Abstract
Background
Depression or Major depressive disorder (MDD) is a prolonged condition of sadness. MDD is the most common mental disorder that affects more than 264 million people worldwide. According to the monoamine hypothesis, serotonin (5-hydroxy tryptamine, 5-HT), dopamine (DA) and norepinephrine (NE) are the major neurotransmitters (NTs) involved in depression.
Methods
The methodology adopted for writing this review article is essentially based on the secondary literature search through a systematic literature review. This review mainly focussed on the role of 5-HT3 receptor antagonists (5-HT3RA) in depression and comorbid disorders like anxiety.
Results
Out of three major NTs mentioned above, serotonin has a predominant role in the pathophysiology of depression. The serotonin type-3 receptors (5-HT3R) are well renowned to be expressed in the central nervous system (CNS) in regions which have significance in the vomiting reflex, perception of pain, the reward system, cognition, depression and anxiety control. 5-HT3R are the receptors of serotonergic family that belong to ligand-gated ion channel. 5-HT3RA inhibit the binding of serotonin to postsynaptic 5-HT3R and increases its availability to other receptors like 5-HT1A, 1B and 1D as well as 5-HT2 receptors and produces anti-depressant-like effect. 5-HT3RA also have an important role in mood and stress disorders. Some of the studies have shown the effectiveness of these agents in stress disorder.
Conclusion
The present article focussed on the role of 5-HT3R and their antagonists in the treatment of depression and anxiety. Further studies are warranted to prove their efficacy with respect to other standard anti-depressants.
Keywords: Co-morbid, depression, ion channel, serotonin, 5-HT3 receptors, cognition
1. INTRODUCTION
According to the latest report from the World Health Organization (WHO), MDD is the most common mental disorder that affects more than 264 million people worldwide [1]. The diagnostic and statistical manual for mental disorder-IV (DSM-IV) has given nine symptoms for assessment of depression. Out of these nine symptoms, if any five are present for more than 2 weeks, then the patient is said to be depressed, however, warrants further confirmation [2, 3]. The hormones like estrogen and progesterone may modulate the functioning of 5HT3R as women are more susceptible to be affected with depression as compared to men [4-6].
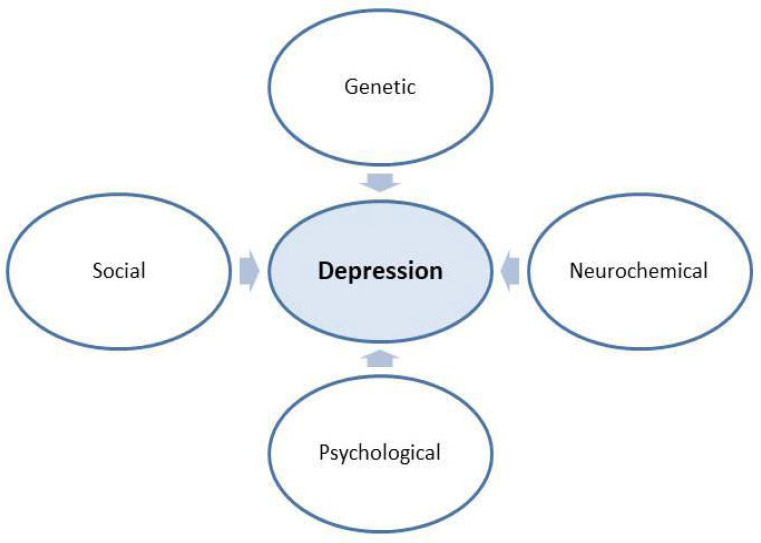
According to this monoamine, the hypothesis level of three NTs, namely 5-HT, NE and DA, is decreased in depression [7, 8]. In addition, γ-amino butyric acid (GABA) and glutamate also have an important role in the pathophysiology of depression [9]. Moreover, recent studies also relate depression with alterations in the physiology of the brain, neuronal plasticity and reduced volume of the frontal cortex and the hippocampus [10]. Now, genetic involvement in the development of depression has also been identified. Genes such as SLC6A4 (previously known as SERT), DRDR4, SLC6A4 or 5-HTT and TPH2 are also found to have a predominant role in the pathological progression of depression [11]. Various important causes of depression have are in (Fig. 1). Moreover, dysregulation of the hypothalamic-pituitary-adrenal (HPA)-axis and increased oxidative stress also has a predominant role in the development of MDD [12, 13]. Imbalance in antioxidant and oxidant enzyme levels in the brain and plasma levels of the depressed patient has also been frequently observed [13, 14]. Various studies indicated that MDD demonstrates increased levels of various peripheral inflammatory biomarkers when compared with non-depressed individuals. Increased levels of C-reactive protein, TNF- α, Interferon-α have been observed in depressed patients [15].
Fig. (1).
Causes of depression. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Various pharmacological therapies are available for the treatment of depression and anxiety, including selective serotonin reuptake inhibitors (SSRI), noradrenaline dopamine reuptake inhibitors (NDRI), dopamine reuptake inhibitors (DARI), etc. [16]. However, these treatment approaches are successful to treat the symptoms of depression to some extent, but they have the drawback of ineffectiveness against treatment-resistant depression. In addition, most of the antidepressants show their effect after 1-2 months of treatment as they act through modification of the receptors [17].
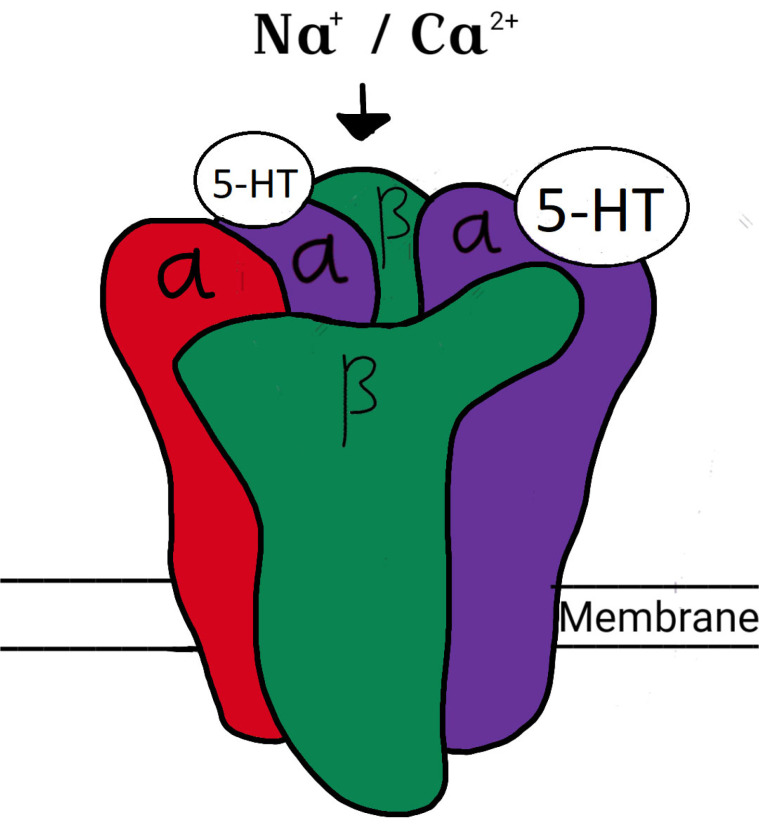
Serotonin is an important neurotransmitter having a role in many physiological processes such as platelet aggregation, pain, sleep, appetite, muscle contraction, emotions and obsessions and compulsions. Targeting serotonin is an interesting strategy for the development of newer potential anti-depressants. 5- HT and its receptors are distributed in CNS, peripheral nervous system (PNS), as well as in a number of non-neuronal tissues in the gut, cardiovascular system and blood. Based on the signal transduction and amino acid sequence, now the serotonin receptors are classified into seven major types (5-HT1-7) [18, 19]. All the serotonin receptors belong to the superfamily of G-protein coupled receptor (GPCR), except 5-HT3R, which is a superfamily of ligand- gated cation channel receptor. The structure of 5-HT3R is shown in (Fig. 2).
Fig. (2).
Structure of the 5-HT3 receptor. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Certain drugs and research molecules also target other 5-HT receptors. The blockade of 5-HT2A receptors also seems to improve the clinical effects of SSRIs. These receptors are located postsynaptically to 5-HT axons, mainly in the neocortex. Moreover, antidepressant drugs like nefazodone, trazodone, mirtazapine are antagonists of 5-HT2A or α2-adrenoceptors [20]. Some preclinical studies have demonstrated 5-HT1A receptor mediated hippocampal transmission after the chronic treatment with SSRIs as well as other antidepressant drugs [21]. However, 5HT1A receptor agonists failed to demonstrate clinical significance in depression despite preclinical evidence. Even the effectiveness of buspirone, a 5-HT1A partial agonist, is far behind as compared to standard antidepressants [22]. The other receptor is 5-HT1B that may also act as a potential target for antidepressant drugs and a key determinant of stress activity. Administration of SSRIs in mice lacking 5-HT1B autoreceptors exhibits increases in 5-HT levels in the ventral hippocampus (vHPC) and leads to a decrease in anxiety like behavior [23].
The expression of 5-HT3R has been confirmed in regions having a role in the vomiting reflex, perception of pain, the reward system, memory and control of anxiety. This underlines their relevance in emesis, migraine, drug addiction, neurodegenerative and psychiatric disorders. Various behavioral and biochemical preclinical studies have reported the effectiveness of 5-HT3R modulators in comorbid models of depression and anxiety. In addition, few clinical evidence have also shown the significance of 5-HT3RA in CNS disorders. The effect comes in acute dose levels as well as showed inhibition of treatment resistance [24, 25]. Hence in this review, we discussed the role of 5-HT3RA in depression comorbid with anxiety.
2. CHEMISTRY OF 5-HT3RA
5-HT3RA are classified into two types on the basis of binding pattern towards serotonin type 3 receptors, namely competitive and non-competitive 5-HT3RA. The antagonists which compete with the serotonin sites are called competitive antagonists and compounds which target allosteric sites are called non-competitive antagonists [26].
3. COMPETITIVE ANTAGONISTS
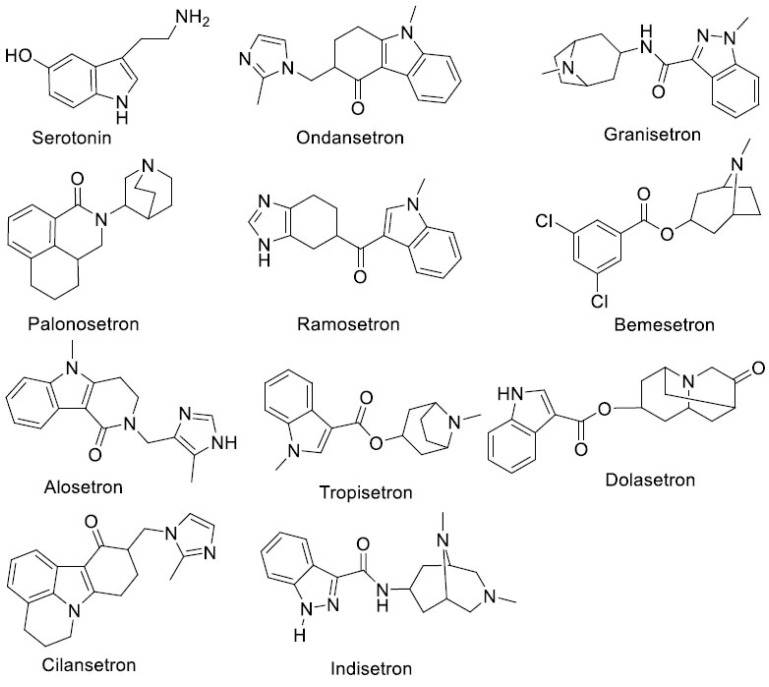
The chemical structures of competitive antagonists have similarities with serotonin. The chemical structures of serotonin and competitive 5-HT3RA are shown in (Fig. 3). The clinically available 5-HT3 receptor antagonists are highly selective towards 5-HT3 receptors than other receptors [27]. Most of the currently available competitive antagonists are in the form of salts as most of these drugs contain basic nitrogen atom(s). For example, granisetron, ramosetron, ondansetron, and palonosetron are available in the form of hydrochloride salt, while dolasetron is available in the mesylate salt form.
Fig. (3).
Chemical structures of serotonin and competitive 5-HT3 receptor antagonists.
In old literature, as well as in recent literature, the competitive antagonists are classified into two types, namely first generation and second generation 5-HT3RA. Ondansetron, dolasetron, tropisetron, and granisetron are classified as first-generation 5-HT3RA, while palonosetron was categorized in second-generation 5-HT3RA [26, 27]. In the first generation antagonists, heterocyclic systems are mostly indole or indole-like derivative, while palonosetron consists of a benzoisoquinoline (tricyclic) system.
Most of the competitive antagonists are metabolized to form the hydroxyl derivative. These metabolites are formed as the result of hydroxylation on the aryl/alicyclic system or by the reduction of the carbonyl group. For example, ondansetron, alosetron, tropisetron, granisetron, palonosetron follow hydroxylation at the aryl/alicyclic system while the dolasetron afforded hydroxyl derivative by the reduction of the ketone carbonyl group [28-32]. The formed hydroxyl metabolites are active or inactive or more active than the parent molecule. Another common pathway involved in the metabolism of competitive antagonists is demethylation, which means the removal of the methyl group from the parent molecule [32].
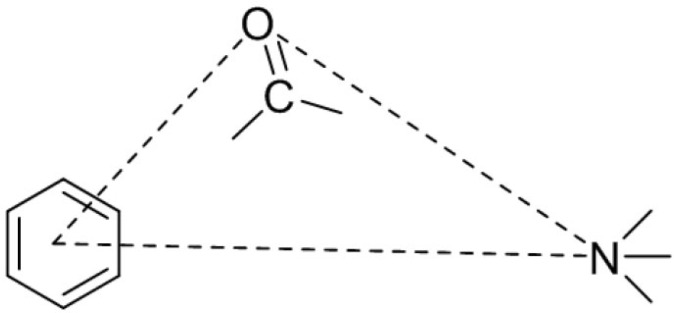
4. PHARMACOPHORE OF COMPETITIVE 5-HT3RA
The pharmacophore of clinically available 5-HT3RA and the reported molecules possesses three necessary elements [33, 34], which consists of an aromatic ring, carbonyl linking group, and a nitrogen atom. The basic pharmacophore is depicted in (Fig. 4).
Fig. (4).
Basic pharmacophore of 5-HT3 receptor antagonists.
Several investigators have explored the role of the carbonyl group (as hydrogen bond acceptor) and the nitrogen atom (as basic nitrogen atom) in 5-HT3RA for their interaction with 5-HT3R [34-39]. Though a wide range of aromatic systems have been studied as 5-HT3 receptor antagonists viz. benzothiazole, benzoxazole [40],isoquinoline [41], quinolone [42], quinoxaline [43], and indole [41], unfortunately, none of the researchers explored the role of an aromatic group in 5-HT3RA for interaction with the receptor. In our previous study, we proposed that the possible interaction between the aromatic group and 5-HT3R takes place through hydrophobic interaction.
Hibert et al. (1990) [36] proposed pharmacophoric distances between the elements: the distance between the centroid of aromatic to carbonyl oxygen ~ 3.3 Å, between the centroid of aromatic to basic nitrogen ~ 6.7 Å and the distance between carbonyl oxygen to basic nitrogen ~ 5.2 Å. In a few studies [38, 44, 45], compounds displayed potent 5-HT3 receptor antagonism even though they deviated from the model proposed by Hibert et al. (1990) [36]. Similarly, in our previous study [46], many of the synthesized compounds displayed good antagonism even though the distances between the pharmacophoric elements have deviated from the model proposed by Hibert et al. (1990) [36].
Replacing the carbonyl group with suitable bioisotere is well tolerated; Rosen et al. (1990) demonstrated thiazole as hydrogen bond acceptor instead of the carbonyl group [39]. The obtained compounds maintained the 5-HT3 antagonism, some compounds displayed antagonism greater than the standard drugs. A similar kind of results was observed when nitrogen was used as a source of hydrogen bond acceptor [47, 48].
5. NON-COMPETITIVE ANTAGONISTS
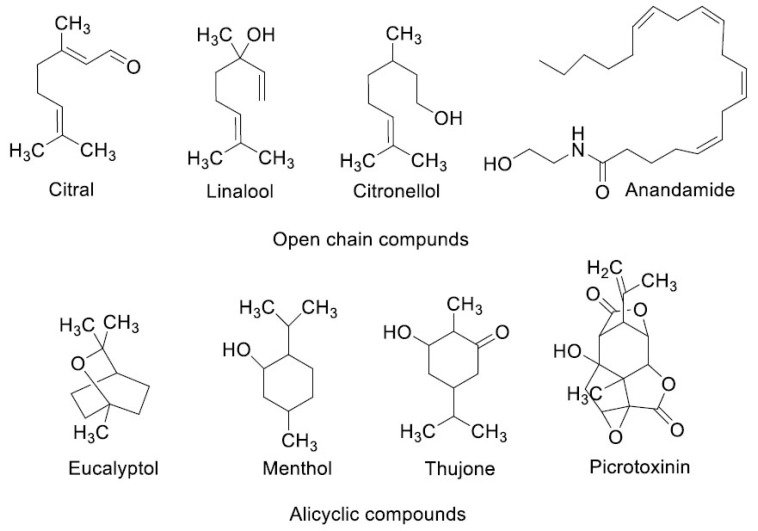
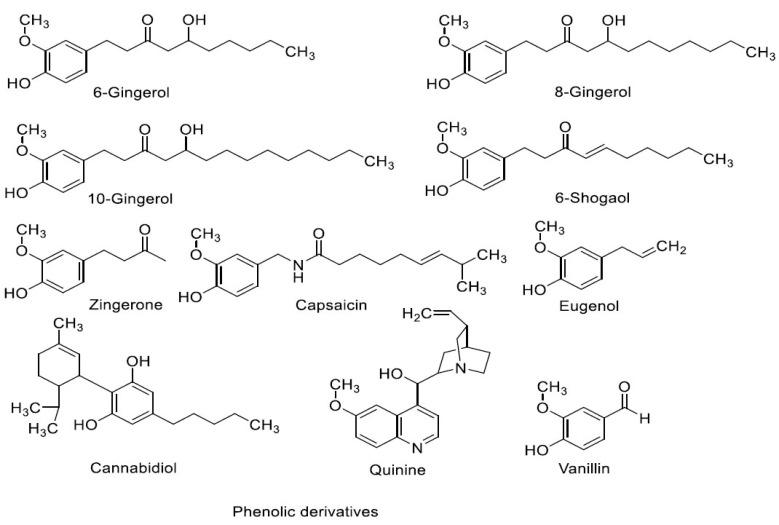
Most of the non-competitive 5-HT3RA are obtained from natural sources [49]. The chemical structures of non-competitive 5-HT3RA are shown in (Figs. 5A and 5B). The chemical structures of the non-competitive antagonists lack the similarity with serotonin.
Fig. (5A).
Chemical structures of non-competitive antagonists.
Fig. (5B).
Chemical structures of non-competitive antagonists.
6. PHARMACOPHORE OF NON-COMPETITIVE 5-HT3RA
To the best of our knowledge, there is no pharmacophore model proposed/developed for the non-competitive 5-HT3RA. Based on our observation, we identified a few common features present in non-competitive 5-HT3RA. The common features are aliphatic residue (except vanillin) and the presence of at least one oxygen atom in the molecule. The oxygen atom may exist in the form of alcohol, aldehyde, ester, ether, amide, ketone, or phenolic functional groups. Furthermore, a set of common features is observed while comparing the phenolic derivatives. Most of the compounds except quinine contain an alkoxy or alkyl substituent present at ortho to a phenolic hydroxyl group and alkyl (except vanillin) substituent is located at para or meta position with respect to the phenolic hydroxyl group.
Based on this observation, we may consider these elements are necessary components (pharmacophoric elements) of non-competitive 5-HT3RA. However, to make a conclusive statement on the pharmacophore model, an extensive study with a wide range of non-competitive 5-HT3RA is required.
7. STRUCTURE, EXPRESSION AND PRIMARY FUNCTIONS OF 5-HT3R
Serotonin exerts its effect through seven subfamilies of receptors, i.e., 5-HT1 to 5-HT7 [50]. Out of these seven subtypes, only the 5-HT3R subtype is a pentameric ion channel belonging to the superfamily of Cys-loop receptors. Long back before 50 years, ‘5-HT3R’ was described as the so- called ‘M receptor’ in the guinea-pig gut as 5-HT stimulated contractions could be blocked by the antagonist morphine [51]. It is made up of five monomer subtypes, the 5-HT3A–E subunits, which exhibit differences in the amino-terminal and the transmembrane region. Architecture is more or less similar for 5-HT3A, 3B, 3C, 3E subunits, whereas the 5-HT3D subunit lacks most of the N-terminal domain, including the Cys-loop [52]. The functional relevance of different receptor compositions is still not clarified. These receptors work through fast synaptic transmission. Using different methods such as autoradiography, immunohistochemistry and in situ hybridization, the distribution of 5-HT3R has been largely explained with some variance between species [53]. They are expressed in many brain regions, including the hippocampus, entorhinal cortex, frontal cortex, cingulate cortex, amygdala, nucleus accumbens (NAc), substantia nigra, and ventral tegmental area (VTA), with the highest densities in the area postrema and the nucleus tractus solitaries, regions responsible for the vomiting reflex [54-57]. The animal studies conducted to determine the expression of 5-HT3R revealed that around 70-80% of these receptors are mainly are located in presynaptic nerve endings [58]. 5-HT3R found within the PNS in location that includes vagal afferents from the heart and GI tract are also of physiological importance [59].
In CNS, 5-HT3R are located in the regions involved in vomiting, pain perception, rewarding, memory and regulation of anxiety, while in the peripheral system, they are expressed on various nerve and immune cells [59, 60]. In the CNS, the density of 5-HT3R appeared to be lower as compared to other 5-HT receptors. These receptors have their important role in emesis, particularly cancer chemotherapy induced nausea and vomiting, pain sensation, addiction, psychiatric and gastrointestinal disorders [61, 62]. Moreover, the preferential localization on nerve endings is consistent with a functional role of 5-HT3R in the control of the release of NTs such as DA, cholecystokinin, glutamate, acetylcholine, GABA, substance P, or 5-HT itself [63].
A typical subunit exhibits a large extracellular N-terminus, four TMs and a short extracellular C-terminus. Further characteristics are the large intracellular domain (ICD) between TM 3 and 4 and the Cys-loop in the N-terminus [64, 65, 66]. The transmembrane region of the channel pore is formed by the TM 2 domains of the five subunits [67]. The five subunits of 5-HT3R cover central cation permeable pore, which facilitates the influx of Na+/K+/Ca+2 ions via the opening of ion channel followed by fast desensitization [68]. These 5-HT3R have a predominant role in the stimulation of nausea and vomiting and 5-HT3RA are well recognized for their role in the reduction of cancer chemotherapy induced nausea and vomiting (CINV) and post-operative nausea and vomiting (PONV) [69]. Ondansetron, granistetron, tropisetron, dolasetron, palonosetron, etc. are the important examples in this category [70]. Binding of three agonist molecules to homomeric 5-HT3R leads to a fully activated ion channel [71, 72].
8. PHARMACOLOGY AND PHYSIOLOGY OF : 5-HT3R
5-HT3R is selective for permeation of Na+, K+ and Ca2+ ions [68]. Activation of these receptors leads to the opening of ion channels and the influx of these cations, followed by depolarization of the membrane. The 5-HT3R activation leads to the fast synaptic transmission of various NTs like 5-HT, DA or GABA [60, 73]. This activation is dependent on the location of these receptors, i.e., presynaptic or postsynaptic. In particular, presynaptic 5-HT3R displays a high permeability to Ca2+, whereas postsynaptic receptors display a lower permeability to Ca2+ compared to Na+ and K+ [74, 75].
Homomeric 5-HT3A receptors are permeable to monovalent and divalent ions equally, while heteromeric receptors have lower permeability to Ca+2 [60, 76]. In addition, heteromeric receptors display faster activation and deactivation as compared to homomeric receptors. 5-HT3RA give bell-shaped dose-response curve in case of both preclinical and clinical studies. Generally, the maximum effect is typically observed at a very low dose, in the microgram range, while higher doses are ineffective [53]. The ineffectiveness of these antagonists seen may be due to the desensitization of receptors by internalization.
9. PROBABLE MECHANISM OF 5-HT3RA IN DEPRESSION AND COMORBID ANXIETY
In various preclinical studies, encouraging results have been obtained from the use of 5-HT3RA in depression and comorbid anxiety models [77, 78]. These results validate the significance of 5-HT3R in the progression of these CNS disorders. 5-HT3RA mainly work through modulation of mainly serotonergic neurotransmission. However, it also modulates the release of DA, NE, and GABA neurotransmitter [7, 9]. Various studies conducted on 5-HT3RA give an idea about the probable mechanism of 5-HT3RA in depression and comorbid anxiety disorders. 5-HT3RA, i] reduce the duration of immobility in various preclinical rodent models of depression and anxiety like forced swim test (FST), tail suspension test (TST), elevated plus maze (EPM) test and light and dark (L/D) test; ii] in a mechanistic model of depression, such as reserpine induced hypothermia, these antagonists reduce the hypothermic effect induced by reserpine due to reduction in vesicular uptake of NTs, and in 5-hydroxy tryptophan (5-HTP) induced head twitches in mice, they reduce the number of head twitches in a specified period of time; iii] In rodent models like olfactory bulbectomy and traumatic brain injury, 5-HT3RA also showed their potential as a promising anti-depressant. These mechanisms reveal that 5-HT3 antagonism facilitates signaling of 5-HT neurotransmission [24, 25, 68].
At a lesser concentration range, 5-HT3RA inhibit the postsynaptic 5-HT3R, which are involved in fast excitatory synaptic transmission in the limbic brain regions [79]. Binding of 5-HT3R to postsynaptic receptors leads to an increase in the availability of 5-HT to other postsynaptic receptors such as 5-HT1B [80], 5-HT2A and 5-HT2C, thereby aiding in signaling associated with serotonergic transmission and stimulating adenylyl cyclase followed by the initiation of the transformation of ATP to cAMP, that functions as a second messenger. cAMP further stimulates the phosphorylation enzyme protein kinase-A (PKA) [68]. Once PKA gets activated, phosphorylation of other intracellular protein molecules is initiated, thereby modifying the expression of CREB and BDNF in the nucleus [unpublished data]. This leads to anti-depressant-like effects by improving synaptic plasticity, neuronal survival and neurogenesis. However, at higher dose levels, the presynaptic and somatodendritic 5-HT3 receptor blockade inhibits 5-HT release, eventually reducing the synaptic 5-HT levels that predispose to depression-like effects [81]. In addition to 5-HT, the 5-HT3 (hetero) receptors located on nerve terminals modulate the release of other NTs such as NE, DA, Ach and GABA [68]. Various evidences gathered from previous studies suggested that inhibition of this receptor has a differential effect on the release of these NTs in the synapse. However, according to some reports, 5-HT3RA did not show a uniform effect with respect to antidepressant action. Several studies showed that the antagonism of 5-HT3R leads to the enhancement of dopaminergic activity in mesolimbic, mesocortical and nigrostriatal pathways [27]. These pathways have importance in psychoses and Parkinson’s disease, respectively. Antagonism of 5-HT3R in presynaptic neurons tends to suppress dopaminergic transmission and an increase in depression-like symptoms [53]. Similarly, activation of presynaptic 5-HT3R results in the facilitation of GABA release, while the same effect is inhibited by 5-HT3RA. Hence inhibition of presynaptic receptors tends to produce depression-like effects [68]. The modulatory effect of 5-HT3RA on the cholinergic system emerges from the co-localization of 5-HT3 and nicotinic receptors in striatal nerve terminals of the rat brain [82].
In addition, presynaptic 5-HT3R stimulation inhibits ACh release, mainly in the cortex [83]. There are various evidences available to demonstrate the effect of 5-HT3RA on NTs release or inhibition, however further studies are warranted to establish their role in various CNS disorders due to their distribution pattern and complex signaling transduction.
10. CLINICAL EVIDENCE
5-HT3R agonist or antagonist responses are associated with a bell-shaped dose-response curve in both preclinical and clinical studies. Generally, they show good activity in the low dose range and activity decreases with an increase in dose [27]. Some clinical studies have suggested that 5-HT3 receptors may be a relevant target in the treatment of affective disorders [53]. Evidence for the importance of 5-HT3RA in the treatment of depression stems from clinical trials in which patients suffering from complex disorders such as fibromyalgia and bulimia showed improvement of the comorbid depression [84, 85]. A few clinical trials have exhibited the effectiveness of 5-HT3RA monotherapy or its combination with antipsychotics in patients with psychosis and schizophrenia. Ondansetron (4 mg/day) treatment has been shown to improve the mental state and social behaviour of a schizophrenic patient [68].
11. TREATMENT RESISTANT DEPRESSION (TRD)
Despite an increase in the number of antidepressants, the pharmacotherapy of depression remains inadequate 1. At least 40% of patients do not respond well to antidepressant therapy. In general, antidepressant drugs take 8-12 weeks to show their effect as they are working through the modification of receptors, as well as the synthesis of neurotransmitter requires some time. The antidepressant drugs change the sensitivity of the receptor that, in turn, may cause externalization or internalization, change in expression of genes which involve neurogenesis and synaptic remodeling [86, 87].TRD is a complicated clinical problem caused by various risk factors. The complexities of TRD are addressed with combination strategies, which include medication optimization, a combination of antidepressant treatments, switching of therapy and augmentation with non-antidepressants, psychological therapies and non-pharmacological treatments such as deep brain stimulation, vagal nerve stimulation and trans cranial magnetic stimulation [88].
Long term treatment with classic antidepressants like SSRI, TCA, MAO inhibitors leads to the development of resistance against these drugs over a time period. In this regard, the 5-HT3 receptor antagonists work through the fast receptors that are ligand-gated ion channel and the activation of postsynaptic 5-HT3R is involved in fast synaptic transmission [79, 89]. These antagonists can be used in combination with classic antidepressants and antianxiety drugs [53]. Various interaction studies conducted by Bhatt et al., 2014, also demonstrated the effectiveness of these antagonists in various animal models of depression and comorbid anxiety models. In addition, a study conducted by Bhatt et al., 2013, has also demonstrated that 5-HT3R antagonists also potentiated the effects of various standard drugs such as fluoxetine, bupropion, etc. The effect may also suggest that 5-HT3RA may be used as an effective therapy against treatment resistant depression. 5-HT3RA showed beneficial effect after a single dose in models like FST, TST, EPM and other models [24, 78, 90].
12. ROLE OF 5-HT3RA IN THE REDUCTION OF OXIDATIVE STRESS
Oxidative stress plays a major role in the progression of various neuropsychiatric disorders, including depression. The brain has high metabolic activities, higher oxygen consumption, higher lipid contents, weaker antioxidative defense and more demand for glucose compared to other organs [13]. Oxidative stress or reactive oxygen species (ROS) is the main cause of neurodegeneration and its involvement in the pathogenesis of MDD is unequivocally established [91]. The imbalance between ROS and antioxidative defense leads to the deregulation of the physiology of the brain and abnormalities in nerve signaling. In depression, an imbalance in antioxidant enzymes such as SOD, catalase and reduced glutathione (GSH) and oxidant markers like peroxides and nitrates has been observed. This imbalance leads to an increase in activity of proinflammatory pathways such as TNF-α and IL-1β stimulation and other apoptotic mediators such as Caspase-3, ultimately leading to neuronal death [13, 92]. According to a study conducted by Bhatt et al., 2014, compound '6g', a 5-HT3RA, exerted antidepressant-like effects in behavioral despair paradigm in chronically stressed mice by restoring antioxidant mechanisms. The compound significantly reversed the CUMS-induced behavioral (increased immobility period, reduced sucrose preference and decreased locomotor activity) and biochemical (increased lipid peroxidation; decreased glutathione levels, superoxide dismutase and catalase activities) in mice [93]. Similarly, according to a study conducted by Gupta et al., 2015, 4i, a 5-HT3RA and fluoxetine treatment reversed the corticosterone (CORT) induced depressive-like deficits. Furthermore, 4i and fluoxetine reduced CORT induced oxidative brain insults, which may plausibly demonstrate one of the key mechanisms for antidepressant-like effects of the compounds [94]. 5-HT3RA show neuroprotection in in vitro and in vivo studies. In fact, oxidative stress–induced injury in rat cortical neurons was counteracted through curtailing caspase-3 activation, calcium influx, reactive oxygen species generation, and excitotoxicity. The protective effect is mediated through blockade of 5-HT3R by means of employing selective 5-HT3RA [95]. Moreover, 5-HT3RA tropisetron also acts as a partial agonist of α7 nicotinic acetylcholine receptor (α7nAChR). The activation of α7nAChR leads to inhibition of inflammatory conditions and apoptotic signaling pathways in conditions associated with oxidative stress [96].
13. IMPORTANCE OF 5-HT3RA IN HPA AXIS DYSFUNCTION
HPA axis dysregulation is one of the main predisposing factors for the pathogenesis of depression and other comorbid disorders like anxiety. The HPA axis is involved in the release of cortisol via the involvement of the hypothalamus, pituitary and adrenal gland [12]. This axis works on a negative feedback mechanism where increased cortisol level in the blood conveys signals to the hypothalamus to reduce the release of a cortisol release factor. In case of depression and anxiety, the negative feedback mechanism of the HPA axis fails and increases the cortisol levels in blood [97]. However, in a normal individual, the HPA axis works perfectly fine with normal levels of cortisol. 5-HT3RA are helpful in reducing the levels of cortisol in animal models. According to a study conducted by Kurhe et al., 2015, QCM-4, a 5-HT3RA, ameliorates the plasma HPA axis hyperactivity, leptin resistance and brain oxidative stress in depression and anxiety- like behavior in obese mice [98]. 5-HT3R antagonism on the HPA axis, mice lacking 5HT3A exhibited dampened adrenocorticotropic hormone responses to acute stressors, including lipopolysaccharide and restraint, with no change in pituitary sensitivity to corticotropin-releasing hormone (CRH) [99]. According to Gupta et al., 2014, Ondansetron and fluoxetine treatments significantly increased the percentage of serotonin levels in certain brain regions and attenuated HPA-axis hyperactivity, as evidenced by the low percentage of plasma CORT levels in chronic unpredictable stress (CUS) mice. These findings indicate the potential role of ondansetron (a 5-HT3RA) in reversing CUS-induced depressive behaviour, which is possibly mediated by its modulating effects on the HPA-axis and serotonergic system [100].
14. THERAPEUTIC USE
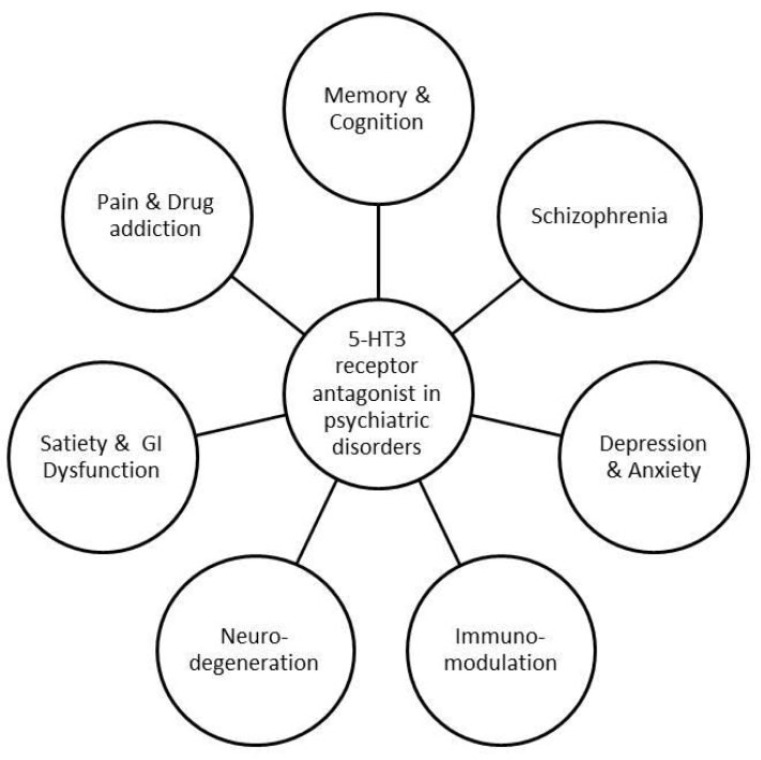
5-HT3RA have a variety of roles in different disorders. Various studies performed in laboratory animals suggested the important role of these antagonists in emotion, cognition, pain perception and memory process, neurodegenerative diseases and GI signaling. The role of 5-HT3 receptor antagonists in various disorders is shown in (Fig. 5A). Due to their availability in various locations in CNS and GIT and their role in the control of emotions and memory, one can see their role in pathophysiological regulation of neurological and gastrointestinal disorders.
As discussed earlier, these 5-HT3RA are well known for their role in CINV and PONV. In this section, we are going to discuss the role of 5-HT3RA in schizophrenia, irritable bowel syndrome (IBS), cognitive dysfunction and substance abuse and dysfunction.
15. COGNITION AND MEMORY
5-HT3RA play a significant role in cognition and memory. Cortex and dorsal hippocampus are the important regions associated with memory function, and antagonism of 5-HT3R at these locations inhibits the 5-HT modulated release of acetylcholine without affecting steady state release [101, 102]. 5-HT3RA have been shown to inhibit 5-HT3 agonist-induced ACh release in the entorhinal cortex of rats and the neocortex of guinea pigs, which are important structures for memory function [103]. A negative influence of 5-HT3R activation on ACh release in the neocortex has also been reported in humans. Tropisetron enhances memory by activation of α7 nAChR [104]. 5-HT3R have a substantial role in the progression of Alzheimer’s disease and Schizophrenia. Overexpression of 5-HT3R in mice has been involved in the enhancement of learning and memory as well as attention [59]. Ondansetron has been found to improve memory in patients over 50 years of age. Administration of 5-HT2A/2C or 5-HT4 receptor agonists or 5-HT1A or 5-HT3 and 5-HT1B receptor antagonists retards impairment in normal memory function and promotes learning in tasks that require a high cognition demand [105]. Moreover, polymorphism in the regulatory portion of 5-HT3A receptor subunit has been associated with lower activity of amygdala and dorsal and medial frontal cortices, and was linked with reduced reaction time at the recognition of face [106].
16. PAIN
Serotonin causes activation of presynaptic 5-HT3R on the central terminal of spinal afferents which are involved in the perception of pain via sensory and nociceptive inputs from the periphery to CNS [107]. Chronic pain in rats has been removed by ondansetron via antagonism at 5-HT3R. Behavioral studies have confirmed the involvement of 5-HT3R in pain and traumatic injury. 5-HT3 knockout mice confirmed the involvement of antagonists in nociception after injury [107, 108]. In humans, the role of 5-HT3RA has been confirmed in migraine and rheumatoid arthritis. The beneficial effects of 5-HT3RA tropisetron in rheumatic diseases such as rheumatoid arthritis, tendinopathies and fibromyalgia seem promising [27, 109]. However, the role of 5-HT3RA in chronic pain still needs to be studied in detail. Moreover, alosetron, a 5-HT3RA, has shown its role in abdominal discomfort and pain in both male and female patients by improving stool consistency [110].
17. GASTROINTESTINAL DYSFUNCTION AND VISCERAL PAIN
5-HT3RA are well known for their role in CINV and PONV. These antagonists block 5-HT peripherally as well as centrally in GI vagal nerve terminals and chemoreceptor trigger zone (CTZ), respectively; this blockade leads to a powerful antiemetic effect [111]. They are involved in the modulation of serotonergic transmission and regulation of GI function. In specific, they are involved in the regulation of GI motility, visceral sensation, secretion processes and perception of visceral pain. 5-HT3RA prevent the activation of 5-HT3R on extrinsic afferent neurons and can decrease the visceral pain associated with IBS [112]. Activation of 5-HT3R may modulate GI excitability and activity of gastrointestinal vagal afferents at various sites and may be involved in various pathological and functional body processes, including distention- and chemical-evoked vagal reflexes, nausea, and vomiting, as well as visceral hypersensitivity. 5-HT3RA relieve painful colonic distention caused by increased cerebral blood flow in 5-HT3R rich areas such as the hippocampus, amygdala and orbitofrontal cortex in IBS patients [113]. Walstab et al., 2014, reported that monoterpene alcohol menthol and the aporphine alkaloid boldine combat symptoms of functional gastrointestinal disorders and work through ligand-gated ion channels [114]. In addition, they also inhibited 5-HT receptors by the 5-HT-induced activation of 5-HT3 receptors in the low and middle micromolar range, respectively. Boldine was a competitive antagonist of both receptors being 6.5- to 10-fold more potent at 5-HT3A- vs 5-HT3AB receptors. Menthol non-competitively and stereoselectively inhibited both 5-HT3A and 5-HT3AB receptors.
18. SCHIZOPHRENIA AND NEURODEGENERATIVE DISORDERS
The role of 5-HT3RA in psychoses is still not very clear that not all the human trials with 5-HT3RA showed marked effect and promising results. Serotonin has a modulatory effect on dopaminergic neurons of the mid-brain area via 5-HT3R and 5-HT3RA that have been shown to decrease the hyperactivity of dopaminergic neurons in rats [115]. In clinics, 5-HT3RA also alleviate symptoms of schizophrenia, particularly tardive dyskinesia and psychosis [116]. Some recent studies have reported the predominant role of ondansetron as a potential adjunctive therapy for the treatment of negative symptoms of schizophrenia. However, 5-HT3RA have a limited role in the effective treatment of positive symptoms of psychoses. Interestingly, several neuroleptics and antidepressants have been shown to block 5-HT3R in a non-competitive manner, possibly via interaction with the receptor–lipid interface [117]. In addition, various studies reported the predominant neuroprotective properties of ondansetron and tropisetron, consistent with their capacity for inhibiting the protein phosphatase calcineurin-involved neurodegenerative cascades [26].
19. SATIETY CONTROL
Preclinical studies suggested an important role of 5-HT3R in the regulation of intake of food. It has been shown that the suppression of food intake by peripheral serotonin release is mediated via 5-HT3R. Cholecystokinin (CCK) and peripheral serotonin suppress food intake synergistically, and blockade of 5-HT3R attenuates the effect of CCK on food intake in combination with gastric distension [118]. Blockade of 5-HT3R antagonised the anorexia induced by methamphetamine [119]. In addition, 5-HT3R located centrally are involved in the regulation of blood glucose levels [120]. The significance of these pharmacological activities for the use of 5-HT3RA in humans has not yet been investigated.
20. DRUG ADDICTION
5-HT3RA influences the ‘reward pathway’. In humans, the administration of ondansetron leads to a reduction in alcohol intake and problems associated with the intake of alcohol. 5-HT3RA have been shown to reduce self-administration of ethanol in wild-type (WT) compared to 5-HT3A KO mice [121] and of morphine in rats [122]. In addition, ondansetron potentiated the methamphetamine induced hyperactivity in rats [119]. They have been involved in attenuation of the effect of morphine and cocaine that leads to an increase in dopaminergic activity in the mesolimbic area. These antagonists also cause locomotor activation, aggression stimulating effects and reduction in consumption of alcohol, as well as self-administration of drugs [59]. In humans, 5-HT3RA were particularly effective in reducing the self-administration of ethanol and morphine, but a less marked effect was seen on self-administration of cocaine [123, 124]. The overall effect of 5-HT3RA on substance abuse is inhibitory and also reduces the self-administration of abusing drugs.
21. IMMUNOMODULATION
5-HT3RA have anti-inflammatory and immunomodulatory properties, which are demonstrated by various in-vitro and in vivo studies. According to a study conducted by Bhatt et al., 2017, 5-HT3RA are able to reduce the inflammation and anxiety induced by lipopolysaccharide in mice. In addition to the anxiolytic effect, 5-HT3RA are able to produce an anti-oxidant effect as well via enhancing the levels of anti-oxidant enzymes such as catalase and superoxide dismutase [125]. Tropisetron found to inhibit lipopolysaccharide induced increased levels of TNF-alpha and IL-1B in monocytes and serotonin induced prostaglandin E2 release from synovial cells [27]. It is also involved in the inhibition of calcineurin induced activation of T-cells as well as modulation of Th1 cytokines in patients with the musculoskeletal disease [126]. 5-HT3RA are also being used as adjunct therapy with intra-articular glucocorticoids for their analgesic and anti-inflammatory effects.
22. STROKE
Stroke is a leading cause of disability and death worldwide. There is a decrease in blood supply to the brain that has been taken place due to thrombus formation in the cerebral artery. The condition requires immediate hospitalization, and till date, no effective treatment requirement except tissue plasminogen activator as the only agents is approved by the U.S. Food and Drug Administration. 5-HT3RA display a potential neuroprotective effect in various in vitro and in vivo activities. In fact, oxidative stress–induced injury in rat cortical neurons was counteracted through curtailing caspase-3 activation, calcium influx, ROS generation, and excitotoxicity [26]. A study conducted by Lee et al., 2005, also observed the protection of neurons mediated through blockade of 5-HT3R. In in vivo models, tropisetron showed a beneficial effect in the embolic stroke model [95].
23. AUTHOR’S INSIGHT
Based on the above discussed literature, we can confirm that 5-HT3RA have well validated role in CINV and PONV. The 5-HT3 receptor antagonists have their role in various CNS and other disorders, including depression and comorbid disorders like anxiety. 5-HT3RA belong to the category of ligand-gated ion channels. The ligand-gated ion channels are the second most important targets for drug discovery only after G protein–coupled receptors. The role of these receptors in these disorders is further confirmed by their expression in the CNS in regions involved in the vomiting reflex, processing of pain, the reward system, cognition, depression and anxiety control. The motivating outcomes from preliminary behavioral tests on 5-HT3RA, their good safety profile further established the role of these drugs in depression and comorbid anxiety. We have performed some preclinical studies with 5-HT3RA in our group and found their efficacy in both acute and chronic models of depression and anxiety. In addition, they have shown effectiveness in various rodent models of comorbidities, namely olfactory bulbectomy, traumatic brain injury, lipopolysaccharide induced depression and chronic unpredictable mild stress models. Moreover, they have also potentiated the effect of various standard drugs like fluoxetine, desipramine, bupropion as represented by various studies conducted in our lab. They worked through fast synaptic transmission effectively in very less time as compared to other standard drugs which work through some different mechanism. On the basis of the above mentioned literature, we may predict the role of these antagonists in depression comorbid with anxiety. They may also be useful and work as effective therapy against treatment resistant depression cases. These agents are very effective in addressing the issue of comorbidity very effectively. However, some detailed studies in clinics are required to prove the efficacy and exact signaling mechanisms of these 5-HT3RA. The pharmacokinetic aspects of these drugs also need to be addressed by conducting relevant studies.
CONCLUSION
Ligand-gated ion channels are important receptors after GPCR via which most of the drugs showed their action. The drugs acting through these receptors have a clear advantage of fast synaptic transmission. 5-HT3RA showed a clear advantage of fast action as well as effectiveness against various neuropsychiatric disorders when compared to other members of the serotonin receptor family. Initially, 5-HT3RA was established as a treatment for CINV and PONV. Setrons like ondansetron, tropisetron, dolasetron, etc. emerged as a gold standard treatment for CINV. Nonetheless, other therapeutic effects of this class were neglected for years until recent investigations demonstrated that these compounds could alleviate the pathology of certain neurodegenerative and neuropsychiatric disorders. In this review, we have seen the role of 5-HT3RA in other conditions like pain, addiction, eating disorders, inflammation, cognition or memory, gastrointestinal problem and schizophrenia. In addition, 5-HT3RA showed effectiveness in depression comorbid with anxiety disorders. Various preclinical studies showed that 5-HT3RA alleviate the symptoms of depression and anxiety in rodent models. They act through modulation of the synaptic transmission in various CNS areas. In addition, they are able to reduce the oxidative stress, cortisol levels in the plasma/brain of mice. They enhance the availability of 5-HT on 5-HT1 and 5-HT2 receptors and showed an anti-depressant effect via increasing the levels of BDNF and CREB in the brain of mice. In clinical trials, these setrons have shown their potential against the most intractable symptoms of schizophrenia like negative and cognitive symptoms. Setrons also have shown their effectiveness against early onset alcohol dependence. The condition is presumably associated with major serotonergic dysfunction, including overexpression of postsynaptic 5-HT3R in the mesolimbic DA system. These 5-HT3RA can also be used in combination with other standard drugs and in this way, they are able to reduce the adverse effects such as abuse liability, sedation, glucose intolerance, weight gain, sexual disturbance and anticholinergic effects. In addition, the use of 5-HT3RA in combination may also be an effective approach against treatment resistant depression cases. Given the advantageous therapeutic profile of 5-HT3RA combined with their broad therapeutic window, more detailed studies on this class of drugs could open avenues for the development of novel pharmacophores with higher efficacy and better compliance for the management of neurologic and neuropsychiatric disorders.
Fig. (6).
Involvement of 5-HT3 receptor antagonists in various disorders.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
References
- 1.WHO. https://www.who.int/news-room/fact-sheets/detail/depression
- 2.Center for substance abuse treatment. Managing depressive symptoms in substance abuse clients during early recovery. 2008. https://www.ncbi.nlm.nih.gov/books/NBK64063/ [PubMed]
- 3.Albert P.R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 2015;40(4):219–221. doi: 10.1503/jpn.150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boivin J.R., Piekarski D.J., Wahlberg J.K., Wilbrecht L. Age, sex, and gonadal hormones differently influence anxiety- and depression-related behavior during puberty in mice. Psychoneuroendocrinology. 2017;85:78–87. doi: 10.1016/j.psyneuen.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shors T.J., Leuner B. Estrogen-mediated effects on depression and memory formation in females. J. Affect. Disord. 2003;74(1):85–96. doi: 10.1016/S0165-0327(02)00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karpinski M., Mattina G.F., Steiner M. Effect of gonadal hormones on neurotransmitters implicated in the pathophysiology of obsessive-compulsive disorder: a critical review. Neuroendocrinology. 2017;105(1):1–16. doi: 10.1159/000453664. [DOI] [PubMed] [Google Scholar]
- 7.Boku S., Nakagawa S., Toda H., Hishimoto A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018;72(1):3–12. doi: 10.1111/pcn.12604. [DOI] [PubMed] [Google Scholar]
- 8.Heninger G.R., Delgado P.L., Charney D.S. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29(1):2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- 9.Lener M.S., Niciu M.J., Ballard E.D., Park M., Park L.T., Nugent A.C., Zarate C.A. Jr. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol. Psychiatry. 2017;81(10):886–897. doi: 10.1016/j.biopsych.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W., Ge T., Leng Y., Pan Z., Fan J., Yang W., Cui R. The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plast. 2017;2017:6871089. doi: 10.1155/2017/6871089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shadrina M., Bondarenko E.A., Slominsky P.A. Genetics factors in major depression disease. Front. Psychiatry. 2018;9:334. doi: 10.3389/fpsyt.2018.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller J., Gomez R., Williams G., Lembke A., Lazzeroni L., Murphy G.M., Jr, Schatzberg A.F. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry. 2017;22(4):527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt S., Nagappa A.N., Patil C.R. Role of oxidative stress in depression. Drug Discov. Today. 2020;25(7):1270–1276. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Michel T.M., Pülschen D., Thome J. The role of oxidative stress in depressive disorders. Curr. Pharm. Des. 2012;18(36):5890–5899. doi: 10.2174/138161212803523554. [DOI] [PubMed] [Google Scholar]
- 15.Raison C.L., Miller A.H. Is depression an inflammatory disorder? Curr. Psychiatry Rep. 2011;13(6):467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunner D.L. Combining antidepressants. Shanghai Jingshen Yixue. 2014;26(6):363–364. doi: 10.11919/j.issn.1002-0829.214177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machado-Vieira R., Baumann J., Wheeler-Castillo C., Latov D., Henter I.D., Salvadore G., Zarate C.A. The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals (Basel) 2010;3(1):19–41. doi: 10.3390/ph3010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols D.E., Nichols C.D. Serotonin receptors. Chem. Rev. 2008;108(5):1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 19.Lanfumey L., Hamon M. 5-HT1 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004;3(1):1–10. doi: 10.2174/1568007043482570. [DOI] [PubMed] [Google Scholar]
- 20.Celada P., Puig M., Amargós-Bosch M., Adell A., Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J. Psychiatry Neurosci. 2004;29(4):252–265. [PMC free article] [PubMed] [Google Scholar]
- 21.Haddjeri N., Blier P., de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J. Neurosci. 1998;18(23):10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blier P., Ward N.M. Is there a role for 5-HT1A agonists in the treatment of depression? Biol. Psychiatry. 2003;53(3):193–203. doi: 10.1016/S0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- 23.Nautiyal K.M., Tritschler L., Ahmari S.E., David D.J., Gardier A.M., Hen R. A lack of serotonin 1B Autoreceptors results in decreased anxiety and depression-related behaviors. Neuropsychopharmacology. 2016;41(12):2941–2950. doi: 10.1038/npp.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatt S., Mahesh R., Devadoss T., Jindal A.K. Anxiolytic-like effect of (4-benzylpiperazin-1-yl)(3-methoxyquinoxalin-2-yl)methanone (6g) in experimental mouse models of anxiety. Indian J. Pharmacol. 2013;45(3):248–251. doi: 10.4103/0253-7613.111923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt S., Mahesh R., Devadoss T., Jindal A.K. Antidepressant-like effect of novel 5-HT3 receptor antagonist N-n-butyl-3-ethoxyquinoxalin-2-carboxamide (6p): an approach using rodent behavioral antidepressant tests. Indian J. Pharmacol. 2013;45(4):348–353. doi: 10.4103/0253-7613.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fakhfouri G., Rahimian R., Dyhrfjeld-Johnsen J., Zirak M.R., Beaulieu J.M. 5-HT3 receptor antagonists in neurologic and neuropsychiatric disorders: The Iceberg still lies beneath the surface. Pharmacol. Rev. 2019;71(3):383–412. doi: 10.1124/pr.118.015487. [DOI] [PubMed] [Google Scholar]
- 27.Faerber L., Drechsler S., Ladenburger S., Gschaidmeier H., Fischer W. The neuronal 5-HT3 receptor network after 20 years of research--evolving concepts in management of pain and inflammation. Eur. J. Pharmacol. 2007;560(1):1–8. doi: 10.1016/j.ejphar.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Somers G.I., Harris A.J., Bayliss M.K., Houston J.B. The metabolism of the 5HT3 antagonists ondansetron, alosetron and GR87442 I: a comparison of in vitro and in vivo metabolism and in vitro enzyme kinetics in rat, dog and human hepatocytes, microsomes and recombinant human enzymes. Xenobiotica. 2007;37(8):832–854. doi: 10.1080/00498250701485575. [DOI] [PubMed] [Google Scholar]
- 29.Bigaud M., Elands J., Kastner P.R., Bohnke R.A., Ernrnert L.W., Galvan M. Pharmacology of the human metabolites of dolasetron, an antiemetic 5-HT3 receptor antagonist. Drug Dev. Res. 1995;34:289–296. doi: 10.1002/ddr.430340306. [DOI] [Google Scholar]
- 30.Kees F., Färber L., Bucher M., Mair G., Mörike K., Grobecker H. Pharmacokinetics of therapeutic doses of tropisetron in healthy volunteers. Br. J. Clin. Pharmacol. 2001;52(6):705–707. doi: 10.1046/j.0306-5251.2001.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoltz R., Parisi S., Shah A., Macciocchi A. Pharmacokinetics, metabolism and excretion of intravenous [l4C]-palonosetron in healthy human volunteers. Biopharm. Drug Dispos. 2004;25(8):329–337. doi: 10.1002/bdd.410. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura H., Ariyoshi N., Okada K., Nakasa H., Nakazawa K., Kitada M. CYP1A1 is a major enzyme responsible for the metabolism of granisetron in human liver microsomes. Curr. Drug Metab. 2005;6(5):469–480. doi: 10.2174/138920005774330666. [DOI] [PubMed] [Google Scholar]
- 33.Swain C.J., Baker R., Kneen C., Moseley J., Saunders J., Seward E.M., Stevenson G., Beer M., Stanton J., Watling K. Novel 5-HT3 antagonists. Indole oxadiazoles. J. Med. Chem. 1991;34(1):140–151. doi: 10.1021/jm00105a021. [DOI] [PubMed] [Google Scholar]
- 34.Rizzi J.P., Nagel A.A., Rosen T., McLean S., Seeger T. An initial three-component pharmacophore for specific serotonin-3 receptor ligands. J. Med. Chem. 1990;33(10):2721–2725. doi: 10.1021/jm00172a007. [DOI] [PubMed] [Google Scholar]
- 35.Bermudez J., Dabbs S., Joiner K.A., King F.D. 5-Hydroxytryptamine (5-HT3) receptor antagonists. 2. 1-Indolinecarboxamides. J. Med. Chem. 1990;33(7):1929–1932. doi: 10.1021/jm00169a017. [DOI] [PubMed] [Google Scholar]
- 36.Hibert M.F., Hoffmann R., Miller R.C., Carr A.A. Conformation-activity relationship study of 5-HT3 receptor antagonists and a definition of a model for this receptor site. J. Med. Chem. 1990;33(6):1594–1600. doi: 10.1021/jm00168a011. [DOI] [PubMed] [Google Scholar]
- 37.Swain C.J., Baker R., Kneen C., Herbert R., Moseley J., Saunders J., Seward E.M., Stevenson G.I., Beer M., Stanton J., et al. Novel 5-HT3 antagonists: indol-3-ylspiro(azabicycloalkane-3,5‘(4’H)-oxazoles). J. Med. Chem. 1992;35(6):1019–1031. doi: 10.1021/jm00084a007. [DOI] [PubMed] [Google Scholar]
- 38.Ohta M., Suzuki T., Koide T., Matsuhisa A., Furuya T., Miyata K., Yanagisawa I. Novel 5-hydroxytryptamine (5-HT3) receptor antagonists. I. Synthesis and structure-activity relationships of conformationally restricted fused imidazole derivatives. Chem. Pharm. Bull. (Tokyo) 1996;44(5):991–999. doi: 10.1248/cpb.44.991. [DOI] [PubMed] [Google Scholar]
- 39.Rosen T., Nagel A.A., Rizzi J.P., Ives J.L., Daffeh J.B., Ganong A.H., Guarino K., Heym J., McLean S., Nowakowski J.T., Schmidt A.W., Seeger T.F., Siok C.J., Vincent L.A. Thiazole as a carbonyl bioisostere. A novel class of highly potent and selective 5-HT3 receptor antagonists. J. Med. Chem. 1990;33(10):2715–2720. doi: 10.1021/jm00172a006. [DOI] [PubMed] [Google Scholar]
- 40.Monge A., Peña M.C., Palop J.A., Calderó J.M., Roca J., García E., Romero G., del Río J., Lasheras B. Synthesis of 2-piperazinylbenzothiazole and 2-piperazinylbenzoxazole derivatives with 5-HT3 antagonist and 5-HT4 agonist properties. J. Med. Chem. 1994;37(9):1320–1325. doi: 10.1021/jm00035a012. [DOI] [PubMed] [Google Scholar]
- 41.Clark R.D., Miller A.B., Berger J., Repke D.B., Weinhardt K.K., Kowalczyk B.A., Eglen R.M., Bonhaus D.W., Lee C.H., Michel A.D., Smith W.L., Wongll E.H.F. 2-(Quinuclidin-3-yl)pyrido[4,3-b]indol-1-ones and isoquinolin-1-ones. Potent conformationally restricted 5-HT3 receptor antagonists. J. Med. Chem. 1993;36(18):2645–2657. doi: 10.1021/jm00070a008. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi H., Miwa Y., Ichikawa S., Yoda N., Miki I. lshii, A.; Kono, M.; Yasuzawa, T.; Suzuki, F. 5-HT3 Receptor Antagonists. 2. 4-Hydroxy-quinoline carboxylic Acid Derivatives. J. Med. Chem. 1993;36:617–626. doi: 10.1021/jm00057a011. [DOI] [PubMed] [Google Scholar]
- 43.Monge A., Palop J.A., Del Castillo J.C., Calderó J.M., Roca J., Romero G., Del Río J., Lasheras B. Novel antagonists of 5-HT3 receptors. Synthesis and biological evaluation of piperazinylquinoxaline derivatives. J. Med. Chem. 1993;36(19):2745–2750. doi: 10.1021/jm00071a005. [DOI] [PubMed] [Google Scholar]
- 44.Heidempergher F., Pillan A., Pinciroli V., Vaghi F., Arrigoni C., Bolis G., Caccia C., Dho L., McArthur R., Varasi M. Phenylimidazolidin-2-one derivatives as selective 5-HT3 receptor antagonists and refinement of the pharmacophore model for 5-HT3 receptor binding. J. Med. Chem. 1997;40(21):3369–3380. doi: 10.1021/jm970060o. [DOI] [PubMed] [Google Scholar]
- 45.Kuroita T., Marubayashi N., Sano M., Kanzaki K., Inaba K., Kawakita T. Benzoxazines. II. Synthesis, conformational analysis, and structure--activity relationships of 3,4-dihydro-2H-1,4-benzoxazine-8-carboxamide derivatives as potent and long-acting serotonin-3 (5-HT3) receptor antagonists. Chem. Pharm. Bull. (Tokyo) 1996;44(11):2051–2060. doi: 10.1248/cpb.44.2051. [DOI] [PubMed] [Google Scholar]
- 46.Mahesh R., Devadoss T., Pandey D.K., Bhatt S., Yadav S.K. Design, synthesis and structure-activity relationship of novel quinoxalin-2-carboxamides as 5-HT3 receptor antagonists for the management of depression. Bioorg. Med. Chem. Lett. 2010;20(22):6773–6776. doi: 10.1016/j.bmcl.2010.08.128. [DOI] [PubMed] [Google Scholar]
- 47.Mahesh R., Perumal R.V., Pandi P.V. Microwave assisted synthesis of 2-(4-substituted piperazin-1-yl)-1,8-naphthyridine-3-carbonitrile as a new class of serotonin 5-HT3 receptor antagonists. Bioorg. Med. Chem. Lett. 2004;14(20):5179–5181. doi: 10.1016/j.bmcl.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 48.Verheij M.H.P., Thompson A.J., van Muijlwijk-Koezen J.E., Lummis S.C.R., Leurs R., de Esch I.J.P. Design, synthesis, and structure-activity relationships of highly potent 5-HT₃ receptor ligands. J. Med. Chem. 2012;55(20):8603–8614. doi: 10.1021/jm300801u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al Kury L.T., Mahgoub M., Howarth F.C., Oz M. Natural negative allosteric modulators of 5-HT₃ Receptors. Molecules. 2018;23(12):3186. doi: 10.3390/molecules23123186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cortes-Altamirano J.L., Olmos-Hernandez A., Jaime H.B., Carrillo-Mora P., Bandala C., Reyes-Long S., Alfaro-Rodríguez A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 receptors and their role in the modulation of pain response in the central nervous system. Curr. Neuropharmacol. 2018;16(2):210–221. doi: 10.2174/1570159X15666170911121027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaddum J.H., Picarelli Z.P. Two kinds of tryptamine receptor. Br. J. Pharmacol. Chemother. 1957;12(3):323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niesler B., Walstab J., Combrink S., Möller D., Kapeller J., Rietdorf J., Bönisch H., Göthert M., Rappold G., Brüss M. Characterization of the novel human serotonin receptor subunits 5-HT3C,5-HT3D, and 5-HT3E. Mol. Pharmacol. 2007;72(1):8–17. doi: 10.1124/mol.106.032144. [DOI] [PubMed] [Google Scholar]
- 53.Betry C., Etievant A., Oosterhof C., Ebert B., Sanchez C., Haddjeri N. Role of 5-HT3 receptors in the antidepressant response. Pharmaceuticals. 2011;4:603–629. doi: 10.3390/ph4040603. [DOI] [Google Scholar]
- 54.Barnes J.M., Barnes N.M., Costall B., Ironside J.W., Naylor R.J. Identification and characterisation of 5-hydroxytryptamine 3 recognition sites in human brain tissue. J. Neurochem. 1989;53(6):1787–1793. doi: 10.1111/j.1471-4159.1989.tb09244.x. [DOI] [PubMed] [Google Scholar]
- 55.Abi-Dargham A., Laruelle M., Wong D.T., Robertson D.W., Weinberger D.R., Kleinman J.E. Pharmacological and regional characterization of [3H]LY278584 binding sites in human brain. J. Neurochem. 1993;60(2):730–737. doi: 10.1111/j.1471-4159.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 56.Bufton K.E., Steward L.J., Barber P.C., Barnes N.M. Distribution and characterization of the [3H]granisetron-labelled 5-HT3 receptor in the human forebrain. Neuropharmacology. 1993;32(12):1325–1331. doi: 10.1016/0028-3908(93)90027-Z. [DOI] [PubMed] [Google Scholar]
- 57.Parker R.M., Barnes J.M., Ge J., Barber P.C., Barnes N.M. Autoradiographic distribution of [3H]-(S)-zacopride-labelled 5-HT3 receptors in human brain. J. Neurol. Sci. 1996;144(1-2):119–127. doi: 10.1016/S0022-510X(96)00211-0. [DOI] [PubMed] [Google Scholar]
- 58.Miquel M.C., Emerit M.B., Nosjean A., Simon A., Rumajogee P., Brisorgueil M.J., Doucet E., Hamon M., Vergé D. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur. J. Neurosci. 2002;15(3):449–457. doi: 10.1046/j.0953-816x.2001.01872.x. [DOI] [PubMed] [Google Scholar]
- 59.Walstab J., Rappold G., Niesler B. 5-HT(3) receptors: role in disease and target of drugs. Pharmacol. Ther. 2010;128(1):146–169. doi: 10.1016/j.pharmthera.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Thompson A.J., Lummis S.C. 5-HT3 receptors. Curr. Pharm. Des. 2006;12(28):3615–3630. doi: 10.2174/138161206778522029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machu T.K. Therapeutics of 5-HT3 receptor antagonists: current uses and future directions. Pharmacol. Ther. 2011;130(3):338–347. doi: 10.1016/j.pharmthera.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Navari R.M. 5-HT3 receptors as important mediators of nausea and vomiting due to chemotherapy. Biochim. Biophys. Acta. 2015;1848(10 Pt B):2738–2746. doi: 10.1016/j.bbamem.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 63.Hannon J., Hoyer D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008;195(1):198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 64.Mukerji J., Haghighi A., Séguéla P. Immunological characterization and transmembrane topology of 5-hydroxytryptamine3 receptors by functional epitope tagging. J. Neurochem. 1996;66(3):1027–1032. doi: 10.1046/j.1471-4159.1996.66031027.x. [DOI] [PubMed] [Google Scholar]
- 65.Spier A.D., Wotherspoon G., Nayak S.V., Nichols R.A., Priestley J.V., Lummis S.C. Antibodies against the extracellular domain of the 5-HT3 receptor label both native and recombinant receptors. Brain Res. Mol. Brain Res. 1999;67(2):221–230. doi: 10.1016/S0169-328X(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 66.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005;346(4):967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 67.Miyazawa A., Fujiyoshi Y., Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423(6943):949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 68.Rajkumar R., Mahesh R. The auspicious role of the 5-HT3 receptor in depression: a probable neuronal target? J. Psychopharmacol. 2010;24(4):455–469. doi: 10.1177/0269881109348161. [DOI] [PubMed] [Google Scholar]
- 69.Smith H.S., Cox L.R., Smith E.J. 5-HT3 receptor antagonists for the treatment of nausea/vomiting. Ann. Palliat. Med. 2012;1(2):115–120. doi: 10.3978/j.issn.2224-5820.2012.07.07. [DOI] [PubMed] [Google Scholar]
- 70.Tricco A.C., Blondal E., Veroniki A.A., Soobiah C., Vafaei A., Ivory J., Strifler L., Cardoso R., Reynen E., Nincic V., Ashoor H., Ho J., Ng C., Johnson C., Lillie E., Antony J., Roberts D.J., Hemmelgarn B.R., Straus S.E. Comparative safety and effectiveness of serotonin receptor antagonists in patients undergoing chemotherapy: a systematic review and network meta-analysis. BMC Med. 2016;14(1):216. doi: 10.1186/s12916-016-0761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mott D.D., Erreger K., Banke T.G., Traynelis S.F. Open probability of homomeric murine 5-HT3A serotonin receptors depends on subunit occupancy. J. Physiol. 2001;535(Pt 2):427–443. doi: 10.1111/j.1469-7793.2001.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rayes D., De Rosa M.J., Sine S.M., Bouzat C. Number and locations of agonist binding sites required to activate homomeric Cys-loop receptors. J. Neurosci. 2009;29(18):6022–6032. doi: 10.1523/JNEUROSCI.0627-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koyama S., Matsumoto N., Kubo C., Akaike N. Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdala neurons. J. Physiol. 2000;529(Pt 2):373–383. doi: 10.1111/j.1469-7793.2000.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nayak S.V., Rondé P., Spier A.D., Lummis S.C., Nichols R.A. Calcium changes induced by presynaptic 5-hydroxytryptamine-3 serotonin receptors on isolated terminals from various regions of the rat brain. Neuroscience. 1999;91(1):107–117. doi: 10.1016/S0306-4522(98)00520-X. [DOI] [PubMed] [Google Scholar]
- 75.Yakel J.L., Shao X.M., Jackson M.B. The selectivity of the channel coupled to the 5-HT3 receptor. Brain Res. 1990;533(1):46–52. doi: 10.1016/0006-8993(90)91793-G. [DOI] [PubMed] [Google Scholar]
- 76.Barnes N.M., Hales T.G., Lummis S.C., Peters J.A. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology. 2009;56(1):273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta D., Devadoss T., Bhatt S., Gautam B., Jindal A., Pandey D., Mahesh R. Anti-depressant-like activity of a novel serotonin type-3 (5-HT3) receptor antagonist in rodent models of depression. Indian J. Exp. Biol. 2011;49(8):619–626. [PubMed] [Google Scholar]
- 78.Bhatt S., Mahesh R., Devadoss T., Jindal A. Anti-depressant like activity of N-n-butyl-3-methoxyquinoxaline-2-carboxamide (6o) a 5-HT3 receptor antagonist. Indian J. Exp. Biol. 2013;51(6):435–443. [PubMed] [Google Scholar]
- 79.Sugita S., Shen K.Z., North R.A. 5-hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala. Neuron. 1992;8(1):199–203. doi: 10.1016/0896-6273(92)90121-S. [DOI] [PubMed] [Google Scholar]
- 80.Bourin M., Redrobe J.P., Baker G.B. Pindolol does not act only on 5-HT1A receptors in augmenting antidepressant activity in the mouse forced swimming test. Psychopharmacology (Berl.) 1998;136(3):226–234. doi: 10.1007/s002130050560. [DOI] [PubMed] [Google Scholar]
- 81.Ramamoorthy R., Radhakrishnan M., Borah M. Antidepressant-like effects of serotonin type-3 antagonist, ondansetron: an investigation in behaviour-based rodent models. Behav. Pharmacol. 2008;19(1):29–40. doi: 10.1097/FBP.0b013e3282f3cfd4. [DOI] [PubMed] [Google Scholar]
- 82.Nayak S.V., Rondé P., Spier A.D., Lummis S.C., Nichols R.A. Nicotinic receptors co-localize with 5-HT(3) serotonin receptors on striatal nerve terminals. Neuropharmacology. 2000;39(13):2681–2690. doi: 10.1016/S0028-3908(00)00109-X. [DOI] [PubMed] [Google Scholar]
- 83.Giovannini M.G., Ceccarelli I., Molinari B., Cecchi M., Goldfarb J., Blandina P. Serotonergic modulation of acetylcholine release from cortex of freely moving rats. J. Pharmacol. Exp. Ther. 1998;285(3):1219–1225. [PubMed] [Google Scholar]
- 84.Haus U., Varga B., Stratz T., Späth M., Müller W. Oral treatment of fibromyalgia with tropisetron given over 28 days: influence on functional and vegetative symptoms, psychometric parameters and pain. Scand. J. Rheumatol. Suppl. 2000;113:55–58. doi: 10.1080/030097400446652. [DOI] [PubMed] [Google Scholar]
- 85.Faris P.L., Eckert E.D., Kim S.W., Meller W.H., Pardo J.V., Goodale R.L., Hartman B.K. Evidence for a vagal pathophysiology for bulimia nervosa and the accompanying depressive symptoms. J. Affect. Disord. 2006;92(1):79–90. doi: 10.1016/j.jad.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 86.Blier P. Optimal use of antidepressants: when to act? J. Psychiatry Neurosci. 2009;34(1):80. [PMC free article] [PubMed] [Google Scholar]
- 87.Taylor C., Fricker A.D., Devi L.A., Gomes I. Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cell. Signal. 2005;17(5):549–557. doi: 10.1016/j.cellsig.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Harbi K.S. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer. Adherence. 2012;6:369–388. doi: 10.2147/PPA.S29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roerig B., Katz L.C. Modulation of intrinsic circuits by serotonin 5-HT3 receptors in developing ferret visual cortex. J. Neurosci. 1997;17(21):8324–8338. doi: 10.1523/JNEUROSCI.17-21-08324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhatt S., Mahesh R., Jindal A., Devadoss T. Neuropharmacological effect of novel 5-HT3 receptor antagonist, N-n-propyl-3-ethoxyquinoxaline-2-carboxamide (6n) on chronic unpredictable mild stress-induced molecular and cellular response: Behavioural and biochemical evidences. Pharmacol. Rep. 2014;66(5):804–810. doi: 10.1016/j.pharep.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 91.Maes M., De Vos N., Pioli R., Demedts P., Wauters A., Neels H., Christophe A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J. Affect. Disord. 2000;58(3):241–246. doi: 10.1016/S0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 92.Maes M., Galecki P., Chang Y.S., Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(3):676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 93.Bhatt S., Radhakrishnan M., Jindal A., Devadoss T., Dhar A.K. Neuropharmacological evaluation of a novel 5-HT3 receptor antagonist (6g) on chronic unpredictable mild stress-induced changes in behavioural and brain oxidative stress parameters in mice. Indian J. Pharmacol. 2014;46(2):191–196. doi: 10.4103/0253-7613.129316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta D., Radhakrishnan M., Kurhe Y. Effect of a novel 5-HT3 receptor antagonist 4i, in corticosterone-induced depression-like behavior and oxidative stress in mice. Steroids. 2015;96:95–102. doi: 10.1016/j.steroids.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 95.Lee H.J., Ban J.Y., Seong Y.H. Blockade of 5-HT(3) receptor with MDL7222 and Y25130 reduces hydrogen peroxide-induced neurotoxicity in cultured rat cortical cells. Life Sci. 2005;78(3):294–300. doi: 10.1016/j.lfs.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 96.Khalifeh S., Fakhfouri G., Mehr S.E., Mousavizadeh K., Dehpour A.R., Khodagholi F., Kazmi S., Rahimian R. Beyond the 5-HT3 receptors: A role for α7nACh receptors in neuroprotective aspects of tropisetron. Hum. Exp. Toxicol. 2015;34(9):922–931. doi: 10.1177/0960327114562034. [DOI] [PubMed] [Google Scholar]
- 97.Spiers J.G., Chen H.J., Sernia C., Lavidis N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015;8:456. doi: 10.3389/fnins.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kurhe Y., Mahesh R., Devadoss T. QCM-4, a 5-HT₃ receptor antagonist ameliorates plasma HPA axis hyperactivity, leptin resistance and brain oxidative stress in depression and anxiety-like behavior in obese mice. Biochem. Biophys. Res. Commun. 2015;456(1):74–79. doi: 10.1016/j.bbrc.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 99.Bhatnagar S., Sun L.M., Raber J., Maren S., Julius D., Dallman M.F. Changes in anxiety-related behaviors and hypothalamic-pituitary-adrenal activity in mice lacking the 5-HT-3A receptor. Physiol. Behav. 2004;81(4):545–555. doi: 10.1016/j.physbeh.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 100.Gupta D., Radhakrishnan M., Kurhe Y. 5HT3 receptor antagonist (ondansetron) reverses depressive behavior evoked by chronic unpredictable stress in mice: modulation of hypothalamic-pituitary-adrenocortical and brain serotonergic system. Pharmacol. Biochem. Behav. 2014;124:129–136. doi: 10.1016/j.pbb.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 101.Harrell A.V., Allan A.M. Improvements in hippocampal-dependent learning and decremental attention in 5-HT(3) receptor overexpressing mice. Learn. Mem. 2003;10(5):410–419. doi: 10.1101/lm.56103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Preston G.C. Racagni, G.; Brunello, N.; Langer, S.Z.; editors. Recent Advances in the treatment of neurodegenerative diorders and cognitive dysfunction. Vol. 7. International Academy of Biomedical Drug Research; Karger, Basel, Switzerland.: 1994. 5-HT3 Antagonists and dosorders of cognition. pp. 89–93. [Google Scholar]
- 103.Ramírez M.J., Cenarruzabeitia E., Lasheras B., Del Río J. Involvement of GABA systems in acetylcholine release induced by 5-HT3 receptor blockade in slices from rat entorhinal cortex. Brain Res. 1996;712(2):274–280. doi: 10.1016/0006-8993(95)01471-3. [DOI] [PubMed] [Google Scholar]
- 104.Callahan P.M., Bertrand D., Bertrand S., Plagenhoef M.R., Terry A.V., Jr Tropisetron sensitizes α7 containing nicotinic receptors to low levels of acetylcholine in vitro and improves memory-related task performance in young and aged animals. Neuropharmacology. 2017;117:422–433. doi: 10.1016/j.neuropharm.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 105.Buhot M.C., Martin S., Segu L. Role of serotonin in memory impairment. Ann. Med. 2000;32(3):210–221. doi: 10.3109/07853890008998828. [DOI] [PubMed] [Google Scholar]
- 106.Iidaka T., Ozaki N., Matsumoto A., Nogawa J., Kinoshita Y., Suzuki T., Iwata N., Yamamoto Y., Okada T., Sadato N. A variant C178T in the regulatory region of the serotonin receptor gene HTR3A modulates neural activation in the human amygdala. J. Neurosci. 2005;25(27):6460–6466. doi: 10.1523/JNEUROSCI.5261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suzuki R., Morcuende S., Webber M., Hunt S.P., Dickenson A.H. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat. Neurosci. 2002;5(12):1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki R., Rahman W., Hunt S.P., Dickenson A.H. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain Res. 2004;1019(1-2):68–76. doi: 10.1016/j.brainres.2004.05.108. [DOI] [PubMed] [Google Scholar]
- 109.Stratz T., Müller W. The use of 5-HT3 receptor antagonists in various rheumatic diseases--a clue to the mechanism of action of these agents in fibromyalgia? Scand. J. Rheumatol. Suppl. 2000;113:66–71. doi: 10.1080/030097400446689. [DOI] [PubMed] [Google Scholar]
- 110.Zheng Y., Yu T., Tang Y., Xiong W., Shen X., Jiang L., Lin L. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2017;12(3):e0172846. doi: 10.1371/journal.pone.0172846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Browning K.N. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front. Neurosci. 2015;9:413. doi: 10.3389/fnins.2015.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fayyaz M., Lackner J.M. Serotonin receptor modulators in the treatment of irritable bowel syndrome. Ther. Clin. Risk Manag. 2008;4(1):41–48. doi: 10.2147/tcrm.s140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mayer E.A., Berman S., Derbyshire S.W., Suyenobu B., Chang L., Fitzgerald L., Mandelkern M., Hamm L., Vogt B., Naliboff B.D. The effect of the 5-HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment. Pharmacol. Ther. 2002;16(7):1357–1366. doi: 10.1046/j.1365-2036.2002.01287.x. [DOI] [PubMed] [Google Scholar]
- 114.Walstab J., Wohlfarth C., Hovius R., Schmitteckert S., Röth R., Lasitschka F., Wink M., Bönisch H., Niesler B. Natural compounds boldine and menthol are antagonists of human 5-HT3 receptors: implications for treating gastrointestinal disorders. Neurogastroenterol. Motil. 2014;26(6):810–820. doi: 10.1111/nmo.12334. [DOI] [PubMed] [Google Scholar]
- 115.Ashby C.R., Minabe Y., Toor A., Fishkin L.D., Granoff M.I., Wang R.Y. Effect produced by acute and chronic administration of the selective 5-HT3 receptor antagonist BRL 46470 on the number of spontaneously active midbrain dopamine cells in the rat. Drug Dev. Res. 1994;31:228–236. doi: 10.1002/ddr.430310310. [DOI] [Google Scholar]
- 116.Sirota P., Mosheva T., Shabtay H., Giladi N., Korczyn A.D. Use of the selective serotonin 3 receptor antagonist ondansetron in the treatment of neuroleptic-induced tardive dyskinesia. Am. J. Psychiatry. 2000;157(2):287–289. doi: 10.1176/appi.ajp.157.2.287. [DOI] [PubMed] [Google Scholar]
- 117.Rammes G., Eisensamer B., Ferrari U., Shapa M., Gimpl G., Gilling K., Parsons C., Riering K., Hapfelmeier G., Bondy B., Zieglgänsberger W., Holsboer F., Rupprecht R. Antipsychotic drugs antagonize human serotonin type 3 receptor currents in a noncompetitive manner. Mol. Psychiatry. 2004;9(9):846–858, 818. doi: 10.1038/sj.mp.4001490. [DOI] [PubMed] [Google Scholar]
- 118.Hayes M.R., Covasa M. Gastric distension enhances CCK-induced Fos-like immunoreactivity in the dorsal hindbrain by activating 5-HT3 receptors. Brain Res. 2006;1088(1):120–130. doi: 10.1016/j.brainres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 119.Ginawi O.T., Al-Majed A.A., Al-Suwailem A.K. Ondansetron, a selective 5-HT3 antagonist, antagonizes methamphetamine-induced anorexia in mice. Pharmacol. Res. 2005;51(3):255–259. doi: 10.1016/j.phrs.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Carvalho F., Barros D., Silva J., Rezende E., Soares M., Fregoneze J., de Castro-E-Silva E. Hyperglycemia induced by pharmacological activation of central serotonergic pathways depends on the functional integrity of brain CRH system and 5-HT3 receptors. Horm. Metab. Res. 2005;37(8):482–488. doi: 10.1055/s-2005-870323. [DOI] [PubMed] [Google Scholar]
- 121.Hodge C.W., Kelley S.P., Bratt A.M., Iller K., Schroeder J.P., Besheer J. 5-HT(3A) receptor subunit is required for 5-HT3 antagonist-induced reductions in alcohol drinking. Neuropsychopharmacology. 2004;29(10):1807–1813. doi: 10.1038/sj.npp.1300498. [DOI] [PubMed] [Google Scholar]
- 122.Hui S.C., Sevilla E.L., Ogle C.W. 5-HT3 antagonists reduce morphine self-administration in rats. Br. J. Pharmacol. 1993;110(4):1341–1346. doi: 10.1111/j.1476-5381.1993.tb13966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johnson B.A. Role of the serotonergic system in the neurobiology of alcoholism: implications for treatment. CNS Drugs. 2004;18(15):1105–1118. doi: 10.2165/00023210-200418150-00005. [DOI] [PubMed] [Google Scholar]
- 124.Johnson B.A., Roache J.D., Ait-Daoud N., Javors M.A., Harrison J.M., Elkashef A., Mojsiak J., Li S-H., Bloch D.A. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of cocaine dependence. Drug Alcohol Depend. 2006;84(3):256–263. doi: 10.1016/j.drugalcdep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 125.Bhatt S., Mahesh R., Devadoss T., Jindal A. Neuropharmacological evaluation of a novel 5-HT3 receptor antagonist (4-benzylpiperazin-1-yl)(3-methoxyquinoxalin-2-yl) methanone (6g) on lipopolysaccharide-induced anxiety models in mice. J. Basic Clin. Physiol. Pharmacol. 2017;28(2):101–106. doi: 10.1515/jbcpp-2016-0083. [DOI] [PubMed] [Google Scholar]
- 126.Vega Lde.L., Muñoz E., Calzado M.A., Lieb K., Candelario-Jalil E., Gschaidmeir H., Färber L., Mueller W., Stratz T., Fiebich B.L. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem. Pharmacol. 2005;70(3):369–380. doi: 10.1016/j.bcp.2005.04.031. [DOI] [PubMed] [Google Scholar]