Abstract
Background and study aims Fujifilm has developed a novel ELUXEO 7000 endoscope system that employs light-emitting diodes (LEDs) at four different wavelengths as light sources that enable blue light imaging (BLI), linked color imaging (LCI), and high-definition white-light endoscopy (HD-WLE). The aim of this study was to address the diagnostic accuracy of real-time polyp characterization using BLI, LCI and HD-WLE (ELUXEO 7000 endoscopy system).
Patients methods This is a prespecified post-hoc analysis of a prospective study in which 22 experienced endoscopists (> 2,000 colonoscopies) from eight international centers participated. Using a combination of BLI, LCI, and HD-WLE, lesions were endoscopically characterized including a high- or low-confidence statement. Per protocol, digital images were created from all three imaging modalities. Histopathology was the reference standard. Endoscopists were familiar with polyp characterization, but did not take dedicated training for purposes of this study.
Results Overall, 341 lesions were detected in 332 patients. Of the lesions, 269 histologically confirmed polyps with an optical diagnosis were included for analysis (165 adenomas, 27 sessile serrated lesions, and 77 hyperplastic polyps). Overall, polyp characterization was performed with high confidence in 82.9 %. The overall accuracy for polyp characterization was 75.1 % (95 % confidence interval [CI] 69.5–80.1 %), compared with an accuracy of 78.0 % (95 % CI 72.0–83.2 %) for high confidence assignments. The accuracy for endoscopic characterization for diminutive polyps was 74.7 % (95 %CI 68.4–80.3 %), compared with an accuracy of 78.2 % (95 % CI 71.4–84.0 %) for high-confidence assignments.
Conclusions The diagnostic accuracy of BLI, LCI, and HD-WLE by experienced endoscopist for real-time polyp characterization seems limited (NCT03344289).
Introduction
Colorectal cancer (CRC) develops from at least two types of precursor lesions, adenomas and serrated polyps, together referred to as polyps. Early detection and removal of these polyps during colonoscopy decreases the incidence of CRC 1 . Current practice is to send all detected and resected colorectal polyps for histopathological assessment by the pathologist to determine the interval for a surveillance colonoscopy. Diminutive polyps (1 to 5 mm), which constitute up to 60 % of all polyps, are rarely malignant 2 3 . Accurate endoscopic characterization of these polyps would allow resecting and discarding diminutive polyps without histopathologic assessment. Furthermore, all diminutive harmless hyperplastic polyps (HPs) in the rectosigmoid could be characterized and left in situ 4 . Application of this strategy could result in a more cost-effective practice 5 6 .
To reach this goal, enhancements in endoscopic imaging have been made in the last two decades. Especially, the development of virtual chromoendoscopy (e. g. blue light imaging [BLI], narrow band imaging [NBI]) revolutionized the field of endoscopic imaging. The main advantage of virtual chromoendoscopy is that it is available in all new generations of endoscopy systems and that it is easy to activate with a button on the handle of the endoscope. Virtual chromoendoscopy improved the endoscopist’s ability to accurately differentiate between diminutive and small adenomas, HPs, and sessile serrated lesions (SSLs) 7 .
Since 2017–2018, Fujifilm has marketed the ELUXEO 7000 endoscope system that employs four different wavelength light-emitting diodes (LEDs) as light sources. By changing the intensity of each of the four LEDs, a BLI, linked color imaging (LCI), and high-definition white-light endoscopy (HD-WLE) can be obtained. The white-light mode is similar to conventional endoscopy using a Xenon lamp. In both BLI and LCI mode, the peak intensity of the LEDs is set at 410 nm ± 10 nm. As this is the peak absorption of light of hemoglobin, microvascular structures at the surface of the mucosa can be distinguished more clearly from blood vessels in the deep mucosa. Thereby, mucosal surface patterns are better visualized and clarify the boundaries of the mucosal pit. In this way, both BLI and LCI could potentially increase the accuracy for polyp characterization. To date, data on LCI and BLI for the characterization of polyps are scarce but promising 8 9 10 11 12 .
The aim of this study was to assess the diagnostic accuracy of real-time colorectal polyp characterization using BLI, LCI and HD-WLE (ELUXEO 7000 endoscope system).
Patient and methods
Study design
This prospective study is a prespecified post-hoc analysis of the LCI-LYNCH trial 13 . The study was conducted in in eight centers in Belgium, Italy, the Netherlands, Poland, Spain, and the United Kingdom. Methods and outcomes of this trial are described in detail elsewhere. The trial is registered at ClinicalTrials.gov (NCT03344289). The Standards for Reporting of Diagnostic Accuracy (STARD) guideline was followed in reporting the diagnostic test accuracies with respect to lesion characterization 14 .
Patients
Patients were considered eligible for participation in the trial if they were aged 18 or older, provided informed consent, were scheduled for polyp surveillance, and had been diagnosed with a Lynch syndrome-associated pathogenic gene variant in one of the MMR genes ( MLH1, MSH2, MSH6, PMS2 ) or deletions in the 3’ region of the EpCAM gene . Exclusion criteria included surveillance colonoscopy within 1 year from current exam, colonoscopy planned for the evaluation of symptoms, total proctocolectomy, known colonic neoplasia (referred patients), or a concurrent diagnosis of (serrated) polyposis syndrome or inflammatory bowel disease. In line with the LCI-LYNCH trial patients with a Boston Bowel Preparation Scale (BBPS) 15 < 6 or an incomplete colonoscopy were excluded.
Endoscopists
All participating endoscopists had extensive experience with colonoscopy (> 2,000 colonoscopies). At the start of the study, participating endoscopists had performed at least 10 procedures with the ELUXEO 7000 system. Endoscopists were not formally trained in the NBI International Colorectal Endoscopic classification (NICE) 16 or the Workgroup on serrAted polypS and Polyposis (WASP) classification 17 for purpose of this study.
Endoscopy equipment
Colonoscopies were performed with the ELUXEO 7000 system, which consists of a light source, a processor, and special scope series developed by Fujifilm (EC-760ZP and EC-760 R, Fujifilm, Tokyo, Japan). Switching between the three different imaging modes (LCI, BLI and HD-WLE) in the ELUXEO 7000 system was performed by pressing a button on the shaft of the colonoscope. High-definition monitor output was used at appropriate viewing distances at the discretion of the endoscopist.
Real-time lesion characterization (index test)
When the patient was eligible for inclusion, the endoscope was advanced to the cecum. Cecal intubation was confirmed by identification of the appendiceal orifice and ileocecal valve or by intubation of the terminal ileum. Upon reaching the cecum, the quality of the bowel preparation was assessed using the BBPS 15 . During withdrawal from the cecum or terminal ileum, the colon was scrutinized for the presence of lesions. If a lesion was detected, the segment of the colon was registered, the size was estimated by using a reference of known diameter, e. g. open biopsy forceps, and the morphology of the lesion was described according to the Paris classification 18 . In addition, digital still images of all detected lesions were taken in LCI, BLI, and HD-WLE imaging mode. The endoscopist could use all three imaging modes (LCI, BLI and HD-WLE) to predict the endoscopic diagnosis of the lesion including a high- or low-confidence statement. Endoscopists could choose between hyperplastic polyp, SSL, adenoma, carcinoma, or other. In addition, endoscopists assessed each lesion using the WASP classification 17 , which includes the NICE classification and the Hazewinkel criteria ( Supplementary Fig. 1 ) 16 19 . First, endoscopists assessed the three criteria of the NICE classification (color/vessel/surface pattern) aiming to differentiate between non-adenomas (type 1), adenomas or superficial carcinoma (type 2) and deep invasive carcinoma (type 3). Using these criteria, the presence of at least one adenoma-like feature was sufficient to diagnose a type 2 polyp. Subsequently, the four Hazewinkel criteria (i. e. clouded surface, indistinctive border, irregular shape, and darks spots inside the crypts) were used to differentiate between SSLs and HPs for type 1 polyps and between SSLs and adenomas for type 2 polyps. The presence of at least two SSL-like features was hereby considered sufficient to diagnose a SSL. Subsequently, all detected lesions and their adjacent mucosa were removed for histopathologic evaluation. Obvious hyperplastic lesions of 1 to 5 mm in the rectosigmoid could be left in situ, at the discretion of the endoscopist.
Histopathology (reference test)
At each center, an experienced gastrointestinal pathologist was designated for this study. Pathologists were blinded for optical diagnosis of the lesions detected during colonoscopy. Histological samples were collected in paraffin and processed using standard procedures. Histological findings were reported according to the Vienna classification of gastrointestinal neoplasia 20 . SSLs were defined as serrated lesions with at least two irregular, dilated crypts, including dilatation of the base of the crypts that often have a boot, L- or inverted T-shape 21 . Adenomas and SSLs were categorized as neoplastic. HPs and SSLs were grouped as serrated polyps. An advanced adenoma was defined as an adenoma ≥ 10 mm, had villous morphology, or contained high-grade dysplasia.
Study outcomes
The primary outcome of this study was the diagnostic test accuracies (e. g. accuracy, sensitivity, specificity, positive and negative predictive values) for endoscopic characterization of colorectal polyps using a combination of BLI, LCI and HD-WLE (ELUXEO 7000 endoscope system). Accuracy was defined as the percentage of correctly predicted endoscopic diagnoses compared to the reference standard pathology. For the calculation of the overall accuracy, adenomas, SSLs and HPs were considered as different histological subtypes.
Secondary outcomes included a description of detected colorectal polyps; diagnostic test accuracies for polyps ≤ 5 mm and > 5mm; diagnostic test accuracies according to the level of confidence; predictors for accurate endoscopic characterization of polyps.
Statistical methods
Diagnostic test accuracies of endoscopic characterization with BLI, LCI and HD-WLE (index test) were calculated with the outcomes of histopathology as the reference standard (reference test). Diagnostic test accuracies included sensitivity, specificity, and predictive values. If histopathology outcome was carcinoma, traditional serrated adenoma, normal mucosa, inflammatory lesion or missing, the lesion was excluded from the diagnostic accuracy analysis. In addition, lesions with no endoscopic characterization were excluded. Answers were dichotomized to a positive outcome for each histological subtype of interest and a negative outcome for the other histological subtypes. For all outcomes on diagnostic test accuracies, 95 % confidence intervals (CIs) were calculated. Diagnostic test accuracy also was calculated for each participating center. Owing to the limited number of histopathology predictions per individual endoscopist, we decided not to separately analyze the diagnostic test accuracies.
Generalized estimating equations modeling using binary logistic regression adjusted for clustering of polyps and patients per endoscopist was performed to evaluate predictors of accuracy. The outcome variable of the model was accurate histology prediction of a polyp. Potential predictors included confidence level, polyp size, polyp block, and polyp location. Polyp block was defined as the number of polyps endoscopically characterized by and endoscopist during the study duration. The association between predictors and accuracy were summarized as odds ratios, including the 95 %CI and corresponding P value. Analysis was performed in statistical software R (version 3.6.1) using the EpiR and lme4 packages, with the function of glmer. P < 0.05 was considered statistically significant.
Ethical approval and role of the funding source
The study was conducted according to the ethical principles of the Declaration of Helsinki and was approved by the ethics committees of each participating center. Fujifilm Europe GmbH provided research equipment on loan for this study and an unrestricted research grant that partially supported a research fellow to help execute the study. The sponsor had no role in the trial design, execution, data analysis, interpretation, decision to submit the paper, or manuscript preparation. All contributing authors had access to the study data and reviewed and approved the final manuscript.
Results
Between January 2018 and March 2020, 357 patients were eligible for randomization. Nineteen patients were excluded because of poor bowel preparation (BBPS < 6) and six because of incomplete colonoscopy. After excluding these patients, the total number of patients was 332 ( Fig. 1 ). Their mean age was 48.4 years (SD 14.1), 141 (42 %) were male and 72 (22 %) had a personal history of CRC. A total of 22 endoscopists from eight centers participated in the study. The number of colonoscopies per center ranged from 15 to 81. Table 1 shows the clinicopathological characteristics of the detected and included lesions polyps. Overall, 341 lesions were detected. Of these 341 lesions, 176 (54 %) were adenomas, 28 (9 %) were SSLs, 79 (24 %) were HPs, four (1 %) were traditional serrated adenomas, five (1 %) were carcinomas, 33 (10 %) lesions were reported as normal mucosa or other non-neoplastic lesions (e. g. inflammatory lesions), and 16 (5 %) lesions were not retrieved for histology.
Fig. 1.
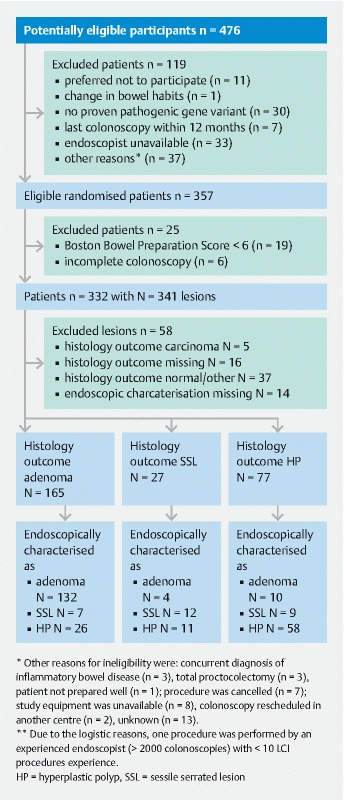
Flow diagram.
Table 1. Characteristics of the detected and included lesions.
|
Detected lesions
(N = 341) |
Included polyps
1
(N = 269) |
Included diminutive polyps
1
(N = 220) |
|
| Location | |||
|
237 (70 %) | 188 (70 %) | 148 (67 %) |
|
104 (30 %) | 81 (30 %) | 72 (33 %) |
| Morphology | |||
|
159 (47 %) | 127 (47 %) | 112 (51 %) |
|
171 (50 %) | 142 (53 %) | 108 (49 %) |
|
11 (3 %) | 0 (0 %) | 0 (0 %) |
| Endoscopic characterisation | |||
|
2 (0 %) | 0 (0 %) | 0 (0 %) |
|
172 (51 %) | 146 (55 %) | 119 (54 %) |
|
31 (9 %) | 28 (15 %) | 12 (5 %) |
|
118 (35 %) | 95 (30 %) | 89 (41 %) |
|
18 (5 %) | 0 (0 %) | 0 (0 %) |
| Confidence level | |||
|
267 (82 %) | 223 (83 %) | 178 (81 %) |
|
69 (17 %) | 46 (17 %) | 42 (19 %) |
|
5 (1 %) | 0 (0 %) | 0 (0 %) |
| Histopathology | |||
|
5 (1 %) | – | – |
|
176 (52 %) | 165 (61 %) | 137 (62 %) |
|
5 | 5 | 4 |
|
0 | 0 | 0 |
|
28 (8 %) | 27 (10 %) | 14 (6 %) |
|
0 | 0 | 0 |
|
4 (1 %) | 0 | 0 |
|
79 (23 %) | 77 (29 %) | 69 (31 %) |
|
27 (8 %) | 0 | 0 |
|
6 (2 %) | 0 | 0 |
|
16 (5 %) | 0 | 0 |
Histologically confirmed adenomas, SSL and hyperplastic polyps with as well an optical diagnosis were included; Data are n or n (%)
Polypoid was defined as Paris classification Ip, Isp and Is.
Non-polypoid was defined as Paris classification IIa and IIb.
In the diagnostic accuracy analysis, only histologically confirmed adenomas, SSLs and HPs for which also an optical diagnosis was recorded were included ( Fig. 1 and Table 1 ). A total of 269 polyps were included, of which 165 (61 %%) were adenoma, 27 (10 %) SSL and 77 (29 %) hyperplastic polyp. Of all polyps included, 220 (82 %) were diminutive (≤ 5 mm), 33 (12%) were small (6–9 mm), and 15 (6 %) were ≥ 10 mm. Of the 220 diminutive polyps, 72 (33 %) were located in the rectosigmoid. Thirty-eight (53 %) of them were neoplastic (i. e. adenoma or SSL) while 34 (47 %) were non-neoplastic (i. e. hyperplastic polyp).Overall, a high-confidence optical diagnosis was made in 223 (82.9 %) of the 269 polyps. Of the 220 diminutive polyps, 179 (81.3 %) were predicted with high confidence. The proportion of polyps with a high-confidence optical diagnosis varied between the participating centers (range 58–100 %, median = 84 %).
Five histologically confirmed adenocarcinomas were detected during the study period. These carcinomas were not included in the diagnostic accuracy analysis, but did have an optical diagnosis. Three of these carcinomas were correctly recognized as a carcinoma, while two carcinomas were not recognized (one was optically diagnosed as an adenoma and one as a hyperplastic polyp). As well, one adenoma was incorrectly optically diagnosed as a carcinoma.
Diagnostic test accuracies for polyp characterization
Diagnostic test accuracies for polyp characterization for overall and high-confidence predictions of polyps are shown in Table 2 and Supplementary Table 1 . The overall accuracy for endoscopic characterization for all polyps was 75.1 % (95 %CI 69.5–80.1 %), compared with an accuracy of 78.0 % (95 % CI 72.0–83.2 %) when endoscopic characterization was assigned with high confidence. The overall sensitivity of characterizing adenomas (adenomas vs. serrated polyps) was 80.0 % (95 % CI 73.1–85.8 %) and increased to 84.9 % (95 % CI 77.8–90.4 %) for high-confidence predictions.
Table 2. Diagnostic test accuracies for endoscopic characterization with histopathology as reference standard.
| Endoscopic characterization | ||||
|
All polyps, % (95 % CI)
(N = 269) |
High confidence all polyps, % (95 % CI)
(N = 223) |
Diminutive polyps, % (95 % CI)
(N = 220) |
Diminutive polyps with high confidence, % (95 %CI)
(N = 178) |
|
| Overall accuracy 1 | 75.1 (69.4–80.1) | 78.0 (72.0–83.2) | 74.7 (68.4–80.3) | 78.2 (71.4–84.0) |
| Adenomas vs serrated polyps | ||||
|
82.5 (77.5–86.9) | 86.1 (80.9–90.3) | 81.9 (76.2–86.7) | 86.0 (80.1–90.8) |
|
80.0 (73.1–85.8) | 84.9 (77.8–90.4) | 79.0 (71.2–85.5) | 84.8 (76.8–90.9) |
|
86.5 (78.4–92.4) | 88.1 (79.2–94.1) | 86.8 (77.5–93.2) | 88.1 (77.8–94.7) |
|
90.4 (84.4–94.7) | 92.2 (86.1–96.2) | 90.8 (84.2–95.3) | 92.2 (95.3–96.6) |
|
73.2 (64.4–80.8) | 77.9 (68.2–85.8) | 71.3 (61.4–79.9) | 77.6 (66.6–86.4) |
| SSLs vs non-SSLs | ||||
|
88.5 (84.0–92.0) | 87.9 (82.9–91.9) | 91.0 (86.4–94.4) | 90.5 (85.2–94.4) |
|
44.4 (25.5–64.7) | 47.8 (26.8–69.4) | 21.4 (4.7–50.8) | 25.0 (5.5–57.2) |
|
93.3 (89.5–96.2) | 92.5 (87.9–95.7) | 95.6 (91.9–98.0) | 95.2 (90.8–97.9) |
|
42.9 (24.5–62.8) | 42.3 (23.3–63.1) | 25.0 (5.5–57.2) | 27.3 (6.0–61.0) |
|
93.8 (90.0–96.5) | 93.9 (89.6–96.8) | 94.7 (90.8–97.3) | 94.6 (90.0–97.5) |
| HPs vs non-HPs | ||||
|
79.2 (73.8–83.9) | 82.1 (76.4–86.9) | 76.5 (70.3–81.9) | 79.9 (73.3–85.5) |
|
75.3 (64.2–84.4) | 73.8 (60.9–84.2) | 76.8 (65.1–86.1) | 76.4 (63.0–86.8) |
|
80.7 (74.4–86.1) | 85.2 (78.8–90.3) | 76.3 (68.8–82.8) | 81.5 (73.5–87.9) |
|
61.1 (50.5–70.9) | 65.2 (52.8–76.3) | 59.6 (48.6–69.8) | 64.6 (51.8–76.1) |
|
89.1 (83.5–93.3) | 89.6 (83.7–93.9) | 87.9 (81.1–92.9) | 88.6 (81.3–93.8) |
PPV, positive predictive value; CI, confidence interval; NPV, negative predictive value; SSL, sessile serrated lesion; HP, hyperplastic polyp.
For the calculation of the overall accuracy adenomas, SSLs and HPs were considered different histological subtypes.
The overall accuracy for endoscopic characterization for all diminutive polyps was 74.7 % (95 % CI 68.4–80.3 %), compared with an accuracy of 78.2 % (95 % CI 71.4–84.0 %) when characterization was assigned with high confidence. Overall sensitivity for diminutive adenomas (adenomas vs. serrated polyps) was 79.0 % (95 % CI 71.2–85.5 %) and increased to 84.8 % (95 % CI 76.8–90.9 %) for high-confidence predictions.
Only 12 of 27 SSLs were assessed with at least two Hazewinkel criteria, corresponding to a sensitivity of 44.4 % (95 % CI% 25.5–64.7 %), specificity of 93.3 % (95 % CI 89.5–96.2 %), PPV of 42.9 % (95 % CI 24.5–62.8 %), NPV of 93.8 % (95 % CI 90.0–96.5 %). When only diminutive SSLs were taken into account the diagnostic accuracies decreased to a sensitivity of 21.4 % (95 % CI% 4.7–50.8 %), specificity of 95.6 % (95 % CI 91.9–98.0%), PPV of 25.0 % (95 % CI 5.5–27.2 %), NPV of 94.7 % (95 % CI 90.8–97.3 %).
Diagnostic test accuracies for polyp characterization per center
Diagnostic test accuracies for polyp characterization for overall and high-confidence predictions of polyps per center are shown in Fig. 2 . This figure shows the wide variation in accuracies for polyp characterization for overall and high-confidence predictions of the individual centers.
Fig. 2.
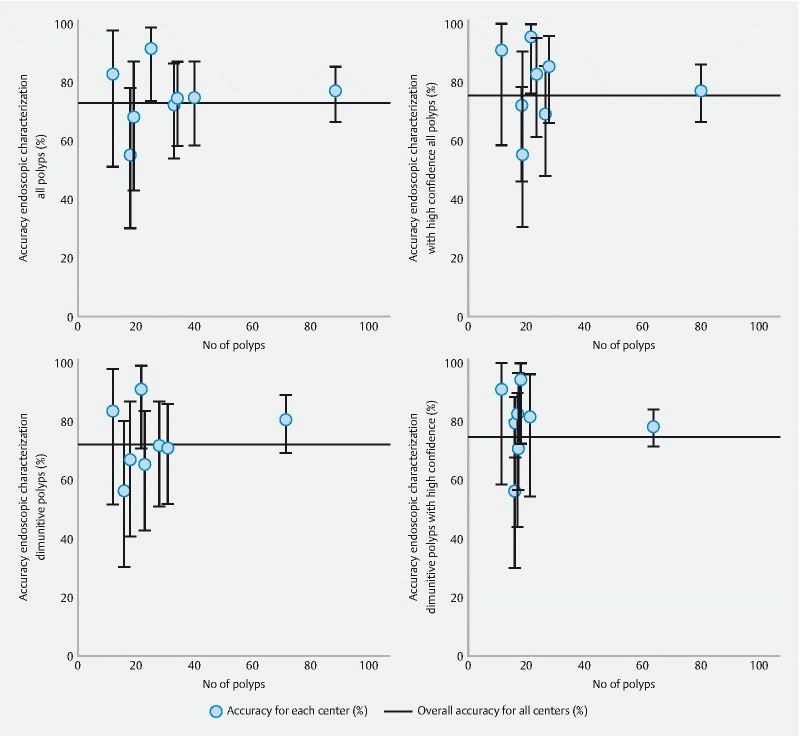
Overall accuracies for endoscopic characterization per center with histopathology as reference standard (overall accuracies along with its 95% confidence intervals). *For the calculation of the overall accuracy adenomas, SSLs and HPs were considered different histological subtypes.
Predictors for accurate endoscopic polyp characterization
Outcomes of univariate and multivariate logistic regression analysis to find predictors for accurate prediction of histology are shown in Table 3 . For accurate prediction of histology, only high-confidence predictions were independently associated with accurate histology prediction in multivariate analysis.
Table 3. Outcomes of multilevel logistic regression analysis to find predictors for accurate endoscopic prediction of histology.
| Predictors |
Univariate logistic regression odds ratio
(95 % CI) |
P | Multivariate logistic regression odds ratio (95 % CI) | P |
| Confidence level | ||||
|
Reference | Reference | ||
|
3.02 (1.20–7.59) | 0.02 | 2.25 (1.13–4.42) | 0.02 |
| Polyp location | ||||
|
Reference | Reference | ||
|
0.53 (0.21–1.34) | 0.29 | 0.87 (0.49–1.52) | 0.55 |
| Polyp size, mm | ||||
|
Reference | Reference | ||
|
1.97 (0.79–4.96) | 0.15 | 1.08 (0.59–2.04) | 0.81 |
| Polyp block 1 | ||||
|
Reference | |||
|
0.90 (0.36–2.25) | 0.89 | ||
|
0.98 (0.41–2.34) | 0.98 | ||
| Morphology | ||||
|
Reference | |||
|
0.93 (0.37–2.33) | 0.85 | ||
Polyp block was defined as the number of polyps an endoscopist had endoscopically characterized during the study.
Polypoid was defined as Paris classification Ip, Isp and Is.
Non-polypoid was defined as Paris classification IIa and IIb
Discussion
In this post-hoc analysis of a prospective multicenter trial, diagnostic accuracy for real-time polyp characterization using BLI, LCI and HD-WLE appears to be suboptimal. Participating endoscopists were experienced endoscopists and familiar with endoscopic polyp characterization, but did not follow a specific training for purpose of this study. Using a combination of BLI, LCI and HD-WLE the lesions were endoscopically characterized with an overall accuracy of 75 %, which increased to 78 % for high-confidence assignments only. The overall sensitivity of characterizing adenomas (adenomas vs. serrated polyps) was 80.0 % compared with an accuracy of 84.8 % when optical diagnosis was assigned with high confidence. For diminutive adenoma characterization (adenomas vs serrated polyps), sensitivities were respectively 79.0 % and 84.8 % for high-confidence assessments only. There are two plausible reasons for these relatively low diagnostic accuracies. First, the different light techniques used in this study were simply not sufficient enough to highlight the specific endoscopic features of the assessed polyps, leading to suboptimal results. In addition, the WASP classification used in this study has not been validated for BLI or LCI 22 . A second explanation could be that the study did not primarily focus on polyp characterization and that endoscopists were not formally trained prior to the start of the study.
As concerns SSLs, the present study shows suboptimal results, with an overall sensitivity of 44 % for all sized SSLs and even a lower sensitivity of 21 % for diminutive SSLs. Almost 90% of the detected SSLs were small in size (< 1 cm). This is in line with a previous post-hoc analysis from prospective multicentre study of NBI characterization by community endoscopists in daily practice. Vleugels and colleagues described that only 24.4.% of 202 diminutive SSLs were accurately diagnosed when using the WASP classification 23 . Of the 202 diminutive SSLs, 83 were misclassified as HPs. Kumar and colleagues described also that up to one third of the SSLs were misclassified as HPs when applying the NICE classification 24 . Apparently, many morphological features typical of SSLs, such as the presence of mucus cap, clouded surface, indistinctive borders or irregular shape cannot be recognized easily in small lesions. This was confirmed by Bustamante-Balén et al, who showed that the presence of two or more Hazewinkel criteria are not reliable for a positive diagnosis of SSL (sensitivity 32.9%, PPV 30.6 %), particularly not for diminutive SSLs (sensitivity 14.7 %, PPV 14.3 %) 25 . Another explanation for the suboptimal accuracy for diagnosing SSL might be that there was no revision of histopathology in this study, while there is considerable interobserver variability in the histopathological diagnosis of SSLs 26 . The discordances in histological evaluation (especially between SSL and HP and in diminutive polyps) may explain low accuracy. However, we performed a sub-analysis for adenomas versus serrated polyps, which showed similar diagnostic accuracies. To increase the probability for correct diagnosing SSLs, it might be considered use the presence of three or more Hazewinkel criteria instead of two or more.
As reported in a recent meta-analysis and reaffirmed by our study, high-confidence assignment is the most significant predictor of optical diagnostic accuracy 27 . High-confidence predictions are a crucial factor for the implementation of optical diagnosis and for cost savings while following the “resect and discard” strategy. Although the concept of high confidence is easy to understand, a uniform definition of high-confidence diagnosis is lacking. Therefore, setting the confidence level is inherently subjective. The ESGE recently insisted on the importance of developing more uniform criteria for high-confidence assignments 22 . As well, little is known about the optimal high-confidence rate. Cost effectiveness analyses showed a cost saving of € 6–22 per individual when 79–84 % of the diminutive polyp are characterized with high confidence 5 6 . In this study, the proportion of high-confidence assignments was reasonably comparable (81.3 %). Therefore, a minimum rate of approximately 75 % of high confidence predictions might be appropriate, although a higher rate would expand the economic benefits of the strategy.
The main strength of our study is that polyp characterization was performed during real-time colonoscopy in eight different hospitals with 22 endoscopists, which allows externalization of results. Several limitations of this study have to be acknowledged. As mentioned before, endoscopists in this study were not formally trained, while dedicated training is required to improve and maintain optical diagnosis competence. On the other hand, a setting without a formal training reflects daily clinical practice and is therefore all the more worthwhile to investigate 22 . Second, diminutive polyps of the rectosigmoid that looked as HPs were not removed and were not included in the analysis. This “under-detection” could have artificially increased the proportion of adenomas and SSLs among diminutive rectosigmoid lesions in our study (53 %), which is higher than the prevalence found in another large cohort of patients with Lynch syndrome (23 %) 28 . If all polyps would have been included leaving no polyps in situ, the results might have been better. Another aspect that could limit generalizability is that this study was performed in patients with Lynch syndrome. However, there is no reason to assume a difference in polyp phenotypes from this patient group compared with polyp phenotypes from sporadic patients 29 . In addition, the polyp subtype distribution in this Lynch cohort is comparable to a screening or polyp surveillance cohort 30 . Therefore, we think that our data can be extrapolated to the general population. Another aspect that may limit generalizability is that endoscopists participating in this study were specialist endoscopists, devoted to high-risk CRC conditions and with interest in performing endoscopy research. This may have also caused selection bias, as it is unclear how less experienced endoscopists would have performed. However, based on previous literature we anticipate that if characterization proves difficult among specialists, this can be extrapolated to endoscopists with less experience in optical diagnosis of lesions. Since no uniform definition of high-confidence diagnosis was available, this might have caused differences in accuracy between endoscopists. As polyps were evaluated with the combination of BLI, LCI and HD-WLE it was not possible to investigate the sensitivity and accuracy of each light modality independently. Last, the number of lesions assessed per single endoscopist participating in our study was quite small. Our study findings should therefore be confirmed in larger sample studies performed in daily clinical practice.
Conclusions
This study illustrates that in a real-time setting experienced endoscopists were not able to achieve a high diagnostic accuracy using a combination of BLI, LCI and HD-WLE. Ongoing monitoring and development of artificial intelligences systems are needed to improve endoscopistsʼ polyp characterization with this system.
Acknowledgements
The authors thank A. Baker, C. Camps, C. Cohen, E. Finati, H. Mues, H. Willekens, J. Dash, P. Wilson, R. Dierx, M. Edmonds, M. van der Ende-van Loon, R. Moreira, S. Arntz, and V. Roos for their invaluable help with patient recruitment and data collection. We thank Dr. M. van Leerdam and Prof. C. Cellier for their effort to set up the trial in their hospitals.
Footnotes
Competing interests Fujifilm Europe GmbH provided research equipment on loan and an unrestricted research grant for this study but had no involvement in the design, recruitment, data collection, analysis or writing of the manuscript.
Dr. Repici has received loan equipment and a consultancy fee from Medtronic and Fujifilm. Dr. Bastiaansen received speaking fees from Olympus, Tillotts Pharma AG, and Ovesco Endoscopy. Dr. Dekker received equipment on loan from Olympus and Fujifilm, a research grant from Fujifilm, a consulting fee for medical advice from Tillots, Olympus, Fujifilm, GI Supply, and CPP-FAP, and a speakersʼ fee from Olympus, Roche, and GI Supply. Dr. Balaguer has endoscopic equipment on loan of Fujifilm, received an honorarium for consultancy from Sysmex, CPP-FAP speaking fees from Norgine, and an editorial fee from Elsevier. Dr. Kamiński has received speaking, teaching, and consultancy fees from Olympus (2017 to present), speaking and teaching fees and a loan of equipment from Fujifilm (2019), and speaking fees from Medtronic (2019), Alfa Sigma (2017–2019), and Norgine (2018–2019). Dr. Pellisé received a research grant from Fujifilm Spain and Casen Recordati, a loan of equipment from Fujifilm, a consultancy fee from Norgine, a speaking fee from Norgine, Olympus, Casen Recordati, and Janssen, and an editorial fee from Thieme. Dr. Bisschops has provided consultancy to and received research grants and speaking fees from Pentax (2008 to present) and Fujifilm (2015 to present); his department has received research grants and equipment from Pentax and Fujifilm (2015 to present). Dr. Bhandari has received grant funding from Norgine, Fujifilm, Olympus, Pentax, and Boston Scientific. Dr. Fockens received research support from Boston Scientific and a consulting fee from Olympus, Cook, and Ethicon Endosurgery.
Supplementary material :
References
- 1.Zauber A G, Winawer S J, OʼBrien M J et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassan C, Pickhardt P J, Kim D H et al. Systematic review: distribution of advanced neoplasia according to polyp size at screening colonoscopy. Aliment Pharmacol Therap. 2010;31:210–217. doi: 10.1111/j.1365-2036.2009.04160.x. [DOI] [PubMed] [Google Scholar]
- 3.Vleugels J LA, Hazewinkel Y, Fockens P et al. Natural history of diminutive and small colorectal polyps: a systematic literature review. Gastrointest Endosc. 2017;85:1169–INF. doi: 10.1016/j.gie.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Rex D K, Kahi C, OʼBrien M et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419–422. doi: 10.1016/j.gie.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Hassan C, Pickhardt P J, Rex D K.A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening Clin Gastroenterol Hepatol 20108865–869., 869.e861–863 [DOI] [PubMed] [Google Scholar]
- 6.Vleugels J LA, Greuter M JE, Hazewinkel Y et al. Implementation of an optical diagnosis strategy saves costs and does not impair clinical outcomes of a fecal immunochemical test-based colorectal cancer screening program. Endosc Int Open. 2017;5:E1197–E1207. doi: 10.1055/s-0043-113565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picot J, Rose M, Cooper K et al. Virtual chromoendoscopy for the real-time assessment of colorectal polyps in vivo: a systematic review and economic evaluation. Health Tech Assess. 2017;21:1–308. doi: 10.3310/hta21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisschops R, Hassan C, Bhandari P et al. BASIC (BLI Adenoma Serrated International Classification) classification for colorectal polyp characterization with blue light imaging. Endoscopy. 2018;50:211–220. doi: 10.1055/s-0043-121570. [DOI] [PubMed] [Google Scholar]
- 9.Neumann H, Neumann Sen H, Vieth M et al. Leaving colorectal polyps in place can be achieved with high accuracy using blue light imaging (BLI) United European Gastroenterol J. 2018;6:1099–1105. doi: 10.1177/2050640618769731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rondonotti E, Paggi S, Amato A et al. Blue-light imaging compared with high-definition white light for real-time histology prediction of colorectal polyps less than 1 centimeter: a prospective randomized study. Gastrointest Endosc. 2019;89:554–INF. doi: 10.1016/j.gie.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Rondonotti E, Hassan C, Andrealli A et al. Clinical Validation of BASIC classification for the resect and discard strategy for diminutive colorectal polyps. Clin Gastroenterol Hepatoln. 2020 doi: 10.1016/j.cgh.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Wu C H, Chen T H, Hsu C M et al. Linked-color imaging combined with the NICE classification system for optical diagnosis of colon polyps: new image-enhanced endoscopic technology for pathological prediction. Ther Clin Risk Manag. 2017;13:1317–1321. doi: 10.2147/TCRM.S147155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houwen B BSL, Hazewinkel Y, Pellisé M et al. Linked colour imaging for the detection of polyps in patients with Lynch syndrome: a multicentre, parallel randomised controlled trial. Gut. 2021 doi: 10.1136/gutjnl-2020-323132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossuyt P M, Reitsma J B, Bruns D E et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calderwood A H, Jacobson B C. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72:686–692. doi: 10.1016/j.gie.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewett D G, Kaltenbach T, Sano Y et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599–INF. doi: 10.1053/j.gastro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 17.IJspert J EG, Bastiaansen B AJ, van Leerdam M E et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut. 2016;65:963–970. doi: 10.1136/gutjnl-2014-308411. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous Anonymous. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 19.Hazewinkel Y, López-Cerón M, East J E et al. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013;77:916–924. doi: 10.1016/j.gie.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Schlemper R J, Riddell R H, Kato Y et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snover D. WHO Classification of Tumours of the Digestive System; 2010. Serrated polyps of the colon and rectum and serrated polyposis. pp. 160–165.
- 22.Dekker E, Houwen B, Puig I et al. Curriculum for optical diagnosis training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2020 doi: 10.1055/a-1231-5123. [DOI] [PubMed] [Google Scholar]
- 23.Vleugels J LA, IJspeert J EG, Hazewinkel Y et al. Optical diagnosis of sessile serrated polyps: bottleneck for the optical diagnosis paradigm? J Clin Gastroenterol. 2017;51:426–432. doi: 10.1097/MCG.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Fioritto A, Mitani A et al. Optical biopsy of sessile serrated adenomas: do these lesions resemble hyperplastic polyps under narrow-band imaging? Gastrointest Endosc. 2013;78:902–909. doi: 10.1016/j.gie.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bustamante-Balén M, Satorres C, Ramos-Soler D et al. Evaluation of the optical criteria for sessile serrated lesions of the colon: A prospective study on a colorectal cancer screening population. Endosc Int Open. 2021;9:E14–E21. doi: 10.1055/a-1293-7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne S R, Church T R, Wandell M et al. Endoscopic detection of proximal serrated lesions and pathologic identification of sessile serrated adenomas/polyps vary on the basis of center. Clin Gastroenterol Hepatol. 2014;12:1119–1126. doi: 10.1016/j.cgh.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 27.McGill S K, Evangelou E, Ioannidis J P et al. Narrow band imaging to differentiate neoplastic and non-neoplastic colorectal polyps in real time: a meta-analysis of diagnostic operating characteristics. Gut. 2013;62:1704–1713. doi: 10.1136/gutjnl-2012-303965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivero-Sánchez L, Gavric A, Herrero J et al. The “diagnose and leave in” strategy for diminutive rectosigmoid polyps in Lynch syndrome: a post hoc analysis from a randomized controlled trial. Endoscopy. 2020 doi: 10.1055/a-1328-5405. [DOI] [PubMed] [Google Scholar]
- 29.Vleugels J LA, van Neerven S M, van Leerdam M E et al. CD31-positive microvessel density within adenomas of Lynch syndrome patients is similar compared to adenomas of non-Lynch patients. Endosc Int Open. 2019;7:E701–E707. doi: 10.1055/a-0832-8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vleugels J LA, Sahin H, Hazewinkel Y et al. Endoscopic detection rate of sessile serrated lesions in Lynch syndrome patients is comparable with an age- and gender-matched control population: case-control study with expert pathology review. Gastrointest Endosc. 2018;87:1289–1296. doi: 10.1016/j.gie.2017.11.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


