Abstract
Management of biliary tract cancers (BTCs) is rapidly evolving. Curative management relies on surgical resection followed by adjuvant capecitabine for cholangiocarcinoma and gallbladder cancers. Unfortunately relapse rate remains high, and better adjuvant strategies are urgently required. A majority of patients are diagnosed with advanced disease, when chemotherapy with cisplatin and gemcitabine followed by second-line 5-FU and oxaliplatin /irinotecan is the cornerstone of treatment for most patients in the absence of targetable alterations. Targeted therapies, including therapies for tumours with fibroblast growth factor receptor-2 (FGFR-2) fusions, isocitrate dehydrogenase-1 (IDH-1) mutations, B-Raf proto-oncogene serine/threonine kinase (BRAF) V600E mutations, neurotrophic tyrosine receptor kinase (NTRK) fusions, Human epidermal growth factor-2 (HER-2) amplifications, and/or microsatellite instability are rapidly changing the treatment paradigm for many patients with advanced BTC, especially for patients with intrahepatic cholangiocarcinoma. Because of this, molecular profiling should be considered early on patients pathway to allow adequate planning of therapy. Ongoing research is likely to clarify the role of immunotherapy, liver-directed therapy, and liver transplant for BTCs in the future.
Key words: biliary tract cancer, cholangiocarcinoma, gallbladder cancer, ampullary cancer, treatment, chemotherapy, surgery, targeted
Highlights
-
•
Surgical resection remains the mainstay of cure for BTC.
-
•
Adjuvant therapy for 6 months with capecitabine is recommended after surgery.
-
•
Palliative treatment: CisGem followed by FOLFOX/5-FU and irinotecan (FOLFIRI) [nanoliposomal irinotecan (nal-IRI)] or biomarker-selected therapy.
-
•
Targeted therapies against IDH-1, FGFR-2, BRAF, NTRK, HER-2, MSI, and MMR deficiency are of interest.
-
•
FOLFIRI and nal-IRI are additional second-line chemotherapy alternatives to FOLFOX.
-
•
Immunotherapy, liver-directed therapy, and liver transplant may be of benefit in selected patients.
Introduction
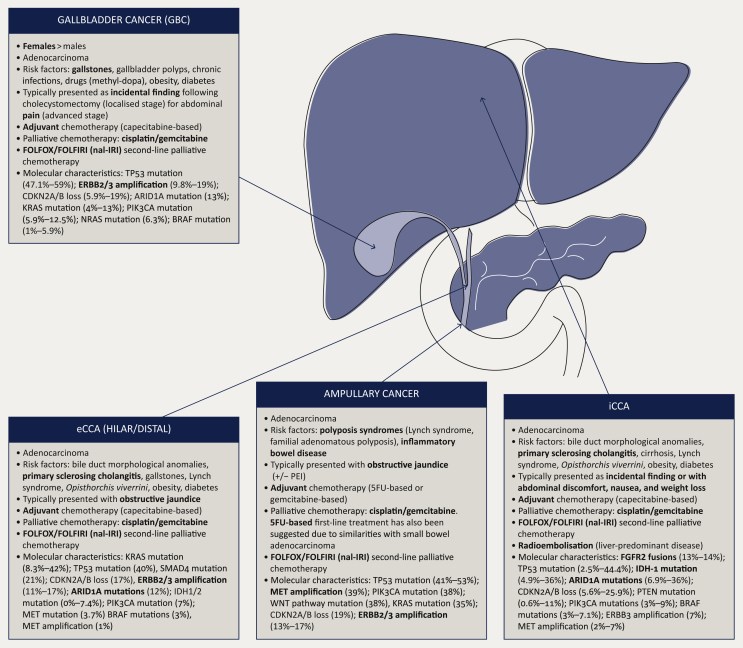
Biliary tract cancers (BTCs) include cholangiocarcinoma (CCA), gallbladder cancer (GBC), and ampullary tumours.1 CCAs can be subdivided depending on their location between intrahepatic CCA (iCCA) and extrahepatic CCA (eCCA). eCCA can be also subdivided between hilar and distal CCA depending on their location.2 It is important to highlight that patients' demographics, mode of presentation, and tumour mutational profiles have both overlap and distinctions within these subgroups (Figure 1).
Figure 1.
Biliary tract cancers (BTCs) are a heterogeneous group of malignancies.
5-FU, 5-fluorouracil; eCCA, extrahepatic cholangiocarcinoma; FOLFOX, 5-fluorouracil and oxaliplatin; FOLFIRI, 5-fluorouracil and irinotecan; iCCA, intrahepatic cholangiocarcinoma; nal-IRI, nanoliposomal irinotecan; PEI, pancreatic exocrine insufficiency. Adapted from Lamarca A, Frizziero M, McNamara MG, Valle JW. Clinical and translational research challenges in biliary tract cancers. Curr Med Chem. 2020;27(29):4756-4777.
BTCs are rare cancers, with estimated incidence of <6 cases per 100 000 habitants.2 However, incidence is increasing, mainly due to an increased incidence of iCCA. BTCs are known to have a poor prognosis, with an estimated 5-year overall survival (OS) rate of <20% when all stages are analysed together. This is attributable to the high rate of diagnosis in the advanced stages, when the disease is mainly managed by palliative therapeutic approaches. In addition, for those patients diagnosed in early stages, relapse rate remains high at >50%. Thus the majority of patients will be ultimately managed with systemic therapy over the course of their disease, regardless of whether they present with advanced disease or develop recurrence after surgery.
Diagnosis and work-up
Diagnosis of CCA has traditionally been difficult due to the paucity of tissue obtained from fine-needle aspirates and bile duct brushings, the lack of viable tumour cells in these often desmoplastic tumours with significant necrosis, and the lack of a pathognomonic immunohistochemical stain or staining pattern.3 Core biopsies are therefore recommended where feasible and safe, for both histologic confirmation and obtaining sufficient tissue for molecular profiling in cases of advanced disease. Cross-sectional imaging with computerised tomography of the chest, abdomen, and pelvis and/or magnetic resonance as needed are recommended for complete staging. 18F-fluorodeoxyglucose positron emission tomography can be used for assessment of lymph node and distant metastases and also to confirm disease recurrence if the identification of occult sites of disease would change management (i.e. surgery/local therapies) or if diagnosis of relapse remains unclear following standard of care imaging.4
Management of resectable disease
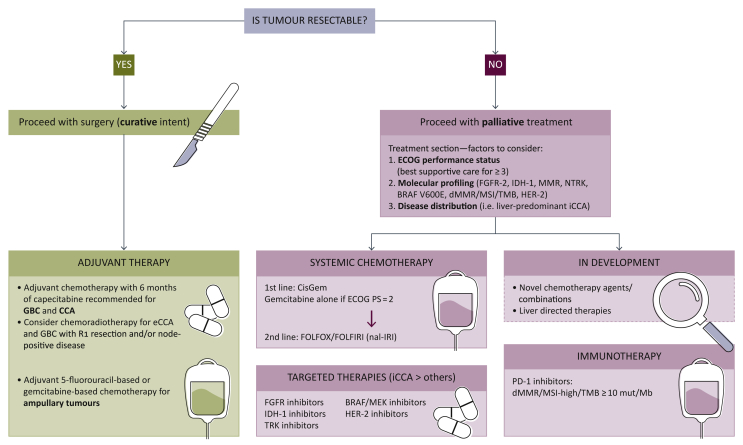
A summary of the management algorithm for patients with BTCs, based on current scientific evidence and latest guidelines recommendations, is provided in Figure 2. Surgery with curative intent is the current gold standard for patients who are diagnosed with resectable disease.1 As mentioned earlier, relapse rate remains high and ranges between 42% and 70% based on latest data.5, 6, 7
Figure 2.
Biliary tract cancers (BTCs): Diagnosis and management algorithm.
BRAF, B-Raf proto-oncogene serine/threonine kinase; CCA, cholangiocarcinoma; dMMR, deficient DNA mismatch repair; eCCA, extrahepatic cholangiocarcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; FGFR, fibroblast growth factor receptor; GBC, gallbladder cancer; HER-2, human epidermal growth factor-2; iCCA, intrahepatic cholangiocarcinoma; IDH-1, isocitrate dehydrogenase-1; MEK, mitogen-activated extracellular signal-regulated kinase; MSI, microsatellite instability; nal-IRI, nanoliposomal irinotecan; NTRK, neurotrophic tyrosine receptor kinase; TMB, tumour mutational burden; TRK, tyrosine receptor kinase. Adapted from2.
Following curative resection, including resections achieving complete margin clearance (R0) or microscopic (R1) margin infiltration, current evidence supports the role of adjuvant treatment for BTCs.7
Since 2017, data from three phase III randomised studies evaluating adjuvant therapy after surgical resection of CCA and GBC have been reported. Two of these studies explored the role of gemcitabine-based adjuvant treatment—gemcitabine and oxaliplatin in the PRODIGE-12 study and single-agent gemcitabine in the BCAT study—and showed no benefit over observation alone.7 A recent meta-analysis of these two studies confirmed such findings.8 However, and based on the phase III randomised BILCAP clinical trial,5 which randomised patients to capecitabine or observation, current guidelines do recommend the use of a 6-month period of adjuvant capecitabine for resected CCA and GBC.9 This recommendation stands despite the fact that the BILCAP study did not meet its primary endpoint, OS in the intention-to-treat population. The study showed a median OS of 51.1 months [95% confidence interval (CI) 34.6-59.1] in the capecitabine arm and 36.4 months (95% CI 29.7-44.5 months) in the control arm [hazard ratio (HR) 0.81, 95% CI 0.63-1.04; P = 0.097] and did show benefit for OS in the prespecified sensitivity analysis [53 months (95% CI 40 months-not reached) versus 36 months (95% CI 30-44 months), HR 0.75 (95% CI 0.58-0.98); P = 0.033]. The latter adjusted the survival analysis for other prognostic factors such as nodal status, grade of disease, and gender. A current ongoing trial is exploring the role of combination chemotherapy with cisplatin and gemcitabine (CisGem) in this setting compared with capecitabine alone (ACTICCA-1; NCT02170090). In addition, there is nonrandomised evidence to consider the use of chemoradiotherapy for eCCA and GBC with R1 resection and/or node positive disease.9,10
For adjuvant therapy for ampullary tumours, the randomised phase III ESPAC-3 clinical trial recruited patients with periampullary malignancies, including tumours from the ampulla of Vater.6 The study had three arms: adjuvant gemcitabine, adjuvant 5-fluorouracil (5-FU), and a third arm with observation alone. The use of 5-FU (compared with observation) did not reach statistical significance [HR 0.79, 95% CI 0.58-18; P = 0.13]. By contrast, the gemcitabine arm showed a reduction in risk of death compared with observation alone (HR 0.70, 95% CI 0.51-0.97; P = 0.03). There are some challenges at the time to interpreting the ESPAC-3 study. First, a variety of tumours besides ampullary malignancies were included in the study. Second, the study was powered to identify an OS benefit when comparing the joined arms with adjuvant chemotherapy versus observation alone; therefore the study was not powered to select the most effective adjuvant chemotherapy. Based on these findings, use of 5-FU-based or gemcitabine-based adjuvant chemotherapy can be considered following surgery for ampullary malignancies. In some centres, choice of chemotherapy may be driven by histological subtype (‘intestinal type’ versus ‘pancreato-biliary type’ versus ‘indeterminate’).
Management of advanced disease
Chemotherapy
In the setting of advanced disease and adequate performance status, CisGem is the current first-line standard of care based on the survival benefit shown with this regimen over gemcitabine alone in the ABC-02 clinical trial.11 Estimated median progression-free survival (PFS) and OS with CisGem in this trial were 8.0 months (versus 5.0 months with gemcitabine alone; HR 0.63, 95% CI 0.51-0.77; P < 0.001) and 11.7 months (versus 8.1 months with gemcitabine alone; HR 0.64, 95% CI 0.52-0.80; P < 0.001), respectively. In the event of performance status of 2, significant comorbidities, or contraindication for cisplatin, single-agent gemcitabine is a reasonable option. Alternative chemotherapy combinations are currently being explored, mainly in the form of triple chemotherapy schedules and combinations of chemotherapy with immunotherapy. The TOPAZ-1 trial comparing CisGem combined with durvalumab/placebo has been recently reported as positive, with full data awaited at the time of this publication.12 The recently presented PRODIGE 38 AMEBICA study compared modified 5-FU, oxaliplatin, and irinotecan (mFOLFIRINOX) with CisGem and did not meet its primary endpoint of improving the 6-month PFS rate (44.6% with mFOLFIRINOX versus 47.3% with CisGem).13 A third triplet regimen, CisGem with nab-paclitaxel, achieved an objective response rate (ORR) of 45% in the evaluable population of a single-arm phase II study,14 and the results of the randomised phase III study (SWOG0809) of this regimen against CisGem in advanced BTC are awaited.
Following progression to first-line CisGem, second-line treatment with 5-FU and oxaliplatin (FOLFOX) is considered current standard of care in patients with advanced BTC in the absence of targetable alterations or biomarkers that could predict response to immunotherapy (see “Targeted treatments” and “Immunotherapy” sections below). The ABC-06 trial showed that FOLFOX improved OS compared with active symptom control alone in patients with progression after CisGem (median OS: 6.2 versus 5.3 months, respectively, adjusted HR 0.69, 95% CI 0.50-0.97; P = 0.031), with benefit also in the 6- (50.6% versus 35.5%) and 12-month (25.9% versus 11.4%) OS rate.15 Promising results were recently presented for the phase II NIFTY study which randomised patients to nanoliposomal irinotecan (nal-IRI) and 5-FU versus 5-FU alone following progression after CisGem treatment. The study met its primary endpoint showing a median PFS of 7.1 months in the combination arm versus 1.4 months with 5-FU alone (HR 0.56, 95% CI 0.39-0.81; P = 0.0019)16; this study was conducted in a Korean population, and confirmatory studies in Western population are likely to be required prior to considering this option as standard of care in view of discrepant results with previous randomised phase II studies comparing FOLFOX and 5-FU and irinotecan (FOLFIRI) in advanced chemorefractory BTC, which showed similar outcomes with both schedules.17 While these results are awaited, FOLFIRI (with irinotecan or nal-IRI if accesible), may be considered as a treatment option if oxaliplatin contraindicated or in the presence of progression to oxaliplatin.
Targeted treatments
Targeted treatments following a precision medicine approach have revolutionised the management of BTCs, especially iCCA.18 As mentioned previously, BTCs include a heterogeneous group of malignancies that also differ in their mutational profiles (Figure 1). In iCCA, isocitrate dehydrogenase-1 (IDH-1) mutations (15%-20% frequency) and fibroblast growth factor receptor-2 (FGFR-2) fusions (10%-15%) are the most common actionable alterations. Human epidermal growth factor-2 (HER-2) amplification is more prevalent in eCCA and GBC but also seen in iCCA (5%-15%). Finally, B-Raf proto-oncogene serine/threonine kinase (BRAF) V600E mutations are seen across BTCs (4%-5%). In addition, some tumour-agnostic approaches such as neurotrophic tyrosine receptor kinase (NTRK) may play a role for a small proportion of patients with BTCs (<1%). To identify these potentially targetable alterations, performing molecular profiling for patients diagnosed with CCA (especially iCCA) is now considered standard of care; to allow adequate time to plan therapeutic strategies based on the results, performing such analyses at time of diagnosis or during first-line treatment of advanced disease is recommended.19
Selective FGFR inhibitors have shown significant activity in patients with refractory advanced CCA harbouring an FGFR-2 fusion or other rearrangement, with multiple ATP-competitive and covalently binding inhibitors in development. Phase II single-arm registrational trials of FGFR inhibitors in this population show an ORR of 23%-42% and a median PFS of 7-9 months.20, 21, 22 Currently, two of these compounds have been approved by regulatory agencies: pemigatinib [Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved in 2020 and 2021, respectively] and infigratinib (FDA approved in 2021).18
For patients with advanced IDH-1-mutant CCA refractory to one or two lines of systemic therapy, the IDH-1 inhibitor ivosidenib has shown activity in the ClarIDHy phase III clinical trial when compared with placebo.23 This study met its primary endpoint, showing an improvement in median PFS from 1.4 months with placebo to 2.7 months with ivosidenib (HR 0.37, 95% CI 0.25-0.54; P = 0.001), gaining it FDA approval in this setting. Median OS in the ivosidenib arm was 10.3 months (95% CI 7.8-12.4 months) versus 7.5 months (95% CI 4.8-11.1 months) with placebo (HR 0.79, 95% CI 0.56-1.12; one-sided P = 0.09). When adjusted for crossover, median OS with placebo was 5.1 months (95% CI 3.8-7.6 months); HR 0.49 (95% CI 0.34-0.70; one-sided P < 0.001).24 Other targetable alterations include BRAF V600E mutations25 and NTRK fusions,26 and also HER-2 amplifications27 and mutations.28 A recent phase II study exploring the combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib in patients with BRAF V600E-mutated BTC reported an independent reviewer-assessed ORR of 51% (95% CI 36%-67%).25 Following a tumour-agnostic approach, targeting NTRK fusions with tropomyosin receptor kinase (TRK) inhibitors such as larotrectinib and entrectinib (both FDA and EMA approved) have reported an ORR of 57%-79% with a complete response rate of 7%-16%.26,29 Finally, recent studies have explored the HER2 pathway in patients with advanced BTC.27,28 The MyPathway study explored the activity of pertuzumab combined with trastuzumab in chemorefractory advanced BTC with HER-2 amplification, HER-2 overexpression, or both, and the ORR was 23% (95% CI 11%-39%).27 For patients with advanced BTCs harbouring an HER-2 mutation, the ORR achieved with the pan-Her inhibitor neratinib was 12% (95% CI 3%-31%), with tumour shrinkage enriched in gallbladder and extrahepatic subtypes of BTC.28
Immunotherapy
Pembrolizumab is indicated for patients with BTC deficient in mismatch repair proteins or with microsatellite instability-high tumours30; it is also FDA approved for tumours with high tumour mutational burden (≥10 mut/Mb).31 In addition, the TOPAZ-1 trial, as mentioned earlier, explored the combinations of CisGem with durvalumab/placebo in the frontline setting for advanced BTC in a biomarker-unselected population. The study was recently reported as positive, with full data awaited at the time of this publication.12 Outside of this, the use of immunotherapy remains limited to clinical trials.2
Other medical scenarios and treatment approaches to take into account
Liver transplant
Liver transplant after neoadjuvant chemoradiation in cases of unresectable, locally advanced perihilar CCA has shown promising results in retrospective series and stands as an option for patients in some countries.32, 33, 34 The role of liver transplant for iCCA has had limited study and remains controversial. A case series demonstrated encouraging survival rates in a series of carefully selected patients with advanced iCCA,35 but definitive evidence to support the role of transplant for this indication remains unclear.
Liver-directed therapies
It is expected that a significant proportion of patients with iCCA may present with multifocal liver disease in the absence of extrahepatic metastases.36 In this scenario, liver radioembolisation (selective internal radiation therapy) has been explored in phase II studies,37 including in combination with CisGem, and shown benefit including conversion to resectable disease in some patients. As it stands, the role of selective internal radiation therapy remains investigational and to be used upon availability and adequate patient selection.
Management of cancer-related complications: biliary obstruction
Especially for patients with eCCA and ampullary malignancies, obstructive jaundice at the time of diagnosis is a common scenario. In addition, recurrence of stent-related events such as stent obstruction or cholangitis during treatment may occur within a median time of 4.4 months from first stent insertion in up to 43% of patients with a metal stent in situ.38 Prompt identification and management of such complications to avoid clinical deterioration and life-threatening complications are critical.
Pancreatic exocrine insufficiency for patients undergoing Whipple resection
A proportion of patients with BTC, mainly patients with distal CCA and ampullary tumours, may undergo a Whipple resection with curative intent. In these scenarios, proactive management of pancreatic exocrine insufficiency and adequate dosing of pancreatic exocrine replacement therapy should be secured to enable a good quality of life and good treatment tolerance and to minimise weight loss.39
Conclusions and future steps
Management of BTCs is rapidly changing, with multiple clinical trials informing best practice. Curative management still relies on surgical resection followed by adjuvant capecitabine for CCA and GBC. However, relapse rates remain high and better adjuvant strategies, including biomarkers for patient selection, are urgently required to increase rate of cure. In the advanced setting, chemotherapy with CisGem followed by second-line FOLFOX is the cornerstone of treatment for the majority of patients in the absence of targetable alterations. Targeted therapies are rapidly changing the treatment paradigm for many patients with advanced BTC, especially for patients with iCCA. Molecular profiling is therefore now the standard of care and should be obtained early in patients' care to allow adequate planning of therapy. Ongoing research is likely to clarify the role of immunotherapy, liver-directed therapy, and liver transplant for BTCs. With the expanding treatment options for advanced BTC, identifying biomarkers to match patients with the highest yield therapies will be critical for maximising survival for patients with this disease.
Acknowledgments
Funding
This work was supported by The Christie Charity and the European Union's Horizon 2020 Research and Innovation Programme [grant number 825510, ESCALON] to AL; this article/publication is based on work from COST Action European Cholangiocarcinoma Network, supported by COST (European Cooperation in Science and Technology (no grant number); www.cost.eu), a funding agency for research and innovation networks; the American Cancer Society Clinical Scientist Development Grant [grant number 134013-CSDG-19-163-01-TBG], the National Institutes of Health/National Cancer Institute Gastrointestinal Cancer SPORE [grant number P50 CA127003], V Foundation for Cancer Research Translational Grant (no grant number), and the Cholangiocarcinoma Foundation Andrea Marie Fuquay Research Fellowship (no grant number) to LG.
Disclosure
AL has received travel and educational support from Ipsen, Pfizer, Bayer, AAA, Sirtex, Novartis, Mylan, and Delcath; speaker honoraria from Merck, Pfizer, Ipsen, Incyte, AAA, QED, Servier, and EISAI; advisory honoraria from EISAI, Nutricia Ipsen, QED, Roche, Servier, and Boston Scientific; is a member of the Knowledge Network and NETConnect Initiatives funded by Ipsen. JE has received honoraria from Servier, Incyte, Basilea, Boston Scientific, MSD, AstraZeneca, Roche, Eisai, Ipsen, and Bayer. LG reports research funding (to the institution) from Adaptimmune, Bayer, Merck, MacroGenics, Genentech, Novartis, Incyte, Loxo Oncology, Relay Therapeutics, QED, Taiho Oncology, Leap Therapeutics, Bristol Myers Squibb, NuCana, and Servier; honoraria (to self) for serving on scientific advisory committees or as a consultant from Alentis Therapeutics AG, Black Diamond, Basilea, Genentech, Exelixis, H3Biomedicine, Incyte Corporation, QED Therapeutics, Sirtex Medical Ltd, The Servier Group, Sirtex, and Taiho Oncology and participation on data safety monitoring boards for AstraZeneca.
References
- 1.Valle J.W., Borbath I., Khan S.A., et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 2.Banales J.M., Marin J.J.G., Lamarca A., et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forner A., Vidili G., Rengo M., Bujanda L., Ponz-Sarvisé M., Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):98–107. doi: 10.1111/liv.14086. [DOI] [PubMed] [Google Scholar]
- 4.Lamarca A., Barriuso J., Chander A., et al. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: systematic review and meta-analysis. J Hepatol. 2019;71(1):115–129. doi: 10.1016/j.jhep.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Primrose J.N., Fox R.P., Palmer D.H., et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 6.Neoptolemos J.P., Moore M.J., Cox T.F., et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. J Am Med Assoc. 2012;308(2):147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 7.Lamarca A., Edeline J., McNamara M.G., et al. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat Rev. 2020;84:101936. doi: 10.1016/j.ctrv.2019.101936. [DOI] [PubMed] [Google Scholar]
- 8.Edeline J. Adjuvant gemcitabine-based chemotherapy for biliary tract cancer: pooled analysis of the BCAT and PRODIGE-12 studies. Ann Oncol. 2020;31(suppl 4):S260–S273. doi: 10.1016/j.ejca.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Shroff R.T., Kennedy E.B., Bachini M., et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37(12):1015–1027. doi: 10.1200/JCO.18.02178. [DOI] [PubMed] [Google Scholar]
- 10.Horgan A.M., Amir E., Walter T., Knox J.J. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30(16):1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- 11.Valle J., Wasan H., Palmer D.H., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 12.Imfinzi plus chemotherapy significantly improved overall survival in 1st-line advanced biliary tract cancer in TOPAZ-1 Phase III trial at interim analysis. https://www.astrazeneca.com/media-centre/press-releases/2021/imfinzi-improved-survival-in-biliary-tract-cancer.html Available at.
- 13.Phelip J.M., Desrame J., Edeline J., et al. Modified FOLFIRINOX versus CISGEM chemotherapy for patients with advanced biliary tract cancer (PRODIGE 38 AMEBICA): a randomized phase II Study. J Clin Oncol. 2021 doi: 10.1200/JCO.21.00679. https://doi.org/10.1200/JCO.21.00679 JCO2100679. In press. [DOI] [PubMed] [Google Scholar]
- 14.Shroff R.T., Javle M.M., Xiao L., et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5(6):824–830. doi: 10.1001/jamaoncol.2019.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamarca A., Palmer D.H., Wasan H.S., et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo C., Kim K.-P., Kim I., et al. Liposomal irinotecan (nal-IRI) in combination with fluorouracil (5-FU) and leucovorin (LV) for patients with metastatic biliary tract cancer (BTC) after progression on gemcitabine plus cisplatin (GemCis): multicenter comparative randomized phase 2b study (NIFTY) J Clin Oncol. 2021;39(suppl_15):4006. [Google Scholar]
- 17.Kim J.W., Suh K.J., Kim J.-W., et al. A randomized phase II study of oxaliplatin/5-FU (mFOLFOX) versus irinotecan/5-FU (mFOLFIRI) chemotherapy in locally advanced or metastatic biliary tract cancer refractory to first-line gemcitabine/cisplatin chemotherapy. J Clin Oncol. 2020;38(suppl 15):4603. [Google Scholar]
- 18.Lamarca A., Barriuso J., McNamara M.G., Valle J.W. Molecular targeted therapies: ready for “prime time” in biliary tract cancer. J Hepatol. 2020;73(1):170–185. doi: 10.1016/j.jhep.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Mosele F., Remon J., Mateo J., et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(11):1491–1505. doi: 10.1016/j.annonc.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Javle M., Lowery M., Shroff R.T., et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36(3):276–282. doi: 10.1200/JCO.2017.75.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abou-Alfa G.K., Sahai V., Hollebecque A., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal L., Meric-Bernstam F., Hollebecque A., et al. Abstract CT010: Primary results of phase 2 FOENIX-CCA2: the irreversible FGFR1-4 inhibitor futibatinib in intrahepatic cholangiocarcinoma (iCCA) with FGFR2 fusions/rearrangements. Cancer Res. 2021;81(13):CT010. [Google Scholar]
- 23.Abou-Alfa G.K., Macarulla T., Javle M.M., et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu A.X., Macarulla T., Javle M.M., et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021;7(11):1669–1677. doi: 10.1001/jamaoncol.2021.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subbiah V., Lassen U., Élez E., et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21(9):1234–1243. doi: 10.1016/S1470-2045(20)30321-1. [DOI] [PubMed] [Google Scholar]
- 26.Hong D.S., DuBois S.G., Kummar S., et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531–540. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javle M., Borad M.J., Azad N.S., et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021;22(9):1290–1300. doi: 10.1016/S1470-2045(21)00336-3. [DOI] [PubMed] [Google Scholar]
- 28.Harding J.J., Cleary J.M., Quinn D.I., et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: results from the phase II SUMMIT ‘basket’ trial. J Clin Oncol. 2021;39(suppl_3):320. [Google Scholar]
- 29.Doebele R.C., Drilon A., Paz-Ares L., et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le D.T., Uram J.N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FDA approves pembrolizumab for adults and children with TMB-H solid tumors. FDA. 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors Available at.
- 32.Rosen C.B., Heimbach J.K., Gores G.J. Liver transplantation for cholangiocarcinoma. Transpl Int. 2010;23(7):692–697. doi: 10.1111/j.1432-2277.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 33.BPT Loveday, Knox J.J., Dawson L.A., et al. Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada. J Surg Oncol. 2018;117(2):213–219. doi: 10.1002/jso.24833. [DOI] [PubMed] [Google Scholar]
- 34.Duignan S., Maguire D., Ravichand C.S., et al. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: a single-centre national experience. HPB (Oxford) 2014;16(1):91–98. doi: 10.1111/hpb.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lunsford K.E., Javle M., Heyne K., et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3(5):337–348. doi: 10.1016/S2468-1253(18)30045-1. [DOI] [PubMed] [Google Scholar]
- 36.Lamarca A., Ross P., Wasan H.S., et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst. 2020;112(2):200–210. doi: 10.1093/jnci/djz071. [DOI] [PubMed] [Google Scholar]
- 37.Edeline J., Touchefeu Y., Guiu B., et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2020;6(1):51–59. doi: 10.1001/jamaoncol.2019.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamarca A., Rigby C., McNamara M.G., Hubner R.A., Valle J.W. Impact of biliary stent-related events in patients diagnosed with advanced pancreatobiliary tumours receiving palliative chemotherapy. World J Gastroenterol. 2016;22(26):6065–6075. doi: 10.3748/wjg.v22.i26.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carnie L.E., Farrell K., Barratt N., et al. Pancreatic enzyme replacement therapy for patients diagnosed with pancreaticobiliary cancer: validation of an algorithm for dose escalation and management. Pancreas. 2021;50(9):1254–1259. doi: 10.1097/MPA.0000000000001906. [DOI] [PubMed] [Google Scholar]