019/). The availability of normative and prospective foot development data, dismisses most flatfoot concerns, and negates continued attention to this topic. The agenda for researching flat feet in children should target children with indisputable foot pathology from discrete diagnoses, namely, foot pain and diagnoses of JIA; syndromes associated with hypermobility (e.g. Down, Ehlers‐Danlos, Marfan, etc); neuromuscular conditions (e.g. cerebral palsy; muscular dystrophy or atrophy), and perhaps conditions with rising public health implications (e.g. physical inactivity, obesity, hypertension, diabetes). In such warranted investigations, the use of similar and validated outcome measures would allow the combination of results, and the ability to pool estimates, to obtain meaningful consensus. Any future trials in relevant cohorts must be adequately powered with participants, and evaluate whether any group differences are both real and clinically worthwhile. Short‐term benefit should be established, in order to justify the considerable resources and ethical implications for lengthy studies. Follow‐up periods of at least five years are needed due to lower limb and foot growth in children. This will avail observation in children that may predict any predisposition to foot and gait problems as adults. If no such differences are observed, there would be no need to treat flat feet in children, regardless of the aetiology.
Dispelling misconceptions regarding paediatric foot posture will save resources in the form of clinical consultations, the cost of unnecessary intervention, and misidentification of 'deficiency' (Evans 2021). There are two ongoing trials addressing children with JIA (ACTRN12616001082493; ISRCTN49672274), and one large scale trial for children with symptomatic flat feet (NCT04104555), but many current trials will add little to the body of evidence, and probably waste research resources.
The agenda for researching asymptomatic flat feet in healthy children is now firmly closed, as there is no justification for wasting research and healthcare resources on flat feet in healthy children that do not hurt. Instead, a new and targeted research agenda, addressing children with indisputable foot pathology and associated diagnoses is definitely indicated, and further, encouraged. Future updates of this review will address only relevant paediatric foot conditions.
Feedback
Feedback, June 2011
Summary
Date of Submission: 20 June 2011
Name: Alan Cooper BSc
Personal Description: Occupation UK Podiatrist
Feedback: When one of my daughters was born 32 years ago, the first thing I noticed (as soon as she was delivered) was her left foot laying flat against the tibia, and fibula ‐ so much so that I observed a clear imprint of the outline of the bones. At the time, I was in my 2nd year at Podiatry college. The condition was undoubtedly T. calcaneovalgus, and in my opinion, severe. The foot was dorisflexed, abducted, which in my view as an adult it would have exhibited severe abnormal pronation, with an everted calcaneum, a flat arch with the whole of the mid‐tarsal joint contacting the ground plantar grade. An orthopaedic surgeon was asked to look at her feet, and as he felt all the components of the foot were there, we were referred to a physiotherapist for treatment. The treatment was of course manipulation of the foot, with splinting for several months. The splint was removed six or seven times a day, and the foot manipulated. Today, looking at my daughter's feet, there is no difference between them, and both stand correctly in their neutral position. Interestingly, on our last appointment with the physiotherapist, she had asked a father to bring in his child with the same condition, who was born at the same time. The child had not received manipulation, and as a consequence, there was no real change in the foot's appearance from birth.
Over my 30 years of practice, I have seen many conditions of pes planus, and wondered if there was a relationship between this and untreated mild, moderate, or severe T. calcaneovalgus: does this go unseen, and untreated? If it was picked up by the podiatrist at birth, is it possible that the condition of pes planus in many folk could be eliminated? Just recently, a toddler was referred to me with bilateral flat feet: heels everted, feet abducted, with metatarsals, and metatarsal joints appearing to be either close, or contacting the ground. The father had almost a cavoid foot, and the mother's foot was quite normal. Bearing in mind the limited evidence for many treatments, I think I shall recommend small orthosis, together with some manipulation. When I have enquired of old, and older clients in the past, the treatment appeared to be just exercises, which seemed to have made no difference to the adult foot at all.
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organisation or entity with a financial interest in the subject matter of my feedback.
Reply
27 June 2011
Dear Mr Cooper
Thank you for your response relating to the Cochrane Review. Dealing with children’s feet is an issue. Dr Evans recently described a traffic light system for treating flat feet that has been strongly debated [1‐3]. From a clinical viewpoint, identifying a foot problem at a very young age is still based upon clinical expertise and personal choice for management [4]. Unfortunately, this is the lowest form of evidence [5], and further work is needed in this area to ensure that clinicians are using an evidence‐base approach to manage flat feet in children.
From the Cochrane Review, we only found reports on the use of foot orthoses for the management of paediatric flat feet [4]. The use of manipulation as a form of treatment was not reported in the literature. However, in a recent critical review [6], we found one article that evaluated a group of children who followed a rehabilitative programme versus a historical group of children who had been treated with insoles and orthopaedic footwear [7]. Over a two‐year period, 300 children (mean age was 3.4 years; 184 male, 116 female) with bilateral flexible flatfoot (600 feet) were recruited and underwent a rehabilitative programme for a mean period of 2.8 years. The rehabilitative programme consisted of simple therapeutic exercises, which could be easily learned by both patients and their caregivers. These children were compared to a historical group of children (674 feet) who had been treated in a paediatric department for infantile flexible flatfoot with the use of foot orthoses. The results demonstrated that in the two groups (children treated with rehabilitation and children treated with foot orthoses), the rehabilitative approach seemed to be more effective. The authors suggested that rehabilitation has a marginal influence on the natural history of paediatric valgus flexible flatfoot, even though it plays a role in maintaining good flexibility of the flatfoot, thus limiting functional impairment.
In summary, the use of exercise is commonly reported, but the evidence is limited. Future studies need to further investigate the potential effect of rehabilitative exercises, and foot manipulation, and continue to investigate the efficacy of foot orthoses, and footwear modifications, and consider the effect of other contemporary clinical interventions.
References
Evans AM. The flat‐footed child ‐ to treat or not to treat: What is the clinician to do? J Am Podiatr Med Ass 2008; 98: 386‐393.
D'Amico JC. The flat‐footed child ‐ to treat or not to treat: What is the clinician to do? J Am Podiatr Med Ass 2009; 99:267.
Bresnahan P. The flat‐footed child ‐ to treat or not to treat: What is the clinician to do? J Am Podiatr Med Ass 2009; 99: 178.
Rome K, Ashford RL, Evans AM. Non‐surgical interventions for pediatric pes planus. Cochrane Database Syst Rev 2010; 7(CD006311):DOI:10.1002
Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB. Evidence‐based Medicine. How to practice and teach EBM. 2000. 2nd edition. Churchill Livingstone, UK.
Evans AM, Rome K. A review of the evidence for non‐surgical interventions for flexible paediatric flat feet. Eur J Phys Rehabil Med 2011; 47: 1‐2.
Riccio I, Gimigliano AE, Gimigliano R, Porpora G, Iolascon G. Rehabilitative treatment in flexible flatfoot: a perspective cohort study Musculoskeletal Surg 2009; 93:101–1
Contributors
Professor Keith Rome, Professor Podiatry, AUT University, New Zealand
Dr Angela Evans, Senior Research Fellow, University of South Australia, Australia
Feedback, May 2012
Summary
Date of Submission: 12 May 2012
Name: A. van Heukelum
Personal Description: Occupation General Practice Resident
Feedback: During our residency, all students are required to make an "critically appraised topic", based on a personal case during practice. The problem of painful flat feet or pes planus came to my attention, so I was very pleased to find this review on the subject, and would like to thank you for your clear, insightful review. Clearly, much more research needs to be done to make evidence. I have one question though, about the included article of Powell (2005). As I understood, the inclusion criterium, amongst others, was patients with pes planus. In the short description of the article by Powell (2005), it was stated that it included cases of pes planus with juvenile idiopatic arthritis, although in this article, this was not clear to me. Could you perhaps give more details about why the participants of this study would have pes planus?
Submitter has modified conflict of interest statement:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Pes planus (or a valgus/pronated rearfoot posture) is by far the most common presentation in juvenile idiopathic arthritis, but hindfoot varus posture can also occur, and is usually associated with significantly reduced joint range of motion. I have included three references that may assist you:
Ferrari J. A review of foot deformities seen in juvenile chronic arthritis. The Foot, 1998; 8: 193‐196.2.
Mavidrou A et al. Conservative management of the hindfoot in juvenile chronic arthritis. The Foot, 1991; 1; 139‐143.3.
Hendry G et al. A survey of foot problems in juvenile idiopathic arthritis. Musculoskeletal Care, 2008; 6: 221‐232.
The Cochrane review team, at the time of reviewing the literature, made a decision to include the Powell (2005) study, based upon the current evidence of pes planus in this population.
One issue that has not been addressed, and is debated in the Cochrane Review and other articles we have published, is the definition of pes planus. Perhaps one day a consensus of opinion will address the problem.
Contributors
Professor Keith Rome, Professor Podiatry, AUT University, New Zealand
What's new
| Date | Event | Description |
|---|---|---|
| 11 November 2021 | New citation required but conclusions have not changed | A total of 19 studies are included in this version of the review, an addition of 16 new studies since the last version was published in 2010. |
| 11 November 2021 | New search has been performed | Review updated, and current until 01 September 2021 |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 7, 2010
| Date | Event | Description |
|---|---|---|
| 25 September 2012 | Feedback has been incorporated | New feedback |
| 10 August 2011 | Feedback has been incorporated | Feedback added |
Acknowledgements
The authors wish to thank Renea Johnston, Managing Editor, Cochrane Musculoskeletal Group, for updating the search strategy, and for helpful advice, and Sheila Cyril, Assistant Managing Editor, Cochrane Musculoskeletal Group for running the updated searches. We would like to thank the following people for translating articles into English: Miranda Cumpston, formerly of the Cochrane Musculoskeletal Group; Gustavo Zanoli, Cochrane Musculoskeletal Group; Anette Bluemle, German Cochrane Centre; Lorenzo Moja, Italian Cochrane Centre; and Malgorzata Bala, Department of Internal Medicine, Jaiellonian University Medical College, Krakow, Poland. We thank the peer reviewers: Dr Andrea Coda, Senior Lecturer in Podiatry, PhD, Hons (BSc) Pod. School of Health Sciences, College of Health, Medicine and Wellbeing, The University of Newcastle. Priority Research Centre Health Behaviour (PRCHB), Hunter Medical Research Institute (HMRI), NSW, Australia, and Dr Helen A Banwell (Ph.D), Allied Health and Human Performance, University of South Australia, Adelaide, South Australia.
Appendices
Appendix 1. CENTRAL, in the Cochrane Library search
Search Name: Cochrane A028‐R limit date 2009 ‐ 300720
Date Run: 02/09/2021 11:00:04
ID Search Hits
#1 MeSH descriptor: [Flatfoot] explode all trees 60
#2 flat next foot*:ti,ab 81
#3 flatfoot:ti,ab 53
#4 flat next feet:ti,ab 103
#5 flatfeet:ti,ab 5
#6 pes next planus:ti,ab 44
#7 painful next foot:ti,ab 26
#8 pes next planovalgus:ti,ab 4
#9 "posterior tibial tendon dysfunction":ti,ab 9
#10 subtalar:ti,ab 163
#11 sub* next talar:ti,ab 4
#12 calcane*:ti,ab 723
#13 heel next bone*:ti,ab 35
#14 medical arch*:ti,ab 1702
#15 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) 2715
#16 MeSH descriptor: [Musculoskeletal Diseases] explode all trees 43202
#17 MeSH descriptor: [Neuromuscular Diseases] explode all trees 10781
#18 MeSH descriptor: [Nervous System Diseases] explode all trees 87486
#19 ehlers‐danlos:ti,ab 51
#20 "down* syndrome":ti,ab 747
#21 trisomy:ti,ab 215
#22 mongolism:ti,ab 2
#23 "inflammatory arthritis":ti,ab 439
#24 juvenile near/3 arthritis:ti,ab 839
#25 (#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) 124705
#26 MeSH descriptor: [Diabetes Mellitus, Type 1] explode all trees 5764
#27 (diabet* or IDDM):ti,ab 95002
#28 MeSH descriptor: [Diabetes Insipidus] explode all trees 73
#29 mellitus:ti,ab 28759
#30 diabet* next insipidus:ti,ab 123
#31 diabet* next mellitus:ti,ab 28693
#32 (#26 OR #27) 95299
#33 # 32 NOT (#26 OR #29 OR #31) 127788
#34 (#32 OR #33) 217218
#35 MeSH descriptor: [Joint Instability] explode all trees 777
#36 ligament* next laxity:ti,ab 48
#37 pronat*:ti,ab 521
#38 malalignment:ti,ab 297
#39 (#35 OR #36 OR #37 OR #38) 1626
#40 (#25 OR #34 OR #39) 329079
#41 (#15 AND #40) 875
#42 child:ti,ab 122707
#43 children:ti,ab 122705
#44 childhood:ti,ab 15011
#45 infant:ti,ab 16230
#46 teenage:ti,ab 481
#47 adolescen*:ti,ab 29258
#48 paediatric:ti,ab 31799
#49 pediatric:ti,ab 31807
#50 (#42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49) 165257
#51 (#41 AND #50) with Publication Year from 2009 to 2020, with Cochrane Library publication date Between Jul 2009 and Jul 2020, in Trials 57
#52 (#41 AND #50) with Publication Year from 2020 to 2021, with Cochrane Library publication date Between Jul 2020 and Sep 2021, in Trials 9
Appendix 2. MEDLINE search
Database: Ovid MEDLINE(R) <1946 to September 01, 2021>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp flatfoot/ (2420)
2 flat foot$.mp. (492)
3 flatfoot$.mp. (2639)
4 flat feet.mp. (314)
5 flatfeet.mp. (140)
6 pes planus.mp. (444)
7 painful foot.mp. (139)
8 pes planovalgus.mp. (178)
9 posterior tibial tendon dysfunction.mp. (407)
10 subtalar.mp. (3432)
11 (sub$ adj talar).mp. (51)
12 calcane$.mp. (12164)
13 heel bone$.mp. (192)
14 medical arch$2.mp. (4)
15 or/1‐14 (16654)
16 exp musculoskeletal diseases/ (1132028)
17 exp neuromuscular diseases/ (321208)
18 exp nervous system diseases/ (2639188)
19 ehlers danlos.mp. (3950)
20 down$ syndrome.mp. (29398)
21 trisomy.mp. (20802)
22 mongolism.mp. (1563)
23 inflammatory arthritis.tw. (4517)
24 (juvenile adj3 arthritis).tw. (9551)
25 or/16‐24 (3553109)
26 exp diabetes mellitus, type 1/ (79337)
27 (diabet$ or IDDM).tw. (589388)
28 26 or 27 (596792)
29 exp diabetes insipidus/ (8046)
30 mellitus.tw. (176625)
31 29 not (26 or 30) (7373)
32 (diabet$ adj (insipidus not mellitus)).tw. (7731)
33 31 or 32 (9648)
34 28 not 33 (588951)
35 joint instability.sh. (21632)
36 ligament$ laxity.mp. (806)
37 pronat$.mp. (6019)
38 malalignment.mp. (5292)
39 or/35‐38 (32599)
40 or/25,34,39 (4079777)
41 15 and 40 (10302)
42 randomized controlled trial.pt. (542072)
43 controlled clinical trial.pt. (94342)
44 randomized.ab. (461416)
45 placebo.ab. (200637)
46 drug therapy.fs. (2367832)
47 randomly.ab. (309518)
48 trial.ab. (488467)
49 groups.ab. (1911898)
50 or/42‐49 (4665240)
51 (animals not (humans and animals)).sh. (4847355)
52 50 not 51 (4000598)
53 41 and 52 (1452)
54 limit 53 to ("infant (1 to 23 months)" or "preschool child (2 to 5 years)" or "child (6 to 12 years)" or "adolescent (13 to 18 years)") (476)
55 child.mp. (2156988)
56 children.mp. (987895)
57 childhood.mp. (243050)
58 infant$.mp. (1278871)
59 teenag$.mp. (20079)
60 adolescen$.mp. (2155197)
61 paediatric.mp. (56044)
62 pediatric.mp. (285434)
63 or/55‐62 (4022120)
64 53 and 6 (70)
65 54 or 64 (512)
66 limit 65 to dt=20090701‐20200728 (236)
67 limit 66 to dt=20200730‐20210901 (16)
Appendix 3. Embase search strategy
Database: Embase Classic+Embase <1947 to 2021 September 01>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Flatfoot/ (4297)
2 flat foot$.mp. (1085)
3 flatfoot$.mp. (4756)
4 flat feet.mp. (638)
5 flatfeet.mp. (234)
6 pes planus.mp. (882)
7 painful foot.mp. (239)
8 pes planovalgus.mp. (390)
9 posterior tibial tendon dysfunction.mp. (604)
10 subtalar.mp. (5576)
11 (sub$ adj talar).mp. (85)
12 calcane$.mp. (18956)
13 heel bone$.mp. (324)
14 medical arch$2.mp. (3)
15 or/1‐14 (26512)
16 exp Musculoskeletal Disease/ (2653609)
17 exp Neuromuscular Disease/ (222845)
18 exp Neurologic Disease/ (4051261)
19 ehlers danlos.mp. (6829)
20 down$ syndrome.mp. (43417)
21 trisomy.mp. (36587)
22 mongolism.mp. (2063)
23 inflammatory arthritis.tw. (10525)
24 (juvenile adj3 arthritis).tw. (18516)
25 or/16‐24 (6050059)
26 Insulin Dependent Diabetes Mellitus/ (124599)
27 (diabet$ or IDDM).tw. (1059361)
28 26 or 27 (1074667)
29 exp Diabetes Insipidus/ (17717)
30 mellitus.tw. (311351)
31 29 not (26 or 30) (16444)
32 (diabet$ adj (insipidus not mellitus)).tw. (12278)
33 31 or 32 (17980)
34 28 not 33 (1061737)
35 joint instability.sh. (12223)
36 ligament$ laxity.mp. (1257)
37 pronat$.mp. (8728)
38 malalignment.mp. (6085)
39 or/35‐38 (27594)
40 or/25,34,39 (6917639)
41 15 and 40 (21433)
42 random$.ti,ab. (1712450)
43 factorial$.ti,ab. (42419)
44 (crossover$ or cross over$ or cross‐over$).ti,ab. (115459)
45 placebo$.ti,ab. (335897)
46 (doubl$ adj blind$).ti,ab. (228318)
47 (singl$ adj blind$).ti,ab. (27619)
48 assign$.ti,ab. (434599)
49 allocat$.ti,ab. (172371)
50 volunteer$.ti,ab. (277903)
51 crossover procedure.sh. (68322)
52 double blind procedure.sh. (189697)
53 randomized controlled trial.sh. (676185)
54 single blind procedure.sh. (43642)
55 or/42‐54 (2581294)
56 exp animal/ or nonhuman/ or exp animal experiment/ (31561864)
57 exp human/ (24025004)
58 56 and 57 (24025004)
59 56 not 58 (7536860)
60 55 not 59 (2270319)
61 41 and 60 (1208)
62 limit 61 to (infant or child or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>) (173)
63 child.mp. (2719502)
64 children.mp. (1537907)
65 childhood.mp. (477947)
66 infant$.mp. (1065321)
67 teenag$.mp. (32051)
68 adolescen$.mp. (1863177)
69 paediatric.mp. (112907)
70 pediatric.mp. (520089)
71 or/63‐70 (4483036)
72 61 and 71 (188)
73 62 or 72 (188)
74 limit 73 to dd=20090701‐20200728 (99)
75 limit 74 to dd=20200730‐20210901 (5)
Appendix 4. CINAHL search strategy
S40 S26 and S39
S39 S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38
S38 TI Allocat* random* or AB Allocat* random* S37 (MH "Quantitative Studies")
S36 (MH "Placebos")
S35 TI Placebo* or AB Placebo*
S34 TI Random* allocat* or AB Random* allocat* S33 (MH "Random Assignment") S32 TI Randomi?ed control* trial* or AB Randomi?ed control* trial*
S31 AB singl* blind* or AB singl* mask* or AB doub* blind* or AB doubl* mask* or AB trebl* blind* or AB trebl* mask* or AB tripl* blind* or AB tripl* mask* S30 TI singl* blind* or TI singl* mask* or TI doub* blind* or TI doubl* mask* or TI trebl* blind* or TI trebl* mask* or TI tripl* blind* or TI tripl* mask*
S29 TI clinical* trial* or AB clinical* trial* S28 PT clinical trial
S27 (MH "Clinical Trials+")
S26 S5 and S25
S25 S10 or S19 or S24
S24 S20 or S21 or S22 or S23
S23 TI malalignment or AB malalignment S22 TI pronat* or AB pronat*
S21 TI ligament* laxity or AB ligament* laxity S20 (MH "Joint Instability+")
S19 S13 not S18
S18 S16 or S17
S17 TI diabet* insipidus or AB diabet* insipidus not TI diabet* mellitus or AB diabet* mellitus
S14 not (S11 or S15)
S15 ti mellitus or ab mellitus
S14 (MH "Diabetes Insipidus") S13 S11 or S12
S12 TI diabet* or AB diabet* or TI IDDM or AB IDDM S11 (MH "Diabetes Mellitus, Insulin‐Dependent")
S10 S6 or S7 or S8 or S9
S9 TI ehlers‐danlos or AB ehlers‐danlos or TI down* syndrome or AB down* syndrome or TI trisomy or AB trisomy or TI mongolism or AB mongolism or TI inflammatory arthritis or AB inflammatory arthritis or TI juvenile N3 arthritis or AB juvenile N3 arthritis
S8 (MH "Nervous System Diseases+") S7 (MH "Neuromuscular Diseases+")
S6 (MH "Musculoskeletal Diseases+") S5 S1 or S2 or S3 or S4
S4 TI subtalar or AB subtalar or TI sub* adj talar or AB sub* adj talar or TI calcane* or AB calcane* or TI heel bone* or AB heel bone* or TI medical arch* or AB medical arch* Search modes ‐ Boolean/Phrase S3 TI pes planus or AB pes planus or TI painful foot or AB painful foot or TI pes planovalgus or AB pes planovalgus or TI posterior tibial tendon dysfunction or AB posterior tibial tendon dysfunction Search S2 TI flat foot* or AB flat foot* or TI flatfoot* or AB flatfoot* or TI flat feet or AB flat feet or TI flatfeet or AB flatfeet
S1 (MH "Flatfoot")
Appendix 5. Dissertation abstracts search
(flatfoot) OR (flat foot*) OR (flatfoot*) OR (flat feet) OR (flatfeet) OR (pes planus) OR (painful foot) in Citation and Abstract
Data and analyses
Comparison 1. Custom foot orthoses (CFOs) versus shoes for asymptomatic flat feet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion without pain | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.07] |
| 1.2 Withdrawal due to adverse events | 3 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.94, 1.19] |
Comparison 2. Prefabricated foot orthoses (PFOs) versus shoes in asymptomatic flat feet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion without pain | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.16] |
| 2.2 Withdrawal due to adverse events | 4 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.23] |
Comparison 3. CFOs versus PFOs in asymptomatic flat feet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pain | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.18] |
| 3.2 Withdrawal due to adverse events | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.12] |
Comparison 4. CFOs versus shoes in juvenile idiopathic arthritis (JIA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.78, ‐0.22] |
| 4.2 Function | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.2.1 foot pain ‐ FFI | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐19.19 [‐35.50, ‐2.88] |
| 4.2.2 activity limitation ‐ FFI | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐19.38 [‐35.54, ‐3.22] |
| 4.2.3 disability ‐ FFI | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐18.55 [‐34.42, ‐2.68] |
| 4.3 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.3.1 PedsQL physical ‐ child‐rated | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 12.10 [‐1.60, 25.80] |
| 4.3.2 Peds QL physical ‐ parent‐rated | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 9.01 [‐4.08, 22.10] |
| 4.4 Treatment success (gait parameters) | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐2.77, 0.11] |
| 4.5 Withdrawal due to adverse events | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.11, 2.94] |
Comparison 5. PFOs versus shoes in JIA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Pain | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐1.94, 1.98] |
| 5.2 Function | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.2.1 foot pain ‐ FFI | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐7.08 [‐27.10, 12.94] |
| 5.2.2 disability ‐ FFI | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐4.17 [‐24.40, 16.06] |
| 5.2.3 activity limitations ‐ FFI | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐7.96 [‐26.79, 10.87] |
| 5.3 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 PedsQL physical ‐ child‐rated | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐3.84 [‐19.01, 11.33] |
| 5.3.2 PedsQL physical ‐ parent‐rated | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐13.22, 11.94] |
| 5.4 Treatment success (Timed walking) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.90, 1.14] |
| 5.5 Withdrawal due to adverse events | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.14, 3.61] |
Comparison 6. CFOs versus PFOs in JIA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Pain | 2 | 87 | Mean Difference (IV, Random, 95% CI) | ‐1.48 [‐3.23, 0.26] |
| 6.2 Function | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.2.1 FFi ‐ disability | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐14.38 [‐30.22, 1.46] |
| 6.2.2 FFI ‐ activity limitation | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐11.42 [‐23.91, 1.07] |
| 6.2.3 FFI ‐ foot pain | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐12.11 [‐28.95, 4.73] |
| 6.3 Quality of life | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.3.1 PedsQL ‐ child‐rated | 2 | 83 | Mean Difference (IV, Random, 95% CI) | 8.64 [‐3.90, 21.18] |
| 6.3.2 PedsQL ‐ parent‐rated | 2 | 84 | Mean Difference (IV, Random, 95% CI) | 2.94 [‐11.00, 16.88] |
| 6.4 Treatment success (timed walking) | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.88, ‐0.02] |
| 6.5 Withdrawal due to adverse events | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.13, 4.87] |
Comparison 7. PFOs versus shoes in symptomatic flat feet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Function | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1.1 PODCI ‐ upper extremity and physical function | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 7.60 [6.87, 8.33] |
| 7.1.2 PODCI ‐ transfer and basic mobility | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 10.60 [9.86, 11.34] |
| 7.1.3 PODCI ‐ sports and physical function | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.90 [3.18, 4.62] |
| 7.1.4 PODCI ‐ global function | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [2.28, 3.72] |
| 7.2 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.2.1 PODCI ‐ pain/comfort | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 3.70 [2.97, 4.43] |
| 7.2.2 PODCI ‐ happiness | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.42, 0.02] |
| 7.2.3 PedsQL ‐ physical | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐4.93, ‐3.47] |
| 7.2.4 PedsQL ‐ psychosocial | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.12, 1.32] |
| 7.2.5 PedsQL ‐ total score | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 1.80 [1.07, 2.53] |
Comparison 8. CFOs versus shoes in DCD flat feet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Gait parameters | 1 | Other data | No numeric data | |
| 8.1.1 six minute walk test (6MWT; m) | 1 | Other data | No numeric data | |
| 8.1.2 cadence (steps/min.) | 1 | Other data | No numeric data | |
| 8.1.3 double support (%) | 1 | Other data | No numeric data | |
| 8.1.4 stride (m) | 1 | Other data | No numeric data |
Comparison 9. Anti‐pronation taping versus sham taping.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Balance test (SEBT) at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1.1 anterior | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.73 [‐4.70, 8.16] |
| 9.1.2 posterior | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐7.49, 8.49] |
| 9.1.3 medial | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐5.95, 6.49] |
| 9.1.4 lateral | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐7.24, 7.34] |
| 9.2 Agility test (Illinois Agility Test) at 4 weeks | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.08, 0.46] |
| 9.3 Vertical jump height (cm) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐1.37, 2.11] |
9.1. Analysis.

Comparison 9: Anti‐pronation taping versus sham taping, Outcome 1: Balance test (SEBT) at 4 weeks
9.2. Analysis.

Comparison 9: Anti‐pronation taping versus sham taping, Outcome 2: Agility test (Illinois Agility Test) at 4 weeks
9.3. Analysis.

Comparison 9: Anti‐pronation taping versus sham taping, Outcome 3: Vertical jump height (cm)
Comparison 10. Neuromuscular electrical stimulation (NMES) versus sham NMES.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Navicular height (mm) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐3.66 [‐4.03, ‐3.28] |
| 10.1.1 right foot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐3.28 [‐3.88, ‐2.68] |
| 10.1.2 left foot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐3.91 [‐4.40, ‐3.42] |
| 10.2 Staheli’s arch index (mm) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.33, ‐0.25] |
| 10.2.1 right foot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.35, ‐0.25] |
| 10.2.2 left foot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.34, ‐0.22] |
| 10.3 Calcaneal inclination angle (degrees) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐2.64 [‐3.99, ‐1.29] |
| 10.3.1 right foot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐2.87 [‐5.06, ‐0.68] |
| 10.3.2 left foot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐4.22, ‐0.78] |
| 10.4 Talus second metatarsal angle (degrees) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐2.53 [‐3.04, ‐2.02] |
| 10.4.1 right foot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐2.45 [‐3.25, ‐1.65] |
| 10.4.2 left foot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐2.58 [‐3.24, ‐1.92] |
| 10.5 Talo‐navicular coverage angle | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.32 [‐1.87, ‐0.77] |
| 10.5.1 right foot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐2.82, ‐1.00] |
| 10.5.2 left foot | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.98 [‐1.67, ‐0.29] |
10.1. Analysis.

Comparison 10: Neuromuscular electrical stimulation (NMES) versus sham NMES, Outcome 1: Navicular height (mm)
10.2. Analysis.

Comparison 10: Neuromuscular electrical stimulation (NMES) versus sham NMES, Outcome 2: Staheli’s arch index (mm)
10.3. Analysis.

Comparison 10: Neuromuscular electrical stimulation (NMES) versus sham NMES, Outcome 3: Calcaneal inclination angle (degrees)
10.4. Analysis.

Comparison 10: Neuromuscular electrical stimulation (NMES) versus sham NMES, Outcome 4: Talus second metatarsal angle (degrees)
10.5. Analysis.

Comparison 10: Neuromuscular electrical stimulation (NMES) versus sham NMES, Outcome 5: Talo‐navicular coverage angle
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abd‐Elmonem 2021.
| Study characteristics | ||
| Methods | Randomised controlled two‐arm parallel trial | |
| Participants | Initially recruited N = 134, excluded = 62 Study included: 72 children with flat feet, 36 in each group Age: 7 to 12 years old male and female Gender: 31 M, 35 F (M/F ‐ 15/18 in intervention group; 16/17 in sham group) Setting: out‐patient Physical Therapy Clinic of Faculty of Physical Therapy, Cairo University, Cairo, Egypt Inclusion criteria: (i) diagnosed (by an orthopedist) with asymptomatic flexible flatfoot, (ii) navicular drop more than 9 mm, (iii) grade III flatfoot Staheli Arch Index (midfoot width exceeding forefoot width) Exclusion criteria: (i) congenital deformities of the lower extremities (e.g. genu valgum, femoral anteversion), (ii) scar or osseous anomalies Baseline characteristics: age, mean (SD): 9.5 (1.02) in intervention group; 9.45 (0.76) in sham group |
|
| Interventions | Group 1: neuromuscular electrical stimulation and corrective exercise ‐ 4 months (3 sessions/week) Group 2: sham neuromuscular electrical stimulation and corrective exercise ‐ 4 months (3 sessions/week) |
|
| Outcomes | Assessments of Staheli’s arch index (through foot print), navicular drop (through navicular drop test), and radiographic indexes (through anterior‐posterior and medio‐lateral X‐ray) of both feet were performed before and after the intervention programmes | |
| Source of funding | This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors | |
| Notes | Trial registration: NCT04410926 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The online Graph Pad software was used to allocate study participants". |
| Allocation concealment (selection bias) | Low risk | "The randomisation was carried out by an independent person, who was unaware of the study protocol and not otherwise in control of the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "All children/legal guardians, radiologist and researchers responsible for evaluations were blinded to participants’ allocation". However it is unclear whether the blinding was successful, as the trialists did not discuss the possibility that participants might have guessed their intervention based on whether they felt any discomfort related to the intervention or not. |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | Low risk | There were no self‐reported outcomes |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/36 (discontinued intervention = 3) in the intervention, and 3/36 in control group (discontinued intervention = 2; femoral fracture = 1) withdrew from the study and not included in the analysis |
| Selective reporting (reporting bias) | Low risk | trial registered, results for all pre‐specified outcomes reported |
| Other bias | Low risk | none apparent |
Aboutorabi 2013.
| Study characteristics | ||
| Methods | Randomised controlled three‐arm parallel trial | |
| Participants | Initially recruited N = 62, 12 excluded Study included: 30 children with flat feet, 20 healthy controls Age: average 7.76 (1.4) years Gender: 30 M, 20 F (flat foot – 18 M, 12 F; healthy – 12 M, 8 F) Location ‐ Hehran, Iran Setting: Iran University of Medical Sciences, Dept Orthopedics and Prosthetics, Faculty of Rehabilitation Sciences Inclusion criteria: flat foot and healthy children; flatfoot FPI > +6 Exclusion criteria: contracture of soft tissue, genu varum or valgum, structural congenital deformity, lower extremity fracture, neurological problems, foot or ankle surgery in past 6 months, leg length discrepancy Baseline characteristics: trial group/flat feet N = 30, 18 M:12 F, control group/non‐flat feet N = 20, 12 M:8 F |
|
| Interventions | 1. functional foot orthoses and regular shoe 2. medical shoes 3. barefoot |
|
| Outcomes | Outcomes were measured as immediate effects at baseline only. Gait parameters for the 3 interventions, i.e. 1. step length 2. step width 3. walking velocity 4. symmetry 5. centre of pressure (CoP) displacement |
|
| Source of funding | none | |
| Notes | ‐ FPI (Foot Posture Index) of healthy group is not defined, versus the flatfoot group FPI +6 to +12. Hence, basis for comparison from baseline unclear ‐ Immediate effects only, hence, we did not include this trial in further analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of low risk or high risk. ‘A randomised method was used for the sampling from the flat footed children who were referred for a new pair of shoes’. ‘The test order was randomised for each child to minimize learning effect.’ |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Each child participates in barefoot, regular shoe, and medical shoe trials. No blinding was performed. Research staff prepared each child for barefoot, regular shoe, and medical shoe trials. No blinding was performed. |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | High risk | N/A |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Unclear risk | Outcomes included assessment of gait parameters. Both the participant and the researcher were considered to be outcome assessors as both could influence the result. The participants were not blinded. No mention was made of blinding of researchers involved in outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of low risk or hHigh risk. |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol. Insufficient information to permit judgement of low risk or high risk. |
| Other bias | Unclear risk | 5 min rest was allowed between testing conditions for each child. It was not clear if this was sufficient to eliminate a carry‐over effect. The random order of testing minimised this effect. |
Ahn 2017.
| Study characteristics | ||
| Methods | Randomised controlled two‐arm parallel trial | |
| Participants | 40 children with flexible flat feet Age: average 10 (4.5) years Gender: 24 M, 16 F Location: Daejeon, Korea Setting: Department of Pediatric Rehabilitation at Chung Nam National University Hospital Inclusion criteria: flexible flatfoot defined as > 4° valgus RCSP, one abnormal x‐ray finding i.e. > 30° AP talo‐calcaneal angle, > 45° lateral talo‐calcaneal angles, > 4° lateral talo‐metatarsal angles, < 10° calcaneal pitch Exclusion criteria: rigid flatfoot, of any cause Baseline characteristics: matched except for age ‐ RFO 10.14 (4.99) years, TCFO 9.59 (4.24) years |
|
| Interventions | 1. rigid foot orthoses (N = 20) 2. talus control foot orthoses (N = 20) |
|
| Outcomes | ‐ RCSP ‐ x‐rays, 4 angles: AP talo‐calcaneal angle, lateral talo‐calcaneal angles, lateral talo‐metatarsal angles, calcaneal pitch Both measures were assessed at baseline and after 12 months |
|
| Source of funding | none reported | |
| Notes | no mention of pain, or gait parameters | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of low risk or high risk. ‘Forty children were randomly prescribed a foot orthosis: 20 a RFO and 20 a TCFO’ |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded Personnel were not blinded |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | High risk | N/A |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Unclear risk | It was not clear if the person who measured resting calcaneal stance position or radiographs was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of children who completed the study, or for whom outcome data were presented was not reported. |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol. Insufficient information to permit judgement of low risk or high risk. |
| Other bias | Unclear risk | Funding source for orthoses was not stated. |
Asgaonkar 2012.
| Study characteristics | ||
| Methods | Randomised controlled two‐arm parallel trial | |
| Participants | 80 children with flat feet, aged 5 to 15 years, in Mumbai, India 1. treatment group N = 45 2. age‐sex matched control group N = 35 Age: 5 to 15 years Gender: not stated, but groups 'age and sex matched' (p 91) Location: primary and secondary schools Setting: Mumbai, India Inclusion criteria: flat feet ‐ from footprint measures (Bleck arch index > 1.15) Exclusion criteria: fixed foot deformity, surgeries for foot deformities, pain injury that had required non‐weight bearing, systemic problems Baseline characteristics: matched, but uneven group sizes |
|
| Interventions | 1. valgus insole, rubber material of average thickness 4 cm (N = 45) 2. no treatment (N = 35) |
|
| Outcomes | Outcome measures at baseline and 12 months 1. Pain ‐ VAS 2. PCI (physical cost index) ‐ average HR and speed for 100 m walk 3. Gait parameters ‐ step length, stride length, cadence, walking velocity |
|
| Source of funding | none reported | |
| Notes | ‐ 20/80 dropouts in 12‐month trial, most from treatment group ‐ pain used VAS – some children were only 5 years old ‐ valgus insole vague; unsure re: footwear used in either group (presume ‘usual’, but not stated) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of low risk or high risk. "Out of the total 80 students, age, and sex, matched two groups were made at random; control group consisting of 35 students and experimental group consisting of 45 students." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and the outcome was likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | High risk | No mention was made of blinding of researchers involved in outcome assessment. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | High risk | No mention was made of blinding of researchers involved in outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in number of dropouts per group. n = 45 in the experimental group, 9 participants withdrew from the study and an additional 6 participants left the school. n = 35 in the control group, it appears 5 participants left the school and none withdrew from the study. Reasons for dropouts from the experimental group were not provided, but are likely related to the intervention. |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol. Insufficient information to permit judgement of low risk or high risk. |
| Other bias | Unclear risk | No funding source reported |
Bok 2016.
| Study characteristics | ||
| Methods | Randomised controlled four‐arm parallel trial | |
| Participants | 21 children with symptomatic flexible flat feet Age: aged 8 to 13 years Gender: 8 M, 13 F Location: Jung‐gu, Daejeon, South Korea Setting: Department of Rehabilitation Medicine, Chungnam National University Hospital Inclusion criteria: severe flat feet with pain, RCSP > 6° everted Exclusion criteria: (i) a fixed foot deformity (ii) reported previous intervention (e.g. orthoses or surgery) (iii) congenital and developmental foot disease (iv) neuromuscular or central nervous system disease Children with a history of overuse or traumatic injury to the lower limb in the past 6 months, bony surgery to the lower limb, or systemic endocrine, neurogenic, or musculoskeletal disorders were also excluded. Baseline characteristics: 8 M:13 F |
|
| Interventions | All children trialled 4 shoes with different degrees of orthotic (N = 21): 1. shoes 2. shoes and 0° orthotic 3. shoes and 15° orthotic 4. shoes and 30° orthotic |
|
| Outcomes | Plantar pressures ‐ peak pressure, maximum force, contact area Reported shoe and orthotic condition, as immediate effects on plantar pressures |
|
| Source of funding | orthoses supplied by Korean company | |
| Notes | ‐ did not include any pain evaluation (yet all participants were symptomatic at baseline) ‐ immediate effects only, hence, we did not include this trial in further analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘The 4 orthotic conditions were tested in a random order to minimize potential sequencing effects.’ Insufficient information about the sequence generation process to permit judgement of low risk or high risk. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Each child participated in each orthotic condition. No blinding was performed. Research staff dispensed the orthoses to the children. It was unclear how involved the research staff were in preparing each child for each trial, for example, fitting the orthoses in the shoes. |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | Unclear risk | Immediate effects only |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Unclear risk | Outcomes included assessment of gait parameters. Both the participant and the researcher were considered to be outcome assessors, as both could influence the result. The participants were not blinded. No mention was made of blinding of researchers involved in outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of low risk or high risk. |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol. Insufficient information to permit judgement of low risk or high risk. |
| Other bias | Unclear risk | It was not clear if participants were allowed an adjustment period for each orthotic design. The more aggressively posted orthoses may require a longer period to adjust to normalise gait. |
Coda 2014.
| Study characteristics | ||
| Methods | Randomised controlled two‐arm parallel trial, single blinded, multi‐centre | |
| Participants | 60 children with JIA Ages ‐ 11.17(3.51) control PFOs, 10.64(3.84) fitted PFOs Genders: control PFOs 6M/23F; fitted PFOs 9M/22F Setting: Paediatric Rheumatology hospital clinics Location: Scotland, UK Inclusion criteria: diagnosed with juvenile idiopathic arthritis (JIA; any subtype) according to International League of Associations for Rheumatology criteria, lower extremity joint involvement with disease onset ranging from 5 to 18 years, previous failure of orthotic management in which the child has not worn any foot orthoses for at least 3 months, ability to walk a minimum of 15 metres or more without assistive devices, at least 6 months after start of disease modifying antirheumatic drug therapy Exclusion criteria: inability to walk barefoot or shod, concomitant musculoskeletal disease, central or peripheral nerve disease and endocrine disorders, previous foot surgery, currently using foot orthoses, supply of foot orthoses is contraindicated Baseline characteristics: CPFO group had higher VAS, 14 versus 6.5 in PFO group (NS); CPFO group had 65% oligoarthritis versus PFO 44.8% |
|
| Interventions | 1. Fitted PFOs, customised in clinic (N = 31) 2. Control PFOs (N = 29) Both PFO types had same black covers to assist blinding |
|
| Outcomes | Baseline, 3 and 6 month outcomes ‐ VAS ‐ PedsQL |
|
| Source of funding | Queen Margaret University, Edinburgh ‐ PhD scholarship | |
| Notes | Foot posture was not defined Validated outcome measures used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “After obtaining informed consent, children were randomised in blocks of 10 each by an online computer random number generator (www.randomization.com).” |
| Allocation concealment (selection bias) | Unclear risk | No specific mention of allocation concealment. Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | low ‐ ‘The control FOs was made with leather board (1 mm) without corrections. Both FOs had the same black‐ethylene vinyl acetate (EVA) top cover (0.75 mm) to allow for blinding and monitoring the level of adherence to wearing the FOs.’ high ‐ study did not blind investigators to the intervention, and the outcome may be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | Low risk | Pain measured by participants HRQoL measured by participants and parents/carers Participants and their parents/carers are blinded |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‘Overall, 179 out of a possible 180 assessments were completed (99.4%) and accounted for statistical analysis.’ No reason for missing data given and it was not clear from which group and time point the data were missing. |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol. Insufficient information to permit judgement of low risk or high risk. |
| Other bias | Unclear risk | It is not clear how the orthoses in this study were funded. |
Gould 1989.
| Study characteristics | ||
| Methods | Randomised controlled four‐arm parallel trial | |
| Participants | 125 normal toddlers (beginner walkers) aged 11 to 14 months Age: 11 to 14 months Gender: not stated, except “boys and girls” Table 9, p 243 – genders/groups at 5 years (27 M, 25 F) Location: University of Vermont, USA Setting: pediatrics dept Inclusion criteria: "clinically normal children... aged 11 to 14 months" Exclusion criteria: none stated Baseline characteristics: sex ratio at baseline not stated |
|
| Interventions | Group 1, N = 50 – straight last shoes Group 2, N = 25 – Group 1 shoes + long arch cookies Group 3, N = 15 – orthopaedic shoes with long counters, solid shanks, Thomas heels, 0.3 cm inside heel wedges Group 4, N = 25 – Group 3 shoes + thin longitudinal arch support |
|
| Outcomes | Baseline, 2,3, and 5 years ‐ x‐ray angles (AP talo‐1st metatarsal, lateral talo‐1st metatarsal, lateral talo‐calcaneal) ‐ biometry (pedoscope ‐ arch appearance) ‐ clinical examination ‐ use of shoes, examination of femoral, tibial, knee configuration ‐ "will be reported in detail subsequently" |
|
| Source of funding | 1. Annual grant from Footwear Association 2. Shoes provided by Sabel shoes |
|
| Notes | Group 1, N = 50 acting as shod control group, across 4 years of the trial High attrition ‐ 52/125 finished the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘divided by lot into four difference footwear groups’. Insufficient information about the sequence generation process to permit judgement of low risk or high risk. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded Personnel were not blinded |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Unclear risk | ‘A determination was made as to whether the arch was slightly or moderately improved. The records were then consulted to determine what type of footwear was worn in each case.’ It was not clear who made this determination, and if they had opportunity prior to looking at the records to know what footwear was worn in each case. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 42% of children completed the trial. Attrition was uneven between groups. Reasons for attrition were not sufficiently reported to permit judgement of low risk or high risk. |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol. Insufficient information to permit judgement of low risk or high risk. |
| Other bias | Unclear risk | Subjective and non‐validated outcome assessments. ‘A determination was made as to whether the arch was slightly or moderately improved’ |
Hsieh 2018.
| Study characteristics | ||
| Methods | Randomised controlled two‐arm parallel trial | |
| Participants | 52 healthy children with symptomatic flexible flatfoot Age: all approximated 6 years ‐ treatment group 6.9 (0.6) years, control group 6.2 (0.4) years Gender: (28 M, 24 F) Location: Shin Kong Wu Ho‐Su Memorial Hospital, Taiwan Setting: Dept Physical Medicine and Rehabilitation Inclusion criteria: symptomatic flexible flatfoot (pain over the foot or calf, fatigue after prolonged walking, and gait disturbances) Exclusion criteria: history of foot injury or surgery, foot abnormalities affecting locomotion or foot mobility, or a confirmed diagnosis of developmental delays, such as developmental co‐ordination disorder and neurological deficits Baseline characteristics: treatment group had significantly lower scores for PODCI (transfer and mobility), PedsQL (physical, psychological, total health) |
|
| Interventions | Treatment group (N = 26): thermoplastic insoles, heat moulded to child's feet (CPFOs) Control group (N = 26): no treatment |
|
| Outcomes | Physical activity: timed 10‐m walk, stair ascent, timed up and go, chair rise Physical function: parent reported PODCI (Chinese version) Psychometric: PODCI, PedsQL (parent report) |
|
| Source of funding | Multiple research grants from Shin Kong Wu Ho‐Su Memoril Hospital, and Ministry of Science and Technology, Taiwan | |
| Notes | Trial ran for 12 weeks Flatfoot assessment: ND test, FPI‐6, lat and AP x‐rays, Beighton score Self‐selected usual footwear Insole use suggested to be 5 hours/day |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly assigned to the treatment group (with customized insoles) or the control group (without customized insoles) according to computer‐generated random numbers (Fig. 1). |
| Allocation concealment (selection bias) | Low risk | Allocation was initially concealed. A sealed envelope was opened for each consecutive participant to reveal the participant’s group allocation when the participant was recruited to the study. One physician enrolled all participants, and another investigator generated the allocation sequence and assigned the participants to their groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control group had no orthotic intervention |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | High risk | Due to nature of intervention |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | High risk | High risk for primary outcome of pain as participants were not blinded. Secondary outcomes that were assessed by a blinded assessor had low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons provided for missing outcome data. Number was small and reason provided unlikely to be due to the intervention. |
| Selective reporting (reporting bias) | High risk | ClinicalTrials.gov (NCT02414087) The published study included more outcomes than are listed in the trial registry. |
| Other bias | Low risk | none apparent |
Jafarnezhadgero 2018.
| Study characteristics | ||
| Methods | Randomised controlled two‐arm parallel trial | |
| Participants | 30 healthy boys, with flexible flat feet Age: 8 to 12 years Gender: all boys Location: not clear ‐ boys 'recruited from orthopaedic specialists in local community' (p 4) Setting: not clear, ethics approval and trial registry within Iran (p 4) Inclusion criteria: boys who volunteered, flat feet assessed using navicular drop, arch height index, RCSP Exclusion criteria: history of bone fractures, signs of functional lower limb instability, ligament injury, reconstruction of ligaments, neuromuscular dysfunction, dysfunction of lower limb muscles, leg length differences larger than 1 cm, and a history of lower extremity trauma or surgery. Baseline characteristics: all were right foot dominant, no significant baseline differences between groups for examined variables |
|
| Interventions | Treatment group (N = 15): medial arch support foot orthoses (custom made medial arch support foot orthoses), CFOs Control group (N = 15): flat 2‐mm thick insoles (sham) |
|
| Outcomes | Gait kinematics and ground reaction forces (kinetic) ‐ joint vectors, moments, and ground reaction forces | |
| Source of funding | Deutsche Forschungsgemeinschaft (DFG), and Open Access Publishing Fund of University of Potsdam, Germany. | |
| Notes | All participants issued with same footwear (New Balance 759) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Says only ‘The block randomization method was used to allocate study participants into experimental groups.’ |
| Allocation concealment (selection bias) | Unclear risk | Says only; ‘Another naïve examiner controlled the allocation of each participant and was responsible for delivering the treatment to both groups.’ Not enough information to determine risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham orthoses used |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | High risk | Due to nature of intervention |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Low risk | Examiners blinded for all outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Iranian Registry of Clinical Trials (IRCT2017082235517N1; URL: www.irct.ir/user/trial/26811/ view) ‐ unable to access the online trial registry. |
| Other bias | Low risk | none apparent |
Kanatli 2016.
| Study characteristics | ||
| Methods | Randomised controlled two‐arm parallel trial | |
| Participants | 45 children, gender (33 boys, 12 girls) ‐ with moderate flexible flatfoot Age: mean age 39.5 months (17 to 72 months) Location: Ankara, Turkey Setting: Gazi University School of Medicine Inclusion criteria: moderate flexible flatfoot (Volpe classification ‐ Jack's test, standing heel position) Exclusion criteria: rigid flatfoot, neuromuscular disorders, genetic disease associated with collagen abnormalities Baseline characteristics: sample sex ratio 33 M: 12 F, not stated/group |
|
| Interventions | 1. orthopaedic shoes, N = 21 2. control group, N = 24 |
|
| Outcomes | Baseline and at follow up, i.e. 34.6 (10.9) months (range 24 to 57 months) ‐ Joint laxity (Wynne‐Davis method) ‐ Arch Index (Staheli method) ‐ x‐ray angles (lateral and AP talo‐1st metatarsal, lateral talo‐horizontal, lateral calcaneal pitch, lateral and AP talo‐calcaneal) |
|
| Source of funding | not stated | |
| Notes | Orthopaedic shoes had internal ‘orthosis’ and Thomas heels | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Systematic, non‐random approach. ‘Patients were randomised and separated in two groups. Randomization was made by weekly basis. Patients that fulfilled the inclusion criteria during even number weeks consisted group 1 and in odd number weeks consisted group 2.’ |
| Allocation concealment (selection bias) | High risk | Inexplicably unconcealed procedure: allocated depending on week odd or even week at enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Children were not blinded. Children received orthopaedic shoes or no intervention. Personnel fitted the orthopaedic shoes or no intervention. |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Unclear risk | It is not clear who assessed the outcomes and if this person was aware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled children completed the study. |
| Selective reporting (reporting bias) | High risk | Arch index scores were not reported for any time point. |
| Other bias | Unclear risk | It was not clear how the orthopaedic shoes in this study were funded. |
Khamooshi 2017.
| Study characteristics | ||
| Methods | Randomised controlled three‐arm parallel trial | |
| Participants | 60/130 girls with flat feet Age: 9 to13 years Gender: all girls Location:Tehran, Iran Setting: Schools of Khalil Abad county Inclusion criteria: female, good general health, flexible flat feet – assessed using tip toe test, navicular collapse Exclusion criteria: lower extremity surgery, fracture, orthopaedic problems Baseline characteristics: all girls, matched re age, BMI |
|
| Interventions | 1. control group, N = 20 2. stretches, strengthening; i.e. Achilles tendon stretching and strengthening, exercises effective on the interior longitudinal foot arch, N = 20 3. stretches, strengthening, core exercises; i.e. exercises related to the muscles, Achilles tendon stretching and strengthening, exercises effective on the interior longitudinal foot arch and activities for core stability, N = 20 Baseline, and after exercise programme: groups 2 and 3 performed the exercises for 8 weeks, three times a week, in the form of three turns with 20 repetitions. |
|
| Outcomes | Pre/post exercise programme (8 weeks apart) ‐ Staheli footprint arch index: the narrowest section of the arch (A) was divided by the broadest section of the foot (B) based on Staheli's formula (AI = A/B) ‐ Navicular collapse test: seated with knee flexed 90 degrees, and subtalar neutral position. The navicular height from ground measured (mm). This is repeated with participant standing, and the difference recorded as the navicular collapse rate. |
|
| Source of funding | Not stated | |
| Notes | Only Staheli index reported, and yet Navicular collapse is stated to improve with exercises (both groups), versus control (p 154) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of low risk or high risk. ‘In the present study, 60 female students with flat feet disorders were selected, who were stochastically assigned to three 20‐individual groups’ |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded Personnel were not blinded |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Unclear risk | It was not clear if the person who measured Staheli arch index was aware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of children who completed the study, or for whom outcome data were presented was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Data for ‘navicular bone collapse’ (also referred to as Navi Loss within the paper) was not reported. |
| Other bias | Unclear risk | The training programmes were inadequately described. |
Morrison 2013.
| Study characteristics | ||
| Methods | Randomised controlled two‐arm parallel trial | |
| Participants | 22 children were approached to participate in the study. All were male, white, British, and FPI > +4 20 children consented to participate, then 5 failed to attend. 1 participant withdrew 14 participants (median age 7.5 years) completed the study Age: aged 6 to 11 years Gender; all white British boys Location: London, UK Setting: children’s physical rehabilitation programme (Children’s Therapy Service at Medway Community Healthcare) Inclusion criteria: diagnosis DCD, flat feet defined by FPI > +4 Exclusion criteria: medical complications likely to affect gait – neuromuscular, orthopaedic conditions Baseline characteristics: all boys, stated that groups were similar for age, foot posture, and hypermobility (but no demographic data) |
|
| Interventions | ‐ CFOs (N = 9) ‐ no CFOs (N = 5) Both groups completed a 7‐week rehabilitation programme |
|
| Outcomes | ‐ Spatio‐temporal gait (Gait‐rite system) ‐ Six‐minute walk test No significant differences from baseline to 7 week completion. |
|
| Source of funding | Canonbury Healthcare funding | |
| Notes | High attrition ‐ 14/21 (68%) finished the trial Small sample Specific cohort Preliminary trend inferred from NS results No true control group – quasi RCT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | All participants entering the study were quasi‐randomised into one of the two groups..’. Method of randomisation unclear |
| Allocation concealment (selection bias) | Low risk | ‘…using a sealed envelope technique. Each envelope was opened after the child consented to taking part in the programme’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants aware of foot orthoses being supplied at the start of end of rehabilitation program. Personnel supplied the orthoses at the start or end of the rehabilitation programme. |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | High risk | The investigator was blind to the treatment group during the initial data collection. It was not clear if investigators were blind at the time of outcome assessment at follow‐up. The gait parameters reported are influenced by the participants who were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons not provided for attrition. |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol. Insufficient information to permit judgement of low risk or high risk |
| Other bias | Low risk | Source of funding declared and independence of funder from research design, conduct and reporting assured. |
Powell 2005.
| Study characteristics | ||
| Methods | Randomised controlled three‐arm parallel trial | |
| Participants | 48 children with JIA, and persistent lower limb pain were enrolled (sample size calculation not stated) Age: 5 to 19 years (mean 12 years, 7 months, SD 3.7) Gender: 30 F, 10 M Setting; outpatients, Southern Californian children's hospitals, Location: San Diego, USA Inclusion criteria: diagnosed juvenile chronic arthritis; over 5 years old, active foot disease, history of foot pain over 1 month, able to walk 50 metres, stable medications Exclusion criteria: previous use of shoe inserts, joint injections in last 6 months, osseous anomaly Baseline characteristics: fewer males in CFO group (13.3% versus 33.3% in PFO, 30.8% in shoes); more polyarthritis in CFO group (73.3% versus 50% in PFOs, and 30.8% in shoes); CFO group had more pain on VAS (5.23) versus PFO (VAS 3.5), versus shoe (VAS 4.7) |
|
| Interventions | 1: custom‐made semi‐rigid foot orthoses (CFO) 2: prefabricated foot orthoses (PFO) 3: new athletic footwear with a medial longitudinal arch and shock‐absorbing sole |
|
| Outcomes | Outcomes measured at baseline and 3 months
|
|
| Source of funding | Foot orthoses supplied by Langer Biomechanics, Neoprene insoles supplied by Spenco Medical | |
| Notes | Pain reported in the trial using two instruments (Pediatric pain questionnaire and foot pain subscale of the Foot Function index) For this review, we extracted data using the Pediatric pain questionnaire for the analysis of pain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of random sequence generation unclear. ‘Once accepted into the study, each subject was randomly placed…’ Insufficient information about the sequence generation process to permit judgement of low risk or high risk. |
| Allocation concealment (selection bias) | Low risk | ‘Sealed envelope containing a predetermined numbered placement card into one of the 3 intervention groups’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome may be influenced by lack of blinding Physical therapist 1 who administered the interventions was not blinded Physical therapist 2 who performed baseline and follow‐up measures was blinded |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | High risk | Pain, level of disability, and activity limitation measured by participants |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | High risk | Speed of ambulation influenced by participant and measured by personnel The outcome measurement may be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals few and similar between groups No child withdrew from the study because of discomfort or lack of efficacy. Children who withdrew were not different from children who completed the study in terms of parental education level, family income, race/ethnicity, or child’s age, gender, or type of arthritis.’ |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol. Insufficient information to permit judgement of low risk or high risk. |
| Other bias | Unclear risk | Orthoses and shoe inserts reported to be donated by medical products companies. No assurance was made of their independence from the research design, conduct, or report. |
Solanki 2020.
| Study characteristics | ||
| Methods | Randomised controlled two‐arm parallel trial | |
| Participants | 166 children screened, with 70 meeting the criteria, 44 randomised into two arms (22 each) and 44 provided final outcome data Age: children were in 8th and 9th standard in the school (probably 13 and 14 year olds) Gender: boys and girls, no other details provided Location: India Setting: schools in Bardoli, Gujarat, India Inclusion criteria: children with flat feet assessed using 'too many toes' sign, calcaneal angle, and navicular height Exclusion criteria: previous trauma or fracture of lower limb, history of previous surgeries of the lower limb during last 3 months, hypersensitive skin, and any allergy to tape Baseline characteristics: not reported |
|
| Interventions | Group A: anti‐pronation taping + conventional treatment for 4 weeks Group B: sham taping + conventional treatment for 4 weeks A common (4 week) intervention program was executed for both groups as conventional therapy, which included strengthening exercise, stretching, & faradic foot bath that was given for 30 minutes per day |
|
| Outcomes | star excursion balance test (SEBT), vertical jump height (VJH), and Illinois agility test (IAT) measured at 1, 2, 3, and 4 weeks | |
| Source of funding | not described | |
| Notes | We used 4 weeks of data for the SEBT scores, VJH, and IAT, as the intervention was given for 4 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "By use of lottery method, 44 children were randomly selected". We presumed this was drawing of lots. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It appeared that from the participants' point of view, sham taping was not distinguishable from the anti‐pronation taping. |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | Low risk | no self‐reported outcomes reported |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Low risk | measurement of the outcomes were unlikely to be affected by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | trial was not registered; baseline characteristics not reported |
| Other bias | Low risk | none apparent |
Wenger 1989.
| Study characteristics | ||
| Methods | Randomised controlled four‐arm parallel trial | |
| Participants | 131 children recruited, with 129 meeting the criteria, 98 provided final outcome data Age: 1 to 6 years; mean age at baseline 29.3 (13.6) months (N = 98) Gender: N = 129, not reported; final N = 98 ‐ male 60, female 38 Location: USA, Dallas Setting: Dept Orthopedic surgery, Children’s Hospital, San Diego Inclusion criteria: 1 to 6 years old and flexible flat feet (observed to have valgus heel and poor arch formation in stance, further assessed by tip toe test, where varus shift of heel used to confirm flexible flat foot) Exclusion criteria: neurological condition (cerebral palsy or muscular disease), excessive laxity (Down or Ehlers‐Danlos syndrome), or previous treatment with foot orthoses or corrective footwear Baseline characteristics: (CFO group had more younger children (12 to 32 months), fewer in 32 to 52 month age range, fewer at 52 to 72 months age. Shoe group had lowest joint laxity, heel cup group had highest laxity, sex ratio ‐ not reported. |
|
| Interventions | Gp 1 ‐ controls Gp 2 – corrective orthopaedic shoes Gp 3 – Helfet heel cups Gp 4 – custom moulded plastic inserts (UCBL) |
|
| Outcomes | ‐ x‐rays (lateral talo‐horizontal angle > 35°, lateral talo‐1st metatarsal > 10°, AP talo‐calcaneal ‐ too difficult to interpret) ‐ photos (all x‐rays and photos recorded every 6 months) ‐ foot progression angle of gait (intoe/straight/out‐toe), heel cord ‐ assessed by passive ankle dorsiflexion, laxity (rated on Wynne‐Davies 1 to 5 scale; low laxity = 0 or 1, high laxity = 2 to 5) ‐ examined every 3 months by flatfoot team (nurse, Orthop Surg, Pedorthist) in unspecified manner. Shoe fit checked every 3 months by pedorthist, and shoes replaced whenever required for correct fitting. Commenced N = 129, completed N = 98 (lost n = 31, over 3 years of trial) Group 1, 31 controls; 21 completed, lost 10 Group 2, 32 shoes; 28 completed, lost 4 Group 3, 35 Helfet; 27 completed, lost 8 Group 4, 31 UCBL; 22 completed, lost 9 |
|
| Source of funding | shoes were provided | |
| Notes | All participants wore specified footwear (some variation between groups) Minimum 3 years treatment and follow‐up Attrition 98/129 (76%), and at completion, boys = 61/98 (61%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were assigned to treatment groups by a nurse who picked numbers randomly.’ It is not clear how this was done. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of intervention group. Personnel were aware of intervention group. |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | Low risk | ‘At the time of three‐year follow‐up, clinical, radiographic, and photographic analyses were completed with the examiners in ignorance of the child’s treatment group.’ |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Unclear risk | 31 participants were excluded from analysis as they were lost to follow up, mostly due to non‐compliance; the trialists did not report which treatment these exclusions received, and this may have biased the results of the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 98/129 completed the trial; 31/129 participants: ‘did not return for all of the follow‐up visits, did not wear the shoes regularly, or moved from the state and were dropped from the study’. Insufficient reporting of attrition/exclusions to permit judgement of low risk or high risk |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol. Insufficient information to permit judgement of low risk or high risk |
| Other bias | Unclear risk | ‘All shoes were provided by the Prescription Footwear Association’. No assurance was made of their independence from the research design, conduct, or report. However, since the report published negative findings, it suggests independence. |
Whitford 2007.
| Study characteristics | ||
| Methods | Randomised clinical three‐arm parallel trial | |
| Participants | 672 children responded to advertisements Recruited 178 with flexible flat feet Age: 7 to 11 years Gender: male 53.4%, female 46.6% Inclusion: bilateral flexible flat feet, as indicated by RCSP > 5° everted, Navicular drop 10 mm or greater Exclusion: unilateral flexible excessive foot pronation, history of lower limb surgery, any serious medical pathology, such as cancer, known neuromuscular motor co‐ordination condition, prior or recent foot orthoses use, intellectual or learning difficulty, or any chromosomal abnormality Location: Adelaide, South Australia Setting: outpatients Baseline characteristics: lower laxity score in CFO group 17.0 versus PFO 26.2, and shoes 30.6 |
|
| Interventions | Gp 1: CFOs Gp 2: PFOs Gp 3: no orthoses (control) |
|
| Outcomes | Assessment at baseline, 3 months, 12 months Outcome included in the trial 1. motor proficiency 2. pain ‐ proportion with pain in subgroup that had pain (post hoc analysis) 3. exercise efficiency 4. self‐perception profile Outcome included in this review is proportion without pain at 12 months |
|
| Source of funding | not stated (NHMRC) | |
| Notes | Most children did not have pain at baseline, pain analysed as subgroup Secondary outcomes measured included ligamentous laxity, tight calf muscles, and body mass index |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was undertaken using computer generated lists of equally sized groups’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | ‘… participants, and parents were not blinded.’ ‘Prescribing podiatrists… were not blinded’. |
| Blinding of outcome assessment (detection bias) ‐ self‐reported outcomes (e.g., pain, function) | High risk | Motor proficiency, pain, Self Perception Profile ‐ outcomes are subjective or influenced by participants, and participants were not blinded |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Low risk | exercise efficiency ‐ ‘As in‐shoe orthoses are concealed within the child’s shoes when worn, raters of outcome measures were blinded to the orthotic status of the child.’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate and reasons similar between groups |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol. The measurement of pain was done using VAS as a continuous measure, but reported only as a dichotomous outcome. |
| Other bias | Unclear risk | Funding source not stated |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benedetti 2011 | Not a randomised controlled trial |
| Ford 2017 | Not a randomised controlled trial |
| Hill 2020 | Review ‐ not an RCT |
| Hurd 2010 | Not a randomised controlled trial |
| MacKenzie 2012 | Review ‐ not a randomised controlled trial |
| Mosca 2010 | Not a randomised controlled trial |
| Okamura 2020 | Participants were university students, not children |
| Perhamre 2011 | Study not addressing flat feet |
| Perhamre 2012 | Study not addressing flat feet |
| Pothrat 2013 | Not a randomised controlled trial |
| Riccio 2009 | Not a randomised controlled trial |
| Uden 2017 | Not a randomised controlled trial |
| Yung 2011 | Participants were not children |
Characteristics of studies awaiting classification [ordered by study ID]
Pandey 2013.
| Methods | Randomised controlled four‐arm parallel trial |
| Participants | 150 symptomatic flat foot, 50 controls (age > 8 years) |
| Interventions |
|
| Outcomes |
|
| Notes | No variance, no age definition, no term of intervention, no randomisation details AE contacted Pandey via email x 3, searched internet for alternative address; unable to contact for additional data |
Sinha 2013.
| Methods | Randomised controlled two‐arm parallel trial |
| Participants | 101 recruited – children with symptomatic flat feet 81 children finished trial 20 lost or non‐compliant |
| Interventions | 1. Foot orthosis N = 55 2. Control N = 26 |
| Outcomes |
|
| Notes |
Unable to contact author – 3 emails; searched for alternative addresses – no contact made |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616001082493.
| Study name | Effectiveness of foot orthoses in children with juvenile idiopathic arthritis: a randomised control trial |
| Methods | Randomised controlled two‐arm trial ‐ 12 months |
| Participants | Children with juvenile idiopathic arthritis (JIA) 1. Diagnosed with JIA according to ILAR (International League of Associations for Rheumatology) criteria 2. Age 5 to 18 years old 3. Active lower‐limb joint arthritis involvement 4. No previous use of foot orthoses, or previous failure of foot orthotic management, where the patient has not worn any foot orthoses (FO) for a period of at least 3 months 5. Ability to walk a minimum of 15 meters without assistive devices 6. If disease‐modifying antirheumatic drugs (DMARD), biological therapy, or both are used, not having started these drug therapies within 6 months of enrolling in the trial |
| Interventions | Customised prefabricated foot orthoses (PFO), sham insole (control) |
| Outcomes | pain, disability, quality of life |
| Starting date | not stated |
| Contact information | www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12616001082493 |
| Notes |
CTRI/2018/07/014989.
| Study name | Effect of adhesive taping and exercises on outward rotation of foot in affected children |
| Methods | Randomised controlled two‐arm cross‐over trial ‐ 4 weeks |
| Participants | Children with pronated feet and neurodevelopmental disorders: 1) Diagnosed cases of non‐progressive neurodevelopmental disorders by paediatrician 2) Foot Posture Index score more than 6 3) Navicular Drop Test more than 10 mm 4) Both male and female 5) Age 6 to 11 years 6) Patient should be able to stand without support or with minimum hand support 7) Flexible pes planus |
| Interventions | Intervention: Group A: kinesio taping for 5 days for 2 weeks, and then group A will be switched to Group B therapy after 2 weeks of window period Control: Group B: exercises for 5 days for 2 weeks, and then group B will be switched to Group A after 2 weeks of window period |
| Outcomes | 1. Foot Posture Index/time point 2. Navicular Drop Test/time point |
| Starting date | not stated |
| Contact information | www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/07/014989 |
| Notes |
CTRI/2019/08/020925.
| Study name | Effects of barefoot walking on the flat foot in school going children: a randomised control trial |
| Methods | Randomised control trial |
| Participants | N = 38, age between 6 and 14 years |
| Interventions | Intervention 1: Barefoot walking and exercises: children have to be barefoot while doing exercises, as follows: 1.Towel gathering exercise for 15 minutes 2.Heel cord stretching (holding for 30 seconds and then relax for 30 seconds; repeat once) 3.Toe spread (5 seconds, than 2 second relax) 4.Posterior tibialis exercises (3 sets, 10 repetitions) Intervention 2: Bare foot exercises and Exercises: Bare foot walking and exercises.<br>Exercises are, A.Towel gathering exercise for 15 minutes B. Heel cord stretching (holding for 30 seconds and then relax for 30 seconds and then repeat for 1times) C. Toe spread [5 sec than 2 sec relax] D. Posterior Tibialis exercises [3sets, 10reps] Control Intervention1: Not applicable |
| Outcomes | The Oxford Ankle Foot Questionnaire for Children (OxAFQ‐C) time point: 8 weeks |
| Starting date | 27 August 2019, not recruiting |
| Contact information | Sharath HV, chippala1979@gmail.com. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=36115 |
| Notes | Nitte institute of physiotherapy, Nitte University, India |
IRCT2017082235517N1.
| Study name | Effects of long‐term use of arch support foot orthoses on lower limb kinematics and kinetics during walking in children with flexible flat foot |
| Methods | Randomised control trial |
| Participants | N = 30 Inclusion criteria: age range between 9 and 12 years; flexible flat feet; male Exclusion criteria: history of lower limb injury; history of surgery |
| Interventions | Intervention 1: intervention group: 4 months use of arch support foot orthoses Intervention 2: control group: long‐term use of placebo orthoses; rehabilitation; placebo; Intervention group: 4 months use of arch support foot orthoses; Control group: long term use of placebo orthoses |
| Outcomes | Walking kinematics Time point: before and after 4 months Method of measurement: vicon cameras; walking kinetics. Timepoint: before and after 4 months. Method of measurement: Force platform. |
| Starting date | 4 September 2017 |
| Contact information | Amir Ali Jafarnezhadgero, amiralijafarnezhad@gmail.com; en.irct.ir/trial/26811 |
| Notes | Mohaghegh Ardabili University, Iran (Islamic Republic of) |
ISRCTN14602568.
| Study name | Orthotics for treatment of symptomatic flat feet in children ‐ the OSTRICH study |
| Methods | Interventional randomised controlled trial including a qualitative study within a trial (treatment) |
| Participants | N = 1055 Inclusion criteria: 1. Aged between 6 and 14 years, inclusive 2. Have one or both symptomatic pes planus* 3. The child, parent, or legal guardian is able to speak, write and understand English 4. The parent or legal guardian is able to give informed consent *Symptomatic pes planus is described as the manifestation of foot and lower limb symptoms, secondary to altered foot alignment (reduced medial longitudinal arch, everted rearfoot, and abducted forefoot). The diagnosis will be made pragmatically, by treating clinicians, in line with current practice. Eligibility for the birthday card SWAT All participants recruited into the host trial will be eligible to take part in this SWAT. |
| Interventions | Participants will be allocated to one of three groups: 1. A package consisting of an exercise programme and advice covering topics, such as typical arch development in children, coping strategies, and footwear advice 2. The exercise and advice package plus a pair of prefabricated, off‐the‐shelf orthoses (i.e. insoles that are mass produced to a generic shape, but can be adapted by a clinician) 3. The exercise and advice package plus a pair of custom‐made foot orthoses, where the shape of the insole is made for a specific person, based on a 3D impression or scan of the patient's foot The participant will be informed which group they have been allocated to at their first trial appointment. Participants will be asked to wear their orthoses every day in their shoes, and to do their exercises. Participants in group 1 and 2 will, on the whole, need one clinic appointment, but those in group 3, may need a total of 2 or 3 appointments. Blinding of participants to the treatment allocation is not possible. Measures will be collected at baseline, weeks 1 to 12, and at three, six, and 12 months after a participant is enrolled into the study. The measures are all self‐reported by either the participant or their parent or legal guardian. Birthday card study: In the birthday card SWAT, the researchers will evaluate whether sending a participant a birthday card increases the number of questionnaires they return to the study team. Participants will be allocated to one of three groups; birthday card, birthday card informed by nudge theory to encourage completion of questionnaires, or no birthday card. |
| Outcomes | Physical domain subscale of the Oxford Ankle Foot Questionnaire for Children (OxAFQ‐C); Time point(s): over the 12‐month follow‐up period |
| Starting date | 18 May 2020 |
| Contact information | David Torgerson, PhD; Sarah Cockayne, MSc; sarah.cockayne@york.ac.uk www.isrctn.com/ISRCTN14602568 |
| Notes | University of York, UK |
ISRCTN49672274.
| Study name | Foot disease in juvenile idiopathic arthritis: foot care trial |
| Methods | Randomised clinical two‐arm trial ‐ 12 months |
| Participants | Children with JIA: 1. juvenile idiopathic arthritis (JIA) diagnosed according to the International League of Associations for Rheumatology (ILAR) 2004 criteria 2. Lower limb arthritis of two or more large joints (hips, knees, ankles, and subtalar joints) 3. Widespread polyarthritis involving large and small joints |
| Interventions | Usual clinical care (standard podiatry) versus individualised care package (foot orthoses (FO), shoes, physiotherapy, podiatry) |
| Outcomes | Juvenile Arthritis Foot Disability Index (JAFI) Function HRQoL (health‐related quality of life) |
| Starting date | not stated |
| Contact information | /www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN49672274 |
| Notes |
KCT0001717.
| Study name | The effects of talus control foot orthosis in children with flexible flatfoot |
| Methods | Randomised control trial |
| Participants | N = 40 Inclusion criteria: diagnosed with flexible flatfoot; visited the Department of Pediatric Rehabilitation over 6 years Exclusion criteria: rigid flatfoot caused by hereditary foot disease or neuromuscular disease, fixed foot deformity, or previous surgery history |
| Interventions | Device: plantar orthoses |
| Outcomes | X‐ray measurements of the angles: talus‐calcaneus, talus‐navicular, and talus‐first metatarsal angle |
| Starting date | 7 December 2015 |
| Contact information | So‐Young Ahn |
| Notes | Chungnam National University Hospital, Republic of Korea |
NCT02414087.
| Study name | Therapeutic effects of customized insoles on children with flatfoot |
| Methods | Randomised controlled trial ‐ two arms ‐ 12 weeks |
| Participants | 52 children with flat feet |
| Interventions | 1. customised full‐length insoles 2. control |
| Outcomes | physical function balance quality of life |
| Starting date | not stated |
| Contact information | clinicaltrials.gov/show/NCT02414087 |
| Notes | Ru‐Lan Hsieh, Shin Kong Wu Ho‐Su Memorial Hospital, Taipei Medical University |
NCT02633566.
| Study name | Clinical trial of the effect of functional orthoses in children with flat feet |
| Methods | randomised, double‐blind, parallel design clinical trial ‐ 1 year |
| Participants | 61 children, aged 3 and 4 years old |
| Interventions | 1. Intervention group ‐ functional plantar orthotics 2. Control group ‐ placebo‐type plantar orthotics |
| Outcomes | X‐rays: talus‐calcaneus, tibionavicular, and talus‐first metatarsal angles were compared pre‐ and post‐treatment |
| Starting date | not stated |
| Contact information | clinicaltrials.gov/show/NCT02633566 |
| Notes | Raquel Lopez Fresno, PhD Student (Podiatrist) |
NCT03151538.
| Study name | Effects on pes planus exercise training mixed with play on pre‐school children |
| Methods | not stated |
| Participants | Pre‐school children with pes planus (flat feet) |
| Interventions | exercise training mixed with play |
| Outcomes | effects on pes planus and femoral anteversion angle |
| Starting date | not stated |
| Contact information | clinicaltrials.gov/show/NCT03151538 |
| Notes | Burcu Talu, Assistant Professor, Inonu University |
NCT04104555.
| Study name | Orthotics for treatment of symptomatic flat feet in children ‐ the OSTRICH study |
| Methods | |
| Participants | ‐ Have pes planus secondary to any systematic condition or syndrome**, malignancy ‐ Have a history of foot or ankle surgery, or both ‐ Require an ankle‐foot orthoses, or other lower limb device, or have received treatment previously for their flat feet ‐ This does not exclude children with hypermobility spectrum disorder (HSD), where the manifestation is non‐syndromic and isolated (L‐HSD), peripheral (P‐HSD), or generalised hypermobility (G‐HSD)(14). OSTRICH pen and signposting to multimedia trial information SWAT inclusion criteria: any potential participant identified as eligible to be sent trial information will be eligible for the study OSTRICH birthday card SWAT inclusion criteria: all participants recruited into the host trial will be eligible to take part in this SWAT. Exclusion criteria: any participant who has withdrawn from the main OSTRICH study, or is not due a follow‐up questionnaire will be excluded. |
| Interventions | Other: Birthday card; Other: Birthday card plus nudge; Device: Prefabricated orthoses; Device: Custom orthoses; Other: Pen;Other: Signposting to multimedia; Other: Pen and signposting to multimedia; Other: Usual supportive care ‐ exercises and footwear advice (the comparator) |
| Outcomes | Physical domain subscale of the Oxford Ankle Foot Questionnaire for Children (OxAFQ‐C) over the 12‐month follow‐up period |
| Starting date | 19 September 2019 |
| Contact information | David Torgerson, PhD; Sarah Cockayne, MSc; sarah.cockayne@york.ac.uk clinicaltrials.gov/ct2/show/NCT04104555 |
| Notes | University of York, UK |
NCT04410926.
| Study name | Corrective exercises with neuromuscular electrical stimulation In children with flexible flat feet |
| Methods | prospective double‐masked randomised controlled trial |
| Participants | 70 school children were recruited, 67 were randomised |
| Interventions | 1. intervention group (corrective exercise and neuromuscular electrical stimulation (NMES)) 2. control group (corrective exercise and placebo NMES) |
| Outcomes | not stated |
| Starting date | 1 September 2018 to 31 December 2019 |
| Contact information | clinicaltrials.gov/show/NCT04410926 |
| Notes | Ethics Statement ‐ study was approved by the Institutional Review Board of the Faculty of Physical Therapy, Cairo University, Egypt (no. P.T.REC/012/0016370) |
Differences between protocol and review
Post hoc, we decided to include only FOs as the main comparisons, viz CFO versus shoes, PFO versus shoes, PFOs versus CFOs. Similarly, we had intended to include any paediatric population, and separate symptomatic versus asymptomatic participants. Post hoc, we decided to address two paediatric populations, namely asymptomatic, juvenile idiopathic arthritis (JIA), or other clinical concerns.
We did not describe risk of bias and summary of findings tables, or GRADE analyses in the original protocol, but we included them in this review update, in accordance with current Cochrane standards.
We extended both outcomes and comparisons, and better defined them in this review update, which includes 16 trials versus the 3 trials available in 2010 (Rome 2010). Further, the updated review is more structured regarding participants, intervention comparisons (three comparisons), and outcomes.
Intended comparisons were: any FOs versus sham; any FOs versus shoes; customised FOs (CFOs) versus prefabricated FOs (PFOs).
Contributions of authors
AE, KR, and FH conceived and designed the review. AE collected and inputted the data. AE, and MC conducted the GRADE analyses. AE, KR, and FH compiled the data, and AE drafted the manuscript. All authors read and approved the final manuscript.
Sources of support
Internal sources
Auckland University of Technology, New Zealand
University of Newcastle, NSW, Australia
La Trobe University, Melbourne, Australia
External sources
No sources of support provided
Declarations of interest
KR, AE are authors of the 2010 systematic review addressing this topic.
AE declares authorship of Evidence Essentials (www.evidenceessentials.com; blog and monograph series), Board Directorship Australian Podiatry Association, Board Directorship AnglicareSA
MC: none known
KR: none known
FH: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abd‐Elmonem 2021 {published data only}
- Abd-Elmonem AM, El-Negamy EH, Mahran MA, Ramadan AT. Clinical and radiological outcomes of corrective exercises and neuromuscular electrical stimulation in children with flexible flatfeet: a randomized controlled trial. Gait & Posture July 2021;88:297-303. [DOI] [PubMed] [Google Scholar]
Aboutorabi 2013 {published data only}
- Aboutorabi A, Saeedi H, Kamali M, Farahmand B, Eshraghi A, Dolagh RS. Immediate effect of orthopedic shoe and functional foot orthosis on center of pressure displacement and gait parameters in juvenile flexible flat foot. Prosthetics and Orthotics International 2014;38(3):218-23. [DOI] [PubMed] [Google Scholar]
Ahn 2017 {published data only}
- Ahn SY, Bok SK, Kim BO, Park IS. The effects of talus control foot orthoses in children with flexible flatfoot. Journal of the American Podiatric Medical Association 2017;107(1):46-53. [DOI: 10.7547/15-045] [DOI] [PubMed] [Google Scholar]
Asgaonkar 2012 {published data only}
- Asgaonkar B, Kadam P. Effectiveness of valgus insole on pain, gait parameters and physiological cost index of walking in flat feet in 5 to15 years. Indian Journal of Physiotherapy and Occupational Therapy 2012;6(2):90-4. [Google Scholar]
Bok 2016 {published data only}
- Bok SK, Lee H, Kim BO, Ahn S, Song Y, Park I. The effect of different foot orthosis inverted angles on plantar pressure in children with flexible flatfeet. PloS One 2016;11(7):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coda 2014 {published data only}
- Coda A, Fowlie PW, Davidson JE, Walsh J, Carline T, Santos D. Foot orthoses in children with juvenile idiopathic arthritis: a randomised controlled trial. Archives of Disease in Childhood 2014;99(7):649-51. [DOI] [PubMed] [Google Scholar]
Gould 1989 {published data only}
- Gould N, Moreland M, Alvarez R, Trevino S, Fenwick J. Development of the child's arch. Foot & Ankle International 1989;9:241-5. [DOI] [PubMed] [Google Scholar]
Hsieh 2018 {published data only}
- Hsieh RL, Peng HL, Lee WC. Short-term effects of customized arch support insoles on symptomatic flexible flatfoot in children. A randomized controlled trial. Medicine 2018;97(20):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jafarnezhadgero 2018 {published data only}
- Jafarnezhadgero A, Madadi-Shad M, Alavi-Mehr SM, Granacher U. The long-term use of foot orthoses affects walking kinematics and kinetics of children with flexible flat feet: A randomized controlled trial. PloS One 2018;13(10):e0205187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanatli 2016 {published data only}
- Kanatlı U, Aktas E, Yetkin H. Do corrective shoes improve the development of the medial longitudinal arch in children with flexible flat feet? Journal of Orthopaedic Science 2016;21(5):662-6. [DOI] [PubMed] [Google Scholar]
Khamooshi 2017 {published data only}
- Khamooshi R, Mohammadieh SM, Rahnama N, Zalani FR. Comparing the effects of simultaneous eight-week stretching/strengthening training with core stability exercises on the flat foot deformity of 9 to 13 year old female students. International Journal of Musculoskeletal Pain Prevention 2016;1(4):149-56. [Google Scholar]
Morrison 2013 {published data only}
- Morrison S, Ferrari J, Smillie S. Assessment of gait characteristics and orthotic management in children with developmental coordination disorder: preliminary findings to inform multidisciplinary care. Research in Developmental Disabilities 2013;34(10):3197-201. [DOI] [PubMed] [Google Scholar]
Powell 2005 {published data only}
- Powell M, Seid M, Szer IS. Efficacy of custom foot orthotics in improving pain and functional status in children with juvenile idiopathic arthritis: a randomised trial. Journal of Rheumatology 2005;32:943-50. [PubMed] [Google Scholar]
Solanki 2020 {published data only}
- Priyanka S, Murugan S. Anti pronation taping and Its effect on balance, explosive power and agility of school going children with flat foot. Indian Journal of Public Health Research & Development 2020;11(7):868-73. [Google Scholar]
Wenger 1989 {published data only}
- Wenger DR, Mauldin D, Spleck G, Morgan D, Lieber RL. Corrective shoes and inserts as treatment for flexible flatfoot in infants and children. Journal of Bone and Joint Surgery 1989;71(6):800-10. [PubMed] [Google Scholar]
Whitford 2007 {published data only}
- Whitford D, Esterman A. A randomised controlled trial of two types of in-shoe orthoses in children with flexible excess pronation of the feet. Foot & Ankle International 2007;28:715-23. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Benedetti 2011 {published data only}
- Benedetti MG, Ceccarelli F, Berti L, Luciani D, Catani F, Boschi M, Giannini S. Diagnosis of flexible flatfoot in children: a systematic clinical approach. Orthopedics 2011;34(2):94. [DOI] [PubMed] [Google Scholar]
Ford 2017 {published data only}
- Ford SE, Scannell BP. Pediatric flatfoot: pearls and pitfalls. Foot and Ankle Clinics 2017;22(3):643-56. [DOI] [PubMed] [Google Scholar]
Hill 2020 {published data only}
- Hill M, Healy A, Chockalingam N. Effectiveness of therapeutic footwear for children: a systematic review. Journal of Foot and Ankle Research 2020;13(23):1-26. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurd 2010 {published data only}
- Hurd WJ, Kavros SJ, Kaufman KR. Comparative biomechanical effectiveness of over-the-counter devices for individuals with a flexible flatfoot secondary to forefoot varus. Clinical Journal of Sport Medicine 2010;20(6):428-35. [DOI] [PubMed] [Google Scholar]
MacKenzie 2012 {published data only}
- MacKenzie AJ, Rome K, Evans AM 2012 Dec 1, 32(8):830-4. The efficacy of nonsurgical interventions for pediatric flexible flat foot: a critical review.. Journal of Pediatric Orthopaedics 2012;32(8):830–4. [DOI] [PubMed] [Google Scholar]
Mosca 2010 {published data only}
- Mosca V. Flexible flatfoot in children and adolescents. Journal of Children's Orthopaedics 2010;4(2):107-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okamura 2020 {published data only (unpublished sought but not used)}
- Okamura K, Fukuda K, Oki S, Ono T, Tanaka S, Kanai S. Effects of plantar intrinsic foot muscle strengthening exercise on static and dynamic foot kinematics: A pilot randomized controlled single-blind trial in individuals with pes planus. Gait Posture 2020 Jan;75:40-45. [DOI] [PubMed] [Google Scholar]
Perhamre 2011 {published data only}
- Perhamre S, Lundin F, Norlin R, Klässbo M. Sever's injury; treat it with a heel cup: a randomized, crossover study with two insole alternatives. Scandinavian Journal of Medicine & Science in Sports 2011;21(6):e42–7. [DOI] [PubMed] [Google Scholar]
Perhamre 2012 {published data only}
- Perhamre S, Lundin F, Klässbo M, Norlin R. A heel cup improves the function of the heel pad in Sever's injury: effects on heel pad thickness, peak pressure and pain. Scandinavian Journal of Medicine & Science in Sports 2012;22(4):516-22. [DOI] [PubMed] [Google Scholar]
Pothrat 2013 {published data only}
- Pothrat C, Authier G, Viehweger E, Rao G. Multifactorial gait analysis of children with flat foot and hind foot valgus deformity. Computer Methods in Biomechanics and Biomedical Engineering 2013;16(Suppl 1):80-1. [DOI] [PubMed] [Google Scholar]
Riccio 2009 {published data only}
- Riccio I, Gimigliano F, Gimigliano R, Porpora G, Iolascon G. Rehabilitative treatment in flexible flatfoot: a perspective cohort study. Musculoskeletal Surgery 2009;93(3):101-7. [DOI] [PubMed] [Google Scholar]
Uden 2017 {published data only}
- Uden H, Scharfbillig R, Causby R. The typically developing paediatric foot: how flat should it be? A systematic review. Journal of Foot and Ankle Research 2017;10(37):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yung 2011 {published data only}
- Jung D-Y, Koh E-K, Kwon O-Y. Effect of foot orthoses and short-foot exercise on the cross-sectional area of the abductor hallucis muscle in subjects with pes planus: a randomized controlled trial. Journal of Back and Musculoskeletal Rehabilitation 2011;24:225–31. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Pandey 2013 {published data only}
- Pandey S, Pal CP, Kumar D, Singh P. Flatfoot in Indian population. Journal of Orthopaedic Surgery 2013;21(1):32-6. [DOI] [PubMed] [Google Scholar]
Sinha 2013 {published data only}
- Sinha S, Song HR, Kim HJ, Park MS, Yoon YC, Song SH. Medial arch orthosis for paediatric flatfoot. Journal of Orthopaedic Surgery 2013;21(1):37-43. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616001082493 {unpublished data only}
- Effectiveness of foot orthoses in children with juvenile idiopathic arthritis: a randomised control trial. Ongoing study. not stated. Contact author for more information.
CTRI/2018/07/014989 {unpublished data only}
- Effect of adhesive taping and exercises on outward rotation of foot in affected children. Ongoing study. not stated. Contact author for more information.
CTRI/2019/08/020925 {published and unpublished data}
- CTRI/2019/08/020925. Bare foot walking effect on flat foot in school going children [Effects of bare foot walking on the flat foot in school going children:a randomised control trial]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=36115 (first registration 27 August 2019).
IRCT2017082235517N1 {unpublished data only}en.irct.ir/trial/26811
- Effects of long-term use of arch support foot orthoses on lower limb kinematics and kinetics during walking in children with flexible flat foot. Ongoing study. 4 September 2017. Contact author for more information.
ISRCTN14602568 {unpublished data only}www.isrctn.com/ISRCTN14602568
- Orthotics for treatment of symptomatic flat feet in children - the OSTRICH study. Ongoing study. 18 May 2020. Contact author for more information.
ISRCTN49672274 {unpublished data only}
- Foot disease in juvenile idiopathic arthritis: foot care trial. Ongoing study. not stated. Contact author for more information.
KCT0001717 {unpublished data only}
- The effects of talus control foot orthosis in children with flexible flatfoot. Ongoing study. 7 December 2015. Contact author for more information.
NCT02414087 {unpublished data only}
- Therapeutic effects of customized insoles on children with flatfoot. Ongoing study. not stated. Contact author for more information.
NCT02633566 {unpublished data only}
- Clinical trial of the effect of functional orthoses in children with flat feet. Ongoing study. not stated. Contact author for more information.
NCT03151538 {unpublished data only}
- Effects on pes planus exercise training mixed with play on pre-school children. Ongoing study. not stated. Contact author for more information.
NCT04104555 {unpublished data only}
- Orthotics for treatment of symptomatic flat feet in children - the OSTRICH study. Ongoing study. 19 September 2019. Contact author for more information.
NCT04410926 {unpublished data only}
- Corrective exercises with neuromuscular electrical stimulation In children with flexible flat feet. Ongoing study. 1 September 2018 to 31 December 2019. Contact author for more information.
Additional references
Aharonson 1992
- Aharonson Z, Arcan M, Steinback TV. Foot-ground pressure pattern of flexible flatfoot in children with and without correction of calcaneovalgus. Clinical Orthopaedics and Related Research 1992;181:177-82. [PubMed] [Google Scholar]
Bauer 2015
- Bauer K, Mosca VS, Zionts LE. What's new in paediatric flatfoot? Journal of Pediatric Orthopedics 2016;36(8):865-9. [DOI] [PubMed] [Google Scholar]
Blitz 2010
- Blitz NM, Stabile RJ, Giorgini RJ, Di Domenico LA. Flexible pediatric and adolescent pes planovalgus: conservative and surgical treatment options. Clinics in Podiatric Medicine and Surgery 2010;27(1):59-77. [DOI] [PubMed] [Google Scholar]
Bresnahan 2009
- Bresnahan P. The flat-footed child – to treat or not to treat: what is the clinician to do? Journal of American Podiatric Medical Association 2009;99(3):178. [DOI] [PubMed] [Google Scholar]
Brooks 1991
- Brooks MH. Flatfeet in children. BMJ 1991;302:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Budiman 1991
- Budiman-Mak E, Conrad KJ, Roach K. The Foot Function Index: a measure of foot pain and disability. Journal of Clinical Epidemiology 1991;44:561-70. [DOI] [PubMed] [Google Scholar]
Capello 1998
- Cappello T, Song KM. Determining treatment of flatfeet in children. Current Opinion in Pediatrics 1998;10:77-81. [DOI] [PubMed] [Google Scholar]
Carli 2012
- Carli A, Saran N, Kruijt J, Alam N, Hamdy R. Physiological referrals for paediatric musculoskeletal complaints: a costly problem that needs to be addressed. Paediatrics and Child Health 2012;17(e):93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2016 [Computer program]
- Visual Rx. Cates C, Version 4. Hertfordshire (UK): Dr Chris Cates, 2016. available at www.nntonline.net.
Choia 2020
- Choi JY, Hong WH, Suh JS, Han JH, Lee DJ, Lee YJ. The long-term structural effect of orthoses for pediatric flexible flat foot: a systematic review. Foot and Ankle Surgery 2020;26(2):181-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
D'Amico 2009
- D'Amico JC. The flat-footed child – to treat or not to treat: what is the clinician to do? Journal of American Podiatric Medical Association 2009;99(3):267-8. [DOI] [PubMed] [Google Scholar]
Daltroy 1998
- Daltroy LH, Liang MH, Fossel AH, Goldberg MJ. The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. Journal of Pediatric Orthopaedics 1998;18(5):561–71. [DOI] [PubMed] [Google Scholar]
Daneshmandi 2011
- Daneshmandi H, Azhdari F, Saki F, Daneshmandi MS. The study of lower limb alignment in athletes with and without ACL reconstruction. Brazilian Journal of Biomotricity 2011;5(4):248-54. [Google Scholar]
Dars 2018
- Dars S, Uden H, Banwell HA, Kumar S. The effectiveness of non-surgical intervention (Foot Orthoses) for paediatric flexible pes planus: a systematic review: update. PloS One 2018;13(2):e0193060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 10. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Evans 2008
- Evans AM. The flat-footed child—to treat or not to treat: what is the clinician to do? Journal of the American Podiatric Medical Association 2008;98(5):386-93. [DOI] [PubMed] [Google Scholar]
Evans 2009
- Evans AM, Nicholson H, Zakarias N. The paediatric flat foot proforma (p-FFP): improved and abridged following a reproducibility study. Journal of Foot and Ankle Research 2009;2(1):1-8. [DOI: 10.1186/1757-1146-2-25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2012
- Evans AM. Screening for foot problems in children: is this practice justifiable? Journal of Foot and Ankle Research 2012;5(1):18. [DOI: 10.1186/1757-1146-5-18] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2015
- Evans A, Menz H, Bourke J, Nikopoulos N, Ferrari J, Wilkinson M, et al. Foot care and people with intellectual disability. International Journal of Child Health & Human Development 2015;8(4):471-91. [Google Scholar]
Evans 2017
- Evans AM. Mitigating clinician and community concerns about children's flatfeet, intoeing gait, knock knees or bow legs. Journal of Paediatric Child Health 2017;53(11):1050-3. [DOI: 10.1111/jpc.13761] [DOI] [PubMed] [Google Scholar]
Evans 2021
- Evans AM. Paediatric flatfeet – a 2020 guide for clinicians to identify the 'Boomerangs'. Journal of the American Podiatric Medical Association 2021;5:20-103. [DOI: 10.7547/20-103] [DOI] [PubMed] [Google Scholar]
Fellas 2017a
- Fellas A, Hawke F, Santos, D, Coda A. Prevalence, presentation and treatment of lower limb pathologies in juvenile idiopathic arthritis: a narrative review. Journal of Paediatric Child Health 2017;53(9):836-40. [DOI] [PubMed] [Google Scholar]
Fellas 2017b
- Fellas A, Coda A, Hawke F. Physical and mechanical therapies for lower-limb problems in juvenile idiopathic arthritis a systematic review with meta-analysis. Journal of the American Podiatric Medical Association 2017;107(5):399-412. [DOI: 10.7547/15-213] [DOI] [PubMed] [Google Scholar]
Garcia‐Rodriguez 1999
- Garcia-Rodriguez A, Martib-Jimenez F, Carnero-Varo M, Gomez-Garcia E, Gomez-Aracena J, Fernanedz-Crehuet J. Flexible flat feet in children: a real problem? Pediatrics 1999;103:84-6. [DOI] [PubMed] [Google Scholar]
Gijon‐Nogueron 2019
- Gijon-Nogueron G, Martinez-Nova A, Alfageme-Garcia P, Montes-Alguacil J, Evans AM. International normative data for paediatric foot posture assessment: a cross-sectional investigation. BMJ Open 2019;9(4):e023341. [DOI: 10.1136/bmjopen-2018-023341] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 11 November 2021. Available at gradepro.org.
Gribble, 2012
- Gribble PA, Hertel J, Plisky P. Using the Star Excursion Balance Test to assess dynamic postural-control deficits and outcomes in lower extremity injury: a literature and systematic review. Journal of Athletic Training 2012;47(3):339–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2004
- Harris EJ, Vanore JV, Thomas JL, Kravitz SR, Mendelson SA, Mendicino RW, et al. Diagnosis and treatment of pediatric flatfoot. The Journal of Foot and Ankle Surgery 2004;43:341-73. [DOI] [PubMed] [Google Scholar]
Harris 2010
- Harris EJ. The natural history and pathophysiology of flexible flatfoot. Clinic in Podiatric Medicine & Surgery 2010;27:1-23. [DOI] [PubMed] [Google Scholar]
Henry 2008
- Hendry G, Gardner-Medwin J, Watt GF, Woodburn J . A survey of foot problems in juvenile idiopathic arthritis. Musculoskeletal Care 2008;6:221-32. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2020b
- Higgins JPT, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Kerr 2015
- Kerr CM, Stebbins J, Theologis T, Zavatsky B. Static postural differences between neutral and flat feet in children with and without symptoms. Clinical Biomechanics 2015;30:314-7. [DOI] [PubMed] [Google Scholar]
Kim 2017
- Kim HY, Shin HS, Ko JH, Cha YH, Ahn JH, Hwang JY. Gait analysis of symptomatic flatfoot in children: an observational study. Clinics in Orthopedic Surgery 2017;9:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klaue 1997
- Klaue K. Planovalgus and cavovarus deformity of the hind foot. Journal of Bone and Joint Surgery. British Volume 1997;79(6):892-5. [DOI: 10.1302/0301-620x.79b6.7195] [DOI] [PubMed] [Google Scholar]
Klepper 2011
- Klepper SE. Measures of pediatric function. Arthritis Care & Research 2011;63(S11):S371–82. [DOI: 10.1002/acr.20635] [DOI] [PubMed] [Google Scholar]
Kutlu 2018
- Kutlu M, Dogan O. Test-retest reliability and validity of three different agility tests for various team sports in young male athletes. Central European Journal of Sport Sciences and Medicine 2018;22(2):33-8. [DOI: 10.18276/cej.2018.2-04] [DOI] [Google Scholar]
Landorf 2008
- Landorf KB, Radford JA. Minimal important difference: values for the Foot Health Status questionnaire, foot function index and visual analogue scale. The Journal of Foot and Ankle Surgery 2008;18(1):15-9. [DOI: 10.1016/j.foot.2007.06.006] [DOI] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from training.cochrane.org/handbook/archive/v5.0.1.
Martinez‐Nova 2018
- Martínez-Nova A, Gijón-Noguerón G, Alfageme-García P, Montes-Alguacil J, Evans AM. Foot posture development in children aged 5 to 11 years: a three-year prospective study. Gait & Posture 2018;62:280–4. [DOI: 10.1016/j.gaitpost.2018.03.032] [DOI] [PubMed] [Google Scholar]
Mereday 1972
- Mereday C, Dolan CM, Lusskin R. Evaluation of the University of California Biomechanics Laboratory shoe insert in 'flexible' pes planus. Clinical Orthopedic Related Research 1972;82:45e58. [PubMed] [Google Scholar]
Moher 2015
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 2015;4(1):1-9. [DOI: 10.1186/2046-4053-4-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montalvo 2021
- Montalvo S, Gonzalez MP, Dietze-Hermosa MS, Eggleston JD, Dorgo S. Common vertical jump and reactive strength index measuring devices: a validity and reliability analysis. Journal of Strength & Conditioning Research 2021;35(5):1234–43. [DOI] [PubMed] [Google Scholar]
Moraleda 2011
- Moraleda L, Mubarak SJ. Flexible flatfoot: differences in the relative alignment of each segment of the foot between symptomatic and asymptomatic patients. Journal of Pediatric Orthopedics 2011;31:421–8. [DOI] [PubMed] [Google Scholar]
Moynihan 2012
- Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ 2012;28:344. [DOI: 10.1136/bmj.e3502] [DOI] [PubMed] [Google Scholar]
Moynihan 2014
- Moynihan R, Henry D, Moons KG. Using evidence to combat overdiagnosis and overtreatment: evaluating treatments, tests, and disease definitions in the time of too much. PLoS Medicine 2014;11(7):e1001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moynihan 2017
- Pathirana T, Clark J, Moynihan R. Mapping the drivers of overdiagnosis to potential solutions.. BMJ 2017;16:358. [DOI: 10.1136/bmj.j3879] [DOI] [PubMed] [Google Scholar]
Page 2020
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Pfeiffer 2006
- Pfeiffer M, Kotz R, Ledl T, Hauser G, Sluga M. Prevalence of flatfoot in preschool-aged children. Pediatrics 2006;118:634-9. [DOI] [PubMed] [Google Scholar]
Rigoldi 2012
- Rigoldi C, Galli M, Cimolin V, Camerota F, Celletti C, Tenore N, et al. Gait strategy in patients with Ehlers-Danlos syndrome hypermobility type and Down syndrome. Research in Developmental Disabilities 2012;33(5):1437–42. [DOI: 10.1016/j.ridd.2012.03.016] [DOI] [PubMed] [Google Scholar]
Schunemann 2020b
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Schünemann 2020a
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Staheli 1987
- Staheli LT, Chew DE, Corbett M. The longitudinal arch: a survey of 802 feet in normal children and adults. Journal of Bone and Joint Surgery. American Volume 1987;69:426-8. [PubMed] [Google Scholar]
Varni 1996
- Varni JW, Waldron SA, Gragg RA, Rapoff MA, Bernstein BH, Lindsley CB, et al. Development of the Waldron/Varni pediatric pain coping inventory. Pain 1996;67(1):141-50. [DOI] [PubMed] [Google Scholar]
Varni 2002
- Varni JW, Seid M, Knight TS, Szer JS. The PedsQL in pediatric rheumatology: reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory, generic core scales and rheumatology module. Arthritis and Rheumatism 2002;46:714-25. [DOI] [PubMed] [Google Scholar]
Weber 2014
- Weber A, Martin K. Efficacy of orthoses for children with hypotonia. Pediatric Physical Therapy 2014;26:38–47. [DOI] [PubMed] [Google Scholar]
Wegener 2011
- Wegener C, Hunt AE, Vanwanseele B, Burns J, Smith RM. Effect of children's shoes on gait: a systematic review and meta-analysis. Journal of Foot and Ankle Research 2011;4(1):3. [DOI: 10.1186/1757-1146-4-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1968
- Wilson JMG, Jungner G, World Health Organization. Principles and Practice of Screening for Disease; Public Health Papers No. 34. France: World Health Organization, 1968. [Google Scholar]
Yan 2013
- Yan G, Yang Z, Lu M, Zhang J, Zhu Z, Guo Y. Relationship between symptoms and weight-bearing radiographic parameters of idiopathic flexible flatfoot in children. Chinese Medical Journal 2013;126(11):2029-33. [PubMed] [Google Scholar]
Yoosefinejad 2014
- Yoosefinejad AK, Ghalamghash R. The evaluation and prevalence of foot problems among Iranian students using 'alfoots' company scanner. Health Science Journal 2014;8(3):393-9. [Google Scholar]
References to other published versions of this review
Evans 2010
- Evans AM, Rome K, Carroll M, Hawke F. Foot orthoses for treating paediatric flat feet. Cochrane Database of Systematic Reviews 2010, Issue 7. Art. No: CD006311. [DOI: 10.1002/14651858.CD006311.pub2] [DOI] [Google Scholar]
Rome 2010
- Rome K, Evans A, Ashford R. Non-surgical interventions for paediatric pes planus. Cochrane Database of Systematic Reviews 2010, Issue 7. Art. No: CD006311. [DOI: 10.1002/14651858.CD006311.pub2] [DOI] [PubMed] [Google Scholar]


