Abstract
Working memory is the ability to keep information in one’s mind and mentally manipulate it. Decrements in working memory play a key role in many behavioral and psychiatric disorders, therefore identifying modifiable environmental risk factors for such decrements is important for mitigating these disorders. There is some evidence that prenatal exposure to individual chemicals may adversely impact working memory among children, but few studies have explored the association of co-exposure to multiple chemicals with this outcome in adolescence, a time when working memory skills undergo substantial development. We investigated the association of organochlorines (DDE, HCB, PCBs) and metals (lead, manganese) measured in cord serum and cord blood, respectively, with working memory measured with the Wide Range Assessment of Memory and Learning, 2nd Edition among 373 adolescents living near a Superfund site in New Bedford, Massachusetts. We used Bayesian Kernel Machine Regression (BKMR) and linear regression analyses and assessed effect modification by sex and prenatal social disadvantage. In BKMR models, we observed an adverse joint association of the chemical mixture with Verbal, but not Symbolic, Working Memory. In co-exposure and covariate-adjusted linear regression models, a twofold increase in cord blood manganese was associated with lower working memory scaled scores, with a stronger association with Verbal Working Memory (difference=−0.75; 95% CI: −1.29, −0.20 points) compared to Symbolic Working Memory (difference=−0.44; 95% CI: −1.00, 0.12 points). There was little evidence of effect modification by sex and some evidence associating organochlorine pesticides with poorer working memory scores among those with greater prenatal social disadvantage. This study provided evidence of an adverse joint association of a chemical mixture with a verbal working memory task among adolescents, as well as an adverse association of prenatal manganese exposure with working memory.
Keywords: Prenatal exposures, chemical mixtures, organochlorines, metals, working memory, adolescent neurodevelopment
1. Introduction
Working memory is the ability to not only keep information in one’s mind temporarily, but also mentally manipulate or work with it.1 It includes verbal and non-verbal memory, both of which are critical to numerous abilities such as mentally ordering information, adapting instructions into action, and updating plans in response to new information.1 Along with inhibition and cognitive flexibility, working memory is a building block of executive function necessary for higher-order skills including planning, reasoning, problem-solving, and decision-making.1 Working memory skills begin to develop in early childhood and young children are able to hold some information in their minds. However, manipulating such information involves recruitment of the dorsolateral prefrontal cortex and superior parietal cortex, anatomical regions of the brain that undergo substantial development in adolescence.2 Working memory deficits are associated with many psychiatric and behavioral disorders. For example, both verbal and spatial working memory impairments are core features of schizophrenia.3,4 In addition, children with ADHD or autism generally perform worse on both verbal and visuospatial working memory tasks than controls.5,6 It is not known whether working memory decrements are risk factors for these disorders or whether their underlying pathology may also impact working memory. However, identifying potential modifiable risk factors, such as environmental chemical exposures, is vital to better understanding and mitigating the functional impacts of these disorders.
Several epidemiologic studies have investigated the association of prenatal exposures to organochlorines such as dichlorodiphenyldichloroethylene (DDE), hexachlorobenzene (HCB), and polychlorinated biphenyls (PCBs) with childhood working memory. Specifically, higher DDE, HCB, and PCB levels measured either in cord serum or maternal serum during pregnancy have been associated with poorer memory scores (including working memory skills) on the McCarthy Scales of Children’s Abilities (MSCA) among 4-year-olds in Spain and Greece, though these adverse associations did not always reach statistical significance.7–9 Associations of working memory with organochlorines have been less consistent across studies of older children (7-to-11-year-olds).10,11 However, in children born to mothers consuming PCB-contaminated fish from Lake Michigan, higher cord serum PCB levels were associated with poorer working memory on the Sternberg Memory Paradigm and the Weschler Intelligence Scale for Children, Revised (WISC-R) digit span task.11 Importantly, previous studies have not assessed the impact of prenatal exposures to organochlorines on working memory among adolescents, despite this being the time when working memory skills undergo substantial development.
Some other studies have focused on the potential adverse impacts of metals on working memory among children and adolescents. Lead (Pb) levels measured in maternal erythrocytes during pregnancy and cord blood have been associated with lower working memory scores on the Behavior Rating Inventory of Executive Function (BRIEF) checklist and psychometric tests of working memory among older children (ages 7–11).12,13 In an Italian cohort of children residing near ferro-manganese plants, adolescent girls with the highest deciduous tooth manganese (Mn) concentrations reflecting prenatal exposure committed more working memory errors on the Virtual Radial Arm Maze (VRAM) than those with intermediate levels of exposure, but tooth Mn was not associated with working memory in boys.14 Only one study has analyzed the relation of prenatal methylmercury (MeHg) exposure with working memory in adolescence and there were no adverse associations observed.15 Finally, several cross-sectional studies have found biomarkers of exposure to arsenic (As) and other metals to be associated with decrements in working memory skills among 6 to 12-year-old children.16–19 However, the design of such studies does not allow for establishing directionality of the association.
Simultaneous exposure to multiple chemical contaminants is common.20 Few studies, however, have examined the association of prenatal exposure to multiple pollutants with working memory among children or adolescents. In one of the few studies that has examined such an association – a prospective study based in the Faroe Islands – low cord blood MeHg concentrations combined with high cord blood Pb concentrations were associated with decrements in working memory among 14-year-olds as measured by Digit Span Backward scores.21 Two cross-sectional studies have also assessed the impact of multiple metals on working memory in children. In an Italian cohort study of 6 to 12-year-olds, proximity to an industrial site from which airborne metals and other contaminants were emitted was used as an exposure proxy for multiple metal exposures, while biomarkers of exposure were used when assessing exposure to specific individual metals.22 Participants who lived closer to the industrial site performed worse on psychometric tests of working memory than those who lived further away; blood Pb and urine cadmium were adversely associated with the working memory indices of the WISC-IV; and hair Mn and blood Pb were associated with more errors on the CANTAB Spatial Working Memory test.22 Finally, in a cross-sectional study of 8 to 11-year-old children from Bangladesh, researchers found that in the presence of As co-exposure, greater blood Mn concentrations were associated with lower WISC-IV Working Memory scores, but Mn-As interactions were not statistically significant.23
In summary, working memory plays a crucial role in higher level cognition and decrements are associated with a number of psychiatric and behavioral disorders of public health importance. Although there are some studies investigating the association of prenatal exposures and working memory in childhood, there are few studies investigating this association in adolescents. Yet substantial working memory development occurs in this age group and therefore this may be a key time when adverse impacts manifest, including from environmental insults that may have taken place during the prenatal time period. In addition, exposure to organochlorines and metals seldom occurs independently, yet there are only a few studies that have assessed the impact of chemical mixtures on working memory, and most are cross-sectional. Therefore, this prospective study addresses a number of limitations in prior literature by assessing the impact of prenatal exposure to a chemical mixture on working memory in adolescents. In the main analysis, we focused on a chemical mixture composed of DDE, HCB, PCBs, Pb, and Mn chosen based on likely neurodevelopmental toxicity, prevalence of exposure, and availability of exposure biomarkers in our study target population. In a number of previous studies, associations of prenatal exposures to neurotoxicants with executive functions varied by sex7,10,14,24,25 and socioeconomic stress indicators.22 Therefore, we also assessed the potential for sex and social disadvantage to modify the impact of exposure mixtures on working memory. Secondary analyses included MeHg and As as additional components of the chemical mixture.
2. Materials and methods
2.1. Study population.
The New Bedford Cohort (NBC) is a longitudinal birth cohort study of 788 mother-infant pairs recruited shortly after the infant’s birth at St. Luke’s Hospital in New Bedford, Massachusetts. The NBC was established to assess the effects of prenatal chemical exposures on child development among families residing in communities near the New Bedford Harbor. Residents of these communities were potentially at increased risk of chemical exposure because of proximity to the New Bedford Harbor which was designated a Superfund site in 1982 due to PCB and metal contamination from local industrial emissions.26 Inclusion criteria for study participation were mothers being at least 18 years old, speaking English or Portuguese, and living in one of the four towns surrounding the New Bedford Harbor for at least the duration of their pregnancy. Exclusion criteria were delivery via cesarean section or having an infant who was too ill to undergo study neonatal examination. Further details on study enrollment have been described previously.27,28 Biomarkers of prenatal chemical exposure were collected on study infants at birth or from mothers in the peripartum period. NBC children have been followed periodically since birth for detailed neurodevelopmental assessments.
The focus of this analysis is the subset of 528 NBC children who participated in follow up during adolescence, which took place between 2008 and 2014 (at a median age 15.5, range 13–17 years). A total of 373 of these adolescents had complete data on working memory outcomes, covariates, and biomarkers of prenatal exposure to DDE, HCB, PCBs, Pb, and Mn. 235 participants had complete data on working memory outcomes, covariates, and biomarkers of prenatal exposure to DDE, HCB, PCBs, Pb, Mn, MeHg, and As.
2.2. Chemical exposure assessment.
DDE, HCB, and PCBs were measured in cord blood samples collected in red top Vacutainer™ tubes at birth. The samples were centrifuged at 2500–3000 RPM for 10 minutes after which the serum fraction was aliquoted with glass transfer pipettes into 6 ml pre-washed glass Wheaton jars with Teflon coated lids, They were then stored at −20 degrees Celsius prior to analysis.28 The cord serum samples were analyzed for DDE, HCB, and 51 PCB congeners at the Harvard T.H. Chan School of Public Health Organic Chemistry Laboratory (Boston, MA) using gas chromatography with electron capture detection.26–28 The sum of the 4 most prevalent PCB congeners (ΣPCB4) including 118, 138, 153, and 180 were used for this analysis due to their minimal measurement error and common usage to assess congener-specific effects in other population-based studies. A procedural blank, a field blank, and two matrix spike samples were included in each analytic batch for quality control. The limit of detection (LOD) of DDE was 0.07 ng/g and the LOD of HCB was 0.016 ng/g. Among the 373 adolescents in this analysis, the percentage of DDE and HCB measures below the LOD were 1.6% and 27.9% respectively. The LODs for the four PCBs used in this analysis ranged from 0.01101 to 0.01414 ng/g serum. Among the 373 adolescents in this analysis, 20.4% had at least one of the four PCB congener measures below the LOD. Quantifiable values below the LOD were included in this analysis.28 For the organochlorines, the within-batch coefficients of variation were 5% for DDE, 6% for HCB, and 7.5% for PCBs reflecting high reproducibility. The between-batch coefficients of variation over the 5 years of analysis were excellent (20%) for DDE and PCBs and acceptable (39%) for HCB.
Pb and Mn were measured in cord whole blood collected at birth. The samples were analyzed by the Harvard T.H. Chan School of Public Health Metals Laboratory (Boston, MA) using isotope dilution inductively coupled plasma mass spectrometry (ICP-MS, Sciex Elan 5000, Perkin Elmer, Norwalk, CT) and external calibration on a dynamic reaction cell-inductively coupled plasma-mass spectrometer (DRC-ICP-MS, Elan 6100, Perkin Elmer, Norwalk, CT), respectively, and concentrations were reported as the mean of 5 replicate measurements. For quality control (QC) monitoring, procedural blanks, duplicates, spiked samples, standard reference material (NIST SRM 955b Pb in blood; NIST SRM 1643d trace elements in water), biological reference material (ICP03B-05 and ICP03B-02 multi-elements in human blood from INSPQ/Laboratoire de Toxicologie, Quebec) and certified reference material (GBW 09101 human hair, Shanghai Institute of Nuclear Research, Academia Sinica, China) were used. Recovery rates for QC and spiked samples were 90–110%, precision was > 95%. The LOD was 0.02 μg/dL with 0.5% of Pb values and no Mn values below the LOD.
Total Hg and As were measured in maternal hair and toenail samples, respectively, collected, on average, 10 days postpartum. Hair samples were cut from the occiput and, where identifiable, the 3 centimeters closest to the scalp were analyzed as reflective of exposures during the last trimester of pregnancy. Clippings from all ten toenails were analyzed. Toenails grow about 1.6 millimeters per month so metals found in distal nail clippings reflect exposures that occurred over approximately the previous year.29 Hair samples were cleaned using sonication for 15 minutes in 10 mL of 1% Triton X-100 solution in 50-mL Falcon tubes, rinsed five times with distilled deionized water, and dried for 24 hours at 60 degrees Celsius prior to analysis. Hair was then analyzed for total Hg, a reasonable proxy for MeHg,30 using a DM-80 Direct Mercury analyzer at the Harvard T.H. Chan School of Public Health Trace Metals Analysis Laboratory (Boston, MA).31,32 Toenails were also sonicated and rinsed, then weighed and digested with 1 ml of HNO3 acid for 24 hours at room temperature. Finally, they were analyzed using an external calibration method on a dynamic reaction cell-inductively coupled plasma-mass spectrometer (Agilent 7700x ICP-MS) at the Dartmouth Trace Elements Analysis Laboratory (Hanover, NH). QC procedures for both analyses included daily calibration verification, procedural blanks, and certified reference material. Recovery rates for QC standards were 90–110% and precision > 95% for hair total Hg, while the coefficients of variation for reference standards were < 15% for toenail As.31,33 The LODs for hair total Hg and toenail As varied as they were dependent on sample size. The average LODs for Hg and As were 50 ng/g of hair and 0.03 ng/g of toenails, respectively, with 0.4% of values below the average LOD of Hg and 4.7% of value below the average LOD of As.
2.3. Working Memory assessment.
A trained study examiner administered the Wide Range Assessment of Memory and Learning, 2nd Edition (WRAML2) Verbal Working Memory and Symbolic Working Memory subtests34 to study participants at the adolescent assessment. For Verbal Working Memory, participants were read a list of words containing both animal and non-animal words. Participants were then asked to recall the animal words in size order from smallest to largest followed by the non-animal words in any order. Next, participants were asked to recall the animal words in size order from smallest to largest, followed by the non-animal words also in size order. For Symbolic Working Memory, the examiner first dictated a series of numbers then asked the participant to identify the listed numbers in correct numerical order on a Number Stimulus Card. Next, the examiner dictated a series of numbers and letters and asked the participant to identify the listed numbers in numerical order and the letters in alphabetical order on the Number-Alphabet Stimulus Card. Performance on each of the tests was based on the total correct raw score that was then age-standardized to a scaled score with a mean of 10 and standard deviation of 3.34 The Verbal and Symbolic Working Memory scaled scores were then combined to create the Working Memory Index, which was age-standardized to a mean of 100 with a standard deviation of 15.34
2.4. Covariate assessment.
The infant’s race/ethnicity, birthweight, gestational age, and newborn exam as well as the mother’s pregnancy and delivery course were obtained via review of hospital medical records from the birth. Ten days postpartum, a questionnaire eliciting information regarding maternal socio-demographics, medical history, diet, smoking, alcohol, and drug use, and infant feeding was completed during a study home visit. Periodic pediatric medical record reviews and parental and child self-reported questionnaires were administered at study follow-up visits to update demographic and health information for study participants. Follow-up assessments at ages 8 and 15 years also included a home visit to assess the quality of the child’s home environment and parenting using the Home Observation for Measurement of the Environment (HOME)35 and assessment of maternal IQ using the Kaufman Brief Intelligence Test (KBIT)36. We also constructed a measure of social disadvantage, the prenatal social disadvantage index (PNSDI), as the sum of five adverse social or economic exposures at the time of the child’s birth where presence of each risk factor was assigned a value of 1, absence a value of 0: mother unmarried, mother’s education as high school graduate or less, father’s education as high school graduate or less, annual household income less than $20,000, and mother’s age at birth less than 20 years.
2.5. Statistical analysis.
For all statistical analyses, to reduce the influence of extreme values, chemical exposures were log2-transformed so associations are based on a two-fold increase in exposure concentrations. For all formal statistical modeling, model covariates were selected using Directed Acyclic Graphs (DAGs). The DAGs were informed by both a literature review regarding potential confounders of the relationship of prenatal organochlorine and metal exposures with cognition as well as covariates that had been previously found to predict cognitive outcomes in the NBC. All models were adjusted for adolescent race/ethnicity, sex, age at exam, year of birth, and 15-year HOME score; test examiner; maternal IQ, maternal pregnancy seafood consumption and smoking; and family characteristics at the child’s birth (maternal marital status, parental education, household income). Characteristics of participants who were included in the analyses were compared to those who were excluded using t-tests and chi-square tests where appropriate.
In exploratory analyses, we examined potential non-linear relationships and interactions of exposure to a chemical mixture composed of DDE, HCB, ΣPCB4, Pb, and Mn with working memory outcomes using Bayesian Kernel Machine Regression (BKMR). BKMR is an exposure-response surface estimation technique that models the relationship between a large number of exposures and an outcome using a flexible exposure-response function.37 In BKMR, this function is estimated using a Gaussian Kernel, which is capable of capturing many underlying functional forms.37 BKMR implements an iterative estimation algorithm (Markov Chain Monte Carlo) to estimate the function. The resulting graphics helped us to identify non-linear exposure-outcome associations and interactions between exposures. To assess non-linearities, we visually inspected plots of the estimated exposure-response functions and 95% credible intervals of DDE, HCB, ΣPCB4, Pb, and Mn with working memory scaled scores while assigning the remaining exposures to their median values. To assess interactions, we visually inspected plots of the estimated exposure-response functions between one of the five main exposures and working memory scaled scores, where a second exposure was fixed at varying levels and all of the remaining exposures were assigned to their median value. We assumed no interaction in plots where the slope of each chemical was similar at varying levels of the second chemical. The results of BKMR analysis informed specification of standard parametric regression models as described below. We also used BKMR to assess the joint association of the chemical mixture with each of the working memory outcomes. BKMR analyses were conducted using the R bkmr package.38
As Verbal Working Memory, Symbolic Working Memory, and the Working Memory Index scores were normally distributed and the exploratory BKMR results supported linear models with no interactions, we fit multivariable linear regression models using Ordinary Least Squares (OLS) to estimate the association between chemical exposures and working memory outcomes. All five (DDE, HCB, ΣPCB4, Pb, Mn) exposures were included in all regression models simultaneously along with the aforementioned covariates. We then assessed potential effect modification of exposure-outcome associations by sex and PNSDI using interaction terms in the models followed by sex- and PNSDI-stratified linear regression models. In PNSDI-stratified models, we compared chemical associations among participants who had a PNSDI of 3 or more to those had a PNSDI of less than 3. This cut-off was selected as it was correlated with other indicators of social disadvantage such as the HOME score and allowed for sufficient numbers of participants in each group to maintain enough power for PNSDI-stratified analyses.
To account for potential selection bias due to loss to follow-up, we used inverse probability weighting (IPW).39 IPW weights participants included in the analyses based on the inverse of the probability of their being in the study given their particular set of exposures and covariates, thereby creating a pseudo-population that represents the original source population. The particular set of exposures and covariates used to create the pseudo-population in this analysis included DDE, HCB, ΣPCB4, and Pb and socio-demographics at birth such as maternal education, household income, and obstetric risk score (a score that summarizes adverse factors that occur throughout pregnancy and the perinatal period)40 and child characteristics such as race/ethnicity and sex. We used stabilized IPW39 to improve efficiency, trimmed at the 2.5th and 97.5th percentile.
Because maternal hair and toenail samples were collected at a home visit ten days postpartum, rather than at birth, there were many participants missing MeHg and As exposure biomarkers. Thus, in a secondary analysis, we added biomarkers of MeHg and As to our chemical mixture and assessed the impact of DDE, HCB, ΣPCB4, Pb, Mn, MeHg, and As on working memory. Once again, we used BKMR to explore non-linear relationships, interactions, and joint associations of this mixture with working memory as well as standard parametric multivariable linear regression models. All statistics were conducted using R version 3.6.0.41
3. Results
3.1. Study population.
Table 1 describes the outcome, exposure, and covariate measures of the NBC subset assessed as adolescents who had complete data on working memory outcomes, biomarkers of exposure to DDE, HCB, ΣPCB4, Pb, and Mn, and covariates (n=373) and those who were excluded from the analysis. Included NBC study participants were socio-demographically diverse with 29% identifying as non-white, 51% with mothers having less than or equal to a high school education at the time of their birth, and 31% in households with an annual income of < $20,000 at the time of their birth (Table 1). Those who were included in the analysis had greater sociodemographic and economic advantage compared to those with missing data. Compared to adolescents excluded from the analyses, mothers of included adolescents were more likely to be married at birth and have a higher IQ. Included parents were more likely to have more than a high school education and an annual household income ≥ $20,000. Participants in the main analytic group had slightly higher cord serum DDE and ΣPCB4 levels and lower cord blood Pb levels than those who were excluded due to loss to follow-up or missing data. They also performed slightly better on tests of working memory than those who were excluded.
Table 1.
Characteristics of New Bedford Cohort (NBC) participants who were included in the main analysis group1 and those who were excluded.
| Descriptive Characteristic | Main analysis group, n=373 | Excluded group, n=415 | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Working Memory Measures3 | n(%) | Mean (SD) | Range | n(%) | Mean (SD) | Range | p-value2 |
| Verbal Working Memory | 373 | 9.0 (2.7) | 1–16 | 155 | 8.3 (2.9) | 1–17 | 0.01* |
| Symbolic Working Memory | 373 | 9.8 (2.8) | 1–19 | 154 | 9.2 (2.6) | 1–14 | 0.02* |
| Working Memory Index | 373 | 96.6 (13.2) | 55–142 | 154 | 93.1 (13.1) | 60–128 | 0.01* |
| Exposure Measures 4,5 | |||||||
| Cord serum DDE (ng/g) | 373 | 0.6 (1.2) | 0.02–14.9 | 378 | 0.4 (0.4) | 0–4.2 | < 0.01* |
| Cord serum HCB (ng/g) | 373 | 0.03 (0.02) | 0–0.1 | 378 | 0.03 (0.05) | 0–0.7 | 0.1 |
| Cord serum ΣPCB4 (ng/g) | 373 | 0.3 (0.3) | 0.01–4.4 | 378 | 0.2 (0.2) | 0.01–1.9 | 0.05 |
| Cord blood Pb (μg/dL) | 373 | 1.4 (0.9) | 0–9.4 | 375 | 1.7 (1.7) | 0–17.4 | < 0.01* |
| Cord blood Mn (μg/dL) | 373 | 4.2 (1.6) | 0.7–14.6 | 335 | 4.3 (2.0) | 0.2–22.1 | 0.6 |
| Covariate Measures 6 | |||||||
| Child Characteristics | |||||||
| Race/Ethnicity | 0.09 | ||||||
| Non-Hispanic White | 263 (70.5) | 268 (64.6) | |||||
| Hispanic | 33 (8.8) | 56 (13.5) | |||||
| Other | 77 (20.6) | 89 (21.4) | |||||
| Missing | 0 | 2 (0.5) | |||||
| Sex | 0.05 | ||||||
| Male | 179 (48.0) | 229 (55.2) | |||||
| Female | 194 (52.0) | 186 (44.8) | |||||
| Age at Exam | 373 | 15.5 (0.6) | 14.4–17.8 | 155 | 15.7 (0.7) | 13.9–17.9 | < 0.01* |
| Home Score | 373 | 43.9 (6.3) | 21–56 | 118 | 42.7 (6.0) | 27–53 | 0.07 |
| Year of Birth | |||||||
| 1993–1994 | 100 (26.8) | 159 (38.3) | < 0.01* | ||||
| 1995–1996 | 153 (41.0) | 147 (35.4) | |||||
| 1997–1998 | 120 (32.2) | 109 (26.3) | |||||
| Maternal Characteristics | |||||||
| Marital status at birth | < 0.01* | ||||||
| Not married | 136 (36.5) | 195 (47.0) | |||||
| Married | 237 (63.5) | 165 (39.8) | |||||
| Missing | 0 | 55 (13.3) | |||||
| Maternal IQ | 373 | 99.4 (10.4) | 57–124 | 262 | 95.8 (10.2) | 72–126 | < 0.01* |
| Seafood during pregnancy (serv/day) | 373 | 0.5 (0.6) | 0–5.3 | 260 | 0.6 (0.7) | 0–6 | 0.6 |
| Smoking during pregnancy | 0.1 | ||||||
| No | 272 (72.9) | 210 (50.6) | |||||
| Yes | 101 (27.1) | 103 (24.8) | |||||
| Missing | 0 | 102 (24.6) | |||||
| Household Characteristics at Birth | |||||||
| Maternal education | < 0.01* | ||||||
| ≤ High School | 190 (50.9) | 231 (55.7) | |||||
| > High School | 183 (49.1) | 127 (30.6) | |||||
| Missing | 0 | 57 (13.7) | |||||
| Paternal Education | < 0.01* | ||||||
| ≤ High School | 246 (66.0) | 266 (64.1) | |||||
| > High School | 127 (34.0) | 81 (19.5) | |||||
| Missing | 0 | 68 (16.4) | |||||
| Annual Household Income | < 0.01* | ||||||
| < $20,000 | 115 (30.8) | 150 (36.1) | |||||
| ≥ $20,000 | 258 (69.2) | 201 (48.4) | |||||
| Missing | 0 | 64 (15.4) | |||||
| Examination Characteristics | |||||||
| Examiner | 0.4 | ||||||
| 1 | 277 (74.3) | 121 (29.2) | |||||
| 2 | 96 (25.7) | 34 (8.2) | |||||
| Missing | 0 | 260 (62.7) | |||||
Main analysis group: complete working memory outcome, covariate and exposure data for DDE, HCB, PCBs, Pb and Mn, n=373.
P-values represent results comparing characteristics between participants included in Set 1 and those excluded from Set 1 using t-tests and chi-square tests. Categorical covariate comparisons based on the distribution of non-missing values.
NBC participants with missing working memory measures: Verbal Working Memory n=260; Symbolic Working Memory n=261; Working Memory Index n=261.
NBC participants with missing exposure measures: DDE n=37; HCB n=37; ΣPCB4 n=37; Pb n= 40; Mn n=80.
NBC participants with missing covariate measures: age at exam n=260; HOME score n= 297; maternal IQ n=153; seafood during pregnancy n= 155.
p < 0.05.
Abbreviations: DDE: dichlorodiphenyldichloroethylene; HCB: hexachlorobenzene; ΣPCB4: Sum of 4 PCB congeners (118, 138, 153, 180); Pb: lead; Mn: manganese.
3.2. Exposure measures.
Among participants in the main analytic group, exposures to DDE, HCB, and ΣPCB4 were moderately correlated (Spearman r=0.4–0.7) and exposure to Pb was weakly correlated with the organochlorines and Mn (Spearman r=0.1–0.2). Mn was not correlated with the organochlorines. Despite the residential proximity of NBC participants to the New Bedford Harbor Superfund site, chemical biomarker levels in the NBC study participants were similar to the general populations of the U.S. and Canada,42–45 with the exception of total hair Hg concentrations which were similar to those observed in high fish-eating populations.46
3.3. Working memory measures.
WRAML2 Verbal and Symbolic Working Memory were moderately correlated with one another (Spearman r=0.6). NBC WRAML2 working memory scores were lower than the standardized sample [mean (SD) of Verbal and Symbolic Working Memory: 10 (3) and Working Memory Index: 100 (3)] (Table 1).
3.4. BKMR model results.
Visual inspection of exploratory BKMR analyses of the association of a chemical mixture composed of DDE, HCB, ΣPCB4, Pb, and Mn with Verbal and Symbolic Working Memory did not indicate any non-linear relationships or interactions between exposures (Figures 1–2). Therefore, we did not include higher-order terms or interactions between chemicals in the parametric models. BKMR results suggested an adverse association of the joint chemical mixture with Verbal Working Memory for joint exposures up to the ~60th percentile when compared to their median levels (Figure 3). There was no evidence of a joint association of the exposures with the Symbolic Working Memory subtest (Figure 3).
Figure 1.
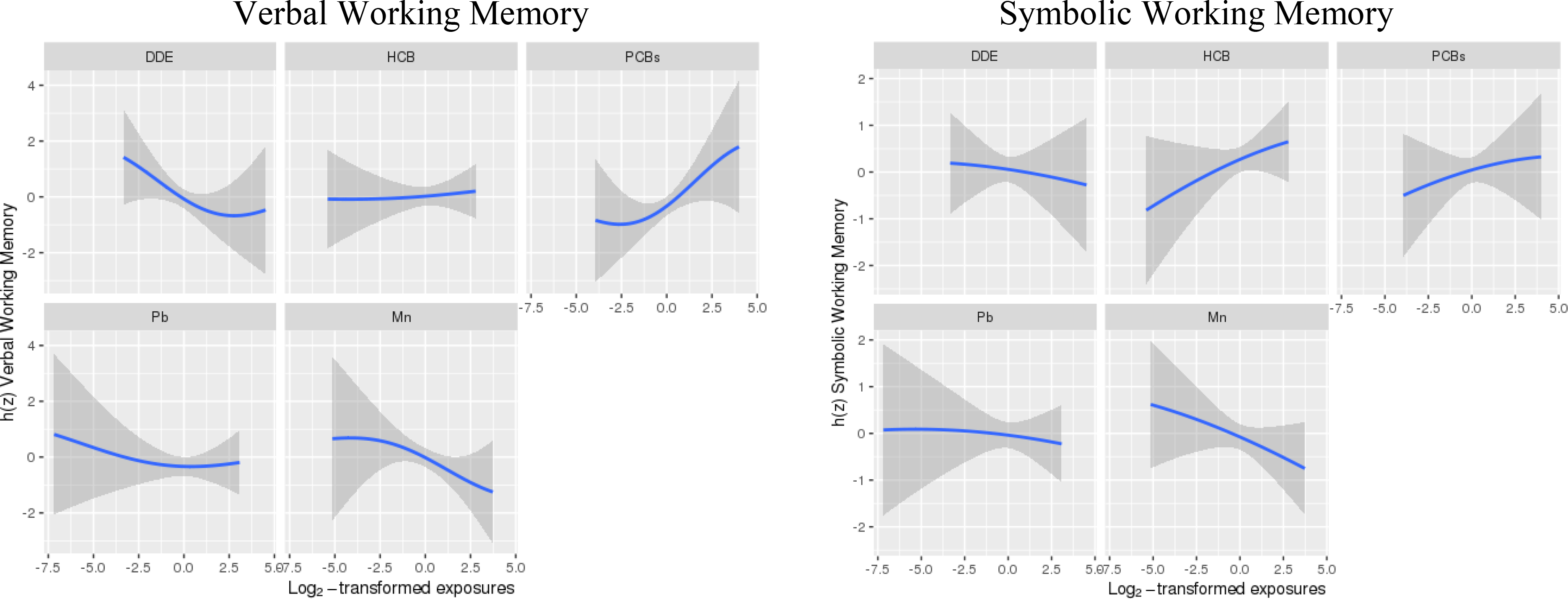
Estimated exposure-response functions and 95% credible intervals1 of each of the 5 main exposures with the Wide Range Assessment of Memory and Learning 2nd Edition working memory scaled scores, where all remaining exposures are assigned to their median value among adolescents in the main analysis group.2
1Exposures have been log2-transformed and models have been adjusted for all listed exposures, child race, sex, age at exam, year of birth, and HOME score; maternal marital status at child’s birth, IQ, seafood consumption during pregnancy, and smoking during pregnancy; maternal and paternal education and annual household income at child’s birth; study examiner. 2Main analysis group: complete working memory outcome, covariate and exposure data for DDE, HCB, PCBs, Pb and Mn, n=373.
Abbreviations: DDE: dichlorodiphenyldichloroethylene; HCB: hexachlorobenzene; PCBs: Sum of 4 PCB congeners (118, 138, 153, 180); Pb: lead; Mn: manganese.
Figure 2.
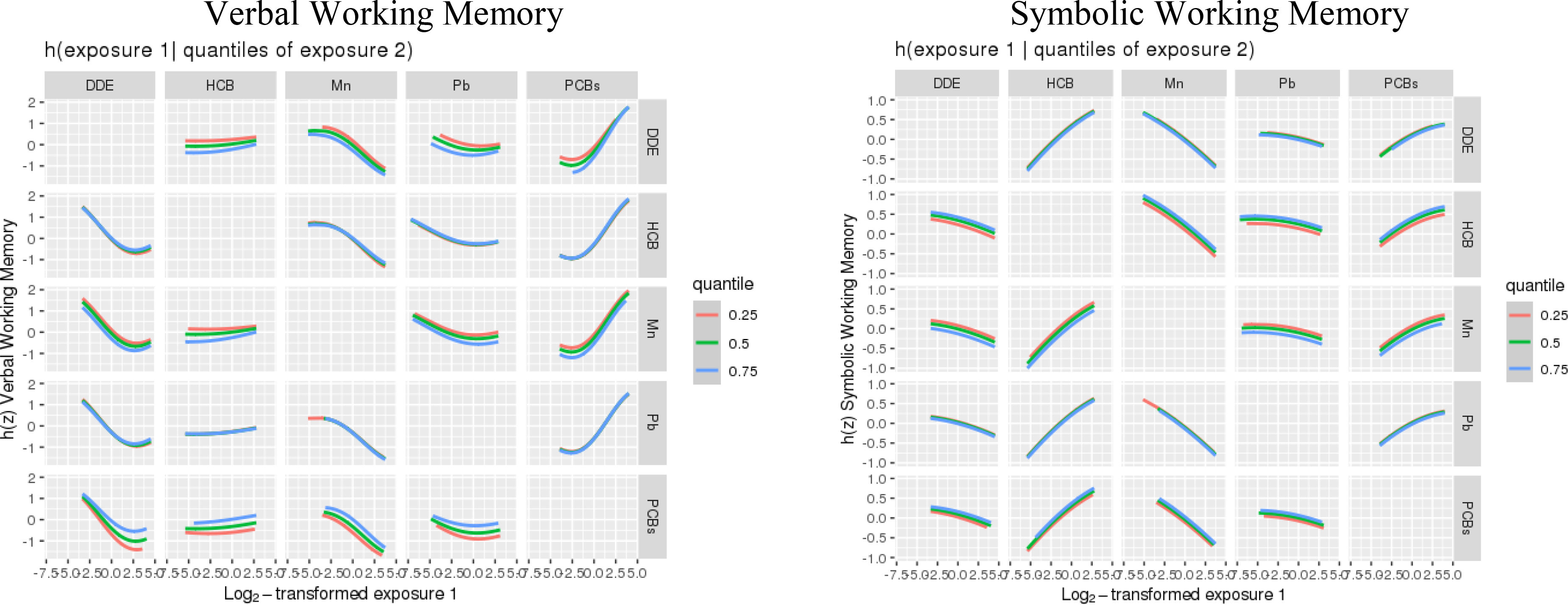
Exposure-response functions1 associating each of the 5 main exposures and a second exposure fixed at various quantiles with the Wide Range Assessment of Memory and Learning 2nd Edition working memory scaled scores, while the remaining exposures are assigned to their median value among adolescents in the main analysis group2.
1Exposures have been log2-transformed and models have been adjusted for all listed exposures, child race, sex, year of birth, age at exam, and HOME score; maternal marital status at child’s birth, IQ, seafood consumption during pregnancy, and smoking during pregnancy; maternal and paternal education and annual household income at child’s birth; study examiner. 2Main analysis group: complete working memory outcome, covariate and exposure data for DDE, HCB, PCBs, Pb and Mn, n=373.
Abbreviations: DDE: dichlorodiphenyldichloroethylene; HCB: hexachlorobenzene; ΣPCB4: Sum of 4 PCB congeners (118, 138, 153, 180); Pb: lead; Mn: manganese.
Figure 3.
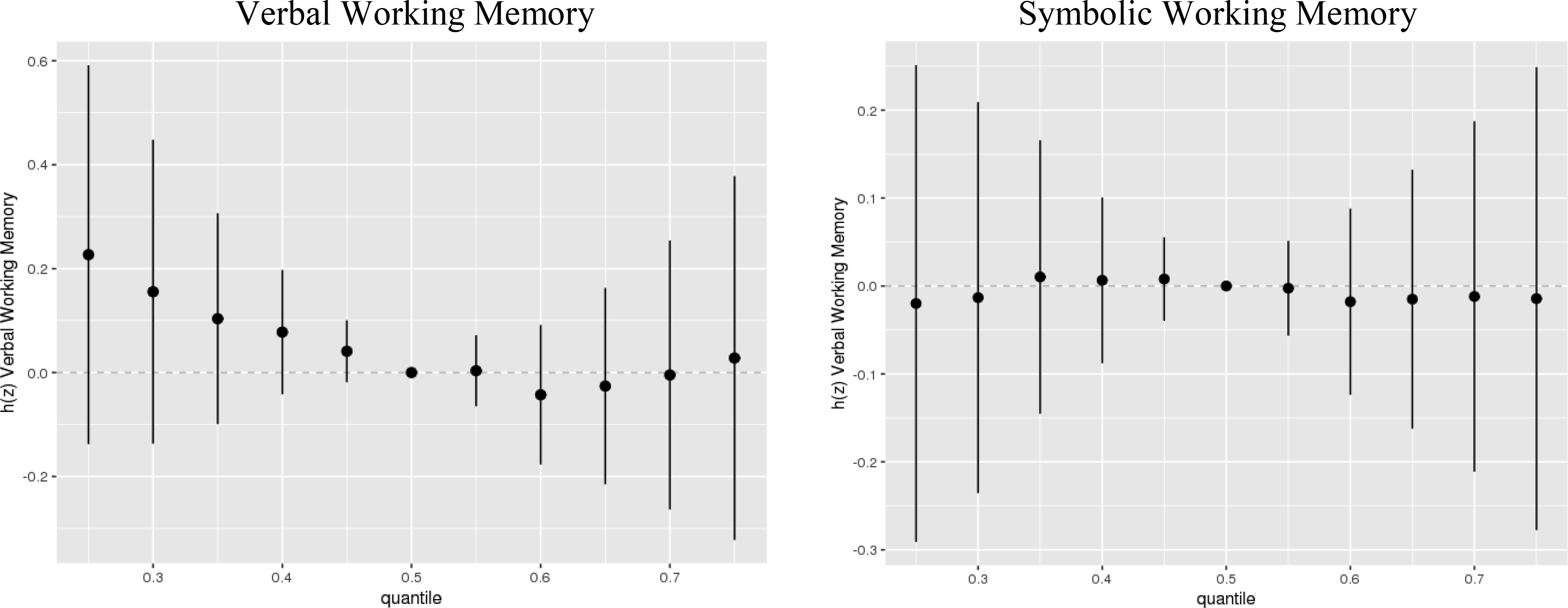
Joint association (estimates and 95% credible intervals1) of the five-chemical mixture with the Wide Range Assessment of Memory and Learning 2nd Edition working memory scaled scores among adolescents in the main analysis group2. Chemical mixture levels at each percentile are compared to each component at its median level.
1Exposures have been log2-transformed and models have been adjusted for all listed exposures, child race, sex, age at exam, year of birth, and HOME score; maternal marital status at child’s birth, IQ, seafood consumption during pregnancy, and smoking during pregnancy; maternal and paternal education and annual household income at child’s birth; study examiner. 2Main analysis group: complete working memory outcome, covariate and exposure data for DDE, HCB, PCBs, Pb and Mn, n=373.
3.5. Linear regression model results.
Table 2 shows the results of covariate-adjusted linear regression analyses of the association of prenatal concentrations of DDE, HCB, ΣPCB4, Pb, and Mn with Verbal Working Memory, Symbolic Working Memory, and the Working Memory Index, where all five exposures were included in the models simultaneously. We found that a twofold increase in cord blood Mn concentration was associated with lower working memory scores (Verbal Working Memory: difference=−0.75; 95% CI: −1.29, −0.20 points; Symbolic Working Memory: difference=−0.44; 95% CI: −1.00, 0.12 points; Working Memory Index: difference=−3.23; 95% CI: −5.87, −0.59 points). Findings also included an unexpected positive association between a twofold increase in ΣPCB4 and Verbal Working Memory (difference=0.32; 95% CI: 0.01, 0.63 points). Associations of the remaining exposures and working memory outcomes were modest and imprecise with confidence limits that included the null.
Table 2.
Complete-case results of multivariable linear regression analyses (difference in scaled scores associated with a twofold increase in exposure and 95% CI)1 assessing the relation of prenatal exposure to a five-chemical mixture with Wide Range Assessment of Memory and Learning, 2nd Edition working memory scaled scores among adolescents in the main analysis group2.
| Exposure | Verbal Working Memory Difference (95% CI) | Symbolic Working Memory Difference (95% CI) | Working Memory Index Difference (95% CI) |
|---|---|---|---|
| Log2 DDE | −0.24 (−0.53, 0.04) | −0.09 (−0.38, 0.21) | −0.91 (−2.28, 0.47) |
| Log2 HCB | 0.12 (−0.19, 0.42) | 0.24 (−0.08, 0.55) | 1.00 (−0.47, 2.48) |
| Log2 ΣPCB4 | 0.32 (0.01, 0.63)* | 0.10 (−0.22, 0.42) | 1.18 (−0.34, 2.69) |
| Log2 Pb | −0.11 (−0.40, 0.18) | −0.08 (−0.38, 0.22) | −0.53 (−1.92, 0.87) |
| Log2 Mn | −0.75 (−1.29, −0.20)* | −0.44 (−1.00, 0.12) | −3.23 (−5.87, −0.59)* |
Exposures have been log2-transformed and models have been adjusted for all listed exposures, child race, sex, age at exam, year of birth and HOME score; maternal marital status at child’s birth, IQ, seafood consumption during pregnancy, and smoking during pregnancy; maternal and paternal education and annual household income at child’s birth; study examiner.
Main analysis group: complete working memory outcome, covariate and exposure data for DDE, HCB, PCBs, Pb and Mn, n=373.
p < 0.05
Abbreviations: DDE: dichlorodiphenyldichloroethylene; HCB: hexachlorobenzene; ΣPCB4: Sum of 4 PCB congeners (118, 138, 153, 180); Pb: lead; Mn: manganese.
3.6. Assessment of effect modification.
When we included chemical-sex interaction terms to each of the overall working memory models, we did not find evidence of any chemical-sex interactions. In sex-stratified analyses (Figure 4; Supplemental Table 1), a doubling of cord blood Mn was associated with lower Verbal Working Memory scores among both sexes, though the association was statistically significant among males (difference = −0.88; 95% CI: −1.70, −0.07 points) but not females (difference = −0.38; 95% CI: −1.14, 0.39 points). However, the confidence intervals for the effect estimates of the two groups overlapped. There was also an unexpected positive association between HCB concentrations and Symbolic Working Memory scores among females (difference=0.48; 95% CI: 0.04, 0.91 points).
Figure 4.
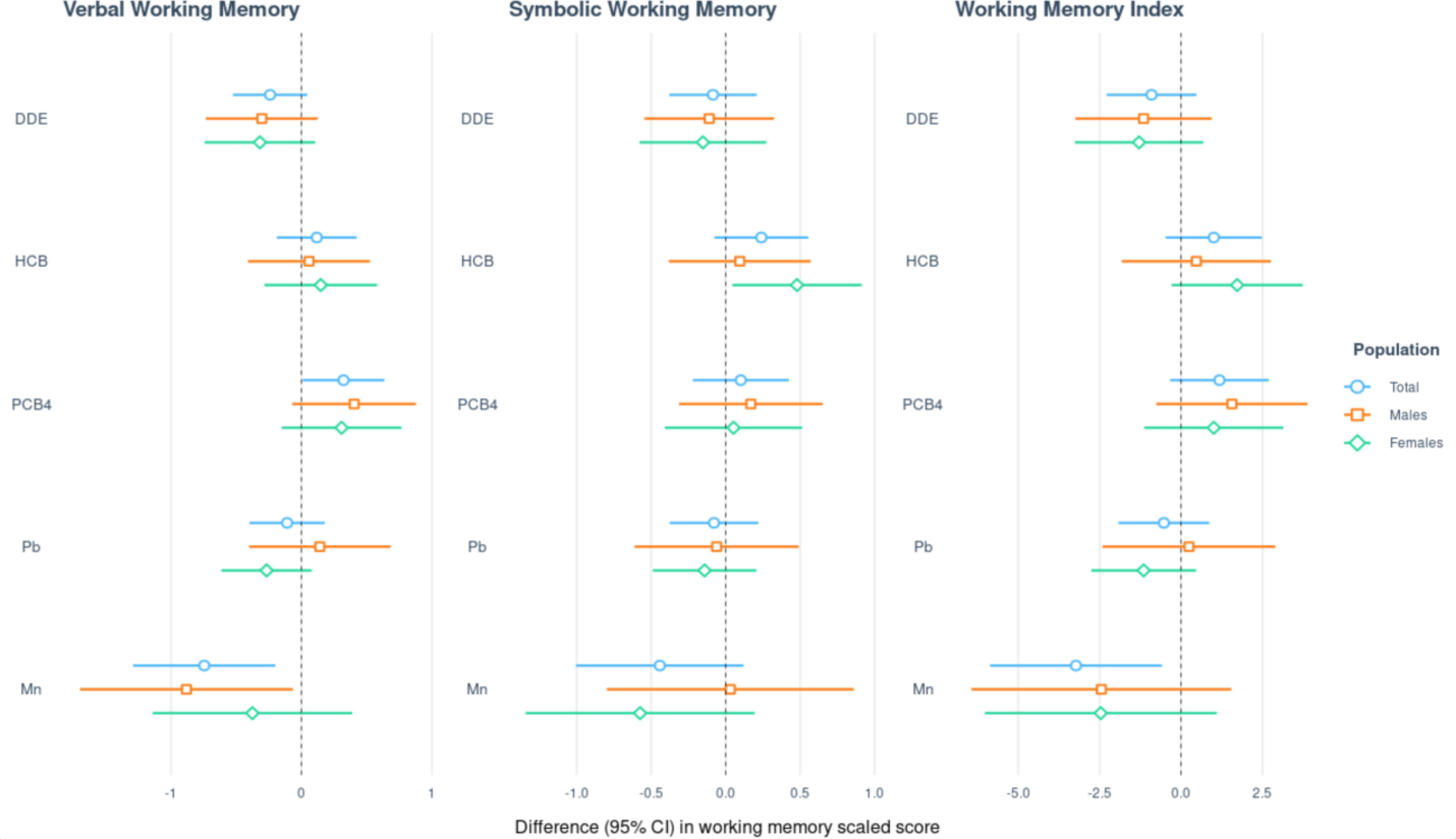
Sex-stratified and overall results of multivariable linear regression analyses (difference in points associated with a twofold increase in exposure and 95% CI)1 assessing the relation of prenatal exposure to a five-chemical mixture with Wide Range Assessment of Memory and Learning, 2nd Edition working memory scaled scores among adolescents in the main analysis group2.
1Exposures have been log2-transformed and models have been adjusted for all listed exposures, child race, sex, age at exam, year of birth, and HOME score; maternal marital status at child’s birth, IQ, seafood consumption during pregnancy, and smoking during pregnancy; maternal and paternal education and annual household income at child’s birth; study examiner. 2Main analysis group: complete working memory outcome, covariate and exposure data for DDE, HCB, PCBs, Pb and Mn. Total n=373; Males n= 179; Females n=194.
Abbreviations: DDE: dichlorodiphenyldichloroethylene; HCB: hexachlorobenzene; ΣPCB4: Sum of 4 PCB congeners (118, 138, 153, 180); Pb: lead; Mn: manganese.
Of the 373 study participants, 241 had a PNSDI < 3 (less prenatal social disadvantage), while 132 had a PNSDI ≥ 3 (more social disadvantage). We found suggestive evidence of an interaction between PNSDI and DDE for Verbal Working Memory, with a stronger negative association among those with more social disadvantage (difference = −0.60; 95% CI: −1.16 points, −0.03 vs. difference = −0.04; 95% CI: −0.37, 0.30 points) (Figure 5; Supplemental Table 2). In general, we noted stronger negative associations of DDE and HCB with the working memory measures among those with a PNSDI ≥ 3; conversely, HCB was positively associated with working memory measures among those with a PNSDI < 3 (Figure 5). Unexpectedly, ΣPCB4 exposure was positively associated with working memory outcomes for both strata of PNSDI (Figure 5; Supplemental Table 2), with the strongest evidence of a positive association with Verbal Working Memory where PNSDI ≥ 3 (difference = 0.73; 95% CI: 0.11, 1.35 points). Also unexpected was suggestive evidence that the strongest negative associations of Mn with working memory were where PNSDI was less than 3. IPW results of the main, sex-stratified, and PNSDI-stratified analyses were similar to the results of complete-case analyses (Supplemental Tables 3–5).
Figure 5.
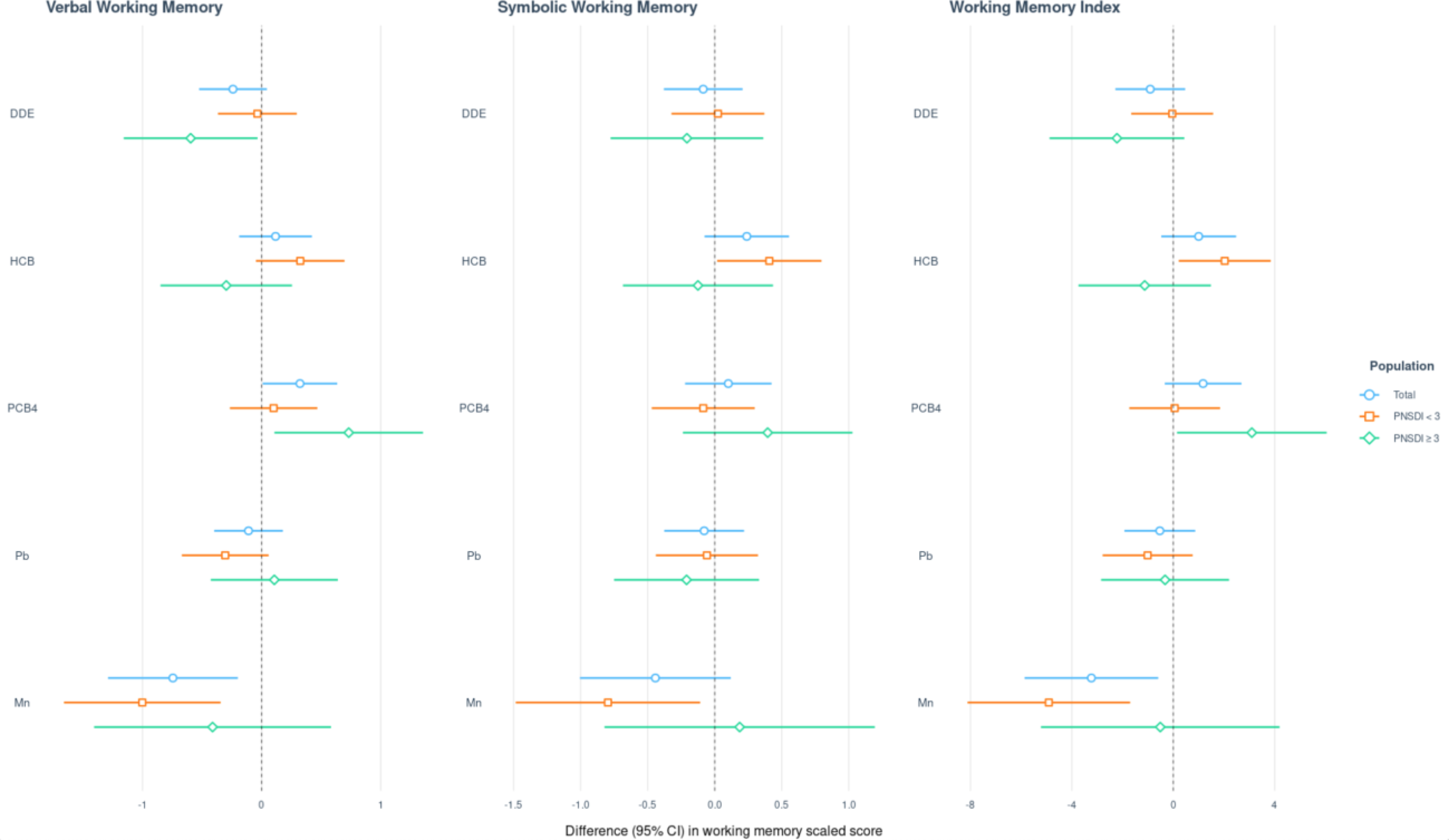
Prenatal social disadvantage index (PNSDI)1-stratified and overall results of multivariable linear regression analyses (difference in points associated with a twofold increase in exposure and 95% CI)2 assessing the relation of prenatal exposure to a five-chemical mixture with Wide Range Assessment of Memory and Learning, 2nd Edition working memory scaled scores among adolescents in the main analysis group3.
1Prenatal social disadvantage index (PNSDI) was constructed as the sum of five adverse social or economic exposures at the time of the child’s birth where presence of each risk factor was assigned a value of 1, absence a value of 0: mother unmarried, mother’s education as high school graduate or less, father’s education as high school graduate or less, annual household income less than $20,000, and mother’s age at birth less than 20 years.2Exposures have been log2-transformed and models have been adjusted for all listed exposures, child race, sex, age at exam, year of birth, and HOME score; maternal marital status at child’s birth, IQ, seafood consumption during pregnancy, and smoking during pregnancy; maternal and paternal education and annual household income at child’s birth; study examiner. 3Main analysis group: complete working memory outcome, covariate and exposure data for DDE, HCB, PCBs, Pb and Mn. Total n=373; PNSDI < 3 n= 241; PNSDI ≥ 3 n=132.
Abbreviations: DDE: dichlorodiphenyldichloroethylene; HCB: hexachlorobenzene; ΣPCB4: Sum of 4 PCB congeners (118, 138, 153, 180); Pb: lead; Mn: manganese.
3.7. Secondary analyses.
In secondary analyses, we added biomarkers of MeHg and As to our chemical mixture and assessed associations of DDE, HCB, ΣPCB4, Pb, Mn, MeHg, and As with working memory (n= 235). Similar to participants in the main analyses, those who were included in the secondary analyses performed better on all tests of working memory, had higher cord serum DDE levels and lower cord Pb levels, and had characteristics consistent with greater sociodemographic advantage (Supplemental Table 6). Among participants in the secondary analytic group, MeHg was moderately correlated with the organochlorines (Spearman r=0.2–0.5), while As was not correlated with other exposures.
The results of exploratory BKMR analyses of the association of the seven-chemical exposure mixture with Verbal and Symbolic Working Memory did not indicate any non-linear relationships or interactions between the exposures in their associations with working memory outcomes (Supplemental Figures 1–2). In contrast to the five-chemical models, BKMR did not demonstrate an adverse joint association of the seven-chemical mixture with either working memory subtest (Supplemental Figure 3). The results of covariate-adjusted linear regression showed similar patterns of associations as were observed in the main analyses, though the effect estimates were typically attenuated and less precise (Supplemental Table 7). MeHg and As were not associated with working memory measures. IPW results were similar to complete case (Supplemental Table 8).
4. Discussion
The purpose of this study was to examine the hypothesized association of prenatal exposure to a five-chemical mixture composed of DDE, HCB, ΣPCB4, Pb, and Mn with working memory among adolescents from the NBC study. The most consistent finding across the main linear regression analyses was an association of cord blood Mn with lower working memory scores (Table 2). This association was strongest for Verbal Working Memory and these findings remained when we used IPW to account for potential selection bias due to loss to follow-up. They were also observed when we included MeHg and As in the chemical mixture, though the effect estimates were somewhat attenuated, especially for Symbolic Working Memory (Supplemental Table 7). In sex-stratified results, we found a stronger adverse association of Mn with Verbal Working Memory among males than females, but a stronger adverse association of Mn with Symbolic Working Memory among females than males, though the interaction with sex was not statistically significant (Figure 4). In PNSDI-stratified analyses, we observed Mn to be more strongly adversely associated with all working memory outcomes among those who had a lower PNSDI (Figure 5). This may be due to a type of saturation effect in which it is harder to detect subtle chemical associations when other risk factors such as high PNSDI predominate.
Consistent with our results, high prenatal exposure to Mn measured in teeth was previously found to be associated with working memory errors on the Virtual Radial Arm Maze among adolescent girls in Italy.14 In addition, a small exploratory study found that tooth Mn levels reflecting prenatal exposures were associated with poor inhibition, another executive function related to working memory, among young children ages 36–54 months.47 Two cross-sectional studies of children ages 7–12 in Brazil have also found adverse associations between hair Mn and verbal tasks, specifically verbal working memory and verbal memory.48,49 The brain dopaminergic system is targeted by Mn exposure.50,51 In animal studies, Mn exposure in the neonatal time period has been associated with striatal dopamine levels and altered dopaminergic synaptic environments in brain regions involved in working memory such as the prefrontal cortex, nucleus accumbens, and dorsal striatum.50,51
There is not a well-established point source for Mn exposure located near New Bedford, therefore exposure to Mn was likely via diet and water, the most common sources of Mn in the U.S. general population.52 In this study, Mn was measured in cord blood using ICP-MS. This biomarker and method of measurement may be prone to certain limitations. First, there is some disagreement about which biological specimen is the most valid biomarker of Mn exposure, however cord blood Mn has been found to be a useful measure of fetal exposure and well-correlated with other biomarkers of prenatal exposure.53–55 In addition, a limitation of using ICP-MS to measure cord blood Mn is that an isotope of Mn has a similar mass to two isotopes of iron which may result in an overestimation of Mn concentrations among those with high iron levels.56 However, the laboratory in which these analyses were conducted reported that although some inflation of Mn levels occurred, relative Mn concentrations were unaffected. Therefore, this limitation should not impact the results of our study.
We also observed evidence of an unexpected positive association between ΣPCB4 exposure and Verbal Working Memory scores (Table 2). It remained when accounting for loss to follow-up using IPW and was present (though attenuated) when including MeHg and As in the chemical mixture. The attenuation of this association in secondary analyses when MeHg and As were included in the model may be due to less precision due to a smaller sample. It may also be due to population differences between the two groups – when we accounted for potential loss to follow-up using IPW, we did see a slightly stronger positive association between ΣPCB4 and Verbal Working Memory than in the complete case results (Supplemental Table 8). This positive association may be due to negative confounding by diet.57Although we accounted for seafood consumption in our models as its intake is a source of both dietary PCB exposure and beneficial nutrients, seafood intake was measured with a food frequency questionnaire. Measurement error in the dietary assessment may have resulted in residual confounding by beneficial nutrients. To further investigate this issue, when we stratified by seafood consumption, we found that among those who ate ≤ 2 seafood servings per week, the association between ΣPCB4 and Verbal Working Memory trended negative while among those who ate > 2 seafood servings per week, the association was statistically significantly positive though the confidence intervals for the two groups overlapped (Supplemental Table 9). There was not a significant difference in cord serum PCB levels between the two groups with median concentrations of 0.18 ng/g vs. 0.21 ng/g among those eating ≤ 2 vs. > 2 servings per week. This supports the potential for residual confounding by seafood consumption to contribute to the unexpected positive association of PCBs with working memory. Those who ate less fish were likely exposed to PCBs from other sources and were not exposed to beneficial nutrients simultaneously. Thus, findings supported potential adverse PCB associations in this subset. Meanwhile, among heavy fish consumers who were likely co-exposed to both PCBs and beneficial nutrients, an apparent beneficial association of PCBs with working memory persisted.
Our results were not consistent with those in a Michigan cohort, where researchers found an adverse association between prenatal PCB exposure and working memory among 11-year-olds.11 A number of differences in the two studies may contribute to the discordant findings: PCB exposure levels were much higher in Michigan than in the NBC cohort (e.g., median serum PCB 153 in Michigan = 120 ng/g lipid vs. 30 ng/g lipid in the NBC) 42; Michigan study participants were younger than those in the NBC (mean respective ages of 11 vs. 15.5 years); Michigan participants were selected based on maternal contaminated Great Lakes fish consumption, while in the NBC study, PCB exposure was associated with not only fish consumption, but other dietary and demographic factors as well, which may have led to differing exposure patterns; working memory was assessed using different psychometric tests in the two studies (Sternberg Memory and subtests of the Wechsler Intelligence Scale for Children—Revised (WISC-R) in Michigan vs WRAML2 in the NBC); and the Michigan study did not account for other environmental exposures, such as organochlorine pesticides or other metals as was done in this study, which may have resulted in unmeasured confounding.11,26,42
Although confidence limits included the null, DDE was negatively associated with all working memory outcomes with no evidence of a difference in effect estimates by sex. Our results were consistent with those in California and Greece in which working memory among 10 year-olds and 4-year-olds, respectively, was weakly adversely impacted by prenatal exposure to DDE at levels higher than those in the NBC, without reaching statistical significance.8,10 One Spanish cohort, which also had higher levels of DDE than the NBC cohort, did find an adverse association between prenatal DDE and McCarthy Scales of Children’s Abilities Total Memory score which reflects a number of memory skills in young children, including working memory. However they did not assess working memory as a distinct outcome.7 Of note, while the aforementioned studies focused on just one exposure at a time, our study assessed multiple exposures to both organochlorines and metals in the same models, which accounted for potential confounding by these other exposures.
Pb also trended negative in its association with overall working memory outcomes, though effect estimates were small and confidence intervals were wide and included the null. We did not observe evidence of sexual dimorphism in effect estimates. Our Pb results were consistent with those found in eastern Massachusetts, where associations between prenatal Pb exposure at levels somewhat lower than those found in the NBC and working memory among 7-year-olds trended in an adverse direction without reaching statistical significance.12 A study of 11-year-old children in Quebec found evidence of adverse associations between cord blood Pb and working memory at higher levels of Pb than those found in the NBC study.13 A study in Boston previously found that general executive function outcomes among 10-year-olds were adversely impacted by recent or concurrent Pb exposure, rather than prenatal Pb exposure, so these may be important windows of susceptibility to consider.59
We observed weak positive associations between HCB and working memory, though confidence intervals once again were wide included the null. The one study that examined maternal serum pregnancy HCB concentrations and working memory among four-year-old children in Greece found an adverse association at the highest levels of exposure,8 however the NBC study had lower levels of HCB than those found in Greece (mean (SD): 0.1 (0.1) ng/ml). In secondary analyses when MeHg and As were added into the chemical mixtures, we found that associations of MeHg and As with working memory outcomes were largely null. This may be due to the smaller sample size and therefore less power to detect effects.
The NBC cohort is a diverse population with a substantial proportion of participants exposed to economic and sociodemographic disadvantages (Table 1). When we assessed effect modification by a prenatal social disadvantage index, we observed stronger adverse associations of DDE and HCB with working memory measures among participants with a PNSDI ≥ 3 compared to those who had a lower PNSDI (Figure 5). We also observed stronger positive associations between ΣPCB4 and working memory among participants with more social disadvantage. As previously mentioned, positive PCB-working memory associations may be due to residual negative confounding by diet which cannot be excluded in this study. However, it is unclear why such residual confounding might impact those with a PNSDI ≥ 3 more than those with a PNSDI < 3, therefore further research is needed to better understand these associations. Overall, our results point to the importance of examining effect modification by socio-demographic stressors in the association of prenatal exposure to chemical pollutants with working memory outcomes.
Exploratory BKMR analyses did not support non-linear associations between the prenatal exposures of interest and working memory outcomes, nor did they show evidence of interactions between chemicals (Figures 1–2), though this study may have been underpowered to identify interactions. The BKMR results suggested an adverse joint association of the chemical mixture with Verbal Working Memory but not Symbolic Working Memory (Figure 3).
This study had some limitations. Of the 788 original participants recruited to the NBC, 528 completed the adolescent follow-up assessment and 373 had complete data on exposures, outcomes, and covariates of interest. To account for this loss to follow-up and other missing data, we used IPW to weight participants that were included in our study so they would represent those who were excluded. The IPW results were very similar to the complete-case results, giving reassurance that selection bias due to censoring was unlikely. Due to the limited number of participants who had complete data on exposure, outcomes, and covariates, we may have been underpowered to observe modest interactions between exposures or effect modification by sex and PSNDI. We were particularly limited in the number of participants who had data on prenatal exposure to MeHg and As as samples for these measures required an extra study visit. Therefore, we may have also been underpowered to observe effects of these exposures. In addition, it is possible that we observed some spurious associations as we did not adjust for multiple comparisons in this study. However, decreasing the frequency of type I errors, or rejecting the null hypothesis when it is true, may increase the risk of type II errors, or failing to reject the null hypothesis when it is false.60 Finally, we measured each of the exposures at a single time point and lack data on exposures in childhood or adolescence. Further studies are needed to investigate whether these windows of exposure may also play a role in working memory development during adolescence. This study also had important strengths including extensive prospectively collected biomarkers of prenatal exposure to organochlorines and metals and comprehensive psychometric measures of working memory among adolescents, as well as sociodemographic, dietary, and lifestyle information. This allowed us to conduct an investigation of the association of prenatal exposure to a chemical mixture with working memory among adolescents while adjusting for potential confounders and modification by markers of psychosocial disadvantage.
5. Conclusion
We found suggestive evidence of an adverse impact of prenatal Mn on adolescent working memory, particularly on a Verbal Working Memory task, as well as an adverse joint association of the chemical mixture with Verbal Working Memory. We also observed different associations of prenatal exposures with working memory outcomes among participants who had greater sociodemographic/socioeconomic disadvantage, supporting the importance of social and economic stressors as potential sources of altered susceptibility to chemical exposure risk. Given that working memory undergoes considerable development during adolescence and deficiencies may be associated with numerous psychiatric and behavioral disorders, further research should examine the impact of environmental exposures on working memory in this age group, as well as the potential socioeconomic disparities in vulnerability to environmental chemical exposures.
Supplementary Material
Acknowledgments
Funding sources: NIEHS/NIH P42ES005947, R01ES014864, P30ES000002.
Training Grants: NIOSH ERC T42OH008416, NIEHS T32ES00706941
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Internal Review Board:
IRB Protocol Title: “Metal & organochlorine exposure: impact on adolescent behavior & cognition”
IRB Protocol Number: 2008P001663
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Diamond A Executive Functions. Annu Rev Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crone EA, Wendelken C, Donohue S, Van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci U S A. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glahn DC, Bearden CE, Cakir S, et al. Differential working memory impairment in bipolar disorder and schizophrenia: Effects of lifetime history of psychosis. Bipolar Disord. 2006;8(2):117–123. doi: 10.1111/j.1399-5618.2006.00296.x [DOI] [PubMed] [Google Scholar]
- 4.Lee J Working Memory Impairments in Schizophrenia: A Meta-Analysis. Artic J Abnorm Psychol. 2005. doi: 10.1037/0021-843X.114.4.599 [DOI] [PubMed] [Google Scholar]
- 5.Kasper LJ, Alderson RM, Hudec KL. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clin Psychol Rev. 2012;32(7):605–617. doi: 10.1016/j.cpr.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Habib A, Harris L, Pollick F, Melville C. A meta-analysis of working memory in individuals with autism spectrum disorders. PLoS One. 2019;14(4). doi: 10.1371/journal.pone.0216198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribas-Fitó N, Torrent M, Carrizo D, et al. In Utero Exposure to Background Concentrations of DDT and Cognitive Functioning among Preschoolers. Am J Epidemiol. 2006;164:955–962. doi: 10.1093/aje/kwj299 [DOI] [PubMed] [Google Scholar]
- 8.Kyriklaki A, Vafeiadi M, Kampouri M, et al. Prenatal exposure to persistent organic pollutants in association with offspring neuropsychological development at 4 years of age: The Rhea mother-child cohort, Crete, Greece. Environ Int. 2016;97:204–211. doi: 10.1016/j.envint.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 9.Forns J, Torrent M, Garcia-Esteban R, et al. Prenatal exposure to polychlorinated biphenyls and child neuropsychological development in 4-year-olds: An analysis per congener and specific cognitive domain. Sci Total Environ. 2012;432:338–343. doi: 10.1016/j.scitotenv.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 10.Gaspar FW, Harley KG, Kogut K, et al. Prenatal DDT and DDE exposure and child IQ in the CHAMACOS cohort. Environ Int. 2015;85:206–212. doi: 10.1016/j.envint.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143(6):780–788. doi: 10.1067/S0022-3476(03)00577-8 [DOI] [PubMed] [Google Scholar]
- 12.Fruh V, Rifas-Shiman SL, Amarasiriwardena C, et al. Prenatal lead exposure and childhood executive function and behavioral difficulties in project viva. Neurotoxicology. 2019;75:105–115. doi: 10.1016/j.neuro.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson JL, Muckle G, Ayotte P, Dewailly É, Jacobson SW. Relation of prenatal methylmercury exposure from environmental sources to childhood IQ. Environ Health Perspect. 2015;123(8):827–833. doi: 10.1289/ehp.1408554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anglen Bauer J, Henn BC, Austin C, et al. Manganese in teeth and neurobehavior: sex-specific windows of susceptibility HHS Public Access. Env Int. 2017;108:299–308. doi: 10.1016/j.envint.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debes F, Budtz-Jørgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28(5):536–547. doi: 10.1016/j.ntt.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 16.Calderón J, Navarro ME, Jimenez-Capdeville ME, et al. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ Res. 2001;85(2):69–76. doi: 10.1006/enrs.2000.4106 [DOI] [PubMed] [Google Scholar]
- 17.Carvalho CF, Menezes-Filho JA, Matos VP d., et al. Elevated airborne manganese and low executive function in school-aged children in Brazil. Neurotoxicology. 2014;45:301–308. doi: 10.1016/j.neuro.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 18.Nascimento S, Baierle M, Göethel G, et al. Associations among environmental exposure to manganese, neuropsychological performance, oxidative damage and kidney biomarkers in children. Environ Res. 2016;147:32–43. doi: 10.1016/j.envres.2016.01.035 [DOI] [PubMed] [Google Scholar]
- 19.Wasserman GA, Liu X, LoIacono NJ, et al. A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environ Heal A Glob Access Sci Source. 2014;13(1):23. doi: 10.1186/1476-069X-13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlin DJ, Rider CV., Woychik R, Birnbaum LS. Unraveling the health effects of environmental mixtures: An NIEHS priority. Environ Health Perspect. 2013. doi: 10.1289/ehp.1206182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yorifuji T, Debes F, Weihe P, Grandjean P. Prenatal exposure to lead and cognitive deficit in 7- and 14-year-old children in the presence of concomitant exposure to similar molar concentration of methylmercury. Neurotoxicol Teratol. 2011;33(2):205–211. doi: 10.1016/j.ntt.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucchini RG, Guazzetti S, Renzetti S, et al. Neurocognitive impact of metal exposure and social stressors among schoolchildren in Taranto, Italy. Environ Heal A Glob Access Sci Source. 2019;18(1):67. doi: 10.1186/s12940-019-0505-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasserman GA, Liu X, Parvez F, et al. Arsenic and manganese exposure and children’s intellectual function. Neurotoxicology. 2011;32(4):450–457. doi: 10.1016/j.neuro.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barg G, Daleiro M, Queirolo EI, et al. Association of low lead levels with behavioral problems and executive function deficits in schoolers from Montevideo, Uruguay. Int J Environ Res Public Health. 2018;15(12). doi: 10.3390/ijerph15122735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behforooz B, Newman J, Gallo MV., Schell LM. PCBs and measures of attention and impulsivity on a continuous performance task of young adults. Neurotoxicol Teratol. 2017;64:29–36. doi: 10.1016/j.ntt.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi AL, Levy JI, Dockery DW, et al. Does living near a Superfund site contribute to higher polychlorinated biphenyl (PCB) exposure? Environ Health Perspect. 2006;114(7):1092–1098. doi: 10.1289/ehp.8827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol. 2010;171(5):593–601. doi: 10.1093/aje/kwp427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korrick SA, Altshul LM, Tolbert PE, Burse VW, Needham LL, Monson RR. Measurement of PCBs, DDE, and hexachlorobenzene in cord blood from infants born in towns adjacent to a PCB-contaminated waste site. J Expo Anal Environ Epidemiol. 2000;10(6 II SUPPL.):743–754. doi: 10.1038/sj.jea.7500120 [DOI] [PubMed] [Google Scholar]
- 29.He K Trace elements in nails as biomarkers in clinical research. Eur J Clin Invest. 2011. doi: 10.1111/j.1365-2362.2010.02373.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers GJ, Davidson PW. Prenatal Methylmercury Exposure and Children: Neurologic, Developmental, and Behavioral Research. Environ Health Perspect. 1998;106:841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orenstein STC, Thurston SW, Bellinger DC, et al. Prenatal organochlorine and methylmercury exposure and memory and learning in school-age children in communities near the new bedford harbor superfund site, Massachusetts. Environ Health Perspect. 2014;122(11):1253–1259. doi: 10.1289/ehp.1307804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oken E, Wright RO, Kleinman KP, et al. Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environ Health Perspect. 2005;113(10):1376–1380. doi: 10.1289/ehp.8041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amaral AFS, Porta M, Silverman DT, et al. Pancreatic cancer risk and levels of trace elements. Gut. 2012;61(11):1583–1588. doi: 10.1136/gutjnl-2011-301086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheslow D, Adams W. Wide Range Assessment of Memory and Learning, Second Edition. https://www.pearsonclinical.com/psychology/products/100001702/wide-range-assessment-of-memory-and-learning-second-edition-wraml2.html. Published 2003. Accessed November 7, 2017.
- 35.Caldwell B, Bradley R. Home Observation for Measurement of the Environment. Dorsey, New York; 1985. [Google Scholar]
- 36.Kaufman A, Kaufman N. Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- 37.Bobb JF, Claus Henn B, Valeri L, Coull BA. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Heal A Glob Access Sci Source. 2018;17(1):67. doi: 10.1186/s12940-018-0413-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bobb JF. bkmr: Bayesian Kernel Machine Regression. 2017. https://cran.r-project.org/web/packages/bkmr/index.html. Accessed May 12, 2020. [Google Scholar]
- 39.Hernán MA, Robins JM. How to adjust for selection bias. In: Causal Inference: What If. Boca Raton: Chapman & Hall/CRC; 2020:107–112. [Google Scholar]
- 40.Sokol RJ, Rosen MG, Stojkov J, Chik L. Clinical application of high-risk scoring on an obstetric service. Am J Obstet Gynecol. 1977. doi: 10.1016/0002-9378(77)90212-5 [DOI] [PubMed] [Google Scholar]
- 41.R Core Team. R: The R Project for Statistical Computing. 2019. https://www.r-project.org/. Accessed May 12, 2020.
- 42.Longnecker MP, Wolff MS, Gladen BC, et al. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect. 2003;111(1):65–70. doi: 10.1289/ehp.5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ettinger AS, Egan KB, Homa DM, Brown MJ. Blood lead levels in U.S. women of childbearing age, 1976–2016. Environ Health Perspect. 2020. doi: 10.1289/EHP5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arbuckle TE, Liang CL, Morisset AS, et al. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere. 2016. doi: 10.1016/j.chemosphere.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 45.Ettinger AS, Arbuckle TE, Fisher M, et al. Arsenic levels among pregnant women and newborns in Canada: Results from the Maternal-Infant Research on Environmental Chemicals (MIREC) cohort. Environ Res. 2017. doi: 10.1016/j.envres.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 46.McDowell MA, Dillon CF, Osterloh J, et al. Hair mercury levels in U.S. children and women of childbearing age: Reference range data from NHANES 1999–2000. Environ Health Perspect. 2004;112(11):1165–1171. doi: 10.1289/ehp.7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol Teratol. 2007;29(2):181–187. doi: 10.1016/j.ntt.2006.09.020 [DOI] [PubMed] [Google Scholar]
- 48.Carvalho CF de, Oulhote Y, Martorelli M, et al. Environmental manganese exposure and associations with memory, executive functions, and hyperactivity in Brazilian children. Neurotoxicology. 2018;69:253–259. doi: 10.1016/j.neuro.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 49.Menezes-Filho JA, de Carvalho-Vivas CF, Viana GFS, et al. Elevated manganese exposure and school-aged children’s behavior: A gender-stratified analysis. Neurotoxicology. 2014;45:293–300. doi: 10.1016/j.neuro.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 50.Kern CH, Smith DR. Preweaning Mn exposure leads to prolonged astrocyte activation and lasting effects on the dopaminergic system in adult male rats. Synapse. 2011;65(6):532–544. doi: 10.1002/syn.20873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran TT, Chowanadisai W, Crinella FM, Chicz-DeMet A, Lönnerdal B. Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status. In: NeuroToxicology.; 2002. doi: 10.1016/S0161-813X(02)00091-8 [DOI] [PubMed] [Google Scholar]
- 52.Agency for Toxic Substances & Disease Registry (ATSDR). Toxicological Profile for Manganese.
- 53.Bauer JA, Devick KL, Bobb JF, et al. Associations of a metal mixture measured in multiple biomarkers with IQ: Evidence from italian adolescents living near ferroalloy industry. Environ Health Perspect. 2020. doi: 10.1289/EHP6803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coetzee DJ, McGovern PM, Rao R, Harnack LJ, Georgieff MK, Stepanov I. Measuring the impact of manganese exposure on children’s neurodevelopment: Advances and research gaps in biomarker-based approaches. Environ Heal A Glob Access Sci Source. 2016. doi: 10.1186/s12940-016-0174-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gunier RB, Mora AM, Smith D, et al. Biomarkers of manganese exposure in pregnant women and children living in an agricultural community in California. Environ Sci Technol. 2014. doi: 10.1021/es503866a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilschefski S, Baxter M. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin Biochem Rev. 2019. doi: 10.33176/aacb-19-00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi AL, Cordier S, Weihe P, Grandjean P. Negative confounding in the evaluation of toxicity: The case of methylmercury in fish and seafood. Crit Rev Toxicol. 2008;38(10):877–893. doi: 10.1080/10408440802273164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weisskopf MG, Seals RM, Webster TF. Bias amplification in epidemiologic analysis of exposure to mixtures. Environ Health Perspect. 2018. doi: 10.1289/EHP2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stiles KM, Bellinger DC. Neuropsychological correlates of low-level lead exposure in school-age children: A prospective study. Neurotoxicol Teratol. 1993;15(1):27–35. doi: 10.1016/0892-0362(93)90042-M [DOI] [PubMed] [Google Scholar]
- 60.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990. doi: 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


