Abstract
Breast carcinoma is highly metastatic and invasive. Tumor metastasis is a convoluted and multistep process involving tumor cell disseminating from their primary site and migrating to the secondary organ. Epithelial-mesenchymal transition (EMT) is one of the crucial steps that initiate cell progression, invasion, and metastasis. During EMT, epithelial cells alter their molecular features and acquire a mesenchymal phenotype. The regulation of EMT is centered by several signaling pathways, including primary mediators TGF-β, Notch, Wnt, TNF-α, Hedgehog, and RTKs. It is also affected by hypoxia and microRNAs (miRNAs). All these pathways are the convergence on the transcriptional factors such as Snail, Slug, Twist, and ZEB1/2. In addition, a line of evidence suggested that EMT and cancer stem like cells (CSCs) are associated. EMT associated cancer stem cells display mesenchymal phenotypes and resist to chemotherapy or targeted therapy. In this review, we highlighted recent discoveries in these signaling pathways and their regulation in breast cancer metastasis and invasion. While the clinical relevance of EMT and breast cancers remains controversial, we speculated a convergent signaling network pivotal to elucidating the transition of epithelial to mesenchymal phenotypes and onset of metastasis of breast cancer cells.
Keywords: Breast cancer, EMT, Metastasis, Cancer stem cells, Cancer signaling pathways
Introduction
Breast carcinoma is one of the most common types of cancer in women. It is the fifth most common diseases leading to death worldwide. Invasion and metastasis are responsible for almost 90% of breast cancer death.25 Molecular mechanisms underlying the cancer progression and metastasis have remained largely elusive.23 Therefore, it is of paramount importance to understand these processes. Extensive efforts have been made to unveil the metastatic events in the last two decades.122
Tumor metastasis is a multistep process involving tumor cell disseminating from their primary site and migrating to the secondary organ.59 It accompanies with a series of sequential steps, including cell invasion to a local tissue, intravasation to the blood circulation, transporting through vascularization, and extravasation to a secondary organ or tissue site.13 Mounting evidence suggested that the epithelial-mesenchymal transition (EMT) plays a crucial role in promoting metastasis in breast carcinoma, although the actual mechanisms remain controversial.128
EMT is a convoluted biological event in which epithelial cells lose their adhesiveness ability and gain mesenchymal characteristics.52 Three types of EMT have been discovered. Type 1 EMT is associated with embryogenesis, gastrulation, and neural formation; whereas type 2 is characterized by tissue regeneration and wound healing. Type 3 is linked to malignancy, invasion, and metastasis.55 EMT is considered as one of the hallmarks of cancer invasion.5 Morphologically, EMT program is characterized as an alteration in the phenotype from epithelial to mesenchymal cells.60 The regulation of EMT mechanisms is ensured by several signaling pathways, including primary mediators TGF-β, Notch, and Wnt, and is also affected by hypoxia and expression of microRNAs (miRNAs).91 All these pathways are the convergence on the transcriptional factors such as Snail, Slug, and Twist. Their expression leads to EMT.31
In this review, we first discussed breast cancer subtypes and their relationship with the EMT. We then highlighted EMT markers associated with breast cancer metastasis. Furthermore, we discussed signaling pathways involved in breast cancer EMT. In addition, we examined the effects of other factors such as hypoxia, miRNA, and alternative splicing on the breast cancer EMT. Finally, we stipulated the close relationship between EMT and the cancer stem cell and their impact on breast cancer metastasis.
Heterogeneity of Breast Cancers and EMT
Breast cancer is often a heterogeneous disease. It has long recognized that the diversity of cancer cells determines the progression of the cancer and its therapeutic resistance.26 The molecular classification of breast cancers empowers a design of individual therapies. It will ultimately increase the survival rate.21 It is, therefore, important to characterize the heterogeneity of breast cancer cells.
Subsets of breast cancer cells can be classified in several different ways. For instance, breast tumors can be categorized into six subgroups, i.e., normal-like, epidermal growth factor receptor (HER)-2+, luminal A, luminal B, basal-like, and claudin-low, based on their gene expression profile.17 The normal-like subgroup has a similar expression feature with noncancerous breast cells. The HER-2 has an impact on several signaling pathways and is associated with dysregulated tumor growth, oncogenesis, metastasis and chemoresistance in breast cancer.104 The overexpression of HER-2 is linked to a poor prognosis in chemo and targeted therapy.22 The luminal A and B subgroups often express luminal cytokeratin 8/18 and estrogen receptor in a different manner.28 The luminal A subgroup is associated with a higher estrogen receptor (ER) expression and a low HER-2 expression. The luminal B subgroup, however, is linked to a low ER expression and a high Ki67 index.95 The basal-like subgroup is characterized with the expression of biomarkers, including cadherin, p63, cytokeratin 17, vimentin, cytokeratin 5/6 and cytokeratin in the basal myoepithelial cells of normal breast tissues.92 The claudin-low subtype is associated by a low expression of cell-cell adhesion molecules, such as claudins 3, 4 and 7, occluding, and E-cadherin.18 The claudin-low subtype is linked to the presence of EMT and stem-cell like features.101 The basal-like and claudin-low subtypes are often found in triple-negative breast cancer (TNBC).119 TNBC is characterized by the lack of PR, ER and HER-2 hormone receptors.119 The over expression of estrogen and progesterone receptors are used as a predictive marker in hormone therapy.88 These hormone receptors are used as an adjuvant endocrine therapy in the regulation of breast tumorigenesis.88
These six subsets of breast cancers and their association with EMT displays major differences. For instance, Luminal A and B are the most common breast cancer subtypes discovered in patients. These subtypes express ERα and ERα signals that suppress EMT.117 It has been recognized that ERα expression in luminal A and B subtypes leads to a better prognosis as compared to TNBC subtypes. Ye, et. al. showed that ERα inhibits EMT via the suppression of Slug which leads to an increase in E-Cadherin expression.142 Another study reported that ERα promotes stemness and EMT in breast cancers by suppressing BM1.130 Therefore, ERα signaling is an essential regulating factor in luminal A and B subtypes that inhibit EMT in breast cancers.130
On the other hand, a number of studies suggested that a multipotent cytokine transforming growth factor β (TGF-β) can stimulate EMT in breast cancers. The TGF-β stimulation leads to an increase in Snail, TWIST, and ZEB 1/2 expression in the luminal A and B breast cancer cell lines.146 The TGF-β-induced EMT activates EGFR-, IGF1R-, and MAPK-dependent ERα signaling and increase antiestrogen resistance.146 Another pathway MEK-ERK is also associated with EMT in the luminal A and B subtypes of breast cancer cells.118 MCF7 is a luminal cancer cell line. The VEGFR expression in MCF7 is associated with the expression of Snail and N-CADHERIN.119 Taken together, it can be speculated that the ERα TGF-β, and MEK-ERK signaling pathways promote EMT in the luminal A and B subtypes.
EMT mechanisms of HER2+ breast cancer cells are similar to those in luminal subtypes. They also undergo TGF-β-dependent EMT.73 It was found that TGF-β–SMAD3 pathway is critical to EMT in HER2+ cancers.73 HER2 directly regulates the TGF-β expression and the activation of TGF-β/SMAD3 signaling. Additionally, the upregulation of transcription factors such as Slug and TWIST1 are crucial to EMT in HER+ breast cancer subtypes.78 One study showed that the AKT signaling activation increases the expression of Slug in HER2+ breast cancer cells such as MDA-MB-453 and BT474.78 Another regulator of TWIST1 is phosphorylated on Serine 68 residue in HER2+ invasive ductal carcinomas.10 It promotes breast cancer invasiveness.43 Another study revealed that the overexpression of HER2 in MCF7 cells increased the expression of tumor kinase (Btk)/protein tyrosine kinase 6 (PTK-6) receptors that induce EMT and invasiveness.2 Therefore, the TGF-β dependent pathways and TWIST and Slug transcription factors are crucial to EMT of HER2+ breast cancer cells.
Another subtypes, TNBCs or basal like, claudin-low are the most aggressive breast cancer cells. Due to their lack of hormone-responsive receptors, they have limited therapeutic options.139 TNBCs can be categorized into four groups; basal-like, mesenchymal, immunomodulatory, and luminal androgen receptor (AR)-positive subtypes.139 The basal-like subtype of TNBC is associated with cell cycle and DNA damage pathways.46 The ADP-ribose polymerase (PARP) inhibitors target to these pathways.85 Mesenchymal TNBC tumors are associated with growth factor signaling such as PI3K/AKT and EMT that are inhibited by mTOR and eribulin mesylate.74,96 Immunomodulatory TNBC are associated with immune cell signaling pathways such as NFκB and JAK/STAT pathways.84 Therefore, immune checkpoint inhibitors could give a promising response to the patients with immunomodulatory TNBC. Luminal androgen receptor (AR+) subtype tumors are associated with androgen-signaling and they can be treated by blocking the androgen receptor.97 It has been discovered that the EMT of TNBCs is associated with the Notch, Hedgehog, TGF-β, and Wnt pathways. High Notch expression in TNBC patients is associated with poor survival rates.36 The NUMB protein is used as an antagonist to Notch signaling to inhibit EMT in TNBC.36,147 Notch signaling is important to the TNBC subtypes. More studies are needed to confirm the connection between Notch and EMT in TNBC, however.
Another hedgehog pathway is also critical to EMT in breast cancer.8 It has been found that the hedgehog signaling activates three glioma-associated oncogenes, GLI1, 2, and 3 and GLI1. It is crucial to the EMT of breast cancer cells.8 The hedgehog signaling is also associated with hypoxia-induced EMT invasiveness of MDA-MB-231 TNBC cells.8 Like other types of breast cancer, TGF-β is also crucial to EMT and the stemness of MDA-MB-231 TNBC cells.124 Okita, et al. showed that musculoaponeurotic fibrosarcoma (MAF) oncogene family protein K (MAFK) induces EMT in a TGF-β-dependent manner in TNBC cells.82 It is suggested that TGF-β could be a hallmark regulator of EMT in these tumor cells. The Wnt signaling is another pathway that is crucial to TNBC metastasis. Upregulation of the Wnt/β-catenin signaling is associated with poor clinical outcomes in TNBC subtypes.90
Clearly, different breast cancer subsets display different behaviors under EMT. Breast cancer subtypes are regulated in different manners by EMT signaling pathways. Each pathway plays a different role in each subset. Therefore, targeting these pathways could be beneficial in terms of the drawback part of the breast cancer heterogeneity.
Epithelial-Mesenchymal Transition in Breast Cancer
The EMT is a natural and vital phenomenon in mesenchymal cell differentiation.11 This process is essential for many biological processes, including wound healing, embryonic development, etc.1 The correlation between EMT and cancer was first reported in the early 80s.99 It was discovered that benign tumor cells acquired invasive properties after EMT. Since then, the role of EMT in metastasis of certain carcinogenic tumors has been described in various studies.32 In lieu of increasing interest in understanding the relationship between EMT and cancer metastasis, the exact mechanism underlying EMT during cancer invasion and metastasis in breast cancer remains largely elusive. Nevertheless, signaling pathways involved in EMT and their associated transcription factors have been extensively investigated. Understanding these signaling pathways will help discover new therapeutic targets and the biomarkers for aggressive breast cancers.
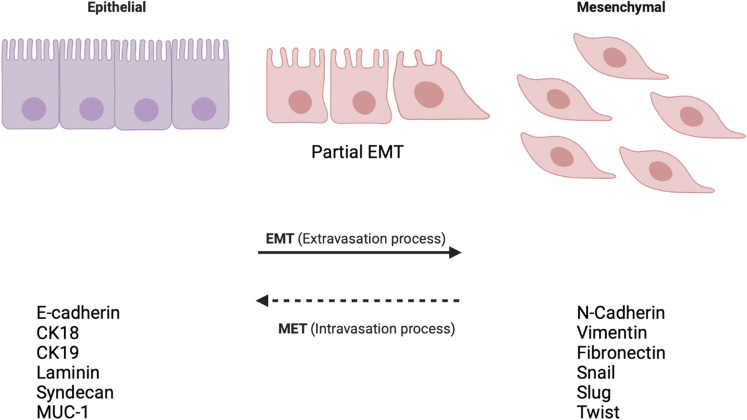
Metastasis starts when tumor cells gain the ability to disseminate from their primary tumor site and invade into the surrounding tissues that are referred to as stromal tissues. The dissemination occurs either as a group or as single cells.30 Epithelial tumor cells are usually connected with their surrounding cells through E-cadherin.141 E-cadherin is an adhesion molecule present in adherent junctions (AJ) that connect epithelial cells tightly.141 To metastasize, tumor cells break these intercellular junctions, migrate as single cells, and invade into neighboring tissues, as shown in Fig. 1. During EMT, epithelial cells lose their epithelial phenotype, cell-cell adhesiveness, and gain a mesenchymal phenotype enabling them to migrate and invade.68 An EMT process includes downregulating the expression of epithelial markers such as E-cadherin and cytokeratins, including CK18, CK19, laminin, Syndecan-1, MUC-1; and upregulating the expression of mesenchymal markers such as N-cadherin, vimentin, and fibronectin, Twist, Snail, and Slug.79 In breast cancer progression, EMT is the early stage of invasiveness, as tumor cells often undergo the EMT program that facilitates cell dissemination.120
Figure 1.
Epithelial cells lose their phenotype and gain a mesenchymal phenotype.
Signaling Pathways Responsible for Breast Cancer EMT
Regulators of EMT Pathways
EMT regulation involves various signaling pathways, including TGF-β, Wnt/β-catenin, Notch, TNF-α/ NF-κB, Hedgehog, and receptor tyrosine kinase (RTKs) signaling pathway.39 These signaling pathways regulate transcription factors, such as Snail, Zeb1/Zeb2, TWIST, miRNA, epigenetic regulators, and alternative splicing during breast cancer progression.75 In addition to promote the transformation of early-stage primary tumors into invasive malignancies, EMT has been implicated in the generation of cancer cells with stem cell-like characteristics, including increased self-renewal, tumor-initiating capabilities, and resistance to apoptosis and chemotherapy.109
TGF-β Pathway in EMT
TGF-β is one of the crucial cytokines inducing EMT programming.131 It plays a critical role in the activation of EMT and interacts with downstream signaling pathways during breast tumorigenesis.45 It is an essential cytokine in cell growth and proliferation. Dysregulation in TGF-β expression leads to various carcinoma including breast carcinogenesis.149 TGF-β signaling pathway is activated by intracellular Smad2/3 transducer proteins in EMT.41 In Smad-dependent signaling, there are three forms of TGF-β: TGF-β1, TGF-β2, and TGF-β3 signaling associated with three different receptors: types I, II, and III. TGF-β binds to TGF-βR-II, leading to the activation of TGF-βR-I, which then induces the Smad2/3-dependent signaling pathways.114 TGF-β receptors activate Smad2 and Smad3, leading to an activated complex of Smad2/3. Smad2/3 then complexes with Smad4.136 This complex regulates several transcription factors that control the expression of many EMT related genes. This regulation includes direct phosphorylation of the receptors of SMAD transcription factors and cell polarity regulation proteins.136 Studies suggested that a decrease in the expression of Smad2 and Smad3 links to the suppression of the invasiveness.132 The upregulation of Smad2 and Smad3 expression, on the other hand, is linked to EMT. In non-Smad signaling pathways, TGF-β triggers the AKT/PI3K, Ras/Raf/MEK/ERK, and Wnt/β-catenin signaling pathways that induce the expression of epithelial proteins.148 Both Smad-dependent and Non-Smad pathways work together to regulate the transcription factors, including Snail, Slug, ZEB1/2, and Twist.60 TGF-β crosstalk with other signaling pathways, including Notch, Wnt/β-catenin, nuclear factor (NF)–κB, and RTKs, induces EMT and plays critical roles in maintaining the mesenchymal phenotype of invasive/metastatic tumor cells.87 During EMT, TGF-β signaling alters the tight junction formation in epithelial cells and activates other signaling pathways, including Wnt, Notch, and Hh MAPK pathways. TGF-β regulates several gene expressions, including the transcription factor, SNAI1 and SNAI2/Slug, ZEB ZEB (ZEB1 and ZEB2/SIP1, Six family of homeobox (Six1), and Twist.112 It then regulates transcription of E-cadherin, occludin and claudin.87 In breast carcinoma, the upregulation of TGF-β is associated with an increase in EMT.39 Recent study revealed its connection with breast cancer stem cells in EMT.
The Wnt/ β-catenin pathway in EMT
The Wnt/β-catenin pathway is considered exclusively crucial to the breast cancer EMT programming. A growing number of studies revealed that the Wnt signaling involves in the breast cancer cells’ metastasis, immune microenvironment, stemness maintenance, and therapeutic resistance.138 There has been 19 Wnt genes, 10 Frizzled (Fzd) receptors, and 2 low density lipoprotein receptor-related protein (LRP) co-receptors reported currently.98
The Wnt signaling is regulated by canonical or non-canonical signaling.56 Canonical signaling is associated with β-catenin-dependent expression. It has been reported that β-catenin accumulation in nucleus is linked to a poor prognosis in breast cancer. On the other hand, non-canonical Wnt signaling is β-catenin-independent and do not lead to β-catenin expression in nucleus.69 GSK-3β has been found to play a role in regulating β-catenin expression. It was reported that upregulation in GSK3β phosphorylation leads to the degradation of β-catenin, altering the Wnt signaling.7Wnt can stabilize the levels of Snail and β-catenin to induce EMT and cancer metastasis by blocking the activity of GSK-3β.153
Three Wnt signaling pathways, including Wnt/β-Catenin, Wnt–planar cell polarity (PCP), and Wnt–Ca2 signaling, have been discovered for their roles in breast cancer progression. The Wnt/β-catenin is activated by the upregulation of SNAI1expression.54 It leads to the downregulation of E-cadherin and upregulation of vimentin in breast cancer cells.54 It has been reported that breast carcinoma subsets are induced by abnormal β-catenin expression and its subcellular localization, linked with the activation of the Wnt signaling pathway.33 Some studies showed that the increase in β-catenin expression plays an important role in the development of breast carcinoma together with alterations in the Wnt pathway.76 While the Wnt pathway is considered to be linked to the EMT in breast carcinoma, β-catenin alone is insufficient to induce EMT.91 One of the studies in spindle lesions of the breast found the aberrant β-catenin expression in metaplastic breast carcinomas, suggesting the activation of the Wnt pathway in at least a subset of breast cancers.58 β-catenin also acts as a molecular bridge to enhance cell-cell adhesion in the tight junctions of epithelial cells.123 The stabilization of β-catenin and the activation of the Wnt signaling pathway are association with a transcription factor (TCF/LEF) and many other factors during EMT.113
Notch Pathway in EMT
In mammal cell development, the Notch pathway regulates cell survive. It plays a critical role in initiating and progressing cancer development.65 Four Notch receptors (Notch1–4) and five ligands (Jagged1, 2 and Delta-like1, 3, 4) have been discovered.125 The Notch signaling activates the NF-κB pathway and modulates TGF-β of the EMT programming.24 Notch signaling is Numb-mediated. Numb is a negative regulator of EMT in both human epithelial cells and breast cancer cells. Reduced Numb expression is found to be associated with the upregulation of EMT.147 Studies have revealed a potential connection between the overexpression of Notch signaling and the overall poor survival rate of breast cancer patients.147 Notch signaling synchronizes with other pathways to induce EMT.126 Notch signaling coordinates Jagged 1, a Notch ligand; and HEY1, a Notch target gene.126
Recent studies reported that the Notch signaling is linked to the maintenance of a cancer stem cell population in breast cancers.144 The Notch signaling regulates the Snail expression either by transcriptionally activating Snail or via lysyl oxidase (LOX).29 The Notch signaling upregulates LOX by activating a hypoxia-inducible factor 1-α (HIF-1α), which leads to stabilize Snail and ultimately results in upregulating EMT programming that causes the invasion of cancer cells.102 It has been shown that hypoxia-induced Notch activation promotes EMT in breast cancer cells by upregulating the expression of HEY2 and HES1, resulting in an increase in cell migration and invasion of breast cancer.110 Recent data suggested that breast carcinoma and increased Notch expression are linked to progression and poor prognosis.57 Additional data implied that positive regulation of Slug by Jagged1-mediated activation of Notch IC leads to the suppression of E-Cadherin, causing EMT induction in breast cancers.126 A number of studies suggested a link between hypoxia and Notch activity. Hypoxia is one of the critical regulators of tumor metastasis. Notch serves as a critical intermediate in conveying the hypoxic response into EMT.47 Another study suggested an interplay between TGF-β and Notch activity. Increased Notch activity through Smad3 boosts Jagged1 and HEY1 expression, resulting in an increase in Slug expression and ultimately leading to the E-cadherin suppression.126 Notch also regulates Snail-1 expression and lysyl oxidase (LOX).102 The association of Snail with LOX expression and hypoxia signaling mechanisms has been speculated.47
TNF-α/ NF-κB signaling pathway in EMT
Tumor necrosis factor-α (TNF-α) is a 26 kDa transmembrane protein. It is cleaved into a soluble 17 kDa by TNF-α-converting enzyme (TACE).44,134 It is an essential cytokine involved in several processes, including inflammation, cellular homeostasis, and tumor progression.64 TNF-α promotes angiogenesis, invasion, and metastasis associated with the EMT reprogramming134, through counteracting with E-cadherin and the activation of MMP9.64 The induction of TNF-α in EMT is associated with the upregulation of Twist-1. The upregulation of TNF-α has shown to be associated with increased metastatic potential and invasiveness of breast cancer cells.72 It has been shown that an increase in Twist-1 expression regulates EMT and cancer stemness properties induced by TNF-α.152 Recent evidence suggested that Twist-1 expression promotes breast cancer cell metastasis in mice.135 It has been discovered recently that a long-time exposure to TNFα activates IKKβ and NF-κB, resulting in the upregulation of the transcriptional repressor Twist1 and EMT as well as cancer stemness properties.64,127 In particular, a study from breast cancer patients showed that there is an direct link between TNF-α production by peripheral blood T lymphocytes and circulating tumor cells expressing EMT markers.63 Evidence suggested that the activation of NF-κB is associated with Snail, Slug, Twist, and ZEB1/ZEB2.64 It was found that Snail can be stabilized via the activation of NF-κB by GSK-3β degradation in TNF-α/NF-κB activation pathway.64 NF-κB is also responsible for the activation of vimentin and matrix metalloproteinases (MMPs) of mesenchymal cell markers.100
The hedgehog pathway in EMT
Another signaling pathway in breast cancer EMT is related to stem cell renewal hedgehog (Hh) pathway. The Hh pathway involves in embryonic development, stem cell renewal, and tissue homeostasis4. In the Hh pathway, three glioma-associated oncogene (GLI) transcription factors, including GLI1, GLI2, and GLI3, are responsible for activating or repressing the transcription of these factors.80 A line of evidence suggested the contribution of the Hh pathways to EMT in breast cancers. Colavito, et al. reported that a high level of expression of GLI1 in breast cancer cells that undergo EMT.15 It was also shown that the Hh pathway is associated with cancer stem cell properties and crosstalk between NFkB and GLI1.105 As classified in the Wnt pathways, there are also canonical and non-canonical signaling in the Hh pathway. A study suggested that non-canonical activation of GLI1 via hypoxia or other inflammatory cytokines induces EMT, drug resistance, and invasion in breast cancer cells.48 GLI1 expression and their contribution to EMT in breast cancers via the Hh pathway was confirmed using mouse models.53
Receptor tyrosine kinase (RTK) signaling pathway in EMT
There are several RTK identified for their contribution to EMT of breast cancer cells.60 Hepatocyte growth factor (HGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF) activate RTKs. HGF signals is associated with epithelial differentiation by downregulating E-cadherin and is linked to tumor metastasis. The HGF pathway is also associated with Snail transcription factor that induces EMT.60
MAPK or PI3K are two signaling that regulate EMT and invasion in breast cancer together with TGF-β.39 Ras-activated MAPK induces EMT and invasion of breast cancer cells by promoting the Twist1 serine 68 phoshorylation and stabilization of PI3K signaling.116 Studies also suggested that the Wnt and EGFR signaling are associated with the RTK pathways.62 Although studies suggested the importance of the RTK signaling and their contribution to EMT, the EMT mechanism is composed of various signaling pathways. The activation of RTK itself may not be sufficient to elicit EMT. The contribution of other pathways, including TGF-β, Wnt, Notch, NF-κB, and ERK/MAPK pathways may lead to the EMT establishment.
Hypoxia in EMT
Hypoxia is a crucial factor in tumor microenvironment (TME) that regulates multiple hallmarks of cancer behaviors, such as angiogenesis, invasion, EMT, stemness and immune evasion.77 Hypoxia is considered to be one of the major physiological causes for EMT.60 It is associated with the HIF1α, hepatocyte growth factor (HGF), SNAI1, and TWIST1; the activation of the Notch or NF-κB pathways; and the induction of DNA hypomethylation.60 In breast cancer development, the hypoxic microenvironment plays a prefunding role in the progression of the disease.35 Hypoxia-related gene expression is linked to a poor prognosis of the disease.51 It has been reported that the expression of HIF-1α leads to endocrine therapy resistance to ERα+ breast cancer cells.51
It has been shown that 3% oxygen levels can induce EMT, by inhibiting the activity of GSK3β and sparing β-catenin from phosphorylation and subsequent destruction.61 In other studies, the activation of Notch signaling is required for hypoxia-induced EMT by activating the Wnt/β-catenin signaling with the resulting stimulation of Snail1.126
In addition to the HIF pathways, recent studies showed that there are also other signaling pathways in hypoxia that may be linked to EMT.115 For example, AMP-activated protein kinase (AMPK), adenosine monophosphate (AMP)/adenosine triphosphate (ATP), or adenosine diphosphate (ADP)/ATP can also be upregulated by hypoxia.115 It has been reported by Saxena, et al. that the AMPK activation could lead to the upregulation of TWIST1 expression that promotes EMT in breast cancer.107 On contrary, Chou, et al. reported that the AMPK activation by another activator OSU-53 suppresses EMT induction by modulating the Akt-MDM2-Foxo3 signaling axis.14 Another signaling pathway is PI3K-Akt-mTOR, a mediator that plays a big role in cell proliferation, nutrient uptake, anabolic reactions and autophagy.40 This pathway is found to be activated by hypoxia.40 PI3K-Akt-mTOR has been considered as a mediator of TGF-β signaling through non-SMAD pathway.121 TGF-β may activate PI3K directly or by activating epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) receptors in various cell types.38 It has been found that PI3K-Akt-mTOR could influence HIF-1α activation, as mTOR is an upstream mediator of HIF-1α.150 mTOR regulates its translation via phosphorylation of 4E-BP1, which in turn inhibits the interaction of eIF4E with translation initiation complex and results in mRNA translation activation that facilitates HIF-1α protein synthesis.150 These controversial research results suggested that more studies are needed to prove hypoxia driven PI3K-Akt-mTOR in EMT.
Another pathway is the MAPK signaling. MAPK factors are hypoxia-related signaling. They are involved in EMT induction through non-SMAD TGF-β signaling.115,83 There are three MAPKs, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MARK. Among them, ERK-mediated hypoxia inducts EMT through E-cadherin regulation.20 Further studies suggested that type 1 and 3 ERK’s along with other type of MAPKs including (JNK) and p38 MAPK are found to be able to stabilize the phosphorylation site of TWIST1 for EMT induction in breast cancer cells.20 While hypoxia is always considered to be in a HIF-mediated manner, recent studies showed the potential inducement of EMT by AMPK, PI3K-Akt-mTOR and MAPKs, suggesting a non-HIF manner EMT induction. More studies are need to reveal the potential of mediation behind EMT.
MicroRNAs (MiRNA) in EMT
miRNAs are small noncoding RNAs that regulate gene expression by binding to and destroying a target mRNA, resulting in a reduction in protein expression encoded by the mRNAs.108 For instance, miRNA-200c and MiR-9 have been found to regulate transcription factors, ZEB1 and ZEB2 and E-cadherin expression in non-small lung cancers to promote or suppress EMT.81 These dual functions increase the complexity of revealing the miRNA’s actual roles played in EMT programming. miRNA-200c is a member of the tumor suppressive family of miRNAs.27 A line of evidence suggested that EMT is suppressed in triple negative breast cancer cells that do not express miRNA-200c.89 Another miR-335 was found to be linked to the suppression of breast cancer.143 A correlation between the expression of miR-335 and 20 primary breast tumors has been investigated extensively. It was reported that miR-335 downregulated metastatic genes in breast cancer, and the loss of miR-335 may serve as a negative prognostic indicator.143 miR-300 was found to target to Twist and downregulate EMT and invasion of breast cancer.151 The extensive review on the roles of miRNAs played in EMT programing of breast cancer can be found in the literature.151,145
The discovery of unique roles of miRNAs played in either suppressing or promoting breast cancer cells’ EMT makes them a good target for drug development.50 Nevertheless, the miRNA–mRNA interactions are complicated. Each miRNA may potentially target to hundreds of mRNAs, or vice versa. Since each small interfering RNA can interact with millions of target mRNAs, their impact on gene expression can be significant, however .34 Therefore, more comprehensive analyses are necessary to understanding the miRNAs and their signaling pathways.34,106
Alternative Splicing and EMT
A line of evidence suggested that the alternation of mRNA splicing during EMT leads to the production of diverse protein isoforms in turned mesenchymal cells.129 Such alternative splicing has been found in p120 catenin, CD44, the RTK, FGFR2 (REFS), etc. that regulate EMT.129 Studies revealed that these alternative splicing are regulated by splicing regulatory proteins 1 (ESRP1) and 2 (ESRP2), two RNA proteins that control the splicing of many gene transcripts.129 The downregulation of these two proteins results in changes in mesenchymal protein isoforms, including adhesion, motility, and signaling pathways.129 During EMT, other changes in splicing also occur, leading to an increase in EMT associated protein expression, including FOX1 homologue2 (RBFOX2), another RNA binding protein that promotes EMT and cell invasion.3 There is another splicing factor, Ser-Arg-rich splicing factor 1 (SRSF1) that promotes EMT by splicing the mRNA encoding receptor of RTK, (RON1) and the GTPase RAC1.37
Snail /Slug/Twist
Slug, Snail, and Twist are transcription factors that regulate the expression of tumor suppression proteins.71 Studies suggested that abnormal expression of these three transcription factors in breast cancer cells. It has been speculated that they play a key role in breast cancer progression.140 Twist is indeed one of the transcription factors that play a role in cell migration and tissue development during embryogenesis.140 It is also an important regulator in the EMT mechanism.140 Twist expression is correlated to an increase in the expression of mesenchymal markers, such as fibronectin, vimentin, αSMA, and N-cadherin.12 The upregulation of Twist expression is correlated to a poor prognosis in cancers.93
Snail and Slug are the members of Snail family sharing a SNAG domain at the N and C terminal regions that bind to E-boxes in the regulatory regions of target genes.19 However, Snail-mediated histone modifications lead to the repressing of E-cadherin expression. The mechanism underlying these modification remains unknown.86 Posttranscriptional modifications affect activity of the Snail1 and Snail2. These modifications involve many signaling factors, including TGFβ, Notch, tumor necrosis factor-α (TNF-α), EGF, FGF, Wnt, Shh, SCF/c-kit, hypoxia, and estrogens in breast cancer.137
Cancer Stem Cells (CSCs) and EMT
A growing evidence links EMT to CSCs, as more and more studies suggested that CSCs phenotype contributes to the metastasis and drug resistance.94 Mani, et al . first detected presence of the stem cell CD44highCD24low population in epithelial cells that undergo EMT.70 More studies have shown that cells undergoing EMT can acquire stem cell-like characteristics.111 However, the molecular connection between EMT and CSCs has not been explicated yet.6 It has been suggested that breast epithelial cells undergoing EMT has similar gene expression profile as of mesenchymal stem cells.133 Creighton, et al. demonstrated that breast cancers are found to be enriched in EMT and stem cell phenotype after endocrine (letrozole) and chemotherapy (docetaxel).16 It is postulated that these cells may be tumor initiators and responsible for disease persistence, spreading, and refractoriness to chemotherapy.16
CSCs are able to undergo invasion, migration, survival, colonization, and metastasis.67 In tumor development, CSCs are the ones that can self-renew and trigger tumorigenesis. They are CD44+/CD24−.49 Breast cancer stem cells display undergoing EMT at the site of primary tumors. It has been understood that EMT-related genes in CD44+/CD24− cells are expressed higher in primary breast cancer cells with stem cell features.70
CSCs are crucial to EMT. Their stem cell phenotype shows an increased resistance to apoptosis.112 It has been reported that breast cancer patients undergoing chemotherapy/neoadjuvant therapy have a higher CD44+/CD24− cell expressing EMT-associated genes that were found in the posttreatment biopsy.103 Recent evidences suggested that the EMT may facilitate the generation of cancer cells with the mesenchymal features needed for dissemination and the self-renewal properties needed for initiating secondary tumors.42 Another study suggested that the expression of Snail and Twist increases the tumor sphere formation in immortalized human mammary epithelial cells (HMLE) and yields more cells with a CD44high/CD24low stem cell feature.128
Studies also suggested that there may be a direct link between EMT and CSCs-like properties, which may be prerequisites for cancer cell metastasis. A major driving force for these processes is the TGF-β signaling pathway.112 Another signaling pathway, the ZEB/miR-200 is also associated with EMT-CSC linkage. This feedback loop of ZEB/miR-200 is a driving force for cancer progression towards metastasis by controlling the state of CSCs.9 Recent evidences showed that the activation of β-catenin and the Akt pathways by Twist are critical for the maintenance of EMT-associated and CSC-like features.66
Remarks
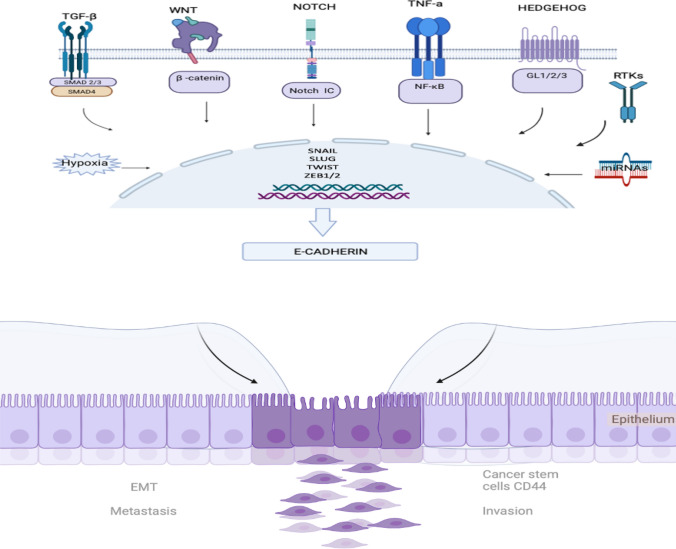
EMT mechanism manifests itself through various signal pathways, including TGF-β pathway, Wnt/β-catenin pathway, Notch pathway, and TNF-α/ NF-κB signaling pathway. These pathways orchestrate EMT and metastasis events in breast cancers. Each signal is a chain of cascades that affect one another. Examining these pathways leads to a hypothesis that the convergence of all these pathways on the transcription factors such as Snail, Zeb, and Twist leads to EMT, as illustrated in Fig. 2.
Figure 2.
The convergence of EMT signaling pathways.
A growing evidence suggested that breast cancer stem cells affect EMT. Examining cancer stem cells in more detail revealed their relationship with the EMT and onset of the metastasis. Further study along these lines would shed light on the treating and/or preventing metastasis of breast cancer. In this context, understanding EMT mechanisms and determining external causes will help develop a better treatment for breast cancer cells.
Conflict of Interest
B.B., S.J. and K.Y. have no conflicts of interest to disclose.
Ethical Approval
No human subjects or animal research was performed as part of this study. The images were created in Biorender.com
Abbreviations
- AJ
Adherent junctions
- AS
Alternative splicing
- CSCs
Cancer stem cells
- CT
Chemotherapy
- EMT
Epithelial Mesenchymal Cell Transition
- ER
Estrogen receptor
- HER-2
Human epidermal growth factor receptor 2
- miRNA
MicroRNA
- PR
Progesterone receptor
- TJ
Tight junctions
- TGF
Transforming growth factor
- TNBC
Triple-negative breast cancer
- TNF
Tumor necrosis factor
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J. Clin. Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai M, Liang K, Lu Y, Qiu S, Fan Z. Brk/PTK6 cooperates with HER2 and Src in regulating breast cancer cell survival and epithelial-to-mesenchymal transition. Cancer Biol. Ther. 2013;14:237–245. doi: 10.4161/cbt.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiello NM, Kang Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 2019;216:1016–1026. doi: 10.1084/jem.20181827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armas-Lopez L, Zuniga J, Arrieta O, Avila-Moreno F. The Hedgehog-GLI pathway in embryonic development and cancer: implications for pulmonary oncology therapy. Oncotarget. 2017;8:60684–60703. doi: 10.18632/oncotarget.19527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barriere G, Fici P, Gallerani G, Fabbri F, Rigaud M. Epithelial mesenchymal transition: a double-edged sword. Clin. Transl. Med. 2015;4:14. doi: 10.1186/s40169-015-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battula VL, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–1445. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol. Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhateja, P., Cherian, M., Majumder, S. & Ramaswamy, B. The Hedgehog Signaling Pathway: A Viable Target in Breast Cancer? Cancers (Basel)11 (2019). [DOI] [PMC free article] [PubMed]
- 9.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop–a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter RL, Paw I, Dewhirst MW, Lo HW. Akt phosphorylates and activates HSF-1 independent of heat shock, leading to Slug overexpression and epithelial-mesenchymal transition (EMT) of HER2-overexpressing breast cancer cells. Oncogene. 2015;34:546–557. doi: 10.1038/onc.2013.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T, You Y, Jiang H, Wang ZZ. Epithelial-mesenchymal transition (EMT): a biological process in the development, stem cell differentiation, and tumorigenesis. J. Cell Physiol. 2017;232:3261–3272. doi: 10.1002/jcp.25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang C, Ayyanathan K. Snail/Gfi-1 (SNAG) family zinc finger proteins in transcription regulation, chromatin dynamics, cell signaling, development, and disease. Cytokine Growth Factor Rev. 2013;24:123–131. doi: 10.1016/j.cytogfr.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang SP, Cabrera RM, Segall JE. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 2016;311:C1–C14. doi: 10.1152/ajpcell.00238.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou CC, et al. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res. 2014;74:4783–4795. doi: 10.1158/0008-5472.CAN-14-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colavito SA, Zou MR, Yan Q, Nguyen DX, Stern DF. Significance of glioma-associated oncogene homolog 1 (GLI1) expression in claudin-low breast cancer and crosstalk with the nuclear factor kappa-light-chain-enhancer of activated B cells (NFkappaB) pathway. Breast Cancer Res. 2014;16:444. doi: 10.1186/s13058-014-0444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creighton CJ, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai X, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015;5:2929–2943. [PMC free article] [PubMed] [Google Scholar]
- 18.Dias K, et al. Claudin-low breast cancer; clinical & pathological characteristics. PLoS ONE. 2017;12:e0168669. doi: 10.1371/journal.pone.0168669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz VM, Vinas-Castells R, Garcia de Herreros A. Regulation of the protein stability of EMT transcription factors. Cell. Adh Migr. 2014;8:418–428. doi: 10.4161/19336918.2014.969998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do HTT, Cho J. Involvement of the ERK/HIF-1alpha/EMT pathway in XCL1-induced migration of MDA-MB-231 and SK-BR-3 breast cancer cells. Int. J. Mol. Sci. 2020;22:89. doi: 10.3390/ijms22010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eliyatkin N, Yalcin E, Zengel B, Aktas S, Vardar E. Molecular classification of breast carcinoma: from traditional, old-fashioned way to a new age, and a new way. J. Breast Health. 2015;11:59–66. doi: 10.5152/tjbh.2015.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.English DP, Roque DM, Santin AD. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Mol. Diagn. Ther. 2013;17:85–99. doi: 10.1007/s40291-013-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct. Target Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farnie G, Clarke RB. Mammary stem cells and breast cancer–role of Notch signalling. Stem Cell Rev. 2007;3:169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 25.Fedele M, Cerchia L, Chiappetta G. The epithelial-to-mesenchymal transition in breast cancer: focus on basal-like carcinomas. Cancers (Basel) 2017;9:134. doi: 10.3390/cancers9100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Y, et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106. doi: 10.1016/j.gendis.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng X, Wang Z, Fillmore R, Xi Y. MiR-200, a new star miRNA in human cancer. Cancer Lett. 2014;344:166–173. doi: 10.1016/j.canlet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg. Oncol. Clin. N.Am. 2018;27:95–120. doi: 10.1016/j.soc.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Francesco EM, Maggiolini M, Musti AM. Crosstalk between Notch, HIF-1alpha and GPER in breast cancer EMT. Int. J. Mol. Sci. 2018;19:2011. doi: 10.3390/ijms19072011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Garg M. Epithelial-mesenchymal transition - activating transcription factors - multifunctional regulators in cancer. World J. Stem Cells. 2013;5:188–195. doi: 10.4252/wjsc.v5.i4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georgakopoulos-Soares I, Chartoumpekis DV, Kyriazopoulou V, Zaravinos A. EMT factors and metabolic pathways in cancer. Front. Oncol. 2020;10:499. doi: 10.3389/fonc.2020.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geyer FC, et al. beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod. Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 34.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilkes DM, Semenza GL. Role of hypoxia-inducible factors in breast cancer metastasis. Future Oncol. 2013;9:1623–1636. doi: 10.2217/fon.13.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giuli MV, Giuliani E, Screpanti I, Bellavia D, Checquolo S. Notch Signaling Activation as a Hallmark for Triple-Negative Breast Cancer Subtype. J Oncol. 2019;2019:8707053. doi: 10.1155/2019/8707053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goncalves V, Pereira JFS, Jordan P. Signaling pathways driving aberrant splicing in cancer cells. Genes (Basel) 2017;9:9. doi: 10.3390/genes9010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao Y, Baker D, Ten Dijke P. TGF-beta-Mediated epithelial-mesenchymal transition and cancer metastasis. Int. J. Mol. Sci. 2019;20:2767. doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassan B, Akcakanat A, Holder AM, Meric-Bernstam F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surg. Oncol. Clin. N. Am. 2013;22:641–664. doi: 10.1016/j.soc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hata A, Chen YG. TGF-beta Signaling from receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016;8:a022061. doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol. Neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 43.Hong J, et al. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res. 2011;71:3980–3990. doi: 10.1158/0008-5472.CAN-10-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 2010;49:1215–1228. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hua W, Ten Dijke P, Kostidis S, Giera M, Hornsveld M. TGFbeta-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer. Cell Mol. Life Sci. 2020;77:2103–2123. doi: 10.1007/s00018-019-03398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hubalek M, Czech T, Muller H. Biological subtypes of triple-negative breast cancer. Breast Care (Basel) 2017;12:8–14. doi: 10.1159/000455820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishida T, Hijioka H, Kume K, Miyawaki A, Nakamura N. Notch signaling induces EMT in OSCC cell lines in a hypoxic environment. Oncol. Lett. 2013;6:1201–1206. doi: 10.3892/ol.2013.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeng KS, Sheen IS, Leu CM, Tseng PH, Chang CF. The role of smoothened in cancer. Int. J. Mol. Sci. 2020;21:6863. doi: 10.3390/ijms21186863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji J, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin. Oncol. 2012;39:461–472. doi: 10.1053/j.seminoncol.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang W, Peng J, Zhang Y, Cho WC, Jin K. The implications of cancer stem cells for cancer therapy. Int. J. Mol. Sci. 2012;13:16636–16657. doi: 10.3390/ijms131216636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jogi A, Ehinger A, Hartman L, Alkner S. Expression of HIF-1alpha is related to a poor prognosis and tamoxifen resistance in contralateral breast cancer. PLoS ONE. 2019;14:e0226150. doi: 10.1371/journal.pone.0226150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasper M, Jaks V, Fiaschi M, Toftgard R. Hedgehog signalling in breast cancer. Carcinogenesis. 2009;30:903–911. doi: 10.1093/carcin/bgp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katz E, et al. An in vitro model that recapitulates the epithelial to mesenchymal transition (EMT) in human breast cancer. PLoS ONE. 2011;6:e17083. doi: 10.1371/journal.pone.0017083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim DH, et al. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: a comprehensive overview. J. Clin. Med. 2017;7:1. doi: 10.3390/jcm7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kontomanolis EN, et al. The notch pathway in breast cancer progression. Sci. World J. 2018 doi: 10.1155/2018/2415489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lacroix-Triki M, et al. beta-catenin/Wnt signalling pathway in fibromatosis, metaplastic carcinomas and phyllodes tumours of the breast. Mod. Pathol. 2010;23:1438–1448. doi: 10.1038/modpathol.2010.141. [DOI] [PubMed] [Google Scholar]
- 59.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SY, et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol. Cancer. 2017;16:10. doi: 10.1186/s12943-016-0577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leone K, Poggiana C, Zamarchi R. The interplay between circulating tumor cells and the immune system: from immune escape to cancer immunotherapy. Diagnostics (Basel) 2018;8:59. doi: 10.3390/diagnostics8030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li CW, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, et al. Regulation of EMT by Notch signaling pathway in tumor progression. Curr. Cancer Drug Targets. 2013;13:957–962. doi: 10.2174/15680096113136660101. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Zhou BP. Activation of beta-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer. 2011;11:49. doi: 10.1186/1471-2407-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao WT, Ye YP, Deng YJ, Bian XW, Ding YQ. Metastatic cancer stem cells: from the concept to therapeutics. Am. J. Stem Cells. 2014;3:46–62. [PMC free article] [PubMed] [Google Scholar]
- 68.Lindsey S, Langhans SA. Epidermal growth factor signaling in transformed cells. Int. Rev. Cell Mol. Biol. 2015;314:1–41. doi: 10.1016/bs.ircmb.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann. Surg. Oncol. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 72.Mercogliano MF, Bruni S, Elizalde PV, Schillaci R. Tumor necrosis factor alpha blockade: an opportunity to tackle breast cancer. Front. Oncol. 2020;10:584. doi: 10.3389/fonc.2020.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mezencev R, Matyunina LV, Jabbari N, McDonald JF. Snail-induced epithelial-to-mesenchymal transition of MCF-7 breast cancer cells: systems analysis of molecular changes and their effect on radiation and drug sensitivity. BMC Cancer. 2016;16:236. doi: 10.1186/s12885-016-2274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mezi S, et al. Standard of care and promising new agents for the treatment of mesenchymal triple-negative breast cancer. Cancers (Basel) 2021;13:1080. doi: 10.3390/cancers13051080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moyret-Lalle C, Ruiz E, Puisieux A. Epithelial-mesenchymal transition transcription factors and miRNAs: "Plastic surgeons" of breast cancer. World J. Clin. Oncol. 2014;5:311–322. doi: 10.5306/wjco.v5.i3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mukherjee N, et al. Subtype-specific alterations of the Wnt signaling pathway in breast cancer: clinical and prognostic significance. Cancer Sci. 2012;103:210–220. doi: 10.1111/j.1349-7006.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- 77.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nami B, Wang Z. HER2 in breast cancer stemness: a negative feedback loop towards Trastuzumab resistance. Cancers (Basel) 2017;9:40. doi: 10.3390/cancers9050040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu. Rev. Cell Dev. Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 80.Niewiadomski P, et al. Gli proteins: regulation in development and cancer. Cells. 2019;8:147. doi: 10.3390/cells8020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nourmohammadi B, et al. Expression of miR-9 and miR-200c, ZEB1, ZEB2 and E-cadherin in non-small cell lung cancers in Iran. Asian Pac. J. Cancer Prev. 2019;20:1633–1639. doi: 10.31557/APJCP.2019.20.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okita, Y. et al. The transcription factor MAFK induces EMT and malignant progression of triple-negative breast cancer cells through its target GPNMB. Sci Signal10 (2017). [DOI] [PubMed]
- 83.Olea-Flores M, et al. Extracellular-signal regulated kinase: a central molecule driving epithelial-mesenchymal transition in cancer. Int. J. Mol. Sci. 2019;20:2885. doi: 10.3390/ijms20122885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Owen KL, Brockwell NK, Parker BS. JAK-STAT signaling: a double-edged sword of immune regulation and cancer progression. Cancers (Basel) 2019;11:2002. doi: 10.3390/cancers11122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel M, Nowsheen S, Maraboyina S, Xia F. The role of poly(ADP-ribose) polymerase inhibitors in the treatment of cancer and methods to overcome resistance: a review. Cell Biosci. 2020;10:35. doi: 10.1186/s13578-020-00390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol. Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pelullo M, et al. Wnt, Notch, and TGF-beta pathways impinge on hedgehog signaling complexity: an open window on cancer. Front. Genet. 2019;10:711. doi: 10.3389/fgene.2019.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther. Adv. Med. Oncol. 2019;11:1758835919833519. doi: 10.1177/1758835919833519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piasecka D, Braun M, Kordek R, Sadej R, Romanska H. MicroRNAs in regulation of triple-negative breast cancer progression. J. Cancer Res. Clin. Oncol. 2018;144:1401–1411. doi: 10.1007/s00432-018-2689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pohl SG, et al. Wnt signaling in triple-negative breast cancer. Oncogenesis. 2017;6:e310. doi: 10.1038/oncsis.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 92.Prat A, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Puisieux A, Valsesia-Wittmann S, Ansieau S. A twist for survival and cancer progression. Br. J. Cancer. 2006;94:13–17. doi: 10.1038/sj.bjc.6602876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Radisky DC, LaBarge MA. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008;2:511–512. doi: 10.1016/j.stem.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 95.Rajc J, Frohlich I, Mrcela M, Tomas I, Flam J. Prognostic impact of low estrogen and progesterone positivity in luminal B (Her2 Negative) breast cancer. Acta Clin. Croat. 2018;57:425–433. doi: 10.20471/acc.2018.57.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rajput S, Guo Z, Li S, Ma CX. PI3K inhibition enhances the anti-tumor effect of eribulin in triple negative breast cancer. Oncotarget. 2019;10:3667–3680. [PMC free article] [PubMed] [Google Scholar]
- 97.Rampurwala M, Wisinski KB, O'Regan R. Role of the androgen receptor in triple-negative breast cancer. Clin Adv Hematol Oncol. 2016;14:186–193. [PMC free article] [PubMed] [Google Scholar]
- 98.Ren Q, Chen J, Liu Y. LRP5 and LRP6 in wnt signaling: similarity and divergence. Front. Cell Dev. Biol. 2021;9:670960. doi: 10.3389/fcell.2021.670960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ribatti D, Tamma R, Annese T. Epithelial-mesenchymal transition in cancer: a historical overview. Transl. Oncol. 2020;13:100773. doi: 10.1016/j.tranon.2020.100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rinkenbaugh AL, Baldwin AS. The NF-kappaB pathway and cancer stem cells. Cells. 2016;5:16. doi: 10.3390/cells5020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sabatier R, et al. Claudin-low breast cancers: clinical, pathological, molecular and prognostic characterization. Mol. Cancer. 2014;13:228. doi: 10.1186/1476-4598-13-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santamaria PG, Moreno-Bueno G, Cano A. Contribution of epithelial plasticity to therapy resistance. J. Clin. Med. 2019;8:676. doi: 10.3390/jcm8050676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sareyeldin RM, et al. Gene expression and miRNAs profiling: function and regulation in Human Epidermal Growth Factor Receptor 2 (HER2)-positive breast cancer. Cancers (Basel) 2019;11:646. doi: 10.3390/cancers11050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sari IN, et al. Hedgehog signaling in cancer: a prospective therapeutic target for eradicating cancer stem cells. Cells. 2018;7:208. doi: 10.3390/cells7110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Savan R. Post-transcriptional regulation of interferons and their signaling pathways. J. Interferon Cytokine Res. 2014;34:318–329. doi: 10.1089/jir.2013.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saxena M, et al. AMP-activated protein kinase promotes epithelial-mesenchymal transition in cancer cells through Twist1 upregulation. J. Cell Sci. 2018;131:jcs208314. doi: 10.1242/jcs.208314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwerk J, Savan R. Translating the untranslated region. J. Immunol. 2015;195:2963–2971. doi: 10.4049/jimmunol.1500756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scimeca M, et al. Emerging prognostic markers related to mesenchymal characteristics of poorly differentiated breast cancers. Tumour Biol. 2016;37:5427–5435. doi: 10.1007/s13277-015-4361-7. [DOI] [PubMed] [Google Scholar]
- 110.Shao S, et al. Notch1 signaling regulates the epithelial-mesenchymal transition and invasion of breast cancer in a Slug-dependent manner. Mol. Cancer. 2015;14:28. doi: 10.1186/s12943-015-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singha PK, et al. Increased Smad3 and reduced Smad2 levels mediate the functional switch of TGF-beta from growth suppressor to growth and metastasis promoter through TMEPAI/PMEPA1 in triple negative breast cancer. Genes Cancer. 2019;10:134–149. doi: 10.18632/genesandcancer.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun Y, Zhang J, Ma L. alpha-catenin. A tumor suppressor beyond adherens junctions. Cell Cycle. 2014;13:2334–2339. doi: 10.4161/cc.29765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Talyor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J. Mammary Gland Biol. Neoplasia. 2010;12:169–190. doi: 10.1007/s10911-010-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tam SY, Wu VWC, Law HKW. Hypoxia-induced epithelial-mesenchymal transition in cancers: HIF-1alpha and beyond. Front. Oncol. 2020;10:486. doi: 10.3389/fonc.2020.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tan EJ, Olsson AK, Moustakas A. Reprogramming during epithelial to mesenchymal transition under the control of TGFbeta. Cell Adh. Migr. 2015;9:233–246. doi: 10.4161/19336918.2014.983794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Testa U, Castelli G, Pelosi E. Breast cancer: a molecularly heterogenous disease needing subtype-specific treatments. Med. Sci. (Basel) 2020;8:18. doi: 10.3390/medsci8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tian M, Schiemann WP. TGF-beta stimulation of EMT programs elicits non-genomic ER-alpha activity and anti-estrogen resistance in breast cancer cells. J. Cancer Metastasis Treat. 2017;3:150–160. doi: 10.20517/2394-4722.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Toft DJ, Cryns VL. Minireview: basal-like breast cancer: from molecular profiles to targeted therapies. Mol. Endocrinol. 2011;25:199–211. doi: 10.1210/me.2010-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tzavlaki K, Moustakas A. TGF-beta signaling. Biomolecules. 2020;10:487. doi: 10.3390/biom10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wahdan-Alaswad R, et al. Metformin attenuates transforming growth factor beta (TGF-beta) mediated oncogenesis in mesenchymal stem-like/claudin-low triple negative breast cancer. Cell Cycle. 2016;15:1046–1059. doi: 10.1080/15384101.2016.1152432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang MM. Notch signaling and Notch signaling modifiers. Int. J. Biochem. Cell Biol. 2011;43:1550–1562. doi: 10.1016/j.biocel.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Z, Li Y, Kong D, Sarkar FH. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr. Drug Targets. 2010;11:745–751. doi: 10.2174/138945010791170860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang W, Nag SA, Zhang R. Targeting the NFkappaB signaling pathways for breast cancer prevention and therapy. Curr. Med. Chem. 2015;22:264–289. doi: 10.2174/0929867321666141106124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y, Zhou BP. Epithelial-mesenchymal transition in breast cancer progression and metastasis. Chin. J. Cancer. 2011;30:603–611. doi: 10.5732/cjc.011.10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Warzecha CC, Carstens RP. Complex changes in alternative pre-mRNA splicing play a central role in the epithelial-to-mesenchymal transition (EMT) Semin. Cancer Biol. 2012;22:417–427. doi: 10.1016/j.semcancer.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wei XL, et al. ERalpha inhibits epithelial-mesenchymal transition by suppressing Bmi1 in breast cancer. Oncotarget. 2015;6:21704–21717. doi: 10.18632/oncotarget.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wendt MK, Allington TM, Schiemann WP. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol. 2009;5:1145–1168. doi: 10.2217/fon.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu Y, et al. Decreased levels of active SMAD2 correlate with poor prognosis in gastric cancer. PLoS ONE. 2012;7:e35684. doi: 10.1371/journal.pone.0035684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu Y, Sarkissyan M, Vadgama JV. Epithelial-mesenchymal transition and breast cancer. J. Clin. Med. 2016;5:13. doi: 10.3390/jcm5020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br. J Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu Y, et al. Twist1 promotes breast cancer invasion and metastasis by silencing Foxa1 expression. Oncogene. 2017;36:1157–1166. doi: 10.1038/onc.2016.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu P, Liu J, Derynck R. Post-translational regulation of TGF-beta receptor and Smad signaling. FEBS Lett. 2012;586:1871–1884. doi: 10.1016/j.febslet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu R, Won JY, Kim CH, Kim DE, Yim H. Roles of the phosphorylation of transcriptional factors in epithelial-mesenchymal transition. J. Oncol. 2019;2019:5810465. doi: 10.1155/2019/5810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu X, Zhang M, Xu F, Jiang S. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol. Cancer. 2020;19:165. doi: 10.1186/s12943-020-01276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yam C, Mani SA, Moulder SL. Targeting the molecular subtypes of triple negative breast cancer: understanding the diversity to progress the field. Oncologist. 2017;22:1086–1093. doi: 10.1634/theoncologist.2017-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang J, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 141.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 142.Ye Y, et al. ERalpha signaling through slug regulates E-cadherin and EMT. Oncogene. 2010;29:1451–1462. doi: 10.1038/onc.2009.433. [DOI] [PubMed] [Google Scholar]
- 143.Ye L, et al. Functions and targets of miR-335 in cancer. Onco Targets Ther. 2021;14:3335–3349. doi: 10.2147/OTT.S305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yuan X, et al. Expression of Notch1 correlates with breast cancer progression and prognosis. PLoS ONE. 2015;10:e0131689. doi: 10.1371/journal.pone.0131689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zaravinos A. The regulatory role of microRNAs in EMT and cancer. J. Oncol. 2015;2015:865816. doi: 10.1155/2015/865816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zarzynska JM. Two faces of TGF-beta1 in breast cancer. Mediators Inflamm. 2014;2014:141747. doi: 10.1155/2014/141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang J, et al. NUMB negatively regulates the epithelial-mesenchymal transition of triple-negative breast cancer by antagonizing Notch signaling. Oncotarget. 2016;7:61036–61053. doi: 10.18632/oncotarget.11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang YE. Non-Smad Signaling Pathways of the TGF-beta Family. Cold Spring Harb Perspect Biol. 2017;9:a022129. doi: 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y, Alexander PB, Wang XF. TGF-beta family signaling in the control of cell proliferation and survival. Cold Spring. Harb. Perspect. Biol. 2017;9:a022145. doi: 10.1101/cshperspect.a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Z, Yao L, Yang J, Wang Z, Du G. PI3K/Akt and HIF1 signaling pathway in hypoxiaischemia (Review) Mol. Med. Rep. 2018;18:3547–3554. doi: 10.3892/mmr.2018.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao M, Ang L, Huang J, Wang J. MicroRNAs regulate the epithelial-mesenchymal transition and influence breast cancer invasion and metastasis. Tumour Biol. 2017;39:1010428317691682. doi: 10.1177/1010428317691682. [DOI] [PubMed] [Google Scholar]
- 152.Zhao Z, Rahman MA, Chen ZG, Shin DM. Multiple biological functions of Twist1 in various cancers. Oncotarget. 2017;8:20380–20393. doi: 10.18632/oncotarget.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zheng H, et al. Glycogen synthase kinase-3beta: a promising candidate in the fight against fibrosis. Theranostics. 2020;10:11737–11753. doi: 10.7150/thno.47717. [DOI] [PMC free article] [PubMed] [Google Scholar]