Abstract
This prospective multicenter study, established by the Japanese Ministry of Health, Labour and Welfare and involving 27 institutions, aimed to compare postoperative outcomes between laminoplasty (LM) and posterior fusion (PF) for cervical ossification of the posterior longitudinal ligament (OPLL), in order to address the controversy surrounding the role of instrumented fusion in cases of posterior surgical decompression for OPLL. 478 patients were considered for participation in the study; from among them, 189 (137 and 52 patients with LM and PF, respectively) were included and evaluated using the Japanese Orthopaedic Association (JOA) scores, the JOA Cervical Myelopathy Evaluation Questionnaire (JOACMEQ), and radiographical measurements. Basic demographic and radiographical data were reviewed, and the propensity to choose a surgical procedure was calculated. Preoperatively, there were no significant differences among the participants in terms of patient backgrounds, radiographical measurements (K-line or cervical alignment on X-ray, OPLL occupation ratio on computed tomography, increased signal intensity change on magnetic resonance imaging), or clinical status (JOA score and JOACMEQ) after adjustments. The overall risk of perioperative complications was found to be lower with LM (odds ratio [OR] 0.40, p = 0.006), and the rate of C5 palsy occurrence was significantly lower with LM (OR 0.11, p = 0.0002) than with PF. The range of motion (20.91° ± 1.05° and 9.38° ± 1.24°, p < 0.0001) in patients who had PF was significantly smaller than in those who had LM. However, multivariable logistic regression analysis showed no significant difference among the participants in JOA score, JOA recovery rate, or JOACMEQ improvement at two years. In contrast, OPLL progression was greater in the LM group than in the PF group (OR 2.73, p = 0.0002). Both LM and PF for cervical myelopathy due to OPLL had resulted in comparable postoperative outcomes at 2 years after surgery.
Subject terms: Neurological disorders, Outcomes research
Introduction
Ossification of the posterior longitudinal ligament (OPLL), defined as heterotopic bone formation in the posterior longitudinal ligament1, is a common cause of degenerative cervical myelopathy (DCM)2. Surgical decompression is indicated in cases of moderate and severe myelopathy (modified Japanese Orthopaedic Association (JOA) score ≤ 14)3; however, patients with OPLL are at a higher risk of perioperative complications than patients with other forms of DCM4. Surgical intervention includes anterior, posterior, or combined approaches, but the posterior approach is predominantly chosen for surgical treatment with multilevel decompression (≥ 3 segments), because of the high rate of complications with the anterior approach and combined approaches5.
Posterior surgeries include laminoplasty (LP) and laminectomy with fusion (PF); however, the optimal technique remains debatable6,7. LP is recognized as a standard technique for the treatment of cervical multi-segment DCM, and a more satisfactory long-term outcome has been reported with LP in cases of cervical OPLL than with PF8–10. In contrast, however, some patients demonstrated poor results11, especially those who were K-line (−), and/or those with a high percentage of ossification occupation rate12–14. In addition, long-term follow-up has revealed increased ossification after LP, leading to reoperation15,16. PF, meanwhile, is another posterior procedure that has become widely used with the development of instruments17. PF has a low risk of kyphotic change and ossification progression after surgery, and physicians believe that PF is preferable to LP in cases with severe ossification and/or in those that are K-line (−)6, but sufficient scientific evidence has not been accumulated. Systematic review and meta-analysis comparing these procedures have shown equivalent postoperative results; however, the details are unclear because there is data only from a limited number of small-scale prospective studies6. In particular, the size and types of OPLL have a considerable impact on the severity of myelopathy and the postoperative course, which makes comparisons difficult18,19. Put simply, the two techniques must be compared under equal conditions, and with the fact that severe cases are more commonly dealt with via PF taken into account.
Thus, the objective of the present study was to compare postoperative outcomes between LP and PF for cervical OPLL in a propensity score-matched analysis adjusted for baseline factors and radiographical characteristics of spinal cord compression.
Methods
This nationwide, multicenter, longitudinal study involved 28 academic institutions affiliated with the Japanese Multicenter Research Organization for Ossification of the Spinal Ligament formed by the Japanese Ministry of Health, Labour and Welfare. This study was approved by the Ethics Committee of Tokyo Medical and Dental University (M2000-1963), and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent when registered for possible participation. In total, 478 Japanese patients with cervical OPLL were prospectively enrolled between April 2015 and July 2017. Data were analyzed after obtaining approval from the Ethics Committee of all participating institutions. Patients were eligible for inclusion in the present study if they (1) were 20 years old or older; (2) had imaging evidence of OPLL on computed tomography (CT) and spinal cord compression on magnetic resonance imaging (MRI); and (3) had undergone surgery. All patients had surgical decompression for the cervical OPLL performed on them, and the attending surgeon determined the surgical approach and the number of levels to decompress. A total of 369 cases were initially enrolled in this study, among which 260 and 109 underwent LP and PF, respectively. We excluded 113 patients with preoperative comorbidities affecting neurological and lower-limb function, including 28 patients with a thoracolumbar spine surgery, 15 with limb joint surgery, 10 with mental illnesses requiring medications, 7 with spinal cord injury, 3 with Parkinson’s disease, 3 with cervical spondylotic amyotrophy, 2 with radiculopathy without myelopathy, and 45 other neurological or locomotive diseases. In addition, 39 patients with incomplete questionnaire data and 28 patients with incomplete radiographical followed-up data were excluded. Finally, the remaining 189 patients were included in the current study (Fig. 1 and Table 1).
Figure 1.
Patient selection flowchart.
Table 1.
Demographic parameters, preoperative radiographical parameters and quality of life in the original sample (before weighting) of patients undergoing laminoplasty or posterior spinal fusion.
| Laminoplasty (n = 137) | Posterior spinal fusion (n = 52) | p | |
|---|---|---|---|
| Age (years) | 64.2 ± 11.6 | 63.9 ± 10.6 | 0.85 |
| Sex (male ratio: %) | 70.1 | 73.1 | 0.68 |
| Body mass index | 25.0 ± 3.7 | 26.4 ± 4.6 | 0.03 |
| Smoking history (%) | 32.8 | 44.2 | 0.15 |
| Duration of symptoms (months) | 46.5 ± 74.7 | 40.9 ± 55.7 | 0.63 |
| Levels of OPLL | |||
| C1–2 (%) | 3.6 | 1.9 | |
| C2–3 (%) | 32.1 | 50.0 | |
| C3–4 (%) | 60.6 | 71.1 | |
| C4–5 (%) | 84.7 | 84.6 | |
| C5–6 (%) | 84.7 | 80.8 | |
| C6–7 (%) | 43.8 | 46.2 | |
| C7–T1 (%) | 5.1 | 26.9 | 0.009 |
| Levels of surgery | 3.4 ± 0.9 | 4.9 ± 1.5 | < 0.0001 |
| Comorbidities | |||
| Diabetes mellitus (%) | 29.2 | 40.4 | 0.14 |
| Hypertension (%) | 40.9 | 30.8 | 0.20 |
| Malignancy (%) | 5.8 | 5.8 | 0.99 |
| Cerebrovascular disease (%) | 7.3 | 3.8 | 0.39 |
| Myocardial infarction (%) | 2.9 | 7.7 | 0.16 |
| Collagen disease (%) | 0.7 | 3.8 | 0.17 |
| Drug | |||
| Anticoagulant | 11.7 | 13.5 | 0.74 |
| Radiographical measurements | |||
| Cervical lordosis (°) | 11.5 ± 11.4 | 6.5 ± 11.4 | 0.008 |
| Range of motion (°) | 26.3 ± 12.5 | 24.9 ± 14.7 | 0.50 |
| K-line (+/−) (%) | 73 | 59.6 | 0.08 |
| Thickness of ossification (mm) | 4.9 ± 1.6 | 6.3 ± 1.7 | < 0.0001 |
| Spinal canal occupation ratio > 60% (%) | 6.6 | 34.6 | < 0.0001 |
| Increased signal intensity on MRI (%) | 85.4 | 90.4 | 0.37 |
| JOA score | 11.1 ± 2.5 | 9.9 ± 3.2 | 0.007 |
| JOACMEQ | |||
| Cervical function | 63.0 ± 27.7 | 53.8 ± 31.0 | 0.0501 |
| Upper limb function | 71.9 ± 21.9 | 64.1 ± 30.2 | 0.051 |
| Lower limb function | 56.3 ± 29.2 | 49.2 ± 30.6 | 0.14 |
| Bladder function | 73.3 ± 20.0 | 72.0 ± 26.7 | 0.71 |
| Quality of life | 44.6 ± 17.3 | 43.1 ± 20.0 | 0.62 |
| VISUAL analog scale | |||
| Pain or stiffness in the neck or shoulder | 37.7 ± 32.0 | 50.0 ± 30.6 | 0.02 |
| Tightness in the chest | 9.7 ± 20.9 | 13.0 ± 20.3 | 0.03 |
| Pain or numbness in the arms or hands | 58.4 ± 31.1 | 68.8 ± 25.8 | 0.046 |
| Pain or numbness from chest to toe | 40.8 ± 33.4 | 51.4 ± 29.8 | 0.33 |
Summary statistics for continuous variables are means and standard deviations. OPLL ossification of posterior longitudinal ligament, JOA Japanese Orthopaedic Association, JOACMEQ Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire, MRI magnetic resonance imaging.
Surgical procedures
Laminoplasty
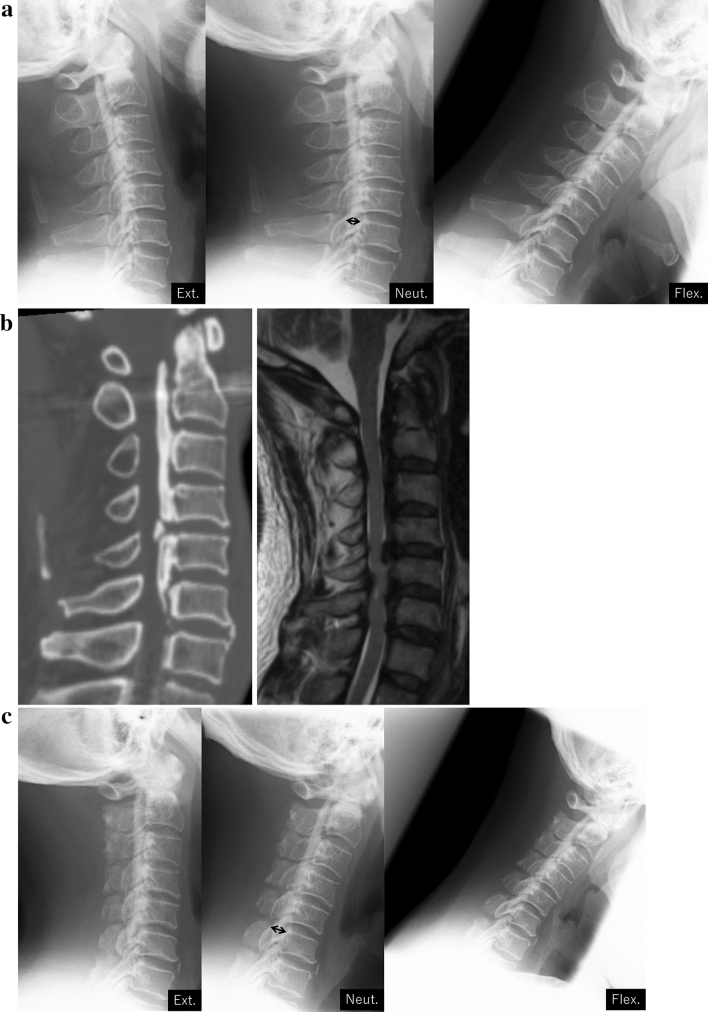
Open- (25 cases) or French-door (112 cases) laminoplasty was performed20,21. A midline incision was made directly above the laminae, followed by detachment of the bilateral paravertebral muscles from the spinous processes. After cutting away the spinous processes, gutters were created on the bilateral laminae using a high-speed drill at the border of the laminae and facets. In the open-door laminoplasty cases, laminae on one side were completely cut to create an opening, and those on the other side were partially cut, preserving the ventral cortex, to prepare for a hinge. After this, the laminae were gradually opened. In the French-door laminoplasty cases, the center of the laminae was cut using a high-speed drill, in addition to bilateral gutters. After the halves of the laminae were elevated, a graft material was tied to bridge the bilateral edges of the laminae (Fig. 2).
Figure 2.
A representative case of cervical laminoplasty. (a): preoperative functional X-rays. The thickness of OPLL was 7.1 mm (double-arrow). The neutral position and range of motion at C2-C7 were 9° and 40°. (b) Preoperative CT and MRI sagittal images. (c) Two years postoperative functional X-rays. The thickness of OPLL was 9.4 mm (double-arrow). The neutral position and range of motion at C2–C7 were 3° and 25°.
Posterior spinal fusion
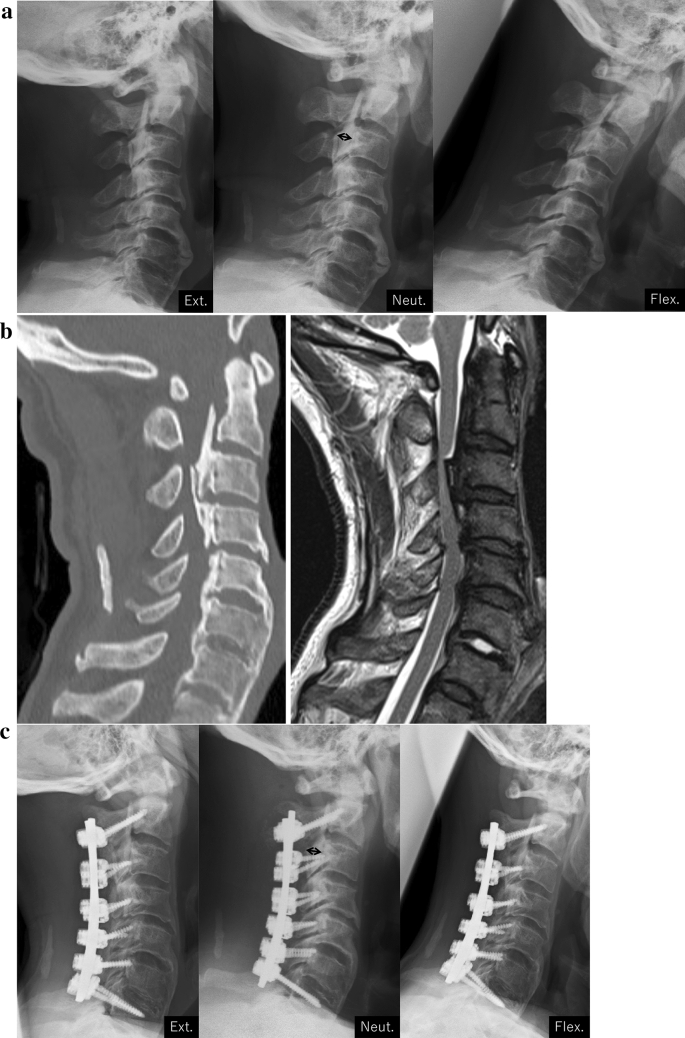
Posterior spinal fusion using the pedicle screw and/or lateral mass screw system was performed22. With respect to surgical decompression, laminectomy or laminoplasty was performed in accordance with each facility’s policies (Fig. 3).
Figure 3.
A representative case of cervical posterior fusion. (a) Preoperative functional X-rays. The thickness of OPLL was 8.0 mm (double-arrow). The neutral position and range of motion at C2–C7 were 11° and 20°. (b) Preoperative CT and MRI sagittal images. (c) 2 years postoperative functional X-rays. The thickness of OPLL was 7.4 mm (double-arrow). The neutral position and range of motion at C2–C7 were 19° and 0°.
Data collection
Data were collected for each participant, including demographic information, symptomatology, causative pathology, and surgical summary. Functional impairment, disability, and quality of life (QOL) were also evaluated preoperatively and at 24 months postoperatively. Basic demographic and clinical data, including age, sex, diabetes status, body mass index (BMI), smoking history, and disease duration, were collected for each patient.
Evaluation
Clinical assessments
Clinical status was evaluated using the Japanese Orthopaedic Association (JOA) cervical score and the Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ) preoperatively and at two years post-surgery. The recovery rate based on JOA scores was calculated as follows: (postoperative JOA − preoperative JOA)/(17 − preoperative JOA) × 10023. The minimum clinically important difference (MCID) for the JOA score and the JOA recovery rate was defined as 2.5 points and 52.8%, respectively24. The JOACMEQ, meanwhile, includes 24 questions covering the domains of cervical function, upper limb function, lower limb function, bladder function, and QOL25; scores for each domain were calculated according to official guidelines and range from 0 to 100 points, with a higher score indicating better health status. The JOACMEQ also incorporates visual analog scale (VAS) scores for pain or stiffness in the neck or shoulder, tightness in the chest, pain or numbness in the arms or hands, and pain or numbness from chest to toe. We evaluated improvement in each of the five JOACMEQ domains. Clinically significant improvement was confirmed if (1) the patient answered all the questions necessary to calculate the functional score for a domain and an increase of ≥ 20 points was obtained for that score, or (2) the functional score after treatment was > 90 points, even if answers for any unanswered questions were assumed to be the worst possible answers.
Each attending surgeon was also required to record all adverse events throughout the study period. A central panel of investigators classified each adverse event in relation to the surgery; any discrepancies among reviewers were resolved by consulting source documents. Perioperative complications were defined as surgery-related events occurring within 30 days of surgery.
Radiographical assessments
The most compressed level and the presence of a signal intensity change in the spinal cord were also investigated on mid-sagittal MRI.
The number of ossification levels (longitudinal extent of OPLL), K-line (positive or negative)7, JOA welfare classification (continuous, segmental, mixed, and circumscribed)4, spinal canal occupation ratio of OPLL on axial CT at the maximum cord compression level, and signal intensity change on T2 weight MRI were investigated.
The cervical lordotic angle (C2–7 angle) and range of motion (ROM) in flexion–extension were also measured, using tangential lines drawn on the posterior edge of the C2 and C7 vertebral bodies on lateral radiographs taken in a neutral position.
Statistical analysis
Propensity score methods were used to estimate treatment effects from the observational data in the present study. A backward elimination logistic regression model was created to estimate the probability of treatment assignment, including all the relevant baseline variables with p-values less than 0.25. In order to balance the distribution of baseline variables between treatment groups, a pseudo sample was created by weighting standardized inverse probability of treatment, in which we replaced extreme values of weight with those of the 1st and 99th percentiles. The Student t-test and Chi-square test were used to compare differences in means and proportions, respectively, of baseline covariates in the weighted sample. The covariates that were found to be different between the two groups with p-values less than 0.05 were further adjusted in the generalized linear model to compare treatment effects 30 days and 2 years after the operation. We calculated odds ratios (ORs) and the 95% confidence intervals (95% CIs) for the dichotomized indices, taking PF as the reference, and mean differences for the continuous variables adjusted for age, sex, and unbalanced variables. A two-sided p-value less than 0.05 was considered statistically significant. Statistical analysis was conducted using SAS version 9.4 (SAS Institute. Inc, Cary, NC, USA).
Results
Table 2 shows demographic parameters, preoperative radiographical parameters, and QOL in the weighed sample (post propensity score matching) of patients undergoing LP or PF. There was no significant difference in age, sex, BMI, duration of symptoms, smoking history, or anticoagulant drug use. Concerning comorbidities, although there was no significant difference between the two groups with regard to diabetes mellitus, hypertension, malignancy, myocardial infarction, or collagen disease, the frequency of cerebrovascular disease was higher in the LP group than in the PF group (7.4% and 1.4%, respectively, p = 0.04). Baseline radiographical measurements were not significantly different between the groups, as assessed by confirming degree of cervical lordosis, ROM, K-line, thickness of ossification, spina canal occupation ratio of the OPLL > 60%, and increased signal intensity on MRI. Baseline functional status and QOL were also not significantly different, as assessed by JOA score or JOACMEQ. The two exceptions were the bladder function score in JOACMEQ, which was significantly higher in the PF group than in the LP group (mean (standard deviation, SD) of 79.7 (33.6) compared with 72.8 (20.5); p = 0.03) and VAS pain or numbness level in the arms or hands in JOACMEQ, which was significantly higher in the PF group than in the LP group (mean (SD) of 68.0 (32.8) compared with 59.9 (32.4); p = 0.048).
Table 2.
Demographic parameters, preoperative radiographical parameters, and quality of life in the weighed sample of patients undergoing laminoplasty or posterior spinal fusion.
| Laminoplasty | Posterior spinal fusion | p | |
|---|---|---|---|
| Age (year) | 64.3 ± 11.9 | 66.7 ± 14.7 | 0.13 |
| Sex (male ratio: %) | 70.7 | 67.4 | 0.55 |
| Body mass index | 25.0 ± 3.7 | 24.8 ± 5.4 | 0.75 |
| Smoking history (%) | 33.2 | 37.2 | 0.50 |
| Duration of symptoms (months) | 44.4 ± 75.1 | 41.9 ± 81.7 | 0.80 |
| Levels of OPLL | |||
| C1–2 (%) | 2.2 | 3.8 | |
| C2–3 (%) | 35.8 | 38.5 | |
| C3–4 (%) | 62.8 | 67.3 | |
| C4–5 (%) | 83.2 | 84.6 | |
| C5–6 (%) | 86.1 | 90.4 | |
| C6–7 (%) | 38.0 | 48.1 | |
| C7–T1 (%) | 6.6 | 13.5 | 0.86 |
| Levels of surgery | 3.5 ± 0.9 | 3.6 ± 2.3 | 0.96 |
| Comorbidities | |||
| Diabetes mellitus (%) | 30.6 | 35.3 | 0.43 |
| Hypertension (%) | 41.6 | 36.9 | 0.45 |
| Malignancy (%) | 6.1 | 4.1 | 0.47 |
| Cerebrovascular disease (%) | 7.4 | 1.4 | 0.04 |
| Myocardial infarction (%) | 3.2 | 4.6 | 0.55 |
| Collagen disease (%) | 1.5 | 1.7 | 0.87 |
| Drug | |||
| Anticoagulant | 11.8 | 7.2 | 0.21 |
| Radiographical measurements | |||
| Cervical lordosis (°) | 11.6 ± 11.8 | 10.4 ± 14.4 | 0.42 |
| Range of motion (°) | 26.2 ± 12.7 | 25.6 ± 24.0 | 0.80 |
| K-line (+/−) (%) | 73.6 | 65.2 | 0.14 |
| Thickness of ossification (mm) | 5.1 ± 1.7 | 5.3 ± 2.8 | 0.34 |
| Spinal canal occupation ratio > 60% (%) | 8.6 | 13.2 | 0.24 |
| Increased signal intensity on MRI (%) | 86.2 | 92.0 | 0.14 |
| JOA score | 10.9 ± 2.6 | 10.6 ± 4.4 | 0.55 |
| JOACMEQ | |||
| Cervical function | 61.8 ± 28.0 | 57.0 ± 44.1 | 0.24 |
| Upper limb function | 70.5 ± 22.3 | 65.1 ± 45.6 | 0.15 |
| Lower limb function | 54.9 ± 29.6 | 50.7 ± 47.4 | 0.35 |
| Bladder function | 72.8 ± 20.5 | 79.7 ± 33.6 | 0.03 |
| Quality of life | 44.0 ± 17.5 | 41.8 ± 32.6 | 0.43 |
| Visual analog scale | |||
| Pain or stiffness in the neck or shoulder | 39.0 ± 33.0 | 41.4 ± 46.1 | 0.59 |
| Tightness in the chest | 9.7 ± 21.9 | 8.3 ± 26.2 | 0.63 |
| Pain or numbness in the arms or hands | 59.9 ± 32.4 | 68.0 ± 32.8 | 0.048 |
| Pain or numbness from chest to toe | 42.2 ± 34.4 | 47.5 ± 52.9 | 0.29 |
Summary statistics for continuous variables are means and standard deviations. JOA Japanese Orthopedic Association, JOACMEQ Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire, MRI magnetic resonance imaging.
Overall, perioperative complications were significantly less common in the LP group than in the PF group (17.0% and 29.7%, respectively, p = 0.02). In particular, C5 palsy was significantly less frequent in the LP group than in the PF group (3.9% and 19.9%, p = 0.0002); however, there was no significant difference in dural tear, wound disruption, wound infection, or revision surgery. Multivariable logistic regression analysis showed that overall complications (OR 0.40, 95% CI 0.21–0.77, p = 0.006) and C5 palsy (OR 0.11, 95% CI 0.03–0.34, p = 0.0002) were significantly less common in the LP group than in the PF group (Table 3).
Table 3.
Logistic regression analysis for complications and revision surgery in the weighted sample of patients undergoing laminoplasty or posterior spinal fusion.
| OR | 95% CI | p | |
|---|---|---|---|
| Overall complications | 0.40 | 0.21–0.77 | 0.006 |
| C5 palsy | 0.11 | 0.03–0.34 | 0.0002 |
| Dural tear | 1.99 | 0.28–14.1 | 0.49 |
| Wound disruption | 0.26 | 0.02–3.62 | 0.32 |
| Deep wound infection | 0.54 | 0.11–2.60 | 0.44 |
| Superficial wound infection | 0.24 | 0.03–1.73 | 0.16 |
| Revision surgery | 0.31 | 0.01–11.10 | 0.52 |
Weighted for IPTW and further adjusted for baseline age, sex, VAS (pain or numbness in the arms or hands), preoperative JOACMEQ (bladder function), preoperative comorbidity (cerebrovascular disease).
IPTW inverse probability of treated weighting, JOACMEQ the Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire, OR odds ratio, CI confidence intervals, VAS visual analogue scale.
Patients achieved similar postoperative functional and QOL outcomes, judging by JOA scores, JOA recovery rates, and JOACMEQ scores (Table 4). There were also no significant radiographical differences between the groups (Table 4). ROM and JOACMEQ cervical function score were significantly lower in the PF group than in the LP group (20.91 ± 1.05 and 9.38 ± 1.24, p < 0.0001; 67.64 ± 2.78 and 55.46 ± 3.29, p = 0.005, respectively) (Table 4). Multivariable logistic regression analysis showed no significant difference in the relationship between JOA score and MCID (JOA score > MCID in both groups) or JOA recovery rate and MCID (JOA recovery rate > MCID in both groups), or clinically significant improvement in JOACMEQ (Table 5). The percentage of patients with progression in the thickness of OPLL was significantly higher in the LP group than in the PF group (56.0% vs. 30.5% in PF; OR 2.73, 95% CI 1.60–4.67, p = 0.0002) (Table 5).
Table 4.
Two years postoperative radiographical parameters and quality of life in the weighted sample of patients undergoing laminoplasty or posterior spinal fusion.
| Laminoplasty | Posterior spinal fusion | p | |
|---|---|---|---|
| Radiographical measurements | |||
| Cervical lordosis (°) | 9.69 ± 1.10 | 7.66 ± 1.30 | 0.24 |
| Range of motion (°) | 20.91 ± 1.05 | 9.38 ± 1.24 | < 0.0001 |
| Thickness of ossification (mm) | 6.11 ± 0.54 | 4.80 ± 0.64 | 0.12 |
| JOA score | 13.73 ± 0.21 | 13.72 ± 0.25 | 0.97 |
| JOA RR | 45.84 ± 3.08 | 51.15 ± 3.65 | 0.27 |
| JOACMEQ | |||
| Cervical function | 67.64 ± 2.78 | 55.46 ± 3.29 | 0.005 |
| Upper limb function | 80.45 ± 1.76 | 77.53 ± 2.08 | 0.29 |
| Lower limb function | 63.44 ± 2.44 | 56.19 ± 2.89 | 0.058 |
| Bladder function | 77.19 ± 1.62 | 76.12 ± 1.92 | 0.67 |
| Quality of life | 53.63 ± 1.64 | 50.77 ± 1.94 | 0.27 |
| Visual analog scale | |||
| Pain or stiffness in the neck or shoulder | 35.85 ± 2.78 | 38.18 ± 3.29 | 0.59 |
| Tightness in the chest | 9.94 ± 1.80 | 7.80 ± 2.13 | 0.89 |
| Pain or numbness in the arms or hands | 39.84 ± 2.70 | 44.20 ± 3.20 | 0.30 |
| Pain or numbness from chest to toe | 33.10 ± 2.83 | 34.78 ± 3.35 | 0.70 |
Values are means and standard-error. Values are weighted for IPTW and further adjusted for baseline age, sex, VAS (pain or numbness in the arms or hands), preoperative CMEQ (bladder function), preoperative comorbidity (cerebrovascular disease).
JOA Japanese Orthopaedic Association, JOACMEQ Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire, IPTW inverse probability of treated weighting, VAS visual analogue scale.
Table 5.
Logistic regression analysis for postoperative functional outcome and progression of OPLL in in the weighted sample of patients undergoing laminoplasty or posterior spinal fusion.
| OR | 95% CI | p | |
|---|---|---|---|
| JOA score > MCID | 0.98 | 0.57–1.68 | 0.94 |
| JOA RR > MCID | 0.88 | 0.50–1.53 | 0.64 |
| Clinical improvement of JOACMEQ | |||
| Cervical function | 1.05 | 0.58–1.87 | 0.88 |
| Upper limb function | 1.04 | 0.57–1.89 | 0.90 |
| Lower limb function | 1.75 | 0.89–3.44 | 0.11 |
| Bladder function | 1.16 | 0.59–2.28 | 0.67 |
| Quality of life | 1.88 | 0.95–3.70 | 0.07 |
| Progression of OPLL | 2.73 | 1.60–4.67 | 0.0002 |
Weighted for IPTW and further adjusted for baseline age, sex, VAS (pain or numbness in the arms or hands), preoperative JOACMEQ (bladder function), preoperative comorbidity (cerebrovascular disease).
JOA Japanese Orthopaedic Association, JOACMEQ Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire, OR odds ratio, CI confidence intervals, OPLL ossification of the posterior longitudinal ligament, MCID minimum clinically important difference, IPTW inverse probability of treated weighting, VAS visual analogue scale.
Discussion
This nationwide, multicenter, prospective study provides a comprehensive evaluation of the comparative efficacy of LP and PF for patients with cervical OPLL. There have been reports to date comparing laminoplasty and posterior fusion, but there have been only 3 prospective studies: Lee et al. (laminoplasty 21, posterior fusion 21), Liu et al. (laminoplasty 32, posterior fusion 35), and Bai et al. (laminoplasty 32, posterior fusion 32)6. Therefore, we thought it necessary to evaluate the results in a multicenter study. In addition, there have been no evaluations of QOL in this connection, and so we compared the JOACMEQ results as patient-reported outcome measurement (PROM) for cervical, upper limb, lower limb, bladder function, quality of life, and neck pain. The results indicated comparable improvement among patients with OPLL experiencing both surgical procedures at two years post-operation. The exceptions to this were the JOACMEQ cervical function scores and the cervical ROM on X-ray (Table 4), which were lower and smaller, respectively, in the patients with PF than in those with LP. In contrast, logistic regression analysis revealed no clinically significant difference in the improvement of JOACMEQ cervical function (Table 5), suggesting that the choice of PF or LP might have only a limited effect on the outcome for cervical function.
A relatively high incidence of surgical complications in cases of cervical OPLL compared with other forms of DCM has been reported; neurologic deficit and neck pain are particularly common in posterior procedures4,26. In the current study, the overall complication rate (OR 0.40) and the rate of C5 palsy occurrence (OR 0.11) were significantly lower with LP than with PF. There was no significant difference between the two techniques in the laterality of C5 palsy (16% of C5 palsy in laminoplasty cases was bilateral, but all other C5 palsy, including the C5 palsy that occurred with posterior fixation, was unilateral) or postoperative recovery (16% and 25% of patients experienced incomplete recovery in laminoplasty- and posterior fusion cases, respectively), and pathological differences in C5 palsy could not be clarified in this study. However, iatrogenic foraminal stenosis and larger posterior shift of the spinal cord might be associated with a higher risk of C5 palsy in PF27,28. Takemitsu et al. reported that the risk of postoperative C5 palsy with posterior instrumentation was 11.6 times greater than without instrumentation29, and this relative risk was similar to the current result. However, the actual incidence of C5 palsy after posterior procedures remains unclear, with some retrospective studies having reported it as 0%–30% and 2.6%–50% with LP and PF, respectively27–31. This prospective study, however, demonstrated that the risk of C5 palsy was 10 times higher with posterior instrumentation; thus, prophylactic foraminotomy might be recommended to reduce C5 palsy in cases with PF30,31. Since this study did not investigate whether or not prophylactic foraminotomy was performed, further investigation is needed to determine the usefulness of concomitant foraminotomy.
Neck pain was reported as another common postoperative complication in cases of cervical OPLL, and is more common in such cases than in cases of other forms of DCM4. Postoperative cervical kyphotic alignment might be associated with neck pain. Few studies have compared these complications between different posterior procedures in cases of cervical OPLL. However, in the present study, pain or stiffness in the neck or shoulder was comparable with both procedures (Table 4). Prior to the current study, we hypothesized that postoperative kyphotic alignment change was more frequent with LP than with PF, something which might be associated with postoperative neck pain. There were, however, no differences in this study in radiographical alignment or degree of postoperative neck pain between the two groups. Machino et al. reported that the kyphotic alignment change after laminoplasty was observed in 7.2% of 457 cases with preoperative lordotic alignment, and the incidence of postoperative kyphotic change was relatively low32. Although kyphotic change after LP was reported in another report33, cervical alignment is maintained in the majority of cases if patient selection is appropriate. It has also been reported that the angle of the T1 slope is important as an indicator of appropriate patient selection, i.e. for choosing patients who are less likely to display kyphotic changes after laminoplasty, but the relationship between T1 slope and postoperative kyphosis is still debated33,34. Recent studies have shown that postoperative kyphosis occurs in patients with low cervical extension function35,36. The risk of kyphosis may be higher in patients with extension range of motion (ROM) (extension—neutral C2-7 Cobb’s angle) of less than 14°35 or a gap ROM (flexion ROM—extension ROM) of greater than 27°36. Therefore, preoperative functional imaging of the cervical spine should be evaluated, and laminoplasty should be indicated in patients with a low risk of kyphosis, as reported by Lee and Fujishiro et al.35,36.
With respect to functional and QOL outcomes at two years post-surgery, there were significant differences in the average scores for the JOACMEQ cervical function domain section and cervical ROM on X-ray (Table 4). The JOACMEQ cervical function score is based on answers to four questions about daily life activities that require up-and-down movement and rotation of the neck37. PF was found to have reduced ROM, which might be associated with lower JOACMEQ cervical function score. Although JOACMEQ has criteria for determining whether these differences are clinically meaningful, multivariate analysis showed no significant differences in postoperative improvement of cervical function. Therefore, the effect on QOL was considered to be limited, although there was a numerical difference. Furthermore, although the perioperative complication of C5 palsy was more frequent in cases when PF was performed, the majority of C5 palsy cases showed improvement at two-years post-surgery. Therefore, it can be said that this complication was not associated with significant negative outcomes at two years post-surgery.
Although reoperation for the progression of OPLL after LP was not observed in the two postoperative years reviewed in the current study, revision surgery around 10 years after LP has been reported15,16. OPLL progression is commonly observed after LP8,9; 70% of patients in one study showed an increase in OPLL size 10 years after surgery16. The size of ossification commonly increases in young adults, those with continuous- or mixed-type OPLL, and those with kyphotic alignment change15,16. Takatsu et al. reported that mechanical stress after laminectomy may be the cause of OPLL progression38, and Ando et al. reported that micromotion at the site of ossification is involved in ossification progression, because fixation halted ossification progression39. Therefore, based on the current results as well, it is highly likely that micromotion after laminoplasty will promote ossification, and that fixation will inhibit ossification within the area of fixation. However, since there is a possibility that ossification may develop outside the area of fixation, further study is needed to determine the usefulness of the surgical technique in long-term follow-up.
Strengths and limitations
The findings of this study are likely to be more generalizable than findings from single-center studies, since patients were prospectively enrolled at 24 multicenter sites. The large number of recruitment sites allowed us to evaluate outcomes for 189 patients with OPLL who received LP or PF. In addition, we evaluated outcomes using different radiographical and questionnaire tools, allowing for a comprehensive assessment of surgical outcomes in patients with OPLL.
This study has several limitations, however. First, a 26% attrition rate was observed at the two-year post-surgery. This was a result of the exclusion of cases with even one missing response to a quality-of-life questionnaire item, or missing x-ray or CT data. If clinical research coordinators could be appointed in each hospital to conduct detailed checks of the radiographical assessments and response results of research subjects, it would be possible to reduce the amount of missing data, but the high cost of doing so would be a major problem. Second, this is not an international study, and clinical outcomes might differ depending on race and ethnicity. Third, a standardized surgical protocol was not utilized across centers, and decisions about the approach, number of decompressed levels, and use of instrumentation and fusion were made at the discretion of the attending surgeon. Some baseline covariates were unbalanced between the two treatment groups in the weighted sample, and there may be residual systematic differences for other measured and unmeasured baseline covariates, which may yield a biased estimation of treatment effects. Although a backward elimination logistic regression model was used for baseline adjustment, PF was performed in more severe cases with less cervical lordosis or with larger spinal canal occupation of OPLL compared to LP. Therefore, a future prospective large-scale multicenter international study is needed to validate the current results. Fourth, the Open- and French-door laminoplasty techniques are grouped as the same laminoplasty in the current study, but complications and functional improvement may vary20. Although there was no significant difference in clinical outcomes and complications using either procedure in our previous paper which involved the same cohort40, future large cohort studies need a separate analysis for the two procedures. Fifth, the work in the present study, conducted at 28 academic institutions, was performed by highly experienced surgeons, but the number of years of experience of each of the surgeons is unknown. We cannot deny the possibility that the difference in experience of the surgeons between the two techniques may have affected the results. Lastly, in considering the usefulness of surgical treatment, a comparison with the natural course of cervical OPLL progression must be made. However, the present data set was not compared with data for the natural course. We are currently investigating the natural course of cervical OPLL in a multicenter study, which will make it possible to report on comparative results for quality of life in the future.
In conclusion, cervical LP and PF provide almost comparable functional and QOL improvements at two years after surgery, although perioperative complications were more numerous in cases where PF was performed.
Acknowledgements
This work was supported by Health and Labour Science Research grants (H29-nanchi(nan)-ippan-040) and by a grant from the Japan Agency for Medical Research and Development (16ek0109136h0002). There are no other financial associations that may be relevant or seen as relevant to this work.
Author contributions
H.N., S.I., T.Y., M.K., Y.L., and H.Y. designed the study; H.N., S.I., T.Y., S.E., K.S., K.K., Y.N., T.H., K.W., K.K., K.F., A.K., T.F., T.K., Y.N., Y.O., N.N., K.A., M.T., K.M., H.N., K.M., S.M., T.K., K.Y., S.K., S.K., T.O., S.I., S.F., H.K., H.K., and M.K. collected the data; H.N., S.I., T.Y., M.K., Y.L., and H.Y. analyzed and interpreted the data; H.N., S.I., T.Y., M.K., Y.L., and H.Y. wrote the initial draft of the manuscript; H.N., Y.L., and H.Y. performed the statistical analyses. M.Y., A.O., and Y.K. supervised the study. M.M., M.Y., and A.O. acquired the funding. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Hiroaki Nakashima, Email: hirospine@med.nagoya-u.ac.jp.
Japanese Multicenter Research Organization for Ossification of the Spinal Ligament:
Hiroaki Nakashima, Shiro Imagama, Toshitaka Yoshii, Satoru Egawa, Kenichiro Sakai, Kazuo Kusano, Yukihiro Nakagawa, Takashi Hirai, Kanichiro Wada, Keiichi Katsumi, Kengo Fujii, Atsushi Kimura, Takeo Furuya, Tsukasa Kanchiku, Yukitaka Nagamoto, Yasushi Oshima, Narihito Nagoshi, Kei Ando, Masahiko Takahata, Kanji Mori, Hideaki Nakajima, Kazuma Murata, Shunji Matsunaga, Takashi Kaito, Kei Yamada, Sho Kobayashi, Satoshi Kato, Tetsuro Ohba, Satoshi Inamia, Shunsuke Fujibayashi, Hiroyuki Katoh, Haruo Kanno, Masao Koda, Yoshiharu Kawaguchi, Katsushi Takeshita, Morio Matsumoto, Masashi Yamazaki, and Atsushi Okawa
References
- 1.Hashizume Y. Pathological studies on the ossification of the posterior longitudinal ligament (opll) Acta Pathol. Jpn. 1980;30:255–273. doi: 10.1111/j.1440-1827.1980.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 2.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology genetics and pathogenesis. Spine. 2015 doi: 10.1097/brs.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 3.Fehlings MG, et al. Change in functional impairment, disability, and quality of life following operative treatment for degenerative cervical myelopathy: A systematic review and meta-analysis. Glob. Spine J. 2017;7:53s–69s. doi: 10.1177/2192568217710137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakashima H, et al. Comparison of outcomes of surgical treatment for ossification of the posterior longitudinal ligament versus other forms of degenerative cervical myelopathy. J. Bone Joint Surg. Am. 2016;98:370–378. doi: 10.2106/JBJS.O.00397. [DOI] [PubMed] [Google Scholar]
- 5.Kato S, Ganau M, Fehlings MG. Surgical decision-making in degenerative cervical myelopathy: Anterior versus posterior approach. J. Clin. Neurosci. 2018;58:7–12. doi: 10.1016/j.jocn.2018.08.046. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, et al. Comparison of laminoplasty versus laminectomy and fusion in the treatment of multilevel cervical ossification of the posterior longitudinal ligament: A systematic review and meta-analysis. Medicine. 2018;97:e11542. doi: 10.1097/md.0000000000011542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan X, et al. Comparison of laminectomy and fusion vs laminoplasty in the treatment of multilevel cervical spondylotic myelopathy: A meta-analysis. Medicine. 2019;98:e14971. doi: 10.1097/md.0000000000014971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchi Y, et al. Minimum 10-year followup after en bloc cervical laminoplasty. Clin. Orthop. Relat. Res. 2003;411:129–139. doi: 10.1097/01.blo.0000069889.31220.62. [DOI] [PubMed] [Google Scholar]
- 9.Chiba K, et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy: Average 14-year follow-up study. Spine. 2006;31:2998–3005. doi: 10.1097/01.brs.0000250307.78987.6b. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa Y, et al. Long-term results after expansive open-door laminoplasty for the segmental-type of ossification of the posterior longitudinal ligament of the cervical spine: A comparison with nonsegmental-type lesions. J. Neurosurg. Spine. 2005;3:198–204. doi: 10.3171/spi.2005.3.3.0198. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima H, et al. Prediction of outcome following surgical treatment of cervical myelopathy based on features of ossification of the posterior longitudinal ligament: A systematic review. JBJS Rev. 2017;5:01874474. doi: 10.2106/jbjs.rvw.16.00023. [DOI] [PubMed] [Google Scholar]
- 12.Fujiyoshi T, et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: The K-line. Spine. 2008;33:E990–E993. doi: 10.1097/BRS.0b013e318188b300. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki M, Kawaguchi Y, Kimura T, Yonenobu K. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J. Neurosurg. Spine. 2002;96:180–189. doi: 10.3171/spi.2002.96.2.0180. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki M, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: Part 1: Clinical results and limitations of laminoplasty. Spine. 2007;32:647–653. doi: 10.1097/01.brs.0000257560.91147.86. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima H, et al. Reoperation for late neurological deterioration after laminoplasty in individuals with degenerative cervical myelopathy: Comparison of cases of cervical spondylosis and ossification of the posterior longitudinal ligament. Spine. 2020;45:909–916. doi: 10.1097/BRS.0000000000003408. [DOI] [PubMed] [Google Scholar]
- 16.Chiba K, et al. Multicenter study investigating the postoperative progression of ossification of the posterior longitudinal ligament in the cervical spine: A new computer-assisted measurement. J. Neurosurg. Spine. 2005;3:17–23. doi: 10.3171/spi.2005.3.1.0017. [DOI] [PubMed] [Google Scholar]
- 17.Fehlings MG, et al. Laminectomy and fusion versus laminoplasty for the treatment of degenerative cervical myelopathy: Results from the AOSpine North America and International prospective multicenter studies. Spine J. 2017;17:102–108. doi: 10.1016/j.spinee.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Fujimori T, et al. Long-term results of cervical myelopathy due to ossification of the posterior longitudinal ligament with an occupying ratio of 60% or more. Spine. 2014;39:58–67. doi: 10.1097/BRS.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima H, et al. What are the important predictors of postoperative functional recovery in patients with cervical OPLL? Results of a multivariate analysis. Glob. Spine J. 2018;9:315–320. doi: 10.1177/2192568218794665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakashima H, et al. Comparative effectiveness of open-door laminoplasty versus French-door laminoplasty in cervical compressive myelopathy. Spine. 2014;39:642–647. doi: 10.1097/brs.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 21.Duetzmann S, Cole T, Ratliff JK. Cervical laminoplasty developments and trends, 2003–2013: A systematic review. J. Neurosurg. Spine. 2015;23:24–34. doi: 10.3171/2014.11.spine14427. [DOI] [PubMed] [Google Scholar]
- 22.Mikhail CM, Dowdell JE, 3rd, Hecht AC. Posterior fusion for the subaxial cervical spine: A review of the major techniques. HSS J. 2020;16:188–194. doi: 10.1007/s11420-019-09722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6:354–364. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Kato S, et al. Does posterior scoliosis correction improve respiratory function in adolescent idiopathic scoliosis? A systematic review and meta-analysis. Glob. Spine J. 2019;9:866–873. doi: 10.1177/2192568218811312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukui M, et al. Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ): Part 4. Establishment of equations for severity scores. Subcommittee on low back pain and cervical myelopathy, evaluation of the clinical outcome committee of the Japanese Orthopaedic Association. J. Orthop. Sci. 2008;13:25–31. doi: 10.1007/s00776-007-1194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Dai LY. A systematic review of complications in cervical spine surgery for ossification of the posterior longitudinal ligament. Spine J. 2011;11:1049–1057. doi: 10.1016/j.spinee.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Nakashima H, et al. Multivariate analysis of C-5 palsy incidence after cervical posterior fusion with instrumentation. J. Neurosurg. Spine. 2012;17:103–110. doi: 10.3171/2012.4.spine11255. [DOI] [PubMed] [Google Scholar]
- 28.Hojo Y, et al. A late neurological complication following posterior correction surgery of severe cervical kyphosis. Eur. Spine J. 2011;20:890–898. doi: 10.1007/s00586-010-1590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takemitsu M, Cheung KMC, Wong YW, Cheung WY, Luk KDK. C5 nerve root palsy after cervical laminoplasty and posterior fusion with instrumentation. J. Spinal Disord. Tech. 2008;21:267–272. doi: 10.1097/BSD.0b013e31812f6f54. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima H, et al. Complications of cervical pedicle screw fixation for nontraumatic lesions: A multicenter study of 84 patients. J. Neurosurg. Spine. 2012;16:238–247. doi: 10.3171/2011.11.spine11102. [DOI] [PubMed] [Google Scholar]
- 31.Katsumi K, Yamazaki A, Watanabe K, Ohashi M, Shoji H. Can prophylactic bilateral C4/C5 foraminotomy prevent postoperative C5 palsy after open-door laminoplasty? A prospective study. Spine. 2012;37:748–754. doi: 10.1097/BRS.0b013e3182326957. [DOI] [PubMed] [Google Scholar]
- 32.Machino M, et al. Cervical alignment and range of motion after laminoplasty: Radiographical data from more than 500 cases with cervical spondylotic myelopathy and a review of the literature. Spine. 2012;37:E1243–1250. doi: 10.1097/BRS.0b013e3182659d3e. [DOI] [PubMed] [Google Scholar]
- 33.Kim TH, Lee SY, Kim YC, Park MS, Kim SW. T1 slope as a predictor of kyphotic alignment change after laminoplasty in patients with cervical myelopathy. Spine. 2013;38:E992–997. doi: 10.1097/BRS.0b013e3182972e1b. [DOI] [PubMed] [Google Scholar]
- 34.Kim B, et al. Relationship between T1 slope and loss of lordosis after laminoplasty in patients with cervical ossification of the posterior longitudinal ligament. Spine J. 2016;16:219–225. doi: 10.1016/j.spinee.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, et al. Does extension dysfunction affect postoperative loss of cervical lordosis in patients who undergo laminoplasty? Spine. 2019;44:E456–E464. doi: 10.1097/brs.0000000000002887. [DOI] [PubMed] [Google Scholar]
- 36.Fujishiro T, et al. Gap between flexion and extension ranges of motion: A novel indicator to predict the loss of cervical lordosis after laminoplasty in patients with cervical spondylotic myelopathy. J. Neurosurg. Spine. 2021;35:8–17. doi: 10.3171/2020.10.spine201723. [DOI] [PubMed] [Google Scholar]
- 37.Ando K, et al. Outcomes of surgery for thoracic myelopathy owing to thoracic ossification of the ligamentum flavum in a nationwide multicenter prospectively collected study in 223 patients: Is instrumented fusion necessary? Spine. 2020;45:E170–E178. doi: 10.1097/brs.0000000000003208. [DOI] [PubMed] [Google Scholar]
- 38.Takatsu T, Ishida Y, Suzuki K, Inoue H. Radiological study of cervical ossification of the posterior longitudinal ligament. J. Spinal Disord. 1999;12:271–273. [PubMed] [Google Scholar]
- 39.Ando K, et al. Comparative study of surgical treatment and nonsurgical follow up for thoracic ossification of the posterior longitudinal ligament: radiological and clinical evaluation. Spine. 2017;42:407–410. doi: 10.1097/brs.0000000000001769. [DOI] [PubMed] [Google Scholar]
- 40.Nagoshi N, et al. Comparison of surgical outcomes after open- and double-door laminoplasties for patients with cervical ossification of the posterior longitudinal ligament: A prospective multicenter study. Spine. 2021;46:E1238–e1245. doi: 10.1097/brs.0000000000004094. [DOI] [PubMed] [Google Scholar]