Abstract
Food demand of growing population can only be met by finding solutions for sustaining the crop yield. The understanding of basic mechanisms employed by microorganisms for the establishment of parasitic relationship with plants is a complex phenomenon. Symbionts and biotrophs are dependent on living hosts for completing their life cycle, whereas necrotrophs utilize dead cells for their growth and establishment. Hemibiotrophs as compared to other microbes associate themselves with plants in two phase’s, viz. early bio-phase and later necro-phase. Plants and microbes interact with each other using receptors present on host cell surface and elicitors (PAMPs and effectors) produced by microbes. Plant–microbe interaction either leads to compatible or incompatible reaction. In response to various biotic and abiotic stress factors, plant undergoes programmed cell death which restricts the growth of biotrophs or hemibiotrophs while necrotrophs as an opportunist starts growing on dead tissue for their own benefit. PCD regulation is an outcome of plant–microbe crosstalk which entirely depends on various biochemical events like generation of reactive oxygen species, nitric oxide, ionic efflux/influx, CLPs, biosynthesis of phytohormones, phytoalexins, polyamines and certain pathogenesis-related proteins. This phenomenon mostly occurs in resistant and non-host plants during invasion of pathogenic microbes. The compatible or incompatible host–pathogen interaction depends upon the presence or absence of host plant resistance and pathogenic race. In addition to host–pathogen interaction, the defense induction by beneficial microbes must also be explored and used to the best of its potential. This review highlights the mechanism of microbe- or symbiont-mediated PCD along with defense induction in plants towards symbionts, biotrophs, necrotrophs and hemibiotrophs. Here we have also discussed the possible use of beneficial microbes in inducing systemic resistance in plants against pathogenic microbes.
Keywords: Programmed cell death, Compatibility, Resistance, Necrosis, Systemic acquired resistance, Reactive oxygen species, Microbes
Introduction
Programmed cell-death (PCD) or apoptosis (mostly used for cell death in animals) is the death of cell in any form, regulated by an intracellular program leading to selective elimination of unwanted or damaged cells during an organism's life-cycle (Cooper 2000; Alberts et al. 2002; Elmore 2007; Kaczanowski 2016; Arcy 2019; Tang et al. 2019). Plants suffer from various stresses or injuries which leads to poorly characterized form of cell-death, i.e., necrosis (Rock and Kono 2008; Van Doorn et al. 2011; Escobar et al. 2015). Plant cell death differs from animal cell death with respect to presence of phagocytes and presence of rigid cell wall which blocks the engulfment and removal of apoptotic cells (Dickman et al. 2017). Based on this, the term Apoptosis is generally used for animals whereas for plant cell death, the term “apoptotic like” is used (Dickman et al. 2017). The cell or tissue death is essential for growth and development of plants and also for withstanding the adverse effect of various biotic and abiotic stress factors (Duque-Parra 2005). Programmed cell death is categorized as developmental PCD (dPCD) (based on its role in growth and development of plant) and environmental PCD (ePCD) in response to biotic/abiotic stress factors (Sychta et al. 2021). Programmed cell death is a regulated process of all unicellular or multicellular eukaryotes and prokaryotes (Lord and Gunawardena 2012; Locato and De Gara 2018). With the evolution of complex multicellular organisms including plants, PCD has been adapted to fulfill diverse functions involved in cell differentiation, cell number homeostasis, tissue development and activation of immune system (Gavrilescu and Denkers 2003; Devarenne and Martin 2007; Reape et al. 2008; Wituszynska and Karpinski 2013). The regulation of cell number in multicellular organisms is under the control of cell division and cell death rate. If the cells are not required further, then the plants undergo activation of intracellular death program known as PCD (Palavan et al. 2005). The harmonization between cell death and its proliferation (growth and differentiation) is important for sustaining the tissue and organ homeostasis (Van Breusegem and Dat 2006). PCD involves condensation, shrinkage and fragmentation of cells and it is generally considered as an early event heading towards the tolerance of plants to various abiotic (salinity, extreme temperatures and pollutants) and biotic stresses (Wituszynska and Koukalova et al. 1997; Overmyer et al. 2000; Swidzinski et al. 2002; Wituszynska and Karpinski 2013; Petrov et al. 2015). Plant PCD is also associated with embryogenesis, aleurone layer degeneration during the germination of monocot seeds, tracheary elements differentiation, formation of root aerenchyma, trichome formation, abscission of floral parts, self-incompatibility of pollen grains and leaf senescence, etc. (Thomas and Franklin-Tong 2004; Gechev et al. 2006; Xu and Zhang 2009). The effect of PCD and signalling cascade activation within the plants varies from one stress factor to another and even from one organism to another depending on its mode of survival. In plants infected by biotrophs, PCD activates defense related signalling molecules while in infection with necrotrophs, pathogen starts utilizing dead tissues (Navarre and Wolpert 1999; Coffeen and Wolpert 2004; Laluk and Mengiste 2010; Balint-Kurti 2019). Hemibiotrophs, as compared to necrotrophs and biotrophs, undergo transition from biotrophy to necrotrophy (Lee and Rose 2010). Defense mechanism activated during biotrophic phase arrests the growth and development of pathogen by PCD and after completion of bio-phase, whereas hemibiotroph starts growing on dead tissue. PCD is not always positive for plant growth, i.e. extensive PCD in response to abiotic stress factors may lead to crop yield losses (Burke et al. 2020).
Along with defense activation by different microbial communities, several forms of PCD are known, among which hypersensitivity in plants is quite similar to developmental PCD/apoptosis. Hypersensitive response (HR) occurs at the point of pathogen ingress and it is a form of cell death which mostly occurs during incompatible interaction and sometimes in compatible host–pathogen interaction. Various biochemical events like generation of ROS, nitric oxide (NO), ionic efflux/influx, caspase-like protease, biosynthesis of phytohormones, phytoalexins, polyamines and certain pathogenesis-related proteins are activated within or in the surrounding plant cells in response to invading plant pathogen. Among phytohormones, SA-mediated defense pathway is activated against biotrophs and JA/ethylene-mediated pathway is activated in response to necrotrophs (Glazebrook 2005). In some plants along with the activation of phytohormones, camalexin- a phytoalexin is also known to play role in defence (Khare et al. 2017; Li et al. 2019). Additionally, few genes related to enhanced susceptibility are as well reported to have involvement in the regulation of SA-mediated pathway during PTI which ultimately suppress the pathogen growth (Zhang et al. 2018). Moreover, transcription factors (TFs) like members of NAC (NAM, ATAF, CUC), ERF (ethylene responsive element binding factors), MYB (myeloblastosis), bZIP (Basic leucine zipper domain), bHLH (Basic helixloop-helix), NF-Y (Nuclear Factor Y), CAMTA (CaM-binding transcription activator) and WRKY families also play fundamental roles in plant responses to different stress factors (Yuan et al. 2019; Burke et al. 2020).
The concept of programmed cell death and its other forms has already been discussed by many workers (Formigli et al. 2000; Sperandio et al. 2000; Reape et al. 2008; Duca et al. 2014). In the current compilation, the information on basic plant cell death mechanism and defence activation against different plant–microbe interactions has been discussed. The assembled information will help scientists to understand and identify novel underlying signals responsible for stress resistance or susceptibility and it could be utilized further for the identification of exact reason behind the physiological changes occurring in plants with the ultimate objective of formulating effective stress management strategies.
Mechanism of cell death
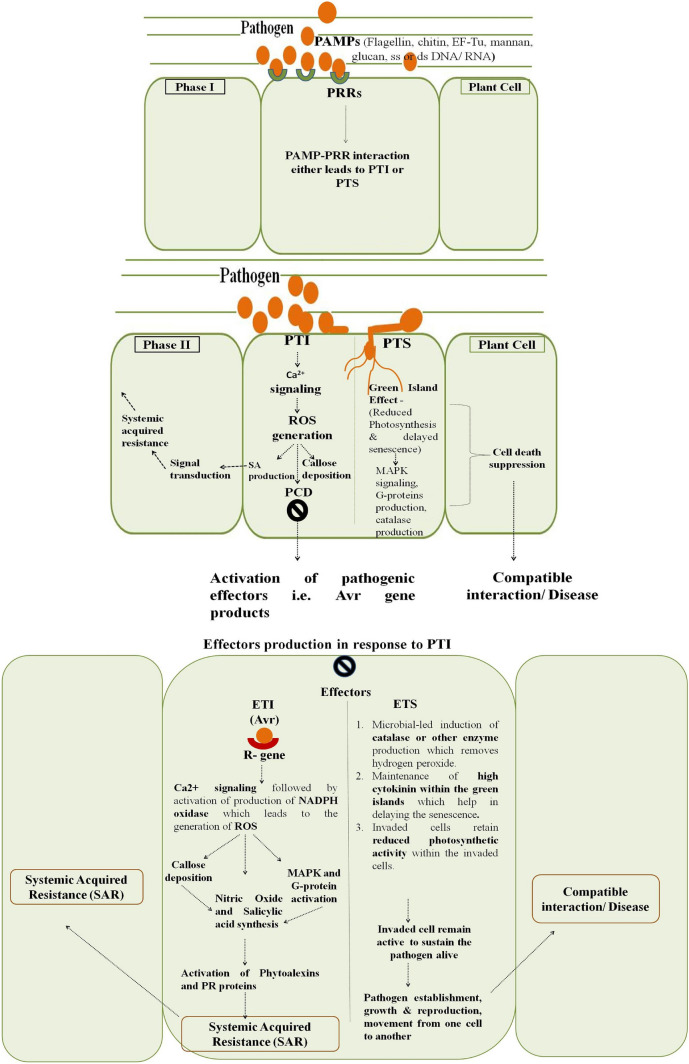
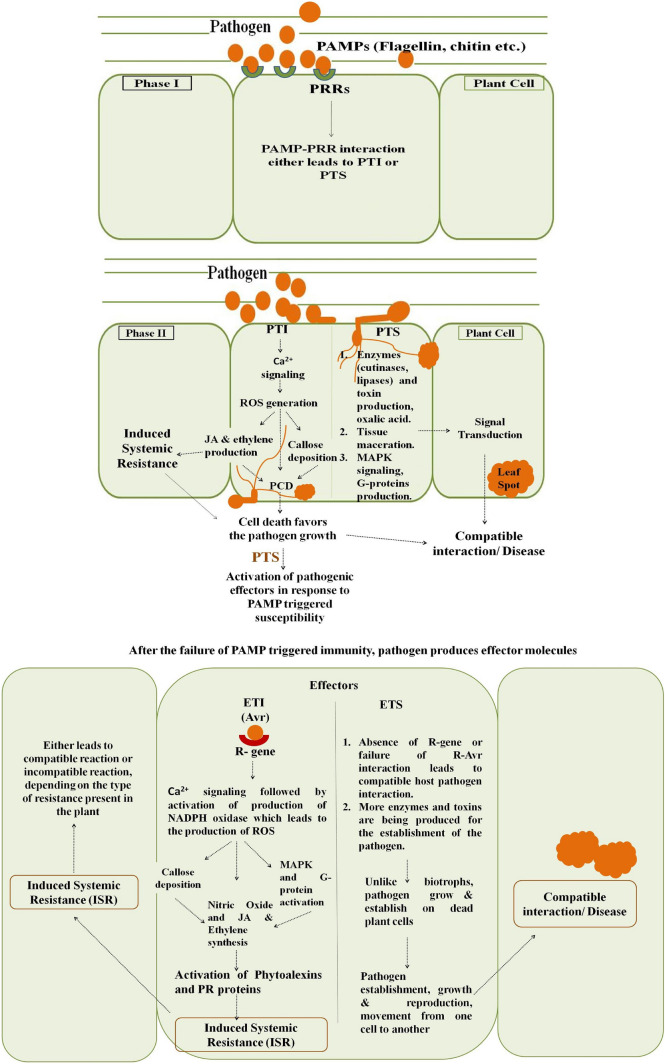
Cell death in response to growth and development is necessary for the plant to complete its life cycle and to protect itself from the effect of various stress factors (Woo et al. 2013; Petrov et al. 2015). Intrinsically, plants have different barriers to restrict the entry or growth of microbes and also to withstand the effect of abiotic stress factors. Among structural barriers, plant cell wall plays a significant role (Freeman and Beattie 2008). If the basal defense fails, then plants starts utilizing the resistance gene products which leads to effector triggered immunity or susceptibility. The microbes interact with plants following a sequence of events which occurs in a tandem manner. These events are perception, signalling, signal transduction and signal termination (Berne and Javornik 2016). Initially, host and pathogen components are recognized by the specific receptors present on plant or pathogen’s surface and following perception, the plant undergoes generation of defense signals (Prell and Day 2001; Ponzio et al. 2016). Once the successful recognition occurs, the permeability of cell membrane changes which activates the opening of Ca2+ channels and ultimately leads to increase in the amount of free Ca2+ concentration in the cytosol (White and Broadley 2003; Tuteja and Mahajan 2007). The free calcium activates the protein kinases which are involved in the phosphorylation of various regulatory proteins. There are reports in which ROS also induces the increase in cytosolic Ca2+ concentration which leads to the activation of other defense responses like production of phytoalexins, PR proteins, callose deposition, etc. (Mori and Schroeder 2004; Ellinger and Voigt 2014). The plant–pathogen interaction at molecular level is a mutual interplay, in which either calcium signalling pathways leads to the activation of ROS pathway or vice versa (Gorlach et al. 2015). The high cytosolic calcium ion concentrations have effect on the production of various enzymes like NADPH oxidase which is involved in the generation of ROS (Sagi and Fluhr 2006; Gorlach et al. 2015; Fananas et al. 2020). The common ROS produced in response to biotic and abiotic factors are superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl radical (OH−) or singlet oxygen (Pogany et al. 2006). In addition to these, nitric oxide (NO) is also produced within the cell which acts like a secondary messenger for the activation of plant defense genes. Sometimes, NO reacts with superoxide to result in the formation of reactive species peroxynitrite (Romero-Puertas et al. 2004). Further, the ROS attributes to the activation of mitogen activated protein kinases (MAPK) cascades and trimeric G protein signalling pathways and also helps in removing the damaged or stressed cells from the plant (Jalmi and Sinha 2015). The raised levels of cytosolic Ca2+ due to ROS results in opening of mitochondrial permeability transition pore and releases the cytochrome c, which ultimately induces transcription of PCD genes (Rantong and Gunawardena 2015). Along with these signalling molecules, some hormones like salicylic acid (SA), jasmonic acid (JA), ethylene, auxin, gibberellic acid, cytokinin and others are also known to play role in host–pathogen interaction (Li et al. 2019). Among these, the production of SA plays a lead role in the activation of local or systemic acquired resistance. Against biotrophs, SA is involved in the activation of defense while in hemibiotrophs, it plays important role only during the biotrophic stage of the pathogen (Figs. 1, 3). Other hormones such as JA and ethylene are important in activating defense against various stress factors but in case of necrotrophs (Fig. 2) they are common ones (Zhang et al. 2017).
Fig. 1.
PCD induction during interaction between biotrophic microbes and host. Phase I 1.Contact of host and pathogen. 2. Activation and recognition of pathogen components by receptors present on the surface of host. Phase II 1. Spore germination and germtube formation. 2. Appressoria development in response to chemical stimuli received from host. 3. Release of either virulence or avirulence gene products
Fig. 3.
PCD induction during interaction between hemibiotrophic microbes and host
Fig. 2.
PCD induction during interaction between necrotrophic microbes and host
Additionally, polyamines (PAs) present in free or bound form are also known to have direct or indirect involvement in PCD. Polyamines are present in all living organisms. Most commonly found PAs in plants are putrescine (PUT), spermidine (SPD), spermine (SPM) and thermospermine (t-SPM absent in animals). Indirectly, the metabolic derivatives (catabolites & interconversion products/intermediates like H2O2 or aminoaldehydes) of PAs regulate the PCD (Moschou and Roubelakis-Angelakis 2014). The catabolism of PA by amine oxidases (polyamine oxidases and diamine oxidases) induces accumulation of H2O2 which plays important role as a plant defence signalling molecule (Pal and Janda 2017). Directly PAs and their analogues induce regulatory effect on ion channels. Efflux of K + ions activates caspases and nucleases which promote the production of reactive oxygen species involved in the resistance mechanism (Zepeda-Jazo et al. 2011). In various plant-pathogen interactions such as barley/wheat- Blumeria graminis, wheat- Fusarium graminearum, oat- Puccinia coronata f. sp. avenae, tobacco- TMV, pepper- Xanthomonas campestris, the induction of PAs production has been reported and relationship between host resistance and accumulation of PA was observed (Negrel et al. 1984; Asthir et al. 2004; Gardiner et al. 2010; Kim et al. 2013; Montilla-Bascon et al. 2016). Activation of ROS production also induces ca2+ influx which executes hypersensitive response in plants and activates various defence related signals (Moschou and Roubelakis-Angelakis 2014). Different PAs analogues have already been used by scientists to establish their role in PCD in Humans but in plant sciences it is still to be unexplored (Moschou and Roubelakis-Angelakis 2014). Moreover, many intrinsic (phytoanticipins) or induced secondary metabolites (phytoalexins) with antimicrobial properties are also reported in different plant species (Piasecka et al. 2015).
There are reports in which lipids and their metabolites produced by host or pathogenic microbes are also known to play role in development, pathogenesis and protection of plants (Reina-Pinto and Yephremov 2009; Shah 2005). In response to invading pathogens, herbivores or wounds, the production of JA (a lipid derived signalling molecule) starts within the plant (Zhang et al. 2017). Expression of genes encoding for JA signalling is useful for the plants to tackle the plant pathogenic necrotrophs (Zhang et al. 2017). In addition to plant defense, lipid transfer proteins like OsC6 are involved in developmental processes such as pollen coat formation (Zhang et al. 2010). The role of one class of lipids, i.e., sphingolipids in plant cell death is known but still the generated information is not enough (Liang et al. 2003). Cerebroside- a sphingolipid in different pathosystems like rice blast, Fusarium oxysporum, Pythium, Botrytis pathosystem,etc. serves as a non-specific defence elicitor (Umemura et al. 2004). In addition to lipids, other host components like cutin/cutin monomers also initiates the expression of genes entailed in pathogenesis. In Alfaalfa-Colletotrichum species interaction, the presence of cutin constituents induces the expression of genes which encodes for protein kinase C (PKC) and LIPK (lipid-induced protein kinase). These encoded products either leads to the production of more infection structures or in the suppression of disease development (Dickman et al. 2003).
Generally, healthy plant produces sucrose during photosynthesis which is transported to other plant parts and helps plants to fulfil their growth demands, whereas during infection, pathogens (especially biotrophs) start using the sugars for their own needs and force plants to increase sugar content in the cell which leads to activation of defense-related mechanisms within the cells. Likewise, the expression of cell wall invertase and transporters of hexose increases the hexose content which leads to PR genes activation, down regulation of photosynthesis and establishment of various barriers to stop pathogen from invading other plant cells (Fotopoulos et al. 2003; Tauzin and Giardina 2014; Proels and Huckelhoven 2014). The activity of various signalling molecules or defense regulators leads to chromatin condensation, cysteine proteases activation, release of cytochrome c, membrane potential loss and shrinkage of cytoplasm (Chibucos et al. 2009). Overall, produced signals leads to the death of a localized cell (hypersensitive response) and after HR, the perceived signals are transferred from one cell to another (Yang et al. 1997; Liu and Lam 2019). During transduction of defense signals, receptor-like kinases (RLKs) play an important role. Following signal transduction, the perception of signals by neighboring cells either leads to compatible or incompatible reaction and at the end of plant-pathogen battle, the signal production is terminated.
Forms of cell death
After successful recognition of pathogen, plant responds rapidly in the form of hypersensitive response which results in hasty cell death at the site of infection. Hypersensitive response (HR) activates various signalling molecules involved in the activation of systemic acquired resistance (SAR) within the infected plants and is a specialized form of PCD (Morel ad Dangl 1997). The host pathogen interaction could be compatible or incompatible depending on the host resistance or pathogen virulence. During incompatible interaction, cell death occurs at early stages of infection due to pathogen specific R–Avr gene(s) interactions while during compatible interaction, cell death occurs late in the infection process (Pontier et al. 1998). This Gene-for-Gene interaction plays significant role in the host–pathogen crosstalk. According to this hypothesis, the inheritance of resistance to pathogens and the ability of pathogens to cause disease in plants are under the control of corresponding gene pairs (Flor 1942).
Hypersensitivity and developmental PCD/apoptosis in plants are similar mechanisms with considerable variations in induction and expression of cell death (Heath 1998). This HR is useful for necrotrophic pathogens but in case of specific biotrophic interaction, it restricts the growth and development of pathogen (Balint-Kurti 2019). It has been known to possess many features of apoptosis/autophagy and other forms of PCD but it should be considered as the special form of PCD in which specific R-Avr interaction is involved (Balint-Kurti 2019). Important forms of plant cell death are autophagy, autophagy-related genes (AL-PCD) and necrosis (Reape et al. 2008). Autophagy plays an imperative role in the overall development of plants, cellular homeostasis during plant growth, senescence, and in the response of plants to biotic and abiotic stress factors and in various other metabolic activities (Su et al. 2020). This process plays supreme role in plant fitness and immunity (Bozhkov 2018). Autophagy is an intracellular process which leads to the degradation or recycling of cytoplasmic materials by vacuoles or lysosomes and could be in suppressed or overexpressed form. Plants with suppressed autophagy have altered anthocyanin levels, HR and decreased fitness to various stress factors, while plants with overexpressed autophagy have increased resistance to necrotophs and positive effect on plant growth and development (Janse van Rensburg et al. 2019). Number of autophagy-related genes (ATG genes) are known which are responsible for the suppression or overexpression of autophagy (Tang and Bassham 2018; Su et al. 2020). Other form of cell death in plants is apoptosis-like programmed cell death (AL-PCD). Generally in apoptosis, cells break up into apoptotic bodies which are further engulfed by phagocytes and in animals it is one of the common form of cell death. In plants, AL-PCD has been recorded in some in vitro experiments but it is not a common feature of plant cell death (Reape et al. 2008). In some reports chromatin condensation and DNA fragmentation were quoted as the results of apoptosis but these are not specific to apoptosis, i.e., they could be observed during autophagy or necrosis also (Van Doorn et al. 2011). Classical apoptosis was not recorded in plants like animals or other warm blooded anilmals because of absence of phagocytic cells (Van Doorn et al. 2011). The other important form of PCD is necrosis, in which cells swell and lose their ability to osmoregulate, results in the inflow of water and other ions into the cell. In addition to swelling of mitochondria, it involves early rupture of plasma membrane and protoplast shrinkage (Van Doorn et al. 2011). Unlike other forms of cell death, necrosis is not associated with the developmental processes of plants and it hardly needs signalling transduction pathways (Pennell and Lamb 1997). Earlier necrosis was considered to be as unprogrammed and uncontrolled form of cell death but recently it has been classified as the type III form of cell death. It is still not characterized properly at biochemical and genetic level (Van Doorn et al. 2011).
In mammals, Bax protein localization at mitochondria leads to cytochrome c activation and release of caspases which are responsible for the cleavage of proteins required for survival of cell (Wang and Youle 2009). Some of the mechanisms or processes of apoptosis/PCD are similar in plants and animals but several key regulators like canonical caspases (Cysteine-containing Aspartate-specific proteases) and Bcl-2 related proteins are absent in plants. However, some caspase-like protease (CLP) activities are activated in plants against various stress factors. Caspases and caspase-like proteases involved in apoptosis and AL-PCD are known to induce cell death in same manner (Mea et al. 2007). The programmed mechanism similar to animal apoptosis is present in plants but the involved signals or regulators are distinct (Watanabe and Lam 2009; Xu and Zhang 2009; Cai and Gallois 2015). Although many features of PCD are similar in plants and animals but at molecular level, cell death mechanism in plants and animals is non-homologous and the research on this aspect is still at its infancy (Mea et al. 2007; Collazo et al. 2006).
PCD in response to microorganisms
Plants interact with various microorganisms which includes fungi, bacteria, viruses, etc. Such kind of interactions might be beneficial or detrimental to the plant and the damaging entities causing harm to plants are referred to as pathogens (Spanu and Panstruga 2017). Among different interactions, the interactions involving the transfer of nutrients or various resources are important. There are various interactions between host and microbes as per the hierarchy of evolution of parasitism. According to literature we can place different microbes into four groups’ viz. biotrophs, necrotrophs, hemibiotrophs (Pusztahelyi et al. 2016) and symbionts. But with the advances in research, there are some microbes which could be kept in two or more categories and here in this review, general host–pathogen interaction mechanisms followed by microbes of different groups is given. Microbes produce various kinds of effectors which interact with plant components to result in susceptibility or resistance (Table 1). Each microbe competes with its host for survival and establishment using different infection behaviours, the details of which are given below:
Table 1.
Various molecules/effectors produced by micro-organisms during host–pathogen interaction
| Sr. No | Name of the microorganism | Biotrophy associated effectors/molecules | Necrotrophy associated effectors/molecules | Nature of pathogen reaction with plant | References |
|---|---|---|---|---|---|
| 1 | Phytophthora infestans and P. sojae | SNE1, IpiO-4 and PsCRN115 (suppresses the action of necrosis inducing effectors) | PiNPP1.1, PsojNIP, INF1, IpiO-1 and PsCRN63 (activated during necrosis phase and suppresses SNE1 activities) | Hemibiotroph | Lee and Rose (2010), Kelley et al. (2010), Halterman et al. (2010), Zhang et al. (2015), Mukhtar et al. (2016), Kroner et al. (2019) |
| 2 | Colletotrichum orbiculare and C. gloeosporioides | Co-DN3, CgDN3 | NIS1, MC69 | Hemibiotroph | Stephenson et al. (2000), Saitoh et al. (2012), Irieda et al. (2014) |
| 3 | Magnaporthe oryzae | Not known yet | BAS1, BAS2, BAS3 and BAS4 | Hemibiotroph (BAS4 induces necrotic lesions on rice during late stages of infection) | Mosquera et al. (2009), Wang et al. (2019) |
| 4 | Alternaria alternata | – | AAL mycotoxin |
Necrotroph (Mycotoxin induces death of plant cells) |
Gonçalves et al. (2017) |
| 5 | Stagonospora nodorum | – | SnTox1 | Necrotroph (SnTox1 interacts with Snn1 and induce necrosis) | Liu et al. (2012) |
| 6 | Fusarium oxysporum | – | Secreted-in-Xylem” (Six) proteins/effectors Six1, Six3, Six5 and Six6 |
Necrotroph (Six-required for pathogenicity) |
Cao et al. (2018) |
| 7 | Sclerotinia sclerotiorum | – | SsSSVP1 | Necrotroph | Lyu et al. (2016) |
| 8 | Erysiphe pisi | Ep CSEP (candidate secreted effector proteins)/CSPs | – | Biotroph (Ep CSEP plays role in pathogenesis) | Sharma et al. (2019) |
| 9 | Blumeria graminis f. sp. hordei and Gaeumannomyces graminis var. Tritici | Blumeria effector candidate (BEC) 1019, Avra10 and AvrK1 used for host colonization | – | Biotroph | Ridout et al. (2006), Zhang et al. (2019) |
| 10 | Hyaloperonospora parasitica | ATR1 and ATR13 | – | Biotroph (promotes susceptibility in Arabidopsis thaliana) | Sohn et al. (2007) |
| 11 | Pseudomonas syringae pv. tomato | AvrPto (suppresses cell wall-based defences) and AvrPtoB (promotes the virulence of Pto) | Hemibiotroph | Wang et al. (2019) | |
| 12 | Trichoderma virens | TVHYDII1(for plant root colonization and induce antagonistic activity against Rhizoctonia solani) | Symbiosis with plants and antagonist to pathogen | Guzman-Guzman et al. (2017) | |
| 13 | Plant viruses | Nuclear Shuttle Protein Interacting Kinase 1 (NIK 1) (for signalling pathway which ultimately suppresses the translation of host plant) | PAMPs of viral origin are known to regulate host machinery | Teixeira et al. (2019) | |
PCD induction during interaction between biotrophic microbe and plant:
Biotrophs are completely dependent on living host plants for their growth and development (Glazebrook 2005). Like necrotrophs and hemibiotrophs, these microbes cannot survive without living host (Spanu and Panstruga 2017). Biotrophic pathogens enter into the plant cells, survive within the apoplastic space and obtain nutrients from the live plant (Mukhtar et al. 2016). Initially pathogen-associated molecular patterns (PAMPs) present on the pathogen’s surface are perceived by receptors present on the host plant surface and their interaction leads to PAMP-triggered immunity (PTI) within the plants (Jones and Dangl 2006) in case of incompatible interaction. Further, PTI results in the activation of reactive oxygen species (ROS), hormonal biosynthesis, phytoalexins production and activation of other defense mechanisms (Jwa and Hwang 2017). The reaction of pathogen varies from resistant to susceptible depending on the crop plant varieties. Some plants have resistance genes against the invading pathogens and these plants recognize the pathogen effectors and activate effector-triggered immunity (ETI). This ETI finally results in PCD which restricts the pathogens from obtaining food from the host plant (Fig. 1). But in case of compatible interactions, plants develop green islands in which the site of infection remains green with reduced photosynthesis and rest of the leaf senesces (Coghlan and Walters 1992). In green islands, the cytokinin-like activity is comparatively high which contributes towards the maintenance of sugar level in the vicinity of infection site (Angra and Mandahar 1991). The production of cytokinins within the cell helps in delaying the senescence, negatively regulating the ROS generation and maintaining the photosynthetic activity within the invading plant cell which ultimately helps in keeping plant cells alive (Chanclud et al. 2016). Generally, green islands form when the disease is not severe and pathogenic propagules are present in less number on the host surface (Walters et al. 2008). Like biotrophs, the green island formation is common in the biotrophic phase of hemibiotrophs also. Some biotrophs and hemibiotrophs like Blumeria graminis f.sp. hordei (powdery mildew of barley) and Septoria tritici (septoria blotch of wheat), respectively, are also known to secrete catalase enzyme which remove or scavenge the hydrogen peroxide molecules from the host plant to establish itself within the cells (Zhang et al. 2004; Shetty et al. 2007).
Another important biotrophic disease incitants are plant viruses and during their interaction with plants, protein–protein or protein-nucleic acid based signalling is triggered which help plants to produce antiviral substances and help pathogen to cope up with the basal defense expressed within the invaded plant cells (Wu et al. 2019). Different defense mechanisms activated after the interaction of host and virus components are RNA interference, mRNA degradation, autophagy-mediated protein degradation, etc. Most importantly virus coat protein (CP) components interacts with plant (host) components and subsequent interaction leads to either susceptibility or resistance depending on the host genetics (Calil and Fontes 2017; Wu et al. 2019). The PAMPs of viral origin are known to activate the Nuclear Shuttle Protein Interacting Kinase 1 (NIK 1) derived signalling pathway which ultimately suppresses the translation of host plant (Teixeira et al. 2019). Like fungal biotrophs, dark green islands (DGI) also developed in systemic viral infection especially as a result of post transcriptional gene silencing (Moore et al. 2001; Moore and MacDiarmid 2006). But unlike fungal biotrophs, the green islands formed in viral infections are the recovered cells and these cells have fewer virus particles as compared to surrounding cells (Burundukova et al. 2009; Chen et al. 2015). As biotrophs need living host cells to fulfill their etiological requirements henceforth a strategy of host resistnace can be explored and brought in use by gene(s) pyramiding or transfer in high yielding susceptible host to achieve durable host resistant and ecofriendly management.
PCD induction during interaction between necrotrophic microbe and plant
Necrotrophic microbes with the help of toxins and enzymes kill their host cells and grow on dead plant parts. Based on the type of toxins produced by them, they are classified as either host-specific or non host-specific necrotrophs (broad host range) (Coll et al. 2011; Wen 2013; Abdullah and Akhtar 2016). Host-specific necrotrophs produce host specific toxins which serve as effectors for the activation of defense and in this case, the virulence target is R-proteins. Broad host range necrotrophs uses cell wall-degrading enzymes (CWDEs), various toxins or other compounds singly or in combination to attack the plant. Unlike host-specific necrotrophs, these microorganisms can cause disease in a range of plants (Laluk and Mengiste 2010). To both types of necrotrophs, plants activate defense mechanisms by activating common crosstalk which involves the generation of ROS molecules, which directly or indirectly causes death of plant tissue (Wen 2013). The early induction of ROS accumulation in the plant cells could induce resistance against necrotrophs but unlike biotrophs in later stages, these microbes use ROS induced dead cells for their own establishment and growth (Sobiczewsk et al. 2017). If plant succeeds in detoxifying or not recognizing the pathogenic toxins, then there will be toxin triggered immunity (also called as pathogen triggered immunity) which not benefits the pathogen by providing the substrate for its growth (Fig. 2). Some necrotrophs like Botrytis cinerea, Cochliobolus victoriae take advantages of host hypersensitivity machinery to induce cell death in plants despite of presence of NBR-LRR motif (Govrin and Levine 2000; Lorang et al. 2007). Necrotrophs after attachment with host plant penetrate the plant tissues with the help of appressoria or enzymatic action or through wounds (Laluk and Mengiste 2010; Bellincampi et al. 2014). The enzymes, phytotoxic metabolites and toxins produced by the pathogen cause lysis and decomposition of plant cells and thereafter the dead cells serve as a reservoir of food for invading microbes (Cho 2015). Some necrotrophs induce the production of phytohormones like JA/Ethylene within the plant cells which leads to defense activation against them in case of resistant plants (Glazebrook 2005). In some cases, both biotrophs and necrotrophs suppress the SA accumulation but in case of certain broad host range necrotrophs, the SA accumulation is being induced which ultimately suppresses the JA signalling (Ma and Ma 2016).
Sclerotinia sclerotiorum, a broad host range necrotroph initially activates the accumulation of oxalic acid (OA) and Ss-Rhs1 for successful colonization within the plant cell (Kim et al. 2008; Liang and Rollins 2018). After this, tissue maceration is achieved with the help of secreted toxins, enzymes and different cell death elicitors. Generally, oligogalaturonides produced by Botrytis cinerea and S. sclerotiorum are recognized by wall-associated kinases present on plant surface and this interaction activates a cascade of events which lead to defense activation. Along with MAPK, the Ca2+ signalling pathway also plays role in the activation of defense against S. sclerotiorum (Wang et al. 2019). All these signals finally lead to ROS production and necrosis of tissue, which for the sake of survival is used by necrotrophs like Botrytis cinerea and S. sclerotiorum, etc. (Govrin and Levine 2000).
PCD induction during interaction between hemibiotrophic microbes and plant
Hemibiotrophs attack plants in two phases, i.e., initial biotrophic and later necrotrophic phase (Chowdhury et al. 2017). The hemibiotrophs starts proliferating within the host plant without producing any symptoms and after its establishment, it switches from biotrophic to necrotrophic phase (Fig. 3). During the second phase of its growth and development, it starts the production of certain enzymes and toxins to kill the invaded cells and starts growing on the dead decaying parts (Lee and Rose 2010). Invading hemibiotrophs like other microbes, during the initial hours of infection activates the plant defense mechanism which is later on suppressed by the effectors produced by pathogen to complete its bio-phase (Vargas et al. 2012). The plants remain symptomless in the initial biotrophic phase which helps pathogen to remain undetectable during early establishment (Spanu and Panstruga 2017). The biotrophic to necrotrophic phase is characterized by transition of intercellular thick primary hyphae to thin filamentous secondary hyphae within the host cell. Hemibiotrophs during an early developmental phase form swollen vesicles in the root cortex tissues of resistant and susceptible cultivars which later on develop into penetrating structures. There are reports in which the hemibiotrophic pathogens form runner hyphae to colonize the root surface (Chowdhury et al. 2017). Generally, the completion of bio-phase and transition of biotrophy to necrotrophy takes longer duration in case of resistant host cultivars. Following the transition phase, pathogen undergoes the production of secondary hyphae which leads to tissue necrosis using necrosis inducing proteins (NIPs). The defense in hemibiotrophs is dependent on the activation of SA (during biotrophic phase) and JA (during necrotrophic phase) signalling and the response varies from resistant to susceptible genotypes (Haddadi et al. 2016). Hemibiotoroph Macrophomina phaseolina is known to cause charcoal rot of various host plants. This pathogen attacks some of the plants following necrotrophic mode of action, whereas in some crops like sesame it acts like a hemibiotroph (Chowdhury et al. 2017). In sesame, sequential expression of biotrophy- and necrotrophy-related genes, i.e., BAS3 (biotrophy marker gene) and NIP (necrotrophy marker gene) at respective stages of pathogen development is reported (Chowdhury et al. 2017). The expression of these pathogenicity genes varies from resistant to susceptible plant varieties of sesame. In the early bio-phase, there was not any difference in the activation and production of defense signalling molecules. During the early growth stage, hemibiotrophs suppresses the activation of defense signals, especially the ROS generation, which ultimately leads to successful bio-phase, while in later stages of infection, pathogen utilizes ROS-mediated death of cells for their successful parasitic establishment. In Nirmala, a charcoal rot resistant cultivar less H2O2 was accumulated and the ROS scavenging activity was high which delayed the disease progression (Chowdhury et al. 2017). Along with this, the phenylpropanoid ammonia lyase activity was also high which led to the production of phenols and flavonoids. Additionally, lignin biosynthesis and callose deposition in resistant cultivar reduce the damage induced by necrosis.
Another hemibiotroph, Colletotrichum graminicola is known to cause anthracnose or stalk rot of maize (Nicoli et al. 2016). Most of the Colletotrichum spp. are hemibiotrophic in nature, i.e., they follow an initial short biotrophic phase and later necrotrophic phase in which plant cells are killed (da Silva et al. 2020). Initially, the fungal conidia comes in contact with host and produces a short germ tube which further differentiates into a specialized structure known as appressorium (Fig. 3). Followed by melanization of appresoria and production of osmolytes, the turgor pressure in appressoria is generated during pathogenesis (Ludwig et al. 2014). Primarily, the invading pathogen hides itself from the plant by deacetylation which helps pathogen to avoid degradation of chitin by chitinases produced by the plants. The chitin to chitosan conversion and apposition of protein layer reduces the chances of early defense activation which helps pathogen to complete its biotrophic phase (Oliveira and Deising 2016). The biotrophic phase is followed by necrotrophy in which defense signalling molecules activate the cell death in response to pathogen. The green islands reminiscent are also reported in C. graminicola infection which was very much similar with green islands obseved in biotrophs (Behr et al. 2010).
PCD induction owing to Plant pathogenic bacteria in plants
The classification of plant pathogenic bacteria into biotrophic, necrotrophic and hemibiotrophic category is not so clear (Kraepiel and Barny 2016). Hence, in this review the PCD induction by plant pathogenic bacteria is discussed separately. Biotrophic or hemibiotrophic bacterial pathogens generally use type III secretion system to transfer effectors into the plant cell. These injected effectors further suppress the plant defences and help pathogen to establish within the invaded cells. In contrast to this, necrotrophic soft rot-causing bacterial pathogens use cell wall degrading enzymes which lead to maceration of plant tissue. Thereafter starts the utilization of macerated dead cells for nutrients. In addition to enzymes, there are bacterial species which takes the help of toxins for their establishment within the plant cells (Kraepiel and Barny 2016). The production of enzymes, toxins or effectors, etc. is under the control of cell to cell communication phenomenon known as quorum sensing (QS) (Katoch et al. 2020) and the QS-mediated regulation of various virulence determinants is involved in the suppression of defense (Kraepiel and Barny 2016). During pathogenesis of bacterial pathogens and in antibiotic resistance against various antibiotics, the QS plays main role. Therefore, effective management of plant diseases caused by bacterial pathogens could be done by inhibiting the QS signalling between bacterial cells and the inhibition of QS signals is known as quorum quenching (QQ) (Rutherford and Bassler 2012; Katoch et al. 2020).
Interaction of symbionts & biocontrol agents (BCAs) with plants
Different biotic and abiotic factors are known to cause serious yield losses in crop plants and various disease management strategies could be integrated to sustain the crop yield without having any adverse effect on human health and environment (Khoury and Makkouk 2010; Liliane and Charles 2020). In addition to detrimental microbes, there are species of microbes which have beneficial effects on plant growth and development and their non-pathogenic strains have been used in the management of plant diseases by many workers (Ganeshan and Kumar 2005; Kaur et al. 2010; Kumar et al. 2016; Bubici et al. 2019). BCAs act directly by parasitizing the pathogens or indirectly modulating the plants as inducing systemic resistance (Kohl et al. 2019). Unlike systemic resistance induced in response to plant pathogens, BCAs induce host plant resistance without any hypersensitive response (Liu et al. 2007). After initial contact with the host roots, the BCAs start colonizing them, and in response plant activates its defense mechanism to prevent further entry of BCAs upto cortical cell (Fig. 4) which helps it to deal with other invading pathogenic microbes (Nishad et al. 2020). The strategy used by some biocontrol agents is given below.
Fig. 4.
PCD induction during interaction between symbiont & biocontrol agents and host
Trichoderma and other biocontrol agents
Trichoderma is a well-known bio-control agent (BCA) which like other BCAs not only parasitizes the plant pathogenic microorganism directly but also known to induce systemic defense indirectly by colonizing the plant roots (Viterbo et al. 2005). In addition to Trichoderma, other non-pathpogenic fungal species like Fusarium, Rhizoctonia, etc. are also reported to enhance the growth and productivity of plants along with their protection to invading pathogenic incitants (Harman et al. 2004). Trichoderma comes in contact with plant roots, coils around the roots and starts producing appressoria-like structures and certain enzymes like endopolygalacturonase (Moran et al. 2009) which help BCA to penetrate the root cortex (Fig. 4). The intercellular hypha of Trichoderma induces rapid ion fluxes, oxidative burst, cell wall depositions and synthesis of phenolic compounds within the plant which ultimately limits the growth of Trichoderma upto cortical cells and indirectly activates defense mechanism which is effective against pathogenic microbes (Shoresh et al. 2010; Mukherjee et al. 2012). Trichoderma, upon interaction releases many compounds, particularly proteins, which leads to biosynthesis of phytoalexins or other defense-related compounds along with enhanced root development and good plant establishment (Ramirez-Valdespino et al. 2019). Presence or absence of T. harzianum affected the entire suite of chitinolytic enzymes during interaction with maize plants, which alters the plant proteome and transcriptome and also enhanced plant growth and resistance to diseases. The formation of a dimer between endo- and exochitinase was reported during plant–BCA interaction. The dimer originating from Trichoderma inoculated plants was reported to have a higher antifungal activity as compared to uninoculated control plants (Shoresh and Harman 2010). Additionally, the Avr gene products produced by various microorganisms as well as BCAs also induce defense mechanism in plants (Vinale et al. 2008). The influx or efflux of various ions, signalling molecules, and production of SA/JA/phytoalexins/PR proteins leads to systemic resistance against pathogenic microorganisms (Bisen et al. 2016).
Additionally, a Basidiomycetes- Piriformospora indica, being a mycorrhizal fungus is also known to induce systemic resistance against Blumeria graminis f. sp hordei causing powdery mildew of barley and various abiotic stress factors like high salt concentrations (Deshmukh et al. 2006). P. indica even produces giberellin within the invaded cells or tissues which modulates the plant defense against various biotic and abiotic stresses (Shoresh et al. 2010).
Plant growth promoting rhizobacteria (PGPRs)
In addition to fungal BCAs, PGPRs are also recognized to have stimulatory effects on the plant growth and development. Like fungal BCAs, these microorganisms are known to have direct antagonistic effect or indirect induction of systemic resistance against plant pathogens (Patel et al. 2016). These bioagents colonize the plant roots and induce jasmonic acid (JA) mediated induced systemic resistance within the plant system which ultimately help plants in checking the growth of number of invading plant pathogens (Mandal and Ray 2011; Ahemad and Kibret 2014). The rhizobacteria-induced systemic resistance (RISR) like SAR is dependent on transcription factor NPR1 which is also known as PR gene 1 (Harman et al. 2004).
Bio-control agents (BCAs) are not known to cause PCD in plants, but there are some Trichoderma species which are known to induce PCD in invading plant pathogens. For example, Trichoderma pseudokoningii peptaibol, i.e., trichokonin VI has been reported to induce PCD in Fusarium oxysporum (Shi et al. 2012; Sood et al. 2020). Despite this, there are reports in which PGPRs positively regulates the induction of PCD in rice plants and helping plants under saline water conditions (Jha and Subramanian 2014).
Future prospects and conclusions
Plant growth and development is being adversely affected by several biotic and abiotic stress factors. In response to these factors, plants react by activating intracellular signalling which either leads to defense activation or failure of plant survival. During plant–pathogen interaction, there are four main events viz. perception, signalling, signal transduction and signal termination which occurs in a tandem manner. Recognition either leads to compatible or incompatible host pathogen interaction depending on the presence or absence of resistance gene(s). Generally, plants undergo genetically controlled programmed cell death (PCD) or hypersensitivity to tackle different stress factors. Entire process of defense activation is dependent on invading pathogen and their infection strategies. Both plant and pathogen co-evolve in nature for their survival. First line of defense is PTI during interaction to resist the pathogen attack on host, while once it fails, then it leads to the activation of ETI. Microbes interacting with plants are generally categorized as biotrophs (dependent on living organisms, i.e., obligate), necrotrophs (capable of growing on dead matter), hemibiotrophs (have early biotrophic phase and later necrotrophic phase) and beneficial microbes (like symbiont and PGPRs having mutualistic relationship with organisms). This classification is not necessarily valid for all the plant pathogens, i.e., one organism could be kept in one or more categories. Among above mentioned classes of pathogens, necrotrophs and hemibiotrophs are comparatively more difficult to manage as they are capable of completing their life cycle on dead plant tissues. For an effective management of plant diseases, it is important to have knowledge about infection strategies followed by pathogenic microbes and this review highlights the differences in pathogenesis among different pathogens as well as mechanism of PCD induction as per nature and behaviour of microbes. Along with this, the importance of using symbionts and biocontrol agents, and their mode of action have also been discussed. The identification of novel underlying signals responsible for stress resistance or susceptibility could be used for formulating effective stress management strategies. The potential of symbionts and biocontrol agents in managing the plant diseases must be harnessed fully and for this, the basic information like plant protection strategy or molecules used by plants for defense must be known to us.
For sustaining crop production, scientists are focussing on using eco-friendly approaches over chemical pesticides. The detailed studies on gene regulatory networks involved in defense activation have scope in crop protection. Many transcription factors (TFs) as regulators of plant immunity within the plants have already been identified/characterized and to protect plants from adversities, focus must be kept on the identification of new TFs using advanced approaches like knockout/knockdown and overexpression. The expression of TFs could be manipulated by modifying the binding sites in promoters of TFs through CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats). Additionally, for analyzing the biological functions of TFs, various advanced techniques like CRES-T, tag line, RNAi, and antisense technologies must also be brought into use (Yuan et al. 2019). In addition to this, genome-wide DNA mapping and high throughput approaches could also be used in future for the identification of immunity-related TFs and to characterize gene regulatory networks involved in defense activation. While using TFs based approach for plant genetic improvement, the pleiotropic effects of TFs must also be considered especially while using NAC TFs. Detailed study of defense-related regulatory network will be helpful in future for dealing with stress factors without having adverse effect on human and animal health as done by overuse of pesticides.
Abbreviations
- PAMP
Pathogen-associated molecular patterns
- PRRs
Pattern recognition receptors
- PCD
Programmed cell death
- ROS
Reactive oxygen species
- SA
Salicylic acid
- JA
Jasmonic acid
- HR
Hypersensitive response
- ETI
Effector triggered immunity
- PTI
PAMP triggered immunity
- NBS-LRR
Nucleotide binding site-Leucine rich repeat
- Ss-Rhs1
Sclerotinia sclerotiorum Rearrangement hotspot 1
Author contributions
LP conceived the idea of review. LP, SK and SS wrote and designed the manuscript. All authors read and approved the manuscript.
Funding
There is no financial support for this manuscript.
Declarations
Conflict of interest
There is no conflict of interest regarding the publication of this article.
References
- Abdullah SNA, Akhtar MS. Plant and necrotrophic fungal pathogen interaction: mechanism and mode of action. In: Hakeem K, Akhtar M, Abdullah S, editors. Plant, soil and microbes. Cham: Springer; 2016. [Google Scholar]
- Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci. 2014;26:1–20. doi: 10.1016/j.jksus.2013.05.001. [DOI] [Google Scholar]
- Alberts B, Johnson A, Lewis J, et al (2002) Molecular biology of the cell. 4th edition. Garland Science, New York; 2002. Programmed Cell Death (Apoptosis). https://www.ncbi.nlm.nih.gov/books/NBK26873/
- Angra R, Mandahar CL. Pathogenesis of barley leaves by Helminthosporium teres I: green island formation and the possible involvement of cytokinins. Mycopathologia. 1991;114:21–27. [Google Scholar]
- Arcy D. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- Asthir B, Spoor W, Duffus C. Involvement of polyamines, diamine oxidase and polyamine oxidase in resistance of barley to Blumeria graminis f. sp. hordei. Euphytica. 2004;136:307–312. [Google Scholar]
- Balint-kurti P. The plant hypersensitive response: concepts, control and consequences. Mol Plant Pathol. 2019;20:1163–1178. doi: 10.1111/mpp.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr M, Humbeck K, Hause G, Deising HB, Wirsel SGR. The hemibiotroph Colletotrichum graminicola locally induces photosynthetically active green islands but globally accelerates senescence on aging maize leaves. MPMI. 2010;23:879–892. doi: 10.1094/MPMI-23-7-0879. [DOI] [PubMed] [Google Scholar]
- Bellincampi D, Cervone F, Lionetti V. Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front Plant Sci. 2014;5:1–8. doi: 10.3389/fpls.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne S, Javornik B. Signalling crosstalk of plant defence responses to xylem-invading pathogens. Abiotic and biotic stress in plants- Recent advances and future perspectives. Rijeka: InTech; 2016. pp. 411–440. [Google Scholar]
- Bisen K, Keswani C, Patel JS, Sarma BK, Singh HB. Trichoderma spp.: efficient inducers of systemic resistance in plants. In: Choudhary D, Varma A, editors. Microbial-mediated induced systemic resistance in plants. Singapore: Springer; 2016. [Google Scholar]
- Bozhkov PV. Plant autophagy: mechanisms and functions. J Exp Bot. 2018;69:1281–1285. doi: 10.1093/jxb/ery070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubici G, Kaushal M, Prigigallo MI, Gomez-Lama Cabanas C, Mercado-Blanco J. Biological control agents against Fusarium wilt of banana. Front Microbiol. 2019;10:616. doi: 10.3389/fmicb.2019.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R, Schwarze J, Sherwood OL, Jnaid Y, McCabe PF, Kacprzyk J. Stressed to death: the role of transcription factors in plant programmed cell death induced by abiotic and biotic stimuli. Front Plant Sci. 2020;11:1235. doi: 10.3389/fpls.2020.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burundukova OL, Sapotsky MV, Kochetov AV, Trifonova EA, Malinovsky VI. Dark and light green tissues of tobacco leaves systemically infected with tobacco mosaic virus. Biol Plant. 2009;53:294–300. [Google Scholar]
- Cai Y, Gallois P. Programmed cell death regulation by plant proteases with caspase-like activity. In: Gunawardena A, McCabe P, editors. Plant programmed cell death. Cham: Springer; 2015. pp. 191–202. [Google Scholar]
- Calil IP, Fontes EPB. Plant immunity against viruses: antiviral immune receptors in focus. Ann Bot. 2017;119:711–723. doi: 10.1093/aob/mcw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Blekemolen MC, Tintor N, Cornelissen BJC, Takken FLW. The Fusarium oxysporum Avr2-Six5 effector pair alters plasmodesmatal exclusion selectivity facilitates cell-to-cell movement of Avr2. Mol Plant. 2018;11(5):691–705. doi: 10.1016/j.molp.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Chanclud E, Kisiala A, Emery NRJ, Chalvon V, Ducasse A, Romiti-Michel C, et al. Cytokinin production by the rice blast fungus is a pivotal requirement for full virulence. PLoS Pathog. 2016;12:e1005457. doi: 10.1371/journal.ppat.1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-J, Liu J, Zhao F-F, Li J-Y, Wang S-X, Lin H-H, Xi D-H. Characterisation of the dark green islands of cucumber mosaic virus infected Nicotiana tabacum. Plant Cell Rep. 2015;34:1225–1238. doi: 10.1007/s00299-015-1781-1. [DOI] [PubMed] [Google Scholar]
- Chibucos MC, Collmer CW, Torto-Alalibo T, Gwinn-Giglio M, Lindeberg M, Li D, Tyler BM. Programmed cell death in host-symbiont associations, viewed through the Gene Ontology. BMC Microbiol. 2009;9:S5. doi: 10.1186/1471-2180-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y. How the necrotrophic fungus Alternaria brassicicola kills plant cells remains an enigma. Eukaryot Cell. 2015;14:335–344. doi: 10.1128/EC.00226-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Basu A, Kundu S. Biotrophy-necrotrophy switch in pathogen evoke differential response in resistant and susceptible sesame involving multiple signaling pathways at different phases. Sci Rep. 2017;7:17251. doi: 10.1038/s41598-017-17248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffeen WC, Wolpert TJ. Purification and characterization of serine proteases that exhibit caspase-like activity and are associated with programmed cell death in Avena sativa. Plant Cell. 2004;16:857–873. doi: 10.1105/tpc.017947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan SE, Walters DR. Photosynthesis in green-islands on powdery mildew infected barley leaves. Physiol Mol Plant Pathol. 1992;40:31–38. [Google Scholar]
- Coll NS, Epple P, Dang JL. Programmed cell death in the plant immune system. Cell Death Differ. 2011;18:1247–1256. doi: 10.1038/cdd.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo C, Chacun O, Borras O. Programmed cell death in plants resembles apoptosis of animals. Biotechnol Appl. 2006;23:1–10. [Google Scholar]
- Cooper GM. The cell: a molecular approach. 2. Sunderland: Sinauer Associates; 2000. [Google Scholar]
- Da Silva LL, Moreno HLA, Correia HLN, Santana MF, de Queiroz MV. Colletotrichum: species complexes, lifestyle, and peculiarities of some sources of genetic variability. Appl Microbiol Biotechnol. 2020;104:1891–1904. doi: 10.1007/s00253-020-10363-y. [DOI] [PubMed] [Google Scholar]
- Deshmukh S, Huckelhoven R, Schafer P, Imani J, Sharma M, Weiss M, Waller F, Kogel KH. The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. PNAS. 2006;103:18450–18457. doi: 10.1073/pnas.0605697103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarenne TP, Martin GB. Manipulation of plant programmed cell death pathways during plant-pathogen interactions. Plant Signal Behav. 2007;2:188–189. doi: 10.1038/sj.emboj.7600910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman MB, Ha YS, Yang Z, Adams B, Huang C. A protein kinase from Colletotrichum trifolii is indued by plant cutin and is required for appressorium formation. Mol Plant-Microbe Interact. 2003;16:411–421. doi: 10.1094/MPMI.2003.16.5.411. [DOI] [PubMed] [Google Scholar]
- Dickman M, Williams B, Li Y, de Figueiredo P, Wolpert T. Reassessing apoptosis in plants. Nat Plants. 2017;3(10):773–779. doi: 10.1038/s41477-017-0020-x. [DOI] [PubMed] [Google Scholar]
- Duca SD, Serafini-Fracassini D, Cai G. Senescence and programmed cell death in plants: polyamine action mediated by Transglutaminase. Front Plant Sci. 2014;5:1–17. doi: 10.3389/fpls.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Parra JE. Note on the origin and history of the term “Apoptosis”. Anat Rec Part B. 2005;283:2–4. doi: 10.1002/ar.b.20047. [DOI] [PubMed] [Google Scholar]
- Ellinger D, Voigt CA. Callose biosynthesis in arabidopsis with a focus on pathogen response: what we have learned within the last decade. Ann Bot. 2014;114:1349–1358. doi: 10.1093/aob/mcu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar ML, Echeverria OM, Vazquez-Nin GH. Necrosis as programmed cell death. Rijeka: Intech; 2015. pp. 419–433. [Google Scholar]
- Fananas EM, Todesca S, Sicorello A, Masino L, Pompach P, Magnani F, Pastore A, Mattevi A. On the mechanism of calcium-dependent activation of NADPH oxidase 5 (NOX5) FEBS J. 2020;287:2486–2503. doi: 10.1111/febs.15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, Capaccioli S, Orlandini SZ. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J Cell Physiol. 2000;182:41–49. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanan AJ, Sauer N, Hall JL, Williams LE. The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atβfruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol. 2003;132:821–829. doi: 10.1104/pp.103.021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Beattie GA. An overview of plant defenses against pathogens and herbivores. Plant Health Instr. 2008 doi: 10.1094/PHI-I-2008-0226-01. [DOI] [Google Scholar]
- Ganeshan G, Kumar AM. Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases. J Plant Interact. 2005;1:123–134. doi: 10.1080/17429140600907043. [DOI] [Google Scholar]
- Gardiner DM, Kazan K, Praud S, Torney FJ, Rusu A, et al. Early activation of wheat polyamine biosynthesis during Fusarium head blight implicates putrescine as an inducer of trichothecene mycotoxin production. BMC Plant Biol. 2010;10:289. doi: 10.1186/1471-2229-10-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilescu LC, Denkers EY. Apoptosis and the balance of homeostatic and pathologic responses to protozoan infection. Infect Immun. 2003;71:6109–6115. doi: 10.1128/IAI.71.11.6109-6115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays. 2006;28:1091–1101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- Gonçalves AP, Heller J, Daskalov A, Videira A, Glass NL. Regulated Forms of Cell Death in Fungi. Front Microbiol. 2017;8:1837. doi: 10.3389/fmicb.2017.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- Guzman-Guzman P, Aleman-Duarte MI, Delaye L, Herrera-Estrella A, Olmedo-Monfil V. Identification of effector-like proteins in Trichoderma spp. and role of hydrophobin in the plant-fungus interaction and mycoparasitism. BMC Genet. 2017;18:16. doi: 10.1186/s12863-017-0481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadi P, Ma L, Wang H, Borhan MH. Genome-wide transcriptomic analyses provide insights into the lifestyle transition and effector repertoire of Leptosphaeria maculans during the colonization of Brassica napus seedlings. Mol Plant Pathol. 2016;17:1196–1210. doi: 10.1111/mpp.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman DA, Chen Y, Sopee J, Berduo-Sandoval Sanchez-Perez A. Competition between Phytophthora infestans effectors leads to increased aggressiveness on plants containing broad-spectrum late blight resistance. PLoS ONE. 2010;5(5):e10536. doi: 10.1371/journal.pone.0010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman G, Howell C, Viterbo A, Chet I, Lorito M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- Heath MC. Apoptosis, programmed cell death and the hypersensitive response. Eur J Plant Pathol. 1998;104:117–124. [Google Scholar]
- Irieda H, Maeda H, Akiyama K, Hagiwara A, Saitoh H, Uemura A, et al. Colletotrichum orbiculare secretes virulence effectors to a biotrophic interface at the primary hyphal neck via exocytosis coupled with SEC22-mediated traffic. Plant Cell. 2014;26:2265–2281. doi: 10.1105/tpc.113.120600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalmi SK, Sinha AK. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front Plant Sci. 2015;6:769. doi: 10.3389/fpls.2015.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse van Rensburg HC, Van den Ende W, Signorelli S. Autophagy in plants: both a puppet and a puppet master of sugars. Front Plant Sci. 2019;10:14. doi: 10.3389/fpls.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha Y, Subramanian RB. PGPR regulate caspase-like activity, programmed cell death and antioxidant enzyme activity in paddy under salinity. Physiol Mol Biol Plants. 2014;20(2):201–207. doi: 10.1007/s12298-014-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Jwa NS, Hwang BK. Convergent evolution of pathogen effectors toward reactive oxygen species signaling networks in plants. Front Plant Sci. 2017;8:1687. doi: 10.3389/fpls.2017.01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczanowski S. Apoptosis: its origin, history, maintenance and the medical implications for cancer and aging. Phys Biol. 2016;13:031001. doi: 10.1088/1478-3975/13/3/031001. [DOI] [PubMed] [Google Scholar]
- Katoch S, Kumari N, Salwan R, Sharma V, Sharma PN. Recent developments in social network disruption approaches to manage bacterial plant diseases. Biol Control. 2020;150:104376. doi: 10.1016/j.biocontrol.2020.104376. [DOI] [Google Scholar]
- Kaur R, Kaur J, Singh RS. Nonpathogenic Fusarium as a biological control agent. Plant Pathol J. 2010;9:79–91. doi: 10.3923/ppj.2010.79.91. [DOI] [Google Scholar]
- Kelley BS, Lee SJ, Damasceno CM, Chakravarthy S, Kim BD, Martin GB, Rose JKC. A secreted effector protein (SNE1) from Phytophthora infestans is a broadly acting suppressor of programmed cell death. Plant J. 2010;62:357–366. doi: 10.1111/j.1365-313X.2010.04160.x. [DOI] [PubMed] [Google Scholar]
- Khare D, Choi H, Huh SU, Bassin B, Kim J, Martinoia E, Sohn KH, Paek KH, Lee Y. Arabidopsis ABCG34 contributes to defense against necrotrophic pathogens by mediating the secretion of camalexin. PNAS. 2017;114:E5712–E5720. doi: 10.1073/pnas.1702259114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury WE, Makkouk K. Integrated plant disease management in developing countries. Plant Pathol J. 2010;92:S4.35–S4.42. [Google Scholar]
- Kim KS, Min JY, Dickman MB. Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. MPMI. 2008;21:605–612. doi: 10.1094/MPMI-21-5-0605. [DOI] [PubMed] [Google Scholar]
- Kim NH, Kim BS, Hwang BK. Pepper arginine decarboxylase is required for polyamine and gamma-aminobutyric acid signaling in cell death and defense response. Plant Physiol. 2013;162:2067–2083. doi: 10.1104/pp.113.217372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, Kolnaar R, Ravensberg WJ. Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front Plant Sci. 2019;10:845. doi: 10.3389/fpls.2019.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukalova B, Kovarík A, Fajkus J, Siroky J. Chromatin fragmentation associated with apoptotic changes in tobacco cells exposed to cold stress. FEBS Lett. 1997;414(2):289–292. doi: 10.1016/s0014-5793(97)01008-9. [DOI] [PubMed] [Google Scholar]
- Kraepiel Y, Barny M-A. Gram-negative phytopathogenic bacteria, all hemibiotrophs after all? Mol Plant Pathol. 2016;17:313–316. doi: 10.1111/mpp.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A, Mabon R, Corbiere R, Montarry J, Andrivon D. The level of specialization of Phytophthora infestans to potato and tomato is a biotrophy-related, stable trait. bioRxiv. 2019 doi: 10.1101/571596. [DOI] [Google Scholar]
- Kumar SK, Fan L, Fu K, Yu C, Wang M, Xia H, Sun J, Li Y, Chen J. Cellulase from Trichoderma harzianum interacts with roots and triggers induced systemic resistance to foliar disease in maize. Sci Rep. 2016;6:35543. doi: 10.1038/srep35543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laluk K, Mengiste T. Necrotroph attacks on plants: wanton destruction or covert extortion? Arabidopsis Book. 2010;8:e0136. doi: 10.1199/tab.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Rose JKC. Mediation of the transition from biotrophy to necrotrophy in hemibiotrophic plant pathogens by secreted effector proteins. Plant Signal Behav. 2010;5:769–772. doi: 10.4161/psb.5.6.11778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Han X, Feng D, Yuan D, Huang LJ. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: do we understand what they are whispering. Int J Mol Sci. 2019;20:671. doi: 10.3390/ijms20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Rollins JA. Mechanisms of broad host range necrotrophic pathogenesis in Sclerotinia sclerotiorum. Phytopathology. 2018;108:1128–1140. doi: 10.1094/PHYTO-06-18-0197-RVW. [DOI] [PubMed] [Google Scholar]
- Liang H, Yao N, Song JT, Luo S, Lu H, Greenberg JT. Ceramide phosphorylation modulates programmed cell death in plants. Genes Dev. 2003;17:2636–2641. doi: 10.1101/gad.1140503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liliane TN, Charles MS. Factors affecting yield of crops. Agronomy-climate change and food security. Rijeka: InTech; 2020. pp. 1–16. [Google Scholar]
- Liu JZ, Lam HM. Signal transduction pathways in plants for resistance against pathogens. Int J Mol Sci. 2019;20:2335. doi: 10.3390/ijms20092335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XG, Gao KX, Kang ZS, He BL. Systemic resistance induced by biocontrol agents in plants and its biochemical and cytological mechanisms. Chin J Appl Ecol. 2007;18:1861–1868. [PubMed] [Google Scholar]
- Liu Z, Zhang Z, Faris JD, Oliver RP, Syme R, et al. The cysteine rich necrotrophic effector SnTox1 produced by Stagonospora nodorum triggers susceptibility of wheat lines harbouring Snn1. PLoS Pathog. 2012;8:e1002467. doi: 10.1371/journal.ppat.1002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locato V, Gara LD. Programmed cell death in plants: an overview. Methods Mol Biol. 2018;1743:1–8. doi: 10.1007/978-1-4939-7668-3_1. [DOI] [PubMed] [Google Scholar]
- Lorang JM, Sweat TA, Wolpert TJ. Plant disease susceptibility conferred by a ‘‘resistance’’ gene. PNAS. 2007;104:14861–14866. doi: 10.1073/pnas.0702572104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CE, Gunawardena AH. Programmed cell death in C. elegans, mammals and plants. Eur J Cell Biol. 2012;91(8):603–613. doi: 10.1016/j.ejcb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Ludwig N, Lohrer M, Hempel M, Mathea S, Schliebner I, Menzel M, Kiesow A, Schaffrath U, Deising HB, Horbach R. Melanin is not required for turgor generation but enhances cell-wall rigidity in appressoria of the corn pathogen Colletotrichum graminicola. MPMI. 2014;27:315–327. doi: 10.1094/MPMI-09-13-0267-R. [DOI] [PubMed] [Google Scholar]
- Lyu X, Shen C, Fu Y, Xie J, Jiang D, Li G, Cheng J. A small secreted virulence-related protein is essential for the necrotrophic interactions of Sclerotinia sclerotiorum with its host plants. PLoS Pathog. 2016;12:e1005435. doi: 10.1371/journal.ppat.1005435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma KW, Ma W. Phytohormone pathways as targets of pathogens to facilitate infection. Plant Mol Biol. 2016;91:713–725. doi: 10.1007/s11103-016-0452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Ray RC. Induced systemic resistance in biocontrol of plant diseases. In: Singh A, editor. Tomatoes: agricultural procedures, pathogen reactive oxygen species and antioxidative. Berlin, Heidelberg: Springer-Verlag; 2011. pp. 241–260. [Google Scholar]
- Mea MD, Serafini-Fracassini D, Duca SD. Programmed cell death: similarities and differences in animals and plants. A Flower Paradigm. Amino Acids. 2007;33:395–404. doi: 10.1007/s00726-007-0530-3. [DOI] [PubMed] [Google Scholar]
- Montilla-Bascón G, Rubiales D, Altabella T, Prats E. Free polyamine and polyamine regulation during pre-penetration and penetration resistance events in oat against crown rust (Puccinia coronata f. sp. avenae) Plant Pathol. 2016;65:392–401. [Google Scholar]
- Moore CJ, MacDiarmid RM. Dark Greens Islands: the phenomenon. In: Loebenstein G, Carr JP, editors. Natural resistance mechanisms of plants to viruses. Dordrecht: Springer; 2006. [Google Scholar]
- Moore CJ, Sutherland PW, Forster RLS, Gardner RC, MacDiarmid RM. Dark Green Islands in Plant Virus infection are the result of posttranscriptional gene silencing. MPMI. 2001;14:939–946. doi: 10.1094/MPMI.2001.14.8.939. [DOI] [PubMed] [Google Scholar]
- Moran DE, Hermosa R, Ambrosino P, Cardoza RE, Gutierrez S, Lorito M, Monte E. The ThPG1 Endopolygalacturonase is required for the Trichoderma harzianum–plant beneficial interaction. Mol Plant Microbe Interact. 2009;22:1021–1031. doi: 10.1094/MPMI-22-8-1021. [DOI] [PubMed] [Google Scholar]
- Morel JB, Dangl JL. The hypersensitive response and the induction of cell death in plants. Cell Death Differ. 1997;4:671–683. doi: 10.1038/sj.cdd.4400309. [DOI] [PubMed] [Google Scholar]
- Mori IC, Schroeder JI. Reactive oxygen species activation of plant Ca2+ channels. A signalling mechanism in polar growth, hormone transduction, stress signalling, and hypothetically mechanotransduction. Plant Physiol. 2004;135:702–708. doi: 10.1104/pp.104.042069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou PN, Roubelakis-Angelakis KA. Polyamines and programmed cell death. J Exp Bot. 2014;65:1285–1296. doi: 10.1093/jxb/ert373. [DOI] [PubMed] [Google Scholar]
- Mosquera G, Giraldo MC, Khang CH, Coughlan S, Valent B. Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as biotrophy-associated secreted proteins in rice blast disease. The Plant Cell. 2009;21(4):1273–1290. doi: 10.1105/tpc.107.055228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee M, Mukherjee PK, Horwitz BA, Zachow C, Berg G, Zeilinger S. Trichoderma-plant-pathogen interactions: advances in genetics of biological control. Indian J Microbiol. 2012;52:522–529. doi: 10.1007/s12088-012-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar MS, McCormack ME, Argueso CT, Pajerowska-Mukhtar KM. Pathogen tactics to manipulate plant cell death. Curr Biol. 2016;26:R608–R619. doi: 10.1016/j.cub.2016.02.051. [DOI] [PubMed] [Google Scholar]
- Navarre DA, Wolpert TJ. Victorin induction of an apoptosis/senescence-like response in oats. Plant Cell. 1999;11:237–249. doi: 10.1105/tpc.11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrel J, Vallee JC, Martin C. Ornithine decarboxylase activity and the hypersensitive reaction to tobacco mosaic-virus in Nicotiana tabacum. Phytochemistry. 1984;23:2747–2751. [Google Scholar]
- Nicoli A, Zambolim L, da Costa RV, Guimaraes LJM, Lanza FE, da Silva DD, Cota LV. Identification of sources of resistance to anthracnose stalk rot in maize. Ciencia Rural Santa Maria. 2016;46:1885–1890. [Google Scholar]
- Nishad R, Ahmed T, Rahman VJ, Kareem A. Modulation of plant defense system in response to microbial interactions. Front Microbiol. 2020;11:1298. doi: 10.3389/fmicb.2020.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira GE, Deising HB. Attenuation of PAMP-triggered immunity in maize requires down-regulation of the key b-1,6-glucan synthesis genes KRE5 and KRE6 in biotrophic hyphae of Colletotrichum graminicola. Plant J. 2016;87:355–375. doi: 10.1111/tpj.13205. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann HJ, Kangasjarvi J. The ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signalling pathways in regulating superoxide-dependent cell death. Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal M, Janda T. Role of polyamine metabolism in plant pathogen interactions. J Plant Sci Phytopathol. 2017;1:095–0100. [Google Scholar]
- Palavan UN, Buyuktuncer ED, Tufekci MA. Programmed cell death in plants. J Cell Mol Biol. 2005;4:9–23. [Google Scholar]
- Patel S, Sayyed R, Saraf M. Bacterial determinants and plant defense induction: their role as biocontrol agents in sustainable. In: Hakeem KR, Akhtar MS, editors. Plant soil and microbes. Singapore: Springer-Nature; 2016. pp. 187–204. [Google Scholar]
- Pennell RI, Lamb C. Programmed cell death in plants. Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov V, Hille J, Mueller-Roeber B, Gechev TS. ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci. 2015;6:1–16. doi: 10.3389/fpls.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecka A, Jedrzejczak-Rey N, Bednarek P. Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol. 2015;206:948–964. doi: 10.1111/nph.13325. [DOI] [PubMed] [Google Scholar]
- Pogany M, Harrach BD, Hafez YM, Barna B, Kiraly Z, Paldi E. Role of reactive oxygen species in abiotic and biotic stresses in plants. Acta Phytopathol Entomol Hung. 2006;41:23–35. doi: 10.1556/APhyt.41.2006.1-2.4. [DOI] [Google Scholar]
- Pontier D, Balagu C, Robya D. The hypersensitive response. A programmed cell death associated with plant resistance. La réponse hypersensible. Un cas de mort cellulaire programmée associée à la résistance des plantes. Comptes Rendus De L'académie Des Sciences. 1998;321(9):721–734. doi: 10.1016/s0764-4469(98)80013-9. [DOI] [PubMed] [Google Scholar]
- Ponzio C, Weldegergis BT, Dicke M, Gols R. Compatible and incompatible pathogen-plant interactions differentially affect plant volatile emissions and the attraction of parasitoid wasps. Funct Ecol. 2016;30:1779–1789. doi: 10.1111/1365-2435.12689. [DOI] [Google Scholar]
- Prell HH, Day P. Recognition, the first step in interaction between plant and pathogen. Plant-fungal pathogen interaction. Berlin, Heidelberg: Springer; 2001. [Google Scholar]
- Proels RK, Huckelhoven R. Cell-wall invertases, key enzymes in the modulation of plant metabolism during defence responses. Mol Plant Pathol. 2014;15:858–864. doi: 10.1111/mpp.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztahelyi T, Holb IJ, Pocsi I. Plant-fungal interactions: special secondary metabolites of the biotrophic, necrotrophic, and other specific interactions. In: Merillon JM, Ramawat K, editors. Fungal metabolites. Reference series in phytochemistry. Cham: Springer; 2016. [Google Scholar]
- Ramirez-Valdespino CA, Casas-Flores S, Olmedo-Monfil V. Trichoderma as a model to study effector-like molecules. Front Microbiol. 2019;10:1030. doi: 10.3389/fmicb.2019.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantong G, Gunawardena AHLAN. Programmed cell death: genes involved in signalling, regulation, and execution in plants and animals. Botany. 2015;93:193–210. doi: 10.1139/cjb-2014-0152. [DOI] [Google Scholar]
- Reape TJ, Molony EM, McCabe PF. Programmed cell death in plants: distinguishing between different modes. J Exp Bot. 2008;59(3):435–444. doi: 10.1093/jxb/erm258. [DOI] [PubMed] [Google Scholar]
- Reina-Pinto JJ, Yephremov A. Surface lipids and plant defenses. Plant Physiol Biochem. 2009;47:540–549. doi: 10.1016/j.plaphy.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Ridout CJ, Skamnioti P, Porritt O, Sacristan S, Jones JDG, Brown JKM. Multiple avirulence paralogues in cereal powdery mildew fungi may contribute to parasite fitness and defeat of plant resistance. Plant Cell. 2006;18:2402–2414. doi: 10.1105/tpc.106.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Puertas MC, Perazzolli M, Zago ED, Delledonne M. Nitric oxide signalling functions in plant-pathogen interactions. Cell Microbiol. 2004;6:795–803. doi: 10.1111/j.1462-5822.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh H, et al. Large-scale gene disruption in Magnaporthe oryzae identifies MC69, a secreted protein required for infection by monocot and dicot fungal pathogens. PLoS Pathog. 2012;8:e1002711. doi: 10.1371/journal.ppat.1002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006;141:336–340. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. Lipids, lipases, and lipid-modifying enzymes in plant disease resistance. Annu Rev Phytopathol. 2005;43:229–260. doi: 10.1146/annurev.phyto.43.040204.135951. [DOI] [PubMed] [Google Scholar]
- Sharma G, Aminedi R, Saxena D, Gupta A, Banerjee P, Jain D, Chandran D. Effector mining from the Erysiphe pisi haustorial transcriptome identifies novel candidates involved in pea powdery mildew pathogenesis. Mol Plant Pathol. 2019;20:1506–1522. doi: 10.1111/mpp.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty NP, Mehrabi R, Lutken H, Haldrup A, Kema GHJ, Collinge DB, Jorgensen HJL. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol. 2007;174:637–647. doi: 10.1111/j.1469-8137.2007.02026.x. [DOI] [PubMed] [Google Scholar]
- Shi M, Lei Chen L, Wang XW, Zhang T, Zhao PB, Song XY, Sun CY, Chen XL, Zhou BC, Zhang YZ. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology. 2012;158:166–175. doi: 10.1099/mic.0.052670-0. [DOI] [PubMed] [Google Scholar]
- Shoresh M, Harman GE. Differential expression of maize chitinases in the presence or absence of Trichoderma harzianum strain T22 and indications of a novel exo- endo-heterodimeric chitinase activity. BMC Plant Biol. 2010;10:136. doi: 10.1186/1471-2229-10-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- Sobiczewskia P, Iakimova ET, Mikicinski A, Wezgrzynowicz-Lesiak E, Dyki B. Necrotrophic behaviour of Erwinia amylovora in apple and tobacco leaf tissue. Plant Pathol. 2017;66:842–855. doi: 10.1111/ppa.12631. [DOI] [Google Scholar]
- Sohn KH, Lei R, Nemri A, Jones JD. The downy mildew effector proteins ATR1 and ATR13 promote disease susceptibility in Arabidopsis thaliana. Plant Cell. 2007;19:4077–4090. doi: 10.1105/tpc.107.054262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood M, Kapoor D, Kumar V, Sheteiwy MS, Ramakrishnan M, Landi M, Araniti F, Sharma A. Trichoderma: the “Secrets” of a multitalented biocontrol agent. Plants-Basel. 2020;9:762. doi: 10.3390/plants9060762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanu PD, Panstruga R. Editorial: biotrophic plant-microbe interactions. Front Plant Sci. 2017;8:192. doi: 10.3389/fpls.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio S, De BI, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA. 2000;97:14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson SA, Hatfield J, Rusu AG, Maclean DJ, Manners JM. CgDN3: An essential pathogenicity gene of Colletotrichum gloeosporioides necessary to avert a hypersensitive-like response in the host Stylosanthes guianensis. Mol Plant Microbe Interact. 2000;13:929–941. doi: 10.1094/MPMI.2000.13.9.929. [DOI] [PubMed] [Google Scholar]
- Su T, Li X, Yang M, Shao Q, Zhao Y, Ma C, Wang P. Autophagy: an intracellular degradation pathway regulating plant survival and stress response. Front Plant Sci. 2020;11:164. doi: 10.3389/fpls.2020.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidzinski JA, Sweetlove LJ, Leaver CJ. A custom microarray analysis of gene expression during programmed cell death in Arabidopsis thaliana. Plant J. 2002;30:431–446. doi: 10.1046/j.1365-313x.2002.01301.x. [DOI] [PubMed] [Google Scholar]
- Sychta K, Słomka A, Kuta E. Insights into plant programmed cell death induced by heavy metals-discovering a Terra Incognita. Cells. 2021;10:65. doi: 10.3390/cells10010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Bassham DC. Autophagy in crop plants: what’s new beyond Arabidopsis? Open Biol. 2018;8:180162. doi: 10.1098/rsob.180162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauzin AS, Giardina T. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front Plant Sci. 2014;5:1–8. doi: 10.3389/fpls.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira RM, Ferreira MA, Raimundo GAS, Loriato VAP, Reis PAB, Fontes EPB. Virus perception at the cell surface: revisiting the roles of receptor-like kinases as viral pattern recognition receptors. Mol Plant Pathol. 2019;20:1196–1202. doi: 10.1111/mpp.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SG, Franklin-Tong VE. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature. 2004;429:305–309. doi: 10.1038/nature02540. [DOI] [PubMed] [Google Scholar]
- Tuteja N, Mahajan S. Calcium signaling network in plants-an overview. Plant Signal Behav. 2007;2:79–85. doi: 10.4161/psb.2.2.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura K, Tanino S, Nagatsuka T, Koga J, Iwata M, Nagashima K, Amemiya Y. Cerebroside elicitor confers resistance to Fusarium disease in various plant species. Phytopathology. 2004;94:813–818. doi: 10.1094/PHYTO.2004.94.8.813. [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF. reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doorn WG, Beers EP, Dangl JL, Franklin-Tong VE, Gallois P, Hara-Nishimura I, Jones AM, Kawai-Yamada M, Lam E, Mundy J, Mur LAJ, Petersen M, Smertenko A, Taliansky M, Van Breusegem F, Wolpert T, Woltering E, Zhivotovsky B, Bozhkov PV. Morphological classification of plant cell deaths. Cell Death Differ. 2011;18:1241–1246. doi: 10.1038/cdd.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas WA, Martin JMS, Rech GE, Rivera LP, Benito EP, Diaz-Minguez JM, Thon MR, Sukno SA. Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotricum graminicola in maize. Plant Physiol. 2012;158:1342–1358. doi: 10.1104/pp.111.190397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marraa R, Barbettid MJ, Li H, Woo SL, Loritoa M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol Mol Plant Pathol. 2008;72:80–86. doi: 10.1016/j.pmpp.2008.05.005. [DOI] [Google Scholar]
- Viterbo A, Harel M, Horwitz BA, Chet I, Mukherjee PK. Trichoderma mitogen-activated protein kinase signaling is involved in induction of plant systemic resistance. Appl Environ Microbiol. 2005;71:6241–6246. doi: 10.1128/AEM.71.10.6241-6246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters DR, McRoberts N, Fitt BDL. Are green islands red herrings? Significance of green islands in plant interactions with pathogens and pests. Biol Rev. 2008;83:79–102. doi: 10.1111/j.1469-185X.2007.00033.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ma L-Y, Cao J, Li Y-L, Ding L-N, Zhu K-M, Yang Y-H, Tan X-L. Recent advances in mechanisms of plant defense to Sclerotinia sclerotiorum. Front Plant Sci. 2019;10:1314. doi: 10.3389/fpls.2019.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Lam E. Bax Inhibitor-1, a conserved cell death suppressor, is a key molecular switch downstream from a variety of biotic and abiotic stress signals in plants. Int J Mol Sci. 2009;10:3149–3167. doi: 10.3390/ijms10073149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L. Cell death in plant immune response to necrotrophs. J Plant Biochem Physiol. 2013;1:1. doi: 10.4172/jpbp.1000e103. [DOI] [Google Scholar]
- White PJ, Broadley MR. Calcium in plants. Ann Bot. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wituszynska W, Karpinski S. Programmed cell death as a response to high light, UV and drought stress in plants. Abiotic stress - plant responses and applications in agriculture. Rijeka: InTech; 2013. pp. 207–246. [Google Scholar]
- Woo HR, Kim HJ, Nam HG, Lim PO. Plant leaf senescence and death - regulation by multiple layers of control and implications for aging in general. J Cell Sci. 2013;126:4823–4833. doi: 10.1242/jcs.109116. [DOI] [PubMed] [Google Scholar]
- Wu X, Valli A, García JA, Zhou X, Cheng X. The tug-of-war between plants and viruses: great progress and many remaining questions. Viruses. 2019;11:203. doi: 10.3390/v11030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Zhang L. Plant caspase-like proteases in plant programmed cell death. Plant Signal Behav. 2009;4:902–904. doi: 10.4161/psb.4.9.9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, ShahJ KDF. Signal perception and transduction in plant defense responses. Genes Dev. 1997;11:1621–1639. doi: 10.1101/gad.11.13.1621. [DOI] [PubMed] [Google Scholar]
- Yuan X, Wang H, Cai J, Li D, Song F. NAC transcription factors in plant immunity. Phytopathol Res. 2019;1:3. doi: 10.1186/s42483-018-0008-0. [DOI] [Google Scholar]
- Zepeda-Jazo I, Velarde-Buendía AM, Enríquez-Figueroa R, Bose J, Shabala S, Muñiz-Murguía J, Pottosin II. Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membranes. Plant Physiol. 2011;157:2167–2180. doi: 10.1104/pp.111.179671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Henderson C, Gurr SJ. Blumeria graminis secretes an extracellular catalase during infection of barley: potential role in suppression of host defence. Mol Plant Pathol. 2004;5:537–547. doi: 10.1111/J.1364-3703.2004.00251.X. [DOI] [PubMed] [Google Scholar]
- Zhang D, Liang W, Yin C, Zong J, Gu F, Zhang D. OsC6, encoding a lipid transfer protein, is required for postmeiotic anther development in rice. Plant Physiol. 2010;154:149–162. doi: 10.1104/pp.110.158865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Li Q, Liu T, Liu L, Shen D, Zhu Y, et al. Two cytoplasmic effectors of Phytophthora sojae regulate plant cell death via interactions with plant catalases. Plant Physiol. 2015;167:164–175. doi: 10.1104/pp.114.252437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang F, Melotto M, Yao J, He SY. Jasmonate signaling and manipulation by pathogens and insects. J Exp Bot. 2017;68:1371–1385. doi: 10.1093/jxb/erw478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Feng Zhao F, Jiang L, Chen C, Wu L, Liu Z. Different pathogen defense strategies in arabidopsis: more than pathogen recognition. Cells. 2018;7:1–24. doi: 10.3390/cells7120252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu K, Yu D, Liu Z, Peng C, Li X, Zhang J, Dong Y, Zhang Y, Tian P, Guo T, Li C. The highly conserved barley powdery mildew effector BEC1019 confers susceptibility to biotrophic and necrotrophic pathogens in wheat. Int J Mol Sci. 2019;20(18):4376. doi: 10.3390/ijms20184376. [DOI] [PMC free article] [PubMed] [Google Scholar]