Abstract
Purpose:
Exfoliation syndrome (XFS), the most common recognizable cause of open-angle glaucoma worldwide, is a systemic disorder with genetic predisposition due to variations in lysyl oxidase-like 1 (LOXL1) function, leading to altered elastin matrices in ocular and systemic tissues. Obstructive sleep apnea (OSA) is a highly prevalent disorder also involving elastic tissue dysfunction and has been found to be associated with glaucoma. Due to similarities between the disorders, we sought to uncover any relationship in the prevalence of these diagnoses.
Design:
Case-control retrospective cohort study.
Subjects:
A cohort of 81,735 patients diagnosed with OSA at ages 50–90 years were identified from medical records from 1996 to 2017 in the Utah Population Database. Case subjects were matched to random controls on to sex and birth year in a 4:1 ratio.
Methods:
International Classification of Diseases, Ninth Revision (ICD-9) codes or their ICD-10 equivalent were used to define a diagnosis of OSA (ICD-9 327.23) and a diagnosis of XFS (ICD-9 365.52 and 366.11). Conditional logistic regression odds ratios accounting for individual matching on sex and birth year was used to estimate the risk of XFS in OSA patients. Models included adjustment for race, obesity, tobacco use, hypertension, atrial fibrillation, and chronic obstructive pulmonary disease.
Outcome:
Whether OSA patients have an increased risk of diagnosis of XFS compared with non-OSA population controls.
Results:
There was an increased risk of an XFS diagnosis in OSA patients compared with non-OSA controls (OR = 1.27; 95% CI, 1.02–1.59; P = 0.03). In a stratification of patients by hypertension diagnosis history, OSA patients with hypertension exhibited an increased risk of XFS compared to non OSA controls with hypertension (OR = 2.67; 95% CI, 2.06–3.46; P <0.0001).
Conclusions:
Patients with OSA may be at an increased risk of XFS compared to non-OSA diagnosed individuals, particularly in patients with a history of hypertension.
Introduction
Exfoliation syndrome (XFS) is a systemic age-related elastin disorder recognized by the presence of fibrillary material in the anterior segment of the eye. It has a known association with exfoliative glaucoma (XFG),1 as well as zonular weakness, cataract, central retinal vein occulsion, and lens dislocation.1 Systemic diseases associated with this elastin-related disorder have also been reported by our group and others, including pelvic organ prolapse, atrial fibrillation (AF), chronic obstructive pulmonary disease (COPD), inguinal hernia, as well as cardiovascular and cerebro-vascular disease.2,3,4,5,6
XFS has a genetic basis, with the lysyl oxidase-like gene (LOXL1) variants associated with abnormal extracellular matrix (ECM) material production and deposition, and elastin repair.4 XFS has been hypothesized to be associated with chronic low-grade oxidative stress in the anterior segment of the eye with deposition of extracellular microfibrils.7,8 Markers of oxidative stress include increased prooxidant-antioxidant balance8, and endothelin-1.9 The prevalence has been reported to increase in higher latitudes,10 as high as 10% in Scandanavian populations,11 but is also common to those in Greece (11%),12 Celtic populations and throughout the middle east.13 XFS progresses to XFG in some patients and the risk is increased with increasing age.14 XFG has been observed to involve higher baseline intraocular pressure (IOP) and to require more IOP lowering medications than those for primary open angle glaucoma to control progression.15 Additionally, cataract surgical management of patients with XFS and XFG can be complicated by capsular tear, zonular dehiscence, and vitreous loss.16
Obstructive sleep apnea (OSA) is a common disorder affecting at approximately 22% of men and 17% of women worldwide,17 although some analyses have concluded that the prevalence may be much higher.18 Severity increases with age, male sex, and higher body-mass index (BMI).18 Other factors including family history, menopause, craniofacial abnormalities and health behaviors such as cigarette smoking and alcohol use may also play a role.10 OSA is caused by the partial or total collapse of upper airways during sleep. In patients with OSA, airway patency is maintained by the pharyngeal muscles during awake hours, but due to an anatomically small upper airway that relaxes and collapses during sleep, changes in airflow occur, leading to frequent arousals during sleep.19 The gold-standard method for diagnosing OSA is overnight polysomnography.20 Severity of OSA increases with age and increasing BMI.21 OSA is also associated with systemic diseases such as hypertension and atrial fibrillation (AF).22,23 Hypertension and obesity may also influence progression of AF in patients with OSA.24 Untreated OSA is associated with systemic hypertension, cardiovascular disease, and derangements in glucose metabolism.25,26,27
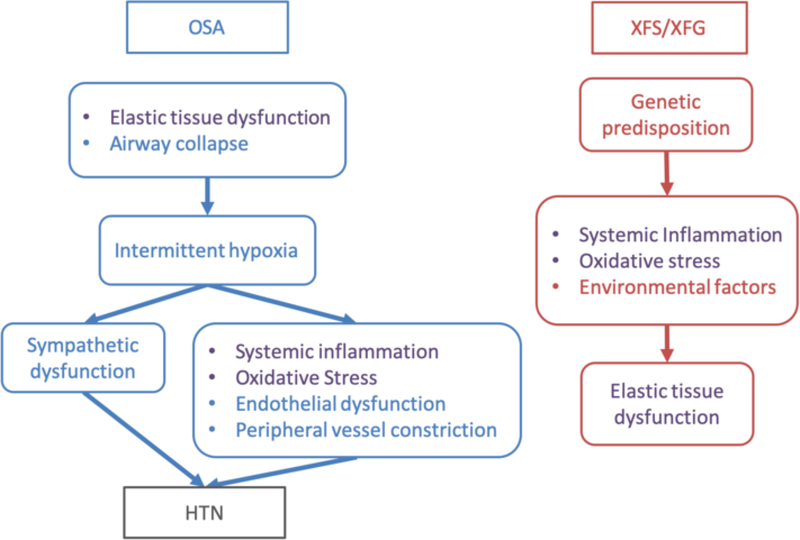
There is a notable and complex association of both XFS and OSA with hypertension (HTN). Population studies have shown a dose dependent relationship between OSA severity and blood pressure, (even when controlling for concurrent diseases such as obesity, age and sex).28,29,30,31,32 The mechanisms linking OSA to HTN are theorized to be the intermittent hypoxia leading to heightened sympathetic nervous system response,33 and oxidative stress, driving subsequent damage to endothelial cells, peripheral arterial constriction and stiffening.34 Other mechanisms involved with OSA and hypertension include increased inflammation, increase in the renin-angiotensin aldosterone system, and increased endothelin,35 all factors now recognized to play a role in glaucoma.36,37,38 Both OSA and glaucoma39 are associated with vascular endothelial dysfunction.40 A healthy functioning vascular endothelium is necessary to maintain vascular homeostasis and the balance of anti-inflammatory, pro-inflammatory, and coagulation pathways. HTN is associated with increased risk of glaucoma41 and exfoliation syndrome42,43 as well as nighttime drops in blood pressure.44,45 Pathogenic mechanisms common to OSA and XFS are shown in Figure 1. While the causal mechanisms linking OSA and HTN are still being investigated, it appears that the interaction is bidirectional with the one reinforcing the other. For the purposes of our current analysis, it is sufficient to recognize that HTN and OSA modulate one another and this may impact and influence the clinical presentation of XFS. We theorize that the underlying oxidative stress and inflammation caused by OSA, alone or in combination with HTN, may place patients at higher risk of presenting with XFS, based on an underlying genetic predispostion.
Figure 1.
Summary of pathogenic mechanisms of OSA and XFS/XFG.
XFS= Exfoliation syndrome, XFG=Exfoliation Glaucoma, OSA=Obstructive sleep apnea, HTN=Systemic hypertension
The left side summarizes some of the proposed mechanisms involved in OSA, focusing on some of the factors contributing to HTN. The right side summarizes pathogenesis of XFS/XFG. The text in purple highlight similarities between OSA and XFS/XFG.
We examined a large population database, which has successfully demonstrated systemic associations in the past, to determine whether there are associations between OSA and XFS, and to report the strength of these associations.
Methods:
Utah Population Database
The Utah Population Database (UPDB) is a research resource located at the University of Utah that contains electronic data records for more than 11 million individuals who currently reside in or have had an event recorded in Utah (e.g. a birth, marriage, death, medical record, or genealogy record).46 Contained within the UPDB are vital records data from birth and death certificates from the early 1900s and electronic medical claims data beginning in 1996 from statewide inpatient hospital and ambulatory facility records, as well as links to the clinical records of a statewide system of hospitals and clinics, University of Utah Health Care (UUHC). Approval to conduct this study was obtained from the University of Utah Institutional Review Board (00081512) and the Resource for Genetic and Epidemiologic Research, the body that governs research use of UPDB data. All data was de-identified and HIPAA compliant per the tenets of the Declaration of Helsinki.
Study Population
Using the UPDB, the electronic medical records (EMR) of 2.2 million UUHC patients and statewide medical discharge data from Utah inpatient and ambulatory facilities were interrogated over an 18-year period (1999–2017) to identify an OSA cohort of 81,735 patients ages 50 through 90 years at time of index diagnosis based on International Classification of Diseases, Ninth Revision (ICD-9) code 327.23 (obstructive sleep apnea) or the ICD-10 equivalent (G47.33) beginning in October, 2015. A comparison (“control”) group of individuals with no diagnosis of any form of sleep apnea were randomly selected from the UPDB and individually matched based on sex and birth year in a 4:1 ratio to OSA patients.
An XFS cohort of 2,943 patients ages 50–90 years diagnosed from 1996 – 2015 was previously identified based on ICD-9 codes 365.52 (exfoliation glaucoma) or 366.11 (exfoliation of lens capsule) as described.5 For comparison, 14,713 control subjects for were individually matched on sex and birth year to XFS patients in a 5:1 ratio. Each control was required to have follow up in Utah that was at least as long as their respective matched study patient, based on the latest event recorded in the UPDB.
Study Outcome
The risk of having had an XFS diagnosis (including exfoliation glaucoma, XFG) in patients with a diagnosis of OSA in comparison to risk of XFS in their respective matched controls without a history of sleep apnea..
Statistical Methods
Odds ratios (OR) were calculated from conditional multivariable logistic regression models to estimate the risk of XFS in OSA patients compared to birth- and sex-matched controls. A model including adjustment covariates for race, obesity, hypertension, tobacco use, hypertension, atrial fibrillation (AF), and chronic obstructive pulmonary disease (COPD) diagnosis history was analyzed. First-order interactions (multiplicative scale) were examined between covariates potentially related to both OSA and XFS, including: obesity, hypertension, tobacco use, AF, and COPD. Race was recorded as as a binary variable (nonwhite vs. white) and obesity according to body mass index (BMI) derived from height and weight in drivers license data in UPDB corresponding to the time that was closest to a case diagnosis of OSA. Body mass index was categorized as non-obese (BMI <30), obese (BMI≥ 30), or BMI unknown. A binary hypertension history variable was identified in the medical record based on a presence of an ICD-9 code (or ICD-10 equivalent) for hypertension (401.0 malignant, 401.1 benign, or 401.9 unspecified) vs. no indication of hypertension. Similarly, tobacco use was designated by the medical record codes, 305.1 and V15.82 which corresponded to any tobacco use disorder and history of tobacco use, respectively, versus no tobacco use. History of AF was determined by a diagnosis of ICD-9 code 427.31 and COPD history was determined by diagnosis code 491.22 in the medical record. The software package SAS 9.4 (SAS Institute Inc, Cary, NC) was used for all statistical analyses.
Continuous positive airway pressure (CPAP) Pilot
Given that the diagnosis of OSA based on diagnosis codes in administrative databases may be misclassified, we sought to identify a pilot subset in our OSA patient cohort, defined by ICD-9/-10 code, who had either a physician order for either a CPAP machine and/or certification of CPAP compliance in UUHC sleep-wake clinic records. Accordingly, a subset of 6,275 OSA patients with CPAP was available to examine the risk of XFS in comparison to their respective, non-OSA controls as a robustness check of our OSA cohort definition..
Results
Study Participants
The characteristics of the OSA patient cohort and matched controls are shown in Table 1. Years of follow-up and race did not differ significantly between patient cohort and sex- and birth year-matched controls. The mean age at index diagnosis for OSA in patients was 64.6 years and mean diagnosis age among all XFS patients was 74.9 years (Supplemental Table S1), which is consistent current published demographic data.18,47 In the OSA cohort, the mean age of onset of XFS was 71.5 (SD ±8.38) years. In the XFS cohort, the mean age of onset of OSA was 71.8 (SD ±9.56) years. Most subjects had at least 10 to 20 or more years of follow-up. At study end, OSA patients were less likely to be living compared to controls. The majority of study subjects were white, consistent with the Utah population. Where BMI was available, OSA patients were more often obese compared to their non-OSA counterparts (45.9% vs 20.3%). Patients with OSA were also more likely than control subjects to have a history of tobacco use (18.5% vs 14.6%). Patients with OSA had a significantly higher likelihood of having AF than their respective controls (9.8% vs. 2.9% respectively) which is consistent with published data.48 For those with OSA, the proportion with a COPD diagnosis was also greater than in their respective controls (5.1% vs. 1.2%), as was of the presence of a hypertension history compared to controls (26.8% vs. 9.3%).
Table 1.
Characteristics of Utah obstructive sleep apnea (OSA) patients ages 50–90y and respective unexposed controls.
| No OSA | OSA patients | ||||
|---|---|---|---|---|---|
| Characteristic | N % | N % | P 1 | ||
| Total | 319,939 | 100.0 | 81,735 | 100.0 | |
| Female | 132,184 | 41.3 | 33,698 | 41.2 | |
| Male | 187,755 | 58.7 | 48,037 | 58.8 | 0.65 |
| Age at diagnosis of OSA case2 | |||||
| Mean y (±SD) | 64.5 | (9.4) | 64.6 | (9.4) | 0.04 |
| Years of followup | |||||
| Mean y (±sd) | 20.9 | (2.2) | 20.6 | (2.6) | <0.001 |
| <1y to 9y | 115 | 0.0 | 52 | 0.1 | |
| 10y to 22y | 319,824 | 100.0 | 81,683 | 99.9 | 0.001 |
| Vital status | |||||
| Alive at 12/31/2017 | 290,165 | 90.7 | 68,168 | 83.4 | |
| Deceased | 29,774 | 9.3 | 13,567 | 16.6 | <0.0001 |
| Race | |||||
| Caucasian | 306,255 | 95.7 | 78,507 | 96.1 | |
| Non-Caucasian | 13,684 | 4.3 | 3,228 | 3.9 | <0.0001 |
| Obesity (BMI≥ 30)3 | |||||
| Not indicated | 254,952 | 79.7 | 44,182 | 54.1 | |
| Indicated | 64,987 | 20.3 | 37,553 | 45.9 | <0.0001 |
| Tobacco use | |||||
| Not indicated | 273,222 | 85.4 | 66,639 | 81.5 | |
| Indicated | 46,717 | 14.6 | 15,096 | 18.5 | <0.0001 |
| AFib history4 | |||||
| Absent | 310,603 | 97.1 | 73,728 | 90.2 | |
| Present | 9,336 | 2.9 | 8,007 | 9.8 | <0.0001 |
| COPD history4 | |||||
| Absent | 316,032 | 98.8 | 77,583 | 94.9 | |
| Present | 3,907 | 1.2 | 4,152 | 5.1 | <0.0001 |
| Hypertension4 | |||||
| Absent | 290,169 | 90.7 | 59,832 | 73.2 | |
| Present | 29,770 | 9.3 | 21,903 | 26.8 | <0.0001 |
| OSA history4 | |||||
| Absent | 319,939 | 100.0 | 0 | 0.0 | |
| Present | 0 | 0.0 | 81,735 | 100.0 | — |
| XFS history4 | |||||
| Absent | 319,190 | 99.8 | 81,487 | 99.7 | |
| Present | 749 | 0.2 | 248 | 0.3 | <0.001 |
Discrete measures, chi-square test; continuous measures, paired t test.
Age at time of OSA patient diagnosis. For controls with no OSA, age at the time of diagnosis of their corresponding matched case.
Body mass index (BMI) as height in meters2 /weight in kg.
ICD-9 diagnosis in the patient medical record from 1996–2015.
Association of XFS with OSA
In Table 2, the odds ratio (OR) estimates from the covariate-adjusted model with 95% confidence intervals (CI-L, lower; CI-U, upper) and P values for XFS risk in OSA patients overall and in OSA patients stratified by hypertension history compared with matched control subjects are shown. After accounting for matching variables of sex and birth year and with covariate adjustment for: race, obesity, tobacco use, AF, COPD, hypertension, and interactions of hypertension with both AF and XFS, we observed a modest increased risk of XFS in OSA patients overall (OR = 1.27; 95% CI, 1.02–1.59; P = 0.03); see Table 2. In the subset of OSA patients with CPAP pilot data, a consistent but non-statistically significant increased risk of XFS was also observed (OR = 2.89; 95% CI 0.77–11.03; P = 0.12; data not shown).
Table 2.
Association of exfoliation glaucoma/syndrome (XFS) and obstructive sleep apnea (OSA) in Utah patients ages 50–90y compared with controls unexposed to OSA.
| Covariate-adjusted model | ||||
|---|---|---|---|---|
| OR | 95%CI-L | 95%CI-U | P 1 | |
| OSA cohort (overall)2 | ||||
| XFS absent | Ref. | — | ||
| XFS present | 1.27 | (1.02 – 1.59) | 0.03 | |
| OSA cohort, hypertension absent3 | ||||
| XFS absent | Ref. | — | ||
| XFS present | 0.85 | (0.68 – 1.05) | 0.12 | |
| OSA cohort, hypertension present3 | ||||
| XFS absent | Ref. | — | ||
| XFS present | 2.67 | (2.06 – 3.46) | <0.0001 | |
Wald chi-square test.
Model accounting for sex and birth year and adjusted for race, obesity, tobacco use, atrial fibrillation history, chronic obstructive pulmonary disease history, hypertension history, and interaction terms for: atrial fibrillation and hypertension; and, XFS and hypertension.
Stratified model accounting for sex and birth year and adjusted for race, obesity, tobacco use, atrial fibrillation history, and chronic obstructive pulmonary disease history.
Sub-analyses by Hypertension History
As history of hypertension appeared to interact with both XFS and AF in the OSA patient cohort, a stratification by the absence or presence of hypertension history in OSA patients was also conducted (Table 2). For patients with no hypertension history, we observed no increased risk of an XFS diagnosis in OSA patients compared with controls. For OSA patients in which a diagnosis of hypertension was present in the medical record, a 2.7-fold increased risk of XFS was observed (OR = 2.67; 95% CI, 2.06–3.46; P < 0.0001); see Table 2.
As a check on the robustness of our finding of an association between OSA and XFS, we performed a parallel set of analyses to examine the association of an OSA diagnosis in our previously identified XFS case and control cohort. We observed no increased risk of OSA in XFS patients compared to non-XFS controls, when stratified by hypertension, those XFS patients with a hypertension history exhibited an increased risk of having an OSA diagnosis (OR = 1.53; 95% CI, 1.22–1.93; P = 0.0003; data not shown). In ourXFS cohort, patients with an XFS diagnosis had an increased risk of a hypertension diagnosis than non-XFS controls (OR = 2.98; 95%CI, 2.70–3.28, P<0.0001 Table 3) which is consistent with prior publications42,43. Furthermore, increased risk of hypertension in XFS patients was observed in both those with or without an OSA diagnosis (Table 3). See Supplemental Table S1 (available online) for a description of the XFS patient and control cohorts.
Table 3.
Association of hypertension (HTN) and exfoliation syndrome (XFS) in 2,943 Utah patients ages 50–90y compared with 5:1 controls unexposed to XFS.
| Covariate-adjusted model | ||||
|---|---|---|---|---|
| OR | 95%CI-L | 95%CI-U | P 1 | |
| XFS cohort (overall)2 | ||||
| HTN absent | Ref. | — | ||
| HTN present | 2.98 | (2.70 – 3.28) | <0.0001 | |
| XFS cohort, OSA absent3 | ||||
| HTN absent | Ref. | — | ||
| HTN present | 2.91 | (2.63 – 3.23) | <0.0001 | |
| XFS cohort, OSA present3 | ||||
| HTN absent | Ref. | — | ||
| HTN present | 4.71 | (2.43 – 9.13) | <0.0001 | |
Wald chi-square test.
Model accounting for sex and birth year and adjusted for race, obesity, tobacco use, atrial fibrillation history, chronic obstructive pulmonary disease history, hypertension history, and interaction terms for: atrial fibrillation and hypertension; and, XFS and hypertension.
Stratified model on obstructive sleep apnea (OSA) accounting for sex and birth year and adjusted for race, obesity, tobacco use, atrial fibrillation diagnosis, and chronic obstructive pulmonary disease diagnosis.
Discuss
We hypothesized that OSA and XFS may be inter-related due to the oxidative stress and inflammatory changes common to both disorders, as well as the implication that OSA has had in glaucoma development. Since patients with OSA are more likely to have a diagnosis of XFS, we infer that XFS and OSA may be involved in a similar disease process and are comorbidities, and that those with OSA may have greater risk of XFS in genetically predisposed individuals, especially if they also have HTN.
Our results indicate that after accounting for multiple risk factors, OSA patients are more likely to have a diagnosis of XFS especially in those with a hypertension history. In an XFS patient cohort, increased risk of OSA with hypertension was corroborated. Given these findings, all patients with XFS should be referred for evaluation for OSA and particulary if they have hypertension. Given that XFS typically presents after 70 years of age, the likelihood of finding OSA is high given the increasing prevalence of OSA with age and therefore all patients with XFS should be screened for OSA and especially if they exhibit glacuomatous progression. The other clinical implication is that in OSA patients with hypertension, particularly those over age 70 (when XFS typically presents) can be considered for ophthalmologic referral for evaluation for XFS.
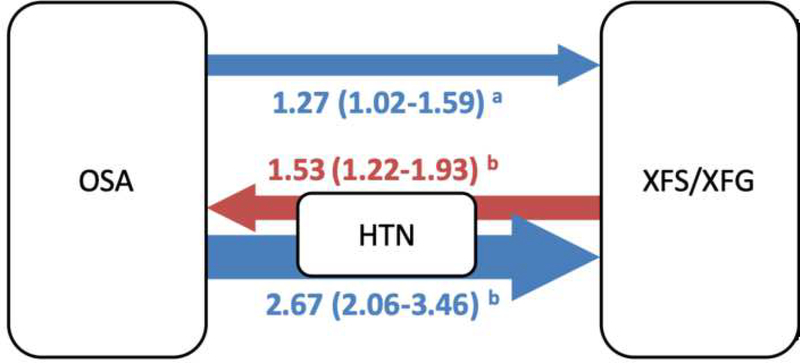
The statistically significant odds ratios representing the relationship between OSA and XFS are summarized in Figure 2, to provide insight as to how hypertension (HTN) may be involved in the disease process. If we compare the risk of an XFS diagnosis for the entire OSA cohort compared to those who also had a HTN diagnosis, the odds ratio appears greater for those with HTN. This was confirmed by the analysis of those in the CPAP cohort.
Figure 2.
Summary of significant* odds ratio evidence in OSA and XFS cohorts
Arrow direction, color, width, and number indicate strength of odds ratio association with comorbid condition, for example: The OSA cohort of patients had a 1.27 odds ratio of having a diagnosis of XFS. Patients with XFS and HTN had a 1.53 odds ratio of having diagnosis of OSA etc. For full list of odds ratios along with confidence intervals see Table 2 and Table 3.
*All numbers in figure are statistically significant with p<0.05, with confidence intervals that do not include 1.
aModel accounting for sex and birth year and adjusted for race, obesity, tobacco use, AF, COPD, hypertension and: interaction of AF and hypertension, XFS and hypertension (OSA cohort)
bModel accounting for sex and birth year, adjusted for race, obesity, tobacco use, AF, and COPD history.
XFS= Exfoliation syndrome, XFG=Exfoliation glaucoma, OSA=Obstructive sleep apnea, HTN=Systemic hypertension
What role does treatment with CPAP in OSA patients, particularly in those with hypertension, play in the incidence of XFS, and what mechanisms may be responsible? CPAP has been shown to decrease daytime and nighttime blood pressure measurements in those with refractory hypertension.29,49,50 Meta-analyses however only show blood pressure drops of 2–3 mmHg.51 It may be useful for further research to focus on the other mechanisms by which CPAP treatment affects XFS and ophthalmic health. Since CPAP lowers the oxidative stress and systemic inflammation in patients with HTN,52 we propose that further research examine how this affects the risk of XFS development and progression.
A lingering question for our study is whether OSA influences the progression of XFS (without glaucomatous damage), to manifestations of glaucoma with an elevation of IOP and development of XFG with optic nerve head damage. Our current dataset did not incorporate all of the clinical factors including UV exposure and altitude that can be taken into consideration when evaluating the conversion of XFS to XFG as well as glaucomatous progression, and we recognize this as a limitation of our analysis, and an area for further inquiry.
Besides HTN, other comorbid conditions associated with OSA include the metabolic syndrome (hyperlipidemia, obesity, and elevated blood glucose)25,26,27,21 as well as increasing age and male sex.18 OSA has increased prevalence in those with atrial fibrillation (AF).22,23 Hypertension and obesity also appear to increase the progression of AF in patients with OSA.24
Although OSA is estimated to occur in 17% of individuals, it is under-recognized and undertreated.32 XFS is also missed on clinical exam more often than it is diagnosed. Many ophthalmologists miss XFS on routine non-mydriatic anterior segment exams, because pupillary dilation is often necessary to assess the central disc of material on the anterior lens and the peripheral granular zone.53 With regard to hypertension, it may be that those with hypertension are more likely to have an eye exam and have XFS diagnosed, which we acknowledge could be a source of bias in our study. It may also be true that those without diagnosis of systemic illness such as OSA or HTN were less likely to get eye exams, and this is recognized as another limitation of our study.
It is important to note that having a CPAP order did not guarantee compliance with CPAP, however 67% of patients with a CPAP order also had certification of compliance. Conversely, as information regarding CPAP orders/certification was not available for most patients in the UPDB, absence of an order/certification could not infer non-use of CPAP.
The UPDB provides a strong foundation for large population database studies. The unique dataset allows a combination of civil government, outpatient clinic, and hospital records to be analyzed in one comprehensive source. Nevertheless, there has been some difficulty in assessing OSA according to administrative diagnosis codes in some academic settings. In another study, a group of approximately 5,000 adults who underwent polysomnography studies, 56% of these met criteria for OSA, however, none of the administrative diagnostic codes or therapeutic interventions by themselves or in combination identified OSA with high sensitivity and specificity.54 Because of this, studies that use diagnosis codes and interventions alone need to be evaluated with caution, and this is a potential limitation to this present study. However, in an effort to better identify and characterize clinical CPAP-treated OSA of potentially greater severity, we performed a pilot OSA patient study within a single healthcare system in which CPAP orders and evidence of CPAP compliance were available from clinic records. While the presence of a CPAP order does not indicate actual daily CPAP use, even the documentation of CPAP compliance at one or more time points does not indicate sustained usage of CPAP for OSA treatment. Therefore the implications of CPAP use should also be interpreted with caution. Interestingly, patients with CPAP orders were much more likely to have a hypertension history (nearly 2/3 of CPAP+ patients) than OSA overall.
For almost a century, XFS was misunderstood as being primarily an ocular phenomenon. The present study further supports the now established and broader understanding of XFS as a genetically predisposed systemic illness that is impacted and correlated with various systemic comorbidities. We expect that future research will continue to enable a better understanding of XFS and its complexities as researchers emphasize the connection with other disease states that can help better manage and influence patient care.
Supplementary Material
Acknowledgements
This work was supported in part by a grant from the Glaucoma Foundation, NY, NY (to B. Wirostko, PI) and an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah. Partial support of the Utah Population Database (UPDB) was provided by: the Huntsman Cancer Institute, University of Utah and Cancer Center Support Grant P30 CA2014 from the National Cancer Institute (N. Ulrich, PI); the University of Utah Program in Personalized Health and Center for Clinical and Translational Science (funded by National Institutes of Health (NIH) Clinical and Translational Science Awards); the Pedigree and Population Resource and University of Utah Information Technology Services for establishing the Master Subject Index between the Utah Population Database and the University of Utah Health Sciences Center; and, a National Center for Research Resources (NCRR) grant, “Sharing Statewide Health Data for Genetic Research” (R01 RR021746 to G. Mineau, PI) with additional support from the Utah State Department of Health. We thank the Pedigree and Population Resource of Huntsman Cancer Institute, University of Utah (funded in part by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance and support of the UPDB. This work was also supported by the Bennett Family Foundation Research Fund of the New York Glaucoma Research Institute and Research Fund of the New York Glaucoma Research Institute. The funding organizations had no role in the design or conduct of this research. We especially thank the Medical Informaticist with University of Utah Health Care, for their assistance in obtaining clinical information for this study.
Financial Support:
Funding Support for this study was provided by a grant from The Glaucoma Foundation, New York, NY, an unrestricted institutional grant from Research to Prevent Blindness, Inc., New York, NY to the John A Moran Eye Center, University of Utah. Support for the Utah Population Database was provided by Cancer Center Support Grant CA- from the National Cancer Institute. P30 CA2014. See acknowledgements for a more detailed attribution of support for the UPDB including datasets used in this research.
Footnotes
Conflicts of Interest: None.
References
- 1.Ritch R Exfoliation syndrome: the most common identifiable cause of open-angle glaucoma. J Glaucoma. 1994;3(2):176–177. doi: 10.1097/00061198-19940032000018 [DOI] [PubMed] [Google Scholar]
- 2.Wirostko B, Allingham R, Wong J, Curtin K. Utah Project on Exfoliation Syndrome (UPEXS). J Glaucoma 2018;27(7):S75–S77. doi: 10.1097/IJG.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 3.Wirostko BM, Curtin K, Ritch R, et al. Risk for exfoliation syndrome in women with pelvic organ prolapse: A Utah Project on Exfoliation Syndrome (UPEXS) study. JAMA Ophthalmol 2016;134(11): 1255–1262. doi: 10.1001/jamaophthalmol.2016.34U [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Yu Y, Fu S, Zhao W, Liu P. LOXL1 gene polymorphism with exfoliation syndrome/exfoliation glaucoma: A meta-analysis. J Glaucoma 2016;25(l):62–94. doi: 10.1097/IJG.0000000000000128 [DOI] [PubMed] [Google Scholar]
- 5.Taylor SC, Bemhisel AA, Curtin K, Allingham RR, Ritch R, Wirostko BM. Association between chronic obstructive pulmonary disease and exfoliation syndrome: The Utah project on exfoliation syndrome. Ophthalmol Glaucoma 2019;2(1):3–10. doi: 10.1016/j.ogla.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 6.Besch BM, Curtin K, Ritch R, Allingham RR, Wirostko BM. Association of Exfoliation Syndrome with Risk of Indirect Inguinal Hernia: The Utah Project on Exfoliation Syndrome. JAMA Ophthalmol 2018;136(12):1368–1374. doi: 10.1001/jamaophthalmol.2018.4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlötzer-Schrehardt U Molecular pathology of pseudoexfoliation syndrome/glaucoma New insights from LOXL1 gene associations. Exp Eye Res 2009;88(4):776–785. doi: 10.1016/j.exer.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 8.Koliakos GG, Befani CD, Mikropoulos D, Ziakas NG, Konstas AGP. Prooxidant-antioxidant balance, peroxide and catalase activity in the aqueous humour and serum of patients with exfoliation syndrome or exfoliative glaucoma. Graefe’s Arch Clin Exp Ophthalmol 2008;246(10):1477–1483. doi: 10.1007/s00417-008-0871-y [DOI] [PubMed] [Google Scholar]
- 9.Koliakos G, Konstas A, Schlötzer-Schrehardt U, et al. Endothelin-1 concentration is increased in the aqueous humour of patients with exfoliation syndrome. Br J Ophthalmol 2004;88(4):523–527. https://bjo.bmj.com/content/88/4/523.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein JD, Pasquale LR, Talwar N, et al. Geographic and Climatic Factors Associated with the Exfoliation Syndrome. Arch Ophthalmol 2011;129(8):1053–1060. doi: 10.1001/archophthalmol.2011.191.Geographic [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnarsson A, Damji KF, Sverrisson T, Sasaki H, Jonasson F. Pseudoexfoliation in the Reykjavik Eye Study: Prevalence and related ophthalmological variables. Acta Ophthalmol Scand 2007;85(8):822–827. doi: 10.1111/j.1600-0420.2007.01051.x [DOI] [PubMed] [Google Scholar]
- 12.Topouzis F, Wilson MR, Harris A, et al. Prevalence of Open-Angle Glaucoma in Greece: The Thessaloniki Eye Study. Am J Ophthalmol 2007;144(4):511–520. doi: 10.1016/j.ajo.2007.06.029 [DOI] [PubMed] [Google Scholar]
- 13.Rao RQ, Arain TM, Ahad MA. The prevalence of pseudoexfoliation syndrome in Pakistan. Hospital based study. BMC Ophthalmol 2006;6:1–5. doi: 10.1186/1471-2415-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang JH, Loomis, Wiggs JL, Stein JD, Pasquale LR Demographic and geographic features of exfoliation glaucoma in 2 United States-based prospective cohorts. Ophthalmology 2012;119(1):27–35. doi: 10.1016/j.ophtha.2011.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon Y, Sung KR, Kim JM, Shim SH, Yoo C, Park JH Risk Factors Associated with Glaucomatous Progression in Pseudoexfoliation Patients. J Glaucoma 2017;26(12):110–1113. doi: 10.1097/IJG.0000000000000791 [DOI] [PubMed] [Google Scholar]
- 16.Scorolli L, Scorolli L, Campos EC, Bassein L, Meduri RA. Pseudoexfoliation syndrome: A cohort study on intraoperative complications in cataract surgery. Ophthalmologica 1998;212(4):278–280. doi: 10.1159/000027307 [DOI] [PubMed] [Google Scholar]
- 17.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-A review on the epidemiology of sleep apnea. J Thorac Dis 2015;7(8):131–1322. doi: 10.3978/j.issn.2072-1439.2015.06.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senaratna CV, Perret JL, Lodge CJ,e tal. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 19.White DP Pathogenesis of obstructive and central sleep apnea. Am J RespirCrit Care Med 2005;172(11):1363–1370. doi: 10.1164/rccm.200412-1631SO [DOI] [PubMed] [Google Scholar]
- 20.Punjabi NM. The Epidemiology of Adult Obstructive Sleep Apnea. Proc Am Thorac Soc 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, & Kales A Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 1998;157(1):144–148. doi: 10.1164/ajrccm.157.1.9706079 [DOI] [PubMed] [Google Scholar]
- 22.Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Gregg C. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation — Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation ( ORBIT-AF ). Am Heart J 2015;169(5):647–654. doi: 10.1016/j.ahj.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 23.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004;110(4):364–367. doi: 10.1161/01.CIR.0000136587.68725.8E [DOI] [PubMed] [Google Scholar]
- 24.Abumuamar AM, Mollayeva T, Sandor P, Newman D, Nanthakumar K, Shapiro CM. Efficacy of continuous positive airway pressure treatment in patients with cardiac arrhythmia and obstructive sleep apnea: what is the evidence? Clin Med Insights 2017;9:1–10. doi: 10.1177/1179559X17734227 [DOI] [Google Scholar]
- 25.Peppard PE, Young T, Palta M, Skatrud J Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2006;342(19):1879–1384. [DOI] [PubMed] [Google Scholar]
- 26.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: A long-term follow-up. Eur Respir J 2006;28(3):596–602. doi: 10.1183/09031936.06.00107805 [DOI] [PubMed] [Google Scholar]
- 27.Punjabi NM; Polotsky VY. Disorders of glucose metabolism in sleep apnea. Journal of Applied Physiology. J Appl Physiol 2005;99(5):1998–2007. doi: 10.1152/japplphysiol.00695.2005 [DOI] [PubMed] [Google Scholar]
- 28.Peppard PE, Young T, Palta M, & Skatrud J Prospective Study of the Association Between Sleep-Disordered Breathing and Hypertension Vol 342 (19).; 2000. [DOI] [PubMed] [Google Scholar]
- 29.Lombardi C, Pengo MF, Parati G. Systemic hypertension in obstructive sleep apnea. J Thorac Dis 2018;10(S34):S4231–S4243. doi: 10.21037/jtd.2018.12.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, & Dempsey J Sleep apnea and hypertension: a population-based study. Ann Intern Med 1994;120(5):382–388. doi: 10.2307/3977030 [DOI] [PubMed] [Google Scholar]
- 31.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: Population study. Br Med J 2000;320(7233):479–482. doi: 10.1136/bmj.320.7233.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin José M., Agusti Alvar, Villar Isabel, Forner Marta, Nieto David, Santiago J Carrizo FB et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012;307(20):2169–2176. doi: 10.1001/jama.2012.3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Cade BE, Chen H, et al. Variants in angiopoietin-2 (ANGPT2) contribute to variation in nocturnal oxyhaemoglobin saturation level. Hum Mol Genet 2016;25(23):5244–5253. doi: 10.1093/hmg/ddw324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai A, Wang L, & Zhou Y Hypertension and obstructive sleep apnea. Hypertension Research, 2016;39(6):391–395. [DOI] [PubMed] [Google Scholar]
- 35.Kario K Obstructive sleep apnea syndrome and hypertension: Mechanism of the linkage and 24-h blood pressure control. Hypertens Res 2009;32(7):537–541. doi: 10.1038/hr.2009.73 [DOI] [PubMed] [Google Scholar]
- 36.Vohra R, Tsai JC, & Kolko M The role of inflammation in the pathogenesis of glaucoma. Surv Ophthalmol 2013;58(4):311–320. [DOI] [PubMed] [Google Scholar]
- 37.Vaajanen A, Vapaatalo H. Local ocular renin-angiotensin system - a target for glaucoma therapy? Basic Clin Pharmacol Toxicol 2011;109(4):217–224. doi: 10.1111/j.1742-7843.2011.00729.x [DOI] [PubMed] [Google Scholar]
- 38.Emre M, Orgül S, Haufschild T, Shaw SG, Flammer J. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br J Ophthalmol 2005;89(1):6–63. doi: 10.1136/bjo.2004.046755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi J, Kook MS. Systemic and Ocular Hemodynamic Risk Factors in Glaucoma. Biomed Res Int 2015;2015. doi: 10.1155/2015/141905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkeson A, Yeh SY, Malhotra A, Jelic S. Endothelial function in obstructive sleep apnea. Prog Cardiovasc Dis 2009;51(5):351–362. doi: 10.1016/j.pcad.2008.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bae HW, Lee N, Lee HS, Hong S, Seong GJ, Kim CY. Systemic hypertension as a risk factor for open-angle glaucoma: A meta-analysis of population-based studies. PLoS One 2014;9(9). doi: 10.1371/journal.pone.0108226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell P, Wang Jie Jin, Smith W Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol 1997;124(5):685–687. doi: 10.1016/S0002-9394(14)70908-0 [DOI] [PubMed] [Google Scholar]
- 43.Miyazaki M, Kubota T, Kubo M,et al. The prevalence of pseudoexfoliation syndrome in a Japanese population: The hisayama study. J Glaucoma 2005;14(6):482–484. doi: 10.1097/01.ijg.0000185436.15675.b3 [DOI] [PubMed] [Google Scholar]
- 44.Melgarejo JD, Lee JH, Petitto M,et al. Glaucomatous optic neuropathy associated with nocturnal dip in blood pressure: findings from the Maracaibo aging study. Ophthalmology 2018;125(6):807–814. doi: 10.1016/j.ophtha.2017.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collignon N, Dewe W, Guillaume S, & Collignon-Brach J Ambulatory blood pressure monitoring in glaucoma patients. The nocturnal systolic dip and its relationship with disease progression. Int Ophthalmol 1998;22(1):19–25. [DOI] [PubMed] [Google Scholar]
- 46.The University of Utah Pedigree and Population Resource: Utah Population Database. http://healthcare.utah.edu/hunts-mancancerinstitute/research/updb/. Accessed February 7, 2019.
- 47.Kozobolis VP, Detorakis ET, Tsilimbaris MK, Vlachonikolis LG, Tsambarlakis LC, Palliharis LG. Correlation between age-related macular degeneration and pseudoexfoliation syndrome in the population of Crete (Greece). Arch Ophthalmol 1999;117(5):664–669. doi: 10.1001/archopht.117.5.664 [DOI] [PubMed] [Google Scholar]
- 48.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am J Respir Crit Care Med 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montesi SB, Edwards BA, Malhotra A, Bakker JP. Effect of continuous positive airway pressure treatment on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012;8(5):587–596. doi: 10.5664/jcsm.2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: Effect of CPAP on blood pressure and baroreflex. Eur Respir J 2003;21(2):241–247. doi: 10.1183/09031936.03.00035402 [DOI] [PubMed] [Google Scholar]
- 51.Fava C, Dorigoni S, Dalle Vedove F, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea: A systematic review and meta-analysis. Chest 2014;145(4):762–771. doi: 10.1378/chest.13-1115 [DOI] [PubMed] [Google Scholar]
- 52.Karamanli H, Özol D, Ugur KS, et al. Influence of CPAP treatment on airway and systemic inflammation in OSAS patients. Sleep Breath 2014;18(2):251–256. doi: 10.1007/s11325-012-0761-8 [DOI] [PubMed] [Google Scholar]
- 53.Konstas AGP, Ringvold A. Epidemiology of exfoliation syndrome. J Glaucoma 2018;27(7):S4–S11. doi: 10.1097/IJG.0000000000000908 [DOI] [PubMed] [Google Scholar]
- 54.McIsaac DI, Gershon A, Wijeysundera D, Bryson GL, Badner N, van Walraven C. Identifying obstructive sleep apnea in administrative data. Anesthesiology 2015;123(2):253–263. doi: 10.1097/ALN.0000000000000692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.