Abstract
Objective
About 1:650–1,000 children are born with an extra X or Y chromosome (XXX; XXY; XYY), which results in a sex chromosome trisomy (SCT). This study aims to cross-sectionally investigate the impact of SCT on early social cognitive skills. Basic orienting toward social cues, joint attention, and theory of mind (ToM) in young children with SCT were evaluated.
Method
About 105 children with SCT (range: 1–7 years old) were included in this study, as well as 96 age-matched nonclinical controls. Eyetracking paradigms were used to investigate the eye gaze patterns indicative of joint attention skills and orienting to social interactions. The ToM abilities were measured using the subtest ToM of the Developmental NEuroPSYchological Assessment, second edition, neuropsychological test battery. Recruitment and assessment took place in the Netherlands and in the United States.
Results
Eyetracking results revealed difficulties in children with SCT in social orienting. These difficulties were more pronounced in children aged 3 years and older, and in boys with 47,XYY. Difficulties in joint attention were found over all age groups and karyotypes. Children with SCT showed impairments in ToM (26.3% in the [well] below expected level), increasing with age. These impairments did not differ between karyotypes.
Conclusions
An impact of SCT on social cognitive abilities was found already at an early age, indicating the need for early monitoring and support of early social cognition. Future research should explore the longitudinal trajectories of social development in order to evaluate the predictive relationships between social cognition and outcome later in life in terms of social functioning and the risk for psychopathology.
Keywords: Sex chromosome trisomy, Young children, Theory of mind, Joint attention, Social orienting, Eyetracking
Introduction
Sex chromosome trisomy (SCT) is a common genetic variation, characterized by an extra X or Y chromosome, which results in the chromosomal patterns 47,XXY (Klinefelter syndrome [KS]), 47,XXX (trisomy X or triple X) or 47,XYY (XYY syndrome), different from the typical 46,XY or 46,XX karyotype in boys and girls. Prevalence estimates vary from 1:650 to 1:1,000 (Berglund et al., 2019; Boyd, Loane, Garne, Khoshnood, & Dolk, 2011). Although the social behavioral phenotype is variable, associated social functioning might include difficulties with social interactions and social adjustment, social anxiety, and shyness. Children and adolescents with SCT also show higher percentages of psychosocial challenges, including difficulties in adaptive social functioning (Urbanus, Swaab, Tartaglia, Cordeiro, & van Rijn, 2020; Van Rijn, 2019; Van Rijn, Stockmann, Van Buggenhout, et al., 2014; Visootsak & Graham Jr., 2009). On average, across studies, 18% of children with XXY, 15% of girls with XXX, and 30% of boys with XYY meet the criteria of a clinical diagnosis of autism spectrum disorder (ASD; see for a review Van Rijn, 2019).
The presence of an extra X or Y chromosome is known to affect the maturation of the brain structures within the networks that are referred to as the “social brain” (Raznahan et al., 2016). Brain areas that are affected by the presence of an extra X or Y chromosome include the medial prefrontal, anterior cingulate, and superior temporal sulcus, which play a key role in complex social cognitive functions such as theory of mind (ToM) abilities (Soto-Icaza, Aboitiz, & Billeke, 2015). Theory of mind (also called “perspective taking” or “cognitive empathy”) is a multifaceted construct defined as “the ability to make the implicit assumption that the behavior of others is determined by their desires, attitudes and beliefs” (Frith & Frith, 2003). It is a core social cognitive ability, crucial in everyday life, needed to fully understand social interactions and is a prerequisite for showing social adaptive behavior, which is responsive of social feedback following interactions. To understand the vulnerabilities of individuals with SCT in the development of adaptive social functioning, it is important to investigate the early impact of SCT on major aspects of social cognition in early childhood (Happé & Frith, 2014): social orienting, joint attention, andToM.
These early social cognitive functions have differential developmental trajectories and unfold at different stages of development, depending on the brain maturation (Soto-Icaza et al., 2015). Typically from the age of 3 onward, the understanding of the complexity of social interactions that lead to another person’s false beliefs develop (Devine & Hughes, 2014; Wellman, Cross, & Watson, 2001). This increasing comprehension of false beliefs is represented in the levels of ToM, such as the level that refers to the understanding of second-order beliefs and the recognition of influence of earlier experiences on mental states (Wellman, 2014).
Fundamental in the development of ToM are more basic social cognitive skills, such as joint attention (Sodian & Kristen-Antonow, 2015). Joint attention is defined as “the capacity to coordinate attention between interactive social partners with respect to objects or events in order to share an awareness of these objects or events” (Nation & Penny, 2008). Joint attention is associated with the early emergence of children’s awareness that others have intentions, and it is crucial for developing perspective-taking skills (Mundy & Newell, 2007). Experiencing the mental state of attention in episodes of shared attention is essential to the understanding of others’ mental states. Joint attention is therefore considered essential in the development toward ToM (Korhonen, Kärnä, & Räty, 2014). It includes the cognitive abilities of sharing attention (i.e., alternating eye gaze), following the attention of another (i.e., following eye gaze or pointing) and directing the attention of another (Dawson et al., 2004). In the current study, we focus on the impact of SCT on the ability to follow eye gaze or pointing of a social partner.
Even before children acquire more complex social cognitive functions like ToM, social cognitive development begins to be expressed in infancy with basic social perceptual abilities, such as the ability to orient toward important social cues (i.e., faces and eyes). This ability is defined as social orienting (Mundy & Neal, 2001). This inborn elemental alignment of sensory receptors to a person or social event is believed to be the initial stage of the later developing more complex ability to think and reason about own or another person’s intentions or mental state. Impairments in social orienting already early in life can deprive a child of social information input, which, in turn, could disrupt brain development and social cognitive development (Mundy & Neal, 2001).
There have been no studies investigating ToM, joint attention, and social orienting in young children with SCT younger than 8 years old. Only a few studies investigated the ToM abilities in school-aged children and adolescents with SCT. These studies showed that ToM in school-aged boys and girls with 47,XXY and 47,XXX was less well developed compared to typically developing controls (Melogno et al., 2019; Van Rijn, Stockmann, Van Buggenhout, et al., 2014). With regard to social orienting, it was reported that some individuals with SCT have deficits in attending to social cues: Research in adult men with 47,XXY and in boys and girls with an extra X chromosome (47,XXX and 47,XXY) showed reduced attention to essential facial features and a missing typical first fixation on the eyes, both during the scanning of static facial expressions (Van Rijn, 2015) and during the dynamic presentation of faces in emotional movie clips (Van Rijn et al., 2014). It is important to evaluate whether the impact of SCT on these social cognitive functions can already be detected early in development when these abilities typically develop (Soto-Icaza et al., 2015). Insight in the early impact of SCT on core social cognitive abilities early in life may help to understand the social difficulties that may emerge in later childhood, adolescence, and adulthood of individuals with SCT. Second, knowledge about the impact of SCT on early social cognitive abilities might eventually be useful in identifying targets for early monitoring and (preventive) support of young children ofSCT.
The aim of the current study was to investigate the early impact of SCT on social orienting, joint attention, and ToM. Cross-sectional age differences were explored to understand the developmental tracks. Objective and sensitive eyetracking measures were used to study social orienting and joint attention, as eye fixations can indicate how social information is processed. Based on the relevance of the X or Y chromosomes for the development of neural networks supportive of the development of social cognition, we hypothesized that young children with SCT would show higher rates of difficulties with ToM, joint attention skills, and social orienting, compared to their typically developing peers.
Materials and Methods
Participants
The present study is part of a larger ongoing longitudinal study (the TRIXY Early Childhood Study - Leiden, The Netherlands), which includes children with SCT and nonclinical controls aged 1–7.5 years. The TRIXY Early Childhood Study aims to identify the neurodevelopmental risk in young children with an extra X or Y chromosome.
A group of 105 children with SCT (range: 1–7.5 years old; Mage = 3.69, SD = 1.95) was included in this cross-sectional study, as well as a population-based sample of 96 nonclinical controls within the same age range (42 boys; Mage = 3.62, SD = 1.63). Mean age did not differ between groups (t(199) = 0.26, p = .792). The SCT group consisted of 34 girls with 47,XXX (32.4%), 49 boys with 47,XXY (46.7%), and 22 boys with 47,XYY (21.0%). Seventy children (66.7%) were diagnosed prenatally (20 girls with XXX, 35 boys with XXY, and 15 boys with XYY) and 35 children (33.3%) postnatally (14 girls with XXX, 13 boys with XXY, and 8 boys with XYY). Twenty-four out of 49 boys with 47,XXY had received testosterone treatment (49.0%). The diagnosis of SCT was defined by trisomy in at least 80% of the cells, which was confirmed by standard karyotyping.
Recruitment and assessment took place on two sites: the Trisomy of the X and Y chromosomes (TRIXY) Expert Center at Leiden University (LUBEC) in Leiden, The Netherlands and the eXtraordinary Kids Clinic in Developmental Pediatrics at Children's Hospital Colorado in Aurora, the USA. Children in the SCT group were recruited in cooperation with the clinical genetics departments (from The Netherlands and Colorado (USA)) as well as through patient-advocacy groups and social media postings. For the SCT group, possible recruitment bias was assessed and three subgroups were identified: “active prospective follow-up,” which included families who were actively followed after prenatal diagnosis (51.4% of the SCT group); “information-seeking parents,” which included families who were actively looking for more information about SCT without having specific concerns about the behavior of their child (29.5% of the SCT group); and “clinically referred cases,” which included families seeking professional help based on specific concerns about their child’s development (19.0% of the SCT group). All participants had Dutch or English as first speaking language, had normal or corrected-to-normal vision, and did not have a history of traumatic brain injury. Nonclinical control children were recruited from the western part of The Netherlands and were approached with information brochures about the study. For ethical reasons, children in the control group were not subjected to genetic screening, as these children were meant to be a representation of the general population. As the prevalence of SCT is ~1 in 1,000, the risk of having one or more children with SCT in the control group was considered minimal and acceptable.
Age of the primary caregiver and parental education were assessed. Age of the primary caregiver ranged from 23 to 50 years. There was a significant difference between the research groups for the age of primary caregiver (p < .001): On average, the primary caregiver of children with SCT was older (M = 38.80, SD = 4.75), compared to the primary caregiver of typically developing peers (M = 35.14, SD = 5.25). About 96% of all parents indicated that their child had a second caregiver. Parental education was assessed according to the criteria of Hollingshead (Hollingshead, 1975). Scores of this scale include: 0 (no formal education), 1 (less than seventh grade), 2 (junior high school), 3 (partial high school), 4 (high school graduate), 5 (partial college or specialized training), 6 (standard college or university graduation), and 7 (graduate or professional training). If two parents were available, the level of education was averaged over both parents. A Pearson χ2 test was performed to investigate the possible differences in parental education distribution between the SCT and the control group. A significant difference was found (χ2(10) = 18.43, p = .048), indicating higher parental education in the SCT group. Average parental education was 5.90 (SD = 0.94) in the SCT group and 5.49 (SD = 1.36) in the control group.
Global Level of Cognitive Development
To measure the global level of intelligence and receptive language, development age appropriate instruments were administrated. The Bayley, third edition (subscale cognitive scale; Bayley III; Bayley, 2006) was administered to 1–2-year-old children. In the older children, four subtests of the Wechsler Preschool and Primary Scales of Intelligence, third edition (WPPSI-III; Wechsler, 2002) were used to estimate the global level of intelligence (children aged 3 years: Block Design, Receptive Vocabulary, Information, Object Assembly; children aged 4 years and older: Block Design, Matrix Reasoning, Vocabulary, and Similarities). For children aged 4 years and older, the total IQ estimates were calculated based on this short form version of the WPPSI-III (Hurks, Hendriksen, Dek, & Kooij, 2016). The Peabody Picture Vocabulary Test, third edition (PPVT-III; Dunn & Dunn, 1997) was used to measure the receptive language level in children aged 3 years and older.
Theory of Mind
The ToM subtest of the Developmental NEuroPSYchological Assessment, second edition (NEPSY-II; Korkman, Kirk, & Kemp, 2007) was used to assess children’s understanding of mental functions and other people’s perspectives. The ToM subtest was administered to all participants aged 3 years and older. Also in this subset of participants, the mean age did not differ between the SCT and control groups (t(126) = 1.70, p = .092).
The ToM subtest of the NEPSY was administrated according to the detailed instructions as described in the manual (Korkman et al., 2007). The ToM subtest consists of two different subtasks: verbal tasks and contextual tasks. In the verbal tasks, the questions are based on verbal scenarios. They measure the understanding of (false) beliefs, intentions, other’s thoughts, ideas, and comprehension of figurative language. Two items aim to measure the child’s verbal and gestural imitation abilities, as imitation abilities are thought to be a basic ability for ToM skills. The child is asked to answer the tasks verbally, with the exception of an imitation question where the child is asked to imitate gestures or words. In all of the items the child can answer very briefly; one word is often sufficient for a correct answer, and in two of the questions, it is also possible to answer by pointing. The contextual tasks of the ToM subtest aim to measure the child’s ability to relate affects to a broader social context. In these items, the child is shown drawings with children in social contexts. In each drawing, there is a target girl whose face is not shown. The child is asked to point to one of four photographs of the same girl’s face with different emotions, selecting the emotion of the girl in the drawing.
The total score range is between 1 and 28 (sum score of the 15 verbal tasks and 6 contextual tasks), with higher scores reflecting better ToM skills. Besides raw scores, percentile scores as compared to norms from the general population can be calculated; depending on the spoken language of the child, the Dutch or English norms were used. Percentile scores were labeled as being in the normal range (percentile score ≥ 26), the borderline range (11 < percentile score > 25), the below expected level (3 < percentile score > 10), and the well below expected level (percentile score ≤ 2).
Joint Attention
Joint attention was assessed during a paradigm similar to Von Hofsten, Dahlström, and Fredriksson (2005) and Falck-Ytter, Fernell, Hedvall, von Hofsten, and Gillberg (2012). The children were shown video clips of a female adult sitting behind a table. On the table, four distinct objects were presented: a bowling set, a train, a camera, and a ball. Because gazing and pointing are both important attention-directing elements of joint attention, these elements are both part of the paradigm in which they were presented separately as well as combined: The adult just looked at the object (gaze; G condition) or just pointed although looking straight ahead (point; P condition), or both looked and pointed (gaze and point; G + P condition). The adult performed the action for 4 s after which she returned her attention to the camera. The clips lasted for 8.5–10 s, did not include speech, and background music was added to keep the children involved in the task. To prevent carry-over effects, a black slide was presented for 2 s between the clips. The order of clips was counterbalanced between participants. See Fig. 1 for screenshots of a trial in the G + P condition. As a measure of the tendency to accurately follow referential cues, a difference score (DS) was calculated. The DS represents the standard measurement of the ability of children to follow gaze (Moore & Corkum, 1998) and reflects the number of gaze shifts from the adult to the specific area of interest (AOI) of the attended object minus the number of gaze shifts from the adult to the AOIs of the unattended objects (Gredebäck, Fikke, & Melinder, 2010).
Fig. 1.
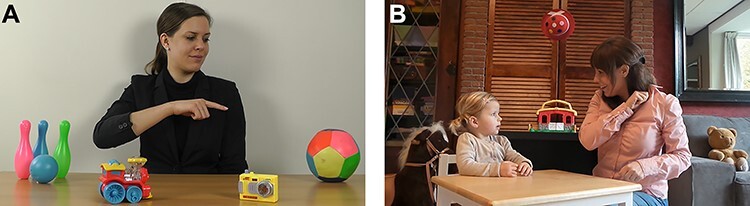
Screenshots of the video clips in the joint attention paradigm (A) and the social orienting paradigm (B).
Social Orienting
Eye gaze fixations toward key sources of social information, namely faces and eyes, were measured during a social orienting paradigm. See Fig. 1 for a screenshot of the video clip. The 30-s video showed a social plot in which social cues are reciprocally exchanged between two persons. A child and adult are seated on chairs with a table in between, with non-social distractors (toys) in the background. The social plot starts with the adult presenting a piece of chocolate to the child; the adult then nonverbally and verbally communicates to the child to wait and not to take the chocolate yet. Then, the adult places the chocolate in one of her closed hands, shows her closed hand, and asks the child to guess in which hand she is holding the chocolate; the child correctly identifies the hand with the chocolate, and the adult shows the chocolate. However, unexpectedly, the adult does not allow the child to take the chocolate. The child shows sadness and disappointment.
In order to preserve ecological validity, all sounds were retained. To prevent interference with language abilities, language used in the clip was not the same as the language of the participants (i.e., Italian vs. Dutch or English). In a group of nonclinical young children aged 3–7 years, this eyetracking paradigm was found to be related to real-life social behaviors and independent of age, IQ, or gender (Van Rijn, Urbanus, & Swaab, 2019).
Eyetracking Equipment and Procedures
Gaze data within specific AOIs were collected using the Tobii X2-60 eyetracker (Tobii Technology AB, Danderyd, Sweden), which records the x- and y-coordinates of the child’s eye position at 60 Hz by using corneal reflection techniques. The computer with eyetracker was placed on a table adapted to the height of the seat, and the child was seated in a car seat at 65-cm viewing distance. A five-point calibration procedure was used, with successful calibration defined as a maximum calibration error of 1° for individual calibration points (i.e., < 1 cm at a distance of 65 cm from the eyetracker). After the calibration procedure, the child was instructed to watch the movie clips and pictures on the computer. The two eyetracking paradigms started with an attention grabber (e.g., a moving picture of an animal, shown on a black background and accompanied by a sound) to direct the attention of the child to the screen.
Gaze data was processed using Tobii Studio (version 3.2.1) using the Tobii Identification by Velocity Threshold (I-VT) fixation filter. This filter controls for the validity of the raw eyetracking data making sure only valid data were used (Olsen, 2012). Only gaze data points with validity code “0” (indicating that high quality data of both eyes was collected) were included in the analysis.
The “Dynamic AOI” tool of Tobii Studio was used to draw AOIs, which were drawn with a 1-cm margin to ensure that the AOIs were sufficiently large outside the defining contours to reliably capture the gaze fixation (Hessels, Kemner, van der Boomen, & Hooge, 2016). Dynamic AOIs were grouped into the following categories: adult, attended and unattended objects, and the whole screen (joint attention paradigm); faces, eyes, and the whole screen (social orienting paradigm). Eyes were included in the face AOI to prevent overlap in terms of reliably distinguishing the impact of SCT on orienting to faces and eyes. In order to evaluate the amount of nonvalid eyetracking data (i.e., attention toward the screen), the total visit duration toward the whole screen was calculated, divided by the duration of the clip, multiplied by 100, reflecting the percentage of valid data collected during each of the eyetracking paradigms.
In the joint attention paradigm, the amount of gaze shifts was based on the individual gaze plots of the participants (provided by Tobii Studio), combined with the amount of fixations on the AOIs (N fixation count). We included gaze shifts from fixations on the AOI adult to fixations on any of the four possible reference AOIs, occurring after the time the adult’s gaze or pointing gesture was first directed at the attended object. In the social orienting paradigm, proportions fixation duration were calculated by taking the total fixation duration within the AOI, divided by the total visit duration toward the whole screen of the individual child, multiplied by 100, reflecting the percentage of time the children were attending to anAOI.
Study Procedures
Signed informed consent was obtained from the parents of all participating children according to the declaration of Helsinki. This study was approved by the Ethical Committee of Leiden University Medical Center, The Netherlands and the Colorado Multiple Institutional Review Board (COMIRB) in Colorado, USA. Assessment took place at various sites (Colorado, USA and The Netherlands) either in a quiet room at the University or at home. To standardize the testing environment, the testing setup and research protocols were identical at both sites. Researchers from Leiden University were responsible for project and data management (i.e., training and supervision of researchers processing and scoring of data). Administration of the NEPSY, WPPSI was performed on a table by trained child psychologists or psychometrists in the Dutch or English language, depending on the first language of the child. The eyetracking procedure and PPVT administration took place during a separate appointment, within 1 week after the NEPSY and WPPSI administration. The laptop with the eyetracker was placed in a small tent to standardize the testing environment and to control for light conditions. The child was seated in a car seat in front of the eyetracker. The examiner was seated beside the child (directing Tobii Studio with a remote keyboard) and started the calibration procedure. Eyetracking paradigms were shown in a fixed order (social orienting or joint attention). Parents were allowed to stay in the room (out of sight) and were asked not to communicate with their child during the procedure.
Data Analyses
Statistical Package for the Social Science (SPSS) version 25 was used for statistical analyses. Pearson’s correlations were used in order to test for associations between social orienting, joint attention, ToM, cognitive functioning, and receptive language abilities. For groupwise (SCT vs. control; research site; and recruitment bias) comparisons of ToM skills, proportions of fixation duration within the AOIs, independent t-tests or MANOVAs were used. 3 × 2 ANOVA were used to assess the main effect of the condition (gaze, point, and gaze + point) on joint attention outcomes in the SCT versus control group. The moderating effect of age on the differences of social orienting, joint attention, and ToM between the SCT and control groups was assessed using a bootstrapping, nonparametric resampling procedure (PROCESS; Hayes, 2009). Bootstrapping analysis with 5,000 resamples was done to test for a significant moderating effect using the SPSS macro developed by Hayes (2017). In this analysis, the moderation effect is significant if the 95% bias-corrected confidence interval (CI) for the moderator effect does not include zero. A Pearson χ2 test was used to explore the distributions of karyotypes across age groups that were created in order to measure the SCT versus control differences in ToM and social orienting. Influence of karyotype on social orienting, joint attention, and ToM was tested by a MANOVA. Statistical analyses were performed one-tailed (SCT vs. control) or two-tailed (influence of research site, karyotype, and recruitment bias), and the level of significance was set at p < .05. In case of significant differences, Cohen’s d or partial η2 were used to calculate the effect sizes.
Results
Differences in Early Social Cognition Between Research Sites
First, to control for the potential impact of the research site on outcomes of the study, the data of the two research sites were compared. No differences between children in the Netherlands and USA were found for ToM (t(61) = 1.32, p = .192), joint attention (t(89) = 0.80, p = .428), and social orienting (faces: (t(89) = 0.234, p = .816); eyes: (t(89) = 0.68, p = .499). Based on this, all SCT data were collapsed across the sites.
Theory of Mind
ToM: differences in SCT versus controls
The NEPSY ToM task was completed by 128 children (9 children were not able to complete the task due to fatigue of the child). Independent t-tests indicated differences between the SCT and control groups for verbal ToM (t(126) = −1.75, p = .041, Cohen’s d = 0.31), contextual ToM (t(126) = −2.42, p = .009, Cohen’s d = 0.43), and total ToM (t(126) = −2.42, p = .013, Cohen’s d = 0.40), indicating lower ToM scores in the SCT group. The effect size (Cohen’s d) on the total score differences between groups indicated medium SCT versus control differences. See Table 1 for the exact mean and standard deviation values for the verbal ToM scores, the contextual ToM scores, and total scores. When evaluating scores normalized for age, for overall ToM skills, 47.5% of the SCT group scored in the average to above-average level, 26.2% scored in the borderline level, 19.7% in the below expected level, and 6.6% in the well below expected level.
Table 1.
Descriptive statistics for SCT and control groups
| Age group | N | Missing | SCT | Control | |||
|---|---|---|---|---|---|---|---|
| Min–Max | M (SD) | Min–Max | M (SD) | ||||
| Cognitive development (norm score; Bayley-III) | 1–2 years | 63 | 1 | 62–125 | 98.52 (13.24) | 70–129 | 99.43 (14.98) |
| Total IQ (WPPSI-III) | 3–7 years | 131 | 6 | 55–138 | 95.17 (19.57) | 72–140 | 109.15 (13.56) |
| Receptive language (standard score; PPVT-III) | 3–7 years | 132 | 5 | 65–129 | 99.46 (14.75) | 74–133 | 108.67 (12.67) |
| Social orienting to faces (proportions fixation duration) | 1–7 years | 186 | 15 | 0.00–0.64 | 0.22 (0.12) | 0.00–0.57 | 0.24 (0.12) |
| Social orienting to eyes (proportions fixation duration) | 1–7 years | 186 | 15 | 0.00–0.38 | 0.05 (0.06) | 0.00–0.28 | 0.08 (0.07) |
| Joint attention (DS gaze shifts) | 1–7 years | 187 | 14 | −6.33 to 4.33 | −0.56 (2.29) | −3.67 to 7.67 | 1.31 (2.27) |
| ToM verbal (raw score; NEPSY-II) | 3–7 years | 128 | 9 | 0–16 | 7.22 (4.03) | 1–21 | 8.60 (4.82) |
| ToM contextual (raw score; NEPSY-II) | 3–7 years | 128 | 9 | 0–6 | 2.41 (1.36) | 0–6 | 3.06 (1.65) |
| ToM total score (raw score; NEPSY-II) | 3–7 years | 128 | 9 | 0–19 | 9.49 (4.71) | 3–24 | 11.66 (6.01) |
Notes: Bayley-III = Bayley, third edition; WPPSI-III = Wechsler Preschool and Primary Scales of Intelligence, third edition; PPVT-III = Peabody Picture Vocabulary Test, third edition; NEPSY-II = NEuroPSYchological Assessment, second edition; SCT = sex chromosome trisomy; DS = difference score; ToM = theory ofmind.
ToM: age-dependent differences in SCT versus controls
Bias-corrected bootstrapping analyses (PROCESS) were conducted to test for a moderating effect of the child’s age on the difference in the total ToM skills between the SCT and control groups. There was a significant moderation effect of child’s age (b = 2.69, SE = 0.57, t = 4.67, p < .001, 95% CI [1.55, 3.83]), revealing that the difference on total ToM skills between the SCT and control groups increases with older ages (not in favor of children with SCT). R2 = 0.59, indicating that this model explained 59% of the variance in ToM skills. See Fig. 2 for a graphical representation of ToM in the SCT versus control group.
Fig. 2.
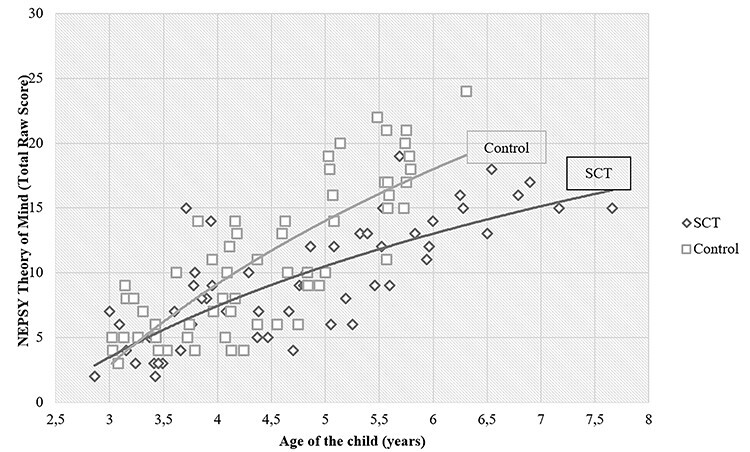
Theory of mind and age for the sex chromosome trisomy and control groups.
Mean raw scores were significantly correlated with general IQ (measured with WPPSI total IQ; Pearson’s r = .25, p = .006) and normed scores on a receptive language skills task (measured with the PPVT; r = .62, p < .001). In order to control for the association between the ToM task performance and cognitive or receptive language skills, norm scores of global intelligence and receptive language skills were added as covariates to the PROCESS analysis. The significant effect of group on ToM skills remained, even when the global intelligence and receptive language skills were added as covariates. A significant moderation effect of child’s age was found (b = 1.32, SE = 0.60, p = .030, 95% CI [0.13, 2.52], R2 = 0.67), revealing that the difference on ToM skills between the SCT and control groups increases with older ages, although controlling for global intelligence and receptive language skills.
ToM: differences in SCT versus controls in two age groups
Because of these age and group effects, participants were divided in two age groups in order to investigate the ToM abilities in different stages during development in early childhood: children who were 3 and 4 years old (n = 75; Mage = 3.91, SDage = 0.59; 34 SCT [10 XXX, 19 XXY, 5 XYY], 41 controls), and children 5, 6 and 7 years old (n = 53; Mage = 5.87, SDage = 0.68; 29 SCT [15 XXX, 9 XXY, 5 XYY], 24 controls). There was no significant association between the type of SCT and age group (χ2(2) = 4.20, p = .122), indicating that the distribution of karyotypes was similar across the age groups.
There was no difference in the total ToM score in children aged 3–4 years in the SCT group (M = 6.85, SD = 3.24) and the control group (M = 8.05, SD = 3.80; t(73) = −1.45, p = .076). In the 5–7-year-old age group, a difference was found between the SCT (M = 12.59, SD = 4.29) and the control group (M = 17.83, SD = 3.54; t(51) = −4.79, p < .001; Cohen’s d = 1.33): 5–7 years old with SCT showed lower ToM abilities compared to their typically developing peers.
Joint Attention
Joint attention: attention toward the screen
The joint attention paradigm was successfully completed by 190 children (11 children were not able to complete the task due to technical issues or fatigue of the child). The total proportion of valid on-screen visit duration was 70.4% for the whole eyetracking paradigm and did not differ between the SCT and the control group (t(188) = −1.25, p = .215). This proportion indicated sufficiently high attention to the screen (>70%; Frank, Amso, & Johnson, 2014). The main outcome measures, that is, DSs for the three conditions (gaze, point, and gaze + point) were not correlated with intellectual functioning (p = .081; Bayley cognition scaled score or WPPSI TotalIQ).
Joint attention: effect of condition
A 3 × 2 mixed ANOVA with DS as the dependent variable with attention-direction condition (gaze or point or gaze + point) as within factor and group (SCT, control) as between subject factor revealed no significant main effect of condition (F(2, 184) = 1.70, p = .186) and no significant interaction effect (F(2, 184) = 1.62, p = .200), indicating no effect of condition on gaze following. Therefore, the three conditions were collapsed in the subsequent analyses.
Joint attention: differences in SCT versus controls
An independent t-test with DS as the dependent variable showed a significant effect of research group (SCT vs. control; t(185) = −5.60, p < .001, Cohen’s d = 0.82). These effects reflect lower joint attention accuracy in the SCT group over all ages (M = −0.56, SD = 2.29), compared to the control group (M = 1.31, SD = 2.27).
Joint attention: age-dependent differences in SCT versus controls
A bias-corrected bootstrapping PROCESS analysis was conducted to test for a moderating effect of the child’s age on the difference in DS between the SCT and the control group. There was no significant moderation effect of child’s age (b = 0.15, SE = 0.16, t = 0.94, p = .349, 95% CI [−0.17, 0.48]), revealing that the difference on DS between the SCT and control groups was not moderated by age. See Fig. 3 for a graphical representation for the SCT versus control group.
Fig. 3.
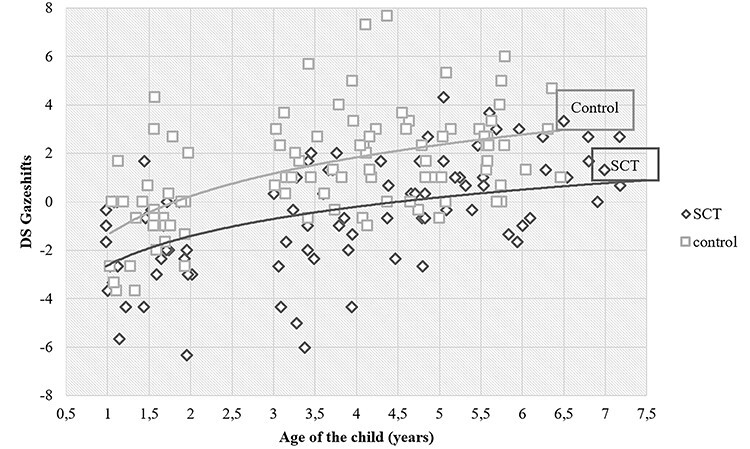
Joint attention: DS gaze shifts and age for the sex chromosome trisomy and control groups. Notes: DS gaze shifts: difference scores between gaze shifts from area of interest (AOI) adult to AOI attended object minus gaze shifts from AOI adult to AOI unattended object.
Social Orienting
Social orienting: attention toward the screen
The social orienting paradigm was successfully completed by 186 children (15 children were not able to complete the task due to technical issues or fatigue of the child). The total proportion valid on-screen visit duration was 94.1%, and it did not significantly differ between the SCT and control groups (t(184) = −1.77, p = .079). The main outcome measures were not correlated with cognitive functioning (proportions of fixation for the AOI faces [p = .495]) and eyes (p = .155; dependent on age: Bayley cognition scaled score or WPPSI TotalIQ).
Social orienting: differences in SCT versus controls
Total eye gaze fixation durations at AOI faces and eyes were measured in order to investigate the SCT versus control differences in social orienting. Independent t-tests on the eye gaze fixation duration at the AOI faces and eyes indicated that, on average, children with SCT showed lower fixation duration to eyes (t(184) = −3.01, p = .002, Cohen’s d = 0.46) but not to faces (t(184) = −1.33, p = .093). See Table 1 for exact M and standard deviations.
Social orienting: age-dependent differences in SCT versus controls
A bias-corrected PROCESS bootstrapping analysis was conducted to test for a moderating effect of child’s age on fixation duration on the AOI faces and eyes between the SCT and control groups. There was a significant moderation effect of child’s age on fixation durations on the AOI Faces (b = 0.02, SE = 0.01, t = 2.32, p = .022, 95% CI [0.004, 0.044]; R2 = 0.05) but not on the AOI Eyes (b = 0.009, SE = 0.006, t = 1.68, p = .094, 95% CI [−0.002, 0,020]).
Social orienting: differences in SCT versus controls in three age groups
Because of these group and age effects, the participants were divided in three age groups in order to further investigate eyetracking outcome measures in different stages during development in early childhood: children aged 1 and 2 years old (n = 59; Mage = 1.48, SDage = 0.33; 29 SCT [6 XXX, 19 XXY, 4 XYY], 30 controls), children aged 3 and 4 years old (n = 74; Mage = 3.91, SDage = 0.60; 34 SCT [11 XXX, 17 XXY, 6 XYY], 40 controls), and children aged 5–7 years old (n = 53; Mage = 5.84, SDage = 0.64; 28 SCT [15 XXX, 8 XXY, 5 XYY], 25 controls). There was no significant association between the type of SCT and age group (χ2(4) = 8.22, p = .084), indicating that the distribution of karyotypes was similar across the age groups.
There were no overall SCT versus controls differences in fixation duration at the faces and eyes AOI in children aged 1–2 years old (V = 0.061, F(2, 56) = 1.81, p = .087) and in the 3–4-year-old age group (F(2, 71) = 1.57, p = .108). In the 5–7 year-old age group, an SCT versus control difference was found (F(2, 50) = 7.313, p = .001; partial η2 = 0.23): 5–7 years old with SCT fixated less at faces and eyes than the control group. See Table 2 for exact M and standard deviation, p-values, and post hoc effects.
Table 2.
Groups differences in social orienting (SCT vs. control) in fixation duration in three age groups (M, SD)
| Phases of development | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1, 2 years old | 3, 4 years old | 5–7 years old | |||||||||||
| n = 59 (29 SCT, 30 control) | n = 74 (34 SCT, 40 control) | n = 53 (28 SCT, 25 control) | |||||||||||
| AOI | SCT | Control | p-value | SCT | Control | p-value | Post hoc effect | Cohen’s d | SCT | Control | p-value | Post hoc effect | Cohen’s d |
| Faces | 0.26 (0.15) | 0.23 (0.16) | .189 | 0.20 (0.11) | 0.23 (0.10) | .156 | 0.19 (0.10) | 0.28 (0.09) | <0.001 | SCT < control | 0.95 | ||
| Eyes | 0.05 (0.08) | 0.06 (0.07) | .283 | 0.05 (0.05) | 0.07 (0.07) | .040 | SCT < control | 0.33 | 0.06 (0.06) | 0.12 (0.07) | <0.001 | SCT < control | 0.92 |
Notes: SCT = sex chromosome trisomy; AOI = area of interest.
Associations Between Social Cognitive Skills Within the SCT Group
To explore the associations between basic social cognitive skills and ToM within the SCT group, Pearson’s correlations were calculated between social orienting, joint attention, and ToM. A significant correlation was found between social orientation to faces and joint attention (r = .217, p = .045), and a correlation was found for joint attention and ToM (r = .331, p = .010). See Table 3 for r-values for all variables.
Table 3.
Intercorrelations for social orienting, joint attention, and ToM in the SCT group
| Measure | 1. | 2. | 3. | 4. |
|---|---|---|---|---|
| 1. Social orienting to faces | — | .626** | .217* | .943 |
| 2. Social orienting to eyes | — | .124 | .611 | |
| 3. Joint attention | — | .331** | ||
| 4. ToM | — |
Notes: SCT = sex chromosome trisomy; ToM = theory of mind. *p < .05; **p < .01.
Karyotype Differences Within the SCT Group
In order to investigate the influence of various karyotypes on the social cognitive outcomes accounting for the effect of age, ANCOVA analyses were carried out with main effect of karyotype (XXX vs. XXY vs. XYY) and age as covariate. For ToM (N = 63; 25 XXX, 28 XXY, 10 XYY), no difference between karyotypes was found (F(2, 59) = 0.47, p = .625). Also for joint attention skills (N = 91; 30 XXX, 46 XXY, 15 XYY), no difference between karyotypes was found (F(2, 87) = 0.48, p = .623). For social orienting (N = 91; 32 XXX, 44 XXY, 15 XYY), no significant difference was found for orienting to eyes (F(2, 87) = 2.06, p = .134). These results indicate that karyotype was not predictive of ToM and joint attention abilities and social orienting to eyes, when accounting for age. However, a significant difference between karyotypes was found on social orienting to faces, (F(2, 87) = 4.09, p = .020, partial η2 = 0.09). Estimated Marginal Means revealed that the XYY subgroup had significantly decreased social orienting to faces compared to the XXY subgroup (p = .041) and XXX subgroup (p = .005).
Recruitment Bias Within the SCT Group
Within the SCT group, we tested for differences on the social cognitive outcomes between the three recruitment groups with MANOVA. There were no significant differences for social cognitive outcomes (see Table 4): How children enrolled in the study did not affect their outcomes on ToM, joint attention, and social orienting abilities.
Table 4.
Differences in social cognition across recruitment groups (M, SD)
| Prospective follow-up | Information-seeking parents | Clinically referred cases | p-value | |
|---|---|---|---|---|
| NEPSY ToM | n = 29 | n = 20 | n = 14 | |
| Total raw score | 8.90 (4.97) | 10.00 (4.46) | 10.00 (4.71) | .658 |
| Joint attention paradigm | n = 46 | n = 28 | n = 17 | |
| DS gaze shifts | 16.33 (9.29) | 20.32 (7.94) | 19.29 (5.51) | .114 |
| Social orienting paradigm | n = 43 | n = 28 | n = 20 | |
| Total fixation duration faces | 0.21 (0.12) | 0.25 (0.14) | 0.20 (0.09) | .308 |
| Total fixation duration eyes | 0.04 (0.05) | 0.06 (0.05) | 0.06 (0.05) | .345 |
Notes: NEPSY = NEuroPSYchological Assessment; ToM = theory of mind; DS = difference score.
Discussion
The present study was designed to evaluate the impact of SCT on early social cognitive development. As an extra X or Y chromosome affects the maturation of brain areas involved in social cognitive processes (Raznahan et al., 2016), and SCT increases the risks of difficulties in adaptive social functioning in childhood, adolescence, and adult life (Van Rijn et al., 2019), we studied one of the core social cognitive skills, namely ToM, in addition to the basic social cognitive skills typically developed during the first years of life, that is, social orienting and joint attention. Insights in the impact of SCT on early social cognitive skills could enhance our understanding of how social behavioral difficulties may be anchored in the altered processing of social information already early in neurodevelopment.
We addressed the question whether the impact of SCT on ToM abilities could already be found in children at 3–7 years of age. In line with our expectations, a difference was found in the overall ToM skills between children with SCT and their age-related peers, suggesting that, on average, young children with SCT have difficulties with ToM. The proportion of young children with SCT who scored in the below expected level (19.7%) and well below expected level (6.6%) revealed that substantial ToM impairments already can be found in a subset of the SCT group early in development across all SCT karyotypes. Next, when age was taken into account, analyses showed a moderating effect of age, indicating more ToM difficulties in older children with SCT. This effect remained significant even after controlling for global intelligence and the receptive language level. From the age of 5 years old, children with SCT as a group showed impairments in ToM with large effect sizes.
From a developmental perspective, it is known that basic social cognitive skills are involved in the maturation of ToM (Hughes & Leekam, 2004; Korhonen et al., 2014). We therefore studied two cognitive functions related to ToM with the help of eyetracking paradigms: joint attention, that is, being able to coordinate attention between a social partner with respect to objects or events; and social orienting, that is, the ability to align sensory receptors to social important cues. First, we found a difference with a large effect size in the joint attention between the SCT and control groups, suggesting difficulties with joint attention in children with SCT. These difficulties in joint attention were irrespective of age. Second, with regard to social orienting, eyetracking results showed that children from the age of 3 years on general are less inclined to orient their attention toward social cues, as compared to their typically developing peers. No social orienting differences between the SCT and control groups were seen in children aged 1–2 yearsold.
The difficulties with ToM and contributing basic mechanisms of social orienting and joint attention as found in this study have implications for our understanding of social-adaptive problems found in young children with SCT (Urbanus et al., 2020) and social (cognitive) difficulties found later in the life of children and adolescents with SCT (Urbanus, van Rijn, & Swaab, 2019). To be more specific, these findings may suggest that challenges in social functioning throughout the life span are likely anchored in social cognitive vulnerabilities that may be present already very early in life. At the age of 1–2, children with SCT, on average, showed intact social orienting; however, processing gaze as part of joint attention seems to be affected. Given that joint attention requires the adequate processing of eye gaze and gestures to follow the intentions of others, it is not only sufficient for young children to orient toward the social value of information but they are also required to have awareness that others have intentions and perceptions (Charman et al., 2000).
At the age of 3–5, on average, both social orienting and joint attention seem to be affected in young children with SCT. Failures to demonstrate the tendency to orient to social stimuli deprive children from access to social information needed to further develop skills in following, understanding, and responding to social directions and interactions of others. Studies with typically developing children have shown that the basic social orienting skills and the ability to coordinate attention with a social partner in relation to a third object (i.e., joint attention) correlates with the development of the ability to decode and reason about others’ mental state (i.e., ToM; Sodian & Kristen-Antonow, 2015; Wellman et al., 2001). Indeed, in our study, we found impaired ToM abilities in children with SCT from the age of 5, irrespective of their level of global intelligence and receptive language. As expected, social orienting, joint attention, and ToM were shown to be associated: In the SCT group, we found that social orienting to faces was significantly correlated with joint attention, and joint attention was correlated withToM.
The phenomenon of increasing impairments of more complex abilities in older age groups of children with SCT in comparison with typically developing peers as found in this study with regard to social orienting and ToM is known as “growing into deficit” (Rourke, 1983). Impairments in fundamental social cognitive abilities may lead to a cascade of negative developmental effects, as vulnerabilities with attending, following, and understanding social information increase, whereas the access to quantitative and qualitative opportunities to learn from social interactions decrease. This negative loop of increasing difficulties may affect the development of social adaptive behavior, which is involved in the forming and maintaining of reciprocal social relationships.
It is known that early impairments in social orienting, joint attention, and ToM influence (social) development through childhood. In typically developing children, the development of social cognitive skills seem to be associated with other domains of neurocognitive development, such as language (Delgado et al., 2002) and executive functioning (Drayton, Turley-Ames, & Guajardo, 2011). These neurocognitive functions have also shown to be vulnerable in individuals with SCT (see for a review: Van Rijn (2019). To illustrate, the increasing ability of young children to use social information such as gaze direction and pointing to locate an object (i.e., joint attention) increases the opportunity for forming correct word–object associations required for the acquisition of communicative competence and language (see Delgado et al., 2002). Studies have shown that difficulties with orienting toward social cues, joint attention, and ToM early in development are also associated with ASDs (Baron-Cohen, Allen, & Gillberg, 1992; Chawarska, Klin, & Volkmar, 2003; Sullivan et al., 2007).
When exploring differences between karyotypes in SCT in the impact on social orienting, joint attention, and ToM at different ages, the results suggest that although social cognition was impaired in all karyotypes, social orienting difficulties might be somewhat more pronounced in boys with 47,XYY compared to boys and girls with 47,XXX and 47,XXY. On a behavioral level, it has already been shown that boys with 47,XYY have a higher risk for ASD compared to boys with 47,XXY (Cordeiro, Tartaglia, Roeltgen, & Ross, 2012; Ross et al., 2012; Tartaglia et al., 2017). This more pronounced vulnerability in the XYY group compared to the XXX, and XXY group was not found in joint attention and ToM, which were similarly affected across all karyotypes.
Considering the importance of social orienting, joint attention, and ToM for a broad range of developmental outcomes, further research will be needed to explore whether difficulties in ToM, joint attention, and social orienting in young children with SCT are predictive of risk for later social adaptive difficulties and neurodevelopmental diagnoses, such as ASD. The detection of early markers in young children with SCT (both in research and clinical practice) is crucial when considering the large heterogeneity in children with SCT (see, e.g., the heterogeneity in the behavioral profile of young children with SCT; Urbanus et al., 2020). Monitoring of children with SCT from infancy to childhood (through testing, relevant questionnaires completing by the surrounding, and observations in natural settings) and, if necessary, preventive support should first focus on the basic ability to attend to social cues and further on the ability to follow triadic communicative exchanges (i.e., joint attention) and complex ToM abilities from around the age of 3. Currently available interventions for children with ASD, which are aimed to increase the motivation to orient toward social stimuli, to be involved in triadic exchanges with a social partner, and to develop ToM abilities, might also benefit children with SCT. Future studies addressing the effectiveness of (preventive) interventions in young children with SCT are warranted.
The current study has both strengths and limitations. Strengths included the relatively large, international sample of the study, that consisted of children varying in time of diagnosis (pre- or postnatal), recruitment strategy, and boys with 47,XXY who did or did not receive testosterone treatment. With the large sample size of this study, we were able to investigate the social cognitive abilities of children at specific ages (i.e., 1–2, 3–5, and 5–7 age groups). Social cognitive outcomes in the SCT group did not differ across the international research sites, indicating a high degree of similarity in the social cognitive function among children with SCT in Western cultures. As it was beyond the scope of this study to investigate the influence of time of diagnosis (i.e., prenatal or postnatal) and testosterone treatment in boys with 47,XXY, future studies with suitable designs (e.g., randomized control trials investigating the influence of testosterone) should study these parameters in relation with general social cognitive functioning in young children with SCT. An important limitation of the current study is the cross-sectional design, which limits cause-effect conclusions. Therefore, future research should further investigate the longitudinal effects of impairments in social orienting, joint attention, and ToM on behavioral outcomes and psychopathology in young children with SCT, which will be explored in this population with prospective follow-up.
Further, these results demonstrate the performance of young children (both SCT and control) on tasks designed to measure early social cognitive skills, which included stimuli presented on paper and screens. Although overall social functioning in naturalistic environments encompasses more than only neurocognitive behavior, the outcomes of the NEPSY task and eyetracking paradigms are found to be predictive for daily life social behavior (respectively: Rosello, Berenguer, Baixauli, García, & Miranda, 2020; Van Rijn et al., 2019).
In this study, the social cognitive performance of young children with SCT was not dependent on the recruitment strategy (i.e., prospective follow-up group, information-seeking parents group, or clinically referred cases group), which suggests that our findings are representative for this group of diagnosed children. However, it is important to take into consideration that SCT is still highly underdiagnosed or diagnosed late in life (Berglund et al., 2019), although it is expected that more individuals will be diagnosed early in life with the introduction of less-invasive screening methods during pregnancy (Samango-Sprouse, Keen, Sadeghin, & Gropman, 2017). Nonetheless, it remains unsure to what degree the findings in this study can be generalized to those who have SCT, but remain undiagnosed.
To conclude, the study presented here shows that already at an early age, SCT affects the ability to orient to social information and to follow and understand the desires, believes, and intentions of others. These difficulties are seen from an early age onward and become increasingly deviant across the age range of 3–7. Knowledge about these early social cognitive abilities is important, as this may help to identify targets for early monitoring and preventive interventions.
Conflict of Interest
None declared.
Acknowledgements
This work was supported by a grant from the Dutch Organization for Scientific Research (NWO funding # 016.165.397 to S.v.R., PhD). Work in Colorado was partially supported by infrastructure of NIH/NCATS Colorado CTSA (grant number UL1 TR002535). Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The authors warmly thank the families that participated in our study and the research assistants and students for their help with data collection and processing.
References
- Baron-Cohen, S., Allen, J., & Gillberg, C. (1992). Can autism be detected at 18 months? The needle, the haystack and the CHAT. British Journal of Psychiatry, 16(1), 839–839. [DOI] [PubMed] [Google Scholar]
- Bayley N. . (2006). Bayley Scales of Infant and Toddler Development. San Antonio, TX: The Psychological Corporation.
- Berglund, A., Viuff, M. H., Skakkebæk, A., Chang, S., Stochholm, K., & Gravholt, C. H. (2019). Changes in the cohort composition of turner syndrome and severe non-diagnosis of Klinefelter, 47, XXX and 47, XYY syndrome: A nationwide cohort study. Orphanet Journal of Rare Diseases, 14(1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, P. A., Loane, M., Garne, E., Khoshnood, B., & Dolk, H. (2011). Sex chromosome trisomies in Europe: Prevalence, prenatal detection and outcome of pregnancy. European Journal of Human Genetics, 19(2), 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman, T., Baron-Cohen, S., Swettenham, J., Baird, G., Cox, A., & Drew, A. (2000). Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cognitive Development, 15(4), 481–498. [Google Scholar]
- Chawarska, K., Klin, A., & Volkmar, F. (2003). Automatic attention cueing through eye movement in 2-year-old children with autism. Child Development, 74(4), 1108–1122. [DOI] [PubMed] [Google Scholar]
- Cordeiro, L., Tartaglia, N., Roeltgen, D., & Ross, J. (2012). Social deficits in male children and adolescents with sex chromosome aneuploidy: A comparison of XXY, XYY and XXYY syndromes. Research in Developmental Disabilities, 33(4), 1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, G., Toth, K., Abbott, R., Osterling, J., Munson, J., Estes, A. et al. (2004). Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology, 40(2), 271–283. [DOI] [PubMed] [Google Scholar]
- Delgado, C. E., Mundy, P., Crowson, M., Markus, J., Yale, M., & Schwartz, H. (2002). Responding to joint attention and language development. Journal of Speech, Language, and Hearing Research, 45(4), 715–719. [DOI] [PubMed] [Google Scholar]
- Devine, R. T., & Hughes, C. (2014). Relations between false belief understanding and executive function in early childhood: A meta-analysis. Child Development, 85(5), 1777–1794. [DOI] [PubMed] [Google Scholar]
- Drayton, S., Turley-Ames, K. J., & Guajardo, N. R. (2011). Counterfactual thinking and false belief: The role of executive function. Journal of Experimental Child Psychology, 108(3), 532–548. [DOI] [PubMed] [Google Scholar]
- Dunn, L. M., & Dunn, L. (1997). M. Peabody picture vocabulary test (3rd ed.). Circle Pines, MN: American Guidance Service. [Google Scholar]
- Falck-Ytter, T., Fernell, E., Hedvall, Å. L., von Hofsten, C., & Gillberg, C. (2012). Gaze performance in children with autism spectrum disorder when observing communicative actions. Journal of Autism and Developmental Disorders, 42(10), 2236–2245. [DOI] [PubMed] [Google Scholar]
- Frank, M. C., Amso, D., & Johnson, S. P. (2014). Visual search and attention to faces during infancy. Journal of Experimental Child Psychology, 118, 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith, U., & Frith, C. D. (2003). Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 358(1431), 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredebäck, G., Fikke, L., & Melinder, A. (2010). The development of joint visual attention: A longitudinal study of gaze following during interactions with mothers and strangers. Developmental Science, 13(6), 839–848. [DOI] [PubMed] [Google Scholar]
- Happé, F., & Frith, U. (2014). Annual research review: Towards a developmental neuroscience of atypical social cognition. Journal of Child Psychology and Psychiatry, 55(6), 553–577. [DOI] [PubMed] [Google Scholar]
- Hayes, A. F. (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76(4), 408–420. [Google Scholar]
- Hayes, A. F. (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press. [Google Scholar]
- Hessels, R. S., Kemner, C., van der Boomen, C., & Hooge, I. T. C. (2016). The area-of-interest problem in eyetracking research: A noise-robust solucation for face and sparse stimuli. Behavior Research Methods, 48(4), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead, A. B. (1975). Four-factor index of social status. Unpublished manuscript. New Haven: Department of Sociology, Yale University. [Google Scholar]
- Hughes, C., & Leekam, S. (2004). What are the links between theory of mind and social relations? Review, reflections and new directions for studies of typical and atypical development. Social Development, 13(4), 590–619. [Google Scholar]
- Hurks, P., Hendriksen, J., Dek, J., & Kooij, A. (2016). Accuracy of short forms of the Dutch Wechsler preschool and primary scale of intelligence. Assessment, 23(2), 240–249. [DOI] [PubMed] [Google Scholar]
- Korhonen, V., Kärnä, E., & Räty, H. (2014). Autism spectrum disorder and impaired joint attention: A review of joint attention research from the past decade. Nordic Psychology, 66(2), 94–107. [Google Scholar]
- Korkman, M., Kirk, U., & Kemp, S. (2007). NEPSY-II: A developmental neuropsychological assessment (2nd ed.). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Melogno, S., Pinto, M. A., Badolato, F., Sist, E., Esposito, A., Orsolini, M. et al. (2019). High-level language competencies and theory of mind in a group of children with Klinefelter syndrome. American Journal of Medical Genetics Part A, 179(2), 183–189. [DOI] [PubMed] [Google Scholar]
- Moore, C., & Corkum, V. (1998). Infant gaze following based on eye direction. British Journal of Developmental Psychology, 16(4), 495–503. [Google Scholar]
- Mundy, P., & Neal, R. (2001). Neural plasticity, joint attention, and a transactional socialorienting model of autism. International Review of Research in Mental, 23, 139–168. [Google Scholar]
- Mundy, P., & Newell, L. (2007). Attention, joint attention, and social cognition. Current Directions in Psychological Science, 16(5), 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation, K., & Penny, S. (2008). Sensitivity to eye gaze in autism: Is it normal? Is it automatic? Is it social? Development and Psychopathology, 20(1), 79–97. [DOI] [PubMed] [Google Scholar]
- Olsen, A. (2012). The Tobii I-VT Fixation Filter: algorithm description. Danderyed, Sweden: Tobii Technology. [Google Scholar]
- Raznahan, A., Lee, N. R., Greenstein, D., Wallace, G. L., Blumenthal, J. D., Clasen, L. S. et al. (2016). Globally divergent but locally convergent X-and Y-chromosome influences on cortical development. Cerebral Cortex, 26(1), 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosello, B., Berenguer, C., Baixauli, I., García, R., & Miranda, A. (2020). Theory of mind profiles in children with autism spectrum disorder: Adaptive/social skills and pragmatic competence. Frontiers in Psychology, 11, 567401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, J. L., Roeltgen, D. P., Kushner, H., Zinn, A. R., Reiss, A., Bardsley, M. Z. et al. (2012). Behavioral and social phenotypes in boys with 47, XYY syndrome or 47, XXY Klinefelter syndrome. Pediatrics, 129(4), 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke, B. P. (1983). Child neuropsychology: Introduction to theory, research, and clinical practice. New York: Guilford Press. [Google Scholar]
- Samango-Sprouse, C., Keen, C., Sadeghin, T., & Gropman, A. (2017). The benefits and limitations of cell-free DNA screening for 47, XXY (Klinefelter syndrome). Prenatal Diagnosis, 37(5), 497–501. [DOI] [PubMed] [Google Scholar]
- Sodian, B., & Kristen-Antonow, S. (2015). Declarative joint attention as a foundation of theory of mind. Developmental Psychology, 51(9), 1190. [DOI] [PubMed] [Google Scholar]
- Soto-Icaza, P., Aboitiz, F., & Billeke, P. (2015). Development of social skills in children: Neural and behavioral evidence for the elaboration of cognitive models. Frontiers in Neuroscience, 9, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, M., Finelli, J., Marvin, A., Garrett-Mayer, E., Bauman, M., & Landa, R. (2007). Response to joint attention in toddlers at risk for autism spectrum disorder: A prospective study. Journal of Autism and Developmental Disorders, 37(1), 37–48. [DOI] [PubMed] [Google Scholar]
- Tartaglia, N. R., Wilson, R., Miller, J. S., Rafalko, J., Cordeiro, L., Davis, S. et al. (2017). Autism spectrum disorder in males with sex chromosome aneuploidy: XXY/Klinefelter syndrome, XYY, and XXYY. Journal of Developmental and Behavioral Pediatrics, 38(3), 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus, E., Swaab, H., Tartaglia, N., Cordeiro, L., & van Rijn, S. (2020). The behavioral profile of children aged 1-5 years with sex chromosome trisomy (47,XXX, 47,XXY, 47,XYY). American Journal of Medical Genetics, 184C, 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus, E., van Rijn, S., & Swaab, H. (2019). A review of neurocognitive functioning of children with sex chromosome trisomies: Identifying targets for early intervention. Clinical Genetics 2020, 97, 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijn, S. (2015). Social attention in 47, XXY (Klinefelter syndrome): Visual scanning of facial expressions using eyetracking. Journal of International Neuropsychological Society, 21(5), 364–372. [DOI] [PubMed] [Google Scholar]
- Van Rijn, S. (2019). A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47, XXY, 47, XXX, 47, XYY). Current Opinion in Psychiatry, 32(2), 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijn, S., Stockmann, L., Van Buggenhout, G., van Ravenswaaij-Arts, C., & Swaab, H. (2014b). Social cognition and underlying cognitive mechanisms in children with an extra X chromosome: A comparison with autism spectrum disorder. Genes, Brain and Behavior, 13(5), 459–467. [DOI] [PubMed] [Google Scholar]
- Van Rijn, S., Urbanus, E., & Swaab, H. (2019). Eyetracking measures of social attention in young children: How gaze patterns translate to real-life social behaviors. Social Development. [Google Scholar]
- Visootsak, J., & Graham, J. M., Jr. (2009). Social function in multiple X and Y chromosome disorders: XXY, XYY, XXYY, XXXY. Developmental Disabilities Research Reviews, 15(4), 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hofsten, C., Dahlström, E., & Fredriksson, Y. (2005). 12-month-old infants’ perception of attention direction in static video images. Infancy, 8(3), 217–231. [Google Scholar]
- Wechsler, D. (2002). Wechsler preschool and primary scale of intelligence (3rd ed.). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wellman, H. M. (2014). Making minds: How theory of mind develops. New York: Oxford University Press. [Google Scholar]
- Wellman, H. M., Cross, D., & Watson, J. (2001). Meta-analysis of theory-of-mind development: The truth about false belief. Child Development, 72(3), 655–684. [DOI] [PubMed] [Google Scholar]


