Abstract
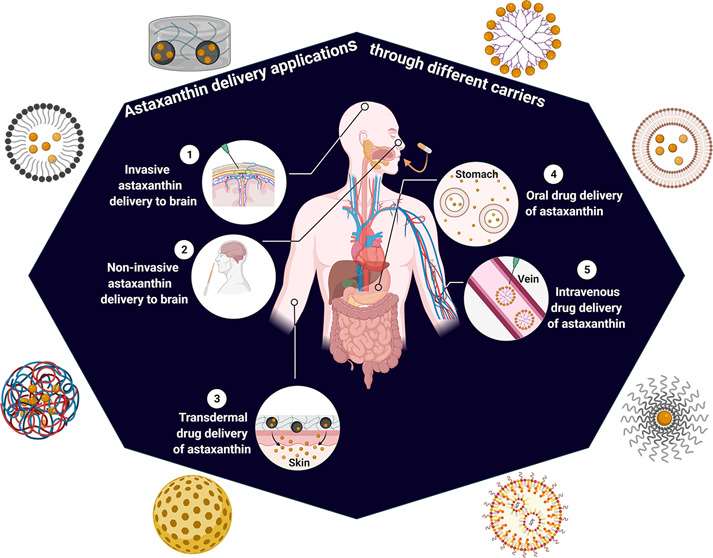
Astaxanthin (AXT) is one of the most important fat-soluble carotenoids that have abundant and diverse therapeutic applications namely in liver disease, cardiovascular disease, cancer treatment, protection of the nervous system, protection of the skin and eyes against UV radiation, and boosting the immune system. However, due to its intrinsic reactivity, it is chemically unstable, and therefore, the design and production processes for this compound need to be precisely formulated. Nanoencapsulation is widely applied to protect AXT against degradation during digestion and storage, thus improving its physicochemical properties and therapeutic effects. Nanocarriers are delivery systems with many advantages—ease of surface modification, biocompatibility, and targeted drug delivery and release. This review discusses the technological advancement in nanocarriers for the delivery of AXT through the brain, eyes, and skin, with emphasis on the benefits, limitations, and efficiency in practice.
1. Introduction
Astaxanthin (AXT), a highly potent xanthophyll, is a red, lipid-soluble carotenoid.1,2 Despite its numerous health-benefits, AXT has limited use in the pharmaceutical and food industries due to its poor solubility in water and lack of stability when exposed to oxygen, light, and high temperatures;3,4 conjugation with fatty acids or proteins promotes its natural stability.5 Notably, the oral intake of AXT is equally limited due to its low rate of dispersion in blood vessels as well as its low cellular absorption. An extensive effort has been made to boost the bioavailability, stability, and solubility of this powerful antioxidant by encapsulation. This method may protect AXT from gastric fluid and allow its gradual release in the intestinal fluids.
Among the various methods of encapsulation, liposomes, spray drying, solvent evaporation, ionic gelation, coacervation, and lyophilization are used in AXT formulation. Controlling the particle size and further purification of the product due to the use of solvents are the limitation of these encapsulation techniques. Recently, supercritical fluid precipitation is an environmentally friendly technology that has been used for the encapsulation of AXT. In a new study, supercritical carbon dioxide (SC-CO2) was employed in contact with the emulsion of AXT, ethyl acetate saturated water, and ethyl cellulose to encapsulate AXT. This method preserved the antioxidant activity of AXT and generated a high production capacity with an encapsulation efficiency of 84%.6 In another study microspheres of AXT were prepared using SC-CO2 technology with an encapsulation efficiency of 91.5%; AXT was dissolved in poly(l-lactic acid), dichloromethane, and acetone and then was evaporated into the bulk SC-CO2.7 The size and structure of capsules are significant factors to be taken into account for encapsulation of AXT. Structures of multiple layers (liposomes, oil-in-water emulsions) with nanometric scale provide higher stability and biological activity and allow controlled release of AXT.8 Not only do these micro-/nanocapsules protect AXT against gastrointestinal digestion and later release in the intestine, but also smaller AXT-loaded carriers (<500 nm) can be absorbed by endocytosis or through Peyer’s patches, thus enhancing the bioavailability of AXT.9 Therefore, the physicochemical properties, such as the size, charge, surface, and composition of the lipidic particles, can protect AXT against enzymatic digestion and enhance its stability and bioavailability.10 These nanoparticles, due to their lipophilic properties, can adhere to membranes and penetrate cells, and therefore, they have been suggested as excellent AXT carriers across the intestinal barrier. Nanostructured lipid carriers seem to be more stable to degradation than liposomes in the presence of gastric acid secretions and pancreatic lipases. For instance, the use of phospholipids, saturated lipids, or phytosterols can enhance the stability of carriers. Also, the surfactant-based delivery systems such as niosomes have resistance to hydrolysis and acid media.11 Other materials such as alginate/gelatin and whey protein/gum Arabic in gastric acidic pH are insoluble and prevent degradation but in intestinal pH facilitate dissolution where the encapsulated AXT is released.6 Therefore, the selected materials for encapsulation modulate the release of AXT in the intestine and cause resistance to its pH, hence preserving micro-/nanocapsules until degradation. Also, the delay in gastrointestinal transit of nanocapsules depends on the mucoadhesive properties of materials and the small particle size. Chitosan-based nanoparticles present advantages for loading AXT, as they are safe, biodegradable, and have high affinity to the cell membrane, thus improving the transport of AXT through the epithelial tight junctions. However, these nanoparticles are degraded under low pH conditions and cannot protect AXT during gastrointestinal digestion. Studies have demonstrated that blended chitosan with casein and oxidized dextran or other nonionic polymers enhance the physicochemical stability of these nanoparticles.12 One major criterion for choosing an efficient and suitable biopolymeric or lipid-based nanoencapsulation system for transportation of astaxanthin is the structure, barriers, and cellular composition of the target organ (brain, skin, and eye, etc.). The choice of an appropriate encapsulant material helps enhance the bioaccessibility, solubility, and long-term stability of astaxanthin in target organs. Overall, based on recent studies, chitosan (carbohydrate biopolymer) in combination with proteins or other carbohydrates is a valuable carrier for astaxanthin, and among lipid-based nanocarriers, nanoniosomal and nanostructured lipid vehicles are efficient systems relative to other lipid-based systems. Added parameters in the selection of a proper encapsulant, are its availability and reasonable price, and also the suitable route of its administration (oral, ocular, parenteral, etc.).13−16
The goal of this review is to highlight the properties and applications of AXT-encapsulated nanocarriers. In this regard, the limitations, advantages, and practicality of recent innovations and developments including nanodelivery systems of AXT for various ailments (e.g., neurological, ocular, and dermal disorders) are deliberated.
2. Source, Structure, and Extraction
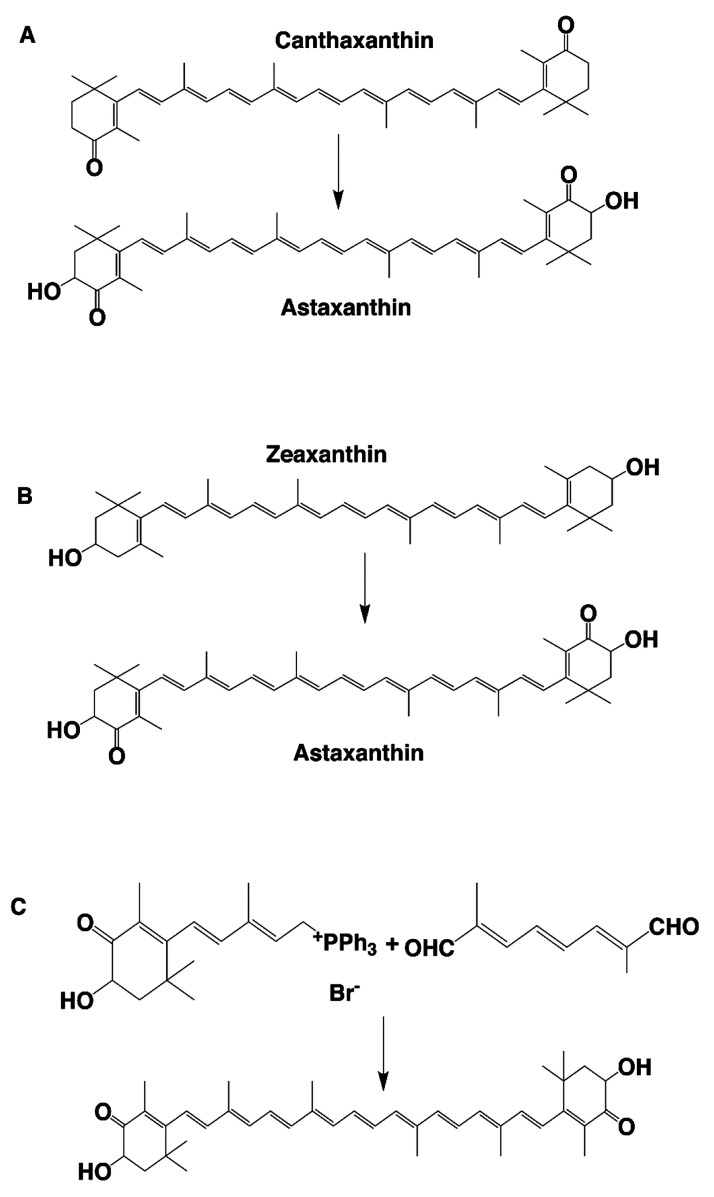
AXT is a xanthophyll, with the molecular formula C40H52O4 and molar mass 596.84 g/mol. It is naturally present in many sea creatures and living organisms, namely salmon, shrimp, krill, lobster, microorganisms, and some plants.17 Synthetic AXT, on the other hand, is produced by petrochemical products following a multistep process. Three different methods are used for the chemical synthesis of AXT: hydroxylation of canthaxanthin (Figure 1A), oxidation of zeaxanthin (Figure 1B), and Wittig reaction (a dialdehyde with two phosphoniums) (Figure 1C). To date, only natural AXT has been approved for human consumption. It is used as an expensive material for various therapeutic applications, whereas the use of the synthetic form falls mainly into aquaculture appliances merely as a feed additive.18 Notably, the antioxidant activity of natural AXT is 20–50 times stronger than that of synthetic AXT. It has exhibited better therapeutic performance and has shown no toxic effects.19 Therefore, the consumption of natural AXT and demand for it have grown more dramatically than those for the synthetic counterpart. Natural AXT is mainly derived from algae (Haematococcus Pluvialis), bacteria (Paracoccus haeundaensis, Paracoccus carotinifaciens), and yeast (Phaffia rhodozyma/Xanthophyllomyces dendrorhous). Haematococcus pluvialis is a freshwater microalgae and is known as a great source of natural astaxanthin.20,21 Many companies are producing natural AXT from algae, due to its mounting importance in the pharmaceutical industry.22−24 A considerable challenge in biotechnological production of AXT is the downstream processes. As AXT is produced intracellularly and high-purity AXT is needed for nutraceutical and pharmaceutical applications, high operating costs are mostly encountered; thus, the cost of downstream processes is nearly 80% of the production cost.25,26 An effective downstream process can reduce production costs and develop productivity.
Figure 1.
Three strategies of the chemical synthesis of AXT: (A) hydroxylation of canthaxanthin; (B) oxidation of zeaxanthin; and (C) Wittig reaction.
AXT consists of two terminal rings joined by a polyene chain. The molecule contains two asymmetric centers located at the 3 and 3′ positions of the β-ionone ring with a hydroxyl group (-OH) on either end of the molecule (Figure 2A). A chain of conjugated double bonds is extended at the center of the molecule which is responsible for the antioxidant activity of AXT.27−30 In view of the presence of oxygen in its rings, AXT possesses a more polar nature, making it a strong antioxidant as it can donate electrons and mop up free radicals. Notably, the configuration of stereogenic carbons at the 3 and 3′ positions in these rings defines AXT spatial isomers as chiral (3S, 3S′) or (3R, 3R′) or as meso (3R, 3′S), with the chiral configuration being the most abundant in nature (Figure 2B).
Figure 2.
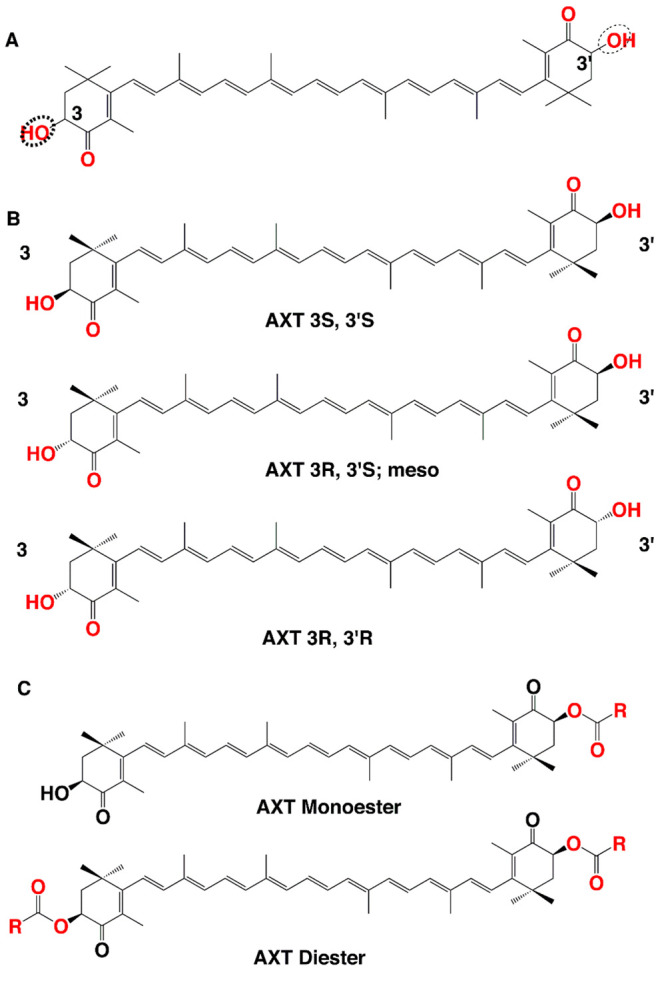
(A) Chemical structure and (B) stereoisomers of AXT. (C) Structures of AXT monoester and diester forms.
The presence of a hydroxyl and carbonyl (C=O) in each ionone ring explains features such as its polar nature and its ability to undergo esterification. Based on its source, AXT can exist in different forms such as optical R/S isomers, geometric isomers, and esterified or free forms.
Although the most predominant form of AXT in nature is the esterified form, the nonesterified form can also be found. AXT is found in three different forms based on its two hydroxyl groups: the nonesterified form (free form), monoesterified form (one hydroxyl group esterified with fatty acid), and diesterified form (two hydroxyl groups esterified with fatty acid) (Figure 2C). Various sources of AXT synthesis contain different ratios of these three forms. For instance, AXT extracted from yeast Xanthophyllomyces dendrorhous is the (3R, 3′R) isomer in the free form, while Haematococcus pluvialis biosynthesizes the (3S, 3′S) isomer in the monoesterified form predominantly (Table 1).31
Table 1. Comparison of the Physicochemical Properties of AXT from Different Sources.
| source | isomers | 3,3′-OH group modification | properties of dominant form | ref |
|---|---|---|---|---|
| Haematococcus pluvialis | 3S, 3′S | 70% monoesterified, 25% diesterified, 5% free form | high stability | (32, 33) |
| Paracoccus carotinifacience | 3S, 3′S | 100% free form | unstable, sensitive to oxidation, higher bioaccessibility | (34, 35) |
| Phaffia rhodozyma | 3R, 3′R | 100% free form | ||
| synthetic | 1(3S, 3′S), 2(3R, 3′S), 2(3S, 3′R), 1(3R, 3′R) | free form |
The ratio of stereoisomers in synthetic and natural AXT is inherently different. Synthetic AXT contains the (1(3R, 3′R):2(3R, 3′S):1(3S, 3′S)) that is the free form, whereas variable ratios of tree stereoisomers exist in natural AXT mainly in a complex with proteins or lipids, or the esterified form. The remarkable bioactivity of AXT originates from the 3S, 3′S isomer which explains a better bioavailability after dietary supplementation with natural AXT than the synthetic form.The research conducted by Yang et al. indicated that diesterified AXT with long-chain and saturated acids has more stability than other forms of AXT. They showed that the stability of AXT directly correlated with the esterification degree, length of the carbon chain, and saturated state of the fatty acid. Furthermore, the decrease in the esterification degree, the decrease in the length of the carbon chain, and the increase in unsaturation of the fatty acid of AXT are beneficial for its bioavailability. During digestion, monoesterified AXT with short-chain and unsaturated fatty acids was easily hydrolyzed. Therefore, the bioavailability of free AXT is considerably higher than that of monoesterified AXT, and that for the monoesterified form is notably greater than that of the diesterified AXT.33 After supplementation with AXT (either free or esterified), the only form found in human blood is the free form. Moreover, studies in humans demonstrated that the free form of AXT is the primary active form and has more bioavailability than the esterified form.36 It is speculated that the amount of esterified AXT at the uptake site is limited due to the need for gastrointestinal hydrolysis of these esters before absorption.37 In the purification step of the downstream process, impurities such as salts, cell debris, other carotenoids, solvents, proteins, esters, and other contaminants are separated and free natural AXT (99% purity or more) is obtained. After removal of ester groups, free AXT and its isomers easily can be analyzed by chromatographic techniques; free AXT would form useful pharmaceutical antioxidants as they can be bound to water-soluble groups.38 Mimoun-Benarroch et al. demonstrated that the absorption of esterified natural AXT from H. pluvialis is slower than free AXT from P. carotinifaciens and P. rhodozyma; hydrolysis of the esterified form in the intestinal lumen before absorption probably contributes to the decrease in the uptake process. Additionally, these esterified AXTs cannot be identified by chromatographic analysis unless their fatty acid chains are removed.39 However, some studies claim that esterification makes AXT more soluble and enhances its stability to oxidation; therefore, it can have better pharmacological properties than free AXT.40 Consequently, some researchers have carried out purification of H. pluvialis AXT and recovered a high percentage of purified free or monoester AXT.41,42 Normal-phase chromatography coupled with reverse-phase chromatography can be used in separation of free and esterified AXT (mono and diesters) in 25 min.43,44 The antioxidant activity of various forms of natural AXT is still debated. It has been claimed that free AXT is more efficient than the esterified AXT,45 while some others have reported the esterified form with better antioxidant activity.46−49 The study of Rao et al. on a skin cancer model in rats showed that esterified AXT has better antioxidant and anticancer potency than the free form.50 Also, comparing these two forms on exercise performance in mice exhibited that esterified AXT significantly promoted muscular endurance, protected erythrocytes from oxidative damage, and increased the running time.51
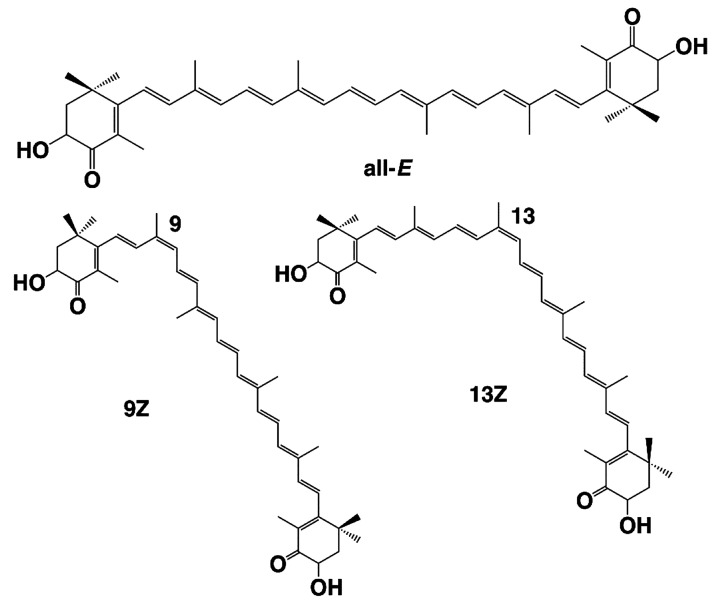
In view of the presence of several conjugated double bonds, two kinds of geometrical isomerization occur in the AXT molecule: Z and all-E isomers (Figure 3). The most representative AXT in nature is the all-E stable isomer when the carbons are located in the E positions at double bonds. Less stable but more beneficial Z isomers (a mixture of the 9Z and 13Z isomers) are obtained when AXT extracts are affected by factors such as the metal ions,52 solvents, heat, or pH of the reaction medium.53 Viazau et al. examined the isomerization of AXT under heat and overlighted conditions and in both in vitro and in vivo (H. pluvialis cells) systems. In the first 5 h of light treatment in the in vitro conditions and in the presence of methanol, both Z-isomers increased to 5% and then decreased, but during the whole period of heat treatment, the amount of accumulated Z-isomers was increased. In H. pluvialis cells, under conditions of intense light and sodium acetate, the accumulation of Z-isomers at first reached 45% and then decreased; reduction of isomers may be due to de novo synthesis of all-E-AXT and the oxidative degradation of AXT. To increase the total production of AXT in H. pluvialis cells, the presence of sodium acetate and long-term light is necessary, and to increase the production of Z-isomers, only short-term light is sufficient.54 Several studies have investigated the beneficial features of Z isomers relative to E isomers of AXT. Yang et al. have noted the selective accumulation of 13Z- AXT in human plasma with the assertion that Z isomers are more fruitful for human health.55 As the Z isomers are more soluble in organic solvents, their extraction is more efficient when Z-isomerization accelerating catalysts are added to the extraction solvent; so, they have better extractability than the all-E isomer.53 As a result of some alternations taking place in the physicochemical properties of AXT in the Z configuration, as they change from a crystalline state to an amorphous (oily) state, processes such as extraction, emulsification, and micronization are facilitated by safe and sustainable solvents.56 Higher dispersibility and solubility of AXT -Z isomers lead to higher bioaccessibility and bioavailability of this molecule; 13Z- AXT has higher bioaccessibility than 9Z- and all-E-AXT in the in vitro-digestion model.55Z-isomerization also effects the anticancer, antioxidant, anti-inflammatory, antiaging, and antiatherosclerotic activities of AXT.56 Yang et al. demonstrated higher inhibition of inflammation for Z-isomers, especially 9Z, by decreasing the expression of NK-κ, IL-8, TNF-α, and COX2 in the Caco-2 cell monolayer model.57 Better antiaging activity of 9Z- AXT was observed when the median life span of Caenorhabditis elegans, fed with it, increased by 59.39% compared to an increase by 30.43% when fed by all-E-isomers.58 All these changes in the function and activity of AXT-Z isomers are due to the altered physicochemical characteristics of this molecule. Some physicochemical properties influencing the E/Z-isomerization are the solubility, color value, stability, crystallinity, and melting point. Changes in the Gibbs free energy affect the stability of the Z-isomer, which in turn affects its antioxidant properties.59 Liu and Osawa have shown the robust antioxidant effects of the Z-isomer (especially 9Z-AXT) in highly efficient radical scavenging activity and also suppressing the production of ROS in neuroblastoma cells as well as the inhibition of induction of hydroperoxides.60 On the other hand, Yang et al., by different antioxidant activity assays, showed that 13Z- AXT has stronger antioxidant activity relative to all-E and 9Z.61Z-Isomers have a higher solubility in organic solvents, vegetable oil, and SC-CO2 which enhances their bioaccessibility. Likewise, the uptake of Z-isomers into bile acids improves, and their internalization to the Caco-2 cells by carotenoid transport proteins is more efficient (Table 2).55
Figure 3.
Structures of E/Z isomers of AXT.
Table 2. Propertes of Different Geometric Isomers of AXTa.
| property | type of isomer | type of assay | ref |
|---|---|---|---|
| antioxidant capacity | 13Z > all-E > 9Z | CAA assays (Caco2-BBe1/HT-29) | (61) |
| 13Z > 9Z > all-E | ORAC-L, PLC assays | (61) | |
| 9Z > 13Z > all-E | DPPH and lipid peroxidation assay (SH-SY5Y cells) | (62) | |
| transport efficiency | 9Z > 13Z > all-E | Caco-2 cell monolayer model | (55) |
| bioavailability/bioaccessibility | Z-isomers> all-E | oral-dosing test (human) | (63) |
| all-E > 13Z > 9Z | oral-dosing test (rainbow trout) | (64) | |
| 13Z > 9Z, all-E | oral-dosing test (human) | (65) | |
| 13Z > 9Z> all-E | digestion model (Caco-2 cells) | (55) | |
| stability | all-E > 9Z > 13Z | storage tests (heating and filtration) | (66) |
| all-E, 13Z > 9Z | pH test | (61) | |
| solubility | Z-isomers > all-E | organic solvents | (53) |
Abbreviations: ORAC-L assay, oxygen radical absorbing capacity assay for lipophilic compounds; PCL assay, photochemiluminescence assay; CAA assay, cellular antioxidant activity assay; DPPH, 2,2-diphenyl-1-picrylhydrazyl; bioaccessibility, the amount of AXT available for absorption in the gut after the digestion process; bioavailability, the amount of AXT which reaches the site of physiological activity after administration.25
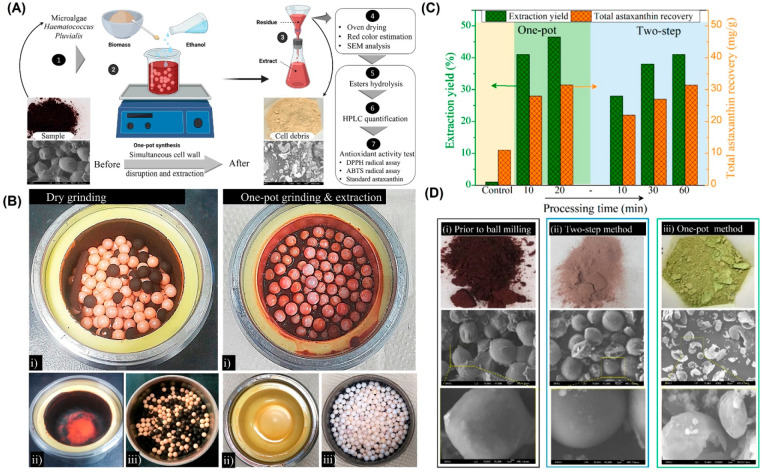
An effective downstream process reduces production costs and develops productivity. Not surprisingly, the natural AXT obtained from Haematococcus pluvialis is expensive and has only 1% of the total AXT market share while the rest goes to the synthetic counterpart.67 However, there are emerging strategies which have the potential to increase the natural AXT’s share in the market. It is known that AXT can be concentrated in Haematococcus pluvialis up to 5 wt % of its dry weight at the aplanospore stage under undesirable conditions among which high salinity, high temperature, and more light can be enumerated. On the other hand, if undesirable conditions prevail, it would culminate in the accumulation of AXT; the increase in the AXT is accompanied by the formation of a acetolysis-resistant wall around the cells with a thickness up to 2.3 μm, an impediment for the extraction process.68,69 Only 5% of AXT in the cells is in the free form and the rest is bound to fatty acids. The extraction of the free form plus its derivatives requires the rupture of the cell wall, but preserving the AXT bioactivity during the process is of vital importance, making it a remarkable challenge in the field.70 A mild one-step strategy has been reported to yield 47 wt % through the recovery of AXT from the mature cysts of Haematococcus pluvialis. In this method, the cell wall of the cyst cells is completely ruptured under mild conditions (200 rpm, room temperature, and atmospheric pressure) in a short time (≤30 min); ensuing extracts are realized using different solvents generally recognized as safe (GRAS), e.g., ethanol, acetone, n-hexane, ethyl acetate, and isopropyl alcohol). Astaxanthin recovery is the highest in ethanol, followed by that in acetone, ethyl acetate, isopropyl alcohol (IPA), and hexane. Figure 4 exhibits the optimized one-pot process together with the usual dry grinding and two-step process to make a comparison. The pretreatment to rupture the cells wall is avoided in the one-step strategy, making the process efficient relative to the previous studies.71,72 The difference between dry and wet methods is discernible in Figure 4B; the dry ball milling, which is adopted widely, causes the formation of cells debris on the balls and the chamber’s wall followed by their aggregation and, hence, is less efficient. In the case of the two-step process, an initial grinding is performed followed by the extraction via Soxhlet, supercritical fluid, or other means with a low yield, while the one-pot process allows the AXT extraction in high yield in a short time at ambient temperature.67
Figure 4.
(A) Schematic indicating the one-pot strategy for extraction of AXT from Haematococcus pluvialis. (B) Comparison between dry and wet techniques by digital camera images: (i) right after the ball milling process, (ii) of the containers wall, and (iii) of the zirconia balls after the process. (C) AXT yield for the control (freeze-dried Haematococcus pluvialis through Soxhlet extraction by acetone without applying ball milling (12 h)), one-pot strategy (up to 20 min), and two-step technique (up to 60 min). (D) Digital camera and SEM images showing the cell debris (i) prior to ball milling, (ii) after the two-step method, and (iii) after the one-pot method. Reprinted with modification from ref (67) with permission from American Chemical Society.
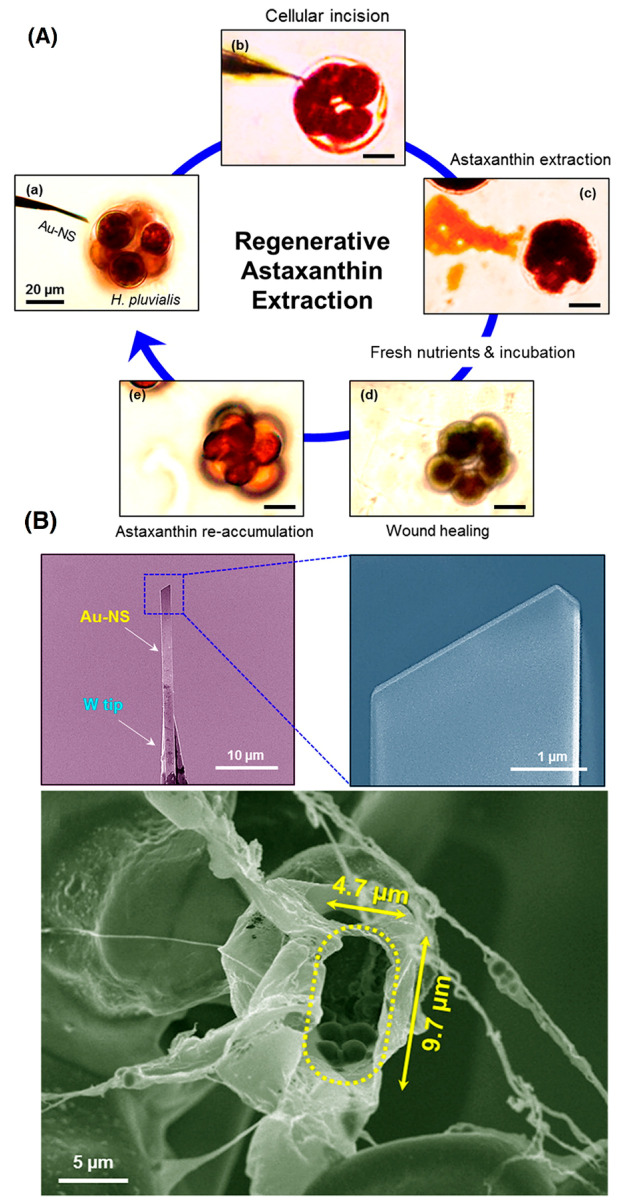
Although the one-step strategy afforded the highest amount of AXT from Haematococcus pluvialis, it is considered an invasive approach as it entails complete disruption of the algae. The biorefinery of microalgae comprised some steps such as cultivation, harvesting, and subsequent extraction, which is a costly and time-consuming endeavor. There is a noninvasive strategy that is capable of reducing both the time and cost-termed microalgae milking.73 The same as milking cows, the idea behind this process is to reuse the biomass for a prolonged production; an innovative strategy has been adopted to extract AXT multiple times from a single Haematococcus pluvialis cell. The process begins with an incision in the cell wall through a gold nanoscalpel followed by extraction of AXT and finally wound healing by providing incubation and nutrients. Importantly, the extraction is synchronized with chlorophyll leakage besides AXT. After the extraction process, the nutrient addition stopped leaking the pigments and the chlorophyll content increased again, which is vital for preserving the cellular metabolism. The relationship between chlorophyll and AXT is found to be inverse; enhancment in the AXT content up to twice that of the control groups was discerned after the first extraction process74 (Figure 5). Of course, more research is required to optimize the milking process, and it is worth researching as the process is reusable multiple times as desired.
Figure 5.
(A) Regenerative AXT extraction from Haematococcus pluvialis through the gold manipulator. (B) SEM micrographs of the gold manipulator and incised cell. Reprinted from ref (74) with permission from American Chemical Society.
Xanthophyll carotenoids, to which AXT belongs, are solubilized in the small intestine after ingestion. This process is carried out in mixed micelles which contain bile acids, phospholipids, cholesterol, and fatty acids. Then, these carotenoids enter the epithelial cells by a simple and facilitated diffusion through their cytoplasmic membranes. Once they are broken up, carotenoids are stored in the liver. They are next resecreted as very low-density lipoproteins, low-density lipoproteins, and high-density lipoproteins into the blood and transported to the tissues. The polar ends of AXT make it more readily absorbable than other nonpolar carotenoids such as lycopene. It has been shown that esterified AXT is hydrolyzed (fatty acids removed from the either ring) before being transported as low-density lipoproteins.75,76 AXT is similar in structure to the β-carotene, with the former having 13 conjugated double bonds, whereas the latter has 11; the ability of carotenoids to neutralize free radicals enhances with increasing conjugated double bonds and the presence of a functional group in its terminal rings.77 Polar AXT spans the membrane, with its polar end groups extending toward the head regions of the membrane bilayer. As a result, AXT stops free radical chain reactions and scavenges lipid peroxyl radicals and ROS (endogenous ROS) on the membrane surface, while its polyene chain can trap ROS in the interior of the membrane.78 The toxicity and efficacy of soft capsules of oil-based AXT have been evaluated by Satoh et al. According to this analysis, no safety issues have been observed while the metabolic syndromes were improved. The United States Food and Drug Administration and the European Food Safety Authority have approved AXT as a dietary supplement, a food ingredient, and an additive. Until now, the AXT extracted from H. Pluvialis and P. carotinifaciens has been authorized for human consumption at dosages ranging from 12 to 24 mg and 6 mg per day, respectively, for up to 30 days.79,80
3. AXT Function in the Human Body: Antioxidant Activity and Signaling Pathways
AXT has exhibited prooxidant properties. It is known that the low ROS amounts are advantageous for gene expression, cellular signaling, and the stimulus of antioxidative defense mechanisms.81 Several studies have demonstrated that AXT is more potent than beta-carotene in scavenging free radicals induced by internal (inflammation, aging, stress, and cancer, among others) or external sources (cigarette smoke, pollutants, UV radiation, etc.)82,83 and conserves unsaturated fatty acid methyl esters by preventing peroxidation. Besides, AXT esters have shown high antilipid peroxidation activity.84,85 The health-promoting impact of AXT on many diseases has been demonstrated in several studies wherein the promising therapeutic effects of AXT were highlighted.86,87 AXT strengthens and modulates the immune system and increases antibody production in a T helper-dependent manner. Thus, it raises the number of antibody secretory cells from spleen cells and the production of immunoglobulins by blood cells.88,89 Its very strong antioxidant activity may have protective impacts on the cardiovascular system.90 Coombes et al. demonstrated that AXT has no effect on enhanced inflammation, oxidative stress, and arterial stiffness in renal transplant recipients.91 Other studies have suggested that AXT has immense effects on cardiac function, buildup joint strength, exercise performance, and postexercise recovery.92 Also in heart failure patients, three month consumption of AXT has an antioxidative stress effect and improves exercise tolerance and cardiac contractility.93 The antitumor effects of AXT including anti-inflammation,94 antiproliferation,95 antioxidation,96 and increasing apoptosis95 have been confirmed in many in vivo and in vitro studies. It also improves the functioning of the brain and can reduce or prevent brain diseases, such as Parkinson’s disease, autism, and Alzheimer’s disease.97,98 AXT reduces wrinkles on the skin and prevents age spots, improves skin’s elasticity, and reduces ultraviolet damage due to sun rays, hence acting as an internal sunscreen.99,100
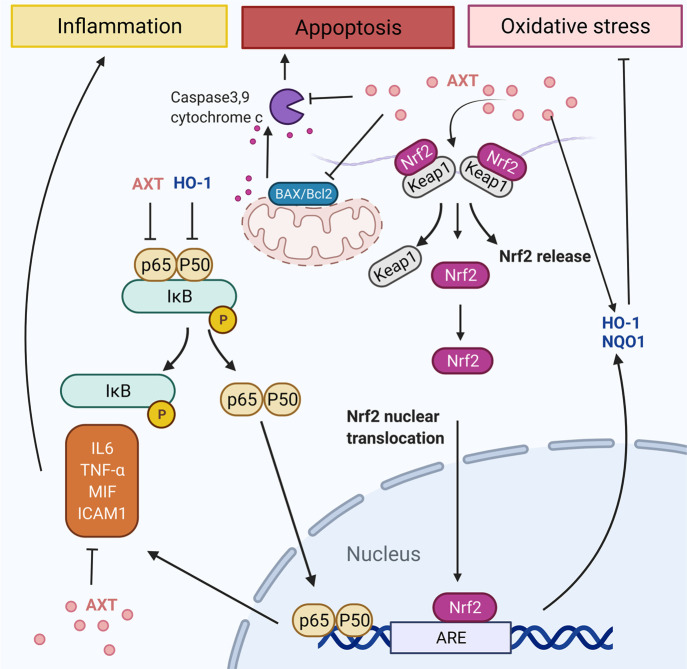
AXT has a significant role on the signaling pathways of inflammation, oxidative stress, and reactive oxygen-dependent apoptosis by interrupting their signaling pathways in neurodegeneration and ocular and skin-related damage.101,102 Though ROS have a significant role in neuronal signaling and function, unwarranted generation of ROS is lethal for neural cell function, with permanent oxidation. AXT showed neuroprotective effects by reducing intracellular ROS and preventing mitochondrial H2O2 generation.103
AXT can prevent inflammation by inhibiting the release of interleukins (ILs), tumor necrosis factor-alpha (TNF-α), and intercellular adhesion molecule 1 (ICAM1) as shown in Figure 6.101,104 The anti-inflammatory properties of AXT were due to its inhibition of the TLR4 pathway beyond TLR4/MyD88/NF-κB pathway regulation,105 downregulation of TLR4 and MyD88 expression, and inhibition of TLR4/MyD88/NF-κB pathway activation, which has a considerable role in regulating burn-induced renal tissue inflammation.106 AXT has ocular anti-inflammatory assets by impeding the NF-kB signaling pathway over suppression of TNF-α, NO, and PGE2 generation.107 Moreover, AXT suppressed the choroidal neovascularization by downregulation of ICAM-1, macrophage-derived VEGF, MCP-1, and IL-6 as inflammatory mediators.108 Also, it can effectively support additional tissue protection by maintaining the oxidant/antioxidant balance associated with its unique structure.109
Figure 6.
Schematic illustration of AXT’s role on signaling pathways of inflammation, oxidative stress, and apoptosis by interrupting their signaling pathways.
To block the oxidative stress, AXT activates Nrf2/antioxidant response elements (Nrf2/ARE), inhibits the phosphorylated extracellular regulated protein kinase/extracellular regulated protein kinase ratio (p-ERK/ERK), and increases the release of NAD(P)H quinine oxidoreductase-1 (NQO-1) and heme oxygenase-1 (HO-1). It has been shown the Kelch-like ECH-associated protein 1 (Keap1)-Nrf2-ARE has a critical function in the antioxidant response of cells.101 AXT antioxidant mechanisms additionally include regulating the PI3K/Akt signaling pathway.110 AXT acting as a shield for photoreceptor cells from oxidative stress reduced apoptosis due to stimulation of the PI3K/Akt/Nrf2 signaling pathway at hyperglycemia conditions. AXT diminished the retinal ganglion cells and Muller cell damage via enhanced HO-1 production. Various signaling pathways are incorporated for increasing the cellular resistance toward oxidative stress. In this way, the Nrf2-ARE pathway plays an essential role and maintains cell function (Figure 6).101,111 One transcription factor attached to the ARE is Nrf2, which encourages Phase II enzyme expression. The interaction of Nrf2 with chaperone Keap1 occurs at the lack of oxidative damage. Contrariwise, in oxidant conditions, Nrf2, detached from Keap1, as its activated form, and translocated to the nucleus, attaches to the ARE and stimulates Phase II enzyme expression, for instance, heme oxygenase-1 (HO-1) and NQO1.81,104
AXT shows prooxidant properties and can create trace quantities of ROS instead of quenching them, which activates the expression of HO-1 and adjusts the GSH-Px expression and activity via the ERK-Nrf-2/HO-1 signaling pathway.81 This generated ROS was innocuous to the cells because pristine AXT endorsed proliferation of cells and improved the activity of GSH-Px and SOD enzyme and showed protective effects against H2O2-induced oxidative stress in HUVECs and reduced the ROS production induced by H2O2.81
Additionally, activation of Nrf2 can support the survival of retinal pericyte. AXT can activate the Nrf2-ARE pathway, thus enhancing the HO-1 and NQO1 expression and decreasing oxidative damage with protective effects from elevated glucose-induced apoptosis in photoreceptor cells (Figure 6).124
AXT’s role against apoptosis was ascertained by blocking caspase3,9 as shown in Figure 6, as well as cytochrome c, p-ERK/ERK, and the Bax/Bcl2 ratio.101,111 AXT has therapeutic effects in ischemia-reperfusion injury of the spinal cord and induced oxidative stress and neural apoptosis by PI3K/Akt/GSK-3β signaling pathway activation.112 The PI3K/Akt/GSK-3β signaling pathway showed neuroprotective function by inhibiting apoptosis and stimulating proliferation of the cell.113
4. Brain Delivery of AXT as a Neurological Drug-Therapy Agent
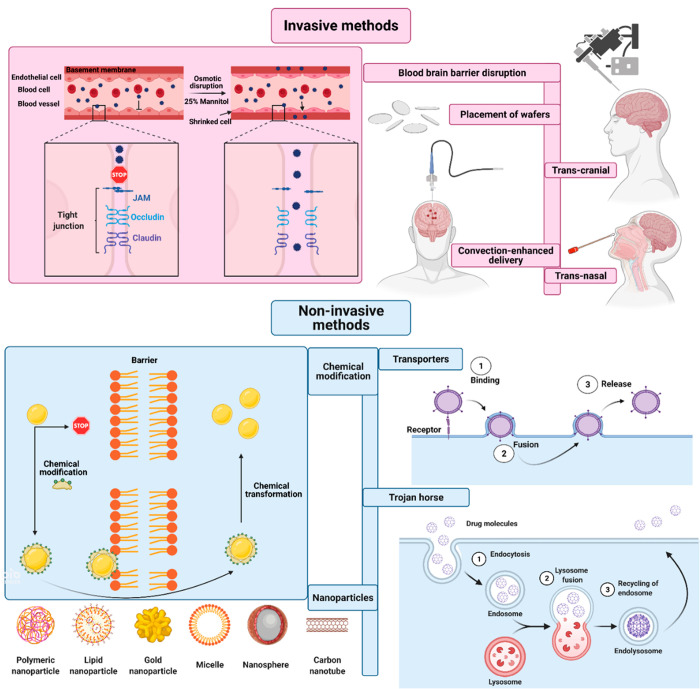
4.1. Blood–Brain Barrier, Anatomy, and Delivey Systems
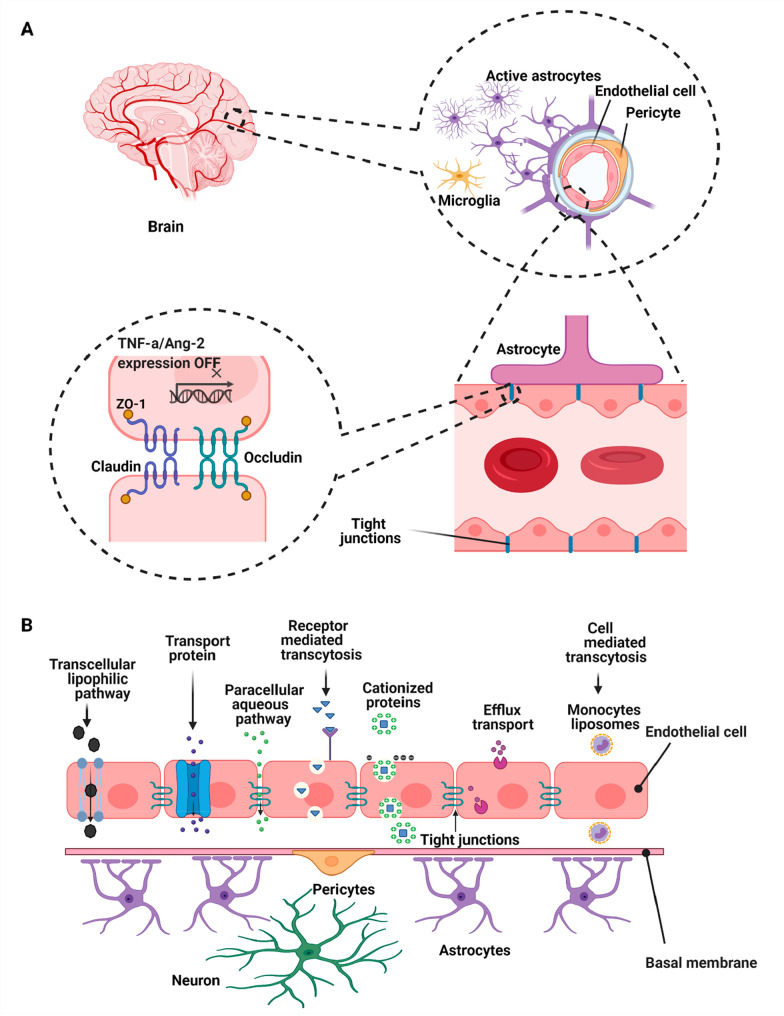
The central nervous system (CNS) contains the brain and spinal cord, with the latter being located inside the spine. It is separated from other parts of the human body through the blood–brain barrier, which is the boundary between the extracellular fluid of the brain in the CNS and the circulatory blood flow in the body (Figure 7A).114 This barrier is made up of specialized capillaries that, unlike the normal structure in capillaries, do not have the usual pores and have a tight intercellular connection. Thus, many molecules cannot pass through them through diffusion and reach the cerebrospinal fluid in the brain.115−118 The endothelial surface of these capillaries is covered with special proteins that allow glucose to enter the brain as well as the exchange of gas between the circulating blood and the brain from the barrier.119 This barrier results from tight junctions between endothelial cells in the CNS artery and restricts the passage of solutes and substances.120 The CNS is capable of activating the immune system in response to several forms of injury including trauma, infection, stroke, and neurotoxins.
Figure 7.
(A) Brain endothelial cells form the cellular barrier and are connected continuously by the means of tight junctions; tight junctions are the main structures of the blood–brain barrier and selectively transfer nutrients between the blood and the brain. The role of the pericytes is controlling the cerebral blood flow while astrocyte end feet are responsible for biochemical support of the endothelial cells.122 (B) Various strategies for diffusion through the blood–brain barrier.127
Neuronal inflammation ensues for a variety of reasons, including infection, concussion, toxic metabolites, deformed proteins, and autoimmunity. Microglia (innate immune cells in the CNS) are activated in response to these factors and initiate the inflammatory process in nerve tissues. Although this response is initiated to protect nerve tissue against infection, it can lead to damage of nerve cells with the occurrence of neurological diseases, if the response is severe and not well controlled.121
Many drugs cannot penetrate through brain cells and thus preclude a therapeutic effect in brain-based diseases. Thus, the following promising strategies have been introduced for drug delivery to the brain.
-
(1)
Transient permeability enhancement in the blood–brain barrier: Disconnection of tight junctions between endothelial cells using ultrasound/microbubbles and osmotic pressure changes, but this method allows uncontrolled entry of nanoparticles into the cell which disrupts the brain’s homeostatic function, causing brain toxicity.123
-
(2)
Diffusion of small lipophilic molecules (<400 Da) through endothelial cells in two forms: paracellular and transcellular.124 Tight junctions hinder the diffusion of hydrophilic or lipid insoluble molecules via paracellular transport. Due to the lipid nature of liposomes and deformable liposomes (solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs)), it is possible for them to pass through the phospholipid bilayer of the BBB endothelial cell membrane by lipid-mediated free diffusion (facilitated diffusion) or lipid-mediated endocytosis.
-
(3)
The transcytosis pathway through absorption, receptors, and various carriers (Figure 7B). In absorptive transcytosis, transfer begins by creating an electrostatic interaction between a positively charged particle and a negatively charged plasma membrane. This pathway is not specific to the brain and is also found in the liver, kidneys, or lungs. In one study, nanoparticles have been prepared using a polylactide polymer bound to a PEG polymer, and the results showed successful adsorption of the generated nanoparticles; the presence of PEG is intended to improve the performance of the formulation and increases the shelf life of the nanoparticles.125,126
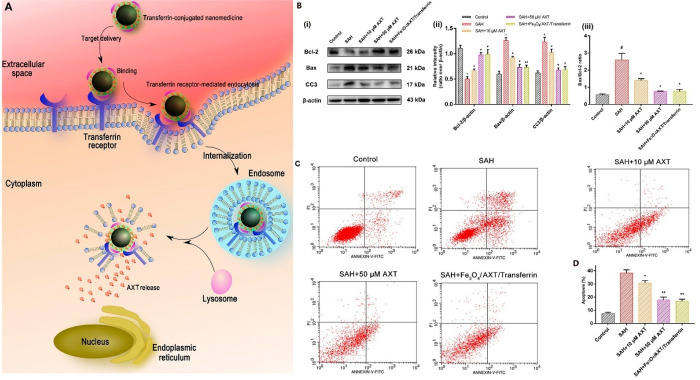
In receptor-mediated transcytosis, various ligands are placed on the surface of a nanoparticle that binds to cell surface receptors and is endocytosed by the cell, with receptors and transporters being used as targets, including GLUT1, LfR, and TfR.128,129 One of the most effective techniques is the use of transferrin, which is highly expressed on the blood–brain barrier and facilitates the nanoparticle’s penetration through the barrier.130 A recent study took advantage of transferrin to facilitate the penetration of Fe3O4-polyethylene glycol-encapsulated AXT nanoparticles through the blood–brain barrier for subarachnoid hemorrhage treatment. Transferrin ligand is comprised of two domains, one of which is α helixes and the other of which is ß sheets, and this ligand has high affinity toward its receptor. The cellular uptake of transferrin-conjugated nanoparticles through primary cortical neurons is significantly better than the nonmodified nanoparticles. Moreover, after exposure to oxyhemoglobin, which provides ROS, the neuronal survival gets improved and the apoptosis markers are reduced because of the AXT release.131Figure 8 illustrates the efficiency of transferrin-modified and -nonmodified nanoparticles for subarachnoid hemorrhage.
Figure 8.
(A) Schematic of the entry mechanism of transferrin-modified and -nonmodified nanoparticles to neurons through receptor-mediation followed by the degradation of nanoparticles and AXT release. (B) Assessment of neural damage after exposure to oxyhemoglobin for pure AXT and the transferrin-modified AXT-loaded nanoparticles as follows: (i) Western blots, (ii) relative intensity analysis of Bax/ß-actin, Bcl-2/ß-actin, and cleaved caspase-3 (CC3)/ß-actin, and (iii) Bax/Bcl-2 ratio for different samples. (C) Cell apoptosis results after oxyhemoglobin exposure. (D) Apoptotic ratio of cells related to each group. #p < 0.05 vs control group; p < 0.05 vs subarachnoid hemorrhage (SAH) group; p < 0.01 vs SAH group. Reprinted from ref (131) with permission from Frontiers.
Various strategies have been developed to increase the permeability of drugs through the blood–brain barrier.132−134 There are mainly two types of drug delivery to the brain, one of which is invasive and the other of which is noninvasive. Invasive methods, such as intracerebroventricular injection, osmotic and ultrasound disruption of the blood–brain barrier, and convection-enhanced delivery, help to deliver the drug directly to the desired location in the brain. Using the intracerebroventricular injection method, the drug is injected directly into the cerebrospinal fluid.135−137 The convection-enhanced delivery method is used to facilitate targeted drug delivery to brain tumors. In this procedure, a small hole is made in the patient’s skull to set one or more thin tubes (cannulas) to the tumor site from different angles. Then the drug is pumped into the tumor through a cannula. In ultrasound technology, the microscopic bubbles are injected into the bloodstream. Using an MRI scan, the injection is given exactly in a specific area of the brain. Then the ultrasound is transmitted to the same point through a cap placed on the head. These waves vibrate the bubbles, helping to open the tight junctions slightly, and allow the drugs to enter the brain through the created pathway.138−140 Also, different mechanisms that include Aβ deposition in cerebrovascular cells (Figure 9) could increase the AXT-efficiency to reduce the side effects of some drugs as well as increase the expression of some types of necessary genes in the brain.141 Osmotic disruption is an invasive route by which hypertonic fluids cause shrinkage of the endothelial cells of the cerebrovascular artery followed by the disruption of tight junctions of the blood–brain barrier (Figure 10).127,142 Another route of drug administration to the brain is by inhalation, but due to the limited absorption level of the olfactory lips, inappropriate amounts of drug molecules may reach the target;117,143,144 the success rate of drug delivery through these methods has been found to be inefficient.145,146 Opening tight connections with osmotic pressure can cause generation of toxins and other unwanted substances to enter the brain in addition to drugs. For this reason, more research has moved toward noninvasive methods. By increasing the lipophilicity of small drug molecules, the possibility of their transfer into the brain increases. As lipophilicity is enhanced, the metabolism and distribution of the drug in the body also increases, which in turn increases the dose of the drug, thus enhancing the side effects.147−149 Large molecules, such as peptides, proteins, or genes, are unable to cross the blood–brain barrier. In addition, these compounds have little stability in the environment, so they are rapidly metabolized and are not released into the brain. Moreover, many drugs, which have optimized molecular weight and lipophilic properties, pass through the blood–brain barrier naturally and easily, but they are quickly returned to the bloodstream by very strong outward pumps.147 The use of nanotechnology to enhance drug delivery to the brain without damaging the blood–brain barrier can be useful in this context and promising for the treatment of brain diseases.150,151 For example, a Trojan horse trick has been used to counteract drug resistance wherein the drug is hidden inside a DNA capsule and enters the cell like a Trojan horse and prevents the drug from being drained by the cell.152 Further, drug-carriers can also bind specifically to receptors on the endothelial cells and enter the brain parenchyma by receptor-mediated transport.153 Two important and effective advantages of nanotechnology-assisted delivery to the target organ are the enhanced efficacy of the drug and the reduction of side effects to other organs. Today, different types of metal, lipid, and polymeric nanoparticles have been used in drug delivery to the brain.154,155 In neurodegenerative disease, the alteration of the blood–brain barrier and the size of nanoparticles are important factors affecting the release of nanoparticles into the brain parenchyma. Evaluation of nanoparticle toxicity on neurons in clinical and in vivo environments is one of the most important challenges pertaining to the deployment of nanotechnology.156,157
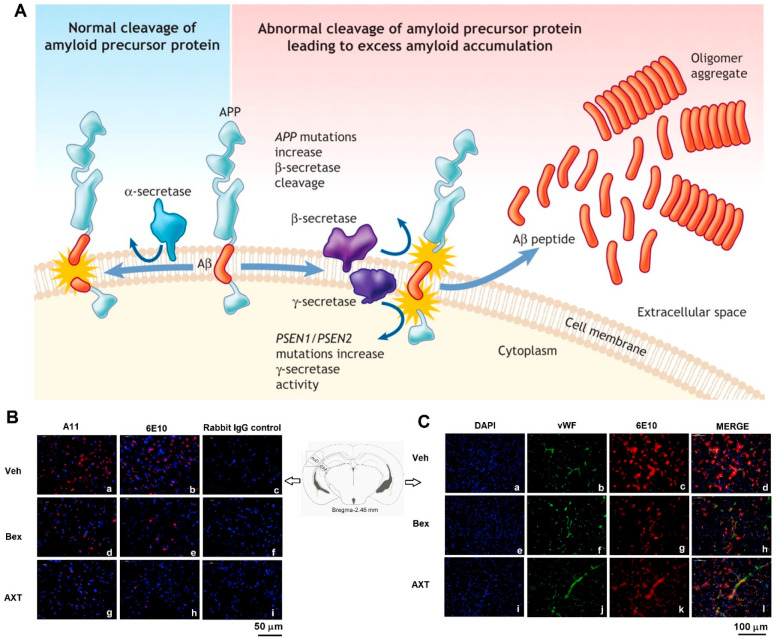
Figure 9.
(A) As a transmembrane protein, the amyloid precursor protein (APP) undergoes a series of proteolytic cleavages by secretase enzymes. It is not amyloidogenic if APP is cleaved through α-secretase in the middle of Aβ, but the cleavage through β- and γ-secretase enzymes is accompanied by the release of neurotoxic Aβ peptides which can accumulate into an oligomer aggregate. The APP gene mutations prevent the cleavage through α-secretase followed by enabling the preferential cleavage through β-secretase. Mutations in the presenilin-1 and presenilin-2 genes (PSEN1 and PSEN2), which are regarded as the components of the γ-secretase complex, raise the cleavage through γ-secretase at this site. Notably, both situations result in the production of excess Aβ peptide. Over time, the oxidative stress causes neuronal death followed by the development of neuritic plaques typical of Alzheimer’s disease. Reprinted from ref (158) with permission from CMAJ. (B) Immunofluorescence staining was conducted on 18 μm sections of the mouse brain. (C) Immunofluorescence double staining was conducted on 18 μm sections of the mouse brains. Vehicle (Veh), bexarotene (Bex), and astaxanthin (AXT). Reprinted from ref (141) with permission of Elsevier.
Figure 10.
Schematic illustration showing invasive and noninvasive approaches used for drug delivery into the brain.127
4.2. Neurological Diseases and Role of AXT
4.2.1. Oxidative Stress and Its Assorted Roles in Neurodegenerative Diseases
Oxidative stress is an imbalance between free radicals and the antioxidants in the body resulting in the generation of ROS. Oxidative stress plays an important role in the development and progression of many degenerative diseases such as autoimmune diseases, cancer, heart disease, and diabetes. Notably, AXT plays a very specific role in neurodegenerative inflammatory diseases such as Alzheimer’s, Parkinson’s, Huntington’s, amyotrophic lateral sclerosis, multiple sclerosis, and other processes related to pathological aging.159,160 With the increase in life expectancy, the prevalence of neurodegenerative diseases is also increasing, which have various symptoms such as altered mitochondrial function, abnormal accumulation of proteins and proteasomes, and reformed iron metabolism affecting different parts of the brain which can lead to a defective cycle and the onset of cell death.161 Factors that produce ROS can damage mitochondria, increase Ca2+ levels, inhibit proteasome function, and ultimately lead to neuronal destruction. For physiological reasons, the CNS is believed to be highly sensitive to oxidative stress. The human brain makes up only a small percentage of the total body weight; however, the brain consumes 20% of its basic oxygen consumption. The major ROS involved in the destruction of neurons are superoxide, hydrogen peroxide, and highly active hydroxyl radicals.162 Nitric oxide as a high-diffusion biological messenger plays an important role in the physiology of the central nervous system. After production, nitric oxide reacts rapidly with superoxide to produce strong peroxynitrite (ONOO–) and hydroxyl radicals; ROS and reactive nitrogen species collectively cause oxidative stress in the nervous system. The CNS is a reservoir of unsaturated lipids that are highly vulnerable to peroxidation and oxidative changes. The double bonds in unsaturated fatty acids are critical sites for attack by free radicals that trigger a chain reaction, thus inflicting damage to their adjacent unsaturated fatty acids.163 The brain’s antioxidant defense system is not adequate enough; brain tissue has relatively lower antioxidant activity than other tissues; for example, the brain has 10% of liver’s antioxidant activity.164
4.2.2. Inflammation and Brain Diseases
Inflammation in the brain is known as nerve inflammation and can be caused by messages from destroyed neurons in the nervous system, invading germs such as viruses and bacteria, harmful chemicals, and also the deformed proteins (such as beta-amyloid peptides) in the brain.165 Two major mechanisms that cause inflammation in the brain are
-
(1)
peripheral inflammation that occurs in the body and can stimulate the brain’s immune system to cause inflammation in the brain tissue and
-
(2)
direct cellular damage to the brain that can trigger inflammation processes.166 Neuritis is seen in many pathological conditions such as stroke, infection, and neurodegenerative disorders.167 This process is characterized by activation of microglia, increased permeability of the blood–brain barrier, and peripheral immune cell permeability to brain tissue, sequestration of inflammatory cytokines, and ultimately the failure to control inflammation with neuronal injury and death. These processes are not only affected by microglia but also by astrocytes, neurons, and endothelial cells of the brain blood vessels, T cells, and peripheral aliens.168 Microglia is part of the immune system and acts like macrophages in other tissues, accounting for ∼10–15% of the brain’s cell population.168 In neurodegenerative diseases, microglial cells resemble the M1 phenotype of peripheral macrophages and produce harmful environments for neurons by producing inflammatory cytokines (TNF-α, IL1β, IL-6, NO) and ROS.169
4.2.3. Effect of Inflammation-Promoting Factors on Cerebrovascular Endothelial Cells
Peripheral inflammation can affect the brain in several distinct ways. Bacterial lipopolysaccharides are a classic example of the pathogen-associated molecular patterns of pathogen recognition and inflammatory signaling that stimulate the innate immune system.170,171 Lipopolysaccharides target cells, express CD14 and TLR4, and by activating intracellular cascades, eventually lead to activation of transcription factors including NFKβ and AP1.172 These factors are transmitted to the cell nucleus and transcriptionally trigger inflammatory factors. The activities of iNOS, COX2, and NADPH oxidase are increased, resulting in enhanced production of NO, PGE2, ROS, inflammatory chemokines, and pro-inflammatory cytokines in cerebral vascular endothelial cells.173,174 This activates microglia and stimulates astrocytes and initiates inflammatory cascades in the brain tissue. By increasing the expression of adhesion molecules and damage to the blood–brain barrier during inflammation, peripheral macrophages can also enter the brain tissue and promote inflammation in the brain.175,176 Systematic injection of lipopolysaccharides also enhances the production and release of aldosterone which overactivates mineralocorticoid receptors in cerebrovascular endothelial cells, thus intensifying the production and release of proinflammatory cytokines.177,178 Inflammatory mechanisms that are triggered by damage to brain tissue cells vary, sometimes due to genetic defects and in most cases due to unknown factors, wherein neurodegenerative or autoimmune diseases can play a role. For example, the amyloid-beta peptide, which accumulates in the brain in Alzheimer’s disease, can stimulate inflammatory processes in brain tissue. Other causes of neuritis include stroke, head injury, and direct infection of the brain tissue.178 The notion that there is a link between systemic inflammation and dementia first emerged when an increase in inflammatory processes had been observed in post-mortem Alzheimer’s patients. Studies have shown a link between dementia and elevated cytokine levels such as IL-1β, acute phase reactive protein, TNFα, and IL-6.179 Furthermore, laboratory studies have shown that the serum and cerebrospinal fluid of Parkinson’s patients have higher levels of IL-1β, TNF-α, and IL-12 as well as CD4+ and CD8+ lymphocytes, which indicate the activation of peripheral lymphocytes.180 The activity of microglia produces large amounts of free radicals, including superoxide, hydrogen peroxide, hydroxyl radicals, and cytokines with cytotoxicity, which damage neurons.181,182
4.2.4. AXT and Brain Protection
The pathways of inflammation, oxidative stress, and apoptosis cause the destruction and death of neuronal cells and eventually result in neurodegenerative disorders.143 Several direct and indirect mechanisms have been proposed regarding the positive effects of antioxidants on cognitive function improvement as they can affect cognitive function through reduced inflammation, NF-κB regulation, and reduced cytokine production. AXT is a powerful antioxidant with restorative, antiseptic, antiaging, and anti-inflammatory properties and is being used in the treatment of many neurological diseases such as neuropathic pain, Alzheimer’s disease, Parkinson’s disease, autism, depression, etc.183 Its unique chemical structure allows it to easily cross the blood–brain barrier and reach the brain, which is the most important target organ for AXT. The ability of AXT to regulate the immune system, reduce inflammation, and treat neurodegenerative diseases has been confirmed.184 There have been reports of increased production of IL-6 in the progression of multiple sclerosis disease,185 which causes demyelination and neuroinflammation due to its destructive effect on the blood–brain barrier. In a study, it has been found that AXT crosses the blood–brain barrier easily, allowing the carotenoid to protect the CNS against chronic and acute neuronal damage.98
Th1 cytokines are involved in the development of MS, and AXT modulates the response of the immune system by shifting the Th1 to Th2 cell response.186 According to the obtained data, it has been concluded that AXT, as an oral supplement, has an effective role in the prevention, healing, and reduction of inflammation and neuronal damage caused by multiple sclerosis. The potential of AXT to reduce ischemic damage in the mammalian brain through preventing apoptosis and suppressing ROS has been reported;187 it protects against injuries caused by high blood pressure, vascular oxidation, and cerebral thrombosis. Moreover, AXT prevents nerve damage and reduces the risk of stroke by suppressing the ROS and activating the Nrf2-ARE route. Therefore, it may be useful for ischemic susceptible patients to have a protective effect against neurological disorders caused by the toxicity of free radicals.188 Accumulation of amyloid-β peptide oligomers decreases the expression of type-2 ryanodine receptors and enhances the production of mitochondrial ROS, which ultimately lead to neuronal cell death and Alzheimer’s disease. AXT is capable of protecting nerve cells against the harmful effects of amyloid-β peptide oligomers by regulating type-2 ryanodine receptor gene expression and thus can be useful in treating Alzheimer’s disease.103 This red carotenoid significantly reduces the levels of amyloid-β peptide oligomers, TNF-α, nitrite, and AChE, the oxidative stress, and the activities of GSK-3β and IRS-S307 in the hippocampus and prevents the insulin resistance of the hippocampus involved in Alzheimer’s disease.189 A study has shed light on the capability of AXT as a protective agent against progressive Alzheimer’s disease. Pure AXT and its combination with docosahexaenoic acid have been administered to APP/PSEN1 double transgenic mice up to 2 months. The results revealed that the combination had a stronger effect on the regulation of oxidative stress, inflammasome expression and activation, plus reduction of Tau hyper-phosphorylation, and suppression of neuroinflammation in mice than the pure AXT by itself.190
High glycosylated hemoglobin levels, acute phase reactive protein, IL-6, and TNF-α increase cognitive impairment in depressed diabetic patients.191 On the other hand, several clinical studies suggest that mood disorders can be a risk factor for Alzheimer’s disease.192 Recent studies propose that preventing inflammatory reactions in the brain and reducing nerve damage can reduce depression in diabetic mice.193 Therefore, reducing inflammatory cytokines appears to be effective in the pathophysiology and treatment of the depressive disorder.194 In many studies, natural ingredients have been studied as supplements to improve mood and reduce anxiety and stress by inhibiting inflammation.195 Animal studies revealed that the severity of depression has been reduced when mice were treated by oral AXT (25 mg/kg) for 10 weeks.196 Also, in some studies, daily intake of 0.2 mg of shrimp oil supplement containing AXT for 7 weeks improved the learning, working memory, and depression.197 An increase in survival and proliferation of human adipose-derived stem cells has been observed when AXT is used. The use of AXT can increase the transplantation efficiency of human adipose-derived stem cells in the treatment of MS, which is a debilitating disease of the brain and spinal cord (central nervous system).198
5. Ocular Delivery System for Medicinal Use of AXT
5.1. Eye Physiology, Diseases, and Challenges
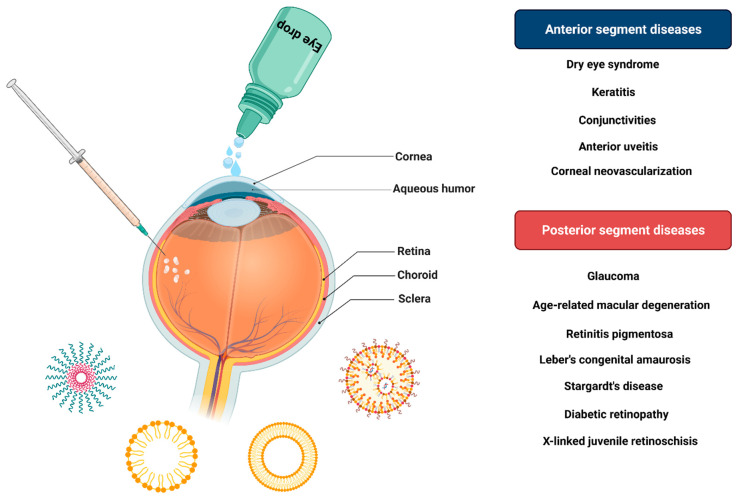
Medications used for eye diseases often affect the surface of the eye or its anterior part. The treatment of some diseases such as glaucoma, retinitis pigmentosa, leber congenital amaurosis, stargardt, x-linked juvenile retinoschisis age-related macular degeneration (AMD), and diabetic retinopathy is related to the posterior or back of the eye. Some anatomical structures, including the cornea, sclera, conjunctiva, and retinal epithelium pigment, challengingly limit the effectiveness of the drug delivery to this portion of the eye.199,200 Due to protective mechanisms such as tearing and reflex blinking, a small percentage of the prescription drug can be absorbed. Tears wipe away microorganisms and waste materials and even remove drugs from the surface of the eye. Besides, a part of the drug binds to the protein in the tears and thus becomes inactive.201 The presence of tight junctions in the corneal epithelium restricts drug delivery to the eye. Because of the 3-layer cornea and also its lipophilic and hydrophilic properties, the drugs that are designed to pass those barriers can reach the target.202,203 The eye contact time is about 5 min, which only accounts for about 5% of prescription drugs.204 Repeated administration may compensate for the short duration of drug exposure to the cells of the eye, but it may increase the risk of cytotoxicity. Besides, intraocular injection of the short-lived drugs for posterior diseases of the eye is problematic because repeated injections increase the risk of eye-bleeding.205 About 40% of the drugs studied for the treatment of eye diseases are low-water-soluble and lipophilic drugs. As a result, it is not possible to use them in the usual formulations with an aqueous base. Therefore, biocompatible and biodegradable nanoparticles are selected for intraocular administration to have an acceptable shelf life and adhesion ability to the mucous membrane (Figure 11).206−208 The results of in vivo studies have revealed that the nanoparticles have bioadhesive ability which increases the drug’s shelf life and enhances the drug uptake. The use of biodegradable polymers is also a very suitable method for drug delivery to the posterior areas and treatment of chronic eye diseases. By optimizing the surface of nanoparticles, the bioavailability and shelf life of drugs in the eye can be improved.
Figure 11.
Diseases related to different parts of the eye and various methods of drug delivery to the eye.
5.2. AXT for Ocular Diseases
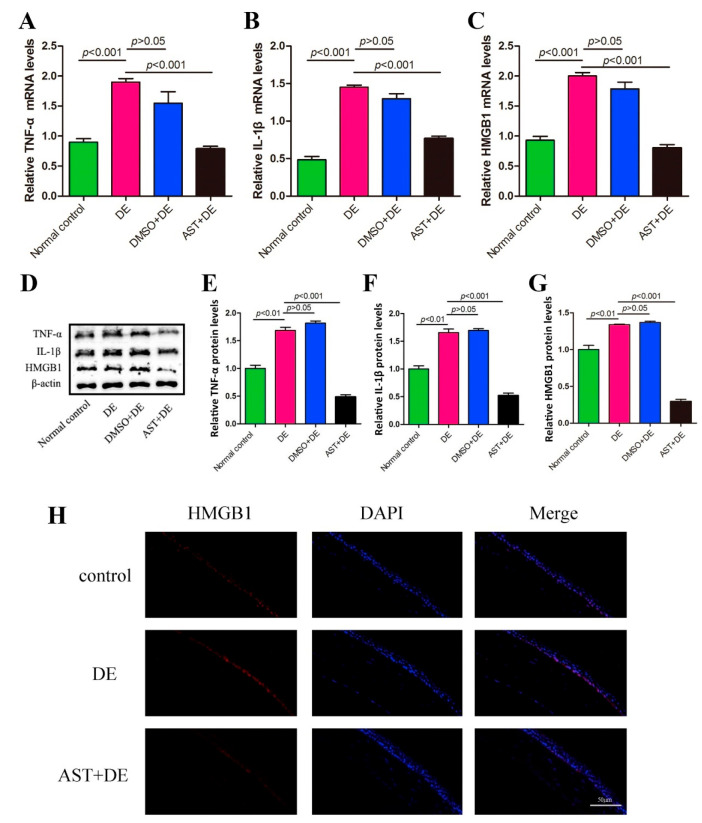
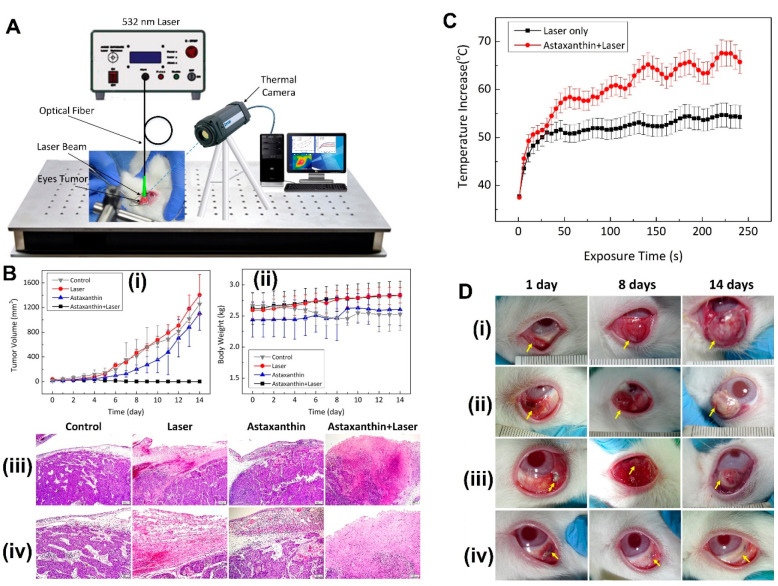
AXT helps protect retinal cells against oxidative damage and UV light and relieves symptoms of eye fatigue108,209 with validation that AXT inhibits ROS production and retinal cell death.210 Retinal ischemia increases NF-κB production and induces retinal inflammation.211 In retinal diseases, glia cells play an essential role in inflammation by producing inflammatory cytokines such as IL1β and TNFα.212 These cytokines activate transcription of COX2 and iNOS genes, leading to the synthesis of NO and PGE2, which are inflammatory mediators.213 AXT inhibits NF-κB activation and expression of COX2 and iNOS.214 The topical use of AXT limits the damage caused by the effects of ultraviolet radiation, and also the level of apoptotic cells was significantly lower in the irradiated coronas treated with AXT eye drops; it is reportedly more effective in protecting the ocular surface from UV than the systemic injection.215 Nonetheless, AXT reduces inflammation in the retina via reduction in the expression of TNF and IL1β.186 This antioxidant reduces apoptosis in retinal ganglion cells as well as retinal pigment epithelium by increasing the expression of p-Akt, p-mTOR, and Nrf2. It also decreases the expression of caspase-3, thus preventing glaucoma and AMD.210,216 AXT has a protective effect on the retina and treats injuries caused by the elevated intraocular pressure217 and hence inhibits the glaucomatous retinal degeneration.209 Nowadays, drug macromolecules as angiogenesis inhibitors including aflibercept, pegaptanib, and ranibizumab with molecular weights of 97, 50, and 48 kDa, respectively, are AMD’s first treatment. These drugs target the endothelial vascular growth factor, which is associated with choroidal neovascularization during AMD.218 For efficient delivery of biomolecules to the posterior segment, intrauterine injections are often performed, which have disadvantages such as eye infections, patient discomfort, high intraocular pressure, and retinal artery occlusion.219 Since macromolecule drug delivery is still in its infancy, alternative delivery strategies are much sought after. Notably, considerable attention is now focused on ocular delivery of small drugs. AXT as a small molecule can be used to treat eye diseases, especially AMD, which must target the posterior part and cross the barriers.220 Besides, AXT could be a potential agent to reduce the ocular inflammation mediators in mice through the mRNA exprssion of TNF-α, IL-1β, and HMGB1 as well as the protein expression of TNF-α, IL-1β, and HMGB1 (Figure 12).221
Figure 12.
(A–C) mRNA expression of TNF-α, IL-1β, and HMGB1. (D–G) Protein expression of TNF-α, IL-1β, and HMGB1. (H) Fluorescence images showing the expression of HMGB1 in the corneal epithelium. Reprinted from ref (221) with permission from Elsevier.
5.3. AXT Delivery for Ocular Health
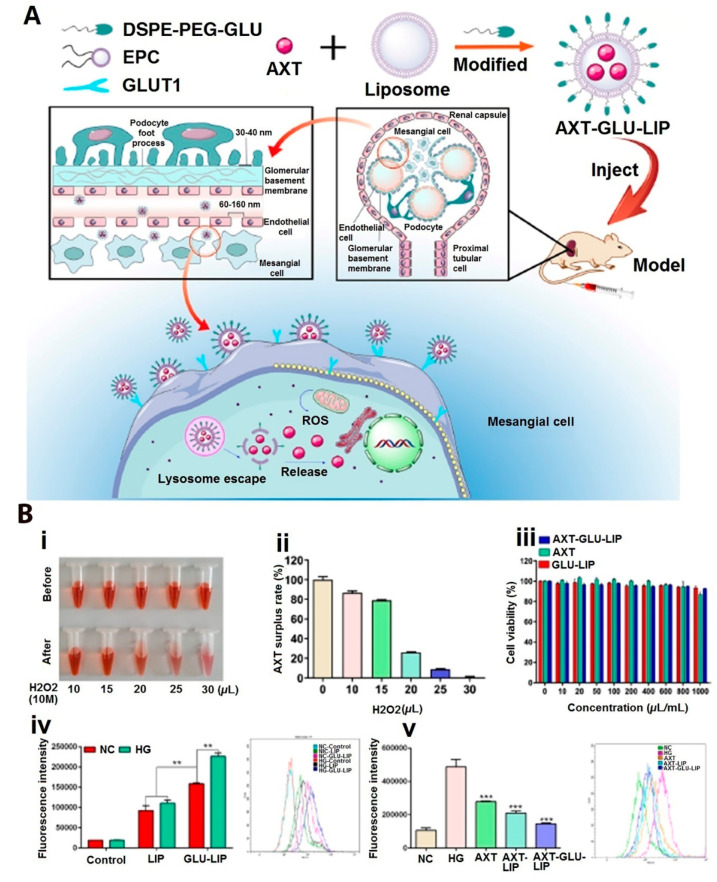
Topical medications such as eye drops, eye ointments, etc. for the treatment of eye diseases have advantages as they are minimally invasive and convenient for patients. However, there are some lingering challenges. Most eye drops are removed within seconds due to obstacles such as limited lacrimal capacity and subsequent tears, particularly in the case of high molecular weight and hydrophilic drugs, which unlike small molecule lipophilic drugs, have very limited permeability. Drug molecules are transported via two pathways (corneal and noncorneal) to reach the anterior and posterior segments, respectively, and both have barriers to drug permeation.222 Thus, some issues should be addressed such as the drug’s molecular size and weight, its permeability, hydrophilicity, and hydrophobicity, and above all its delivery system. Topical application of drugs is a preferred route for diseases of the surface or the anterior portion of the eye that affect the cornea or sclera and the lens. For the drug cargo delivery to the posterior ocular segments, there is a need for further investigation to develop appropriate systems or devices to overcome the barriers within the ocular tissue. Among numerous studies, the use of nanotechnology-based drug formulations has been one of the most successful. Developing novel nanoformulations for in situ delivery and release of therapeutic molecules can circumvent ocular barriers and reduce systemic side effects. AXT, as a lipid-soluble keto-carotenoid, is used in the treatment of oxidative stress-induced ocular diseases including AMD and dry eye due to aging, allergies, inflammations, etc.108,183 Since the retinal epithelial cells are the active site of this drug, delivery to this site is of particular importance. As topical routes, namely eye drops, are more practical and easier to use for patients, it is important to design an appropriate drug delivery system for topical application of AXT, which has poor solubility in aqueous solutions.223 Nanosized liposomes are a good choice because they can cover hydrophobic AXT well and alter the drug’s surface charge, followed by delivering the cargo to the desired position in the posterior ocular tissues. AXT-coated liposomes have been applied in an in vitro dry eye model, and its effect on reducing cell apoptosis and inhibiting ROS production and aging markers is evident. Moreover, it has been revealed that when positively charged liposomes are applied, AXT delivery to the desired location increased locally. Cationic liposomes exhibit higher affinity toward cells than neutral ones. This higher affinity could make them a suitable candidate as a nanocarrier for drugs such as AXT.224 Transportation of drugs via a topical route can be enhanced by mucus-penetrating delivery systems to various ocular tissues beyond the mucus layer; mucus-penetrating nanoparticles have been tested in vivo to discern improvement of drug diffusion. The results implied that it enhanced diffusion not only toward the ocular surface but also toward the posterior segments.225 Furthermore, some biological molecules such as peptides (as cell-penetrating agent), proteins, monoclonal antibodies, genes, and oligonucleotides can be conjugated to nanoparticles for drug transportation to the posterior segment of the eye.226 Nanoparticles and liposomes, nanomicelles, nanosuspensions, and dendrimers are other nanotechnology-based carrier systems which are being studied for ocular delivery therapeutics.227 Nevertheless, nanoformulations seem to help in overcoming various ocular barriers better than other delivery systems for AXT. However, there is still room to discover novel drug delivery systems to increase the stability, solubility, and bioavailability of AXT.
6. Dermal Delivery of AXT for Skin Protection
6.1. Skin Morphology, Barriers, and Penetration Routes
Skin is the initial barrier for living creatures against the environment, and the first obstruction to penetrate it is the stratum corneum, the main barricade for drug penetration.228 There are two main routes through the skin for the permeation of active substances: trans-appendages and trans-epidermal pathways. The trans-epidermal pathway is responsible for skin permeation and comprises two routes, intercellular (paracellular) and transcellular (polar) pathways (Figure 13).229 The intercellular pathway is the major penetration pathway for active antioxidants into the skin and even possibly into deeper areas of the skin.230 Nanotechnology is of immense help for successful skin drug delivery. It can control the release of drugs to enhance performance, provide higher drug loading capacity, help attain the physical and chemical stability of the drugs during the time of storage, and prolong the drug delivery, thus improving the drug concentration.231 The size of the drug molecule is the first challenge for its penetration due to the 10–40 μm thick stratum corneum wherein drugs with relatively low molecular weight (∼ below 500 g/mol) can reach the dermis.228 Besides, the cells present in this layer are haphazardly arranged; therefore, the drug has to travel a long way to cross this layer.232 To date, a variety of different physical and chemical approaches for enhancing drug delivery parameters through the skin have been devised; many of them are costly irritants.233 The novel nanotechnology-based approach for topical drug delivery with controlled drug release has been recognized as an effective strategy especially for drugs with poor water solubility and short half-life.234−236 Besides the role of the nanoparticles and nanocarriers in treatment of skin disorders, they have been widely used in the cosmetic industry; moisturizing creams containing liposomes were first developed ∼40 years ago.237 The skin has the most contact with the external environment, and therefore, it demands more care and maintenance. Daily skin care, deploying cosmetics containing nutraceuticals, enhances the skin’s elasticity, texture, and smoothness, thus promoting skin health.238 Delivering the drug through the skin by transdermal patches or topical formulations is problematic because of the presence of the stratum corneum; this layer of the epidermis limits the delivery of bioactive molecules with relatively low molecular weight. To overcome these limitations in passing biological barriers, microneedle patches are a promising tool to perforate the stratum corneum.239 Microneedles, comprising micro-/miniature-sized needles, are able to deliver cargo into the dermis following a noninvasive route.228 However, to date, no study has been undertaken to deliver AXT via microneedles. Hence, there is room for conducting research in microneedle-mediated delivery of AXT.
Figure 13.
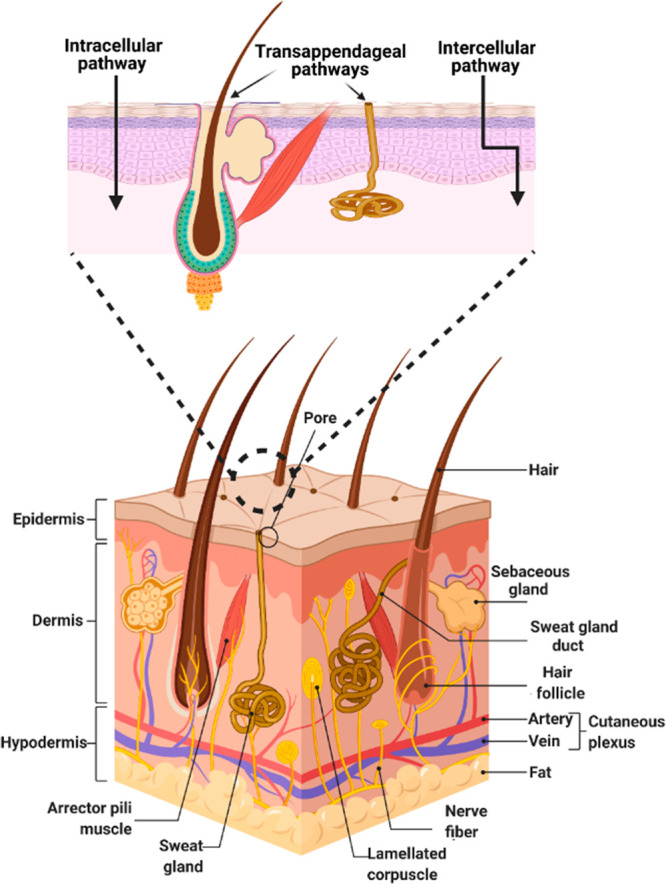
Schematic illustration of skin layers and major skin permeation routes for the delivery of nanoparticles. The first one is the pathway through opening areas of the skin such as sweat glands and hair follicles which leads to a better penetration of the drugs into the skin. Drug molecules diffuse through the phospholipid membranes and cytoplasm of the deceased keratinocytes. In this continuous way bioactive agents pass through the small spaces between the cells of the skin.
6.2. AXT Delivery for Skin Health
There is a balance between reactive oxygen and nitrogen species generation and antioxidant system activity in living cells. The structure and functionality of normal cells changes when any factor leads to the disruption of this balance.240 The disadvantages of excessive oxidative stress for the skin are facial lines, deep wrinkles, dullness and roughness, dry aged skin, and the loss of elasticity.241 UV rays can penetrate the skin and create oxidative stress, followed by DNA, protein, and lipid damage, and errors in DNA repair leading to mutation, collagen degradation, wrinkles, erythema, and skin cancer.242 AXT enhances skin health through several mechanisms including antioxidant properties, anti-inflammatory effects,243 improving immunity,244 and the DNA repair effect.245 Many studies have evaluated the efficacy of AXT on the skin and demonstrated that it improves skin elasticity, texture, and moisture content and decreases wrinkles and visible signs of aging.246,247 Due to the anti-inflammatory and antioxidant properties of AXT, it has been suggested to potentially decrease skin cancer rate;248 the cosmetic benefits of AXT have also been investigated by some researchers. In a topical application, a cream containing AXT was used on 11 females’ skin. After 3 weeks, the skin moisture as well as the elasticity of the majority of applicants’ has increased and three females with fine wrinkles showed improvement in their skins. In another study, a group of 49 women of 45–50 years of age were administered 4 mg of AXT for 6 weeks with over 50% of the participants’ skin features, including elasticity and moisture, being improved.249 AXT initiates the cellular antioxidant defense system and modulates the Nrf2 pathway, leading to antioxidant response.250 The Keap1-Nrf2-ARE signaling pathway is the key antioxidant defense system against oxidative stress. Nrf2 is a key transcription factor that is negatively regulated by Keap1, and its main role is to regulate the cell’s protective responses to oxidative stress. Under basic conditions, most of the Nrf2 molecules in the cytoplasm bind to the Keap1 protein and are destroyed. Oxidative stress reduces Nrf2 degradation by altering specific cysteine codes in Keap1, resulting in the transfer of Nrf2 to the nucleus. It binds to ARE in the promoter region of genes encoding antioxidant enzymes and induces the production of endogenous antioxidant enzymes.81 These enzymes include superoxide dismutase, catalase, peroxiredoxin, etc., which play an important role in combating ROS.251
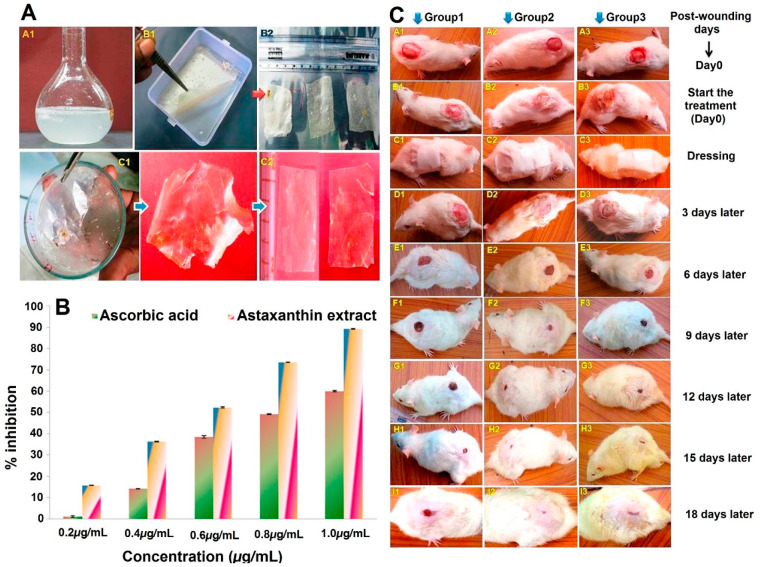
AXT also affects the function of the immune system; for example, in a study of human lymphocytes, AXT enhanced the immunoglobulin production in response to T cell stimulation. In other studies, it has been proven that AXT enhances immune responses and improves the cytotoxic activity of T and NK cells in vivo.(244,252) In a study carried out on healthy female college students, immune system markers including IFN-g and IL-6 production, NK cell cytotoxic activity, and LFA-1 expression have been meaningfully enhanced; cell and humoral immune responses were improved by dietary AXT usage.253 It is worth mentioning that the participants with an average age of 21 received daily AXT for a period of 8 weeks and their immune responses were evaluated during a clinical study. In the middle of the experiment, it has been observed that a DNA damage biomarker is decreased by AXT with improvement in young females’ immune response.253 Ultraviolet radiation induces the production of reactive oxygen species (ROS) and free radicals such as hydroxyl and singlet oxygen, and these being reactive molecules cause DNA strands breakage and the oxidation of its bases.245 Due to AXT’s antioxidant properties, it prevents the accumulation of free radicals, thereby preventing damage to DNA.104 In addition, the effect of AXT in the tissue engineering and wound healing in both the in vitro and in vivo phases showed very promising results. In this manner, combination of AXT with some types of polysaccharides, such as chitosan and collagen, leads to increasing the ratio of wound healing in a fraction of time compared to other types of studies that used only the polysaccharides and/or other types of routine polymeric nanostructures. Comparing the results of the used AXT incorporated collagen with the control group (saline only) and the drug control group (gentamicin incorporated collagen) showed that the AXT could accelerate the wound healing in the rat by up to 50% compared to the two control groups (Figure 14).254 Also, it has been reported that AXT may influence the kinetics of DNA repair.250 In a study, the protective capability of AXT against UV-induced DNA alterations has been assessed; synthetic AXT hindered DNA damage in human melanocytes and intestinal CaCo-2 cells.255 Alterations in extracellular matrix components such as fibrous proteins including collagen, elastin, and glycosaminoglycans lead to skin dryness, wrinkle formation, and the loss of skin elasticity.256 UV-induced ROS production stimulating synthesis of matrix metalloproteinases results in extracellular matrix destruction and the loss of collagen. AXT with its antioxidant ability prevents the growth and accumulation of free radicals, and it has been observed to prevent matrix metalloproteinase expression in different cells.257 The effects of AXT on the promotion of matrix-metalloproteinase-1 and skin fibroblast elastase on UV-treated human dermal fibroblasts of cultured human dermal fibroblasts have been assessed where AXT decreased the effects of UV radiation on skin.99 Pro-inflammatory mediators are reportedly increased during UV radiation, and AXT inhibited the production of inflammatory mediators by blocking NF-κB activation. The effect of AXT on expression of NF-κB p65, IL-6, TNF-α, and IFN-γ has been investigated elsewhere. A total of 32 buffaloes have been supplemented with AXT during a period of 30 days. The inflammatory mediator expression from peripheral blood mononuclear cells is compared to control groups. It turned out that the mRNA expression of IL-6, TNF-α, and IFN-γ decreased in comparison with control groups.258 As has been noted, AXT reduces the level of inducible nitric oxide and cyclooxygenase. This property has an important effect on the development of anti-inflammatory drugs.259
Figure 14.
(A) Images attributed to the preparation process of AXT and drug incorporated collagen films extracted from D. singhalensis as follows: (A1) Collagen solution, (B1 and C1) films containing AXT and gentamycin in the collagen film solution, (B2 and C2) films before treatment modified square shape (5 × 4 cm) formation. (B) Antioxidation activity (DPPH) of AXT extracted from D. singhalensis compared to ascorbic acid in different concentrations. (C) Photographic representation of wound contraction on different postexcision healing days (1–21 days). Group 1 is the control group; group 2 is the AXT incorporated collagen; and group 3 is the gentamicin incorporated collagen. Reprinted from ref (254) with permission from Elsevier.
7. AXT and Treatment of Diabetes
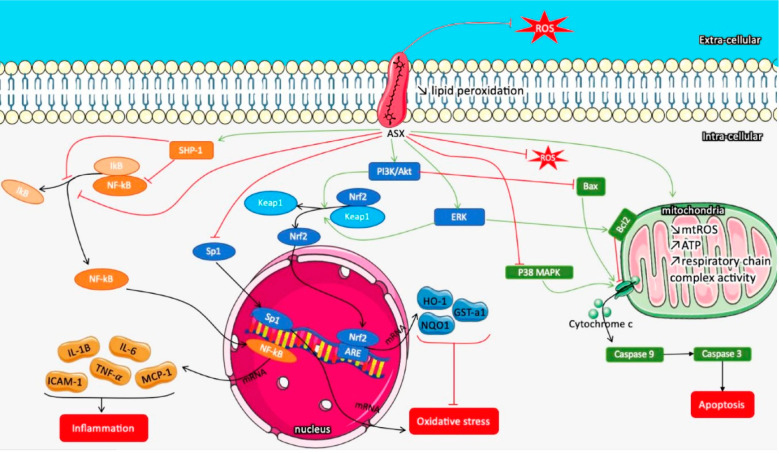
Diabetes mellitus, known as just diabetes among people, refers to a group of metabolic disorders and is recognized with a high blood sugar level over a long time. The number of people dealing with diabetes is about 463 million, and it is expected that this number will increase to 578 million in the next 10 years.260 Oxidative stress caused mainly by hyperglycemia-induced ROS is known to have a detrimental effect on the progression of diabetes. AXT with superior antioxidation activity can compensate oxidative damage through various mechanisms—scavenging of free radicals, hampering the peroxidation of lipids, and quenching singlet oxygen. In contrast to other family members of carotenoids, the polar structure of AXT helps the drug molecule to incorporate itself into the cell membrane without disorganizing it, thus leading to a decrease in the hydroperoxide levels of the lipid layer.261 Moreover, it has been revealed that AXT is capable of enhancing the mitochondrial activity through reduction of the ROS produced in the mitochondry leading to an increase in the ATP and repiratory activities.262Figure 15 indicates the possible mechanisms through which AXT inhibits oxidative-related damages.
Figure 15.
Schematic representation of the molecular pathways implied in the protective potential of astaxanthin (AXT): Due to its membrane penetrance, AXT has both intra- and extracellular ROS scavenging actions. Moreover, in the phospholipid membrane, the AXT polyene chain participates in the reduction of lipid peroxidation. Through the regulation of various pathways, AXT reduces inflammation, oxidative stress, and apoptosis. Red arrows indicate inhibitory action, and green arrows show enhancement action. Reprinted from ref (260) under open access license.
Caused by lesions in the renal tubule and glomeruli, diabetic nephropathy is a microvascular complication of diabetes mellitus (type I and II), and the main symptoms recognized are reduction of the glomerular filtration rate, damage in the epithelial cells of the renal tubules, etc.263 Oxidative stress is a key factor causing diabetic nephropathy, and AXT with its superior antioxidant property is of particular interest for application in this case. Depending on the stage of diabetes, AXT is effective in treating and reducing its complications. The antidiabetic effects of Astaxanthin have been observed by
decrease in serum glucose and fructosamine levels in patients using AXT (8 mg daily for 8 weeks)264
melioration of glucose metabolism and lower blood pressure264
lower fasting blood sugar in mice265
decreased MDA (malondialdehyde) in serum and increased SOD activity with glucose reducing effects266
protection of the pancreatic beta-cells against glucose toxicity by increasing their insulin secretion265
prevention of the ER-stress mediation of beta-cell apoptosis267
increased insulin sensitivity and glucose uptake and decreased insulin resistance in high-fat fructose diet (HFFD)-fed mice using AXT for 45 days (6 mg/kg/day) impressed on the insulin signaling pathway268
increased glucose metabolism and tolerance in muscle and decreased insulin resistance in this tissue as well as augmented mitochondrial biogenesis in muscle cells of (high-fat diet) HFD-treated mice269
improved glucose metabolism by affecting AXT on the liver’s metabolic enzymes and increasing the storage of glycogen in the liver270
reduced inflammatory phenomenon and liver dysfunction due to diabetes in (Streptozotocin) STZ-induced diabetic rats by reducing the levels of ROS and AGEs (advanced glycation end products) and reduction of lipid peroxidation in the liver during 18 days of consumption of 50 mg/kg AXT per day271
Some complications of diabetes include the following:
(1) Retinopathy. A slow-progressive complication of diabetes with increased inflammation, decreased antioxidant enzyme’s functions, numerous metabolic changes in the retina cells, microvascular damage, oxidative stress in the retina and its capillary cells, and activation of the autophagy pathway in retina cells.272−274 In a study, the preventive role of AXT on retinopathy in rats has been examined and a decrease in oxidative stress and inflammatory mediators and an increase in antioxidant enzymes were observed.275 An in vitro experiment on human retinal pigment epithelial cells showed that AXT can reduce the effects of high glucose on cells by decreasing AGEs, ROS, and lipid peroxidation.276 Khedher et al. showed the inhibitory effect of AXT on aldolase reductase activity, which is a key enzyme in the pathogenesis of retinopathy.277
(2) Neuropathy. Adverse effects of this complication are neuronal abnormalities, brain cell apoptosis, hippocampal-based cognitive dysfunction, and neuronal behaviors.278 All these problems are due to the activities of oxidative stress, the presence of inflammatory mediators, and the activation of apoptosis-related molecules. Studies show the the protective and melioration effects of AXT administration on neuropathy include increased antioxidant enzymes’ activity, reduced level of inflammatory molecules, protection of cells from apoptosis,279 improved neuronal behaviors in STZ mice,98 and attenuated cognitive deficit by inhibition of oxidative stress and inflammation in diabetic mice.280
(3) Cardiovascular Effects. They are diabetes-related disorders caused by thrombosis, arteriosclerosis, vascular damage, and platelet aggregation that all are the result of high glucose and oxidative stress.281,282 AXT reduces these effects by reduction of oxidative stress and inflammation as it showed anti-inflammatory and anticoagulatory effects,283 regulation of redox reactions, control and regulation of vasoconstriction, blood pressure, and blood fluidity,284 and reduction of the LDL level.285
(4) Nephropathy. Nephroprotective effects of AXT are observed by increased urinary albumin and decreased oxidative stress markers in db/db mice with 12 weeks of AXT administration,286 inhibition of COX-2, MCP-1, TGFB, and ROS production in glomerular mesangial high-glucose-stimulated cells,287 normalization of creatinine and uric acid levels, reduction of urea and glomerular hypertrophy in diabetic rats and improvement of renal dysfunction,288 increase in the expression of antioxidant enzymes, and maintaining the antioxidant status of the kidneys and plasma, which reduce the renal complications of diabetes289 and prevent renal fibrosis by reducing the accumulation of ECM components and protection against oxidative damage by activation of transcription factor Nrf 2-ARE.290
However, the drug’s poor solubility and stability negatively affect its antioxidation capability and bioavailability. A recent study targeted diabetic nephropathy through a drug delivery system comprising liposome encapsulating AXT with the aim of designing a smart delivery system targeting glomerular mesangial cells based on glucose transporter 1 which reportedly plays a significant role in transporting glucose to glomerular mesangial cells.291 The glucose-modified liposome encapsulating AXT can successfully penetrate through glucose transporter 1 of the glomerular mesangial cell membrane, and the drug delivery system efficiently scavenged ROS generated by oxidative stress.292 Moreover, the AXT release study has been accomplished at different pH’s; the acidic medium exempilified the lysosome environment, while the phosphate buffer saline +10% fetal bovine serum represented the blood environment. The liposomes exhibited a faster release in the acidic environment and a better protection of drug molecules. Figure 16 indicates the physicochemical and biological properties of liposome encapsulating AXT in vitro plus a schematic showing how the glucose ligand drug delivery can reach the mesangial cells.
Figure 16.
(A) Schematic on the targeting of glomerular mesangial cells through the glucose ligand-modified liposome encapsulating AXT. (B) AXT antioxidative activities (i), the surplus rate of AXT in H2O2 scavenging (ii), the cell viability of GLU-LIP, AXT, and AXT-GLU-LIP samples in the exposure of human renal mesangial cells (HRMCs) at different concentrations for 24 h (iii), the cellular uptake of DiO-labeled samples through HRMCs (iv), the level of ROS for different samples in the exposure of HRMCs (v). The p values, including *p < 0.05, **p < 0.01, and ***p < 0.001, represent a significant difference between the samples and HG. Abbreviations: 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine (DSPE), liposome (LIP), glocuse ligand (GLU), yolk lecithin (EPC), diabetic cell model (HG), negative control (NC), and glucose transporter 1 (GLUT1). Reprinted from ref (292) with permission from Elsevier.
8. Therapeutic AXT Delivery for Other Disorders—Cancer
Cancer essentially means the growth of a malignant cell. Human malignancies are the result of a set of distinct genetic events. These changes occur in genes that affect cell cycle control, cell survival, cell movement, and angiogenesis. The entry and progression of a cell through the cell cycle are accompanied by changes in the amount and activity of a family of proteins called cyclins. The amount of different cyclins increases at certain stages of the cell cycle, and due to this enhancement, activation of E/CDK2, D/CDK6, and D/CDK4 cyclins takes place, which causes RB phosphorylation, resulting in cell proliferation. Cell proliferation occurs spontaneously when cell cycle-directing genes are impaired due to mutation or amplification. For instance, activation of cyclin D1, which transpires due to mutation, accelerates cell proliferation by facilitating RB phosphorylation.293 Studies show that AXT stops the cell cycle at the stage of G0/G1 and prevents the expression of cyclin D1, by increasing the expression of p53, p27, and p21WAF-1/CLP1 at the same time. One way cells escape cancer is to choose death, i.e., apoptosis. The degradation of the nuclear membrane and cytoplasm of cells and organelles leads to fragmentation of cells which are then rapidly ingested by phagocytes and abducted from the environment. Several genes play important roles in apoptosis, including Bim, Bcl-2, Bcl-XL, Bak, Bax, Bad, p53, and Mcl-1. The proteins Mcl-1, Bcl-2, and Bcl-XL work together to act against apoptosis, while the proteins Bim, Bad, Bak, and Bax play a function in apoptosis.294−296 Studies have shown that AXT reduces the expression of anti-apoptotic and increases the expression of pro-apoptotic proteins, promoting the release of cytochrome c and Smac/Diablo into the cytoplasm. Bcl-2 causes the release of cytochrome c from mitochondria, which leads to the activation of caspase-9 and then caspase-3. AXT induces mitochondrial apoptosis in cells through caspases, leading to cancer cell death.297,298 AXT exerts antiproliferative effects by increasing the expression of Bax and caspase 3 and decreasing the expression of malondialdehyde and bcl2 in the LS-180 cell line.299,300 AXT can treat prostate cancer by inhibiting alpha-reductase enzyme function.301 Many studies have pointed to the anticancer role of AXT in prostate, liver, colon, lung, breast, and other cancers.302−304 At present, a large number of drug delivery systems comprise nanoparticles, and various materials have been used as drug stimulants or enhancers to improve the effectiveness of the treatment and the durability and stability as well as the safety of anticancer drugs. AXT as a biological molecule can reduce metal salts to form nanoparticles that are suitable for treatment in biological systems; production of gold nanoparticles (Au NPs) with AXT as a natural reducing agent has been assessed. The cytotoxic effect of prepared nanostructures against human breast cancer cells (MDA-MB-231) has been evaluated through a tetrazolium-based assay; AXT-Au NPs display a strong cytotoxic effect against cancer cells, and apoptotic morphology has been detected in the treated cells. The AXT reduced Au NPs, on the other hand, have the potential to act as a promising agent in the field of photobased diagnosis and therapy as they display an interesting UV–vis absorption peak in the near-infrared region that is essential in photobased diagnosis and therapy. A near infrared region laser can penetrate into tissue effectively, and nanoparticles can convert this light into thermal energy, which is applied in photothermal therapy.305 It is interesting to note that AXT alone has a photocatalytic property by which it can turn light into heat without any need for an additional photothermal agent. This property has been exploited to eradicate eye tumors through photothermal therapy. An increase in the local heat of tumors has been observed once the near-infrared is applied. The obtained results clearly showed that the AXT is a very promising candidate for the treatment of any type of cancer through photothermal therapy as depicted in Figure 17.
Figure 17.
(A) Experimental setup for AXT-induced photothermal therapy; (B) the tumor volume (i), rabbits’ body weight (ii), and H&E staining images at ×40 (iii) and ×100 (iv) of the samples; (C) the in vitro assessment of temperature change when NIR with a wavelength of 532 nm had been irradiated; (D) treatment of eye tumors up to 14 days: control (i), tumors treated with the laser alone for 4 min at 532 nm and 0.11 W cm–2 (ii), injected AXT solution (300 μg mL–1) without being exposed to laser irradiation (iii), tumors treated with both AXT injection followed by laser irradiation (532 nm and 0.11 W cm–2 for 4 min) (iv). Reprinted from306 with permission from Public Library of Science.
Early detection of cancer can significantly increase the likelihood of successful treatment. Such imaging tests can have a significant impact on cancer diagnosis. Photoacoustic imaging is a hybrid imaging technique based on the photoacoustic effect with high resolution and sensitivity, and it can be used to diagnose different stages of cancer. Compared to other common methods of tumor imaging, it is more economical and has better contrast in tumor diagnosis.307 AXT, with an absorption peak at 490 nm, can be used as a potential photoabsorbing agent to enhance photoacoustic responses in targeting cancerous tumors.308,309 Nguyen et al. demonstrated that AXT can be employed as an exogenous photoacoustic biocompatible contrast agent to recognize the size and the location of bladder tumors.310 Also, Bharathiraja et al. synthesized polypyrrole nanoparticles using AXT-conjugated bovine serum albumin as an optical contrast agent for photobased therapy and cancer detection. In another study, an AXT-alpha tocopherol nanoemulsion has been synthesized by spontaneous and ultrasonication emulsification methods and its effect examined on three different types of cancer cells; it has significant anticancer potential against different cancer cells and exhibits antimicrobial and wound healing properties.305
Additionally, some researchers have examined the use of solid lipid nanoparticles as oral delivery systems for vitamins and their analogs because they are biocompatible with the lipid matrix (comprising triglycerides, fatty acids, or glycerol esters) and are readily degraded in vivo; AXT, being a natural carotenoid, works against several disorders and is more potent than β-carotene and vitamin E.5 However, its use in oral formulations is limited due to its light sensitivity, decomposition in the presence of oxygen, and poor water solubility. Therefore, AXT has been entrapped into solid lipid nanoparticles to improve its bioavailability.311 A drug delivery system based on Tween 20 esters and glycerol has been developed for AXT delivery with the average diameter of these solid lipid nanoparticles being 163–167 nm, while the encapsulation percentage was ∼89%. The results reveal that solid lipid nanoparticles caused the long-term release of AXT in GI simulated juices.312 In another study, AXT-loaded colloidal particles have been developed to address the limiting factors of AXT for oral drug delivery applications via chitosan oligosaccharide-coated poly(lactic-co-glycolic acid) wherein the drug molecules are loaded. Notably, two types of poly(lactic-co-glycolic acid) with different lactide to glycolide ratios have been tested (50:50 and 25:75, respectively), and the physicochemical, drug delivery potential, and biological properties have been assessed in vitro. Coating of chitosan oligosaccharides made the drug delivery system pH-responsive, and the release rate is increased when the pH of the medium turned to acidic. In contrast to pure AXT and noncoated AXT-loaded poly(lactic-co-glycolic acid) samples, the chitosan oligosaccharide coating led to a good dispersity in water at room temperature and enhanced bioavailability which is highly beneficial for drug delivery applications.313Table 3 presents some examples of AXT-loaded nanocarriers for different biomedical applications.
Table 3. Different AXT-Loaded Nanocarriers Targted for Various Organsa.
| organ | indication | nanocomposite drug delivery | activity | route | ref | |
|---|---|---|---|---|---|---|
| skin | AXT-NANE | enhance transformation of stratum corneum and permeation of AXT | dermal delivery | (314) | ||
| skin | wound in diabetic individuals | AXT-TP-KC NEs | accelerate wound healing/control of hyperglycemia | transdermal administration | (315) | |
| eye | inherited retinal degeneration (RD) | AXT- polysorbate 20 NEs | eliminate the abnormities in visual signal transmission and visual impairments | oral administration | (316) | |
| brain | OxyHb-induced neuronal damage/subarachnoid hemorrhage | AXT-Fe3O4 -Tf-PEG NPs | neuroprotective | not mentioned | (131) | |
| brain | AXT-Tf- PEG-Fe3O4 NPs | neuroprotective | not mentioned | (131) | ||
| brain | neurological disorders | AXT-SLNs | neuroprotective | nasal drug delivery | (317) | |
| liver | alcohol-induced hepatic injury | AXT-DC NPs | mice | hepatoprotective | oral administration | (318) |
| liver | acute hepatotoxicity | nanoliposomes | mice | hepatoprotective | oral administration | (79) |
| liver | alcoholic liver fibrosis | nanoliposomes | mice | hepatoprotective | oral administration | (319) |
Abbreviations: AXTDC NPs, astaxanthin-DNA/chitosan nanoparticles; SLNs, solid lipid nanoparticles; AXT- Fe3O4-Tf- PEG NPs, astaxanthin/Fe3O4/transferrin/PEG nanoparticles; NLCs and CDs, nanoscaled lipid carriers and cyclodextrins; NE, nanoemulsion; NLC, nanostructured lipid carriers; AXT-TP-KC NEs, astaxanthin/alphatocopherol/κ-carrageenan nanoemulsion
9. AXT from Bench to Bedside
In addition to medicine, AXT has many applications in a variety of industrial fields. This major microalgal (Haematococcus) carotenoid is used for the cosmetic, food, nutraceutical, and aqua-food industries, among others. Commercially, there is a high demand and very competitive market among the producer companies for the production of this pigment and its derivatives. The AXT market for animal feed and nutraceuticals was $300 million and $30 million, respectively, in the year 2009.320 In 2018, this market surpassed USD 600 million,321 and it exceeded USD 650 million in 2020 (Global Market Insights: https://www.gminsights.com/industry-analysis/astaxanthin-market). Based on Global Market Insights, the AXT market size is estimated to grow at over 5.5% CAGR (compound annual growth rate) between 2021 and 2027. Synthetic and natural AXT are two sources of this market. Generally, the consumption of synthetic AXT is in poultry, pet food, and aquaculture applications, and almost 95% of the AXT market is produced by chemical synthesis.322 Although the consumption of synthetic AXT is dominant, consumer demand for the effective natural Haematococcus astaxanthin has been growing, especially in the nutraceutical industry. Natural AXT is anticipated to reach US$ 770 million (with the production of 190 t) by 2024, at growth over CAGR of 7.7%.320 The AXT market has displayed steady growth since 2014, and its global market size is predicted to reach 3.4 billion USD by 2027, at a CAGR of 16.2%.323 It is easily obtainable in various forms of dried meal, powder, oil, and biomass, thus presenting an increase in global pigment sales volume, and will have the most significant global market evolution by 2026.324 There is great interest in carotenoids from natural sources, and AXT’s broad applications in food, pharmaceuticals, nutraceuticals, dietary supplements, feed, and personal care products are anticipated to grow.
10. Future Perspective and Remarks
One of the problems for human beings today is dealing with chronic and dangerous diseases. Free radicals and oxidants, in general, are continuously produced in the body of living organisms via various metabolic reactions. In view of the role of free radicals and oxidants in the development and progression of these diseases, their counteractive molecules, antioxidant compounds, are becoming valuable supplements in the human diet. In the last two decades, oxidative stress and antioxidants have become one of the most important and popular research areas among researchers.325 Diet supplemented with synthesized chemical antioxidants is considered a treatment for ROS disorders, but research shows that regular use of synthetic antioxidants increases mortality.326 Therefore, the hypothesis based on the therapeutic effect of antioxidant conditions in vitro does not concur with its effects in vivo.(327) Side effects of chemical drugs and their incompatibility with human nature have created special importance for the accurate identification and study of the chemical compounds in medicinal plants, yeasts, algae, and several bacteria including natural antioxidants. Natural antioxidants appear to be a good alternative to synthetic antioxidants as they can effectively fight inflammation and oxidative stress;328 developed countries have made the development of healthy foods an important priority. By identifying and using these compounds, while improving diet and reducing diseases, they have contributed to enhanced consumer safety and health as affirmed by clinical studies on the health effects of bioactive compounds.329 AXT is a healthy nutrient without toxicity, and due to its strong antioxidant properties, it has been involved in protecting cellular compounds against oxidative damage and in regulating gene expression, inducing cell–cell communication and cell health. On the other hand, its use as a natural antioxidant is limited due to its low bioavailability, sensitivity to environmental conditions, processes, and the gastrointestinal tract, and the lack of a proper drug delivery system. Therefore, considerable research has been undertaken on the use of nanocarriers loaded with AXT for therapeutic applications. Besides, the combination of AXT with other nanomaterials may bring synergistic effect, e.g., antioxidant activity, which can be employed for the treatment of different ailments.330−334
Glossary
Abbreviations Used
- AMD
age-related macular degeneration
- AXT
astaxanthin
- AuNP
gold nanoparticle
- C=O
carbonyl group
- CNS
central nervous system
- COS
chitosan oligosaccharides
- DSPE
distearoyl-sn-glycero-3-phosphatidylethanolamine
- DHA
docosahexaenoic acid
- GLUT1
glucose transporter 1
- HRMCs
human renal mesangial cells
- -OH
hydroxyl group
- LIP
liposome;
- NO
nitric oxide
- NOS
nitric oxide synthase
- ONOO-
peroxynitrit
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SLN
solid lipid nanoparticles
Biographies
Zohreh Jafari holds a Ph.D. degree in medical biotechnology from Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran. Her Ph.D. work was focused on proteomics, Zanjani Viper Venom, and recombinant production of the most effective subunit in blood coagulation. Currently, she works as a research assistant and head of the genetic group at NanoScience Technology and Histogenotech Companies. Her work focuses on cancer nanomedicine and smart drug delivery. Her research interests are recombinant proteins, CRISPR technology, antibody engineering, and nanomedicine.
Ashkan Bigham holds an M.Sc. in Materials Science & Engineering (Ceramics) from Islamic Azad University of Najafabad, Iran. Currently, he works at Institute of Polymers, Composites, and Biomaterials (IPCB) of National Research Council of Italy (CNR) as a Research fellow. His expertise falls into designing and fabrication of various bioceramics and biocomposites for biomedical-related applications, among which tissue engineering and regenerative medicine, drug delivery, and cancer therapy can be enumerated.
Sahar Sadeghi earned her Ph.D. degree in medical biotechnology from Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran. She studied the effectiveness of poly epitope proteins along with chitin and chitosan microparticles as preventive vaccines against Influenza virus and Streptococcus pneumonia in her thesis. Her field of interest is research on therapeutic aspects of natural products (chitin, chitosan, carotenoids, ...), recombinant medical proteins, protein/peptide-based prophylactic and therapeutic vaccines, as well as chitinous biomaterials as adjuvants.
Sayed Mehdi Dehdashti received his Ph.D. degree in Medical Biotechnology from the Gauhati University, India (2016). After one postdoctoral research stint at Indian Institute of Technology Gauhati (2016–2017), he began his second postdoctoral research stay at Cellular and Molecular Biology Research Center (CMBRC), Shahid Beheshti University of Medical Sciences (SBMU), Iran. His research interest and expertise fall into molecular pharming and pharmaceutical biotechnology.
Navid Rabiee, Ph.D., is a Postdoctoral research associate at Sharif University of Technology, Tehran, Iran, and an Invited Visiting Scholar at School of Engineering, Macquarie University, Sydney, New South Wales, Australia. Since 2016, he has been working in several fields with a multidisciplinary approach by blending modern molecular and cellular biology/biochemical sciences with engineering principles to design the next-generation of medical systems and devices for patient treatment. He is a pioneer in the field of BioInorganic Chemistry with emphasis on the CRISPR delivery in assistance of inorganic nanovectors. His work has resulted in publication of over 200 peer-reviewed journal articles in prestigious journals including Nature, Nature Medicine, The Lancet, Nano Today, Biomaterials, Advanced Functional Materials, etc., 6 books, and over 20 chapters.
Alireza Abedivash received a master’s degree in Cellular and Molecular Biology from Sari Agricultural and Natural Resources University, Sari, Iran. He accomplished his master’s thesis project working on the production of recombinant CEL I endonuclease in the prokaryotic host at Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. His professional interests focus on novel mutation detection techniques.
Mojtaba Bagherzadeh, Ph.D., is a professor of the Department of Chemistry at Sharif University of Technology, Tehran, Iran. His current research interests include inorganic chemistry, inorganic catalysis, and bioinorganic chemistry. He has published over 150 ISI papers, with the H-Index of 38.
Behzad Nasseri, Ph.D. (Medical Nanotechnology, Hacettepe University, Turkey), M.Sc. (Medical Nanotechnology, Hacettepe University, TurKey), B.Sc. (Chemical Engineering, Amirkabir University of Technology, Iran), is a nanomedicine researcher. Of his ten year (after B.Sc.) research and teaching career, he has been working in nanomaterial and biomaterial design as a senior researcher and has participated in medical nanotechnology research projects of Shahid Beheshti University of Medical Sciences and Tabriz University of Medical Sciences. Dr. Nasseri has 18 (18) peer reviewed articles and has authored book chapters on nanobiotechnology and nanomedicine and biomaterial fields. He is honorary editor of a few journals in the mentioned fields.
Hassan Karimi-Maleh was born in Sari, Iran (1982), and received his Ph.D. degree in Chemistry from the Isfahan University of Technology (Isfahan, Iran) in 2011. He has published more than 200 research papers (H-INDEX 84), more than 150 conference papers, one book chapter, and many reviews, and he is one of the Top 1% Scientists in Chemistry and Agriculture simultaneously in ISI Essential Science Indicators. He was selected as a highly cited Top researcher by clarivate analytics (cross filed; 2018). He works as an academic staff member at Quchan University of Technology, Iran, and also as Visiting Professor at Johannesburg University South Africa. His research interests include development of electrochemical sensors and DNA and enzymatic biosensors, synthesis of nanomaterials and characterization of them, water treatment and removal processes, methanol and ethanol fuel cell systems, drug delivery, ionic liquids, conductive polymers, surface electrochemistry, and corrosion.
Esmaeel Sharifi is an Assistant Professor of Tissue Engineering and Biomaterials at Hamadan University of Medical Sciences, Iran. He is also a Postcoctoral fellow at Institute of Polymers, Composites and Biomaterials, National Research Council (IPCB-CNR), Naples, Italy. He received his doctoral degree in Tissue Engineering from Tehran University of Medical Sciences, Iran, in 2016. His research interests include bioactive glasses, glass-ceramics, composites, antimicrobial compounds, and nanostructures for biomedical applications.
Rajender Varma (H-Index 117, Highly Cited Res. 2016, 18, 19, 20, 21; Publons Awardee 2018–19) was born in India (Ph.D., Delhi University 1976) and is a senior scientist at U.S. EPA with a visiting position at RCPTM, Palacky University, Olomouc, Czech Republic. He has over 48 years of multidisciplinary research experience ranging from eco-friendly synthetic methods using microwaves, ultrasound, etc. to greener assembly of nanomaterials and sustainable appliances of magnetically retrievable nanocatalysts in benign media. He is a member of the editorial advisory board of several international journals, has published over 760 papers, and has been awarded 17 U.S. Patents, 6 books, 26 book chapters, and 3 encyclopedia contributions with 52,000 plus citations.
Pooyan Makvandi holds two Ph.D. degrees, one in polymer chemistry from University of Mazandaran in Iran and the second in Biomaterials Science and Engineering from University of Naples Federico II, Italy. Currently, he works at Center for Materials Interfaces (CMI), Istituto Italiano di Tecnologia, Pisa, Italy. His work focuses on smart and responsive materials, 3D printing, antimicrobial compounds, and nanostructures for various biomedical applications, including tissue regeneration, drug delivery, and cancer therapy.
The authors declare no competing financial interest.
References
- Baralic I.; Andjelkovic M.; Djordjevic B.; Dikic N.; Radivojevic N.; Suzin-Zivkovic V.; Radojevic-Skodric S.; Pejic S. Effect of Astaxanthin Supplementation on Salivary IgA, Oxidative Stress, and Inflammation in Young Soccer Players. Evidence-Based Complementary and Alternative Medicine 2015, 2015, 783761. 10.1155/2015/783761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Nagarajan D.; Zhang Q.; Chang J. S.; Lee D. J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36 (1), 54–67. 10.1016/j.biotechadv.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Chuyen H. V.; Roach P. D.; Golding J. B.; Parks S. E.; Nguyen M. H. Encapsulation of carotenoid-rich oil from Gac peel: Optimisation of the encapsulating process using a spray drier and the storage stability of encapsulated powder. Powder Technol. 2019, 344, 373–379. 10.1016/j.powtec.2018.12.012. [DOI] [Google Scholar]
- Taksima T.; Limpawattana M.; Klaypradit W. Astaxanthin encapsulated in beads using ultrasonic atomizer and application in yogurt as evaluated by consumer sensory profile. LWT - Food Science and Technology 2015, 62 (1, Part 2), 431–437. 10.1016/j.lwt.2015.01.011. [DOI] [Google Scholar]
- Higuera-Ciapara I.; Félix-Valenzuela L.; Goycoolea F. M. Astaxanthin: a review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46 (2), 185–196. 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- Tirado D. F.; Palazzo I.; Scognamiglio M.; Calvo L.; Della Porta G.; Reverchon E. Astaxanthin encapsulation in ethyl cellulose carriers by continuous supercritical emulsions extraction: A study on particle size, encapsulation efficiency, release profile and antioxidant activity. J. Supercrit. Fluids 2019, 150, 128–136. 10.1016/j.supflu.2019.04.017. [DOI] [Google Scholar]
- Liu G.; Hu M.; Zhao Z.; Lin Q.; Wei D.; Jiang Y. Enhancing the stability of astaxanthin by encapsulation in poly (l-lactic acid) microspheres using a supercritical anti-solvent process. Particuology 2019, 44, 54–62. 10.1016/j.partic.2018.04.006. [DOI] [Google Scholar]
- Qiang M.; Pang X.; Ma D.; Ma C.; Liu F. Effect of membrane surface modification using chitosan hydrochloride and lactoferrin on the properties of astaxanthin-loaded liposomes. Molecules 2020, 25 (3), 610. 10.3390/molecules25030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S. A.; Feng S.-S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30 (10), 2512–2522. 10.1007/s11095-012-0958-3. [DOI] [PubMed] [Google Scholar]
- Sangsuriyawong A.; Limpawattana M.; Siriwan D.; Klaypradit W. Properties and bioavailability assessment of shrimp astaxanthin loaded liposomes. Food Sci. Biotechnol. 2019, 28 (2), 529–537. 10.1007/s10068-018-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostamabadi H.; Falsafi S. R.; Jafari S. M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Controlled Release 2019, 298, 38–67. 10.1016/j.jconrel.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Hu Q.; Hu S.; Fleming E.; Lee J.-Y.; Luo Y. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity. Int. J. Biol. Macromol. 2020, 151, 747–756. 10.1016/j.ijbiomac.2020.02.170. [DOI] [PubMed] [Google Scholar]
- Rabiee N.; Bagherzadeh M.; Ghadiri A. M.; Kiani M.; Fatahi Y.; Tavakolizadeh M.; Pourjavadi A.; Jouyandeh M.; Saeb M. R.; Mozafari M. Multifunctional 3D Hierarchical Bioactive Green Carbon-Based Nanocomposites. ACS Sustainable Chem. Eng. 2021, 9 (26), 8706–8720. 10.1021/acssuschemeng.1c00781. [DOI] [Google Scholar]
- Rabiee N.; Khatami M.; Jamalipour Soufi G.; Fatahi Y.; Iravani S.; Varma R. S. Diatoms with Invaluable Applications in Nanotechnology, Biotechnology, and Biomedicine: Recent Advances. ACS Biomater. Sci. Eng. 2021, 7 (7), 3053–3068. 10.1021/acsbiomaterials.1c00475. [DOI] [PubMed] [Google Scholar]
- Rahimnejad M.; Nasrollahi Boroujeni N.; Jahangiri S.; Rabiee N.; Rabiee M.; Makvandi P.; Akhavan O.; Varma R. S. Prevascularized Micro-/Nano-Sized Spheroid/Bead Aggregates for Vascular Tissue Engineering. Nano-Micro Lett. 2021, 13 (1), 1–24. 10.1007/s40820-021-00697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare H.; Ahmadi S.; Ghasemi A.; Ghanbari M.; Rabiee N.; Bagherzadeh M.; Karimi M.; Webster T. J.; Hamblin M. R.; Mostafavi E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681. 10.2147/IJN.S299448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balietti M.; Giannubilo S. R.; Giorgetti B.; Solazzi M.; Turi A.; Casoli T.; Ciavattini A.; Fattorettia P. The effect of astaxanthin on the aging rat brain: gender-related differences in modulating inflammation. J. Sci. Food Agric. 2016, 96 (2), 615–618. 10.1002/jsfa.7131. [DOI] [PubMed] [Google Scholar]
- Angell A.; de Nys R.; Mangott A.; Vucko M. J. The effects of concentration and supplementation time of natural and synthetic sources of astaxanthin on the colouration of the prawn Penaeus monodon. Algal Res. 2018, 35, 577–585. 10.1016/j.algal.2018.09.031. [DOI] [Google Scholar]
- Jin Y.; Zhang C.; Liu W.; Tang Y.; Qi H.; Chen H.; Cao S. The alcohol dehydrogenase gene family in melon (Cucumis melo L.): bioinformatic analysis and expression patterns. Front. Plant Sci. 2016, 7, 670. 10.3389/fpls.2016.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerkeng B.; Peisker M.; Von Schwartzenberg K.; Ytrestøyl T.; Åsgård T. Digestibility and muscle retention of astaxanthin in Atlantic salmon, Salmo salar, fed diets with the red yeast Phaffia rhodozyma in comparison with synthetic formulated astaxanthin. Aquaculture 2007, 269 (1–4), 476–489. 10.1016/j.aquaculture.2007.04.070. [DOI] [Google Scholar]
- Kanwugu O. N.; Rao A. R.; Ravishankar G. A.; Glukhareva T. V.; Kovaleva E. G.. Astaxanthin from bacteria as a feed supplement for animals. In Global Perspectives on Astaxanthin; Elsevier: 2021; pp 647–667. [Google Scholar]
- https://www.algatech.com/ (Accessed September 25 2020). In.
- https://astareal.com/en/ (Accessed September 25 2020).
- https://www.cyanotech.com/ (Accessed September 25 2020).
- Rodríguez-Sifuentes L.; Marszalek J.; Hernández-Carbajal G.; Chuck-Hernández C. Importance of Downstream Processing of Natural Astaxanthin for Pharmaceutical Application. Front. Chem. Eng. 2021, 2, 601483. 10.3389/fceng.2020.601483. [DOI] [Google Scholar]
- Li X.; Wang X.; Duan C.; Yi S.; Gao Z.; Xiao C.; Agathos S. N.; Wang G.; Li J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. 10.1016/j.biotechadv.2020.107602. [DOI] [PubMed] [Google Scholar]
- Jackson H.; Braun C. L.; Ernst H. The Chemistry of Novel Xanthophyll Carotenoids. Am. J. Cardiol. 2008, 101 (10, Supplement), S50–S57. 10.1016/j.amjcard.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Bagherzadeh M.; Rabiee N.; Fatahi Y.; Dinarvand R. Zn-rich (GaN) 1– x (ZnO) x: a biomedical friend?. New J. Chem. 2021, 45 (8), 4077–4089. 10.1039/D0NJ06310J. [DOI] [Google Scholar]
- Rabiee N.; Bagherzadeh M.; Ghadiri A. M.; Kiani M.; Aldhaher A.; Ramakrishna S.; Tahriri M.; Tayebi L.; Webster T. J. Green synthesis of ZnO NPs via Salvia hispanica: Evaluation of potential antioxidant, antibacterial, mammalian cell viability, H1N1 influenza virus inhibition and photocatalytic activities. J. Biomed. Nanotechnol. 2020, 16 (4), 456–466. 10.1166/jbn.2020.2916. [DOI] [PubMed] [Google Scholar]
- Rabiee N.; Bagherzadeh M.; Kiani M.; Ghadiri A. M.; Zhang K.; Jin Z.; Ramakrishna S.; Shokouhimehr M. High gravity-assisted green synthesis of ZnO nanoparticles via Allium ursinum: Conjoining nanochemistry to neuroscience. Nano Express 2020, 1 (2), 020025. 10.1088/2632-959X/abac4d. [DOI] [Google Scholar]
- Higuera-Ciapara I.; Felix-Valenzuela L.; Goycoolea F. Astaxanthin: a review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46 (2), 185–196. 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- Kamath B. S.; Srikanta B. M.; Dharmesh S. M.; Sarada R.; Ravishankar G. A. Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur. J. Pharmacol. 2008, 590 (1–3), 387–395. 10.1016/j.ejphar.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Yang L.; Qiao X.; Gu J.; Li X.; Cao Y.; Xu J.; Xue C. Influence of molecular structure of astaxanthin esters on their stability and bioavailability. Food Chem. 2021, 343, 128497. 10.1016/j.foodchem.2020.128497. [DOI] [PubMed] [Google Scholar]
- Chen Y.-Y.; Lee P.-C.; Wu Y.-L.; Liu L.-Y. In vivo effects of free form astaxanthin powder on anti-oxidation and lipid metabolism with high-cholesterol diet. PLoS One 2015, 10 (8), e0134733. 10.1371/journal.pone.0134733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y.; Ishikura M.; Maoka T. Bioavailability of astaxanthin in Haematococcus algal extract: the effects of timing of diet and smoking habits. Biosci., Biotechnol., Biochem. 2009, 73 (9), 1928–1932. 10.1271/bbb.90078. [DOI] [PubMed] [Google Scholar]
- Liu Z.-W.; Zhou Y.-X.; Wang L.-H.; Ye Z.; Liu L.-J.; Cheng J.-H.; Wang F.; Bekhit A. E.-D.; Aadil R. M. Multi-spectroscopies and molecular docking insights into the interaction mechanism and antioxidant activity of astaxanthin and β-lactoglobulin nanodispersions. Food Hydrocolloids 2021, 117, 106739. 10.1016/j.foodhyd.2021.106739. [DOI] [Google Scholar]
- Mimoun-Benarroch M.; Hogot C.; Rhazi L.; Niamba C.-N.; Dépeint F. The Bioavailability of Astaxanthin Is Dependent on Both the Source and the Isomeric Variants of the Molecule. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca, Food Sci. Technol. 2016, 73, 61–69. 10.15835/buasvmcn-fst:12350. [DOI] [Google Scholar]
- Su F.; Xu H.; Yang N.; Liu W.; Liu J. Hydrolytic efficiency and isomerization during de-esterification of natural astaxanthin esters by saponification and enzymolysis. Electron. J. Biotechnol. 2018, 34, 37–42. 10.1016/j.ejbt.2018.05.002. [DOI] [Google Scholar]
- Mimoun-Benarroch M.; Hogot C.; Rhazi L.; Niamba C.; Depeint F. The Bioavailability of Astaxanthin Is Dependent on Both the Source and the Isomeric Variants of the Molecule. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca, Food Sci. Technol. 2016, 73, 61. 10.15835/buasvmcn-fst:12350. [DOI] [Google Scholar]
- Rao A. R.; Sarada R.; Shylaja M. D.; Ravishankar G. Evaluation of hepatoprotective and antioxidant activity of astaxanthin and astaxanthin esters from microalga-Haematococcus pluvialis. J. Food Sci. Technol. 2015, 52 (10), 6703–6710. 10.1007/s13197-015-1775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W.; Lin H.; Zhai Y.; Cao L.; Leng K.; Xing L. Separation, Purification, and Identification of (3S, 3′ S)-trans-Astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2015, 50 (9), 1377–1383. 10.1080/01496395.2014.976873. [DOI] [Google Scholar]
- Sun W.; Lin H.; Zhai Y.; Cao L.; Leng K.; Xing L. Separation, Purification, and Identification of (3S,3′S)-trans-Astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2015, 50 (9), 1377–1383. 10.1080/01496395.2014.976873. [DOI] [Google Scholar]
- Gallego R.; Arena K.; Dugo P.; Mondello L.; Ibáñez E.; Herrero M. Application of compressed fluid–based extraction and purification procedures to obtain astaxanthin-enriched extracts from Haematococcus pluvialis and characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry. Anal. Bioanal. Chem. 2020, 412 (3), 589–599. 10.1007/s00216-019-02287-y. [DOI] [PubMed] [Google Scholar]
- Fábryová T.; Tůmová L.; da Silva D. C.; Pereira D. M.; Andrade P. B.; Valentão P.; Hrouzek P.; Kopecký J.; Cheel J. Isolation of astaxanthin monoesters from the microalgae Haematococcus pluvialis by high performance countercurrent chromatography (HPCCC) combined with high performance liquid chromatography (HPLC). Algal Res. 2020, 49, 101947. 10.1016/j.algal.2020.101947. [DOI] [Google Scholar]
- Jaime L.; Rodríguez-Meizoso I.; Cifuentes A.; Santoyo S.; Suarez S.; Ibáñez E.; Señorans F. J. Pressurized liquids as an alternative process to antioxidant carotenoids’ extraction from Haematococcus pluvialis microalgae. LWT - Food Science and Technology 2010, 43 (1), 105–112. 10.1016/j.lwt.2009.06.023. [DOI] [Google Scholar]
- Kobayashi M.; Sakamoto Y. Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnol. Lett. 1999, 21 (4), 265–269. 10.1023/A:1005445927433. [DOI] [Google Scholar]
- Cerón M. C.; García-Malea M. C.; Rivas J.; Acien F. G.; Fernandez J. M.; Del Río E.; Guerrero M. G.; Molina E. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl. Microbiol. Biotechnol. 2007, 74 (5), 1112–1119. 10.1007/s00253-006-0743-5. [DOI] [PubMed] [Google Scholar]
- Régnier P.; Bastias J.; Rodriguez-Ruiz V.; Caballero-Casero N.; Caballo C.; Sicilia D.; Fuentes A.; Maire M.; Crepin M.; Letourneur D.; Gueguen V.; Rubio S.; Pavon-Djavid G.. Astaxanthin from Haematococcus pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Mar. Drugs 2015, 13 ( (5), ).2857. 10.3390/md13052857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier P.; Bastias J.; Rodriguez-Ruiz V.; Caballero-Casero N.; Caballo C.; Sicilia D.; Fuentes A.; Maire M.; Crepin M.; Letourneur D.; Gueguen V.; Rubio S.; Pavon-Djavid G. Astaxanthin from Haematococcus pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Mar. Drugs 2015, 13 (5), 2857–2874. 10.3390/md13052857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. R.; Sindhuja H. N.; Dharmesh S. M.; Sankar K. U.; Sarada R.; Ravishankar G. A. Effective Inhibition of Skin Cancer, Tyrosinase, and Antioxidative Properties by Astaxanthin and Astaxanthin Esters from the Green Alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61 (16), 3842–3851. 10.1021/jf304609j. [DOI] [PubMed] [Google Scholar]
- Aoi W.; Maoka T.; Abe R.; Fujishita M.; Tominaga K. Comparison of the effect of non-esterified and esterified astaxanthins on endurance performance in mice. J. Clin. Biochem. Nutr. 2018, 62 (2), 161–166. 10.3164/jcbn.17-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.; Chen F.; Zhao G.; Wang Z.; Liao X.; Hu X. Isomerization of trans-astaxanthin induced by copper (II) ion in ethanol. J. Agric. Food Chem. 2005, 53 (24), 9620–9623. 10.1021/jf0517750. [DOI] [PubMed] [Google Scholar]
- Honda M.; Kageyama H.; Hibino T.; Sowa T.; Kawashima Y. Efficient and environmentally friendly method for carotenoid extraction from Paracoccus carotinifaciens utilizing naturally occurring Z-isomerization-accelerating catalysts. Process Biochem. 2020, 89, 146–154. 10.1016/j.procbio.2019.10.005. [DOI] [Google Scholar]
- Viazau Y. V.; Goncharik R. G.; Kulikova I. S.; Kulikov E. A.; Vasilov R. G.; Selishcheva A. A. E/Z isomerization of astaxanthin and its monoesters in vitro under the exposure to light or heat and in overilluminated Haematococcus pluvialis cells. Bioresour. Bioprocess. 2021, 8 (1), 1–13. 10.1186/s40643-021-00410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.; Zhang H.; Liu R.; Zhu H.; Zhang L.; Tsao R. Bioaccessibility, cellular uptake, and transport of astaxanthin isomers and their antioxidative effects in human intestinal epithelial Caco-2 cells. J. Agric. Food Chem. 2017, 65 (47), 10223–10232. 10.1021/acs.jafc.7b04254. [DOI] [PubMed] [Google Scholar]
- Honda M.; Kageyama H.; Hibino T.; Zhang Y.; Diono W.; Kanda H.; Yamaguchi R.; Takemura R.; Fukaya T.; Goto M. Improved carotenoid processing with sustainable solvents utilizing Z-isomerization-induced alteration in physicochemical properties: A review and future directions. Molecules 2019, 24 (11), 2149. 10.3390/molecules24112149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.; Hassan Y. I.; Liu R.; Zhang H.; Chen Y.; Zhang L.; Tsao R. Anti-inflammatory effects of different astaxanthin isomers and the roles of lipid transporters in the cellular transport of astaxanthin isomers in Caco-2 cell monolayers. J. Agric. Food Chem. 2019, 67 (22), 6222–6231. 10.1021/acs.jafc.9b02102. [DOI] [PubMed] [Google Scholar]
- Liu X.; Chen X.; Liu H.; Cao Y. Antioxidation and anti-aging activities of astaxanthin geometrical isomers and molecular mechanism involved in Caenorhabditis elegans. J. Funct. Foods 2018, 44, 127–136. 10.1016/j.jff.2018.03.004. [DOI] [Google Scholar]
- Honda M.; Maeda H.; Fukaya T.; Goto M. Effects of Z-isomerization on the bioavailability and functionality of carotenoids: a review. Prog. Carotenoid Res. 2018, 139–159. 10.5772/intechopen.78309. [DOI] [Google Scholar]
- Liu X.; Osawa T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 2007, 357 (1), 187–193. 10.1016/j.bbrc.2007.03.120. [DOI] [PubMed] [Google Scholar]
- Yang C.; Zhang L.; Zhang H.; Sun Q.; Liu R.; Li J.; Wu L.; Tsao R. Rapid and efficient conversion of all-E-astaxanthin to 9 Z-and 13 Z-isomers and assessment of their stability and antioxidant activities. J. Agric. Food Chem. 2017, 65 (4), 818–826. 10.1021/acs.jafc.6b04962. [DOI] [PubMed] [Google Scholar]
- Liu X.; Osawa T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 2007, 357 (1), 187–93. 10.1016/j.bbrc.2007.03.120. [DOI] [PubMed] [Google Scholar]
- Coral-Hinostroza G.; Ytrestøyl T.; Ruyter B.; Bjerkeng B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3 ′ R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2004, 139, 99–110. 10.1016/j.cca.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Bjerkeng B.; Følling M.; Lagocki S.; Storebakken T.; Olli J. J.; Alsted N. Bioavailability of all-E-astaxanthin and Z-isomers of astaxanthin in rainbow trout (Oncorhynchus mykiss). Aquaculture 1997, 157 (1), 63–82. 10.1016/S0044-8486(97)00146-4. [DOI] [Google Scholar]
- Østerlie M.; Bjerkeng B.; Liaaen-Jensen S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin11Preliminary results from the present study were presented at the First International Congress on Pigments in Food Technology, March 24–26, 1999, Seville, Spain1 and the 12th International Symposium on Carotenoids (Book of Abstracts, p. 72), July 18–23, 1999, Cairns, Australia. J. Nutr. Biochem. 2000, 11 (10), 482–490. 10.1016/S0955-2863(00)00104-2. [DOI] [PubMed] [Google Scholar]
- Dizon M.; Tatarko M.; Szabo K.; Hianik T. Application of high-resolution ultrasonic spectroscopy for detection of the plasmin activity toward β-casein. Food Chem. 2021, 353, 129373. 10.1016/j.foodchem.2021.129373. [DOI] [PubMed] [Google Scholar]
- Irshad M.; Myint A. A.; Hong M. E.; Kim J.; Sim S. J. One-pot, simultaneous cell wall disruption and complete extraction of astaxanthin from Haematococcus pluvialis at room temperature. ACS Sustainable Chem. Eng. 2019, 7 (16), 13898–13910. 10.1021/acssuschemeng.9b02089. [DOI] [Google Scholar]
- Damiani M. C.; Leonardi P. I.; Pieroni O. I.; Cáceres E. J. Ultrastructure of the cyst wall of Haematococcus pluvialis (Chlorophyceae): wall development and behaviour during cyst germination. Phycologia 2006, 45 (6), 616–623. 10.2216/05-27.1. [DOI] [Google Scholar]
- Hagen C.; Siegmund S.; Braune W. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. Eur. J. Phycol. 2002, 37 (2), 217–226. 10.1017/S0967026202003669. [DOI] [Google Scholar]
- Kim D.-Y.; Vijayan D.; Praveenkumar R.; Han J.-I.; Lee K.; Park J.-Y.; Chang W.-S.; Lee J.-S.; Oh Y.-K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. 10.1016/j.biortech.2015.08.107. [DOI] [PubMed] [Google Scholar]
- Choi S.-A.; Oh Y.-K.; Lee J.; Sim S. J.; Hong M. E.; Park J.-Y.; Kim M.-S.; Kim S. W.; Lee J.-S. High-efficiency cell disruption and astaxanthin recovery from Haematococcus pluvialis cyst cells using room-temperature imidazolium-based ionic liquid/water mixtures. Bioresour. Technol. 2019, 274, 120–126. 10.1016/j.biortech.2018.11.082. [DOI] [PubMed] [Google Scholar]
- Molino A.; Mehariya S.; Iovine A.; Larocca V.; Di Sanzo G.; Martino M.; Casella P.; Chianese S.; Musmarra D. Extraction of astaxanthin and lutein from microalga Haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction technology with ethanol as co-solvent. Mar. Drugs 2018, 16 (11), 432. 10.3390/md16110432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Cheng L.-H.; Xu X.-H.; Zhang L.; Chen H.-L. Screening of biocompatible organic solvents for enhancement of lipid milking from Nannochloropsis sp. Process Biochem. 2011, 46 (10), 1934–1941. 10.1016/j.procbio.2011.06.024. [DOI] [Google Scholar]
- Praveenkumar R.; Gwak R.; Kang M.; Shim T. S.; Cho S.; Lee J.; Oh Y.-K.; Lee K.; Kim B. Regenerative astaxanthin extraction from a single microalgal (Haematococcus pluvialis) cell using a gold nano-scalpel. ACS Appl. Mater. Interfaces 2015, 7 (40), 22702–22708. 10.1021/acsami.5b07651. [DOI] [PubMed] [Google Scholar]
- Choi H. D.; Youn Y. K.; Shin W. G. Positive Effects of Astaxanthin on Lipid Profiles and Oxidative Stress in Overweight Subjects. Plant Foods Hum. Nutr. 2011, 66 (4), 363–369. 10.1007/s11130-011-0258-9. [DOI] [PubMed] [Google Scholar]
- Tyssandier V.; Choubert G.; Grolier P.; Borel P. Carotenoids, mostly the xanthophylls, exchange between plasma lipoproteins. Int. J. Vitam. Nutr. Res. 2002, 72 (5), 300–308. 10.1024/0300-9831.72.5.300. [DOI] [PubMed] [Google Scholar]
- Miki W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63 (1), 141–146. 10.1351/pac199163010141. [DOI] [Google Scholar]
- Brotosudarmo T. H. P.; Limantara L.; Setiyono E.; Heriyanto Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 2156582. 10.1155/2020/2156582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Álvarez Ó.; Calvo M. M.; Gómez-Estaca J. Recent Advances in Astaxanthin Micro/Nanoencapsulation to Improve Its Stability and Functionality as a Food Ingredient. Mar. Drugs 2020, 18 (8), 406. 10.3390/md18080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A.; Tsuji S.; Okada Y.; Murakami N.; Urami M.; Nakagawa K.; Ishikura M.; Katagiri M.; Koga Y.; Shirasawa T. Preliminary clinical evaluation of toxicity and efficacy of a new astaxanthin-rich Haematococcus pluvialis extract. J. Clin. Biochem. Nutr. 2009, 44 (3), 280–284. 10.3164/jcbn.08-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu T.; Xuan R.; Jiang L.; Wu W.; Zhen Z.; Song Y.; Hong L.; Zheng K.; Zhang J.; Xu Q.; Tan Y.; Yan X.; Chen H. Astaxanthin Induces the Nrf2/HO-1 Antioxidant Pathway in Human Umbilical Vein Endothelial Cells by Generating Trace Amounts of ROS. J. Agric. Food Chem. 2018, 66 (6), 1551–1559. 10.1021/acs.jafc.7b05493. [DOI] [PubMed] [Google Scholar]
- Young I. S.; Woodside J. V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54 (3), 176. 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35 (5), 1147–1150. 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- Ambati R. R.; Sarada R.; Baskaran V.; Gokare R. Identification of carotenoids from green alga Haematococcus pluvialis by HPLC and LC-MS (APCI) and their antioxidant properties. J. Microbiol. Biotechnol. 2009, 19 (11), 1333–41. 10.4014/jmb.0905.03007. [DOI] [PubMed] [Google Scholar]
- Ranga Rao A.; Raghunath Reddy R. L.; Baskaran V.; Sarada R.; Ravishankar G. A. Characterization of Microalgal Carotenoids by Mass Spectrometry and Their Bioavailability and Antioxidant Properties Elucidated in Rat Model. J. Agric. Food Chem. 2010, 58 (15), 8553–8559. 10.1021/jf101187k. [DOI] [PubMed] [Google Scholar]
- Hix L. M.; Frey D. A.; McLaws M. D.; Østerlie M.; Lockwood S. F.; Bertram J. S. Inhibition of chemically-induced neoplastic transformation by a novel tetrasodium diphosphate astaxanthin derivative. Carcinogenesis 2005, 26 (9), 1634–1641. 10.1093/carcin/bgi121. [DOI] [PubMed] [Google Scholar]
- Parisi V.; Tedeschi M.; Gallinaro G.; Varano M.; Saviano S.; Piermarocchi S. Carotenoids and Antioxidants in Age-Related Maculopathy Italian Study: Multifocal Electroretinogram Modifications after 1 Year. Ophthalmology 2008, 115 (2), 324–333. 10.1016/j.ophtha.2007.05.029. [DOI] [PubMed] [Google Scholar]; e2.
- Jyonouchi H.; Zhang L.; Gross M.; Tomita Y. Immunomodulating actions of carotenoids: Enhancement of in vivo and in vitro antibody production to T-dependent antigens. Nutr. Cancer 1994, 21 (1), 47–58. 10.1080/01635589409514303. [DOI] [PubMed] [Google Scholar]
- Lin K.-H.; Lin K.-C.; Lu W.-J.; Thomas P.-A.; Jayakumar T.; Sheu J.-R. Astaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-γ and IL-2 secretion in primary cultured lymphocytes in vitro and ex vivo. Int. J. Mol. Sci. 2016, 17 (1), 44. 10.3390/ijms17010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatoor A. S.; Al Humayed S. Astaxanthin Ameliorates high-fat diet-induced cardiac damage and fibrosis by upregulating and activating SIRT1. Saudi J. Biol. Sci. 2021, 28 (12), 7012–7021. 10.1016/j.sjbs.2021.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J. S.; Sharman J. E.; Fassett R. G. Astaxanthin has no effect on arterial stiffness, oxidative stress, or inflammation in renal transplant recipients: a randomized controlled trial (the XANTHIN trial). Am. J. Clin. Nutr. 2016, 103 (1), 283–289. 10.3945/ajcn.115.115477. [DOI] [PubMed] [Google Scholar]
- Brown D. R.; Gough L. A.; Deb S. K.; Sparks S. A.; McNaughton L. R. Astaxanthin in Exercise Metabolism, Performance and Recovery: A Review. Frontiers in Nutrition 2018, 4, 76 10.3389/fnut.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T.; Kasai T.; Sato A.; Ishiwata S.; Yatsu S.; Matsumoto H.; Shitara J.; Murata A.; Shimizu M.; Suda S.; Hiki M.; Naito R.; Daida H. Effects of 3-Month Astaxanthin Supplementation on Cardiac Function in Heart Failure Patients with Left Ventricular Systolic Dysfunction-A Pilot Study. Nutrients 2020, 12 (6), 1896. 10.3390/nu12061896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza L.; Pesce M.; Patruno A.; Franceschelli S.; Lutiis M. A. d.; Grilli A.; Felaco M. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U937: SHP-1 as a novel biological target. Mar. Drugs 2012, 10 (4), 890–899. 10.3390/md10040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.; Zhang J.-j.; Wang M.-r.; Liu W.-b.; Gu X.-b.; Lv C.-J. Astaxanthin induces mitochondria-mediated apoptosis in rat hepatocellular carcinoma CBRH-7919 cells. Biol. Pharm. Bull. 2011, 34, 839–844. 10.1248/bpb.34.839. [DOI] [PubMed] [Google Scholar]
- Kim K.-N.; Heo S.-J.; Kang S.-M.; Ahn G.; Jeon Y.-J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. In Vitro 2010, 24 (6), 1648–1654. 10.1016/j.tiv.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Al-Amin M. M.; Akhter S.; Hasan A. T.; Alam T.; Nageeb Hasan S. M.; Saifullah A. R. M.; Shohel M. The antioxidant effect of astaxanthin is higher in young mice than aged: a region specific study on brain. Metab. Brain Dis. 2015, 30 (5), 1237–1246. 10.1007/s11011-015-9699-4. [DOI] [PubMed] [Google Scholar]
- Ying C.-j.; Zhang F.; Zhou X.-y.; Hu X.-t.; Chen J.; Wen X.-r.; Sun Y.; Zheng K.-y.; Tang R.-x.; Song Y.-j. Anti-inflammatory effect of astaxanthin on the sickness behavior induced by diabetes mellitus. Cell. Mol. Neurobiol. 2015, 35 (7), 1027–1037. 10.1007/s10571-015-0197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma K.; Nakajima H.; Ohtsuki M.; Imokawa G. Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J. Dermatol. Sci. 2010, 58 (2), 136–142. 10.1016/j.jdermsci.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Tominaga K.; Hongo N.; Karato M.; Yamashita E.. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59 ( (1), ). 10.18388/abp.2012_2168 [DOI] [PubMed] [Google Scholar]
- Fakhri S.; Aneva I. Y.; Farzaei M. H.; Sobarzo-Sánchez E.. The neuroprotective effects of astaxanthin: therapeutic targets and clinical perspective. Molecules 2019, 24 ( (14), ).2640. 10.3390/molecules24142640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasso C.; Orefice I.; Pellone P.; Cirino P.; Miele R.; Ianora A.; Brunet C.; Sansone C.. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16 ( (8), ).247. 10.3390/md16080247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobos P.; Bruna B.; Cordova A.; Barattini P.; Galáz J. L.; Adasme T.; Hidalgo C.; Muñoz P.; Paula-Lima A.. Astaxanthin protects primary hippocampal neurons against noxious effects of Aβ-oligomers. Neural Plast. 2016, 2016.1. 10.1155/2016/3456783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davinelli S.; Nielsen M. E.; Scapagnini G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10 (4), 522. 10.3390/nu10040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L.; Li X.; Huang R.; Luo H. Exploring the mechanism of astaxanthin against lipopolysaccharide-induced acute lung injury by network pharmacology and experimental validation. Research Square 2021, 10, 1–14. 10.21203/rs.3.rs-334157/v1. [DOI] [Google Scholar]
- Guo S.; Guo L.; Fang Q.; Yu M.; Zhang L.; You C.; Wang X.; Liu Y.; Han C. Astaxanthin protects against early acute kidney injury in severely burned rats by inactivating the TLR4/MyD88/NF-κB axis and upregulating heme oxygenase-1. Sci. Rep. 2021, 11 (1), 6679. 10.1038/s41598-021-86146-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y.; Ohgami K.; Shiratori K.; Jin X. H.; Ilieva I.; Koyama Y.; Yazawa K.; Yoshida K.; Kase S.; Ohno S. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-kappaB signaling pathway. Exp. Eye Res. 2006, 82 (2), 275–281. 10.1016/j.exer.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Giannaccare G.; Pellegrini M.; Senni C.; Bernabei F.; Scorcia V.; Cicero A. F. G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18 (5), 239. 10.3390/md18050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaafan M. A.; Abdelhamid A. M. The cardioprotective effect of astaxanthin against isoprenaline-induced myocardial injury in rats: involvement of TLR4/NF-κB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2021, 25 (11), 4099–4105. 10.26355/eurrev_202106_26052. [DOI] [PubMed] [Google Scholar]
- Zarneshan S. N.; Fakhri S.; Farzaei M. H.; Khan H.; Saso L. Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem. Toxicol. 2020, 145, 111714. 10.1016/j.fct.2020.111714. [DOI] [PubMed] [Google Scholar]
- Lai T. T.; Yang C. M.; Yang C. H.. Astaxanthin Protects Retinal Photoreceptor Cells against High Glucose-Induced Oxidative Stress by Induction of Antioxidant Enzymes via the PI3K/Akt/Nrf2 Pathway. Antioxidants (Basel, Switzerland) 2020, 9 ( (8), ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J.; Sun H.; Wei H.; Dong M.; Zhang Y.; Xu W.; Fang Y.; Zhao J. Astaxanthin alleviates spinal cord ischemia-reperfusion injury via activation of PI3K/Akt/GSK-3β pathway in rats. J. Orthop. Surg. Res. 2020, 15 (1), 275. 10.1186/s13018-020-01790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarneshan S. N.; Fakhri S.; Farzaei M. H.; Khan H.; Saso L. Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem. Toxicol. 2020, 145, 111714. 10.1016/j.fct.2020.111714. [DOI] [PubMed] [Google Scholar]
- Reinhold A. K.; Rittner H. L. Barrier function in the peripheral and central nervous system—a review. Pfluegers Arch. 2017, 469 (1), 123–134. 10.1007/s00424-016-1920-8. [DOI] [PubMed] [Google Scholar]
- Berndt P.; Winkler L.; Cording J.; Breitkreuz-Korff O.; Rex A.; Dithmer S.; Rausch V.; Blasig R.; Richter M.; Sporbert A.; Wolburg H.; Blasig I. E.; Haseloff R. F. Tight junction proteins at the blood–brain barrier: far more than claudin-5. Cell. Mol. Life Sci. 2019, 76 (10), 1987–2002. 10.1007/s00018-019-03030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costea L.; Mészáros Á.; Bauer H.; Bauer H.-C.; Traweger A.; Wilhelm I.; Farkas A. E.; Krizbai I. A. The Blood–Brain Barrier and Its Intercellular Junctions in Age-Related Brain Disorders. Int. J. Mol. Sci. 2019, 20 (21), 5472. 10.3390/ijms20215472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdő F.; Denes L.; de Lange E. Age-associated physiological and pathological changes at the blood–brain barrier: a review. J. Cereb. Blood Flow Metab. 2017, 37 (1), 4–24. 10.1177/0271678X16679420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M. D.; Zhao Z.; Montagne A.; Nelson A. R.; Zlokovic B. V. Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 2019, 99 (1), 21–78. 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullahi W.; Davis T. P.; Ronaldson P. T. Functional Expression of P-glycoprotein and Organic Anion Transporting Polypeptides at the Blood-Brain Barrier: Understanding Transport Mechanisms for Improved CNS Drug Delivery?. AAPS J. 2017, 19 (4), 931–939. 10.1208/s12248-017-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo F. A.; Carvalho L. R.; Grinberg L. T.; Farfel J. M.; Ferretti R. E.; Leite R. E.; Filho W. J.; Lent R.; Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513 (5), 532–541. 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Masgrau R.; Guaza C.; Ransohoff R. M.; Galea E. Should We Stop Saying ‘Glia’ and ‘Neuroinflammation’?. Trends Mol. Med. 2017, 23 (6), 486–500. 10.1016/j.molmed.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Wilhelm I.; Krizbai I. A. In Vitro Models of the Blood–Brain Barrier for the Study of Drug Delivery to the Brain. Mol. Pharmaceutics 2014, 11 (7), 1949–1963. 10.1021/mp500046f. [DOI] [PubMed] [Google Scholar]
- Vega R. A.; Zhang Y.; Curley C.; Price R. L.; Abounader R. 370 magnetic resonance-guided focused ultrasound delivery of polymeric brain-penetrating nanoparticle microRNA conjugates in glioblastoma. Neurosurgery 2016, 63 (CN_suppl_1), 210. 10.1227/01.neu.0000489858.08559.c8. [DOI] [Google Scholar]
- Abbott N. J.; Patabendige A. A. K.; Dolman D. E. M.; Yusof S. R.; Begley D. J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37 (1), 13–25. 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Lin Q.; Liang R.; Williams P. A.; Zhong F. Factors affecting the bioaccessibility of β-carotene in lipid-based microcapsules: Digestive conditions, the composition, structure and physical state of microcapsules. Food Hydrocolloids 2018, 77, 187–203. 10.1016/j.foodhyd.2017.09.034. [DOI] [Google Scholar]
- Lin Q.; Liang R.; Zhong F.; Ye A.; Singh H. Effect of degree of octenyl succinic anhydride (OSA) substitution on the digestion of emulsions and the bioaccessibility of β-carotene in OSA-modified-starch-stabilized-emulsions. Food Hydrocolloids 2018, 84, 303–312. 10.1016/j.foodhyd.2018.05.056. [DOI] [Google Scholar]
- Dube T.; Chibh S.; Mishra J.; Panda J. J. Receptor Targeted Polymeric Nanostructures Capable of Navigating across the Blood-Brain Barrier for Effective Delivery of Neural Therapeutics. ACS Chem. Neurosci. 2017, 8 (10), 2105–2117. 10.1021/acschemneuro.7b00207. [DOI] [PubMed] [Google Scholar]
- Makvandi P.; Chen M.; Sartorius R.; Zarrabi A.; Ashrafizadeh M.; Moghaddam F. D.; Ma J.; Mattoli V.; Tay F. R. Endocytosis of Abiotic Nanomaterials and Nanobiovectors: Inhibition of Membrane Trafficking. Nano Today 2021, 40, 101279. 10.1016/j.nantod.2021.101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.-z.; Cheng Y.; Cai R.-q.; Wang B. D. W.-w.; Cui H.; Liu M.; Zhang B.-l.; Mei Q.-b.; Zhou S.-y. The enhancement of siPLK1 penetration across BBB and its anti glioblastoma activity in vivo by magnet and transferrin co-modified nanoparticle. Nanomedicine 2018, 14 (3), 991–1003. 10.1016/j.nano.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Dixit S.; Novak T.; Miller K.; Zhu Y.; Kenney M. E.; Broome A.-M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7 (5), 1782–1790. 10.1039/C4NR04853A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z.-q.; Wu Q.; Zhou X.-m.; Zhang X.-s.; Yuan B.; Wen L.-l.; Xu W.-d.; Cui S.; Tang X.-l.; Zhang X. Receptor-mediated delivery of Astaxanthin-loaded nanoparticles to neurons: An enhanced potential for subarachnoid hemorrhage treatment. Front. Neurosci. 2019, 13, 989. 10.3389/fnins.2019.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjadian F.; Moghoofei M.; Mirkiani S.; Ghasemi A.; Rabiee N.; Hadifar S.; Beyzavi A.; Karimi M.; Hamblin M. R. Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: Set the bugs to work?. Biotechnol. Adv. 2018, 36 (4), 968–985. 10.1016/j.biotechadv.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiee N.; Ahmadi S.; Afshari R.; Khalaji S.; Rabiee M.; Bagherzadeh M.; Fatahi Y.; Dinarvand R.; Tahriri M.; Tayebi L. Polymeric Nanoparticles for Nasal Drug Delivery to the Brain: Relevance to Alzheimer’s Disease. Adv. Therap. 2021, 4 (3), 2000076. 10.1002/adtp.202000076. [DOI] [Google Scholar]
- Rabiee N.; Bagherzadeh M.; Rabiee M. A perspective to the correlation between Brain insulin resistance and Alzheimer: medicinal chemistry approach. Curr. Diabetes Rev. 2019, 15 (4), 255–258. 10.2174/1573399814666181031154817. [DOI] [PubMed] [Google Scholar]
- Siegal T.; Rubinstein R.; Bokstein F.; Schwartz A.; Lossos A.; Shalom E.; Chisin R.; Gomori J. M. In vivo assessment of the window of barrier opening after osmotic blood-brain barrier disruption in humans. J. Neurosurg. 2000, 92 (4), 599–605. 10.3171/jns.2000.92.4.0599. [DOI] [PubMed] [Google Scholar]
- Rabiee N.; Ahmadvand S.; Ahmadi S.; Fatahi Y.; Dinarvand R.; Bagherzadeh M.; Rabiee M.; Tahriri M.; Tayebi L.; Hamblin M. R. Carbosilane dendrimers: Drug and gene delivery applications. J. Drug Delivery Sci. Technol. 2020, 59, 101879. 10.1016/j.jddst.2020.101879. [DOI] [Google Scholar]
- Rabiee N.; Yaraki M. T.; Garakani S. M.; Garakani S. M.; Ahmadi S.; Lajevardi A.; Bagherzadeh M.; Rabiee M.; Tayebi L.; Tahriri M. Recent advances in porphyrin-based nanocomposites for effective targeted imaging and therapy. Biomaterials 2020, 232, 119707. 10.1016/j.biomaterials.2019.119707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K.; McDannold N.; Vykhodtseva N.; Raymond S.; Weissleder R.; Jolesz F. A.; Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J. Neurosurg. 2006, 105 (3), 445–454. 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- Maghsoudi S.; Shahraki B. T.; Rabiee N.; Fatahi Y.; Dinarvand R.; Tavakolizadeh M.; Ahmadi S.; Rabiee M.; Bagherzadeh M.; Pourjavadi A. Burgeoning polymer nano blends for improved controlled drug release: a review. Int. J. Nanomed. 2020, 15, 4363. 10.2147/IJN.S252237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiee N.; Ahmadi S.; Fatahi Y.; Rabiee M.; Bagherzadeh M.; Dinarvand R.; Bagheri B.; Zarrintaj P.; Saeb M. R.; Webster T. J. Nanotechnology-assisted microfluidic systems: from bench to bedside. Nanomedicine 2021, 16 (3), 237–258. 10.2217/nnm-2020-0353. [DOI] [PubMed] [Google Scholar]
- Fanaee-Danesh E.; Gali C. C.; Tadic J.; Zandl-Lang M.; Kober A. C.; Agujetas V. R.; de Dios C.; Tam-Amersdorfer C.; Stracke A.; Albrecher N. M. Astaxanthin exerts protective effects similar to bexarotene in Alzheimer’s disease by modulating amyloid-beta and cholesterol homeostasis in blood-brain barrier endothelial cells. Biochim. Biophys. Acta, Mol. Basis Dis. 2019, 1865 (9), 2224–2245. 10.1016/j.bbadis.2019.04.019. [DOI] [PubMed] [Google Scholar]
- Bellavance M.-A.; Blanchette M.; Fortin D. Recent Advances in Blood–Brain Barrier Disruption as a CNS Delivery Strategy. AAPS J. 2008, 10 (1), 166–177. 10.1208/s12248-008-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabana P.; Bonthagarala B.; Harini A. L.; Dasari V. Nasal drug delivery: a potential route for brain targeting. Int. J. Adv. Sci. Res. 2015, 1, 65–70. 10.7439/ijasr.v1i2.1782. [DOI] [Google Scholar]
- Nasseri B.; Kocum I. C.; Seymen C. M.; Rabiee N. Penetration depth in nanoparticles incorporated radiofrequency hyperthermia into the tissue: comprehensive study with histology and pathology observations. IET Nanobiotechnol. 2019, 13 (6), 634–639. 10.1049/iet-nbt.2019.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M. Molecular Trojan horses for blood–brain barrier drug delivery. Curr. Opin. Pharmacol. 2006, 6 (5), 494–500. 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Delivery of Biologics Across the Blood–Brain Barrier with Molecular Trojan Horse Technology. BioDrugs 2017, 31 (6), 503–519. 10.1007/s40259-017-0248-z. [DOI] [PubMed] [Google Scholar]
- Thassu D.; Pathak Y.; Deleers M.. Nanoparticulate drug-delivery systems: an overview. CRC Press: 2007. [Google Scholar]
- Rabiee N.; Ahmadi S.; Arab Z.; Bagherzadeh M.; Safarkhani M.; Nasseri B.; Rabiee M.; Tahriri M.; Webster T. J.; Tayebi L. Aptamer hybrid nanocomplexes as targeting components for antibiotic/gene delivery systems and diagnostics: a review. Int. J. Nanomed. 2020, 15, 4237. 10.2147/IJN.S248736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiee N.; Bagherzadeh M.; Ghadiri A. M.; Fatahi Y.; Aldhaher A.; Makvandi P.; Dinarvand R.; Jouyandeh M.; Saeb M. R.; Mozafari M. Turning Toxic Nanomaterials into a Safe and Bioactive Nanocarrier for Co-delivery of DOX/pCRISPR. ACS Appl. Bio Mater. 2021, 4 (6), 5336–5351. 10.1021/acsabm.1c00447. [DOI] [PubMed] [Google Scholar]
- Silva G. A. Nanotechnology approaches to crossing the blood-brain barrier and drug delivery to the CNS. BMC Neurosci. 2008, 9 (3), S4. 10.1186/1471-2202-9-S3-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.; Fan W.; Lau J.; Deng L.; Shen Z.; Chen X. Emerging blood–brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48 (11), 2967–3014. 10.1039/C8CS00805A. [DOI] [PubMed] [Google Scholar]
- Tillotson G. S. Trojan Horse Antibiotics-A Novel Way to Circumvent Gram-Negative Bacterial Resistance?. Infect. Dis.: Res. Treat. 2016, 9, 45–52. 10.4137/IDRT.S31567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F.; Zou D.; Wang W.; Yin Y.; Yin T.; Hao S.; Wang B.; Wang G.; Wang Y. Non-invasive approaches for drug delivery to the brain based on the receptor mediated transport. Mater. Sci. Eng., C 2017, 76, 1316–1327. 10.1016/j.msec.2017.02.056. [DOI] [PubMed] [Google Scholar]
- Jain K. Nanobiotechnology-based drug delivery to the central nervous system. Neurodegener. Dis. 2007, 4 (4), 287–291. 10.1159/000101884. [DOI] [PubMed] [Google Scholar]
- Naqvi S.; Panghal A.; Flora S. J. S. Nanotechnology: A Promising Approach for Delivery of Neuroprotective Drugs. Front. Neurosci. 2020, 14, 494–494. 10.3389/fnins.2020.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masserini M.Nanoparticles for brain drug delivery. International Scholarly Research Notices 2013, 2013.1. 10.1155/2013/238428 [DOI] [Google Scholar]
- Pietroiusti A.; Campagnolo L.; Fadeel B. Interactions of engineered nanoparticles with organs protected by internal biological barriers. Small 2013, 9 (9–10), 1557–1572. 10.1002/smll.201201463. [DOI] [PubMed] [Google Scholar]
- Patterson C.; Feightner J. W.; Garcia A.; Hsiung G.-Y. R.; MacKnight C.; Sadovnick A. D. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. Cmaj 2008, 178 (5), 548–556. 10.1503/cmaj.070796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekatsina M.; Paladini A.; Piroli A.; Zis P.; Pergolizzi J. V.; Varrassi G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37 (1), 113–139. 10.1007/s12325-019-01148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiblich H.; Trombly M.; Ramirez A.; Heneka M. T. Neuroimmune Connections in Aging and Neurodegenerative Diseases. Trends Immunol. 2020, 41 (4), 300–312. 10.1016/j.it.2020.02.002. [DOI] [PubMed] [Google Scholar]
- Schüssel K.; Keil U.; Eckert A.. Oxidative stress and neurodegenerative disease. In Oxidative Stress, Disease And Cancer; World Scientific: 2006; pp 627–647. [Google Scholar]
- Melo A.; Monteiro L.; Lima R. M. F.; de Oliveira D. M.; de Cerqueira M. D.; El-Bachá R. S. Oxidative Stress in Neurodegenerative Diseases: Mechanisms and Therapeutic Perspectives. Oxidative Med. Cellular Longevity 2011, 2011, 467180. 10.1155/2011/467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan Butterfield D.; Castegna A.; Lauderback C. M.; Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol. Aging 2002, 23 (5), 655–664. 10.1016/S0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Pan L.; Wang H.; Gu K. Nanoliposomes as Vehicles for Astaxanthin: Characterization, In Vitro Release Evaluation and Structure. Molecules 2018, 23 (11), 2822. 10.3390/molecules23112822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T.; Mucke L. Inflammation in Neurodegenerative Disease—A Double-Edged Sword. Neuron 2002, 35 (3), 419–432. 10.1016/S0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Cervellati C.; Trentini A.; Pecorelli A.; Valacchi G. Inflammation in neurological disorders: the thin boundary between brain and periphery. Antioxid. Redox Signaling 2020, 33 (3), 191–210. 10.1089/ars.2020.8076. [DOI] [PubMed] [Google Scholar]
- González H.; Elgueta D.; Montoya A.; Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J. Neuroimmunol. 2014, 274 (1), 1–13. 10.1016/j.jneuroim.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y.; Chiba K. Role of Microglial M1/M2 Polarization in Relapse and Remission of Psychiatric Disorders and Diseases. Pharmaceuticals 2014, 7 (12), 1028–1048. 10.3390/ph7121028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. D.; Olschowka J. A.; O’Banion M. K. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation 2014, 11 (1), 98. 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaeigoudari A.; Shafei M. N.; Soukhtanloo M.; Sadeghnia H. R.; Reisi P.; Nosratabadi R.; Behradnia S.; Hosseini M. The effects of L-arginine on spatial memory and synaptic plasticity impairments induced by lipopolysaccharide. Adv. Biomed Res. 2015, 4, 202. 10.4103/2277-9175.166138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaeigoudari A.; Soukhtanloo M.; Shafei M. N.; Sadeghnia H. R.; Reisi P.; Beheshti F.; Behradnia S.; Mousavi S. M.; Hosseini M. Neuronal nitric oxide synthase has a role in the detrimental effects of lipopolysaccharide on spatial memory and synaptic plasticity in rats. Pharmacol. Rep. 2016, 68 (2), 243–249. 10.1016/j.pharep.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Fischer C. W.; Elfving B.; Lund S.; Wegener G. Behavioral and systemic consequences of long-term inflammatory challenge. J. Neuroimmunol. 2015, 288, 40–46. 10.1016/j.jneuroim.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Hosseini M.; Zakeri S.; Khoshdast S.; Yousefian F. T.; Rastegar M.; Vafaee F.; Kahdouee S.; Ghorbani F.; Rakhshandeh H.; Kazemi S. A. The effects of Nigella sativa hydro-alcoholic extract and thymoquinone on lipopolysaccharide - induced depression like behavior in rats. J. Pharm. BioAllied Sci. 2012, 4 (3), 219–225. 10.4103/0975-7406.99052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. M. Angiotensin II AT1 Receptor Blockers Ameliorate Inflammatory Stress: A Beneficial Effect for the Treatment of Brain Disorders. Cell. Mol. Neurobiol. 2012, 32 (5), 667–681. 10.1007/s10571-011-9754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D.; Thangavel R.; Selvakumar G. P.; Zaheer S.; Ahmed M. E.; Raikwar S. P.; Zahoor H.; Saeed D.; Natteru P. A.; Iyer S.; Zaheer A. Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216 10.3389/fncel.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankowski R.; Mader S.; Valdés-Ferrer S. I. Systemic Inflammation and the Brain: Novel Roles of Genetic, Molecular, and Environmental Cues as Drivers of Neurodegeneration. Front. Cell. Neurosci. 2015, 9, 28 10.3389/fncel.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benicky J.; Sánchez-Lemus E.; Honda M.; Pang T.; Orecna M.; Wang J.; Leng Y.; Chuang D.-M.; Saavedra J. M. Angiotensin II AT1 Receptor Blockade Ameliorates Brain Inflammation. Neuropsychopharmacology 2011, 36 (4), 857–870. 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. M. Angiotensin II AT1 receptor blockers as treatments for inflammatory brain disorders. Clin. Sci. 2012, 123 (10), 567–590. 10.1042/CS20120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollor J. N.; Smith E.; Agars E.; Kuan S. A.; Baune B. T.; Campbell L.; Samaras K.; Crawford J.; Lux O.; Kochan N. A.; Brodaty H.; Sachdev P. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. AGE 2012, 34 (5), 1295–1308. 10.1007/s11357-011-9301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C. C.; Tarelli R.. Parkinson’s disease and systemic inflammation. Parkinson’s Dis. 2011, 2011.1. 10.4061/2011/436813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I.; Chugh D.; Ekdahl C. T. Role of fractalkine–CX3CR1 pathway in seizure-induced microglial activation, neurodegeneration, and neuroblast production in the adult rat brain. Neurobiol. Dis. 2015, 74, 194–203. 10.1016/j.nbd.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Zychowska M.; Rojewska E.; Makuch W.; Przewlocka B.; Mika J. The influence of microglia activation on the efficacy of amitriptyline, doxepin, milnacipran, venlafaxine and fluoxetine in a rat model of neuropathic pain. Eur. J. Pharmacol. 2015, 749, 115–123. 10.1016/j.ejphar.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Fakhri S.; Abbaszadeh F.; Dargahi L.; Jorjani M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. 10.1016/j.phrs.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Barros M. P.; Poppe S. C.; Bondan E. F. Neuroprotective Properties of the Marine Carotenoid Astaxanthin and Omega-3 Fatty Acids, and Perspectives for the Natural Combination of Both in Krill Oil. Nutrients 2014, 6 (3), 1293–1317. 10.3390/nu6031293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber J. J.; Dhib-Jalbut S. Protective autoimmunity in the nervous system. Pharmacol. Ther. 2009, 121 (2), 147–159. 10.1016/j.pharmthera.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Lin T.-C.; Hung K.-H.; Peng C.-H.; Liu J.-H.; Woung L.-C.; Tsai C.-Y.; Chen S.-J.; Chen Y.-T.; Hsu C.-C. Nanotechnology-based drug delivery treatments and specific targeting therapy for age-related macular degeneration. J. Chin. Med. Assoc. 2015, 78 (11), 635–641. 10.1016/j.jcma.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Shen H.; Kuo C.-C.; Chou J.; Delvolve A.; Jackson S. N.; Post J.; Woods A. S.; Hoffer B. J.; Wang Y.; Harvey B. K. Astaxanthin reduces ischemic brain injury in adult rats. FASEB J. 2009, 23 (6), 1958–1968. 10.1096/fj.08-123281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L.; Zhou Y.; Li X.-f.; Wan Q.-j.; Yu L.-h. Preventive treatment of astaxanthin provides neuroprotection through suppression of reactive oxygen species and activation of antioxidant defense pathway after stroke in rats. Brain Res. Bull. 2017, 130, 211–220. 10.1016/j.brainresbull.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Rahman S. O.; Panda B. P.; Parvez S.; Kaundal M.; Hussain S.; Akhtar M.; Najmi A. K. Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by Aβ peptides in animal model of Alzheimer’s disease. Biomed. Pharmacother. 2019, 110, 47–58. 10.1016/j.biopha.2018.11.043. [DOI] [PubMed] [Google Scholar]
- Che H.; Li Q.; Zhang T.; Wang D.; Yang L.; Xu J.; Yanagita T.; Xue C.; Chang Y.; Wang Y. Effects of astaxanthin and docosahexaenoic-acid-acylated astaxanthin on Alzheimer’s disease in APP/PS1 double-transgenic mice. J. Agric. Food Chem. 2018, 66 (19), 4948–4957. 10.1021/acs.jafc.8b00988. [DOI] [PubMed] [Google Scholar]
- Gorska-Ciebiada M.; Saryusz-Wolska M.; Borkowska A.; Ciebiada M.; Loba J. Serum levels of inflammatory markers in depressed elderly patients with diabetes and mild cognitive impairment. PLoS One 2015, 10 (3), e0120433. 10.1371/journal.pone.0120433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrego P. J.; Fayed N.; Pina M. A. Conversion from mild cognitive impairment to probable Alzheimer’s disease predicted by brain magnetic resonance spectroscopy. Am. J. Psychiatry 2005, 162 (4), 667–675. 10.1176/appi.ajp.162.4.667. [DOI] [PubMed] [Google Scholar]
- Kessing L. V.; Rytgaard H. C.; Ekstrøm C. T.; Knop F. K.; Berk M.; Gerds T. A. Antidiabetes Agents and Incident Depression: A Nationwide Population-Based Study. Diabetes Care 2020, 43 (12), 3050–3060. 10.2337/dc20-1561. [DOI] [PubMed] [Google Scholar]
- Fonseka T. M.; McIntyre R. S.; Soczynska J. K.; Kennedy S. H. Novel investigational drugs targeting IL-6 signaling for the treatment of depression. Expert Opin. Invest. Drugs 2015, 24 (4), 459–475. 10.1517/13543784.2014.998334. [DOI] [PubMed] [Google Scholar]
- Mazza M.; Pomponi M.; Janiri L.; Bria P.; Mazza S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: An overview. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31 (1), 12–26. 10.1016/j.pnpbp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Baker J. S.; Chen X.; Wang Y.; Chen H.; Davison G. W.; Yan X. High-Dose Astaxanthin Supplementation Suppresses Antioxidant Enzyme Activity during Moderate-Intensity Swimming Training in Mice. Nutrients 2019, 11 (6), 1244. 10.3390/nu11061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibrand K.; Berge K.; Messaoudi M.; Duffaud A.; Panja D.; Bramham C. R.; Burri L. Enhanced cognitive function and antidepressant-like effects after krill oil supplementation in rats. Lipids Health Dis. 2013, 12 (1), 6. 10.1186/1476-511X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi N. The Evaluation of Astaxanthin Effects on Differentiation of Human Adipose Derived Stem Cells into Oligodendrocyte Precursor Cells. Avicenna J. Med. Biotechnol. 2018, 10 (2), 69–74. [PMC free article] [PubMed] [Google Scholar]
- Ameeduzzafar; Ali J.; Fazil M.; Qumbar M.; Khan N.; Ali A. Colloidal drug delivery system: amplify the ocular delivery. Drug Delivery 2016, 23 (3), 700–716. 10.3109/10717544.2014.923065. [DOI] [PubMed] [Google Scholar]
- Joseph R. R.; Venkatraman S. S. Drug delivery to the eye: what benefits do nanocarriers offer?. Nanomedicine 2017, 12 (6), 683–702. 10.2217/nnm-2016-0379. [DOI] [PubMed] [Google Scholar]
- Jumelle C.; Gholizadeh S.; Annabi N.; Dana R. Advances and limitations of drug delivery systems formulated as eye drops. J. Controlled Release 2020, 321, 1–22. 10.1016/j.jconrel.2020.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karla P. K.; Earla R.; Boddu S. H.; Johnston T. P.; Pal D.; Mitra A. Molecular Expression and Functional Evidence of a Drug Efflux Pump (BCRP) in Human Corneal Epithelial Cells. Curr. Eye Res. 2009, 34 (1), 1–9. 10.1080/02713680802518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katragadda S.; Talluri R. S.; Mitra A. K. Modulation of P-Glycoprotein–Mediated Efflux by Prodrug Derivatization: An Approach Involving Peptide Transporter–Mediated Influx Across Rabbit Cornea. J. Ocul. Pharmacol. Ther. 2006, 22 (2), 110–120. 10.1089/jop.2006.22.110. [DOI] [PubMed] [Google Scholar]
- Barar J.; Javadzadeh A. R.; Omidi Y. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin. Drug Delivery 2008, 5 (5), 567–581. 10.1517/17425247.5.5.567. [DOI] [PubMed] [Google Scholar]
- Ghasemi Falavarjani K.; Nguyen Q. D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye 2013, 27 (7), 787–794. 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.; Suresh P. K.. Drug delivery to eye: Special reference to nanoparticles. Int. J. Drug Delivery 2010, 2 ( (1), ).12. 10.5138/ijdd.2010.0975.0215.02007 [DOI] [Google Scholar]
- Tiwari R.; Pandey V.; Asati S.; Soni V.; Jain D. Therapeutic challenges in ocular delivery of lipid based emulsion. Egyptian Journal of Basic and Applied Sciences 2018, 5 (2), 121–129. 10.1016/j.ejbas.2018.04.001. [DOI] [Google Scholar]
- Zhao R.; Li J.; Wang J.; Yin Z.; Zhu Y.; Liu W. Development of Timolol-Loaded Galactosylated Chitosan Nanoparticles and Evaluation of Their Potential for Ocular Drug Delivery. AAPS PharmSciTech 2017, 18 (4), 997–1008. 10.1208/s12249-016-0669-x. [DOI] [PubMed] [Google Scholar]
- Kikuchi K.; Dong Z.; Shinmei Y.; Murata M.; Kanda A.; Noda K.; Harada T.; Ishida S. Cytoprotective Effect of Astaxanthin in a Model of Normal Intraocular Pressure Glaucoma. J. Ophthalmol. 2020, 2020, 9539681. 10.1155/2020/9539681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T.; Shimazawa M.; Inoue Y.; Nakano Y.; Ojino K.; Izawa H.; Tsuruma K.; Ishibashi T.; Hara H. Astaxanthin Protects Against Retinal Damage: Evidence from In Vivo and In Vitro Retinal Ischemia and Reperfusion Models. Curr. Eye Res. 2016, 41 (11), 1465–1472. 10.3109/02713683.2015.1127392. [DOI] [PubMed] [Google Scholar]
- Rivera J. C.; Dabouz R.; Noueihed B.; Omri S.; Tahiri H.; Chemtob S. Ischemic Retinopathies: Oxidative Stress and Inflammation. Oxid. Med. Cell. Longevity 2017, 2017, 3940241. 10.1155/2017/3940241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semeraro F.; Cancarini A.; dell’Omo R.; Rezzola S.; Romano M. R.; Costagliola C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 582060. 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tha K. K.; Okuma Y.; Miyazaki H.; Murayama T.; Uehara T.; Hatakeyama R.; Hayashi Y.; Nomura Y. Changes in expressions of proinflammatory cytokines IL-1β, TNF-α and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res. 2000, 885 (1), 25–31. 10.1016/S0006-8993(00)02883-3. [DOI] [PubMed] [Google Scholar]
- Choi S.-K.; Park Y.-S.; Choi D.-K.; Chang H.-I. Effects of astaxanthin on the production of NO and the expression of COX-2 and iNOS in LPS-stimulated BV2 microglial cells. J. Microbiol. Biotechnol. 2008, 18 (12), 1990–1996. 10.4014/JMB.0800.489. [DOI] [PubMed] [Google Scholar]
- Lennikov A.; Kitaichi N.; Fukase R.; Murata M.; Noda K.; Ando R.; Ohguchi T.; Kawakita T.; Ohno S.; Ishida S. Amelioration of ultraviolet-induced photokeratitis in mice treated with astaxanthin eye drops. Mol. Vis 2012, 18, 455–464. [PMC free article] [PubMed] [Google Scholar]
- Yamagishi R.; Aihara M. Neuroprotective effect of astaxanthin against rat retinal ganglion cell death under various stresses that induce apoptosis and necrosis. Mol. Vis 2014, 20, 1796–1805. [PMC free article] [PubMed] [Google Scholar]
- Cort A.; Ozturk N.; Akpinar D.; Unal M.; Yucel G.; Ciftcioglu A.; Yargicoglu P.; Aslan M. Suppressive effect of astaxanthin on retinal injury induced by elevated intraocular pressure. Regul. Toxicol. Pharmacol. 2010, 58 (1), 121–130. 10.1016/j.yrtph.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Schwartz S. D.; Regillo C. D.; Lam B. L.; Eliott D.; Rosenfeld P. J.; Gregori N. Z.; Hubschman J.-P.; Davis J. L.; Heilwell G.; Spirn M.; Maguire J.; Gay R.; Bateman J.; Ostrick R. M.; Morris D.; Vincent M.; Anglade E.; Del Priore L. V.; Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 2015, 385 (9967), 509–516. 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- Rowe-Rendleman C. L.; Durazo S. A.; Kompella U. B.; Rittenhouse K. D.; Di Polo A.; Weiner A. L.; Grossniklaus H. E.; Naash M. I.; Lewin A. S.; Horsager A. Drug and gene delivery to the back of the eye: from bench to bedside. Invest. Ophthalmol. Vis. Sci. 2014, 55 (4), 2714–2730. 10.1167/iovs.13-13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak K.; Misra M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. 10.1016/j.biopha.2018.08.138. [DOI] [PubMed] [Google Scholar]
- Li H.; Li J.; Hou C.; Li J.; Peng H.; Wang Q. The effect of astaxanthin on inflammation in hyperosmolarity of experimental dry eye model in vitro and in vivo. Exp. Eye Res. 2020, 197, 108113. 10.1016/j.exer.2020.108113. [DOI] [PubMed] [Google Scholar]
- Agrahari V.; Mandal A.; Agrahari V.; Trinh H. M.; Joseph M.; Ray A.; Hadji H.; Mitra R.; Pal D.; Mitra A. K. A comprehensive insight on ocular pharmacokinetics. Drug Delivery Transl. Res. 2016, 6 (6), 735–754. 10.1007/s13346-016-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratter A.; Biagi D.; Cicero A. F. G. Sublingual Delivery of Astaxanthin through a Novel Ascorbyl Palmitate-Based Nanoemulsion: Preliminary Data. Mar. Drugs 2019, 17 (9), 508. 10.3390/md17090508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa T.; Yoshida M.; Fukuta T.; Tanaka T.; Inagi T.; Kogure K. Efficacy of high-affinity liposomal astaxanthin on up-regulation of age-related markers induced by oxidative stress in human corneal epithelial cells. Journal of clinical biochemistry and nutrition 2019, 64, 27–35. 10.3164/jcbn.18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf L. R.; Popov A. M.; Enlow E. M.; Bourassa J. L.; Ong W. Z.; Nowak P.; Chen H. Topical ocular drug delivery to the back of the eye by mucus-penetrating particles. Translational vision science & technology 2015, 4 (3), 11–11. 10.1167/tvst.4.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y.; Liu J.; Jin S.; Guo W.; Liang X.; Hu Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7 (3), 281–291. 10.1016/j.apsb.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.; Cholkar K.; Agrahari V.; Mitra A. K. Ocular drug delivery systems: An overview. World J. Pharmacol 2013, 2 (2), 47–64. 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makvandi P.; Jamaledin R.; Chen G.; Baghbantaraghdari Z.; Zare E. N.; Di Natale C.; Onesto V.; Vecchione R.; Lee J.; Tay F. R.; et al. Stimuli-Responsive Transdermal Microneedle Patches. Mater. Today 2021, 47, 206–222. 10.1016/j.mattod.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzinger M.-A.; Briançon S.; Pelletier J.; Chevalier Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17 (3), 156–165. 10.1016/j.cocis.2012.02.001. [DOI] [Google Scholar]
- Iqbal B.; Ali J.; Baboota S. Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int. J. Dermatol. 2018, 57 (6), 646–660. 10.1111/ijd.13902. [DOI] [PubMed] [Google Scholar]
- Chamundeeswari M.; Jeslin J.; Verma M. L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17 (2), 849–865. 10.1007/s10311-018-00841-1. [DOI] [Google Scholar]
- Dayan N. Pathways for skin penetration. Cosmetics and toiletries 2005, 120 (6), 67–76. [Google Scholar]
- Zhang H.; Zhai Y.; Yang X.; Zhai G. Breaking the skin barrier: achievements and future directions. Curr. Pharm. Des. 2015, 21 (20), 2713–2724. 10.2174/1381612821666150428124406. [DOI] [PubMed] [Google Scholar]
- Al Shaal L.; Shegokar R.; Müller R. H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. 2011, 420 (1), 133–140. 10.1016/j.ijpharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Batheja P.; Sheihet L.; Kohn J.; Singer A. J.; Michniak-Kohn B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J. Controlled Release 2011, 149 (2), 159–167. 10.1016/j.jconrel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Prow T. W.; Grice J. E.; Lin L. L.; Faye R.; Butler M.; Becker W.; Wurm E. M. T.; Yoong C.; Robertson T. A.; Soyer H. P.; Roberts M. S. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Delivery Rev. 2011, 63 (6), 470–491. 10.1016/j.addr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Ahmadi Ashtiani H. R.; Bishe P.; Lashgari N.-A.; Nilforoushzadeh M. A.; Zare S. Liposomes in cosmetics. J. Skin Stem Cell 2016, 3 (3), e65815. 10.5812/jssc.65815. [DOI] [Google Scholar]
- Tabata N.; O’Goshi K.; Zhen Y. X.; Kligman A. M.; Tagami H. Biophysical Assessment of Persistent Effects of Moisturizers after Their Daily Applications: Evaluation of Corneotherapy. Dermatology 2000, 200 (4), 308–313. 10.1159/000018393. [DOI] [PubMed] [Google Scholar]
- Makvandi P.; Kirkby M.; Hutton A. R. J.; Shabani M.; Yiu C. K. Y.; Baghbantaraghdari Z.; Jamaledin R.; Carlotti M.; Mazzolai B.; Mattoli V.; Donnelly R. F. Engineering Microneedle Patches for Improved Penetration: Analysis, Skin Models and Factors Affecting Needle Insertion. Nano-Micro Lett. 2021, 13 (1), 93. 10.1007/s40820-021-00611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri M.; Validi M.; Gholipour A.; Makvandi P.; Sharifi E. Chitosan Nanofiber Biocomposites for Potential Wound Healing Applications: Antioxidant Activity with Synergic Antibacterial Effect. Bioeng. Transl. Med. 2021, e10254. 10.1002/btm2.10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visioli F.; Artaria C. Astaxanthin in cardiovascular health and disease: mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct. 2017, 8 (1), 39–63. 10.1039/C6FO01721E. [DOI] [PubMed] [Google Scholar]
- Svobodova A.; Walterova D.; Vostalova J. Ultraviolet light induced alteration to the skin. Biomed. Pap. 2006, 150 (1), 25. 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- Rafi M. M.; Yadav P. N.; Reyes M. Lycopene inhibits LPS-induced proinflammatory mediator inducible nitric oxide synthase in mouse macrophage cells. J. Food Sci. 2007, 72 (1), S069–S074. 10.1111/j.1750-3841.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- Chew B. P.; Mathison B. D.; Hayek M. G.; Massimino S.; Reinhart G. A.; Park J. S. Dietary astaxanthin enhances immune response in dogs. Vet. Immunol. Immunopathol. 2011, 140 (3), 199–206. 10.1016/j.vetimm.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Santocono M.; Zurria M.; Berrettini M.; Fedeli D.; Falcioni G. Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells. J. Photochem. Photobiol., B 2006, 85 (3), 205–215. 10.1016/j.jphotobiol.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Chalyk N. E.; Klochkov V. A.; Bandaletova T. Y.; Kyle N. H.; Petyaev I. M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. (N. Y., NY, U. S.) 2017, 48, 40–48. 10.1016/j.nutres.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Ito N.; Seki S.; Ueda F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10 (7), 817. 10.3390/nu10070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall B.; McPartland C. K.; Moore R.; Frank-Kamenetskii A.; Booth B. W. Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells In Vitro. Antioxidants 2018, 7 (10), 135. 10.3390/antiox7100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita E. The effects of a dietary supplement containing astaxanthin on skin condition. Food Style 21 2005, 9 (9), 72. [Google Scholar]
- Singh K. N.; Patil S.; Barkate H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. Journal of cosmetic dermatology 2020, 19 (1), 22–27. 10.1111/jocd.13019. [DOI] [PubMed] [Google Scholar]
- Tavassolifar M. j.; Vodjgani M.; Salehi Z.; Izad M. The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Dis. 2020, 2020, 5793817. 10.1155/2020/5793817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennedsen M.; Wang X.; Willén R.; Wadström T.; Andersen L. P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 2000, 70 (3), 185–189. 10.1016/S0165-2478(99)00145-5. [DOI] [PubMed] [Google Scholar]
- Park J. S.; Chyun J. H.; Kim Y. K.; Line L. L.; Chew B. P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7 (1), 18. 10.1186/1743-7075-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeruraj A.; Liu L.; Zheng J.; Wu J.; Arumugam M. Evaluation of astaxanthin incorporated collagen film developed from the outer skin waste of squid Doryteuthis singhalensis for wound healing and tissue regenerative applications. Mater. Sci. Eng., C 2019, 95, 29–42. 10.1016/j.msec.2018.10.055. [DOI] [PubMed] [Google Scholar]
- Lyons N. M.; O’Brien N. M. Modulatory effects of an algal extract containing astaxanthin on UVA-irradiated cells in culture. J. Dermatol. Sci. 2002, 30 (1), 73–84. 10.1016/S0923-1811(02)00063-4. [DOI] [PubMed] [Google Scholar]
- Poljšak B.; Dahmane R. G.; Godić A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat 2012, 21 (2), 33–36. [PubMed] [Google Scholar]
- Kishimoto Y.; Tani M.; Uto-Kondo H.; Iizuka M.; Saita E.; Sone H.; Kurata H.; Kondo K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2010, 49 (2), 119–126. 10.1007/s00394-009-0056-4. [DOI] [PubMed] [Google Scholar]
- Priyadarshini L.; Aggarwal A. Astaxanthin inhibits cytokines production and inflammatory gene expression by suppressing IκB kinase-dependent nuclear factor κB activation in pre and postpartum Murrah buffaloes during different seasons. Vet. World 2018, 11 (6), 782–788. 10.14202/vetworld.2018.782-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa Y.; Rehman M. U.; Shimizu T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Experimental dermatology 2014, 23 (3), 178–183. 10.1111/exd.12347. [DOI] [PubMed] [Google Scholar]
- Landon R.; Gueguen V.; Petite H.; Letourneur D.; Pavon-Djavid G.; Anagnostou F. Impact of astaxanthin on diabetes pathogenesis and chronic complications. Mar. Drugs 2020, 18 (7), 357. 10.3390/md18070357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty H. P.; Byun J.; Lockwood S. F.; Jacob R. F.; Mason R. P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta, Biomembr. 2007, 1768 (1), 167–174. 10.1016/j.bbamem.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Kim S. H.; Kim H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction-a mini-review. Nutrients 2018, 10 (9), 1137. 10.3390/nu10091137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.; Zhao Z.; Zhang X.; Wu A.; Huang Y.; Miao Y.; Yang M. Effect of berberine on the renal tubular epithelial-to-mesenchymal transition by inhibition of the Notch/snail pathway in diabetic nephropathy model KKAy mice. Drug Des., Dev. Ther. 2017, 11, 1065. 10.2147/DDDT.S124971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhadi N. S.; Zakerkish M.; Mohammadiasl J.; Zarei M.; Mohammadshahi M.; Haghighizadeh M. H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27 (2), 341–346. 10.6133/apjcn.052017.11. [DOI] [PubMed] [Google Scholar]
- Uchiyama K.; Naito Y.; Hasegawa G.; Nakamura N.; Takahashi J.; Yoshikawa T. Astaxanthin protects β-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002, 7 (5), 290–293. 10.1179/135100002125000811. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Tao J.; Xie X. Astaxanthin promotes Nrf2/ARE signaling to inhibit HG-induced renal fibrosis in GMCs. Mar. Drugs 2018, 16 (4), 117. 10.3390/md16040117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara A.; Takahashi K.; Morita N.; Murashima T.; Onuma H.; Sumitani Y.; Tanaka T.; Kondo T.; Hosaka T.; Ishida H. The novel mechanisms concerning the inhibitions of palmitate-induced proinflammatory factor releases and endogenous cellular stress with astaxanthin on MIN6 β-cells. Mar. Drugs 2017, 15 (6), 185. 10.3390/md15060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunkumar E.; Bhuvaneswari S.; Anuradha C. V. An intervention study in obese mice with astaxanthin, a marine carotenoid–effects on insulin signaling and pro-inflammatory cytokines. Food Funct. 2012, 3 (2), 120–126. 10.1039/C1FO10161G. [DOI] [PubMed] [Google Scholar]
- Nishida Y.; Nawaz A.; Kado T.; Takikawa A.; Igarashi Y.; Onogi Y.; Wada T.; Sasaoka T.; Yamamoto S.; Sasahara M. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J. Cachexia, Sarcopenia Muscle 2020, 11 (1), 241–258. 10.1002/jcsm.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari S.; Anuradha C. V. Astaxanthin prevents loss of insulin signaling and improves glucose metabolism in liver of insulin resistant mice. Can. J. Physiol. Pharmacol. 2012, 90 (11), 1544–1552. 10.1139/y2012-119. [DOI] [PubMed] [Google Scholar]
- Park C. H.; Xu F. H.; Roh S.-S.; Song Y. O.; Uebaba K.; Noh J. S.; Yokozawa T. Astaxanthin and Corni Fructus protect against diabetes-induced oxidative stress, inflammation, and advanced glycation end product in livers of streptozotocin-induced diabetic rats. J. Med. Food 2015, 18 (3), 337–344. 10.1089/jmf.2014.3174. [DOI] [PubMed] [Google Scholar]
- Dehdashtian E.; Mehrzadi S.; Yousefi B.; Hosseinzadeh A.; Reiter R. J.; Safa M.; Ghaznavi H.; Naseripour M. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018, 193, 20–33. 10.1016/j.lfs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Kowluru R. A.; Kowluru A.; Mishra M.; Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retinal Eye Res. 2015, 48, 40–61. 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S.; Kern T. S.; Song B.; Stuebe C. Mechanistic insights into pathological changes in the diabetic retina: implications for targeting diabetic retinopathy. Am. J. Pathol. 2017, 187 (1), 9–19. 10.1016/j.ajpath.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh P.-T.; Huang H.-W.; Yang C.-M.; Yang W.-S.; Yang C.-H. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS One 2016, 11 (1), e0146438. 10.1371/journal.pone.0146438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.; Liu J.; Zeng X.; Huangfu J.; Jiang Y.; Wang M.; Chen F. Protective actions of microalgae against endogenous and exogenous advanced glycation endproducts (AGEs) in human retinal pigment epithelial cells. Food Funct. 2011, 2 (5), 251–258. 10.1039/c1fo10021a. [DOI] [PubMed] [Google Scholar]
- Benlarbi-Ben Khedher M.; Hajri K.; Dellaa A.; Baccouche B.; Hammoum I.; Boudhrioua-Mihoubi N.; Dhifi W.; Ben Chaouacha-Chekir R. Astaxanthin inhibits aldose reductase activity in Psammomys obesus, a model of type 2 diabetes and diabetic retinopathy. Food Sci. Nutr. 2019, 7 (12), 3979–3985. 10.1002/fsn3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohbakhsh A.; Karimi G.; Iranshahi M. Carotenoids in the treatment of diabetes mellitus and its complications: A mechanistic review. Biomed. Pharmacother. 2017, 91, 31–42. 10.1016/j.biopha.2017.04.057. [DOI] [PubMed] [Google Scholar]
- Xu L.; Zhu J.; Yin W.; Ding X. Astaxanthin improves cognitive deficits from oxidative stress, nitric oxide synthase and inflammation through upregulation of PI3K/Akt in diabetes rat. Int. J. Clin. Exp. Pathol. 2015, 8 (6), 6083. [PMC free article] [PubMed] [Google Scholar]
- Feng Y.; Chu A.; Luo Q.; Wu M.; Shi X.; Chen Y. The protective effect of astaxanthin on cognitive function via inhibition of oxidative stress and inflammation in the brains of chronic T2DM rats. Front. Pharmacol. 2018, 9, 748. 10.3389/fphar.2018.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirban A.; Gawlowski T.; Roden M. Vascular effects of advanced glycation endproducts: clinical effects and molecular mechanisms. Mol. Metab. 2014, 3 (2), 94–108. 10.1016/j.molmet.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain W. D.; Paldánius P. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17 (1), 1–10. 10.1186/s12933-018-0703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. c.; Pen P. J.; Yin M. c. Anticoagulatory and antiinflammatory effects of astaxanthin in diabetic rats. J. Food Sci. 2012, 77 (2), H76–H80. 10.1111/j.1750-3841.2011.02558.x. [DOI] [PubMed] [Google Scholar]
- Hussein G.; Goto H.; Oda S.; Sankawa U.; Matsumoto K.; Watanabe H. Antihypertensive potential and mechanism of action of astaxanthin: III. Antioxidant and histopathological effects in spontaneously hypertensive rats. Biol. Pharm. Bull. 2006, 29 (4), 684–688. 10.1248/bpb.29.684. [DOI] [PubMed] [Google Scholar]
- Iwamoto T.; Hosoda K.; Hirano R.; Kurata H.; Matsumoto A.; Miki W.; Kamiyama M.; Itakura H.; Yamamoto S.; Kondo K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Thromb. 2000, 7 (4), 216–222. 10.5551/jat1994.7.216. [DOI] [PubMed] [Google Scholar]
- Naito Y.; Uchiyama K.; Aoi W.; Hasegawa G.; Nakamura N.; Yoshida N.; Maoka T.; Takahashi J.; Yoshikawa T. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. BioFactors 2004, 20 (1), 49–59. 10.1002/biof.5520200105. [DOI] [PubMed] [Google Scholar]
- Manabe E.; Handa O.; Naito Y.; Mizushima K.; Akagiri S.; Adachi S.; Takagi T.; Kokura S.; Maoka T.; Yoshikawa T. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J. Cell. Biochem. 2008, 103 (6), 1925–1937. 10.1002/jcb.21583. [DOI] [PubMed] [Google Scholar]
- Sila A.; Ghlissi Z.; Kamoun Z.; Makni M.; Nasri M.; Bougatef A.; Sahnoun Z. Astaxanthin from shrimp by-products ameliorates nephropathy in diabetic rats. Eur. J. Nutr. 2015, 54 (2), 301–307. 10.1007/s00394-014-0711-2. [DOI] [PubMed] [Google Scholar]
- Penislusshiyan S.; Chitra L.; Ancy I.; Kumaradhas P.; Palvannan T. Novel antioxidant astaxanthin-s-allyl cysteine biconjugate diminished oxidative stress and mitochondrial dysfunction to triumph diabetes in rat model. Life Sci. 2020, 245, 117367. 10.1016/j.lfs.2020.117367. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Chen Y.; Chen Q.; Yang H.; Xie X.. Astaxanthin promotes Nrf2/ARE signaling to alleviate renal fibronectin and collagen IV accumulation in diabetic rats. J. Diabetes Res. 2018, 2018.1. 10.1155/2018/6730315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Schin M.; Saha J.; Burke K.; Holzman L. B.; Filipiak W.; Saunders T.; Xiang M.; Heilig C. W.; Brosius F. C. III Podocyte-specific overexpression of GLUT1 surprisingly reduces mesangial matrix expansion in diabetic nephropathy in mice. American Journal of Physiology-Renal Physiology 2010, 299 (1), F91–F98. 10.1152/ajprenal.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Li W.; Shi L.; Jiang L.; Li M.; Zhang C.; Peng H. Kidney-targeted astaxanthin natural antioxidant nanosystem for diabetic nephropathy therapy. Eur. J. Pharm. Biopharm. 2020, 156, 143–154. 10.1016/j.ejpb.2020.09.005. [DOI] [PubMed] [Google Scholar]
- Utikal J.; Udart M.; Leiter U.; Kaskel P.; Peter R. U.; Krähn G. Numerical abnormalities of the Cyclin D1 gene locus on chromosome 11q13 in non-melanoma skin cancer. Cancer Lett. 2005, 219 (2), 197–204. 10.1016/j.canlet.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Susin S. A.; Daugas E.; Ravagnan L.; Samejima K.; Zamzami N.; Loeffler M.; Costantini P.; Ferri K. F.; Irinopoulou T.; Prévost M.-C. Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 2000, 192 (4), 571–580. 10.1084/jem.192.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267 (5203), 1456–1462. 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Zhou G. P.; Doctor K. Subcellular location prediction of apoptosis proteins. Proteins: Struct., Funct., Genet. 2003, 50 (1), 44–48. 10.1002/prot.10251. [DOI] [PubMed] [Google Scholar]
- Fesik S. W.; Shi Y. Controlling the Caspases. Science 2001, 294 (5546), 1477–1478. 10.1126/science.1062236. [DOI] [PubMed] [Google Scholar]
- Murphy K. M.; Ranganathan V.; Farnsworth M. L.; Kavallaris M.; Lock R. B. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000, 7 (1), 102–111. 10.1038/sj.cdd.4400597. [DOI] [PubMed] [Google Scholar]
- Tang G.; Yang J.; Minemoto Y.; Lin A. Blocking Caspase-3-Mediated Proteolysis of IKKβ Suppresses TNF-α-Induced Apoptosis. Mol. Cell 2001, 8 (5), 1005–1016. 10.1016/S1097-2765(01)00380-X. [DOI] [PubMed] [Google Scholar]
- Tang X.; Liu B.; Wang X.; Yu Q.; Fang R. Epidermal growth factor, through alleviating oxidative stress, protect IPEC-J2 cells from lipopolysaccharides-induced apoptosis. Int. J. Mol. Sci. 2018, 19 (3), 848. 10.3390/ijms19030848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. L. A Preliminary Investigation of the Enzymatic Inhibition of 5α-Reductase and Growth of Prostatic Carcinoma Cell Line LNCap-FGC by Natural Astaxanthin and Saw Palmetto Lipid Extract In Vitro. J. Herb. Pharmacother. 2005, 5 (1), 17–26. 10.1080/J157v05n01_03. [DOI] [PubMed] [Google Scholar]
- Chen Y.-T.; Kao C.-J.; Huang H.-Y.; Huang S.-Y.; Chen C.-Y.; Lin Y.-S.; Wen Z.-H.; Wang H.-M. D. Astaxanthin reduces MMP expressions, suppresses cancer cell migrations, and triggers apoptotic caspases of in vitro and in vivo models in melanoma. J. Funct. Foods 2017, 31, 20–31. 10.1016/j.jff.2017.01.005. [DOI] [Google Scholar]
- Liao K.-S.; Wei C.-L.; Chen J.-C.; Zheng H.-Y.; Chen W.-C.; Wu C.-H.; Wang T.-J.; Peng Y.-S.; Chang P.-Y.; Lin Y.-W. Astaxanthin enhances pemetrexed-induced cytotoxicity by downregulation of thymidylate synthase expression in human lung cancer cells. Regul. Toxicol. Pharmacol. 2016, 81, 353–361. 10.1016/j.yrtph.2016.09.031. [DOI] [PubMed] [Google Scholar]
- Nagaraj S.; Rajaram M. G.; Arulmurugan P.; Baskaraboopathy A.; Karuppasamy K.; Jayappriyan K. R.; Sundararaj R.; Rengasamy R. Antiproliferative potential of astaxanthin-rich alga Haematococcus pluvialis Flotow on human hepatic cancer (HepG2) cell line. Biomedicine & Preventive Nutrition 2012, 2 (3), 149–153. 10.1016/j.bionut.2012.03.009. [DOI] [Google Scholar]
- Bharathiraja S.; Manivasagan P.; Oh Y. O.; Moorthy M. S.; Seo H.; Bui N. Q.; Oh J. Astaxanthin conjugated polypyrrole nanoparticles as a multimodal agent for photo-based therapy and imaging. Int. J. Pharm. 2017, 517 (1–2), 216–225. 10.1016/j.ijpharm.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Nguyen V. P.; Kim S. W.; Kim H.; Kim H.; Seok K. H.; Jung M. J.; Ahn Y.-c.; Kang H. W. Biocompatible astaxanthin as a novel marine-oriented agent for dual chemo-photothermal therapy. PLoS One 2017, 12 (4), e0174687. 10.1371/journal.pone.0174687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 2009, 3 (9), 503–509. 10.1038/nphoton.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E. C.; Glaus C.; Chen J.; Welch M. J.; Xia Y. Inorganic nanoparticle-based contrast agents for molecular imaging. Trends Mol. Med. 2010, 16 (12), 561–73. 10.1016/j.molmed.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. M.; Mancini M. C.; Nie S. Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4 (11), 710–711. 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V. P.; Park S.; Oh J.; Wook Kang H. Biocompatible astaxanthin as novel contrast agent for biomedical imaging. Journal of biophotonics 2017, 10 (8), 1053–1061. 10.1002/jbio.201600159. [DOI] [PubMed] [Google Scholar]
- Wang T.; Hu Q.; Lee J.-Y.; Luo Y. Solid lipid–polymer hybrid nanoparticles by in situ conjugation for oral delivery of astaxanthin. J. Agric. Food Chem. 2018, 66 (36), 9473–9480. 10.1021/acs.jafc.8b02827. [DOI] [PubMed] [Google Scholar]
- Li M.; Zahi M. R.; Yuan Q.; Tian F.; Liang H. Preparation and stability of astaxanthin solid lipid nanoparticles based on stearic acid. Eur. J. Lipid Sci. Technol. 2016, 118 (4), 592–602. 10.1002/ejlt.201400650. [DOI] [Google Scholar]
- Liu C.; Zhang S.; McClements D. J.; Wang D.; Xu Y. Design of astaxanthin-loaded core–shell nanoparticles consisting of chitosan oligosaccharides and poly (lactic-co-glycolic acid): enhancement of water solubility, stability, and bioavailability. J. Agric. Food Chem. 2019, 67 (18), 5113–5121. 10.1021/acs.jafc.8b06963. [DOI] [PubMed] [Google Scholar]
- Sun R.; Xia N.; Xia Q. Non-aqueous nanoemulsions as a new strategy for topical application of astaxanthin. J. Dispersion Sci. Technol. 2020, 41 (12), 1777–1788. 10.1080/01932691.2019.1635027. [DOI] [Google Scholar]
- Shanmugapriya K.; Kim H.; Kang H. W. A new alternative insight of nanoemulsion conjugated with κ-carrageenan for wound healing study in diabetic mice: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2019, 133, 236–250. 10.1016/j.ejps.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Xu L.; Yu H.; Sun H.; Yu X.; Tao Y. Optimized nonionic emulsifier for the efficient delivery of astaxanthin nanodispersions to retina: in vivo and ex vivo evaluations. Drug Delivery 2019, 26 (1), 1222–1234. 10.1080/10717544.2019.1682718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P. C.; Srivastava P.; Pandey P.; Khan W.; Panda B. P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016, 6 (12), 10001–10010. 10.1039/C5RA19113K. [DOI] [Google Scholar]
- Han J. H.; Ju J. H.; Lee Y. S.; Park J. H.; Yeo I. J.; Park M. H.; Roh Y. S.; Han S. B.; Hong J. T. Astaxanthin alleviated ethanol-induced liver injury by inhibition of oxidative stress and inflammatory responses via blocking of STAT3 activity. Sci. Rep. 2018, 8 (1), 1–10. 10.1038/s41598-018-32497-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuluaga M.; Gueguen V.; Letourneur D.; Pavon-Djavid G. Astaxanthin-antioxidant impact on excessive Reactive Oxygen Species generation induced by ischemia and reperfusion injury. Chem.-Biol. Interact. 2018, 279, 145–158. 10.1016/j.cbi.2017.11.012. [DOI] [PubMed] [Google Scholar]
- MU N.; Mehar J. G.; Mudliar S. N.; Shekh A. Y. Recent advances in microalgal bioactives for food, feed, and healthcare products: commercial potential, market space, and sustainability. Comp. Rev. Food Sci. Food Safety 2019, 18 (6), 1882–1897. 10.1111/1541-4337.12500. [DOI] [PubMed] [Google Scholar]
- Silva S. C.; Ferreira I. C.; Dias M. M.; Barreiro M. F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25 (15), 3406. 10.3390/molecules25153406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorzelska E.; Hamulka J.; Wawrzyniak A.. Astaksantyna–budowa, właściwości i możliwości zastosowania w żywności funkcjonalnej. Żywność Nauka Technologia Jakość 2016, 23 ( (1), ). [Google Scholar]
- Borowiak D.; Lenartowicz P.; Grzebyk M.; Wiśniewski M.; Lipok J.; Kafarski P. Novel, automated, semi-industrial modular photobioreactor system for cultivation of demanding microalgae that produce fine chemicals—The next story of H. pluvialis and astaxanthin. Algal Res. 2021, 53, 102151. 10.1016/j.algal.2020.102151. [DOI] [Google Scholar]
- Mulders K. J.; Lamers P. P.; Martens D. E.; Wijffels R. H. Phototrophic pigment production with microalgae: biological constraints and opportunities. J. Phycol. 2014, 50 (2), 229–242. 10.1111/jpy.12173. [DOI] [PubMed] [Google Scholar]
- Tan B. L.; Norhaizan M. E.; Liew W.-P.-P.; Sulaiman Rahman H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic G.; Nikolova D.; Gluud C. Antioxidant supplements and mortality. Curr. Opin. Clin. Nutr. Metabol. Care 2013, 17 (1), 40–44. 10.1097/MCO.0000000000000009. [DOI] [PubMed] [Google Scholar]
- Ndhlala A. R.; Moyo M.; Van Staden J. Natural Antioxidants: Fascinating or Mythical Biomolecules?. Molecules 2010, 15 (10), 6905–6930. 10.3390/molecules15106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan P.; Arulselvan P.; Choi D.-K. Phytobioactive compound-based nanodelivery systems for the treatment of type 2 diabetes mellitus - current status. Int. J. Nanomed. 2017, 12, 1097–1111. 10.2147/IJN.S124601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78 (s1), A18–A25. 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- González-Ballesteros N.; Rodríguez-Argüelles M. C.; Lastra-Valdor M.; González-Mediero G.; Rey-Cao S.; Grimaldi M.; Cavazza A.; Bigi F. Synthesis of Silver and Gold Nanoparticles by Sargassum Muticum Biomolecules and Evaluation of Their Antioxidant Activity and Antibacterial Properties. J. Nanostruct. Chem. 2020, 10 (4), 317–330. 10.1007/s40097-020-00352-y. [DOI] [Google Scholar]
- Shin S.; Saravanakumar K.; Mariadoss A. V. A.; Hu X; Sathiyaseelan A.; Wang M.-H. Functionalization of Selenium Nanoparticles Using the Methanolic Extract of Cirsium Setidens and Its Antibacterial, Antioxidant, and Cytotoxicity Activities. J. Nanostruct. Chem. 2021, 0123456789 10.1007/s40097-021-00397-7. [DOI] [Google Scholar]
- Ashrafi M.; Bayat M.; Mortazavi P.; Hashemi S. J.; Meimandipour A Antimicrobial Effect of Chitosan–Silver–Copper Nanocomposite on Candida Albicans. J. Nanostruct. Chem. 2020, 10 (1), 87–95. 10.1007/s40097-020-00331-3. [DOI] [Google Scholar]
- Gulla S.; Lomada D.; Araveti P. B.; Srivastava A.; Murikinati M. K.; Reddy K. R.; Inamuddin; Reddy M. C.; Altalhi T. Titanium Dioxide Nanotubes Conjugated with Quercetin Function as an Effective Anticancer Agent by Inducing Apoptosis in Melanoma Cells. J. Nanostruct. Chem. 2021, 11 (4), 721–734. 10.1007/s40097-021-00396-8. [DOI] [Google Scholar]
- Magne T. M.; de Oliveira Vieira T.; Alencar L. M. R.; Junior F. F. M.; Gemini-Piperni S.; Carneiro S. V.; Fechine L. M. U. D.; Freire R. M.; Golokhvast K.; Metrangolo P. Graphene and its derivatives: understanding the main chemical and medicinal chemistry roles for biomedical applications. J. Nanostruct. Chem. 2021, 10.1007/s40097-021-00444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]