Significance Statement
Hyperkalemia is common after treatment with a mineralocorticoid receptor antagonist (MRA). In the FIDELIO-DKD randomized trial, the nonsteroidal MRA finerenone improved cardiorenal outcomes, but was associated with a twofold higher risk of hyperkalemia versus placebo, consistent across patient subgroups. Short-term increases in serum potassium and decreases in eGFR with finerenone or placebo were associated with subsequent hyperkalemia; at month 4, the magnitude of the increased hyperkalemia risk for any given change from baseline was smaller with finerenone than with placebo. Routine potassium monitoring, with temporary treatment interruption and dose reduction in the event of hyperkalemia, is necessary for the safe use of finerenone to protect the kidneys and cardiovascular system of patients with CKD and T2D.
Keywords: chronic kidney disease, randomized controlled trials, diabetic nephropathy, hyperkalemia, mineralocorticoid receptor antagonist
Abstract
Background
Finerenone reduced risk of cardiorenal outcomes in patients with CKD and type 2 diabetes in the FIDELIO-DKD trial. We report incidences and risk factors for hyperkalemia with finerenone and placebo in FIDELIO-DKD.
Methods
This post hoc safety analysis defined hyperkalemia as ≥mild or ≥moderate based on serum potassium concentrations of >5.5 or >6.0 mmol/L, respectively, assessed at all regular visits. Cumulative incidences of hyperkalemia were based on the Aalen–Johansen estimator using death as competing risk. A multivariate Cox proportional hazards model identified significant independent predictors of hyperkalemia. Restricted cubic splines assessed relationships between short-term post-baseline changes in serum potassium or eGFR and subsequent hyperkalemia risk. During the study, serum potassium levels guided drug dosing. Patients in either group who experienced ≥mild hyperkalemia had the study drug withheld until serum potassium was ≤5.0 mmol/L; then the drug was restarted at the 10 mg daily dose. Placebo-treated patients underwent sham treatment interruption and downtitration.
Results
Over 2.6 years’ median follow-up, 597 of 2785 (21.4%) and 256 of 2775 (9.2%) patients treated with finerenone and placebo, respectively, experienced treatment-emergent ≥mild hyperkalemia; 126 of 2802 (4.5%) and 38 of 2796 (1.4%) patients, respectively, experienced moderate hyperkalemia. Independent risk factors for ≥mild hyperkalemia were higher serum potassium, lower eGFR, increased urine albumin-creatinine ratio, younger age, female sex, β-blocker use, and finerenone assignment. Diuretic or sodium-glucose cotransporter–2 inhibitor use reduced risk. In both groups, short-term increases in serum potassium and decreases in eGFR were associated with subsequent hyperkalemia. At month 4, the magnitude of increased hyperkalemia risk for any change from baseline was smaller with finerenone than with placebo.
Conclusions
Finerenone was independently associated with hyperkalemia. However, routine potassium monitoring and hyperkalemia management strategies employed in FIDELIO-DKD minimized the impact of hyperkalemia, providing a basis for clinical use of finerenone.
Drugs that interrupt the renin-angiotensin system (RAS) are the backbone of therapy in patients with CKD and type 2 diabetes (T2D).1–3 Above the kidney protection offered by a single RAS inhibitor, dual-agent RAS inhibition was evaluated for kidney protection in patients with CKD and T2D in several randomized controlled trials.4–6 No kidney or cardiovascular (CV) benefits were apparent but an increased risk of adverse events (AEs), including AKI and hyperkalemia, was seen.4–6
The FInerenone in reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease (FIDELIO-DKD) trial evaluated the effects of the novel, selective, nonsteroidal mineralocorticoid receptor (MR) antagonist (MRA) finerenone, in addition to standard of care, including a maximum tolerated dose of a single RAS inhibitor, to slow CKD progression and reduce the risk of CV outcomes in patients with CKD and T2D. Finerenone significantly reduced the relative risk versus placebo of the primary composite kidney-specific outcome by 18% (absolute risk reduction of 3.3%) and the key secondary composite CV outcome by 14% (absolute risk reduction of 1.8%).7 Consistent with the known role of the MR in the regulation of electrolyte and fluid homeostasis,8 MR antagonism with finerenone increased the incidence of any hyperkalemia (an investigator-reported AE) compared with placebo in a patient population at high intrinsic risk of hyperkalemia.7
Here, we report the incidence and risk factors associated with hyperkalemia defined quantitatively by central laboratory assessment of serum potassium concentration ([K+]) measured at every study visit (using thresholds of >5.5 mmol/L for ≥mild hyperkalemia and >6.0 mmol/L for ≥moderate hyperkalemia, in accordance with the latest Kidney Disease: Improving Global Outcomes [KDIGO] guidance on severity of acute hyperkalemia9). We describe the cumulative incidence of hyperkalemia, the associated risk factors, the interaction of finerenone with these risk factors, and measures taken to mitigate the risk of hyperkalemia during the study. Lastly, we contextualize the risk of hyperkalemia with finerenone compared with the risk associated with the use of dual RAS inhibition and steroidal MR antagonism.
Methods
The FIDELIO-DKD trial (NCT02540993) design and details have been published previously.7 Briefly, FIDELIO-DKD was a phase 3, randomized, double-blind, placebo-controlled, multicenter clinical trial testing the efficacy and safety of finerenone in patients with advanced CKD and T2D. The trial was performed in accordance with the principles of the Declaration of Helsinki and was approved by International Review Boards, independent Ethics Committees, and competent authorities according to national and international regulations.
Patients and Study Design
Patients were included in the trial if they were treated with a maximum tolerated labeled dose of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB), but not both, with a serum [K+] ≤4.8 mmol/L at both the run-in and screening visits. CKD was defined as either: (1) persistent, moderately elevated albuminuria (urine albumin-creatinine ratio [UACR] ≥30–<300 mg/g), an eGFR≥25–<60 ml/min per 1.73 m2, and a history of diabetic retinopathy; or (2) persistent severely elevated albuminuria (UACR≥300–≤5000 mg/g) and an eGFR≥25–<75 ml/min per 1.73 m2. Exclusion criteria included treatment with a steroidal MRA, renin inhibitor, or potassium-sparing diuretic that could not be discontinued ≥4 weeks before the screening visit.
Eligible patients were randomized 1:1 to receive oral finerenone or placebo using a computer-generated randomizations schedule and stratified by region (North America, Europe, Asia, Latin America, other), albuminuria at screening (moderately increased, severely increased), and eGFR at screening (25–<45, 45–<60, ≥60 ml/min per 1.73 m2). All patients, investigators, and study personnel (except for the independent data monitoring committee) were masked to treatment allocation.
The study consisted of run-in, screening, and double-blind treatment periods. During the run-in period (4–16 weeks), RAS inhibitor therapy was uptitrated to the maximum tolerated labeled dose, maintained for ≥4 weeks before the screening visit. Patients meeting eligibility criteria at the screening visit were subsequently randomized within 2 weeks. Patients received an initial dose of study drug of 10 or 20 mg once daily (od), on the basis of an eGFR at the screening visit of 25–<60 or ≥60 ml/min per 1.73 m2, respectively. After the start of treatment, study drug dose reduction or temporary treatment interruption was allowed at any time for safety reasons.
Serum [K+] Assessment and Management of Hyperkalemia
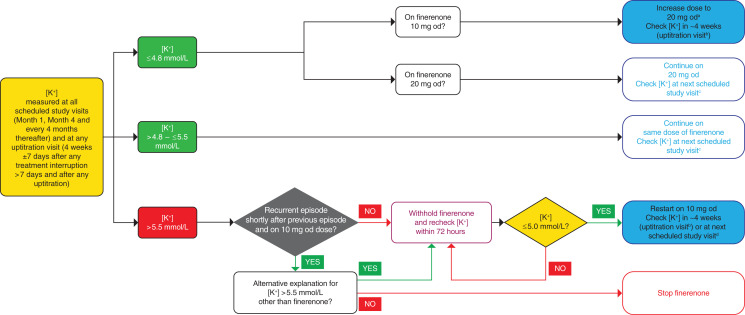
At regular study visits (month 1, month 4, and every 4 months thereafter), study drug dosing was on the basis of serum [K+] and eGFR, assessed at local laboratories (Figure 1). If serum [K+] was ≤4.8 mmol/L, the dose of study drug was either uptitrated from 10 mg to 20 mg od (provided any eGFR decrease was <30% from the last measured value) or maintained at the 20-mg-od dose. If serum [K+] was >4.8–≤5.5 mmol/L, treatment was continued with the same dose of study drug. When serum [K+] was >5.5 mmol/L, study drug was temporarily withheld and serum [K+] rechecked within 72 hours—at this point, if serum [K+] was ≤5.0 mmol/L, study drug was restarted at the 10-mg-od dose; otherwise, study drug continued to be withheld until serum [K+] was ≤5.0 mmol/L. Because this was a double-blind trial, placebo recipients underwent sham up- and downtitration depending on their serum [K+], and had temporary placebo interruption if serum [K+] was >5.5 mmol/L, in the same manner as for the finerenone group.
Figure 1.
Potassium management algorithm in FIDELIO-DKD. aIf eGFR is stable (i.e., ≤30% decrease since last available measurement). bUptitration visits were performed at 4 weeks ±7 days after any treatment interruption >7 days and after any uptitration. cRegular study visits were scheduled at month 1, month 4, and every 4 months thereafter. dIf treatment interruption ≤7 days.
The protocol specified that serum [K+] should be measured within 4 weeks (±7 days) after a temporary treatment interruption of ≥7 days or after dose adjustments or uptitration of study drug. If the treatment interruption was <7 days, serum [K+] was measured within 4 months, at the next scheduled study visit. Permanent discontinuation of study drug was recommended if a patient on the 10-mg-od dose experienced a recurrent hyperkalemia event soon after a previous event with interruption of study drug (provided the only explanation for the recurring hyperkalemia event was the study drug), or if, in the opinion of the investigator, continuation of treatment was harmful to the patient (Figure 1).
Except for temporary treatment interruption and subsequent dose reductions of study drug in response to elevations in serum [K+], management of hyperkalemia was at the investigator’s discretion on the basis of local guidance. There were no restrictions on the use of potassium supplements or potassium binders during the trial, and a low-potassium diet was not mandated by the protocol. Irrespective of serum [K+], investigators were instructed to maintain standard-of-care therapy; dose reduction of concomitant RAS inhibitor therapy was not allowed solely to facilitate the maintenance of study drug.
Outcomes
The primary outcome of this post hoc safety analysis was ≥mild or ≥moderate hyperkalemia, defined using serum [K+] thresholds of >5.5 and >6.0 mmol/L, respectively, as assessed quantitatively by the central laboratory at every study visit. Hyperkalemia was also reported as an investigator-reported AE using the Medical Dictionary for Regulatory Activities (MedDRA) preferred terms ‘hyperkalemia’ and ‘blood potassium increased’, as reported previously.7 AEs, including elevations in serum [K+], were considered as treatment emergent if they started or worsened during study drug intake or up to 3 days after any temporary or permanent interruption.
As reported previously, the primary efficacy outcome of the FIDELIO-DKD study was a composite of time to first onset of kidney failure, a sustained ≥40% decrease in eGFR from baseline over ≥4 weeks, or renal death. The key secondary efficacy outcome was a composite of time to first onset of CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure.7,10
Statistical Analyses
Safety analyses were performed in the safety analysis set (all randomized patients without critical Good Clinical Practice [GCP] violations who took ≥1 dose of study drug). Cumulative incidences of hyperkalemia were on the basis of the Aalen–Johansen estimator using death as a competing risk; for the serum [K+] analyses, patients with elevated baseline values (>5.5 and >6.0 mmol/L, respectively) were censored at day 1. The following baseline variables were used in a multivariate Cox proportional hazards model (model 1) to identify significant independent predictors of ≥mild or ≥moderate hyperkalemia. The variables were selected after a review of the literature and were those deemed biologically plausible by the study investigators. They included the following: age, sex, eGFR categories, serum [K+] categories, log2-transformed UACR, RAS inhibitor dosing, diuretic use, β-blocker use, sodium-glucose co-transporter–2 inhibitor (SGLT-2i) use, and study drug assignment. A significance threshold of P<0.05 was used to identify significant, independent predictors. Schoenfeld residuals were used to check if the proportional hazards assumption was satisfied for each baseline variable used in the model. Another multivariate Cox proportional hazards model (model 2) was used to describe the interaction of the study drug with each of the baseline variables included in model 1 described above. Model 2 evaluated whether the treatment effect of finerenone on risk of ≥mild or ≥moderate hyperkalemia differed within the subgroups; the likelihood ratio test of the nested models was reported as the P value of the interaction. Restricted cubic splines were used to capture any possible nonlinear effects of the short-term change in serum [K+] or eGFR (from baseline to month 1 or 4) and the subsequent risk of hyperkalemia. The likelihood ratio test was used for the nested models with and without using the spline function to assess whether the model using splines was a better fit to the data than the standard linear model. The hazard ratio and 95% confidence intervals were plotted for the variable where the spline function had been applied. These values compared a change in the relevant variable with that of a zero change within each treatment group.
Efficacy analyses were performed in the full analysis set, which included all randomized patients excepting 60 patients with critical GCP violations. In time-to-event analyses, the superiority of finerenone versus placebo was tested via a stratified log-rank test; stratification factors were region, eGFR category at screening, and albuminuria category at screening; the statistical assumptions of FIDELIO-DKD have been published previously.7 All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Serum [K+] at Run-In, Screening, and Baseline
Between September of 2015 and June of 2018, 13,911 patients were enrolled, of whom 8177 did not meet eligibility criteria at the run-in or screening visits; 5734 patients were subsequently randomized. At the run-in visit, potassium values were available for 12,010 of 13,911 patients, of whom 2181 (18.2%) had a serum [K+] >4.8 mmol/L and were excluded. At the screening visit, 640 of 7114 (9.0%) patients had a serum [K+] >4.8 mmol/L and were screen-failed. At baseline, the mean serum [K+] was 4.37±0.46 mmol/L in the finerenone group and 4.38±0.46 mmol/L in the placebo group. The proportion of patients with a baseline serum [K+] ≤4.8 mmol/L versus >4.8 mmol/L was 4889 of 5658 (86.4%) and 769 of 5658 (13.6%), respectively. A total of 390 (6.9%) patients had a baseline serum [K+] >5.0 mmol/L (Supplemental Figure 1).
Changes in Serum [K+] during the Study
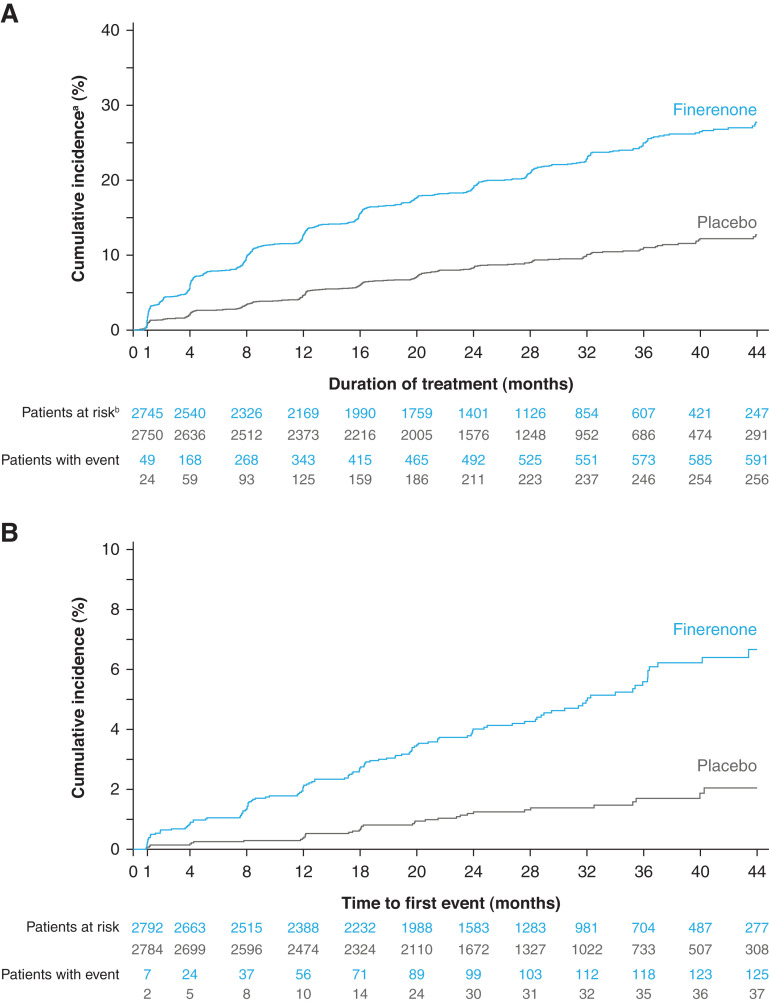
Patients treated with finerenone had higher mean serum [K+] compared with placebo recipients. The maximum difference between treatment groups was 0.23 mmol/L at month 4 and was consistent across predefined screening eGFR categories (Supplemental Figure 2). Over 2.6 years of median follow-up, 597 of 2785 (21.4%) and 256 of 2775 (9.2%) patients in the finerenone and placebo groups, respectively, experienced treatment-emergent ≥mild hyperkalemia. A total of 126 of 2802 (4.5%) patients in the finerenone group and 38 of 2796 (1.4%) patients in the placebo group had treatment-emergent ≥moderate hyperkalemia. At month 1, 86 of 2742 (3.1%) and 14 of 2757 (0.5%) patients in the finerenone group and 34 of 2730 (1.2%) and four of 2749 (0.1%) patients in the placebo group had serum [K+] >5.5 and >6.0 mmol/L, respectively; hyperkalemia events accumulated gradually throughout the study in both treatment groups (Figure 2).
Figure 2.
Cumulative incidence of elevated serum [K+]. (A) Shows time to treatment-emergent serum [K+] >5.5 mmol/L. (B) Shows time to treatment-emergent serum [K+] >6.0 mmol/L. aCumulative incidence calculated by Aalen–Johansen estimator using all-cause death as a competing risk. bIncidence calculated as n/N over 2.6 years’ median follow-up. cPatients at risk must have both a baseline and postbaseline treatment-emergent value and the baseline value must be below the threshold.
Compared with patients without ≥mild hyperkalemia, patients with treatment-emergent ≥mild hyperkalemia tended to have lower eGFR, higher albuminuria, and higher serum [K+] at baseline, and were more likely to be treated with a potassium binder but less likely to be treated with a diuretic or an SGLT-2i (Table 1 and Supplemental Table 1). Similar differences were observed between patients with and without treatment-emergent ≥moderate hyperkalemia (Supplemental Tables 2 and 3).
Table 1.
Baseline demographics in patients with versus without any serum [K+] >5.5 mmol/L
| Baseline Demographicsa | No Serum [K+] >5.5 mmol/L (n=4604) | Any Serum [K+] >5.5 mmol/L (n=1054) |
|---|---|---|
| Sex, male, n (%) | 3279 (71.2) | 694 (65.8) |
| Age, yr, mean±SD. | 65.81±9.02 | 64.45±9.09 |
| Age category, n (%) | ||
| <65 yr | 1879 (40.8) | 493 (46.8) |
| 65–74 yr | 1960 (42.6) | 434 (41.2) |
| ≥75 yr | 765 (16.6) | 127 (12.0) |
| Serum [K+], mmol/L, mean±SD. | 4.31±0.43 | 4.65±0.48 |
| Serum [K+], mmol/L, n (%) | ||
| ≤4.1 | 1577 (34.3) | 131 (12.4) |
| >4.1–≤4.5 | 1706 (37.1) | 323 (30.6) |
| >4.5–≤4.8 | 878 (19.1) | 274 (26.0) |
| >4.8–≤5.0 | 247 (5.4) | 132 (12.5) |
| >5.0 | 196 (4.3) | 194 (18.4) |
| eGFR, ml/min per 1.73 m2, mean±SD. | 44.83±12.43 | 42.20±12.86 |
| eGFR category, ml/min per 1.73 m2, n (%) | ||
| <25 | 102 (2.2) | 33 (3.1) |
| 25–<45 | 2334 (50.7) | 638 (60.5) |
| 45–<60 | 1611 (35.0) | 286 (27.1) |
| ≥60 | 557 (12.1) | 97 (9.2) |
| UACR, mg/g, median (IQR) | 820.45 (439.79–1565.00) |
956.94 (479.18–1917.93) |
| UACR category, mg/g, n (%) | ||
| <30 | 18 (0.4) | 5 (0.5) |
| 30–<300 | 547 (11.9) | 137 (13.0) |
| ≥300 | 4038 (87.7) | 912 (86.5) |
| eGFR 25–<45 ml/min per 1.73 m2 and serum [K+] >4.5 mmol/L, n (%) | ||
| No | 3897 (84.6) | 699 (66.3) |
| Yes | 707 (15.4) | 355 (33.7) |
| Label-recommended dose of RASi, n (%) | ||
| ≤Minimum | 1368 (29.7) | 331 (31.4) |
| >Minimum to <maximum | 1125 (24.4) | 263 (25.0) |
| ≥Maximum | 2096 (45.5) | 458 (43.5) |
| β-blocker, n (%) | 2405 (52.2) | 560 (53.1) |
| Diuretic, n (%) | 2677 (58.1) | 527 (50.0) |
| Loop | 1338 (29.1) | 275 (26.1) |
| Thiazide | 1146 (24.9) | 205 (19.4) |
| Potassium binders, n (%) | 91 (2.0) | 45 (4.3) |
| Potassium supplements, n (%) | 156 (3.4) | 14 (1.3) |
| Glucose-lowering therapies, n (%) | 4481 (97.3) | 1027 (97.4) |
| SGLT-2i | 240 (5.2) | 19 (1.8) |
Percentages are rounded to the nearest decimal place. IQR, interquartile range; RASi, RAS inhibitor.
Information on RASi dosing was missing in 15 patients without any serum [K+] >5.5 mmol/L and two patients with any serum [K+] >5.5 mmol/L.
Multivariate Analysis of Risk Factors for ≥Mild and ≥Moderate Hyperkalemia
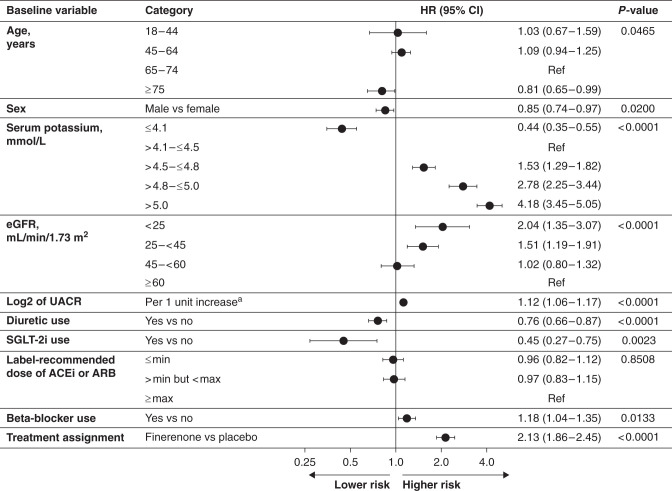
A multivariate Cox proportional hazards regression model identified baseline risk predictors for ≥mild (Figure 3) and ≥moderate (Supplemental Figure 3) hyperkalemia. Higher baseline serum [K+] was associated with an increased risk of ≥mild hyperkalemia; the risk was increased 1.5-, 2.8-, and 4.2-fold in patients with serum [K+] of 4.5–<4.8, 4.8–5.0, and >5.0 mmol/L at baseline, respectively, compared with a serum [K+] of 4.1–4.5 mmol/L. Likewise, lower eGFR was an independent predictor of hyperkalemia; compared with an eGFR≥60 ml/min per 1.73 m2, the risk of ≥mild hyperkalemia increased 1.5-fold and twofold as eGFR dropped below 45 and 25 ml/min per 1.73 m2, respectively. Patients with an eGFR of 45–<60 ml/min per 1.73 m2 had a similar risk to those with an eGFR≥60 ml/min per 1.73 m2. Every doubling of UACR was associated with a 1.1-fold increased risk of hyperkalemia. Finally, compared with placebo, assignment to finerenone doubled the risk of hyperkalemia, after adjustment for all other risk factors included in the model. Baseline diuretic or SGLT-2i use and advanced age were associated with lower risk of ≥mild hyperkalemia; baseline RAS inhibitor dosing did not modify hyperkalemia risk.
Figure 3.
Multivariate analysis of time to any serum [K+] >5.5 mmol/L. aUACR is modeled as a continuous variable; 1 unit change in log2 UACR denotes doubling of UACR. 95% CI, 95% confidence interval; Ref, reference category.
The magnitude of the increased risk of ≥mild and ≥moderate hyperkalemia with finerenone versus placebo was consistent across subgroups, including sex, baseline CKD severity, and baseline medication use (Supplemental Figures 4 and 5). Significant treatment interactions were noted for the risk of ≥mild hyperkalemia by baseline serum [K+] and age (Supplemental Figure 4).
Short-Term Changes in Serum [K+] and eGFR and the Future Risk of Hyperkalemia
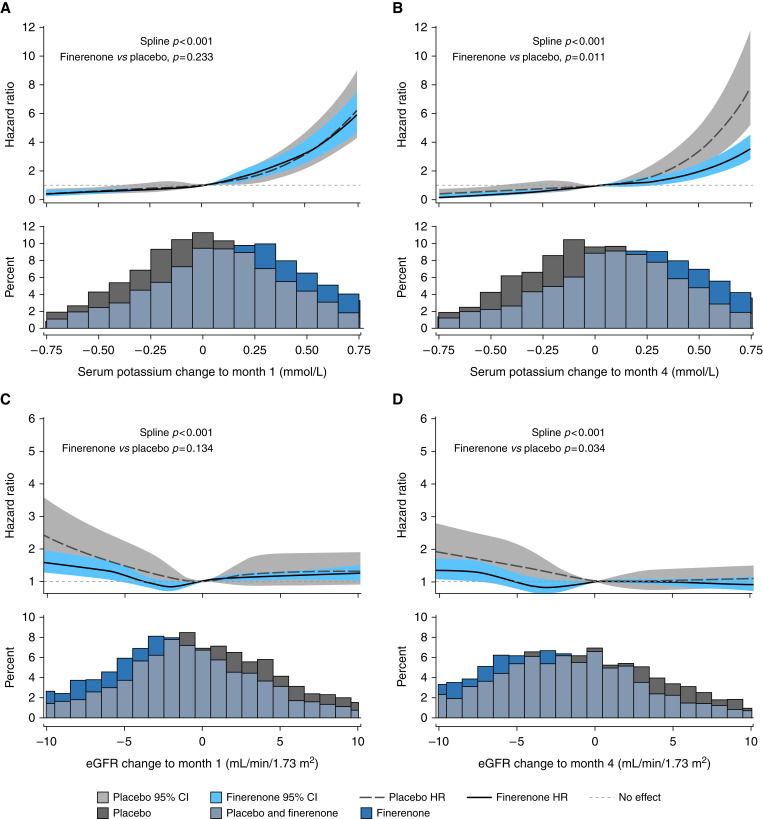
Irrespective of treatment assignment, the risk of ≥mild hyperkalemia was higher in patients with larger increases in serum [K+] between baseline and month 1 (Figure 4A) or month 4 (Figure 4B) compared with those with no change in serum [K+] (P<0.001 for both time points). At month 1, for any given increase in serum [K+], the magnitude of the increased risk of ≥mild hyperkalemia was similar between treatment groups, compared with no change in each respective group (P=0.233; Figure 4A). However, at month 4, the relationship between changes in serum [K+] and subsequent risk of ≥mild hyperkalemia was significantly different between groups (P=0.011; Figure 4B); a placebo recipient with a 0.5-mmol/L increase in serum [K+] had an approximately 3.4-fold higher risk of hyperkalemia than a patient with no change in serum [K+], whereas with finerenone, a 0.5-mmol/L increase in serum [K+] was associated with a twofold higher risk of hyperkalemia compared with no change. A similar pattern was observed for changes in eGFR at month 1 (Figure 4C) and month 4 (Figure 4D) and the subsequent risk of ≥mild hyperkalemia. At month 1, patients with a greater decrease in eGFR from baseline were at higher risk of subsequent hyperkalemia (P<0.001) than patients with no change in eGFR, and the magnitude of the increased risk for any given decrease in eGFR was similar between treatment groups (P=0.134; Figure 4C). A greater decrease in eGFR from baseline to month 4 compared with no change in eGFR was also associated with an increased risk of developing hyperkalemia during the study (P<0.001); however, the relationship was significantly different between treatment groups (P=0.034; Figure 4D). A placebo-treated patient with a 5-ml/min per 1.73 m3 decrease in eGFR had a 1.4-fold higher risk of hyperkalemia than a patient with no change in eGFR, whereas a finerenone recipient with a 5-ml/min per 1.73 m3 decrease in eGFR had the same risk of hyperkalemia as one with no change in eGFR.
Figure 4.
Short-term changes in serum [K+] and eGFR and the future risk of hyperkalemia ([K+] >5.5 mmol/L). (A) Changes in serum [K+] from baseline to month 1. (B) Changes in serum [K+] from baseline to month 4. (C) Changes in eGFR from baseline to month 1. (D) Changes in eGFR from baseline to month 4. 95% CI, 95% confidence interval; HR, hazard ratio.
Management of Hyperkalemia during the Study
Management of hyperkalemia in the FIDELIO-DKD trial was at the investigator’s discretion, following local clinical guidelines, except for the temporary discontinuation of study drug in patients with a serum [K+] >5.5 mmol/L. At baseline, 136 (2.4%) patients were on a potassium binder; most of these patients were treated with calcium or sodium polystyrene sulphonate. During the trial, 307 (10.9%) and 183 (6.5%) patients in the finerenone and placebo groups, respectively, started treatment with a potassium binder (Supplemental Table 4 and Supplemental Figure 6). Only one patient (in the placebo group) received dialysis while hospitalized for hyperkalemia during the study. In patients with investigator-reported, treatment-emergent, hyperkalemia-related AEs, the most frequent action reported by the investigator as having the highest effect to reduce serum [K+] was the temporary withdrawal of study drug, consistent with the protocol-recommended action (Table 2).
Table 2.
Management of investigator-reported hyperkalemia (actions reported by the investigator as having the highest effect on hyperkalemia)
| Action | Finerenone (n=2827) | Placebo (n=2833) |
|---|---|---|
| Drug withdrawn, n (%) | 64 (2.3) | 25 (0.9) |
| Drug interrupted, n (%) | 310 (11.0) | 146 (5.2) |
| Dose reduced, n (%) | 9 (0.3) | 6 (0.2) |
| Dose not changed, n (%) | 127 (4.5) | 74 (2.6) |
| Not applicable or unknown, n (%) | 6 (0.3) | 4 (0.1) |
In total, 516 (18.3%) patients in the finerenone group and 255 (9.0%) patients in the placebo group had an investigator-reported hyperkalemia-related AE. Actions were considered in the following order: drug withdrawn, drug interrupted, dose reduced, dose not changed, and not applicable and unknown.
Efficacy Outcomes in Patient Subgroups According to Risk of Hyperkalemia
Prespecified subgroup analyses demonstrated that the effects of finerenone versus placebo on the primary composite kidney, key secondary composite CV, and all-cause mortality outcomes were similar among patients with low (<4.8 mmol/L), mid (≥4.8–5.0 mmol/L), or high (>5.0 mmol/L) serum [K+] at baseline and among patients with moderate-to-severe eGFR impairment at baseline (Supplemental Figure 7).
Discussion
Patients in FIDELIO-DKD had a high intrinsic risk of hyperkalemia because of their advanced CKD, T2D, and treatment with optimized doses of an ACEi or ARB. This was reflected by the high incidence of hyperkalemia in the placebo arm during the study. Hyperkalemia (defined quantitatively by central laboratory assessment of serum [K+] measured at every study visit) was about twofold higher with finerenone than placebo, including across multiple patient subgroups. Notably, hyperkalemia was the only AE or serious AE to demonstrate excess cases with finerenone; it was an expected side effect of MR antagonism,11 and the kidney and CV benefits of finerenone seen in the overall population were maintained in patients at highest risk of hyperkalemia.7
These analyses describe the risk factors that independently predicted the development of hyperkalemia in FIDELIO-DKD, including baseline serum [K+], baseline eGFR, and baseline UACR. Although lower eGFR (<45 ml/min per 1.73 m2) and higher baseline [K+] (>4.5 mmol/L) are well-established risk factors for hyperkalemia,9,12 it is also the case that higher UACR is associated with increased hyperkalemia,13 although this appears to be less widely recognized; in this analysis, a strong and robust relationship emerged between higher UACR and subsequent occurrence of hyperkalemia. Unlike previous studies that have found male sex and advanced age to be hyperkalemia risk factors,13–15 we found the converse, and the reasons are unclear. Notably, optimizing RAS inhibition before starting treatment with finerenone did not increase the risk of hyperkalemia, perhaps because RAS inhibitor dosing was individualized during the run-in period (uptitrated to the highest dose that each patient could safely tolerate). The use of diuretics and an SGLT-2i may be prudent strategies to reduce the risk of hyperkalemia. However, because few patients were treated with an SGLT-2i at baseline (partly because SGLT-2i use at the time of the study was restricted to patients with higher eGFR values) these results should be interpreted with caution.
Short-term increases in serum [K+] after the start of treatment were predictive of subsequent risk of hyperkalemia; this risk was shared between placebo and finerenone. However, for any given increase in serum [K+] between baseline and month 4 versus no change, the increased risk of hyperkalemia was smaller with finerenone than placebo. These findings may appear counterintuitive but can be explained by the following considerations: finerenone is expected to increase serum [K+] through MR antagonism, which may have triggered changes in management, such as finerenone treatment interruption and dose reduction, or adding other therapies (such as diuretics or potassium binders) that might mitigate the subsequent risk of hyperkalemia.9 On the other hand, increases in serum [K+] provoked by placebo may reflect processes in the kidney that reduce its ability to secrete potassium, such as AKI, tubulointerstitial inflammation, or obstruction16; these are less amenable to treatment interventions (evidence on the effectiveness of potassium binders and loop diuretics in an acute setting are lacking) and are unaffected by reducing the dose of placebo.9
Short-term decreases in eGFR after the start of treatment were associated with an increased risk of hyperkalemia during the study, and the magnitude of the increased risk for any given reduction in eGFR versus no change in eGFR was smaller with finerenone than with placebo. Provoked by natriuresis or modest BP reduction, the decrease in eGFR induced by finerenone is hemodynamic (in contrast to a tubular cause) and is less likely to impair the ability to secrete potassium. Therefore, temporary treatment interruption and finerenone dose reduction are likely to restore eGFR toward normal and normalize serum [K+]. On the other hand, short-term decreases in eGFR in patients receiving placebo may be due to tubular factors or obstructive uropathy, which also impair the ability to secrete potassium,9 thus increasing the subsequent risk of hyperkalemia. Moreover, because finerenone slows eGFR decline versus placebo, this may reduce the risk of subsequent hyperkalemia.7
Relative to the incidence of hyperkalemia in FIDELIO-DKD, a higher incidence was reported in trials using dual RAS inhibition.4–6 In VA Diabetes iN Nephropathy Study (VA NEPHRON-D), 32 of 724 (4.4%) patients in the losartan group, and 72 of 724 (9.9%) patients in the losartan plus lisinopril group, experienced severe hyperkalemia (defined as serum [K+] >6.0 mmol/L, or hyperkalemia requiring a visit to the emergency room, hospitalization, or dialysis). Thus, in the VA NEPHRON-D study, 18 patients would have needed to have been prescribed dual RAS blockade for one patient to develop severe hyperkalemia.5 Similarly, looking at hyperkalemia events leading to permanent treatment discontinuation in Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (Core and Extension Phases) and Olmesartan Reducing Incidence of End Stage Renal Disease in Diabetic Nephropathy Trial, a small number of patients (45 and 26 patients, respectively) would have needed to be treated with dual RAS blockade before one patient would have needed to stop treatment because of hyperkalemia.4,6 In contrast, in FIDELIO-DKD, 71 patients would have needed to be prescribed finerenone before one patient permanently stopped the drug.7 Furthermore, in FIDELIO-DKD, finerenone significantly reduced the risk of the composite kidney and CV outcomes (by 18% and 14%, respectively) compared with placebo, whereas the dual RAS inhibitor studies failed to demonstrate kidney or CV benefits.4–6
A common comparator for a nonsteroidal MRA, albeit not indicated for patients with CKD, is the steroidal MRA spironolactone. A Cochrane Database Systematic Review of 27 small studies including patients with CKD stages 1–4 with and without diabetes (n=1549) noted that spironolactone plus an ACEi or ARB decreased albuminuria and BP, doubled the risk of hyperkalemia, and increased the risk of gynecomastia fivefold, with insufficient data to analyze benefits on clinical outcomes such as ESKD.17 The active metabolites of spironolactone have a long half-life.11,18 In the Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease (AMBER) study, which included patients with CKD (eGFR 25–45 ml/min per 1.73 m2) and resistant hypertension, an exploratory analysis showed that 75% of patients still had detectable urinary metabolites of spironolactone 2 weeks after stopping treatment. Moreover, approximately two in three patients developed ≥mild hyperkalemia and approximately one in four patients had to discontinue spironolactone because of hyperkalemia within 12 weeks. Even when given the potassium-binding drug patiromer, >30% of patients developed ≥mild hyperkalemia and 6.8% of patients stopped spironolactone because of hyperkalemia.19 These high rates of hyperkalemia and discontinuation of spironolactone in AMBER suggest that the incidence of hyperkalemia with finerenone may be lower than with spironolactone in comparable patient populations. In addition, in a head-to-head phase 2 study in patients with heart failure with reduced ejection fraction and CKD, the incidence of hyperkalemia was 11.1% with spironolactone (25–50 mg od) compared with 4.8% with finerenone (10 mg od).20 Once-daily oral administrations of 5 mg and 10 mg of finerenone were at least as effective as spironolactone (25 mg or 50 mg/day) in decreasing cardiorenal biomarker levels, but resulted in smaller increases in serum potassium, lower incidences of hyperkalemia and worsening renal function, and smaller reductions in systolic BP.20 These observations suggest that some pharmacodynamic effects mediated by MRAs, including BP control or serum potassium changes, are the consequence of a significant drug exposure over a long period (long half-life, area-under-the-curve driven), whereas others (e.g., anti-inflammatory, antihypertrophic, and antifibrotic effects) are the consequences of relatively short drug exposure (short half-life, maximum serum concentration [of drug] driven) triggered by different signaling cascades. Strikingly, the rise in serum potassium with finerenone 5 mg twice daily was larger in comparison with finerenone 10 mg od, whereas reductions of N-terminal pro–B-type natriuretic peptide or albuminuria were similar.20 This differential effect of once- versus twice-daily dosing of finerenone illustrates the importance of consideration of both pharmacokinetics and physiology when considering hyperkalemia rates. It is likely that the low absolute risk of hyperkalemia with finerenone is because of its unique attributes: the short half-life of the drug, absence of active metabolites, balanced kidney-heart distribution (in rodent models), and the novel mechanism of action involving distinct blockade of the MR and different effects on subsequent gene expression.11 Notably, because of the short half-life of finerenone (2–3 hours in patients with CKD) and lack of active metabolites,11,21 finerenone-associated hyperkalemia can be effectively managed by treatment interruption, as demonstrated in FIDELIO-DKD. It is important to highlight that these properties contrast with the long half-life and multiple active metabolites of spironolactone, as well as the fact that spironolactone has a kidney versus heart tissue distribution of approximately 6:1 (approximately 1:1 for finerenone) in rodent models, and that spironolactone interacts with the MR in a different manner to finerenone.11,21
Esaxerenone is a potent and highly selective nonsteroidal MRA with a much longer half-life (approximately 19 hours) than finerenone.22 In a patient population with less advanced CKD and T2D, 52 weeks of treatment with esaxerenone led to discontinuation due to elevated serum [K+] in 4.0% of patients compared with 0.4% of patients receiving placebo.23,24 Another steroidal MRA, eplerenone, which is more selective than spironolactone, approximately doubles the risk of hyperkalemia in patients with heart failure,25,26 and is contraindicated in patients with T2D and moderately elevated albuminuria without heart failure (in the USA).27
Results from the FIDELIO-DKD trial provide insights into requirements for serum [K+] monitoring in patients treated with finerenone. The earliest time point after which serum [K+] was measured was 1 month after treatment initiation. Very few patients developed hyperkalemia at this time: 86 of 2742 (3.1%) and 34 of 2730 (1.2%) patients in the finerenone and placebo groups, respectively. The second scheduled serum [K+] assessment was at month 4 after treatment initiation, and at 4-monthly intervals thereafter. The frequency of potassium monitoring employed in FIDELIO-DKD was similar to the frequency of monitoring recommended for patients with CKD in the 2012 KDIGO guidelines (3–4 times a year for patients with UACR>300 mg/g and eGFR<60 ml/min per 1.73 m2, and 2–3 times a year for patients with UACR 30–300 mg/g and eGFR 15–59 ml/min per 1.73 m2).3 Evidence from routine clinical practice suggests that physicians often intervene and reduce the dose of RAS inhibitors when serum [K+] rises above 5.0 mmol/L28; in FIDELIO-DKD, RAS inhibitor dose reduction was not permitted and finerenone was continued with no dose adjustments in patients with a serum [K+] of 5.0–5.5 mmol/L. It was only when serum [K+] rose to >5.5 mmol/L that finerenone was temporarily withheld. Treatment was resumed (at the 10-mg dose) when serum [K+] was ≤5.0 mmol/L. The occurrence of serum [K+] >5.5 or >6.0 mmol/L accumulated gradually over time and could occur months or years after starting finerenone. This may be related to two important determinants of hyperkalemia: declining kidney function and increasing serum [K+], or a combination of both.29 Thus, potassium monitoring at each clinical follow-up visit would be a prudent strategy, in addition to both physicians and patients being aware of conditions or triggers that may precipitate a hyperkalemia event, such as medications (e.g., trimethoprim), acute illness, volume depletion, and AKI.29,30 The FIDELIO-DKD protocol has established a reliable potassium management algorithm, aligned to current guidelines,3 that may serve as a framework for use in clinical practice, taking into consideration patient characteristics (i.e., eGFR and baseline serum [K+]) that may increase their risk of hyperkalemia.
In summary, these analyses from FIDELIO-DKD characterize the risk of hyperkalemia in patients with CKD and T2D, reporting that finerenone was associated with a low absolute risk of clinically relevant hyperkalemia, with only a small proportion of events having a clinical effect. These analyses contribute to the understanding of how clinical characteristics, established risk factors, and treatment with finerenone may interact. Notably, elevated serum [K+] and lower eGFR when starting treatment with finerenone did not abrogate kidney or CV benefits of finerenone. These data provide a robust basis for incorporation of finerenone into clinical practice, with routine potassium monitoring for patients with CKD and T2D considered appropriate to manage the risk of hyperkalemia.
Disclosures
R. Agarwal reports personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals Inc., during the conduct of the study; he also reports personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Fresenius, Janssen, Relypsa/Vifor Pharma, and Sanofi; he has received personal fees from Ironwood Pharmaceuticals, Lexicon, Merck & Co., and Reata Pharmaceuticals, and nonfinancial support from E.R. Squibb & Sons, Opko Pharmaceuticals, and Otsuka America Pharmaceutical; he is a member of data safety monitoring committees for Amgen, AstraZeneca, and Celgene; a member of steering committees for randomized trials for Akebia Therapeutics, Bayer, Janssen, and Relypsa; a member of adjudication committees for AbbVie, Bayer, Boehringer Ingelheim, and Janssen; he has served as Associate Editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation and has been an author for UpToDate; Consultancy Agreements: Abbvie, Akebia, Amgen, AstraZeneca, Bayer, Birdrock Bio, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Ironwood Pharmaceuticals, Johnson & Johnson, Merck, Novartis, Opko, Otsuka, Reata, Relypsa, Sandoz, Sanofi, Takeda, ZS Pharma; Honoraria: Abbvie, Akebia, Amgen, AstraZeneca, Bayer, Birdrock Bio, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Ironwood Pharmaceuticals, Johnson & Johnson, Merck, Novartis, Opko, Otsuka, Reata, Relypsa, Sandoz, Sanofi, Takeda, ZS Pharma; Scientific Advisor or Membership: Kidney Disease: Improving Global Outcomes, Hypertension, the American Journal of Nephrology, the Journal of the American Society of Hypertension, Seminars in Dialysis, Eli Lilly, Ironwood Pharmaceuticals, Johnson & Johnson, Reata, and Sanofi; and he has received research grants from the US Veterans Administration and the National Institutes of Health. S.D. Anker has received research support from Abbott Vascular and Vifor Pharma; personal fees from Abbott Vascular, Bayer, Boehringer Ingelheim, BRAHMS, Cardiac Dimensions, Impulse Dynamics, Novartis, Servier, and Vifor Pharma; Consultancy Agreements: Abbott Vascular, Bayer, Boehringer Ingelheim, BRAHMS, Cardiac Dimensions, Cordio, Novartis, Servier, and Vifor Pharma; and Scientific Advisor or Membership: Cardiac Dimensions and Novartis. G.L. Bakris reports research funding paid to the University of Chicago Medicine from Bayer during the conduct of the study; he also reports research funding paid to the University of Chicago Medicine from Novo Nordisk and Vascular Dynamics; he acted as a consultant for and received personal fees from Alnylam, Alnylam Pharmaceuticals, Astra-Zeneca, Bayer, Boeringher-Ingelheim, Cyclerion Therapeutics, Horizon Pharma, Ionis, Janssen, KBP Biosciences, Medscape, Merck, Novo Nordisk, Relypsa, Vascular Dynamics, and Vifor; he is an Editor of the American Journal of Nephrology, Nephrology, and Hypertension, and Section Editor of UpToDate; and he is an Associate Editor of Diabetes Care and Hypertension Research; Honoraria: Alnylam, Astra Zeneca, Ionis, KBP Biosciences, Merck, Novo Nordisk, Teijin, and Vifor; Scientific Advisor or Membership: American Heart Association, KBP Biosciences, Merck, Teijin, and Vifor; and Other Interests/Relationships: the American Diabetes Association, the American Heart Association, the Blood Pressure Council, and the National Kidney Foundation. G. Filippatos reports lectures fees and/or that he is a committee member of trials and registries sponsored by Amgen, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor Pharma. He is a Senior Consulting Editor for JACC Heart Failure and he has received research support from the European Union; Scientific Advisor or Membership: the European Heart Journal and the European Journal of Heart Failure; and Speakers Bureau: Bayer and Boehringer Ingelheim. A. Joseph and R. Lawatscheck are full-time employees of Bayer AG, Division Pharmaceuticals, Germany. P. Kolkhof is a full-time employee of Bayer AG, Division Pharmaceuticals, Germany. He is the coinventor of finerenone and holds US and European patents relating to finerenone (US8436180B2 and EP2132206B1) and patents and inventions with Bayer AG. B. Pitt reports consultancy fees from Ardelyx, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, Merck, Phasebio, Protonintel, Relypsa/Vifor Pharma, Sanofi/Lexicon, Sarfez Pharmaceuticals, scPharmaceuticals, SQ Innovation, and Tricida; he has stock options for Ardelyx, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, Protonintel, Relypsa/Vifor Pharma, Sarfez Pharmaceuticals, scPharmaceuticals, SQ Innovation, and Tricida; Honoraria: Astra Zeneca, Bayer, Boehringer Ingelheim/Lilly, Cereno Scientific, KBP Biosciences, Phasebio, Sanofi/Lexicon, and Sarfez; he also holds a patent for site-specific delivery of eplerenone to the myocardium (US patent #9931412) and a provisional patent for histone-acetylation–modulating agents for the treatment and prevention of organ injury (provisional patent US 63/045,784). P. Rossing reports personal fees from Bayer during the conduct of the study; he has received research support and personal fees from AstraZeneca and Novo Nordisk, and personal fees from Astellas, Boehringer Ingelheim, Eli Lilly and Company, Gilead, Mundipharma, Novo Nordisk, Sanofi, and Vifor Pharma; Scientific Advisor or Membership: Astellas, Astra Zeneca, Bayer, Gilead, MSD, Mundipharma, and Novo Nordisk. All fees are given to Steno Diabetes Center, Copenhagen. L.M. Ruilope reports receipt of consultancy fees from Astra-Zeneca, Bayer, Daiichi-Sankyo, Medtronic, Novartis, Recor, Sanofi, and Vifor; Honoraria: Astra-Zeneca, Bayer, Daiichi-Sankyo, Medtronic, Novartis, Pfizer, Sanofi, and Vifor; Scientific Advisor or Membership: Astra-Zeneca, Bayer, Daiichi-Sankyo, Medtronic, Novartis, Pfizer, Sanofi, and Vifor; and Speakers Bureau: Astra-Zeneca, Bayer, Daiichi-Sankyo, and Novartis. C. Scott is a full-time employee of Bayer PLC, Data Science and Analytics, United Kingdom. D.J. Wilson is a full-time employee of Bayer AG, Cardiorenal Medical Affairs, United States; and Other Interests/Relationships: Senior Director—Nephrology, US Medical Affairs.
Funding
The FIDELIO-DKD trial was sponsored by Bayer AG.
Supplementary Material
Acknowledgments
Medical writing assistance was provided by Laura Johnstone, PhD, of Chameleon Communications International, and was funded by Bayer AG.
Data included in this manuscript were presented at the World Congress of Nephrology 2021. The sponsor of the FIDELIO-DKD trial, Bayer AG, designed and oversaw conduct of the trial, in collaboration with the FIDELIO-DKD Executive Committee. Bayer AG conducted all statistical analyses and all authors participated in data interpretation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Data Sharing Statement
Data from this study will be made available in the public domain—the electronic repository and date of data availability will be confirmed by Bayer AG.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021070942/-/DCSupplemental.
Supplemental Table 1. Baseline demographics in patients with versus without any serum potassium concentration >5.5 mmol/L, by treatment group.
Supplemental Table 2. Baseline demographics in patients with versus without any serum potassium concentration >6.0 mmol/L.
Supplemental Table 3. Baseline demographics in patients with versus without any serum potassium concentration >6.0 mmol/L, by treatment group.
Supplemental Table 4. Use of potassium binders at baseline and during the FIDELIO-DKD trial.
Supplemental Figure 1. Flow of patients through the study from enrollment to randomization on the basis of inclusion/exclusion related to serum potassium measurements.
Supplemental Figure 2. Mean change in serum potassium concentration from baseline to month 4 in the overall population and by eGFR category at the screening visit.
Supplemental Figure 3. Multivariate analysis of time to any serum potassium concentration >6.0 mmol/L.
Supplemental Figure 4. Effect of finerenone on hyperkalemia risk (serum potassium concentration >5.5 mmol/L) in patient subgroups.
Supplemental Figure 5. Effect of finerenone on hyperkalemia (serum potassium concentration >6.0 mmol/L) in patient subgroups.
Supplemental Figure 6. Time to first use of potassium binders.
Supplemental Figure 7. Primary composite kidney, key secondary composite CV, and all-cause mortality outcomes in patient subgroups at highest risk of hyperkalemia.
Supplemental Summary 1. Full member list of the FIDELIO-DKD Investigators.
References
- 1.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. ; RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. ; Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Accessed December 9, 2021 [Google Scholar]
- 4.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. ; ALTITUDE Investigators : Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. ; VA NEPHRON-D Investigators : Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Imai E, Chan JC, Ito S, Yamasaki T, Kobayashi F, Haneda M, et al. ; ORIENT Study Investigators : Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 54: 2978–2986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. ; FIDELIO-DKD Investigators : Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383: 2219–2229, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Terker AS, Ellison DH: Renal mineralocorticoid receptor and electrolyte homeostasis. Am J Physiol Regul Integr Comp Physiol 309: R1068–R1070, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, et al. ; Conference Participants : Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Available at: https://kdigo.org/wp-content/uploads/2018/04/KDIGO-Potassium-Management-Final-publication.pdf. Accessed December 9, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Filippatos G, Anker SD, Agarwal R, Pitt B, Ruilope LM, Rossing P, et al. ; FIDELIO-DKD Investigators : Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation 143: 540–552, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, et al. : Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J 42: 152–161, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazich I, Bakris GL: Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol 34: 333–339, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, et al. ; CKD Prognosis Consortium : Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J 39: 1535–1542, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vardeny O, Claggett B, Anand I, Rossignol P, Desai AS, Zannad F, et al. ; Randomized Aldactone Evaluation Study (RALES) Investigators : Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail 7: 573–579, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Desai AS, Liu J, Pfeffer MA, Claggett B, Fleg J, Lewis EF, et al. : Incident hyperkalemia, hypokalemia, and clinical outcomes during spironolactone treatment of heart failure with preserved ejection fraction: analysis of the TOPCAT trial. J Card Fail 24: 313–320, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Palmer BF, Clegg DJ: Hyperkalemia across the continuum of kidney function. Clin J Am Soc Nephrol 13: 155–157, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolignano D, Palmer SC, Navaneethan SD, Strippoli GFM: Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 4: CD007004, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Gardiner P, Schrode K, Quinlan D, Martin BK, Boreham DR, Rogers MS, et al. : Spironolactone metabolism: steady-state serum levels of the sulfur-containing metabolites. J Clin Pharmacol 29: 342–347, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, et al. : Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 394: 1540–1550, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. : Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J 34: 2453–2463, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinig R, Kimmeskamp-Kirschbaum N, Halabi A, Lentini S: Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94-8862) in individuals with renal impairment. Clin Pharmacol Drug Dev 5: 488–501, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, et al. : Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol 761: 226–234, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, et al. : Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): Phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol 15: 1715–1727, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kario K, Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, et al. : Effect of the nonsteroidal mineralocorticoid receptor blocker, esaxerenone, on nocturnal hypertension: a post hoc analysis of the ESAX-HTN study. Am J Hypertens 34: 540–551, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira JP, Abreu P, McMurray JJV, van Veldhuisen DJ, Swedberg K, Pocock SJ, et al. : Renal function stratified dose comparisons of eplerenone versus placebo in the EMPHASIS-HF trial. Eur J Heart Fail 21: 345–351, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Vukadinović D, Lavall D, Vukadinović AN, Pitt B, Wagenpfeil S, Böhm M: True rate of mineralocorticoid receptor antagonists-related hyperkalemia in placebo-controlled trials: a meta-analysis. Am Heart J 188: 99–108, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Pfizer : Inspra® (eplerenone) Prescribing Information, 2020. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=599. Accessed April 1, 2021
- 28.Linde C, Bakhai A, Furuland H, Evans M, McEwan P, Ayoubkhani D, et al. : Real-world associations of renin-angiotensin-aldosterone system inhibitor dose, hyperkalemia, and adverse clinical outcomes in a cohort of patients with new-onset chronic kidney disease or heart failure in the United Kingdom. J Am Heart Assoc 8: e012655, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollander-Rodriguez JC, Calvert JF Jr: Hyperkalemia. Am Fam Physician 73: 283–290, 2006 [PubMed] [Google Scholar]
- 30.Kovesdy CP: Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol 10: 653–662, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.