Abstract
The human microflora is a complex ecosystem composed of diverse microorganisms mainly distributed in the epidermal and mucosal habitats of the entire body, including the mouth, lung, intestines, skin, and vagina. These microbial communities are involved in many essential functions, such as metabolism, immunity, host nutrition, and diseases. Recent studies have focused on the microbiota associated with cancers, particularly the oral and intestinal microbiota. Radiotherapy, the most effective cytotoxic modality available for solid tumors, contributes to the treatment of cancer patients. Mounting evidence supports that the microbiota plays pivotal roles in the efficacy and prognosis of tumor radiotherapy. Here, we review current research on the microbiota and cancer development, and describe knowledge gaps in the study of radiotherapy and the microbiota. Better understanding of the effects of the microbiome in tumorigenesis and radiotherapy will shed light on future novel prevention and treatment strategies based on modulating the microbiome in cancer patients.
Keywords: Oral microbiome, gut microbiome, cancer, radiotherapy
Introduction
The human microbiota, including bacteria, fungi, and viruses, is widely present on the epithelial barrier surfaces of the human body1. After a long period of coevolution, commensal microorganisms act as “friends” or “safeguards” that maintain human physiological processes; however, some microbes act as “evildoers” involved in the development of various diseases, including neurologic diseases, metabolic disorders, cardiovascular disorders, infectious diseases, and gastrointestinal complications2–6. Cancer morbidity and mortality rank second among all noncommunicable diseases. Through next-generation sequencing technologies, research on the roles of the microbiome, particularly those in the digestive tract, in cancer has experienced a renaissance, and the understanding of the configurations of human flora in tumor development and treatment has gradually been clarified7–13. Microbes appear to be involved in approximately 20% of human cancers, and the relationship between cancer and the microbiome is complex14,15. Microbes not only promote tumorigenesis but also affect the efficacy and prognosis of therapies11,16. For instance, previous studies have indicated that total abdominal irradiation influences gut microbiota configurations, and gut flora and the derived metabolites can be used as novel remedies to protect against radiation-induced toxicity to the hematopoietic system and gastrointestinal tract17–19. Currently, the symbiotic microbiome has become a hotspot of research in tumor occurrence and therapy.
In this review, we provide an overview of the relationship between commensal microbiota and carcinogenesis, with a focus on the effects of digestive tract microbiota on radiotherapy. Importantly, modulation of oral or intestinal microorganisms might be a potential auxiliary method in tumor radiotherapy.
Overview of the oral microbiome and cancer development
The oral microflora, the microbial community existing in the human oral cavity, comprises more than 700 bacterial species that are crucial players in resisting pathogens and maintaining oral homeostasis20. However, the oral microbiota is also responsible for a variety of oral diseases, including periodontal diseases, dental caries, and oral cancer21,22. Increasing evidence suggests that oral microorganisms are associated with carcinogenesis in distant organs, such as colorectal cancer, esophageal cancer, and pancreatic cancer23.
Head and neck tumors
High-throughput sequencing has shed light on the complex functions of microorganisms, thus providing an in-depth understanding of the mechanisms underlying head and neck cancers. Head and neck cancers are malignancies that mainly arise in the oral cavity, hypopharynx, oropharynx, and larynx24. Periodontitis, poor oral hygiene, and oral flora imbalance are potential risk factors for the development of head and neck squamous cell carcinoma, primarily oral squamous cell carcinoma (OSCC) and oropharyngeal squamous cell carcinoma (OPSCC)24,25. Smoking and excessive drinking are considered “time bombs” that negatively affect health. In OSCC, in the presence of other known risk factors, tobacco and alcohol consumption disrupt the oral microbiota, thus resulting in oral cancer development26. The oral microbiota converts alcohol into acetaldehyde, a mutagen, thereby promoting carcinogenesis of the head and neck mucosa24,27,28.
A prototypic example of a condition associated with bacterial infection is human papilloma virus (HPV), an independent risk factor for OPSCC, which induces the overexpression of the protein P16 and the oncogenes E6 and E7, thereby driving carcinogenesis29–31. People with HPV infection also have elevated abundance of the genera Gemellaceae and Leuconostoc32. In addition, Porphyromonas gingivalis (P. gingivalis) and Fusobacterium nucleatum (F. nucleatum) infection activate IL-6/STAT3 signaling, spur periodontitis, and participate in the pathogenesis of OSCC12,33. To date, substantial evidence on how periodontal pathogens induce OSCC is lacking. Further studies, particularly clinical studies, are warranted to improve knowledge of cancer-provoking microbial characteristics.
Accompanying the insights into the pathogenic factors in oral cancers, mounting and compelling evidence indicates that patients with cancer have a distinctive oral microbiome compared with that in healthy controls. The oral epithelium in patients with head and neck squamous cell carcinoma has a lower abundance and diversity of microbiota32, thus validating that an oral flora imbalance promotes the development of tumors. In detail, species of some salivary bacterial genera, such as Prevotella melaninogenica (P. melaninogenica), Capnocytophaga gingivalis (C. gingivalis), and Streptococcus mitis (S. mitis), are correlated with OSCC34. In addition, dysbiosis at tumor sites may show a signature of markedly decreased abundance of the phyla Firmicutes and Actinobacteria compared with that in paired normal tissue35,36. Previous studies have revealed only a correlation between some special microbes and oral tumors; however, the complexity is less well characterized and requires further investigation. Such research cannot be limited to the study of a single pathogen37.
Colorectal cancer
The digestive tract is a tubular passage typically extending from the mouth to the anus or cloaca. Microorganisms in the upper digestive tract may migrate to and colonize the lower digestive tract. In colorectal cancer (CRC), some oral bacteria emerge in the lower digestive tract, particularly at tumor sites. For example, periodontal pathogens including Fusobacterium and Porphyromonas are detectable in samples from patients with CRC. Other oral pathogens, such as Treponema denticola and Prevotella intermedia, are also associated with an increased risk of CRC38–41. In addition, Fusobacterium, particularly F. nucleatum, is a genus frequently observed in CRC42,43. Colon ectopic F. nucleatum is a CRC-promoting bacterium, and scientists have further explored the mechanism underlying how F. nucleatum promotes CRC carcinogenesis, including immune modulation, virulence factors, microRNAs, and bacterial metabolism44. In addition to Fusobacterium, orally originating microorganisms, such as Gemella, Peptostreptococcus, and Parvimonas, are found in the lower digestive tract and are associated with the progression of CRC40; however, additional studies are needed to explore the virulence factors and carcinogenic potential of these genera.
Pancreatic cancer
Multiple publications have demonstrated an association between increased pancreatic cancer risk and poor oral health45,46. Oral health is strongly affected by the activities of oral flora, and scientists have hypothesized that the bacterial conditions in the oral cavity may be associated with a high pancreatic cancer risk47,48. In many of these studies, a higher frequency of the genera Bacteroides and Granulicatella adiacens is more common in patients with pancreatic cancer than in healthy people; however, some bacteria, including Neisseria elongata and S. mitis, are present at lower concentrations49,50. A previous study has confirmed that elevated blood serum antibodies to P. gingivalis, an oral pathogen, may contribute to a higher risk of pancreatic cancer51. Given this evidence, researchers have compared oral bacterial samples between pancreatic cancer patients and healthy controls, and found that the presence of Aggregatibacter actinomycetemcomitans and P. gingivalis in the oral cavity is associated with pancreatic carcinogenesis, whereas the phylum Fusobacteria and the genus Leptotrichia are protective and decrease the risk13. Although dysbiosis of the oral microbiota has been implicated in pancreatic cancer13,49, direct evidence defining a causal relationship between oral dysbiosis and early distal cancer is lacking. However, in light of the clear relationship between periodontal pathogens and pancreatic cancer, the therapeutic or prophylactic implications in preventing periodontal pathogens might potentially decrease the risk of pancreatic cancer.
Gut microbiome and cancer development
The human intestinal flora consists of more than 1,000 types of bacterial species, and the gut exists in homeostasis in healthy individuals through interactions between the host and microbiome, thus preventing the invasion of pathogens52. However, intestinal dysbiosis leads to unfavorable host effects, such as tumorigenesis53. The mechanisms through which the gut microbiome affects tumor development are diverse, including the creation of a local chronic inflammatory state, as well as genotoxic effects27,54. Here, we summarize available studies and characterize the gut microflora features in human cancers.
Colorectal cancer
Similarly to other diseases, CRC results from various multifactorial genetic factors and environmental stimuli55,56. Genetic predisposition syndromes for CRC account for a minority of cases57. Family studies have shown that heritability as the etiological agent for CRC accounts for only 12%–35% of the total incidence55,58, thus suggesting that environmental factors play crucial roles in CRC development. The roles of the commensal microbiome in CRC, particularly the microbiota dwelling in the intestine, are increasingly being recognized.
As early as the 1960s, scientists studied the effects of intestinal microflora on the CRC susceptibility in mouse models59. Later evidence identified the relationship between gut flora dysbiosis and colorectal tumorigenesis, and most studies indicate that shifts in the gut microbiome composition contribute to CRC development60. A significant discrepancy in microbiota configuration between the tumor site and peritumoral milieu has been reported61,62. Fecal particles carry substantial physiological and pathological information and have the potential to be used for noninvasive diagnosis. Focusing on fecal samples from patients with CRC, researchers have discovered that microbiota diversity and Clostridia frequency are lower than those in the non-CRC population, whereas the abundance of Porphyromonas and Fusobacterium at the genus level is higher42. Wong and colleagues63 have collected and transplanted the stool microbiome from patients with CRC to experimental animals, and found that the patients’ fecal flora promoted colorectal carcinogenesis in mice, thus suggesting that irregular gut microbiota might be a driver of CRC. The mechanisms through which gut dysbiosis promotes CRC are presumed to involve disorders in host metabolism and mucosal immune responses induced by microorganisms64,65. High-throughput sequencing has allowed researchers to identify specific gut pathogens positively associated with CRC. For instance, infection with Streptococcus bovis (S. bovis) has been determined to indicate a high risk of colonic tumors66, and enterotoxigenic Bacteroides fragilis (ETBF) is significantly enriched in patients with CRC compared with the healthy population. Several of the malgenic mechanisms, including the production of reactive oxygen species that induce DNA damage and the activation of pathways involving the proinflammatory cytokine IL-17 and Wnt, are closely associated with the induction of CRC by ETBF and have been comprehensively reviewed67–69. Alternatively, chronic inflammation targets the intestinal microbiota, disturbs the metabolome, and produces metabolites with direct genotoxicity. For example, pks+ Escherichia coli (E. coli) drives tumorigenesis by inducing DNA mutagens70, and Campylobacter jejuni promotes CRC occurrence through the direct genotoxic action of cytolethal distending toxin71.
Liver cancer
Specific enteric microbes and microbial dysbiosis affect the status of distant organs in a sophisticated and orchestrated manner. For example, gut microbiota-derived heterogeneous metabolites enter the blood circulation and affect the physiological and pathological processes of nonenteric tissues such as the liver. A gut flora imbalance potentiates hepatocellular carcinoma by damaging DNA, activating oncogenic signaling pathways, and releasing tumor-promoting compounds72,73. The intestine and the liver are anatomically and physiologically linked via the portal vein, thus forming the “intestinal-liver axis”74–76, and the gut microbiome is a critical factor mediating this axis75. Intestinal dysbiosis and increased intestinal permeability facilitate the transfer of microorganisms from the intestine to the liver, including gut microbial components called microbe-associated molecular patterns, metabolites, and the microbiota itself. These elements cause an inflammatory response and may potentially lead directly to carcinogenesis77,78. Bile acids (BAs) are active biocomponents that regulate the metabolic pathways of hepatocytes and intestinal epithelial cells. However, enteric microorganisms modify primary BAs into secondary BAs such as deoxycholic acid, thereby leading to DNA damage, hepatotoxicity, and carcinogenesis79,80. Furthermore, the production of BAs may alter immune function, for example, through natural killer T cells’ influencing tumor growth81. Gram-negative or gram-positive-bacteria-derived lipopolysaccharide or lipoteichoic acid interacts with Toll-like receptor (TLR) 4 or TLR2 and increases inflammatory status through the innate immune response, thus promoting liver fibrosis and cancer82–84. Additionally, the gut microbiota is associated with obesity, infectious hepatitis, and nonalcoholic steatohepatitis, all of which may lead to cirrhosis and consequently hepatocellular carcinoma72.
Breast cancer
Given the lack of substantial evidence, integrating the gut microbiota into an understanding of breast cancer occurrence is difficult. However, studies have shown that the intestinal flora in patients with breast cancer is different from that in healthy controls85, and mounting studies indicate that the gut microbiota is involved in the metabolism of estrogen, which promotes carcinogenesis. The possible mechanisms through which the gut microbiota affects breast cancer include regulating circulating estrogen and phytoestrogen profiles, interfering with energy metabolism and obesity, and impeding antitumor immune function86,87. Strikingly, recent studies have shown that deletion of the gut microbiome by using an antibiotic cocktail increases breast cancer cell metastasis in mouse models, thus highlighting the importance of gut microbes in breast cancer88. Given the success of preliminary outcomes, further preclinical, clinical, and epidemiological studies are urgently needed to elucidate the relationship between the gut microbiome and breast cancer.
The microbiome and cancer radiotherapy
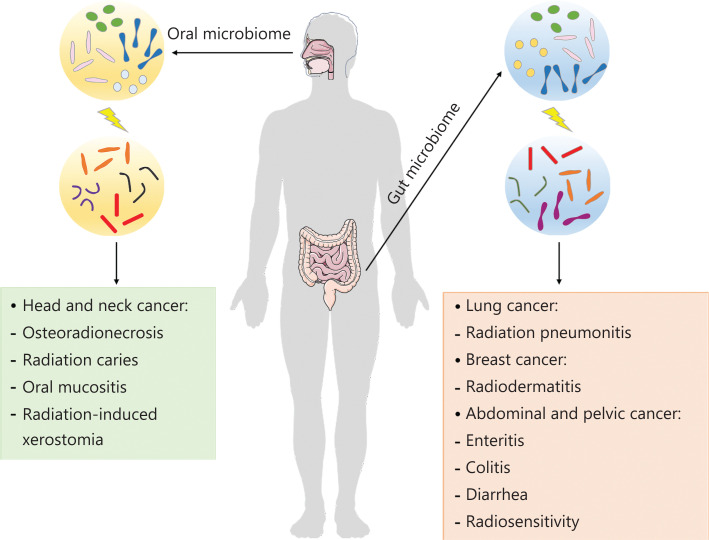
Radiotherapy is one of the major remedies for cancer and is regarded as a milestone for oncotherapy89. Approximately 50% of patients newly diagnosed with cancer and many patients with recurrent or persistent tumors undergo radical or palliative radiotherapy with the explicit goal of reducing tumor growth and inhibiting tumor metastasis90. Compelling evidence suggests that the microbiome is strongly affected by radiotherapy (Figure 1), including efficacy, prognosis, and adverse effects91,92.
Figure 1.
The influence of the microbiota on cancer radiotherapy. Oral microbes contribute to radiation-induced chronic complications including osteoradionecrosis, caries, oral mucositis, and xerostomia in head and neck tumors. Gut microbes can influence the adverse effects and efficacy of radiotherapy for various cancers.
The microbiome in radiotherapy for head and neck tumors
Radiotherapy is the first-line option for head and neck cancers (HNCs). Although surgery is the oldest and most traditional treatment for HNC, modern radiotherapy has been demonstrated to be on par with surgery in treating HNC, particularly when lesions are in early stages. Iatrogenic irradiation of the head and neck region is inevitably associated with deleterious effects to many normal organs and precipitates irreversible oral complications, such as osteoradionecrosis93, radiation caries94, mucositis95,96, and low saliva97, thus decreasing patient quality of life. The human oral cavity is home to a wide range of microbiota, with more than 700 species of microorganisms identified to date98,99. In patients receiving radiation therapy, the dynamic and stable oral ecosystem is undermined. In 1975, Brown and colleagues100 found that radiotherapy-reduced saliva flow is accompanied by pronounced shifts in some microbial populations, thus linking radiation exposure to the oral microbiota for the first time. Subsequently, studies on oral bacteria and radiotherapy have gradually become a novel research focus in studies on HNC. For example, studies on the oral microbial changes have demonstrated that radiotherapy increases the Candida frequency in the oral cavity in patients with squamous cell carcinoma101. At 6–8 months after radiotherapy, the abundance of specific microflora, such as Candida albicans, Enterococci, Lactobacillus spp., and Streptococcus mutans, are considerably higher than those in patients without radiotherapy102. Saliva serves as an essential safeguard in the oral cavity that helps maintain a “healthy mouth”; for example, saliva flow reduction is an emblematic “red flag” suggesting dental caries. Saliva lubricates oral surfaces and maintains the structural integrity of teeth by attenuating demineralization, clearing food, facilitating remineralization, and bolstering the adaptive and innate arms of the host defenses. More recently, exposure to 20 Gy has been found to decrease salivary function by as much as 80%103. Owing to the high radiosensitivity of salivary glands, iatrogenic irradiation of the head and neck region harms salivary glands and retards saliva secretion104. Saliva consists of a spectrum of primary nutrients for the resident oral microbiota; thus, the radiotherapy-induced decrease in saliva impairs the equilibrium of the oral microbiota. In addition, prolonged hyposalivation and xerostomia significantly affect the microflora environment, thus further influencing the oral microbiota configurations105.
Before next-generation sequencing technologies, the traditional methods to assess microorganisms included screening and culturing any microbes or target microbes on special culture media. The emergence of next-generation sequencing technologies has enabled discovery of the diversity of microbial gene repertoires8, thus enabling further study of how radiotherapy affects oral microorganisms. Previous studies have reported that a total of 140 species of bacteria belonging to 13 phyla are found in the dental plaques in patients with head and neck tumors before and during radiotherapy, and a negative relationship exists between the number of OTUs and radiation dose106,107. Beyond research focusing on the changes in microorganisms, studies are increasingly demonstrating the important roles of oral microbes in the pathogenesis of radiation-induced chronic long-term complications, including caries and oral mucositis. For example, Mougeot and colleagues108 have reported a potential protective role of Abiotrophia defectiva (A. defectiva) and a cariogenic role of Prevotella melaninogenica (P. melaninogenica), on the basis of analysis of the oral microbiome profiles of patients with HNC. However, whether the oral microbiota can be used as a biomarker to predict the incidence and severity of oral toxicity remains uncertain. Several studies have demonstrated the emergence of pathogenic bacteria associated with mucositis in patients with HNC during radiotherapy, thus suggesting that oral microbes may contribute to the onset and severity of oral mucositis109,110. Emerging data describe the dynamic variation in oral microbiota during radiotherapy and its association with the incidence and severity of oral mucositis in patients with HNC 111. The results show that several bacteria, including Haemophilus, Fusobacterium, Tannerella, Porphyromonas, and Eikenella, contribute to patient susceptibility to oral mucositis. Some bacteria can also serve as probiotics that mitigate radiation-induced oral injuries. For instance, Lactobacillus brevis CD2 lozenges have been suggested to be a safe and effective treatment that decreases the incidence of mucositis in patients with HNC during radiotherapy112. Furthermore, increasing attention is being paid to the intriguing therapeutic possibility of regulating salivary microbiota for oral mucositis induced by radiotherapy113. One study has shown that a probiotic combination significantly ameliorates radiation-induced oral mucositis by improving the immune response114. However, further studies are needed to discover the effects of specific bacterial strains on oral toxicity mediated by radiation therapy for HNC and to provide mechanistic insight.
The microbiome in radiotherapy for lung cancer and breast cancer
Emerging evidence indicates that the lung has a microbiome of its own. Sputum samples from patients with lung cancer, compared with those from non-lung cancer patients, have higher relative abundance of isolated Granulicatella, Abiotrophia, and Streptococcus115. Furthermore, the microbiota in lung tumor tissues shows higher taxa-taxa interactions and lower species richness116. One recent study has reported that patients with periodontal disease are at increased risk of developing lung carcinoma, but the biological mechanisms remain undetermined, and whether oral microbes play a key role in tumorigenesis requires further detailed study117. However, the enteric counterparts of the oral flora and gut microbiome have been demonstrated to have prognostic value for lung cancer patients118,119. Despite the above evidence linking the microbiota to lung cancer, the role of local (lung) or distal (oral/gut) microbiota in lung cancer has not been clarified. In terms of microflora and lung cancer radiotherapy, a recent study has provided evidence that the intestinal microbiota protects against radiation pneumonitis, as demonstrated through fecal microbiome transplantation into irradiated mice; therefore, the gut-lung axis may be an innovative therapeutic avenue for protecting against radiation-induced lung injury120. Nevertheless, the protective potential of special gut microbiota in lung cancer during radiotherapy is not yet well defined. Longitudinal epidemiological and prospective studies are needed to determine the effects of microbial communities on lung cancer radiotherapy.
Chan and colleagues121 have used 16S rRNA gene amplicon sequencing to explore the effects of the local breast ductal microbiome on breast cancer. The authors have presented the first evidence of a lower incidence of a genus from the family Sphingomonadaceae and higher incidence of the genus Alistipes in nipple aspirate fluid collected from patients with breast cancer. Additional studies have reported that bacterial species, including those from Bacillus, Enterobacteriaceae, and Staphylococcus, are enriched in breast tissue from patients with breast cancer122. Strikingly, recent evidence has further demonstrated a functional relationship between the gut microbiome and breast cancer88. For example, a study has indicated that gut microbiome disorder promotes breast cancer metastasis in mouse models, thus suggesting that greater attention should be paid to the association between the gut microbiome and breast cancer123. Radiation therapy, a major therapeutic modality in the treatment of breast cancer, can be used in breast conservation and postmastectomy settings. Patients with breast cancer experience acute or chronic dermatitis in response to radiotherapy, consisting of atrophy, fibrosis, or pigmentation alterations, which can be mitigated by topical steroids and skin hygiene124. The mechanisms of radiation-induced dermatitis include the activation of T cells and the prevention of epidermal repair by bacterial superantigens, particularly those from Staphylococcus aureus (S. aureus)125. Recently, the microbiome has been widely considered an indirect factor that affects the immune system by shifting the balance of glucose utilization and fatty acid oxidation during radiotherapy126. M1 macrophages promote the radiosensitivity of breast cancer cells. In contrast, M2 macrophages cause radioresistance in breast cancer cells, an effect attributed to STAT6 phosphorylation and M2 polarization mediated by IL-4/IL-13127,128. To date, a comprehensive understanding of the causal relationship between microbiota and radiotherapy for breast cancer remains lacking.
The microbiome in radiotherapy for abdominal and pelvic cancer
Radiotherapy is an effective treatment for abdominal and pelvic cancer, on the basis of its genotoxic effect on tumor cells. However, a healthy bowel is inevitably exposed to radiation, thus causing adverse sequelae that manifest as gastrointestinal toxicity129. As an organ, the intestine comprises many different cell types, including vascular, enteric immune, and nervous system cells, all of which are influenced by irradiation to various degrees130. Radiation exposure damages the intestinal barrier, alters the abundance and composition of gut microbiota, and causes apoptosis in intestinal crypts17,19,131,132. The pathogenesis of enteritis, colitis, and diarrhea in mouse models and patients receiving radiation therapy has been found to be associated with a gut flora imbalance, which limits therapy completion133. Gut dysbiosis is typically characterized by decreased microbial abundance and/or significant shifts in composition. Evidence suggests that gut microbial dysbiosis may be a biomarker predicting radiation enteropathy. Patients with pelvic radiotherapy-induced diarrhea possess a significantly higher relative Firmicutes/Bacteroides ratio as well as differences in the relative abundance of Clostridia cluster XVIII134. Studies support that Clostridia cluster XVIII promotes the expansion and differentiation of T cells, which fight against colitis and allergic diarrhea135. Another clinical study has shown that patients receiving pelvic radiotherapy show prominent changes in their gut microbiota composition after radiotherapy, particularly in Firmicum and Fusobacterium, which have been found to decrease by 10% and increase by 3%, respectively136. Evidence from murine models indicates that rectal irradiation can induce local microbial dysbiosis, which in turn increases mucosal IL-1β secretion, thus resulting in radiation-induced colonic damage137. The above studies clearly show that the gut microbiota composition is associated with the pathogenesis of radiation-induced damage, but larger studies are needed to confirm these findings.
Our team has long focused on gut microbiota and radiation toxicity, and has demonstrated that gut microbiota configurations relate to radiation injuries in hosts, and that modulation of the intestinal microbiome mitigates hematopoietic system and gastrointestinal tract toxicity after irradiation challenge17–19,138,139. Intuitively, certain gut microbiota configurations significantly ameliorate the response and toxicity to acute radiation syndrome18. We propose that rehabilitation strategies for patients undergoing radiotherapy for cancer should take the sex of patients into account17. In terms of mechanism, our studies of gut microbiota-produced metabolites, including valeric acid and indole 3-propionic acid, provide a new perspective regarding microbiome-based remedies for radioactive diseases19,139. Recently, a clinical study has provided the first evidence that fecal microbiota transplantation might be safe and effective in improving intestinal symptoms and mucosal injury in patients with chronic radiation enteritis within a certain period of time140. However, the relevance of the biological mechanisms through which fecal microbiota transplantation might serve as a therapeutic mitigating radiation-induced toxicity remains to be investigated.
In addition to their roles in pathogenicity, microorganisms have been demonstrated to play key roles in the response to protect the intestinal mucosa against radiotherapy-induced toxicity. For example, probiotic Lactobacillus rhamnosus GG has been shown to prevent radiation-induced injury, by decreasing epithelial injury and improving crypt survival, which depends on TLR-2/MyD88/COX-2 signaling141. In some clinical studies, probiotics have been shown to decrease the risk of some severe consequences of radiation therapy, such as diarrhea133,142. All the above findings support the possibility that probiotics could be used as adjuvant agents during radiotherapy. However, the findings of a meta-analysis show that current evidence does not indicate beneficial effects of probiotics for the prevention of radiotherapy-induced diarrhea; consequently, research efforts should focus on the specific forms of gastrointestinal toxicity and certain microbial phenotypes to develop targeted microbiota manipulation143.
The effects of the intestinal microbiota have also been investigated in the setting of radiotherapy efficacy for abdominal and pelvic cancer. Crawford and Gordon144 have found that intestinal flora increases the radiosensitivity of endothelial cells and lymphocytes in the mesenchymal cores of the small intestinal villi in germ-free mice receiving total body irradiation, thus providing new viewpoints regarding the relationship between intestinal flora and radiosensitivity. In addition, perturbations in the circadian rhythm elicit alterations in gut bacterial configurations that worsen radiation-induced injuries, as compared with those in mice housed under 12 h dark/12 h light cycles138. Antibiotic-treated mouse models have provided further evidence supporting this association18. These studies have been further supplemented by published clinical studies demonstrating that circadian rhythm may affect radiotherapy local control and toxicities145. Even if the gut microbiome may be a key player in mitigating radiation therapy-associated complications, the direct effects of the intestinal microbiota on the efficacy of radiotherapy remain to be understood, but might be an important and interesting field in radiomedicine.
Conclusion
In the past few years, knowledge of the microbiome in cancers has rapidly grown, and the mechanisms of tumorigenesis that are attributable to the microbiota are progressively being understood (summarized in Table 1). The microbiome is relevant in cancer development and therapy through many routes, via bacterial infection or crucially through bacterial dysbiosis; however, the underlying mechanisms remain poorly understood. Moreover, some challenges regarding how the microbiome affects tumor radiotherapy remain to be solved. In general, insights have been gained into the relationship between the oral/gut microbiome and cancer radiotherapy. Specifically, radiotherapy influences the commensal microbiota of hosts, and conversely, the commensal microbiota affects the efficacy and prognosis of radiotherapy. However, much remains to be learned about the mechanisms underlying these events. Furthermore, with advances in cancer radiotherapy, we must consider the factors modulating commensal flora, such as diet, antibiotic usage, and hygiene management. Only by fully understanding these interactions can we know how to optimally modulate the microbiome to enhance radiotherapy efficiency and limit radiation-induced adverse effects.
Table 1.
The influence of microbes on cancer development
| Malignancy | Methods | Main results | References |
|---|---|---|---|
| Head and neck cancer | Meta-analysis | Oral microbiota converts alcohol into acetaldehyde, thus causing cancer. | 26 |
| Case-control study | HPV is widely considered an independent risk factor inducing OPSCC. | 30 | |
| Murine model | P. gingivalis and F. nucleatum infection promotes OSCC via the IL-6-STAT3 pathway. | 33 | |
| Case-control study | P. melaninogenica, C. gingivalis, and S. mitis are elevated in the saliva of individuals with OSCC. | 34 | |
| Case-control study | Actinomyces and Firmicutes are significantly depleted in tumor tissue relative to normal tissue. | 35 | |
| Colorectal cancer (CRC) | Case-control study | Fusobacterium and Porphyromonas are detectable in samples from patients with CRC. | 40 |
| Case-control study | Treponema denticola and Prevotella intermedia are associated with increased risk of CRC. | 41 | |
| Murine model | F. nucleatum precipitates CRC carcinogenesis via immune modulation, virulence factors, microRNAs, and bacterial metabolism. | 44 | |
| Case-control study | Gemella, Peptostreptococcus, and Parvimonas are found in CRC. | 40 | |
| Case-control study | Gut microbial dysbiosis contributes to the development of CRC. | 61 | |
| Case-control study | Lower relative abundance of Clostridia and higher relative abundance of Porphyromonas and Fusobacterium are found in patients with CRC. | 42 | |
| Murine model | Stool microbiota from patients with CRC promotes colorectal carcinogenesis in mice. | 63 | |
| Case-control study | Streptococcus bovis is a risk factor for colonic tumors. | 66 | |
| Murine model | ETBF is highly expressed in patients with CRC compared with healthy people. | 67,68 | |
| Murine model | E. coli induces tumorigenesis through generating DNA mutagens. | 70 | |
| Murine model | Campylobacter jejuni promotes CRC through the genotoxic action of cytolethal distending toxin. | 71 | |
| Pancreatic cancer | Case-control study | The Bacteroides genus and Granulicatella adiacens are more common in patients with pancreatic cancer than healthy people; however, Neisseria elongata and Streptococcus mitis are present in lower concentrations in pancreatic cancer. | 49 |
| Case-control study | P. gingivalis may contribute to a higher risk of pancreatic cancer. | 51 | |
| Case-control study | Aggregatibacter actinomycetemcomitans and P. gingivalis in the oral cavity are associated with pancreatic carcinogenesis, whereas the phylum Fusobacteria and its genus Leptotrichia are protective and decrease the risk. | 13 | |
| Liver cancer | Murine model | Gut bacterial metabolites cause DNA damage and carcinogenesis. | 79 |
| Murine model | Gut bacteria-controlled bile acids may alter immune function, thus influencing tumor growth. | 81 | |
| Murine model | Intestinal microbiota and lipopolysaccharide accelerate hepatocarcinogenesis. | 82 | |
| Murine model | A gram-positive gut microbial component increases the risk of cancer development through creating a tumor-promoting microenvironment. | 83 | |
| Breast cancer | Case-control study | The intestinal flora in patients with breast cancer is different from that in healthy controls. | 85 |
| Murine model | Gut dysbiosis affects mammary tumor dissemination. | 88 |
Grant support
This work was supported by grants from the Science Foundation for Distinguished Young Scholars of Tianjin (Grant No. 20JCJQJC00100), the National Natural Science Foundation of China (Grant Nos. 81572969, 81730086 and 81872555), the Drug Innovation Major Project of China (Grant No. 2018ZX09711001-007–008), and the PUMC Graduate Innovation Fund (Grant No. 2018-1001-04 and 2019-1001-06).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Jiali Dong and Ming Cui.
Collected the data: Jiali Dong, Yuan Li and Huiwen Xiao.
Performed the analysis: Jiali Dong and Ming Cui.
Wrote the paper: Jiali Dong.
Oversaw the entire project and revised the paper: Ming Cui and Saijun Fan.
References
- 1.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336:1255–62. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czesnikiewicz-Guzik M, Muller DN. Scientists on the spot: salt, the microbiome, and cardiovascular diseases. Cardiovasc Res. 2018;114:e72–e3. doi: 10.1093/cvr/cvy171. [DOI] [PubMed] [Google Scholar]
- 3.Khanna S, Montassier E, Schmidt B, Patel R, Knights D, Pardi DS, et al. Gut microbiome predictors of treatment response and recurrence in primary Clostridium difficile infection. Aliment Pharmacol Ther. 2016;44:715–27. doi: 10.1111/apt.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim D, Zeng MY, Nunez G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp Mol Med. 2017;49:e339. doi: 10.1038/emm.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–81. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 6.Hansen JJ, Sartor RB. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr Treat Options Gastroenterol. 2015;13:105–20. doi: 10.1007/s11938-014-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–21. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–16. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 10.Rajagopala SV, Vashee S, Oldfield LM, Suzuki Y, Venter JC, Telenti A, et al. The Human Microbiome and Cancer. Cancer Prev Res (Phila) 2017;10:226–34. doi: 10.1158/1940-6207.CAPR-16-0249. [DOI] [PubMed] [Google Scholar]
- 11.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271–85. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 12.Chattopadhyay I, Verma M, Panda M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol Cancer Res Treat. 2019;18:1533033819867354. doi: 10.1177/1533033819867354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67:120–7. doi: 10.1136/gutjnl-2016-312580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 15.Elinav E, Garrett WS, Trinchieri G, Wargo J. The cancer microbiome. Nat Rev Cancer. 2019;19:371–6. doi: 10.1038/s41568-019-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The Influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570–80. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui M, Xiao H, Li Y, Zhang S, Dong J, Wang B, et al. Sexual dimorphism of gut microbiota dictates therapeutics efficacy of radiation injuries. Adv Sci (Weinh) 2019;6:1901048. doi: 10.1002/advs.201901048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui M, Xiao H, Li Y, Zhou L, Zhao S, Luo D, et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol Med. 2017;9:448–61. doi: 10.15252/emmm.201606932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Dong J, Xiao H, Zhang S, Wang B, Cui M, et al. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes. 2020;11:1–18. doi: 10.1080/19490976.2019.1709387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69:137–43. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Le Bars P, Matamoros S, Montassier E, Le Vacon F, Potel G, Soueidan A, et al. The oral cavity microbiota: between health, oral disease, and cancers of the aerodigestive tract. Can J Microbiol. 2017;63:475–92. doi: 10.1139/cjm-2016-0603. [DOI] [PubMed] [Google Scholar]
- 22.Healy CM, Moran GP. The microbiome and oral cancer: more questions than answers. Oral Oncol. 2019;89:30–3. doi: 10.1016/j.oraloncology.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Niu Q, Fan W, Huang F, He H. Oral microbiota and gastrointestinal cancer. Onco Targets Ther. 2019;12:4721–8. doi: 10.2147/OTT.S194153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galvao-Moreira LV, da Cruz MC. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol. 2016;53:17–9. doi: 10.1016/j.oraloncology.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt BL, Kuczynski J, Bhattacharya A, Huey B, Corby PM, Queiroz EL, et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS One. 2014;9:e98741. doi: 10.1371/journal.pone.0098741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma – an update. CA Cancer J Clin. 2015;65:401–21. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 27.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–12. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Homann N, Jousimies-Somer H, Jokelainen K, Heine R, Salaspuro M. High acetaldehyde levels in saliva after ethanol consumption: methodological aspects and pathogenetic implications. Carcinogenesis. 1997;18:1739–43. doi: 10.1093/carcin/18.9.1739. [DOI] [PubMed] [Google Scholar]
- 29.Ma Y, Madupu R, Karaoz U, Nossa CW, Yang L, Yooseph S, et al. Human papillomavirus community in healthy persons, defined by metagenomics analysis of human microbiome project shotgun sequencing data sets. J Virol. 2014;88:4786–97. doi: 10.1128/JVI.00093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tezal M, Scannapieco FA, Wactawski-Wende J, Hyland A, Marshall JR, Rigual NR, et al. Local inflammation and human papillomavirus status of head and neck cancers. Arch Otolaryngol Head Neck Surg. 2012;138:669–75. doi: 10.1001/archoto.2012.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirghani H, Amen F, Moreau F, Lacau St Guily J. Do high-risk human papillomaviruses cause oral cavity squamous cell carcinoma? Oral Oncol. 2015;51:229–36. doi: 10.1016/j.oraloncology.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Guerrero-Preston R, Godoy-Vitorino F, Jedlicka A, Rodriguez-Hilario A, Gonzalez H, Bondy J, et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget. 2016;7:51320–34. doi: 10.18632/oncotarget.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binder Gallimidi A, Fischman S, Revach B, Bulvik R, Maliutina A, Rubinstein AM, et al. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget. 2015;6:22613–23. doi: 10.18632/oncotarget.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3:27. doi: 10.1186/1479-5876-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Funchain P, Bebek G, Altemus J, Zhang H, Niazi F, et al. Microbiomic differences in tumor and paired-normal tissue in head and neck squamous cell carcinomas. Genome Med. 2017;9:14. doi: 10.1186/s13073-017-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong H, Shi Y, Zhou X, Wu C, Cao P, Xu C, et al. Microbiota in the throat and risk factors for laryngeal carcinoma. Appl Environ Microbiol. 2014;80:7356–63. doi: 10.1128/AEM.02329-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee WH, Chen HM, Yang SF, Liang C, Peng CY, Lin FM, et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci Rep. 2017;7:16540. doi: 10.1038/s41598-017-16418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zackular JP, Rogers MA, Ruffin MTt, Schloss PD. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res (Phila) 2014;7:1112–21. doi: 10.1158/1940-6207.CAPR-14-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang Q, Chiu J, Chen Y, Huang Y, Higashimori A, Fang J, et al. Fecal bacteria act as novel biomarkers for noninvasive diagnosis of colorectal cancer. Clin Cancer Res. 2017;23:2061–70. doi: 10.1158/1078-0432.CCR-16-1599. [DOI] [PubMed] [Google Scholar]
- 40.Koliarakis I, Messaritakis I, Nikolouzakis TK, Hamilos G, Souglakos J, Tsiaoussis J. Oral bacteria and intestinal dysbiosis in colorectal cancer. Int J Mol Sci. 2019;20:4146. doi: 10.3390/ijms20174146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Cai Q, Shu XO, Steinwandel MD, Blot WJ, Zheng W, et al. Prospective study of oral microbiome and colorectal cancer risk in low-income and African American populations. Int J Cancer. 2019;144:2381–9. doi: 10.1002/ijc.31941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907–11. doi: 10.1093/jnci/djt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 44.Hashemi Goradel N, Heidarzadeh S, Jahangiri S, Farhood B, Mortezaee K, Khanlarkhani N, et al. Fusobacterium nucleatum and colorectal cancer: a mechanistic overview. J Cell Physiol. 2019;234:2337–44. doi: 10.1002/jcp.27250. [DOI] [PubMed] [Google Scholar]
- 45.Wei MY, Shi S, Liang C, Meng QC, Hua J, Zhang YY, et al. The microbiota and microbiome in pancreatic cancer: more influential than expected. Mol Cancer. 2019;18:97. doi: 10.1186/s12943-019-1008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33:1055–8. doi: 10.1093/carcin/bgs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pendyala G, Joshi S, Chaudhari S, Gandhage D. Links demystified: periodontitis and cancer. Dent Res J (Isfahan) 2013;10:704–12. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Ogrendik M. Periodontal pathogens in the etiology of pancreatic cancer. Gastrointest Tumors. 2017;3:125–7. doi: 10.1159/000452708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61:582–8. doi: 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ertz-Archambault N, Keim P, Von Hoff D. Microbiome and pancreatic cancer: a comprehensive topic review of literature. World J Gastroenterol. 2017;23:1899–908. doi: 10.3748/wjg.v23.i10.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres PJ, Fletcher EM, Gibbons SM, Bouvet M, Doran KS, Kelley ST. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ. 2015;3:e1373. doi: 10.7717/peerj.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 53.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25:377–88. doi: 10.1038/s41591-019-0377-7. [DOI] [PubMed] [Google Scholar]
- 54.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 56.Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin Gastroenterol Hepatol. 2019;17:275–89. doi: 10.1016/j.cgh.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med. 2008;359:2143–53. doi: 10.1056/NEJMra0802968. [DOI] [PubMed] [Google Scholar]
- 58.Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int J Cancer. 2002;99:260–6. doi: 10.1002/ijc.10332. [DOI] [PubMed] [Google Scholar]
- 59.Laqueur GL, McDaniel EG, Matsumoto H. Tumor induction in germfree rats with methylazoxymethanol (MAM) and synthetic MAM acetate. J Natl Cancer Inst. 1967;39:355–71. [PubMed] [Google Scholar]
- 60.Dejea C, Wick E, Sears CL. Bacterial oncogenesis in the colon. Future Microbiol. 2013;8:445–60. doi: 10.2217/fmb.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakatsu G, Li X, Zhou H, Sheng J, Wong SH, Wu WK, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727. doi: 10.1038/ncomms9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y, Chen J, Zheng J, Hu G, Wang J, Huang C, et al. Mucosal adherent bacterial dysbiosis in patients with colorectal adenomas. Sci Rep. 2016;6:26337. doi: 10.1038/srep26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 2017;153:1621–33 e6. doi: 10.1053/j.gastro.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 64.Johnson CH, Dejea CM, Edler D, Hoang LT, Santidrian AF, Felding BH, et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015;21:891–7. doi: 10.1016/j.cmet.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–72. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 66.Gupta A, Madani R, Mukhtar H. Streptococcus bovis endocarditis, a silent sign for colonic tumour. Colorectal Dis. 2010;12:164–71. doi: 10.1111/j.1463-1318.2009.01814.x. [DOI] [PubMed] [Google Scholar]
- 67.Thiele Orberg E, Fan H, Tam AJ, Dejea CM, Destefano Shields CE, Wu S, et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2017;10:421–33. doi: 10.1038/mi.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, Murray-Stewart TR, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A. 2011;108:15354–9. doi: 10.1073/pnas.1010203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–22. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–3. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He Z, Gharaibeh RZ, Newsome RC, Pope JL, Dougherty MW, Tomkovich S, et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 2019;68:289–300. doi: 10.1136/gutjnl-2018-317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mima K, Nakagawa S, Sawayama H, Ishimoto T, Imai K, Iwatsuki M, et al. The microbiome and hepatobiliary-pancreatic cancers. Cancer Lett. 2017;402:9–15. doi: 10.1016/j.canlet.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 73.Huang H, Ren Z, Gao X, Hu X, Zhou Y, Jiang J, et al. Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in HBV-related hepatocellular carcinoma. Genome Med. 2020;12:102. doi: 10.1186/s13073-020-00796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527–39. doi: 10.1038/nrgastro.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C, Yang M, Ericsson AC. The potential gut microbiota-mediated treatment options for liver cancer. Front Oncol. 2020;10:524205. doi: 10.3389/fonc.2020.524205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seo YS, Shah VH. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin Mol Hepatol. 2012;18:337–46. doi: 10.3350/cmh.2012.18.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67:1084–103. doi: 10.1016/j.jhep.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 80.Payne CM, Weber C, Crowley-Skillicorn C, Dvorak K, Bernstein H, Bernstein C, et al. Deoxycholate induces mitochondrial oxidative stress and activates NF-kappaB through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis. 2007;28:215–22. doi: 10.1093/carcin/bgl139. [DOI] [PubMed] [Google Scholar]
- 81.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–16. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, et al. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 2017;7:522–38. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 84.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 85.Goedert JJ, Jones G, Hua X, Xu X, Yu G, Flores R, et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv147. djv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw029. djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shapira I, Sultan K, Lee A, Taioli E. Evolving concepts: how diet and the intestinal microbiome act as modulators of breast malignancy. ISRN Oncol. 2013;2013:693920. doi: 10.1155/2013/693920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buchta Rosean C, Bostic RR, Ferey JCM, Feng TY, Azar FN, Tung KS, et al. Preexisting commensal dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor-positive breast cancer. Cancer Res. 2019;79:3662–75. doi: 10.1158/0008-5472.CAN-18-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shevtsov M, Sato H, Multhoff G, Shibata A. Novel approaches to improve the efficacy of immuno-radiotherapy. Front Oncol. 2019;9:156. doi: 10.3389/fonc.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin. 2017;67:65–85. doi: 10.3322/caac.21358. [DOI] [PubMed] [Google Scholar]
- 91.Kumagai T, Rahman F, Smith AM. The microbiome and radiation induced-bowel injury: evidence for potential mechanistic role in disease pathogenesis. Nutrients. 2018;10:1405. doi: 10.3390/nu10101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang S, Wang Q, Zhou C, Chen K, Chang H, Xiao W, et al. Colorectal cancer, radiotherapy and gut microbiota. Chin J Cancer Res. 2019;31:212–22. doi: 10.21147/j.issn.1000-9604.2019.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jalali G, Unkel JH, Reed JA. Management of dental complications in a child with rhabdomyosarcoma. Pediatr Dent. 2012;34:506–9. [PubMed] [Google Scholar]
- 94.de Oliveira Mota CC, Gueiros LA, Maia AM, Santos-Silva AR, Gomes AS, Alves Fde A, et al. Optical coherence tomography as an auxiliary tool for the screening of radiation-related caries. Photomed Laser Surg. 2013;31:301–6. doi: 10.1089/pho.2012.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chattopadhyay S, Saha A, Azam M, Mukherjee A, Sur PK. Role of oral glutamine in alleviation and prevention of radiation-induced oral mucositis: a prospective randomized study. South Asian J Cancer. 2014;3:8–12. doi: 10.4103/2278-330X.126501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khaw A, Liberali S, Logan R, Keefe D, Bartold PM. Influence of periodontitis on the experience of oral mucositis in cancer patients undergoing head and neck radiotherapy: a pilot study. Support Care Cancer. 2014;22:2119–25. doi: 10.1007/s00520-014-2186-3. [DOI] [PubMed] [Google Scholar]
- 97.Pimentel MJ, Filho MM, Araujo M, Gomes DQ, LJ DAC. Evaluation of radioprotective effect of pilocarpine ingestion on salivary glands. Anticancer Res. 2014;34:1993–9. [PubMed] [Google Scholar]
- 98.Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–25. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zaura E, Nicu EA, Krom BP, Keijser BJ. Acquiring and maintaining a normal oral microbiome: current perspective. Front Cell Infect Microbiol. 2014;4:85. doi: 10.3389/fcimb.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brown LR, Dreizen S, Handler S, Johnston DA. Effect of radiation-induced xerostomia on human oral microflora. J Dent Res. 1975;54:740–50. doi: 10.1177/00220345750540040801. [DOI] [PubMed] [Google Scholar]
- 101.Sonalika WG, Amsavardani Tayaar S, Bhat KG, Patil BR, Muddapur MV. Oral microbial carriage in oral squamous cell carcinoma patients at the time of diagnosis and during radiotherapy – a comparative study. Oral Oncol. 2012;48:881–6. doi: 10.1016/j.oraloncology.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 102.Almstahl A, Wikstrom M, Fagerberg-Mohlin B. Microflora in oral ecosystems in subjects with radiation-induced hyposalivation. Oral Dis. 2008;14:541–9. doi: 10.1111/j.1601-0825.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 103.Hou J, Zheng H, Li P, Liu H, Zhou H, Yang X. Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother Oncol. 2018;129:44–51. doi: 10.1016/j.radonc.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 104.Schuurhuis JM, Stokman MA, Roodenburg JL, Reintsema H, Langendijk JA, Vissink A, et al. Efficacy of routine pre-radiation dental screening and dental follow-up in head and neck oncology patients on intermediate and late radiation effects. A retrospective evaluation. Radiother Oncol. 2011;101:403–9. doi: 10.1016/j.radonc.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 105.Guobis Z, Kareiviene V, Baseviciene N, Paipaliene P, Niedzelskiene I, Sabalys G, et al. Microflora of the oral cavity in patients with xerostomia. Medicina (Kaunas) 2011;47:646–51. [PubMed] [Google Scholar]
- 106.Hu YJ, Shao ZY, Wang Q, Jiang YT, Ma R, Tang ZS, et al. Exploring the dynamic core microbiome of plaque microbiota during head-and-neck radiotherapy using pyrosequencing. PLoS One. 2013;8:e56343. doi: 10.1371/journal.pone.0056343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao L, Hu Y, Wang Y, Jiang W, He Z, Zhu C, et al. Exploring the variation of oral microbiota in supragingival plaque during and after head-and-neck radiotherapy using pyrosequencing. Arch Oral Biol. 2015;60:1222–30. doi: 10.1016/j.archoralbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 108.Mougeot JC, Stevens CB, Almon KG, Paster BJ, Lalla RV, Brennan MT, et al. Caries-associated oral microbiome in head and neck cancer radiation patients: a longitudinal study. J Oral Microbiol. 2019;11:1586421. doi: 10.1080/20002297.2019.1586421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Laheij AM, de Soet JJ. Can the oral microflora affect oral ulcerative mucositis? Curr Opin Support Palliat Care. 2014;8:180–7. doi: 10.1097/SPC.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 110.Orlandi E, Iacovelli NA, Tombolini V, Rancati T, Polimeni A, De Cecco L, et al. Potential role of microbiome in oncogenesis, outcome prediction and therapeutic targeting for head and neck cancer. Oral Oncol. 2019;99:104453. doi: 10.1016/j.oraloncology.2019.104453. [DOI] [PubMed] [Google Scholar]
- 111.Vesty A, Gear K, Biswas K, Mackenzie BW, Taylor MW, Douglas RG. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support Care Cancer. 2020;28:2683–91. doi: 10.1007/s00520-019-05084-6. [DOI] [PubMed] [Google Scholar]
- 112.Sharma A, Rath GK, Chaudhary SP, Thakar A, Mohanti BK, Bahadur S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: a randomized double-blind placebo-controlled study. Eur J Cancer. 2012;48:875–81. doi: 10.1016/j.ejca.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 113.V DES, Belgioia L, Cante D, MR LAP, Caspiani O, Guarnaccia R, et al. Lactobacillus brevis CD2 for prevention of oral mucositis in patients with head and neck tumors: a multicentric randomized study. Anticancer Res. 2019;39:1935–42. doi: 10.21873/anticanres.13303. [DOI] [PubMed] [Google Scholar]
- 114.Jiang C, Wang H, Xia C, Dong Q, Chen E, Qiu Y, et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer. 2019;125:1081–90. doi: 10.1002/cncr.31907. [DOI] [PubMed] [Google Scholar]
- 115.Hosgood HD, 3rd, Sapkota AR, Rothman N, Rohan T, Hu W, Xu J, et al. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environ Mol Mutagen. 2014;55:643–51. doi: 10.1002/em.21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mao Q, Ma W, Wang Z, Liang Y, Zhang T, Yang Y, et al. Differential flora in the microenvironment of lung tumor and paired adjacent normal tissues. Carcinogenesis. 2020;41:1094–103. doi: 10.1093/carcin/bgaa044. [DOI] [PubMed] [Google Scholar]
- 117.Zeng XT, Xia LY, Zhang YG, Li S, Leng WD, Kwong JS. Periodontal disease and incident lung cancer risk: a meta-analysis of cohort studies. J Periodontol. 2016;87:1158–64. doi: 10.1902/jop.2016.150597. [DOI] [PubMed] [Google Scholar]
- 118.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 119.Nagasaka M, Sexton R, Alhasan R, Rahman S, Azmi AS, Sukari A. Gut microbiome and response to checkpoint inhibitors in non-small cell lung cancer-A review. Crit Rev Oncol Hematol. 2020;145:102841. doi: 10.1016/j.critrevonc.2019.102841. [DOI] [PubMed] [Google Scholar]
- 120.Nie X, Li L, Yi M, Qin W, Zhao W, Li F, et al. The intestinal microbiota plays as a protective regulator against radiation pneumonitis. Radiat Res. 2020;194:52–60. doi: 10.1667/RR15579.1. [DOI] [PubMed] [Google Scholar]
- 121.Chan AA, Bashir M, Rivas MN, Duvall K, Sieling PA, Pieber TR, et al. Characterization of the microbiome of nipple aspirate fluid of breast cancer survivors. Sci Rep. 2016;6:28061. doi: 10.1038/srep28061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. 2016;82:5039–48. doi: 10.1128/AEM.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miko E, Kovacs T, Sebo E, Toth J, Csonka T, Ujlaki G, et al. Microbiome-microbial metabolome-cancer cell interactions in breast cancer-familiar, but unexplored. Cells. 2019;8:293. doi: 10.3390/cells8040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bray FN, Simmons BJ, Wolfson AH, Nouri K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther (Heidelb) 2016;6:185–206. doi: 10.1007/s13555-016-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hill A, Hanson M, Bogle MA, Duvic M. Severe radiation dermatitis is related to Staphylococcus aureus. Am J Clin Oncol. 2004;27:361–3. doi: 10.1097/01.coc.0000071418.12121.c2. [DOI] [PubMed] [Google Scholar]
- 126.Eslami SZ, Majidzadeh AK, Halvaei S, Babapirali F, Esmaeili R. Microbiome and breast cancer: new role for an ancient population. Front Oncol. 2020;10:120. doi: 10.3389/fonc.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McGee HM, Jiang D, Soto-Pantoja DR, Nevler A, Giaccia AJ, Woodward WA. Targeting the tumor microenvironment in radiation oncology: proceedings from the 2018 ASTRO-AACR research workshop. Clin Cancer Res. 2019;25:2969–74. doi: 10.1158/1078-0432.CCR-18-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rahal OM, Wolfe AR, Mandal PK, Larson R, Tin S, Jimenez C, et al. Blocking interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2018;100:1034–43. doi: 10.1016/j.ijrobp.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 129.Larsen A, Reitan JB, Aase ST, Hauer-Jensen M. Long-term prognosis in patients with severe late radiation enteropathy: a prospective cohort study. World J Gastroenterol. 2007;13:3610–3. doi: 10.3748/wjg.v13.i26.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shukla PK, Gangwar R, Manda B, Meena AS, Yadav N, Szabo E, et al. Rapid disruption of intestinal epithelial tight junction and barrier dysfunction by ionizing radiation in mouse colon in vivo: protection by N-acetyl-l-cysteine. Am J Physiol Gastrointest Liver Physiol. 2016;310:G705–15. doi: 10.1152/ajpgi.00314.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–25. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y, Xiao H, Dong J, Luo D, Wang H, Zhang S, et al. Gut microbiota metabolite fights against dietary polysorbate 80-aggravated radiation enteritis. Front Microbiol. 2020;11:1450. doi: 10.3389/fmicb.2020.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis – current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40:409–21. doi: 10.1111/apt.12878. [DOI] [PubMed] [Google Scholar]
- 134.Wang A, Ling Z, Yang Z, Kiela PR, Wang T, Wang C, et al. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study. PLoS One. 2015;10:e0126312. doi: 10.1371/journal.pone.0126312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 136.Nam YD, Kim HJ, Seo JG, Kang SW, Bae JW. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS One. 2013;8:e82659. doi: 10.1371/journal.pone.0082659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gerassy-Vainberg S, Blatt A, Danin-Poleg Y, Gershovich K, Sabo E, Nevelsky A, et al. Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut. 2018;67:97–107. doi: 10.1136/gutjnl-2017-313789. [DOI] [PubMed] [Google Scholar]
- 138.Cui M, Xiao H, Luo D, Zhang X, Zhao S, Zheng Q, et al. Circadian rhythm shapes the gut microbiota affecting host radiosensitivity. Int J Mol Sci. 2016;17:1786. doi: 10.3390/ijms17111786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xiao HW, Cui M, Li Y, Dong JL, Zhang SQ, Zhu CC, et al. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome. 2020;8:69. doi: 10.1186/s40168-020-00845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ding X, Li Q, Li P, Chen X, Xiang L, Bi L, et al. Fecal microbiota transplantation: a promising treatment for radiation enteritis? Radiother Oncol. 2020;143:12–8. doi: 10.1016/j.radonc.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 141.Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61:829–38. doi: 10.1136/gutjnl-2011-300367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Drago L. Probiotics and colon cancer. Microorganisms. 2019;7:66. doi: 10.3390/microorganisms7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wardill HR, Van Sebille YZA, Ciorba MA, Bowen JM. Prophylactic probiotics for cancer therapy-induced diarrhoea: a meta-analysis. Curr Opin Support Palliat Care. 2018;12:187–97. doi: 10.1097/SPC.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 144.Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A. 2005;102:13254–9. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chan S, Rowbottom L, McDonald R, Bjarnason GA, Tsao M, Danjoux C, et al. Does the time of radiotherapy affect treatment outcomes? a review of the literature. Clin Oncol (R Coll Radiol) 2017;29:231–8. doi: 10.1016/j.clon.2016.12.005. [DOI] [PubMed] [Google Scholar]