Abstract
Sleep is entwined across many physiologic processes in the brain and periphery, thereby exerting tremendous influence on our well-being. Yet sleep exists in a social-environmental context. Contextualizing sleep health with respect to its determinants—from individual- to societal-level factors—would enable neuroscientists to more effectively translate sleep health into clinical practice. Key challenges and opportunities pertain to (i) recognizing and exploring sleep’s functional roles, (ii) clarifying causal mechanisms in relation to key outcomes, (iii) developing richer model systems, (iv) linking models to known contextual factors, and (v) leveraging advances in multisensory technology. Meeting these challenges and opportunities would help transcend disciplinary boundaries such that social-environmental considerations related to sleep would become an ever-greater presence in the clinic.
Sleep is generally defined as a naturally recurring, reversible state of perceptual disengagement, reduced consciousness, and relative immobility, the propensity of which is patterned by homeostatic and circadian factors. Many studies have described the neurophysiology that contributes to sleep regulation, including the neuroanatomy of sleep and arousal-promoting circuitry (1); the diffuse, interconnected neurotransmitter systems that transition between non–rapid eye movement sleep (NREM), REM sleep, and wakefulness (2); and the core molecular clock mechanism and circadian pacemaker activity within the brain (3). Additional work has delineated how sleep serves as a prerequisite for optimizing the performance of critical biological functions, including those related to memory (4), waste clearance from the brain (5), and nutrient metabolism (6). Over the past 70 years, sleep has been increasingly conceptualized with deference to these findings, promoting an understanding of sleep as a set of interrelated physiological processes sometimes devoid of greater biological and social-environmental context.
Sleep is not just a collection of physiologic processes that occur together at the intersection of rest and activity and of circadian light-dark rhythms. Sleep is a non-negotiable biological state required for the maintenance of human life. It is associated with behaviors, but sleep itself is not a volitional behavior. It involves physiologic processes but is more than an amalgam of them. Sleep is a foundational aspect of our biology—our need for sleep parallels those for air, food, and water.
Total sleep deprivation results in death in animal models (7). For obvious ethical reasons, no previous scientific work has empirically tested the amount of sleep deprivation necessary to induce organ failure and death in humans. Prolonged sleep deprivation in humans has been studied, though. When sleep is curtailed, sleep propensity increases, such that individuals kept from sleep for extended periods will fall asleep faster and more deeply when sleep is no longer restricted (8). Continued wakefulness for several days results in the occurrence of involuntary sleep, where individuals fall asleep even while trying to stay awake. Although there are no formal studies of human mortality related to in-lab sleep loss, there are many reported cases in which sleep propensity was sufficiently high to induce involuntary sleep initiation, even during activities when such an event would likely result in death (such as while driving a car) (9). Thus, sleep is not a luxury for people with too much free time; it is a biological necessity whose many functions are increasingly the interest of study.
Yet, modern society has a complicated relationship with sleep health. The drive for productivity has led to spoken and unspoken pressures for minimizing sleep. Incentives can be both direct (e.g., rewards for staying awake to perform tasks) or indirect (e.g., being perceived as more industrious). Existing evidence suggests that Americans trade sleep hours for work or leisure hours (10) and that this trade-off is exacerbated by modern sedentary lifestyles and use of electronic media at night. This larger discrepancy between the physiologic importance of sleep and its societal devaluing has deleterious consequences for health and well-being at the level of the individual, with collective effects that ripple across the population.
The field of neuroscience is well positioned to improve our basic understanding of how sleep is dynamically regulated and how it relates to other functions in the brain and periphery. Neuroscience is also well suited to leverage the translational potential of sleep to improve society. The fact that sleep is a fixture within so many aspects of our biology presents challenges and complications for the field. However, at the same time, it offers a range of opportunities for improving clinical outcomes in areas of medicine that are not accustomed to considering the influence of sleep on disease processes or the influence of social-environmental factors in shaping sleep health.
Conceptualizing sleep health
Sleep health, like proper nutrition, is not a unitary construct. Borrowing from paradigms historically used to define general health, Buysse’s RU-SATED model outlines six dimensions of sleep health (11). These include:
1) Regularity. Regularity refers to the degree to which sleep occurs at approximately the same time each day. Irregular sleep schedules are an independent predictor of adverse health outcomes (12). These associations are thought to arise from decreased circadian synchronization across organ and tissue systems, disorganized patterns of cellular and organismal stress, and weekday-to-weekend fluctuations in sleep timing that may induce repetitive episodes of circadian disruption and occasional sleep insufficiency.
2) Satisfaction. Subjective sleep satisfaction remains an important indicator of sleep health. Although considered an imprecise measure, it predicts clinical outcomes (13), may reflect the presence of sleep impairments that are otherwise difficult to detect and assess, and may represent underlying physiologic processes for which there are no validated assessment tools, such as subcortical activation and local wakefulness in the brain (14).
3) Alertness. The ability to maintain attentive wakefulness during the day carries important safety implications, as well as implications for cardiometabolic health (15). Laboratory studies indicate that dulled alertness that stems from sleep loss can be profound and cumulative (16).
4) Timing. The circadian system partitions many aspects of sleep to the “biological night,” which represents the portion of the 24-hour day that is behaviorally and physiologically conducive for sleep and sleep-related restorative activities organized within and between the brain and body (17).
5) Efficiency. Sleep efficiency refers to the ratio between the time spent asleep and the time available for sleeping. It is a standard metric used in the assessment and treatment of insomnia (18). Low sleep efficiency is associated with daytime dysfunction and adverse longitudinal health outcomes (19).
6) Duration. Consensus statements from several scientific, medical, and public health organizations recommend that adults, in general, should obtain at least 7 hours of sleep per night (20). Some statements have further advised that adults should not aim for more than 9 hours (20). Recommendations for children and adolescents have also been published (20, 21), providing suggested ranges of daily sleep that scale inversely with age (i.e., infants require the most sleep, toddlers less sleep than infants, and so on until late adolescence when adultlike levels of sleep are recommended). For more than 50 years, self-reported sleep duration has been associated with mortality, including 7 of the 15 leading causes of death in the US (22).
Sleep and health outcomes
Cardiovascular health
Habitual sleep duration outside of the normative 7- to 8-hour range contributes to an increased incidence of hypertension, cardiovascular disease, and cardiovascular mortality (23, 24). Cross-sectionally, habitual sleep duration and quality are associated with hypertension, hyperlipidemia, coronary disease, vascular disease, myocardial infarction, and stroke (23, 25). Primary sleep disorders such as sleep apnea (26), insomnia (24, 27), sleep-related movement disorders (28), and narcolepsy (29) also influence cardiovascular health. The mechanisms that bridge sleep to cardiovascular health remain elusive but seem to implicate sympathetic activation, cellular stress, inflammation, and metabolic dysregulation (23).
Metabolic health
Extensive investigation has revealed a cross-sectional relationship between habitual sleep duration outside the normative range and obesity-related outcomes (30). Meta-analyses of longitudinal studies suggest that the incidence of obesity is elevated in habitual short sleepers (≤6 hours) relative to 7- to 8-hour sleepers (31). This is consistent with other work showing that sleep loss impairs insulin and glucose homeostasis, disrupts metabolic hormones (e.g., leptin and ghrelin), alters adipose tissue function, and contributes to the incidence of type 2 diabetes mellitus (32).
Immunologic health
Sleep deprivation generates a proinflammatory state, as ascertained by tests performed in the lab or in the field (33). In laboratory settings, acute deprivation alters the expression, production, and/or function of cytokines, antibodies, and leukocytes. Field-based investigations suggest that overly short or long sleep duration promotes plasma-level markers of inflammation (34). Accordingly, habitual insufficient sleep has been linked to increased rates of infection, reduced recovery capacity, and blunted vaccine responses (35). The presence of primary sleep disorders also factors into immune health: Sleep apnea and insomnia are each associated with constitutive elevations in circulating immune markers (36, 37). The relationship between sleep and the immune system is likely bidirectional (via homeostatic and neuroimmune interactions), with a deficiency in either poised to negatively affect the quality of the other (38).
Mental health
Insomnia is a well-established risk factor for depression and is implicated across several neuropsychiatric conditions, including anxiety, psychotic, and attention-deficit/hyperactivity disorders (39). Elevated suicide risk is associated with increased insomnia, nightmares, and habitual sleep duration outside of the normative range (40). Nocturnal wakefulness—simply being awake in the middle of the night—may also represent a risk factor for suicide (41, 42). In general, sleep disruption produces basic functional impairments in emotion regulation, recognition, reasoning, and memory (43). This may be driven by neurophysiologic changes to cortical and subcortical structures that are unduly affected by acute or chronic sleep loss.
Behavioral health
Several studies have correlated differences in sleep to differences in the willingness of individuals to engage in unhealthy behaviors (especially those with an impulse-control component). For example, insufficient sleep and poor sleep quality have been associated with suboptimal dietary health at the population level (44). In the laboratory, acute sleep loss provokes increased calorie consumption, particularly late at night (45). Translational work in human and animal models suggests that eating during the night impairs metabolism (46). Insufficient sleep and poor sleep quality are further associated with more sedentary behavior, less exercise, more habitual smoking, and greater use of alcohol (47). Adding sleep health components to intervention strategies may aid in efforts such as smoking cessation (48).
Cognitive health
Connections between sleep and neurocognitive health are documented across the scientific literature. In addition to its acute effects on executive function and declarative memory (49), poor sleep has been prospectively linked with the development and progression of Alzheimer’s disease (AD) (50). A plausible mechanism linking sleep to AD is the function of the glymphatic system, which operates in a sleep-dependent fashion to clear AD-related proteins such as amyloid-b (Ab) and tau from the brain’s interstitial space. Sleep contributes to the regulation of Ab and tau proteins and discourages their buildup into pathological aggregates (51). In the wider population, those who report habitual insufficient and/or poor sleep quality evince less work productivity (52), more drowsy driving (53), and increased propensity for injuries or errors (54).
Intersecting and interacting influences
Literature that links sleep to health is frequently circumscribed in that individual studies typically focus on a singular aspect of sleep health paired to one domain of interest. For example, studies of sleep duration and metabolic health do not typically quantify other sleep metrics (such as sleep quality, satisfaction, or daytime alertness) or assess multiple outcome domains (such as emotion regulation and decision-making). Yet, interactions between sleep health variables have been repeatedly demonstrated. For example, many individuals who experience insufficient sleep duration also experience insomnia symptoms (55). Understanding the isolated and combined effects of multiple domains of sleep health can potentially enhance our mechanistic understanding of clinical outcomes in depression, obesity, and those pertaining to cardiovascular events such as heart attack or stroke. Furthermore, although cardiometabolic and psychological domains are rarely assessed together, poor mental health can adversely affect cardiovascular, metabolic, and immune system activity, which are also influenced by neurocognitive processes such as health-related decision-making. Part of the mechanistic pathway that links poor sleep to cardiometabolic health may indeed be associated with mental health [e.g., emotional eating (56)] and/or altered executive function [e.g., decision-making about food choices (57)].
Conceptualizing sleep health in context
As outlined above, sleep health makes important contributions to a wide array of clinical outcomes. Additional work has conceptualized determinants of sleep itself. Understanding the factors that shape sleep health has the potential to improve intervention strategies and identify therapeutic targets applicable to cardiovascular, neurocognitive or neurodegenerative, and immune-related disorders such as cancer.
Individual-level factors
The most proximal influences on sleep are individual-level factors. These are factors that are embedded within the everyday life of the person—their demographic and personality traits, along with their beliefs, attitudes, and habits.
Age. Older adults experience systematic declines in sleep efficiency and time spent in slow-wave sleep (58). Age-related increases are observed in the prevalence of primary sleep disorders such as sleep apnea (59), chronic insomnia (60), nocturia (61), and movement-related nocturnal disorders [e.g., restless legs syndrome (62)]. Paradoxically, subjective measures of general sleep disturbance (63), perceived sleep insufficiency (64), and sleep debt (65) all tend to decrease. In a similar vein, short sleep duration is less strongly associated—or not associated at all—with obesity and hypertension in later life (66, 67). Laboratory studies indicate that the cognitive performance of older individuals may also be more resilient to the detrimental effects of sleep loss (68).
Sex at birth. Females are at greater risk for insomnia and will report more dissatisfaction with sleep (69), whereas males are at relatively greater risk of sleep apnea (59). Specific to females are prolonged episodes of sleep disturbance and daytime fatigue associated with menstruation, pregnancy, the postpartum period, perimenopause, and menopause (70, 71).
Race and ethnicity. Sleep disparities are well characterized in the US population (72–75). Black and African American individuals are significantly more likely to report insufficient sleep, as well as long sleep duration, relative to non-Hispanic white individuals. Various disparities related to sleep health are also seen in Hispanic and Latinx individuals, Asian and Asian American people, and multiracial individuals. These associations are reliable even after adjustment for socioeconomics and other demographic considerations (76). Notably, the relationship between sleep and cardiometabolic health that pervades the general population is differentiated by race and ethnicity, which suggests that disparities in sleep health may be integral to larger health care disparities (77). Future research priorities will aim to elucidate and address the racial and ethnic sleep disparities that figure more and more prominently in the literature (78).
Beliefs, attitudes, and habits. Work in this area is still emerging, though evidence suggests that individuals with more-positive beliefs and attitudes about sleep or who adopt sleep-protective behaviors (e.g., good sleep hygiene) are more likely to experience better sleep health (79).
Social-level factors
Individual-level factors are embedded within social-level factors. Examples of social-level factors that can potentially influence sleep are work and occupation, family and home, neighborhood, religion, social networks, and culture. Of these, several have been specifically evaluated with respect to sleep health.
Socioeconomic position. Associations abound between socioeconomic position and sleep health. Poverty (75, 80) and the trappings of poverty, such as food insecurity (76, 81), housing insecurity (82), and lower educational attainment (72, 80), are all associated with worse measures of sleep, including insufficient sleep duration and decreased sleep quality.
Work and occupation. Shift work is a well-established impediment to healthy sleep (83). For non-shift workers, long commute times, stressful work conditions, and longer work hours impair sleep (84, 85). Job loss and unemployment likewise moderate sleep health (86).
Home and family. Caregiving responsibilities present barriers to sleep health (87). Larger households may also contribute to patterns of insufficient sleep (64).
Neighborhood. Increased neighborhood light at night, increased traffic, and noise pollution are associated with worse sleep outcomes (88). People who live in neighborhoods that are more disordered (e.g., with more graffiti and crime) or perceived as less safe experience poorer sleep overall (e.g., shorter duration, less efficiency, and more daytime sleepiness) (89). Notably, individuals who perceive their neighborhood to be less socially cohesive also experience reduced sleep quality (90).
Societal-level factors
Societal-level factors are built from social-level factors. Societal factors include technology, public policy (91), geography (92), racism and discrimination (93), globalization and “24/7” society (i.e., general lifestyles of work and leisure in which people may be active at any hour and do not necessarily sleep consistently at night). Although these factors are not readily manipulable, recognizing their influence on sleep health can inform our understanding of how sleep is shaped and how this influence has the potential to shape other health- and disease-related outcomes.
Social-ecological model of sleep health
The embedded model of individual-, social-, and societal-level factors of sleep health, and the mental and physical health effects of sleep, are depicted in Fig. 1. This social-ecological model of sleep health was originally described in 2010 (22) and has since been conceptually refined (94). This model provides a framework for understanding sleep health in a broader biological and social-environmental context. The framework can be leveraged to inform better diagnosis, monitoring, and treatment of chronic diseases whose progression and treatment receptibility are often influenced by sleep.
Fig. 1. Social-ecological model of sleep health.
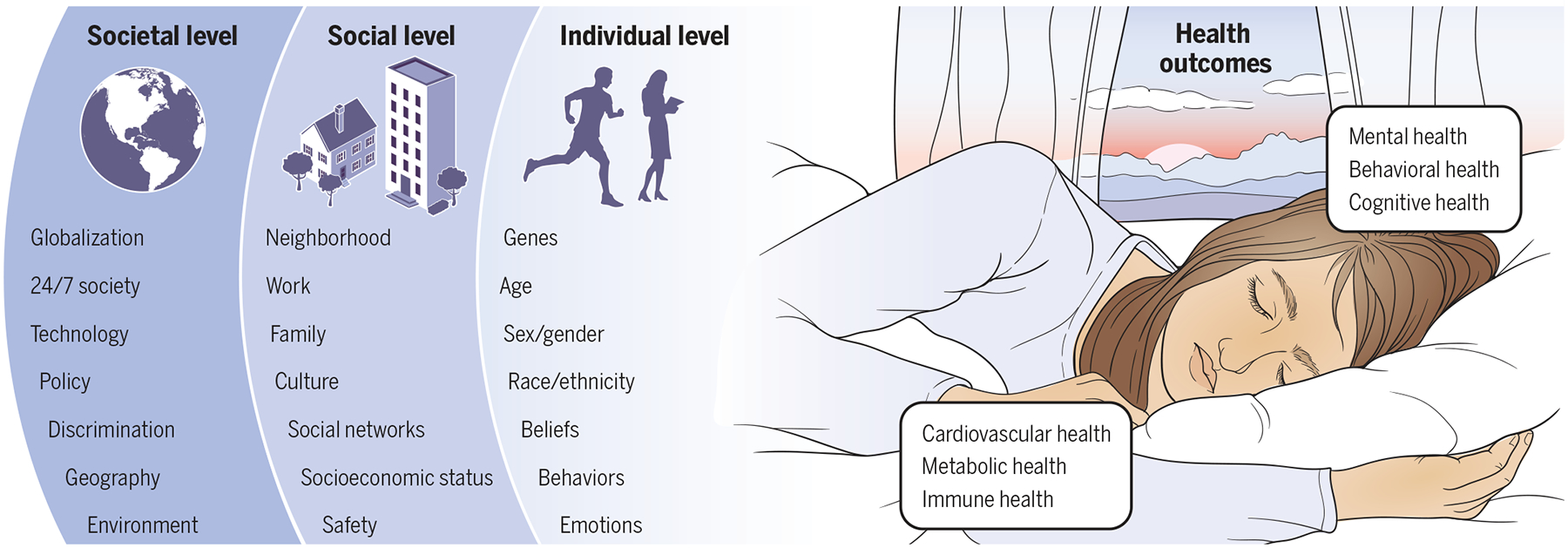
The right side of the image, which depicts a sleeping person, reinforces the importance of situational context in sleep physiology (94). The external environment is shown as a dawn-to-dusk transition, reinforcing the importance of day-night circadian context in sleep physiology (6). Health outcomes associated with sleep—cardiovascular health (23), metabolic health (31), immune health (38), mental health (39), behavioral health (24), and cognitive health (50)—are listed. On the left side of the image, the levels of the social-ecological model are depicted. Individual-level factors that influence sleep include age (58), genetics (105), sex and gender (69), beliefs (94), feelings and attitudes (94), race and ethnicity (78), and behaviors (94). These are embedded within social-level factors, which include neighborhood (88), work and occupation (84), family and home (101), culture (80), social networks (90), socioeconomics (76), and safety and security (89). These factors are further embedded within those at the societal level, such as such as technology (95), globalization (94), racism and discrimination (93), public policy (91), geography (92), the physical environment (94), and “24/7” society (83).
Sleep health technology
One of the societal-level factors that has profoundly affected population sleep health is technological progress, particularly with re gard to the proliferation of portable sleep-assessment technology, the ubiquitous access to digital media at night, and the cultivation of “big data” sources enabling access to sleep health information.
Portable sleep-assessment technology
Over the past few decades, wearable sleep actigraphy has undergone notable innovation, with newly built capabilities for multiaxial accelerometry and microelectromechanical systems enabling high-resolution detection of movement, as well as detection strategies for other peripheral signals (such as heart rate) that can improve assessments of sleep when computed alongside movement information (95). These devices are now available as consumer devices, some of which may be more accurate than their traditional scientific counterparts (96). The increasing use of these devices to assess sleep has led to clinical issues such as orthosomnia (97).
Electronic media use at night
Many people bring electronic devices into the bedroom and use them in bed during the nighttime hours that overlap with their routine sleep schedule. These trends are a cause for concern, because electronic media consumption at night prior to sleep can delay sleep onset, reduce sleep duration, impair sleep quality, worsen circadian health, and decrease daytime functioning (98). The mechanisms behind these disturbances range from exposure to short-wavelength light (99) to the cognitive and emotional stimulation caused by engaging content (100). These effects are pronounced in younger individuals, who have delayed sleep phases (101).
Big data and internet of things
As wearable sensor technology becomes increasingly inexpensive and sensors capable of inferring aspects of sleep-wake behavior become better integrated within the built environment, broader datasets will be collected to provide actionable knowledge about real-world sleep and circadian health. For example, large datasets curated from consumer apps have been used to describe changing sleep patterns during the COVID-19 pandemic (102); electronic medical records are providing insights at the provider, network, and national levels (103); and deep repositories of sleep-assessment data are being used to train artificial intelligence to better interpret sleep-wake physiology in relation to health (104).
Challenges and opportunities for translating sleep considerations into clinical study and practice
Challenges
1) Define the deeper biological context of sleep. Hallmarks for defining sleep include electrophysiological changes in the frequency, amplitude, and synchronicity of brain activity measured via electroencephalography (EEG) in humans and other mammals or local field potential recordings in invertebrates. Implicit within these definitions is that sleep is a phenomenon that can be objectively quantified only through networks of cells coordinated, in animals, with centralized nervous systems. Thus, these criteria cannot be applied universally across simpler organisms, such as those that populate the gut microbiome, or to single cells and cell masses involved with tumor growth and metastasis. There may be ways to define sleep at the single-cell level within the nucleus that have yet to be fully considered (105). The interphase nucleus of cells is a vibrant area, where chromosomes exhibit a polarized, multilayered spatial architecture on the basis of their proximity to structural elements of the nucleus, subnuclear structures, and one another along topologically associating domains and long-range chromatin interactions. These organization motifs—and many finer ones—contribute to how genomic expression is circadian-regulated and may provide insights into how sleep is expressed at the single-cell level or dysregulated in cancer cells or cells situated within various neurodegenerative disease contexts. These changes can become treatment targets in their own right.
2) Clarify the neuroscientific mechanisms that underlie different facets of sleep health. Although many studies have linked different aspects of sleep to outcomes, there is relatively little work that delineates the specific pathways by which this occurs. Perhaps answers lie in genetic studies. For example, people with an autosomal dominant mutation in the b1-adrenergic receptor gene (ADRB1-A187V) (106), a missense mutation in the DEC2 gene (BHLHE41) (107), or a point mutation in neuropeptide S receptor 1 gene (NPSR1) (108) seem to require less sleep than those in the general population. Further characterization of these genes may help uncover presently unknown mechanistic pathways for sleep duration and increase the impetus to identify genes that influence other dimensions of sleep health, such as sleep efficiency and satisfaction. Improved understanding of the overarching “genetics of sleep health” would enable clinical investigators to develop genetic (along with social-environmental) profiles for the sleep health of trial participants and patients, as well as to consider when stronger or weaker associations might be expected between sleep and health outcomes of interest.
3) Identify translational models that link sleep and circadian neuroscience to clinical outcomes with wider population relevance. Efforts to study other generalizable contexts that connect sleep to health are needed. One example is in neurodevelopment: A mother’s relative inability to sleep during pregnancy may be linked to increased adiposity in offspring (109), along with increased infant snoring (110), worse temperament (mood regulation) (110, 111), and more nighttime awakenings before consolidation of the sleep schedule (111). Given these observations, work in animal models might explore the biological mechanisms by which sleep disruption during pregnancy can influence long-term risk prevalence for physical and mental health conditions. Perhaps maternal sleep deficiency leads to changes in the uterine environment, thus provoking epigenetic reprogramming of sleep and arousal in offspring (112). Consequences related to maternal sleep deprivation have already been reported in rodents (113). Greater study of prenatal sleep in parents and neonatal sleep in infants may produce insights into conditions with important neurodevelopmental components, such as intellectual disability, autism, and schizophrenia.
4) Integrate the behavioral, social, and environmental determinants of sleep health into neuroscientific models that leverage a social-ecological framework. A future challenge in the sleep field will be to incorporate what we are learning at the population level, by monitoring morbidity reports and the effects of public health policy, and then translate it back to preclinical animal models. Such an effort would require closer collaboration between human and animal researchers to create reliable paradigms for translating findings between species with overlapping, but in many cases separate, determinants of sleep health. This issue is as difficult as it is urgent. Attempts at addressing it might start with the simple premise that rodent sleep in the lab is often socially impoverished relative to sleep in the wild or the sleep of humans. Cosleeping might be a factor that better unmasks relationships between sleep and health in lab animals, thus enabling more reliable application of findings to people.
5) Develop better technological strategies for tracking sleep health and translating these data into actionable insights (with all due legal and ethical considerations). Many of the challenges associated with translating real-world sleep into personalized health interventions involve engineering and computational challenges, such as developing better sensors, developing better strategies for combining and integrating multimodal time-series data, and applying artificial intelligence to predict changes in sleep metrics that will have tangible effects on cardiovascular, immune, and brain function. Additional challenges include reconceptualizing the role of measurement in interventions, as well as reconceptualizing the measurements themselves (95). For example, a better understanding of existing signals (such as EEG or photoplethysmography) could lead to additional physiological insights about sleep under real-world conditions and—within the home environment—could delineate how sleep trajectories change upon disease onset and progression, as well as during intervention attempts timed to different disease stages.
Opportunities
1) Sleep plays a fundamental role in human physiology. Knowledge about sleep can potentially improve pain management, chronic disease treatment, and cognitive outcomes in neurodegenerative disorders, along with coordinating drug bioavailability. For these indications and others, a new technology model can be envisioned for the bedroom as a treatment site within the home that becomes operational as we fall asleep. Applied sleep neuroscience should explore how sleep-wake rhythms can empower individuals to better respond to clinical interventions, as well as help individuals recover from each day’s pressures, thereby preparing them for tomorrow’s disease risk.
2) Sleep is associated with many aspects of mental and physical well-being. Although sleep is acknowledged as a major contributor to mental and physical health outcomes, this growing understanding has yet to be codified into NIH and other federal guidelines that would encourage clinical trial designers to incorporate sleep as a common variable of interest and to formalize the scheduling, col lection, and reporting of biosample collection with respect to time of day and an individual’s sleep phase. With such mandates in place along with open science practices, biomedical research could synthesize information from multiple levels of analysis to provide insights into the fundamental contributions of sleep to individual organ systems and emergent physiology, as well as how sleep modifies the course of disease and response to experimental treatments.
3) Sleep health itself is multidimensional. Many investigations concerning human sleep are limited by an overly narrow focus on sleep deprivation and primary sleep disorders, thus creating a false dichotomy between typical and atypical sleep. The very concept of sleep health, which moves beyond clinical disorders to emphasize the positive contributions of sleep to mental and physical well-being, suggests that all sleep gradations are relevant to health outcomes. To the extent that this is the case, each aspect of sleep can become a “lever arm” in neuroscience to perpetuate good health or improve disease-related outcomes.
4) Sleep health may represent a pathway for reducing health disparities. Structural barriers, working independently or collectively, are known to have adverse effects on sleep, with likely effects on mental and physical health outcomes that enhance racial and ethnic disparities in health care. Reductions in health disparities may be aided with community-level engagement, investments in social services, and data-driven policies that (i) increase awareness of the importance of sleep, (ii) create greater vigilance for primary sleep disorders, and (iii) encourage discussion of sleep problems with medical professionals. Public campaigns such as these offer the opportunity for sleep neuroscience to make a tangible difference in the day-to-day lives of people in greatest need of help.
5) Sleep is becoming easier and less expensive to assess in the real world. The evolution of sleep measurement outside the confines of the laboratory presents many opportunities to harness an individual’s own data in the service of personalized medical approaches that can improve the ease with which a person’s sleep is conceptualized in relation to their health. Artificial intelligence and other big-data analytics can also examine sleep at the cohort or population scale, thus improving the availability and utility of data gathered across multiple naturalistic contexts (104).
Conclusion
Future neuroscience research on sleep health presents challenges and opportunities that will be difficult to untangle. For instance, many social-environmental determinants of sleep health are not presently considered by medical professionals when placing an individual’s sleep in context in the clinic, thus creating ambiguity as to whether sleep differences are the product of disease or reflect the aggregated circumstances of the individual (e.g., age, race, gender, socioeconomic status, work and home environment). Moreover, although sleep is acknowledged in the scientific literature as making decisive contributions to normal and abnormal physiology, it has yet to be standardized in clinical trial design. Perhaps nowhere is this more salient than in AD trials, which exemplify lost opportunities to benchmark the effects of amyloid-lowering drugs with respect to a person’s previous and recent sleep history. Similar lost opportunities may be realized (in hindsight) for other medical conditions. In the present Review, we have attempted to itemize the core aspects of sleep health, describe the social-environmental determinants that contribute to each, and summarize how sleep ultimately factors into cardiovascular, metabolic, immunologic, mental, cognitive, and behavioral health. Along the way, we have pointed out areas of inquiry in neuroscience with the potential to accelerate the translation of sleep research from the lab to clinical practice. From distinguishing sleep states in single cells to understanding the genetics of sleep health, as well as from creating coherent translational bridges between lab animal and human sleep to creating credible and accessible sleep tracking technology in the home environment, neuroscience will be pivotal for integrating sleep health considerations into how we diagnose, monitor, treat, and test interventions for human brain disorders and other diseases. With any luck, the “chimera” of sleep can ultimately be leveraged at multiple interconnected levels to improve the mind and body of the individual, the collective well-being of society, and the essentially valuable parts of human existence.
Sleep health in context.
There are several dimensions to sleep health, such as regularity, satisfaction, alertness, timing, efficiency, and duration. Alone and in combination, these dimensions interact with many aspects of our general health, including cardiovascular, metabolic, immune, mental, behavioral, and cognitive health. Interactions between sleep and health are further shaped by individual- and social-level factors operating within larger societal factors. All occur in the context of the day-night cycle.
ACKNOWLEDGMENTS
The authors wish to thank L. Hale and C. Jackson for input on Fig. 1.
Funding:
This work was supported by grants from the National Institute on Drug Abuse (R01DA051321) and the National Institute on Minority Health and Health Disparities (R01MD011600).
Competing interests:
M.A.G. reports receiving grants from Jazz Pharmaceuticals, Kemin Foods, and CeraZ and performing consulting activities for Jazz Pharmaceuticals, Idorsia, Merck, Fitbit, Natrol, Smartypants Vitamins, Athleta, Nightfood, and Simple Habit in the past 3 years. F.-X.F. reports no potential conflicts of interest.
REFERENCES AND NOTES
- 1.Joiner WJ, Physiology 33, 317–327 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE, Neuron 68, 1023–1042 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franken P, Curr. Opin. Neurobiol 23, 864–872 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Rasch B, Born J, Physiol. Rev 93, 681–766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yankova G, Bogomyakova O, Tulupov A, Rev. Neurosci 10.1515/revneuro-2020-0106 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Allada R, Bass J, Engl N. J. Med 384, 550–561 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rechtschaffen A, Bergmann BM, Behav. Brain Res 69, 55–63 (1995). [DOI] [PubMed] [Google Scholar]
- 8.Dinges DF et al. , Sleep 20, 267–277 (1997). [PubMed] [Google Scholar]
- 9.Tefft BC, Accid. Anal. Prev 45, 180–186 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Basner M et al. , Sleep 30, 1085–1095 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buysse DJ, Sleep 37, 9–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakanishi-Minami T, Kishida K, Funahashi T, Shimomura I, Diabetol. Metab. Syndr 4, 18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edinger JD et al. , J. Clin. Sleep Med 11, 311–334 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandner MA, Perlis ML, JAMA Netw. Open 2, e1918214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkin TJ, Rupp T, Picchioni D, Wesensten NJ, Chest 134, 653–660 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Banks S, Van Dongen HP, Maislin G, Dinges DF, Sleep 33, 1013–1026 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster RG, Interface Focus 10, 20190098 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M, Clin J. Sleep Med 4, 487–504 (2008). [PMC free article] [PubMed] [Google Scholar]
- 19.Cespedes Feliciano EM et al. , Pediatrics 142, e20174085 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirshkowitz M et al. , Sleep Health 1, 233–243 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Paruthi S et al. , J. Clin. Sleep Med 12, 785–786 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandner MA, Hale L, Moore M, Patel NP, Sleep Med. Rev 14, 191–203 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandner MA et al. , Curr. Opin. Cardiol 31, 551–565 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng L, Zheng Y, Hui R, Hypertens. Res 36, 985–995 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St-Onge MP et al. , Circulation 134, e367–e386 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bock JM, Vungarala S, Karim S, Somers VK, Can. J. Cardiol 37, 756–765 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sofi F et al. , Eur. J. Prev. Cardiol 21, 57–64 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Hwang IC, Na KS, Lee YJ, Kang SG, Psychiatry Investig 15, 701–709 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jennum PJ, Plazzi G, Silvani A, Surkin LA, Dauvilliers Y, Sleep Med. Rev 58, 101440 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Grandner MA, Sleep Health 3, 393–400 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Zhai L, Zhang D, Sleep Med 15, 1456–1462 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Koren D, Taveras EM, Metabolism 84, 67–75 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Besedovsky L, Lange T, Born J, Pflugers Arch 463, 121–137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN, Nat. Sci. Sleep 5, 93–107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prather AA, Pressman SD, Miller GE, Cohen S, Int. J. Behav. Med 28, 151–158 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Pak VM, Grandner MA, Pack AI, Sleep Med. Rev 18, 25–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin MR, Olmstead R, Carroll JE, Biol. Psychiatry 80, 40–52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Besedovsky L, Lange T, Haack M, Physiol. Rev 99, 1325–1380 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hertenstein E et al. , Sleep Med. Rev 43, 96–105 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Perlis ML et al. , Sleep Med. Rev 29, 101–107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tubbs AS et al. , J. Clin. Psychiatry 81, 19m12964 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tubbs AS, Fernandez FX, Johnson DA, Perlis ML, Grandner MA, Clin J. Psychiatry 82, 20m13820 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Goldstein AN, Walker MP, Annu. Rev. Clin. Psychol 10, 679–708 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji X, Grandner MA, Liu J, Public Health Nutr 20, 687–701 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markwald RR et al. , Proc. Natl. Acad. Sci. U.S.A 110, 5695–5700 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaix A, Manoogian ENC, Melkani GC, Panda S, Annu. Rev. Nutr 39, 291–315 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson F, Malone SK, Lozano A, Grandner MA, Hanlon AL, Ann. Behav. Med 50, 715–726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patterson F et al. , Nicotine Tob. Res 21, 139–148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Killgore WDS, in Human Sleep and Cognition, Part I: Basic Research, Kerkhof GA, Van Dongen HPA, Eds. (Elsevier, 2010), chap. 7, pp. 105–129. [Google Scholar]
- 50.Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K, Curr. Opin. Psychiatry 27, 478–483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C, Holtzman DM, Neuropsychopharmacology 45, 104–120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hui SK, Grandner MA, J. Occup. Environ. Med 57, 1031–1038 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maia Q, Grandner MA, Findley J, Gurubhagavatula I, Accid. Anal. Prev 59, 618–622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uehli K et al. , Sleep Med. Rev 18, 61–73 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Grandner MA, Kripke DF, Psychosom. Med 66, 239–241 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghani SB et al. , Behav. Sleep Med 10.1080/15402002.2021.1902814 (2021). [DOI] [Google Scholar]
- 57.Greer SM, Goldstein AN, Walker MP, Nat. Commun 4, 2259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV, Sleep 27, 1255–1273 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Peppard PE et al. , Am. J. Epidemiol 177, 1006–1014 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ancoli-Israel S, Sleep Med 10 (suppl. 1), S7–S11 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Asplund R, in Principles and Practice of Geriatric Sleep Medicine, Pandi-Perumal SR, Monti JR, Monjan AA, Eds. (Cambridge Univ. Press, 2010), chap. 14, pp. 150–159. [Google Scholar]
- 62.Yeh P, Walters AS, Tsuang JW, Sleep Breath 16, 987–1007 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Zilli I, Ficca G, Salzarulo P, Sleep Med 10, 233–239 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Grandner MA et al. , Front. Neurol 6, 112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fox EC et al. , Sleep Health 4, 317–324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grandner MA, Schopfer EA, Sands-Lincoln M, Jackson N, Malhotra A, Obesity 23, 2491–2498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grandner M et al. , J. Clin. Sleep Med 14, 1031–1039 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Philip P et al. , J. Sleep Res 13, 105–110 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Hall MH, Kline CE, Nowakowski S, F1000Prime Rep 7, 63 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaver JL, Woods NF, Menopause 22, 899–915 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Sweet L, Arjyal S, Kuller JA, Dotters-Katz S, Obstet. Gynecol. Surv 75, 253–262 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Etindele Sosso FA, Holmes SD, Weinstein AA, Sleep Health 7, 417–428 (2021). [DOI] [PubMed] [Google Scholar]
- 73.Ahn S et al. , Sleep Med 81, 169–179 (2021). [DOI] [PubMed] [Google Scholar]
- 74.Hale L, Do DP, Sleep 30, 1096–1103 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grandner MA et al. , Sleep Med 11, 470–478 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA, Sleep 37, 601–611 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grandner MA et al. , Sleep 36, 769–779 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jackson CL, Walker JR, Brown MK, Das R, Jones NL, Sleep 43, zsaa037 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH, Sleep Med. Rev 22, 23–36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G, Sleep Med 18, 7–18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez SM, Grandner MA, Nazmi A, Canedo ER, Ritchie LD, Nutrients 11, 1419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bozick R, Troxel WM, Karoly LA, Sleep 44, zsab005 (2021). [DOI] [PubMed] [Google Scholar]
- 83.Pallesen S, Bjorvatn B, Waage S, Harris A, Sagoe D, Front. Psychol 12, 638252 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grandner MA, Am. J. Health Promot 32, 1629–1634 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barnes CM, Watson NF, Sleep Med. Rev 47, 112–118 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Mai QD, Hill TD, Vila-Henninger L, Grandner MA, J. Sleep Res 28, e12763 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perez E, Perrin PB, Lageman SK, Villaseñor T, Dzierzewski JM, Disabil. Rehabil 10.1080/09638288.2020.1814878 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hunter JC, Hayden KM, Public Health 162, 126–134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fuller-Rowell TE et al. , Sleep Med 81, 341–349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robbins R et al. , Sleep Med 60, 165–172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barnes CM, Drake CL, Perspect. Psychol. Sci 10, 733–737 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Grandner MA et al. , Sleep Health 1, 158–165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grandner MA et al. , Behav. Sleep Med 10, 235–249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grandner MA, Sleep Med. Clin 15, 319–340 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Grandner MA, Lujan MR, Ghani SB, Sleep 44, zsab071 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chinoy ED et al. , Sleep 44, zsaa291 (2021).33378539 [Google Scholar]
- 97.Baron KG, Abbott S, Jao N, Manalo N, Mullen R, Clin J. Sleep Med 13, 351–354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hale L et al. , Child Adolesc. Psychiatr. Clin. N. Am 27, 229–245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Phillips AJK et al. , Proc. Natl. Acad. Sci. U.S.A 116, 12019–12024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weaver E, Gradisar M, Dohnt H, Lovato N, Douglas P, Clin J. Sleep Med 6, 184–189 (2010). [PMC free article] [PubMed] [Google Scholar]
- 101.Carskadon MA, Pediatr. Clin. North Am 58, 637–647 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rezaei N, Grandner MA, Sleep Health 7, 303–313 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wickwire EM, Morin CM, Sleep Med. Rev 54, 101387 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Perez-Pozuelo I et al. , NPJ Digit. Med 3, 42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pacheco-Bernal I, Becerril-Pérez F, Aguilar-Arnal L, Clin. Epigenetics 11, 79 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi G et al. , Neuron 103, 1044–1055.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hirano A et al. , Proc. Natl. Acad. Sci. U.S.A 115, 3434–3439 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xing L et al. , Sci. Transl. Med 11, eaax2014 (2019).31619542 [Google Scholar]
- 109.Brener A et al. , Sci. Rep 10, 13979 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tauman R et al. , Am. J. Obstet. Gynecol 212, 656.e1–656.e7 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Nakahara K et al. , Sci. Rep 10, 11084 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Palagini L, Biber K, Riemann D, Sleep Med. Rev 18, 225–235 (2014). [DOI] [PubMed] [Google Scholar]
- 113.Pires GN et al. , J. Sleep Res 30, e13135 (2021). [DOI] [PubMed] [Google Scholar]


