Summary
Background
The early-onset sepsis calculator (EOSC) reduces unnecessary antibiotic treatment in newborns. However, its performance in identifying cases with early-onset disease (EOD) is unclear. We compared the sensitivity of the EOSC to the current Dutch and National Institute for Health and Care Excellence (NICE) guidelines when applied to a cohort of newborns with culture-positive early-onset sepsis and meningitis.
Methods
Culture-positive Streptococcus agalactiae (GBS) and Escherichia coli (E. coli) sepsis and meningitis patients ≤3 days old with a gestational age ≥34 weeks, identified between 1/1/2018 and 31/1/2021 in a Dutch prospective nationwide cohort study were included. Cases were identified by treating physicians and microbiological surveillance. Primary outcome was the proportion of patients that would have been treated according to the EOSC, the Dutch, and the NICE EOD prevention guidelines. Differences between proportions were analysed using McNemar's test.
Findings
We included 81 GBS and 7 E. coli EOD cases. At 4 h after birth, the EOSC would have recommended antibiotic treatment in 32 (36%) patients, compared to 44 (50%) by the Dutch (p<0·01) and 48 (55%) by the NICE guideline (p<0·01). The EOSC would have initially recommended routine care for 52% of patients compared to 31% and 30% for the Dutch and NICE guidelines (p<0·01). At 24 h after birth, the EOSC would have recommended antibiotic treatment in 54 (61%) infants compared to 64 (73%) by the Dutch (p = 0·02) and 63 (72%) by the NICE guidelines (p = 0·06).
Interpretation
The sensitivity of the EOSC in identifying cases of EOD is lower compared to both Dutch and NICE guidelines, especially directly after birth. The EOSC relies more on clinical symptoms and results in less overtreatment of healthy newborns at the cost of later antibiotic treatment in initially well-appearing EOD patients.
Funding
This work was supported by grants received from Netherlands Organization for Health Research and Development (ZonMw; NWO-Vidi-Grant (grant number 917·17·308); NWO-Vici-Grant (grant number 918·19·627)), the Academic Medical Centre (AMC Innovative Impulse Grant) and Steun Emma Foundation Grant.
Key words: early-onset disease (EOD), sepsis, meningitis, sepsis calculator, EOS calculator, newborns, neonates, cohort study
Research in context.
Evidence before this study
We searched PubMed for studies published from 2011 (introduction of the neonatal early-onset sepsis calculator [EOSC]) onwards by searching all fields for (“early-onset sepsis calculator” OR “EOS risk calculator” OR “sepsis risk calculator” or “Kaiser sepsis calculator”) and title and abstract fields for (”early onset sepsis” or ”neonatal sepsis”), with no language restrictions.
A limited number of studies of which most were retrospective, have shown that both the EOSC and the former National Institute for Health and Care Excellence (NICE) guideline have poor sensitivity in identifying early-onset disease (EOD) cases. No studies have compared the EOSC to the current Dutch (2017) and NICE (2021) guidelines.
Added value of this study
To our knowledge, our study used the largest prospective cohort of culture-positive EOD cases to compare the sensitivity of the EOSC to the Dutch and the NICE guidelines. It is also the first study comparing the sensitivity of the EOSC to the new (2021) NICE guideline. In our cohort of 88 culture-positive EOD cases, the EOSC would have recommended antibiotic treatment in 36% directly after birth, which was lower than the sensitivity of the Dutch (50%, p<0·01) and NICE guideline (55%, p<0·01). In 52% of EOD cases, the EOSC would have recommended routine care directly after birth, compared to 31% and 30% for the Dutch (p<0.01) and NICE guidelines (p<0·01), respectively.
Implications of all the available evidence
Our results show that neither the EOSC nor current national guidelines are able to predict EOD with high sensitivity. Although current guidelines result in more antibiotic overtreatment, the EOSC would likely result in later recognition and treatment of EOD patients that initially are asymptomatic. Therefore, better EOD risk stratification methods are needed.
Alt-text: Unlabelled box
Introduction
Neonatal early-onset sepsis and meningitis (early-onset disease; EOD) are important causes of neonatal death.1,2 The predominant pathogen is Group B Streptococcus (GBS; Streptococcus agalactiae), causing one-third to half of all EOD cases.3,4 Survivors of invasive GBS disease are at increased risk of long-term neurodevelopmental impairment.1 In the United Kingdom (UK), the National Institute for Health and Care Excellence (NICE) guideline, intended for the management of EOD, was updated (NG195) in 2021.5 It provides recommendations for intrapartum antibiotic prophylaxis (IAP) and for empiric antibiotic treatment of newborns with clinical signs or maternal risk factors. In the Netherlands, the NICE guideline was adapted with the last update in 2017.6
These national EOD prevention guidelines come with two important limitations. First, the incidence of early-onset GBS disease in both countries is increasing.7,8 In 46% of culture-positive EOD cases, maternal risk factors are absent, making it difficult to identify these patients when clinical signs are not (yet) present.9 Second, the non-specific clinical and biochemical manifestations of EOD cause substantial antibiotic overtreatment in healthy newborns. In the UK, 13–20% of infants on the postnatal wards receive empiric antibiotic treatment for suspected EOD and in the Netherlands a percentage of 5% has been reported.10,11 Antibiotic use in newborns results in separation of parents and child and is associated with various adverse outcomes, such as disruption of the infant gut microbiome and asthma and obesity in later life.12,13
The Kaiser Permanente neonatal early-onset sepsis risk calculator (EOSC) is an online tool for EOD risk assessment in newborns ≥34 weeks of gestation. Treatment recommendations are based on the evaluation of maternal risk factors and neonatal clinical findings. The new NICE guideline endorses the use of the EOSC as an alternative framework for antibiotic management decisions, under the condition that it is part of a prospective audit.5 A meta-analysis showed that implementation of the EOSC reduces the use of empirical antibiotic treatment with a relative risk of 56% compared to conventional strategies.14 A recent meta-analysis on 234 EOD cases showed that the EOSC did not recommend immediate initiation of antibiotic treatment, but close clinical observation or routine care, in 59% of newborns with culture-confirmed EOD.15 Although it was not a primary outcome for these meta-analyses, there were no short-term adverse consequences when using the EOSC. However, little is known about the sensitivity of the EOSC to detect early-onset sepsis.
The aim of this study was to compare the sensitivity of the EOSC to the Dutch and NICE guidelines, using culture-positive EOD patients from a Dutch nationwide prospective cohort study.
Methods
Study design
All patients with a positive blood- or cerebrospinal fluid (CSF) culture <72 h of life and a gestational age of ≥34 weeks from “the Netherlands observational study on GBS disease, bacterial virulence and protective serology (NOGBS)” that were included between 1/1/2018 and 31/1/2021 were used for the present study. The NOGBS study was approved by the medical ethical committee of the Amsterdam University Medical Centre, Amsterdam, The Netherlands (protocol no. NL-63,123·018·17, approved on October 12th, 2017). Written, informed consent was provided by the patient's caregivers. The NOGBS study is an ongoing Dutch multicentre prospective cohort study that includes infants aged 0–3 months with culture-positive GBS or Escherichia coli (E. coli) sepsis or meningitis. Patients were prospectively identified through the surveillance system of the Netherlands Reference Laboratory for Bacterial meningitis (NRLBM).16 The NRLBM receives approximately 90% of all CSF isolates of patients with a bacterial meningitis in the Netherlands. Additionally, the NRLBM receives blood isolates of infants under the age of 1 year with GBS or E. coli sepsis and/or meningitis.16 Based on these microbiological surveillance data and approximately 170,000 yearly live births in the Netherlands, approximately 130 cases of culture-positive GBS and E. coli infections (<1 week of life) would be expected during the study period.17,18 Physicians could also contact the investigators at any time to include a patient. This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Data collection and definitions
EOD was defined as a positive blood or CSF culture obtained <72 h of life. The Dutch and the NICE guidelines recommend clinical observation when one maternal or neonatal non-red flag is present and antibiotic treatment when two or more non-red flags or one or more red flags are present (Supplementary Table 1).5,6 The majority of risk factors were collected prospectively. For the NICE guideline, three additional risk factors were obtained retrospectively: the presence or absence of oliguria, unexplained excessive bleeding or thrombocytopenia and altered muscle tone.5 For the EOSC, GBS status was considered positive if the result of the rectovaginal swab or urine culture was positive for GBS and available at the day of birth. In the Netherlands, intravenous penicillin is the first choice for IAP. Two additional risk factors were collected retrospectively for the EOSC: highest maternal intrapartum temperature and duration of rupture of membranes in hours. Highest maternal temperature was missing for 30 (34%) of 88 patients. Each infant was retrospectively scored as well appearing, equivocal, or clinically ill at 4, 12 and 24 h after birth (Table 1). Regarding the clinical course and outcome, we defined severe disease as any of the following: meningitis, seizures, brain lesions at hospital discharge, need for catecholamine support, mechanical ventilation or death.19
Table 1.
. EOSC classification of infant's clinical presentation.
| Clinical exam | Description |
|---|---|
| Clinical illness | 1. Persistent need for NCPAP/HFNC*/mechanical ventilation (outside of the delivery room) 2. Hemodynamic instability requiring vasoactive drugs 3. Neonatal encephalopathy/perinatal depression - Seizure - Apgar Score at 5 min <5 4. Need for supplemental O2 ≥2 h to maintain oxygen saturations >90% (outside of the delivery room) |
| Equivocal | 1. Persistent physiologic abnormality ≥4 hrs - Tachycardia (HR ≥160) - Tachypnea (RR ≥60) - Temperature instability (≥100·4˚F or <97·5˚F) - Respiratory distress (grunting, flaring, or retracting) not requiring supplemental O2 2. Two or more physiologic abnormalities lasting for ≥2 hrs - Tachycardia (HR ≥160) - Tachypnea (RR ≥60) - Temperature instability (≥100·4˚F or <97·5˚F) - Respiratory distress (grunting, flaring, or retracting) not requiring supplemental O2 |
| Well appearing | No persistent physiologic abnormalities |
Abbreviations: NCPAP: nasal continuous positive airway pressure, HFNC: high flow nasal cannula.
The EOSC prediction model was recreated in SPSS using the beta-coefficients reported by Kuzniewicz et al.20 The prior estimated EOD risk at birth is based on overall EOD incidence and five maternal risk factors (gestational age, highest intrapartum temperature, duration of rupture of membranes, GBS status and IAP). Although the EOSC offers the possibility to specify the baseline incidence for the target population, a recent article has argued that the EOSC performs best when the incidence from the developmental set is used, independent of the true EOD incidence in a particular population.21 Following this advice, we used an incidence of 0·6 per 1000 live births. The prior odds were multiplied by the likelihood ratio of the newborn's clinical examination to calculate the posterior odds. The posterior odds were converted to the posterior risk per 1000 live births. Following the decision diagram for the EOSC,21 different treatment recommendations were defined: routine care (posterior risk <1/1000 live births), monitoring vital signs every four hours (posterior risk <1/1000 live births, but prior risk >1/1000 live births), monitoring vital signs every four hours and blood culture assessment (posterior risk 1–3/1000 live births) and antibiotic treatment (posterior risk >3/1000 live births or clinical signs of illness).
For each patient, treatment advice was determined retrospectively at 4, 12 and 24 h after birth. These time points were chosen based on previously described and recommended timeframes for EOSC use.20,22 In addition, 12 and 24 h are commonly used observation periods in clinical practice for newborns at risk of EOD. Treatment advice was classified into three groups: routine care, clinical observation (vitals every 4 h) and antibiotic treatment. Sensitivity was based on the proportion of newborns with antibiotic treatment recommendation. We compared the sensitivities of both guidelines to the EOSC.
Statistical analyses
The proportion of patients with antibiotic treatment advice by the EOSC was compared to that of the Dutch and NICE guidelines using the McNemar's test.23 Differences were considered statistically significant at a p-value of 0·05 or less. Continuous data were reported as median with interquartile range (IQR). Analyses were performed using IBM SPSS Statistics version 26·0 (SPSS Inc., Chicago, IL). In cases where the highest maternal temperature was missing, we used multiple linear regression to estimate the highest maternal temperature as suggested by Puopolo et al.24 This study was the basis for the development of the EOSC and provides a linear regression model that uses duration of rupture of membranes, administration of IAP and the presence of epidural anaesthesia as predictors of highest maternal temperature. As a sensitivity analysis, we assessed the hypothetical situation of universal GBS screening, assuming all mothers of GBS patients would have been identified as GBS carriers. Based on this situation, recommendations concerning IAP for the mother and antibiotic treatment for the infants were determined based on the Dutch and NICE guideline.
Role of the funding source
The funding source did not have any involvement in study design; data collection, data analysis or interpretation, or in the writing of the article.
Results
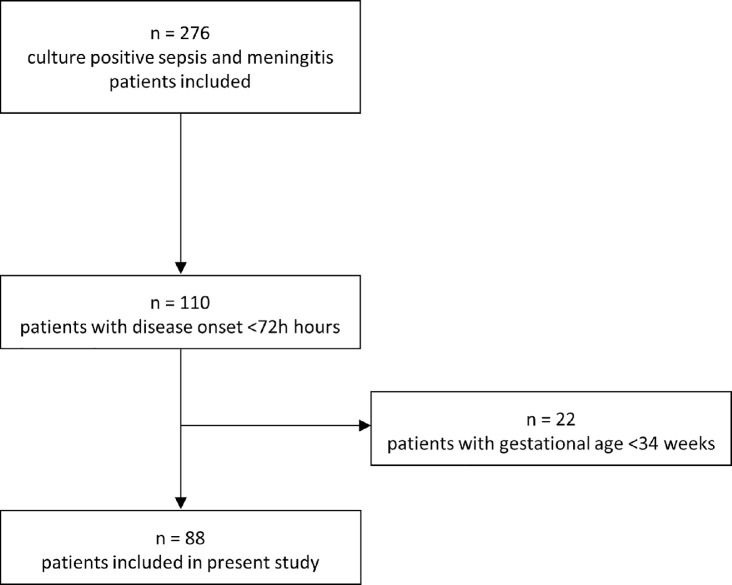
We identified 88 EOD patients born at a gestational age ≥34 weeks from the NOGBS study (Figure 1). All patients had a positive blood culture and 11 (13%) also had a positive CSF culture (Table 2). GBS was the causative pathogen in 81 (92%) patients. Median gestational age was 39 3 + 7 weeks (IQR 37+3–40+4 weeks, range 34+1–41+6). Sixty-one (69%) patients developed symptoms within 12 h after birth. In 67 (76%) patients symptoms were present at 24 h after birth, of whom 24 (36%) were classified as clinically ill directly after birth. Three (3%) of 88 patients did not develop any symptoms. However, early antibiotic treatment might have prevented the development of symptoms, as these patients were treated directly after birth based on the presence of maternal risk factors incorporated in the Dutch guideline. We classified 25 (28%) patients as having severe disease. One patient died.
Figure 1.
Inclusion of patients with early-onset disease.
Table 2.
Characteristics of patients with early-onset disease.
| All patients (n=88) | |
|---|---|
| Gestational age (weeks) | 39+3 (37+3–40+4) |
| Female | 42 (47·7) |
| Apgar score at 5 min | 10 (9–10) |
| Apgar score <7 at 5 min | 12 (13·6) |
| Birth weight (kg) | 3·4 (2·9–3·8) |
| Day of culture | 0 (0–1) |
| Onset of symptoms <24 h | 67 (76·1) |
| Diagnosis | |
| Sepsis | 77 (87·5) |
| Meningitis | 11 (12·5) |
| Causative pathogen | |
| GBS | 81 (92·0) |
| E. coli | 7 (8·0) |
| Positive CSF culture | 11/64 (17·2) |
| CSF leukocytes (106/L) | 5991 (3218–9587) |
| <1000 | 0/8 (0) |
| 1000–10 000 | 6/8 (75·0) |
| ≥10 000 | 2/8 (25·0) |
| CSF Glucose (mmol/L) | 0.2 (0·1–1·2) |
| CSF Protein (g/L) | 4.0 (2.8–5.0) |
| ≥1 Spanos criterion* | 8/8 (100) |
| Highest CRP (mg/L) | 103 (46–147) |
| Transfer to intensive care unit | 20 (22·7) |
| Deaths | 1 (1·1) |
Numbers are presented as n (%) or median (IQR). *Spanos criteria: CSF glucose <1·9 mmol/L; CSF-blood glucose ratio <0·23; CSF protein >2·2 g/L; CSF WBC count >2000 × 106/L; CSF neutrophil count >1180 106/L.
Sensitivity of the EOSC compared to the Dutch and NICE guidelines
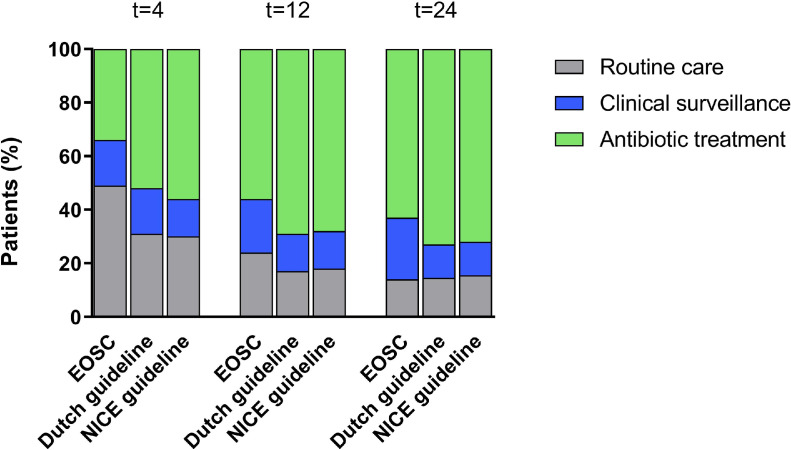
In the first 4 h after birth, EOSC application would have resulted in antibiotic treatment in 32 (36%) of 88 patients, compared to 44 (50%) by the Dutch guideline (p<0·01) and 48 (55%) by the NICE guideline (p<0·01, Figure 2, Supplementary Table 2). Of the 32 patients with treatment recommendation according to the EOSC, 24 (75%) were scored as clinically ill. Only one (3%) patient was scored as well-appearing and would have been treated based on the presence of maternal risk factors only. In 46 (52%) of 88 patients, the EOSC would have recommended routine care, compared to 27 (31%) by the Dutch guideline (p<0·01) and 26 (30%) by the NICE guideline (p<0·01).
Figure 2.
Treatment advice at different ages by the EOSC, the Dutch and the NICE guidelines.
N = 88. EOSC: early-onset sepsis calculator, t = 4: 4 h after birth, t = 12: 12 h after birth, t = 24: 24 h after birth.
During the first 24 h of life, differences between the EOSC and the guidelines decreased as clinical symptoms persisted or became more severe (Figure 2, Supplementary Table 2). At 12 h after birth, the EOSC would have recommended antibiotic treatment in 48 (55%) patients, compared to 61 (69%) by the Dutch guideline (p<0·01) and 60 (68%) by the NICE guideline (p = 0·02). At 24 h after birth, 54 (61%) patients would have been treated according to the EOSC, compared to 64 (73%) by the Dutch guideline (p = 0·02) and 63 (72%) by the NICE guideline (p = 0·06).
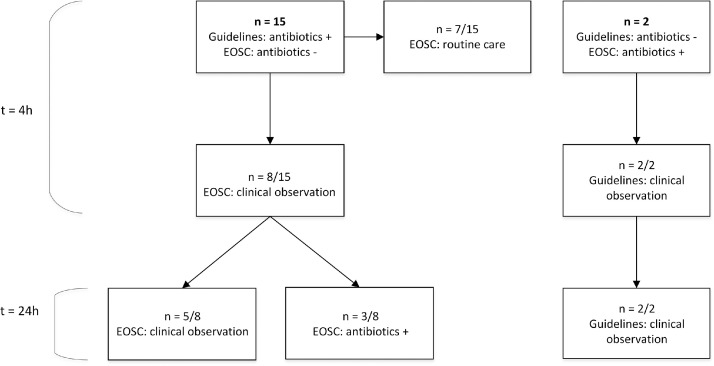
In 17 (19%) of 88 patients, antibiotic treatment recommendation by the EOSC differed from both guidelines (Figure 3). In 15 (88%) of these patients, both guidelines would have recommended antibiotic treatment directly after birth, while the EOSC would not. However, following EOSC guidance, these 15 patients would have initially been scored as “well appearing” or “equivocal”, resulting in routine care (7 [47%]) or close clinical observation (8 [53%]). At 24 h after birth, 3 (38%) of 8 patients with a clinical observation recommendation had developed symptoms that would initiate antibiotic treatment according to the EOSC. In 2 (12%) of 17 patients, EOSC application would result in antibiotic treatment directly after birth, contrary to both guidelines. Both patients had no maternal risk factors and were scored as clinically ill due to respiratory problems. These symptoms accounted for one non-red flag in both guidelines, resulting in clinical observation only.
Figure 3.
Patients with a discrepancy between the EOSC and guideline recommendations to start antibiotic treatment shortly after birth.
N=number of cases. EOSC: early-onset sepsis calculator, t = 4: 4 h after birth, t = 24: 24 h after birth. All recommendations at 12 and 24 h were the same.
We classified 25 (28%) patients as having severe disease. In 10 (40%) of these patients, the EOSC would have recommended antibiotic treatment directly after birth, compared to 11 (44%) by the Dutch guideline (p = 0·50) and 12 (48%) by the NICE guideline (p = 0·25). When comparing the group of patients where antibiotics were started within 24 h to patients where antibiotics were started after 24 h, the proportion of meningitis cases in the latter group was higher: 1 (2%) of 65 and 10 (43%) of 23 (p<0.01), respectively. However, Lumbar puncture was performed more than 24 h after start of antibiotic treatment in 38 (83%) in the 46 patients who were treated early, compared to 5 (26%) of the 19 patients who were started on antibiotic after 24 h of life (p<0.01, Supplementary Table 3).
Sensitivity of the updated nice guideline compared to the previous nice guideline
Compared to the former guideline, the sensitivity of the new NICE guideline increased with an absolute 1% at 4 h after birth and decreased with an absolute 2% at 12 and 24 h after birth. For one patient, the advice changed from clinical observation to antibiotic treatment, due to the need for cardiopulmonary resuscitation. This was a non-red flag in the former guideline and a red flag in the new guideline. On the other hand, the former guideline would have recommended antibiotic treatment in three patients, while the new guideline would have recommended clinical observation. All three patients had respiratory distress with an onset >4 h after birth, which accounted for a red flag in the old guideline and a non-red flag in the new guideline. The other changes in risk factors did not affect antibiotic treatment advice.
Sensitivity analysis
We assessed the hypothetical situation of universal GBS screening, assuming that all 81 mothers of GBS patients would have been identified as GBS carriers. In this scenario, 33 (41%) of 81 mothers would have received IAP according to the Dutch guideline due to additional risk factors, and IAP would have been considered in the remaining 48 (59%). In the real situation, only 11 (14%) of 81 mothers of GBS patients in our cohort received IAP, and in 2 (18%) prophylaxis was considered adequate (at least two doses of intravenous antibiotics prior to delivery). The NICE guideline would have offered IAP to all pregnant women with GBS colonization. Concerning the newborns, the Dutch guideline would have recommended antibiotic treatment in 53 (65%) of 81 patients and the NICE guideline in 54 (67%) patients, compared to 41 (51%) and 45 (56%) in the current situation (p<0·01 and p<0·01, respectively).
Discussion
The primary aim of the present study was to compare the sensitivity of the EOSC to the current Dutch and NICE guidelines. In our cohort of 88 culture-positive EOD patients, both national risk-based guidelines and the EOSC had poor sensitivity directly after birth. However, the EOSC performed significantly worse, especially in identifying EOD patients without signs of clinical illness. During the first day of life, differences between the guidelines and the EOSC became smaller due to clinical deterioration of newborns.
Our findings are in line with the retrospective study by Morris et al., who found a sensitivity of 39% for the EOSC compared to 56% for the former NICE guideline in the first hours after birth.25 Our results also correspond to those of a recent meta-analysis on 234 EOD patients, which reported that the EOSC recommends antibiotics in 41% of EOD patients shortly after birth.15
The EOSC relies more heavily on signs of clinical illness, resulting in a more specific approach and a substantial reduction in unnecessary EOD treatments. This comes at the expense of sensitivity, as can be expected. However, with the EOSC, more initially well-appearing patients will receive delayed treatment. Previous studies showed no short-term adverse consequences when waiting for clinical signs to develop before starting antibiotic treatment.25,26 However, studies on long-term outcomes are lacking.
The benefits of the EOSC in reducing antibiotic overexposure are well-established.14 Future studies should focus on improving the sensitivity of the EOSC while minimizing additional overtreatment. Current Dutch and UK prevention guidelines and the EOSC are based on decades of research into clinical risk factors for EOD. It seems unlikely that new clinical risk factors will be found that will both increase sensitivity and specificity. This presumption is exemplified by our finding that the new NICE guideline has similar poor sensitivity compared to the old version.
Implementation of screening for rectovaginal GBS colonization will likely improve sensitivity, but has been shown to increase antibiotic overtreatment even more. However, there also studies that do not show an increase in antibiotic treatment when using screening instead of risk based strategies.27 Furthermore, not all EOD cases are due to GBS and in countries with universal GBS screening, 61–73% of GBS EOD patients are born to mothers who tested GBS negative.28,29
Potential strategies to improve EOD identification in well-appearing newborns are the evaluation of acute phase reactant biomarkers like C-reactive protein (CRP) and ferritin, and the molecular detection of EOD-specific pathogens in cord blood.30,31 Although many potential biomarkers have been identified, there is currently no diagnostic test for the detection of neonatal EOD with an acceptable sensitivity and specificity. However, some studies have shown that adding blood biomarkers, like CRP and procalcitonin, to the EOSC improves diagnostic accuracy.32
In half of newborns ultimately diagnosed with EOD, the EOSC would have initially recommended ‘routine care’. This indicates that clinical observation might be required for all newborns. A recent approach to manage newborns at risk for early-onset sepsis is based on serial physical examinations. Two studies from Italy and Norway showed that serial examination, performed by midwives, bedside nursing staff and physicians, may reduce antibiotic overexposure even more than the EOSC, with no evidence of worse short-term outcomes.19,33 However, as with the EOSC, studies on long term outcomes are lacking. Due to the observational nature of this study, our finding that meningitis was more common in patients that were started on antibiotics >24 h after birth, cannot be interpreted as proof for a causal relationship between later initiation of antibiotics and development of meningitis. The Dutch guideline does not recommend routine lumbar puncture in newborns that qualify for antibiotic treatment due to maternal risk factors only.6 Fewer lumbar punctures were performed in this subgroup that received early antibiotic treatment, and most lumbar punctures were done more than 24 h after starting antibiotic treatment (Supplementary Table 3). This has likely resulted in some false negative culture results in these patients. Also, many studies have shown that late-onset GBS disease is strongly associated with meningitis.34 It might be that the children who became clinically ill after 24 h resemble the late-onset population more than children who were started on antibiotics within 24 h.
This is the first study that directly compares the sensitivity of the Dutch guideline for the prevention of neonatal early-onset infections with the EOSC. It is also the first study determining the sensitivity of the new NICE guideline and comparing it to the EOSC. Another strength is the prospective character of the cohort study and the relatively high number of culture-positive EOD cases. Our study has several limitations. First, we retrospectively determined the clinical condition of each newborn. It was occasionally difficult to determine the exact duration of symptoms that is required for the ‘equivocal’ category of infant's clinical condition for the EOSC. Second, in some patients, antibiotics were started shortly after birth. This early treatment could have influenced the clinical condition of the newborn at 24 h of age, leading to a lower sensitivity of both guidelines, but especially the EOSC at that time point. Third, the prediction model that the EOSC is based on, has not been validated in the Dutch population with a lower incidence of EOD.7,17
In conclusion, the current Dutch guideline, the current NICE guideline, and the EOSC all suffer from poor sensitivity. These differences become smaller during the first day of life, as the EOSC depends heavily on signs of clinical illness. Other reports have found that the higher sensitivity of both guidelines comes at the cost of more antibiotic overtreatment of healthy newborns.10,11 Vice versa, the more specific approach of the EOSC results in less antibiotic overtreatment at the cost of later treatment of actual EOD patients. Better EOD risk stratification is needed.
Contributors
LS, MNvK, FBP, NBA and MWB designed the study. LS, MNvK, JFK, FBP, NBA, NMvS, MCB, DvdB and MWB reviewed and helped to revise the manuscript. All authors reviewed and agreed on the final version. NMvS provided notifications of isolates received by the NRLBM. LS, MNvK, JFK and MWB collected and verified the underlying data. LS and MWB analysed and interpret the data, prepared the first draft of the manuscript and created the figures and tables. LS and MWB had final responsibility for the decision to submit for publication.
Declaration of interests
MCB participates on the Advisory Board for the ENCEPH-IG trial. All the other authors declare no conflicts.
Acknowledgments
Acknowledgments
We thank the NOGBS study group (see Supplement for study group details) and all Dutch physicians and patients who participated in the study. We also thank the NRLBM for providing daily updates of the received isolates, thereby facilitating the identification of patients for the NOGBS study.
Funding
This work was supported by grants received from Netherlands Organization for Health Research and Development (ZonMw; NWO-Vidi-Grant (grant number 917·17·308); NWO-Vici-Grant (grant number 918·19·627)), the Academic Medical Centre (AMC Innovative Impulse Grant) and Steun Emma Foundation Grant.
Data sharing statement
Individual participant data underlying the results reported in this article will be available on reasonable request, after de-identification. The study protocol will also be available. Proposals should be directed to the corresponding author. A data access agreement should be signed by data requestors.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101270.
Appendix. Supplementary materials
References
- 1.Horváth-Puhó E., van Kassel M.N., Goncalves B.P., et al. Mortality, neurodevelopmental impairments, and economic outcomes after invasive group B streptococcal disease in early infancy in Denmark and the Netherlands: a national matched cohort study. Lancet Child Adolesc Health. 2021;5(6):398–407. doi: 10.1016/S2352-4642(21)00022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seale A.C., Bianchi-Jassir F., Russell N.J., et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin Infect Dis. 2017;65(suppl 2):S200–SS19. doi: 10.1093/cid/cix664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrag S.J., Farley M.M., Petit S., et al. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics. 2016;138(6) doi: 10.1542/peds.2016-2013. [DOI] [PubMed] [Google Scholar]
- 4.Vergnano S., Menson E., Kennea N., et al. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F9–F14. doi: 10.1136/adc.2009.178798. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Clinical Excellence: neonatal infection (early onset): antibiotics for prevention and treatment. NG195. London: national Institute for Health and Clinical Excellence; 2021.
- 6.The Dutch Society of Obstetrics and Gynaecology, the Dutch Paediatrics Association. Prevention and treatment of early-onset neonatal infection (Adapted from NICE guidelines). 2017: 1–97.
- 7.Bekker V., Bijlsma M.W., van de Beek D., et al. Incidence of invasive group B streptococcal disease and pathogen genotype distribution in newborn babies in the Netherlands over 25 years: a nationwide surveillance study. Lancet Infect Dis. 2014;14(11):1083–1089. doi: 10.1016/S1473-3099(14)70919-3. [DOI] [PubMed] [Google Scholar]
- 8.Lamagni T.L., Keshishian C., Efstratiou A., et al. Emerging trends in the epidemiology of invasive group B streptococcal disease in England and Wales, 1991-2010. Clin Infect Dis. 2013;57(5):682–688. doi: 10.1093/cid/cit337. [DOI] [PubMed] [Google Scholar]
- 9.Trijbels-Smeulders M., de Jonge G.A., Pasker-de Jong P.C., et al. Epidemiology of neonatal group B streptococcal disease in the Netherlands before and after introduction of guidelines for prevention. Arch Dis Child Fetal Neonatal Ed. 2007;92(4):F271–F276. doi: 10.1136/adc.2005.088799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goel N., Shrestha S., Smith R., et al. Screening for early onset neonatal sepsis: NICE guidance-based practice versus projected application of the Kaiser Permanente sepsis risk calculator in the UK population. Arch Dis Child Fetal Neonatal Ed. 2020;105(2):118–122. doi: 10.1136/archdischild-2018-316777. [DOI] [PubMed] [Google Scholar]
- 11.Kerste M., Corver J., Sonnevelt M.C., et al. Application of sepsis calculator in newborns with suspected infection. J Matern Fetal Neonatal Med. 2016;29(23):3860–3865. doi: 10.3109/14767058.2016.1149563. [DOI] [PubMed] [Google Scholar]
- 12.Azad M.B., Bridgman S.L., Becker A.B., et al. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes (Lond) 2014;38(10):1290–1298. doi: 10.1038/ijo.2014.119. [DOI] [PubMed] [Google Scholar]
- 13.Russell S.L., Gold M.J., Hartmann M., et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13(5):440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achten N.B., Klingenberg C., Benitz W.E., et al. Association of Use of the Neonatal Early-Onset Sepsis Calculator With Reduction in Antibiotic Therapy and Safety: a Systematic Review and Meta-analysis. JAMA Pediatr. 2019;173(11):1032–1040. doi: 10.1001/jamapediatrics.2019.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achten N.B., Plotz F.B., Klingenberg C., et al. Stratification of Culture-Proven Early-Onset Sepsis Cases by the Neonatal Early-Onset Sepsis Calculator: an Individual Patient Data Meta-Analysis. J Pediatr. 2021;234:77–84. doi: 10.1016/j.jpeds.2021.01.065. [DOI] [PubMed] [Google Scholar]
- 16.Netherlands Reference Laboratory For Bacterial Meningitis (AMC/RIVM) University of Amsterdam; Amsterdam: 2021. Bacterial Meningitis in The Netherlands: Annual Report 2020. [Google Scholar]
- 17.van Kassel M.N., de Boer G., Teeri S.A.F., et al. Molecular epidemiology and mortality of Group B streptococcal meningitis and infant sepsis in The Netherlands: a 30-year nationwide surveillance study. Lancet Microbe. 2021;2(1):E32–E40. doi: 10.1016/S2666-5247(20)30192-0. [DOI] [PubMed] [Google Scholar]
- 18.Statistics Netherlands. Available online: www.cbs.nl (accessed on 1 December 2021).
- 19.Berardi A., Spada C., Reggiani M.L.B., et al. Group B Streptococcus early-onset disease and observation of well-appearing newborns. PLoS ONE. 2019;14(3) doi: 10.1371/journal.pone.0212784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuzniewicz M.W., Walsh E.M., Li S., et al. Development and Implementation of an Early-Onset Sepsis Calculator to Guide Antibiotic Management in Late Preterm and Term Neonates. Jt Comm J Qual Patient Saf. 2016;42(5):232–239. doi: 10.1016/s1553-7250(16)42030-1. [DOI] [PubMed] [Google Scholar]
- 21.Benitz W.E., Achten N.B. Technical assessment of the neonatal early-onset sepsis risk calculator. Lancet Infect Dis. 2020;21(5):e134–e140. doi: 10.1016/S1473-3099(20)30490-4. [DOI] [PubMed] [Google Scholar]
- 22.Escobar G.J., Puopolo K.M., Wi S., et al. Stratification of Risk of Early-Onset Sepsis in Newborns ≥34 Weeks’ Gestation. Pediatrics. 2014;133(1):30–36. doi: 10.1542/peds.2013-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S., Lee W. Does McNemar's test compare the sensitivities and specificities of two diagnostic tests? Stat Methods Med Res. 2017;26(1):142–154. doi: 10.1177/0962280214541852. [DOI] [PubMed] [Google Scholar]
- 24.Puopolo K.M., Draper D., Wi S., et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011;128(5):e1155–e1163. doi: 10.1542/peds.2010-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris R., Jones S., Banerjee S., et al. Comparison of the management recommendations of the Kaiser Permanente neonatal early-onset sepsis risk calculator (SRC) with NICE guideline CG149 in infants >/=34 weeks' gestation who developed early-onset sepsis. Arch Dis Child Fetal Neonatal Ed. 2020;105(6):581–586. doi: 10.1136/archdischild-2019-317165. [DOI] [PubMed] [Google Scholar]
- 26.Kuzniewicz M.W., Puopolo K.M., Fischer A., et al. A Quantitative, Risk-Based Approach to the Management of Neonatal Early-Onset Sepsis. JAMA Pediatr. 2017;171(4):365–371. doi: 10.1001/jamapediatrics.2016.4678. [DOI] [PubMed] [Google Scholar]
- 27.Hasperhoven G.F., Al-Nasiry S., Bekker V., et al. Universal screening versus risk-based protocols for antibiotic prophylaxis during childbirth to prevent early-onset group B streptococcal disease: a systematic review and meta-analysis. BJOG. 2020;127(6):680–691. doi: 10.1111/1471-0528.16085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Dyke M.K., Phares C.R., Lynfield R., et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009;360(25):2626–2636. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 29.Verani J.R., Spina N.L., Lynfield R., et al. Early-onset group B streptococcal disease in the United States: potential for further reduction. Obstet Gynecol. 2014;123(4):828–837. doi: 10.1097/AOG.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 30.Mithal L.B., Palac H.L., Yogev R., et al. Cord Blood Acute Phase Reactants Predict Early Onset Neonatal Sepsis in Preterm Infants. PLoS ONE. 2017;12(1) doi: 10.1371/journal.pone.0168677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mithal LB, Qi C, Green S, et al. Umbilical Cord Blood Diagnostics for Early Onset Sepsis in Premature Infants: detection of Bacterial DNA and Systemic Inflammatory Response 2017: 10.1101/200337. [DOI]
- 32.He Y., Chen J., Liu Z., et al. Efficacy and safety of applying a neonatal early-onset sepsis risk calculator in China. J Paediatr Child Health. 2020;56(2):237–243. doi: 10.1111/jpc.14572. [DOI] [PubMed] [Google Scholar]
- 33.Vatne A., Klingenberg C., Oymar K., et al. Reduced Antibiotic Exposure by Serial Physical Examinations in Term Neonates at Risk of Early-onset Sepsis. Pediatr Infect Dis J. 2020;39(5):438–443. doi: 10.1097/INF.0000000000002590. [DOI] [PubMed] [Google Scholar]
- 34.Berardi A., Trevisani V., Di Caprio A., et al. Understanding Factors in Group B Streptococcus Late-Onset Disease. Infect Drug Resist. 2021;14:3207–3218. doi: 10.2147/IDR.S291511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.